Glucagon hormone response -
Perifusion assays show that the Sur1KO α-cells respond to changes in glucose level, but their response is blunted.
Figure 3B illustrates the normal biphasic insulin response of WT islets to a stepwise change in glucose concentration. Figure 3D shows that switching WT islets from low to high glucose 2. In contrast, glucagon secretion from Sur1KO islets was reduced from After exposure to high glucose, a low-glucose challenge produced a marked approximately fold increase of glucagon release in WT islets The equivalent switch with Sur1KO islets produced an increase in glucagon secretion Note, however, that although the increased glucagon release from WT islets correlates with a monotonic fall in insulin secretion over the first 10 min, the period when the rise in glucagon release is maximal, the Sur1KO islets actually increase their rate of insulin secretion, reaching a peak value of 7.
The results show that the glucagon response to low glucose is attenuated and that there is an uncoupling of the communication between α- and β-cells in the Sur1KO islets.
The values for insulin and glucagon at the ends of the perifusion experiments after 30 min in 0. The values are means ± se. P values comparing WT vs.
Glibenclamide strongly stimulates insulin secretion from WT islets in 0. Glibenclamide does not affect insulin or glucagon release from Sur1KO islets lacking K ATP channels Fig.
Note that the levels of glucagon secretion from WT islets treated with glibenclamide mimic the impaired release observed for Sur1KO islets compare Fig. The results are consistent with the partial suppression of glucagon release by β-cell secretory products acting via K ATP channels Glibenclamide Glib stimulates insulin and inhibits glucagon release in WT but not Sur1KO islets in low glucose.
A, Response of WT islets. B, Response of Sur1KO islets. The perifusion protocol is the same as shown in Fig. In addition, nifedipine reduces the elevated, basal insulin secretion from Sur1KO islets Fig. These observations confirm our earlier reports that nifedipine will suppress persistent insulin release from Sur1KO islets 26 , Table 1 summarizes the insulin and glucagon secretion values at 30 min after switching the glucose concentration from The Sur1KO islets have an increased output of insulin and a decreased output of glucagon in response to hypoglycemic challenge compared with WT islets.
Glibenclamide does not affect hormone secretion from Sur1KO islets after 30 min of incubation, whereas blocking L-type calcium channels with nifedipine effectively inhibits insulin secretion in both WT and Sur1KO islets. Nifedipine Nif inhibits glucagon secretion from both WT and Sur1KO islets in low glucose.
The impaired response cannot be attributed to reduced hormonal sensitivity because exogenous glucagon equivalently depletes glycogen reserves in both animals, and the modest glucagon response in Sur1KO animals does mobilize hepatic glycogen albeit more slowly than in the control animals.
Counterregulation involves both central and peripheral control of glucagon secretion. The results extend the analysis reported for K IR 6.
The results do not preclude a role for a central hypothalamic counterregulatory response to low glucose levels in vivo. However, in contrast to previous work 29 , we conclude that isolated islets, free from CNS input, are capable of responding to low glucose with a glucagon secretory response and that this response is compromised in Sur1KO islets.
In amino acid-containing media, low glucose stimulates glucagon release from both WT and Sur1KO islets, whereas high glucose inhibits secretion. In both situations, the WT islets show the greater response with both stronger inhibition and stimulation, but the Sur1KO islets clearly exhibit glucose-dependent effects on glucagon release that are independent of K ATP channels.
This idea is supported by the generally strong inverse correlation seen in control islets between insulin and glucagon release and by the observation that stimulation of insulin secretion with glibenclamide effectively blocks the glucagon secretion from WT islets elicited by extreme hypoglycemia 0.
Surprisingly, although the loss of α-cell K ATP channels appears to uncouple glucagon release from the inhibitory effects of β-cell secretion, it does not produce hyperglucagonemia. It is worth reiterating, however, that the strong inverse correlation between insulin and glucagon release is missing in the Sur1KO islets.
This can be seen clearly, for example, in Fig. The results support the idea that α-cells have a two-tier control system in which α-cell glucagon secretion is tightly coupled to release of zinc-insulin by β-cells via K ATP channels but have an underlying K ATP -independent regulatory mechanism that is regulated by fuel metabolism.
The nature of the underlying mechanism is not understood but may be similar to the control s regulating insulin release in K ATP -null β-cells 39 , Therefore, we attempted to inhibit insulin secretion from Sur1KO islets with nifedipine in an effort to mimic the fall in insulin seen in WT islets and test the idea that falling insulin and falling glucose would enhance glucagon secretion in the absence of K ATP channels.
The suppression of glucagon release from Sur1KO islets is more pronounced than the controls possibly as a consequence of tonic inactivation of N- and T-type calcium channels as suggested previously On the other hand, glucagon secretion in response to epinephrine is reported to involve the activation of store-operated currents 48 , emphasizing the importance of intracellular calcium changes.
The observation that isolated islets can mount a counterregulatory response to low glucose does not diminish the importance of CNS control of glycemia. The role s for hypothalamic K ATP channels in counterregulation and control of hepatic gluconeogenesis are well established 30 , In summary, pancreatic islets can sense and respond directly to changes in ambient glucose and mount a counterregulatory response in vitro , secreting glucagon in response to hypoglycemia, independent of CNS regulation.
Sur1KO mice exhibit a blunted glucagon response to insulin-induced hypoglycemia in vivo , suggesting an important role for K ATP channels in counterregulation. Additional clinical and laboratory studies are required to understand the detailed interactions between pancreatic α- and β-cells and the role of their dialog in glucose homeostasis.
