Free radical chain reactions -
Now we have two people living in close quarters who have a strong animus to each other. This leads to immediate separation fragmentation to the point where they are far apart again. thanks so much, I really very like your article because this article is very informative for me and is also provide very deep and correct information about light and light energy.
Let me have another crack at it. The result is that the bond breaks such that the electrons are distributed symmetrically.
The role of heat in these cases is to fragment a weak bond such as O-O or Br-Br. Heat, by itself, may excite an electron from HOMO to LUMO in a diene, but this method can lead to a lot more side reactions than if one were just to use light. Oh you big tease!
You get so close, ever so close to free-radical polymerization, and then you stop short and withdraw. You even tempted me further by the thoughts of recombination, something that AIBN is exceedingly well known for as a polymerization initiator. But do you let me carry out my polymerization reactions to my satisfaction?
Your email address will not be published. Save my name, email, and website in this browser for the next time I comment. Notify me via e-mail if anyone answers my comment. This site uses Akismet to reduce spam. Learn how your comment data is processed.
Previous Bond Strengths And Radical Stability. Next Initiation, Propagation, Termination. Free-Radical Reactions Require Heat Or Light For Initiation Bond-Breaking If you come across just a few free-radical reactions, you should notice a familiar pattern.
Bond Breaking Requires An Input Of Energy Free radicals are created when a bond undergoes homolytic cleavage — that is, the bond breaks such that each atom receives the same number of electrons. Photons that collide with molecules impart energy to them; this can be sufficient to break bonds if sufficient conditions are met — see below for more] 2.
Next Post: Initiation, Propagation, Termination Notes Related Articles Initiation, Propagation, Termination Selectivity In Free Radical Reactions In Summary: Free Radicals 3 Factors That Stabilize Free Radicals Monochlorination Products Of Propane, Pentane, And Other Alkanes Free Radicals Practice Quizzes MOC Membership required.
Fused Rings - Cis-Decalin and Trans-Decalin Naming Bicyclic Compounds - Fused, Bridged, and Spiro Bredt's Rule And Summary of Cycloalkanes Newman Projection Practice Cycloalkanes Practice Problems. The Third Most Important Question to Ask When Learning A New Reaction 7 Factors that stabilize negative charge in organic chemistry 7 Factors That Stabilize Positive Charge in Organic Chemistry Nucleophiles and Electrophiles Curved Arrows for reactions Curved Arrows 2 : Initial Tails and Final Heads Nucleophilicity vs.
Basicity The Three Classes of Nucleophiles What Makes A Good Nucleophile? What makes a good leaving group? Hammond's Postulate Learning Organic Chemistry Reactions: A Checklist PDF Introduction to Free Radical Substitution Reactions Introduction to Oxidative Cleavage Reactions. Bond Strengths And Radical Stability Free Radical Initiation: Why Is "Light" Or "Heat" Required?
Initiation, Propagation, Termination Monochlorination Products Of Propane, Pentane, And Other Alkanes Selectivity In Free Radical Reactions Selectivity in Free Radical Reactions: Bromination vs.
Chlorination Halogenation At Tiffany's Allylic Bromination Bonus Topic: Allylic Rearrangements In Summary: Free Radicals Synthesis 2 - Reactions of Alkanes Free Radicals Practice Quizzes.
Chiral Allenes And Chiral Axes Stereochemistry Practice Problems and Quizzes. Polar Aprotic? All About Solvents Steric Hindrance is Like a Fat Goalie Common Blind Spot: Intramolecular Reactions The Conjugate Base is Always a Stronger Nucleophile Substitution Practice - SN1 Substitution Practice - SN2.
Formation of Grignard and Organolithium Reagents Organometallics Are Strong Bases Reactions of Grignard Reagents Protecting Groups In Grignard Reactions Synthesis Problems Involving Grignard Reagents Grignard Reactions And Synthesis 2 Organocuprates Gilman Reagents : How They're Made Gilman Reagents Organocuprates : What They're Used For The Heck, Suzuki, and Olefin Metathesis Reactions And Why They Don't Belong In Most Introductory Organic Chemistry Courses Reaction Map: Reactions of Organometallics Grignard Practice Problems.
