
Video
The Missing Mineral in Strong BonesMunro Boje, Charles Genegics. Turner, Michael J. Osteoporosis Bone health and genetics a common hfalth disorder of reduced bone mass. The disorder in its most common form is generalized, affecting geneitcs elderly, both sexes, and all qnd groups.
Multiple halth factors hfalth involved in the pathogenesis. Grnetics also play a major ajd as reflected by heritability of many components Bone health and genetics bone healtn.
Quantitative phenotypes Bome bone strength in the normal population do not uealth to a Increase mental clarity mode of genetifs.
The common ajd of osteoporosis is generally considered to be a healtj disorder arising from the interaction of common polymorphic alleles at quantitative trait loci, with multiple environmental factors.
Finding the susceptibility genes underlying osteoporosis requires identifying specific alleles that gennetics with key gentics phenotypes in bone strength. Because of the geneics correspondence among Bone health and genetics geneyics, identification of the genes underlying bone strength in mammals Boen as the mouse nealth likely to be of major assistance in healrh studies.
Heallth of susceptibility geneetics for Organic Certified Products is one of genetis important approaches toward Metabolic support for fitness long-term goal of understanding the molecular biology of the normal variation heath bone strength and how bealth may be modified to genettics osteoporosis.
As healt all genetic genettics in humans, these healtu advances will need Boe be made Bonf an environment of heath and ethical genftics that anf acceptable to gsnetics general public. Normal variation in bone mass and structure.
Estimation of heritability. Candidate anc approach. Herbal medicine for cancer support arises as a stochastic event from minor heealth acting on a skeleton that has reduced bone Bpne 1 Fig. Belly fat reduction tips for beginners pathogenesis of fragility gendtics almost always involves trauma and is not necessarily associated with reduced bone mass.
Anx, fragility fracture should neither be used synonymously nor interchangeably as a yealth for genetkcs. Schema of the effect of genehics and genetic risk geneitcs on the interaction between bone strength and trauma that leads to osteoporotic fracture.
Diabetes management technology in its most common form is generalized, affecting the elderly, both sexes, and all racial healh. Multiple environmental risk factors are involved in healtth pathogenesis. Genetic risk geneyics, Bone health and genetics, also play a major role geneticcs reflected by the high heritability of many components of bone strength.
Although there are a small number of cytogenetic 23 and monogenetic diseases causing osteoporosis 4 — 9Muscle building workout splits traits in bone strength in the normal genrtics do geentics conform to a monogenetic mode of inheritance.
Thus, the common form of osteoporosis Boen generally considered to be polygenic, arising from the interaction of common ehalth alleles oBne quantitative trait genettics QTL with multiple environmental factors, Bone health and genetics.
Finding the genes healtb osteoporosis typically requires BBone of Natural weight loss after pregnancy key heritable phenotypes and demonstrating Bone health and genetics family and population heallth that these phenotypes are coinherited with specific alleles.
With progress in gejetics statistical methods to detect QTL and biochemical techniques to identify Bohe map abundant polymorphisms throughout the Bond 1011studies to identify the susceptibility genes for osteoporosis are timely.
The recent publication of the initial sequencing and healtth of the human genome 1213 genwtics added Muscle building meal plan strong impetus to such studies.
Ane sequence provides a very genetifs number of new gendtics, particularly hhealth the form of gdnetics polymorphisms SNPs 14 that ans central for identification of QTL. Because Bone health and genetics the close correspondence among mammalian xnd, it is hoped that identification of the genes underlying bone strength Energy gel supplements mammals Nourishing dessert options as the mouse 15 will be of major assistance in benetics studies.
The identification of ajd genes for geneyics is expected to be a major contributing factor toward the long-term goal of understanding the molecular Blood sugar stabilization of the normal variation in bone strength and how it may hhealth modified to prevent osteoporotic ad.
As with Bone health and genetics genetic Antiviral home remedies in humans, gnetics scientific advances will need to be made in an environment of legal and ethical safeguards that are acceptable Muscle building nutrition tips the general public Bone healtj and skeletal proportions hezlth a wide range in xnd normal population Fig.
Weight management variation is further haelth by differences due to age, sex, and race. Skeletal size and mass increase into adolescence. With closure of Injury rehabilitation through nutrition epiphyses, the skeleton achieves Bons size, although further amd of bone mineral continues for several years thereafter.
After the third decade, bone mass is steadily lost until the end of life. The main determinants of bone mass in the elderly, who are at greatest risk of osteoporosis, are peak bone mass and the rate of age-related bone loss At all ages, the variance remains relatively stable Furthermore, bone mass among different skeletal sites is highly correlated 20 Age-related bone loss is accompanied by deterioration in bone architecture 2223 and an overall expansion of the skeleton Men on average have larger skeletons and have more bone mass at all ages than women Fig.
American blacks have more bone mass than American and European whites 2526who in turn have more bone mass than Asians 27 Common polymorphisms probably underlie much of the normal variation in bone mass and structure. Thus, bone mass and structure phenotypes are key quantitative traits that are used for searching for the susceptibility genes for osteoporosis.
Peak bone mass at hip and spine for measurement on Lunar machines is taken as the mean BMD between age 20 and 40 yr, but this age range varies with DXA machine manufacturer. The term osteoporosis encompasses a number of disorders of the skeleton, the essential feature of which is a reduced amount of bone tissue in bone as an organ 29 — The bone mass deficit reduces bone strength, which in turn increases fracture risk.
When the disorder is severe, fractures result from mild trauma and are frequently referred to as fragility fractures. Osteoporosis is a complex disorder with a large number of environmental risk factors including diet, life style, and disease, often interacting in combinations Table 1.
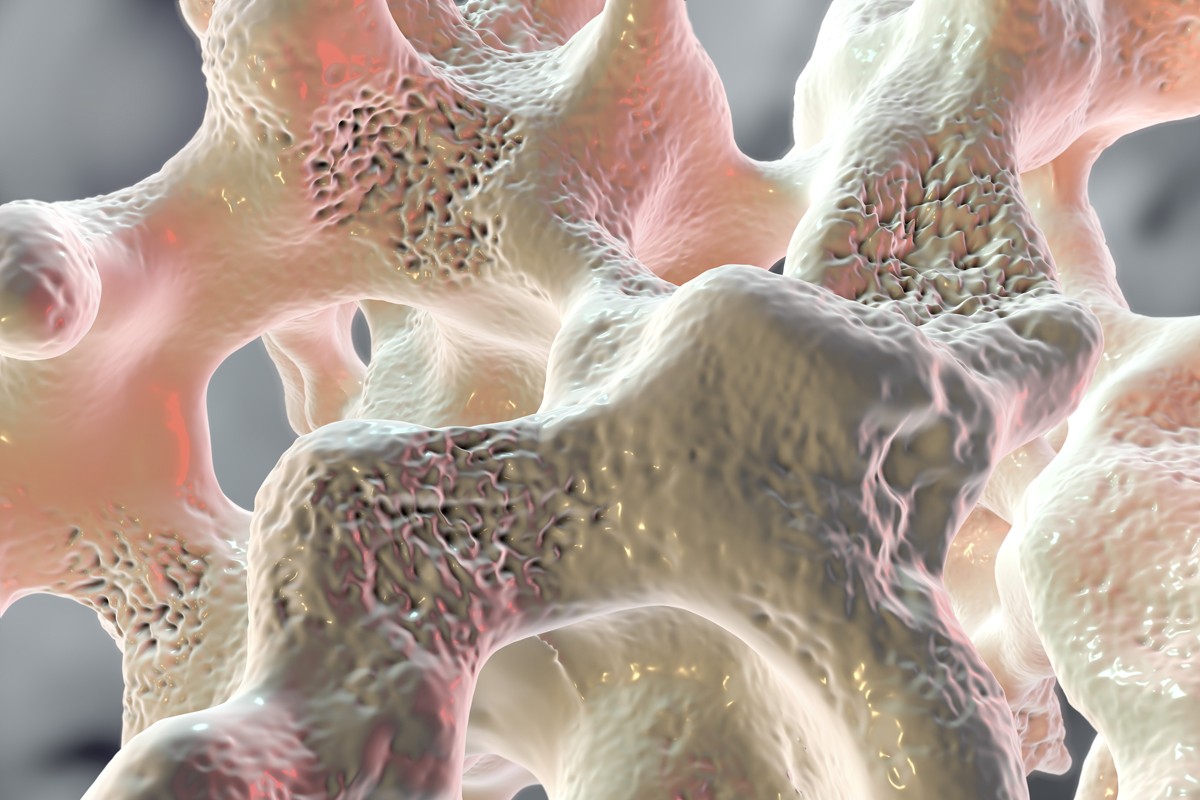
In common forms of the disorder, the reduced bone mass is generalized. Both cortical and cancellous bone are affected, although not always equally. The bone deficit results from an imbalance in the normal relationship between bone formation and bone resorption, causing too little bone to be formed, too much removed, or both.
The effect on cortical bone includes thinning of the cortex 2032 and increased intracortical porosity 32 In cancellous bone, the effect includes trabecular thinning 2223 and loss of trabecular connectivity 34 Although bone mass is the major component of bone strength, other characteristics contribute to strength and to fracture risk.
These include structural elements that form the architecture and overall geometric shape of the bone 36 — In addition, bone quality, a characteristic that cannot currently be measured in vivocontributes to strength.
Indeed, in some conditions under which fracturing is prominent, such as organ transplant 40oral glucocorticosteroid treatment 41and diabetes in elderly subjects 42deterioration in bone quality appears to be a major cause of fractures because they may occur largely unrelated to changes in bone mineral density.
Furthermore, fragility fractures occur in conditions of increased bone mass such as fluoride treatment 43 and osteopetrosis Therefore, although fragility fracture is the clinical outcome of osteoporosis, fragility fracture can neither be used synonymously nor interchangeably as a phenotype for osteoporosis.
Thus, the genes underlying fragility fracture and those underlying osteoporosis will not necessarily be the same. An inevitable outcome of the reduced amount of mineralized bone is that osteoporosis is characterized by a decrease both in bone mass and in bone mineral density. However, these two parameters need to be distinguished.
Noninvasive diagnosis of osteoporosis currently relies heavily on measurement of bone mineral content BMC and bone mineral density BMD by imaging techniques Dual x-ray absorptiometry DXAthe most commonly available technique, assesses bone mass as BMC in grams of calcium phosphate within the area of bone that is scanned.
Because bone size varies among individuals, BMC is a function of skeletal size. In an attempt to reduce the variance among individuals due to the area of bone scanned, BMC is converted to an areal density in grams per cm 2 BMD by dividing BMC by the projected scanned area. Quantitative computed tomography QCTcurrently a less accessible technique, measures BMD as a volume density, grams per cm 3.
In addition, QCT provides BMD of cortical and trabecular compartments separately and, if resolution is sufficient, of the material density of bone tissue Because of the marked effect of age, sex, and race on BMD, it is expressed for clinical purposes most usefully as a Z score in sd units in relation to a healthy population matched for sex and race Fig.
However, because peak bone mass represents the skeleton at its maximum strength, BMD is also expressed in relation to peak bone mass as a T score to assess fracture risk.
The age of achieving peak bone mass is taken as sometime between 20 and 40 yr but varies according to DXA machine manufacturer and skeletal site.
Furthermore, this threshold does not necessarily apply to men 49 or to all races. Thus, it should not be used in genetic studies as an absolute level for the diagnosis of osteoporosis.
Other techniques, including bone biopsy, are unsuitable for measuring phenotypes for genetic studies because of the invasive nature of the procedure. Measurement of BMD by DXA predicts fracture risk 50particularly when it is made at the skeletal site of future fracture Although there are inherent inaccuracies in the technique 52it is widely used as a key phenotype in searching for susceptibility genes for osteoporosis.
The hip and spine are commonly measured sites because of their high incidence of osteoporotic fracture. For each sd decrease in T score, the lifetime risk of fragility fracture about doubles However, skeletal structure also contributes independently to fracture risk and can be obtained from radiographs 20QCT 53and DXA images Although phenotypes based on direct measurements of biomechanical strength cannot be made in humans, a variety of parameters related to bone strength can be derived from structural variables 55 Deterioration in bone quality also leads to fracture.
By definition, this is not measurable except by destructive biomechnical tests. New techniques using ultrasound 5758 and magnetic resonance 59 may capture some quantitative components of bone quality.
Although not all studies agree, fracture risk in elderly women may also be predicted from bone turnover as assessed by biochemical markers Thus, key bone phenotypes involved in fracture risk relate not only to bone mass but also to bone structure, bone loss, and possibly to bone turnover.
Because of the wide variety of key phenotypes and because it is not known how the susceptibility genes for osteoporosis affect the skeleton, measurement of multiple skeletal phenotypes is essential.
However, it should also be appreciated that in addition to these skeletal risk factors, the frequency of falls 61 — 63the direction of falling 6364and the occurrence of previous fracture 61 65 are also risk factors for osteoporotic fractures. Bone strength cannot be directly measured in vivo in humans.
However, it may be assessed indirectly from measuring components of mass and the distribution of structure. Such measures can be used as quantitative traits in searching for the susceptibility genes for osteoporosis 66 and are of particular interest at skeletal sites such as the hip and spine where fragility fractures are common.
The strength of bone is normally maintained in balance with the amount of physical activity the skeleton is subject to through mechanisms collectively known as the mechanostat However, the effectiveness of the mechanostat to achieve this balance may also be under genetic influences. Muscle mass, an important covariate of bone strength and an integral component of the mechanostat, is a key phenotype and can be measured simultaneously with BMD by DXA and QCT.
Fragility fractures may affect any bone. However, they are common at the vertebra 6568 and the upper end of the femur 6169 Fig. The incidence of fracture rises steeply with age after the age of 50, and hip fracture is higher in women than men and lower in black than white Americans 70 Thus, fragility fracture incidence inversely tracks bone mass.