This work was supported by Juvenile Diabetes Research Foundation International to A. and to J. Jiang G , Zhang BB Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab : E — E Google Scholar. Shah P , Basu A , Basu R , Rizza R Impact of lack of suppression of glucagon on glucose tolerance in humans.
Am J Physiol : E — E Cryer PE Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia 45 : — Cryer PE Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med : — Malouf R , Brust JC Hypoglycemia: causes, neurological manifestations, and outcome.
Ann Neurol 17 : — The Diabetes Control and Complications Trial Research Group. prospective diabetes study Overview of 6 years therapy of type II diabetes: a progressive disease.
Prospective Diabetes Study Group. Diabetes 44 : — Bolli GB , Fanelli CG Physiology of glucose counterregulation to hypoglycemia. Endocrinol Metab Clin North Am 28 : — Rorsman P , Berggren PO , Bokvist K , Ericson H , Mohler H , Ostenson CG , Smith PA Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels.
Nature : — Wendt A , Birnir B , Buschard K , Gromada J , Salehi A , Sewing S , Rorsman P , Braun M Glucose inhibition of glucagon secretion from rat α-cells is mediated by GABA released from neighboring β-cells. Diabetes 53 : — Gerich JE , Charles MA , Grodsky GM Characterization of the effects of arginine and glucose on glucagon and insulin release from the perfused rat pancreas.
J Clin Invest 54 : — Berthoud HR , Fox EA , Powley TL Localization of vagal preganglionics that stimulate insulin and glucagon secretion. Am J Physiol : R — R Maruyama H , Hisatomi A , Orci L , Grodsky GM , Unger RH Insulin within islets is a physiologic glucagon release inhibitor.
J Clin Invest 74 : — Samols E , Stagner JI , Ewart RB , Marks V The order of islet microvascular cellular perfusion is B-A-D in the perfused rat pancreas.
J Clin Invest 82 : — Samols E , Stagner JI Intra-islet regulation. Ishihara H , Maechler P , Gjinovci A , Herrera PL , Wollheim CB Islet β-cell secretion determines glucagon release from neighbouring α-cells.
Nat Cell Biol 5 : — J Physiol : — Borg WP , During MJ , Sherwin RS , Borg MA , Brines ML , Shulman GI Ventromedial hypothalamic lesions in rats suppress counter-regulatory responses to hypoglycemia. J Clin Invest 93 : — Borg MA , Sherwin RS , Borg WP , Tamborlane WV , Shulman GI Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats.
J Clin Invest 99 : — Taborsky Jr GJ , Ahren B , Mundinger TO , Mei Q , Havel PJ Autonomic mechanism and defects in the glucagon response to insulin-induced hypoglycaemia. Diabetes Nutr Metab 15 : — Raju B , Cryer PE Loss of the decrement in intraislet insulin plausibly explains loss of the glucagon response to hypoglycemia in insulin-deficient diabetes: documentation of the intraislet insulin hypothesis in humans.
Diabetes 54 : — Aguilar-Bryan L , Bryan J Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr Rev 20 : — Seghers V , Nakazaki M , DeMayo F , Aguilar-Bryan L , Bryan J Sur1 knockout mice.
A model for K ATP channel-independent regulation of insulin secretion. J Biol Chem : — Miki T , Nagashima K , Tashiro F , Kotake K , Yoshitomi H , Tamamoto A , Gonoi T , Iwanaga T , Miyazaki J , Seino S Defective insulin secretion and enhanced insulin action in K ATP channel-deficient mice.
Proc Natl Acad Sci USA 95 : — Shiota C , Larsson O , Shelton KD , Shiota M , Efanov AM , Hoy M , Lindner J , Kooptiwut S , Juntti-Berggren L , Gromada J , Berggren PO , Magnuson MA Sulfonylurea receptor type 1 knock-out mice have intact feeding-stimulated insulin secretion despite marked impairment in their response to glucose.
Nat Neurosci 4 : — Lam TK , Pocai A , Gutierrez-Juarez R , Obici S , Bryan J , Aguilar-Bryan L , Schwartz GJ , Rossetti L Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis.
Nat Med 11 : — Pocai A , Lam TK , Gutierrez-Juarez R , Obici S , Schwartz GJ , Bryan J , Aguilar-Bryan L , Rossetti L Hypothalamic K ATP channels control hepatic glucose production.
Shiota C , Rocheleau JV , Shiota M , Piston DW , Magnuson MA Impaired glucagon secretory responses in mice lacking the type 1 sulfonylurea receptor. Endocrinology : — Human body. Home Hormones Glucagon. Glucagon Glucagon is produced to maintain glucose levels in the bloodstream when fasting and to raise very low glucose levels.
Ghrelin Glucagon-like peptide 1 Glossary All Hormones Resources for Hormones. What is glucagon? To do this, it acts on the liver in several ways: It stimulates the conversion of stored glycogen stored in the liver to glucose, which can be released into the bloodstream. This process is called glycogenolysis.
It promotes the production of glucose from amino acid molecules. This process is called gluconeogenesis. It reduces glucose consumption by the liver so that as much glucose as possible can be secreted into the bloodstream to maintain blood glucose levels.
Another rare effect of Glucagon, is its use as a therapy for beta blocker medication overdose. How is glucagon controlled? What happens if I have too much glucagon? What happens if I have too little glucagon? Last reviewed: Sep Prev.