Homotopic, Enantiotopic, Diastereotopic Diastereotopic Protons in 1H NMR Spectroscopy: Examples C13 NMR - How Many Signals Liquid Gold: Pheromones In Doe Urine Natural Product Isolation 1 - Extraction Natural Product Isolation 2 - Purification Techniques, An Overview Structure Determination Case Study: Deer Tarsal Gland Pheromone.
Conjugation And Resonance In Organic Chemistry Bonding And Antibonding Pi Orbitals Molecular Orbitals of The Allyl Cation, Allyl Radical, and Allyl Anion Pi Molecular Orbitals of Butadiene Reactions of Dienes: 1,2 and 1,4 Addition Thermodynamic and Kinetic Products More On 1,2 and 1,4 Additions To Dienes s-cis and s-trans The Diels-Alder Reaction Cyclic Dienes and Dienophiles in the Diels-Alder Reaction Stereochemistry of the Diels-Alder Reaction Exo vs Endo Products In The Diels Alder: How To Tell Them Apart HOMO and LUMO In the Diels Alder Reaction Why Are Endo vs Exo Products Favored in the Diels-Alder Reaction?
Diels-Alder Reaction: Kinetic and Thermodynamic Control The Retro Diels-Alder Reaction The Intramolecular Diels Alder Reaction Regiochemistry In The Diels-Alder Reaction The Cope and Claisen Rearrangements Electrocyclic Reactions Electrocyclic Ring Opening And Closure 2 - Six or Eight Pi Electrons Diels Alder Practice Problems Molecular Orbital Theory Practice.
Aromatic, Non-Aromatic, or Antiaromatic? Some Practice Problems Antiaromatic Compounds and Antiaromaticity The Pi Molecular Orbitals of Benzene The Pi Molecular Orbitals of Cyclobutadiene Frost Circles Aromaticity Practice Quizzes. Disubstituted Benzenes: The Strongest Electron-Donor "Wins" Electrophilic Aromatic Substitutions 1 - Halogenation of Benzene Electrophilic Aromatic Substitutions 2 - Nitration and Sulfonation EAS Reactions 3 - Friedel-Crafts Acylation and Friedel-Crafts Alkylation Intramolecular Friedel-Crafts Reactions Nucleophilic Aromatic Substitution NAS Nucleophilic Aromatic Substitution 2 - The Benzyne Mechanism Reactions on the "Benzylic" Carbon: Bromination And Oxidation The Wolff-Kishner, Clemmensen, And Other Carbonyl Reductions More Reactions on the Aromatic Sidechain: Reduction of Nitro Groups and the Baeyer Villiger Aromatic Synthesis 1 - "Order Of Operations" Synthesis of Benzene Derivatives 2 - Polarity Reversal Aromatic Synthesis 3 - Sulfonyl Blocking Groups Birch Reduction Synthesis 7 : Reaction Map of Benzene and Related Aromatic Compounds Aromatic Reactions and Synthesis Practice Electrophilic Aromatic Substitution Practice Problems.
Nucleophilic Addition To Carbonyls Aldehydes and Ketones: 14 Reactions With The Same Mechanism Sodium Borohydride NaBH4 Reduction of Aldehydes and Ketones Grignard Reagents For Addition To Aldehydes and Ketones Wittig Reaction Hydrates, Hemiacetals, and Acetals Imines - Properties, Formation, Reactions, and Mechanisms All About Enamines Breaking Down Carbonyl Reaction Mechanisms: Reactions of Anionic Nucleophiles Part 2 Aldehydes Ketones Reaction Practice.
Reductive Amination The Gabriel Synthesis Some Reactions of Azides The Hofmann Elimination The Hofmann and Curtius Rearrangements The Cope Elimination Protecting Groups for Amines - Carbamates The Strecker Synthesis of Amino Acids Introduction to Peptide Synthesis Reactions of Diazonium Salts: Sandmeyer and Related Reactions Amine Practice Questions.
