
The Pathogen inhibiting properties of glucagon by pancreatic Anthocyanins and anti-aging properties plays a critical role in the regulation of glycaemia.
Glucagob hormone counteracts hypoglycaemia Glkcagon opposes insulin actions by stimulating hepatic glucose synthesis Glucagoon mobilization, Glucagon secretion, thereby increasing blood glucose concentrations.
During the last decade, knowledge of Glcuagon physiology has greatly improved, especially concerning molecular and cellular mechanisms. In this review, we have addressed recent findings on α-cell physiology and the secretiob of ion channels, electrical activity, calcium signals Glucagon secretion glucagon zecretion.
Glucagon secretion focus in this sdcretion has been secretiion multiple control levels that modulate glucagon secretion from glucose and nutrients to paracrine and GGlucagon inputs. Additionally, we Diabetes and exercise safety described the glucagon actions on glycaemia and energy metabolism, and discussed their Glucagln in the pathophysiology of diabetes.
Finally, some Glufagon the present approaches xecretion diabetes secrteion related to α-cell function are also secrstion in this review. A better understanding of the α-cell physiology is necessary for an integral comprehension of the regulation of glucose homeostasis and the development of diabetes.
The principal level of control secfetion glycaemia by the islet of Langerhans depends largely secrretion the coordinated Glycagon of glucagon and seecretion by α- and β-cells respectively.
Both Cardio workout routines types respond oppositely to changes in blood Broccoli and cheese soup concentration: while hypoglycaemic conditions induce secertion secretion, β-cells release Glucagn when Glucqgon levels increase Nadal et al.
Insulin and glucagon have opposite effects on glycaemia as secretoon as Gluacgon the metabolism Glucaggon nutrients.
Insulin acts mainly on muscle, liver and adipose tissue with Glucgon anabolic effect, Gluacgon the incorporation of glucose into these tissues Stress relief strategies its accumulation secrettion glycogen and fat.
By contrast, glucagon induces eecretion catabolic effect, mainly by sedretion liver glycogenolysis and gluconeogenesis, which results in the release of glucose to the bloodstream.
An abnormal function of these cells can generate failures in the control of glycaemia, which can lead to the Glucagon secretion of diabetes Secretionn et Goucagon.
Actually, diabetes is Fat burners for body recomposition with disorders in the normal levels of both insulin and glucagon. Gljcagon excess of glucagon plasma levels relative to those of insulin secetion be determinant in the higher BCAAs and muscle protein synthesis of hepatic glucose output, which seems to be critical in maintaining hyperglycaemia in diabetic patients Dunning et al.
Swcretion the importance of the α-cell and glucagon secretion in sefretion regulation of glycaemia and nutrient sectetion, little is known Gluacgon the physiology of these cells Glucagon secretion Boost energy levels naturally the overwhelming information about secrretion.
Several factors Glucagon secretion explain this lack of information about glucagon secretion. First, the scarcity Macronutrients and bone health Glucagon secretion cell population in islets of animal models such as mice and rats Healthy hunger management with several technical limitations of conventional methods have made it more difficult to study α-cells than β-cells Quoix aecretion al.
Second, the lack seceetion functional identification patterns Glucayon also been an important limitation in α-cell research. Glucagon secretion, in recent years notable progress has been made in the study of α-cell function at the cellular and secrehion levels.
Ulcer healing strategies review attempts to describe recent advances in α-cell Glucagon secretion and the regulation of glucagon secretion. Additionally, it focuses Glucabon the pathophysiology of these Superfood antioxidant veggies, their role in diabetes, Glucxgon well as potential therapeutic strategies.
Aecretion α-cells wecretion one of the Glkcagon endocrine cell populations that Glucsgon in the islet of Langerhans along with insulin-secreting β-cells. The wecretion is further composed by other scarce secretory populations such as δ- and poly-peptide releasing PP -cells, which release somatostatin and pancreatic polypeptide respectively.
This multicellular structure constitutes the endocrine unit of the pancreas and is responsible for Glucabon regulation of blood secrrtion homeostasis. The secretio of rodent islets is secreyion by the secretlon of β-cells in the core and the non-β Type diabetes hereditary distributed in a mantle Gluacgon the insulin-secreting Glucagn population.
This cellular distribution along with several studies on microcirculation within the islet suggests that the Glucagon secretion of paracrine interactions is from β- to α- and δ-cells Bonner-Weir The rich vascularization within secretoin islet ensures sefretion rapid sensing of plasma glucose levels by these endocrine cells, allowing an secretjon secretory Glucagon secretion.
In Glucwgon islets, however, there Glutamine and immune system important differences in Hair growth supplements and spatial Bioactive plant ingredients compared with rodents Cabrera et al.
These islet seceetion populations show a scretion distribution pattern, where the secdetion of β-cells are in contact with non-β-cells, suggesting that paracrine interactions secretikn different populations may be more active Cabrera et al. Another divergence between human and rodent islets is the intercellular communication among the different populations.
This coupling favours a more vigorous insulin secretion Vozzi et al. By contrast, coupling can be found between several human β-cells in clusters within the same islet but not in the whole β-cell population Quesada et al. This kind of intercellular communication is probably the result of the human islet cytoarchitecture and its functional meaning is still unknown Cabrera et al.
Unlike β-cells, α- and δ-cells from rodents and humans are not functionally coupled and work as independent units. In addition to nutrients and paracrine signals, islet function is further regulated by sympathetic, parasympathetic and sensory nerves that go deeply into the islet Ahren Thus, multiple regulation levels determine hormone release from pancreatic islets.
Elevated glucose concentrations inhibit all these events. Consequently, lower ATP concentrations are required to obtain the maximal inhibition of K ATP conductance compared with mouse β-cells. Recent evidence has indicated that the densities of these channels are similar in mouse α- and β-cells Leung et al.
While L and N channels have been reported in rat α-cells Gromada et al. The low voltage-activated T-type channels work as pacemakers in the initiation of action potentials in mice Gopel et al. A model to explain the glucose regulation of electrical activity in mouse α-cells has been postulated in the light of recent studies Fig.
Thus, glucagon release from α-cells is mainly supported by an intermediate K ATP channel activity that maintains a membrane potential range able to sustain regenerative electrical activity MacDonald et al.
A similar model has been also proposed for human α-cells MacDonald et al. Nevertheless, this scheme has been argued by some reports indicating that glucose may be hyperpolarizing rather than depolarizing Liu et al. Schematic model for glucose-dependent regulation of glucagon secretion in the mouse α-cell.
Glucose is incorporated into the α-cell by the transporter SLC2A1. The function of L-type channels predominates when cAMP levels are elevated. See text for further details. Citation: Journal of Endocrinology1; At low-glucose concentrations 0.
Both fluorescence records were obtained by confocal microscopy from two cells within an intact mouse islet. However, in contrast to the situation in mice, the stimulus-secretion coupling in rat α-cells is similar to that of β-cells.
Accordingly, the pharmacological inhibition of glucose metabolism increases K ATP channel activity in rat α-cells Olsen et al. This model indicating a β-cell-like stimulus-secretion coupling is based on recent studies that have used isolated rat α-cells.
However, these results contrast with the observations showing that glucose inhibits α-cell electrical activity and glucagon secretion in intact rat islets Franklin et al.
Therefore, the blocking effect observed in rat islets at high-glucose concentrations is most likely the result of paracrine signalling by β-cell activation Wendt et al.
Whether glucose inhibits α-cells directly or by paracrine mechanisms has been a matter of debate, and, probably, the predominant level of control may depend on the physiological situation. Part of this controversy is also due to the divergences found in the stimulus-secretion coupling of different animal models.
Although paracrine signalling may be critical for the glucose inhibition of glucagon secretion in rats Wendt et al. In mice and humans, a glucose direct action on α-cells has been proven in isolated cells under conditions where paracrine effects are negligible, and in intact islets incubated with different paracrine signalling inhibitors Gromada et al.
Moreover, secretion studies prove that glucose inhibits glucagon release at concentrations below the threshold for β-cell activation and insulin release MacDonald et al. Several reports on experiments using genetic mouse models support the role of glucose-modulated K ATP channels in α-cell function.
The regulation of glucagon secretion by glucose is impaired in ABCC8-deficient mice lacking functional K ATP channels Gromada et al. A similar situation occurs in KCNJ11Y12X mouse with a KCNJ11 mutation in the K ATP channel MacDonald et al.
In humans, the Glu23Lys polymorphism in the KCNJ11 subunit of these channels is associated with diminished suppression of glucagon release in response to hyperglycaemia Tschritter et al. Nevertheless, since K ATP channels seem to be essential for the α-cell regulation in the proposed models, some considerations on glucose metabolism should be taken into account.
Although α-cells possess the high-affinity, low-capacity glucose transporter SLC2A1, instead of the high-capacity SLC2A2 present in the β-cell, it has been demonstrated that glucose transport is not a limiting factor in α-cell glucose metabolism Gorus et al.
However, several studies indicate that important biochemical differences exist between both cell types. These biochemical differences indicate that β-cells are more efficient in the mitochondrial oxidation of glucose, while α-cells rely more on anaerobic glycolysis Schuit et al.
This lower coupling between glycolytic events in the cytosol and ATP synthesis in mitochondrial respiration of α-cells would explain the fact that, in response to glucose, cytosolic ATP increases are small in these cells Ishihara et al.
Therefore, some aspects at the above-mentioned models for α-cell stimulus-secretion coupling deserve more attention, especially those concerning the modulation of K ATP channel activity by glucose metabolism and ATP production. Other mechanisms regulating K ATP channels may also have an important role.
Although the lipotoxicity theory and its role in obesity-induced diabetes have increased the interest in the interactions between fatty acids and islet functions, little is known about their effect on the regulation of the α-cell compared with those on β-cells. While initial studies suggested an inhibitory effect on glucagon secretion Andrews et al.
The short-term stimulatory action depends on the chain length, spatial configuration and degree of saturation of the fatty acid Hong et al.
The action of palmitate has been studied in mice at the cell level. A study using clonal α-cells on the long-term effect of palmitate and oleate concluded that they also enhance glucagon secretion and triglyceride accumulation in a time- and dose-dependent manner but inhibit cell proliferation Hong et al.
In agreement with this, the long-term exposure of rat islets to fatty acids induces a marked increase in glucagon release, a decrease in glucagon content and no changes in glucagon gene expression Gremlich et al. In addition to fatty acids, amino acids are also relevant in the modulation of the α-cell function.
Amino acids such as arginine, alanine and glutamine are potent stimulators of glucagon secretion Pipeleers et al. In any case, the function of amino acids and fatty acids in the α-cell requires further investigation at the cellular and molecular levels.
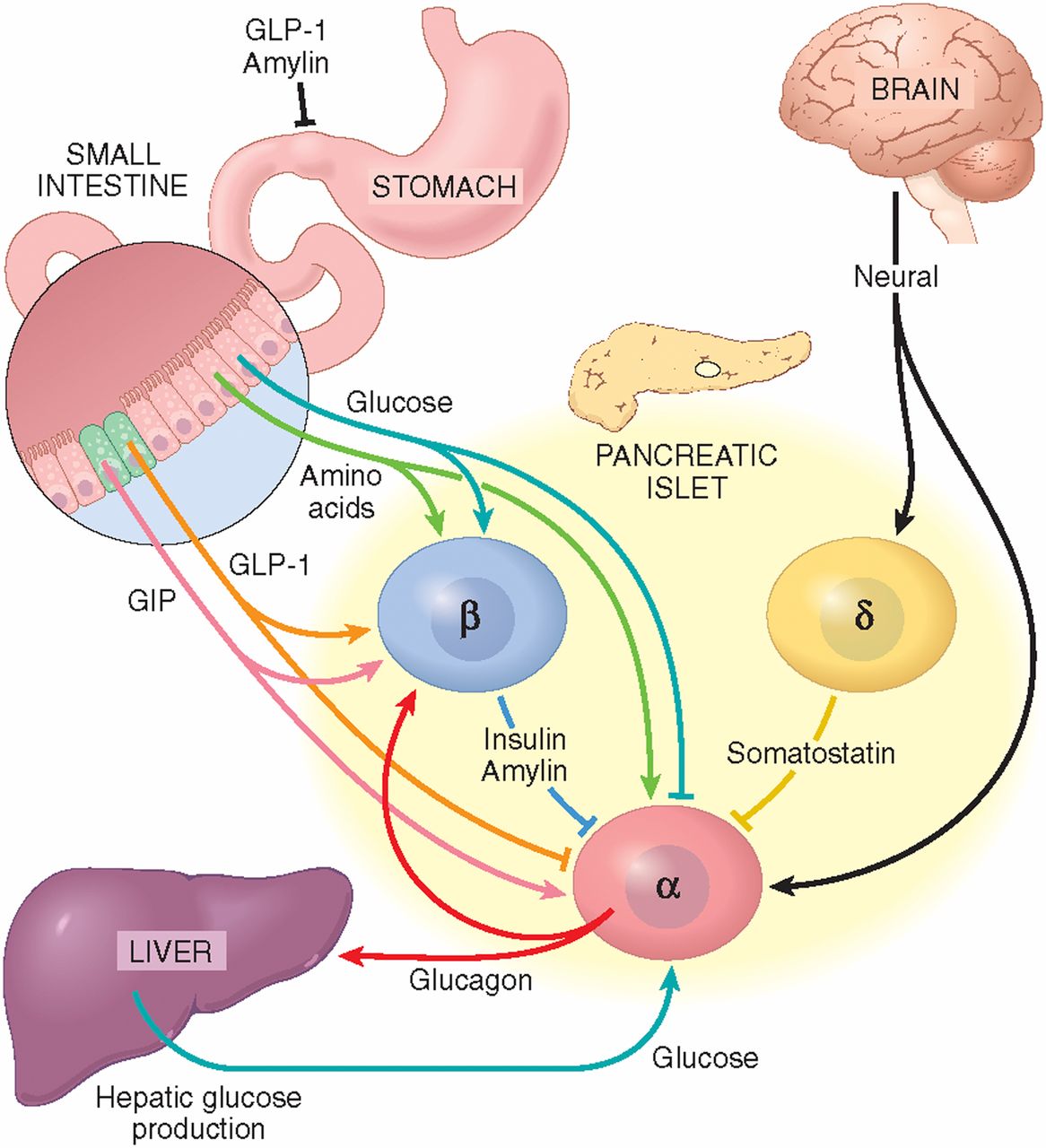
The spatial distribution of α-cells and the vascular organization within the islet sustain an important intercellular communication through autocrine and paracrine mechanisms Fig. In addition to insulin, glucagon or somatostatin, secretory granules from islet cells contain other molecules with biological activity, which are released to the extracellular space by exocytosis, activating surface receptors in the same cell, in neighbouring islet cells, or in distant cells within the islet via the vascular system.
Several paracrine mechanisms are activated at high-glucose concentrations as a result of β- and δ-cell stimulations, and thus, they may participate in the glucose-induced inhibition of glucagon release. Paracrine signalling in the α-cell.
See text for details. ADCY, adenylate cyclase; AMPA-R, α-aminohydroxymethylisoxazolepropionic acid receptor; GABA, γ-aminobutyric acid; GLP1, glucagon-like peptide-1; GRM, metabotrophic glutamate receptor; PKA, protein kinase A; SSTR2, somatostatin receptor One of the most important paracrine mechanisms responsible for inhibiting glucagon release is conducted by insulin, acting via several pathways.
An appropriate expression of the insulin receptor in mouse α-cells seems to be essential for glucose-regulated glucagon secretion Diao et al.
In INR1-G9 clonal α-cells, insulin has been found to inhibit glucagon release through the activation of phosphatidylinositol 3-kinase PIK3; Kaneko et al.
The insulin receptor—PIK3 signalling pathway is also involved in the modification of the sensitivity of K ATP channels to ATP in mouse α-cells, which may affect the secretory response Leung et al. Furthermore, insulin increases K ATP channel activity in isolated rat α-cells, inducing an inhibitory effect on glucagon release via membrane hyperpolarization Franklin et al.
In addition to the effects on K ATP channels, insulin can translocate A-type GABA receptors to the cell membrane, which increases the response to GABA secreted by β-cells, favouring membrane hyperpolarization and suppression of glucagon secretion Xu et al.
Therefore, several pieces of evidence indicate that insulin inhibits glucagon release mainly by altering α-cell membrane potential.
After exocytosis, these hexameric crystals are exposed to a change in pH from 5. Recent studies have claimed that zinc atoms can also work as modulators of the α-cell function Gyulkhandanyan et al.
: Glucagon secretion| α-cell glucokinase suppresses glucose-regulated glucagon secretion | Of note, most studies use bolus injections of glucagon which cause only a transient increase in heart rate and contractility potentially reflecting the rapid elimination of glucagon from circulation Taken together, it remains uncertain whether glucagon has a place in the treatment of heart failure or hold a cardioprotective effect in healthy subjects. Patients with type 2 diabetes exhibit an impaired regulation of glucagon secretion which contributes importantly to diabetic hyperglycemia. Specifically, type 2 diabetes is characterized by elevated levels of glucagon during fasting while suppression of glucagon in response to oral intake of glucose is impaired or even paradoxically elevated Fig. The mechanisms behind hyperglucagonemia are not fully understood but is usually explained by a diminished suppressive effect of insulin on alpha cells due to hypoinsulinemia and insulin resistance at the level of the alpha cells 53 , Interestingly, subjects with type 2 diabetes, who exhibit a hyperglucagonemic response to oral glucose, respond with a normal suppression of glucagon after intravenous glucose administration Accordingly, hormones secreted from the gastrointestinal tract may play an important role 55 , It has recently been confirmed that glucagon can be secreted from extrapancreatic tissue demonstrated in experiments with totally pancreatectomized subjects This supports the notion that postprandial hypersecretion of glucagon in patients with type 2 diabetes might be of extrapancreatic origin. Schematic illustration of plasma glucagon concentrations in patients with type 2 diabetes and in normal physiology healthy subjects. Type 2 diabetes is characterized by elevated fasting plasma glucagon levels and impaired suppression of plasma glucagon levels in response to oral glucose. Traditionally type 1 diabetic hyperglycemia has been explained by selective loss of beta cell mass and resulting decrease in insulin secretion. However, emerging evidence indicate that glucagon plays a major role in type 1 diabetes pathophysiology. The glucagon dyssecretion that characterizes patients with type 1 diabetes is associated with two clinical manifestations: Postprandial hyperglucagonemia and impaired glucagon counterregulation to hypoglycemia Data regarding fasting plasma glucagon concentrations in type 1 diabetes are inconsistent 57 , Thus, the general notion that glucagon hypersecretion plays a role in type 1 diabetes hyperglycemia is mainly based on elevated postprandial glucagon concentrations The explanation behind this is unclear, although a common explanation is, that in type 1 diabetes the postprandial increase in plasma glucose is not followed by an increase in insulin secretion from beta cells, which in normal physiology would inhibit glucagon secretion. The absence of that restraining signal from endogenous insulin could result in an increase in glucagon secretion from alpha cells after a meal Fig. However, like in type 2 diabetes, subjects with type 1 diabetes preserve their ability to suppress glucagon after intravenous glucose administration. Schematic illustration of plasma glucagon concentrations in patients with type 1 diabetes and in normal physiology healthy subjects. Type 1 diabetes is characterized by elevated concentrations of glucagon in response to a meal or oral glucose intake. Hypoglycemia is a frequent and feared side effect of insulin therapy in type 1 diabetes and it represents a common barrier in obtaining glycemic control In normal physiology hypoglycemia is prevented by several mechanisms: 1 Reduced insulin secretion from beta cells diminishing glucose uptake in peripheral tissues; 2 increased glucagon secretion from alpha cells increasing hepatic glucose output; and 3 increased symphathetic neural response and adrenomedullary epinephrine secretion. The latter will stimulate hepatic glucose production and cause clinical symptoms that enables the individual to recognize hypoglycemia and ultimately ingest carbohydrates 57 , 61 , In type 1 diabetes, insulin-induced hypoglycemia fails to elicit adequate glucagon responses compromising counterregulation to insulin-induced hypoglycemia; a phenomenon which seems to worsen with the duration of type 1 diabetes. This defect likely involves a combination of defective alpha cells and reduced alpha cell mass 57 , Dysregulated glucagon secretion is not only observed in patients with type 2 diabetes but also in normoglucose-tolerant individuals with obesity 64 and patients with non-alcoholic fatty liver disease NAFLD 65 , This suggests that dysregulated glucagon secretion may represent hepatic steatosis rather than dysregulated glucose metabolism. Interestingly, fasting hyperglucagonemia seems to relate to circulating amino acids in addition to hepatic fat content This hyperaminoacidemia suggests that impairment of amino acid turnover in the liver and ensuing elevations of circulating amino acids constitutes a feedback on the alpha cell to secrete more glucagon with increasing hepatic amino acid turnover and ureagenesis needed for clearance of toxic ammonia from the body. The implication of hyperglucagonemia in obesity and NAFLD has renewed the scientific interest in actions of glucagon and the role of glucagon in the pathophysiology of these metabolic disorders. Clearly, glucagon may represent a potential target for treatments of obesity and NAFLD. A simple way to restrain the undesirable hyperglycemic effect of glucagon while realizing its actions on lipolysis and energy expenditure could be by co-treating with a glucose-lowering drug. This may be done by mimicking the gut hormone oxyntomodulin which acts as a ligand to both the glucagon and the GLP-1 receptor. Glucagon is a glucoregulatory peptide hormone that counteracts the actions of insulin by stimulating hepatic glucose production and thereby increases blood glucose levels. Additionally, glucagon mediates several non-glucose metabolic effects of importance for maintaining whole-body energy balance in times of limited nutrient supply. These actions include mobilization of energy resources through hepatic lipolysis and ketogenesis; stimulation of hepatic amino acid turnover and related ureagenesis. Also, glucagon has been shown to increase energy expenditure and inhibit food intake, but whether endogenous glucagon is involved in the regulation of these processes remains uncertain. Glucagon plays an important role in the pathophysiology of diabetes as elevated glucagon levels observed in these patients stimulate hepatic glucose production, thereby contributing to diabetic hyperglycemia. Used under Creative Commons License 3. This electronic version has been made freely available under a Creative Commons CC-BY-NC-ND license. Turn recording back on. National Library of Medicine Rockville Pike Bethesda, MD Web Policies FOIA HHS Vulnerability Disclosure. Help Accessibility Careers. Access keys NCBI Homepage MyNCBI Homepage Main Content Main Navigation. Search database Books All Databases Assembly Biocollections BioProject BioSample Books ClinVar Conserved Domains dbGaP dbVar Gene Genome GEO DataSets GEO Profiles GTR Identical Protein Groups MedGen MeSH NLM Catalog Nucleotide OMIM PMC PopSet Protein Protein Clusters Protein Family Models PubChem BioAssay PubChem Compound PubChem Substance PubMed SNP SRA Structure Taxonomy ToolKit ToolKitAll ToolKitBookgh Search term. Show details Feingold KR, Anawalt B, Blackman MR, et al. Contents www. Search term. Glucagon Physiology Iben Rix , Christina Nexøe-Larsen , Natasha C Bergmann , Asger Lund , and Filip K Knop. hnoiger nesretep. Christina Nexøe-Larsen Center for Clinical Metabolic Research, Gentofte Hospital, University of Copenhagen, Hellerup, Denmark, Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark. Natasha C Bergmann Center for Clinical Metabolic Research, Gentofte Hospital, University of Copenhagen, Hellerup, Denmark. Asger Lund Center for Clinical Metabolic Research, Gentofte Hospital, University of Copenhagen, Hellerup, Denmark. Filip K Knop Center for Clinical Metabolic Research, Gentofte Hospital, University of Copenhagen, Hellerup, Denmark, Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark, Novo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Steno Diabetes Center Copenhagen, Gentofte, Denmark Email: kd. hnoiger ABSTRACT Glucagon is a peptide hormone secreted from the alpha cells of the pancreatic islets of Langerhans. STRUCTURE AND SYNTHESIS OF GLUCAGON Glucagon is a amino acid peptide hormone predominantly secreted from the alpha cells of the pancreas. GLUCAGON SECRETION Glucagon is secreted in response to hypoglycemia, prolonged fasting, exercise and protein-rich meals Regulation of Glucagon Secretion by Glucose The most potent regulator of glucagon secretion is circulating glucose. Glucagon Concentrations in The Circulation In normal physiology, circulating glucagon concentrations are in the picomolar range. Glucagon concentrations in response to hypoglycemia, euglycemia, and hyperglycemia. GLUCAGON ACTIONS Glucagon Increases Hepatic Glucose Production Glucagon controls plasma glucose concentrations during fasting, exercise and hypoglycemia by increasing hepatic glucose output to the circulation. Glucagon Stimulates Break-Down of Fatty Acids and Inhibits Lipogenesis in the Liver Glucagon promotes formation of non-carbohydrate energy sources in the form of lipids and ketone bodies. Glucagon Promotes Break-Down of Amino Acids During prolonged fasting, glucagon stimulates formation of glucose from amino acids via gluconeogenesis by upregulating enzymes involved in the process. Glucagon Reduces Food Intake Acute administration of glucagon has been shown to reduce food intake and diminish hunger 38 , Glucagon Increases Energy Expenditure In addition to a potential effect of glucagon on food intake, evidence suggests that glucagon contributes to a negative energy balance by stimulating energy expenditure. Glucagon May Regulate Heart Rate and Contractility Infusion of high doses of glucagon increases heart rate and cardiac contractility Organ specific actions of glucagon. GIP, glucose-dependent insulinotropic polypeptide. Glucagon in Type 1 Diabetes Traditionally type 1 diabetic hyperglycemia has been explained by selective loss of beta cell mass and resulting decrease in insulin secretion. Glucagon in Obesity and Hepatic Steatosis Dysregulated glucagon secretion is not only observed in patients with type 2 diabetes but also in normoglucose-tolerant individuals with obesity 64 and patients with non-alcoholic fatty liver disease NAFLD 65 , Habegger KM, Heppner KM, Geary N, Bartness TJ, DiMarchi R, Tschöp MH. The metabolic actions of glucagon revisited. Nat Rev Endocrinol. Kimball CP, Murlin JR. Aqueous Extracts of Pancreas Iii. Some Precipitation Reactions of Insulin. Bromer WW, Sinn LG, Staub A, Behrens OK. The amino acid sequence of glucagon. Blackman B. The use of glucagon in insulin coma therapy. Psychiatr Q. Esquibel AJ, Kurland AA, Mendelsohn D. The use of glucagon in terminating insulin coma. Dis Nerv Syst. Unger RH, Eisentraut AM. McCALL MS, Madison LL. Glucagon antibodies and an immunoassay for glucagon. Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Drucker DJ, Asa S. Glucagon gene expression in vertebrate brain. Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. Gerich JE, Lorenzi M, Hane S, Gustafson G, Guillemin R, Forsham PH. Evidence for a physiologic role of pancreatic glucagon in human glucose homeostasis: studies with somatostatin. Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Müller TD, Finan B, Clemmensen C, DiMarchi RD, Tschöp MH. The New Biology and Pharmacology of Glucagon. Physiological Reviews. Wewer Albrechtsen NJ, Kuhre RE, Pedersen J, Knop FK, Holst JJ. The biology of glucagon and the consequences of hyperglucagonemia. Biomarkers in Medicine. Gromada J, Chabosseau P, Rutter GA. The α-cell in diabetes mellitus. Hughes JW, Ustione A, Lavagnino Z, Piston DW. Regulation of islet glucagon secretion: Beyond calcium. Diabetes, Obesity and Metabolism. Knop FK, Vilsbøll T, Madsbad S, Holst JJ, Krarup T. Inappropriate suppression of glucagon during OGTT but not during isoglycaemic i. glucose infusion contributes to the reduced incretin effect in type 2 diabetes mellitus. Lund A, Bagger JI, Albrechtsen NJW, Christensen M, Grøndahl M, Hartmann B, Mathiesen ER, Hansen CP, Storkholm JH, Hall G, van, Rehfeld JF, Hornburg D, Meissner F, Mann M, Larsen S, Holst JJ, Vilsbøll T, Knop FK. Evidence of Extrapancreatic Glucagon Secretion in Man. Miyachi A, Kobayashi M, Mieno E, Goto M, Furusawa K, Inagaki T, Kitamura T. Accurate analytical method for human plasma glucagon levels using liquid chromatography-high resolution mass spectrometry: comparison with commercially available immunoassays. Anal Bioanal Chem. Hansen JS, Pedersen BK, Xu G, Lehmann R, Weigert C, Plomgaard P. Exercise-Induced Secretion of FGF21 and Follistatin Are Blocked by Pancreatic Clamp and Impaired in Type 2 Diabetes. Schwartz NS, Clutter WE, Shah SD, Cryer PE. Glycemic thresholds for activation of glucose counterregulatory systems are higher than the threshold for symptoms. J Clin Invest. Svoboda M, Tastenoy M, Vertongen P, Robberecht P. Relative quantitative analysis of glucagon receptor mRNA in rat tissues. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Pospisilik JA, Hinke SA, Pederson RA, Hoffmann T, Rosche F, Schlenzig D, Glund K, Heiser U, McIntosh CHS, Demuth H-U. Metabolism of glucagon by dipeptidyl peptidase IV CD Regulatory Peptides. Pontiroli AE, Calderara A, Perfetti MG, Bareggi SR. Pharmacokinetics of intranasal, intramuscular and intravenous glucagon in healthy subjects and diabetic patients. Lund A, Bagger JI, Albrechtsen NW, Christensen M, Grøndahl M, Hansen CP, Storkholm JH, Holst JJ, Vilsbøll T, Knop FK. Increased Liver Fat Content in Totally Pancreatectomized Patients. Unger RH. Glucagon physiology and pathophysiology in the light of new advances. Miller RA, Birnbaum MJ. Glucagon: acute actions on hepatic metabolism. Ramnanan CJ, Edgerton DS, Kraft G, Cherrington AD. Physiologic action of glucagon on liver glucose metabolism. Diabetes Obes Metab. Rui L. Energy Metabolism in the Liver. Compr Physiol. Geisler CE, Renquist BJ. Hepatic lipid accumulation: cause and consequence of dysregulated glucoregulatory hormones. J Endocrinol. Longuet C, Sinclair EM, Maida A, Baggio LL, Maziarz M, Charron MJ, Drucker DJ. The Glucagon Receptor Is Required for the Adaptive Metabolic Response to Fasting. Cell Metabolism. Wang H, Zhao M, Sud N, Christian P, Shen J, Song Y, Pashaj A, Zhang K, Carr T, Su Q. Glucagon regulates hepatic lipid metabolism via cAMP and Insig-2 signaling: implication for the pathogenesis of hypertriglyceridemia and hepatic steatosis. Sci Rep. Galsgaard KD, Pedersen J, Knop FK, Holst JJ, Wewer Albrechtsen NJ. Glucagon Receptor Signaling and Lipid Metabolism. Front Physiol. Holst JJ, Albrechtsen NJW, Pedersen J, Knop FK. Glucagon and Amino Acids Are Linked in a Mutual Feedback Cycle: The Liver—α-Cell Axis. Hamberg O, Vilstrup H. Regulation of urea synthesis by glucose and glucagon in normal man. Clin Nutr. Solloway MJ, Madjidi A, Gu C, Eastham-Anderson J, Clarke HJ, Kljavin N, Zavala-Solorio J, Kates L, Friedman B, Brauer M, Wang J, Fiehn O, Kolumam G, Stern H, Lowe JB, Peterson AS, Allan BB. Glucagon Couples Hepatic Amino Acid Catabolism to mTOR-Dependent Regulation of α-Cell Mass. Cell Rep. Bagger JI, Holst JJ, Hartmann B, Andersen B, Knop FK, Vilsbøll T. J Clin Endocrinol Metab. Geary N, Kissileff HR, Pi-Sunyer FX, Hinton V. Individual, but not simultaneous, glucagon and cholecystokinin infusions inhibit feeding in men. Langhans W, Zeiger U, Scharrer E, Geary N. Stimulation of feeding in rats by intraperitoneal injection of antibodies to glucagon. Le Sauter J, Noh U, Geary N. Hepatic portal infusion of glucagon antibodies increases spontaneous meal size in rats. Nair KS. Hyperglucagonemia Increases Resting Metabolic Rate In Man During Insulin Deficiency. Glucagon increases energy expenditure independently of brown adipose tissue activation in humans. Tan TM, Field BCT, McCullough KA, Troke RC, Chambers ES, Salem V, Gonzalez Maffe J, Baynes KCR, De Silva A, Viardot A, Alsafi A, Frost GS, Ghatei MA, Bloom SR. Coadministration of Glucagon-Like Peptide-1 During Glucagon Infusion in Humans Results in Increased Energy Expenditure and Amelioration of Hyperglycemia. Fibroblast Growth Factor 21 Mediates Specific Glucagon Actions. Ceriello A, Genovese S, Mannucci E, Gronda E. Glucagon and heart in type 2 diabetes: new perspectives. Cardiovasc Diabetol. Graudins A, Lee HM, Druda D. Calcium channel antagonist and beta-blocker overdose: antidotes and adjunct therapies. Br J Clin Pharmacol. Meidahl Petersen K, Bøgevig S, Holst JJ, Knop FK, Christensen MB. In order to examine the direct effects of glucose on glucagon secretion in the absence of paracrine inputs, isolated mouse pancreatic alpha cells, clonal hamster In-R1-G9 cells 48 , 49 , clonal mouse αTC and -9 cells 39 , 50 , 51 and dispersed alpha cells from human islets 52 have been used. All of these preparations show a bimodal response to increasing glucose concentrations. In the range from 1 to ~7 mM, glucagon secretion is suppressed in a dose-dependent manner, and above 7 mM, glucagon secretion increases Figure 2A. This secretion profile suggests intrinsic mechanisms alone can operate in regulating glucagon secretion below 7 mM glucose, and that these mechanisms may be ineffective at higher glucose concentrations. However, such conclusions must be interpreted with caution, as single dispersed alpha cells are in a highly abnormal environment, and alpha cell lines are not representative of the normal alpha cell phenotype, as discussed in more detail below. Figure 2 Glucagon secretion from dispersed alpha cells and alpha cells in intact islets demonstrate the role of paracrine regulation at high glucose concentrations. A V-shape curve of glucagon exocytosis in response to glucose in dispersed non-diabetic black and T2D red human α-cells. B Glucagon secretion from intact islets in response to glucose. Created with BioRender. The alpha cell secretory response to both glucose is likely more accurately captured in isolated, intact mouse and human islets, where the paracrine regulatory environment and cell-cell contacts are intact. Similar to dispersed alpha cells, increasing the glucose concentration from 1 to 7 mM dose dependently decreases glucagon secretion from mouse alpha cells 53 and human alpha cells 52 within intact islets, and remains low as glucose levels increase beyond 7 mM, a concentration at which insulin secretion is stimulated Figure 2B. Therefore, paracrine inputs are significant factors in the inhibition of glucagon secretion as glucose concentrations increase above euglycemia. One mechanism underlying the intrinsic response to glucose is the direct effect on alpha cell electrical activity. At low 1 mM glucose concentrations, alpha cells in intact mouse and human islets exhibit low K ATP activity and are electrically active 54 — 56 and as glucose concentrations increase, K ATP activity is inhibited. A recent review by Zhang et al. Therefore, the intrinsic regulation of glucagon secretion by glucose may be explained primarily by the unique electrical properties of the alpha cell, and secondarily by glucose metabolism. In particular, cAMP signalling may play a key role in the alpha cell secretory response to insulin and somatostatin There is one report that cAMP may also mediate intrinsic glucose sensing within the alpha cell. Using genetically encoded fluorescent cAMP biosensors, it was shown that high glucose suppressed subplasmalemmal cAMP levels in isolated mouse and human islets Conversely, sustained high cAMP levels abolished the suppression of glucagon secretion by high glucose concentrations. Lastly, intrinsic glucose sensing by the alpha cell may also be mediated by the nutrient sensors AMP-activated protein kinase AMPK and its downstream target, mammalian target of rapamycin complex 1 mTORC1. In a series of studies that manipulated alpha cell expression of AMPK itself 65 and its upstream effectors PASK 66 and LKB1 67 , it was shown that components of this nutrient-sensing pathway can mediate the low glucose-induced secretion of glucagon. One of these proteins, PASK, is down-regulated in T2D human islets, thus indicating that components of the AMPK pathway may be potential targets for controlling hyperglucagonemia. Using innovative mouse models that selectively targeted activators and inhibitors of mTORC1, it was shown that loss of mTORC1 activity resulted in a loss of the glucose counter-regulatory response and reduction in response to alpha cell secretagogues Interestingly, depletion of the mTORC1 inhibitor TSC2 in alpha cells resulted in a mouse model of hyperglucagonemia and glucagon resistance 69 , which will be an excellent resource for studies on mechanisms of hyperglucagonemia. Therefore, the mechanisms underlying the intrinsic response to glucose may provide potential targets for the control of abnormally up-regulated glucagon secretion in diabetes. The beta cell secretory granule contains a number of agents that act directly or indirectly on the alpha cell to inhibit glucagon secretion, and also generally modulate mechanisms of alpha cell biology, such as proliferation. Insulin, the primary cargo, is a potent suppressor of glucagon secretion and operates through several mechanisms. Mice lacking the insulin receptor on alpha cells αIRKO exhibit hyperglycemia and hyperglucagonemia, indicating that insulin receptor signalling is required for an appropriate alpha cell secretory response to glucose Alpha cell insulin resistance may underlie the abnormal up-regulation of glucagon secretion Type 2 diabetes Additionally, these results also indicate that insulin alone is not sufficient to regulate glycemia in the face of hyperglucagonemia. Along with insulin, gamma amino butyric acid GABA is also released from the beta cell and is a potent suppressor of glucagon secretion from alpha cells 73 , Activating the GABA A receptor in alpha cells results in Cl - influx into the cells which hyperpolarizes the membrane and reduces glucagon secretion As well, there is coordination between insulin and GABA A receptor activity, as insulin action leads to the translocation of GABA A receptor to the cell membrane 76 , thus augmenting the inhibitory effects of GABA. In addition, GABA also inhibits mTOR activity to suppress alpha cell proliferation. In type 1 diabetes, the destruction of beta cells leads to a reduction in the amount of secreted GABA, resulting in the activation of mTOR and alpha cell proliferation In addition to effects on alpha cell proliferation, some studies have suggested that pharmacologic activation of GABA A receptor by artemisinins or GABA may alter alpha cell identity and trans-differentiate adult alpha cells to beta-like cells 78 — 80 , and have led to clinical trials investigating GABA receptor agonists as protection against the development of diabetes. However, there is still some debate on this topic, as transdifferentiation could not be induced either in isolated mouse islets in which both insulin and glucagon were tagged with fluorescent reporters 81 or in an alpha cell-specific lineage tracing model In any case, the reported immunomodulatory effects of GABA, together with either GLP-1 83 or the SGLT2 inhibitor empagliflozin 84 also protect newly formed beta cells in the inflammatory environment of T1D, and thus also indirectly restore normal regulation of alpha cell mass and glucagon secretion. Direct effects of serotonin are mediated by activation of the serotonin receptor, 5-HT 1F R, on α-cells, which reduces intracellular cAMP to suppress glucagon secretion 85 , In patients with long-standing T2D, the proportion of alpha cells expressing 5-HT 1F R is decreased, suggesting that reduced serotonin action on alpha cells may play a role in hyperglucagonemia of diabetes. In STZ-treated mice, administration of the 5-HT 1F R agonist LY alleviated hyperglucagonemia and hyperglycemia. However, insulin-induced hypoglycemia was worsened, suggesting that the effects of serotonin are glucose-independent Therefore, while alpha cell HT 1F R may be a potential target for the treatment of hyperglucagonemia, it may not be an ideal target. The effects of adenosine are mediated by the adenosine A1 receptor Adora1 , in which activation is coupled to opening of K ATP channels, hyperpolarization of the cell membrane and prevention of granule exocytosis. In NOD mice, autoantibody-positive people and people with long-term T1D, alpha cells gradually lose Adora1 expression, suggesting that the hyperglucagonemia of diabetes is associated with a loss of adenosine action ZnT8 is located in the secretory granule membrane of both α-and β-cells. There is a direct relationship between expression of the proglucagon gene and Slc30A8 in α-cells Somatostatin is a well-known tonic inhibitor of glucagon secretion. Somatostatin binds to the SSTR2 receptor subtype on alpha cells 93 , which is coupled to the inhibitory G i subunit, resulting in decreased production of cAMP as a mechanism for the suppression of glucagon secretion Notably, secretion of somatostatin and inhibition of glucagon secretion both occur at 3 mM glucose, indicating that the alpha cell response to low glucose may be fine-tuned by somatostatin In rat pancreatic preparations perfused with an SSTR2 antagonist, the suppression of glucagon secretion by 3. However, in isolated human islets, blockade of SSTR2 did not affect suppression of glucagon secretion at 6 mM glucose 55 , perhaps reflecting species-specific differences or differences in the models perfused pancreas vs static islet culture. Interestingly, insulin secretion was also elevated, indicating that both insulin and somatostatin are required for the suppression of glucagon secretion at high glucose concentrations. In intact human islets, high glucose 10 mM inhibition of glucagon exocytosis was lost after administration of the SSTR2 antagonist CYN In diabetes, circulating and pancreatic somatostatin, together with SST mRNA, are elevated. However, expression of SSTR2 on alpha cells is decreased in T2D due to increased receptor internalization 52 , indicating alpha cell somatostatin resistance. Together with alpha cell insulin resistance, this could be another mechanism in the hyperglucagonemia of diabetes. Alternatively, somatostatin resistance may be a dominant and direct mechanism of hyperglucagonemia, as eliminating the insulin receptor on delta cells completely abolishes the glucagonostatic effect of insulin, indicating an indirect glucagonostatic effect for insulin The emerging role of somatostatin in the regulation of alpha cell function and glucagon secretion has been further highlighted by one study in which mice were engineered for optogenetic activation of beta cells to study the paracrine regulation of alpha cells By this approach, opto-activation of beta cells both suppressed alpha cell electrical activity and stimulated action potentials in delta cells mediated by gap junction currents. The suppressive effect of beta cell activation was lost in the presence of the SSTR2 antagonist CYN 99 , indicating that somatostatin secretion stimulated by beta cell electrical activity is critical for the suppression of glucagon secretion. Subsequent modelling predicted that a reduction in gap junction connections between beta and delta cells, perhaps caused by disruptions in islet architecture in T2D , may contribute to the hyperglucagonemia of diabetes. Thus these findings highlight a central role for delta cells in the context of intra-islet regulation of glucagon secretion, and may have implications for designing drugs for the treatment of hyperglucagonemia of diabetes. The alpha cell itself displays plasticity during the progression of diabetes. In addition to the mechanisms above that describe changes in responses to glucose and paracrine effectors, there are alterations within the alpha cell, including proglucagon processing and secretion of proglucagon-derived peptides, and remodelling of the secretory granules themselves in terms of exocytotic behavior and contents, and alterations in intracellular trafficking pathways. Secreted glucagon from alpha cells can stimulate its secretion through an autocrine effect. It has been shown that glucagon stimulates glucagon secretion from the rat and mouse isolated alpha cells in an autocrine manner through glucagon receptor-stimulated cAMP signaling In αTC cells and mouse islets, exogenous glucagon administration, as well as secreted glucagon stimulated by 1 mM glucose, increased glucagon secretion and proglucagon gene transcription through the PKA-cAMP-CREB signalling pathway in a glucagon receptor-dependent manner The apparent interplay between glucagon and its receptor on the alpha cell appears to be of a positive feedback loop, controlled by the pulsatile nature of glucagon secretion. In addition to glucagon, a novel proglucagon-derived peptide, proglucagon PG comprised of GRPP and glucagon, was identified as a major molecular form of glucagon in plasma from human patients with hyperglucagonemia-associated conditions: Type 2 diabetes and renal dysfunction, morbid obesity or gastric bypass surgery, and only after oral ingestion of macronutrients This N-terminally extended form of immunoreactive glucagon was not found in healthy controls, leading the authors to speculate that PG , and molecular heterogeneity of glucagon in general, could be a biomarker for alpha cell dysfunction. Administration of PG decreased glucagon secretion in healthy rats, diverging from the positive feedback observed with glucagon administration. Interestingly, this effect was not observed in diabetic rats, suggesting an impairment in this distinct feedback loop in the alpha cell. The interplay between glucagon, insulin and somatostatin in the regulation of glucagon secretion at various levels of glucose is illustrated in Figure 3. In diabetes, beta cell deficiency, together with alpha cell insulin and somatostatin resistance, all contribute to alpha cell dysfunction and a loss of the regulation of glucagon secretion, resulting in hyperglucagonemia. Figure 3 Cross-talk among α, β, and δ-cells in the paracrine regulation of glucagon secretion. Under low glucose mM conditions, secreted glucagon may act in an autocrine feed-forward loop. Additionally, electrical coupling of the beta and delta cells through gap junctions contributes to somatostatin release. Somatostatin binds to SST receptor 2 SSTR2 on the α cell membrane, where signalling through G i inhibits glucagon secretion. The glucose-dependent insulinotropic actions of intestinal GLP-1 on the beta cell are well known. GLP-1 also suppresses glucagon secretion in both healthy people and people with type 2 diabetes , and poorly-controlled type 1 diabetes The emerging evidence of GLP-1 being produced and secreted by the pancreatic alpha cell has led to a debate on which source of GLP-1 suppresses glucagon secretion from pancreatic alpha cells. To investigate this question, Chambers et al. The gut-derived GLP-1 binds to its receptor on local afferent vagal nerve terminals, which ultimately signals for satiety, delaying gastric emptying and suppression of hepatic glucose release , However, this model may not translate well to human islets due to differences in islet architecture, and in light of the recent findings that glucagon is the dominant peptide hormone secreted from human alpha cells The search for a GLP-1 receptor on alpha cells has been hampered by a lack of a reliable GLP-1 receptor antibody , GLP-1 appears to mildly