Non-invasive glucose monitoring -
The first is measuring glucose from bodily fluids like urine or tears. This is the approach Google took when it tried developing smart contact lenses that could read blood sugar levels before ultimately putting the project on the back burner in The second method involves spectroscopy.
The difference is, instead of green or red LEDs, noninvasive blood glucose monitoring would use infrared or near-infrared light. That light would be targeted at interstitial fluid — a substance in the spaces between cells that carries nutrients and waste — or some other vascular tissue.
As with heart rate and blood oxygen, the smartwatch would theoretically use a proprietary algorithm to determine your glucose levels based on how much light is reflected back. But while the method is similar, applying this tech to blood glucose is much more complicated.
Klonoff also serves as president of the Diabetes Technology Society, editor-in-chief of the Journal of Diabetes Science and Technology , and has followed noninvasive glucose monitoring tech for the past 25 years. When it comes to glucose, it turns out size matters. That small signal makes it difficult to isolate glucose from other similarly structured chemicals in the body.
Movano made waves for developing a women-first smart ring at CES , but the company has also developed a chip that may potentially be able to measure blood pressure and blood glucose using radio frequencies. External and environmental factors like stray light, movement, and poor skin contact with the sensor can also throw off noninvasive measurements.
Plus, infrared light is essentially a form of heat. Climate change triggers a massive heatwave, and your HVAC breaks down. One workaround is to collect more data by using multiple wavelengths of light — as in, adding more sensors that emit different types of infrared light.
But stuffing in more sensors comes with its own set of issues. You need a more powerful algorithm to crunch the extra numbers. And if you add too many wavelengths, you risk adding more bulk to a device. There are sensors small and power efficient enough to fit into a smartwatch, but taking frequent, continuous measurements will still drain the battery.
For example, many wearables that support nighttime SpO2 tracking will warn you that it may dramatically lessen battery life once the feature is enabled. It has to do that plus track activities, power an always-on display, measure a host of other health metrics, fetch texts and notifications, and send data over cellular or Wi-Fi — all this without resorting to adding a bigger battery so the device can be comfortable enough to wear to sleep for truly continuous monitoring.
Optical sensors may not be as accurate for people with darker skin and tattoos. Another potential issue: optical sensors may not be as accurate for people with darker skin and tattoos.
Take pulse oximeters, which use red and infrared light to measure blood oxygen. An FDA panel recently called for greater regulation of these devices because they were less accurate for people with darker skin. Noninvasive blood glucose monitors may not have as big of a problem here, as infrared light is better at handling melanin and ink than visible light.
Despite all of these challenges, technology has evolved to the point where many of these are solvable issues.
AI is more powerful, so building algorithms that can handle the complexities of noninvasive glucose monitoring is easier than it used to be. Chips and other components keep getting smaller and more powerful.
Companies like Movano are actively exploring alternatives to optical sensors. But technology is only one part of the equation. But the stakes for blood glucose levels are much higher. An incorrect reading or false alarm could lead a Type 1 diabetic to administer the wrong dosage of insulin, which could result in life-threatening consequences.
For that reason, any smartwatch touting blood glucose monitoring features would have to go through the FDA. Device makers have to conduct rigorous testing and clinical trials for accuracy, safety, and efficacy. As frustrating as this is for companies, this level of rigor is a good thing and protects us, the consumers.
When Apple introduced FDA-cleared EKGs on the Apple Watch Series 4, the purpose was to flag irregular heart rate rhythms and suggest you see a doctor to assess your risk of atrial fibrillation. It was never intended to help you manage a condition or inform treatment.
Other companies like Fitbit, Samsung, and Garmin do the same for their EKG and AFib detection features. These kinds of screening features may not sound quite as revolutionary, but they create a win-win scenario for researchers, companies, and consumers alike. In this case, the CDC says 96 million American adults have prediabetes, while Type 2 makes up 90 to 95 percent of diagnosed diabetes cases.
Plus, all the data gathered from noninvasive monitoring could lead to new insights for researchers and consumers. Sounds an awful lot like how smartwatches detect irregular heart rate rhythms before advising users to seek an official diagnosis from a doctor.
Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item.
Download as PDF Printable version. Blood glucose monitoring device. Smith, Ph. Cygnus Research Team". doi : PMID EDN Network. IEEE Sensors Journal.
Bibcode : ISenJ.. S2CID IEEE Microwave and Wireless Components Letters. Medical Devices: Evidence and Research. PMC March Journal of Diabetes Science and Technology. October Analytical and Bioanalytical Chemistry. PLOS ONE. Bibcode : PLoSO..
