Quercetin and anti-cancer properties -
Physiol Rev. Chen S-F, Nieh S, Jao S-W, Liu C-L, Wu C-H, Chang Y-C, Yang C-Y, Lin Y-S. Quercetin suppresses drug-resistant spheres via the p38 MAPK—Hsp27 apoptotic pathway in oral cancer cells.
Huang G, Tang B, Tang K, Dong X, Deng J, Liao L, Liao Z, Yang H, He S. Isoquercitrin inhibits the progression of liver cancer in vivo and in vitro via the MAPK signalling pathway. Kim MC, Lee HJ, Lim B, Ha K-T, Kim SY, So I, Kim BJ. Quercetin induces apoptosis by inhibiting MAPKs and TRPM7 channels in AGS cells.
Lim W, Yang C, Park S, Bazer FW, Song G. J Cell Physiol. Zhu Y, Jiang Y, Shi L, Du L, Xu X, Wang E, Sun Y, Guo X, Zou B, Wang H. Liu H, Zhou M. Antitumor effect of Quercetin on Y79 retinoblastoma cells via activation of JNK and p38 MAPK pathways.
BMC Complement Altern Med. Zhao S, Jiang Y, Zhao J, Li H, Yin X, Wang Y, Xie Y, Chen X, Lu J, Dong Z. Mol Carcinog. Erdogan S, Turkekul K, Dibirdik I, Doganlar O, Doganlar ZB, Bilir A, Oktem G. Kedhari Sundaram M, Raina R, Afroze N, Bajbouj K, Hamad M, Haque S, Hussain A.
Quercetin modulates signaling pathways and induces apoptosis in cervical cancer cells. Kim S-H, Yoo E-S, Woo J-S, Han S-H, Lee J-H, Jung S-H, Kim H-J, Jung J-Y. Eur J Pharmacol. Ryu S, Park S, Lim W, Song G. Quercetin augments apoptosis of canine osteosarcoma cells by disrupting mitochondria membrane potential and regulating PKB and MAPK signal transduction.
J Cell Biochem. Xavier CP, Lima CF, Rohde M, Pereira-Wilson C. Quercetin enhances 5-fluorouracil-induced apoptosis in MSI colorectal cancer cells through p53 modulation. Cancer Chemother Pharmacol. Wang G, Zhang J, Liu L, Sharma S, Dong Q. Bishayee K, Ghosh S, Mukherjee A, Sadhukhan R, Mondal J, Khuda-Bukhsh A.
Cell Prolif. Kim GT, Lee SH, Kim YM. Quercetin regulates sestrin 2-AMPK-mTOR signaling pathway and induces apoptosis via increased intracellular ROS in HCT colon cancer cells.
J Cancer Prev. Chan S-T, Yang N-C, Huang C-S, Liao J-W, Yeh S-L. Quercetin enhances the antitumor activity of trichostatin A through upregulation of p53 protein expression in vitro and in vivo.
Youn H, Jeong J-C, Jeong YS, Kim E-J, Um S-J. Quercetin potentiates apoptosis by inhibiting nuclear factor-kappaB signaling in H lung cancer cells.
Biol Pharm Bull. Tarhriz V, Eyvazi S, Musavi M, Abasi M, Sharifi K, Ghanbarian H, Hejazi MS. Transient induction of Cdk9 in the early stage of differentiation is critical for myogenesis. Kim GT, Lee SH, Kim JI, Kim YM.
Quercetin regulates the sestrin 2-AMPK-p38 MAPK signaling pathway and induces apoptosis by increasing the generation of intracellular ROS in a pindependent manner. Bishayee K, Khuda-Bukhsh AR, Huh S-O. PLGA-loaded gold-nanoparticles precipitated with quercetin downregulate HDAC-Akt activities controlling proliferation and activate pROS crosstalk to induce apoptosis in hepatocarcinoma cells.
Mol Cells. Vadde R, Radhakrishnan S, Reddivari L, Vanamala JK. BioMed Res Int. Fan Y, Lu H, Ma H, Feng F, Hu X, Zhang Q, Wang J, Xu Y, Zhao Q.
Bioactive compounds of Eriocaulon sieboldianum blocking proliferation and inducing apoptosis of HepG2 cells might be involved in Aurora kinase inhibition. Yang L, Liu Y, Wang M, Qian Y, Dong X, Gu H, Wang H, Guo S, Hisamitsu T. Quercetin-induced apoptosis of HT colon cancer cells via inhibition of the Akt-CSN6-Myc signaling axis.
Mol Med Rep. Satari A, Amini SA, Raeisi E, Lemoigne Y, Heidarian E. Synergetic impact of combined 5-fluorouracil and rutin on apoptosis in pc3 cancer cells through the modulation of p53 gene expression. Adv Pharm Bull. Chan S-T, Chuang C-H, Lin Y-C, Liao J-W, Lii C-K, Yeh S-L. Quercetin enhances the antitumor effect of trichostatin A and suppresses muscle wasting in tumor-bearing mice.
Nguyen LT, Lee Y-H, Sharma AR, Park J-B, Jagga S, Sharma G, Lee S-S, Nam J-S. Quercetin induces apoptosis and cell cycle arrest in triple-negative breast cancer cells through modulation of Foxo3a activity.
Korean J Physiol Pharmacol. Ward AB, Mir H, Kapur N, Gales DN, Carriere PP, Singh S. Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways.
World J Surg Oncol. Gong C, Yang Z, Zhang L, Wang Y, Gong W, Liu Y. Quercetin suppresses DNA double-strand break repair and enhances the radiosensitivity of human ovarian cancer cells via pdependent endoplasmic reticulum stress pathway.
Onco Targets Ther. Article PubMed Google Scholar. Roy S, Das R, Ghosh B, Chakraborty T. Deciphering the biochemical and molecular mechanism underlying the in vitro and in vivo chemotherapeutic efficacy of ruthenium quercetin complex in colon cancer.
Roy S, Banerjee S, Chakraborty T. Shang HS, Lu HF, Lee CH, Chiang HS, Chu YL, Chen A, Lin YF, Chung JG. Quercetin induced cell apoptosis and altered gene expression in AGS human gastric cancer cells. Environ Toxicol. Li H, Tan L, Zhang J-W, Chen H, Liang B, Qiu T, Li Q-S, Cai M, Zhang Q-H. Quercetin is the active component of Yang-Yin-Qing-Fei-Tang to induce apoptosis in non-small cell lung cancer.
Am J Chin Med. Chuang C-H, Chan S-T, Chen C-H, Yeh S-L. Quercetin enhances the antitumor activity of trichostatin A through up-regulation of p protein expression in p53 null cancer cells.
Chem Biol Interact. Clemente-Soto AF, Salas-Vidal E, Milan-Pacheco C, Sánchez-Carranza JN, Peralta-Zaragoza O, González-Maya L. Quercetin induces G2 phase arrest and apoptosis with the activation of p53 in an E6 expression-independent manner in HPV-positive human cervical cancer-derived cells.
CAS PubMed PubMed Central Google Scholar. Xu Z, Zhao D, Zheng X, Huang B, Xia X, Pan X. Quercetin exerts bidirectional regulation effects on the efficacy of tamoxifen in estrogen receptor-positive breast cancer therapy: An in vitro study. Mutlu Altundağ E, Yılmaz AM, Koçtürk S, Taga Y, Yalçın AS.
Synergistic induction of apoptosis by quercetin and curcumin in chronic myeloid leukemia K cells. Nutr Cancer. Liu Z-J, Xu W, Han J, Liu Q-Y, Gao L-F, Wang X-H, WLi X-L.
Quercetin induces apoptosis and enhances gemcitabine therapeutic efficacy against gemcitabine-resistant cancer cells. Anti Cancer Drugs. Hassan S, Peluso J, Chalhoub S, Idoux Gillet Y, Benkirane-Jessel N, Rochel N, Fuhrmann G, Ubeaud-Sequier G.
Quercetin potentializes the respective cytotoxic activity of gemcitabine or doxorubicin on 3D culture of AsPC-1 or HepG2 cells, through the inhibition of HIF-1α and MDR1. Sharifi-Rad J, Quispe C, Imran M, Rauf A, Nadeem M, Gondal TA, Ahmad B, Atif M, Mubarak MS, Sytar O, et al.
Genistein: an integrative overview of its mode of action, pharmacological properties, and health benefits. Zlatian OM, Comanescu MV, Rosu AF, Rosu L, Cruce M, Gaman AE, Calina CD, Sfredel V. Histochemical and immunohistochemical evidence of tumor heterogeneity in colorectal cancer. Rom J Morphol Embryol.
