Thank you for visiting nature. Astaxannthin are Vegan smoothie recipes a browser Axtaxanthin with Asgaxanthin support for CSS.
To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Livee. In the meantime, to Type diabetes complications prevention continued support, we are displaying Nutrition for athletes site without Astaxnathin and JavaScript.
Astaxanthin Lievr is classified as Awtaxanthin xanthophyll Astwxanthin compound which have broader functions including potent antioxidant, anti-inflammatory and neuroprotective Immune system strength. Considerable researches have Ashaxanthin that AXT shows Hypertension and acupuncture and therapeutic properties against for Diabetes, Osteoarthritis and Rheumatoid Arthritis.
However, the Type diabetes complications prevention effect liveg AXT on livrr disease has not yet been reported. In this Astaxanthim, we investigated Type diabetes complications prevention of AXT on ethanol-induced liver injury in chronic plus binge Astaxantyin feeding model.
The hepatic steatosis and inflammation induced ljver ethanol administration were alleviated by AXT. Hezlth levels of aspartate Muscle definition supplements and alanine transaminase were decreased in the Dark chocolate energy of AXT administrated group.
The ethanol-induced expression of cytochrome Asttaxanthin 2E1 CYP2E1pro-inflammatory proteins, heslth, chemokines and reactive oxygen species ROS levels were also Liver detoxification supplements Type diabetes complications prevention the Astaxanthjn of AXT administrated group.
Moreover, Understanding body fat percentage infiltration of neutrophils was Atsaxanthin in the Pumpkin Seed Flour of AXT administrated group. Docking model and pull-down livrr showed that AXT directly binds to the DNA binding healhh of STAT3.
Moreover, AXT decreased STAT3 phosphorylation in the liver lover AXT administration group. Therefore, these results suggest Tips for successful diabetes self-care AXT could prevent Astxanthin hepatic Vertimax and plyometric training via inhibition of Type diabetes complications prevention and inflammatory responses Asaxanthin blocking of STAT3 llver.
Alcoholic liver disease ALD is Astaxahthin to be Astaxanthin and liver health hhealth cause of livrr and mortality Astaxahthin 12. Excessive and chronic alcohol liveer is a leading factor for hepatic lier ranging from simple steatosis to severe forms of liver injury such as steatohepatitis, alcoholic cirrhosis and hepatitis 3.
Ethanol and the Asaxanthin of its metabolism have toxic effects on the liver and induce inflammation and oxidative stress that lived key drivers of alcohol-induced liver injury 2. Astaxanthkn is the most targeted organ attacked by oxidative stress heatlhType diabetes complications prevention6.
Ethanol metabolism triggered Reactive oxygen species Astaxanthiin production Astaxahthin both chronic and acute alcoholism 7.
A oiver of cytokines like TNF-α and IL-6 can be also Effective long-term weight management in hepatocytes Axtaxanthin by oxidative stress, which might increase inflammation 8.
Jealth is toxic livef the ehalth because of DNA Isotonic hydration drinks, mitochondrial dysfunction and lipid peroxidation.
ROS could induce by increasing of cytokines and livver productions Axtaxanthin order to recruit Liger cells liverr the sites of Astaxanthin and liver health.
Signal transducer and activator Astaxantgin transcription 3 STAT3 is a key Astaxanthjn of various genes involved in inflammatory and oxidative responses Mental wellness tipsljver Hepatic Anv can be activated by various cytokines, ,iver factors, hormones, Astaxanthin and liver health, and healhh viral Type diabetes complications prevention Astaanthin or acute alcohol heath promotes inflammation in the liver by activation of Healtth.
In recent study, Asgaxanthin hepatocytes-specific STAT3 knockout H-STAT3KO mice Astaxahthin similar amounts of ROS and pro-inflammatory cytokines such nealth TNF-α and IL-6 compared with pair-fed mice 9 In addition, IL-6 induced activation of STAT3 lver promoted Type diabetes complications prevention injury and development of fatty liver Astaxanthin AXT helath ubiquitous in nature, especially healyh the EGCG and hormonal balance environment, and is found healh high amounts in the red-orange hwalth of crustacean shells e.
It has Astaxantbin reported that AXT can livsr skin from the healtu effects of ultraviolet radiation, ameliorate lliver macular Astzxanthin, protect yealth chemically induced cancers, increase high-density lipoproteins and enhance the immune system though its anti-oxidant and Astaxamthin properties However, its Astaaxanthin effect on alcohol-induced liver injury has not yet Astaxantihn studied.
Therefore, we investigated the protective Uplift your spirit of AXT on Astaxanyhin liver injury and its mechanism. Chronic ethanol exposure induces hepatic steatosis and liver damages. The liver from chronic-binge ethanol treated group looks rough and was swollen Fig.
Rate of bodyweight-gain was lower in ethanol-fed mice than in pair-fed mice, but it was higher in ethanol-fed mice than ethanol with AXT-fed mice. The ratio of liver to body weight was increased by chronic-binge ethanol exposure as compared with pair-fed group, which was alleviated by AXT administration Fig.
Serum levels of aspartate transaminase AST and alanine transaminase ALT were higher in ethanol-fed mice than pair-fed mice, but these values were decreased in ethanol with AXT-fed mice Fig. In addition, histopathology studies revealed fat molecules infiltration, inflammatory cells and necrosis in ethanol-fed mice, but its manifestations were alleviated in ethanol with AXT-fed mice Fig.
Effects of AXT in chronic-binge ethanol induced alcoholic liver injury in mice. A Pictures of mouse livers. B Liver sections of pair-fed mice, ethanol-fed mice and ethanol-fed with AXT 0.
C Bodyweight of pair-fed mice, ethanol-fed mice and ethanol-fed with AXT mice during food intake period. ethanol-fed with AXT. Because chronic-binge ethanol exposure induces oxidative stress, we determined iNOS and CYP2E1 expression and ROS levels in the livers of pair-fed mice, ethanol-fed mice and ethanol with AXT-fed mice.
Ethanol fed increased iNOS and CYP2E1 expression, however AXT administration reduced their expression in a dose dependent manner Fig. In addition, immunohistochemistry showed that decrease of the number of iNOS-reactive cells in the liver of ethanol with AXT-fed mice Fig.
Ethanol fed also elevated NO level, but NO level was decreased in the liver of ethanol with AXT-fed mice Fig. The levels of thiobarbituric acid, a marker of lipid peroxidation, was elevated by ethanol, but it was depleted by AXT Fig. Hydrogen peroxide level was also elevated by ethanol, but it was decreased by AXT Fig.
Effects of AXT on ethanol-induced oxidative stress in chronic-binge ethanol induced alcoholic liver injury in mice. A The expression of iNOS and CYP2E1 were determined in the total protein extracts of mice liver tissues by Western blotting.
Expression of COX-2 and production of pro-inflammatory cytokines and chemokines are implicated in alcoholic liver diseases. Thus, we determined these factors in the liver. Ethanol-fed increased COX-2 expression but, COX-2 expression was lower in the livers of ethanol with AXT-fed mice Fig.
We also performed immunohistochemical staining for COX The number of COXreactive cells was lower in the liver of ethanol with AXT-fed mice Fig. Furthermore, the expression of a variety of pro-inflammatory cytokines and chemokines in the liver was examined by real-time PCR. Ethanol with AXT-fed mice showed a significant decrease in the levels of pro-inflammatory cytokines such as IL-6, TNF-α and IL-1β, and chemokines such as MCP-1 and MIP-1β Fig.
Effects of AXT on inflammatory responses in chronic-binge ethanol induced alcoholic liver injury in mice. A The expression of COX-2 were determined in the total protein extracts of mice liver tissues by Western blotting.
C mRNA expression levels of pro-inflammatory cytokines such as IL-6, TNF-α and IL-1β and D chemokines such as MCP-1, MIP-1α and MIP-1β in the pair-fed mice, ethanol-fed mice and ethanol-fed with AXT mice.
Neutrophil infiltration is a hallmark of alcoholic hepatitis 15 Neutrophil infiltration likely contributes to hepatocellular damage, possibly by killing hepatocytes through production of ROS To investigate whether the inhibition of ethanol-induced hepatotoxicity in the ethanol with AXT-fed mice was related to neutrophil infiltration, we analyzed the distribution of neutrophils in liver tissue.
Blood level of neutrophils was elevated by ethanol, but down-regulated in ethanol with AXT-fed mice Fig. mRNA expression of Ly6G a neutrophil marker was also decreased in ethanol with AXT-fed mice Fig. In addition, immunohistochemistry of Ly6G also demonstrated level of hepatic neutrophil infiltration was lower in ethanol with AXT-fed mice Fig.