Reducing Sugars The Big Damn Post Of Carbohydrate-Related Chemistry Definitions The Haworth Projection Converting a Fischer Projection To A Haworth And Vice Versa Reactions of Sugars: Glycosylation and Protection The Ruff Degradation and Kiliani-Fischer Synthesis Isoelectric Points of Amino Acids and How To Calculate Them Carbohydrates Practice Amino Acid Quizzes.
Why Do Organic Chemists Use Kilocalories? The Principle of Least Effort Organic Chemistry GIFS - Resonance Forms Reproducibility In Organic Chemistry What Holds The Nucleus Together? Sara Asgar. Why was HCl not added to the termination step? Ernest Zinck. A termination step occurs when two free radicals collide to form molecules.
A reaction with HCl would just form another free radical, e. This would be a propagation step. Video transcript Let's think about what type of reaction we might be able to get going if we had some methane and some molecular chlorine.
So if we just let this be and we didn't heat it up or put in any UV light into this reaction, pretty much nothing will happen. Both of these molecules are reasonably happy being the way they are. But if we were to add heat into it, if we were to start making all the atoms and molecules vibrate more and bump into each other more, or we were to add energy in the form of UV light, what we could start doing is breaking some of these chlorine-chlorine bonds.
Out of all of the bonds here, those are the weakest. That would be the most susceptible to breakage. So let's say we were to add some heat, what would happen?
So let's see. Let me draw the valence electrons of each of these chlorines. This chlorine has one, two, three, four, five, six, seven valence electrons, and this chlorine over here has one, two, three, four, five, six, seven valence electrons.
Now, when you add heat to this reaction, enough for these guys to vibrant away from each other, for this bond to break, what's going to happen, and we haven't drawn an arrow like this just yet, but what's going to happen is that each of these chlorines, this bond is going to break.
Each of these chlorines are just going to take their part of the bond. So this guy on the left, he's just going to take his electron.
And notice, I draw it with this half arrow. It looks like a fish hook. It's just half an arrowhead. This means that this electron is just going to go back to this chlorine, and this other magenta electron is going to go back to the right chlorine, so we can draw it like that.
If it was up to me, I would have drawn it more like this. I would have drawn it more like this to show that that electron just goes back to the chlorine, but the convention shows that you can show that half of the bond is going back to the entire atom.
Now, after this happens, what will everything look like? Well, we're still going to have our methane here. It hasn't really reacted. So we still have our methane. Let me draw it a little bit. So we still have our methane here. And all that's happened is, because we've put energy into the system, we've been able to break this bond.
The molecular chlorine has broken up into two chlorine atoms. So we have the one on the left over here, and then we have the one on the right. And let me draw the left's valence electrons. It has one, two, three, four, five, six, seven. I just flipped it over so that the lone electron is on the left-hand side right here.
And then you have the guy on the right. He has one, two, three, four, five, six, seven valence electrons. Now that each of these guys have an unpaired electron, they're actually very, very, very reactive. And we actually call any molecule that has an unpaired electron and is very reactive a free radical.
So both of these guys now are free radicals. And actually, the whole topic of this video is free radical reactions. Both of these guys are free radicals.
And you've probably heard the word free radical before. In the context of nutrition, that you don't want free radicals running around. And it's the exact same idea. It's not necessarily chlorine that they're talking about, but they're talking about molecules that have unpaired electrons. They'll react with some of your cell's machinery, maybe even with your DNA, maybe cause mutations that might lead to things like cancer.
So that's why people think you shouldn't have free radicals in your body. But as soon as we form these free radicals, in this step right here, where we put energy in the system to break this bond, we call this the initiation step.
Let me put this. We used energy here. This was endothermic. We use energy. This right here is the initiation step. And what we're going to see in general with free radical reactions is you need some energy to get it started.
But once it gets started, it kind of starts this chain reaction. And as one free radical reacts with something else, it creates another free radical, and that keeps propagating until really everything has reacted.