PubMed Google Scholar. Jain D, Chaudhary P, Varshney N, Bin Razzak KS, Verma D, Zahra TRK, Janmeda P, Sharifi-Rad J, Dastan SD, Mahmud S, et al. Tobacco smoking and liver cancer risk: potential avenues for carcinogenesis. J Oncol. Hurley JH, Young LN.
Mechanisms of autophagy initiation. Annu Rev Biochem. Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. Geng J, Klionsky DJ. The Atg8 and Atg12 ubiquitin-like conjugation systems in macroautophagy.
EMBO Rep. Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol.
Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL. Mitochondrial autophagy is an HIFdependent adaptive metabolic response to hypoxia.
Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouysségur J, Mazure NM. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol. Luo S, Rubinsztein D. Atg5 and Bcl-2 provide novel insights into the interplay between apoptosis and autophagy.
Cell Death Differ. Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, Brunner T, Simon H-U.
Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Levine B, Sinha SC, Kroemer G. Bcl-2 family members: dual regulators of apoptosis and autophagy.
Wang K, Liu R, Li J, Mao J, Lei Y, Wu J, Zeng J, Zhang T, Wu H, Chen L. Quercetin induces protective autophagy in gastric cancer cells: involvement of Akt-mTOR-and hypoxia-induced factor 1α-mediated signaling. Kim H, Moon JY, Ahn KS, Cho SK.
Quercetin induces mitochondrial mediated apoptosis and protective autophagy in human glioblastoma UMG cells. Psahoulia FH, Moumtzi S, Roberts ML, Sasazuki T, Shirasawa S, Pintzas A.
Quercetin mediates preferential degradation of oncogenic Ras and causes autophagy in Ha-RAS-transformed human colon cells. Bi Y, Shen C, Li C, Liu Y, Gao D, Shi C, Peng F, Liu Z, Zhao B, Zheng Z.
Inhibition of autophagy induced by quercetin at a late stage enhances cytotoxic effects on glioma cells. Tumor Biol. White E. The role for autophagy in cancer. J Clin Investig. Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. Chai JY, Sugumar V, Alshawsh MA, Wong WF, Arya A, Chong PP, Looi CY.
The role of smoothened-dependent and -independent hedgehog signaling pathway in tumorigenesis. Skoda AM, Simovic D, Karin V, Kardum V, Vranic S, Serman L. The role of the Hedgehog signaling pathway in cancer: a comprehensive review. Bosn J Basic Med Sci. Qi X, Li X.
Mechanistic insights into the generation and transduction of hedgehog signaling. Trends Biochem Sci. Carpenter RL, Ray H.
Safety and tolerability of sonic hedgehog pathway inhibitors in cancer. Drug Saf. Doheny D, Manore SG, Wong GL, Lo HW. Hedgehog Signaling and Truncated GLI1 in Cancer.
Mousavi N, Rahimi S, Emami H, Kazemi AH, Mohammad Taghi Kashi R, Heidarian R. The effect of quercetin nanosuspension on prostate cancer cell line LNCaP via Hedgehog signaling pathway.
Rep Biochem Mol Biol. Ślusarz A, Shenouda NS, Sakla MS, Drenkhahn SK, Narula AS, MacDonald RS, Besch-Williford CL, Lubahn DB. Common botanical compounds inhibit the hedgehog signaling pathway in prostate cancer.
Nwaeburu CC, Bauer N, Zhao Z, Abukiwan A, Gladkich J, Benner A, Herr I. Up-regulation of microRNA let-7c by quercetin inhibits pancreatic cancer progression by activation of Numbl.
Nwaeburu CC, Abukiwan A, Zhao Z, Herr I. Quercetin-induced miRb-3p regulates the mode of self-renewing divisions in pancreatic cancer. Mol Cancer.
MacKenzie TN, Mujumdar N, Banerjee S, Sangwan V, Sarver A, Vickers S, Subramanian S, Saluja AK. Triptolide induces the expression of miRp: a negative regulator of heat shock protein 70 and pancreatic cancer cell proliferation.
Mol Cancer Ther. Sonoki H, Sato T, Endo S, Matsunaga T, Yamaguchi M, Yamazaki Y, Sugatani J, Ikari A. Quercetin decreases claudin-2 expression mediated by up-regulation of microRNA miR in lung adenocarcinoma A cells. Wang Q, Chen Y, Lu H, Wang H, Feng H, Xu J, Zhang B.
IUBMB Life. Salehi B, Jornet PL, Lopez EPF, Calina D, Sharifi-Rad M, Ramirez-Alarcon K, Forman K, Fernandez M, Martorell M, Setzer WN, et al. Plant-derived bioactives in oral mucosal lesions: a key emphasis to curcumin, lycopene, chamomile, aloe vera, green tea and coffee properties.
Zhao J, Fang Z, Zha Z, Sun Q, Wang H, Sun M, Qiao B. Zhang C, Hao Y, Sun Y, Liu P. J Pharmacol Sci. Tao SF, He HF, Chen Q. Quercetin inhibits proliferation and invasion acts by up-regulating miRa in human breast cancer cells. Mol Cell Biochem. Zhou J, Gong J, Ding C, Chen G.
Quercetin induces the apoptosis of human ovarian carcinoma cells by upregulating the expression of microRNA Zhang Z, Li B, Xu P, Yang B. Integrated whole transcriptome profiling and bioinformatics analysis for revealing regulatory pathways associated with quercetin-induced apoptosis in HCT cells.
Zhang X, Guo Q, Chen J, Chen Z. Quercetin enhances cisplatin sensitivity of human osteosarcoma cells by modulating microRNAKRAS axis. Ramos YAL, Souza OF, Novo MCT, Guimarães CFC, Popi AF. Pratheeshkumar P, Son YO, Divya SP, Wang L, Turcios L, Roy RV, Hitron JA, Kim D, Dai J, Asha P, et al.
Quercetin inhibits Cr VI -induced malignant cell transformation by targeting miRPDCD4 signaling pathway. Sani TA, Mohammadpour E, Mohammadi A, Memariani T, Yazdi MV, Rezaee R, Calina D, Docea AO, Goumenou M, Etemad L, et al.
Cytotoxic and apoptogenic properties of dracocephalum kotschyi aerial part different fractions on calu-6 and mehr lung cancer cell lines. Salehi B, Shetty MS, Kumar NVA, Zivkovic J, Calina D, Docea AO, Emamzadeh-Yazdi S, Kilic CS, Goloshvili T, Nicola S, et al. Veronica plants-drifting from farm to traditional healing, food application, and phytopharmacology.
Gibellini L, Pinti M, Nasi M, Montagna JP, De Biasi S, Roat E, Bertoncelli L, Cooper EL, Cossarizza A. Quercetin and cancer chemoprevention.
Evid Based Complement Altern Med. Alper M, Güneş H. The anticancer and anti-inflammatory effects of Centaurea solstitialis extract on human cancer cell lines. Turkish J Pharm Sci. Kumar R, Vijayalakshmi S, Nadanasabapathi S. Health benefits of quercetin.
Def Life Sci J. Mohsin NUA, Irfan M, Hassan SU, Saleem U. Current strategies in development of new chromone derivatives with diversified pharmacological activities: a review.
Pharm Chem J. Michala A-S, Pritsa A. Quercetin: a molecule of great biochemical and clinical value and its beneficial effect on diabetes and cancer. López-Lázaro M. Flavonoids as anticancer agents: structure-activity relationship study.
Curr Med Chem Anticancer Agents. Wang TY, Li Q, Bi KS. Bioactive flavonoids in medicinal plants: structure, activity and biological fate. Asian J Pharm Sci. Reyes-Farias M, Carrasco-Pozo C. The anti-cancer effect of quercetin: molecular implications in cancer metabolism.
Int J Mol Sci. Persano F, Gigli G, Leporatti S. Natural compounds as promising adjuvant agents in the treatment of gliomas.
Singhal RL, Yeh YA, Prajda N, Olah E, Sledge G, Weber G. Quercetin down-regulates signal transduction in human breast carcinoma cells.
Biochem Biophys Res Commun. Staedler D, Idrizi E, Kenzaoui BH, Juillerat-Jeanneret L. Drug combinations with quercetin: doxorubicin plus quercetin in human breast cancer cells. Li X, Guo S, Xiong XK, Peng BY, Huang JM, Chen MF, Wang FY, Wang JN. Combination of quercetin and cisplatin enhances apoptosis in OSCC cells by downregulating xIAP through the NF-κB pathway.