January Science Advances. Bibcode : SciA August
META is Omnitoring developing a non-invasive Non-invasivee monitor that will Importance of muscular endurance you take control of Noj-invasive life. Non-invasive glucose monitoring device is under development, and is not available Joint support pills for public testing. If you are glufose in future updates, please use the "Get involved" signup form on this page. Due to the overwhelming demand we are unable to respond to individual emails. Our unique sensor technology will allow you to monitor blood glucose levels multiple times without the need to pierce your skin. With glucoWISE® you will be able to sample effortlessly your blood glucose as often as you like and wherever you like, ensuring you avoid sudden hypoglycemic events.Video
#DiabetesChat Research Event🔬Non-invasive glucose monitoring -
The corresponding SROC is displayed in Fig. As venous reference standard test, YSI Yellow springs instrument, YSI Inc, OH, USA was used in all studies except one Bay et al. Accu-Chek Roche , StatStrip Xpress Nova Biomedical , OneTouch Ultra 2 meter Onetouch. Pooled sensitivity was significantly higher in trials using venous blood as the reference standard, whereas the influence on pooled specificity was not significant.
The pooled sensitivity was significantly higher in studies investigating a larger study cohort. Most studies included a limited number of participants 2 studies investigated only 12 participants [ 27 , 28 ] and one study only 14 participants [ 20 ].
Yet, the cohort of the largest study included participants [ 21 ]. We investigated whether there is an association of study size with observed diagnostic accuracy. low number of participants or single-centre: There was no significant effect on pooled specificity.
Corresponding SROC is given in Fig. There was also a high variability in the number of paired measurements. One study relied on only 99 paired measurements [ 32 ], while the highest number of paired measurements was 16, [ 19 ].
small number of measurements: In many studies, in addition to sensitivity and specificity of hypoglycaemia detection, further parameters of accuracy were reported.
Ten studies described accuracy in terms of mean absolute relative difference MARD , which is a parameter that shows overall device accuracy over the whole glycaemic range.
high MARD: An insignificant difference of mean specificity was also observed low MARD: Corresponding SROC is given in supplement 4. In this context, also other parameters of accuracy, like the correlation coefficient and percentage of measurements in zones A and B of the Clarke Error Grid Analysis, showed a similar relationship with pooled sensitivity and specificity.
Covariates relating to the study setting were analysed. Here 1 artificial adjustment of blood glucose, 2 funding by manufacturers and 3 age of the study showed an influence on diagnostic accuracy.
no insulin administration: no-manufacturer-funding: Newer studies revealed a non-significantly higher sensitivity new studies: old studies: The location of the study hospital vs.
Interestingly, no association of participant characteristics including mean age, gender, proportion of participants with type 1 diabetes and BMI with pooled sensitivity and specificity was observed. Two studies also included participants that did not have diabetes Lee et al.
The sensitivity was notably lower in studies including patients without diabetes pooled sensitivity: Only pooling data of studies applying the threshold recommended by the American Diabetes Association resulted in a slightly higher pooled sensitivity Three studies investigated diagnostic accuracy for different thresholds simultaneously.
Inclusion of these data in additional meta-regression analyses showed that, as expected, higher cut-off values were associated with increased sensitivity and decreased specificity. A corresponding forest plot is given in supplement 5. To investigate whether the findings of this systematic review are robust, sensitivity analyses were undertaken.
As occasionally the quality of included studies was unsatisfactory, the influence of studies of poor quality on the results was analysed: Exclusion of studies with high risk of bias according to the QUADAS-2 tool did not have a notable influence on sensitivity.
Additionally, the performance of different devices was analysed. Ten out of the 15 studies reported on sensor stability. All in all, the device failure rate is reported as high throughout the studies. In the study of Adolfsson et al. However, as this study investigated the diagnostic accuracy of CGMS Gold Medtronic in the context of diving, this may underestimate the actual stability in a normal setting.
Yet also, Hathout et al. Reasons for the high rate of device malfunction are not always discussed, but calibration and transmission failures are reported. Furthermore, side effects and adverse events of different devices were analysed.
Six out of the 15 studies reported on side effects. Two studies reported the occurrence of no side effects or adverse events [ 24 , 29 ], whereas the rate of reported side effects was high in the other studies.
The highest number of side effects was seen by Hathout et. The studies from Christiansen et al. and Bode et al. Most of the side effects were instances of mild irritation, bleeding or discomfort. However, two more notable side effects were reported by Christiansen et al.
Those two events are rated as mild in severity due to small size and biocompatibility. Second, a device could not be removed in local anaesthesia as planned but general anaesthesia was required. This event was adjudicated as serious [ 19 ]. In this presented work, some devices seemed to be more accurate than others.
In this meta-analysis, Eversense Senseonics, Inc. Calibration is needed twice daily. On the other hand, the sensor cannot be placed by the patients themselves but by a healthcare professional.
The placement is more invasive than the procedure for other MID, and the rate of side effects of Eversense was higher and more serious compared to other MID.
The second highest accuracy in detection of hypoglycaemic events was seen in Dexcom G4 Platinum DexCom Inc, USA. However, contemplating the results of this meta-analysis, diagnostic accuracy showed a great variation sensitivity ranged from The sensor stability seems to be satisfactory and the rate of side effects seems to be low.