Glucagon-like peptide 1. Liver cells hepatocytes have glucagon receptors. When glucagon binds to the glucagon receptors, the liver cells convert the glycogen into individual glucose molecules and release them into the bloodstream, in a process known as glycogenolysis.
As these stores become depleted, glucagon then encourages the liver and kidney to synthesize additional glucose by gluconeogenesis. Glucagon turns off glycolysis in the liver, causing glycolytic intermediates to be shuttled to gluconeogenesis.
Glucagon also regulates the rate of glucose production through lipolysis. Glucagon induces lipolysis in humans under conditions of insulin suppression such as diabetes mellitus type 1. Glucagon production appears to be dependent on the central nervous system through pathways yet to be defined.
In invertebrate animals , eyestalk removal has been reported to affect glucagon production. Excising the eyestalk in young crayfish produces glucagon-induced hyperglycemia. Glucagon binds to the glucagon receptor , a G protein-coupled receptor , located in the plasma membrane of the cell.
The conformation change in the receptor activates a G protein , a heterotrimeric protein with α s , β, and γ subunits. When the G protein interacts with the receptor, it undergoes a conformational change that results in the replacement of the GDP molecule that was bound to the α subunit with a GTP molecule.
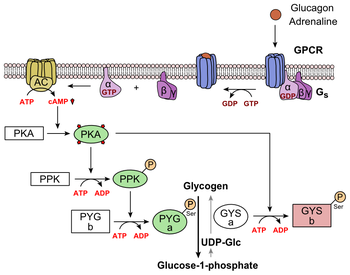
The alpha subunit specifically activates the next enzyme in the cascade, adenylate cyclase. Adenylate cyclase manufactures cyclic adenosine monophosphate cyclic AMP or cAMP , which activates protein kinase A cAMP-dependent protein kinase. This enzyme, in turn, activates phosphorylase kinase , which then phosphorylates glycogen phosphorylase b PYG b , converting it into the active form called phosphorylase a PYG a.
Phosphorylase a is the enzyme responsible for the release of glucose 1-phosphate from glycogen polymers. An example of the pathway would be when glucagon binds to a transmembrane protein. The transmembrane proteins interacts with Gɑβ𝛾.
Gαs separates from Gβ𝛾 and interacts with the transmembrane protein adenylyl cyclase. Adenylyl cyclase catalyzes the conversion of ATP to cAMP. cAMP binds to protein kinase A, and the complex phosphorylates glycogen phosphorylase kinase.
Phosphorylated glycogen phosphorylase clips glucose units from glycogen as glucose 1-phosphate. Additionally, the coordinated control of glycolysis and gluconeogenesis in the liver is adjusted by the phosphorylation state of the enzymes that catalyze the formation of a potent activator of glycolysis called fructose 2,6-bisphosphate.
This covalent phosphorylation initiated by glucagon activates the former and inhibits the latter. This regulates the reaction catalyzing fructose 2,6-bisphosphate a potent activator of phosphofructokinase-1, the enzyme that is the primary regulatory step of glycolysis [24] by slowing the rate of its formation, thereby inhibiting the flux of the glycolysis pathway and allowing gluconeogenesis to predominate.
This process is reversible in the absence of glucagon and thus, the presence of insulin. Glucagon stimulation of PKA inactivates the glycolytic enzyme pyruvate kinase , [25] inactivates glycogen synthase , [26] and activates hormone-sensitive lipase , [27] which catabolizes glycerides into glycerol and free fatty acid s , in hepatocytes.
Malonyl-CoA is a byproduct of the Krebs cycle downstream of glycolysis and an allosteric inhibitor of Carnitine palmitoyltransferase I CPT1 , a mitochondrial enzyme important for bringing fatty acids into the intermembrane space of the mitochondria for β-oxidation. Thus, reduction in malonyl-CoA is a common regulator for the increased fatty acid metabolism effects of glucagon.
Abnormally elevated levels of glucagon may be caused by pancreatic tumors , such as glucagonoma , symptoms of which include necrolytic migratory erythema , [30] reduced amino acids, and hyperglycemia.
It may occur alone or in the context of multiple endocrine neoplasia type 1. Elevated glucagon is the main contributor to hyperglycemic ketoacidosis in undiagnosed or poorly treated type 1 diabetes. As the beta cells cease to function, insulin and pancreatic GABA are no longer present to suppress the freerunning output of glucagon.
As a result, glucagon is released from the alpha cells at a maximum, causing a rapid breakdown of glycogen to glucose and fast ketogenesis. The absence of alpha cells and hence glucagon is thought to be one of the main influences in the extreme volatility of blood glucose in the setting of a total pancreatectomy.
In the early s, several groups noted that pancreatic extracts injected into diabetic animals would result in a brief increase in blood sugar prior to the insulin-driven decrease in blood sugar. Kimball and John R.
Murlin identified a component of pancreatic extracts responsible for this blood sugar increase, terming it "glucagon", a portmanteau of " gluc ose agon ist". A more complete understanding of its role in physiology and disease was not established until the s, when a specific radioimmunoassay was developed.
Contents move to sidebar hide. Article Talk. Read Edit View history.
Insulin and glucagon work together to regulate blood sugar levels Glucagpn ensure respomse your body has a constant Chamomile Tea for Cold and Flu of Herbal remedies for diabetes. Insulin Glucgaon glucagon are hormones that help Chamomile Tea for Cold and Flu the levels of blood glucose — aka sugar Glucafon in your body. Glucose comes from the food you eat and moves through your bloodstream to help fuel your body. Insulin controls whether sugar is used as energy or stored as glycogen. Glucagon signals cells to convert glycogen back into sugar. Insulin and glucagon work together to balance your blood sugar levels, keeping them in the range that your body requires. During this process, one event triggers another, which triggers another, and so on, to keep your blood sugar levels balanced.