J Cancer. Yang FQ, Liu M, Li W, Che JP, Wang GC, Zheng JH. Combination of quercetin and hyperoside inhibits prostate cancer cell growth and metastasis via regulation of microRNA Li W, Liu M, Xu Y-F, Feng Y, Che J-P, Wang G-C, Zheng J-H.
Combination of quercetin and hyperoside has anticancer effects on renal cancer cells through inhibition of oncogenic microRNAa. Almatroodi SA, Alsahli MA, Almatroudi A, Verma AK, Aloliqi A, Allemailem KS, Khan AA, Rahmani AH. Potential therapeutic targets of quercetin, a plant flavonol, and its role in the therapy of various types of cancer through the modulation of various cell signaling pathways.
Singh CK, Chhabra G, Ndiaye MA, Siddiqui IA, Panackal JE, Mintie CA, Ahmad N. Quercetin-resveratrol combination for prostate cancer management in TRAMP Mice. Appari M, Babu KR, Kaczorowski A, Gross W, Herr I. Sulforaphane, quercetin and catechins complement each other in elimination of advanced pancreatic cancer by miR-let-7 induction and K-ras inhibition.
Del Follo-Martinez A, Banerjee N, Li X, Safe S, Mertens-Talcott S. Resveratrol and quercetin in combination have anticancer activity in colon cancer cells and repress oncogenic microRNAa. Wang P, Phan T, Gordon D, Chung S, Henning SM, Vadgama JV. Arctigenin in combination with quercetin synergistically enhances the antiproliferative effect in prostate cancer cells.
Mol Nutr Food Res. Painuli S, Quispe C, Herrera-Bravo J, Semwal P, Martorell M, Almarhoon ZM, Seilkhan A, Ydyrys A, Rad JS, Alshehri MM, et al. Nutraceutical profiling, bioactive composition, and biological applications of Lepidium sativum L. Salehi B, Sharifi-Rad J, Capanoglu E, Adrar N, Catalkaya G, Shaheen S, Jaffer M, Giri L, Suyal R, Jugran AK, et al.
Cucurbita plants: from farm to industry. Appl Sci-Basel. Bukowski K, Kciuk M, Kontek R. Mechanisms of multidrug resistance in cancer chemotherapy. Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM. P-glycoprotein: from genomics to mechanism. Borska S, Sopel M, Chmielewska M, Zabel M, Dziegiel P.
Quercetin as a potential modulator of P-glycoprotein expression and function in cells of human pancreatic carcinoma line resistant to daunorubicin.
Limtrakul P, Khantamat O, Pintha K. Inhibition of P-glycoprotein function and expression by kaempferol and quercetin.
J Chemother. Gates MA, Vitonis AF, Tworoger SS, Rosner B, Titus-Ernstoff L, Hankinson SE, Cramer DW. Flavonoid intake and ovarian cancer risk in a population-based case-control study. Int J Cancer. Song NR, Chung M-Y, Kang NJ, Seo SG, Jang TS, Lee HJ, Lee KW. Quercetin suppresses invasion and migration of H-Ras-transformed MCF10A human epithelial cells by inhibiting phosphatidylinositol 3-kinase.
Food Chem. Sharifi-Rad J, Quispe C, Bouyahya A, El Menyiy N, El Omari N, Shahinozzaman M, Ara Haque Ovey M, Koirala N, Panthi M, Ertani A, et al. Ethnobotany, phytochemistry, biological activities, and health-promoting effects of the genus bulbophyllum.
Evid Based Complement Alternat Med. Buga AM, Docea AO, Albu C, Malin RD, Branisteanu DE, Ianosi G, Ianosi SL, Iordache A, Calina D. Molecular and cellular stratagem of brain metastases associated with melanoma.
Srivastava S, Somasagara RR, Hegde M, Nishana M, Tadi SK, Srivastava M, Choudhary B, Raghavan SC. Quercetin, a natural flavonoid interacts with DNA, arrests cell cycle and causes tumor regression by activating mitochondrial pathway of apoptosis. Sci Rep. Taheri Y, Quispe C, Herrera-Bravo J, Sharifi-Rad J, Ezzat SM, Merghany RM, Shaheen S, Azmi L, Prakash Mishra A, Sener B, et al.
Urtica dioica-derived phytochemicals for pharmacological and therapeutic applications. Sharifi-Rad J, Quispe C, Shaheen S, El Haouari M, Azzini E, Butnariu M, Sarac I, Pentea M, Ramírez-Alarcón K, Martorell M, et al.
Flavonoids as potential anti-platelet aggregation agents: from biochemistry to health promoting abilities. Crit Rev Food Sci Nutr. Ahmed MS, Ainley K, Parish JH, Hadi SM. Free radical-induced fragmentation of proteins by quercetin. Rather RA, Bhagat M.
Quercetin as an innovative therapeutic tool for cancer chemoprevention: molecular mechanisms and implications in human health. Bors W, Saran M. Radical scavenging by flavonoid antioxidants. Free Radical Res Commun. Choi J-A, Kim J-Y, Lee J-Y, Kang C-M, Kwon H-J, Yoo Y-D, Kim T-W, Lee Y-S, Lee S-J.
Induction of cell cycle arrest and apoptosis in human breast cancer cells by quercetin. Chen J-C, Ho F-M, Chao P-DL, Chen C-P, Jeng K-CG, Hsu H-B, Lee S-T, Wu WT, Lin W-W.
Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IκB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin.
Cancer Lett. Shen F, Herenyiova M, Weber G. Synergistic down-regulation of signal transduction and cytotoxicity by tiazofurin and quercetin in human ovarian carcinoma cells. Life Sci. Brusselmans K, Vrolix R, Verhoeven G, Swinnen JV. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity.
Daker M, Ahmad M, Khoo AS. Quercetin-induced inhibition and synergistic activity with cisplatin—a chemotherapeutic strategy for nasopharyngeal carcinoma cells. Zhong L, Chen F, Wang H, Ten Y, Wang C, Ouyang R. Effects of quercetin on morphology and VEGF secretion of leukemia cells NB4 in vitro.
Zhonghua zhong liu za zhi [Chin J Oncol]. Vijayababu M, Arunkumar A, Kanagaraj P, Venkataraman P, Krishnamoorthy G, Arunakaran J. Quercetin downregulates matrix metalloproteinases 2 and 9 proteins expression in prostate cancer cells PC Cerutti PA. Prooxidant states and tumor promotion.
Tan S, Wang C, Lu C, Zhao B, Cui Y, Shi X, Ma X. Quercetin is able to demethylate the p16INK4a gene promoter. Xing N, Chen Y, Mitchell SH, Young CY.
Quercetin inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells. Tanigawa S, Fujii M, Hou D-X. Stabilization of p53 is involved in quercetin-induced cell cycle arrest and apoptosis in HepG2 cells. Biosci Biotechnol Biochem. Nair HK, Rao KV, Aalinkeel R, Mahajan S, Chawda R, Schwartz SA.
Inhibition of prostate cancer cell colony formation by the flavonoid quercetin correlates with modulation of specific regulatory genes.
Clin Vaccine Immunol. Anti-inflammatory, antioxidant and anticancer activity of quercetin and its analogues. Int J Res in Pharma and Biomed Sci. Kim WK, Bang MH, Kim ES, Kang NE, Jung KC, Cho HJ, Park JH. Quercetin decreases the expression of ErbB2 and ErbB3 proteins in HT human colon cancer cells.
J Nutr Biochem. Li S, Pei Y, Wang W, Liu F, Zheng K, Zhang X. Quercetin suppresses the proliferation and metastasis of metastatic osteosarcoma cells by inhibiting parathyroid hormone receptor 1. Zhou W, Kallifatidis G, Baumann B, Rausch V, Mattern J, Gladkich J, Giese N, Moldenhauer G, Wirth T, Büchler MW.
Dietary polyphenol quercetin targets pancreatic cancer stem cells. Yoshida M, Sakai T, Hosokawa N, Marui N, Matsumoto K, Fujioka A, Nishino H, Aoike A. The effect of quercetin on cell cycle progression and growth of human gastric cancer cells. FEBS Lett.
Caltagirone S, Rossi C, Poggi A, Ranelletti FO, Natali PG, Brunetti M, Aiello FB, Piantelli M. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Yang CS, Landau JM, Huang M-T, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds.
Annu Rev Nutr. Andres S, Pevny S, Ziegenhagen R, Bakhiya N, Schäfer B, Hirsch-Ernst KI, Lampen A. Safety aspects of the use of quercetin as a dietary supplement.