reduce action potentials in the alpha cell membrane at 1 mM glucose in isolated mouse alpha cells, and this effect is blocked by the GLP-1R antagonist exendin , therefore suggesting the presence of GLP-1R, perhaps at a very low density, on a small proportion of alpha cells. The development of near infra-red and fluorescent analogues of GLP-1R ligands has enabled both in vivo , and high-resolution tissue imaging , of GLP-1R with high specificity, sensitivity, and reproducibility. Given the already small proportion of alpha cells in the mouse islet, the contribution of direct alpha cell action to the glucagonostatic effect of GLP-1 is likely very small. Islet GLP-1 may also exert its effects through receptors on delta cells , resulting in stimulation of somatostatin secretion and inhibition of glucagon secretion via SSTR2 on alpha cells , This paracrine effect could not be detected in isolated normal human islets ; nonetheless, this mechanism may be clinically relevant in the treatment of T2D, as experiments in human islets showed that the GLP-1R agonist liraglutide enhanced somatostatin secretion to reduce hyperglucagonemia induced by the SGLT2 inhibitor dapagliflozin As drugs targeted to the control of glucagon secretion are now being developed for the treatment of hyperglucagonemia, a deeper understanding of the dynamics of the alpha cell secretory granule is critical for identifying effective targets. However, the study of glucagon granule trafficking and exocytosis presents several technological challenges. Commonly used cell lines such as InR1-G9, αTC and αTC, while useful for preliminary studies on trafficking and secretion, as a rule do not exhibit robust secretory responses to glucose or other secretagogues. The αTC cell line in particular differs from primary alpha cells in their complement of transcriptional, epigenetic and metabolic factors , which may explain the blunted secretory response to glucose. Dispersed primary alpha cells may offer a slightly better alternative, but as discussed above, both cell lines and dispersed primary alpha cells exhibit aberrant glucagon exocytosis patterns at high glucose levels, likely due to the absence of paracrine inputs and juxtamembrane contacts. The greatest advances in gleaning the mechanisms of glucagon granule exocytosis have been made using patch-clamp approaches in isolated rodent or human islets. In such preparations, alpha cells can identified by their unique electrophysiological signature under low glucose conditions or, in the case of mouse islets, by genetically-encoded fluorescence reporters such as YFP , or tdTomato After proglucagon processing and granule maturation, glucagon is stored in the alpha cell secretory granule until a stimulus triggers exocytosis. As in beta cells, there may be different functional pools of secretory granules: a reserve pool and a readily releasable pool that is primed and situated at the sites of exocytosis. Quantitative ultrastructural analysis of murine islets has shown that, in the presence of 1mM glucose, the mouse α-cell contains ~ secretory granules, of which ~ are in close proximity to the plasma membrane, or primed This means that the reserve pool is large and can resupply the readily releasable pool to maintain euglycemia over extended periods of time. In the presence of Following docking, secretory granules are primed through the action of the SNARE protein complex. This complex contains two subsets of proteins; i the t-SNAREs syntaxin 1A and SNAP, located in the plasma membrane; and ii the v-SNAREs VAMP2 and synaptotagmin VII, which are located in the granule membrane Under low glucose conditions, SNAP and syntaxin 1A are translocated to the plasma membrane. SNAP itself may play a role in the transportation of granules from the releasable pool to the readily releasable pool, and then mediates their fusion with plasma membrane via interaction with syntaxin 1A , Live imaging of exocytosis using a proglucagon-luciferase reporter showed spatial clustering of glucagon secretion sites in αTC cells Future studies may reveal some interesting dynamics with SNARE proteins that may fine-tune the alpha cell secretory response to glucose and paracrine inputs. Could disruption of these molecular mechanisms contribute to the hyperglucagonemia of diabetes? However, neither membrane potential nor exocytosis was responsive to insulin or to a greater extent somatostatin, in contrast to normal alpha cells in which both were significantly reduced. Therefore, in T2D, hyperglucagonemia may result from insulin and somatostatin resistance at the level of the readily releasable pool of granules. In alpha cells of patients with T1D, expression levels of genes encoding SNARE proteins, ion channels and cAMP signalling molecules were disrupted , which could explain the impaired glucose counter-regulatory response and the inappropriately elevated levels of postprandial glucagon in T1D. Combining patch-clamp electrophysiological measurements with single-cell RNA sequencing patch-seq in human islets has given high-resolution insight into mechanisms underlying impairments in alpha cell function in diabetes at the level of granule exocytosis. Further characterization of the link between electrophysiological signatures and the genes regulating the dynamics of granule exocytosis will reveal new mechanisms of alpha cell dysfunction in diabetes. Identifying new pathways or networks that control glucagon granule biogenesis and trafficking may identify novel targets for the control of hyperglucagonemia in addition to yielding a greater understanding of alpha cell biology in both health and disease. There is an emerging hypothesis that glucagon secretion can be controlled by trafficking through the endosomal-lysosomal pathway, similar to insulin , and below, we highlight some recent studies that suggest glucagon may regulated through such an alternate trafficking pathway. Brefeldin A-inhibited guanine nucleotide exchange protein 3 BIG3 is a member of the Arf-GEF family of proteins, and was initially found in a database search and found to inhibit insulin granule biogenesis and insulin secretion A subsequent study found that it had a similar role in regulating glucagon granule production and exocytosis Whether BIG3 can mediate glucagon trafficking through lysosomes remains to be investigated. The composition and cargo of the alpha cell secretory granule may also hold some determinants of glucagon secretion. While it is known that granule contents and composition are modified during normal granule maturation, a more complete picture of granule remodeling and heterogeneity in the context of intracellular trafficking networks in normal physiology and in diabetes is required. In an effort to identify networks of secretory granule proteins that interact with glucagon and regulate its trafficking and secretion, proteomic analysis was conducted on αTC cell secretory granule lysates immunoprecipitated with tagged glucagon This qualitative study demonstrated the plasticity in the network of proteins interacting with glucagon in response to insulin or GABA under high 25 mM or low 5. Stathmin-2, a member of the family of neuronal phosphoproteins that associates with the secretory pathway in neurons, was identified as a candidate protein for the regulation of glucagon secretion and subsequently shown to modulate glucagon secretion through the lysosomal pathway and may be down-regulated in diabetes in humans and in mice Therefore, disruptions in the routing of glucagon through the lysosomal pathway may contribute to the hyperglucagonemia of diabetes Figure 4. Figure 4 Stathminmediated lysosomal trafficking modulates glucagon secretion. Glucagon dark blue and stathmin-2 light blue are normally sorted to secretory granules from the Golgi in alpha cells. Stathmin-2 overexpression diverts glucagon-containing secretory granules to lysosomes black arrows , thus reducing glucagon secretion. Additionally, secretion from secretory granules is also enhanced solid red arrow. Glucagon trafficking and exocytosis may also be controlled through nutrient-driven pathways. The nutrient sensor O-GlcNAc transferase OGT catalyses the O-glycosylation of several proteins including those involved in the conventional secretory pathway and autophagosome-lysosome fusion In mice lacking OGT specifically in alpha cells, glucagon secretion, cell content and alpha cell mass are reduced Possible mechanisms include lack of O-glycosylation of FOXA1 and FOXA2, which regulate genes encoding proteins involved in proglucagon processing and glucagon secretion Whether other trafficking proteins are affected, and how alpha cell function is affected in diabetes in these mice, is not yet known. So what are the implications of glucagon trafficking through the lysosomal pathway in diabetes? Lysosomal trafficking and autophagy in the beta cell may be a possible mechanism of insulin secretory defects in diabetes, with a recent study providing evidence for impairment of lysosomal function in human T1D How does lysosomal function contribute to defects in alpha cell function? It is tempting to hypothesize that impairments in lysosomal biogenesis and trafficking result in both reduced insulin secretion in the beta cell and unregulated glucagon secretion from the alpha cell. Further investigation into the altered dynamics of glucagon trafficking in the alpha cell in diabetes may reveal key roles for the lysosome in the regulation of glucagon secretion, thus identifying a potential new target for the treatment of hyperglucagonemia. Finally, some excellent single-cell transcriptomics and epigenomics databases are being generated that reveal the dynamics of intracellular trafficking networks at the transcriptional level in human pancreatic alpha cells in both health and diabetes — The mapping of T2D-associated genetic variants with RNA-seq of human islets may reveal risk factors associated with defects in alpha cell function A novel immunocompromised mouse model in which glucagon-encoding codons were deleted while preserving both GLP-1 and GLP-2 will provide an innovative and much-needed resource for the study of the regulation of glucagon secretion from human islets in vivo In this study, transplantation of islets from people with T2D resulted in hyperglucagonemia with apparent alpha cell insulin resistance, revealing intrinsic alpha cell defects in T2D. Moreover, defects in alpha cell function were more apparent than in isolated islets, thus emphasizing the utility of such an in vivo system to investigate the molecular mechanisms of glucagon secretion in human islets, and the testing of possible treatments for hyperglucagonemia. While the development of glucagon receptor antagonists and other inhibitors of glucagon action has provided some possibilities for the treatment of hyperglucagonemia, there are significant side effects that result from impaired hepatic metabolism and potentially uncontrolled alpha cell proliferation. The advantage to developing such drugs, however, lie in the fact that the glucagon receptor is an easily available target. In contrast, targeting glucagon secretion as a means to treat hyperglucagonemia may alleviate concerns about effects on the liver and alpha cell mass; however, there are potentially many more targets within the alpha cell secretory pathway, and many of those may not be easily accessible for drug treatment. The ongoing discovery of novel proteins and networks that regulate the secretion of glucagon will shed further light on alpha cell biology in health and disease while also searching for improved means to control hyperglucagonemia and hyperglycemia of diabetes. SD and FA co-wrote the manuscript. All authors contributed to the article and approved the submitted version. This work was funded by a Natural Sciences and Engineering Research Council Discovery Grant to SD. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Stanley S, Moheet A, Seaquist ER. Central Mechanisms of Glucose Sensing and Counterregulation in Defense of Hypoglycemia. Endocr Rev — doi: PubMed Abstract CrossRef Full Text Google Scholar. DCCT Research Group. Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes — Unger R, Orci L. The Essential Role of Glucagon in the Pathogenesis of Diabetes Mellitus. Lancet —6. Unger RH, Cherrington AD. Glucagonocentric Restructuring of Diabetes: A Pathophysiologic and Therapeutic Makeover. J Clin Invest — Lee Y, Wang M-Y, Du XQ, Charron MJ, Unger RH. Glucagon Receptor Knockout Prevents Insulin-Deficient Type 1 Diabetes in Mice. Diabetes —7. Conarello SL, Jiang G, Mu J, Li Z, Woods J, Zycband E, et al. Glucagon Receptor Knockout Mice are Resistant to Diet-Induced Obesity and Streptozotocin-Mediated Beta Cell Loss and Hyperglycaemia. Diabetologia — Neumann UH, Ho JSS, Mojibian M, Covey SD, Charron MJ, Kieffer TJ. Glucagon Receptor Gene Deletion in Insulin Knockout Mice Modestly Reduces Blood Glucose and Ketones But Does Not Promote Survival. Mol Metab —6. Damond N, Thorel F, Moyers JS, Charron MJ, Vuguin PM, Powers AC, et al. Blockade of Glucagon Signaling Prevents or Reverses Diabetes Onset Only If Residual β-Cells Persist. Elife — CrossRef Full Text Google Scholar. Kazda CM, Ding Y, Kelly RP, Garhyan P, Shi C, Lim CN, et al. Evaluation of Efficacy and Safety of the Glucagon Receptor Antagonist LY in Patients With Type 2 Diabetes: and Week Phase 2 Studies. Diabetes Care —9. Yang B, Gelfanov VM, Perez-Tilve D, DuBois B, Rohlfs R, Levy J, et al. Optimization of Truncated Glucagon Peptides to Achieve Selective, High Potency, Full Antagonists. J Med Chem — Lee CY, Choi H, Park EY, Nguyen TTL, Maeng HJ, Mee Lee K, et al. Synthesis and Anti-Diabetic Activity of Novel Biphenylsulfonamides as Glucagon Receptor Antagonists. Chem Biol Drug Des — Okamoto H, Cavino K, Na E, Krumm E, Kim SY, Cheng X, et al. Glucagon Receptor Inhibition Normalizes Blood Glucose in Severe Insulin-Resistant Mice. Proc Natl Acad Sci —8. Liang Y, Osborne MC, Monia BP, Bhanot S, Gaarde WA, Reed C, et al. Kim J, Okamoto H, Huang ZJ, Anguiano G, Chen S, Liu Q, et al. Amino Acid Transporter Slc38a5 Controls Glucagon Receptor Inhibition-Induced Pancreatic α Cell Hyperplasia in Mice. Cell Metab — Wei R, Gu L, Yang J, Yang K, Liu J, Le Y, et al. Antagonistic Glucagon Receptor Antibody Promotes α-Cell Proliferation and Increases β-Cell Mass in Diabetic Mice. iScience — Galsgaard KD, Winther-Sørensen M, Ørskov C, Kissow H, Poulsen SS, Vilstrup H, et al. Disruption of Glucagon Receptor Signaling Causes Hyperaminoacidemia Exposing a Possible Liver-Alpha-Cell Axis. Am J Physiol Metab E93—E Wewer Albrechtsen NJ, Pedersen J, Galsgaard KD, Winther-Sørensen M, Suppli MP, Janah L, et al. The Liver—α-Cell Axis and Type 2 Diabetes. Guan H-P, Yang X, Lu K, Wang S-P, Castro-Perez JM, Previs S, et al. Glucagon Receptor Antagonism Induces Increased Cholesterol Absorption. J Lipid Res — Tooze S. Biogenesis of Secretory Granules in the Trans-Golgi Network of Neuroendocrine and Endocrine Cells. Biochim Biophys Acta — Cool DR, Fenger M, Snell CR, Loh YP. Identification of the Sorting Signal Motif Within Pro-Opiomelanocortin for the Regulated Secretory Pathway. J Biol Chem —9. Dhanvantari S, Shen F, Adams T, Snell CR, Zhang C, Mackin RB, et al. Disruption of a Receptor-Mediated Mechanism for Intracellular Sorting of Proinsulin in Familial Hyperproinsulinemia. Mol Endocrinol — Zhang C-F, Dhanvantari S, Lou H, Loh YP. Sorting of Carboxypeptidase E to the Regulated Secretory Pathway Requires Interaction of its Transmembrane Domain With Lipid Rafts. Biochem J — Dikeakos JD, Mercure C, Lacombe M-J, Seidah NG, Reudelhuber TL. FEBS J — Dikeakos JD, Di Lello P, Lacombe M-J, Ghirlando R, Legault P, Reudelhuber TL, et al. Proc Natl Acad Sci USA — Dhanvantari S, Loh YP. Lipid Raft Association of Carboxypeptidase E Is Necessary for Its Function as a Regulated Secretory Pathway Sorting Receptor. J Biol Chem — Cool DR, Normant E, Shen F, Chen H, Pannell L, Zhang Y, et al. Carboxypeptidase E Is a Regulated Secretory Pathway Sorting Receptor: Genetic Obliteration Leads to Endocrine Disorders in Cpe Fat Mice. Cell — Irminger JC, Verchere CB, Meyer K, Halban PA. J Biol Chem —4. McGirr R, Guizzetti L, Dhanvantari S. The Sorting of Proglucagon to Secretory Granules is Mediated by Carboxypeptidase E and Intrinsic Sorting Signals. J Endocrinol — Hosaka M, Watanabe T, Sakai Y, Kato T, Takeuchi T. Interaction Between Secretogranin III and Carboxypeptidase E Facilitates Prohormone Sorting Within Secretory Granules. J Cell Sci — Plá V, Paco S, Ghezali G, Ciria V, Pozas E, Ferrer I, et al. Brain Pathol — Arvan P, Halban PA. Sorting Ourselves Out: Seeking Consensus on Trafficking in the Beta-Cell. Traffic — Guizzetti L, McGirr R, Dhanvantari S. Two Dipolar α-Helices Within Hormone-Encoding Regions of Proglucagon are Sorting Signals to the Regulated Secretory Pathway. Dey A, Lipkind GM, Rouillé Y, Norrbom C, Stein J, Zhang C, et al. Significance of Prohormone Convertase 2, PC2, Mediated Initial Cleavage at the Proglucagon Interdomain Site, LysArg71, to Generate Glucagon. Endocrinology — Rouille Y, Westermark G, Martin SK, Steiner DF. Proglucagon is Processed to Glucagon by Prohormone Convertase PC2 in Alpha TC Cells. Proc Natl Acad Sci —6. Dhanvantari S, Seidah NG, Brubaker PL. Role of Prohormone Convertases in the Tissue-Specific Processing of Proglucagon. Furuta M, Zhou A, Webb G, Carroll R, Ravazzola M, Orci L, et al. Severe Defect in Proglucagon Processing in Islet Alpha-Cells of Prohormone Convertase 2 Null Mice. Campbell SA, Golec DP, Hubert M, Johnson J, Salamon N, Barr A, et al. Human Islets Contain a Subpopulation of Glucagon-Like Peptide-1 Secreting α Cells That is Increased in Type 2 Diabetes. Mol Metab Nie Y, Nakashima M, Brubaker PL, Li QL, Perfetti R, Jansen E, et al. Regulation of Pancreatic PC1 and PC2 Associated With Increased Glucagon-Like Peptide 1 in Diabetic Rats. McGirr R, Ejbick CE, Carter DE, Andrews JD, Nie Y, Friedman TC, et al. Glucose Dependence of the Regulated Secretory Pathway in αtc Cells. Liu P, Song J, Liu H, Yan F, He T, Wang L, et al. Insulin Regulates Glucagon-Like Peptide-1 Secretion by Pancreatic Alpha Cells. Endocrine — Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, et al. Interleukin-6 Enhances Insulin Secretion by Increasing Glucagon-Like Peptide-1 Secretion From L Cells and Alpha Cells. Nat Med —9. Progressive Change of Intra-Islet GLP-1 Production During Diabetes Development. Diabetes Metab Res Rev —8. Kilimnik G, Kim A, Steiner DF, Friedman TC, Hara M. Islets — Wideman RD, Gray SL, Covey SD, Webb GC, Kieffer TJ. Mol Ther —8. Wideman RD, Covey SD, Webb GC, Drucker DJ, Kieffer TJ. Galvin SG, Kay RG, Foreman R, Larraufie P, Meek CL, Biggs E, et al. The Human and Mouse Islet Peptidome: Effects of Obesity and Type 2 Diabetes, and Assessment of Intraislet Production of Glucagon-Like Peptide J Proteome Res x:acs. Runge S, Wulff BS, Madsen K, Bräuner-Osborne H, Knudsen LB. Different Domains of the Glucagon and Glucagon-Like Peptide-1 Receptors Provide the Critical Determinants of Ligand Selectivity. Br J Pharmacol — Salehi A, Vieira E, Gylfe E. Paradoxical Stimulation of Glucagon Secretion by High Glucose Concentrations. Gylfe E. Ups J Med Sci — Whalley NM, Pritchard LE, Smith DM. White a. Processing of Proglucagon to GLP-1 in Pancreatic α-Cells: Is This a Paracrine Mechanism Enabling GLP-1 to Act on β-Cells? Asadi F, Dhanvantari S. Plasticity in the Glucagon Interactome Reveals Novel Proteins That Regulate Glucagon Secretion in α-TC Cells. Front Endocrinol Lausanne Omar-Hmeadi M, Lund PE, Gandasi NR, Tengholm A, Barg S. Paracrine Control of α-Cell Glucagon Exocytosis is Compromised in Human Type-2 Diabetes. Nat Commun — Le Marchand SJ, Piston DW. Glucose Suppression of Glucagon Secretion: Metabolic and Calcium Responses From Alpha-Cells in Intact Mouse Pancreatic Islets. Quoix N, Cheng-xue R, Mattart L, Zeinoun Z, Guiot Y, Beauvois M, et al. Ramracheya R, Ward C, Shigeto M, Walker JN, Amisten S, Zhang Q, et al. Membrane Potential-Dependent Inactivation of Voltage-Gated Ion Channels in α-Cells Inhibits Glucagon Secretion From Human Islets. Zhang Q, Ramracheya R, Lahmann C, Tarasov A, Bengtsson M, Braha O, et al. Role of KATP Channels in Glucose-Regulated Glucagon Secretion and Impaired Counterregulation in Type 2 Diabetes. Zhang Q, Dou H, Rorsman P. J Physiol — Liu Y-J, Vieira E, Gylfe E. A Store-Operated Mechanism Determines the Activity of the Electrically Excitable Glucagon-Secreting Pancreatic α-Cell. Cell Calcium — Tian G, Tepikin AV, Tengholm A, Gylfe E. Watts M, Sherman A. |