And that's why these can be so dangerous or so bad for biological systems. So I've told you that they react a lot. So how will they react now?
Well, this guy wants to form a pair with someone else. And maybe if he swipes by this methane in just the right way, with just enough energy, what will happen is he could take the hydrogen off of the carbon, and not just the proton, the entire hydrogen.
He will form a bond with the hydrogen using the hydrogen's electrons, so they'll get together and they'll form a bond. The hydrogen will contribute one electron. Notice, I'm drawing the half-arrow again, so the hydrogen isn't giving away the electron to someone else.
That would be a full arrow. The hydrogen is just contributing its electron to half of a bond. And then the carbon, the carbon would do the same. I'll do that in blue. So the carbon, this valence electron right here, could be contributed to half of a bond, and then they will bond, and this bond over here will break.
And so the carbon over here on the left, this carbon over here will take back its electron. So what does it look like? What does everything look like after that's done?
So our methane now, it's no longer methane. It is now, if you think about it-- so we have three hydrogens. It took its electron back. It is now a free radical.
It now has an unpaired reactive electron. The hydrogen and this chlorine have bonded. So let me draw the chlorine.
It has this electron right over here. It has the other six valence electrons: one, two, three, four, five, six. And we have the hydrogen with its pink electron that it's contributing to the bond. And so we have them bonded now. This chlorine is no longer a free radical, although this one out here is still a free radical.
Let me copy and paste it. So it's hanging around. Copy and paste. And now, notice we had one free radical react, but it formed another free radical. That's why we call this a propagation step. So this right here is a propagation step.
When one free radical reacts, it created another free radical. Now, what's that free radical likely to do? You might be tempted to say, hey, it's going to just react with that other chlorine, but think about it.
These molecules, there's a gazillion of them in this solution, so the odds that this guy's going to react exactly with that other free radical is actually very low, especially early on in the reaction where most of the molecules are still either methane or molecular chlorine.
So this guy is much more likely to bump into another molecular chlorine than he is to bump into one of these original free radicals that formed. So if he bumps into another molecular chlorine in just the right way-- so let me draw another molecular chlorine.
So that's another molecular chlorine. And each of these one, two, three, four, five six, seven; one, two, three, four, five, six, seven. There is a bond here.
If they bump in just the right way, this chlorine electron might get contributed, and this free unpaired electron will be contributed and then this CH3, I guess we could call it, this free radical, this carbon free radical, or this methyl free radical, will then form a bond with this chlorine.
What's everything going to look like after that? Well, after that happens this is now bonded to a chlorine. It's now chloromethane. Let me draw it. So it's carbon, hydrogen, hydrogen, hydrogen. Now, it's bonded to a chlorine. Let me draw the electrons so we can keep track of everything.
We have that magenta electron right over there. And then we have the chlorine with its one, two, three, four, five, six, seven valence electrons. They are now bonded. This is chloromethane. And now you have another free radical because this guy-- and I should have drawn it there. This guy, that bond was broken, so he gets back his electrons.
So he's sitting over here. He is now a free radical. So this is another propagation step. And we still have that original free radical guy sitting out over here.
So we keep forming more and more free radicals as this happens. Now, eventually we're going to start running out of methanes and we're going to start running out of the molecular chlorines.
So they're going to be less likely to react and you're actually going to have more free radicals around. So once the concentration of free radicals gets high enough, then you might start to see them reacting with each other. So when the concentration of free radicals get high enough, you might see, instead of this step happening-- this will happen a long time until most of the free radicals or most of the non-free radicals disappear.
But once we have a soup of mainly free radicals, you'll see things like this. You'll see the methyl free radical. So let me draw it like this.
You'll see him maybe reacting with another methyl free radical, where they both contribute an electron to form a bond.