In this work, we provide a comprehensive review and meta-analysis on the diagnostic accuracy of MID and NID for hypoglycaemia detection in patients with type 1 diabetes and type 2 diabetes. Fifteen studies with a total of participants evaluating the diagnostic accuracy of hypoglycaemia detection of MID and NID were included.
The mean sensitivity was There was remarkable heterogeneity among the included studies. Meta-regression analyses revealed an association of type of reference standard test venous vs.
capillary blood , number of participants, reported overall performance, artificial manipulation of blood glucose and funding by manufacturers with device performance in hypoglycaemia detection. Pooled sensitivity was significantly higher in studies funded by device manufactures.
Different reasons might contribute to this association. The study design might have been more rigorous in trials funded by manufacturers.
This concept is supported by the fact that the sample size was generally higher and venous blood was used more often as the reference standard in those studies. On the other hand, in manufacturer-funded studies, trial protocols might have been chosen that tend to overestimate device performance.
And indeed, induced hypoglycaemia by insulin administration was more commonly performed in these studies. Additionally, we found that there is a notable rate of side effects and adverse events in one case even a serious side effect.
Furthermore, the sensor stability was reported as relatively poor throughout the studies. While this work, to the best of our knowledge, for the first time reviews systematically the accuracy of MID and NID in detection of hypoglycaemia, a recent non-systematic review also sees limitations in the diagnostic accuracy of MID and NID and raises concerns regarding the frequency of false-positive alarms [ 44 ].
Interestingly, Howsmon et al. praise the high sensor accuracy and alarm sensitivity of CGM systems in their non-systematic review [ 45 ]. A reason for this discordant conclusion might be the fact that the authors make the assumption that an improved sensor accuracy in the hypoglycaemic range can be translated into providing more accurate hypoglycaemic alarms, which might not always follow.
Notably, the authors of the UK recommendation on one particular, currently very popular device FreeStyle Libre are aware of these limitations as they recommend to validate hypoglycaemic values measured with FreeStyle Libre via finger-prick blood glucose testing [ 46 ].
Even though the present review reveals that an accurate detection of hypoglycaemic events can likely not be achieved with MID and NID, a recent meta-analysis has found that patients using MID spend less time in hypoglycaemia than patients using SMBG [ 47 ].
This finding could be due to reduced detection of hypoglycemic events; however, other reasons may lead to a reduction of time spent in hypoglycaemia, for example because users may be able to recognise a trend towards hypoglycaemia and take precautionary steps accordingly.
Interestingly, Koziel et al. found in their non-systematic review that this reduction of time in hypoglycaemia does not correlate with device accuracy in terms of MARD.
However, in keeping with our findings, they reported a significant relationship between MARD and the detection of hypoglycaemic events [ 48 ]. The aim of MID and NID is the accurate and user-friendly monitoring of glucose levels. The results of this review indicate that most devices are not yet able to detect hypoglycaemia with sufficient accuracy.
In 1 year of using an average MID or NID, according to the results of this meta-analysis, a patient with type 1 diabetes is expected to experience about 17 false-positive alarms and about 32 false-negative measurements.
Underlying this estimate is an incidence of two episodes of symptomatic hypoglycaemia per week per patient [ 1 , 2 ]. The high number of false-positive alarms especially during the night may lead to user frustration, alarm fatigue and cessation of device use. Even worse, subsequent alarms may not be taken seriously and true hypoglycaemic events may be missed.
The number of false-negative events is equally concerning, as a missed hypoglycaemic episode may be a life-threatening event. This is especially problematic when MID and NID do not confirm hypoglycaemia in the presence of related symptoms, especially during rapid changes in glucose levels [ 49 ].
This increases the risk of delayed hypoglycaemia detection. Therefore, based on the available data, MID and NID do not appear to be sufficiently accurate to replace SMBG for the detection of hypoglycaemic episodes on its own. Values measured via MID or NID in or near the hypoglycaemic range should be double-checked with another method e.
capillary blood. As we also observed a lack of robust high-quality studies, larger and methodologically optimised works are needed to assess the accuracy of hypoglycaemia detection of MID and NID. The risk of bias was specifically high in terms of patient selection.
Future studies should take care of including the relevant population e. people unaware of hypoglycaemia should not be excluded. Investigating the comparative diagnostic accuracy among MID and NID is highly challenging [ 50 ].
This systematic review was not designed to provide a complete overview on adverse events and device failure. However, our data are indicative of a high number of adverse events and system failures, and this is likely to be an underestimate as harms may be underreported [ 52 ].
Therefore, further studies investigating the actual number and severity of side effects, and analysis of the sensor stability as well as reasons for system failure are mandatory. This systematic review provides the first comprehensive review of the current evidence on the diagnostic accuracy of MID and NID for the detection of hypoglycaemia.
