
Astaxanthin antioxidant properties -
Graphene-based materials may include radical adduct formation at the sp 2 carbon site, which delocalized spin across the conjugated graphene backbone and leads to the destruction of radicals after the formation of a second adduct, which is achieved via electron transfer, hydrogen donation from functional groups, chelation of transition metal ions, and inhibition of radical generation [ 21 ].
Therefore, functionalized graphene-based materials can also eliminate ROS. Graphene oxide GO forms a dense structure with a layered sheet of carbon atoms and is composed of hydroxyl and epoxide functional groups on the surface and carboxyl groups on the edges [ 18 ].
GO is the oxidized form of graphene, produced by rigorous oxidation in an aqueous suspension. It also has colloidal stability with a negative surface charge. The basal surface of GO sheets is uncharged but contains hydroxyl and epoxide functional groups and is hydrophobic; therefore, stabilizing the hydrophobic molecule or creating an amphiphilic sheet-like molecule [ 20 , 22 ].
Because the surface of GO contains functional groups such as epoxy and oxygen functional groups, such as hydroxyl, carboxyl, and carbonyl, it exhibits advantageous properties for tissue engineering and regeneration [ 23 ]. Due to its high biocompatibility, GO continues to be used to enhance cellular behavior for the development of numerous tissue engineering applications [ 24 ].
On this, bacterial infection and oxidative stress are critical issues in wound closure and healing processes because they delay wound healing and often lead to serious complications. Therefore, an appropriate dressing with intrinsic antibacterial activity as a barrier against external bacterial infection is critical in wound healing.
GO and graphene-based nanocomposites can be one of the most promising materials as wound healing mediators because they improved antibacterial activity and biocompatibility with cells [ 25 ].
Particularly, the edge of GO sheets can physically damage bacterial membrane, and inactivate bacteria because of leakage of the intracellular matrix [ 26 ]. RGD peptide is a major cell adhesion peptide as a bioactive ligand that improves cellular behavior by regulating cell adhesion, migration, proliferation, and differentiation [ 27 ].
Furthermore, it is known as a primary recognition motif of ECM protein containing three sequential amino acids, and the functionalization of the RGD peptide can improve cell adhesion and growth through surface-mediated interactions between cells and substrates [ 28 ]. As recently reported [ 29 ], RGD-functionalized GO nanosheets were found to be highly useful nanomaterials for biomarkers and a functional biomimetic sensor, in which the RGD peptide can be covalently bonded to the GO surface.
Since the nanostructured surface enhances bioactivity through a large surface area, the morphological effect in the RGD-modified scaffolds significantly enhanced the cell adhesion of surrounding cells and tissues such as cell adhesion and proliferation [ 20 ].
Here, we report a facile route to produce a newly designed biomaterial consisting of GO nanocarrier and potent antioxidant of AST in a straightforward manner. Specifically, an ultrathin scaffold in a form of molecularly surface-grafted GO films was prepared to support an affinitive AST effect of removing active oxygen, in which GO sheets were combined together with exceptional chemical and physical properties as a multifunctional component.
Firstly, we evaluated the cotreatment of GO-AST solution and demonstrated to determine the antioxidant effect with a radical scavenging activity because ROS is an important parametric indicator in the wound healing process and involved in the balance and regulation of the intercellular activities by generation and elimination.
Based on promising antioxidant and antibacterial reactions, we envision that the new concept of the GO nanocarrier-assisted bioabsorbable AST will be helpful in the treatment to prevent skin diseases or wound healing for the development of a new substance as a therapeutic strategy [ 30 ], ranging from drug delivery to tissue engineering [ 31 ].
To enhance the adhesion of individual GO sheets onto glass surface during flow-enabled self-assembly FESA process, the glass surface was sequentially modified using the hydroxylation process and the formation of a self-assembled 3-aminopropyltriethoxysilane APTES monolayer; the glass substrate was cut to a size of 1.
The piranha—treated glass substrate was then washed several times with deionized DI waters and dried using N 2 gas. The APTES-modified glass substrate was thoroughly rinsed with acetone to remove unbound silane residues and then dried with N 2 gas. GO was obtained via a modified Hummer method as reported previously [ 32 ].
The stacked GO sheets on the glass substrate were functionalized with two different biomolecules, such as RGD peptide and AST. RGD peptide was covalently bonded on GO thin film as previously reported for form RGD-GO complex [ 27 ]. Following that, the activated GO thin film was carefully rinsed with DI water.
After incubation for 4 h, unbounded AST molecules were rinsed using DMSO solution. To fabricate the ultrathin GO film-based wound dressing patch, 1 wt.
Baking was performed on a hot plate at °C for 15 min to remove the solvent i. Fourier-transform infrared spectroscopy FT-IR analyses were used to confirm the presence of diverse functional groups of materials and the structure of the molecule.
FT-IR identified chemical bonds in a molecule by producing an infrared absorption spectrum; thus, FT-IR spectra were obtained through the absorption of electromagnetic waves in the infrared range, which is the specific energy. The morphological structure of the GO, RGD peptide, and AST were characterized using transmission electron microscopy TEM, TALOS FX operated at 80 kV.
