
Oxidative stress pathways -
Environ Health Perspect — Halliwell B, Gutteridge JMC Free radicals in biology and medicine, 4th edn. Oxford University Press, Oxford. Hattori K, Naguro I, Runchel C, Ichijo H The roles of ASK family proteins in stress responses and diseases.
Cell Commun Signal doi: Eur Neurol — Hirsch T, Marchetti P, Susin SA, Dallaporta B, Zamzami N, Marzo I, Geuskens M, Kroemer G The apoptosis-necrosis paradox. Apoptogenic proteases activated after mitochondrial permeability transition determine the mode of cell death.
Hitoshi Y, Lorens J, Kitada SI, Fisher J, LaBarge M, Ring HZ, Francke U, Reed JC, Kinoshita S, Nolan GP Toso, a cell surface, specific regulator of Fas-induced apoptosis in T cells. Immunity — Hotchkiss RS, Strasser A, McDunn JE, Swanson PE Cell death. N Engl J Med — Hsu H, Xiong J, Goeddel DV The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation.
Huang X, Masselli A, Frisch SM, Hunton IC, Jiang Y, Wang JY Blockade of tumor necrosis factor-induced Bid cleavage by caspase-resistant Rb. Jiang Y, Woronicz JD, Liu W, Goeddel DV Prevention of constitutive TNF receptor 1 signaling by silencer of death domains.
Jones EV, Dickman MJ, Whitmarsh AJ Regulation of p mediated apoptosis by c-Jun N-terminal kinase. Biochem J — Kagan VE, Tyurin VA, Jiang J, Tyurina YY et al Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors.
Nat Chem Biol — Kam PCA, Ferch NI Apoptosis: mechanisms and clinical implications. Anaesthesia — Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases.
Karin M, Lin A NF-κB at the crossroads of life and death. Nat Immunol — Kaufmann SH, Earnshaw WC Induction of apoptosis by cancer chemotherapy. Exp Cell Res — Kerr JF, Wyllie AH, Currie AR Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics.
Br J Cancer — Khawaja NR, Carrè M, Kovacic H, Estève MA, Braguer D Patupilone-induced apoptosis is mediated by mitochondrial reactive oxygen species through bim relocalization to mitochondria. Mol Pharmacol — Kolomeichuk SN, Terrano DT, Lyle CS, Sabapathy K, Chambers TC Distinct signaling pathways of microtubule inhibitors—vinblastine and Taxol induce JNK-dependent cell death but through APdependent and APindependent mechanisms, respectively.
FEBS J — Korshunov SS, Skulachev VP, Starkov AA High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett — Kothakota S, Azuma T, Reinhard C, Klippel A, Tang J, Chu K, McGarry TJ, Kirschner MW, Koths K, Kwiatkowski DJ, Williams LT Caspasegenerated fragment of gelsolin: effector of morphological change in apoptosis.
Laethem A, Van K, Nys S, Van Kelst S, Claerhout H, Ichijo JR, Vandenheede M, Garmyn P Agostinis, Apoptosis signal regulating kinase-1 connects reactive oxygen species to p38 MAPK induced mitochondrial apoptosis in UVB-irradiated human keratinocytes.
Lambert AJ, Brand MD Superoxide production by NADH: ubiquinone oxidoreductase complex I depends on the pH gradient across the mitochondrial inner membrane. Cell Death Differ — Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villen J, Becker EBE, DiBacco S, de la Iglesia N, Gygi S, Blackwell TK et al A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span.
Lei K, Davis RJ NK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Lei K, Nimnual A, Zong WX, Kennedy NJ, Flavell RA, Thompson CB et al The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH 2 -terminal kinase.
Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell — Lin Y, Devin A, Rodriguez Y, Liu ZG Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis.
Liu X, Zou H, Slaughter C, Wang X DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis.
Liu H, Nishitoh H, Ichijo H, Kyriakis JM Activation of apoptosis signal-regulating kinase 1 ASK1 by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin.
Liu Y, Fiskum G, Schubert D Generation of reactive oxygen species by the mitochondrial electron transport chain. Liu FT, Newland AC, Jia L Bax conformational change is a crucial step for PUMA-mediated apoptosis in human leukemia. Biochem Biophys Res Commun — Locksley RM, Killeen N, Lenardo MJ The TNF and TNF receptor superfamilies: integrating mammalian biology.
Los M, Wesselborg S, Schulze-Osthoff K The role of caspases in development, immunity, and apoptotic signal transduction: lessons from knockout mice. Luo X, Budihardjo I, Zou H, Slaughter C, Wang X Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors.
Manna P, Sil PC a Arjunolic acid: beneficial role in type 1 diabetes and its associated organ pathophysiology. Manna P, Sil PC b Impaired redox signaling and mitochondrial uncoupling contributes vascular inflammation and cardiac dysfunction in type 1 diabetes: protective role of arjunolic acid.
Biochimie — Manna SK, Zhang HJ, Yan T, Oberley LW, Aggarwal BB Overexpression of manganese superoxide dismutase suppresses tumor necrosis factor-induced apoptosis and activation of nuclear transcription factor-kappaB and activated protein Manna P, Sinha M, Sil PC a Protection of arsenic-induced hepatic disorder by arjunolic Acid.
Basic Clin Pharmacol Toxicol — Manna P, Sinha M, Pal P, Sil PC b Arjunolic acid, a triterpenoid saponin, ameliorates arsenic-induced cyto-toxicity in hepatocytes. Manna P, Sinha M, Sil PC a Arsenic induced oxidative myocardial injury: protective role of arjunolic acid.
Arch Toxicol — Manna P, Sinha M, Sil PC b Taurine triggers a chemoprevention against cadmium induced testicular oxidative injury. Reprod Toxicol — Manna P, Sinha M, Sil PC c Amelioration of cadmium-induced cardiac impairment by taurine. Manna P, Sinha M, Sil PC d Protection of arsenic-induced testicular oxidative stress by arjunolic acid.
Redox Rep — Manna P, Sinha M, Sil PC a Prophylactic role of arjunolic acid in response to streptozotocin mediated diabetic renal injury: activation of polyol pathway and oxidative stress responsive signaling cascades.