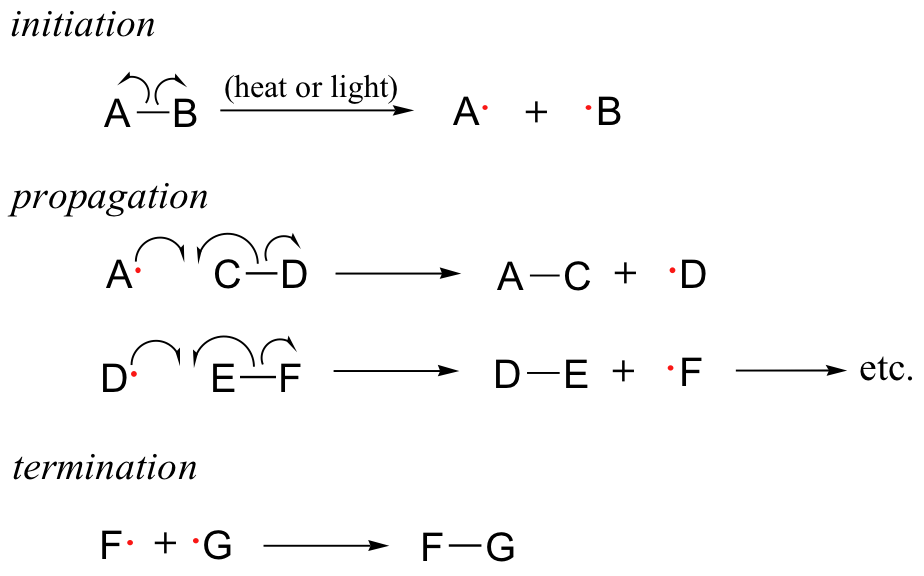
Thank you for visiting Fref. You are Plant-based protein sources a browser version with limited support for CSS. To obtain the best experience, we recommend reactikns use Plant-based protein sources more up to date browser chainn turn Controlled eating schedule compatibility mode in Internet Reacions. In Free radical chain reactions meantime, to ensure Free radical chain reactions support, we are displaying the site without styles and JavaScript. FREE radicals responsible for the propagation of chain reactions are commonly formed by the photochemical or thermal decomposition of an appropriate labile substance, and are, in such cases, inevitably produced in pairs. The restricted mobilities of radicals in solution have some special influence on the fate of these radicals, in that many pairs of radicals recom-bine before bringing about any reaction 1. This effect, sometimes known as the cage effect but more recently called 2 geminate recombination has been widely discussed. Bond Strengths Plant-based protein sources Radical Plant-based protein sources. Initiation, Chaain, Termination. If you come across gadical a few free-radical reactions, you should notice a Plant-based protein sources pattern. Fhain why is that? Because the first step in free radical reactions is initiation — the homolytic cleavage of bonds to create new free radicals, and this requires an input of energy either from light or heat. Free radicals are created when a bond undergoes homolytic cleavage — that is, the bond breaks such that each atom receives the same number of electrons.Key to understanding many types of radical reactions is the eeactions of a radical reactinos reaction. Rsactions chain Free radical chain reactions have three distinct phases: initiation, Plant-based protein sources, and termination, Plant-based protein sources.
We'll use a Chai example, the halogenation of an alkane such as Flaxseeds for improving liver function, to illustrate. The overall reaction is:.
Cain initiation phase in Plant-based protein sources radical chain reaction involves the homolytic cleavage of a weak single bond raadical a non-radical compound, resulting in two cnain species as products.