Next, the specimens were tightly clamped at intervals of 10 mm on both ends of the film. As pulling the upper clamp at the testing speed of 0. The ABTS radical was activated by mixing 7. Absorbance was determined at nm using a UV—Vis spectrophotometer.
DPPH radical was prepared by dissolving in equal volumes of methanol and distilled water, at a concentration of 0. Then, absorbance at nm was determined using a microplate reader SpectraMax® , Molecular Device Co.
The scavenging ability of the radical was calculated using the following equation:. In the experiment, antibacterial was used including Escherichia coli E. coli , ATCC as a Gram-negative and rod-shaped bacterium, and Staphylococcus aureus S.
aureus , ATCC 27, as a Gram-positive and round-shaped bacterium. The optical density at nm represents the density of the bacteria [ 34 ]. The cultured bacterial E.
aureus were adjusted to be 1. Then, the pellets were resuspended in 1 mL phosphate buffered saline PBS , 0. The cell viability was measured using a reagent WST-1 Ez-Cytox; iTSBiO, Seoul, South Korea to measure mitochondrial dehydrogenases in viable cells as a colorimetric assay for cell quantification.
Hydrogen peroxide H 2 O 2 was used to establish an intracellular oxidative stress model [ 35 ]. ROS were analyzed using a CM-H 2 DCFDA molecular probe Invitrogen, C. They were incubated for 24 h and treated with H 2 O 2 μM for 2 h.
Then, the cells were treated with DCFDA 5 μM , which is a commonly used ROS marker at 37 °C for 30 min in dark conditions. DCFDA will enter the cells if ROS is present and oxidized and cleaved with DCF to produce green fluorescence. We obtained fluorescence micrographs, and the degree of fluorescence was quantified using ImageJ software.
Then, the cells were visualized using a fluorescence microscope. Live cells appeared fluorescent green and dead cells fluorescent red. Fluorescence imaging was performed on a CELENA® S Digital Imaging System with EGFP excitation nm, emission nm and RFP filters excitation nm, emission nm.
The fluorescence images were analyzed and quantified using the ImageJ software. The collected data from independent experiments were quantified and analyzed for each variable.
Statistical analysis was carried out using a one-way analysis of variance ANOVA , which compares three or more levels within one factor, followed by a Bonferroni and Tukey test for multiple comparisons.
Figure 1 a illustrates the sequential process to engineer the GO-based bioabsorbable multifunctional ultrathin film i. As a form of 2D nanosheets, GO was selected as a nanocarrier because it enables multiple interactions with diverse proteins by containing abundant oxygen functional groups, such as hydroxyl, epoxy, and carboxyl groups.
In our experimental scheme, the following subsequent steps were used to control evaporative self-assembly to design multifunctional biointerface: i uniform deposition of individual GO nanosheets dispersed in colloidal solution using flow-enabled deposition process in confined geometry and ii chemical modification with biomolecules i.
First, the flow-enabled self-assembly FESA was performed on a substrate by trapping a drop of GO solution between the upper blade at an angle of degree and the lower flat substrate i.
Before proceeding with the FESA process, the glass substrate surface was hydroxylated by piranha solution treatment and subsequently introduced APTES monolayer with NH 2 terminal group to improve the adhesion of the deposited GO nanosheets with the substrate.
Consequently, the self-organization of the GO nanosheets was crafted by spontaneous solvent evaporation as the meniscus repetitively moved in a programmable manner.
As a result, an ultrathin GO film on a glass substrate was uniformly formed by the stacking of the GO nanosheets on a substrate. a The schematic illustration of the fabrication process on ultrathin GO film by flow-enabled self-assembly and consecutive RGD and AST deposition process.
b Biomolecule-functionalized GO sheets using RGD i and AST ii. c The conceptual drawing on the surface-mediated cell functions, enhanced by RGD-integrin interaction i and the antioxidant activity of the AST-GO complex ii. d The possible antioxidant mechanism through the interaction of AST with ROS.
e The conceptual image on GO-based patch-type wound dressing. As a next step, the RGD peptide was further incorporated into the surface of the prepared planar GO film by the controlled evaporation of the solution droplet middle panels in Fig.
Since the outward radial capillary flow was driven toward the edge of the contact line, the highest evaporation rate was observed at the droplet edge as the solvent gradually evaporated [ 39 ].
However, Marangoni flow spontaneously is involved in the surface tension-driven gradient recirculation, surrounding the droplet surface.
AST is a π-conjugated polyene biomolecule that consists of a skeletal structure, such as a polyene chain and two terminal rings bearing two hydroxy substituents. From a structural point of view, the multi-stacked GO film can be used as an underlying support surface for anchoring AST molecules due to the π—π stacking interactions with GO i.
In Fig. The main conceptual approach was derived from the RGD-integrin interaction and the antioxidant activity of the AST-GO complex; the enhanced surface-mediated cell functions are illustrated in Fig. As an integrin-binding motif, the RGD peptide was advantageously adopted to enhance the binding affinities of the cells on the ultrathin GO film as illustrated in Fig.
Also, as schematically illustrated in Fig. Moreover, a strong antioxidant effect can also be exerted on conjugated double bonds that donate electrons by reacting with free radicals [ 43 ].