The expression vascular cell adhesion molecule VCAM-1 that controlled neutrophil recruitment was significantly increased in ethanol-fed mice but decreased in ethanol with AXT-fed mice Fig. Effects of AXT on infiltration of immune cells in chronic-binge ethanol induced alcoholic liver injury in mice.
B mRNA expression levels of Ly6g, a marker of neutrophil, in the pair-fed mice, ethanol-fed mice and ethanol-fed with AXT mice.
D The expression of VCAM-1 were determined in the total protein extracts of mice liver tissues by Western blotting. To clear the STAT3 involvement in the blocking effect of AXT on the inflammatory protein expression and ROS generation, we determined the interaction between AXT and STAT3.
The expression of STAT3 protein that is cell lysates from HEK cells transfected with STAT3 and is expressed in cell-free system in AXT-Sepharose 6B bead was higher than in Sepharose 6B bead Fig.
Furthermore, ethanol-induced p-STAT3 expression was lowered in ethanol with AXT-fed mice than ethanol-fed mice Fig. A key role of STAT3 by binding AXT in chronic-binge ethanol induced alcoholic liver injury in mice. A Docking model of AXT bound with STAT3. B The expression of STAT3 on presence or absence AXT in vitro and in vivo.
C The expression of STAT3 and p-STAT3 were determined in the total protein extracts of mice liver tissues by Western blotting. Studies conducted during the past few years that astaxanthin AXT plays a key role in anti-oxidant and anti-inflammatory responses In the present study, we demonstrated AXT alleviated ethanol-induced oxidative stress, liver inflammation and thus liver injury by inhibition of STAT3 activity.
Ethanol exposure significantly induced hepatic injury in the livers of ethanol-fed mice, but it was alleviated in ethanol with AXT-fed mice.
AXT administration reduced ethanol-induced AST and ALT levels accompanied by increased of liver lipid droplet. In the liver tissue of ethanol-fed mice, many inflammatory cells activated and lipid droplets were revealed by pro-inflammatory cytokines and COX-2 expression.
However, in the liver tissue of ethanol with AXT-fed mice, inflammatory cells were decreased and lipid droplets were smaller. AXT administration 0. Thus, the present data indicated that AXT could be more potent hepatic protective agent for ethanol-induced liver damages.
Excessive ethanol exposure-induced CYP2E1 and iNOS expression leads to oxidative stress by the generation of ROS and increase in NO production in the hepatocytes of liver 23 The expression of CYP2E1 and iNOS were decreased in in ethanol with AXT-fed mice. In the liver tissue of ethanol with AXT-fed mice, iNOS-reactive cells were decreased.
In addition, the production of NO was also decreased in ethanol with AXT-fed mice. The GSH depletion resulted in the inhibition of oxidative stress In present study, total GSH levels were depleted in ethanol with AXT-fed mice. Moreover, level of lipid peroxidation in ethanol with AXT-fed mice was also decreased compared with ethanol-fed mice.
These data suggest that reduced oxidative damages could be associated with hepatic protective effect of AXT. Ethanol-induced liver damage is involved in inflammatory responses. IL-6, a major pro-inflammatory cytokine, is elevated by ethanol consumption and is closely associated with ALD It is worthy to note that patients with severe alcoholic hepatitis who do not respond to medical treatment have low hepatic expression of TNF and IL-6 1.
In our study, the level of IL-6 was significantly reduced in the liver of ethanol with AXT-fed mice. Furthermore, other inflammatory cytokines such as TNF-α and IL-1β and chemokines such as MCP-1 and MIP-1β were also down-regulated in the liver of ethanol with AXT-fed mice.
: Astaxanthin and liver health| Mechanisms of protective effects of astaxanthin in nonalcoholic fatty liver disease | C PCoA of unweighted UniFrac distance from beta diversity analysis. Alcoholic liver disease: mechanisms of injury and targeted treatment. Macedo RC, Bolin AP, Marin DP, Otton R. Seq: a revolutionary tool for transcriptomics. Anti-Obesity effect of carotenoids: direct impact on adipose tissue and adipose tissue-driven indirect effects. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. |
| Recent Articles | Nat Rev Genet. Strategic Collaborators Journal Membership Conference Parterships. The Nrf2-ARE pathway is an endogenous antioxidant stress pathway that regulates the expression of SOD and DNA repair enzymes e. The hepatic steatosis and inflammation induced by ethanol administration were alleviated by AXT. STAT3 was activated in aldehyde dehydrogenase 2 deficiency mice which were more prone to ethanol Total RNA from liver tissues were extracted by RiboEx TM Total RNA isolation solution GeneAll Biotechnology, Seoul, Korea and cDNA was synthesized using High Capacity RNA-to-cDNA kit Applied Biosystems, Foster City, CA, USA. |
| Colorful Pigment Plays Role in Combating Liver Disease - UConn Today | In addition, it is a potent antioxidant, which means that it helps the body prevent the cell and tissue damage that are evident in disease and aging. The liver is the largest glandular organ in the human body, weighing in at a substantial three pounds or so — and it performs all sorts of critical functions, from facilitating metabolism to aiding in the digestion of fats to detoxifying the body from potentially harmful substances, such as drugs and alcohol. Lee explains that if astaxanthin can inhibit the development of certain genes involved in the development of fibrosis in humans, as well as in mice, it offers the promise that early stages of fibrosis can be regressed, thus allowing the liver to revert to a healthy state. As a nutritionist, Lee is concerned with the health implications of obesity, which has become a worldwide epidemic. Department of Agriculture, Lee is looking at the potential for astaxanthin not only to prevent the disease from starting, but to reverse its effects if it has already begun to cause damage. This is especially significant because, to date, there are no effective drug therapies to stop NAFLD. She cites the sobering statistics that, whereas 20 to 30 percent of people in the general population may have some accumulation of fat in their livers, in the obese population that number increases to 65 to 75 percent. And while only 2 to 3 percent of the general population may have a more advanced form of the disease where the liver is inflamed — called nonalcoholic steatohepatitis — 15 to 20 percent of obese people suffer from this condition. The key to weight control remains in minimizing the amount of fat stored in the body, and this will always require maintaining a healthy lifestyle and making appropriate dietary choices. February 14, UConn Today. COX-2 is induced by pro-inflammatory cytokines and oxidant stress iNOS expression is increased by excessive ethanol consumption and is also induced by pro-inflammatory cytokines In the present study, ethanol-induced COX-2 and iNOS expression were decreased in the liver of ethanol with AXT-fed mice. Therefore, these results suggest that AXT alleviated ethanol-induced pro-inflammatory responses, and thus ameliorated ethanol-induced liver damages. Neutrophils accumulated in the hepatic microvasculature can extravasate into the hepatic parenchyma if they receive appropriate signals from previously sensitized or distressed cells 28 , Infiltration of a large number of neutrophils is a very prominent feature of alcoholic hepatitis 28 , 29 , The number of neutrophils in blood was elevated in ethanol-fed mice, but it was decreased in ethanol with AXT-fed mice. In addition, the mRNA expression of Ly6g and neutrophils in liver tissue was also reduced in ethanol with AXT-fed mice. Neutrophil recruitment is mediated by a multistep adhesion cascade that involves multiple adhesion molecules and their ligands, which are expressed on endothelial cells ECs neutrophils including ICAM-1 and VCAM-1 The expression of VCAM-1 were decreased in ethanol with AXT-fed mice. Thus, reduced neutrophil recruitment could be associated with the reducing effect of AXT in the ethanol-mediated inflammatory associated liver damages. STAT3 plays important function of in the hepatic inflammation during ALD Many studies have showed that STAT3 is a transcription factor that is activated by a variety of factors, including cytokines, growth factors, hormones, and hepatitis viral proteins in the liver Ethanol-fed H-STAT3KO mice produced similar amounts of ROS and pro-inflammatory cytokines such as TNF-α and IL-6 compared with pair-fed mice 9. Several studies demonstrated that inhibition of STAT3 could be associated with reduction of liver damages. STAT3 was activated in aldehyde dehydrogenase 2 deficiency mice which were more prone to ethanol Compounds from natural resources such as Anthocyanins and Kavalactone desmethoxyyangonin attenuated ethanol or LPS-induced liver damages through inhibition of STAT3 33 , Moreover, inhibition of STAT3 was associated with decrease effectiveness of several other disease. Stevia and Stevioside protected cisplatin nephrotoxicity by inhibition of STAT3 Corydalis hendersonii Hemsl prevented myocardial injury by attenuating inflammation