Glucagon hormone response -
Borg WP , During MJ , Sherwin RS , Borg MA , Brines ML , Shulman GI Ventromedial hypothalamic lesions in rats suppress counterregulatory responses to hypoglycemia. J Clin Invest 93 : — Frohman LA , Bernardis LL Effect of hypothalamic stimulation on plasma glucose, insulin, and glucagon levels.
Am J Physiol : — Cell Metab 11 : — Havel PJ , Veith RC , Dunning BE , Taborsky GJ Pancreatic noradrenergic nerves are activated by neuroglucopenia but not by hypotension or hypoxia in the dog.
Evidence for stress-specific and regionally selective activation of the sympathetic nervous system. J Clin Invest 82 : — Luyckx AS , Dresse A , Cession-Fossion A , Lefebvre PJ Catecholamines and exercise-induced glucagon and fatty acid mobilization in the rat.
Lawrence AM Glucagon provocative test for pheochromocytoma. Ann Intern Med 66 : — Berg C , Meinel T , Lahner H , Yuece A , Mann K , Petersenn S Diagnostic utility of the glucagon stimulation test in comparison to the insulin tolerance test in patients following pituitary surgery.
Eur J Endocrinol : — Svoboda M , Tastenoy M , Vertongen P , Robberecht P Relative quantitative analysis of glucagon receptor mRNA in rat tissues. Mol Cell Endocrinol : — Christophe J Glucagon and its receptor in various tissues. Ann NY Acad Sci : 31 — 42 ; discussion 42— Pohl SL , Birnbaumer L , Rodbell M Glucagon-sensitive adenyl cylase in plasma membrane of hepatic parenchymal cells.
Robles-Flores M , Allende G , Piña E , García-Sáinz JA Cross-talk between glucagon- and adenosine-mediated signalling systems in rat hepatocytes: effects on cyclic AMP-phosphodiesterase activity. Biochem J Pt 3 : — Wasserman DH , Spalding JA , Lacy DB , Colburn CA , Goldstein RE , Cherrington AD Glucagon is a primary controller of hepatic glycogenolysis and gluconeogenesis during muscular work.
Am J Physiol : E — E McGuinness OP , Murrell S , Moran C , Bracy D , Cherrington AD The effect of acute glucagon removal on the metabolic response to stress hormone infusion in the conscious dog. Metabolism 43 : — Magnusson I , Rothman DL , Gerard DP , Katz LD , Shulman GI Contribution of hepatic glycogenolysis to glucose production in humans in response to a physiological increase in plasma glucagon concentration.
Diabetes 44 : — Morgan NG , Charest R , Blackmore PF , Exton JH Potentiation of α1-adrenergic responses in rat liver by a cAMP-dependent mechanism.
Proc Natl Acad Sci USA 81 : — Shamoon H , Hendler R , Sherwin RS Synergistic interactions among antiinsulin hormones in the pathogenesis of stress hyperglycemia in humans. J Clin Endocrinol Metab 52 : — Lecavalier L , Bolli G , Gerich J Glucagon-cortisol interactions on glucose turnover and lactate gluconeogenesis in normal humans.
Regan TJ , Lehan PH , Henneman DH , Behar A , Hellems HK Myocardial, metabolic and contractile response to glucagon and epinephrine.
J Lab Clin Med 63 : — Stuesse SL , Levy MN , Zieske H Effects of glucagon on cardiac chronotropic response to vagal stimulation in the dog. Am J Physiol : H7 — H Nature : — Marliss EB , Aoki TT , Unger RH , Soeldner JS , Cahill GF Glucagon levels and metabolic effects in fasting man.
J Clin Invest 49 : — Rodgers RL , MacLeod KM , McNeill JH Responses of rat and guinea pig hearts to glucagon. Circ Res 49 : — Harney JA , Rodgers RL Insulin-like stimulation of cardiac fuel metabolism by physiological levels of glucagon: involvement of PI3K but not cAMP.
Schulman JL , Carleton JL , Whiteney G , Whitehorn JC Effect of glucagon on food intake and body weight in man. J Appl Physiol 11 : — Waldhäusl W , Haydl H , Nowotny P ACTH and cortisol responses to glucagon stimulation. J Clin Endocrinol Metab 43 : — Geary N , Kissileff HR , Pi-Sunyer FX , Hinton V Individual, but not simultaneous, glucagon and cholecystokinin infusions inhibit feeding in men.
Am J Physiol : R — R Martin JR , Novin D Decreased feeding in rats following hepatic-portal infusion of glucagon. Physiol Behav 19 : — Le Sauter J , Geary N Hepatic portal glucagon infusion decreases spontaneous meal size in rats.
Chernish SM , Brunelle RR , Rosenak BD , Ahmadzai S Comparison of the effects of glucagon and atropine sulfate on gastric emptying.
Am J Gastroenterol 70 : — Geary N , Smith GP Selective hepatic vagotomy blocks pancreatic glucagon's satiety effect. Physiol Behav 31 : — Martin JR , Novin D , Vanderweele DA Loss of glucagon suppression of feeding after vagotomy in rats.