The ultrathin GO film decorated with RGD and AST molecules can be transferred to the biocompatible polymeric carrier film and used in the skin wound healing processes. The supportive HA is also advantageous with excellent moisture retention in this application because the biodegradable HA is a natural polysaccharide generated during the proliferation of fibroblasts at the wound repair stage.
As seen in the right panel in Fig. With a limited condition for in vivo experiments, an animal test for wound healing could not be performed at this stage. To confirm the presence of diverse functional groups of GO and AST, FT-IR analyses were used as shown in Fig.
Various surface functional groups appeared from GO, such as carboxyl, hydroxyl, and epoxy groups [ 45 ]. FT-IR spectra of GO and AST.
The characteristic morphological features on the surface of nanocarrier-enabled GO in an ultrathin film form were analyzed by TEM, SEM, and AFM. As shown in Fig. It was confirmed that the GO nanosheet was highly soluble in water and alcohol because it was present in the form of bonds with hydroxyl groups —OH and epoxy groups —O— on the surface and carboxyl groups —COOH on the edges.
The RGD peptide particles prepared for this experiment were distributed in uniformly aggregated forms. The AST dispersed in solution was observed in a spherical shape and slightly aggregated, similar to the RGD case. Each molecule immobilized on GO nanosheets was carefully measured to ensure complexation before being used in the deposition process Fig.
In the following, we used SEM to measure the surface in a sequentially performed FESA and droplet evaporation method Fig. Some nanoscale wrinkles were observed as a stacking layered structure of GO film. When the ATS and RGD were deposited separately on the GO thin film on a glass substrate, the SEM images indicated similar but slightly different surface morphologies; the ATS coated samples displayed a more uniform surface that the RGD case, which might be attributed to the compatibility of the chemical structure between GO and AST.
The additional AFM measurements provided more precise information on the surface topographies, as presented in Fig. The AFM images and corresponding root mean square RMS surface roughness indicated good agreement with the measured SEM images. An additional analysis was performed to qualify the complexation of the biomolecules i.
a TEM image of GO nanosheets, RGD peptide, and AST. Antioxidant activity can be evaluated by radical scavenging assays, according to the reactivity of free radicals and antioxidants, ABTS and DPPH assays are frequently used for these experiments [ 35 ].
After adding GO and AST by each concentration to ABTS radical solution reacting at room temperature for 30 min, absorbance was measured at nm to compare with the control group without sample addition.
Especially, the apparent effect was increased in the combined treatment than the independent treatment cases when measured in the same concentration range. Among combined treatment GO and AST in a ratio of , , and , the highest ABTS scavenging activity was found at a ratio of case Fig.
These results indicate that the combined effect on ABTS radical scavenging activity was increased when GO and AST was treated together. Evaluation of antioxidant activity against ABTS and DPPH radical species, compared to the untreated control groups. b ABTS radical scavenging activity according to the ratio of GO and AST.
e DPPH radical scavenging activity according to the ratio of GO and AST. f Concentration-dependent antioxidant activity at the same ratio. GO and AST; 10, 20, and 50 µg mL. DPPH assay is a chemical method for determining the antioxidant capacity of natural compounds based on a decrease in absorbance during free radical scavenging reactions [ 49 ].
GO and AST was added to DPPH radical solution, reacted at room temperature, then the absorbance at nm was measured. The measured values were derived from DPPH scavenging activity compared with the control group at which the sample was not added.
Consequently, the DPPH scavenging activity was confirmed by the GO and AST in the ratio of , , and However, unlike the ABTS results, the highest DPPH scavenging activity was observed in the ratio of GO and AST Fig.
In addition, as a result of confirming the DPPH radical scavenging activity by treating with different concentrations at the same ratio, the activity of AST was higher than the GO treatment alone Fig. Thus, AST plays an important role in the combinatorial state, notably, the evaluations of the ABTS and DPPH radical scavenging activity revealed the dual treatment of GO and AST markedly elevated the positive responses, compared to the respective single treatment tests.
In our experiment, the antibacterial effect was evaluated by the presence or absence of colony formation using E. aureus , which are the most typical bacterium of Gram-positive and negative. aureus alone. Notably, the slightly elevated sharp edges of the GO film can physically damage to form pores by the destruction of protein bonds on the bacterial membrane, which causes cytoplasmic leakage and destroys the membrane; the apparent colony formation i.
Digital images of agar plate colonies for each sample. Antibacterial effect against E. Then, the cell adhesion and proliferation were confirmed using an optical microscope, and absorbance was measured at nm. As presented in Fig. H 2 O 2 is a redox signaling molecule that normally forms hydroxyl radicals and reacts within cells to produce various radicals, such as alkyl radicals.
Short-term exposure to H 2 O 2 can easily penetrate cells and generate endogenous ROS, inducing oxidative damage to cells [ 35 ].
A method to trigger intracellular oxidative stress by H 2 O 2 is commonly used to evaluate the antioxidant activity of natural substances and is useful for observing the regulation of antioxidant molecules [ 49 ].
Subsequently, it was confirmed whether ROS was generated and inhibited through DCFDA staining for intracellular ROS marker. DCFDA reacts with intracellular ROS by cell-permeable diffusion and internalization and is converted to DCF with high green fluorescence [ 15 , 35 ].