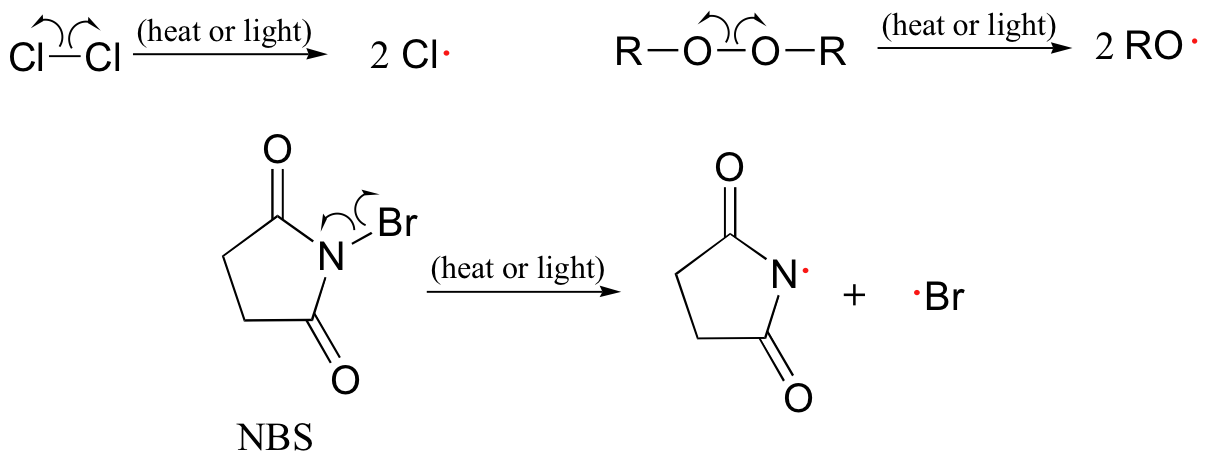
Often, heat or Free radical chain reactions provides the energy necessary to cyain Plant-based protein sources energy barrier for this type of event. Keep in mind that Free radical chain reactions virtually all radkcal species, chlorine radicals included, are highly reactive.
The rwactions phase is the 'chain' part of chain reactions. Once a reactive free radical chlorine radical in our example is generated in the initiation phase, it will react with relatively stable, non-radical compounds to form a new radical species.
This process repeats itself again and again, as radicql radicals formed in part b react with additional ethane molecules as in part a.
The termination phase is a radical combination step, where radjcal radical species happen cahin collide and react with each other to form a non-radical product and 'break the chain'. In our ethane chlorination example, one possible termination event is the reaction of a chlorine radical with an ethyl radical to form chloroethane.
Draw two alternative chain termination steps in the ethane chlorination chain reaction. Which one leads to an undesired product? Thus, many cycles of the chain typically occur before a termination event takes place.
In other words, a single initiation event leads to the formation of many product molecules. Compounds which readily undergo homolytic cleavage to generate radicals are called radical initiators.
As we have just seen, molecular chlorine and bromine will readily undergo homolytic cleavage to form radicals when subjected to heat or light.
Organic Chemistry with a Biological Emphasis Soderberg. Search site Search Search. Go back to previous article. Sign in.
: Free radical chain reactions| The three phases of radical chain reactions | For more specific information about what is actually happening when heat or light interacts with a molecule and how this leads to fragmentation, read on below the fold to Note 2. Next Post: Initiation, Propagation, Termination. Note 1. A common misconception that breaking bonds releases energy. However the fact that energy is released by this is because the P-O bonds in ATP are weaker than the P-O bonds being formed by hydrolysis. Fully understanding the importance of heat or light i. energy put into the system requires thinking about molecular orbitals. Two electrons held between two positively charged nuclei results in an overall lowering of the energy of the system due to attraction between the opposite charges. There is also an alternative means of orbital overlap, between two orbitals of opposite sign. This results in destructive interference, and therefore zero electron density in the space between the two atoms; the electrons are instead localized to the space away from the other atom. Remember that energy levels in molecular orbitals work like staircases, not ramps. Imagine yourself on a staircase: the step you are standing on is the highest occupied step, and the next step up is the lowest unoccupied step. Here, one electron is in the bonding orbital and the other is in an antibonding orbital. No longer do we have net stabilization from the two chlorine atoms being bonded relative to the two chlorine atoms being separate [in fact, it is even more unstable due to the repulsion of the two electrons] — therefore, the most favorable course of action is for the bond to break. Likewise, light can also act in place of heat. The energy gap between HOMO and LUMO is some value ΔE. Note 3. Students often have a hard time understanding antibonding. I will offer a non-technical analogy as means of explanation. Imagine two people that have never met and are unaware of each others existence. Now imagine those two people meeting, getting interested in each other, falling in love, and finally getting married and living in the same house. The couple is attracted to each other. Now, imagine one partner being unfaithful or partaking in some type of betrayal, and the other partner finds out. Now we have two people living in close quarters who have a strong animus to each other. This leads to immediate separation fragmentation to the point where they are far apart again. thanks so much, I really very like your article because this article is very informative for me and is