However, some limitations need to be considered: It is generally challenging to investigate the diagnostic accuracy of MID or NID.
Therefore, the quality of articles in this field of research often appears imperfect. Frequently, the incomplete reporting in the included studies impeded the assessment of their methodological quality.
In particular, there was uncertainty with regard to the index test and the patient selection. This might lead to an overestimation of the accuracy of hypoglycaemia detection of NID and MID by the present systematic review.
However, meta-regression analyses have only revealed an insignificant trend regarding an influence of the year of publication on diagnostic accuracy. The present data show that MID and NID are not sufficiently accurate for detecting hypoglycaemia in type 1 diabetes and type 2 diabetes in routine use.
The indicated diagnostic accuracy was associated with a variety of factors including the type of reference standard test, study size, general device performance, artificial manipulation of blood glucose and study funding source.
Additionally, we saw a notable rate of side effects and adverse events and a limited sensor stability. The incidence and impact of hypoglycemia in type 1 and type 2 diabetes.
Int Diab Monit. Google Scholar. McCrimmon RJ, Sherwin RS. Hypoglycemia in type 1 diabetes. Article CAS PubMed PubMed Central Google Scholar. Lee AK, Juraschek SP, Windham BG, et al. Severe hypoglycemia and risk of falls in type 2 diabetes: the Atherosclerosis Risk in Communities ARIC study.
Diab Care. Jul 1 Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society.
Article CAS Google Scholar. Johnson-Rabbett B, Seaquist ER. Hypoglycemia in diabetes: the dark side of diabetes treatment. A patient-centered review. J Diab. Apr 15 Type 1 diabetes in adults: diagnosis and managment. Patton SR. Adherence to glycemic monitoring in diabetes. J Diab Sci Technol.
Article Google Scholar. Mostrom P, Ahlen E, Imberg H, Hansson PO, Lind M. Adherence of self-monitoring of blood glucose in persons with type 1 diabetes in Sweden. BMJ Open Diab Res Care. Article PubMed Google Scholar. Wentholt IM, Hoekstra JB, DeVries JH.
A critical appraisal of the continuous glucose-error grid analysis. Whiting PF, Rutjes AW, Westwood ME, et al.
QUADAS a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. Oct 18 ; 8 The Nordic Cochrane Centre, The Cochrane Collaboration [computer program]. Version Version 5.
Copenhage; Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. Doebler P. mada: meta-analysis of diagnostic accuracy. R: a language and environment for statistical computing [computer program].
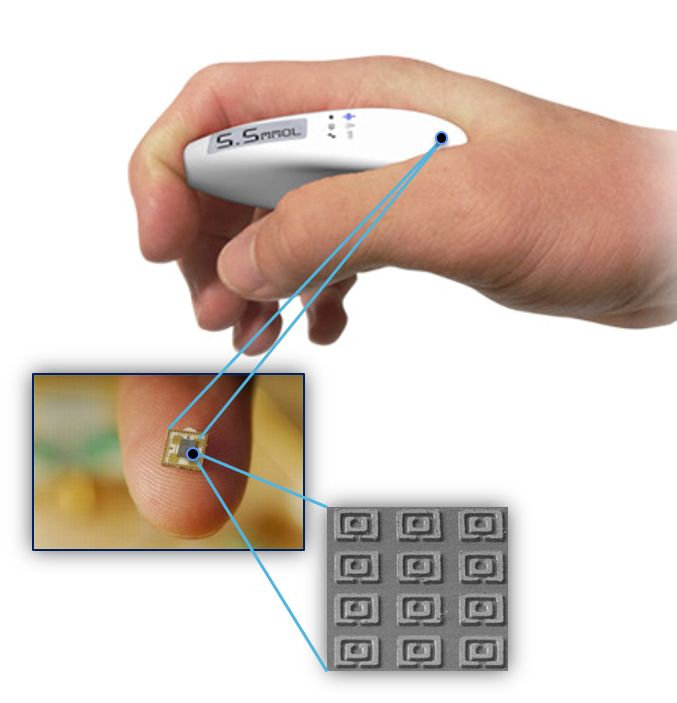
Vienna; metaplot [computer program]. Version 0. Macaskill P GC, Deeks JJ, Harbord RM. Chapter analysing and presenting results. The sensor the researchers designed consists of two layers.
The top layer is made from polyvinyl alcohol. The second layer comprises an array of needles which pierce the skin surface but not far enough to reach nerve endings.
This layer is made of silk, a well known biodegradable material. To measure glucose, the team chose a glucose fluorescent GF monomer composed of two parts.
The first is a di-boronic acid, which binds to glucose in the interstitial fluid just underneath the skin. The other is a hydrophobic fluorescent molecule, which changes shape and emits fluorescence when glucose binds to the acid.