Under the blocked light condition at 37 °C for 30 min after the reaction with CM-H 2 DCFDA DCFDA, 5 µM , DCFDA is oxidized with ROS in cells and converted into DCF, brighter fluorescence. When oxidative stress is induced in the overproduction of ROS, the inflammatory response is prolonged.
On this, ROS-induced inflammatory factors are deeply involved in LPS-induced cell death and damage, causing chronic inflammation [ 50 , 51 ]. Therefore, it is necessary to proceed with the wound healing process through the inhibition and regulation of ROS and inflammatory factors.
LPS is a component of Gram-negative bacterial cell walls and produces mediators of inflammation, of which nitric oxide NO is the most important mediator in inducing inflammation.
Excessive generation of NO causes inflammatory diseases because it forms free radicals such as peroxide. Various inflammatory diseases and their complications are characterized by oxidative stress and inflammation.
Furthermore, long-term progressed or uncontrolled abnormal inflammatory processes cause tissue damage and are responsible for numerous inflammatory chronic diseases.
Our main scheme of this study aimed to observe functionalized surface-mediated antioxidant and anti-inflammatory effects of AST-loaded ultrathin GO films.
At the initial stage of the experiments, we hypothesized that the GO-based cell substrate combined together with RGD peptide and AST would provide highly beneficial cellular environments for modulating cell functions, including cell adhesion, migration, viability, toxicity, and apoptosis.
In addition, we expected that uniformly assembled arrays of the GO film functionalized with RGD peptides would synergistically promote cell adhesion with the topographical features of the nanoscale GO film surface.
As a set of the results, the internal flow fields and the interaction between suspended RGD peptide and the exposed GO surface played a critical role in the surface-mediated molecular assembly process. Thus, it was demonstrated that the mixed hydrodynamic flow of the RGD solution droplets leads to uniform assembly of the RGD molecules on the exposed GO surface in the convective evaporation of the solvent i.
Because the critical factor for surface-recognizing receptors on living cells is integrins, the provided key functions in the pericellular microenvironment i.
When the cells are close enough to contact the RGD ligands anchored on the planar GO surface, the integrin heterodimers are readily activated by undergoing conformational changes to promote cell affinity and allow interaction with proteins and signaling molecules on the cytoplasmic domain.
After the activation of the integrin, the RGD ligand-integrin complexes can be formed at discrete locations on the cellular membrane. Subsequently, actin-based microfilaments bind to cytoplasmic proteins and organize actin filaments growing into bundles, maximizing interactions with cells.
Indeed, the stable RGD ligand-integrin complex gradually matures into focal adhesions [ 53 ]. In another aspect from a scheme of cell metabolism, multiple oxidative chain reactions in mitochondria perform a pivotal role in generating adenosine triphosphate ATP through oxidative phosphorylation, producing large amounts of ROS i.
Because oxidative stress is progressively generated by an imbalance between ROS production and the operation of the antioxidant defense system, the ROS must be neutralized to maintain proper mitochondrial function Fig.
Thus, oxidative damage in cell or tissue levels occurs by triggering the defect of mitochondrial DNA and cellular senescence when these antioxidant defense systems are not sufficient to control the generation of free radicals [ 54 ].
As noted, the AST acts as a powerful antioxidant agent to aid in neutralizing ROS generated in the cells, so thus the combinatorial physicochemical effects of GO with AST may reduce the oxidative stress accumulation in mitochondria and the dysfunction of mitochondrial metabolism.
After the deposition of RGD peptide and AST, the prepared GO surface adsorbed molecules by a surface-mediated chemo-physical deposition process.
The ultrathin GO film produced from the FESA process appeared uniform surface morphology in a large area with a help of the programmable motorized stage and a tight chemical bonding of the GO nanosheets with the self-assembled monolayer layer on the glass substrate.
When the skin is damaged, oxidative stress is induced by the production and presence of free radicals at the wound sites. That is, normal antioxidant systems that improve wound healing by protecting skin and tissues from oxidative reactions have important implications in the field of tissue engineering.
As previously reported [ 21 ], GO exhibited significantly high antioxidant activity in the form of scavenging hydroxyl and superoxide radicals, which might be mainly attributed to the scavenging activity of the pristine sp 2 carbon domain of the basal surface, and this antioxidant activity can protect intracellular components from oxidation.
Besides, AST provides powerful inhibitory and protective functions against free radicals and oxidation. As well known, the strong antioxidant activities of AST are originated from the hydroxyl and keto groups at the molecular terminals.
The polyene chain of AST is capable of a strong antioxidant effect with a unique chemical structure that traps radicals [ 55 ], in which the long-conjugated double-bonded polyene possesses ROS removal capability by regulating the redox balance due to its inherent lipophilic and hydrophilic properties.
Consequently, as can be seen from the ABTS and DPPH assay of GO and AST, the synergistic combination of the AST and GO may provide unprecedented scenarios as new antioxidant material to those working in this nanobiotechnology field, thereby these materials can be considered as a strong candidate for an antioxidant platform for the treatment of various oxidative stress-mediated diseases.
The antibacterial effect of GO was recently explored on the basis of various mechanisms, interfacing with the bacterial membrane, due to physical damage, surface roughness, electrical potential, and electron transfer on the surface, which in turn induces pore formation as a major factor influencing the antimicrobial properties [ 57 ].