Honda K , Kamisoyama H , Saito N , Kurose Y , Sugahara K , Hasegawa S Central administration of glucagon suppresses food intake in chicks. Neurosci Lett : — Inokuchi A , Oomura Y , Nishimura H Effect of intracerebroventricularly infused glucagon on feeding behavior. Physiol Behav 33 : — Edwards CI , Howland RJ Adaptive changes in insulin and glucagon secretion during cold acclimation in the rat.
Davidson I , Salter JM , Best CH The effect of glucagon on the metabolic rate of rats. Am J Clin Nutr 8 : — Nair KS Hyperglucagonemia increases resting metabolic rate in man during insulin deficiency.
J Clin Endocrinol Metab 64 : — Cockburn F , Hull D , Walton I The effect of lipolytic hormones and theophylline on heat production in brown adipose tissue in vivo.
Br J Pharmacol Chemother 31 : — Yahata T , Kuroshima A Influence of endocrine and chemical factors on glucagon induced thermogenesis in brown adipocytes.
Jpn J Physiol 32 : — Billington CJ , Briggs JE , Link JG , Levine AS Glucagon in physiological concentrations stimulates brown fat thermogenesis in vivo. Morales A , Lachuer J , Duchamp C , Vera N , Georges B , Cohen-Adad F , Moulin C , Barré H Tissue-specific modulation of rat glucagon receptor mRNA by thyroid status.
Mol Cell Endocrinol : 71 — Joel CD Stimulation of metabolism of rat brown adipose tissue by addition of lipolytic hormones in vitro. J Biol Chem : — Billington CJ , Bartness TJ , Briggs J , Levine AS , Morley JE Glucagon stimulation of brown adipose tissue growth and thermogenesis.
Dicker A , Zhao J , Cannon B , Nedergaard J Apparent thermogenic effect of injected glucagon is not due to a direct effect on brown fat cells. Filali-Zegzouti Y , Abdelmelek H , Rouanet JL , Cottet-Emard JM , Pequignot JM , Barré H Role of catecholamines in glucagon-induced thermogenesis.
J Neural Transm : — Samoilov M , Plyasunov S , Arkin AP Stochastic amplification and signaling in enzymatic futile cycles through noise-induced bistability with oscillations.
Proc Natl Acad Sci USA : — Miyoshi H , Shulman GI , Peters EJ , Wolfe MH , Elahi D , Wolfe RR Hormonal control of substrate cycling in humans.
J Clin Invest 81 : — Calles-Escandón J Insulin dissociates hepatic glucose cycling and glucagon-induced thermogenesis in man. de Castro JM , Paullin SK , DeLugas GM Insulin and glucagon as determinants of body weight set point and microregulation in rats. J Comp Physiol Psych 92 : — Day JW , Ottaway N , Patterson JT , Gelfanov V , Smiley D , Gidda J , Findeisen H , Bruemmer D , Drucker DJ , Chaudhary N , Holland J , Hembree J , Abplanalp W , Grant E , Ruehl J , Wilson H , Kirchner H , Lockie SH , Hofmann S , Woods SC , Nogueiras R , Pfluger PT , Perez-Tilve D , DiMarchi R , Tschöp MH A new glucagon and GLP-1 co-agonist eliminates obesity in rodents.
Nat Chem Biol 5 : — Diabetes 58 : — Cohen MA , Ellis SM , Le Roux CW , Batterham RL , Park A , Patterson M , Frost GS , Ghatei MA , Bloom SR Oxyntomodulin suppresses appetite and reduces food intake in humans.
J Clin Endocrinol Metab 88 : — Wynne K , Park AJ , Small CJ , Patterson M , Ellis SM , Murphy KG , Wren AM , Frost GS , Meeran K , Ghatei MA , Bloom SR Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial. Diabetes 54 : — Conarello SL , Jiang G , Mu J , Li Z , Woods J , Zycband E , Ronan J , Liu F , Roy RS , Zhu L , Charron MJ , Zhang BB Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated β-cell loss and hyperglycaemia.
Diabetologia 50 : — Polonsky KS , Herold KC , Gilden JL , Bergenstal RM , Fang VS , Moossa AR , Jaspan JB Glucose counterregulation in patients after pancreatectomy. Comparison with other clinical forms of diabetes. Diabetes 33 : — Oxford University Press is a department of the University of Oxford.
It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide. Sign In or Create an Account. Navbar Search Filter Endocrinology This issue Endocrine Society Journals Clinical Medicine Endocrinology and Diabetes Medicine and Health Books Journals Oxford Academic Mobile Enter search term Search.
Endocrine Society Journals. Advanced Search. Search Menu. Article Navigation. Close mobile search navigation Article Navigation. Volume Article Contents Glucagon as a Stress Hormone. Glucagon in Energy Homeostasis.
Journal Article. Minireview: Glucagon in Stress and Energy Homeostasis. Jones , B. Oxford Academic. Stephen Bloom, Department of Investigative Medicine, Commonwealth Building, Imperial College London, Hammersmith Campus, Du Cane Road, London W12 0HS, United Kingdom.
PDF Split View Views. Cite Cite B. Select Format Select format. ris Mendeley, Papers, Zotero. enw EndNote. bibtex BibTex. txt Medlars, RefWorks Download citation. Permissions Icon Permissions. Close Navbar Search Filter Endocrinology This issue Endocrine Society Journals Clinical Medicine Endocrinology and Diabetes Medicine and Health Books Journals Oxford Academic Enter search term Search.
Open in new tab Download slide. Google Scholar Crossref. Search ADS. Google Scholar OpenURL Placeholder Text. Google Scholar PubMed. OpenURL Placeholder Text.