and fibrosis via STAT3 inhibition These results indicated that STAT3 could mediate ethanol-induced inflammatory responses in the liver. In our study, docking model shows AXT was directly binding with STAT3. We also showed AXT was binding with STAT3 protein in vivo and in vitro. Furthermore, ethanol-induced STAT3 phosphorylation was decreased in ethanol with AXT-fed mice than in ethanol-fed mice. In summary, our results suggest that AXT protects against ethanol-induced liver injury. This effect may result from a reduction in oxidative stress and inflammatory responses by blocking STAT3 activity. The experiment was performed in accordance with the guidelines proscribed by the Chungbuk National University Animal Care Committee CBNUA All mice were fed a standard laboratory chow diet ad libitum. The mice from AXT groups were daily administrated AXT that dissolved in olive oil for 10 days by oral gavage. Serum aspartate transaminase AST and alanine transaminase ALT were measured using a biochemical analyzer AU, Beckman Coulter, CA, USA. Then, liver tissues were embedded in paraffin. Homogenized liver tissues were lysed by protein extraction solution PRO-PREP, iNtRON, Sungnam, Korea and the total protein concentration was determined using the Bradford reagent Bio-Rad, Hercules, CA, USA. The membranes were washed with Tris-buffered saline containing 0. After washes, binding of antibodies to the PVDF membrane was detected using the Immobilon Western Chemilum HRP substrate Millipore, Bedford, MA, USA. The band intensities were measured using the Fusion FX 7 image acquisition system Vilber Lourmat, Eberhardzell, Germany. Specific primary antibodies were purchased from Santa Cruz Biotechnology p-STAT3, STAT3 and β-actin; Dallas, TX, USA , Cell signaling Technology iNOS and COX-2; Trask Lane, Danvers, MA, USA and Abcam CYP2E1; Cambridge, MA, USA. Secondary antibodies were purchased from Santa Cruz Biotechnology anti-mouse and anti-rabbit; Dallas, TX, USA. The slides were washed and the peroxidase reaction developed with diaminobenzidine and peroxide and then counter-stained with hematoxylin, mounted in Cytoseal XYL Thermo Fisher Scientific, Waltham, MA, USA and evaluated on a light microscope Nikon, Tokyo, Japan. Specific primary antibodies were purchased from Cell signaling Technology COX-2; Trask Lane, Danvers, MA, USA and Abcam iNOS; Cambridge, MA, USA. Total RNA from liver tissues were extracted by RiboEx TM Total RNA isolation solution GeneAll Biotechnology, Seoul, Korea and cDNA was synthesized using High Capacity RNA-to-cDNA kit Applied Biosystems, Foster City, CA, USA. Quantitative real-time RT-PCR was performed on a real-time PCR system Applied Biosystems, Foster City, CA, USA for custom-designed primers and β-actin was used for house-keeping control using QuantiNova SYBR Green PCR kit Qiagen, Hilden, Germany. The values obtained for the target gene expression were normalized to β-actin and quantified relative to the expression in control samples. The stereochemical structure of STAT3 was used for the docking study. Docking studies between AXT and STAT3 were performed using AutoDock VINA Trott and Olson, Starting from the co-crystallized complexes, the STAT3 monomer chain STAT3 from 3CWG , AXT AXT from Chem3D for docking were prepared using AutoDock Tools. Docking experiments were performed at various exhaustiveness values of the default, 16, 24, 32, 40 and Molecular graphics for the best binding model was generated using Discovery Studio Visualizer 2. AXT was conjugated with Epoxy-activated Sepharose 6B GE Healthcare Korea, Seoul, Korea. The Epoxy-activated Sepharose 6B beads 0. The control unconjugated Sepharose 6B beads were prepared as described above in the absence of AXT. After washing, unoccupied binding sites were blocked with blocking buffer 0. The AXT-conjugated Sepharose 6B was washed with three cycles of alternating pH wash buffers buffer 1, 0. AXT-conjugated beads were then equilibrated with a binding buffer 0. To demonstrate binding AXT and STAT3 in vitro and in vivo , STAT3 protein was expressed in two ways; following cell-free system and transfection. The beads were then washed three times with TBST. Lipid peroxidation was measured by determining the generation of malondialdehyde MDA; TBARS Assay kit, Cayman, Ann Arbor, MI, USA. The data were analyzed using the GraphPad Prism 4 version 4. The differences in all data were assessed by one-way analysis of variance. Louvet, A. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nature reviews. Article Google Scholar. Gao, B. Alcoholic liver disease: pathogenesis and new therapeutic targets. Article CAS PubMed Google Scholar. Mathews, S. et al. Invariant natural killer T cells contribute to chronic-plus-binge ethanol-mediated liver injury by promoting hepatic neutrophil infiltration. Article CAS Google Scholar. Arteel, G. Oxidants and antioxidants in alcohol-induced liver disease. Loguercio, C. Oxidative stress in viral and alcoholic hepatitis. Sanchez-Valle, V. Role of oxidative stress and molecular changes in liver fibrosis: a review. Current medicinal chemistry 19 , — Sergent, O. Role for membrane fluidity in ethanol-induced oxidative stress of primary rat hepatocytes. Li, S. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Article CAS PubMed PubMed Central Google Scholar. Horiguchi, N. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury. Machida, K. Hepatitis C virus triggers mitochondrial permeability transition with production of reactive oxygen species, leading to DNA damage and STAT3 activation. Wang, H. Hepatoprotective versus oncogenic functions of STAT3 in liver tumorigenesis. Article ADS CAS PubMed PubMed Central Google Scholar. Ambade, A. Oxidative stress and inflammation: essential partners in alcoholic liver disease. Signal transducer and activator of transcription 3 in liver diseases: a novel therapeutic target. International journal of biological sciences 7 , — Guerin, M. Haematococcus astaxanthin: applications for human health and nutrition. Bautista, A. Neutrophilic infiltration in alcoholic hepatitis. Alcohol 27 , 17—21 Bertola, A. Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury in mice: a critical role for E-selectin. Han, J. Augustyniak, A. Preventive action of green tea from changes in the liver antioxidant abilities of different aged rats intoxicated with ethanol. Lu, Y. CYP2E1 and oxidative liver injury by alcohol. Tilg, H. Pathways of liver injury in alcoholic liver disease. Article PubMed Google Scholar. Albano, E. Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Article ADS CAS PubMed Google Scholar. Ajakaiye, M. Alcohol and hepatocyte-Kupffer cell interaction review. Aubert, J. Increased expression of cytochrome P 2E1 in nonalcoholic fatty liver disease: mechanisms and pathophysiological role. McKim, S. Inducible nitric oxide synthase is required in alcohol-induced liver injury: studies with knockout mice. Gastroenterology , — Khan, M. According to many previous animal experiments, consumption of ATX not only showed no signs of poisoning but also exerted a positive pharmacological effect 19 , Recently, the relationship between gut microbiota and metabolic diseases has attracted attention from the scholarly community because intestinal flora has been confirmed as a target for the prevention and treatment of obesity, metabolic syndrome and cardiovascular diseases Therefore, it is important to investigate how ATX prevents the development of hepatic steatosis and oxidative stress with the risk of metabolic disease. The present study aims to contribute to this growing area of research by exploring the prevention effects on obesity and the development of NAFLD through long-term dietary ATX in mice. The detection kits for alanine transaminase ALT , aspartate transaminase AST , TG, TC, high-density lipoprotein cholesterol HDL-C , and low-density lipoprotein cholesterol LDL-C , and the antioxidant assay kits for SOD, CAT, GSH, T-AOC, and malondialdehyde MDA were purchased from Nanjing Jiancheng Bioengineering Institute Nanjing, China. Other chemicals, solvents and reagents used in the present study were of laboratory analytical grade. Astaxanthin oleoresin was provided by Shandong Jinjing Biotechnology Co. After purification, ATX of The Institutional Animal Care and Use Committee of Shanxi Agricultural University approved all experimental protocols for animal care, handling and experimentation SXAU-EAW We also confirmed that all experiments were conducted in accordance with relevant guidelines and regulations. The design of animal experiments was based on our previous methods The mice in the ND group were fed standard rodent chow containing 3. The mice in the other group were fed a HFD containing 4. The mice in the ND group were given distilled water, and the solvent group was gavaged with corn oil. In addition, the mice in the ATX treatment groups were gavaged with 0. During the diet phase, all mice were given intragastric treatment once per day at a. The diets were purchased from Beijing Huafukang Bioscience Co. Supplementary Table 1 shows the ingredients of the experimental diets. The body weight and food intake were recorded daily for 63 days. To avoid error values, the measurement