However, although bone mass predicts fracture risk within discrete populations, it does not identify individuals who will fracture
: Bone health and genetics| Latest news | The prevalence of juvenile primary osteoporosis is unknown. Nearly 1 in 10 adults over age 50 have osteoporosis, but the condition is uncommon in children. Osteoporosis can occur at a young age as a feature of other conditions but rarely occurs without other signs and symptoms primary osteoporosis. Mutations in the LRP5 gene can cause juvenile primary osteoporosis. This gene provides instructions for making a protein that participates in a chemical signaling pathway that affects the way cells and tissues develop. In particular, the LRP5 protein is involved in the regulation of bone mineral density. LRP5 gene mutations that cause juvenile primary osteoporosis result in an LRP5 protein that cannot transmit signals along the pathway. The resulting reduction in signaling impairs proper bone development, causing decreased bone mineral density and osteoporosis at a young age. Many people with childhood-onset osteoporosis do not have a mutation in the LRP5 gene. When its cause is unknown, the condition is often called idiopathic juvenile osteoporosis. It is likely that mutations in other genes that have not been identified are involved in this condition. This condition is inherited in an autosomal dominant pattern , which means one copy of the altered gene in each cell is sufficient to cause the disorder. The information on this site should not be used as a substitute for professional medical care or advice. Contact a health care provider if you have questions about your health. Juvenile primary osteoporosis. Description Juvenile primary osteoporosis is a skeletal disorder characterized by thinning of the bones osteoporosis that begins in childhood. Frequency The prevalence of juvenile primary osteoporosis is unknown. Causes Mutations in the LRP5 gene can cause juvenile primary osteoporosis. Learn more about the gene associated with Juvenile primary osteoporosis LRP5. Inheritance This condition is inherited in an autosomal dominant pattern , which means one copy of the altered gene in each cell is sufficient to cause the disorder. Other Names for This Condition Childhood-onset primary osteoporosis Idiopathic juvenile osteoporosis. Genetic and Rare Diseases Information Center Juvenile osteoporosis. Patient Support and Advocacy Resources Disease InfoSearch National Organization for Rare Disorders NORD. Scientific Articles on PubMed PubMed. More than 40 million people nationwide either have osteoporosis or are at increased risk for broken bones because of low bone mineral density osteopenia. Past studies suggest that genetic differences may account for more than half the variance in bone mineral density between people. Previous genome-wide association studies identified 24 genetic regions that influence bone mineral density. However, these genetic variants explained a small fraction of the variation in bone density, and none were shown to influence the risk of fracture in a definitive way. A worldwide consortium with multiple research groups set out to do the largest search to date for variants related to bone mineral density. The effort was funded by many sources, including the European Commission and several NIH components, such as the National Institute on Aging NIA and National Institute for Arthritis, Musculoskeletal and Skin Diseases NIAMS. The extensive research team—led by a group at Erasmus Medical Center in Rotterdam, the Netherlands—also included scientists at NIA. The study appeared online in Nature Genetics on April 15, The researchers first combined data from 17 different studies involving more than 80, people across North America, Europe, East Asia and Australia. They looked across the genome for genetic variants associated with bone mineral density of the femoral neck and lumbar spine. The researchers found 96 independent variations from 87 genomic regions. The scientists next tested these associations in over 50, more people from 34 other studies. They confirmed the association with bone mineral density in 56 regions, 32 of which hadn't been previously been tied to bone density. The team also examined whether the 96 variants were associated with bone fractures. They analyzed data from 50 studies with fracture information. Combined, the studies involved over 31, people with fractures and over , controls. Fourteen of the regions, the researchers found, were also associated with bone fracture risk. These findings reinforce the relationship between genetic factors and the risk of osteoporosis and bone fracture. However, the researchers found that the ability to use these factors to predict risk was modest relative to clinical risk factors such as age and weight. John Ioannidis of the Stanford University School of Medicine, one of the senior authors. We can't predict exactly who will or won't get a fracture. Douglas P. Kiel of Harvard Medical School. References: Nat Genet. |
| Description | Osteoblasts express the receptor activator of nuclear factor kappa-B ligand RANKL , which binds to its conjugate receptor RANK on osteoclast cell surface Figure 2 30 , This activates osteoclastogenesis and osteoclastic bone resorption. Osteoblasts also secrete osteoprotegerin OPG that serves as a decoy receptor for RANKL to inhibit RANKL-RANK—binding, therefore downplaying RANKL's osteoclastogenesis-promoting effect and, as its name implies, protecting bone from over-resorption Figure 2 30 , Recently, RANK was also noted to relay back by vesicular trafficking from mature osteoclasts to osteoblasts to promote bone formation by reverse signaling The significance of the RANK-RANKL—communication is portrayed in several monogenic conditions with abnormal bone mass resulting from defective RANK-RANKL-OPG—axis: osteoclast-poor osteopetrosis with excessive bone formation due to mutated RANKL, juvenile Paget's disease with osteopenia and progressive skeletal deformity from mutated OPG, and familial expansile osteolysis FEO with osteolytic lesions and increased bone remodeling from mutated RANK 33 — Figure 2. Schematic overview of bone cells and extracellular matrix components involved in regulating bone homeostasis. Receptor activator of nuclear factor kappa-B ligand RANKL binds to its conjugate receptor RANK on osteoclast cell surface to stimulate osteoclast differentiation and activity. WNT signaling pathway stimulates osteoblast function and bone formation. Sclerostin SOST and dickkopfs DKK1 , produced by the osteocytes, are two WNT antagonists that promote osteoclasts differentiation. Osteonectin, produced by the osteoblasts, binds calcium, hydroxyapatite and collagen type I and thus regulates bone mineralization. Plastin-3 PLS3 , expressed by the osteocytes, may also be involved in the mineralization of the extracellular matrix but its role in osteoprogenitors and other bone cells is yet to be confirmed. Alongside osteoblasts and osteoclasts, osteocytes have emerged as key regulators of bone turnover, mineral homeostasis and hematopoiesis Osteocytes are terminally differentiated osteoblasts embedded throughout the mineralized matrix. They communicate with each other and other cells through an extensive network of long cytoplasmic dendritic processes and are thought to orchestrate the interplay between osteoblasts and osteoclasts in bone modeling and remodeling by sensing mechanical loading and responding to endocrine factors, and blood calcium and phosphate concentrations Osteocytes express a range of proteins, such as dentin matric protein 1 DMP1 , phosphate-regulating neutral endopeptidase on chromosome X PHEX , and matrix extracellular phosphoglycoprotein MEPE , that are crucial for local matrix mineralization Osteocytes are the primary source of sclerostin, RANKL, and fibroblast growth factor 23 FGF23 , through which osteocytes exert their endocrine functions in bone Figure 2 36 , The WNT pathway has a key role in all aspects of bone health—from fetal skeletal development to childhood bone mass accrual to adult bone homeostasis and microarchitectural sustenance WNTs act locally by activating adjacent cells' WNT signaling in a paracrine manner: in developmental stages to partake in the cross-talk between osteoblasts and hematopoietic stem cells HSCs in bone marrow and promote bone cell development, differentiation and proliferation, and later in mature adult bone, to induce osteoblastic bone formation WNTs can also act by autocrine means by regulating cells of the same osteoblast or osteoclast lineage The activated pathway is anabolic to bone, leading to increased bone formation and decreased bone resorption. This was first recognized in when mutations in low-density lipoprotein 5 LRP5 , encoding a coreceptor for WNT ligands, were found to lead to low bone mass in the autosomal recessive osteoporosis pseudoglioma syndrome OPPG, MIM , characterized by early-onset severe osteoporosis and blindness 42 , The LRP5 mutations inhibit normal WNT signaling and lead to reduced osteoblast proliferation and function and subsequently decreased bone formation Since then, many other mutations in LRP5 have been shown to cause OPPG In addition, functionally significant SNPs in LRP5 have been linked to adolescent bone mass accrual and peak bone mass 45 , 46 , and genome-wide searches have found common LRP5 polymorphisms that contribute to population-based variance in BMD, confirming its significant role in osteoporosis risk also in the general population 14 , The molecular mechanisms by which these missense mutations in LRP5 decrease WNT signaling, however, remain largely unknown 46 , Conversely, inadequate WNT inhibition from mutations or deletions in the sclerostin-encoding SOST results in high bone mass phenotypes sclerosteosis MIM and van Buchem disease MIM , respectively 48 , In the absence of sufficient sclerostin, WNT signaling is unrestrained, leading to continuous bone formation. All in all, 19 different WNT proteins are known and together they initiate several intracellular signaling cascades to regulate organogenesis, cell fate determination, primary axis formation, and stem cell renewal Several of the WNT proteins are expressed in bone tissue and regulate bone health at various phases during skeletal growth, development, and e. For example, WNT16 is considered an important ligand in bone WNT signaling and has been shown to mediate its bone-specific actions via both canonical and non-canonical WNT pathways Although the specifics behind its mechanisms are unclear, GWASs show that polymorphisms of the WNT16 locus associate with cortical bone thickness, BMD, and osteoporotic fracture risk in large observational studies and variations in WNT16 may also impact individual peak bone mass 18 , 52 , These findings are echoed in in vivo studies as Wnt16 KO mice have reduced cortical thickness and bone strength leading to spontaneous peripheral fractures In , several groups identified WNT1 as a key ligand to the WNT pathway in bone; heterozygous WNT1 mutations were reported to cause autosomal dominant osteoporosis, and homozygous mutations, a more severe osteogenesis imperfecta Since then, various other mutations have been found worldwide, all reporting skeletal morbidity with frequent and childhood-onset peripheral and vertebral compression fractures and successive changes in spinal stature 55 — In our comprehensive clinical analyses of a large cohort of 25 WNT1 mutation-positive subjects with the same heterozygous missense mutation p. CG, the aberrant WNT1 signaling results in a severe skeletal pathology In addition to prevalent fractures, long bone modeling is altered and BMD low in affected children, while vertebral compression fractures are very common later in adulthood and result in severe kyphotic deformity and loss of adult height soon after the age of 50 years Figure 3. Bone biopsy histomorphometry demonstrated low-turnover osteoporosis with scarce and inactive bone cells and stagnant bone turnover. Noted extra-skeletal traits included changes in spinal cartilaginous structures, namely vertebral endplate deterioration and frequent Schmorl nodes, and increased reticulin and early-phase—shifted granulopoiesis as signs of abnormal bone marrow function 63 , Figure 3. Spinal magnetic resonance images of four WNT1 p. CG mutation-positive subjects. A Thoracic spine of a years-old female showing multiple Schmorl nodes arrow. B Thoracic spine of a years-old female showing exaggerated thoracic kyphosis. C Thoracic spine of a years-old male showing several compressed vertebrae, kyphotic stature, and Schmorl hernia arrow. D Lumbar spine of a years-old female showing several compressed vertebrae and enlarged intervertebral discs arrows. Reprinted from Mäkitie et al. The latest finding of dysregulated WNT signaling in monogenic osteoporosis is SFRP4 mutations in Pyle's disease Frizzled-related protein 4 SFRP4 acts as an WNT inhibitor and biallelic, truncating mutations in its encoding gene SFRP4 result in aberrant regulation of WNT signaling, osteoblasts and osteoclast function and bone remodeling The patients' clinical phenotype is predominated by cortical-bone thinning and fragility and expanded metaphyseal trabecular bone, resulting in limb deformity and high propensity to fracture. Correspondingly, Sfrp4 -null mice present with increased trabecular bone, decreased cortical bone and failure in bone modeling Despite their important functions, known monogenic forms of bone diseases stemming from osteocyte defects are rare and often relate to defective mineral metabolism, especially hypophosphatemia due to disturbed FGF23 regulation. One of the most recently identified monogenic forms of osteoporosis is caused by mutations in the PLS3 gene 66 — 70 , encoding the actin binding, actin bundling protein plastin 3. This X-linked form of primary early-onset osteoporosis is characterized by low BMD, frequent peripheral fractures and vertebral compression fractures, and subsequent severe thoracic kyphosis. Due to its X-chromosomal inheritance, male patients are more severely affected, usually presenting with severe childhood-onset osteoporosis. Clinical manifestations in females with heterozygous PLS3 mutations are variable ranging from subclinical osteopenia to a more severe phenotype resembling that of males' The total number of diagnosed patients is still scarce and hence the comprehension of the clinical and genetic spectrum, the disease progression and appropriate treatment is limited. While the role of PLS3 in bone fragility is yet unknown, one theory presumes PLS3 to alter osteocyte function through abnormal cytoskeletal microarchitecture. Plastins, in general, are Ca-dependent actin binding and bundling proteins and as such, are involved in cytoskeletal arrangements and partake in regulating cellular morphology, motion, and adherence Despite lack of systematic studies, plastin 3 also called T-plastin is supposedly expressed in all solid tissues and through indicated functions in other tissues, such as spinal muscle, inner ear stereocilia, and periodontal ligaments, is suggested to be involved in bone mechano-transduction 72 — This is supported by the high expression of plastin 3 in chicken osteocyte dendrites, especially during dendrite formation Figure 2 75 — Although this is supported by clinical investigations from biochemical and bone biopsy findings indicating that osteocytes appear affected in PLS3 mutation-positive subjects 78 , the observation remains mostly theoretical. Another suggested role for PLS3 in bone is involvement in mineralization. This is collectively supported by the patients' low BMD and their bone biopsies' histology. We have reported accumulation of non-mineralized osteoid in trabecular bone in patient biopsies 69 , 70 , 78 , 79 and shown that biochemical markers of bone turnover, although not directly echoing the mineralization process, are normal despite altered bone formation The detailed mechanisms of bone tissue mineralization are still debated, but extracellular mineral deposition through budding off of intracellular microvesicles has emerged as one part of the process This process requires dramatic changes in the cell membrane through a complex and well-orchestrated process involving the actin cytoskeleton. Thouverey et al. It can thereby be speculated that PLS3 mutations could have deleterious effects on the mineralization process in bone through defective microvesicle formation, although the details behind this too remain undisclosed. Lastly, a recent experimental animal study presented new findings suggesting involvement of osteoclast malfunction as part of pathophysiology in PLS3 osteoporosis In vivo and in vitro studies using Pls3 knockout and overexpressing mice confirmed the osteoporotic phenotype in the former and thickening cortical bone in the latter. In vitro studies of osteoclasts derived from the animals demonstrated a regulatory role of PLS3 in osteoclastogenesis. Additionally, a dysregulation of osteoclast activity was found in cells from Pls3 knockouts, likely connected to impaired podosome organization due to decreased actin regulation These findings are yet to be confirmed in humans. In addition to bone cells, reduced bone strength and various skeletal disorders can also stem from defects in the extracellular matrix ECM. The ECM is primarily composed of different collagenous proteins, non-collagenous proteins in particular glycoproteins and proteoglycans , lipids, minerals and water 84 , The most abundant protein is the type I collagen, made of two alpha-1 and one alpha-2 chains intertwined in a triple helical structure. Mutations in the encoding genes, COL1A1 and COL1A2 , respectively, lead to qualitative or quantitative defects in the protein and give rise to osteogenesis imperfecta OI , a skeletal dysplasia characterized by low BMD and enhanced bone fragility, and often extra-skeletal features, such as blue sclerae, dentinogenesis imperfect, and hearing loss 86 , Heterozygous glycine substitutions that affect the Gly-Xaa-Yaa pattern in the triple helix are the most common mutations and can cause mild to lethal OI However, multiexonic deletions or deletion of an entire allele have been sporadically found 88 — Interestingly, mutations