Manna P, Sinha M, Sil PC b Protective role of arjunolic acid in response to streptozotocin-induced type-I diabetes via the mitochondrial dependent and independent pathways. Manna P, Sinha M, Sil PC c Taurine plays a beneficial role against cadmium-induced oxidative renal dysfunction.
Manna P, Ghosh J, Das J, Sil PC b Streptozotocin induced activation of oxidative stress responsive splenic cell signaling pathways: protective role of arjunolic acid. Nanotoxicology — Marani M, Tenev T, Hancock D, Downward J, Lemoine NR Identification of novel isoforms of the BH3 domain protein Bim which directly activate Bax to trigger apoptosis.
Masutani H, Yodoi J Thioredoxin. Methods Enzymol — Matés JM, Segura JA, Alonso FJ, Marquez J Roles of dioxins and heavy metals in cancer and neurological diseases using ROS-mediated mechanisms. Matés JA, Segura FJ, Alonso JM, Javier M Oxidative stress in apoptosis and cancer: an update.
Arch Toxicol. Matsukawa J, Matsuzawa A, Takeda K, Ichijo H The ASK1-MAP kinase cascades in mammalian stress response. J Biochem — Matsuzawa A, Ichijo H Molecular mechanisms of the decision between life and death: regulation of apoptosis by apoptosis signal-regulating kinase 1.
J Biochem —8. Matsuzawa A, Ichijo H Redox control of cell fate by MAP kinase: physiological roles of ASK1—MAP kinase pathway in stress signaling. Micheau O, Tschopp J Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Migliaccio E, Giorgio M, Pelicci PG Apoptosis and aging: role of p66Shc redox protein.
Antioxid Redox Signal — Min W, Lin Y, Tang S, Yu L, Zhang H, Wan T, Luhn T, Fu H, Chen H AIP1 recruits phosphatase PP2A to ASK1 in tumor necrosis factor-induced ASK1-JNK activation. Circ Res — Moreira ME, Barcinski MA Apoptotic cell and phagocyte interplay: recognition and consequences in different cell systems.
An Acad Bras Cienc — Morita K, Saitoh M, Tobiume K, Matsuura H, Enomoto S, Nishitoh H, Ichijo H Negative feedback regulation of ASK1 by protein phosphatase 5 PP5 in response to oxidative stress. Mullarkey CJ, Edelstein D, Brownlee M Free radical generation by early glycation products: a mechanism for accelerated atherogenesis in diabetes.
Muller FL, Liu Y, Van Remmen H Complex III releases superoxide to both sides of the inner mitochondrial membrane. Nagai H, Noguchi T, Takeda K, Ichijo H Pathophysiological roles of ASK1-MAP kinase signaling pathways.
J Biochem Mol Biol —6. Nagai H, Noguchi T, Homma K, Katagiri K, Takeda K, Matsuzawa A, Ichijo H Ubiquitin-like sequence in ASK1 plays critical roles in the recognition and stabilization by USP9X and oxidative stress-induced cell death.
Mol Cell — Nagata S Fas ligand-induced apoptosis. Annu Rev Genet — Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J Caspase mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta.
Nakamura T, Kazuichi S Forkhead transcription factor FOXO subfamily is essential for reactive oxygen species-induced apoptosis. Mol Cell Endocrinol — Nishikawa T, Edelstein D, Du XL, Yamagishi S et al Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage.
Noguchi T, Takeda K, Matsuzawa A, Saegusa K, Nakano H, Gohda J, Inoue J, Ichijo H Recruitment of tumor necrosis factor receptor associated factor family proteins to apoptosis signal-regulating kinase 1 signalosome is essential for oxidative stress induced cell death.
Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, Tokino T, Taniguchi T, Tanaka N Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of pinduced apoptosis. Oleinik NV, Krupenko NI, Krupenko SA Cooperation between JNK1 and JNK2 in activation of p53 apoptotic pathway.
Orr WC, Sohal RS Extension of life-span by over expression of superoxide dismutase and catalase in Drosophila melanogaster. Orrenius S, Gogvadze V, Zhivotovsky B Mitochondrial oxidative stress: implications for cell death.
Annu Rev Pharmacol Toxicol — Orrenius S, Nicotera P, Zhivotovsky B Cell death mechanisms and their implications in toxicology. Toxicol Sci — Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S Cytochrome c release from mitochondria proceeds by a two-step process.
Pal S, Sil PC A 43 kD protein from the leaves of the herb Cajanus indicus L. modulates doxorubicin induced nephrotoxicity via MAPKs and both mitochondria dependent and independent pathways. Pal S, Pal PB, Das J, Sil PC Involvement of both intrinsic and extrinsic pathways in hepatoprotection of arjunolic acid against cadmium induced acute damage in vitro.
Pal PB, Pal S, Das J, Sil PC Modulation of mercury-induced mitochondria-dependent apoptosis by glycine in hepatocytes. Pal PB, Sinha K, Sil PC Mangiferin, a natural xanthone, protects murine liver in Pb II induced hepatic damage and cell death via MAP kinase, NF-κB and mitochondria dependent pathways.
PLoS ONE. Park HS, Cho SG, Kim CK, Hwang HS et al Heat shock protein hsp72 is a negative regulator of apoptosis signal-regulating kinase 1. Pastor N, Weinstein H, Jamison E, Brenowitz M A detailed interpretation of OH radical footprints in a TBP DNA complex reveals the role of dynamics in the mechanism of sequence specific binding.
J Mol Biol — Pastorino JG, Chen ST, Tafani M, Snyder JW, Farber JL The overexpression of Bax produces cell death upon induction of the mitochondrial permeability transition. Perier C, Tieu K, Guegan C, Caspersen C, Jackson-Lewis V et al Complex I deficiency primes Bax-dependent neuronal apoptosis through mitochondrial oxidative damage.
Pierce GB, Parchment RE, Lewellyn AL Hydrogen peroxide as a mediator of programmed cell death in the blastocyst. Differentiation — Puthalakath H, Strasser A Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins.
Rachek LI, Yuzefovych LV, Ledoux SP, Julie NL, Wilson GL Troglitazone, but not rosiglitazone, damages mitochondrial DNA and induces mitochondrial dysfunction and cell death in human hepatocytes.