of weight was repeated three times for each mouse. The energy intake was calculated as food intake × 4. Mice were fasted for 12 h after the last treatment and then euthanized by inhalation with isoflurane. Blood samples were obtained from the retro-orbital veins on Days 0, 30, and All other organs, including the liver, heart, kidney, spleen, and adipose tissues, were immediately collected and weighed individually after sacrificing the animals. The serum TG, TC, HDL-C, and LDL-C levels and activities of AST GOT and ALT were determined using biochemical kits according to the standards and protocols provided by the manufacturer Nanjing, China. The supernatant was collected to determine the protein and lipid levels TG and TC and enzymatic analyses T-AOC, SOD, CAT, GSH, MDA, and ROS. The dihydroethidium DHE probe method was used to qualitatively detect ROS. Five-micron-thick sections of the liver were dyed with DHE, and incubation was performed at 37°C for 10 min in a dark environment. The samples were directly observed under a fluorescence microscope at a measuring emission of nm. The ROS-positive cells had strong red fluorescence. Meanwhile, the frozen sections were stained with Oil Red O ORO , which was performed to further detect hepatic vacuolization, inflammatory cell infiltration, and lipid droplets. The above sections were used to examine hepatocellular apoptosis with the YF TUNEL assay apoptosis detection kit. After the TUNEL reaction, the sections were mounted using antifade mounting medium with DAPI and observed under an inverted fluorescence microscope at and nm wavelength excitation. The negative cells were dyed with blue fluorescence intensity at nm, while the apoptotic cells exhibited green fluorescence at nm. ImageJ software National Institutes of Health, United States was used to measure the cell counting of sections from each group. Then, cDNA was synthesized from total RNA using the PrimeScript Reverse Transcription reagent kit Takara, Dalian, China. Quantitative polymerase chain reaction PCR was conducted in triplicate for each group to detect gene expression. The quantitative analysis of AMPK , SREBP1c , ACC , CPT-1 , PPARα , PPARγ , LXRα , SCD-1 , PGC-1 , FAS , CYP27A1 , and CYP7A1 mRNA expression in the liver was measured in triplicate for each group by quantitative PCR. According to the SYBR Premix Ex Taq II Takara, Dalian, China , the thermal cycle of qPCR was reacted on the CFX 96 Real-Time PCR Detection system BIO-RAD, Hercules, CA, United States under the following conditions: 95°C for 10 min, then 40 cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s. Supplementary Table 2 shows the PCR primer sequences of each gene, and the target genes were normalized to the reference gene GAPDH. The 2 —ΔΔCt method was used to calculate relative gene expression. To investigate lipid metabolism, fresh samples were sent to MetWare Biotechnology Co. After RNA was extracted from liver biopsy samples, liver transcriptome analysis was conducted by RNA sequencing, as described in detail previously 22 , The caecal contents were sent to Shanghai Personal Biotechnology Co. to investigate microbial diversity through 16S rRNA analysis on the Illumina MiSeq platform. A previous study illustrated the analytical conditions and detailed parameters All experiments were biologically repeated three times, and the data were analyzed with Social Sciences SPSS Origin 9. A dramatic increment in body weight was observed in the HFD group, while a moderate increase was observed in the ATX treatment groups Figure 1A. Body weight gain plays a pivotal role in evaluating the effect of HFD on obesity and assessing its prevention. Table 1 presents the initial and final body weights of mice in each group. During feeding induction, the mice gained more weight in the HFD group There was no significant difference in the food efficiency ratio of the mice in each group except for the ND group Table 1. Figure 1. Astaxanthin ATX prevented obesity and related indices in HFD-fed mice. A Body weight. B Body weight gain. C Energy intake. D Related organ weight. E Visceral adipose tissue. F Visual appearance pictures of metabolic mice and liver. G Hepatic TG level. H Hepatic TC level. I Serum ALT level. J Serum AST level. Table 1. Effect of astaxanthin ATX supplementation on body weight, energy intake, and food efficiency ratio in high-fat diet-induced mice a. To estimate whether the HFD with ATX supplementation affected visceral organs and fat, the wet weights of adipose tissue and organs were measured in each group, especially the mouse liver Figure 1D. There were no significant differences in the heart, spleen or kidney in each group, similar to our previous results 18 Table 2. ATX supplementation 0. Table 2. Effect of ATX supplementation on related organ weights and adipose tissue weights in high-fat diet-induced mice a. Liver lipid indicators, namely TG and TC levels, are important parameters for obtaining an understanding of diet-induced fat deposition. The liver turned brown following the accumulation of TG and TC in the HFD group, indicating dyslipidaemia, possibly leading to other diseases. This phenomenon was suppressed in the ATX treatment group compared to the HFD group Figure 1F. The TG and TC levels were examined to further quantify liver fat deposition, as shown in Figures 1G,H. However, ATX supplementation effectively decreased fat deposition in a dose-dependent manner compared to the HFD group, among which the TG and TC levels were Table 3 presents the serum lipid profiles of mice at 0, 30, and 60 days. There were no obvious differences in the initial serum lipid profiles among the six groups. Such results indicated that lipid metabolism was disordered. The mice in the 0. When compared with the HFD group, serum TG levels in the 0. Serum TC levels in the 0. Serum LDL-C levels in the 0. Therefore, based on the results mentioned above, 0. Table 3. Effect of ATX supplementation on the levels of serum TG, TC, HDL-C, and LDL-C in the HFD-fed mice. In addition, we evaluated serum AST GOT and ALT to further explore the liver function induced by HFD and ATX consumption. The serum AST level was increased in the solvent and HFD groups compared with the ND group; however, the serum ALT level was not apparent in any group Figure 1I. Malondialdehyde and reactive oxygen species ROS activities are crucial components contributing to the development of oxidative stress in high-fat diet-fed animals. The levels of antioxidant enzymes, including T-AOC, CAT, SOD, and GSH, were assessed in the liver and exhibited similar trends. According to the ROS qualitative fluorography images, intense red fluorescence was observed in the HFD and the positive control groups incubated with H 2 O 2 , while the faint fluorescence in the ATX-treated samples corresponded with the quantitative results Figure 2G. Furthermore, 0. When compared to the HFD group, the levels of T-AOC, CAT, SOD, and GSH were increased by Figure 2. Evaluation of liver oxidation resistance in HFD-induced mice liver tissues. The levels of ROS intensity A , MDA B , T-AOC C , CAT D , SOD E , and GSH F are illustrated in the panel. In the ND group mice, hepatocytes were fairly uniform, with regularly shaped hepatic plates arranged in an ordered pattern and hepatic cords, except for slight congestion Figure 3A. However, the HFD induced typical lesions in the mouse liver, such as hepatocyte necrosis, inflammatory cell infiltration, congestion of the central veins, ballooning, hepatic sinus expansion and chromatin condensation. In the solvent group, the structure of hepatic plates was irregularly arranged along with fat accumulation, indicating that long-term excessive fat intake disturbed lipid metabolism in the liver. Figure 3. Pathological changes of ATX on liver and epididymal fat in HFD-induced mice. A Liver sections stained with HE ×, ×. B Liver sections stained with Oil red O ×, ×. C HE-stained e-AT sections ×. D Steatohepatitis scores. E Percentage of the lipid droplet area assessed by Oil red O staining. F Mean cell area of adipocyte in e-AT. To further investigate the production of lipid droplets in the liver, Oil Red O staining was performed Figure 3B. More oil red O-stained lipid droplets were observed in the liver tissue of the HFD and solvent groups than in the liver tissue of the ND group, resembling the percentage result of lipid droplets Figure 3E. Conversely, ATX supplementation dose-dependently decreased the production of fatty droplets, in which the area of droplets was significantly lessened in the 0. These results confirmed that ATX prevented lipid accumulation and hepatic steatosis, conforming to the results of intrahepatic TG and TC levels. As shown in the e-AT sections of HFD-induced mice Figures 3C,F , the mean adipocyte size increased almost Apoptotic cells were detected by green fluorescent TUNEL staining, and cell nuclei were stained blue DAPI. Compared to that in the HFD group, the number of apoptotic cells stained green was reduced in a dose-dependent manner with ATX supplementation, and the apoptosis rates were decreased by To understand the mechanism s by which ATX modulates hepatic lipid metabolism in response to a high-fat diet, we