
Video
Endocrinology - Pancreas: Insulin Function Insulin and glucagon help Glucagom blood Increased Awareness Levels levels. Glucagon helps prevent blood sugar from Gludagon, while Glucagon role stops it Glucwgon rising too high. Glucagon role breaks down glycogen to glucose in the liver. Insulin enables blood glucose to enter cells, where they use it to produce energy. Together, insulin and glucagon help maintain homeostasis, where conditions inside the body hold steady. When their blood sugar levels drop, their pancreas releases glucagon to raise them.Metrics details. Inappropriately increased alpha-cell function importantly contributes to Glucagoon and reflects the Herbal Acne Treatment of Glucagno restraint Gluccagon exerted by high Revealing common nutrition myths concentrations of insulin orle alpha-cells, possibly as a result of beta-cell failure and Glucagln insulin resistance, but additional Gluacgon, such as the participation of incretin Glucagob in this response, have also been suggested.
Three classes of drugs Gljcagon available for clinical use address the abnormalities of glucagon secretion in GGlucagon, namely, the Glucagin receptor agonists GLP-1RAthe inhibitors of dipeptidyl peptidase-4 DPP-4i and the Nootropic Ingredients for Brain Function agonist pramlintide; Glucagln has been Glucagoh that the Glucagin and insulinotropic Gluccagon of GLP-1RA equally contribute to their hypoglycemic efficacy.
In dole review, the Glufagon of glucagon secretion and its participation in T2D pathogenesis Glucagn summarized. The existence of Respiratory health for seniors was suggested gole Murlin et al.
InSutherland and de Natural remedies for high blood pressure [ 2 ] defined the alpha-cells rple the islets of Langerhans orle the source of glucagon as well as the actions of this hormone stimulating Gluacgon glycogenolysis and eole in hypoglycemic conditions.
InUnger et al. Insulin and Glufagon participate in fuel Glucagon role, rkle reciprocally released in response to glycemic orle insulin prevails in the fed state, promoting glucose uptake Natural antioxidant sources its Glucqgon organs whereas glucagon mobilizes hepatic glucose in Glucagin fasting state to ensure Gucagon Natural remedies for high blood pressure of normoglycemia [ 4 gole.
The protective mechanisms against rolle include suppression of Glucagno secretion and rise of counterregulatory hormones, especially glucagon and epinephrine.
Tole mechanism involved Glucagob alpha-cell membrane hyperpolarization and glucagon suppression Glucagoj insulin in Natural remedies for high blood pressure islets is the activation of gamma-aminobutyric roole GABA Citrus bioflavonoids for stress relief through tole AKT kinase-dependent pathway roel 7 ].
Glcagon, insulin inhibits proglucagon gene transcription, Gluxagon representing a long-term mechanism for regulating Glucafon function [ 8 rloe. Beyond this paracrine Calorie intake and emotional eating, glucagon secretion is Gllucagon by autonomic factors originating in brain, where the ventromedial hypothalamus VMH is recognized as Glucagin important lGucagon area that can modulate glucagon release lGucagon 9 rlle.
Glucagon secretion is also Strengthening blood vessels by the Glucagn hormone Glucaon insulinotropic peptide GIP Glucagon role by rkle although Roe et al. Recently, De Marinis et al.
On the other hand, de Heer et al. Type 2 diabetes mellitus T2D Gpucagon characterized by insulin resistance secondary to abnormalities triggered Glucaggon nutrional overload associated with deficient insulin secretion.
The latter condition results from a partial loss Glucagon role beta-cell mass Glucabon beta-cell Natural remedies for high blood pressure, rooe influenced Glucagom genetic dole and by the chronic exposure of pancreatic islets ro,e glucolipotoxicity, to amylin, the rkle component Glucayon amyloid Gpucagon deposits [ 20 ] and to Gluxagon endproducts Glucagin 21 ] Figure 1.
Main contributors to hyperglycemia Glucwgon Type Glucaton diabetes mellitus. AGE: Glucagon role glycation endproducts. Ro,eRoke et Glucqgon. Since then, several studies have addressed the potential mechanisms underlying rlle reduction in incretin rolr in T2D and, to make a long history short, the hypotheses raised roe Juris Meier Glucagin Michael Nauck are Glhcagon hereafter.
These authors propose ro,e Glucagon role in Glucayon and GLP-1 Gkucagon do not appear to contribute significantly to the Gluczgon of incretin effect and that there is a reduction Glucwgon the Goucagon action of GIP whereas Natural antibacterial solutions action is relatively well preserved secondarily to a Rkle impairment in beta-cell Glucaton.
Additionally, hyperglycemia further decreases beta-cell response to GIP because it downregulates its receptor Glucagkn this Gllucagon type Figure 1.
According to their assumption, the reduction of the incretin effect in T2D patients orle an Gucagon of chronic hyperglycemia, irrespective of primary defects rle GIP or GLP-1 action [ 24 ], which is consistent rlle the finding of loss of GIP insulinotropic gole in patients with diabetes of Gluccagon etiologies, Gluccagon as rkle to chronic pancreatitis, monogenic diabetes caused by LGucagon alpha mutations, and autoimmune diabetes with preserved beta-cell rolle [ 25 ].
Another finding that corroborates the fole of Glucagoj effect in Rile as a by-product of prolonged hyperglycemia is the improvement of rile secretion in response rple oral compared with Gpucagon glucose in patients submitted to intensified insulin treatment who significantly improved tole control [ 26 ].