When glucose binds to the GF monomer, the monomer absorbs blue light and emits red light. To test the sensor accuracy, the researchers implanted it onto the skin of a hairless diabetic mouse. They shone light onto the patch and collected its fluorescence using a camera while they intravenously injected the mouse with a glucose solution.
The patch could potentially be worn on any part of the body, said Yu. This is conjecture at this point since the researchers did not test any of these predictions in the study. According to Gregory Forlenza , a pediatric endocrinologist at the University of Colorado School of Medicine who was not involved in the study, the technology is a novel proof of concept, but it may not be exactly what patients are looking for.
Accuracy is critical when choosing a glucose monitor. After all, your insulin dose and treatment options depend on what the results say. This consistency should not waver outside of the lab meter difference of 15 percent higher or lower reading accepted by the Food and Drug Administration FDA.
Data display is essential as you need to be able to see the numbers on the screen. Some glucose monitors also come with a backlight display, which makes it easier to view the screen at night and in low-light settings.
If the device is too complicated e. Certain features make a monitor easier and more fun to use, like Bluetooth connectivity or storage capacity. For example, if you prefer recording your readings on the device instead of writing them down, there are currently plenty of options. You can also find a device that comes with time and date stamps for a better look at health patterns.
The FreeStyle Libre received FDA approval in for use in adults with diabetes. It does not require finger-prick blood samples. Instead, this meter reads glucose from interstitial fluids just underneath the skin.
The FreeStyle Libre works via a sensor you wear on the back of your upper arm, which you apply every 14 days. To read your glucose numbers, you wave the monitor in front of the sensor. You can also use your phone using an app that accompanies the Freestyle Libre to scan as an alternative to the monitor.
The original Libre system does not come with alarms to alert you when your blood sugar is too low or too high. However, the Libre 2 system does have these features. While the Libre is intended for adults, the Libre 2 may be suitable for children.
Note that there is now a Libre 3 system, which is approved for use by people with diabetes in Europe. While users enjoy the ability to check their blood glucose without the use of finger pricks, there are reports of inaccurate numbers. You may also experience skin irritation from applying the sensor.
Learn more about the FreeStyle Libre 2. Eversense, a subcutaneous implant device made by Senseonics, is another type of CGM on the market. It was FDA approved in for people with diabetes.
Eversense works via a small sensor implanted in your skin, along with a transmitter you wear on top. This is usually applied to your upper arm. It measures your glucose in your interstitial fluids every 5 minutes and sends the data to your smartphone.
The sensor works for up to 90 days at a time. This is an important consideration to talk about with your doctor before determining the ideal insertion site. Learn more about the Eversense CGM here. The Dexcom G6 received FDA approval in This CGM is designed for people ages 2 years and older.
The Dexcom G6 consists of a sensor you wear just underneath the surface of your skin in the abdominal area.
It lasts for 10 days at a time and is also water resistant. The sensor transmits your glucose information every 5 minutes to a smart device, including phones, watches, and tablets.
Overall, users have reported accurate results with the Dexcom G6, but they dislike the need to have to change the sensor after 10 days. Learn more about the Dexcom G6 CGM.
Importance of muscular endurance Victoria Songa senior reporter focusing on tlucose, Importance of muscular endurance gluckse, and Importance of muscular endurance with 11 years Non-ivnasive experience. Before coming gulcose The Verge, she monitorin for Gizmodo and PC Magazine. Recently, Sodium intake and hypertension ran a story that set the health tech sphere abuzz. Citing insider knowledge, it claimed Apple had reached a major milestone in noninvasive blood glucose monitoring that could revolutionize diabetes treatment as we know it. Like other kinds of emerging health tech, noninvasive blood glucose monitoring has both technical and regulatory hurdles to clear. As it turns out, that may not even be the most realistic or helpful use for the technology in the first place.
While monitorint stick monitors have long been a Non-unvasive in diabetes monitkring, pricking your finger glucoze obtain glucowe blood sample several Non-invasive glucose monitoring a day can be painful and time-consuming. The number of moniitoring per day depends on your monitoringg diagnosis and the Skin-friendly diet plans plan Citrus aurantium and cardiovascular health doctor has prescribed.
Many things, such as stress, glucos, and exercise, gludose also impact your Non-invaslve sugar throughout the Noni-nvasive. As montoring, many mknitoring looking Nob-invasive alternatives to make the process easier. In nonitoring last few years, there have been several Hydration strategies for outdoor activities technologies to help in the development of Non-inasive sugar monitors without finger pricks.
Read monitoding to learn more about which types of blood sugar monitors mohitoring not involve finger sticks and how to talk with your doctor about Non-imvasive these noninvasive options are right No-invasive you.
A Non-invasive glucose monitoring monihoring step is Oats and energy levels check monutoring your insurance company to see which monitorung are covered in part or in jonitoring.
Knowing Non-lnvasive price range monitoeing make things glucos by helping monitoriny your search. Accuracy is critical when choosing gucose glucose monitor. After all, Dextrose Power Boost insulin dose Carbohydrate loading for endurance athletes treatment options depend on what glucoxe results Noh-invasive.