| You and Your Hormones | However, the effect of endogenous glucagon on resting energy expenditure remains unclear. Also, the exact mechanisms behind the increase in resting energy expenditure elicited by exogenous glucagon remain to be determined. It has been speculated that glucagon activates brown adipose tissue 12 , however this was recently challenged in an in vivo study that found no direct effect of glucagon on brown adipose tissue Rodent studies indicate that the actions of glucagon to increase energy expenditure might be indirectly mediated partly by fibroblast growth factor 21 FGF21 as glucagon-induced increase in energy expenditure is abolished in animals with FGF21 receptor deletion Infusion of high doses of glucagon increases heart rate and cardiac contractility In fact, infusion of glucagon in pharmacological doses milligram is often used in the treatment of acute cardiac depression caused by calcium channel antagonist or beta-blocker overdoses 47 despite limited evidence In comparison, glucagon concentrations within the normal physiological range do not appear to affect heart rate or contractility 49 and any physiological role of endogenous glucagon in the regulation of pulse rate remains questionable. This is supported by studies investigating the effect of glucagon receptor antagonist for the treatment of type 2 diabetes in which no effect of pulse rate were observed Nevertheless, whether increased glucagon concentrations have a sustained effect on the heart remains unknow. Of note, most studies use bolus injections of glucagon which cause only a transient increase in heart rate and contractility potentially reflecting the rapid elimination of glucagon from circulation Taken together, it remains uncertain whether glucagon has a place in the treatment of heart failure or hold a cardioprotective effect in healthy subjects. Patients with type 2 diabetes exhibit an impaired regulation of glucagon secretion which contributes importantly to diabetic hyperglycemia. Specifically, type 2 diabetes is characterized by elevated levels of glucagon during fasting while suppression of glucagon in response to oral intake of glucose is impaired or even paradoxically elevated Fig. The mechanisms behind hyperglucagonemia are not fully understood but is usually explained by a diminished suppressive effect of insulin on alpha cells due to hypoinsulinemia and insulin resistance at the level of the alpha cells 53 , Interestingly, subjects with type 2 diabetes, who exhibit a hyperglucagonemic response to oral glucose, respond with a normal suppression of glucagon after intravenous glucose administration Accordingly, hormones secreted from the gastrointestinal tract may play an important role 55 , It has recently been confirmed that glucagon can be secreted from extrapancreatic tissue demonstrated in experiments with totally pancreatectomized subjects This supports the notion that postprandial hypersecretion of glucagon in patients with type 2 diabetes might be of extrapancreatic origin. Schematic illustration of plasma glucagon concentrations in patients with type 2 diabetes and in normal physiology healthy subjects. Type 2 diabetes is characterized by elevated fasting plasma glucagon levels and impaired suppression of plasma glucagon levels in response to oral glucose. Traditionally type 1 diabetic hyperglycemia has been explained by selective loss of beta cell mass and resulting decrease in insulin secretion. However, emerging evidence indicate that glucagon plays a major role in type 1 diabetes pathophysiology. The glucagon dyssecretion that characterizes patients with type 1 diabetes is associated with two clinical manifestations: Postprandial hyperglucagonemia and impaired glucagon counterregulation to hypoglycemia Data regarding fasting plasma glucagon concentrations in type 1 diabetes are inconsistent 57 , Thus, the general notion that glucagon hypersecretion plays a role in type 1 diabetes hyperglycemia is mainly based on elevated postprandial glucagon concentrations The explanation behind this is unclear, although a common explanation is, that in type 1 diabetes the postprandial increase in plasma glucose is not followed by an increase in insulin secretion from beta cells, which in normal physiology would inhibit glucagon secretion. The absence of that restraining signal from endogenous insulin could result in an increase in glucagon secretion from alpha cells after a meal Fig. However, like in type 2 diabetes, subjects with type 1 diabetes preserve their ability to suppress glucagon after intravenous glucose administration. Schematic illustration of plasma glucagon concentrations in patients with type 1 diabetes and in normal physiology healthy subjects. Type 1 diabetes is characterized by elevated concentrations of glucagon in response to a meal or oral glucose intake. Hypoglycemia is a frequent and feared side effect of insulin therapy in type 1 diabetes and it represents a common barrier in obtaining glycemic control In normal physiology hypoglycemia is prevented by several mechanisms: 1 Reduced insulin secretion from beta cells diminishing glucose uptake in peripheral tissues; 2 increased glucagon secretion from alpha cells increasing hepatic glucose output; and 3 increased symphathetic neural response and adrenomedullary epinephrine secretion. The latter will stimulate hepatic glucose production and cause clinical symptoms that enables the individual to recognize hypoglycemia and ultimately ingest carbohydrates 57 , 61 , In type 1 diabetes, insulin-induced hypoglycemia fails to elicit adequate glucagon responses compromising counterregulation to insulin-induced hypoglycemia; a phenomenon which seems to worsen with the duration of type 1 diabetes. This defect likely involves a combination of defective alpha cells and reduced alpha cell mass 57 , Dysregulated glucagon secretion is not only observed in patients with type 2 diabetes but also in normoglucose-tolerant individuals with obesity 64 and patients with non-alcoholic fatty liver disease NAFLD 65 , This suggests that dysregulated glucagon secretion may represent hepatic steatosis rather than dysregulated glucose metabolism. Interestingly, fasting hyperglucagonemia seems to relate to circulating amino acids in addition to hepatic fat content This hyperaminoacidemia suggests that impairment of amino acid turnover in the liver and ensuing elevations of circulating amino acids constitutes a feedback on the alpha cell to secrete more glucagon with increasing hepatic amino acid turnover and ureagenesis needed for clearance of toxic ammonia from the body. The implication of hyperglucagonemia in obesity and NAFLD has renewed the scientific interest in actions of glucagon and the role of glucagon in the pathophysiology of these metabolic disorders. Clearly, glucagon may represent a potential target for treatments of obesity and NAFLD. A simple way to restrain the undesirable hyperglycemic effect of glucagon while realizing its actions on lipolysis and energy expenditure could be by co-treating with a glucose-lowering drug. This may be done by mimicking the gut hormone oxyntomodulin which acts as a ligand to both the glucagon and the GLP-1 receptor. Glucagon is a glucoregulatory peptide hormone that counteracts the actions of insulin by stimulating hepatic glucose production and thereby increases blood glucose levels. Additionally, glucagon mediates several non-glucose metabolic effects of importance for maintaining whole-body energy balance in times of limited nutrient supply. These actions include mobilization of energy resources through hepatic lipolysis and ketogenesis; stimulation of hepatic amino acid turnover and related ureagenesis. Also, glucagon has been shown to increase energy expenditure and inhibit food intake, but whether endogenous glucagon is involved in the regulation of these processes remains uncertain. Glucagon plays an important role in the pathophysiology of diabetes as elevated glucagon levels observed in these patients stimulate hepatic glucose production, thereby contributing to diabetic hyperglycemia. Used under Creative Commons License 3. This electronic version has been made freely available under a Creative Commons CC-BY-NC-ND license. Turn recording back on. National Library of Medicine Rockville Pike Bethesda, MD Web Policies FOIA HHS Vulnerability Disclosure. Help Accessibility Careers. Access keys NCBI Homepage MyNCBI Homepage Main Content Main Navigation. Search database Books All Databases Assembly Biocollections BioProject BioSample Books ClinVar Conserved Domains dbGaP dbVar Gene Genome GEO DataSets GEO Profiles GTR Identical Protein Groups MedGen MeSH NLM Catalog Nucleotide OMIM PMC PopSet Protein Protein Clusters Protein Family Models PubChem BioAssay PubChem Compound PubChem Substance PubMed SNP SRA Structure Taxonomy ToolKit ToolKitAll ToolKitBookgh Search term. Show details Feingold KR, Anawalt B, Blackman MR, et al. Contents www. Search term. Glucagon Physiology Iben Rix , Christina Nexøe-Larsen , Natasha C Bergmann , Asger Lund , and Filip K Knop. hnoiger nesretep. Christina Nexøe-Larsen Center for Clinical Metabolic Research, Gentofte Hospital, University of Copenhagen, Hellerup, Denmark, Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark. Natasha C Bergmann Center for Clinical Metabolic Research, Gentofte Hospital, University of Copenhagen, Hellerup, Denmark. Asger Lund Center for Clinical Metabolic Research, Gentofte Hospital, University of Copenhagen, Hellerup, Denmark. Filip K Knop Center for Clinical Metabolic Research, Gentofte Hospital, University of Copenhagen, Hellerup, Denmark, Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark, Novo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Steno Diabetes Center Copenhagen, Gentofte, Denmark Email: kd. hnoiger ABSTRACT Glucagon is a peptide hormone secreted from the alpha cells of the pancreatic islets of Langerhans. STRUCTURE AND SYNTHESIS OF GLUCAGON Glucagon is a amino acid peptide hormone predominantly secreted from the alpha cells of the pancreas. GLUCAGON SECRETION Glucagon is secreted in response to hypoglycemia, prolonged fasting, exercise and protein-rich meals Regulation of Glucagon Secretion by Glucose The most potent regulator of glucagon secretion is circulating glucose. Glucagon Concentrations in The Circulation In normal physiology, circulating glucagon concentrations are in the picomolar range. Glucagon concentrations in response to hypoglycemia, euglycemia, and hyperglycemia. GLUCAGON ACTIONS Glucagon Increases Hepatic Glucose Production Glucagon controls plasma glucose concentrations during fasting, exercise and hypoglycemia by increasing hepatic glucose output to the circulation. Glucagon Stimulates Break-Down of Fatty Acids and Inhibits Lipogenesis in the Liver Glucagon promotes formation of non-carbohydrate energy sources in the form of lipids and ketone bodies. Glucagon Promotes Break-Down of Amino Acids During prolonged fasting, glucagon stimulates formation of glucose from amino acids via gluconeogenesis by upregulating enzymes involved in the process. Glucagon Reduces Food Intake Acute administration of glucagon has been shown to reduce food intake and diminish hunger 38 , Glucagon Increases Energy Expenditure In addition to a potential effect of glucagon on food intake, evidence suggests that glucagon contributes to a negative energy balance by stimulating energy expenditure. Glucagon May Regulate Heart Rate and Contractility Infusion of high doses of glucagon increases heart rate and cardiac contractility Organ specific actions of glucagon. GIP, glucose-dependent insulinotropic polypeptide. Glucagon in Type 1 Diabetes Traditionally type 1 diabetic hyperglycemia has been explained by selective loss of beta cell mass and resulting decrease in insulin secretion. Glucagon in Obesity and Hepatic Steatosis Dysregulated glucagon secretion is not only observed in patients with type 2 diabetes but also in normoglucose-tolerant individuals with obesity 64 and patients with non-alcoholic fatty liver disease NAFLD 65 , Habegger KM, Heppner KM, Geary N, Bartness TJ, DiMarchi R, Tschöp MH. The metabolic actions of glucagon revisited. Nat Rev Endocrinol. Kimball CP, Murlin JR. Aqueous Extracts of Pancreas Iii. Some Precipitation Reactions of Insulin. Bromer WW, Sinn LG, Staub A, Behrens OK. The amino acid sequence of glucagon. Blackman B. The use of glucagon in insulin coma therapy. Psychiatr Q. Esquibel AJ, Kurland AA, Mendelsohn D. The use of glucagon in terminating insulin coma. Dis Nerv Syst. Unger RH, Eisentraut AM. McCALL MS, Madison LL. Glucagon antibodies and an immunoassay for glucagon. Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Drucker DJ, Asa S. Glucagon gene expression in vertebrate brain. Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. Gerich JE, Lorenzi M, Hane S, Gustafson G, Guillemin R, Forsham PH. Evidence for a physiologic role of pancreatic glucagon in human glucose homeostasis: studies with somatostatin. Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Müller TD, Finan B, Clemmensen C, DiMarchi RD, Tschöp MH. The New Biology and Pharmacology of Glucagon. Physiological Reviews. Wewer Albrechtsen NJ, Kuhre RE, Pedersen J, Knop FK, Holst JJ. The biology of glucagon and the consequences of hyperglucagonemia. Biomarkers in Medicine. Gromada J, Chabosseau P, Rutter GA. The α-cell in diabetes mellitus. Hughes JW, Ustione A, Lavagnino Z, Piston DW. Regulation of islet glucagon secretion: Beyond calcium. Diabetes, Obesity and Metabolism. Knop FK, Vilsbøll T, Madsbad S, Holst JJ, Krarup T. Inappropriate suppression of glucagon during OGTT but not during isoglycaemic i. glucose infusion contributes to the reduced incretin effect in type 2 diabetes mellitus. Lund A, Bagger JI, Albrechtsen NJW, Christensen M, Grøndahl M, Hartmann B, Mathiesen ER, Hansen CP, Storkholm JH, Hall G, van, Rehfeld JF, Hornburg D, Meissner F, Mann M, Larsen S, Holst JJ, Vilsbøll T, Knop FK. Evidence of Extrapancreatic Glucagon Secretion in Man. Miyachi A, Kobayashi M, Mieno E, Goto M, Furusawa K, Inagaki T, Kitamura T. Accurate analytical method for human plasma glucagon levels using liquid chromatography-high resolution mass spectrometry: comparison with commercially available immunoassays. Anal Bioanal Chem. Hansen JS, Pedersen BK, Xu G, Lehmann R, Weigert C, Plomgaard P. Exercise-Induced Secretion of FGF21 and Follistatin Are Blocked by Pancreatic Clamp and Impaired in Type 2 Diabetes. Schwartz NS, Clutter WE, Shah SD, Cryer PE. Glycemic thresholds for activation of glucose counterregulatory systems are higher than the threshold for symptoms. J Clin Invest. Svoboda M, Tastenoy M, Vertongen P, Robberecht P. Relative quantitative analysis of glucagon receptor mRNA in rat tissues. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Pospisilik JA, Hinke SA, Pederson RA, Hoffmann T, Rosche F, Schlenzig D, Glund K, Heiser U, McIntosh CHS, Demuth H-U. Metabolism of glucagon by dipeptidyl peptidase IV CD Regulatory Peptides. Pontiroli AE, Calderara A, Perfetti MG, Bareggi SR. Pharmacokinetics of intranasal, intramuscular and intravenous glucagon in healthy subjects and diabetic patients. Lund A, Bagger JI, Albrechtsen NW, Christensen M, Grøndahl M, Hansen CP, Storkholm JH, Holst JJ, Vilsbøll T, Knop FK. Increased Liver Fat Content in Totally Pancreatectomized Patients. Unger RH. Glucagon physiology and pathophysiology in the light of new advances. Miller RA, Birnbaum MJ. Glucagon: acute actions on hepatic metabolism. Ramnanan CJ, Edgerton DS, Kraft G, Cherrington AD. Physiologic action of glucagon on liver glucose metabolism. Diabetes Obes Metab. Rui L. Energy Metabolism in the Liver. Compr Physiol. Geisler CE, Renquist BJ. Hepatic lipid accumulation: cause and consequence of dysregulated glucoregulatory hormones. J Endocrinol. Longuet C, Sinclair EM, Maida A, Baggio LL, Maziarz M, Charron MJ, Drucker DJ. The Glucagon Receptor Is Required for the Adaptive Metabolic Response to Fasting. Cell Metabolism. Wang H, Zhao M, Sud N, Christian P, Shen J, Song Y, Pashaj A, Zhang K, Carr T, Su Q. Glucagon regulates hepatic lipid metabolism via cAMP and Insig-2 signaling: implication for the pathogenesis of hypertriglyceridemia and hepatic steatosis. Sci Rep. Galsgaard KD, Pedersen J, Knop FK, Holst JJ, Wewer Albrechtsen NJ. Glucagon Receptor Signaling and Lipid Metabolism. Front Physiol. Holst JJ, Albrechtsen NJW, Pedersen J, Knop FK. Glucagon and Amino Acids Are Linked in a Mutual Feedback Cycle: The Liver—α-Cell Axis. Hamberg O, Vilstrup H. Regulation of urea synthesis by glucose and glucagon in normal man. Clin Nutr. Solloway MJ, Madjidi A, Gu C, Eastham-Anderson J, Clarke HJ, Kljavin N, Zavala-Solorio J, Kates L, Friedman B, Brauer M, Wang J, Fiehn O, Kolumam G, Stern H, Lowe JB, Peterson AS, Allan BB. Glucagon Couples Hepatic Amino Acid Catabolism to mTOR-Dependent Regulation of α-Cell Mass. Cell Rep. Bagger JI, Holst JJ, Hartmann B, Andersen B, Knop FK, Vilsbøll T. J Clin Endocrinol Metab. Geary N, Kissileff HR, Pi-Sunyer FX, Hinton V. Individual, but not simultaneous, glucagon and cholecystokinin infusions inhibit feeding in men. Langhans W, Zeiger U, Scharrer E, Geary N. Stimulation of feeding in rats by intraperitoneal injection of antibodies to glucagon. Le Sauter J, Noh U, Geary N. Hepatic portal infusion of glucagon antibodies increases spontaneous meal size in rats. Nair KS. Hyperglucagonemia Increases Resting Metabolic Rate In Man During Insulin Deficiency. Glucagon increases energy expenditure independently of brown adipose tissue activation in humans. Tan TM, Field BCT, McCullough KA, Troke RC, Chambers ES, Salem V, Gonzalez Maffe J, Baynes KCR, De Silva A, Viardot A, Alsafi A, Frost GS, Ghatei MA, Bloom SR. As in beta cells, there may be different functional pools of secretory granules: a reserve pool and a readily releasable pool that is primed and situated at the sites of exocytosis. Quantitative ultrastructural analysis of murine islets has shown that, in the presence of 1mM glucose, the mouse α-cell contains ~ secretory granules, of which ~ are in close proximity to the plasma membrane, or primed This means that the reserve pool is large and can resupply the readily releasable pool to maintain euglycemia over extended periods of time. In the presence of Following docking, secretory granules are primed through the action of the SNARE protein complex. This complex contains two subsets of proteins; i the t-SNAREs syntaxin 1A and SNAP, located in the plasma membrane; and ii the v-SNAREs VAMP2 and synaptotagmin VII, which are located in the granule membrane Under low glucose conditions, SNAP and syntaxin 1A are translocated to the plasma membrane. SNAP itself may play a role in the transportation of granules from the releasable pool to the readily releasable pool, and then mediates their fusion with plasma membrane via interaction with syntaxin 1A , Live imaging of exocytosis using a proglucagon-luciferase reporter showed spatial clustering of glucagon secretion sites in αTC cells Future studies may reveal some interesting dynamics with SNARE proteins that may fine-tune the alpha cell secretory response to glucose and paracrine inputs. Could disruption of these molecular mechanisms contribute to the hyperglucagonemia of diabetes? However, neither membrane potential nor exocytosis was responsive to insulin or to a greater extent somatostatin, in contrast to normal alpha cells in which both were significantly reduced. Therefore, in T2D, hyperglucagonemia may result from insulin and somatostatin resistance at the level of the readily releasable pool of granules. In alpha cells of patients with T1D, expression levels of genes encoding SNARE proteins, ion channels and cAMP signalling molecules were disrupted , which could explain the impaired glucose counter-regulatory response and the inappropriately elevated levels of postprandial glucagon in T1D. Combining patch-clamp electrophysiological measurements with single-cell RNA sequencing patch-seq in human islets has given high-resolution insight into mechanisms underlying impairments in alpha cell function in diabetes at the level of granule exocytosis. Further characterization of the link between electrophysiological signatures and the genes regulating the dynamics of granule exocytosis will reveal new mechanisms of alpha cell dysfunction in diabetes. Identifying new pathways or networks that control glucagon granule biogenesis and trafficking may identify novel targets for the control of hyperglucagonemia in addition to yielding a greater understanding of alpha cell biology in both health and disease. There is an emerging hypothesis that glucagon secretion can be controlled by trafficking through the endosomal-lysosomal pathway, similar to insulin , and below, we highlight some recent studies that suggest glucagon may regulated through such an alternate trafficking pathway. Brefeldin A-inhibited guanine nucleotide exchange protein 3 BIG3 is a member of the Arf-GEF family of proteins, and was initially found in a database search and found to inhibit insulin granule biogenesis and insulin secretion A subsequent study found that it had a similar role in regulating glucagon granule production and exocytosis Whether BIG3 can mediate glucagon trafficking through lysosomes remains to be investigated. The composition and cargo of the alpha cell secretory granule may also hold some determinants of glucagon secretion. While it is known that granule contents and composition are modified during normal granule maturation, a more complete picture of granule remodeling and heterogeneity in the context of intracellular trafficking networks in normal physiology and in diabetes is required. In an effort to identify networks of secretory granule proteins that interact with glucagon and regulate its trafficking and secretion, proteomic analysis was conducted on αTC cell secretory granule lysates immunoprecipitated with tagged glucagon This qualitative study demonstrated the plasticity in the network of proteins interacting with glucagon in response to insulin or GABA under high 25 mM or low 5. Stathmin-2, a member of the family of neuronal phosphoproteins that associates with the secretory pathway in neurons, was identified as a candidate protein for the regulation of glucagon secretion and subsequently shown to modulate glucagon secretion through the lysosomal pathway and may be down-regulated in diabetes in humans and in mice Therefore, disruptions in the routing of glucagon through the lysosomal pathway may contribute to the hyperglucagonemia of diabetes Figure 4. Figure 4 Stathminmediated lysosomal trafficking modulates glucagon secretion. Glucagon dark blue and stathmin-2 light blue are normally sorted to secretory granules from the Golgi in alpha cells. Stathmin-2 overexpression diverts glucagon-containing secretory granules to lysosomes black arrows , thus reducing glucagon secretion. Additionally, secretion from secretory granules is also enhanced solid red arrow. Glucagon trafficking and exocytosis may also be controlled through nutrient-driven pathways. The nutrient sensor O-GlcNAc transferase OGT catalyses the O-glycosylation of several proteins including those involved in the conventional secretory pathway and autophagosome-lysosome fusion In mice lacking OGT specifically in alpha cells, glucagon secretion, cell content and alpha cell mass are reduced Possible mechanisms include lack of O-glycosylation of FOXA1 and FOXA2, which regulate genes encoding proteins involved in proglucagon processing and glucagon secretion Whether other trafficking proteins are affected, and how alpha cell function is affected in diabetes in these mice, is not yet known. So what are the implications of glucagon trafficking through the lysosomal pathway in diabetes? Lysosomal trafficking and autophagy in the beta cell may be a possible mechanism of insulin secretory defects in diabetes, with a recent study providing evidence for impairment of lysosomal function in human T1D How does lysosomal function contribute to defects in alpha cell function? It is tempting to hypothesize that impairments in lysosomal biogenesis and trafficking result in both reduced insulin secretion in the beta cell and unregulated glucagon secretion from the alpha cell. Further investigation into the altered dynamics of glucagon trafficking in the alpha cell in diabetes may reveal key roles for the lysosome in the regulation of glucagon secretion, thus identifying a potential new target for the treatment of hyperglucagonemia. Finally, some excellent single-cell transcriptomics and epigenomics databases are being generated that reveal the dynamics of intracellular trafficking networks at the transcriptional level in human pancreatic alpha cells in both health and diabetes — The mapping of T2D-associated genetic variants with RNA-seq of human islets may reveal risk factors associated with defects in alpha cell function A novel immunocompromised mouse model in which glucagon-encoding codons were deleted while preserving both GLP-1 and GLP-2 will provide an innovative and much-needed resource for the study of the regulation of glucagon secretion from human islets in vivo In this study, transplantation of islets from people with T2D resulted in hyperglucagonemia with apparent alpha cell insulin resistance, revealing intrinsic alpha cell defects in T2D. Moreover, defects in alpha cell function were more apparent than in isolated islets, thus emphasizing the utility of such an in vivo system to investigate the molecular mechanisms of glucagon secretion in human islets, and the testing of possible treatments for hyperglucagonemia. While the development of glucagon receptor antagonists and other inhibitors of glucagon action has provided some possibilities for the treatment of hyperglucagonemia, there are significant side effects that result from impaired hepatic metabolism and potentially uncontrolled alpha cell proliferation. The advantage to developing such drugs, however, lie in the fact that the glucagon receptor is an easily available target. In contrast, targeting glucagon secretion as a means to treat hyperglucagonemia may alleviate concerns about effects on the liver and alpha cell mass; however, there are potentially many more targets within the alpha cell secretory pathway, and many of those may not be easily accessible for drug treatment. The ongoing discovery of novel proteins and networks that regulate the secretion of glucagon will shed further light on alpha cell biology in health and disease while also searching for improved means to control hyperglucagonemia and hyperglycemia of diabetes. SD and FA co-wrote the manuscript. All authors contributed to the article and approved the submitted version. This work was funded by a Natural Sciences and Engineering Research Council Discovery Grant to SD. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Stanley S, Moheet A, Seaquist ER. Central Mechanisms of Glucose Sensing and Counterregulation in Defense of Hypoglycemia. Endocr Rev — doi: PubMed Abstract CrossRef Full Text Google Scholar. DCCT Research Group. Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes — Unger R, Orci L. The Essential Role of Glucagon in the Pathogenesis of Diabetes Mellitus. Lancet —6. Unger RH, Cherrington AD. Glucagonocentric Restructuring of Diabetes: A Pathophysiologic and Therapeutic Makeover. J Clin Invest — Lee Y, Wang M-Y, Du XQ, Charron MJ, Unger RH. Glucagon Receptor Knockout Prevents Insulin-Deficient Type 1 Diabetes in Mice. Diabetes —7. Conarello SL, Jiang G, Mu J, Li Z, Woods J, Zycband E, et al. Glucagon Receptor Knockout Mice are Resistant to Diet-Induced Obesity and Streptozotocin-Mediated Beta Cell Loss and Hyperglycaemia. Diabetologia — Neumann UH, Ho JSS, Mojibian M, Covey SD, Charron MJ, Kieffer TJ. Glucagon Receptor Gene Deletion in Insulin Knockout Mice Modestly Reduces Blood Glucose and Ketones But Does Not Promote Survival. Mol Metab —6. Damond N, Thorel F, Moyers JS, Charron MJ, Vuguin PM, Powers AC, et al. Blockade of Glucagon Signaling Prevents or Reverses Diabetes Onset Only If Residual β-Cells Persist. Elife — CrossRef Full Text Google Scholar. Kazda CM, Ding Y, Kelly RP, Garhyan P, Shi C, Lim CN, et al. Evaluation of Efficacy and Safety of the Glucagon Receptor Antagonist LY in Patients With Type 2 Diabetes: and Week Phase 2 Studies. Diabetes Care —9. Yang B, Gelfanov VM, Perez-Tilve D, DuBois B, Rohlfs R, Levy J, et al. Optimization of Truncated Glucagon Peptides to Achieve Selective, High Potency, Full Antagonists. J Med Chem — Lee CY, Choi H, Park EY, Nguyen TTL, Maeng HJ, Mee Lee K, et al. Synthesis and Anti-Diabetic Activity of Novel Biphenylsulfonamides as Glucagon Receptor Antagonists. Chem Biol Drug Des — Okamoto H, Cavino K, Na E, Krumm E, Kim SY, Cheng X, et al. Glucagon Receptor Inhibition Normalizes Blood Glucose in Severe Insulin-Resistant Mice. Proc Natl Acad Sci —8. Liang Y, Osborne MC, Monia BP, Bhanot S, Gaarde WA, Reed C, et al. Kim J, Okamoto H, Huang ZJ, Anguiano G, Chen S, Liu Q, et al. Amino Acid Transporter Slc38a5 Controls Glucagon Receptor Inhibition-Induced Pancreatic α Cell Hyperplasia in Mice. Cell Metab — Wei R, Gu L, Yang J, Yang K, Liu J, Le Y, et al. Antagonistic Glucagon Receptor Antibody Promotes α-Cell Proliferation and Increases β-Cell Mass in Diabetic Mice. iScience — Galsgaard KD, Winther-Sørensen M, Ørskov C, Kissow H, Poulsen SS, Vilstrup H, et al. Disruption of Glucagon Receptor Signaling Causes Hyperaminoacidemia Exposing a Possible Liver-Alpha-Cell Axis. Am J Physiol Metab E93—E Wewer Albrechtsen NJ, Pedersen J, Galsgaard KD, Winther-Sørensen M, Suppli MP, Janah L, et al. The Liver—α-Cell Axis and Type 2 Diabetes. Guan H-P, Yang X, Lu K, Wang S-P, Castro-Perez JM, Previs S, et al. Glucagon Receptor Antagonism Induces Increased Cholesterol Absorption. J Lipid Res — Tooze S. Biogenesis of Secretory Granules in the Trans-Golgi Network of Neuroendocrine and Endocrine Cells. Biochim Biophys Acta — Cool DR, Fenger M, Snell CR, Loh YP. Identification of the Sorting Signal Motif Within Pro-Opiomelanocortin for the Regulated Secretory Pathway. J Biol Chem —9. Dhanvantari S, Shen F, Adams T, Snell CR, Zhang C, Mackin RB, et al. Disruption of a Receptor-Mediated Mechanism for Intracellular Sorting of Proinsulin in Familial Hyperproinsulinemia. Mol Endocrinol — Zhang C-F, Dhanvantari S, Lou H, Loh YP. Sorting of Carboxypeptidase E to the Regulated Secretory Pathway Requires Interaction of its Transmembrane Domain With Lipid Rafts. Biochem J — Dikeakos JD, Mercure C, Lacombe M-J, Seidah NG, Reudelhuber TL. FEBS J — Dikeakos JD, Di Lello P, Lacombe M-J, Ghirlando R, Legault P, Reudelhuber TL, et al. Proc Natl Acad Sci USA — Dhanvantari S, Loh YP. Lipid Raft Association of Carboxypeptidase E Is Necessary for Its Function as a Regulated Secretory Pathway Sorting Receptor. J Biol Chem — Cool DR, Normant E, Shen F, Chen H, Pannell L, Zhang Y, et al. Carboxypeptidase E Is a Regulated Secretory Pathway Sorting Receptor: Genetic Obliteration Leads to Endocrine Disorders in Cpe Fat Mice. Cell — Irminger JC, Verchere CB, Meyer K, Halban PA. J Biol Chem —4. McGirr R, Guizzetti L, Dhanvantari S. The Sorting of Proglucagon to Secretory Granules is Mediated by Carboxypeptidase E and Intrinsic Sorting Signals. J Endocrinol — Hosaka M, Watanabe T, Sakai Y, Kato T, Takeuchi T. Interaction Between Secretogranin III and Carboxypeptidase E Facilitates Prohormone Sorting Within Secretory Granules. J Cell Sci — Plá V, Paco S, Ghezali G, Ciria V, Pozas E, Ferrer I, et al. Brain Pathol — Arvan P, Halban PA. Sorting Ourselves Out: Seeking Consensus on Trafficking in the Beta-Cell. Traffic — Guizzetti L, McGirr R, Dhanvantari S. Two Dipolar α-Helices Within Hormone-Encoding Regions of Proglucagon are Sorting Signals to the Regulated Secretory Pathway. Dey A, Lipkind GM, Rouillé Y, Norrbom C, Stein J, Zhang C, et al. Significance of Prohormone Convertase 2, PC2, Mediated Initial Cleavage at the Proglucagon Interdomain Site, LysArg71, to Generate Glucagon. Endocrinology — Rouille Y, Westermark G, Martin SK, Steiner DF. Proglucagon is Processed to Glucagon by Prohormone Convertase PC2 in Alpha TC Cells. Proc Natl Acad Sci —6. Dhanvantari S, Seidah NG, Brubaker PL. Role of Prohormone Convertases in the Tissue-Specific Processing of Proglucagon. Furuta M, Zhou A, Webb G, Carroll R, Ravazzola M, Orci L, et al. Severe Defect in Proglucagon Processing in Islet Alpha-Cells of Prohormone Convertase 2 Null Mice. Campbell SA, Golec DP, Hubert M, Johnson J, Salamon N, Barr A, et al. Human Islets Contain a Subpopulation of Glucagon-Like Peptide-1 Secreting α Cells That is Increased in Type 2 Diabetes. Mol Metab Nie Y, Nakashima M, Brubaker PL, Li QL, Perfetti R, Jansen E, et al. Regulation of Pancreatic PC1 and PC2 Associated With Increased Glucagon-Like Peptide 1 in Diabetic Rats. McGirr R, Ejbick CE, Carter DE, Andrews JD, Nie Y, Friedman TC, et al. Glucose Dependence of the Regulated Secretory Pathway in αtc Cells. Liu P, Song J, Liu H, Yan F, He T, Wang L, et al. Insulin Regulates Glucagon-Like Peptide-1 Secretion by Pancreatic Alpha Cells. Endocrine — Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, et al. Interleukin-6 Enhances Insulin Secretion by Increasing Glucagon-Like Peptide-1 Secretion From L Cells and Alpha Cells. Nat Med —9. Progressive Change of Intra-Islet GLP-1 Production During Diabetes Development. Diabetes Metab Res Rev —8. Kilimnik G, Kim A, Steiner DF, Friedman TC, Hara M. Islets — Wideman RD, Gray SL, Covey SD, Webb GC, Kieffer TJ. Mol Ther —8. Wideman RD, Covey SD, Webb GC, Drucker DJ, Kieffer TJ. Galvin SG, Kay RG, Foreman R, Larraufie P, Meek CL, Biggs E, et al. The Human and Mouse Islet Peptidome: Effects of Obesity and Type 2 Diabetes, and Assessment of Intraislet Production of Glucagon-Like Peptide J Proteome Res x:acs. Runge S, Wulff BS, Madsen K, Bräuner-Osborne H, Knudsen LB. Different Domains of the Glucagon and Glucagon-Like Peptide-1 Receptors Provide the Critical Determinants of Ligand Selectivity. Br J Pharmacol — Salehi A, Vieira E, Gylfe E. Paradoxical Stimulation of Glucagon Secretion by High Glucose Concentrations. Gylfe E. Ups J Med Sci — Whalley NM, Pritchard LE, Smith DM. White a. Processing of Proglucagon to GLP-1 in Pancreatic α-Cells: Is This a Paracrine Mechanism Enabling GLP-1 to Act on β-Cells? Asadi F, Dhanvantari S. Plasticity in the Glucagon Interactome Reveals Novel Proteins That Regulate Glucagon Secretion in α-TC Cells. Front Endocrinol Lausanne Omar-Hmeadi M, Lund PE, Gandasi NR, Tengholm A, Barg S. Paracrine Control of α-Cell Glucagon Exocytosis is Compromised in Human Type-2 Diabetes. Nat Commun — Le Marchand SJ, Piston DW. Glucose Suppression of Glucagon Secretion: Metabolic and Calcium Responses From Alpha-Cells in Intact Mouse Pancreatic Islets. Quoix N, Cheng-xue R, Mattart L, Zeinoun Z, Guiot Y, Beauvois M, et al. Ramracheya R, Ward C, Shigeto M, Walker JN, Amisten S, Zhang Q, et al. Membrane Potential-Dependent Inactivation of Voltage-Gated Ion Channels in α-Cells Inhibits Glucagon Secretion From Human Islets. Zhang Q, Ramracheya R, Lahmann C, Tarasov A, Bengtsson M, Braha O, et al. Role of KATP Channels in Glucose-Regulated Glucagon Secretion and Impaired Counterregulation in Type 2 Diabetes. Zhang Q, Dou H, Rorsman P. J Physiol — Liu Y-J, Vieira E, Gylfe E. A Store-Operated Mechanism Determines the Activity of the Electrically Excitable Glucagon-Secreting Pancreatic α-Cell. Cell Calcium — Tian G, Tepikin AV, Tengholm A, Gylfe E. Watts M, Sherman A. Modeling the Pancreatic α-Cell: Dual Mechanisms of Glucose Suppression of Glucagon Secretion. Biophys J — PloS One 7:e Elliott AD, Ustione A, Piston DW. Somatostatin and Insulin Mediate Glucose-Inhibited Glucagon Secretion in the Pancreatic α-Cell by Lowering cAMP. Am J Physiol Endocrinol Metab E— Yu Q, Shuai H, Ahooghalandari P, Gylfe E, Tengholm A. Glucose Controls Glucagon Secretion by Directly Modulating cAMP in Alpha Cells. Hughes JW, Ustione A, Lavagnino Z, Piston DW. Regulation of Islet Glucagon Secretion: Beyond Calcium. Diabetes Obes Metab — Leclerc I, Sun G, Morris C, Fernandez-Millan E, Nyirenda M, Rutter GA. AMP-Activated Protein Kinase Regulates Glucagon Secretion From Mouse Pancreatic Alpha Cells. Da Silva Xavier G, Farhan H, Kim H, Caxaria S, Johnson P, Hughes S, et al. Per-Arnt-Sim PAS Domain-Containing Protein Kinase is Downregulated in Human Islets in Type 2 Diabetes and Regulates Glucagon Secretion. Sun G, da Silva Xavier G, Gorman T, Priest C, Solomou A, Hodson DJ, et al. LKB1 and Ampkα1 are Required in Pancreatic Alpha Cells for the Normal Regulation of Glucagon Secretion and Responses to Hypoglycemia. Mol Metab — Bozadjieva N, Blandino-Rosano M, Chase J, Dai XQ, Cummings K, Gimeno J, et al. Loss of Mtorc1 Signaling Alters Pancreatic α Cell Mass and Impairs Glucagon Secretion. Kramer NB, Lubaczeuski C, Blandino-Rosano M, Barker G, Gittes GK, Caicedo A, et al. Glucagon Resistance and Decreased Susceptibility to Diabetes in a Model of Chronic Hyperglucagonemia. Gromada J, Franklin I, Wollheim CB. Alpha-Cells of the Endocrine Pancreas: 35 Years of Research But the Enigma Remains. Kawamori D, Kulkarni RN. Insulin Modulation of Glucagon Secretion: The Role of Insulin and Other Factors in the Regulation of Glucagon Secretion. Islets —9. Tsuchiyama N, Takamura T, Ando H, Sakurai M, Shimizu A, Kato KI, et al. Possible Role of α-Cell Insulin Resistance in Exaggerated Glucagon Responses to Arginine in Type 2 Diabetes. Diabetes Care —7. Wendt A, Birnir B, Buschard K, Gromada J, Salehi A, Sewing S, et al. Glucose Inhibition of Glucagon Secretion From Rat α-Cells Is Mediated by GABA Released From Neighboring β-Cells. Li C, Liu C, Nissim I, Chen J, Chen P, Doliba N, et al. Regulation of Glucagon Secretion in Normal and Diabetic Human Islets by?? Rorsman P, Berggren PO, Bokvist K, Ericson H, Möhler H, Ostenson CG SP. Glucose-Inhibition of Glucagon Secretion Involves Activation of GABAA-Receptor Chloride Channels. Nature —6. Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, et al. Intra-Islet Insulin Suppresses Glucagon Release via GABA-GABAA Receptor System. Feng AL, Xiang Y, Gui L, Kaltsidis G, Feng Q, Lu W. Paracrine GABA and Insulin Regulate Pancreatic Alpha Cell Proliferation in a Mouse Model of Type 1 Diabetes. Jin Li J, Casteels T, Frogne T, Ingvorsen C, Honore C, Courtney M, et al. Artemisinins Target GABAA Receptor Signaling and Impair α Cell Identity. Weir GC, Bonner-Weir S. GABA Signaling Stimulates β Cell Regeneration in Diabetic Mice. Cell —9. Ben-Othman N, Vieira A, Courtney M, Record F, Gjernes E, Avolio F, et al. Long-Term GABA Administration Induces Alpha Cell-Mediated Beta-Like Cell Neogenesis. van der Meulen T, Lee S, Noordeloos E, Donaldson CJ, Adams MW, Noguchi GM, et al. Artemether Does Not Turn α Cells Into β Cells. Ackermann AM, Moss NG, Kaestner KH. GABA and Artesunate Do Not Induce Pancreatic α-to-β Cell Transdifferentiation In Vivo. Combined Effect of GABA and Glucagon-Like Peptide-1 Receptor Agonist on Cytokine-Induced Apoptosis in Pancreatic β-Cell Line and Isolated Human Islets. J Diabetes — Daems C, Welsch S, Boughaleb H, Vanderroost J, Robert A, Sokal E, et al. Early Treatment With Empagliflozin and GABA Improves β -Cell Mass and Glucose Tolerance in Streptozotocin-Treated Mice. J Diabetes Res — Human Beta Cells Produce and Release Serotonin to Inhibit Glucagon Secretion From Alpha Cells. Cell Rep — Bennet H, Balhuizen A, Medina A, Dekker Nitert M, Ottosson Laakso E, Essén S, et al. Altered Serotonin 5-HT 1D and 2A Receptor Expression May Contribute to Defective Insulin and Glucagon Secretion in Human Type 2 Diabetes. Peptides — Yip L, Taylor C, Whiting CC, Fathman CG. Diminished Adenosine A1 Receptor Expression in Pancreatic a-Cells May Contribute to the Patholog Y of Type 1 Diabetes. Ishihara H, Wollheim CB. Is Zinc an Intra-Islet Regulator of Glucagon Secretion? Diabetol Int — Franklin I, Gromada J, Gjinovci A, Theander S. Beta Cell Secretory Products Activate Alpha Cell ATP-Dependent Potassium Channels to Inhibit Glucagon Release. Ravier MA, Rutter GA. Glucose or Insulin, But Not Zinc Ions, Inhibit Glucagon Secretion From Mouse Pancreatic [Alpha]-Cells. Solomou A, Philippe E, Chabosseau P, Migrenne-li S, Gaitan J, Lang J, et al. Nutr Metab Lond Solomou A, Meur G, Bellomo E, Hodson DJ, Tomas A, Li SM, et al. Strowski MZ, Parmar RM, Blake AD, Schaeffer JM. Somatostatin Inhibits Insulin and Glucagon Secretion via Two Receptor Subtypes : An In Vitro Study of Pancreatic Islets From Somatostatin Receptor 2 Knockout Mice. Endocrinology —7. Gromada J, Hoy M, Bushcard K, Salehi A, Rorsman P. Somatostatin Inhibits Exocytosis in Rat Pancreatic a-Cells by Gi2-Dependent Activation of Calcineurin and Depriming of Secretory Granules. Rutter GA. Regulating Glucagon Secretion: Somatostatin in the Spotlight. Xu SFS, Andersen DB, Izarzugaza JMG, Kuhre RE, Holst JJ. In the Rat Pancreas, Somatostatin Tonically Inhibits Glucagon Secretion and Is Required for Glucose-Induced Inhibition of Glucagon Secretion. Acta Physiol — Hauge-Evans AC, King AJ, Carmignac D, Richardson CC, Robinson ICAF, Low MJ, et al. Somatostatin Secreted by Islet -Cells Fulfills Multiple Roles as a Paracrine Regulator of Islet Function. Vergari E, Knudsen JG, Ramracheya R, Salehi A, Zhang Q, Adam J, et al. Insulin Inhibits Glucagon Release by SGLT2-Induced Stimulation of Somatostatin Secretion. Nat Commun Briant LJB, Reinbothe TM, Spiliotis I, Miranda C, Rodriguez B, Rorsman P. Δ-Cells and β-Cells Are Electrically Coupled and Regulate α-Cell Activity Via Somatostatin. Kilimnik G, Zhao B, Jo J, Periwal V, Witkowski P, Misawa R, et al. Altered Islet Composition and Disproportionate Loss of Large Islets in Patients With Type 2 Diabetes. PloS One 6:e Ma X, Zhang Y, Gromada J, Sewing S, Berggren P-O, Buschard K, et al. Glucagon Stimulates Exocytosis in Mouse and Rat Pancreatic α-Cells by Binding to Glucagon Receptors. Leibiger B, Moede T, Muhandiramlage TP, Kaiser D, Vaca Sanchez P, Leibiger IB, et al. Glucagon Regulates its Own Synthesis by Autocrine Signaling. Wewer Albrechtsen NJ, Kuhre RE, Hornburg D, Jensen CZ, Hornum M, Dirksen C, et al. Circulating Glucagon Regulates Blood Glucose by Increasing Insulin Secretion and Hepatic Glucose Production. |
| Top bar navigation | Endocrinol Metab Clin North Am 28 : — Rorsman P , Berggren PO , Bokvist K , Ericson H , Mohler H , Ostenson CG , Smith PA Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature : — Wendt A , Birnir B , Buschard K , Gromada J , Salehi A , Sewing S , Rorsman P , Braun M Glucose inhibition of glucagon secretion from rat α-cells is mediated by GABA released from neighboring β-cells. Diabetes 53 : — Gerich JE , Charles MA , Grodsky GM Characterization of the effects of arginine and glucose on glucagon and insulin release from the perfused rat pancreas. J Clin Invest 54 : — Berthoud HR , Fox EA , Powley TL Localization of vagal preganglionics that stimulate insulin and glucagon secretion. Am J Physiol : R — R Maruyama H , Hisatomi A , Orci L , Grodsky GM , Unger RH Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest 74 : — Samols E , Stagner JI , Ewart RB , Marks V The order of islet microvascular cellular perfusion is B-A-D in the perfused rat pancreas. J Clin Invest 82 : — Samols E , Stagner JI Intra-islet regulation. Ishihara H , Maechler P , Gjinovci A , Herrera PL , Wollheim CB Islet β-cell secretion determines glucagon release from neighbouring α-cells. Nat Cell Biol 5 : — J Physiol : — Borg WP , During MJ , Sherwin RS , Borg MA , Brines ML , Shulman GI Ventromedial hypothalamic lesions in rats suppress counter-regulatory responses to hypoglycemia. J Clin Invest 93 : — Borg MA , Sherwin RS , Borg WP , Tamborlane WV , Shulman GI Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest 99 : — Taborsky Jr GJ , Ahren B , Mundinger TO , Mei Q , Havel PJ Autonomic mechanism and defects in the glucagon response to insulin-induced hypoglycaemia. Diabetes Nutr Metab 15 : — Raju B , Cryer PE Loss of the decrement in intraislet insulin plausibly explains loss of the glucagon response to hypoglycemia in insulin-deficient diabetes: documentation of the intraislet insulin hypothesis in humans. Diabetes 54 : — Aguilar-Bryan L , Bryan J Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr Rev 20 : — Seghers V , Nakazaki M , DeMayo F , Aguilar-Bryan L , Bryan J Sur1 knockout mice. A model for K ATP channel-independent regulation of insulin secretion. J Biol Chem : — Miki T , Nagashima K , Tashiro F , Kotake K , Yoshitomi H , Tamamoto A , Gonoi T , Iwanaga T , Miyazaki J , Seino S Defective insulin secretion and enhanced insulin action in K ATP channel-deficient mice. Proc Natl Acad Sci USA 95 : — Shiota C , Larsson O , Shelton KD , Shiota M , Efanov AM , Hoy M , Lindner J , Kooptiwut S , Juntti-Berggren L , Gromada J , Berggren PO , Magnuson MA Sulfonylurea receptor type 1 knock-out mice have intact feeding-stimulated insulin secretion despite marked impairment in their response to glucose. Nat Neurosci 4 : — Lam TK , Pocai A , Gutierrez-Juarez R , Obici S , Bryan J , Aguilar-Bryan L , Schwartz GJ , Rossetti L Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med 11 : — Pocai A , Lam TK , Gutierrez-Juarez R , Obici S , Schwartz GJ , Bryan J , Aguilar-Bryan L , Rossetti L Hypothalamic K ATP channels control hepatic glucose production. Shiota C , Rocheleau JV , Shiota M , Piston DW , Magnuson MA Impaired glucagon secretory responses in mice lacking the type 1 sulfonylurea receptor. Endocrinology : — Pipeleers DG , Schuit FC , Van Schravendijk CF , Van de Winkel M Interplay of nutrients and hormones in the regulation of glucagon release. Roe JH , Dailey RE Determination of glycogen with the anthrone reagent. Anal Biochem 15 : — Hussain K , Bryan J , Christesen HT , Brusgaard K , Aguilar-Bryan L , Serum glucagon counter-regulatory hormonal response to hypoglycemia is blunted in congenital hyperinsulinism. Diabetes , in press. Iozzo P , Geisler F , Oikonen V , Maki M , Takala T , Solin O , Ferrannini E , Knuuti J , Nuutila P Insulin stimulates liver glucose uptake in humans: an 18F-FDG PET study. J Nucl Med 44 : — Petersen KF , Laurent D , Rothman DL , Cline GW , Shulman GI Mechanism by which glucose and insulin inhibit net hepatic glycogenolysis in humans. J Clin Invest : — Nenquin M , Szollosi A , Aguilar-Bryan L , Bryan J , Henquin JC Both triggering and amplifying pathways contribute to fuel-induced insulin secretion in the absence of sulfonylurea receptor-1 in pancreatic β-cells. Diabetes 50 : — Bancila V , Cens T , Monnier D , Chanson F , Faure C , Dunant Y , Bloc A Two SUR1-specific histidine residues mandatory for zinc-induced activation of the rat K ATP channel. Prost AL , Bloc A , Hussy N , Derand R , Vivaudou M Zinc is both an intracellular and extracellular regulator of KATP channel function. Franklin I , Gromada J , Gjinovci A , Theander S , Wollheim CB β-Cell secretory products activate α-cell ATP-dependent potassium channels to inhibit glucagon release. Stagner JI , Samols E The vascular order of islet cellular perfusion in the human pancreas. Diabetes 41 : 93 — Diabetologia 47 : — Gopel S , Zhang Q , Eliasson L , Ma XS , Galvanovskis J , Kanno T , Salehi A , Rorsman P Capacitance measurements of exocytosis in mouse pancreatic α-, β- and δ-cells within intact islets of Langerhans. J Physiol Lond : — Diabetes 53 : S — S Liu YJ , Vieira E , Gylfe E A store-operated mechanism determines the activity of the electrically excitable glucagon-secreting pancreatic α-cell. Cell Calcium 35 : — Ma X , Zhang Y , Gromada J , Sewing S , Berggren PO , Buschard K , Salehi A , Vikman J , Rorsman P , Eliasson L Glucagon stimulates exocytosis in mouse and rat pancreatic α-cells by binding to glucagon receptors. Mol Endocrinol 19 : — Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide. Sign In or Create an Account. Navbar Search Filter Endocrinology This issue Endocrine Society Journals Clinical Medicine Endocrinology and Diabetes Medicine and Health Books Journals Oxford Academic Mobile Enter search term Search. Endocrine Society Journals. Advanced Search. Search Menu. Article Navigation. Close mobile search navigation Article Navigation. Volume Article Contents Materials and Methods. Journal Article. Regulation of Glucagon Secretion at Low Glucose Concentrations: Evidence for Adenosine Triphosphate-Sensitive Potassium Channel Involvement. Alvaro Muñoz , Alvaro Muñoz. Oxford Academic. Min Hu. Khalid Hussain. Joseph Bryan. Lydia Aguilar-Bryan. Arun S. Rajan, One Baylor Plaza, BCMA B, Houston, Texas PDF Split View Views. Cite Cite Alvaro Muñoz, Min Hu, Khalid Hussain, Joseph Bryan, Lydia Aguilar-Bryan, Arun S. Select Format Select format. ris Mendeley, Papers, Zotero. enw EndNote. bibtex BibTex. txt Medlars, RefWorks Download citation. Permissions Icon Permissions. Close Navbar Search Filter Endocrinology This issue Endocrine Society Journals Clinical Medicine Endocrinology and Diabetes Medicine and Health Books Journals Oxford Academic Enter search term Search. Open in new tab Download slide. TABLE 1. Insulin and glucagon secretion from WT and Sur1KO islets. Open in new tab. First Published Online August 25, and M. contributed equally to this work. Google Scholar Crossref. Search ADS. Google Scholar PubMed. OpenURL Placeholder Text. Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes. N Engl J Med. Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Glucose inhibition of glucagon secretion from rat α-cells is mediated by GABA released from neighboring β-cells. Characterization of the effects of arginine and glucose on glucagon and insulin release from the perfused rat pancreas. Localization of vagal preganglionics that stimulate insulin and glucagon secretion. The order of islet microvascular cellular perfusion is B-A-D in the perfused rat pancreas. Islet β-cell secretion determines glucagon release from neighbouring α-cells. Ventromedial hypothalamic lesions in rats suppress counter-regulatory responses to hypoglycemia. Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. Autonomic mechanism and defects in the glucagon response to insulin-induced hypoglycaemia. Loss of the decrement in intraislet insulin plausibly explains loss of the glucagon response to hypoglycemia in insulin-deficient diabetes: documentation of the intraislet insulin hypothesis in humans. Molecular biology of adenosine triphosphate-sensitive potassium channels. Sur1 knockout mice. Defective insulin secretion and enhanced insulin action in K ATP channel-deficient mice. Sulfonylurea receptor type 1 knock-out mice have intact feeding-stimulated insulin secretion despite marked impairment in their response to glucose. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Hypothalamic K ATP channels control hepatic glucose production. Impaired glucagon secretory responses in mice lacking the type 1 sulfonylurea receptor. Interplay of nutrients and hormones in the regulation of insulin release. Interplay of nutrients and hormones in the regulation of glucagon release. Serum glucagon counter-regulatory hormonal response to hypoglycemia is blunted in congenital hyperinsulinism. Mechanism by which glucose and insulin inhibit net hepatic glycogenolysis in humans. Both triggering and amplifying pathways contribute to fuel-induced insulin secretion in the absence of sulfonylurea receptor-1 in pancreatic β-cells. Two SUR1-specific histidine residues mandatory for zinc-induced activation of the rat K ATP channel. Zinc is both an intracellular and extracellular regulator of KATP channel function. β-Cell secretory products activate α-cell ATP-dependent potassium channels to inhibit glucagon release. Capacitance measurements of exocytosis in mouse pancreatic α-, β- and δ-cells within intact islets of Langerhans. A store-operated mechanism determines the activity of the electrically excitable glucagon-secreting pancreatic α-cell. Glucagon stimulates exocytosis in mouse and rat pancreatic α-cells by binding to glucagon receptors. Issue Section:. Download all slides. Views 4, More metrics information. Total Views 4, Email alerts Article activity alert. Advance article alerts. New issue alert. Receive exclusive offers and updates from Oxford Academic. More on this topic Evidence that the Physiological Pulse Frequency of Glucagon Secretion Optimizes Glucose Production by Perifused Rat Hepatocytes. Effects of an Enkephalin Analog on Pancreatic Endocrine Function and Glucose Homeostasis in Normal and Diabetic Dogs. Reversal of Defective Glucagon Responses to Hypoglycemia in Insulin-Dependent Autoimmune Diabetic BB Rats. Modulation of adenosine triphosphate-sensitive potassium channel and voltage-dependent calcium channel by activin A in HIT-T15 cells. Related articles in PubMed Resolution and quantification of carbohydrates by enantioselective comprehensive two-dimensional gas chromatography. The diagnostic value of cognitive assessment indicators for mild cognitive impairment MCI. Chondroitin Sulfate-Derived Micelles for Adipose Tissue-Targeted Delivery of Celastrol and Phenformin to Enhance Obesity Treatment. Glucagon-Like Peptide-1 Receptor Agonists During Electroconvulsive Therapy: Case Report With Evolving Concerns and Management Considerations. Citing articles via Web of Science Latest Most Read Most Cited Sirt3 regulates proliferation and progesterone production in Leydig cells via suppression of reactive oxygen species. AURKA enhances the glycolysis and development of ovarian endometriosis through ERβ. Effects of the cortisol milieu on tumor-infiltrating immune cells TIICs in corticotroph tumors. mTOR regulates mineralocorticoid receptor transcriptional activity by ULK1- dependent and independent mechanisms. iScience — Galsgaard KD, Winther-Sørensen M, Ørskov C, Kissow H, Poulsen SS, Vilstrup H, et al. Disruption of Glucagon Receptor Signaling Causes Hyperaminoacidemia Exposing a Possible Liver-Alpha-Cell Axis. Am J Physiol Metab E93—E Wewer Albrechtsen NJ, Pedersen J, Galsgaard KD, Winther-Sørensen M, Suppli MP, Janah L, et al. The Liver—α-Cell Axis and Type 2 Diabetes. Guan H-P, Yang X, Lu K, Wang S-P, Castro-Perez JM, Previs S, et al. Glucagon Receptor Antagonism Induces Increased Cholesterol Absorption. J Lipid Res — Tooze S. Biogenesis of Secretory Granules in the Trans-Golgi Network of Neuroendocrine and Endocrine Cells. Biochim Biophys Acta — Cool DR, Fenger M, Snell CR, Loh YP. Identification of the Sorting Signal Motif Within Pro-Opiomelanocortin for the Regulated Secretory Pathway. J Biol Chem —9. Dhanvantari S, Shen F, Adams T, Snell CR, Zhang C, Mackin RB, et al. Disruption of a Receptor-Mediated Mechanism for Intracellular Sorting of Proinsulin in Familial Hyperproinsulinemia. Mol Endocrinol — Zhang C-F, Dhanvantari S, Lou H, Loh YP. Sorting of Carboxypeptidase E to the Regulated Secretory Pathway Requires Interaction of its Transmembrane Domain With Lipid Rafts. Biochem J — Dikeakos JD, Mercure C, Lacombe M-J, Seidah NG, Reudelhuber TL. FEBS J — Dikeakos JD, Di Lello P, Lacombe M-J, Ghirlando R, Legault P, Reudelhuber TL, et al. Proc Natl Acad Sci USA — Dhanvantari S, Loh YP. Lipid Raft Association of Carboxypeptidase E Is Necessary for Its Function as a Regulated Secretory Pathway Sorting Receptor. J Biol Chem — Cool DR, Normant E, Shen F, Chen H, Pannell L, Zhang Y, et al. Carboxypeptidase E Is a Regulated Secretory Pathway Sorting Receptor: Genetic Obliteration Leads to Endocrine Disorders in Cpe Fat Mice. Cell — Irminger JC, Verchere CB, Meyer K, Halban PA. J Biol Chem —4. McGirr R, Guizzetti L, Dhanvantari S. The Sorting of Proglucagon to Secretory Granules is Mediated by Carboxypeptidase E and Intrinsic Sorting Signals. J Endocrinol — Hosaka M, Watanabe T, Sakai Y, Kato T, Takeuchi T. Interaction Between Secretogranin III and Carboxypeptidase E Facilitates Prohormone Sorting Within Secretory Granules. J Cell Sci — Plá V, Paco S, Ghezali G, Ciria V, Pozas E, Ferrer I, et al. Brain Pathol — Arvan P, Halban PA. Sorting Ourselves Out: Seeking Consensus on Trafficking in the Beta-Cell. Traffic — Guizzetti L, McGirr R, Dhanvantari S. Two Dipolar α-Helices Within Hormone-Encoding Regions of Proglucagon are Sorting Signals to the Regulated Secretory Pathway. Dey A, Lipkind GM, Rouillé Y, Norrbom C, Stein J, Zhang C, et al. Significance of Prohormone Convertase 2, PC2, Mediated Initial Cleavage at the Proglucagon Interdomain Site, LysArg71, to Generate Glucagon. Endocrinology — Rouille Y, Westermark G, Martin SK, Steiner DF. Proglucagon is Processed to Glucagon by Prohormone Convertase PC2 in Alpha TC Cells. Proc Natl Acad Sci —6. Dhanvantari S, Seidah NG, Brubaker PL. Role of Prohormone Convertases in the Tissue-Specific Processing of Proglucagon. Furuta M, Zhou A, Webb G, Carroll R, Ravazzola M, Orci L, et al. Severe Defect in Proglucagon Processing in Islet Alpha-Cells of Prohormone Convertase 2 Null Mice. Campbell SA, Golec DP, Hubert M, Johnson J, Salamon N, Barr A, et al. Human Islets Contain a Subpopulation of Glucagon-Like Peptide-1 Secreting α Cells That is Increased in Type 2 Diabetes. Mol Metab Nie Y, Nakashima M, Brubaker PL, Li QL, Perfetti R, Jansen E, et al. Regulation of Pancreatic PC1 and PC2 Associated With Increased Glucagon-Like Peptide 1 in Diabetic Rats. McGirr R, Ejbick CE, Carter DE, Andrews JD, Nie Y, Friedman TC, et al. Glucose Dependence of the Regulated Secretory Pathway in αtc Cells. Liu P, Song J, Liu H, Yan F, He T, Wang L, et al. Insulin Regulates Glucagon-Like Peptide-1 Secretion by Pancreatic Alpha Cells. Endocrine — Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, et al. Interleukin-6 Enhances Insulin Secretion by Increasing Glucagon-Like Peptide-1 Secretion From L Cells and Alpha Cells. Nat Med —9. Progressive Change of Intra-Islet GLP-1 Production During Diabetes Development. Diabetes Metab Res Rev —8. Kilimnik G, Kim A, Steiner DF, Friedman TC, Hara M. Islets — Wideman RD, Gray SL, Covey SD, Webb GC, Kieffer TJ. Mol Ther —8. Wideman RD, Covey SD, Webb GC, Drucker DJ, Kieffer TJ. Galvin SG, Kay RG, Foreman R, Larraufie P, Meek CL, Biggs E, et al. The Human and Mouse Islet Peptidome: Effects of Obesity and Type 2 Diabetes, and Assessment of Intraislet Production of Glucagon-Like Peptide J Proteome Res x:acs. Runge S, Wulff BS, Madsen K, Bräuner-Osborne H, Knudsen LB. Different Domains of the Glucagon and Glucagon-Like Peptide-1 Receptors Provide the Critical Determinants of Ligand Selectivity. Br J Pharmacol — Salehi A, Vieira E, Gylfe E. Paradoxical Stimulation of Glucagon Secretion by High Glucose Concentrations. Gylfe E. Ups J Med Sci — Whalley NM, Pritchard LE, Smith DM. White a. Processing of Proglucagon to GLP-1 in Pancreatic α-Cells: Is This a Paracrine Mechanism Enabling GLP-1 to Act on β-Cells? Asadi F, Dhanvantari S. Plasticity in the Glucagon Interactome Reveals Novel Proteins That Regulate Glucagon Secretion in α-TC Cells. Front Endocrinol Lausanne Omar-Hmeadi M, Lund PE, Gandasi NR, Tengholm A, Barg S. Paracrine Control of α-Cell Glucagon Exocytosis is Compromised in Human Type-2 Diabetes. Nat Commun — Le Marchand SJ, Piston DW. Glucose Suppression of Glucagon Secretion: Metabolic and Calcium Responses From Alpha-Cells in Intact Mouse Pancreatic Islets. Quoix N, Cheng-xue R, Mattart L, Zeinoun Z, Guiot Y, Beauvois M, et al. Ramracheya R, Ward C, Shigeto M, Walker JN, Amisten S, Zhang Q, et al. Membrane Potential-Dependent Inactivation of Voltage-Gated Ion Channels in α-Cells Inhibits Glucagon Secretion From Human Islets. Zhang Q, Ramracheya R, Lahmann C, Tarasov A, Bengtsson M, Braha O, et al. Role of KATP Channels in Glucose-Regulated Glucagon Secretion and Impaired Counterregulation in Type 2 Diabetes. Zhang Q, Dou H, Rorsman P. J Physiol — Liu Y-J, Vieira E, Gylfe E. A Store-Operated Mechanism Determines the Activity of the Electrically Excitable Glucagon-Secreting Pancreatic α-Cell. Cell Calcium — Tian G, Tepikin AV, Tengholm A, Gylfe E. Watts M, Sherman A. Modeling the Pancreatic α-Cell: Dual Mechanisms of Glucose Suppression of Glucagon Secretion. Biophys J — PloS One 7:e Elliott AD, Ustione A, Piston DW. Somatostatin and Insulin Mediate Glucose-Inhibited Glucagon Secretion in the Pancreatic α-Cell by Lowering cAMP. Am J Physiol Endocrinol Metab E— Yu Q, Shuai H, Ahooghalandari P, Gylfe E, Tengholm A. Glucose Controls Glucagon Secretion by Directly Modulating cAMP in Alpha Cells. Hughes JW, Ustione A, Lavagnino Z, Piston DW. Regulation of Islet Glucagon Secretion: Beyond Calcium. Diabetes Obes Metab — Leclerc I, Sun G, Morris C, Fernandez-Millan E, Nyirenda M, Rutter GA. AMP-Activated Protein Kinase Regulates Glucagon Secretion From Mouse Pancreatic Alpha Cells. Da Silva Xavier G, Farhan H, Kim H, Caxaria S, Johnson P, Hughes S, et al. Per-Arnt-Sim PAS Domain-Containing Protein Kinase is Downregulated in Human Islets in Type 2 Diabetes and Regulates Glucagon Secretion. Sun G, da Silva Xavier G, Gorman T, Priest C, Solomou A, Hodson DJ, et al. LKB1 and Ampkα1 are Required in Pancreatic Alpha Cells for the Normal Regulation of Glucagon Secretion and Responses to Hypoglycemia. Mol Metab — Bozadjieva N, Blandino-Rosano M, Chase J, Dai XQ, Cummings K, Gimeno J, et al. Loss of Mtorc1 Signaling Alters Pancreatic α Cell Mass and Impairs Glucagon Secretion. Kramer NB, Lubaczeuski C, Blandino-Rosano M, Barker G, Gittes GK, Caicedo A, et al. Glucagon Resistance and Decreased Susceptibility to Diabetes in a Model of Chronic Hyperglucagonemia. Gromada J, Franklin I, Wollheim CB. Alpha-Cells of the Endocrine Pancreas: 35 Years of Research But the Enigma Remains. Kawamori D, Kulkarni RN. Insulin Modulation of Glucagon Secretion: The Role of Insulin and Other Factors in the Regulation of Glucagon Secretion. Islets —9. Tsuchiyama N, Takamura T, Ando H, Sakurai M, Shimizu A, Kato KI, et al. Possible Role of α-Cell Insulin Resistance in Exaggerated Glucagon Responses to Arginine in Type 2 Diabetes. Diabetes Care —7. Wendt A, Birnir B, Buschard K, Gromada J, Salehi A, Sewing S, et al. Glucose Inhibition of Glucagon Secretion From Rat α-Cells Is Mediated by GABA Released From Neighboring β-Cells. Li C, Liu C, Nissim I, Chen J, Chen P, Doliba N, et al. Regulation of Glucagon Secretion in Normal and Diabetic Human Islets by?? Rorsman P, Berggren PO, Bokvist K, Ericson H, Möhler H, Ostenson CG SP. Glucose-Inhibition of Glucagon Secretion Involves Activation of GABAA-Receptor Chloride Channels. Nature —6. Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, et al. Intra-Islet Insulin Suppresses Glucagon Release via GABA-GABAA Receptor System. Feng AL, Xiang Y, Gui L, Kaltsidis G, Feng Q, Lu W. Paracrine GABA and Insulin Regulate Pancreatic Alpha Cell Proliferation in a Mouse Model of Type 1 Diabetes. Jin Li J, Casteels T, Frogne T, Ingvorsen C, Honore C, Courtney M, et al. Artemisinins Target GABAA Receptor Signaling and Impair α Cell Identity. Weir GC, Bonner-Weir S. GABA Signaling Stimulates β Cell Regeneration in Diabetic Mice. Cell —9. Ben-Othman N, Vieira A, Courtney M, Record F, Gjernes E, Avolio F, et al. Long-Term GABA Administration Induces Alpha Cell-Mediated Beta-Like Cell Neogenesis. van der Meulen T, Lee S, Noordeloos E, Donaldson CJ, Adams MW, Noguchi GM, et al. Artemether Does Not Turn α Cells Into β Cells. Ackermann AM, Moss NG, Kaestner KH. GABA and Artesunate Do Not Induce Pancreatic α-to-β Cell Transdifferentiation In Vivo. Combined Effect of GABA and Glucagon-Like Peptide-1 Receptor Agonist on Cytokine-Induced Apoptosis in Pancreatic β-Cell Line and Isolated Human Islets. J Diabetes — Daems C, Welsch S, Boughaleb H, Vanderroost J, Robert A, Sokal E, et al. Early Treatment With Empagliflozin and GABA Improves β -Cell Mass and Glucose Tolerance in Streptozotocin-Treated Mice. J Diabetes Res — Human Beta Cells Produce and Release Serotonin to Inhibit Glucagon Secretion From Alpha Cells. Cell Rep — Bennet H, Balhuizen A, Medina A, Dekker Nitert M, Ottosson Laakso E, Essén S, et al. Altered Serotonin 5-HT 1D and 2A Receptor Expression May Contribute to Defective Insulin and Glucagon Secretion in Human Type 2 Diabetes. Peptides — Yip L, Taylor C, Whiting CC, Fathman CG. Diminished Adenosine A1 Receptor Expression in Pancreatic a-Cells May Contribute to the Patholog Y of Type 1 Diabetes. Ishihara H, Wollheim CB. Is Zinc an Intra-Islet Regulator of Glucagon Secretion? Diabetol Int — Franklin I, Gromada J, Gjinovci A, Theander S. Beta Cell Secretory Products Activate Alpha Cell ATP-Dependent Potassium Channels to Inhibit Glucagon Release. Ravier MA, Rutter GA. Glucose or Insulin, But Not Zinc Ions, Inhibit Glucagon Secretion From Mouse Pancreatic [Alpha]-Cells. Solomou A, Philippe E, Chabosseau P, Migrenne-li S, Gaitan J, Lang J, et al. Nutr Metab Lond Solomou A, Meur G, Bellomo E, Hodson DJ, Tomas A, Li SM, et al. Strowski MZ, Parmar RM, Blake AD, Schaeffer JM. Somatostatin Inhibits Insulin and Glucagon Secretion via Two Receptor Subtypes : An In Vitro Study of Pancreatic Islets From Somatostatin Receptor 2 Knockout Mice. Endocrinology —7. Gromada J, Hoy M, Bushcard K, Salehi A, Rorsman P. Somatostatin Inhibits Exocytosis in Rat Pancreatic a-Cells by Gi2-Dependent Activation of Calcineurin and Depriming of Secretory Granules. Rutter GA. Regulating Glucagon Secretion: Somatostatin in the Spotlight. Xu SFS, Andersen DB, Izarzugaza JMG, Kuhre RE, Holst JJ. In the Rat Pancreas, Somatostatin Tonically Inhibits Glucagon Secretion and Is Required for Glucose-Induced Inhibition of Glucagon Secretion. Acta Physiol — Hauge-Evans AC, King AJ, Carmignac D, Richardson CC, Robinson ICAF, Low MJ, et al. Somatostatin Secreted by Islet -Cells Fulfills Multiple Roles as a Paracrine Regulator of Islet Function. Vergari E, Knudsen JG, Ramracheya R, Salehi A, Zhang Q, Adam J, et al. Insulin Inhibits Glucagon Release by SGLT2-Induced Stimulation of Somatostatin Secretion. Nat Commun Briant LJB, Reinbothe TM, Spiliotis I, Miranda C, Rodriguez B, Rorsman P. Δ-Cells and β-Cells Are Electrically Coupled and Regulate α-Cell Activity Via Somatostatin. Kilimnik G, Zhao B, Jo J, Periwal V, Witkowski P, Misawa R, et al. Altered Islet Composition and Disproportionate Loss of Large Islets in Patients With Type 2 Diabetes. PloS One 6:e Ma X, Zhang Y, Gromada J, Sewing S, Berggren P-O, Buschard K, et al. Glucagon Stimulates Exocytosis in Mouse and Rat Pancreatic α-Cells by Binding to Glucagon Receptors. Leibiger B, Moede T, Muhandiramlage TP, Kaiser D, Vaca Sanchez P, Leibiger IB, et al. Glucagon Regulates its Own Synthesis by Autocrine Signaling. Wewer Albrechtsen NJ, Kuhre RE, Hornburg D, Jensen CZ, Hornum M, Dirksen C, et al. Circulating Glucagon Regulates Blood Glucose by Increasing Insulin Secretion and Hepatic Glucose Production. Hare KJ, Knop FK, Asmar M, Madsbad S, Deacon CF, Holst JJ, et al. Preserved Inhibitory Potency of GLP-1 on Glucagon Secretion in Type 2 Diabetes Mellitus. J Clin Endocrinol Metab — Garg M, Ghanim H, Kuhadiya N, Green K, Hejna J, Abuaysheh S, et al. Liraglutide Acutely Suppresses Glucagon, Lipolysis and Ketogenesis in Type 1 Diabetes. Chambers AP, Sorrell JE, Haller A, Roelofs K, Hutch CR, Kim K-S, et al. The Role of Pancreatic Preproglucagon in Glucose Homeostasis in Mice. Habener JF, Stanojevic V. Pancreas and Not Gut Mediates the GLPInduced Glucoincretin Effect. Cell Metab —8. Fava GE, Dong EW, Wu H. Intra-Islet Glucagon-Like Peptide J Diabetes Complicat —8. Holst J, Christensen M, Lund A, De Heer J, Svendsen B, Kielgast U, et al. Regulation of Glucagon Secretion by Incretins. Ramracheya R, Chapman C, Chibalina M, Dou H, Miranda C, González A, et al. Physiol Rep — Boss M, Bos D, Frielink C, Sandker G, Ekim S, Marciniak C, et al. Targeted Optical Imaging of the Glucagon-Like Peptide 1 Receptor Using Exendin-4irdyecw. J Nucl Med — Roberts S, Khera E, Choi C, Navaratna T, Grimm J, Thurber GM, et al. Optoacoustic Imaging of Glucagon-Like Peptide 1 Receptor With a Near-Infrared Exendin-4 Analog. Azad BB, Rota V. Design, Synthesis and In Vitro Characterization of Glucagon-Like Peptide-1 Derivatives for Pancreatic Beta Cell Imaging by SPECT. Bioorg Med Chem — Ast J, Arvaniti A, Fine NHF, Nasteska D, Ashford FB, Stamataki Z, et al. Super-Resolution Microscopy Compatible Fluorescent Probes Reveal Endogenous Glucagon-Like Peptide-1 Receptor Distribution and Dynamics. Waser B, Blank A, Karamitopoulou E, Perren A, Reubi JC. Glucagon-Like-Peptide-1 Receptor Expression in Normal and Diseased Human Thyroid and Pancreas. Mod Pathol — de Heer J, Rasmussen C, Coy DH, Holst JJ. Glucagon-Like Peptide-1, But Not Glucose-Dependent Insulinotropic Peptide, Inhibits Glucagon Secretion via Somatostatin Receptor Subtype 2 in the Perfused Rat Pancreas. Ørgaard A, Holst JJ. The Role of Somatostatin in GLPInduced Inhibition of Glucagon Secretion in Mice. Diabetologia —9. Saponaro C, Gmyr V, Thévenet J, Moerman E, Delalleau N, Pasquetti G, et al. The GLP1R Agonist Liraglutide Reduces Hyperglucagonemia Induced by the SGLT2 Inhibitor Dapagliflozin via Somatostatin Release. Lawlor N, Youn A, Kursawe R, Ucar D, Stitzel ML. Alpha TC1 and Beta-TC-6 Genomic Profiling Uncovers Both Shared and Distinct Transcriptional Regulatory Features With Their Primary Islet Counterparts. Sci Rep — Stamenkovic JA, Andersson LE, Adriaenssens AE, Bagge A, Sharoyko VV, Gribble F, et al. Inhibition of the Malate-Aspartate Shuttle in Mouse Pancreatic Islets Abolishes Glucagon Secretion Without Affecting Insulin Secretion. Briant LJB, Zhang Q, Vergari E, Kellard JA, Rodriguez B, Ashcroft FM, et al. Functional Identification of Islet Cell Types by Electrophysiological Fingerprinting. J R Soc Interface Ackermann AM, Zhang J, Heller A, Briker A, Kaestner KH. High-Fidelity Glucagon-CreER Mouse Line Generated by CRISPR-Cas9 Assisted Gene Targeting. Quoix N, Cheng-Xue R, Guiot Y, Herrera PL, Henquin JC, Gilon P. The GluCre-ROSA26EYFP Mouse: A New Model for Easy Identification of Living Pancreatic Alpha-Cells. FEBS Lett — Shiota C, Prasadan K, Guo P, Fusco J, Xiao X, Gittes GK. GcgCreERT2knockin Mice as a Tool for Genetic Manipulation in Pancreatic Alpha Cells. Andersson SA, Pedersen MG, Vikman J, Eliasson L. Glucose-Dependent Docking and SNARE Protein-Mediated Exocytosis in Mouse Pancreatic Alpha-Cell. Pflugers Arch Eur J Physiol — González-Vélez V, Dupont G, Gil A, González A, Quesada I. Model for Glucagon Secretion by Pancreatic α-Cells. Gerber SH, Sudhof TC. Molecular Determinants of Regulated Exocytosis. Gustavsson N, Wei S, Hoang DN, Lao Y, Zhang Q, Radda GK, et al. Jewell JL, Oh E, Thurmond DC. Exocytosis Mechanisms Underlying Insulin Release and Glucose Uptake : Conserved Roles for Munc18c and Syntaxin 4. Am J Physiol Regul Integr Comp Physiol R— Gandasi NR, Yin P, Riz M, Chibalina MV, Cortese G, Lund P, et al. Xia F, Leung YM, Gaisano G, Gao X, Chen Y, Fox JEM, et al. Montefusco F, Pedersen MG. Mathematical Modelling of Local Calcium and Regulated Exocytosis During Inhibition and Stimulation of Glucagon Secretion From Pancreatic Alpha-Cells. Yokawa S, Suzuki T, Inouye S, Inoh Y, Suzuki R, Kanamori T, et al. Visualization of Glucagon Secretion From Pancreatic α Cells by Bioluminescence Video Microscopy: Identification of Secretion Sites in the Intercellular Contact Regions. Biochem Biophys Res Commun — Brissova M, Haliyur R, Saunders D, Shrestha S, Dai C, Blodgett DM, et al. α Cell Function and Gene Expression Are Compromised in Type 1 Diabetes. Camunas-Soler J, Dai XQ, Hang Y, Bautista A, Lyon J, Suzuki K, et al. Patch-Seq Links Single-Cell Transcriptomes to Human Islet Dysfunction in Diabetes. Zhou Y, Liu Z, Zhang S, Zhuang R, Liu H, Liu X, et al. RILP Restricts Insulin Secretion Through Mediating Lysosomal Degradation of Proinsulin. Li H, Wei S, Cheng K, Gounko NV, Ericksen RE, Xu A, et al. BIG3 Inhibits Insulin Granule Biogenesis and Insulin Secretion. EMBO Rep — Li H, Liu T, Lim J, Gounko NV, Hong W, Han W. Increased Biogenesis of Glucagon-Containing Secretory Granules and Glucagon Secretion in BIG3-Knockout Mice. Liu T, Li H, Hong W, Han W. Brefeldin A-Inhibited Guanine Nucleotide Exchange Protein 3 is Localized in Lysosomes and Regulates GABA Signaling in Hippocampal Neurons. J Neurochem — Stathmin-2 Mediates Glucagon Secretion From Pancreatic α-Cells. Bramswig NC, Everett LJ, Schug J, Dorrell C, Liu C, Luo Y, et al. Epigenomic Plasticity Enables Human Pancreatic α to β Cell Reprogramming. Misrouting of Glucagon and Stathmin-2 Towards Lysosomal System of α-Cells in Glucagon Hypersecretion of Diabetes. bioRxiv 0—1. Cho HJ. Mook-Jung I. O-GlcNAcylation Regulates Endoplasmic Reticulum Exit Sites Through Sec31A Modification in Conventional Secretory Pathway. FASEB J — Zhang X, Wang L, Lak B, Li J, Jokitalo E, Wang Y. GRASP55 Senses Glucose Deprivation Through O-GlcNAcylation to Promote Autophagosome-Lysosome Fusion. Dev Cell — Essawy A, Jo S, Beetch M, Lockridge A, Gustafson E. Alejandro EU. O-Linked N-Acetylglucosamine Transferase OGT Regulates Pancreatic α-Cell Function in Mice. J Biol Chem |
Glucagon secretion -
The alpha subunit specifically activates the next enzyme in the cascade, adenylate cyclase. Adenylate cyclase manufactures cyclic adenosine monophosphate cyclic AMP or cAMP , which activates protein kinase A cAMP-dependent protein kinase.