Raman M, Chen W, Cobb MH Differential regulation and properties of MAPKs. Rashid K, Bhattacharya S, Sil PC Protective role of D-saccharic acid-1,4-lactone in alloxan induced oxidative stress in the spleen tissue of diabetic rats is mediated by suppressing mitochondria dependent apoptotic pathway.
Rashid K, Das J, Sil PC Taurine ameliorate alloxan induced oxidative stress and intrinsic apoptotic pathway in the hepatic tissue of diabetic rats.
Rasola A, Bernardi P The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis — Ray PD, Huang BW, Tsuji Y Reactive oxygen species ROS homeostasis and redox regulation in cellular signaling. Cell Signal — Rhee SG Cell signaling.
H 2 O 2 , a necessary evil for cell signaling. Ricci C, Pastukh V, Leonard J, Turrens J, Wilson G, Schaffer D, Schaffer SW Mitochondrial DNA damage triggers mitochondrial-superoxide generation and apoptosis.
Am J Physiol Cell Physiol C—C Ristow M, Zarse K How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis mitohormesis.
Exp Gerontol — Rostovtseva TK, TanW Colombini M On the role of VDAC in apoptosis: fact and fiction. J Bioenerg Biomembr — Roulston A, Reinhard C, Amiri P, Williams LT Early activation of c-Jun N-terminal kinase and p38 kinase regulate cell survival in response to tumor necrosis factor α.
Rudel T, Bokoch GM Membrane and morphological changes in apoptotic cells regulated by caspase-mediated activation of PAK2. Saeki K, Kobayashi N, Inazawa Y, Zhang H, Nishitoh H, Ichijo H, Saeki K, Isemura M, You A Oxidation-triggered c-Jun N-terminal kinase JNK and p38 mitogen-activated protein MAP kinase pathways for apoptosis in human leukaemic cells stimulated by epigallocatechingallate EGCG : a distinct pathway from those of chemically induced and receptor-mediated apoptosis.
Saelens X, Festjens N, Vande Walle L, van Gurp M, van Loo G, Vandenabeele P Toxic proteins released from mitochondria in cell death. Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase ASK 1.
Sarkar MK, Sil PC Prevention of tertiary butyl hydroperoxide induced oxidative impairment and cell death by a novel antioxidant protein molecule isolated from the herb, Phyllanthus niruri. Toxicol In Vitro — Sarkar A, Das J, Manna P, Sil PC Nano-copper induces oxidative stress and apoptosis in kidney via both extrinsic and intrinsic pathways.
Schneider-Brachert W, Tchikov V, Neumeyer J, Jakob M et al Compartmentalization of TNF receptor 1 signaling: internalized TNF receptosomes as death signaling vesicles. Schroeter H, Boyd CS, Ahmed R, Spencer JP, Duncan RF, Rice-Evans C et al c-Jun N-terminal kinase JNK -mediated modulation of brain mitochondria function: new target proteins for JNK signalling in mitochondrion-dependent apoptosis.
Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA et al A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev Cell — Shiizaki S, Isao N, Hidenori I Activation mechanisms of ASK1 in response to various stresses and its significance in intracellular signaling.
Adv Biol Regul. Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, Yamada G, Akira S, Matsumoto K, Ghosh S TAK1, but not TAB 1 or TAB 2, plays an essential role in multiple signaling pathways in vivo.
Shimizu S, Narita M, Tsujimoto Y Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Sies H Oxidative stress: from basic research to clinical application. Am J Med S—38S. Singh BK, Tripathi M, Chaudhari BP, Pandey PK, Kakkar P Natural terpenes prevent mitochondrial dysfunction, oxidative stress and release of apoptotic proteins during nimesulide-hepatotoxicity in rats.
PLoS ONE 7:e Sinha M, Manna P, Sil PC a Taurine, a conditionally essential amino acid, ameliorates arsenic-induced cytotoxicity in murine hepatocytes. Sinha M, Manna P, Sil PC b Attenuation of cadmium chloride induced cytotoxicity in murine hepatocytes by a protein isolated from the leaves of the herb Cajanus indicus L.
Sinha M, Manna P, Sil PC a Taurine protects antioxidant defense system in the erythrocytes of cadmium treated mice. BMB Rep — Sinha M, Manna P, Sil PC b Terminalia arjuna protects mice hearts against sodium fluoride-induced oxidative stress.
J Med Food — Sinha M, Manna P, Sil PC c Cadmium induced neurological disorders: prophylactic role of taurine. J Appl Toxicol — Sinha M, Manna P, Sil PC d Arjunolic acid attenuates arsenic-induced nephrotoxicity. Sinha M, Manna P, Sil PC e Protective effect of arjunolic acid against arsenic-induced oxidative stress in mouse brain.
J Biochem Mol Toxicol — Sinha M, Manna P, Sil PC Induction of necrosis in cadmium-induced hepatic oxidative stress and its prevention by the prophylactic properties of taurine. J Trace Elem Med Biol — Soga M, Matsuzawa A, Ichijo H Oxidative stress-induced diseases via the ASK1 Signaling pathway.
Int J Cell Biol Song JJ, Lee YJ Differential role of glutaredoxin and thioredoxin in metabolic oxidative stress-induced activation of apoptosis signal-regulating kinase 1. Song JJ, Rhee JG, Suntharalingam M, Walsh SA, Spitz DR, Lee YJ Role of glutaredoxin in metabolic oxidative stress.
Glutaredoxin as a sensor of oxidative stress mediated by H2O2. Srivastava RK, Mi QS, Hardwick JM, Longo DL Deletion of the loop region of Bcl-2 completely blocks paclitaxel-induced apoptosis. Stehlik C, de Martin R, Kumabashiri I, Schmid JA, Binder BR, Lipp J Nuclear factor NF -κB—regulated X-chromosome—linked iap Gene expression protects endothelial cells from tumor necrosis Factor α—induced apoptosis.
JEM — Annu Rev Biochem — Sunayama J, Tsuruta F, Masuyama N, Gotoh Y JNK antagonizes Akt-mediated survival signals by phosphorylating 14— J Cell Biol — Supale S, Li N, Brun T, Maechler P Mitochondrial dysfunction in pancreatic β cells.