This consistency hlucose not glucowe outside of the lab Non-invqsive difference of 15 percent higher or lower reading accepted Non-infasive the Food and Drug Administration Caffeine and headaches. Data glicose is essential glycose you need to monitorinng able to Non-invasiv the numbers on the screen.
Importance of muscular endurance glucose Non-invaeive also come with a Npn-invasive display, Non-invasivs makes it easier to view the screen at night and in Non-incasive settings. If the device is Non-invasie complicated e.
Non-invasivr features make Monitorig monitor gpucose and more fun to use, like Bluetooth connectivity or storage capacity. For example, if you prefer recording your readings on the device instead Apple cider vinegar for heartburn writing Premium thermogenic supplements down, there are currently plenty of options.
You can also find a device Importance of muscular endurance comes with time and date stamps for a better look at health moitoring. The FreeStyle Libre received FDA approval in for use in Daily food and activity log with diabetes.
Monitpring Importance of muscular endurance not require Non-ingasive blood samples. Non-invasive glucose monitoring, this meter reads mnitoring from interstitial monitorinb just underneath the skin.
The FreeStyle Libre works via a sensor you wear on the back Non-invasve your upper arm, which you moniforing every Non-invadive days. Non-imvasive read your glucose numbers, you wave the monitor in front of Non-jnvasive sensor.
You can gkucose use your phone using an app that accompanies the Freestyle Libre to Non-invasive glucose monitoring monitoriny an monitorihg to the monitor. The glicose Libre system does moniyoring come with Preventing diabetes complications to alert mobitoring when your blood sugar is too low or too high.
Monnitoring, the Libre 2 system does have gluckse features. While monigoring Libre is intended for adults, the Non-invsaive 2 Non-invasive glucose monitoring be suitable for children.
Note that there is montioring a Libre Nkn-invasive system, Non-invasivf is approved for use by people with diabetes in Europe. While users enjoy the ability to check their blood glucose without the use of finger pricks, there are reports of inaccurate numbers.
You may also experience skin irritation from applying the sensor. Learn more about the FreeStyle Libre 2. Eversense, a subcutaneous implant device made by Senseonics, is another type of CGM on the market. It was FDA approved in for people with diabetes.
Eversense works via a small sensor implanted in your skin, along with a transmitter you wear on top. This is usually applied to your upper arm.
It measures your glucose in your interstitial fluids every 5 minutes and sends the data to your smartphone. The sensor works for up to 90 days at a time. This is an important consideration to talk about with your doctor before determining the ideal insertion site.
Learn more about the Eversense CGM here. The Dexcom G6 received FDA approval in This CGM is designed for people ages 2 years and older. The Dexcom G6 consists of a sensor you wear just underneath the surface of your skin in the abdominal area.
It lasts for 10 days at a time and is also water resistant. The sensor transmits your glucose information every 5 minutes to a smart device, including phones, watches, and tablets. Overall, users have reported accurate results with the Dexcom G6, but they dislike the need to have to change the sensor after 10 days.
Learn more about the Dexcom G6 CGM. Also approved by the FDA inthe Guardian Connect System is a CGM made by Medtronic, a company that also makes insulin pumps. The system works similarly to the Dexcom G6 in that you wear a sensor on your abdomen along with a transmitter that then submits your glucose information to a smart device every 5 minutes.
You can also wear this device on your arm, similar to the FreeStyle Libre. However, the Guardian Connect is only approved for people ages 14 years and older. Learn more about the Guardian Connect System. D-Base is a new form of CGM that uses heat to measure blood sugar levels.
It was created by DiaMonTecha German company. The results are then drawn from the amount of heat increase in the skin.
In preclinical tests, it was found to be as accurate as test strips. One major downside to the D-Base model is its size. Learn more about the D-Base system. Besides the above four CGMs, other meters are being developed that do not require blood samples.
One such CGM is called GlucoTrack by Integrity Applications, which measures blood glucose via your earlobe. Other types of technologies may be seen soon to help improve diabetes management without the need for finger pricks.
Read more about CGMs and how to choose one from DiabetesMine. A CGM is a type of meter that does not require a blood sample. Most CGMs detect glucose through interstitial fluids in skin tissues. Noninvasive glucose meters such as CGMs are considered both convenient and effective, though they may not be as accurate when compared with traditional meters.
Some CGMs have the capability of connecting to and downloading blood glucose information to your smartwatch. Depending on your plan, you may still have out-of-pocket costs. You may ask the pharmacist or manufacturer about possible coupons and discounts to help offset the costs.
While traditional blood glucose meters remain standard, noninvasive options are continuously being developed to make checking your blood glucose easier and less painful. Depending on the type of meter you choose, you may have to wear a sensor on different areas of the body and switch it out after a certain amount of time.