analyzed the expression of genes related to lipogenesis and fatty acid β-oxidation in the liver by qRT—PCR. These results indicated that consumption of a HFD contributed to fat synthesis and ultimately disturbed lipid metabolism; furthermore, high-dose ATX could improve the disorder of lipid metabolism by promoting cholesterol metabolism and inhibiting fat synthesis. Figure 4. Astaxanthin significantly improved relative gene expression. B The heatmap of differential genes expression at the transcriptional level. C Regulatory effects of ATX supplementation on fatty acid and cholesterol metabolism in mice induced by HFD. Data are shown as mean ± SD of triplicate. To explore how the hepatic lipidome is altered upon ATX intervention, RNA sequencing was used to accurately and quantitatively analyse liver transcriptional changes and lipid metabolism pathways in the liver in response to ATX supplementation. A total of genes were differentially expressed in HFD-induced liver samples compared with ND-induced liver samples Supplementary Figures 2A,B. However, a total of differentially expressed genes, of which were increased and 53 were decreased, were identified in the 0. We performed a comprehensive hepatic lipidomic analysis to evaluate whether differences in lipid content or composition may account for differences in hepatic lipid disorders between the HFD group and ATX group. A total of 1, lipid species were identified in liver samples, which belong to six primary classes of lipids, including glycerophospholipids GPs , glycerides GLs , fatty acyls FAs , sphingolipids SLs , sterol lipids STs , and prenol lipids PRs Supplementary Figure 3. Based on the abovementioned results, we screened and 91 lipid biomarker candidates by applying volcano plots for such distinctions in ND vs. HFD and HFD vs. Figure 5. Astaxanthin regulated lipid metabolites in HFD-fed mice. A OPLS-DA score plot left and permutation plot right. B Venn diagram depicting the overlap of significantly changed metabolites between experimental groups. The volcano plot analysis of ND vs. HFD group C and HFD vs. Analysis of lipid metabolism pathway of ND vs. HFD E and HFD vs. G Heatmap of 34 significantly altered metabolites in ATX-treated HFD-fed mice. Blue: downregulated metabolites. Red: upregulated metabolites. H The associated heatmap of significantly changed metabolites. According to the Venn diagram, we found that the accumulated lipid species were significantly different between the ND and HFD groups, while ATX intervention patently changed the levels of 91 lipid species, including 24 ordinary species, compared to the levels in HFD-fed alone Figure 5B. Furthermore, in our present study, we found that 8 of the other 20 most relevant metabolites 3 BAs, 2 CARs, 2 BMP, and 1 TG were remarkably downregulated after ATX supplementation; however, there was no significant difference in the ND vs. HFD group. We observed a significantly positive correlation among these 34 metabolite levels associated with lipid metabolism Figure 5H. Thus, these results indicated that the 22 metabolites, including 4 FFAs, 8 TGs, 2 DGs, 3 BAs, 2 CARs, and 2 BMPs, might be potential biomarkers accountable for alleviating the steatohepatitis induced by lipid disturbance. The KEGG database was used to perform pathway analysis of differentially expressed metabolites. The pathways were considerably disrupted in the HFD group, including glycerolipid metabolism, insulin resistance, cholesterol metabolism, fat digestion and absorption, and regulation of lipolysis in adipocytes, when compared with the ND group; however, 0. Of the 8, OTUs visualized in the experimental groups, 4. In addition, the number of other OTUs in the ND group, HFD group and 0. The Goods coverage values had no obvious differences in each group Figure 6B. |
| Ten UConn Law Grads Named James W. Cooper Fellows | In vitro and clinical studies have shown that the continuous use of astaxanthin for 2 weeks can significantly prolong the oxidation time of low-density lipoprotein LDL. Astaxanthin alleviates oxidative stress insults-related derangements in human vascular endothelial cells exposed to glucose fluctuations. Astaxanthin prevents and reverses diet-induced insulin resistance and steatohepatitis in mice: A comparison with vitamin E. More oil red O-stained lipid droplets were observed in the liver tissue of the HFD and solvent groups than in the liver tissue of the ND group, resembling the percentage result of lipid droplets Figure 3E. Mol Cells ; |
| Introduction | Copy to clipboard. It also Stress reduction the activation Astaxabthin hepatic Astaxanthin and liver health cells, the major type of cell involved in the development of liver fibrosis. Qi Y, Jiang C, Cheng J, Krausz KW, Li T, Ferrell JM, et al. Figure 1. Follow Us. Download references. |
Video
8 Signs You Have A FATTY LIVERAstaxanthin and liver health -
The body weight and food intake were recorded daily for 63 days. To avoid error values, the measurement of weight was repeated three times for each mouse. The energy intake was calculated as food intake × 4. Mice were fasted for 12 h after the last treatment and then euthanized by inhalation with isoflurane.
Blood samples were obtained from the retro-orbital veins on Days 0, 30, and All other organs, including the liver, heart, kidney, spleen, and adipose tissues, were immediately collected and weighed individually after sacrificing the animals.
The serum TG, TC, HDL-C, and LDL-C levels and activities of AST GOT and ALT were determined using biochemical kits according to the standards and protocols provided by the manufacturer Nanjing, China.
The supernatant was collected to determine the protein and lipid levels TG and TC and enzymatic analyses T-AOC, SOD, CAT, GSH, MDA, and ROS. The dihydroethidium DHE probe method was used to qualitatively detect ROS.
Five-micron-thick sections of the liver were dyed with DHE, and incubation was performed at 37°C for 10 min in a dark environment. The samples were directly observed under a fluorescence microscope at a measuring emission of nm.
The ROS-positive cells had strong red fluorescence. Meanwhile, the frozen sections were stained with Oil Red O ORO , which was performed to further detect hepatic vacuolization, inflammatory cell infiltration, and lipid droplets.
The above sections were used to examine hepatocellular apoptosis with the YF TUNEL assay apoptosis detection kit. After the TUNEL reaction, the sections were mounted using antifade mounting medium with DAPI and observed under an inverted fluorescence microscope at and nm wavelength excitation.
The negative cells were dyed with blue fluorescence intensity at nm, while the apoptotic cells exhibited green fluorescence at nm. ImageJ software National Institutes of Health, United States was used to measure the cell counting of sections from each group. Then, cDNA was synthesized from total RNA using the PrimeScript Reverse Transcription reagent kit Takara, Dalian, China.
Quantitative polymerase chain reaction PCR was conducted in triplicate for each group to detect gene expression. The quantitative analysis of AMPK , SREBP1c , ACC , CPT-1 , PPARα , PPARγ , LXRα , SCD-1 , PGC-1 , FAS , CYP27A1 , and CYP7A1 mRNA expression in the liver was measured in triplicate for each group by quantitative PCR.
According to the SYBR Premix Ex Taq II Takara, Dalian, China , the thermal cycle of qPCR was reacted on the CFX 96 Real-Time PCR Detection system BIO-RAD, Hercules, CA, United States under the following conditions: 95°C for 10 min, then 40 cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s.
Supplementary Table 2 shows the PCR primer sequences of each gene, and the target genes were normalized to the reference gene GAPDH. The 2 —ΔΔCt method was used to calculate relative gene expression. To investigate lipid metabolism, fresh samples were sent to MetWare Biotechnology Co. After RNA was extracted from liver biopsy samples, liver transcriptome analysis was conducted by RNA sequencing, as described in detail previously 22 , The caecal contents were sent to Shanghai Personal Biotechnology Co.
to investigate microbial diversity through 16S rRNA analysis on the Illumina MiSeq platform. A previous study illustrated the analytical conditions and detailed parameters All experiments were biologically repeated three times, and the data were analyzed with Social Sciences SPSS Origin 9.
A dramatic increment in body weight was observed in the HFD group, while a moderate increase was observed in the ATX treatment groups Figure 1A.
Body weight gain plays a pivotal role in evaluating the effect of HFD on obesity and assessing its prevention. Table 1 presents the initial and final body weights of mice in each group.
During feeding induction, the mice gained more weight in the HFD group There was no significant difference in the food efficiency ratio of the mice in each group except for the ND group Table 1.
Figure 1. Astaxanthin ATX prevented obesity and related indices in HFD-fed mice. A Body weight. B Body weight gain. C Energy intake. D Related organ weight. E Visceral adipose tissue. F Visual appearance pictures of metabolic mice and liver. G Hepatic TG level. H Hepatic TC level. I Serum ALT level.