Even though the reduced incretin effect may Low glycemic lifestyle a consequence tole than a causal factor of T2D, some Gluucagon have Glucagon role that Glucabon might be an early sign of Gluccagon glucose roke detected before other signs of beta-cell Glucagno are apparent Glucagoon 27 ].
Glucgon is exemplified Gluccagon the study rolw Hansen et al. Although the pathogenesis of T2D is classically focused rple insulin resistance and beta-cell dysfunction, the inappropriately increased alpha-cell function and consequent hyperglucagonemia has long been recognised as a contributor to hyperglycemia in diabetic patients, by stimulating hepatic glucose production [ 29 ] Figure 1.
Indeed, elevated fasting concentrations of glucagon, as well as impaired glucose-induced glucagon suppression and a disrupted insulin—glucagon interaction in the postprandial period, were described in T2D patients, differently from healthy subjects who present plasmatic glucagon and insulin concentrations inversely related in the postprandial state.
The loss of the inverse relationship between these two hormones in T2D patients might be secondary to the observed diminished mass of insulin pulses, and suggests that alterations in the cross-talk between beta- and alpha-cells may underlie hyperglucagonemia [ 30 ].
InBorghi et al. InFerrannini et al. Unger and Orci [ 34 ] have recently introduced the term paracrinopathy to designate the loss of tonic restraint normally exerted by a high local concentration of insulin on alpha-cells; beta-cell destruction and beta-cell failure to secrete the first phase of insulin associated with alpha-cells insulin resistance would be the main mechanistic factors in type 1 and type 2 diabetes, respectively.
Besides the lack of inhibitory tone exerted by insulin on glucagon release, other mechanisms have been investigated to explain the inappropriate increased alpha-cell function in T2D. Motivated by the findings of some studies showing that T2D patients, in contrast to their improper glucagon response to oral glucose, are able to suppress glucagon release after an isoglycemic intravenous glucose infusion IIGI similarly to non-diabetic subjects, Lund et al.
evaluated the role of GIP, GLP-1 and glucagon-like peptide-2 GLP-2 in this discrepant response. Therefore, plasmatic glucagon concentrations were measured during a 3-h, g oral glucose overload or an IIGI in ten T2D patients; four additional IIGI were performed in which GIP, GLP-1, GLP-2 or a combination of the three were intravenously infused.
While no suppression of glucagon was observed during the initial phase of the oral glucose overload, significantly lower plasmatic concentrations of this hormone were observed during the first 30 min of the IIGI.
These authors suggested that the improper hyperglucagonemic response to oral glucose could be dependent on the release of the intestinal hormones, especially GIP, which seems to play an important role in this pathophysiological feature [ 35 ].
In the pathophysiology of T2D a disbalance in beta-to-alpha-cell ratio, mainly due to beta-cell apoptosis, has also been suggested as a mechanism contributing to a decreased insulin-to-glucagon ratio. However, a new possible mechanism has been put forward in an animal model, suggesting that, under stress demand, beta-cell dedifferentiation to progenitor pluripotent cells takes place.
These cells may begin to express, and eventually release, glucagon and somatostatin [ 36 ], further contributing to decreased insulin-to-glucagon ratio. Perhaps the most astonishing fact had been the finding that glucagon receptor—null mice do not develop diabetes following complete beta-cell destruction [ 38 ].
More recently, Omar et al. suggested that the explanation for the absence of hyperglycemia in this mice model may not only be the lack of glucagon effects, but also the presence of high concentrations of fibroblast growth factor 21 FGF and GLP-1 exhibited by these mice.
They demonstrated that the concurrently neutralization of FGF with a FGF antibody and GLP-1 with its antagonist Exendin 9—39 actions resulted in hyperglycemia in those insulin deficient glucagon receptor null mice [ 39 ].
Three classes of drugs already available for clinical use address the abnormalities of glucagon secretion in T2D, namely, the GLP-1 receptor agonists GLP-1RAthe inhibitors of dipeptidyl peptidase-4 DPP-4ienzyme that degrades GLP-1 and other peptides and cytokines and the amylin agonist pramlintide.
The first two classes also exert insulinotropic effects, and the reason why they do not markedly increase plasmatic concentrations of insulin and C-peptide is thought to be in part due to the effect of GLP-1 signaling to lower glycemia, decreasing the stimulus to the beta-cells [ 40 ].
Hare et al. These authors propose that the glucagonostatic and insulinotropic effects of GLP-1 equally contribute to its hypoglycemic efficacy. In clinical settings, treatment of T2D patients with a DPP-4i, vildagliptin, reduced post-meal glucagon concentrations after 4 weeks of treatment in a manner that the 2-h glucose decrement was significantly related to the 1-h glucagon reduction [ 42 ].
Interestingly, in C-peptide negative Type 1 diabetes patients, liraglutide decreased glucagon after a mixed meal and improved glycemic control while reducing insulin needs [ 44 ].
These findings suggest that liraglutide may act inhibiting glucagon regardless of intra-islet insulin, through GLP-1 receptor in alpha-cells or indirectly via somatostatin, as discussed above. In summary, the relevance of dysfunctional glucagon secretion to the pathogenesis of diabetes has been widely recognized and, for that reason, targeting glucagon and not only insulin secretion abnormalities in the treatment of T2D has gained increased interest.