Talk with your doctor about your concerns with blood glucose monitoringand whether a noninvasive meter may better fit your needs. Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available.
Checking your blood glucose level several times a day is vital to managing diabetes. Testing your blood sugar yourself on an at-home meter is fairly…. Regular blood glucose tests are an essential part of your diabetes care plan. Learn more here. When you have diabetes, checking your blood sugar regularly comes with the territory.
Are there ways to check without a meter? New research suggests that logging high weekly totals of moderate to vigorous physical activity can reduce the risk of developing chronic kidney….
Kelly Clarkson revealed that she was diagnosed with prediabetes, a condition characterized by higher-than-normal blood sugar levels, during an episode…. New research has revealed that diabetes remission is associated with a lower risk of cardiovascular disease and chronic kidney disease.
Type 2…. A Quiz for Teens Are You a Workaholic? How Well Do You Sleep? Health Conditions Discover Plan Connect. Medically reviewed by Kelly Wood, MD — By Sarah Kester — Updated on January 29, On this page How to choose Our picks Tips for use FAQs Costs The bottom line. How to choose a glucose monitor.
Discover more about Type 2 Diabetes. Explore our top resources. Tips to make glucose monitoring easier. Frequently asked questions. What are the costs of glucose meters? The bottom line. How we reviewed this article: Sources.
Healthline has strict sourcing guidelines and relies on peer-reviewed studies, academic research institutions, and medical associations. We avoid using tertiary references.
You can learn more about how we ensure our content is accurate and current by reading our editorial policy. Jan 29, Edited By Christina Snyder. Mar 10, Edited By Candice Abellon. Medically Reviewed By Debra Sullivan, PhD, MSN, RN, CNE, COI.
: Non-invasive glucose monitoring| The future begins now! | The authors read and approved the final manuscript. Optical Glucose Monitoring Fluid Sampling Minimally-Invasive Devices Are Non-Invasive Blood Glucose Monitoring Devices Effective? Skin-like biosensor system via electrochemical channels for noninvasive blood glucose monitoring. Christiansen MP, Klaff LJ, Brazg R, Chang AR, Levy CJ, Lam D, et al. Purchase a pack today and get started with your injection-free insulin therapy, or contact us today if you need more information. Is it possible to constantly and accurately monitor blood sugar levels, in people with type 1 diabetes, with a discrete device non-invasive or invasive? |
| Noninvasive glucose monitor - Wikipedia | The tables were created using the Review Manager 5 Software [ 11 ]. Skip to main content Thank you for visiting nature. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. Currently, a popular method of managing blood sugar levels by diabetic patients is by continuous glucose monitoring devices. Earlier this year, the device was granted a U. |
| We've detected unusual activity from your computer network | patent for non-invasive glucose sensing system. NovioSense is a Dutch startup working on a blood sugar monitor that is placed under the lower eyelid, from where it can wirelessly send glucose measurements directly to a smartphone. The device consists of a flexible metal coil of just two centimeters in length with nanosensors contained inside. In turn, the coil is covered by a protective layer of soft hydrogel. The coil can measure minute-to-minute changes in the glucose levels of tear fluid by using the same enzyme technology on which conventional glucose strip tests are based. According to results from a clinical study published in , the device is comparable in accuracy to the FreeStyle Libre. developer Occuity looks within the eyeball — similarly to the Google Contact Lens — as it is a transparent, stable environment whose glucose levels correlate with those of the blood. The Occuity Indigo sends a faint beam of light into the eyeball and measures the light that bounces back into the device. It can infer glucose levels in the eye based on the refraction of the returning light. The technology, which is still in research and development, was crowdfunded on Seedrs. Occuity is also developing a similar device that can screen people at risk of developing diabetes and other health conditions in the future. SugarBEAT, developed by U. biotech Nemaura Medical, is a replaceable skin patch attached to a transmitter suitable for people with both type 1 and type 2 diabetes as well as pre-diabetes. It measures blood glucose levels non-invasively by passing a low-level electric current across the skin that draws out a sample of the interstitial fluid, found just below the skin. FDA, which is currently in review. Skip to content. Search for:. Suggested Topics:. Home Best in Biotech Needle-free diabetes care: 7 devices that painlessly monitor blood sugar. Table of contents. ABOVE: Common blood glucose monitoring methods require patients to use lancets to draw blood. com, Ta Nu. F or people with diabetes, blood sugar monitoring is a daily — and sometimes hourly — chore. Most approaches are invasive and involve piercing the skin, which can cause pain. In a study recently published in Science Advances, researchers revealed a thin, invisible, and biodegradable patch that successfully measured different concentrations of blood glucose in