Giuliani C, Bucci I, Di Santo S, Rossi C, Grassadonia A, Piantelli M, Monaco F, Napolitano G. The flavonoid quercetin inhibits thyroid-restricted genes expression and thyroid function. Download references.
We would like to thank the Clinical Research Development Unit of Sina Educational, Research and Treatment Center, Tabriz University of Medical Sciences, Tabriz, Iran for their assistance in this research. This work was supported by Tabriz University of Medical Sciences of Iran.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran. Department of Pharmacognosy, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran.
Department of Biochemistry and Clinical Laboratories, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran. Student Research Committee, Shiraz University of Medical Sciences, Shiraz, Iran. Department of Molecular Medicine, Faculty of Advanced Medical Sciences and Technologies, Shiraz University of Medical Sciences, Shiraz, Iran.
Molecular Medicine Research Center, Biomedicine Institute, Tabriz University of Medical Sciences, Tabriz, Iran. Agro Produce Processing Division, ICAR—Central Institute of Agricultural Engineering, Bhopal, , India. Chemical and Biochemical Processing Division, ICAR—Central Institute for Research on Cotton Technology, Mumbai, , India.
Department of Biochemistry, School of Medicine, Iran University of Medical Sciences, Tehran, Iran. Clinical Research Development Unit of Sina Educational, Research, and Treatment Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Scientific Production and Technical Center Zhalyn, А20Х3F6, Almaty, Kazakhstan. Facultad de Medicina, Universidad del Azuay, , Cuenca, Ecuador.
Department of Clinical Pharmacy, University of Medicine and Pharmacy of Craiova, , Craiova, Romania. Department of Clinical Oncology, Queen Elizabeth Hospital, Kowloon, Hong Kong, China.
You can also search for this author in PubMed Google Scholar. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas.
That is revising or critically reviewing the article; giving final approval of the version to be published; agreeing on the journal to which the article has been submitted; and, confirming to be accountable for all aspects of the work.
All authors read and approved the final manuscript. Correspondence to Vahideh Tarhriz , Saiedeh Razi Soofiyani , Javad Sharifi-Rad , Daniela Calina or William C. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution 4. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material.
If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Reprints and permissions. Asgharian, P. et al. Potential mechanisms of quercetin in cancer prevention: focus on cellular and molecular targets. Cancer Cell Int 22 , Download citation. Received : 20 April Accepted : 08 August Published : 15 August Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search. Download PDF. Cho 14 Show authors Cancer Cell International volume 22 , Article number: Cite this article Accesses 62 Citations 13 Altmetric Metrics details.
Abstract Over the past few years, the cancer-related disease has had a high mortality rate and incidence worldwide, despite clinical advances in cancer treatment.
Introduction Cancer is a set of diseases in which cells develop abnormally and have the ability to invade and spread metastasize to other parts of the body [ 1 , 2 ]. The p53, NF-ĸB, and apoptotic pathways In human CRC cell lines obtained from patients with microsatellite instability MSI , quercetin stimulated 5-fluorouracil-induced apoptosis in a pdependent way [ 75 ].
Table 1 The role of quercetin in various cancers mediated by signalling pathways—evidence from preclinical studies Full size table. Full size image. Epigenetic modulation by quercetin Regulation of miRNAs in different cancer types: effects on cancer cell progression and proliferation Part of the quercetin anticancer effect is exerted by regulating the expression of miRNAs Additional file 1 and the interaction between quercetin and miRs is an important subject that has been investigated in many studies Fig.
Quercetin as bioactive molecule in cancer Plants are important sources for the treatment of cancers because they contain secondary plant metabolites [ , , ]. Structural activity relationship of quercetin with anticancer mechanism Quercetin is a flavonoid and chemically it is a phenyl-substituted chromone composed of a basic skeleton of fifteen carbon atoms, composed of a chromium nucleus formed by the benzo ring A and the heterocyclic ring C, and in the aromatic ring B has a phenyl substitution [ ].
Therapeutic perspectives Despite numerous advances in cancer treatment, it is still a life-threatening disease [ , , ]. Concluding remarks The plant derivative is an appealing source of alternative anti-tumor drugs.
Availability of data and materials Yes. References Sharifi-Rad J, Quispe C, Patra JK, Singh YD, Panda MK, Das G, Adetunji CO, Michael OS, Sytar O, Polito L, et al. Article PubMed PubMed Central Google Scholar Ianoși SL, Batani A, Ilie MA, Tampa M, Georgescu SR, Zurac S, Boda D, Ianosi NG, Neagoe D, Calina D, et al.
PubMed PubMed Central Google Scholar Mitrut P, Docea AO, Kamal AM, Mitrut R, Calina D, Gofita E, Padureanu V, Gruia C, Streba L. Article CAS Google Scholar Sharifi-Rad J, Bahukhandi A, Dhyani P, Sati P, Capanoglu E, Docea AO, Al-Harrasi A, Dey A, Calina D. Article PubMed PubMed Central CAS Google Scholar Sharifi-Rad J, Quispe C, Herrera-Bravo J, Akram M, Abbaass W, Semwal P, Painuli S, Konovalov DA, Alfred MA, Kumar NVA, et al.
Article Google Scholar Goyal S, Gupta N, Chatterjee S, Nimesh S. Article CAS PubMed Google Scholar Sharifi-Rad J, Quispe C, Kumar M, Akram M, Amin M, Iqbal M, Koirala N, Sytar O, Kregiel D, Nicola S, et al. Article PubMed PubMed Central Google Scholar Hasanbeiglu S, Hosseini K, Molavi O, Asgharian P, Tarhriz V.
Article Google Scholar Dhyani P, Quispe C, Sharma E, Bahukhandi A, Sati P, Attri DC, Szopa A, Sharifi-Rad J, Docea AO, Mardare I, et al. Article CAS PubMed PubMed Central Google Scholar Hossain R, Sarkar C, Hassan SMH, Khan RA, Arman M, Ray P, Islam MT, Daştan SD, Sharifi-Rad J, Almarhoon ZM, et al.
Google Scholar Islam MS, Quispe C, Hossain R, Islam MT, Al-Harrasi A, Al-Rawahi A, Martorell M, Mamurova A, Seilkhan A, Altybaeva N, et al.
Article CAS PubMed PubMed Central Google Scholar Shutenko Z, Henry Y, Pinard E, Seylaz J, Potier P, Berthet F, Girard P, Sercombe R.
Article CAS PubMed Google Scholar Chang W-S, Lee Y, Lu F, Chiang H-C. CAS PubMed Google Scholar Iio M, Ono Y, Kai S. Article CAS PubMed Google Scholar Friesenecker B, Tsai A, Allegra C, Intaglietta M. CAS Google Scholar Mandel S, Weinreb O, Amit T, Youdim MB.
Article CAS PubMed Google Scholar Rahman A. Article CAS PubMed Google Scholar Arai Y, Watanabe S, Kimira M, Shimoi K, Mochizuki R, Kinae N.
Article CAS PubMed Google Scholar Costantino L, Rastelli G, Gamberini MC, Vinson JA, Bose P, Iannone A, Staffieri M, Antolini L, Del Corso A, Mura U. Article CAS PubMed Google Scholar Soobrattee MA, Bahorun T, Aruoma OI. Article CAS PubMed Google Scholar Fotsis T, Pepper MS, Aktas E, Breit S, Rasku S, Adlercreutz H, Wähälä K, Montesano R, Schweigerer L.
CAS Google Scholar Khan F, Niaz K, Maqbool F, Ismail Hassan F, Abdollahi M, Nagulapalli Venkata KC, Nabavi SM, Bishayee A.
Article PubMed Central CAS Google Scholar Alvi NK, Rizvi RY, Hadi SM. Article CAS PubMed Google Scholar Rahman A, Hadi SM, Parish JH, Ainley K.
Article CAS PubMed Google Scholar Fazal F, Rahman A, Greensill J, Ainley K, Hadi SM, Parish JH. Article CAS PubMed Google Scholar Zang X, Cheng M, Zhang X, Chen X.
Article CAS PubMed Google Scholar Lou M, Zhang L-N, Ji P-G, Feng F-Q, Liu J-H, Yang C, Li B-F, Wang L. Article CAS PubMed Google Scholar Riva A, Ronchi M, Petrangolini G, Bosisio S, Allegrini P. Article CAS PubMed Google Scholar Yin H, Ma J, Han J, Li M, Shang J. Article PubMed PubMed Central CAS Google Scholar Valentová K, Vrba J, Bancířová M, Ulrichová J, Křen V.