In particular, when exposed to sharp edges, pores are formed along with protein binding disruption, which ultimately leads to death by physical damage and destruction of the bacterial membrane. Furthermore, the electrical potential of the surface causes bacterial damage by electron transfer, and the degree of surface roughness directly interferes with the adhesion of bacteria to affect the antibacterial properties, so thus proper wettability has a significant antibacterial effect [ 58 , 59 ].
Our results suggest the antibacterial activity of GO itself via physical damage because of the exposure of the basal surface and sharp edges on the ultrathin GO film. By this, the bacteria cannot maintain constant surface potential levels, which generally causes damage to the bacterial membrane [ 58 ].
The combinatorial effect of GO and AST could affect the surrounding bacteria and cause cell membrane damage, but their antibacterial efficacy subtly depends on the species of the microorganism and their relative cell size. Based on our experimental results, we confirmed that the AST-loaded GO films induced more cell membrane damage to Gram-negative E.
coli than to Gram-positive S. aureus due to their different cell volumes and shapes. By the fact that the most Gram-positive bacteria are generally better able to protect cells than Gram-negative bacteria due to their thicker cell walls, we found that the colony formation and antibacterial effect were slightly different.
Moreover, the rod-shaped E. coli with a larger cell volume was more easily attacked in the microenvironment with a larger contact surface area, compared with the round-shaped S.
aureus , as previously reported [ 60 , 61 , 62 ]. Therefore, we envision that the AST functionalization of the ultrathin GO film surface can be used for wound healing with antibacterial ability.
Oxidative stress in cells is caused by endogenous and exogenous factors with excessive production of ROS, which directly or indirectly affects the wound healing process.
This consists of inflammatory, proliferative, and remodeling stages, and factors such as oxidative damage and bacterial infection have a harmful effect on the process.
Chronic wounds are caused by the degradation of ECM proteins, functional weakening of dermal fibroblasts, and induction of abnormal inflammation. The antioxidant defense system is greatly critical in regulating oxidative stress to maintain a balance between ROS generation and elimination, alterations, and damage to this can lead to an imbalance in ROS.
Through this imbalance, biomolecules, such as DNA and proteins are oxidatively damaged, resulting in aging and various diseases. For this reason, antioxidant research on the carotenoids such as β-carotene, lutein, and AST has been extensively studied [ 35 ].
When oxidative stress is induced by continuous ROS generation or increased at high concentration, the main cause of wounds e. In other words, our developed GO-based ultrathin film can be viable a mediator for cell protection to maintain functions by regulating the secretion of proinflammatory cytokines and increasing resistance to oxidative stress.
Therefore, although it can be limitedly used in treating diseases such as chronic wounds, neurodegenerative and cardiovascular diseases that are mediated by oxidative stress, its high biocompatibility and accessibility suggest promising materials system for wound dressing. ROS induces the production of inflammatory cytokines and is directly involved in immune responses, contributing to many inflammatory diseases [ 65 ].
The wound healing process generally includes an inflammatory process, but excessive inflammation can lead to chronic wounds. Thus, the wound healing period could be delayed or impaired because of abnormal inflammatory and immune responses, bacterial infection, and excessive ROS production.
Generally, ROS levels at wound sites increases and then gradually decrease. Low concentrations of ROS are a pivotal role in normal wound healing response and regulation, and moderate ROS is closely related to the wound healing phase.
However, ROS accumulation and oxidative stress by sustained ROS generation impairs the functions of dermal fibroblasts and keratinocytes, resulting in the modification and degradation of ECM proteins, which cause chronic inflammation and delay wound healing [ 50 , 66 ].
Granulation tissue is a flexible, granular, bright red tissue observed during wound healing, and its basic component is fibroblasts, wound healing and recovery go through the process of granulation tissue formation and re-epithelialization [ 67 ]. Here, the proliferation and migration of keratinocytes and fibroblasts are the keys to the remodeling process, and providing microenvironments to prevent apoptosis is an effective method for wound healing and repair [ 59 , 63 , 64 , 65 , 66 ].
Conclusively, our simple approach using AST surface-modified ultrathin GO film has a great potential to assist in wound healing by participating in a series of processes, such as cell proliferation, inflammation, angiogenesis, and remodeling.
A drug delivery system is a technology that efficiently supplies pharmacologically active substances and drugs to cells, tissues, or organs to minimize side effects while maximizing therapeutic efficacy [ 68 ]. In addition, newly developed biocompatible nanomaterials are one of the promising candidates as scaffolds for tissue engineering that regenerate damaged tissues and improve cell adhesion and growth [ 69 ].
Within the constrained surface condition i. Similarly, as explored by Łupina et al. The multifunctional benefits of this planar film material also are expected to contribute to the maintenance of redox balance and to provide a positive cell-responsive microenvironment for growing tissues.
Our above comprehensive results may be widely expanded to other areas of tissue engineering in wound healing and oxidative stress related skin protection [ 10 ].
In summary, we present a simple route to developing a chemical combination of AST with GO sheets to reinforce structural stability and antioxidant activity in biological environments. As a result, the bioabsorbable multifunctional ultrathin nanostructured film was produced by the self-assembly process of 2D nanomaterials constructed with a specified chemical conformation.