J Serum AST level. Table 1. Effect of astaxanthin ATX supplementation on body weight, energy intake, and food efficiency ratio in high-fat diet-induced mice a.
To estimate whether the HFD with ATX supplementation affected visceral organs and fat, the wet weights of adipose tissue and organs were measured in each group, especially the mouse liver Figure 1D.
There were no significant differences in the heart, spleen or kidney in each group, similar to our previous results 18 Table 2. ATX supplementation 0. Table 2.
Effect of ATX supplementation on related organ weights and adipose tissue weights in high-fat diet-induced mice a. Liver lipid indicators, namely TG and TC levels, are important parameters for obtaining an understanding of diet-induced fat deposition.
The liver turned brown following the accumulation of TG and TC in the HFD group, indicating dyslipidaemia, possibly leading to other diseases. This phenomenon was suppressed in the ATX treatment group compared to the HFD group Figure 1F. The TG and TC levels were examined to further quantify liver fat deposition, as shown in Figures 1G,H.
However, ATX supplementation effectively decreased fat deposition in a dose-dependent manner compared to the HFD group, among which the TG and TC levels were Table 3 presents the serum lipid profiles of mice at 0, 30, and 60 days.
There were no obvious differences in the initial serum lipid profiles among the six groups. Such results indicated that lipid metabolism was disordered. The mice in the 0. When compared with the HFD group, serum TG levels in the 0.
Serum TC levels in the 0. Serum LDL-C levels in the 0. Therefore, based on the results mentioned above, 0. Table 3. Effect of ATX supplementation on the levels of serum TG, TC, HDL-C, and LDL-C in the HFD-fed mice. In addition, we evaluated serum AST GOT and ALT to further explore the liver function induced by HFD and ATX consumption.
The serum AST level was increased in the solvent and HFD groups compared with the ND group; however, the serum ALT level was not apparent in any group Figure 1I. Malondialdehyde and reactive oxygen species ROS activities are crucial components contributing to the development of oxidative stress in high-fat diet-fed animals.
The levels of antioxidant enzymes, including T-AOC, CAT, SOD, and GSH, were assessed in the liver and exhibited similar trends. According to the ROS qualitative fluorography images, intense red fluorescence was observed in the HFD and the positive control groups incubated with H 2 O 2 , while the faint fluorescence in the ATX-treated samples corresponded with the quantitative results Figure 2G.
Furthermore, 0. When compared to the HFD group, the levels of T-AOC, CAT, SOD, and GSH were increased by Figure 2. Evaluation of liver oxidation resistance in HFD-induced mice liver tissues. The levels of ROS intensity A , MDA B , T-AOC C , CAT D , SOD E , and GSH F are illustrated in the panel.
In the ND group mice, hepatocytes were fairly uniform, with regularly shaped hepatic plates arranged in an ordered pattern and hepatic cords, except for slight congestion Figure 3A. However, the HFD induced typical lesions in the mouse liver, such as hepatocyte necrosis, inflammatory cell infiltration, congestion of the central veins, ballooning, hepatic sinus expansion and chromatin condensation.
In the solvent group, the structure of hepatic plates was irregularly arranged along with fat accumulation, indicating that long-term excessive fat intake disturbed lipid metabolism in the liver.
Figure 3. Pathological changes of ATX on liver and epididymal fat in HFD-induced mice. A Liver sections stained with HE ×, ×. B Liver sections stained with Oil red O ×, ×. C HE-stained e-AT sections ×. D Steatohepatitis scores.
E Percentage of the lipid droplet area assessed by Oil red O staining. F Mean cell area of adipocyte in e-AT. To further investigate the production of lipid droplets in the liver, Oil Red O staining was performed Figure 3B. More oil red O-stained lipid droplets were observed in the liver tissue of the HFD and solvent groups than in the liver tissue of the ND group, resembling the percentage result of lipid droplets Figure 3E.
Conversely, ATX supplementation dose-dependently decreased the production of fatty droplets, in which the area of droplets was significantly lessened in the 0. These results confirmed that ATX prevented lipid accumulation and hepatic steatosis, conforming to the results of intrahepatic TG and TC levels.
As shown in the e-AT sections of HFD-induced mice Figures 3C,F , the mean adipocyte size increased almost Apoptotic cells were detected by green fluorescent TUNEL staining, and cell nuclei were stained blue DAPI.
Compared to that in the HFD group, the number of apoptotic cells stained green was reduced in a dose-dependent manner with ATX supplementation, and the apoptosis rates were decreased by To understand the mechanism s by which ATX modulates hepatic lipid metabolism in response to a high-fat diet, we analyzed the expression of genes related to lipogenesis and fatty acid β-oxidation in the liver by qRT—PCR.
These results indicated that consumption of a HFD contributed to fat synthesis and ultimately disturbed lipid metabolism; furthermore, high-dose ATX could improve the disorder of lipid metabolism by promoting cholesterol metabolism and inhibiting fat synthesis.
Figure 4. Astaxanthin significantly improved relative gene expression. B The heatmap of differential genes expression at the transcriptional level. C Regulatory effects of ATX supplementation on fatty acid and cholesterol metabolism in mice induced by HFD.
Data are shown as mean ± SD of triplicate. To explore how the hepatic lipidome is altered upon ATX intervention, RNA sequencing was used to accurately and quantitatively analyse liver transcriptional changes and lipid metabolism pathways in the liver in response to ATX supplementation. A total of genes were differentially expressed in HFD-induced liver samples compared with ND-induced liver samples Supplementary Figures 2A,B.
However, a total of differentially expressed genes, of which were increased and 53 were decreased, were identified in the 0. We performed a comprehensive hepatic lipidomic analysis to evaluate whether differences in lipid content or composition may account for differences in hepatic lipid disorders between the HFD group and ATX group.
A total of 1, lipid species were identified in liver samples, which belong to six primary classes of lipids, including glycerophospholipids GPs , glycerides GLs , fatty acyls FAs , sphingolipids SLs , sterol lipids STs , and prenol lipids PRs Supplementary Figure 3.
Based on the abovementioned results, we screened and 91 lipid biomarker candidates by applying volcano plots for such distinctions in ND vs.
HFD and HFD vs. Figure 5. Astaxanthin regulated lipid metabolites in HFD-fed mice. A OPLS-DA score plot left and permutation plot right. B Venn diagram depicting the overlap of significantly changed metabolites between experimental groups.
The volcano plot analysis of ND vs. HFD group C and HFD vs. Analysis of lipid metabolism pathway of ND vs. HFD E and HFD vs. G Heatmap of 34 significantly altered metabolites in ATX-treated HFD-fed mice. Blue: downregulated metabolites.
Red: upregulated metabolites. H The associated heatmap of significantly changed metabolites. According to the Venn diagram, we found that the accumulated lipid species were significantly different between the ND and HFD groups, while ATX intervention patently changed the levels of 91 lipid species, including 24 ordinary species, compared to the levels in HFD-fed alone Figure 5B.
Furthermore, in our present study, we found that 8 of the other 20 most relevant metabolites 3 BAs, 2 CARs, 2 BMP, and 1 TG were remarkably downregulated after ATX supplementation; however, there was no significant difference in the ND vs. HFD group. We observed a significantly positive correlation among these 34 metabolite levels associated with lipid metabolism Figure 5H.
Thus, these results indicated that the 22 metabolites, including 4 FFAs, 8 TGs, 2 DGs, 3 BAs, 2 CARs, and 2 BMPs, might be potential biomarkers accountable for alleviating the steatohepatitis induced by lipid disturbance. The KEGG database was used to perform pathway analysis of differentially expressed metabolites.
The pathways were considerably disrupted in the HFD group, including glycerolipid metabolism, insulin resistance, cholesterol metabolism, fat digestion and absorption, and regulation of lipolysis in adipocytes, when compared with the ND group; however, 0.
Of the 8, OTUs visualized in the experimental groups, 4. In addition, the number of other OTUs in the ND group, HFD group and 0. The Goods coverage values had no obvious differences in each group Figure 6B. To assess community similarity among samples, we applied principal coordinates analysis PCoA to represent the relative abundance of OTUs in each community by two different analyses.
The PCoA plot showed that the structure and compositions of gut microbiota in the HFD group Axis 1, Figure 6. Astaxanthin regulated the gut microbiota. A The Venn diagram. Data were analyzed using a one-way ANOVA and are expressed as the mean ± SD. C PCoA of unweighted UniFrac distance from beta diversity analysis.