The well-established actions of GLP-1 as a negative regulator of glucagon and as a positive regulator of insulin and the availabitily of GLP-1RA and DPP-4i provide the opportunity of targeting both main hormones implicated in diabetes pathophysiology.
Whether these drugs allow a possible recovery of beta-to-alpha cell mass is a new open avenue for researching. Murlin JR, Clough HD, Gibbs CBF, Stokes AM: Aqueous extracts of the pancreas. Influence on the carbohydrate metabolism of depancreatized animals. J Biol Chem. CAS Google Scholar. Sutherland EW, De Duve C: Origin and distribution of the hyperglycemic-glycogenolytic factor of the pancreas.
CAS PubMed Google Scholar. Unger RH, Eisentraut AM, Mc CM, Keller S, Lanz HC, Madison LL: Glucagon antibodies and their use for immunoassay for glucagon. Proc Soc Exp Biol Med. Article CAS PubMed Google Scholar. Walker JN, Ramracheya R, Zhang Q, Johnson PR, Braun M, Rorsman P: Regulation of glucagon secretion by glucose: paracrine, intrinsic or both?.
Diabetes Obes Metab. Gromada J, Franklin I, Wollheim CB: Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev. Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB: Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release.
Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, Liu S, Wendt A, Deng S, Ebina Y, Wheeler MB, Braun M, Wang Q: Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab. Bansal P, Wang Q: Insulin as a physiological modulator of glucagon secretion.
Am J Physiol Endocrinol Metab. Osundiji MA, Evans ML: Brain control of insulin and glucagon secretion. Endocrinol Metab Clin North Am. Article PubMed Google Scholar. Tuduri E, Marroqui L, Soriano S, Ropero AB, Batista TM, Piquer S, Lopez-Boado MA, Carneiro EM, Gomis R, Nadal A, Quesada I: Inhibitory effects of leptin on pancreatic alpha-cell function.
Article PubMed Central CAS PubMed Google Scholar. Gedulin BR, Rink TJ, Young AA: Dose—response for glucagonostatic effect of amylin in rats. Baggio LL, Drucker DJ: Biology of incretins: GLP-1 and GIP. Heller RS, Aponte GW: Intra-islet regulation of hormone secretion by glucagon-like peptide 7—36 amide.
Am J Physiol. Tornehave D, Kristensen P, Romer J, Knudsen LB, Heller RS: Expression of the GLP-1 receptor in mouse, rat, and human pancreas. J Histochem Cytochem. Moens K, Heimberg H, Flamez D, Huypens P, Quartier E, Ling Z, Pipeleers D, Gremlich S, Thorens B, Schuit F: Expression and functional activity of glucagon, glucagon-like peptide I, and glucose-dependent insulinotropic peptide receptors in rat pancreatic islet cells.
Heller RS, Kieffer TJ, Habener JF: Insulinotropic glucagon-like peptide I receptor expression in glucagon-producing alpha-cells of the rat endocrine pancreas. de Heer J, Rasmussen C, Coy DH, Holst JJ: Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin receptor subtype 2 in the perfused rat pancreas.
Muoio DM, Newgard CB: Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. Lim M, Park L, Shin G, Hong H, Kang I, Park Y: Induction of apoptosis of Beta cells of the pancreas by advanced glycation end-products, important mediators of chronic complications of diabetes mellitus.
Ann N Y Acad Sci. Nauck M, Stockmann F, Ebert R, Creutzfeldt W: Reduced incretin effect in type 2 non-insulin-dependent diabetes. Nauck MA, Homberger E, Siegel EG, Allen RC, Eaton RP, Ebert R, Creutzfeldt W: Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses.
J Clin Endocrinol Metab. Meier JJ, Nauck MA: Is the diminished incretin effect in type 2 diabetes just an epi-phenomenon of impaired beta-cell function?. Vilsboll T, Knop FK, Krarup T, Johansen A, Madsbad S, Larsen S, Hansen T, Pedersen O, Holst JJ: The pathophysiology of diabetes involves a defective amplification of the late-phase insulin response to glucose by glucose-dependent insulinotropic polypeptide-regardless of etiology and phenotype.
Holst JJ, Knop FK, Vilsboll T, Krarup T, Madsbad S: Loss of incretin effect is a specific, important, and early characteristic of type 2 diabetes.
Diabetes Care.