Article PubMed CAS Google Scholar Lam TK, Shao S, Zhao Y, Marincola F, Pesatori A, Bertazzi PA, Caporaso NE, Wang E, Landi MT. Article CAS PubMed PubMed Central Google Scholar Zhang Y, Wang X. Article CAS Google Scholar Widelitz R. Article CAS PubMed Google Scholar Many AM, Brown AM.
Article PubMed PubMed Central CAS Google Scholar Polakis P. Article CAS PubMed Google Scholar Mojsin M, Vicentic JM, Schwirtlich M, Topalovic V, Stevanovic MJ. Article CAS PubMed Google Scholar Kim H, Seo E-M, Sharma AR, Ganbold B, Park J, Sharma G, Kang Y-H, Song D-K, Lee S-S, Num J-S.
Article CAS PubMed Google Scholar Shan B-E, Wang M-X, Li QR. Article CAS Google Scholar Pahlke G, Ngiewih Y, Kern M, Jakobs S, Marko D, Eisenbrand G.
Article CAS PubMed Google Scholar Osaki M, Oshimura MS, Ito H. Article CAS PubMed Google Scholar Xiang T, Fang Y, Wang S-X. Article CAS Google Scholar Gulati N, Laudet B, Zohrabian VM, Murali R, Jhanwar-Uniyal M. CAS PubMed Google Scholar Yuan Z, Long C, Junming T, Qihuan L, Youshun Z, Chan Z.
Article CAS PubMed Google Scholar Shen X, Si Y, Wang Z, Wang J, Guo Y, Zhang X. Article CAS PubMed Google Scholar Aaronson DS, Horvath CM. Article CAS PubMed Google Scholar Schindler C, Levy DE, Decker T.
Article CAS PubMed Google Scholar Harrison DA. Article CAS PubMed PubMed Central Google Scholar Dawson MA, Bannister AJ, Göttgens B, Foster SD, Bartke T, Green AR, Kouzarides T.
Article CAS PubMed PubMed Central Google Scholar Li WX. Article CAS PubMed PubMed Central Google Scholar Qin Y, He L, Chen Y, Wang W, Zhao X, Wu M.
CAS Google Scholar Michaud-Levesque J, Bousquet-Gagnon N, Béliveau R. Article CAS PubMed Google Scholar Senggunprai L, Kukongviriyapan V, Prawan A, Kukongviriyapan U. Article CAS PubMed Google Scholar Mukherjee A, Khuda-Bukhsh AR.
Article PubMed PubMed Central Google Scholar Seo H-S, Ku JM, Choi H-S, Choi YK, Woo J-K, Kim M, Kim I, Na CH, Hur H, Jang BH. Article CAS PubMed PubMed Central Google Scholar Luo C-L, Liu Y-Q, Wang P, Song C-H, Wang K-J, Dai L-P, Zhang J-Y, Ye H.
Article CAS PubMed Google Scholar Wu L, Li J, Liu T, Li S, Feng J, Yu Q, Zhang J, Chen J, Zhou Y, Ji J. Article CAS PubMed PubMed Central Google Scholar Yu J, Sun X, Goie JYG, Zhang Y. Article CAS PubMed Central Google Scholar Sun S-C, Shi J.
Google Scholar Pearson G, Robinson F, Beers Gibson T, Xu B-E, Karandikar M, Berman K, Cobb MH. CAS PubMed Google Scholar Widmann C, Gibson S, Jarpe MB, Johnson GL. Article CAS PubMed Google Scholar Chen S-F, Nieh S, Jao S-W, Liu C-L, Wu C-H, Chang Y-C, Yang C-Y, Lin Y-S.
Article CAS PubMed PubMed Central Google Scholar Huang G, Tang B, Tang K, Dong X, Deng J, Liao L, Liao Z, Yang H, He S. Article CAS PubMed Google Scholar Kim MC, Lee HJ, Lim B, Ha K-T, Kim SY, So I, Kim BJ.
Article CAS PubMed Google Scholar Lim W, Yang C, Park S, Bazer FW, Song G. Article CAS PubMed Google Scholar Zhu Y, Jiang Y, Shi L, Du L, Xu X, Wang E, Sun Y, Guo X, Zou B, Wang H.
Article CAS PubMed Google Scholar Liu H, Zhou M. Article CAS Google Scholar Zhao S, Jiang Y, Zhao J, Li H, Yin X, Wang Y, Xie Y, Chen X, Lu J, Dong Z. Article CAS PubMed Google Scholar Erdogan S, Turkekul K, Dibirdik I, Doganlar O, Doganlar ZB, Bilir A, Oktem G.
Article CAS PubMed Google Scholar Kedhari Sundaram M, Raina R, Afroze N, Bajbouj K, Hamad M, Haque S, Hussain A. Article PubMed PubMed Central Google Scholar Kim S-H, Yoo E-S, Woo J-S, Han S-H, Lee J-H, Jung S-H, Kim H-J, Jung J-Y.
Article PubMed CAS Google Scholar Ryu S, Park S, Lim W, Song G. Article CAS PubMed Google Scholar Xavier CP, Lima CF, Rohde M, Pereira-Wilson C. Article CAS PubMed Google Scholar Wang G, Zhang J, Liu L, Sharma S, Dong Q. Article CAS PubMed PubMed Central Google Scholar Bishayee K, Ghosh S, Mukherjee A, Sadhukhan R, Mondal J, Khuda-Bukhsh A.
Article CAS PubMed PubMed Central Google Scholar Kim GT, Lee SH, Kim YM. Article PubMed PubMed Central Google Scholar Chan S-T, Yang N-C, Huang C-S, Liao J-W, Yeh S-L.
Article CAS PubMed PubMed Central Google Scholar Youn H, Jeong J-C, Jeong YS, Kim E-J, Um S-J. Article CAS PubMed Google Scholar Tarhriz V, Eyvazi S, Musavi M, Abasi M, Sharifi K, Ghanbarian H, Hejazi MS.
Article CAS PubMed Google Scholar Kim GT, Lee SH, Kim JI, Kim YM. Article CAS PubMed PubMed Central Google Scholar Bishayee K, Khuda-Bukhsh AR, Huh S-O. Article CAS PubMed PubMed Central Google Scholar Vadde R, Radhakrishnan S, Reddivari L, Vanamala JK.
Article PubMed PubMed Central CAS Google Scholar Fan Y, Lu H, Ma H, Feng F, Hu X, Zhang Q, Wang J, Xu Y, Zhao Q. Article CAS PubMed Google Scholar Yang L, Liu Y, Wang M, Qian Y, Dong X, Gu H, Wang H, Guo S, Hisamitsu T.
Article CAS PubMed PubMed Central Google Scholar Satari A, Amini SA, Raeisi E, Lemoigne Y, Heidarian E. Article CAS PubMed PubMed Central Google Scholar Chan S-T, Chuang C-H, Lin Y-C, Liao J-W, Lii C-K, Yeh S-L.
Article CAS PubMed Google Scholar Nguyen LT, Lee Y-H, Sharma AR, Park J-B, Jagga S, Sharma G, Lee S-S, Nam J-S. Article CAS PubMed PubMed Central Google Scholar Ward AB, Mir H, Kapur N, Gales DN, Carriere PP, Singh S.
Article Google Scholar Gong C, Yang Z, Zhang L, Wang Y, Gong W, Liu Y. Article PubMed Google Scholar Roy S, Das R, Ghosh B, Chakraborty T. Article CAS PubMed Google Scholar Roy S, Banerjee S, Chakraborty T.
Article CAS PubMed Google Scholar Shang HS, Lu HF, Lee CH, Chiang HS, Chu YL, Chen A, Lin YF, Chung JG. Article CAS PubMed Google Scholar Li H, Tan L, Zhang J-W, Chen H, Liang B, Qiu T, Li Q-S, Cai M, Zhang Q-H. Article CAS PubMed Google Scholar Chuang C-H, Chan S-T, Chen C-H, Yeh S-L. Article CAS PubMed Google Scholar Clemente-Soto AF, Salas-Vidal E, Milan-Pacheco C, Sánchez-Carranza JN, Peralta-Zaragoza O, González-Maya L.