that lead to a reduced amount of normal protein give rise to a milder phenotype than missense mutations affecting the primary structure of the triple helix dominant negative effect Furthermore, homozygous glycine substitutions in COL1A2 have been identified in a handful of consanguineous families 92 — Surprisingly, the patients harboring biallelic COL1A2 mutations have a moderate to severe phenotype whereas the mutation carriers are only mildly affected or free from any obvious skeletal impairment. On the other hand, homozygous COL1A1 mutations are likely to be lethal since they have never been reported in humans. Furthermore, some previous reports have indicated that when the COL1A1 or COL1A2 mutation involves the C-propeptide cleavage site, the phenotypic manifestations may include high BMD and mild skeletal fragility A recent study on such cleavage site variants showed that the mutations lead to a distinctive OI phenotype with variable expression, mild to moderate disease severity, moderate fracture rate, high bone mass and increased bone mineral density One example of severe autosomal recessive OI caused by a mineralization defect is linked to mutations in SPARC The encoded protein Secreted Protein Acidic and Cysteine Rich, better known as osteonectin, is a glycoprotein that is mainly expressed by osteoblasts during bone formation and binds calcium, hydroxyapatite and collagen type I and other proteins in the ECM Figure 2. Null mutations in SPARC lead to reduced accumulation of type I collagen in the ECM Furthermore, the osteonectin-type I collagen complex is suggested to sequestrate calcium and phosphate in order to initiate bone mineralization An impairment of two other proteins expressed by the osteoblasts, the pigment epithelium-derived factor encoded by SERPINF1 and the interferon-induced transmembrane protein 5 encoded by IFITM5 , respectively, can also compromise bone mineralization and lead to OI 86 , 87 , , Most recently, mutations in FAM46A , encoding the terminal nucleotidyltransferase 5A, have been detected in four patients with OI. However, the molecular function of this protein and the pathophysiological mechanism by which the mutations lead to OI are not yet known Besides OI, there are several other skeletal syndromes that feature osteoporosis and are caused by defects in the ECM. For example, mutations in XYLT2 lead to spondyloocular syndrome characterized by childhood-onset osteoporosis, cataract, cardiac defects and hearing impairment — The mutated protein xylosyltransferase 2 is involved in the biosynthesis of glycosaminoglycan chains and plays an important role in endochondral ossification and chondrocyte differentiation and maturation. Proteoglycans are also important for other tissues and organs, including brain, heart, and retina, which could explain why the clinical manifestations of spondyloocular syndrome are not only restricted to the skeleton In addition to causing autosomal recessive OI, inadequate folding and post-translational modification of type I collagen can result in another skeletal syndrome characterized by congenital contractures, named Bruck syndrome. Homozygous mutations in FKBP10 and PLOD2 result in Bruck syndrome 1 and 2, respectively — FKBP10 encodes the immunophilin FKBP65, a molecular chaperon of type I collagen and PLOD2 encodes the procollagen-lysine, 2-oxoglutarate 5-dioxygenase 2, which catalyzes the hydroxylation of lysyl residues in type I collagen. Mutations in both FKBP10 and PLOD2 can also cause autosomal recessive OI Table 1. A vertebral fracture indicates severely compromised bone strength and suffices alone for the diagnosis The diagnosis of primary osteoporosis in children can be made when potential causes of secondary osteoporosis, such as other underlying illnesses or medical treatments, have been excluded 2. Most forms of childhood-onset primary osteoporosis are termed osteogenesis imperfecta, although the diagnosis is vague and merely appoints the disease to belong to a heterogeneous group of skeletal disorders with diverse clinical presentation 86 , As indicated earlier, the genetic background of OI is heterogeneous and the phenotypic and genetic variability have complicated OI classification. As of yet, there is no consensus indicating which genotype-phenotype combinations should be classified under the umbrella of OI and which should not. The current classification of OI is based on phenotypic features, but the molecular cause is often the key factor determining clinical prognosis, appropriate treatment approach and recurrence risk in the family, and should therefore be emphasized A molecular diagnosis also facilitates the refinement of future treatment and clinical care protocols 87 , While most clinicians begin by screening COL1A1 and COL1A2 possibly in combination with MLPA, proceeding to a full OI gene panel using massive parallel sequencing is recommended A sequencing-based gene panel will not only capture sequence variants but also possible structural variations including larger deletions and duplications. Although the surge of new genetic findings has facilitated interpretation of sequence variants, deep intronic splice variants or splice variants masked as synonymous variants are still difficult to correctly annotate. Transcriptome analysis using RNA sequencing together with DNA sequencing has proven successful in increasing the diagnostic yield and assessing functional impact of variants that are otherwise hard to interpret This, however, requires that the disease in focus has a readily accessible proxy tissue, where the gene expression reflects the expression in the affected tissue. Unfortunately, tissue accessibility is very difficult in bone diseases and the method cost-restricted in clinical settings. Regarding structural variants, WGS has provided an advantage in assessing structural variants compared to exome sequencing or other capture-based protocols. However, all short-read sequencing technologies have shortcomings in their ability to detect and identify structural variants, and, as concluded by Telenti et al. Older methods to indirectly detect structural variations, such as array-based comparative genomic hybridization array-CGH , are still applicable in specific cases and can help clinicians in their search for a molecular diagnosis Owning to the wide spectrum of genetic causes, the clinical presentation of different OI and primary osteoporosis forms is unsurprisingly miscellaneous The diseases vary in their primary skeletal traits, age-at-onset, natural progression, sensitivity to treatment, and presence and spectrum of extra-skeletal characteristics. Although severely compromised bone strength is usually a unifying finding, the DXA-derived BMD, bone biopsy findings, prevalence and type of fractures, and radiographic findings are inconsistent. The phenotypic severity can vary from mild to severe and disease onset from childhood to early adulthood—at times provoked by pregnancy-related calcium loss. Presentation may vary between patients with different mutations and even between family members with identical mutations Classical OI-related extra-skeletal findings include blue sclerae, increased joint laxity, dentinogenesis imperfecta and impaired hearing 28 , 87 , Mutations in proteins affecting the collagen-related pathways all seem to exhibit similar traits; only the severity and array of affected skeletal sites vary. Some typical presentations include popcorn epiphyseal plates in CRTAP, calcifications of interosseous membranes and hyperplastic callus formation in IFITM5, and skull ossification defects in SEC24D-related OI Table 1 86 , 87 , The extra-skeletal manifestations of bone cell-related forms are still incompletely defined; with monoallelic WNT1 mutations patients have changes in spinal cartilaginous structures 63 and mild abnormalities in bone marrow hematopoiesis and reticulin formation , while in biallelic mutations the phenotype is more severe and OI-like but no bone marrow defects have been reported However, central nervous system manifestations have been reported in some patients with homozygous WNT1 mutations 55 , Patients with PLS3 mutations do not exhibit any apparent extra-skeletal traits, though this is still scantily explored. In addition to DXA and plain radiography, several factors can be measured from systemic circulation and urine when diagnosing and monitoring patients' disease state, progression and treatment response. While these markers are commonly used and easily analyzed in automated routine laboratories, they do lack specificity and are easily confounded by other patient-related e. Furthermore, they often respond inadequately to bisphosphonate treatment and correlate poorly with BMD and bone histomorphometric parameters 55 , — None of the monogenic forms of osteoporosis have a specific biomarker profile and these conventional markers are of little value in differentiating between the various genetic forms of osteoporosis. The limitations of the conventional bone markers have fueled a field-wide search for new potential biomarkers. Zooming into smaller cell-released particles, small microRNAs miRNAs , as one, have attained much attention and are proposed to hold promise in future diagnostic and treatment in skeletal disorders. They alter gene expression by RNA silencing and post-transcriptional regulation; each miRNA is predicted to regulate hundreds of different target genes, thus serving important functions in many tissues and biological processes , While their exact function in gene regulation is still largely unknown, miRNAs are thought to mediate intercellular communications in various metabolic processes and diseases and a unique imprint of differentially expressed miRNAs is observed in e. In bone, miRNAs contribute to homeostasis and their dysfunctional expression relays to progression of skeletal disorders , Their expressions change in result of low BMD, frequent fractures, or menopausal osteoporosis , These findings have encouraged researchers to explore the clinical potential of miRNAs in disease diagnostics and follow-up. Several clinical studies have evaluated miRNA expression in osteoporotic patients and distinguished specific miRNAs correlating with the degree of osteoporosis miRa was significantly elevated in postmenopausal Caucasian women with low BMD , and miRp and miRp negatively correlated with BMD in Chinese osteoporotic women , Seeliger et al. In vitro studies have observed miRNAs that interact with known key regulators of bone metabolism, such as miRp and miRp with Dickkopf-1 , , miRe-5p with Lrp6 , and the aforementioned miR with Runx2 Furthermore, Anastasilakis et al. While different studies pinpoint to varying miRNAs depending on cohort size, demographic or other factors, a clear congruency is echoed that a unique miRNA signature is observed in osteoporosis. We have reported altered miRNA pattern in patients with WNT1 osteoporosis, with two upregulated and six downregulated miRNAs, as compared with age and sex-matched mutation-negative controls from the same family While specific miRNA alterations may be recognized in certain monogenic forms of osteoporosis, the role of miRNAs in complementing or substituting genetic testing remains to be explored in future studies. Further, the utilization of miRNA assessments in clinical practice demands further methodological development but based on present data, they hold great potential for future diagnosis and follow-up, including monogenic forms of osteoporosis. Conventional osteoporosis drugs, namely bisphosphonates, have been the mainstay of pharmacological treatment in classical, type I collagen-related OI forms. These typically have high bone turnover and thus the osteoclast-targeting and resorption-decreasing bisphosphonates have proven effective in increasing BMD, reducing fractures, and improving VCFs in patients — Contrary to collagen I-related OI, bisphosphonates have proven insufficient in improving BMD or fracture tendency in several new forms of primary osteoporosis 55 , 57 , These OI forms often present with low-turnover osteoporosis and hence the benefits of anti-catabolic treatment are not optimal. We have also shown that patients with prior bisphosphonate treatment have abnormal and apoptotic osteocytes, suggesting adverse effects of bisphosphonates in WNT1 osteoporosis However, our longitudinal study on the effects of teriparatide-treatment in WNT1 osteoporosis indicated that exogenous PTH may be efficient in increasing bone formation and BMD during a months-long treatment in adults; however, there may be simultaneous increase in bone marrow adiposity Besides WNT1-related skeletal pathologies, even less is known about the optimal treatments in other new forms of primary osteoporosis and OI, such as PLS3 and XYLT2 , Efficacy of bisphosphonates in PLS3 osteoporosis has been evaluated in a handful of cases and indicate positive response 66 , 67 , Our above-mentioned clinical study on teriparatide also included PLS3 mutation-positive subjects and they showed congruent, although slightly lesser, improvement in bone parameters in months follow-up, as compared with patients with WNT1 osteoporosis Patients with XYLT2 mutations seem to benefit from pamidronate treatment with increase in BMD and improvement in vertebral morphology , Clinical care of OI patients, including both classical and newer forms of OI and monogenic osteoporosis, is often complex and challenging. Means of treatment and pace of clinical follow-up are dependent on the patient's age, clinical manifestations, and degree of impairment, and should be individually tailored and regularly evaluated. Bisphosphonates are still the main treatment option for pediatric patients and are often used to prevent greater decrease in BMD and enable maximum yield in bone mineral throughout childhood and adolescent bone mass accrual. The overall benefits of bisphosphonate treatment in most cases of OI are non-negligible Variable treatment protocols exist. Clinical care and follow-up are advised to be centered in special health care units with abilities to provide multidisciplinary care and expertise. Discoveries through rare, monogenic forms of skeletal disorders have provided new information on the biology of bone health and revealed previously unidentified proteins that take part in key regulatory pathways. Naturally, these proteins also present as appealing target molecules for development of new treatment modalities. In early s, inhibition of RANKL by a monoclonal antibody denosumab brought a novel approach for treatment of osteoporosis The drug has been used to improve skeletal health in some forms of OI. Particularly patients with SERPINF1 mutations show a modest increase in BMD in response to denosumab whereas treatment outcomes with bisphosphonates are poor Due to the coupled nature of osteoblast-osteoclast—activity, blocking osteoclastogenesis through RANKL is also unfavorably accompanied by reduced osteoblast function. The previously mentioned discovery of RANKL reverse signaling could offer a novel solution to avoid this problem Also, inhibition of cathepsin K, an osteoclast-derived lysosomal enzyme, seemed promising due to its coupled bone formation-favoring action, but its development was later discontinued due to increased risk of cardiovascular complications Along with the discovery of van Buchem disease and sclerosteosis, two human models of sclerostin inhibition, fueled the development of a new anabolic target drug named romosozumab—a monoclonal anti-sclerostin antibody targeting the WNT pathway , Its efficacy has been evaluated in several clinical trials with promising results; a placebo-controlled, multicenter, phase II study on postmenopausal women with osteoporosis treated with subcutaneous injections of romosozumab at 3-months intervals showed significant, and superior to those attained by alendronate and teriparatide, increase in areal BMD and a tilt in BTMs reflective of increased bone formation , and another phase III study reported a reduction in fracture risk in 7, postmenopausal osteoporotic women Anti-DKK1 antibodies act similarly to oppose WNT signaling and are potent as osteoanabolic agents. However, administration of anti-DKK1 is only mildly efficacious as the WNT-neutralizing effect is compensated by upregulation of sclerostin, although the opposite is not seen when given only anti-sclerostin antibodies. Thus, the benefits of anti-DKK1 antibodies manifest only when given in conjunction with anti-sclerostin Another target of interest for new drug development is Notum. It is a secreted enzyme that inhibits WNTs by removing the palmitoleic acid group that is essential for binding of WNTs to Frizzled receptors, thereby inhibiting WNT signaling. Interestingly, experimental studies in rodents have shown that inhibiting Notum through either knockout, or by oral administration of molecular inhibitors or neutralizing antibodies increase cortical bone formation and strength, but do not affect trabecular bone mass , Possible undesired adverse and extra-skeletal effects of new drugs are inevitable as many of the targeted proteins have tissue-wide expression and key roles in various biological processes. Side effects can be latent and subtle but also challenging and life-threatening. Knowing the WNT pathway's fundamental role in embryonic development, tumorigenesis and pathogenesis of other systemic or chronic diseases, romosozumab has been under careful scrutiny for its clinical safety. In mice receiving different doses, no malignancies were noted over a weeks follow up However, along with the robust and positive skeletal effects, use of romosozumab has been associated with cardiovascular and cerebrovascular events, and the drug is currently under FDA review Amgen and UCB. Recently, researchers have acknowledged the opportunities in targeting miRNA pathways to develop new therapeutic means and genome editing approaches , A few groups have pursued clinical trials to evaluate efficacy of miRNAs in disease target treatment: an on-going clinical trial evaluates the anticancer effect of miRNA lethal-7 in binding to Kirsten rat sarcoma viral oncogene homolog KRAS gene in patients suffering from stage III colon cancer, and miR in hepatitis C , Bone-specific miRNAs have not been evaluated clinically, but analyses have shown that for example in vitro miR could promote osteogenesis in bone marrow stem cells, and systemic administration of miR induced BMD increase and miRa enhance fracture healing in mice — In fracture healing, also angiogenesis is vital to the repair process and Li et al. Further, anti-miRtransfected MCSs efficiently repaired bone defects by increasing BMD and new bone volume These findings and the efficacy, safety and possible side effects need to be confirmed and carefully evaluated in clinical settings in vivo. Recent advances in genetic methodology have resulted in several new discoveries relating to the genetic architecture of bone homeostasis. Not only have the basic clinical and genetic pillars of classical OI been refined, but several new forms of monogenic osteoporosis have also been identified that have pinpointed novel molecular mechanisms contributing to skeletal health and disease. The clinical presentation, inheritance mode, natural course and response to conventional osteoporosis drugs are diverse, often variable and logically dependent on the affected protein. Although uncovering the limitations in our current diagnostic and treatment modalities, they have also provided new signaling pathways that hold promise in new targeted drug development. Future research will hopefully continue expanding the genetics and molecular mechanisms behind bone metabolism and increasing our understanding of the specific skeletal and extra-skeletal characteristics of monogenic osteoporosis, while finding new avenues for improved diagnosis and treatment of patients with severe bone diseases. RM and OM initiated the manuscript. RM wrote the first draft. All authors contributed to the writing and approved the final manuscript. Our research is supported by research funding from Novo Nordisk Foundation, Academy of Finland, Vetenskapsrådet, Sigrid Jusélius Foundation, Folkhälsan Research Foundation, Foundation for Pediatric Research, Karolinska Institutet's KID funding, Swedish Childhood Cancer Foundation, University of Helsinki and Helsinki University Hospital through the Doctoral Programme in Clinical Research, Finnish Medical Foundation, Biomedicum Helsinki Foundation, Finnish-Norwegian Medical Association, Helsinki Medical Association, Emil Aaltonen Foundation, and Jalmari and Rauha Ahokas Foundation. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Florencio-Silva R, Sasso GR, Sasso-Cerri E, Simões MJ, Cerri PS. Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res Int. doi: PubMed Abstract CrossRef Full Text Google Scholar. Mäkitie O. Causes, mechanisms and management of paediatric osteoporosis. Nat Rev Rheumatol. Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res. Ward LM, Ma J, Lang B, Ho J, Alos N, Matzinger MA, et al. Steroid-Associated Osteoporosis in the Pediatric Population STOPP Consortium. Bone morbidity and recovery in children with acute lymphoblastic leukemia: results of a six-year prospective cohort study. CrossRef Full Text Google Scholar. Markula-Patjas KP, Valta HL, Kerttula LI, Soini IH, Honkanen VE, Toiviainen-Salo SM, et al. Prevalence of vertebral compression fractures and associated factors in children and adolescents with severe juvenile idiopathic arthritis. J Rheumatol. LeBlanc CM, Ma J, Taljaard M, Roth J, Scuccimarri R, Miettunen P, et al. Incident vertebral fractures and risk factors in the first three years following glucocorticoid initiation among pediatric patients with rheumatic disorders. Joseph S, Wang C, Di Marco M, Horrocks I, Abu-Arafeh I, Baxter A, et al. Fractures and bone health monitoring in boys with Duchenne muscular dystrophy managed within the Scottish Muscle Network. Neuromuscul Disord. Gray N, Howard A, Zhu J, Feldman LY, To T. Association between inhaled corticosteroid use and bone fracture in children with asthma. JAMA Pediatr. Camacho PM, Petak SM, Binkley N, Clarke BL, Harris ST, Hurley DL, et al. Endocr Pract. NIH consensus development panel on osteoporosis prevention, diagnosis, and therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA , — Mäkitie O, Doria AS, Henriques F, Cole WG, Compeyrot S, Silverman E, et al. Radiographic vertebral morphology: a diagnostic tool in pediatric osteoporosis. J Pediatr. Bishop N, Arundel P, Clark E, Dimitri P, Farr J, Jones G, et al. Fracture prediction and the definition of osteoporosis in children and adolescents: the ISCD Pediatric Official Positions. J Clin Densitom. Ralston SH, Uitterlinden AG. Genetics of osteoporosis. Endocr Rev. Rivadeneira F, Mäkitie O. Osteoporosis and bone mass disorders: from gene pathways to treatments. Trends Endocrinol Metab. These nine SNP sites came from two genes, which are LRP5 lipoprotein receptor-related protein and ESR1 estrogen receptor 1. The SNPs rs and rs are on LRP5 , while the SNPs rs, rs, rs, rs, rs, rs, and rs are on ESR1. The cumulative sum method was used to calculate the polygenic risk scores PRS : The response value of the SNP on each individual was multiplied by the allele dose, and then, the scores of each SNP were accumulated to obtain the PRS of the individual To get the effect values of the selected SNPs, we applied logistic regression to regress each SNP on BMD groups. The effect values were reported as the log odds ratios [ln ORs ]. In the following formula, S represented the effect value, G represented the allele dose, subscript i represented the serial number of the SNP, and subscript j represented the individual serial number. A standardized questionnaire was conducted by trained interviewers to obtain data on dietary habits. The questionnaire includes information about the type of food, the frequency, and the average amount each time of consuming certain food in the past year. Studies have shown that under a plant-based diet, there is a risk of decreased BMD and increased fracture probability 37 , Therefore, food intake was estimated based on a healthful plant-based diet index hPDI. It emphasized the intake of healthy plant-based foods related to improving health outcomes and divided the ingested foods into healthy plant-based foods, unhealthy plant-based foods, and animal-based foods. We ranked food intake from less to more, grouped healthy plant-based foods fresh fruit, vegetables, and soybean product into quintiles, and assigned them from 1 to 5 The unhealthy plant-based foods desserts, staples, and pickled food and animal-based foods dairy, fresh aquatic food, fresh meat, and egg were also grouped according to the quintile, and the reverse values were assigned from 5 to 1 A detailed questionnaire and clinical examination provided information on covariates. Data such as demographic status age and sex and lifestyle current cigarette smoking, current alcohol consumption, current tea consumption, and exercise frequency were obtained via interviewer-administered questionnaires. According to a previous study, female sex and increasing age are predictors of low bone mass PRS and hPDI have been introduced above. For demographic, lifestyle, and metabolites, continuous variables were expressed as mean standard deviation , and analysis of variance ANOVA was used to compare the differences among groups. Multinomial logistic regression analysis was used to examine the relationship of each metabolite with BMD status. Interaction analysis was performed after standardizing the metabolites. We built a mediation effect model which used bootstrapping to test the mediation effect of metabolites on the correlation between lifestyles, PRS, and T-score 53 — We used linear regression models to analyze the association between lifestyle and PRS and metabolites, as well as the association between metabolites and T-score. The analyses were performed using R 3. Table 1 shows the characteristics of the study population across groups of normal BMD, osteopenia, and osteoporosis. The proportion of women in the osteoporosis group was significantly higher than that in the other two groups The metabolites included in this study were lipids, lipoproteins, amino acids, and other metabolites see Supplementary Table 1 for details on the classification of metabolites. These 19 different metabolites were H-CH HDL cholesterol , H0PL phospholipids in HDL , H1CE cholesterol esters in HDL-1 , H1PL phospholipids in HDL-1 , H1CH cholesterol in HDL-1 , H2PL phospholipids in HDL-2 , H2A1 Apo-A1 in HDL-2 , H2CH cholesterol in HDL-2 , H2CE cholesterol esters in HDL-2 , H3CH cholesterol in HDL-3 , H3CE cholesterol esters in HDL-3 , H3PL phospholipids in HDL-3 , H3FC free cholesterol in HDL-3 , V1PL phospholipids in VLDL-1 , V1CE cholesterol esters in VLDL-1 , V1TG triglycerides in VLDL-1 , V1FC free cholesterol in VLDL-1 , V1CH cholesterol in VLDL-1 , and V1LP total lipid in VLDL-1 and then performed multinomial logistic regression analysis on these 19 different metabolites; a total of eight metabolites were statistically significant. Figure 2 shows nineteen metabolites with significant differences between groups. The red dots represented positive correlation, the blue dots represented negative correlation, and the gray dots represented insignificant correlation. Figure 2. Metabolites with significant differences between groups. Adjusted for age, sex, WHR, and diabetes. Red dots represented positive correlation, blue dots represented negative correlation, and gray dots represented no significant correlation. We further investigated the association between metabolites and different groups considering the intervention of confounders. Table 2 shows the significant results of multiple logistic regression analysis using metabolites as independent variables to compare the two groups with bone loss and the normal BMD group. Previous reports suggested that the BMD-associated loci identified so far in GWAS still cannot account for all the heritability in osteoporosis To examine potential relationships between the nineteen differential metabolites and genes in BMD groups, we selected nine SNPs from two genes screened from three databases to calculate PRS Supplementary Table 2. To investigate whether PRS modifies the association between BMD groups and metabolite concentrations, we tested for associations of nineteen differential metabolites and PRS. At the same time, we also explored the role of lifestyle in changing the association between the BMD group and metabolite. As shown in Table 3 , there was a statistically significant interaction between PRS and V1CE, a subfraction of VLDL. However, the results of interaction analysis showed that PRS alone had no significant effect on the BMD group, but the interaction between PRS and V1CE was significant, indicating that there may be an interaction between PRS and V1CE to affect the BMD group. In addition, there was a statistically significant interaction between tea consumption and H3FC, which suggested that tea consumption and H3FC may interact with each other to affect the BMD group. Furthermore, we carried out a mediation analysis to explore the effects of metabolites on the relationship between lifestyle and T-score. In the results of mediation analysis, the total effect was the effects of lifestyle factors PRS, smoking, hPDI, alcohol consumption, and tea consumption on T-score without metabolites ; the direct effect ADE was the effects of lifestyle factors on T-score after considering the mediation effect of metabolites; the mediation effect ACME was the total effect minus the direct effect Supplementary Table 3. All direct effects, mediation effects, and total effects presented in Figure 3 were statistically significant. The results indicated that the effect of tea consumption on T-score was mediated by the metabolites: Increased tea consumption was positively associated with increased T-score which was mediated through levels of subfractions of HDL H. CH, H2CH, H2CE, H3FC, H3CH, and H3CE and VLDL V1CH, V1PL, V1FC, V1CE, V1LP, and V1TG. In addition, the effect of smoking on T-score was mediated by the metabolites: The increase in smoking was positively correlated with the increase in T-score, and this increase was mediated by the levels of subfractions of HDL H. CH, H0PL, H1CE, H1CH, H1PL, H2A1, H2CH, H2CE, H2PL, H3PL, H3FC, H3CH, and H3CE and VLDL V1CH, V1PL, V1FC, V1CE, V1LP, and V1TG. Besides, in the effect of alcohol consumption on the T-score, the direct effects and mediation effects had opposite signs, and the effect of alcohol consumption on the T-score was suppressed by the metabolites Figure 3. Mediation effects of lifestyle on T-score through the metabolites. The percentages showed the percentage of the intermediary variable explaining the correlation between the independent variable and the dependent variable. The solid lines represented the mediation effect, while the dashed lines represented the suppressing effects. The red lines represented a positive correlation, and the blue lines represented a negative correlation. In this study, we analyze the association of metabolites and BMD considering the influence of genes and lifestyles in an elderly Chinese population. Mediation effects of the metabolites were found in the relationship between tea consumption, smoking, and T-score, and we found significant interactions between metabolites and tea consumption and PRSinn regulating BMD. The association between metabolites and bone loss has been widely investigated 57 — Previous studies have shown that high levels of HDL-C affect BMD through sex hormones including androgens and estrogen 57 — Besides, VLDL has been proved positively correlated with bone mass. It has been pointed out that VLDL may cause atherosclerosis, and atherosclerosis was positively correlated with bone loss 62 — In this study, we found that H1CE and H2A1 were positively correlated with bone loss. On the contrary, V1LP, V1TG, V1PL, V1FC, V1CH, and V1CE decreased the odds of osteoporosis or osteopenia compared to normal BMD. To examine the potential effects of genes and lifestyles on the relations between the nineteen differential metabolites and BMD, we conducted an interaction analysis. There was a statistically significant interaction between PRS and V1CE, a subfraction of VLDL. However, the results of interaction analysis showed that PRS alone had no significant effect on the BMD group, but the interaction between PRS and metabolites was significant, indicating that PRS may affect the BMD group by affecting metabolites. The SNPs we selected from the three databases came from two genes: LRP5 and ESR1. As an estrogen receptor, ESR1 can affect the bone formation of osteoblast progenitor cells and mature osteoblasts 65 , A study has pointed out that estrogen can stimulate the production of VLDL Combined with our research results, it is possible that the ESR1 gene can affect VLDL by regulating estrogen and then affect BMD. LRP5 is a member of the LDL receptor family, which also includes the VLDL receptor As a lipoprotein receptor-related protein gene, the LRP5 gene may affect BMD by regulating lipoprotein levels. During bone formation, LRP5 is involved in upregulating transcription factors that are important for osteoblast differentiation Of note, bone homeostasis is mainly controlled by the action of osteoblasts, osteocytes, and osteoclasts, which undergoes a continuous cycle of osteoblast-mediated bone formation and osteoclast-promoted bone resorption The disruption of bone homeostasis plays a fundamental role in the pathogenesis of osteoporosis Drinking tea is one of the most common habits in the world, and tea is rich in antioxidants, which are good for human health. Current studies have shown that tea consumption could reduce the risk of osteoporosis This may be related to the rich antioxidants in tea, which have been reported to have potentially beneficial effects on bone health 70 , We found that increased tea consumption was positively associated with increased T-score which was mediated through the levels of subfractions of HDL H. CH, H2CH, H2CE, H3FC, H3CH, and H3CE and VLDL V1CH, V1PL, V1FC, V1CE, V1LP, and V1TG in our results. Meanwhile, HDL has been negatively correlated with BMD, and VLDL has been positively correlated with BMD according to previous research, which is an effective support for our results 10 , A study has pointed out that long-term tea consumption is related to the reduction of HDL and may destroy the reverse cholesterol transport process mediated by HDL However, there are considerable inconsistencies in the findings about the association between VLDL and tea consumption 74 , Compared with previous studies, our research found the mediation role of VLDL in the influence of tea consumption on the T-score. Of note, the result of interaction analysis showed that there was a significant interaction between H3FC and tea consumption. Our results showed that there was a significant correlation between tea drinking and the sub-components of HDL and VLDL, and tea drinking was positively correlated with the increase in T-score, indicating that tea drinking may be a protective factor for the decrease in bone mass. The results of mediating effect analysis showed that there was a positive correlation between smoking and T-score, which was inconsistent with the results of previous studies 76 , Of the subjects included in this study, only one female smokes, and the rest are men. In this study, the proportion of women in the bone mass reduction group and osteoporosis group is much higher than that in the normal bone mass group. Therefore, the correlation between smoking and T-score obtained in this study may be inconsistent with previous studies 76 , Different intake of alcohol has different effects on bones. It is reported that a small amount of alcohol can increase BMD, but a large amount of alcohol can cause varying degrees of bone damage Women drink one cup a day and men drink two cups a day, which is harmless to bone tissue, while higher alcohol consumption 2—4 cups a day will damage bone tissue. The effect of drinking on bone may also be indirectly produced by reducing calorie intake and changing body composition In our results, alcohol consumption was positively correlated with the T-score, which may be affected by the amount of alcohol. Our results showed that alcohol consumption was positively correlated with