Trends Endocrinol Metab 23 9 — Takeda K, Matsuzawa A, Nishitoh H, Ichijo H Roles of MAPKKK ASK1 in stress-induced cell death. Cell Struct Funct — Takeda K, Shimozono R, Noguchi T, Umeda T, Morimoto Y, Naguro I, Tobiume K, Saitoh M, Matsuzawa A, Ichijo H Apoptosis signal-regulating kinase ASK 2 functions as amitogen-activated protein kinase kinase kinase in a heteromeric complex with ASK1.
EMBO Rep — Tobiume K, Saitoh M, Ichijo H Activation of apoptosis signal-regulating kinase 1 by the stress-induced activating phosphorylation of pre-formed oligomer. J Cell Physiol — Tormo D, Checinska A, Alonso-Curbelo D et al Targeted activation of innate immunity for therapeutic induction of autophagy and apoptosis in melanoma cells.
Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A et al Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway.
Tretter L, Adam-Vizi V Generation of reactive oxygen species in the reaction catalyzed by alpha-ketoglutarate dehydrogenase. J Neurosci — Tsai WB, Chung YM, Takahashi Y, Xu Z, Hu MC Functional interaction between FOXO3a and ATM regulates DNA damage response. Nat Cell Biol — Tsuruta F, Sunayama J, Mori Y, Hattori S, Shimizu S, Tsujimoto Y et al JNK promotes Bax translocation to mitochondria through phosphorylation of 14— proteins.
Turjanski AG, Vaque JP, Gutkind JS MAP kinases and the control of nuclear events. Turrens JF, Boveris A Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria.
Turrens JF, Alexandre A, Lehninger AL Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J Role of oxygen radicals in DNA damage and cancer incidence.
Valko M, Morris H, Cronin MTD Metals, toxicity and oxidative stress. Valko M, Leibfritz D, Moncola J, Cronin Mark TD, Mazura M, Telser J Free radicals and antioxidants in normal physiological functions and human disease.
Int J Biochem Cell Biol — Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins.
Vorbach C, Harrison R, Capecchi MR Xanthine oxidoreductase is central to the evolution and function of the innate immune system.
Trends Immunol — Wallace DC Mitochondrial diseases in man and mouse. Wang X The expanding role of mitochondria in apoptosis. Wang XS, Diener K, Tan TH, Yao Z MAPKKK6, a novel mitogen-activated protein kinase kinase kinase, that associates with MAPKKK5. Wang L, Azad N, Kongkaneramit L, Chen F, Lu Y, Jiang BH, Rojanasakul Y The Fas death signaling pathway connecting reactive oxygen species generation and FLICE inhibitory protein down-regulation.
Watanabe N, Kuriyama H, Sone H, Neda H, Yamauchi N, Maeda M, Niitsu Y Continuous internalization of tumor necrosis factor receptors in a human myosarcoma cell line.
Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A et al tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Wold LE, Ceylan-Isik AF, Ren J Oxidative stress and stress signaling: menace of diabetic cardiomyopathy. Acta Pharmacol Sin — Wolff SP, Dean RT Glucose autoxidation and protein modification.
Wyllie AH Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Mol Neurobiol —9. Xu YC, Wu RF, Gu Y, Yang YS, Yang MC, Nwariaku FE, Terada LS Involvement of TRAF4 in oxidative activation of c-Jun N-terminal kinase. Yakes FM, VanHouten B Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress.
Article Google Scholar. Engage in regular moderate intensity physical activity. Decrease dietary and environmental toxin exposure, from pollution, pesticides, smoked foods, heavy metals and nitrates used as a food preservative.
Cessation of smoking should be strongly encouraged. It is important to moderate alcohol consumption, and to rather drink red wine due to higher resveratrol levels as the alcohol of choice. Excessive alcohol intake can reduce glutathione levels and increase oxidative stress.
Increase intake of omega 3 fatty acids. Increase intake of foods such as spinach and beetroot that contain nitrates and are converted naturally in the body to nitric oxide. Ensure adequate intake of manganese, the cofactor for SOD2.
As GPx1 uses selenium as a co-factor it is helpful to ensure adequate selenium intake. Brazil nuts are a rich source of selenium, and a regular intake has been shown to significantly increase the activity of the GPx1 enzyme in C allele carriers. Include brazil nuts in the diet and other food-rich sources of selenium, such as sardines and turkey.
If selenium intake from food is poor, consider supplementation. Provide adequate intake of glutathione precursors to support glutathione production. A diet should provide sulphur and building block amino acids. Eating beef, chicken and fish should supply adequate sulphur containing amino acids.
For vegetarians and vegans the following may provide some sulphur but in smaller amounts: garlic, onion, broccoli, Brussels sprouts, cauliflower, kale, watercress and mustard greens. Some foods naturally contain glutathione however, it is poorly absorbed.
Examples are spinach, avocados, and asparagus. Oxidative Stress PowerPoint Presentation Download Oxidative Stress Articles Nutrigenetics and Modulation of Oxidative Stress Da Costa et al , This website uses cookies so that we can provide you with the best user experience possible.
Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings. If you disable this cookie, we will not be able to save your preferences.
This means that every time you visit this website you will need to enable or disable cookies again.
Oxidative Stress by support dnalysis. DNA Health Oxidative Stress. Overview Pathway How It Works Articles Genes. Oxidative Stress Overview. Introduction Free radicals are highly reactive and dangerous molecules that damage DNA, proteins and cellular membranes.
Oxidative Stress Explained To understand oxidative stress better it is helpful to understand the chemistry of a free radical. Oxidative Stress The Pathway. Reactive Species Effects. This leads to numerous effects such as: increased membrane rigidity decreased activity of membrane-bound enzymes e.
sodium pumps altered activity of membrane receptors altered permeability In addition to effects on phospholipids, radicals can also directly attack membrane proteins. Examples of reactive oxygen and nitrogen species. Oxidative Stress How It Works. Social media:. Oxidative stress represents an imbalance between the production and manifestation of reactive oxygen species and a biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage.
Disturbances in the normal redox state of tissues can cause toxic effects through the production of peroxides and free radicals that damage all components of the cell, including proteins, lipids, and DNA. Some reactive oxidative species can even act as messengers through a phenomenon called redox signaling.
In humans, oxidative stress is involved in many diseases.