This enzyme, in turn, activates phosphorylase kinase , which then phosphorylates glycogen phosphorylase b PYG b , converting it into the active form called phosphorylase a PYG a. Phosphorylase a is the enzyme responsible for the release of glucose 1-phosphate from glycogen polymers.
An example of the pathway would be when glucagon binds to a transmembrane protein. The transmembrane proteins interacts with Gɑβ𝛾. Gαs separates from Gβ𝛾 and interacts with the transmembrane protein adenylyl cyclase. Adenylyl cyclase catalyzes the conversion of ATP to cAMP.
cAMP binds to protein kinase A, and the complex phosphorylates glycogen phosphorylase kinase. Phosphorylated glycogen phosphorylase clips glucose units from glycogen as glucose 1-phosphate. Additionally, the coordinated control of glycolysis and gluconeogenesis in the liver is adjusted by the phosphorylation state of the enzymes that catalyze the formation of a potent activator of glycolysis called fructose 2,6-bisphosphate.
This covalent phosphorylation initiated by glucagon activates the former and inhibits the latter. This regulates the reaction catalyzing fructose 2,6-bisphosphate a potent activator of phosphofructokinase-1, the enzyme that is the primary regulatory step of glycolysis [24] by slowing the rate of its formation, thereby inhibiting the flux of the glycolysis pathway and allowing gluconeogenesis to predominate.
This process is reversible in the absence of glucagon and thus, the presence of insulin. Glucagon stimulation of PKA inactivates the glycolytic enzyme pyruvate kinase , [25] inactivates glycogen synthase , [26] and activates hormone-sensitive lipase , [27] which catabolizes glycerides into glycerol and free fatty acid s , in hepatocytes.
Malonyl-CoA is a byproduct of the Krebs cycle downstream of glycolysis and an allosteric inhibitor of Carnitine palmitoyltransferase I CPT1 , a mitochondrial enzyme important for bringing fatty acids into the intermembrane space of the mitochondria for β-oxidation. Thus, reduction in malonyl-CoA is a common regulator for the increased fatty acid metabolism effects of glucagon.
Abnormally elevated levels of glucagon may be caused by pancreatic tumors , such as glucagonoma , symptoms of which include necrolytic migratory erythema , [30] reduced amino acids, and hyperglycemia.
It may occur alone or in the context of multiple endocrine neoplasia type 1. Elevated glucagon is the main contributor to hyperglycemic ketoacidosis in undiagnosed or poorly treated type 1 diabetes.
As the beta cells cease to function, insulin and pancreatic GABA are no longer present to suppress the freerunning output of glucagon. As a result, glucagon is released from the alpha cells at a maximum, causing a rapid breakdown of glycogen to glucose and fast ketogenesis.
The absence of alpha cells and hence glucagon is thought to be one of the main influences in the extreme volatility of blood glucose in the setting of a total pancreatectomy. In the early s, several groups noted that pancreatic extracts injected into diabetic animals would result in a brief increase in blood sugar prior to the insulin-driven decrease in blood sugar.
Kimball and John R. Murlin identified a component of pancreatic extracts responsible for this blood sugar increase, terming it "glucagon", a portmanteau of " gluc ose agon ist".
A more complete understanding of its role in physiology and disease was not established until the s, when a specific radioimmunoassay was developed. Contents move to sidebar hide. Article Talk.
Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item.
Download as PDF Printable version. In other projects. Wikimedia Commons. Peptide hormone. This article is about the natural hormone. For the medication, see Glucagon medication.
Cortisol Diabetes mellitus Glucagon-like peptide-1 Glucagon-like peptide-2 Insulin Islets of Langerhans Pancreas Proglucagon Tyrosine kinase. Biochemistry 4th ed. New York: Wiley. San Francisco: Benjamin Cummings. Isolated Sur1KO islets have an attenuated response to low glucose.
Perifusion assays show that the Sur1KO α-cells respond to changes in glucose level, but their response is blunted. Figure 3B illustrates the normal biphasic insulin response of WT islets to a stepwise change in glucose concentration. Figure 3D shows that switching WT islets from low to high glucose 2.
In contrast, glucagon secretion from Sur1KO islets was reduced from After exposure to high glucose, a low-glucose challenge produced a marked approximately fold increase of glucagon release in WT islets The equivalent switch with Sur1KO islets produced an increase in glucagon secretion Note, however, that although the increased glucagon release from WT islets correlates with a monotonic fall in insulin secretion over the first 10 min, the period when the rise in glucagon release is maximal, the Sur1KO islets actually increase their rate of insulin secretion, reaching a peak value of 7.
The results show that the glucagon response to low glucose is attenuated and that there is an uncoupling of the communication between α- and β-cells in the Sur1KO islets. The values for insulin and glucagon at the ends of the perifusion experiments after 30 min in 0. The values are means ± se.
P values comparing WT vs. Glibenclamide strongly stimulates insulin secretion from WT islets in 0. Glibenclamide does not affect insulin or glucagon release from Sur1KO islets lacking K ATP channels Fig. Note that the levels of glucagon secretion from WT islets treated with glibenclamide mimic the impaired release observed for Sur1KO islets compare Fig.
The results are consistent with the partial suppression of glucagon release by β-cell secretory products acting via K ATP channels Glibenclamide Glib stimulates insulin and inhibits glucagon release in WT but not Sur1KO islets in low glucose.
A, Response of WT islets. B, Response of Sur1KO islets. The perifusion protocol is the same as shown in Fig. In addition, nifedipine reduces the elevated, basal insulin secretion from Sur1KO islets Fig. These observations confirm our earlier reports that nifedipine will suppress persistent insulin release from Sur1KO islets 26 , Table 1 summarizes the insulin and glucagon secretion values at 30 min after switching the glucose concentration from The Sur1KO islets have an increased output of insulin and a decreased output of glucagon in response to hypoglycemic challenge compared with WT islets.
Glibenclamide does not affect hormone secretion from Sur1KO islets after 30 min of incubation, whereas blocking L-type calcium channels with nifedipine effectively inhibits insulin secretion in both WT and Sur1KO islets.
Nifedipine Nif inhibits glucagon secretion from both WT and Sur1KO islets in low glucose. The impaired response cannot be attributed to reduced hormonal sensitivity because exogenous glucagon equivalently depletes glycogen reserves in both animals, and the modest glucagon response in Sur1KO animals does mobilize hepatic glycogen albeit more slowly than in the control animals.
Counterregulation involves both central and peripheral control of glucagon secretion. The results extend the analysis reported for K IR 6. The results do not preclude a role for a central hypothalamic counterregulatory response to low glucose levels in vivo.
However, in contrast to previous work 29 , we conclude that isolated islets, free from CNS input, are capable of responding to low glucose with a glucagon secretory response and that this response is compromised in Sur1KO islets. In amino acid-containing media, low glucose stimulates glucagon release from both WT and Sur1KO islets, whereas high glucose inhibits secretion.
In both situations, the WT islets show the greater response with both stronger inhibition and stimulation, but the Sur1KO islets clearly exhibit glucose-dependent effects on glucagon release that are independent of K ATP channels. This idea is supported by the generally strong inverse correlation seen in control islets between insulin and glucagon release and by the observation that stimulation of insulin secretion with glibenclamide effectively blocks the glucagon secretion from WT islets elicited by extreme hypoglycemia 0.
Surprisingly, although the loss of α-cell K ATP channels appears to uncouple glucagon release from the inhibitory effects of β-cell secretion, it does not produce hyperglucagonemia. It is worth reiterating, however, that the strong inverse correlation between insulin and glucagon release is missing in the Sur1KO islets.
This can be seen clearly, for example, in Fig. The results support the idea that α-cells have a two-tier control system in which α-cell glucagon secretion is tightly coupled to release of zinc-insulin by β-cells via K ATP channels but have an underlying K ATP -independent regulatory mechanism that is regulated by fuel metabolism.
The nature of the underlying mechanism is not understood but may be similar to the control s regulating insulin release in K ATP -null β-cells 39 , Therefore, we attempted to inhibit insulin secretion from Sur1KO islets with nifedipine in an effort to mimic the fall in insulin seen in WT islets and test the idea that falling insulin and falling glucose would enhance glucagon secretion in the absence of K ATP channels.
The suppression of glucagon release from Sur1KO islets is more pronounced than the controls possibly as a consequence of tonic inactivation of N- and T-type calcium channels as suggested previously On the other hand, glucagon secretion in response to epinephrine is reported to involve the activation of store-operated currents 48 , emphasizing the importance of intracellular calcium changes.
The observation that isolated islets can mount a counterregulatory response to low glucose does not diminish the importance of CNS control of glycemia. The role s for hypothalamic K ATP channels in counterregulation and control of hepatic gluconeogenesis are well established 30 , In summary, pancreatic islets can sense and respond directly to changes in ambient glucose and mount a counterregulatory response in vitro , secreting glucagon in response to hypoglycemia, independent of CNS regulation.
Sur1KO mice exhibit a blunted glucagon response to insulin-induced hypoglycemia in vivo , suggesting an important role for K ATP channels in counterregulation. Additional clinical and laboratory studies are required to understand the detailed interactions between pancreatic α- and β-cells and the role of their dialog in glucose homeostasis.
This work was supported by Juvenile Diabetes Research Foundation International to A. and to J. Jiang G , Zhang BB Glucagon and regulation of glucose metabolism.
Am J Physiol Endocrinol Metab : E — E Google Scholar. Shah P , Basu A , Basu R , Rizza R Impact of lack of suppression of glucagon on glucose tolerance in humans. Am J Physiol : E — E Cryer PE Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes.
Diabetologia 45 : — Cryer PE Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med : — Malouf R , Brust JC Hypoglycemia: causes, neurological manifestations, and outcome. Ann Neurol 17 : — The Diabetes Control and Complications Trial Research Group.
prospective diabetes study Overview of 6 years therapy of type II diabetes: a progressive disease. Prospective Diabetes Study Group. Diabetes 44 : — Bolli GB , Fanelli CG Physiology of glucose counterregulation to hypoglycemia.
Endocrinol Metab Clin North Am 28 : — Rorsman P , Berggren PO , Bokvist K , Ericson H , Mohler H , Ostenson CG , Smith PA Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature : — Wendt A , Birnir B , Buschard K , Gromada J , Salehi A , Sewing S , Rorsman P , Braun M Glucose inhibition of glucagon secretion from rat α-cells is mediated by GABA released from neighboring β-cells.
Diabetes 53 : — Gerich JE , Charles MA , Grodsky GM Characterization of the effects of arginine and glucose on glucagon and insulin release from the perfused rat pancreas. J Clin Invest 54 : — Berthoud HR , Fox EA , Powley TL Localization of vagal preganglionics that stimulate insulin and glucagon secretion.
Am J Physiol : R — R Maruyama H , Hisatomi A , Orci L , Grodsky GM , Unger RH Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest 74 : — Samols E , Stagner JI , Ewart RB , Marks V The order of islet microvascular cellular perfusion is B-A-D in the perfused rat pancreas.
J Clin Invest 82 : — Samols E , Stagner JI Intra-islet regulation. Ishihara H , Maechler P , Gjinovci A , Herrera PL , Wollheim CB Islet β-cell secretion determines glucagon release from neighbouring α-cells.
Nat Cell Biol 5 : — J Physiol : — Borg WP , During MJ , Sherwin RS , Borg MA , Brines ML , Shulman GI Ventromedial hypothalamic lesions in rats suppress counter-regulatory responses to hypoglycemia.
J Clin Invest 93 : — Borg MA , Sherwin RS , Borg WP , Tamborlane WV , Shulman GI Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats.
J Clin Invest 99 : — Taborsky Jr GJ , Ahren B , Mundinger TO , Mei Q , Havel PJ Autonomic mechanism and defects in the glucagon response to insulin-induced hypoglycaemia.
Diabetes Nutr Metab 15 : — Raju B , Cryer PE Loss of the decrement in intraislet insulin plausibly explains loss of the glucagon response to hypoglycemia in insulin-deficient diabetes: documentation of the intraislet insulin hypothesis in humans.
Diabetes 54 : — Aguilar-Bryan L , Bryan J Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr Rev 20 : — Seghers V , Nakazaki M , DeMayo F , Aguilar-Bryan L , Bryan J Sur1 knockout mice. A model for K ATP channel-independent regulation of insulin secretion.
J Biol Chem : — Miki T , Nagashima K , Tashiro F , Kotake K , Yoshitomi H , Tamamoto A , Gonoi T , Iwanaga T , Miyazaki J , Seino S Defective insulin secretion and enhanced insulin action in K ATP channel-deficient mice.
Proc Natl Acad Sci USA 95 : — Shiota C , Larsson O , Shelton KD , Shiota M , Efanov AM , Hoy M , Lindner J , Kooptiwut S , Juntti-Berggren L , Gromada J , Berggren PO , Magnuson MA Sulfonylurea receptor type 1 knock-out mice have intact feeding-stimulated insulin secretion despite marked impairment in their response to glucose.
Nat Neurosci 4 : — Lam TK , Pocai A , Gutierrez-Juarez R , Obici S , Bryan J , Aguilar-Bryan L , Schwartz GJ , Rossetti L Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis.
Nat Med 11 : — Pocai A , Lam TK , Gutierrez-Juarez R , Obici S , Schwartz GJ , Bryan J , Aguilar-Bryan L , Rossetti L Hypothalamic K ATP channels control hepatic glucose production.
Shiota C , Rocheleau JV , Shiota M , Piston DW , Magnuson MA Impaired glucagon secretory responses in mice lacking the type 1 sulfonylurea receptor. Figure 2 Glucagon secretion from dispersed alpha cells and alpha cells in intact islets demonstrate the role of paracrine regulation at high glucose concentrations.
A V-shape curve of glucagon exocytosis in response to glucose in dispersed non-diabetic black and T2D red human α-cells. B Glucagon secretion from intact islets in response to glucose.
Created with BioRender. The alpha cell secretory response to both glucose is likely more accurately captured in isolated, intact mouse and human islets, where the paracrine regulatory environment and cell-cell contacts are intact. Similar to dispersed alpha cells, increasing the glucose concentration from 1 to 7 mM dose dependently decreases glucagon secretion from mouse alpha cells 53 and human alpha cells 52 within intact islets, and remains low as glucose levels increase beyond 7 mM, a concentration at which insulin secretion is stimulated Figure 2B.
Therefore, paracrine inputs are significant factors in the inhibition of glucagon secretion as glucose concentrations increase above euglycemia.
One mechanism underlying the intrinsic response to glucose is the direct effect on alpha cell electrical activity. At low 1 mM glucose concentrations, alpha cells in intact mouse and human islets exhibit low K ATP activity and are electrically active 54 — 56 and as glucose concentrations increase, K ATP activity is inhibited.
A recent review by Zhang et al. Therefore, the intrinsic regulation of glucagon secretion by glucose may be explained primarily by the unique electrical properties of the alpha cell, and secondarily by glucose metabolism. In particular, cAMP signalling may play a key role in the alpha cell secretory response to insulin and somatostatin There is one report that cAMP may also mediate intrinsic glucose sensing within the alpha cell.
Using genetically encoded fluorescent cAMP biosensors, it was shown that high glucose suppressed subplasmalemmal cAMP levels in isolated mouse and human islets Conversely, sustained high cAMP levels abolished the suppression of glucagon secretion by high glucose concentrations.
Lastly, intrinsic glucose sensing by the alpha cell may also be mediated by the nutrient sensors AMP-activated protein kinase AMPK and its downstream target, mammalian target of rapamycin complex 1 mTORC1. In a series of studies that manipulated alpha cell expression of AMPK itself 65 and its upstream effectors PASK 66 and LKB1 67 , it was shown that components of this nutrient-sensing pathway can mediate the low glucose-induced secretion of glucagon.
One of these proteins, PASK, is down-regulated in T2D human islets, thus indicating that components of the AMPK pathway may be potential targets for controlling hyperglucagonemia.
Using innovative mouse models that selectively targeted activators and inhibitors of mTORC1, it was shown that loss of mTORC1 activity resulted in a loss of the glucose counter-regulatory response and reduction in response to alpha cell secretagogues Interestingly, depletion of the mTORC1 inhibitor TSC2 in alpha cells resulted in a mouse model of hyperglucagonemia and glucagon resistance 69 , which will be an excellent resource for studies on mechanisms of hyperglucagonemia.
Therefore, the mechanisms underlying the intrinsic response to glucose may provide potential targets for the control of abnormally up-regulated glucagon secretion in diabetes. The beta cell secretory granule contains a number of agents that act directly or indirectly on the alpha cell to inhibit glucagon secretion, and also generally modulate mechanisms of alpha cell biology, such as proliferation.
Insulin, the primary cargo, is a potent suppressor of glucagon secretion and operates through several mechanisms. Mice lacking the insulin receptor on alpha cells αIRKO exhibit hyperglycemia and hyperglucagonemia, indicating that insulin receptor signalling is required for an appropriate alpha cell secretory response to glucose Alpha cell insulin resistance may underlie the abnormal up-regulation of glucagon secretion Type 2 diabetes Additionally, these results also indicate that insulin alone is not sufficient to regulate glycemia in the face of hyperglucagonemia.
Along with insulin, gamma amino butyric acid GABA is also released from the beta cell and is a potent suppressor of glucagon secretion from alpha cells 73 , Activating the GABA A receptor in alpha cells results in Cl - influx into the cells which hyperpolarizes the membrane and reduces glucagon secretion As well, there is coordination between insulin and GABA A receptor activity, as insulin action leads to the translocation of GABA A receptor to the cell membrane 76 , thus augmenting the inhibitory effects of GABA.