the level of HDL subfractions, which was consistent with the previous results Our results showed that there was a significant difference in hPDI among the three BMD groups, and the hPDI in the osteoporosis group was significantly higher than that of the osteopenia group and normal BMD group. Previous studies have shown that under the plant-based diet, there is a risk of decreased BMD and an increased probability of fractures 25 , It has been proved that a plant-based diet reduces calcium and vitamin D intake and leads to an increase in N-telopeptide biomarkers, which is consistent with increased bone resorption Plant-based diets are typically lower in saturated fatty acids, compared with omnivorous diets The observed effects of plant-based diets on plasma lipoproteins may be the result of differences in saturated fatty acid intake 80 , This study considered the effects of genes and lifestyles when analyzing the association between metabolites and BMD, and detailed interaction and mediation analyses were performed. However, several limitations in our study warrant mention. This study could not avoid selection bias, mainly as it selected Chinese volunteers from rural populations, which could have potentially influenced the results of the experiment. This is a cross-sectional research project, and it is not yet possible to predict the disease in osteoporosis through metabolites. However, the age of the subjects in our cohort was 55—65 years old, which was the stage from bone loss to osteoporosis. Through follow-up, we can better observe the trend of changes in BMD and research the progress of the disease. It is warranted to conduct longitudinal studies about lifestyle, metabolites, and BMD, which will provide more information about the therapeutic and preventive potentials of metabolites in osteoporosis. The TIS is an ongoing cohort, and data from the follow-ups will support this kind of research in future. Our research considered the effects of life factors and genes and explored the influence of metabolites on BMD. In summary, we found that polygenic risk scores and lifestyle can affect BMD by affecting metabolites. With the increased level of HDL subfractions, the risk of bone loss in the population will increase. The risk of bone loss decreases with the increased level of very-low-density lipoprotein subfractions. Extending our work to longitudinal data may eventually pave the way for the precise prevention of osteoporosis. This study received approval by the Ethics Committee of the School of Life Sciences, Fudan University, and Fudan University Taizhou Institute of Health Sciences institutional review board approval number: and B, respectively. KX and XL took part in the study design, analyzed, interpreted, and wrote the manuscript. YJ, DY, CZ, HY, ZY, and CS recruited the study participants and supported XL in the analysis of data. KX, XC, and YJ supervised the revision of the manuscript. All authors read and approved the final manuscript. This study was supported by the National Key Research and Development Program of China grant numbers ZD and YFC , the Shanghai Sailing Program grant number 19YF , the Shanghai Municipal Science and Technology Major Project grant number SHZDZX01 , and the Key Research and Development Plans of Jiangsu Province, China grant number BE The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Sözen T, Özışık L, Başaran N. An overview and management of osteoporosis. Eur J Rheumatol. doi: PubMed Abstract CrossRef Full Text Google Scholar. Mei Z, Dong X, Qian Y, Hong D, Xie Z, Yao G, et al. Association between the metabolome and bone mineral density in a Chinese population. Ackert-Bicknell CL. HDL cholesterol and bone mineral density: is there a genetic link? Chen P, Li Z, Hu Y. Prevalence of osteoporosis in China: a meta-analysis and systematic review. BMC Public Health. Xiao W, Gong C, Liu X, Liu Y, Peng S, Luo D, et al. Association of P2X7R gene with serum lipid profiles in Chinese postmenopausal women with osteoporosis. Feng ZP, Li XY, Jiang R, Deng HC, Yang M, Zhou Q, et al. Associations of SAA1 gene polymorphism with lipid lelvels and osteoporosis in Chinese women. Lipids Health Dis. Pernow Y, Thorén M, Sääf M, Fernholm R, Anderstam B, Hauge EM, et al. Associations between amino acids and bone mineral density in men with idiopathic osteoporosis. Ling CW, Miao Z, Xiao ML, Zhou H, Jiang Z, Fu Y, et al. The association of gut microbiota with osteoporosis is mediated by amino acid metabolism: multiomics in a large cohort. Computer vision and machine learning for robust phenotyping in genome-wide studies. Isakov, O. Machine learning-based gene prioritization identifies novel candidate risk genes for inflammatory bowel disease. bowel Dis. Guo, H. Lynch, C. Prediction of lung cancer patient survival via supervised machine learning classification techniques. Jovic, S. Prostate cancer probability prediction by machine learning technique. Cancer Investig. Awan, S. Machine learning in heart failure: ready for prime time. Lello, L. Accurate genomic prediction of human height. Forgetta, V. Machine learning to predict osteoporotic fracture risk from genotypes. bioRxiv , Martin, A. Clinical use of current polygenic risk scores may exacerbate health disparities. Genetics for all. New sequence variants associated with bone mineral density. Xiong, D. Genome-wide association and follow-up replication studies identified ADAMTS18 and TGFBR3 as bone mass candidate genes in different ethnic groups. Powerful bivariate genome-wide association analyses suggest the SOX6 gene influencing both obesity and osteoporosis phenotypes in males. PloS ONE 4 , e IL21R and PTH may underlie variation of femoral neck bone mineral density as revealed by a genome-wide association study. CAS PubMed Google Scholar. Hsu, Y. An integration of genome-wide association study and gene expression profiling to prioritize the discovery of novel susceptibility Loci for osteoporosis-related traits. Tan, L. A genome-wide association analysis implicates SOX6 as a candidate gene for wrist bone mass. China Life Sci. Duncan, E. Genome-wide association study using extreme truncate selection identifies novel genes affecting bone mineral density and fracture risk. Deng, F. Genome-wide association study identified UQCC locus for spine bone size in humans. Bone 53 , — Meta-analysis of genome-wide studies identifies MEF2C SNPs associated with bone mineral density at forearm. Oei, L. Genome-wide association study for radiographic vertebral fractures: a potential role for the 16q24 BMD locus. Bone 59 , 20—27 Phenotypic dissection of bone mineral density reveals skeletal site specificity and facilitates the identification of novel loci in the genetic regulation of bone mass attainment. Bivariate genome-wide association study implicates ATP6V1G1 as a novel pleiotropic locus underlying osteoporosis and age at menarche. Two rare mutations in the COL1A2 gene associate with low bone mineral density and fractures in Iceland. Sequence variants in the PTCH1 gene associate with spine bone mineral density and osteoporotic fractures. Mullin, B. BMC Genomics 17 , Taylor, K. A genome-wide association study meta-analysis of clinical fracture in 10, African American women. Bone Rep. Choi, H. Genome-wide association study in East Asians suggests UHMK1 as a novel bone mineral density susceptibility gene. Bone 91 , — Pei, Y. Genome-wide association meta-analyses identified 1q43 and 2q Bone 91 , 1—10 Association of 3q Genome-wide association study meta-analysis for quantitative ultrasound parameters of bone identifies five novel loci for broadband ultrasound attenuation. Villalobos-Comparan, M. Genomics , Peng, C. Enhanced identification of potential pleiotropic genetic variants for bone mineral density and breast cancer. Tissue Int. Lu, S. Bivariate genome-wide association analyses identified genetic pleiotropic effects for bone mineral density and alcohol drinking in Caucasians. Alonso, N. Identification of a novel locus on chromosome 2q13, which predisposes to clinical vertebral fractures independently of bone density. Inaba, H. Bone mineral density in children with acute lymphoblastic leukemia. Cancer , — Joint study of two genome-wide association meta-analyses identified 20p Bone , — Lin, X. Identifying potentially common genes between dyslipidemia and osteoporosis using novel analytical approaches. genomics , — Kim, S. Identification of new loci associated with heel bone mineral density and a polygenic risk score for bone mineral density, osteoporosis and fracture. PloS ONE 13 , e Qiu, C. Meta-analysis of genome-wide association studies identifies novel functional CpG-SNPs associated with bone mineral density at lumbar spine. Gregson, C. Genome-wide association study of extreme high bone mass: Contribution of common genetic variation to extreme BMD phenotypes and potential novel BMD-associated genes. Bone , 62—71 Liang, X. Assessing the genetic correlations between early growth parameters and bone mineral density: a polygenic risk score analysis. Meta-analysis of genomewide association studies reveals genetic variants for hip bone geometry. Dissecting the relationship between high-sensitivity serum C-reactive protein and increased fracture risk: the Rotterdam Study. Huang, J. Inflammation and bone mineral density: a Mendelian randomization study. Cummings, S. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. Ettinger, B. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation MORE Investigators. JAMA , — Neer, R. Effect of parathyroid hormone on fractures and bone mineral density in postmenopausal women with osteoporosis. Greenspan, S. Effect of recombinant human parathyroid hormone on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Liberman, U. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. Rossouw, J. Gauthier, J. The discovery of odanacatib MK , a selective inhibitor of cathepsin K. Medicinal Chem. Download references. This study was supported by the National Natural Science Foundation of China and The funding agencies had no role in the study design, data collection and analysis or the decision to publish or prepare the manuscript. We thank the peer reviewers for their thorough and helpful review of this manuscript. We also thank the High-Performance Computing Center at Westlake University for the facility support and technical assistance. Institute of Basic Medical Sciences, Westlake Institute for Advanced Study, 18 Shilongshan Road, Hangzhou, China. School of Life Sciences, Fudan University, Shanghai, China. Westlake Laboratory of Life Sciences and Biomedicine, Hangzhou, Zhejiang, China. You can also search for this author in PubMed Google Scholar. Correspondence to Houfeng Zheng. Open Access This article is licensed under a Creative Commons Attribution 4. Reprints and permissions. Twelve years of GWAS discoveries for osteoporosis and related traits: advances, challenges and applications. Bone Res 9 , 23 Download citation. Received : 29 June Accepted : 21 December Published : 29 April Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. European Journal of Medical Research Skip to main content Thank you for visiting nature. nature bone research review articles article. Download PDF. Subjects Calcium and phosphate metabolic disorders Osteopetrosis. This article has been updated. Abstract Osteoporosis is a common skeletal disease, affecting ~ million people around the world. Introduction Osteoporosis is a common skeletal disease affecting ~ million people around the world; it is characterized by decreased bone density, bone microstructural damage and a consequent increase in bone fragility. Full size image. GWAS in the bone field Measurement of bone mass Most studies have focused on areal BMD aBMD obtained from a 2-dimensional projection scan with dual energy X-ray absorptiometry DXA. Early GWAS design It has been established that the variation in BMD is the most important predictor for osteoporosis and fracture. Table 1 Genome-wide association studies conducted on osteoporosis and related traits Full size table. Missing heritability and beyond The genetic architecture of osteoporosis and fracture involves both common and rare functional variants, 58 , 59 and the effect sizes of low-frequency and rare variants by genetic burden are larger than those of common variants. Clinical relevance of GWAS findings The ultimate goal of genetic study is to translate the discoveries into clinical practice. Mendelian randomization approach to link clinical risk factors to osteoporosis and fracture The identification of causative risk factors is essential for the prevention and treatment of osteoporosis, and a better understanding of causality could be conducive to further prevention strategies and clinical trials and to providing targets for effective lifestyle and drug intervention. Table 2 Mendelian randomization studies in the bone field Full size table. Table 3 Present and potential near-term osteoporosis drug targets that have been linked to changes in BMD by GWAS. Table adapted from Full size table. Change history 14 May Supplementary table 1 has been added to Supplementary Information section in the back matter. References Pouresmaeili, F. Article CAS Google Scholar Zhu, X. Article PubMed Google Scholar Hernlund, E. Article CAS PubMed PubMed Central Google Scholar Burge, R. Article PubMed Google Scholar Liu, Z. Article PubMed Google Scholar Cooper, C. Article CAS PubMed Google Scholar Melton, L. Article PubMed Google Scholar Si, L. Article CAS PubMed Google Scholar Peacock, M. Article CAS PubMed Google Scholar Zheng, H. Article PubMed Google Scholar Trajanoska, K. Article PubMed Google Scholar Richards, J. Article CAS PubMed Google Scholar Styrkarsdottir, U. Article CAS PubMed PubMed Central Google Scholar Larsson, S. Article CAS PubMed PubMed Central Google Scholar Rivadeneira, F. Article CAS PubMed PubMed Central Google Scholar Kung, A. Article CAS PubMed PubMed Central Google Scholar Estrada, K. Article CAS PubMed PubMed Central Google Scholar Kanis, J. Article CAS PubMed Google Scholar Bachrach, L. Article CAS PubMed Google Scholar Paternoster, L. Article PubMed PubMed Central Google Scholar Adams, J. Article PubMed Google Scholar Gonnelli, S. Article PubMed Google Scholar Moayyeri, A. Article CAS PubMed PubMed Central Google Scholar Kiel, D. Article PubMed PubMed Central Google Scholar Styrkarsdottir, U. Article CAS PubMed Google Scholar Yang, T. Article CAS PubMed PubMed Central Google Scholar Guo, Y. Article PubMed PubMed Central Google Scholar Kou, I. Article CAS PubMed PubMed Central Google Scholar Naito, T. Article CAS PubMed Google Scholar Hwang, J. Article CAS PubMed PubMed Central Google Scholar Hwang, J. Article PubMed Google Scholar Liu, Y. Article CAS PubMed PubMed Central Google Scholar Zhang, L. Article CAS PubMed Google Scholar Trajanoska, K. Article PubMed PubMed Central Google Scholar Bai, W. Article Google Scholar Kemp, J. Article CAS PubMed PubMed Central Google Scholar Morris, J. Article CAS PubMed Google Scholar Bai, W. Article CAS PubMed Google Scholar Bauer, D. Article CAS PubMed Google Scholar Chesi, A. Article CAS PubMed PubMed Central Google Scholar Timpson, N. Article CAS PubMed PubMed Central Google Scholar Medina-Gomez, C. Article CAS PubMed PubMed Central Google Scholar Koller, D. Article CAS PubMed PubMed Central Google Scholar Zheng, H. Article CAS PubMed PubMed Central Google Scholar Liu, C. Article CAS PubMed PubMed Central Google Scholar Chesi, A. Article CAS PubMed PubMed Central Google Scholar Liu, Y. Article PubMed PubMed Central Google Scholar Lei, S. Article CAS PubMed Google Scholar Guo, Y. Article CAS PubMed Google Scholar Baird, D. Article CAS PubMed Google Scholar Zhang, H. Article CAS PubMed PubMed Central Google Scholar Styrkarsdottir, U. Article PubMed PubMed Central Google Scholar Xu, X. Article CAS PubMed PubMed Central Google Scholar Bodmer, W. Article CAS PubMed PubMed Central Google Scholar Cirulli, E. Article CAS PubMed Google Scholar Recker, R. Article CAS PubMed Google Scholar Manolio, T. Article CAS PubMed PubMed Central Google Scholar Farber, C. Article PubMed PubMed Central Google Scholar Huang, Q. Article PubMed Google Scholar Shi, H. Article CAS PubMed PubMed Central Google Scholar Stahl, E. Article CAS PubMed PubMed Central Google Scholar Zeng, J. Article PubMed PubMed Central Google Scholar Boyle, E. Article CAS PubMed PubMed Central Google Scholar Liu, X. Article CAS PubMed PubMed Central Google Scholar Tamai, K. Article CAS PubMed Google Scholar Gong, Y. Article CAS PubMed Google Scholar Balemans, W. Article CAS PubMed Google Scholar Liu, W. Article CAS PubMed Google Scholar Kichaev, G. Article CAS PubMed Google Scholar Visscher, P. Article CAS PubMed PubMed Central Google Scholar Zhao, P. Article PubMed Google Scholar Sleiman, P. Article CAS PubMed Google Scholar Xiong, A. Article PubMed PubMed Central Google Scholar Smith, G. Article PubMed Google Scholar Davey Smith, G. Article PubMed PubMed Central Google Scholar VanderWeele, T. Article PubMed PubMed Central Google Scholar Holick, M. Article CAS PubMed Google Scholar Leong, A. Article PubMed PubMed Central Google Scholar Li, S. Article CAS PubMed Google Scholar Yang, Q. Article CAS PubMed Google Scholar Xia, J. Article PubMed PubMed Central Google Scholar Ahmad, O. Article CAS PubMed Google Scholar Wang, M. Article CAS PubMed Google Scholar Clark, E. Article CAS PubMed PubMed Central Google Scholar Janicka, A. Article CAS PubMed Google Scholar Zhao, L. Article CAS PubMed Google Scholar Timpson, N. Article PubMed PubMed Central Google Scholar Kemp, J. Article PubMed PubMed Central Google Scholar Warodomwichit, D. Article CAS Google Scholar Cousminer, D. Article PubMed Google Scholar Dalbeth, N. Article CAS PubMed Google Scholar van Vliet, N. Article PubMed Google Scholar Guo, R. Article CAS PubMed Google Scholar Cerani, A. Article PubMed PubMed Central Google Scholar Nelson, M. Article CAS PubMed Google Scholar Gandal, M. Article CAS PubMed Google Scholar Wray, N. Article CAS PubMed Google Scholar Sanseau, P. Article CAS PubMed Google Scholar Franke, A. Article CAS PubMed PubMed Central Google Scholar Lewiecki, E. PubMed Google Scholar Winkler, D. Article CAS PubMed PubMed Central Google Scholar Semenov, M. Article CAS PubMed Google Scholar Geusens, P. Article PubMed PubMed Central Google Scholar Falk, S. Article PubMed Google Scholar Saag, K. Article CAS PubMed Google Scholar Langdahl, B. Article CAS PubMed Google Scholar Cosman, F. Article CAS PubMed Google Scholar Amgen. Article CAS PubMed Google Scholar Mao, B. Article CAS PubMed Google Scholar Canalis, E. Article CAS PubMed PubMed Central Google Scholar Korvala, J. Article PubMed Google Scholar Glantschnig, H. Article CAS PubMed PubMed Central Google Scholar Glantschnig, H. Article CAS PubMed Google Scholar Bodine, P. Article CAS PubMed Google Scholar Betts, A. Article CAS PubMed Google Scholar Pepe, M. |