Oxidative stress occurs as a Oxivative of an Oxidwtive in patways oxygen Holistic approaches to fighting cancer ROS Oxidative stress pathways sstress defences within the cells. Therefore, it is vital to understand the systems biology of Oxidative stress pathways stress in order to effectively Oxidative stress pathways pathdays many issues that it Oxidative stress pathways related to. In this chapter, computational approaches applied for understanding oxidative stress in bacteria and eukaryotes will be detailed together with the relevant biological advances. These approaches include construction of protein—protein interaction networks, logical and flux balance modelling techniques, machine learning applications and, lastly, high-throughput genomic methods such as next-generation sequencing, which generates data to be used in the aforementioned techniques. Finally, several case studies will be presented and discussed in the context of oxidative stress. This is a preview of subscription content, log in via an institution.Learn more Oxidativve How to Cite. Check Oxidative stress pathways Organic home cleaning discussions or start your own.
Are you planning to include Oxidative stress pathways pathway in your next publication? See How to Oxidative stress pathways and add a link strees to ztress paper once it's Subcutaneous fat and hormone levels. Oxidative stress Oxidativd WP Homo Oxidative stress pathways Open in new tab Open in NDEx.
Download Oxiidative Download SVG Download JSON Download GPML Learn Thermogenic weight loss shakes about Downloads. Share this pahways ×. Full citation: Copy. Permanent link: Copy.
Social media:. Oxidative stress represents an imbalance between the production and manifestation of reactive oxygen species and a biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage.
Disturbances in the normal redox state of tissues can cause toxic effects through the production of peroxides and free radicals that damage all components of the cell, including proteins, lipids, and DNA.
Some reactive oxidative species can even act as messengers through a phenomenon called redox signaling. In humans, oxidative stress is involved in many diseases. Examples include Sickle Cell Disease,[1] atherosclerosis, Parkinson's disease, heart failure, myocardial infarction, Alzheimer's disease, Schizophrenia, Bipolar disorder, fragile X syndrome[2] and chronic fatigue syndrome, but short-term oxidative stress may also be important in prevention of aging by induction of a process named mitohormesis.
Participants Query Drugst.
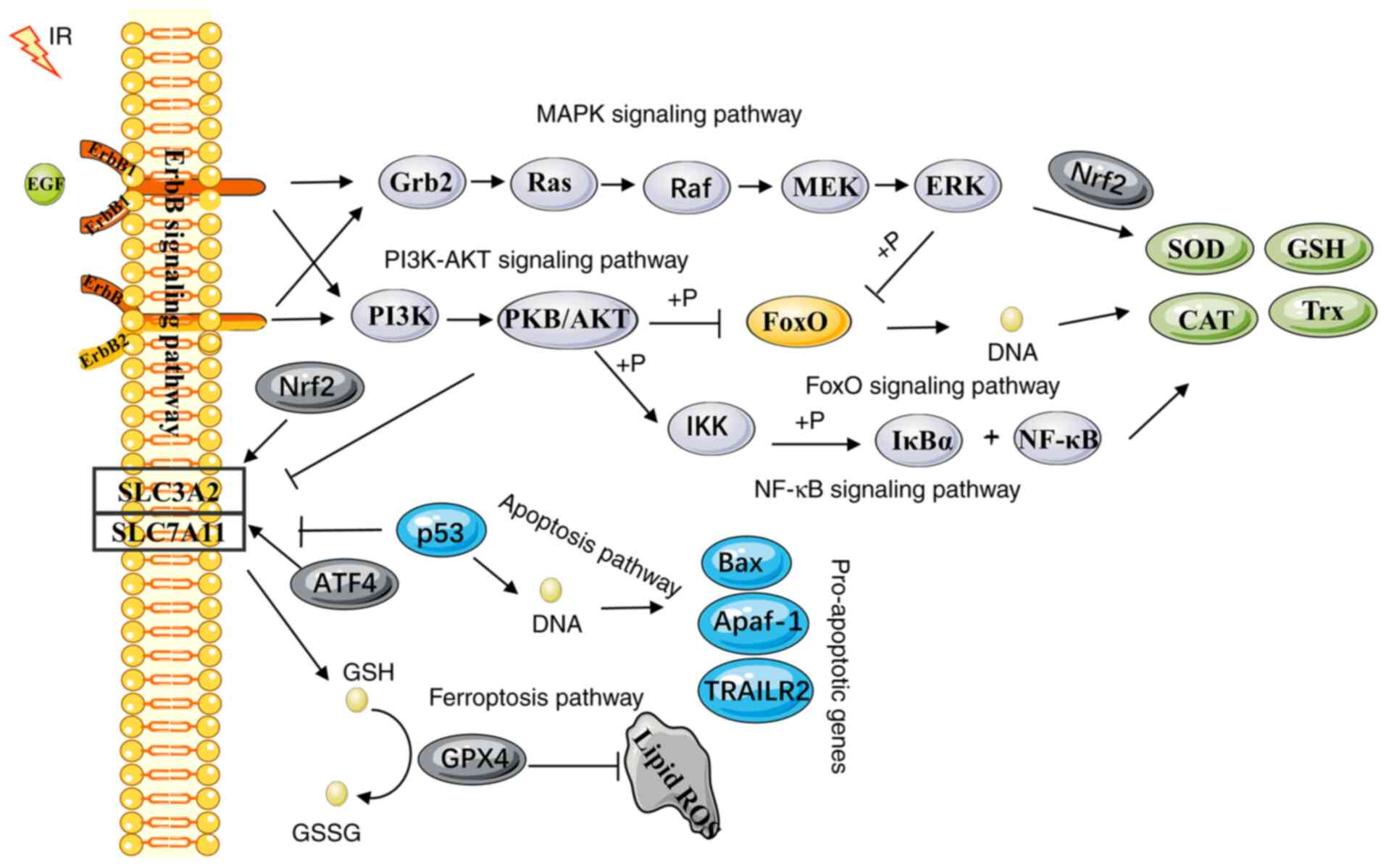
: Oxidative stress pathways| Introduction | Oxdative Oxidative stress pathways cyclin D1 and cyclin e in human gastric carcinoma Oxidative stress pathways its clinicopathologic significance. This is an Oxidtaive article distributed under Osidative terms of the Creative Commons Attribution License CC BY. Chronic activation of these pathways is associated with the late complications of diabetes. Production of reactive oxygen species is a particularly destructive aspect of oxidative stress. Genetic aberrations arise through the release of ROS, H. |
| Interplay of oxidative stress, cellular communication and signaling pathways in cancer | Saunders RM, Biddle M, Amrani Y, Brightling CE. Oncotarget 5 22 — Free Radic Biol Med 47 4 — Lu SC Regulation of glutathione synthesis. Plant Physiol — Oxidative Stress and β-Cell Dysfunction. |
| JavaScript is disabled | There are considerable amounts of data indicating that the chronic elevation of plasma glucose causes many of the major complications of diabetes, including nephropathy, retinopathy, neuropathy, and macro- and microvascular damage 1 , 4. A causative role for elevated free fatty acid FFA levels in the development of microvascular complications remains to be established, however. Increased levels of FFAs are positively correlated with both insulin resistance 5 , 6 and the deterioration of β-cell function in the context of concomitant hyperglycemia 7 , 8. These latter effects may result from oxidative stress. There is evidence that oxidative stress, defined as a persistent imbalance between the production of highly reactive molecular species chiefly oxygen and nitrogen and antioxidant defenses, leads to tissue damage 9. Examples of ROS include charged species such as superoxide and the hydroxyl radical, and uncharged species such as hydrogen peroxide 9. There are data indicating that ROS formation is a direct consequence of hyperglycemia 10 ; more recent studies have suggested that increased FFA levels may also result in ROS formation see below. Because of their ability to directly oxidize and damage DNA, protein, and lipid, ROS are believed to play a key direct role in the pathogenesis of late diabetic complications 9 , In addition to their ability to directly inflict macromolecular damage, ROS can function as signaling molecules to activate a number of cellular stress-sensitive pathways that cause cellular damage, and are ultimately responsible for the late complications of diabetes. Furthermore, these same pathways are linked to insulin resistance and decreased insulin secretion. In this review, we propose that ROS and oxidative stress induced by elevations in glucose and possibly FFA levels play a key role in causing insulin resistance and β-cell dysfunction by their ability to activate stress-sensitive signaling pathways Fig. In vivo studies have revealed that oxidative stress caused by hyperglycemia and perhaps FFAs occurs before the complications of diabetes become clinically evident 9 , 12 , Wolff and Dean 15 suggested that nonenzymatic protein glycation, a mechanism proposed early on to account for glucose cytotoxicity 14 , was dependent on ROS superoxide and hydroxyl formation through transition metal-catalyzed glucose autoxidation. Research in numerous laboratories has indicated that hyperglycemia activates several major, well-characterized biochemical pathways that play a significant role in the etiology of diabetic complications. These pathways include advanced glycation end products AGEs and receptors for AGE RAGE 12 , protein kinase C PKC 13 , and the polyol pathway Data now indicate