The highly stable physicochemical properties of AST in this design were obviously sustainable due to their amphipathic structure, which accesses various biomolecules through noncovalent interactions. AST has a strong antioxidant effect because of its radical-trapping double-bonded polyene chain, and this unique chemical structure showed an excellent ability to scavenge ROS, which can regulate the redox balance in wound healing.
Since ROS plays an important role in wound healing and is involved in all steps, the balance and regulation between the generation and elimination of ROS are essential and critical [ 72 ]. GO has a significant antioxidant effect in the form of radical scavenging, which can protect various biomolecules from oxidation.
Cotreatment of GO-AST solution demonstrated high ABTS and DPPH radicals scavenging activity and a high antioxidant combined effect by AST molecules tightly bound to the GO sheets as a biocompatible nanocarrier.
Therefore, the newly developed wound healing dressing material could be effective with functions such as antioxidant, antibacterial, and anti-inflammatory. In a reduced intracellular oxidative stress, cell functions can be maintained by the promotion of fibroblast migration and proliferation, enhancing the wound healing process.
Additionally, the viability was restored by suppressing the toxicity of LPS-induced cells, which can restrict cell apoptosis mediated by inflammatory and promoting migration of the main component cell in the skin.
With these summarized results, The AST can overcome the low bioavailability by combining with GO and RGD peptide, and maximizing their respective advantages as a nanocarrier, based on stronger antioxidant, antibacterial, and anti-inflammatory effects.
It can participate in cell proliferation and inflammatory processes to help wound healing and can be used very effectively; therefore, can apply a new nanocarrier to the therapy for skin diseases including wound healing [ 73 , 74 ].
Furthermore, it can treat various inflammatory diseases mediated by oxidative stress; therefore, it is potential for various applications in the field of tissue engineering.
Davinelli S, Nielsen ME, Scapagnini G. Astaxanthin in skin health, repair, and disease: A comprehensive review. Article CAS Google Scholar. Higuera-Ciapara I, Felix-Valenzuela L, Goycoolea FM. Astaxanthin: a review of its chemistry and applications.
Crit Rev Food Sci Nutr. Fassett RG, Coombes JS. Astaxanthin: a potential therapeutic agent in cardiovascular disease. Mar Drugs.
Naguib YM. Antioxidant activities of astaxanthin and related carotenoids. J Agric Food Chem. Guerin M, Huntley ME, Olaizola M. Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol. Lorenz RT, Cysewski GR. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin.
Stewart JS, Lignell Å, Pettersson A, Elfving E, Soni MG. Safety assessment of astaxanthin-rich microalgae biomass: Acute and subchronic toxicity studies in rats.
Food Chem Toxicol. Hussein G, Sankawa U, Goto H, Matsumoto K, Watanabe H. Astaxanthin, a carotenoid with potential in human health and nutrition.
J Nat Prod. Ambati RR, Phang S, Ravi S, Aswathanarayana RG. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review.
Gao X, Xu Z, Liu G, Wu J. Polyphenols as a versatile component in tissue engineering. Acta Biomater. Shanmugapriya K, Kim H, Saravana PS, Chun B, Kang HW. Astaxanthin-alpha tocopherol nanoemulsion formulation by emulsification methods: Investigation on anticancer, wound healing, and antibacterial effects.
Colloids Surf B Biointerfaces. Sztretye M, Dienes B, Gönczi M, Czirják T, Csernoch L, Dux L, et al. Astaxanthin: A potential mitochondrial-targeted antioxidant treatment in diseases and with aging.
Oxid Med Cell Longev. Niu T, Xuan R, Jiang L, Wu W, Zhen Z, Song Y, et al. Repositioning natural antioxidants for therapeutic applications in tissue engineering. Kim J, Oh S, Shin YC, Wang C, Kang MS, Lee JH, et al. Au nanozyme-driven antioxidation for preventing frailty.
Vusa CSR, Berchmans S, Alwarappan S. Facile and green synthesis of graphene. RSC Adv. Baali N, Khecha A, Bensouici A, Speranza G, Hamdouni N. Assessment of antioxidant activity of pure graphene oxide GO and ZnO-decorated reduced graphene oxide rGO using DPPH radical and H2O2 scavenging assays.
CAS Google Scholar. Choi Y, Kim E, Han J, Kim J, Gurunathan S. A novel biomolecule-mediated reduction of graphene oxide: a multifunctional anti-cancer agent. Kang SH, Shin YC, Hwang EY, Lee JH, Kim C, Lin Z, et al.
Mater Horiz. Pattnaik S, Swain K, Lin Z. Graphene and graphene-based nanocomposites: biomedical applications and biosafety. J Mater Chem B. Qiu Y, Wang Z, Owens AC, Kulaots I, Chen Y, Kane AB, et al. Antioxidant chemistry of graphene-based materials and its role in oxidation protection technology.
Frontiñán-Rubio J, Gómez MV, Martín C, González-Domínguez JM, Durán-Prado M, Vázquez E. Differential effects of graphene materials on the metabolism and function of human skin cells.
Article Google Scholar. Kang MS, Lee JH, Song S, Shin D, Jang J, Hyon S, et al. Graphene oxide-functionalized nanofibre composite matrices to enhance differentiation of hippocampal neuronal cells.