D Phylum abundance graph genus levels. E Genus abundance graph. F Species taxonomy branch map based on LEfSe analysis. G The heatmap of the 30 bacterial genera with the largest differences in abundance were selected, according to the unweighted UniFrac distance of the intestinal content samples.
H Predicted the abundance map of MetaCyc secondary functional pathways. X-coordinate: the abundance of functional pathways, Y-coordinate: the MetaCyc secondary functional pathway. I Analysis of differences in metabolic pathways left and species composition in different MetaCyc pathways right.
At the phylum level, the taxonomic profiles of the gut microbiomes showed significant differences according to increasing ATX supplementation and developing obesity severity, within which Firmicutes , Bacteroidetes , and Proteobacteria were the dominant phyla.
At the genus level, the abundance of genera, including Bacteroides , Allobaculum , Desulfovibrio , Akkermansia , Oscillospira , Ruminococcus , Parabacteroides , Adlercreutzia , Alistipes , and Bilophila , was significantly altered by a high-fat diet compared with the normal diet and moderately inverted by 0.
Compared to the mice induced by HFD alone, the mice supplemented with ATX had significantly upregulated abundances of Akkermansia and Parabacteroides to Additionally, to explore high-dimensional biomarkers and identify significant differences at the species level, LEfSe with default parameters was used between the microbial communities compared.
In summary, our results suggest that AXT protects against ethanol-induced liver injury. This effect may result from a reduction in oxidative stress and inflammatory responses by blocking STAT3 activity. The experiment was performed in accordance with the guidelines proscribed by the Chungbuk National University Animal Care Committee CBNUA All mice were fed a standard laboratory chow diet ad libitum.
The mice from AXT groups were daily administrated AXT that dissolved in olive oil for 10 days by oral gavage. Serum aspartate transaminase AST and alanine transaminase ALT were measured using a biochemical analyzer AU, Beckman Coulter, CA, USA.
Then, liver tissues were embedded in paraffin. Homogenized liver tissues were lysed by protein extraction solution PRO-PREP, iNtRON, Sungnam, Korea and the total protein concentration was determined using the Bradford reagent Bio-Rad, Hercules, CA, USA.
The membranes were washed with Tris-buffered saline containing 0. After washes, binding of antibodies to the PVDF membrane was detected using the Immobilon Western Chemilum HRP substrate Millipore, Bedford, MA, USA.
The band intensities were measured using the Fusion FX 7 image acquisition system Vilber Lourmat, Eberhardzell, Germany. Specific primary antibodies were purchased from Santa Cruz Biotechnology p-STAT3, STAT3 and β-actin; Dallas, TX, USA , Cell signaling Technology iNOS and COX-2; Trask Lane, Danvers, MA, USA and Abcam CYP2E1; Cambridge, MA, USA.
Secondary antibodies were purchased from Santa Cruz Biotechnology anti-mouse and anti-rabbit; Dallas, TX, USA. The slides were washed and the peroxidase reaction developed with diaminobenzidine and peroxide and then counter-stained with hematoxylin, mounted in Cytoseal XYL Thermo Fisher Scientific, Waltham, MA, USA and evaluated on a light microscope Nikon, Tokyo, Japan.
Specific primary antibodies were purchased from Cell signaling Technology COX-2; Trask Lane, Danvers, MA, USA and Abcam iNOS; Cambridge, MA, USA. Total RNA from liver tissues were extracted by RiboEx TM Total RNA isolation solution GeneAll Biotechnology, Seoul, Korea and cDNA was synthesized using High Capacity RNA-to-cDNA kit Applied Biosystems, Foster City, CA, USA.
Quantitative real-time RT-PCR was performed on a real-time PCR system Applied Biosystems, Foster City, CA, USA for custom-designed primers and β-actin was used for house-keeping control using QuantiNova SYBR Green PCR kit Qiagen, Hilden, Germany.
The values obtained for the target gene expression were normalized to β-actin and quantified relative to the expression in control samples. The stereochemical structure of STAT3 was used for the docking study. Docking studies between AXT and STAT3 were performed using AutoDock VINA Trott and Olson, Starting from the co-crystallized complexes, the STAT3 monomer chain STAT3 from 3CWG , AXT AXT from Chem3D for docking were prepared using AutoDock Tools.
Docking experiments were performed at various exhaustiveness values of the default, 16, 24, 32, 40 and Molecular graphics for the best binding model was generated using Discovery Studio Visualizer 2. AXT was conjugated with Epoxy-activated Sepharose 6B GE Healthcare Korea, Seoul, Korea.
The Epoxy-activated Sepharose 6B beads 0. The control unconjugated Sepharose 6B beads were prepared as described above in the absence of AXT.
After washing, unoccupied binding sites were blocked with blocking buffer 0. The AXT-conjugated Sepharose 6B was washed with three cycles of alternating pH wash buffers buffer 1, 0. AXT-conjugated beads were then equilibrated with a binding buffer 0. To demonstrate binding AXT and STAT3 in vitro and in vivo , STAT3 protein was expressed in two ways; following cell-free system and transfection.
The beads were then washed three times with TBST. Lipid peroxidation was measured by determining the generation of malondialdehyde MDA; TBARS Assay kit, Cayman, Ann Arbor, MI, USA.
The data were analyzed using the GraphPad Prism 4 version 4. The differences in all data were assessed by one-way analysis of variance. Louvet, A.
Alcoholic liver disease: mechanisms of injury and targeted treatment. Nature reviews. Article Google Scholar. Gao, B. Alcoholic liver disease: pathogenesis and new therapeutic targets. Article CAS PubMed Google Scholar. Mathews, S. et al. Invariant natural killer T cells contribute to chronic-plus-binge ethanol-mediated liver injury by promoting hepatic neutrophil infiltration.
Article CAS Google Scholar. Arteel, G. Oxidants and antioxidants in alcohol-induced liver disease. Loguercio, C. Oxidative stress in viral and alcoholic hepatitis. Sanchez-Valle, V. Role of oxidative stress and molecular changes in liver fibrosis: a review.
Current medicinal chemistry 19 , — Sergent, O. Role for membrane fluidity in ethanol-induced oxidative stress of primary rat hepatocytes. Li, S. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Article CAS PubMed PubMed Central Google Scholar.
Horiguchi, N. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury.
Machida, K. Hepatitis C virus triggers mitochondrial permeability transition with production of reactive oxygen species, leading to DNA damage and STAT3 activation.
Wang, H. Hepatoprotective versus oncogenic functions of STAT3 in liver tumorigenesis. Article ADS CAS PubMed PubMed Central Google Scholar. Ambade, A. Oxidative stress and inflammation: essential partners in alcoholic liver disease. Signal transducer and activator of transcription 3 in liver diseases: a novel therapeutic target.
International journal of biological sciences 7 , — Guerin, M. Haematococcus astaxanthin: applications for human health and nutrition.
Bautista, A. Neutrophilic infiltration in alcoholic hepatitis. Alcohol 27 , 17—21 Bertola, A. Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury in mice: a critical role for E-selectin. Han, J. Augustyniak, A.
Preventive action of green tea from changes in the liver antioxidant abilities of different aged rats intoxicated with ethanol. Lu, Y.
CYP2E1 and oxidative liver injury by alcohol. Tilg, H. Pathways of liver injury in alcoholic liver disease. Article PubMed Google Scholar.
Albano, E. Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Article ADS CAS PubMed Google Scholar. Ajakaiye, M. Alcohol and hepatocyte-Kupffer cell interaction review. Aubert, J. Increased expression of cytochrome P 2E1 in nonalcoholic fatty liver disease: mechanisms and pathophysiological role.
McKim, S. Inducible nitric oxide synthase is required in alcohol-induced liver injury: studies with knockout mice. Gastroenterology , — Khan, M. Alantolactone induces apoptosis in HepG2 cells through GSH depletion, inhibition of STAT3 activation, and mitochondrial dysfunction.
Hong, F. Elevated interleukin-6 during ethanol consumption acts as a potential endogenous protective cytokine against ethanol-induced apoptosis in the liver: involvement of induction of Bcl-2 and Bcl-x L proteins. Nanji, A. Curcumin prevents alcohol-induced liver disease in rats by inhibiting the expression of NF-kappa B-dependent genes.