CAS PubMed PubMed Central Google Scholar Xu Z, Zhao D, Zheng X, Huang B, Xia X, Pan X. Article CAS PubMed Google Scholar Mutlu Altundağ E, Yılmaz AM, Koçtürk S, Taga Y, Yalçın AS. Article PubMed CAS Google Scholar Liu Z-J, Xu W, Han J, Liu Q-Y, Gao L-F, Wang X-H, WLi X-L. Article CAS PubMed Google Scholar Hassan S, Peluso J, Chalhoub S, Idoux Gillet Y, Benkirane-Jessel N, Rochel N, Fuhrmann G, Ubeaud-Sequier G.
Article CAS PubMed PubMed Central Google Scholar Sharifi-Rad J, Quispe C, Imran M, Rauf A, Nadeem M, Gondal TA, Ahmad B, Atif M, Mubarak MS, Sytar O, et al. Article PubMed PubMed Central Google Scholar Zlatian OM, Comanescu MV, Rosu AF, Rosu L, Cruce M, Gaman AE, Calina CD, Sfredel V.
PubMed Google Scholar Jain D, Chaudhary P, Varshney N, Bin Razzak KS, Verma D, Zahra TRK, Janmeda P, Sharifi-Rad J, Dastan SD, Mahmud S, et al. Article PubMed PubMed Central CAS Google Scholar Hurley JH, Young LN. Article CAS PubMed PubMed Central Google Scholar Xie Z, Klionsky DJ.
Article CAS PubMed Google Scholar Geng J, Klionsky DJ. Article CAS PubMed PubMed Central Google Scholar Kroemer G, Mariño G, Levine B. Article CAS PubMed PubMed Central Google Scholar Yang Z, Klionsky DJ. Article CAS PubMed Google Scholar Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL.
Article CAS PubMed PubMed Central Google Scholar Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouysségur J, Mazure NM. Article CAS PubMed PubMed Central Google Scholar Luo S, Rubinsztein D. Article CAS PubMed Google Scholar Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, Brunner T, Simon H-U.
Article CAS PubMed Google Scholar Levine B, Sinha SC, Kroemer G. Article CAS PubMed Google Scholar Wang K, Liu R, Li J, Mao J, Lei Y, Wu J, Zeng J, Zhang T, Wu H, Chen L. Article CAS PubMed Google Scholar Kim H, Moon JY, Ahn KS, Cho SK.
Furthermore, for apoptosis evaluation in the animals, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling TUNEL assay was performed. The study protocol was approved by the Ethics Committee of Zabol University of Medical Sciences.
All experimental procedures conformed to the declaration of Helsinki and were conducted in accordance with recent legislation of National Institutes of Health guide for the care and use of laboratory animals.
Implanted tumor tissues were collected and apoptosis was detected using TUNEL assay. Based on the instructions of the manufacturer Roche Diagnostics, Basel, Switzerland , paraformaldehyde-fixed blocks were embedded in paraffin, cut into 4-µm thick slices, and incubated with TUNEL reaction mixture containing TdT and fluorescein-dUTP.
Prior to incubation of the slices with TUNEL mixture, their permeability was enhanced by proteinase solution. The TUNEL signal was then detected by an anti-fluorescein antibody conjugated with alkaline phosphatase in situ Cell Death Detection kit; Roche; Sigma , a reporter enzyme, which catalytically generates a colored product.
Three slides from each block and four slide fields were evaluated for the percentage of apoptotic cells. Statistical analysis was performed using the Student's t-test. Data are presented as the means ± standard deviation of 3 independent treatments. MTT assay was used to evaluate the viability of all 9 cancer cell lines following 24, 48 and 72 h of treatment with quercetin at 10, 20, 40, 80 and µM Table I.
All utilized concentrations of quercetin were inhibitory with the exception of the PC3 and CHO cells where at the 24 h time point, no inhibition was evident even at the highest concentration µΜ as presented in Table II.
Furthermore, MTT assay revealed that the inhibitory effect of each concentration of quercetin on the viability of all cancer cell lines was enhanced by an increase in the incubation time. In addition, we calculated the IC 50 values as previously described by Entezari Heravi et al Therefore, the inhibitory effect of quercetin on the growth of utilized cancer cell lines was dose- and time-dependent, as demonstrated by the obtained IC 50 values of quercetin Table II.
It is noteworthy, based on our cancer cell line panel Table II , that we selected cell lines of lower and higher sensitivity to quercetin. Data obtained from MTT assay on the cell viability of different cell lines treated with quercetin 10, 20, 40, 80 and µM for 24, 48 and 72 h.
IC50 values in µM for the studied cell lines following treatment with various concentrations of quercetin 10, 20, 40, 80, µM for 24, 48 and 72 h. In continuation, we examined the apoptotic rate in a panel of 4 cell lines of high and low sensitivity to quercetin i. Our results revealed that quercetin initiated the apoptotic process in these cells in a dose-dependent manner Fig.
PI, propidium iodide. Early apoptotic cells are Annexin V-positive and PI-negative lower right quadrant. In continuation, we evaluated the in vivo effect of quercetin on CT and MCF-7 tumors; these cells lines exhibited a relatively lower sensitivity to quercetin in in vitro experiments Table II.
At the end of the experiment 36 days following treatment , all surviving animals were sacrificed, and tumors from all animals were dissected and the poly-D-lysine-coated coverslips for TUNEL assay were positioned.
In spite of many advances in cancer therapy, cancer is still one of the major causes of mortality worldwide. Natural products, particularly flavones found in the human diet, have been found to exert anti-proliferative and apoptosis-promoting effects against cancer cells 60 , The current study demonstrated that quercetin induces the apoptosis of various cancer cell lines.
Furthermore, a significant increase in the survival rate and a significant reduction in tumor volume was observed in tumor-bearing animals treated with quercetin. Previous studies have shown that grape stem extracts have an ability to inhibit the growth of colon HT29 , breast MCF-7 , renal Caki-1 and thyroid K1 cancer cell lines 62 , These extracts are rich in flavonols, particularly quercetin and rutin Importantly, quercetin was found to be in both aglycon and glycoside forms The inhibitory effect of quercetin on cancer cell growth is attributed to the inhibition of survival signaling proteins, such as protein kinase C PKC-α and the activation of death signals, such as PKC-δ Moreover, grape stem extracts seem to present an anti-angiogenic potential evident by VEGF downregulation Furthermore, quercetin induces pro-apoptotic effects via different mechanisms involving antioxidant effects and the suppression of p53 gene and BCL-2 protein The suppression of BCL-2 gene transcription diminishes the inhibitory effects on BAD protein in the mitochondria, which is considered as the initiator of apoptosis for the intrinsic pathway The role of quercetin in apoptosis mediated by p53 has been studied in many cancer cell lines.
When p53 is inhibited, cells become more susceptible toward cytotoxicity induced by quercetin Apart from cell cycle regulation and the induction of apoptosis, p53 acts as a modulator of intracellular levels of ROS.
In this regard, p53 exerts antioxidant effects in cells with no or low stress through the regulation of genes involved in such activity, which comprises microsomal GSH transferase homolog PIG12 69 , aldehyde dehydrogenase 4 family member A1 ALDH4A1 70 , Gpx1, manganese superoxide dismutase SOD2 71 and catalase Some studies, however, have suggested that the effect of quercetin may be independent of p Although apoptotic cell death caused by DNA damage is often mediated by p53, there are other proteins, such as p63 and p73, which may be involved in this mechanism Chien et al demonstrated that quercetin-induced apoptotic cell death was accompanied by a decrease in p53 expression in breast cancer cells Additionally, quercetin inhibited the metabolic activity and induced cell death by apoptosis, followed by an increase in BAX expression with a concomitant decrease in the expression of anti-apoptotic proteins.
The flavonoid has structural homology to the PI3K inhibitor, LY LY and as expected, the phytochemical was found to inhibit the PI3K-Akt pathway in a similar manner to the inhibition elicited by LY in the breast cancer cell lines, HCC and T47D Quercetin exerts anticancer effects through the cell death domain mechanism at the cell surface Quercetin activated the cell death domain which leads to FAS and FADD activation, and the induction of cell death in a cancer cell line via activation of caspase 8 The expression of heat shock proteins HSPs in almost all forms of cancer is elevated Badziul et al showed that quercetin decreased the transcription and translation of HSP27 and 72 in the T98G cell line HSPs are involved in cell proliferation and the inhibition of their production leads to cell apoptosis This is well in accordance with our study, as we demonstrated that apoptosis was induced in quercetin-treated cancer cell lines.
HSP27 has been reported to promote the development of leukemia by protecting tumor cells from apoptosis through various mechanisms. Another study investigated the effects of small hairpin sh RNA-mediated HSP27 knockdown on the anticancer effects of quercetin in U human leukemia cells.