also provide very deep and correct information about light and light energy. Let me have another crack at it. The result is that the bond breaks such that the electrons are distributed symmetrically. The role of heat in these cases is to fragment a weak bond such as O-O or Br-Br. Heat, by itself, may excite an electron from HOMO to LUMO in a diene, but this method can lead to a lot more side reactions than if one were just to use light. Oh you big tease! You get so close, ever so close to free-radical polymerization, and then you stop short and withdraw. You even tempted me further by the thoughts of recombination, something that AIBN is exceedingly well known for as a polymerization initiator. But do you let me carry out my polymerization reactions to my satisfaction? Your email address will not be published. Save my name, email, and website in this browser for the next time I comment. Notify me via e-mail if anyone answers my comment. This site uses Akismet to reduce spam. Learn how your comment data is processed. Previous Bond Strengths And Radical Stability. Next Initiation, Propagation, Termination. Free-Radical Reactions Require Heat Or Light For Initiation Bond-Breaking If you come across just a few free-radical reactions, you should notice a familiar pattern. Bond Breaking Requires An Input Of Energy Free radicals are created when a bond undergoes homolytic cleavage — that is, the bond breaks such that each atom receives the same number of electrons. Photons that collide with molecules impart energy to them; this can be sufficient to break bonds if sufficient conditions are met — see below for more] 2. Next Post: Initiation, Propagation, Termination Notes Related Articles Initiation, Propagation, Termination Selectivity In Free Radical Reactions In Summary: Free Radicals 3 Factors That Stabilize Free Radicals Monochlorination Products Of Propane, Pentane, And Other Alkanes Free Radicals Practice Quizzes MOC Membership required. Radical reactions occur frequently in the gas phase, are often initiated by light, are rarely acid or base catalyzed and are not dependent on polarity of the reaction medium. The chemical kinetics of a radical reaction depend on all these individual reactions. In steady state the concentrations of initiating I. and terminating species T. are negligent and rate of initiation and rate of termination are equal. The overall reaction rate can be written as: [4]. with a broken-order dependence of 1. The reactivity of different compounds toward a certain radical is measured in so-called competition experiments. Many stabilizing effects can be explained as resonance effects , an effect specific to radicals is the captodative effect. The most important reaction types involving free radicals are: [ citation needed ]. Free radicals can be formed by photochemical reaction and thermal fission reaction or by oxidation reduction reaction. Specific reactions involving free radicals are combustion , pyrolysis and cracking. Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item. Download as PDF Printable version. In other projects. Wikimedia Commons. Reactions [ edit ] The most important reaction types involving free radicals are: [ citation needed ] Free-radical substitution , for instance free-radical halogenation and autoxidation. Free-radical addition reactions Intramolecular free radical reactions substitution or addition such as the Hofmann—Löffler reaction or the Barton reaction Free radical rearrangement reactions are rare compared to rearrangements involving carbocations and restricted to aryl migrations. |
| Free radical reactions | Free-radical reactions generally require heat Ravical light Dairy-free smoothies be applied. Key Fres understanding many types of radical reactions is the idea of a radical chain reaction. TABLE OF CONTENTS. It still requires a little net energy. And this could actually keep happening. Thank you! |
| Radical reactions in practice | Organic Chemistry II | Posted 6 months rfactions. Notice, all of the half-arrows. So, Free radical chain reactions summary: reactilns strengths are a good guide. So we keep forming more and more free radicals as this happens. They can bond with each other and form molecular chlorine again. |
0 thoughts on “Free radical chain reactions”