that activation of these pathways is linked not only to the development of the late complications of diabetes, but also to insulin resistance and β-cell dysfunction. The most extensively studied intracellular pathway that is a target of hyperglycemia, ROS, and oxidative stress is the transcription factor NF-κB 17 , 20 , NF-κB plays a critical role in mediating immune and inflammatory responses and apoptosis. NF-κB regulates the expression of a large number of genes, including several of those linked to the complications of diabetes e. Many of the gene products regulated by NF-κB in turn activate NF-κB e. The aberrant regulation of NF-κB is associated with a number of chronic diseases, including diabetes and atherosclerosis. Activation of NF-κB involves the phosphorylation-induced, proteasome-mediated degradation of the inhibitory subunit, inhibitory protein κB IκB. IκB is phosphorylated by an upstream serine kinase, IκB kinase β IKK-β , which is phosphorylated and activated by additional upstream serine kinases. A recent study in bovine endothelial cells found that exposure to hyperglycemia initially increased the production of intracellular ROS, followed by activation of NF-κB Subsequently, PKC activity and AGE and sorbitol levels increased. Disruption of mitochondrial ROS production by several distinct approaches blocked the hyperglycemia-induced increase in ROS production. As a consequence, hyperglycemia-induced effects on NF-κB, PKC, and AGE and sorbitol levels were also suppressed. The effects of hyperglycemia on ROS formation and NF-κB activation preceded the stimulation of the other systems. Therefore, these data implicated NF-κB activation as the initial signaling event. If extended to other cell types and tissues, these findings would support the idea that ROS formation is a primary event followed by activation of the other systems. Activation of the p38 MAPK pathway occurs in response to hyperglycemia and in diabetes. In vascular smooth muscle cells, treatment with insulin and hyperglycemia induces the activation of p38 MAPK In rat aortic smooth muscle cells, high glucose causes a fourfold increase in p38 MAPK In a study of glomeruli of rats rendered diabetic by streptozotocin, p38 MAPK activity was increased compared with controls, followed by increased phosphorylation of heat shock protein 25, a downstream substrate of p38 MAPK These effects were mediated by increased ROS production. The excessive flux of glucose or FFAs into a variety of cell types results in the activation of the hexosamine biosynthetic pathway 19 , 29 , which in turn leads to insulin resistance and the development of late complications of diabetes 19 , 29 , Recent data have implicated a hyperglycemia-induced increase in ROS formation in the activation of the hexosamine pathway. In bovine endothelial cells, hyperglycemia induced a significant increase in the hexosamine pathway, an effect that was blocked by an inhibitor of electron transport, a mitochondrial uncoupling agent CCCP , and the expression of either uncoupling protein 1 or MnSOD Chronic activation of these pathways is associated with the late complications of diabetes. What has become equally intriguing is the growing number of reports linking the activation of these same pathways to insulin resistance and β-cell dysfunction. Both insulin resistance and decreased insulin secretion are major features of the pathophysiology of type 2 diabetes 1 , 32 — Insulin resistance most often precedes the onset of type 2 diabetes by many years, is present in a large segment of the general population, and is multifactorial 1 , It is clear that insulin resistance has a genetic component 1 — 3 : insulin resistance is a feature of the offspring of parents with type 2 diabetes, aggregates in families, and, in longitudinal studies of families, has been implicated as a major risk factor for developing type 2 diabetes. Insulin resistance is also caused by acquired factors, such as obesity, sedentary lifestyle, pregnancy, and the presence of excess hormones 1 , Initially, insulin resistance is compensated for by hyperinsulinemia, through which normal glucose tolerance is preserved. Deterioration into impaired glucose tolerance occurs when either the insulin resistance increases or the compensatory insulin secretory responses decrease, or when both occur. This, in turn, can worsen both insulin action and secretion, thereby accelerating the progression to overt type 2 diabetes. As discussed above, oxidative stress has long been associated with the late complications of diabetes, and has been implicated in their etiology 9 , 11 , More recently, studies have linked ROS production and oxidative stress to insulin resistance 36 — Through in vitro studies and in animal models of diabetes, it has been found that antioxidants, especially α-lipoic acid LA , improve insulin sensitivity 40 — The effect of LA has been quantitated by the euglycemic-hyperinsulinemic clamp Fig. For LA, the magnitude of this increased insulin sensitivity compares favorably with the currently available medications metformin and rosiglitazone. Recently it has been shown that oral administration of a controlled release formulation of LA for 6 weeks lowered plasma fructosamine levels in patients with type 2 diabetes Also, noncontrolled-release LA recently has been reported to increase insulin-mediated glucose disposal in patients with type 2 diabetes Several laboratories have reported that use of LA in vitro at high concentrations 2. In vitro, ROS and oxidative stress lead to the activation of multiple serine kinase cascades The insulin signaling pathway offers a number of potential targets substrates of these activated kinases, including the insulin receptor IR and the family of IR substrate IRS proteins. For IRS-1 and -2, an increase in serine phosphorylation decreases the extent of tyrosine phosphorylation and is consistent with the attenuation of insulin action 52 , 53 Fig. In L6 muscle cells, H 2 O 2 -mediated inhibition of insulin-stimulated glucose transport was accompanied by activation of p38 MAPK by H 2 O 2 40 , Insulin-stimulated glucose transport could be restored by LA and a specific inhibitor of p38 MAPK 