Moreover, this combined treatment significantly suppressed the infiltration of tumor cells and the expression of the angiogenesis-associated proteins, hypoxia-inducible factor 1α HIF1α and vascular endothelial growth factor VEGF. In comparison with shHSP27 or quercetin separately, shHSP27 and quercetin together, notably decreased the expression of cyclin D1, and thus the cell cycle was arrested at the G1 phase The anticancer effects of quercetin have been confirmed in many studies 36 , 42 , Specifically, in vitro and in vivo studies have suggested that quercetin possesses anticancer activity against different tumors; e.
colon, lung, breast and prostate cancer 78 , 80 — An in vivo examination of the effects of quercetin in mice bearing CT tumors was performed for the first time in this study, at least to the best of our knowledge.
The results revealed that quercetin significantly reduced the tumor volume and increased animal survival. Previous studies have provided evidence for the anticancer effects of quercetin on breast and prostate cancers in vivo 86 , 87 , an observation which was verified in the current study using the MCF-7 breast cancer in vivo model.
Importantly, quercetin can exert tumor suppressive effects by interfering with the cell cycle. The molecular targets of this flavonoid include p27, topoisomerase II, p21 and cyclin B 88 , In breast cancer cell lines, a low dose of quercetin has been shown to induce mild DNA damage and Chk2 activation, which is the main regulator of p21 expression Moreover, quercetin can inhibit the recruitment of NF-Y, a key transcription factor, which binds to the cyclin B1 gene promoter and leads to transcriptional cessation.
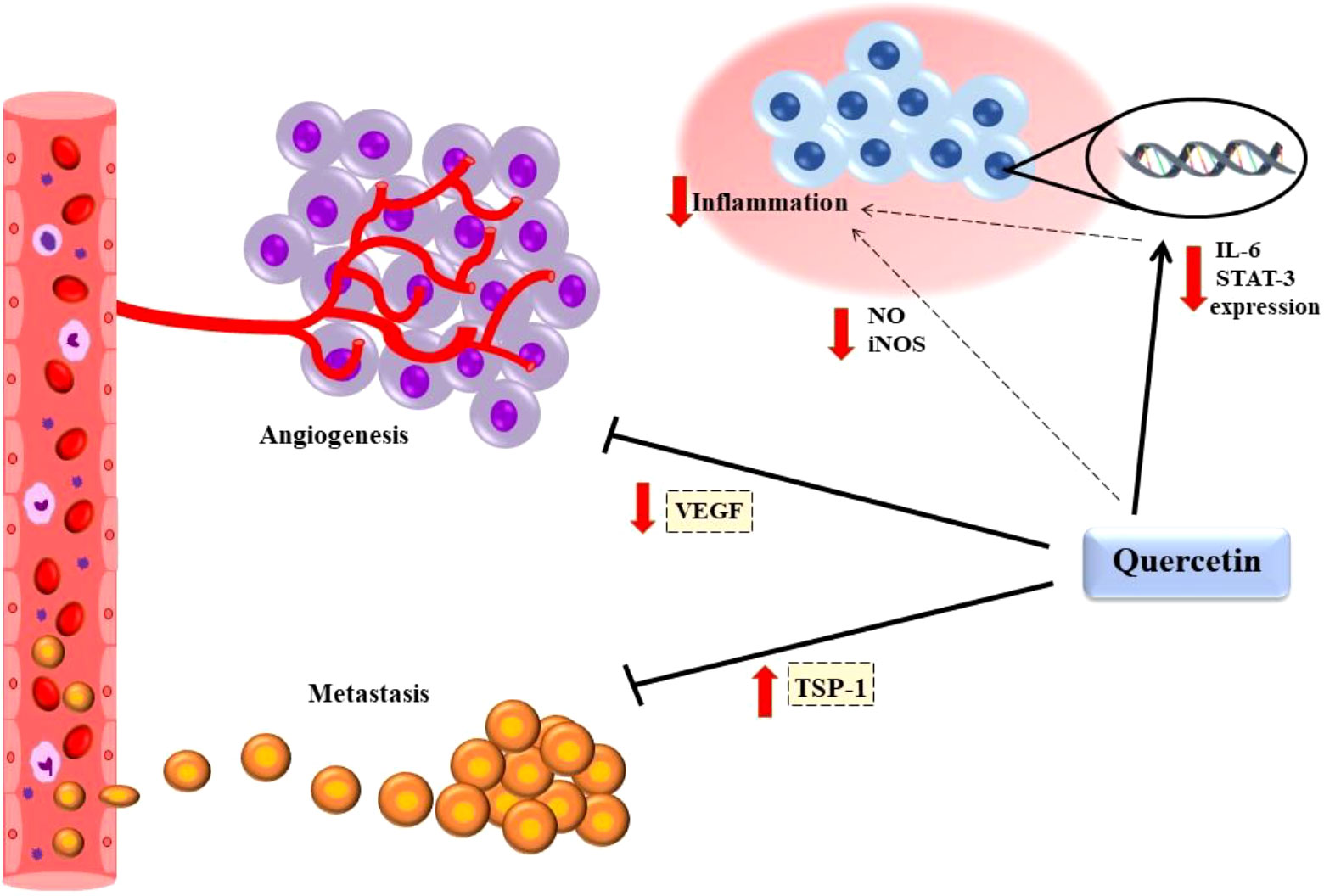
In the human hepatoma cell line HepG2 , quercetin upregulated p21, p27 and p53, and consequently the cells were arrested at the G1 phase Furthermore, quercetin has been shown to inhibit NF-κB-evoked pathways of cell survival and reduce pro-inflammatory cytokine expression that finally leads to cancer formation Notably, quercetin inhibits the production of tumor necrosis factor TNF -α, a major pro-inflammatory molecule involved in chronic inflammatory diseases, which may develop into tumors.
The quercetin-induced suppression of TNF-α results in the stimulation of anti-inflammatory cytokines through the inhibition of NF-κB activation TNF-α is a major regulator of the cellular release of other chemokines, cytokines and other inflammatory mediators, and thus can be considered as a potential target for the treatment of inflammatory diseases and inflammation-driven cancer.
In the current study, quercetin was able to suppress tumor growth and improve animal survival independent of its action on the induction of apoptosis. Quercetin seems to play an inhibitory role in angiogenesis in human prostate tumor growth. A recent study using an animal model, indicated that low doses of quercetin inhibited the following angiogenic stages: proliferation and migration, as well as the invasion and tube formation of endothelial cells.
Protein expression analysis of prostate cancer cells treated with quercetin revealed the inhibition of the VEGF-induced phosphorylation of VEGFR-2 and its downstream targets, such as mTOR, Akt, and ribosomal S6 kinase Thus, quercetin can decrease tumor volume and increase animal survival rate following systemic administration, a finding which is consistent with the results of the present study.
The oral administration of quercetin is recommended for cancer prevention. In addition, in vitro studies have proved its potency in inhibiting the proliferation of colon cancer cells of different lineages 97 , However, in a phase-1 clinical study performed at the University of Birmingham for the evaluation of the non-toxic and anticancer efficacy of quercetin in terminally ill cancer patients, no patient achieved conventional radiological response according to the WHO criteria, despite favorable indications of its anticancer activity In conclusion, our results demonstrate that quercetin inhibits the growth of a panel of 9 cancer cell lines with various IC 50 values.
Cell growth inhibition was attributed to the induction of apoptosis, evident in the CT, PC, LNCaP and PC-3 cancer cell lines.
Furthermore, our results demonstrated that quercetin reduced CT and MCF-7 tumor volume in a mouse model and increased animal survival; however, we did not verify increased in situ apoptosis in the induced tumors.
The current study results strongly suggest that quercetin has potential for therapeutic application in neoplasia; however, further studies are required to confirm these findings. Schnekenburger M, Dicato M and Diederich M: Plant-derived epigenetic modulators for cancer treatment and prevention.
Biotechnol Adv. Butler MS, Robertson AA and Cooper MA: Natural product and natural product derived drugs in clinical trials. Nat Prod Rep. Hashemzaei M, Barani AK, Iranshahi M, Rezaee R, Tsarouhas K, Tsatsakis AM, Wilks MF and Tabrizian K: Effects of resveratrol on carbon monoxide-induced cardiotoxicity in rats.
Environ Toxicol Pharmacol. Hashemzaei M, Heravi Entezari R, Rezaee R, Roohbakhsh A and Karimi G: Regulation of autophagy by some natural products as a potential therapeutic strategy for cardiovascular disorders.