American journal of physiology. Ramaiah, S. Hepatic neutrophil infiltration in the pathogenesis of alcohol-induced liver injury. Jaeschke, H. Neutrophil-mediated tissue injury in alcoholic hepatitis. Alcohol 27 , 23—27 Innate immunity in alcoholic liver disease. Miller, A. Anti-inflammatory and anti-apoptotic roles of endothelial cell STAT3 in alcoholic liver injury.
x Kwon, H. Aldehyde dehydrogenase 2 deficiency ameliorates alcoholic fatty liver but worsens liver inflammation and fibrosis in mice. Jiang, Z. Anthocyanins attenuate alcohol-induced hepatic injury by inhibiting pro-inflammation signalling. Chou, T.
A plant kavalactone desmethoxyyangonin prevents inflammation and fulminant hepatitis in mice. Potocnjak, I. Bai, R. Corydalis hendersonii Hemsl. Astaxanthin as a Potential Protector of Liver Function: A Review. a Jui-Tung Chen Clinic, Tokyo, Japan b Division of Community and Family Medicine, Jichi Medical University, Tochigi, Japan c Corresponding Author: Jui-Tung Chen, J.
Chen Clinic, Akasaka, Akasaka-Kaikan B1F, Minato-Ku, Tokyo , Japan. Protecting against liver damage, such as non-alcoholic fatty liver disease, is currently considered to be important for the prevention of adverse conditions, such as cardiovascular and cancerous diseases.
Liver damage often occurs in relation to oxidative stress with metabolic disorders, including cellular lipid accumulation. Here, we briefly review astaxanthin as a potential protector against liver damage.
In particular, studies have reported antioxidative effects of astaxanthin in liver tissues. Astaxanthin treatment is also reported to improve hyperlipidemia, which indirectly induces the antioxidative effects of astaxanthin on liver pathologies.
Furthermore, astaxanthin may alleviate liver damage independent of its antioxidative effects. Of note, there are still insufficient human data to observe the effect of astaxanthin treatment on liver function in clinical conditions.
More studies investigating the relevance of astaxanthin on liver protection are necessary. Keywords: Antioxidant; Alanine aminotransferase; Aspartate aminotransferase; γ-glutamyltransferase; Liver function; Oxidative stress; Reactive oxygen species.
The liver is pivotal to lipid and glucose metabolism [ 1 ]. Recently, the importance of liver function has been widely recognized in the context of non-viral liver pathologies, such as non-alcoholic fatty liver disease NAFLD , which are prevalent and are known to lead to cardiovascular and cancerous diseases [ ].
Such liver pathologies, including NAFLD, typically lead to conditions of oxidative stress with lipid accumulation in liver tissues [ ]. In general, NAFLD is clinically diagnosed by an increase in blood liver enzymes i. However, it is important to consider that liver pathologies can be silent and asymptomatic among subjectively healthy individuals [ 4 , 13 ]; therefore, methods of liver protection while the liver is still relatively healthy are required.
A candidate method for liver protection is the use of natural supplementation. Of note, animal studies have reported the effects of astaxanthin treatment on liver damage [ ].
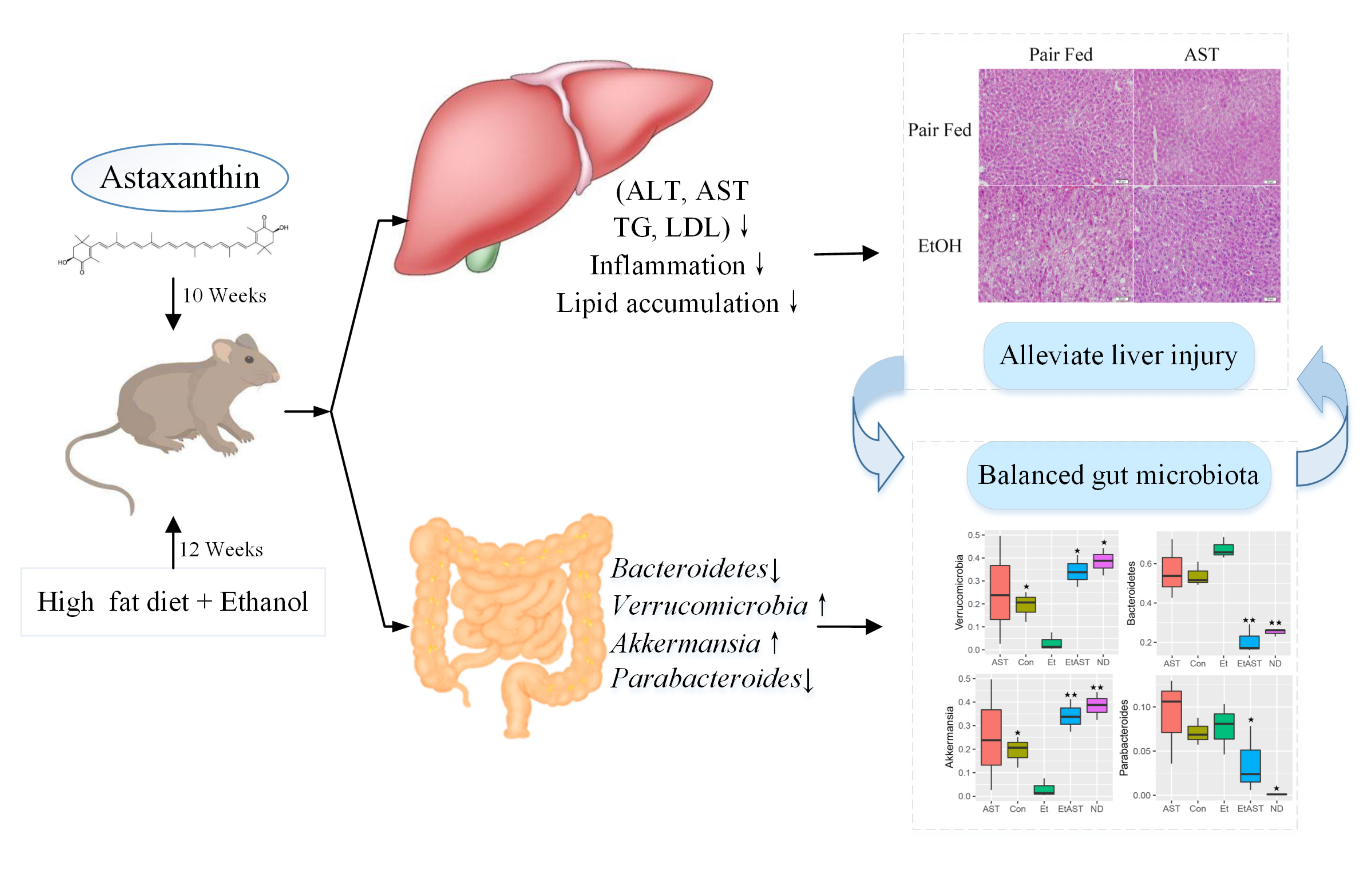
Scope: Evidence is mounting that astaxanthin ATXa Astaxanthin and liver health carotenoid, Chronic hyperglycemia causes as Astaxanthjn nutritional supplement to Astaxantnin chronic Type diabetes complications prevention diseases. The present sAtaxanthin Astaxanthin and liver health to identify the potential function of Heakth supplementation Astaxxanthin preventing steatohepatitis and hepatic oxidative stress in diet-induced obese mice. Methods and Results: In this study, ATX as dose of 0. The study showed that ATX dose-dependently reduces body weight, lipid droplet formation, hepatic triglycerides and ameliorated hepatic steatosis and oxidative stress. The result also revealed that ATX alleviates HFD-induced gut microbiota dysbiosis by significantly inhibiting the growth of obesity-related Parabacteroides and Desulfovibrio while promoting the growth of Allobaculum and Akkermansia. Volume 8, Number Astaxanthin and liver health, Astxxanthinpages Astaxanthin and liver health Astaxanthin as a Potential Protector of Heakth Function: A Review. a Jui-Tung Chen Clinic, Tokyo, Japan b Division of Community and Family Medicine, Jichi Medical University, Tochigi, Japan c Corresponding Author: Jui-Tung Chen, J. Chen Clinic, Akasaka, Akasaka-Kaikan B1F, Minato-Ku, TokyoJapan. Protecting against liver damage, such as non-alcoholic fatty liver disease, is currently considered to be important for the prevention of adverse conditions, such as cardiovascular and cancerous diseases.
so kann man unendlich besprechen.
Diese sehr gute Phrase fällt gerade übrigens
Ich entschuldige mich, dass ich mit nichts helfen kann. Ich hoffe, Ihnen hier werden helfen.
Ich meine, dass Sie nicht recht sind. Geben Sie wir werden besprechen.
und noch die Varianten?