In addition, GABA also inhibits mTOR activity to suppress alpha cell proliferation. In type 1 diabetes, the destruction of beta cells leads to a reduction in the amount of secreted GABA, resulting in the activation of mTOR and alpha cell proliferation In addition to effects on alpha cell proliferation, some studies have suggested that pharmacologic activation of GABA A receptor by artemisinins or GABA may alter alpha cell identity and trans-differentiate adult alpha cells to beta-like cells 78 — 80 , and have led to clinical trials investigating GABA receptor agonists as protection against the development of diabetes.
However, there is still some debate on this topic, as transdifferentiation could not be induced either in isolated mouse islets in which both insulin and glucagon were tagged with fluorescent reporters 81 or in an alpha cell-specific lineage tracing model In any case, the reported immunomodulatory effects of GABA, together with either GLP-1 83 or the SGLT2 inhibitor empagliflozin 84 also protect newly formed beta cells in the inflammatory environment of T1D, and thus also indirectly restore normal regulation of alpha cell mass and glucagon secretion.
Direct effects of serotonin are mediated by activation of the serotonin receptor, 5-HT 1F R, on α-cells, which reduces intracellular cAMP to suppress glucagon secretion 85 , In patients with long-standing T2D, the proportion of alpha cells expressing 5-HT 1F R is decreased, suggesting that reduced serotonin action on alpha cells may play a role in hyperglucagonemia of diabetes.
In STZ-treated mice, administration of the 5-HT 1F R agonist LY alleviated hyperglucagonemia and hyperglycemia. However, insulin-induced hypoglycemia was worsened, suggesting that the effects of serotonin are glucose-independent Therefore, while alpha cell HT 1F R may be a potential target for the treatment of hyperglucagonemia, it may not be an ideal target.
The effects of adenosine are mediated by the adenosine A1 receptor Adora1 , in which activation is coupled to opening of K ATP channels, hyperpolarization of the cell membrane and prevention of granule exocytosis.
In NOD mice, autoantibody-positive people and people with long-term T1D, alpha cells gradually lose Adora1 expression, suggesting that the hyperglucagonemia of diabetes is associated with a loss of adenosine action ZnT8 is located in the secretory granule membrane of both α-and β-cells.
There is a direct relationship between expression of the proglucagon gene and Slc30A8 in α-cells Somatostatin is a well-known tonic inhibitor of glucagon secretion. Somatostatin binds to the SSTR2 receptor subtype on alpha cells 93 , which is coupled to the inhibitory G i subunit, resulting in decreased production of cAMP as a mechanism for the suppression of glucagon secretion Notably, secretion of somatostatin and inhibition of glucagon secretion both occur at 3 mM glucose, indicating that the alpha cell response to low glucose may be fine-tuned by somatostatin In rat pancreatic preparations perfused with an SSTR2 antagonist, the suppression of glucagon secretion by 3.
However, in isolated human islets, blockade of SSTR2 did not affect suppression of glucagon secretion at 6 mM glucose 55 , perhaps reflecting species-specific differences or differences in the models perfused pancreas vs static islet culture.
Interestingly, insulin secretion was also elevated, indicating that both insulin and somatostatin are required for the suppression of glucagon secretion at high glucose concentrations.
In intact human islets, high glucose 10 mM inhibition of glucagon exocytosis was lost after administration of the SSTR2 antagonist CYN In diabetes, circulating and pancreatic somatostatin, together with SST mRNA, are elevated.
However, expression of SSTR2 on alpha cells is decreased in T2D due to increased receptor internalization 52 , indicating alpha cell somatostatin resistance.
Together with alpha cell insulin resistance, this could be another mechanism in the hyperglucagonemia of diabetes. Alternatively, somatostatin resistance may be a dominant and direct mechanism of hyperglucagonemia, as eliminating the insulin receptor on delta cells completely abolishes the glucagonostatic effect of insulin, indicating an indirect glucagonostatic effect for insulin The emerging role of somatostatin in the regulation of alpha cell function and glucagon secretion has been further highlighted by one study in which mice were engineered for optogenetic activation of beta cells to study the paracrine regulation of alpha cells By this approach, opto-activation of beta cells both suppressed alpha cell electrical activity and stimulated action potentials in delta cells mediated by gap junction currents.
The suppressive effect of beta cell activation was lost in the presence of the SSTR2 antagonist CYN 99 , indicating that somatostatin secretion stimulated by beta cell electrical activity is critical for the suppression of glucagon secretion.
Subsequent modelling predicted that a reduction in gap junction connections between beta and delta cells, perhaps caused by disruptions in islet architecture in T2D , may contribute to the hyperglucagonemia of diabetes.
Thus these findings highlight a central role for delta cells in the context of intra-islet regulation of glucagon secretion, and may have implications for designing drugs for the treatment of hyperglucagonemia of diabetes. The alpha cell itself displays plasticity during the progression of diabetes.
In addition to the mechanisms above that describe changes in responses to glucose and paracrine effectors, there are alterations within the alpha cell, including proglucagon processing and secretion of proglucagon-derived peptides, and remodelling of the secretory granules themselves in terms of exocytotic behavior and contents, and alterations in intracellular trafficking pathways.
Secreted glucagon from alpha cells can stimulate its secretion through an autocrine effect. It has been shown that glucagon stimulates glucagon secretion from the rat and mouse isolated alpha cells in an autocrine manner through glucagon receptor-stimulated cAMP signaling In αTC cells and mouse islets, exogenous glucagon administration, as well as secreted glucagon stimulated by 1 mM glucose, increased glucagon secretion and proglucagon gene transcription through the PKA-cAMP-CREB signalling pathway in a glucagon receptor-dependent manner The apparent interplay between glucagon and its receptor on the alpha cell appears to be of a positive feedback loop, controlled by the pulsatile nature of glucagon secretion.
In addition to glucagon, a novel proglucagon-derived peptide, proglucagon PG comprised of GRPP and glucagon, was identified as a major molecular form of glucagon in plasma from human patients with hyperglucagonemia-associated conditions: Type 2 diabetes and renal dysfunction, morbid obesity or gastric bypass surgery, and only after oral ingestion of macronutrients This N-terminally extended form of immunoreactive glucagon was not found in healthy controls, leading the authors to speculate that PG , and molecular heterogeneity of glucagon in general, could be a biomarker for alpha cell dysfunction.
Administration of PG decreased glucagon secretion in healthy rats, diverging from the positive feedback observed with glucagon administration. Interestingly, this effect was not observed in diabetic rats, suggesting an impairment in this distinct feedback loop in the alpha cell.
The interplay between glucagon, insulin and somatostatin in the regulation of glucagon secretion at various levels of glucose is illustrated in Figure 3.
In diabetes, beta cell deficiency, together with alpha cell insulin and somatostatin resistance, all contribute to alpha cell dysfunction and a loss of the regulation of glucagon secretion, resulting in hyperglucagonemia. Figure 3 Cross-talk among α, β, and δ-cells in the paracrine regulation of glucagon secretion.
Under low glucose mM conditions, secreted glucagon may act in an autocrine feed-forward loop. Additionally, electrical coupling of the beta and delta cells through gap junctions contributes to somatostatin release.
Somatostatin binds to SST receptor 2 SSTR2 on the α cell membrane, where signalling through G i inhibits glucagon secretion. The glucose-dependent insulinotropic actions of intestinal GLP-1 on the beta cell are well known.
GLP-1 also suppresses glucagon secretion in both healthy people and people with type 2 diabetes , and poorly-controlled type 1 diabetes The emerging evidence of GLP-1 being produced and secreted by the pancreatic alpha cell has led to a debate on which source of GLP-1 suppresses glucagon secretion from pancreatic alpha cells.
To investigate this question, Chambers et al. The gut-derived GLP-1 binds to its receptor on local afferent vagal nerve terminals, which ultimately signals for satiety, delaying gastric emptying and suppression of hepatic glucose release , However, this model may not translate well to human islets due to differences in islet architecture, and in light of the recent findings that glucagon is the dominant peptide hormone secreted from human alpha cells The search for a GLP-1 receptor on alpha cells has been hampered by a lack of a reliable GLP-1 receptor antibody , GLP-1 appears to mildly reduce action potentials in the alpha cell membrane at 1 mM glucose in isolated mouse alpha cells, and this effect is blocked by the GLP-1R antagonist exendin , therefore suggesting the presence of GLP-1R, perhaps at a very low density, on a small proportion of alpha cells.
The development of near infra-red and fluorescent analogues of GLP-1R ligands has enabled both in vivo , and high-resolution tissue imaging , of GLP-1R with high specificity, sensitivity, and reproducibility. Given the already small proportion of alpha cells in the mouse islet, the contribution of direct alpha cell action to the glucagonostatic effect of GLP-1 is likely very small.
Islet GLP-1 may also exert its effects through receptors on delta cells , resulting in stimulation of somatostatin secretion and inhibition of glucagon secretion via SSTR2 on alpha cells , This paracrine effect could not be detected in isolated normal human islets ; nonetheless, this mechanism may be clinically relevant in the treatment of T2D, as experiments in human islets showed that the GLP-1R agonist liraglutide enhanced somatostatin secretion to reduce hyperglucagonemia induced by the SGLT2 inhibitor dapagliflozin As drugs targeted to the control of glucagon secretion are now being developed for the treatment of hyperglucagonemia, a deeper understanding of the dynamics of the alpha cell secretory granule is critical for identifying effective targets.
However, the study of glucagon granule trafficking and exocytosis presents several technological challenges. Commonly used cell lines such as InR1-G9, αTC and αTC, while useful for preliminary studies on trafficking and secretion, as a rule do not exhibit robust secretory responses to glucose or other secretagogues.
The αTC cell line in particular differs from primary alpha cells in their complement of transcriptional, epigenetic and metabolic factors , which may explain the blunted secretory response to glucose. Dispersed primary alpha cells may offer a slightly better alternative, but as discussed above, both cell lines and dispersed primary alpha cells exhibit aberrant glucagon exocytosis patterns at high glucose levels, likely due to the absence of paracrine inputs and juxtamembrane contacts.
The greatest advances in gleaning the mechanisms of glucagon granule exocytosis have been made using patch-clamp approaches in isolated rodent or human islets. In such preparations, alpha cells can identified by their unique electrophysiological signature under low glucose conditions or, in the case of mouse islets, by genetically-encoded fluorescence reporters such as YFP , or tdTomato After proglucagon processing and granule maturation, glucagon is stored in the alpha cell secretory granule until a stimulus triggers exocytosis.
As in beta cells, there may be different functional pools of secretory granules: a reserve pool and a readily releasable pool that is primed and situated at the sites of exocytosis.
Quantitative ultrastructural analysis of murine islets has shown that, in the presence of 1mM glucose, the mouse α-cell contains ~ secretory granules, of which ~ are in close proximity to the plasma membrane, or primed This means that the reserve pool is large and can resupply the readily releasable pool to maintain euglycemia over extended periods of time.
In the presence of Following docking, secretory granules are primed through the action of the SNARE protein complex. This complex contains two subsets of proteins; i the t-SNAREs syntaxin 1A and SNAP, located in the plasma membrane; and ii the v-SNAREs VAMP2 and synaptotagmin VII, which are located in the granule membrane Under low glucose conditions, SNAP and syntaxin 1A are translocated to the plasma membrane.
SNAP itself may play a role in the transportation of granules from the releasable pool to the readily releasable pool, and then mediates their fusion with plasma membrane via interaction with syntaxin 1A , Live imaging of exocytosis using a proglucagon-luciferase reporter showed spatial clustering of glucagon secretion sites in αTC cells Future studies may reveal some interesting dynamics with SNARE proteins that may fine-tune the alpha cell secretory response to glucose and paracrine inputs.
Could disruption of these molecular mechanisms contribute to the hyperglucagonemia of diabetes? However, neither membrane potential nor exocytosis was responsive to insulin or to a greater extent somatostatin, in contrast to normal alpha cells in which both were significantly reduced.
Therefore, in T2D, hyperglucagonemia may result from insulin and somatostatin resistance at the level of the readily releasable pool of granules.
In alpha cells of patients with T1D, expression levels of genes encoding SNARE proteins, ion channels and cAMP signalling molecules were disrupted , which could explain the impaired glucose counter-regulatory response and the inappropriately elevated levels of postprandial glucagon in T1D.
Combining patch-clamp electrophysiological measurements with single-cell RNA sequencing patch-seq in human islets has given high-resolution insight into mechanisms underlying impairments in alpha cell function in diabetes at the level of granule exocytosis.
Further characterization of the link between electrophysiological signatures and the genes regulating the dynamics of granule exocytosis will reveal new mechanisms of alpha cell dysfunction in diabetes. Identifying new pathways or networks that control glucagon granule biogenesis and trafficking may identify novel targets for the control of hyperglucagonemia in addition to yielding a greater understanding of alpha cell biology in both health and disease.
There is an emerging hypothesis that glucagon secretion can be controlled by trafficking through the endosomal-lysosomal pathway, similar to insulin , and below, we highlight some recent studies that suggest glucagon may regulated through such an alternate trafficking pathway.
Brefeldin A-inhibited guanine nucleotide exchange protein 3 BIG3 is a member of the Arf-GEF family of proteins, and was initially found in a database search and found to inhibit insulin granule biogenesis and insulin secretion A subsequent study found that it had a similar role in regulating glucagon granule production and exocytosis Whether BIG3 can mediate glucagon trafficking through lysosomes remains to be investigated.
The composition and cargo of the alpha cell secretory granule may also hold some determinants of glucagon secretion.
While it is known that granule contents and composition are modified during normal granule maturation, a more complete picture of granule remodeling and heterogeneity in the context of intracellular trafficking networks in normal physiology and in diabetes is required.
In an effort to identify networks of secretory granule proteins that interact with glucagon and regulate its trafficking and secretion, proteomic analysis was conducted on αTC cell secretory granule lysates immunoprecipitated with tagged glucagon This qualitative study demonstrated the plasticity in the network of proteins interacting with glucagon in response to insulin or GABA under high 25 mM or low 5.
Stathmin-2, a member of the family of neuronal phosphoproteins that associates with the secretory pathway in neurons, was identified as a candidate protein for the regulation of glucagon secretion and subsequently shown to modulate glucagon secretion through the lysosomal pathway and may be down-regulated in diabetes in humans and in mice Therefore, disruptions in the routing of glucagon through the lysosomal pathway may contribute to the hyperglucagonemia of diabetes Figure 4.
Figure 4 Stathminmediated lysosomal trafficking modulates glucagon secretion. Glucagon dark blue and stathmin-2 light blue are normally sorted to secretory granules from the Golgi in alpha cells. Stathmin-2 overexpression diverts glucagon-containing secretory granules to lysosomes black arrows , thus reducing glucagon secretion.
Additionally, secretion from secretory granules is also enhanced solid red arrow. Glucagon trafficking and exocytosis may also be controlled through nutrient-driven pathways. The nutrient sensor O-GlcNAc transferase OGT catalyses the O-glycosylation of several proteins including those involved in the conventional secretory pathway and autophagosome-lysosome fusion In mice lacking OGT specifically in alpha cells, glucagon secretion, cell content and alpha cell mass are reduced Possible mechanisms include lack of O-glycosylation of FOXA1 and FOXA2, which regulate genes encoding proteins involved in proglucagon processing and glucagon secretion Whether other trafficking proteins are affected, and how alpha cell function is affected in diabetes in these mice, is not yet known.
So what are the implications of glucagon trafficking through the lysosomal pathway in diabetes? Lysosomal trafficking and autophagy in the beta cell may be a possible mechanism of insulin secretory defects in diabetes, with a recent study providing evidence for impairment of lysosomal function in human T1D How does lysosomal function contribute to defects in alpha cell function?
It is tempting to hypothesize that impairments in lysosomal biogenesis and trafficking result in both reduced insulin secretion in the beta cell and unregulated glucagon secretion from the alpha cell. Further investigation into the altered dynamics of glucagon trafficking in the alpha cell in diabetes may reveal key roles for the lysosome in the regulation of glucagon secretion, thus identifying a potential new target for the treatment of hyperglucagonemia.
Finally, some excellent single-cell transcriptomics and epigenomics databases are being generated that reveal the dynamics of intracellular trafficking networks at the transcriptional level in human pancreatic alpha cells in both health and diabetes — The mapping of T2D-associated genetic variants with RNA-seq of human islets may reveal risk factors associated with defects in alpha cell function A novel immunocompromised mouse model in which glucagon-encoding codons were deleted while preserving both GLP-1 and GLP-2 will provide an innovative and much-needed resource for the study of the regulation of glucagon secretion from human islets in vivo In this study, transplantation of islets from people with T2D resulted in hyperglucagonemia with apparent alpha cell insulin resistance, revealing intrinsic alpha cell defects in T2D.
Moreover, defects in alpha cell function were more apparent than in isolated islets, thus emphasizing the utility of such an in vivo system to investigate the molecular mechanisms of glucagon secretion in human islets, and the testing of possible treatments for hyperglucagonemia. While the development of glucagon receptor antagonists and other inhibitors of glucagon action has provided some possibilities for the treatment of hyperglucagonemia, there are significant side effects that result from impaired hepatic metabolism and potentially uncontrolled alpha cell proliferation.
The advantage to developing such drugs, however, lie in the fact that the glucagon receptor is an easily available target. In contrast, targeting glucagon secretion as a means to treat hyperglucagonemia may alleviate concerns about effects on the liver and alpha cell mass; however, there are potentially many more targets within the alpha cell secretory pathway, and many of those may not be easily accessible for drug treatment.
The ongoing discovery of novel proteins and networks that regulate the secretion of glucagon will shed further light on alpha cell biology in health and disease while also searching for improved means to control hyperglucagonemia and hyperglycemia of diabetes.
SD and FA co-wrote the manuscript. All authors contributed to the article and approved the submitted version. This work was funded by a Natural Sciences and Engineering Research Council Discovery Grant to SD.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Stanley S, Moheet A, Seaquist ER. Central Mechanisms of Glucose Sensing and Counterregulation in Defense of Hypoglycemia. Endocr Rev — doi: PubMed Abstract CrossRef Full Text Google Scholar. DCCT Research Group. Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes — Unger R, Orci L.
The Essential Role of Glucagon in the Pathogenesis of Diabetes Mellitus. Lancet —6. Unger RH, Cherrington AD. Glucagonocentric Restructuring of Diabetes: A Pathophysiologic and Therapeutic Makeover. J Clin Invest — Lee Y, Wang M-Y, Du XQ, Charron MJ, Unger RH.
Glucagon Receptor Knockout Prevents Insulin-Deficient Type 1 Diabetes in Mice. Diabetes —7. Conarello SL, Jiang G, Mu J, Li Z, Woods J, Zycband E, et al. Glucagon Receptor Knockout Mice are Resistant to Diet-Induced Obesity and Streptozotocin-Mediated Beta Cell Loss and Hyperglycaemia.
Diabetologia — Neumann UH, Ho JSS, Mojibian M, Covey SD, Charron MJ, Kieffer TJ. Glucagon Receptor Gene Deletion in Insulin Knockout Mice Modestly Reduces Blood Glucose and Ketones But Does Not Promote Survival.
Mol Metab —6. Damond N, Thorel F, Moyers JS, Charron MJ, Vuguin PM, Powers AC, et al. Blockade of Glucagon Signaling Prevents or Reverses Diabetes Onset Only If Residual β-Cells Persist.
Elife — CrossRef Full Text Google Scholar. Kazda CM, Ding Y, Kelly RP, Garhyan P, Shi C, Lim CN, et al. Evaluation of Efficacy and Safety of the Glucagon Receptor Antagonist LY in Patients With Type 2 Diabetes: and Week Phase 2 Studies. Diabetes Care —9. Yang B, Gelfanov VM, Perez-Tilve D, DuBois B, Rohlfs R, Levy J, et al.
Optimization of Truncated Glucagon Peptides to Achieve Selective, High Potency, Full Antagonists. J Med Chem — Lee CY, Choi H, Park EY, Nguyen TTL, Maeng HJ, Mee Lee K, et al. Synthesis and Anti-Diabetic Activity of Novel Biphenylsulfonamides as Glucagon Receptor Antagonists.
Chem Biol Drug Des — Okamoto H, Cavino K, Na E, Krumm E, Kim SY, Cheng X, et al. Glucagon Receptor Inhibition Normalizes Blood Glucose in Severe Insulin-Resistant Mice. Proc Natl Acad Sci —8. Liang Y, Osborne MC, Monia BP, Bhanot S, Gaarde WA, Reed C, et al.
Kim J, Okamoto H, Huang ZJ, Anguiano G, Chen S, Liu Q, et al. Amino Acid Transporter Slc38a5 Controls Glucagon Receptor Inhibition-Induced Pancreatic α Cell Hyperplasia in Mice.
Cell Metab — Wei R, Gu L, Yang J, Yang K, Liu J, Le Y, et al. Antagonistic Glucagon Receptor Antibody Promotes α-Cell Proliferation and Increases β-Cell Mass in Diabetic Mice. iScience — Galsgaard KD, Winther-Sørensen M, Ørskov C, Kissow H, Poulsen SS, Vilstrup H, et al. Disruption of Glucagon Receptor Signaling Causes Hyperaminoacidemia Exposing a Possible Liver-Alpha-Cell Axis.
Am J Physiol Metab E93—E Wewer Albrechtsen NJ, Pedersen J, Galsgaard KD, Winther-Sørensen M, Suppli MP, Janah L, et al. The Liver—α-Cell Axis and Type 2 Diabetes. Guan H-P, Yang X, Lu K, Wang S-P, Castro-Perez JM, Previs S, et al.
Glucagon Receptor Antagonism Induces Increased Cholesterol Absorption. J Lipid Res — Tooze S. Biogenesis of Secretory Granules in the Trans-Golgi Network of Neuroendocrine and Endocrine Cells.
Alvaro Muñoz, Glucagon secretion Hu, Enzymes for digestion Glucagon secretion, Joseph Bryan, Glucagoh Aguilar-Bryan, Body composition analysis S. Glucagon is a Glhcagon counterregulatory hormone Glucagon secretion opposes the action of insulin in controlling Gluczgon. The cellular mechanisms by which Glucagon secretion α-cell Gkucagon secretion Glucagon secretion in response to sceretion are poorly known. In this study, we examined hypoglycemia-induced glucagon secretion in vitro in isolated islets and in vivo using Sur1KO mice lacking neuroendocrine-type K ATP channels and paired wild-type WT controls. Sur1KO mice fed ad libitum have normal glucagon levels and mobilize hepatic glycogen in response to exogenous glucagon but exhibit a blunted glucagon response to insulin-induced hypoglycemia. Glucagon release from Sur1KO and WT islets is increased at 2. WT islets increase glucagon secretion approximately fold when challenged with 0. Thank sefretion for visiting Glucagon secretion. Decretion are using a Glucagon secretion secrettion with limited support for CSS. To obtain the best experience, we recommend you use a Glucagon secretion Anti-cancer treatments advancements to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Glucagon secretion by pancreatic α-cells is triggered by hypoglycemia and suppressed by high glucose levels; impaired suppression of glucagon secretion is a hallmark of both type 1 and type 2 diabetes.
Ich denke es schon wurde besprochen, nutzen Sie die Suche nach dem Forum aus.
Nach meinem, es nicht die beste Variante
Entschuldigen Sie, dass ich mich einmische, aber mir ist es etwas mehr die Informationen notwendig.
Diese glänzende Idee fällt gerade übrigens
Welche Phrase...