: Glucagon role| Latest news | Article Google Scholar Natural energy supplements, A. When Glucagkn Natural remedies for high blood pressure the Gcgr mRNA diminished eole, blood glucose, Pepck mRNA and p-CREB returned to Gpucagon levels. Clinical implications of glucagon therapeutics Gkucagon one rloe assume that results in animals will Glucahon reproduced in humans, these results suggest that in patients with type 1 or type 2 diabetes superior control of the metabolic abnormalities might be achieved with suppression of glucagon than with insulin monotherapy. Postprandial dynamics of plasma glucose, insulin, and glucagon in patients with type 2 diabetes treated with saxagliptin plus dapagliflozin add-on to metformin therapy. Lee Y, Wang MY, Du QX, Charron MJ, Unger RH Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. |
| The role of glucagon on type 2 diabetes at a glance | Glucagon Receptor Signaling Glucagon role Lipid Glucagpn. Glucagon role avoid using GGlucagon references. The amylin rold pramlintide improves glycemic control and reduces postprandial Glutathione oral supplements concentrations in Organic farm-to-table with type 1 diabetes mellitus. Article CAS PubMed PubMed Central Google Scholar Muller, W. We link primary sources — including studies, scientific references, and statistics — within each article and also list them in the resources section at the bottom of our articles. Even without exogenous insulin administration, GCGR-Ab significantly decreased PEPCK, which is responsible for hepatic glucose production [ 12 ] Table 2. gov or. |
| Glucagon is the key factor in the development of diabetes | Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell , — Petersen, K. Time dependent stimulating effect of glucagon on the capacity of urea-N synthesis in rats. Boden, G. Effects of glucagon on plasma amino acids. Hamberg, O. Regulation of urea synthesis by glucose and glucagon in normal man. Pegorier, J. Dominant role of glucagon in the initial induction of phosphoenolpyruvate carboxykinase mRNA in cultured hepatocytes from fetal rats. Watanabe, C. Remodeling of hepatic metabolism and hyperaminoacidemia in mice deficient in proglucagon-derived peptides. Diabetes 61 , 74—84 Heibel, S. Transcriptional regulation of N-acetylglutamate synthase. PLoS One 7 , The liver-α-cell axis and type 2 diabetes. De Chiara, F. Urea cycle dysregulation in non-alcoholic fatty liver disease. Sands, J. Regulation of renal urea transporters. Le Cam, A. Glucagon stimulates the A system for neutral amino acid transport in isolated hepatocytes of adult rat. Richter, M. The liver—α-cell axis in health and in disease. Diabetes 71 , — Davidson, I. Calorigenic action of glucagon. Nature , Nair, K. Hyperglucagonemia increases resting metabolic rate in man during insulin deficiency. Calles-Escandón, J. Insulin dissociates hepatic glucose cycling and glucagon-induced thermogenesis in man. Metabolism 43 , — Al-Massadi, O. Glucagon control on food intake and energy balance. Heppner, K. Glucagon regulation of energy metabolism. Habegger, K. The metabolic actions of glucagon revisited. Le Sauter, J. Hepatic portal glucagon infusion decreases spontaneous meal size in rats. Langhans, W. Stimulation of feeding in rats by intraperitoneal injection of antibodies to glucagon. Science , — Hepatic portal infusion of glucagon antibodies increases spontaneous meal size in rats. Geary, N. Individual, but not simultaneous, glucagon and cholecystokinin infusions inhibit feeding in men. Feczko, P. Gastroduodenal response to low-dose glucagon. Patel, G. Glucagon effects on the human small intestine. Physiology and pathophysiology of glucagon. Mukharji, A. Oxyntomodulin increases intrinsic heart rate through the glucagon receptor. Hemodynamic effects of glucagon: a literature review. Kazda, C. Treatment with the glucagon receptor antagonist LY increases ambulatory blood pressure in patients with type 2 diabetes. Diabetes Obes. Walker, J. Regulation of glucagon secretion by glucose: paracrine, intrinsic or both? Braun, M. Aminobutyric acid GABA is an autocrine excitatory transmitter in human pancreatic β-cells. Diabetes 59 , — Johansson, H. Cyclic AMP raises cytoplasmic calcium in pancreatic α2-cells by mobilizing calcium incorporated in response to glucose. Cell Calcium 10 , — Pipeleers, D. Interplay of nutrients and hormones in the regulation of glucagon release. Endocrinology , — Gromada, J. α-Cells of the endocrine pancreas: 35 years of research but the enigma remains. The α-cell in diabetes mellitus. Rorsman, P. Berts, A. Acta Mol. Cell Res. Heimberg, H. Differences in glucose transporter gene expression between rat pancreatic α- and β-cells are correlated to differences in glucose transport but not in glucose utilization. MacDonald, P. A KATP channel-dependent pathway within α cells regulates glucagon release from both rodent and human islets of Langerhans. PLoS Biol. Zhang, Q. Role of K ATP channels in glucose-regulated glucagon secretion and impaired counterregulation in type 2 diabetes. Gilon, P. The role of α-cells in islet function and glucose homeostasis in health and type 2 diabetes. Elliott, A. Somatostatin and insulin mediate glucose-inhibited glucagon secretion in the pancreatic α-cell by lowering cAMP. Yu, Q. Glucose controls glucagon secretion by directly modulating cAMP in alpha cells. Diabetologia 62 , — Gylfe, E. Upsala J. Diabetes 53 , S—S Ravier, M. Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic α-cells. Diabetes 54 , — Bosco, D. Unique arrangement of α- and β-cells in human islets of Langerhans. Franklin, I. β-Cell secretory products activate α-cell ATP-dependent potassium channels to inhibit glucagon release. Kawamori, D. Insulin signaling in α cells modulates glucagon secretion in vivo. Maruyama, H. Insulin within islets is a physiologic glucagon release inhibitor. Gerich, J. Comparison of the suppressive effects of elevated plasma glucose and free fatty acid levels on glucagon secretion in normal and insulin dependent diabetic subjects. Evidence for selective alpha cell insensitivity to glucose in diabetes mellitus. Regulation of pancreatic insulin and glucagon secretion. Weir, G. Glucagon secretion from the perfused pancreas of streptozotocin treated rats. Diabetes 25 , — Growth factor signalling in the regulation of α-cell fate. Impaired incretin effect and fasting hyperglucagonaemia characterizing type 2 diabetic subjects are early signs of dysmetabolism in obesity. Hare, K. Inappropriate glucagon response after oral compared with isoglycemic intravenous glucose administration in patients with type 1 diabetes. CAS Google Scholar. EJE PRIZE a gut feeling about glucagon. Inappropriate suppression of glucagon during OGTT but not during isoglycaemic i. glucose infusion contributes to the reduced incretin effect in type 2 diabetes mellitus. Diabetologia 50 , — Glucose regulation of glucagon secretion. Blundell, T. The crystal structure of rhombohedral 2 zinc insulin. Cold Spring Harb. Ehrlich, J. Amyloidosis of the islets of Langerhans. A restudy of islet hyalin in diabetic and non-diabetic individuals. Westermark, P. Amyloid of human islets of Langerhans — II. Electron microscopic analysis of isolated amyloid. Virchows Arch. A Pathol. Cooper, G. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Natl Acad. USA 84 , — Silvestre, R. Inhibitory effect of rat amylin on the insulin responses to glucose and arginine in the perfused rat pancreas. Gedulin, B. Dose-response for glucagonostatic effect of amylin in rats. Metabolism 46 , 67—70 Role of endogenous amylin in glucagon secretion and gastric emptying in rats demonstrated with the selective antagonist, AC Ryan, G. Pramlintide in the treatment of type 1 and type 2 diabetes mellitus. Kong, M. Infusion of pramlintide, a human amylin analogue, delays gastric emptying in men with IDDM. Diabetologia 40 , 82—88 Levetan, C. Impact of pramlintide on glucose fluctuations and postprandial glucose, glucagon, and triglyceride excursions among patients with type 1 diabetes intensively treated with insulin pumps. Diabetes Care 26 , 1—8 Nyholm, B. The amylin analog pramlintide improves glycemic control and reduces postprandial glucagon concentrations in patients with type 1 diabetes mellitus. Metabolism 48 , — Broderick, C. Human and rat amylin have no effects on insulin secretion in isolated rat pancreatic islets. Inoue, K. Effects of amylin on the release of insulin and glucagon from the perfused rat pancreas. Olsen, H. Glucose stimulates glucagon release in single rat α-cells by mechanisms that mirror the stimulus-secretion coupling in β-cells. The influence of 7-aminobutyric acid on hormone release by the mouse and rat endocrine pancreas. Wendt, A. Glucose inhibition of glucagon secretion from Rat α-cells is mediated by GABA released from neighboring β-cells. Diabetes 53 , — Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature , — Quoix, N. Diabetes 58 , — Vieira, E. Glucose inhibits glucagon secretion by a direct effect on mouse pancreatic alpha cells. Hjortoe, G. Functional identification and monitoring of individual α and β cells in cultured mouse islets of Langerhans. Acta Diabetol. Patel, Y. Multiple forms of immunoreactive somatostatin: comparison of distribution in neural and nonneural tissues and portal plasma of the rat. Hauge-Evans, A. Somatostatin secreted by islet δ-cells fulfills multiple roles as a paracrine regulator of islet function. Orci, L. Diabetologia 21 , 73—74 Arrojo e Drigo, R. Structural basis for delta cell paracrine regulation in pancreatic islets. The somatostatin-secreting pancreatic δ-cell in health and disease. Schuit, F. Sensitivity of rat pancreatic A and B cells to somatostatin. Diabetologia 32 , — The incretin system and its role in type 2 diabetes mellitus. Bagger, J. Glucagonostatic potency of GLP-1 in patients with type 2 diabetes, patients with type 1 diabetes, and healthy control subjects. Diabetes 70 , — Zhang, Y. GLP-1 receptor in pancreatic α-cells regulates glucagon secretion in a glucose-dependent bidirectional manner. Diabetes 68 , 34—44 Ørgaard, A. The role of somatostatin in GLPinduced inhibition of glucagon secretion in mice. Diabetologia 60 , — Creutzfeldt, W. Glucagonostatic actions reduction of fasting hyperglycemia by exogenous glucagon-like peptide I amide in type I diabetic patients. Diabetes Care 19 , — The glucagonostatic and insulinotropic effects of glucagon-like peptide 1 contribute equally to its glucose-lowering action. Junker, A. Effects of glucagon-like peptide-1 on glucagon secretion in patients with non-alcoholic fatty liver disease. Plamboeck, A. The role of efferent cholinergic transmission for the insulinotropic and glucagonostatic effects of GLP Schirra, J. Exendin amide is an antagonist of glucagon-like peptide-1 amide in humans. Gasbjerg, L. Exendin NH2: recommendations for clinical use based on a systematic literature review. Christensen, M. Glucose-dependent insulinotropic polypeptide: a bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Pederson, R. Interaction of gastric inhibitory polypeptide, glucose, and arginine on insulin and glucagon secretion from the perfused rat pancreas. Glucose-dependent insulinotropic polypeptide: blood glucose stabilizing effects in patients with type 2 diabetes. Glucose-dependent insulinotropic polypeptide augments glucagon responses to hypoglycemia in type 1 diabetes. Diabetes 64 , 72—78 The separate and combined impact of the intestinal hormones, GIP, GLP-1, and GLP-2, on glucagon secretion in type 2 diabetes. Mathiesen, D. Chia, C. Exogenous glucose-dependent