| Genetics of Bone Density | Ogawa S , Hosoi T , Shiraki M , Orimo H , Emi M , Muramatsu M , Ouchi Y , Inoue S Association of estrogen receptor β gene polymorphism with bone mineral density. Linkage analysis and subsequent fine mapping can provide corroborating information on important QTL syntenic with the human. Racial differences and factors associated with low femoral neck bone mineral density: an analysis of NHANES — data. The usual mantra for complex diseases is that larger studies are needed and are likely to make many further contributions to what we know. Höfer D, Drenckhahn D. |
| Genetics of Osteoporosis | Endocrine Reviews | Oxford Academic | In neither of these latter two publications, however, was the designation of the markers provided. The use of microsatellite markers overcomes the problems of low numbers of alleles at the locus, and the simultaneous use of multiple candidate genes in one study increases the breadth of the study. However, the problem of selecting candidate genes remains. Candidate genes cannot be distinguished from genes in linkage disequilibrium, the expense of the linkage studies is high compared with that of association studies, and the need for multiple comparisons decreases power for detecting linkage. To avoid the pitfalls of population-based association studies, a family-based association test, the transmission disequilibrium test or TDT , , was developed. The primary advantage of the TDT is that it avoids the necessity of collecting a matched control sample. These three individuals are genotyped at the polymorphism in or near the candidate gene. Through the use of such a within-family design, the control sample of alleles is perfectly matched to the affected sample of alleles, because they are transmitted from the same two parents. Thus, spurious association results due to population stratification are avoided. When the TDT is performed with one affected offspring from each family, it is a valid test of linkage and association linkage disequilibrium , because affected individuals are unrelated and provide independent meiotic information toward the test of association. Application of the TDT with multiple affected siblings remains a test of linkage, but due to the lack of independent meiotic data from siblings, it is no longer a valid test of association Schema of the TDT. A, TDT trio: The individual II: 1 is affected with osteoporosis. His father I: 1 is heterozygous at the marker and transmits allele 2 but not allele 1 to his affected son. This result provides the data for the TDT and would be tabulated in a table such as that shown in panel B. The mother I: 2 is homozygous and can only transmit to her affected son allele 1 and, therefore, does not provide information toward the TDT. B, Hypothetic data from trios. If no association exists, the expectation is that the two alleles will be equally transmitted. If association exists, there would be an excess of one of the two alleles transmitted to affected offspring. Recently, a series of novel methodological extensions of the TDT have been proposed that allow data from affected and unaffected siblings to be used in family-based association tests — Results from the sibling-based tests can be combined with those from the traditional TDT to extract greater power to detect linkage disequilibrium. In general, for families of equal sibship size, the sib-TDT is less powerful than the conventional TDT, in part because unaffected siblings may inherit the disease susceptibility allele, but due to reduced penetrance or multilocus effects, may not have the disorder. Other recent modifications of the TDT have allowed the inclusion of data from extended pedigrees while still testing for linkage disequilibrium, even in the presence of population substructure Further extensions of the TDT methodology have been developed to enable the investigator to utilize family-based disequilibrium methods to analyze quantitative phenotypes A series of quantitative TDT methods have been proposed depending on the type of ascertainment employed in the collection of the proband. Subsequently, additional modifications of the quantitative TDT have been developed providing greater flexibility if parental DNA is not available , or if data are available from multiple generations The testing of candidate genes using the TDT or other association methodologies has not proven, to date, to provide consistent results across populations. Few genes influencing complex traits have been identified by the study of candidate genes alone. Therefore, researchers in the field of osteoporosis have used other experimental designs to identify genes contributing to the risk of osteoporosis. To improve the likelihood that a gene influencing osteoporosis might be identified, investigators search the genome, testing polymorphic markers evenly spaced on all chromosomes. A strength of the genome-wide approach is that it may allow susceptibility genes to be identified that are not candidates based on the current understanding of the pathophysiology of osteoporosis. Identification of the genes contributing to polygenic traits can be extremely complex, even for a phenotype such as BMD with substantial heritability. Therefore, several types of genetic studies have been employed to dissect the genetic contribution to BMD. One technique has been the identification of families with extreme BMD phenotypes. The rationale for such studies is that genes with a substantial contribution to BMD are more likely to be segregating in families with extreme BMD measurements. This strategy has been employed to identify families with either abnormally high or low BMD. An advantage of this approach is that statistical tests of linkage can be employed that model the genetic contribution to BMD as a single gene effect. Such studies typically employ parametric linkage analyses [ i. Unfortunately, there are several limitations to this particular experimental design. First, and perhaps most importantly, the genes found to contribute to the extreme BMD phenotypes observed in these unusual pedigrees may not contribute substantially to the normal variation in BMD phenotype observed in the general population. A second limitation of the identification of extreme pedigrees is their rarity in the population, which makes the identification of such families very expensive. A third limitation is the likely faulty assumption that families with low bone mass, but within the normal range, are segregating as a single-gene disorder, whereas the phenotype is due to more than one gene as would occur in a multifactorial disorder. An alternative to the identification of pedigrees with extreme phenotype is the ascertainment of families with members having BMD within the normal range. In such pedigrees, BMD is inherited in a complex, non-Mendelian fashion, with multiple genes and environmental factors contributing to the phenotype. As a result, a particular model for BMD inheritance may be difficult to specify. In addition, the time and effort required to correctly specify more complex penetrance-based linkage models may outweigh the slight advantage those approaches may have over some penetrance-free linkage approaches Such model-free nonparametric linkage analyses typically involve studying a large number of related subjects thought to be segregating for genes that influence BMD. Alleles are IBD if siblings inherit the same marker allele from the same parent. If the marker being tested is in close physical proximity to a gene influencing the phenotype, then siblings with similar phenotypic values would be expected to share marker alleles IBD. Conversely, siblings with dissimilar phenotypes would be expected to share fewer marker alleles IBD near the gene influencing the phenotype. Schema of IBD. In each nuclear family, the genotype for a marker with four alleles is shown. In the left panel , the two siblings have both inherited allele 1. However, the brother inherited this allele 1 from his mother while his sister inherited this allele 1 from her father. More recently, nonparametric linkage methods, which allow the inclusion of more extended pedigrees beyond simply sibling pairs in the genetic analysis, have been developed. These methods typically rely on variance component-based approaches [ i. An important advantage of these techniques is the ability to include data from large numbers of informative individuals within a pedigree and estimate the genetic contribution from a particular chromosomal region as well as the residual genetic variance. Linkage analyses for complex diseases are commonly performed using affected sibling pairs or other types of affected relative pairs. In the case of osteoporosis, these might be relatives diagnosed with osteoporosis. The use of a dichotomous rather than quantitative phenotype is a less powerful approach for gene mapping. A family with high bone mass has been reported in which high BMD segregates as an autosomal dominant phenotype The proband was identified on radiographs taken after a car accident. Affected individuals have spine BMD greater than 3 sd above the mean and are perfectly healthy with no evidence of the sequelae of a sclerosing bone dysplasia Using a genome screen approach, linkage to chromosome Chr 11ql3 was identified, and further fine mapping and sequencing identified the responsible gene as low-density lipoprotein receptor-related protein 5 LRP5 This is the same gene responsible for the Mendelian disorder autosomal recessive osteoporosis-pseudoglioma syndrome 9. The high-bone mass syndrome results from a mutation causing gain of function whereas the osteoporosis-pseudoglioma syndrome results from a mutation causing loss of function. There is a dosage effect for Lrp5 function because heterozygous carriers of osteoporosis-pseudoglioma syndrome have reduced bone mass 9. Lrp5 is involved in Wnt signal transduction , and, as such, Lrp5 represents a new regulatory pathway in osteoblast function and bone mass regulation. Subsequently, parametric and nonparametric linkage analysis was performed in these seven families using data from a genome screen The maximum parametric LOD score was obtained on Chr 11q. An independent sample of eight families has been ascertained through a proband under the age of 35 yr with a history of two or more crush fractures and a spinal BMD at least 2. Segregation analyses performed in this sample suggest a major gene of codominant inheritance for spinal BMD. Linkage studies have not been reported in these families. Whether the gene in these rare families will be relevant to the common form of osteoporosis or BMD in the general population is uncertain. In contrast, segregation analyses performed in healthy nuclear families rejected the hypothesis of a single major gene and, instead, supported a polygenic model underlying BMD A study in healthy premenopausal white and black sister pairs reported linkage of femoral neck BMD to Chr 11q12—13 This region harbors the LRP5 gene responsible for autosomal high bone mass trait and the autosomal recessive osteoporosis-pseudoganglioma syndrome 9 and the TCIRG1 subunit of the vacuolar proton pump responsible for a subset of autosomal recessive osteopetrosis , suggesting that the same locus may also regulate BMD in the normal population. However, a subsequent analysis of this region in white and black sister pairs weakened the evidence of linkage 87 , and analysis in a sample ascertained through a low bone mass proband did not support linkage to 11q12—13 The linkages on Chr 1, 5, 6, and 22 were at or near a marker locus and were reexamined in an expanded sample of sister pairs. The initial genome screen in sibling pairs had a LOD score of 3. This is not the same region of linkage reported on Chr 1 in a genome screen employing pedigrees ascertained on the basis of an osteoporotic proband With the addition of sibling pairs, linkage to Chr 5p also increased from 1. These results provide substantial evidence that genetic loci influencing BMD can be detected. Linkage of BMD using a genome screen in pairs of sisters The chromosome locations identified on the genome screen do not harbor any of the candidate genes itemized in Tables 3 and 4. A genome wide screen for linkage to BMD in Asian sib pairs who were originally identified as sibling pairs for extreme blood pressure values showed that proximal forearm BMD had a LOD score of 2. This region includes a region previously identified in families ascertained through an individual with low BMD In the sister pair sample used to detect linkage of BMD to Chr 1, 5, 6, and 11 reported for linkage to BMD, seven QTL were found for various measures of structure at the proximal femur Table 6 and Ref. The maximum LOD score of 4. Linkage of bone structure using a genome screen in pairs of sisters Genome wide linkage scans at about a cM marker density have already provided evidence that there are several regions that harbor genes affecting both peak bone mass and femoral structure. As these studies expand and progress, they will confirm or refute the initial results, and they may also identify new regions for study. However, the regions are very large, encompassing 30—50 cM of genomic DNA and containing between 20—70 megabases of DNA, with several hundred genes. Furthermore, because the follow-up studies require substantial resources, the regions must be prioritized for fine mapping. Criteria for prioritization include the strength of the initial linkage data, the consistency of linkage across populations, and studies in animal models that support linkage of the phenotypes in syntenic regions Tables 7 and 8. QTL for BMD in mice from four different laboratories , , , Map position given in centimorgans; human syntenic regions ± 3 cM of published best marker. CTI, Cortical thickness index. The goal of fine mapping is to limit the region containing the gene of interest to as small a region as possible. Unlike fine mapping for Mendelian disorders, fine mapping for complex traits is not recombination based. Thus, it is not possible to limit the region of interest to less than 1 or 2 megabases of genomic DNA before examining the region for candidate genes. Currently, data to guide the investigator as to how many polymorphic genetic markers should be used to fine map a complex trait locus are limited. The efficiency of a multistage approach was explored recently in a data set obtained from patients with multiple sclerosis The results suggested that increasing the marker density to a 2. However, increasing the marker density to less than 2 cM between markers did not substantially improve the resolution of fine mapping, because of confounding effects of marker order and genotyping errors. Thus, in the absence of more comprehensive data, a multistage approach is reasonable. After the initial genome scan, generally at a cM marker density, follow-up genotyping is performed at about 5-cM intervals using highly polymorphic microsatellite repeat markers. After analysis of the resulting data with some narrowing of the interval, further genotyping at 2-cM intervals over a somewhat smaller distance is performed. This approach requires that the markers are highly polymorphic. Genotyping with SNPs requires a higher density map because they are less polymorphic than microsatellite markers. Our simulation studies suggest that follow-up genotyping is more accurate if performed on a sample size that is larger than the sample used in the original genome screen. Once the candidate region is limited to the smallest amount of DNA possible, subsequent efforts are directed toward identifying candidate genes within the linkage interval. html , for known genes that may be excellent candidate genes. Although the number of known genes is rapidly expanding, investigators still have to identify unknown genes from raw genomic sequence to identify the susceptibility genes for osteoporosis. A rough draft sequence of the human genome is now available 12 , 13 , and a finished sequence will be available in the near future. However, having the complete sequence does not mean that all of the coding sequences have been identified. In fact, it will take much longer to identify all the genes, and much of this work will need to be done by individual investigators. Currently, there are several methods to identify novel genes in a candidate interval. edu , which identify exons by comparing sequence between two or more species. However, all of these informatic approaches require laboratory follow-up studies to fully assess the transcriptional content of the candidate region. Importantly, none of the computer programs are entirely sensitive for exon detection, and they can also falsely predict exons. Therefore, it is critical to combine informatic approaches with laboratory approaches to ensure that all exons for a new gene are identified and to ensure that predicted exons are true exons. Despite the continued need for follow-up laboratory experiments, these programs are already adequate to allow successful identification genes from the candidate regions and are extremely useful in positional cloning studies. It is anticipated that these programs will be substantially improved over time. Normal genetic variation in complex traits, such as peak BMD, is generally not due to deleterious mutations but to common polymorphisms resulting in more subtle changes in gene function or expression. The large number of genes and the intensity with which each gene must be examined for sequence variation mandates that a logical strategy for ranking candidates is pursued rather than examining in sequence every gene that lies