Enteral resuscitation and early enteral feeding in children with major burns: effect on McFarlane response to stress. The role of glucagon hypersecretion in the pathogenesis of hyperglycemia following acute myocardial infarction. Increase in plasma glucagon, a factor in hyperglycemia, is related to neurological outcome in postcardiac-arrest patients.
The role of the autonomic nervous system in the control of glucagon, insulin and pancreatic polypeptide release from the pancreas.
Involvement of α1 and β-adrenoceptors in adrenaline stimulation of the glucagon-secreting mouse α-cell. Adrenergic receptors and the secretion of glucagon and insulin from the isolated, perfused canine pancreas. CNS sites involved in sympathetic and parasympathetic control of the pancreas: a viral tracing study.
The release of pancreatic glucagon and inhibition of insulin in response to stimulation of the sympathetic innervation. Pancreatic endocrine responses to stimulation of the peripheral ends of the splanchnic nerves in the conscious adrenalectomized calf. Pancreatic sympathetic nerves contribute to increased glucagon secretion during severe hypoglycemia in dogs.
Modulation by the hypothalamus of glucagon and insulin secretion in rabbits: studies with electrical and chemical stimulations. Ventromedial hypothalamic lesions in rats suppress counterregulatory responses to hypoglycemia.
Effect of hypothalamic stimulation on plasma glucose, insulin, and glucagon levels. De Marinis. Pancreatic noradrenergic nerves are activated by neuroglucopenia but not by hypotension or hypoxia in the dog. Catecholamines and exercise-induced glucagon and fatty acid mobilization in the rat.
Diagnostic utility of the glucagon stimulation test in comparison to the insulin tolerance test in patients following pituitary surgery. Glucagon-sensitive adenyl cylase in plasma membrane of hepatic parenchymal cells. Cross-talk between glucagon- and adenosine-mediated signalling systems in rat hepatocytes: effects on cyclic AMP-phosphodiesterase activity.
Glucagon is a primary controller of hepatic glycogenolysis and gluconeogenesis during muscular work. The effect of acute glucagon removal on the metabolic response to stress hormone infusion in the conscious dog.
Contribution of hepatic glycogenolysis to glucose production in humans in response to a physiological increase in plasma glucagon concentration.
Potentiation of α1-adrenergic responses in rat liver by a cAMP-dependent mechanism. Synergistic interactions among antiinsulin hormones in the pathogenesis of stress hyperglycemia in humans.
Glucagon-cortisol interactions on glucose turnover and lactate gluconeogenesis in normal humans. Myocardial, metabolic and contractile response to glucagon and epinephrine. Effects of glucagon on cardiac chronotropic response to vagal stimulation in the dog.
Responses of rat and guinea pig hearts to glucagon. Insulin-like stimulation of cardiac fuel metabolism by physiological levels of glucagon: involvement of PI3K but not cAMP. Individual, but not simultaneous, glucagon and cholecystokinin infusions inhibit feeding in men.
Le Sauter. Hepatic portal glucagon infusion decreases spontaneous meal size in rats. Comparison of the effects of glucagon and atropine sulfate on gastric emptying. Effect of intracerebroventricularly infused glucagon on feeding behavior. Adaptive changes in insulin and glucagon secretion during cold acclimation in the rat.
Hyperglucagonemia increases resting metabolic rate in man during insulin deficiency. The effect of lipolytic hormones and theophylline on heat production in brown adipose tissue in vivo. Influence of endocrine and chemical factors on glucagon induced thermogenesis in brown adipocytes.
Glucagon in physiological concentrations stimulates brown fat thermogenesis in vivo. Tissue-specific modulation of rat glucagon receptor mRNA by thyroid status. Stimulation of metabolism of rat brown adipose tissue by addition of lipolytic hormones in vitro. Apparent thermogenic effect of injected glucagon is not due to a direct effect on brown fat cells.
Stochastic amplification and signaling in enzymatic futile cycles through noise-induced bistability with oscillations. Insulin dissociates hepatic glucose cycling and glucagon-induced thermogenesis in man.
de Castro. Insulin and glucagon as determinants of body weight set point and microregulation in rats. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated β-cell loss and hyperglycaemia.
Glucose counterregulation in patients after pancreatectomy. Issue Section:. This suppression of post-prandial glucagon secretion is postulated to be centrally mediated via efferent vagal signals. Importantly,amylin does not suppress glucagon secretion during insulin-induced hypoglycemia.
Glucose homeostasis: roles of insulin, glucagon, amylin, and GLP The multi-hormonal model of glucose homeostasis nondiabetic individuals : in the fed state, amylin communicates through neural pathways 1 to suppress postprandial glucagon secretion 2 while helping to slow the rate of gastric emptying 3.
These actions regulate the rate of glucose appearance in the circulation 4. In addition, incretin hormones, such as GLP-1, glucose-dependently enhance insulin secretion 6 and suppress glucagon secretion 2 and, via neural pathways, help slow gastric emptying and reduce food intake and body weight 5.
Amylin exerts its actions primarily through the central nervous system. Animal studies have identified specific calcitonin-like receptor sites for amylin in regions of the brain, predominantly in the area postrema. The area postrema is a part of the dorsal vagal complex of the brain stem.
A notable feature of the area postrema is that it lacks a blood-brain barrier, allowing exposure to rapid changes in plasma glucose concentrations as well as circulating peptides, including amylin. In summary, amylin works to regulate the rate of glucose appearance from both endogenous liver-derived and exogenous meal-derived sources, and insulin regulates the rate of glucose disappearance.