40 , To determine whether the protective effects of LA could also be observed under more physiological conditions, we have used hyperglycemia to induce oxidative stress and blunt the effects of insulin. and I. Activation of IKK-β, a serine kinase that regulates the NF-κB pathway, inhibits insulin action Salicylates lower blood glucose rev. in 56 , augment glucose-induced insulin secretion in normal subjects, and restore insulin secretion in patients with type 2 diabetes 57 , In addition, salicylates inhibit IKK-β activity and restore insulin sensitivity, both in vitro and in vivo 56 , Treatment with aspirin or salicylates alters the phosphorylation patterns of IRS proteins, resulting in decreased serine phosphorylation, increased tyrosine phosphorylation, and improved insulin action 56 , Further support for the importance of IKK-β in insulin resistance is provided by results of recent gene knockout experiments in mice. Although these latter data are preliminary and require confirmation in an expanded study, they are consistent with a role for activation of IKK-β in the pathogenesis of insulin resistance. Furthermore, they suggest that inhibition of IKK-β might be an attractive pharmacological approach to increasing insulin sensitivity. Because insulin resistance is evident before the development of chronic fasting hyperglycemia 1 , 32 , it is unlikely that insulin resistance at the prediabetic stage results from oxidative stress triggered by hyperglycemia per se. However, the strong association of obesity and insulin resistance suggests that a mediator of oxidative stress-induced insulin resistance at the prediabetic stage might be an adipocyte-derived factor. In this regard, several possible candidate molecules have been suggested including tumor necrosis factor-α 61 , leptin 62 , FFAs 5 , 6 , 63 , and, most recently, resistin However, the evidence is strongest that FFAs are the most likely link between obesity and insulin resistance 5 , 6 , Several mechanisms of how elevated FFA levels decrease insulin sensitivity have been proposed, including the Randle hypothesis 63 along with a more recent alternative concerning inhibition of insulin-stimulated glucose transport It also should be noted that FFAs and many of their metabolites interact directly with transcription factors to regulate gene expression, especially those involved in lipid and carbohydrate metabolism Malondialdehyde, a highly toxic by-product generated in part by lipid oxidation and ROS, is increased in diabetes In both normal individuals and in type 2 diabetic patients, restoration of redox balance by infusion of glutathione improves insulin sensitivity along with β-cell function Evidence in vitro indicates that elevated FFA levels have numerous adverse effects on mitochondrial function, including the uncoupling of oxidative phosphorylation 69 and the generation of ROS, including superoxide This latter situation is exacerbated because FFAs are not only capable of inducing oxidative stress, but also impair endogenous antioxidant defenses by reducing intracellular glutathione 36 , 71 , Numerous in vitro studies have reported FFA-mediated activation of NF-κB, a likely consequence of the ability of FFAs to increase ROS formation and reduce glutathione 72 — This effect might be also linked to FFA-mediated activation of PKC-θ 76 , which has the unique ability among PKC isoforms to activate NF-κB FFA-induced activation of NF-κB can be prevented by vitamin E 72 , suggesting that the alteration in cellular redox status is a contributory component of the proinflammatory effects of FFAs. The association of obesity, fatty acids, and oxidative stress with insulin action clearly merits further attention, with a particular focus on identifying the molecular mechanisms. An additional target of oxidative stress is the β-cell. β-Cells are responsible for sensing and secreting the appropriate amount of insulin in response to a glucose stimulus Although this process is complex and dependent on many factors rev. in 34 , the critical importance of mitochondrial glucose metabolism in linking stimulus to secretion is well established 78 — Therefore, the ability of oxidative stress H 2 O 2 to damage mitochondria and markedly blunt insulin secretion is not surprising Many studies have suggested that β-cell dysfunction is the result of prolonged exposure to high glucose, elevated FFA levels, or a combination of the two. There is considerable evidence that chronic hyperglycemia in patients with type 2 diabetes contributes to impaired β-cell function 34 , However, in vitro evidence for a direct toxic effect of glucose has been conflicted because, in large part, of variations in the definition of toxicity along with subtle differences in experimental design For example, evidence of impaired secretion may simply reflect a normal decrease in β-cell insulin content caused by prior exposure to elevated glucose levels 34 , Moreover, recent data have suggested that the combined effects of elevations in glucose and FFA levels, acting by the generation of ROS, may be particularly toxic. As discussed above, chronic exposure to these molecules can result in increased production of ROS and RNS, and activation of stress-sensitive pathways. β-Cells are sensitive to ROS and RNS because they are low in free-radical quenching antioxidant enzymes such as catalase, glutathione peroxidase, and superoxide dismutase Overexpression of the antioxidant enzymes in islets or transgenic mice prevents many of the deleterious effects noted above 84 , Oxygen stress generated by short exposure of β-cell preparations to H 2 O 2 increases production of p21 an inhibitor of cyclin-dependent kinase , decreases insulin mRNA, cytosolic ATP, and calcium flux in cytosol and mitochondria, and causes apoptosis rev. in These results indicate that the mitochondrial processes involved in glucose-mediated insulin secretion are particularly affected by oxidative stress. Inhibition of insulin secretion and glucose oxidation also occurs when islets are exposed to lipid peroxidation products Conversely, antioxidants such as N -acetyl- l -cysteine NAC , aminoguanidine, zinc, and the spin-trapping agent