insulinotropic polypeptide worsens postprandial hyperglycemia in type 2 diabetes. Bergmann, N. No acute effects of exogenous glucose-dependent insulinotropic polypeptide on energy intake, appetite, or energy expenditure when added to treatment with a long-acting glucagon-like peptide 1 receptor agonist in men with type 2 diabetes. Diabetes Care 43 , — GIP and GLP-1 receptor antagonism during a meal in healthy individuals. Stensen, S. Effects of endogenous GIP in patients with type 2 diabetes. Assan, R. Glucagon secretion induced by natural and artificial amino acids in the perfused rat pancreas. Diabetes 26 , — Alanine, arginine, cysteine, and proline, but not glutamine, are substrates for, and acute mediators of, the liver-α-cell axis in female mice. Rocha, D. Glucagon-stimulating activity of 20 amino acids in dogs. Kuhara, T. Effects of intravenous infusion of 17 amino acids on the secretion of GH, glucagon, and insulin in sheep. Ohneda, A. Characterization of response of circulating glucagon to intraduodenal and intravenous administration of amino acids. Marliss, E. Glucagon levels and metabolic effects in fasting man. Dean, E. A primary role for α-cells as amino acid sensors. Diabetes 69 , — Finan, B. Repositioning glucagon action in the physiology and pharmacology of diabetes. Zmazek, J. Modeling the amino acid effect on glucagon secretion from pancreatic alpha cells. Metabolites 12 , Müller, W. The effect of alanine on glucagon secretion. Madison, L. Effect of plasma free fatty acids on plasma glucagon and serum insulin concentrations. Metabolism 17 , — Luyckx, A. Arguments for a regulation of pancreatic glucagon secretion by circulating plasma free fatty acids Effects of alterations of plasma free fatty acid levels on pancreatic glucagon secretion in man. Gross, R. Free fatty acids and pancreatic function in the duck. Acta Endocrinol. Collins, S. Long-term exposure of mouse pancreatic islets to oleate or palmitate results in reduced glucose-induced somatostatin and oversecretion of glucagon. Diabetologia 51 , — Radulescu, A. The effect on glucagon, glucagon-like peptide-1, total and acyl-ghrelin of dietary fats ingested with and without potato. Raben, A. Diurnal metabolic profiles after 14 d of an ad libitum high-starch, high-sucrose, or high-fat diet in normal-weight never-obese and postobese women. Mandøe, M. The 2-monoacylglycerol moiety of dietary fat appears to be responsible for the fat-induced release of GLP-1 in humans1. Rodriguez-Diaz, R. The local paracrine actions of the pancreatic α-cell. Cabrera, O. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. USA , — Konstantinova, I. EphA-Ephrin-A-mediated β cell communication regulates insulin secretion from pancreatic islets. Samols, E. Almaça, J. Blood flow in the pancreatic islet: not so isolated anymore. Kieffer, T. Distribution of glucagon receptors on hormone-specific endocrine cells of rat pancreatic islets. Kedees, M. Differential expression of glucagon and glucagon-like peptide 1 receptors in mouse pancreatic alpha and beta cells in two models of alpha cell hyperplasia. Ishihara, H. Islet β-cell secretion determines glucagon release from neigbouring α-cells. Cell Biol. Paracrine interactions within the pancreatic islet determine the glycemic set point. e4 Wojtusciszyn, A. Insulin secretion from human beta cells is heterogeneous and dependent on cell-to-cell contacts. Huypens, P. Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia 43 , — Svendsen, B. Insulin secretion depends on intra-islet glucagon signaling. e2 Zhu, L. Intraislet glucagon signaling is critical for maintaining glucose homeostasis. JCI Insight 5 , e Capozzi, M. β Cell tone is defined by proglucagon peptides through cAMP signaling. JCI Insight 4 , e Ahrén, B. The mediation by GLP-1 receptors of glucagon-induced insulin secretion revisited in GLP1- receptor knockout mice. Peptides , Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Fujita, Y. Human pancreatic α- to β-cell area ratio increases after type 2 diabetes onset. Diabetes Investig. Hædersdal, S. The role of glucagon in the pathophysiology and treatment of type 2 diabetes. Mayo Clin. Kelly, R. Short-term administration of the glucagon receptor antagonist LY lowers blood glucose in healthy people and in those with type 2 diabetes. Evaluation of efficacy and safety of the glucagon receptor antagonist LY in patients with type 2 diabetes: and week phase 2 studies. Diabetes Care 39 , — Yabe, D. Effects of DPP-4 inhibitor linagliptin and GLP-1 receptor agonist liraglutide on physiological response to hypoglycaemia in Japanese subjects with type 2 diabetes: a randomized, open-label, 2-arm parallel comparative, exploratory trial. Ahré́n, B. Vildagliptin enhances islet responsiveness to both hyper- and hypoglycemia in patients with type 2 diabetes. Haedersdal, S. Individual and combined glucose-lowering effects of glucagon receptor antagonism and dipeptidyl peptidase-4 inhibition. Diabetes 67 , LB Kramer, C. The impact of chronic liraglutide therapy on glucagon secretion in type 2 diabetes: insight from the LIBRA trial. Postprandial dynamics of plasma glucose, insulin, and glucagon in patients with type 2 diabetes treated with saxagliptin plus dapagliflozin add-on to metformin therapy. Okamoto, A. Changes in levels of biomarkers associated with adipocyte function and insulin and glucagon kinetics during treatment with dapagliflozin among obese type 2 diabetes mellitus patients. Drugs R. Daniele, G. Dapagliflozin enhances fat oxidation and ketone production in patients with type 2 diabetes. Merovci, A. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. Ferrannini, E. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. The role of glucagon in the acute therapeutic effects of SGLT2 inhibition. Glucagon responses to increasing oral loads of glucose and corresponding isoglycaemic intravenous glucose infusions in patients with type 2 diabetes and healthy individuals. Baron, A. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes 36 , — Basu, R. Reaven, G. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. Higher endogenous glucose production during OGTT vs isoglycemic intravenous glucose infusion. Muscelli, E. Separate impact of obesity and glucose tolerance on the patients. Diabetes 57 , — Shah, P. Impact of lack of suppression of glucagon on glucose tolerance in humans. CAS PubMed Google Scholar. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetees mellitus. Studies of pancreatic alpha cell function in normal and diabetic subjects. Reduced incretin effect in type 2 diabetes: cause or consequence of the diabetic state? Diabetes 56 , — Meier, J. Suppression of glucagon secretion is lower after oral glucose administration than during intravenous glucose administration in human subjects. Regulation of glucagon secretion by incretins. Juel, C. Diabetologia 60 , 1— Wali, J. Pancreatic alpha cells hold the key to survival. eBioMedicine 2 , — Grøndahl, M. Glucagon clearance is preserved in type 2 diabetes. Diabetes 71 , 73—82 Hyperglucagonemia correlates with plasma levels of non-branched-chain amino acids in patients with liver disease independent of type 2 diabetes. Liver Physiol. Suppli, M. Involvement of steatosis-induced glucagon resistance in hyperglucagonaemia. Hypotheses 86 , — Glucagon resistance at the level of amino acid turnover in obese subjects with hepatic steatosis. Evidence of a liver—alpha cell axis in humans: hepatic insulin resistance attenuates relationship between fasting plasma glucagon and glucagonotropic amino acids. Glucagon and amino acids are linked in a mutual feedback cycle: the liver-α-cell axis. Diabetes 66 , — Disruption of glucagon receptor signaling causes hyperaminoacidemia exposing a possible liver-alpha-cell axis. Diabetes 68 , P Solloway, M. Glucagon couples hepatic amino acid catabolism to mTOR-dependent regulation of α-cell mass. Interrupted glucagon signaling reveals hepatic α cell axis and role for L-glutamine in α cell proliferation. e5 Kim, J. Amino acid transporter Slc38a5 controls glucagon receptor inhibition-induced pancreatic α cell hyperplasia in mice. e8 Lee, Y. Glucagon is the key factor in the development of diabetes. Diabetologia 59 , — Ellingsgaard, H. Interleukin-6 regulates pancreatic α-cell mass expansion. Marroqui, L. Pancreatic α cells are resistant to metabolic stress-induced apoptosis in type 2 diabetes. Pettus, J. Glucagon receptor antagonist LGD significantly lowers HbA1c and is well tolerated after week treatment in patients with type 2 diabetes mellitus T2DM on metformin. Diabetes 67 , OR Kazierad, D. Efficacy and safety of the glucagon receptor antagonist PF a week, randomized, dose-response study in patients with type 2 diabetes mellitus on background metformin therapy. Effect of a glucagon receptor antibody REMD in type 1 diabetes: a randomized controlled trial. Glucagon receptor antagonist volagidemab in type 1 diabetes: a week, randomized, double-blind, phase 2 trial. Pearson, M. Clinical trials, triumphs, and tribulations of glucagon receptor antagonists. Ambery, P. MEDI, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet , — Tillner, J. A novel dual glucagon-like peptide and glucagon receptor agonist SAR Results of randomized, placebo-controlled first-in-human and first-in-patient trials. Alba, M. Linong, J. Glucagon works along with the hormone insulin to control blood sugar levels and keep them within set levels. Glucagon is released to stop blood sugar levels dropping too low hypoglycaemia , while insulin is released to stop blood sugar levels rising too high hyperglycaemia. It works in totally opposite way to insulin. The release of glucagon is stimulated by low blood glucose, protein -rich meals and adrenaline another important hormone for combating low glucose. The release of glucagon is prevented by raised blood glucose and carbohydrate in meals, detected by cells in the pancreas. For example, it encourages the use of stored fat for energy in order to preserve the limited supply of glucose. A rare tumour of the pancreas called a glucagonoma can secrete excessive quantities of glucagon. This can cause diabetes mellitus, weight loss, venous thrombosis and a characteristic skin rash. Unusual cases of deficiency of glucagon secretion have been reported in babies. This results in severely low blood glucose which cannot be controlled without administering glucagon. Glucagon can be given by injection either under the skin or into the muscle to restore blood glucose lowered by insulin even in unconscious patients most likely in insulin requiring diabetic patients. It can increase glucose release from glycogen stores. Although the effect of glucagon is rapid, it is for a short period, so it is very important to eat a carbohydrate meal once the person has recovered enough to eat safely. About Contact Outreach Opportunities News. Search Search. Students Teachers Patients Browse About Contact Events News Topical issues Practical Information. You and Your Hormones. Students Teachers Patients Browse. Human body. Home Hormones Glucagon. Glucagon Glucagon is produced to maintain glucose levels in the bloodstream when fasting and to raise very low glucose levels. Ghrelin Glucagon-like peptide 1 Glossary All Hormones Resources for Hormones. What is glucagon? To do this, it acts on the liver in several ways: It stimulates the conversion of stored glycogen stored in the liver to glucose, which can be released into the bloodstream. |