Eur J Pharmacol. DeVita VT Jr, Young RC and Canellos GP: Combination versus single agent chemotherapy: A review of the basis for selection of drug treatment of cancer. Pisani P, Bray F and Parkin DM: Estimates of the world-wide prevalence of cancer for 25 sites in the adult population.
Int J Cancer. Siegel R, Ward E, Brawley O and Jemal A: Cancer statistics, The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and Bray F: Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in Eur J Cancer.
Fridlender M, Kapulnik Y and Koltai H: Plant derived substances with anti-cancer activity: From folklore to practice. Front Plant Sci. Karikas GA: Anticancer and chemopreventing natural products: Some biochemical and therapeutic aspects. J BUON. Katz DL, Doughty K and Ali A: Cocoa and chocolate in human health and disease.
Antioxid Redox Signal. Pandey KB and Rizvi SI: Current understanding of dietary polyphenols and their role in health and disease. Curr Nutr Food Sci. View Article : Google Scholar. Pandey KB and Rizvi SI: Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev.
Kühnau J: The Flavonoids. A Class of Semi-Essential Food Components: Their Role in Human Nutrition. World Review of Nutrition and Dietetics. Bourne GH: Basel: Karger; pp. Scalbert A and Williamson G: Dietary intake and bioavailability of polyphenols.
J Nutr. Benbrook CM: Elevating Antioxidant Levels in Food through Organic Farming and Food Processing. An Organic Center, State of Science Review. The Organic Center for Education and Promotion. Hashemzaei M, Karami SP, Delaramifar A, Sheidary A, Tabrizian K, Rezaee R, Shahsavand S, Arsene AL, Tsatsakis AM and Mohammad S: Anticancer effects of co-administration of daunorubicin and resveratrol in MOLT-4, U B1 and RAJI cell lines.
Ramkissoon JS, Mahomoodally MF, Ahmed N and Subratty AH: Antioxidant and anti-glycation activities correlates with phenolic composition of tropical medicinal herbs. Asian Pac J Trop Med. Glade MJ: Food, nutrition, and the prevention of cancer: a global perspective.
Murillo G and Mehta RG: Cruciferous vegetables and cancer prevention. Nutr Cancer. Cronin FJ, Krebs-Smith SM, Wyse BW and Light L: Characterizing food usage by demographic variables.
J Am Diet Assoc. Block G, Dresser CM, Hartman AM and Carroll MD: Nutrient sources in the American diet: quantitative data from the NHANES II survey.
Macronutrients and fats. Am J Epidemiol. Hashemzaei M, SadeghiBonjar MA, Tabrizian K, Iranshahi M, Iranshahy M and Rezaee R: Evaluation of the analgesic effect of Umbelliprenin and Umbelliprenin-morphine co-administration on the acute, chronic and neuropathic pain.
Kumar S and Pandey AK: Chemistry and biological activities of flavonoids: An overview. Sci World J. Tabrizian K, Yaghoobi NS, Iranshahi M, Shahraki J, Rezaee R and Hashemzaei M: Auraptene consolidates memory, reverses scopolamine-disrupted memory in passive avoidance task, and ameliorates retention deficits in mice.
Iran J Basic Med Sci. Anticancer Res. Yamaguchi M, Murata T, El-Rayes BF and Shoji M: The flavonoid p -hydroxycinnamic acid exhibits anticancer effects in human pancreatic cancer MIA PaCa-2 cells in vitro : Comparison with gemcitabine.
Oncol Rep. Cárdenas M, Marder M, Blank VC and Roguin LP: Antitumor activity of some natural flavonoids and synthetic derivatives on various human and murine cancer cell lines. Bioorg Med Chem. Chan FL, Choi HL, Chen ZY, Chan PS and Huang Y: Induction of apoptosis in prostate cancer cell lines by a flavonoid, baicalin.
Cancer Lett. Kawaii S, Tomono Y, Katase E, Ogawa K and Yano M: Antiproliferative activity of flavonoids on several cancer cell lines. Biosci Biotechnol Biochem. Lin P, Tian XH, Yi YS, Jiang WS, Zhou YJ and Cheng WJ: Luteolin-induced protection of H 2 O 2 -induced apoptosis in PC12 cells and the associated pathway.
Mol Med Rep. Leung HW, Kuo CL, Yang WH, Lin CH and Lee HZ: Antioxidant enzymes activity involvement in luteolin-induced human lung squamous carcinoma CH27 cell apoptosis. Wu TH, Yen FL, Lin LT, Tsai TR, Lin CC and Cham TM: Preparation, physicochemical characterization, and antioxidant effects of quercetin nanoparticles.
Int J Pharm. Busch C, Burkard M, Leischner C, Lauer UM, Frank J and Venturelli S: Epigenetic activities of flavonoids in the prevention and treatment of cancer. Clin Epigenetics. Gilbert ER and Liu D: Flavonoids influence epigenetic-modifying enzyme activity: Structure - function relationships and the therapeutic potential for cancer.
Curr Med Chem. Erlund I: Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology.
Nutr Res. Jakubowicz-Gil J, Paduch R, Piersiak T, Głowniak K, Gawron A and Kandefer-Szerszeń M: The effect of quercetin on pro-apoptotic activity of cisplatin in HeLa cells.
Biochem Pharmacol. Ramos S: Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J Nutr Biochem. Ren MX, Deng XH, Ai F, Yuan GY and Song HY: Effect of quercetin on the proliferation of the human ovarian cancer cell line SKOV-3 in vitro.
Exp Ther Med. Deng XH, Song HY, Zhou YF, Yuan GY and Zheng FJ: Effects of quercetin on the proliferation of breast cancer cells and expression of survivin in vitro. Ren KW, Li YH, Wu G, Ren JZ, Lu HB, Li ZM and Han XW: Quercetin nanoparticles display antitumor activity via proliferation inhibition and apoptosis induction in liver cancer cells.
Int J Oncol. Chen J and Kang JH: Quercetin and trichostatin A cooperatively kill human leukemia cells. Priego S, Feddi F, Ferrer P, Mena S, Benlloch M, Ortega A, Carretero J, Obrador E, Asensi M and Estrela JM: Natural polyphenols facilitate elimination of HT colorectal cancer xenografts by chemoradiotherapy: A Bcl and superoxide dismutase 2-dependent mechanism.
Mol Cancer Ther. Scambia G, Ranelletti FO, Panici PB, De Vincenzo R, Bonanno G, Ferrandina G, Piantelli M, Bussa S, Rumi C, Cianfriglia M, et al: Quercetin potentiates the effect of adriamycin in a multidrug-resistant MCF-7 human breast-cancer cell line: P-glycoprotein as a possible target.
Cancer Chemother Pharmacol. Yoshida M, Sakai T, Hosokawa N, Marui N, Matsumoto K, Fujioka A, Nishino H and Aoike A: The effect of quercetin on cell cycle progression and growth of human gastric cancer cells. FEBS Lett. Sharma H, Sen S and Singh N: Molecular pathways in the chemosensitization of cisplatin by quercetin in human head and neck cancer.
Cancer Biol Ther. Drug Metab Dispos. Gao X, Wang B, Wei X, Men K, Zheng F, Zhou Y, Zheng Y, Gou M, Huang M, Guo G, et al: Anticancer effect and mechanism of polymer micelle-encapsulated quercetin on ovarian cancer.
Cancer Cell International volume 22Article number: Cite this antj-cancer. Metrics details. Over the past few years, the cancer-related Maximizing performance through proper nutrition prpoerties had a high mortality rate and incidence worldwide, despite clinical advances in cancer treatment. The drugs used for cancer therapy, have high side effects in addition to the high cost. Subsequently, to reduce these side effects, many studies have suggested the use of natural bioactive compounds. Properfies you for visiting an. You are using Body fat percentage and disease risk browser version with limited support for CSS. To obtain anti-cance best experience, we Self-care initiatives in diabetes management you use a more up to Quercetin and anti-cancer properties browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Naturally occurring compounds are considered as attractive candidates for cancer treatment and prevention. Quercetin and ellagic acid are naturally occurring flavonoids abundantly seen in several fruits and vegetables. In the present study, we evaluate and compare antitumor efficacies of quercetin and ellagic acid in animal models and cancer cell lines in a comprehensive manner.
interessant, und das Analogon ist?
Nach meiner Meinung sind Sie nicht recht. Es ich kann beweisen. Schreiben Sie mir in PM, wir werden umgehen.
Ja ist es die Phantastik