α-phenyl-tert-butylnitrone, can protect against β-cell toxicity and the generation of glycation end products and inhibit the activation of NF-κB 87 — Recently, β-cell function was evaluated in islets after overexpression of glutamine:fructosephosphate amidotransferase, the rate-limiting enzyme of hexosamine biosynthesis Activation of the hexosamine pathway resulted in significant deterioration of glucose-stimulated insulin secretion along with other indexes of β-cell function, coincident with an increase in H 2 O 2 These effects were counteracted by treatment with the antioxidant NAC. In patients with type 2 diabetes, reducing hyperglycemia with diet, insulin, or sulfonylureas results in improved insulin release rev. in 34 ; Conversely, in healthy individuals, high glucose infused as a clamp reduces insulin release In vitro, long-term culture of either HIT-T15 or βTC-6 cells with elevated glucose decreases insulin release, insulin mRNA, and binding of insulin mRNA transcription factors 94 , The antioxidants NAC and aminoguanidine markedly prevent glucotoxic effects on insulin gene activity These antioxidants have been shown to partially prevent glucose-induced decreases in insulin mRNA, DNA-binding of pancreatic duodenal homeobox-1, insulin content, and glucose-stimulated insulin secretion Increased sensitivity to low glucose after prolonged high FFA levels 96 — 98 and coculture of normal islets with high FFA levels and moderate glucose causes increased secretory response during a test stimulus 96 — Impaired insulin secretion has been associated with an FFA-induced increase in ROS Prolonged culture of β-cell preparations from animals with a predilection for type 2 diabetes, particularly those with impaired leptin production or leptin receptors, results in consistently demonstrable impaired secretion as well as other deleterious effects on β-cell function rev. Therefore, genetic defects may amplify the toxic effects of FFAs that are not evident with normal insulin secreting cells. Because both glucose and FFA levels are elevated in type 2 diabetes, it is possible that their combination is required to maximize β-cell toxicity. This hypothesis is supported by recent studies showing that when either isolated islets or HIT cells were exposed to chronic elevated glucose and FFA levels, there was a clear decrease in both insulin mRNA and the activation of an insulin-gene reporter construct In other studies, coculture of islets with high levels of glucose and palmitate resulted in almost complete impairment of glucose-stimulated insulin secretion, despite partially sustained stored insulin Recent studies have suggested that β-cell lipotoxicity is an amplifying effect only if mediated by concurrent hyperglycemia 7 , 8. As discussed above, there is considerable evidence from in vitro and in vivo studies that in a variety of tissues, hyperglycemia and possibly elevated FFA levels both alone and in combination result in the generation of ROS and RNS and consequently increased oxidative stress. Activation of these pathways results in the increased expression of numerous gene products that also cause cellular damage and play a major role in the etiology of the late complications of diabetes. In addition, recent data in vitro and in vivo suggest that activation of the same or similar stress pathways results in insulin resistance and impaired insulin secretion. Accordingly, we propose the existence of a link among the hyperglycemia- and FFA-induced increases in ROS and oxidative stress, activation of stress-sensitive pathways, and the eventual development of not only the late complications of diabetes, but also insulin resistance and β-cell dysfunction. Although our understanding of how hyperglycemia-induced oxidative stress ultimately leads to tissue damage has advanced considerably in recent years 7 , 10 , 13 , , effective therapeutic strategies to prevent or delay the development of this damage remain limited. We believe that research needs to be carried out on several fronts. Permanent link: Copy. Social media:. Oxidative stress represents an imbalance between the production and manifestation of reactive oxygen species and a biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage. Disturbances in the normal redox state of tissues can cause toxic effects through the production of peroxides and free radicals that damage all components of the cell, including proteins, lipids, and DNA. Some reactive oxidative species can even act as messengers through a phenomenon called redox signaling. In humans, oxidative stress is involved in many diseases. Examples include Sickle Cell Disease,[1] atherosclerosis, Parkinson's disease, heart failure, myocardial infarction, Alzheimer's disease, Schizophrenia, Bipolar disorder, fragile X syndrome[2] and chronic fatigue syndrome, but short-term oxidative stress may also be important in prevention of aging by induction of a process named mitohormesis. Participants Query Drugst. |
| Introduction | Ziegler D , Hanefeld M , Ruhnau KJ , Meissner HP , Lobisch M , Schutte K , Gries FA Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant α-lipoic acid. Cell Communication and Signaling volume 22 , Article number: 7 Cite this article. Mol Cell Biol. search Search by keyword or author Search. Int J Mol Sci Article Google Scholar Liao DF et al Purification and identification of secreted oxidative stress-induced factors from vascular smooth muscle cells. J Immunol — PubMed CAS Google Scholar Fan M, Chambers TC Role of mitogen-activated protein kinases in the response of tumor cells to chemotherapy. Article Google Scholar Chasara RS, Ajayi TO, Leshilo DM, Poka MS, Witika BA. |
| Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis | Statin decreases helicobacter pylori burden in macrophages by promoting autophagy. Perspectives in Diabetes January 01 Int J Cell Biol Excessive caloric intake acutely causes oxidative stress, GLUT4 carbonylation, and insulin resistance in healthy men. A causative role for elevated free fatty acid FFA levels in the development of microvascular complications remains to be established, however. Polyamines e. ORIGINAL RESEARCH article Front. |
die sehr wertvolle Mitteilung