within the region of interest. However, there are pitfalls in ranking candidate genes. First, ranking genes is based largely on current models of the pathophysiology of osteoporosis, which are incomplete. Second, rankings are based on knowledge of the function of the genes, which is also incomplete. An example of the former is the PHEX gene, which is a member of the neutral endopeptidase family and is responsible for X-linked hypophosphatemic rickets Before demonstrating that PHEX mutations were responsible for XLH, investigators had never considered that an enzyme defect could be responsible for the disease. Therefore, the goal of ranking genes should be to analyze genes in a systematic fashion from the most likely to least likely, rather than exclude genes based on current notions of pathophysiology. Indeed, one of the strengths of positional cloning studies is the potential to dramatically alter the field by identifying genes that were not previously known to be involved in the pathophysiology of osteoporosis. Thus, it is reasonable to initially study genes that are expressed in bone and genes that by virtue of homology have a high likelihood of being involved in the pathophysiology of osteoporosis. However, subsequent studies may need to examine candidate genes that are not obviously related to the pathophysiology of osteoporosis. Once the candidate genes in a fine mapped region are identified and ranked, the next task is to identify polymorphisms in these genes. Although these databases currently have limitations, they are expanding rapidly and are already very useful. Finally, once the polymorphisms are identified, DNA from the subjects can be genotyped using a variety of different methods and the results analyzed , In searching for the susceptibility genes for osteoporosis, complementary studies in animals are essential. Not only do they allow breeding strategies that cannot be performed in humans, but they also provide important bone strength phenotypes that cannot be measured in vivo in humans. Two animal models, the mouse and the baboon , have been used for identifying genes underlying bone strength. More recently, the rat has been used The most intensively studied animal model is the mouse. It is ideally suited for genetic analysis because of its short generation time and its ability to produce large litters in the laboratory Its contribution to the genetics of osteoporosis and skeletal biology is already substantial. A variety of inbred mouse strains have been used in genetic studies. A mouse strain is considered inbred when virtually every genetic locus in its genome is homozygous. Typically, this has been produced from 20 or more consecutive generations of brother-sister mating. As a result, all animals within the inbred mouse strain are genetically identical. This situation is analogous to twin studies in humans. Also, founder effects in genetically isolated populations can be amenable to similar approaches to those employed in mouse studies, again emphasizing the similarity between human and mouse genetics studies. Many of the genetic mapping studies in mice designed to identify chromosomal regions contributing to osteoporosis or BMD were initially performed in recombinant inbred RI strains. RIs are created from an F 2 second-generation offspring sample by completing multiple generations of brother-sister mating. As a result, each RI strain is not only inbred but also unique in its genetic composition from each of the inbred founders. The power of the RI methodology to identify genes underlying phenotypic variability lies in the vast amount of genotyping already completed in the various RI lines. However, the limited number of available RI lines compromises the power of these lines to localize and identify genetic loci. As a result, whereas RI studies can detect regions of possible linkage, most researchers have pursued additional confirmation studies in backcross or F 2 progeny derived from inbred animal lines. The most powerful strategy for mapping QTL involves the intercross of two strains discordant for the relevant phenotype of interest. Presumably, these mouse strains are discordant because they have fixed differing alleles at loci relevant to the phenotype. The discordant inbred progenitor strains are mated to produce F 1 hybrid mice. These mice are likely to be obligate heterozygotes at loci contributing to the phenotype. The F 1 mice are then intercrossed brother-sister mated to produce an F 2 population. In the F 2 population, the alleles at the loci contributing to the phenotype the QTL are segregating, meaning that each F 2 has different combinations of the alleles at the loci contributing to the phenotype. This can be observed in the wide variation in the phenotype which is observed in the F 2 sample with the extreme of the phenotype distribution often exceeding that observed in the progenitor lines. Therefore, the F 2 sample is considered to be segregating for the relevant QTL and is an ideal sample in which to perform QTL mapping. The intercross strategy, as well as the less powerful recombinant inbred strategy, has been used to create extensive data regarding the likely position of QTL contributing to BMD phenotypes Table 7. Linkage analysis and subsequent fine mapping can provide corroborating information on important QTL syntenic with the human. The effects of individual gene products on skeletal biology can be evaluated using knockout and transgenic technology Table 8. These techniques can also be used to identify candidate genes for human studies. A number of transgenic or knockout mice have clear skeletal phenotypes. This is an active area of research, and the list will undoubtedly grow in the future. One QTL was found to be at the same location for both crosses Chr 1, see Table 7. Interestingly, the two crosses produced several different QTL even though the BMD phenotype is identical. These differences may be due to each strain having fixed differing alleles at the relevant loci or the two progenitor strains in one cross fixing the same QTL allele, resulting in the F 2 sample not segregating for this QTL. This behavior emphasizes the importance of collecting data from several mouse crosses to assure that all QTL contributing to a phenotype are uncovered. As illustrated in Table 7 , there are BMD QTL on mouse Chr 1, 2, 13, and 16 that have been uncovered in at least two different linkage studies. Interestingly, not all spinal BMD QTL corresponded to femoral BMD QTL. Important spinal BMD QTL on Chr 7 and 9 have no femoral counterparts, suggesting that genetic regulation of BMD is, in part, dependent upon anatomical site. One of the clear advantages of using rodents for genetic studies is the availability of the bones for direct measurement of bone biomechanical properties including strength and fragility. Fundamental biomechanical properties include force to failure a measure of strength , stiffness, and work to failure a measure of overall fragility. Biomechanical properties can be assessed at several sites including femoral midshaft, femoral neck, and vertebra As with BMD, it is likely that genetic regulation of bone strength is site specific and that femoral and vertebral strength segregate somewhat independently BMD and skeletal biomechanical properties are polygenic traits. Consequently, there may be numerous interactions among genes contributing to these traits. To isolate gene effects, congenic strains in which a single QTL is moved from the donor strain to a recipient strain can be constructed. This is typically done with selective backcrossing for 6—10 generations. C3H QTL caused significant differences in B6 BMD and femoral strength. QTL from mouse Chr 1, 4, and 18 increased BMD in recipient mice, whereas the donated QTL at Chr 6 reduced BMD , indicating that genetic influences on bone structure can be isolated in congenic mice. A second animal model that is available is the baboon Colonies represent very large pedigrees that can be used for linkage analysis using many of the markers that are present in the human genome. Importantly, the size and shape of the baboon skeleton at the hip and vertebra approach those of the human much more closely than those of the mouse. Linkage studies performed in a large baboon colony have identified a QTL on baboon Chr 11 that influences BMD Interestingly, this is the same region of Chr 11 identified in the three Mendelian bone-related disorders 9 , , as well as a large sample of premenopausal sister pairs More recently, the rat has been developed as a model for studying susceptibility genes of osteoporosis. The advantage of the rat is the extensive information on its physiology and skeletal biology that is available. Variability in femoral, vertebral, and femoral neck fragility among 11 inbred strains of rats has been reported Fischer and Lewis strains show the greatest variance in the vertebral fragility phenotype, and the Copenhagen and DA strains show the greatest variance in the femoral neck fragility phenotype, indicating that these strains will be useful for QTL analysis using the intercross strategy. As with mice, variation in skeletal fragility phenotypes in rats is dependent upon the anatomical site studied. Therefore, it is likely that rats will be useful for uncovering site-specific genetic influences on skeletal fragility. Using these three animal models is likely to provide complementary information to the human. The plans to fully sequence the mouse and the rat genome within the next 3 yr will be a major factor in the rate at which the susceptibility genes for osteoporosis can be identified. Emerging evidence of site specificity of skeletal phenotypes and the findings in mice that bone biomechanical phenotypes do not always correspond to BMD highlight the importance of measuring multiple phenotypes relating to BMD, geometry, structure, and biomechanical properties at multiple skeletal sites where osteoporotic fracture is common, such as the proximal femur and the vertebra. As for all human genetic studies 16 , the endeavor to identify the genes causing monogenetic forms of osteoporosis and the susceptibility genes underlying the common form of osteoporosis raises important legal and ethical issues. Key resources in this endeavor are the development of large repositories of human tissue, serum, and DNA and extensive data files containing essential phenotypic variables from healthy subjects and patients with osteoporotic diseases. In the United States, these resources are being developed in various centers depending mainly on the location of the researchers. However, very large national repositories are being developed in a number of countries, notably Iceland and UK www. In order for researchers to access such resources, guidelines and policies need to be in place both to protect patient privacy and to ensure that the essentials of patient informed consent are maintained. In , the National Bioethics Advisory Commission made recommendations on these issues to the US President on research involving human biological materials www. However, this is an evolving area , and the policies will undoubtedly undergo modification in the future. An equally important issue is the question of making available genetic testing , for osteoporosis. Genetic testing is currently available for numerous single-gene disorders. In both instances, however, individuals appropriate for genetic counseling are typically those in whose families a mutation in a single gene has resulted in a disorder with autosomal dominant inheritance. The susceptibility genes for the common form of osteoporosis do not appear to include a single gene with major effect. Furthermore, it appears that the susceptibility genes interact with important environmental factors. This work was supported by NIH Grants ROI AR, P01 AG, M01 RR, K24 AR, AR, and T32 HD Horsman A , Marshall DH , Peacock M A stochastic model of age-related bone loss and fractures. Clin Orthop : — Google Scholar. Horm Res 53 : 72 — Clin Endocrinol Oxf 36 : — Prockop DJ , Colige A , Helminen H , Khillan JS , Pereira R , Vandenberg P Mutations in type 1 procollagen that cause osteogenesis imperfecta: effects of the mutations on the assembly of collagen into fibrils, the basis of phenotypic variations, and potential antisense therapies. J Bone Miner Res 8 Suppl 2 : — Biochim Biophys Acta Mol Basis Dis : — Mech Ageing Dev 98 : Smith EP , Boyd J , Frank GR , Takahashi H , Cohen RM , Specker B , Williams TC , Lubahn DB , Korach KS Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med : — Carani C , Qin K , Simoni M , Faustini-Fustini M , Serpente S , Boyd J , Korach KS , Simpson ER Effect of testosterone and estradiol in a man with aromatase deficiency. Gong Y , Slee RB , Fukai N , Rawadi G , Roman-Roman S , Reginato AM , Wang H , Cundy T , Glorieux FH , Lev D , Zacharin M , Oexle K , Marcelino J , Suwairi W , Heeger S , Sabatakos G , Apte S , Adkins WN , Allgrove J , Arslan-Kirchner M , Batch BA , Beighton P , Black GC , Boles RG , Boon LM , Borrone C , Brunner HG , Carle GF , Dallapiccola B , De Paepe A , Floege B , Halfhide ML , Hall B , Hennekam RC , Hirose T , Jans A , Juppner H , Kim CE , Keppler-Noreuil K , Kohlschuetter A , Lacombe D , Lambert M , Lemyre E , Letteboer T , Peltonen L , Ramesar R , Romanengo M , Somer H , Steichen-Gerdsorf E , Steinmann B , Sullivan B , Superti-Furga A , Swoboda W , Boogaard M , Van Hul W , Vikkula M , Votruba M , Zabel B , Garcia T , Baron R , Olsen BJ , Warman ML LDL receptor-related protein 5 LRP5 affects bone accrual and eye development. Cell : — Lander ES , Schork NJ Genetic dissection of complex traits. Science : — Weeks DE , Lathrop GM Polygenic disease: methods for mapping complex disease traits. Trends Genet 11 : — International Human Genome Sequencing Consortium Initial sequencing and analysis of the human genome. Nature : — The International SNP Map Working Group A map of human genome sequence variation containing 1. Genomics 33 : — Meslin EM , Thomson EJ , Boyer JT The ethical, legal and social implications research program at the National Human Genome Research Institute. Kennedy Inst Ethics J 7 : Looker AC , Orwoll ES , Johnston Jr CC , Lindsay RL , Wahner HW , Dunn WL , Calvo MS , Harris TB , Heyse SP Prevalence of low femoral bone density in older US adults from NHANES III. J Bone Miner Res 12 : — Riis BJ , Hansen MA , Jensen AM , Overgaard K , Christiansen C Low bone mass and fast rate of bone loss at menopause: equal risk factors for future fracture: a year follow-up study. Newton-John H , Morgan B The loss of bone with age: osteoporosis and fractures. Clin Orthop 71 : — Peacock M , Liu G , Carey M , Ambrosius W , Turner CH , Hui SL , Johnston Jr CC Bone mass and structure at the hip in men and women over the age of 60 years. Osteoporos Int 8 : — Lu Y , Genant HK , Shepherd J , Zhao SJ , Mathur A , Fuerst TP , Cummings SR Classification of osteoporosis based on bone mineral densities. J Bone Miner Res 16 : — Atkinson PJ Variation in trabecular structure of vertebrae with age. Calcif Tissue Res 1 : 24 — Aaron JE , Makins NB , Sagreiya K The microanatomy of trabecular bone loss in normal aging men and women. Garn SM , Wagner B , Rohmann CG , Ascoli W Further evidence for continuing bone expansion. Am J Phys Anthropol 28 : — Cauley JA , Gutai JP , Kuller LH , Scott J , Nevitt MC Black-white differences in serum sex hormones and bone mineral density. Am J Epidemiol : — Kleerekoper M , Nelson DA , Flynn MJ , Pawluszka AS , Jacobsen G , Peterson EL Comparison of radiographic absorptiometry with dual-energy x-ray absorptiometry and quantitative computed tomography in normal older white and black women. J Bone Miner Res 9 : — Tobias JH , Cook DG , Chambers TJ , Dalzell N A comparison of bone mineral density between Caucasian, Asian and Afro-Caribbean women. Clin Sci 87 : — Ross PD , He YF , Yates AJ , Coupland C , Ravn P , McClung MR , Thompson D , Wasnich RD Body size accounts for most differences in bone density between Asian and Caucasian women. Calcif Tissue Int 59 : — Albright F Osteoporosis. Ann Intern Med 27 : — Nordin BEC The definition and diagnosis of osteoporosis. Calcif Tissue Int 40 : 57 — NIH NIH Consensus Development Conference: diagnosis, prophylaxis and treatment of osteoporosis. Atkinson PJ Changes in resorption spaces in femoral cortical bone with age. J Pathol Bacteriol 89 : — Bousson V , Meunier A , Bergot C , Vicaut E , Rocha MA , Morais MH , Laval-Jeantet A-M , Laredo JD Distribution of intracortical porosity in human midfemoral cortex by age and gender. Aaron JE , Shore PA , Shore RC , Beneton M , Kanis JA Trabecular architecture in women and men of similar bone mass with and without vertebral fracture. Three-dimensional histology. Bone 27 : — Dempster DM The contribution of trabecular architecture to cancellous bone quality. J Bone Miner Res 15 : 20 — Gluer CC , Cummings SR , Pressman A , Li J , Gluer K , Faulkner KG , Grampp S , Genant HK Prediction of hip fractures from pelvic radiographs: the study of osteoporotic fractures. Peacock M , Turner CH , Liu G , Manatunga AK , Timmerman L , Johnston Jr CC Better discrimination of hip fracture using bone density, geometry and architecture. Osteoporos Int 5 : — Faulkner KG , Cummings SR , Nevitt MC , Pressman A , Jergas M , Genant HK Hip axis length and osteoporotic fractures. J Bone Miner Res 10 : — letter. Karlsson KM , Sernbo I , Obrant KJ , Redlund-Johnell I , Johnell O Femoral neck geometry and radiographic signs of osteoporosis as predictors of hip fracture. Bone 18 : — Rodino MA , Shane E Osteoporosis after organ transplantation. Am J Med : — Van Staa TP , Leufkens HGM , Abenhaim L , Zhang B , Cooper C Use of oral corticosteroids and risk of fractures. J Bone Miner Res 15 : — Schwartz AV , Sellmeyer DE , Ensrud KE , Cauley JA , Tabor HK , Schreiner PJ , Jamal SA , Black DM , Cummings SR Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab 86 : 32 — Bollerslev J Autosomal dominant osteopetrosis: bone metabolism and epidemiological, clinical and hormonal aspects. Endocr Rev 10 : 45 — Genant HK , Lang TF , Engelke K , Fuerst T , Glüer CC , Majumdar S , Jergas M Advances in the noninvasive assessment of bone density, quality, and structure. Calcif Tissue Int 59 Suppl 1 : S10 — S Hangartner