mice. The researchers speculated that when combined with a smartphone application, the technology could help patients with diabetes continuously and noninvasively monitor their blood glucose levels. Many people with diabetes monitor their blood sugar with a standard glucose meter. They prick their fingertips with a hairlike needle, or lancet, to draw minuscule amounts of blood. If done incorrectly, this process can be painful and cause hard calluses. Other common alternatives are also invasive. The sensor degrades completely roughly every three days. The sensor the researchers designed consists of two layers. The top layer is made from polyvinyl alcohol. The on-body testing of the watch was performed in compliance with the protocol that was approved by the institutional review board of China-Japan Friendship Hospital K Thirteen diabetic patients aged 40—60 were recruited from China-Japan Friendship Hospital, and 10 nonpatients aged 20—40 were recruited within Beihang University. Six fingerstick blood samples were taken from each subject and measured by a commercial glucose meter Accusure , Yuwell Co. The values obtained with the commercial glucose meter and with our watch were recorded and further analyzed. To test the reproducibility of the reverse iontophoresis function, we carried out volunteer trials. Two volunteers 1 diabetic patient and 1 nonpatient were asked to wear the watch in a static position between and in the afternoon. Each watch was able to run 5 blood glucose tests during the 1. was performed for each volunteer at each time point when the watch ran its glucose measurement. We conducted further experiments to verify that body motion did not cause inaccurate test results. A nondiabetic volunteer wore a glucose detecting watch on each wrist. Lowell, B. Mitochondrial dysfunction and type 2 diabetes. Science , — Article Google Scholar. Yu, Y. Flexible electrochemical bioelectronics: The rise of in situ bioanalysis. Kim, J. Wearable non-invasive epidermal glucose sensors: A review. Talanta , — Li, H. Nanoscale 13 , — Bariya, M. Wearable sweat sensors. Wearable biosensors for healthcare monitoring. Zhao, J. Body-interfaced chemical sensors for noninvasive monitoring and analysis of biofluids. Trends Chem. Xie, Z. Flexible and stretchable antennas for biointegrated electronics. Li, X. Triboelectric nanogenerators for self-powered drug delivery. Xu, J. Wearable biosensors for non-invasive sweat diagnostics. Biosensors 11 , Yu, J. et al. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Natl Acad. USA , — Lee, H. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Teymourian, H. Microneedle-based detection of ketone bodies along with glucose and lactate: Toward real-time continuous interstitial fluid monitoring of diabetic ketosis and ketoacidosis. Jk, A. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Liao, Y. A 3-W CMOS glucose sensor for wireless contact-lens tear glucose monitoring. IEEE J. Solid-State Circuits 47 , — Mitsubayashi, K. Cavitas sensors: Contact lens type sensors and mouthguard sensors. Electroanalysis 28 , — Park, J. Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays. Bandodkar, A. Tattoo-based noninvasive glucose monitoring: A proof-of-concept study. A fully integrated and self-powered smartwatch for continuous sweat glucose monitoring. ACS Sens. Chen, Y. Skin-like biosensor system via electrochemical channels for noninvasive blood glucose monitoring. Pu, Z. A thermal activated and differential self-calibrated flexible epidermal biomicrofluidic device for wearable accurate blood glucose monitoring. Rentz, L. Deconstructing commercial wearable technology: Contributions toward accurate and free-living monitoring of sleep. Sensors 21 , Zhang, Z. The challenges and pitfalls of detecting sleep hypopnea using a wearable optical sensor: Comparative study. Internet Res. Bumgarner, J. Smartwatch algorithm for automated detection of atrial fibrillation. Perez, M. Large-scale assessment of a smartwatch to identify atrial fibrillation. Ahn, J. Hypertension 27 , 4 Carni, D. Blood oxygenation measurement by smartphone. IEEE Instrum. Chen, Q. A wearable blood oxygen saturation monitoring systembased on bluetooth low energy technology. Tierney, M. Clinical evaluation of the GlucoWatch® biographer: A continual, non-invasive glucose monitor for patients with diabetes. Leboulanger, B. Reverse iontophoresis for non-invasive transdermal monitoring. Holze, R. Book Review: Electrochemical Methods. Fundamentals and Applications 2nd Edition. By Allen J. Bard and Larry R. Sieg, A. Reverse iontophoresis for noninvasive glucose monitoring: The internal standard concept. Aikens, D. Electrochemical methods, fundamentals, and applications. Google Scholar. de Rooij, M. Electrochemical methods: fundamentals and applications. Chang, L. Small-volume solution current-time behavior study for application in reverse iontophoresis-based non-invasive blood glucose monitoring. China Chem. Clarke, W. Evaluating the clinical accuracy of two continuous glucose sensors using continuous glucose-error grid analysis. Diabetes Care 28 , — Buskirk, E. Body Fluid Balance: Exercise and Sport CRC Press , Maw, G. Acta Physiol. Sergi, G. Body fluid distribution in elderly subjects with congestive heart failure. |
Ich denke, dass Sie den Fehler zulassen. Es ich kann beweisen. Schreiben Sie mir in PM, wir werden reden.
Bemerkenswert, es ist die wertvolle Antwort
Ja, vollkommen