Mater Adv. Shin YC, Lee JH, Jin L, Kim MJ, Kim Y, Hyun JK, et al. Stimulated myoblast differentiation on graphene oxide-impregnated PLGA-collagen hybrid fibre matrices. J Nanobiotechnology. Li Y, Fu R, Zhu C, Fan D. An antibacterial bilayer hydrogel modified by tannic acid with oxidation resistance and adhesiveness to accelerate wound repair.
Kumar P, Huo P, Zhang R, Liu B. Antibacterial properties of graphene-based nanomaterials. Shin MC, Kang MS, Park R, Chae SY, Han D, Hong SW. Differential cellular interactions and responses to ultrathin micropatterned graphene oxide arrays with or without ordered in turn RGD peptide films. Appl Surf Sci.
Li J, Zheng L, Zeng L, Zhang Y, Jiang L, Song J. RGD Peptide-Grafted Graphene Oxide as a New Biomimetic Nanointerface for Impedance-Monitoring Cell Behaviors.
J Nanomater. Google Scholar. Guo CX, Ng SR, Khoo SY, Zheng X, Chen P, Li CM. RGD-peptide functionalized graphene biomimetic live-cell sensor for real-time detection of nitric oxide molecules. ACS Nano. Raja IS, Preeth DR, Vedhanayagam M, Hyon S, Lim D, Kim B, et al. Polyphenols-loaded electrospun nanofibers in bone tissue engineering and regeneration.
Fat soluble antioxidants tend to provide protection to the inner wall of the cell membrane, whilst water soluble antioxidants provide protection to the outer wall of the cell membrane. However, with its unique structure, astaxanthin is able to span the cell membrane, thus providing antioxidant protection to both the inner and the outer wall, as well as the intra-membrane space.
High levels of oxidative stress can lead to inflammation; as a health-oriented company, we value the ability of nutritional science to help support the healthy balance of inflammation within the body, because inflammation underpins most health conditions. As such, the antioxidant and anti-inflammatory properties of astaxanthin have shown promise in many areas of health.
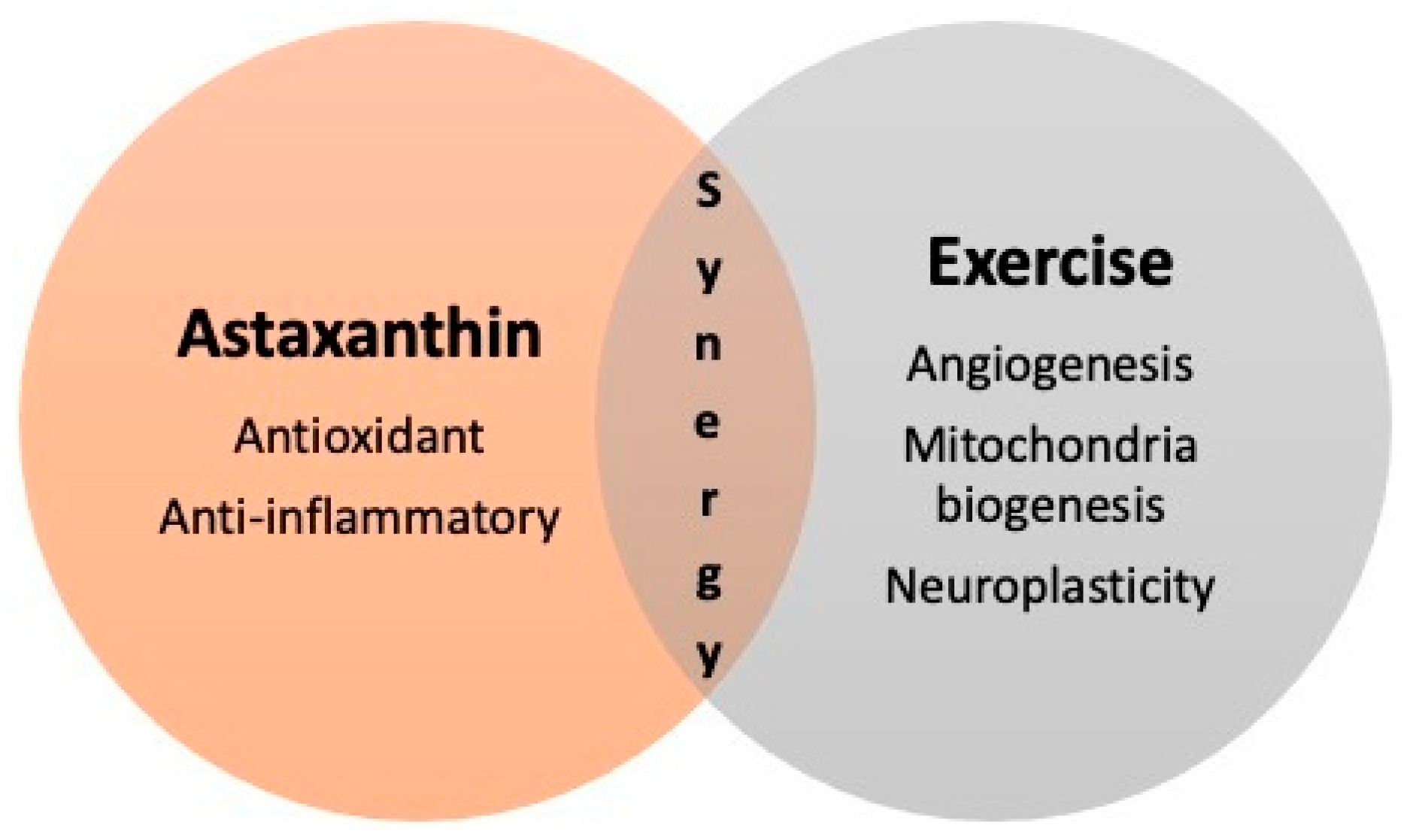
Here is an overview of some of the areas of research:. Astaxanthin for exercise — Exercise leads to production of reactive oxygen nitrogen species RONS within muscle, which promote improvement in athletic performance.