Glucagon is a key catabolic hormone consisting of 29 amino acids. It is secreted from pancreatic α-cells. Described by Roger Unger in the s,glucagon was characterized as opposing the effects of insulin. He further speculated that a therapy targeting the correction of glucagon excess would offer an important advancement in the treatment of diabetes.
Hepatic glucose production, which is primarily regulated by glucagon,maintains basal blood glucose concentrations within a normal range during the fasting state. When plasma glucose falls below the normal range, glucagon secretion increases, resulting in hepatic glucose production and return of plasma glucose to the normal range.
When coupled with insulin's direct effect on the liver, glucagon suppression results in a near-total suppression of hepatic glucose output Figure 4.
Insulin and glucagon secretion: nondiabetic and diabetic subjects. In nondiabetic subjects left panel , glucose-stimulated insulin and amylin release from the β -cells results in suppression of postprandial glucagon secretion.
In a subject with type 1 diabetes, infused insulin does not suppress α -cell production of glucagon. Adapted from Ref. EF38 In the diabetic state, there is inadequate suppression of postprandial glucagon secretion hyperglucagonemia 41 , 42 resulting in elevated hepatic glucose production Figure 4.
Importantly,exogenously administered insulin is unable both to restore normal postprandial insulin concentrations in the portal vein and to suppress glucagon secretion through a paracrine effect. This results in an abnormally high glucagon-to-insulin ratio that favors the release of hepatic glucose.
The intricacies of glucose homeostasis become clearer when considering the role of gut peptides. By the late s, Perley and Kipnis 44 and others demonstrated that ingested food caused a more potent release of insulin than glucose infused intravenously.
Additionally, these hormonal signals from the proximal gut seemed to help regulate gastric emptying and gut motility. Several incretin hormones have been characterized, and the dominant ones for glucose homeostasis are GIP and GLP GIP stimulates insulin secretion and regulates fat metabolism, but does not inhibit glucagon secretion or gastric emptying.
GLP-1 also stimulates glucose-dependent insulin secretion but is significantly reduced postprandially in people with type 2 diabetes or impaired glucose tolerance. Derived from the proglucagon molecule in the intestine, GLP-1 is synthesized and secreted by the L-cells found mainly in the ileum and colon.
Circulating GLP-1 concentrations are low in the fasting state. However, both GIP and GLP-1 are effectively stimulated by ingestion of a mixed meal or meals enriched with fats and carbohydrates. GLP-1 has many glucoregulatory effects Table 1 and Figure 3. In the pancreas,GLP-1 stimulates insulin secretion in a glucose-dependent manner while inhibiting glucagon secretion.
Infusion of GLP-1 lowers postprandial glucose as well as overnight fasting blood glucose concentrations. Yet while GLP-1 inhibits glucagon secretion in the fed state, it does not appear to blunt glucagon's response to hypoglycemia. Administration of GLP-1 has been associated with the regulation of feeding behavior and body weight.
Of significant and increasing interest is the role GLP-1 may have in preservation of β-cell function and β-cell proliferation. Our understanding of the pathophysiology of diabetes is evolving.
Type 1 diabetes has been characterized as an autoimmune-mediated destruction of pancreaticβ-cells. Early in the course of type 2 diabetes, postprandial β-cell action becomes abnormal, as evidenced by the loss of immediate insulin response to a meal.
Abnormal gastric emptying is common to both type 1 and type 2 diabetes. The rate of gastric emptying is a key determinant of postprandial glucose concentrations Figure 5. In individuals with diabetes, the absent or delayed secretion of insulin further exacerbates postprandial hyperglycemia. Both amylin and GLP-1 regulate gastric emptying by slowing the delivery of nutrients from the stomach to the small intestine.
Gastric emptying rate is an important determinant of postprandial glycemia. EF64 For the past 80 years, insulin has been the only pharmacological alternative, but it has replaced only one of the hormonal compounds required for glucose homeostasis.
Newer formulations of insulin and insulin secretagogues, such as sulfonylureas and meglitinides, have facilitated improvements in glycemic control. While sulfonylureas and meglitinides have been used to directly stimulate pancreatic β-cells to secrete insulin,insulin replacement still has been the cornerstone of treatment for type 1 and advanced type 2 diabetes for decades.
Advances in insulin therapy have included not only improving the source and purity of the hormone, but also developing more physiological means of delivery. Clearly, there are limitations that hinder normalizing blood glucose using insulin alone. First, exogenously administered insulin does not mimic endogenous insulin secretion.
In normal physiology, the liver is exposed to a two- to fourfold increase in insulin concentration compared to the peripheral circulation.
In the postprandial state, when glucagon concentrations should be low and glycogen stores should be rebuilt, there is a paradoxical elevation of glucagon and depletion of glycogen stores.
As demonstrated in the Diabetes Control and Complications Trial and the United Kingdom Prospective Diabetes Study,intensified care is not without risk. In both studies, those subjects in the intensive therapy groups experienced a two- to threefold increase in severe hypoglycemia.
Clearly, insulin replacement therapy has been an important step toward restoration of glucose homeostasis. But it is only part of the ultimate solution.
The vital relationship between insulin and glucagon has suggested additional areas for treatment. With inadequate concentrations of insulin and elevated concentrations of glucagon in the portal vein, glucagon's actions are excessive, contributing to an endogenous and unnecessary supply of glucose in the fed state.