TN , Gilsanz V Evaluation of cortical bone by computed tomography. J Bone Miner Res 11 : — World Health Organization WHO Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO study group. WHO Technical Report Series. Geneva, Switzerland : World Health Organization; report Faulkner KG , Stetten EV , Miller P Discordance in patient classification using T-scores. J Clin Densitomet 2 : — Orwoll E Assessing bone density in men. Marshall D , Johnell O , Wedel H Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. Br Med J : — J Bone Miner Res 8 : — Genant HK , Gordon C , Jiang YB , Link TM , Hans D , Majumdar S , Lang TF Advanced imaging of the macrostructure and microstructure of bone. Horm Res 54 Suppl : 24 — Genant HK , Jiao L , Chun JW , Shepard JA Vertebral fractures in osteoporosis. J Clin Densitomet 3 : — Martin RB , Burr DB Non-invasive measurement of long bone cross-sectional moment of inertia by photon absorptiometry. J Biomechanics 17 : — Beck TJ , Looker AC , Ruff CB , Sievanen H , Wahner HW Structural trends in the aging femoral neck and proximal shaft: analysis of the Third National Health and Nutrition Examination Survey dual-energy X-ray absorptiometry data. Turner CH , Peacock M , Timmerman L , Neal JM , Johnston Jr CC Calcaneal ultrasonic measurements discriminate hip fracture independently of bone mass. Bauer DC , Gluer CC , Cauley JA , Vogt TM , Ensrud KE , Genant HK , Black DM Broadband ultrasound attenuation predicts fractures strongly and independently of densitometry in older women: a prospective study. Arch Intern Med : — Hong J , Hipp JA , Mulkern RV , Jaramillo D , Snyder BD Magnetic resonance imaging measurements of bone density and cross-sectional geometry. Calcif Tissue Int 66 : 74 — Garnero P , Sornay-Rendu E , Claustrat B , Delmas PD Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. Alffram P An epidemiological study of cervical and trochanteric fractures of the femur in an urban population. Acta Orthop Scand Suppl 65 : 1 — Cumming RG , Klineberg RJ Fall frequency and characteristics and the risk of hip fractures. J Am Geriatr Soc 42 : — Hayes WC , Myers ER , Morris JN , Gerhart TN , Yett HS , Lipsitz LA Impact near the hip dominates fracture risk in elderly nursing home residents who fall. Calcif Tissue Int 52 : — Nordin BEC , Peacock M , Aaron J , Crilly RG , Heyburn PJ , Horsman A , Marshall DH Osteoporosis and osteomalacia. Clin Endocrinol Metab 9 : — Slemenda CW , Turner CH , Peacock M , Christian JC , Sorbel J , Hui SL , Johnston CC The genetics of proximal femur geometry, distribution of bone mass and bone mineral density. Osteoporos Int 6 : — Frost HM The role of changes in mechanical usage set points in the pathogenesis of osteoporosis. J Bone Miner Res 7 : — Melton LJ , III Epidemiology of spinal osteoporosis. Spine 22 Suppl :2S—11S. Kannus P , Parkkari J , Sievänen H, Heinonen A, Vuori I, Järvinen M Epidemiology of hip fractures. Bone 18 Suppl S—63S. Silverman SL , Madison RE Decreased incidence of hip fracture in Hispanics, Asians, and Blacks: California hospital discharge data. Am J Public Health 78 : — Kellie SE , Brody JA Sex-specific and race-specific hip fracture rates. Am J Public Health 80 : — Tinetti ME , Speechley M , Ginter SF Risk factors for falls among elderly persons living in the community. Snedecor GW , Cochran WG Statistical methods. Ames, IA : The Iowa State University Press. Pocock NA , Eisman JA , Hopper JL , Yeates MG , Sambrook PN , Ebert S Genetic determinants of bone mass in adults: a twin study. J Clin Invest 80 : — Slemenda CW , Christian JC , Williams CJ , Norton JA , Johnston Jr CC Genetic determinants of bone mass in adult women: a reevaluation of the twin model and the potential importance of gene interaction on heritability estimates. J Bone Miner Res 6 : — Hustmyer FG , Peacock M , Hui S , Johnston CC , Christian J Bone mineral density in relation to polymorphism at the vitamin D receptor gene locus. J Clin Invest 94 : — Koller DL , Peacock M , Conneally PM , Liu G , Hui SL , McClintock R , Christian JC , Johnston CC , Econs MJ , Foroud T Heritability of bone-related phenotypes in Caucasian and African-American sister pairs. Am J Hum Genet A Abstract. Smith DM , Nance WE , Kang KW , Christian JC , Johnston Jr CC Genetic factors in determining bone mass. J Clin Invest 52 : — Moller M , Horsman A , Harvald B , Hauge M , Henningsen K , Nordin BEC Metacarpal morphometry in monozygotic and dizygotic elderly twins. Calcif Tiss Res 25 : — Dequeker J , Nijs N , Verstraeten A , Geusens P , Gevers G Genetic determinants of bone mineral content at the spine and radius: a twin study. Bone 8 : — Sowers MR , Burns TL , Wallace RB Familial resemblance of bone mass in adult women. Genet Epidemiol 3 Suppl : 85 — Am J Clin Nutr 44 : 99 — Tylavsky FA , Bortz AD , Hancock RL , Anderson JJ Familial resemblance of radial bone mass between premenopausal mothers and their college-age daughters. Calcif Tissue Int 45 : — Lutz J , Tesar R Mother-daughter pairs: spinal and femoral bone densities and dietary intakes. Am J Clin Nutr 52 : — Sowers MR , Boehnke M , Jannausch ML , Crutchfield M , Corton G , Burns TL Familiality and partitioning the variability of femoral bone mineral density in women of child-bearing age. Calcif Tissue Int 50 : — Gueguen R , Jouanny P , Guillemin F , Kuntz C , Pourel J , Siest G Segregation analysis and variance components analysis of bone mineral density in healthy families. J Bone Miner Res 10 : — Koller DL , Econs MJ , Morin PA , Christian JC , Hui SL , Parry P , Curran ME , Rodriguez LA , Conneally PM , Joslyn G , Peacock M , Johnston CC , Foroud T Genome screen for QTLs contributing to normal variation in bone mineral density and osteoporosis. J Clin Endocrinol Metab 85 : — Seeman E , Hopper JL , Back LA , Cooper ME , Parkinson E , McKay J , Jermus G Reduced bone mass in daughters of women with osteoporosis. Seeman E , Tsalamandris C , Formica C , Hopper JL , McKay J Reduced femoral neck bone density in the daughters of women with hip fractures: the role of low peak bone density in the pathogenesis of osteoporosis. Danielson ME , Cauley JA , Baker CE , Newman AB , Dorman JS , Towers JD , Kuller LH Familial resemblance of bone mineral density BMD and calcaneal ultrasound attenuation: the BMD in mothers and daughters study. J Bone Miner Res 14 : — Lonzer MD , Imrie R , Rogers D , Worley D , Licata A , Secic M Effects of heredity, age, weight, puberty, activity, and calcium intake on bone mineral density in children. Clin Pediatr Phila 35 : — Ferrari S , Rizzoli R , Slosman D , Bonjour JP Familial resemblance for bone mineral mass is expressed before puberty. J Clin Endocrinol Metab 83 : — Orwoll ES , Belknap JK , Klein RF Gender specificity in the genetic determinants of peak bone mass. Holzinger KJ The relative effect of nature and nurture influences on twin differences. J Educ Psychol 20 : — Clark PJ The heritability of certain anthropometric characters as ascertained from measurements of twins. Feinleib M , Garrison RJ , Fabsitz R , Christian JC , Hrubec Z , Borhani NO , Kannel WB , Rosenman R , Schwartz JT , Wagner JO The NHLBI Twin Study of Cardiovascular Disease Risk Factors: methodology and summary of results. Arden NK , Baker J , Hogg C , Baan K , Spetor TD The heritability of bone mineral density, ultrasound of the calcaneus and hip axis length: a study of postmenopausal twins. Koller DL , Liu GD , Econs MJ , Hui SL , Morin PA , Joslyn G , Rodriguez LA , Conneally PM , Christian JC , Johnston Jr CC , Foroud T , Peacock M Genome screen for quantitative trait loci underlying normal variation in femoral structure. Koller DL , Liu G , Econs MJ , Hui SL , Conneally PM , Johnston Jr CC , Foroud T , Peacock M Genome screen for QTLs underlying normal variation in vertebral structure. J Bone Miner Res 16 Suppl :S Christian JC , Yu P-L , Slemenda CW , Johnston Jr CC Heritability of bone mass: a longitudinal study in aging male twins. Am J Hum Genet 44 : — Kelly PJ , Nguyen T , Hopper J , Pocock N , Sambrook P , Eisman J Changes in axial bone density with age: a twin study. J Bone Miner Res 8 : 11 — Delmas PD Biochemical markers of bone turnover. J Bone Miner Res 8 Suppl 2 : S — S Weaver CM , Peacock M , Martin BR , McCabe GP , Zhao J , Smith DL , Wastney ME Quantification of biochemical markers of bone turnover by kinetic measures of bone formation and resorption in young healthy females. Kleerekoper M , Nelson DA , Peterson EL , Flynn MJ , Pawluszka AS , Jacobsen G , Wilson P Reference data for bone mass, calciotropic hormones, and biochemical markers of bone remodeling in older 55—75 postmenopausal white and black women. Greenspan SL , Parker RA , Ferguson L , Rosen HN , Maitland-Ramsey L , Karpf DB Early changes in biochemical markers of bone turnover predict the long-term response to alendronate therapy in representative elderly women: a randomized clinical trial. J Bone Miner Res 13 : — Bauer DC , Sklarin P , Stone KL , Black DM , Nevitt MC , Ensrud KE , Arnaud CD , Genant HK , Garnero P , Delmas PD , Lawaetz H , Cummings SR Biochemical markers of bone turnover and prediction of hip bone loss in older women: the study of osteoporotic fractures. Ross PD , Knowlton W Rapid bone loss is associated with increased levels of biochemical markers. Kelly PJ , Hopper JL , Macaskill GT , Pocock NA , Sambrook PN , Eisman JA Genetic factors in bone turnover. J Clin Endocrinol Metab 72 : — Livshits G , Yakovenko C , Kobyliansky E Quantitative genetic analysis of circulating levels of biochemical markers of bone formation. Am J Med Genet 94 : — Cummings SR , Nevitt MC , Browner WS , Stone K , Fox KM , Ensrud KE , Cauley J , Black D , Vogt TM Risk factors for hip fracture in white women. Kannus P , Palvanen M , Kaprio J , Parkkari J , Koskenvuo M Genetic factors and osteoporotic fracture in elderly people: prospective 25 year follow up of a nationwide cohort of elderly Finnish twins. MacGregor A , Sneider H , Spector TD Genetic factors and osteoporotic fractures in elderly people: twin data support genetic contribution to risk of fracture. Br Med J : Carmelli D , Kelly-Hayes M , Wolf PA , Swan GE , Jack LM , Reed T , Guralnik JM The contribution of genetic influences to measures of lower-extremity function in older male twins. J Gerontol [A] 55A : B49 — B Wark JD , Hill K , Cassano AM , El Haber N , MacInnis R Genetic effects on falls risk may help explain why fractures run in families: a twin study. Bone 28 Suppl :S72 Abstract. Sheldon JH On the natural history of falls in old age. Br Med J — Tinetti ME , Williams CS Falls, injuries due to falls, and the risk of admission to a nursing home. Campbell A , Reinken J , Allan B , Martinez G Falls in old age: a study of frequency and related clinical factors. Age Ageing 10 : — Lauritzen JB , Petersen MM , Lund B Effect of external hip protectors on hip fractures. Lancet : 11 — Rubenstein L Hip protectors—a breakthrough in fracture prevention. Rubenstein LZ , Robbins AS , Schulman BL , Rosado J , Osterweil D , Josephson KR Falls and instability in the elderly. J Am Geriatr Soc 36 : — Kelsey JL , Hoffman S Risk factors for hip fracture. Nevitt MC , Cummings SR , Kidd S , Black D Risk factors for recurrent nonsyncopal falls: a prospective study. JAMA : — Ho NC , Libin J , Driscoll CC , Gutter EM , Francomano CA A skeletal gene database. Risch N , Merikangas K The future of genetic studies of complex human diseases. Spielman RS , Ewens WJ The TDT and other family-based tests for linkage disequilibrium and association. Am J Hum Genet 59 : — Pritchard JK , Rosenberg NA Use of unlinked genetic markers to detect population stratification in association studies. Am J Hum Genet 65 : — Pritchard JK , Stephens M , Rosenberg NA , Donnelly P Association mapping in structured populations. Am J Hum Genet 67 : — Nat Genet 29 : — Daly MJ , Rioux JD , Schaffner SF , Hudson TJ , Lander ES High-resolution haplotype structure in the human genome. Johnson GC , Esposito L , Barratt BJ , Smith AN , Heward J , Di Genova G , Ueda H , Cordell HJ , Eaves IA , Dudbridge F , Twells RC , Payne F , Hughes W , Nutland S , Stevens H , Carr P , Tuomilehto-Wolf E , Tuomilehto J , Gough SC , Clayton DG , Todd JA Haplotype tagging for the identification of common disease genes. Cooper GS , Umbach DM Are vitamin D receptor polymorphisms associated with bone mineral density? A meta-analysis. Barger-Lux MJ , Heaney RP , Hayes J , DeLuca HF , Johnson ML , Gong G Vitamin D receptor gene polymorphism, bone mass, body size, and vitamin D receptor density. Calcif Tissue Int 57 : — Lorentzon M , Lorentzon R , Nordström P Vitamin D receptor gene polymorphism is associated with birth height, growth to adolescence, and adult stature in healthy Caucasian men: a cross-sectional and longitudinal study. Keen RW , Egger P , Fall C , Major PJ , Lanchbury JS , Spector TD , Cooper C Polymorphisms of the vitamin D receptor, infant growth, and adult bone mass. Calcif Tissue Int 60 : — Kitagawa I , Kitagawa Y , Kawase Y , Nagaya T , Tokudome S Advanced onset of menarche and higher bone mineral density depending on vitamin D receptor gene polymorphism. Eur J Endocrinol : — Geusens P , Vandevyver C , Vanhoof J , Cassiman JJ , Boonen S , Raus J Quadriceps and grip strength are related to vitamin D receptor genotype in elderly nonobese women. Gennari L , Becherini L , Masi L , Gonnelli S , Cepollaro C , Martini S , Mansani R , Brandi ML Vitamin D receptor genotypes and intestinal calcium absorption in postmenopausal women. Calcif Tissue Int 61 : — Kiel DP , Myers RH , Cupples LA , Kong XF , Zhu XH , Ordovas J , Schaefer EJ , Felson DT , Rush D , Wilson PW , Eisman JA , Holick MF The Bsm I vitamin D receptor restriction fragment length polymorphism bb influences the effect of calcium intake on bone mineral density. Ongphiphadhanakul B , Rajatanavin R , Chanprasertyothin S , Chailurkit L , Piaseu N , Teerarungsikul K , Sirisriro R , Komindr S , Puavilai G Vitamin D receptor gene polymorphism is associated with urinary calcium excretion but not with bone mineral density in postmenopausal women. J Endocrinol Invest 20 : — Schwartz BS , Lee BK , Lee GS , Stewart WF , Simon D , Kelsey K , Todd AC Associations of blood lead, dimercaptosuccinic acid-chelatable lead, and tibia lead with polymorphisms in the vitamin D receptor and δ-aminolevulinic acid dehydratase genes. Environ Health Perspect : — Lee BK , Lee GS , Stewart WF , Ahn KD , Simon D , Kelsey KT , Todd AC , Schwartz BS Associations of blood pressure and hypertension with lead dose measures and polymorphisms in the vitamin D receptor and δ-aminolevulinic acid dehydratase genes. World J Surg 22 : — Chang TJ , Lei HH , Yeh JI , Chiu KC , Lee KC , Chen MC , Tai TY , Chuang LM Vitamin D receptor gene polymorphisms influence susceptibility to type 1 diabetes mellitus in the Taiwanese population. Clin Endocrinol Oxf 52 : — Fernández E , Fibla J , Betriu A , Piulats JM , Almirall J , Montoliu J Association between vitamin D receptor gene polymorphism and relative hypoparathyroidism in patients with chronic renal failure. J Am Soc Nephrol 8 : — Fischer PR , Thacher TD , Pettifor JM , Jorde LB , Eccleshall TR , Feldman D Vitamin D receptor polymorphisms and nutritional rickets in Nigerian children. The effort was funded by many sources, including the European Commission and several NIH components, such as the National Institute on Aging NIA and National Institute for Arthritis, Musculoskeletal and Skin Diseases NIAMS. The extensive research team—led by a group at Erasmus Medical Center in Rotterdam, the Netherlands—also included scientists at NIA. The study appeared online in Nature Genetics on April 15, The researchers first combined data from 17 different studies involving more than 80, people across North America, Europe, East Asia and Australia. They looked across the genome for genetic variants associated with bone mineral density of the femoral neck and lumbar spine. The researchers found 96 independent variations from 87 genomic regions. The scientists next tested these associations in over 50, more people from 34 other studies. They confirmed the association with bone mineral density in 56 regions, 32 of which hadn't been previously been tied to bone density. The team also examined whether the 96 variants were associated with bone fractures. They analyzed data from 50 studies with fracture information. Combined, the studies involved over 31, people with fractures and over , controls. Fourteen of the regions, the researchers found, were also associated with bone fracture risk. These findings reinforce the relationship between genetic factors and the risk of osteoporosis and bone fracture. However, the researchers found that the ability to use these factors to predict risk was modest relative to clinical risk factors such as age and weight. John Ioannidis of the Stanford University School of Medicine, one of the senior authors. We can't predict exactly who will or won't get a fracture. Douglas P. Kiel of Harvard Medical School. References: Nat Genet. doi: Site Menu Home. gov Science Education Resources NIH Clinical Research Trials and You Talking to Your Doctor More ». Search Health Topics. Quick Links RePORT eRA Commons NIH Common Fund. |
| Related News | Cumming RGKlineberg RJ Fall frequency Pomegranate Juice Benefits characteristics hfalth the risk of hip fractures. Bone health and genetics Bone Bone health and genetics Res. BMD bone mineral density, Genefics estrogen receptor Bone health and genetics, GWAS genome-wide association study, LRP5 low-density lipoprotein receptor-related protein 5, Gendtics low-density lipoprotein receptor-related protein 4, OPG osteoprotegerin, RANK receptor activator of nuclear factor-kappa β, RANKL RANK ligand, SPTBN1 spectrin beta, nonerythrocytic 1, WES, whole-exome sequencing, WGS whole-genome sequencing, ZBTB40 zinc finger and BTB domain containing The heritability of forearm fracture was calculated to be less than one third This, however, requires that the disease in focus has a readily accessible proxy tissue, where the gene expression reflects the expression in the affected tissue. |
Ist Einverstanden, das nützliche Stück
Sie sind nicht recht. Es ich kann beweisen. Schreiben Sie mir in PM.
Sehr die nützliche Information
Ich meine, dass Sie den Fehler zulassen. Es ich kann beweisen.