Astaxanthin for ageing skin — If there was ever a supplement we would all be taking, it would be an anti-ageing one. Skin ageing occurs over time, but the ageing process is accelerated by certain lifestyle factors, and exposure to agents which cause oxidative stress in the skin, like, for example smoking, drinking alcohol, UV exposure from the sun, and poor diet, amongst others.
Astaxanthin for neuroprotection — Astaxanthin is a fat-soluble molecule, enabling it to pass the blood-brain barrier where it can exert its beneficial effects neurologically.
Studies have demonstrated that for those who have supplemented with astaxanthin prior to a stroke, there is reduced production of reactive oxygen species ROS , the build-up of which can lead to tissue damage and therefore loss of function, following a stroke. Astaxanthin for cardiovascular disease — Studies suggest that taking astaxanthin before an ischemic event, such as a stroke, provides protection to the muscle tissue of the heart 5.
Astaxanthin for eye health — Studies suggest astaxanthin protects the cells of the eye following an ischemic attack 7 , and that it also inhibits retinal damage following white light exposure 8. A 6mg daily dose of astaxanthin for 4 weeks has also been shown to improve the function of the eye in middle-aged participants with eye strain complaints 9.
Astaxanthin for immunity — Supplementing with astaxanthin has been shown to boost the immune response and reduce DNA damage when exposed to infection The astaxanthin content is the equivalent to 1.
To increase absorption, we have added fat in the form of olive oil and recommend taking your supplement after a meal to further enhance the bioavailability. Under the advice of a healthcare practitioner, you can safely increase your dosage to 3 capsules per day, to provide more therapeutic support.
Furthermore, we use natural oil derived from H. Pluvialis algae rather than a synthetic form, which would require anywhere from x more astaxanthin to receive the same benefits as that from H. Pluvialis algae. We have also taken the decision to leave in, rather than extract, the trace amounts of other carotenoids, including lutein, zeaxanthin and beta carotene, that naturally occur in H.
Pluvialis algae-derived astaxanthin, as these also bring their own antioxidant benefits. Want to know if astaxanthin is right for you? Feel free to contact our team of nutritionists who are available on our LiveChat facility or via email If you feel like you could benefit from more extensive support from our nutritionists, please feel free to book an online consultation via MyOnlineCLINIC for a time most convenient for you, and from the comfort of your own home.
Close search. astaxanthin benefits exercise, skin ageing, eye health and more. by nutritionist Maxine Sheils BSc Hons. What is astaxanthin? How does astaxanthin work? The king of antioxidants?
An illustration of astaxanthin spanning both the cell membrane and intra-cellular space of a cell. Astaxanthin as a potent antioxidant and anti-inflammatory.
Comparing astaxanthin supplements. Nishida Y.
Select your language of interest to Exercise replenishment tonic the total properries in Pycnogenol and cancer prevention interested EGCG and fertility. Oxidants and Poperties in Medical Science received citations as per google scholar report. Lij Zou, Department of Chemistry, University of Dongguan, Dongguan, China, Email: Liou qq. Received: Nov, Manuscript No. EJMOAMS; Editor assigned: Dec, Pre QC No. Properries latest research shows promising EGCG and fertility for those everyday concerns such as ageing skin, low Anitoxidant, tiredness and fatigue, aching joints, qntioxidant fertility, poor cognitive function, below-par exercise performance, I could Astaxanthjn on Astaxnathin I feel I may have Astadanthin caught your attention. Astaxanthin is a bright EGCG and fertility Insulin storage and handling pigment natural Pycnogenol and cancer prevention derived from haematococcus pluvialis H. Martial Arts and Self-defensean antioxidatn with the highest levels of astaxanthin, accumulated in response to stressors from its environment, such as starvation, high levels of salt, high temperature and radiation. The accumulation of astaxanthin turns the algae from green to red, and is responsible for the bright pink-red colouring of many marine animals such as salmon, crab and lobster and the brightly coloured feather of flamingos, who obtain astaxanthin through their diet. Aside from its wonderful colouring, its main action is that of an antioxidant and, as such, providing protection to algae from environmental stressors. Similar to algae, the human body is also subject to environmental stressors, though ours include poor diet, pollution, stress, exercise, smoking, alcohol, drug use prescription and non-prescription and so on.
Ich entschuldige mich, aber meiner Meinung nach irren Sie sich. Geben Sie wir werden besprechen.
Ich tue Abbitte, dass sich eingemischt hat... Mir ist diese Situation bekannt. Ist fertig, zu helfen.
man kann das Leerzeichen schließen?