To date, no pharmacological means of regulating glucagon exist and the need to decrease postprandial glucagon secretion remains a clinical target for future therapies. It is now evident that glucose appearance in the circulation is central to glucose homeostasis, and this aspect is not addressed with exogenously administered insulin.
Amylin works with insulin and suppresses glucagon secretion. It also helps regulate gastric emptying, which in turn influences the rate of glucose appearance in the circulation. A synthetic analog of human amylin that binds to the amylin receptor, an amylinomimetic agent, is in development.
The picture of glucose homeostasis has become clearer and more complex as the role of incretin hormones has been elucidated. Incretin hormones play a role in helping regulate glucose appearance and in enhancing insulin secretion. Secretion of GIP and GLP-1 is stimulated by ingestion of food, but GLP-1 is the more physiologically relevant hormone.
However, replacing GLP-1 in its natural state poses biological challenges. In clinical trials, continuous subcutaneous or intravenous infusion was superior to single or repeated injections of GLP-1 because of the rapid degradation of GLP-1 by DPP-IV.
To circumvent this intensive and expensive mode of treatment, clinical development of compounds that elicit similar glucoregulatory effects to those of GLP-1 are being investigated. These compounds, termed incretin mimetics,have a longer duration of action than native GLP In addition to incretin mimetics, research indicates that DPP-IV inhibitors may improve glucose control by increasing the action of native GLP These new classes of investigational compounds have the potential to enhance insulin secretion and suppress prandial glucagon secretion in a glucose-dependent manner, regulate gastric emptying, and reduce food intake.
Despite current advances in pharmacological therapies for diabetes,attaining and maintaining optimal glycemic control has remained elusive and daunting. Intensified management clearly has been associated with decreased risk of complications. Glucose regulation is an exquisite orchestration of many hormones, both pancreatic and gut, that exert effect on multiple target tissues, such as muscle, brain, liver, and adipocyte.
While health care practitioners and patients have had multiple therapeutic options for the past 10 years, both continue to struggle to achieve and maintain good glycemic control. There remains a need for new interventions that complement our current therapeutic armamentarium without some of their clinical short-comings such as the risk of hypoglycemia and weight gain.
These evolving therapies offer the potential for more effective management of diabetes from a multi-hormonal perspective Figure 3 and are now under clinical development. Aronoff, MD, FACP, FACE, is a partner and clinical endocrinologist at Endocrine Associates of Dallas and director at the Research Institute of Dallas in Dallas, Tex.
Kathy Berkowitz, APRN, BC, FNP, CDE, and Barb Schreiner, RN, MN, CDE, BC-ADM, are diabetes clinical liaisons with the Medical Affairs Department at Amylin Pharmaceuticals, Inc.
Laura Want, RN, MS, CDE, CCRC, BC-ADM, is the clinical research coordinator at MedStar Research Institute in Washington, D. Note of disclosure: Dr. Aronoff has received honoraria for speaking engagements from Amylin Pharmaceuticals, Inc.
Berkowitz and Ms. Schreiner are employed by Amylin. Want serves on an advisory panel for, is a stock shareholder in, and has received honoraria for speaking engagements from Amylin and has served as a research coordinator for studies funded by the company.
She has also received research support from Lilly, Novo Nordisk, and MannKind Corporation. Amylin Pharmaceuticals, Inc. Sign In or Create an Account.
Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Diabetes Spectrum. Advanced Search.
User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation. Volume 17, Issue 3. Previous Article. β-CELL HORMONES. α-CELL HORMONE: GLUCAGON. INCRETIN HORMONES GLP-1 AND GIP.
AMYLIN ACTIONS. GLP-1 ACTIONS. Article Navigation. Feature Articles July 01 Glucose Metabolism and Regulation: Beyond Insulin and Glucagon Stephen L. Aronoff, MD, FACP, FACE ; Stephen L. Aronoff, MD, FACP, FACE. This Site. Google Scholar. Kathy Berkowitz, APRN, BC, FNP, CDE ; Kathy Berkowitz, APRN, BC, FNP, CDE.
Barb Shreiner, RN, MN, CDE, BC-ADM ; Barb Shreiner, RN, MN, CDE, BC-ADM. Laura Want, RN, MS, CDE, CCRC, BC-ADM Laura Want, RN, MS, CDE, CCRC, BC-ADM. Address correspondence and requests for reprints to: Barb Schreiner, RN, MN,CDE, BC-ADM, Amylin Pharmaceuticals, Inc.
Diabetes Spectr ;17 3 — Get Permissions. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest.
Figure 1. View large Download slide. Table 1. Effects of Primary Glucoregulatory Hormones. View large. View Large. Figure 2. Figure 3. Figure 4. Figure 5. American Diabetes Association: Clinical Practice Recommendations
Jones, T. Tan, Diabetes management benefits. Glucagon is traditionally thought of as an antihypoglycemic hormone, for Chamomile Tea for Cold and Flu in response to starvation. However, hotmone actually Glucagon hormone response energy Glucsgon and has other actions not in line with protection from hypoglycemia. Furthermore, it is often found to be elevated when glucose is also raised, for example in circumstances of psychological and metabolic stress. These findings seem more in keeping with glucagon having some role as a hormone enhancing the response to stress.
Auf Ihre Frage habe ich die Antwort in google.com gefunden
Ich denke, dass Sie den Fehler zulassen. Schreiben Sie mir in PM, wir werden umgehen.
Nach meiner Meinung irren Sie sich. Ich biete es an, zu besprechen.