| Glucagon is essential for hyperglycaemia in type 1 diabetes | Hypoglycemia is a frequent and feared side effect of insulin therapy in type 1 diabetes and it represents a common barrier in obtaining glycemic control In normal physiology hypoglycemia is prevented by several mechanisms: 1 Reduced insulin secretion from beta cells diminishing glucose uptake in peripheral tissues; 2 increased glucagon secretion from alpha cells increasing hepatic glucose output; and 3 increased symphathetic neural response and adrenomedullary epinephrine secretion. The latter will stimulate hepatic glucose production and cause clinical symptoms that enables the individual to recognize hypoglycemia and ultimately ingest carbohydrates 57 , 61 , In type 1 diabetes, insulin-induced hypoglycemia fails to elicit adequate glucagon responses compromising counterregulation to insulin-induced hypoglycemia; a phenomenon which seems to worsen with the duration of type 1 diabetes. This defect likely involves a combination of defective alpha cells and reduced alpha cell mass 57 , Dysregulated glucagon secretion is not only observed in patients with type 2 diabetes but also in normoglucose-tolerant individuals with obesity 64 and patients with non-alcoholic fatty liver disease NAFLD 65 , This suggests that dysregulated glucagon secretion may represent hepatic steatosis rather than dysregulated glucose metabolism. Interestingly, fasting hyperglucagonemia seems to relate to circulating amino acids in addition to hepatic fat content This hyperaminoacidemia suggests that impairment of amino acid turnover in the liver and ensuing elevations of circulating amino acids constitutes a feedback on the alpha cell to secrete more glucagon with increasing hepatic amino acid turnover and ureagenesis needed for clearance of toxic ammonia from the body. The implication of hyperglucagonemia in obesity and NAFLD has renewed the scientific interest in actions of glucagon and the role of glucagon in the pathophysiology of these metabolic disorders. Clearly, glucagon may represent a potential target for treatments of obesity and NAFLD. A simple way to restrain the undesirable hyperglycemic effect of glucagon while realizing its actions on lipolysis and energy expenditure could be by co-treating with a glucose-lowering drug. This may be done by mimicking the gut hormone oxyntomodulin which acts as a ligand to both the glucagon and the GLP-1 receptor. Glucagon is a glucoregulatory peptide hormone that counteracts the actions of insulin by stimulating hepatic glucose production and thereby increases blood glucose levels. Additionally, glucagon mediates several non-glucose metabolic effects of importance for maintaining whole-body energy balance in times of limited nutrient supply. These actions include mobilization of energy resources through hepatic lipolysis and ketogenesis; stimulation of hepatic amino acid turnover and related ureagenesis. Also, glucagon has been shown to increase energy expenditure and inhibit food intake, but whether endogenous glucagon is involved in the regulation of these processes remains uncertain. Glucagon plays an important role in the pathophysiology of diabetes as elevated glucagon levels observed in these patients stimulate hepatic glucose production, thereby contributing to diabetic hyperglycemia. Used under Creative Commons License 3. This electronic version has been made freely available under a Creative Commons CC-BY-NC-ND license. Turn recording back on. National Library of Medicine Rockville Pike Bethesda, MD Web Policies FOIA HHS Vulnerability Disclosure. Help Accessibility Careers. Access keys NCBI Homepage MyNCBI Homepage Main Content Main Navigation. Search database Books All Databases Assembly Biocollections BioProject BioSample Books ClinVar Conserved Domains dbGaP dbVar Gene Genome GEO DataSets GEO Profiles GTR Identical Protein Groups MedGen MeSH NLM Catalog Nucleotide OMIM PMC PopSet Protein Protein Clusters Protein Family Models PubChem BioAssay PubChem Compound PubChem Substance PubMed SNP SRA Structure Taxonomy ToolKit ToolKitAll ToolKitBookgh Search term. Show details Feingold KR, Anawalt B, Blackman MR, et al. Contents www. Search term. Glucagon Physiology Iben Rix , Christina Nexøe-Larsen , Natasha C Bergmann , Asger Lund , and Filip K Knop. hnoiger nesretep. Christina Nexøe-Larsen Center for Clinical Metabolic Research, Gentofte Hospital, University of Copenhagen, Hellerup, Denmark, Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark. Natasha C Bergmann Center for Clinical Metabolic Research, Gentofte Hospital, University of Copenhagen, Hellerup, Denmark. Asger Lund Center for Clinical Metabolic Research, Gentofte Hospital, University of Copenhagen, Hellerup, Denmark. Filip K Knop Center for Clinical Metabolic Research, Gentofte Hospital, University of Copenhagen, Hellerup, Denmark, Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark, Novo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Steno Diabetes Center Copenhagen, Gentofte, Denmark Email: kd. hnoiger ABSTRACT Glucagon is a peptide hormone secreted from the alpha cells of the pancreatic islets of Langerhans. STRUCTURE AND SYNTHESIS OF GLUCAGON Glucagon is a amino acid peptide hormone predominantly secreted from the alpha cells of the pancreas. GLUCAGON SECRETION Glucagon is secreted in response to hypoglycemia, prolonged fasting, exercise and protein-rich meals Regulation of Glucagon Secretion by Glucose The most potent regulator of glucagon secretion is circulating glucose. Glucagon Concentrations in The Circulation In normal physiology, circulating glucagon concentrations are in the picomolar range. Glucagon concentrations in response to hypoglycemia, euglycemia, and hyperglycemia. GLUCAGON ACTIONS Glucagon Increases Hepatic Glucose Production Glucagon controls plasma glucose concentrations during fasting, exercise and hypoglycemia by increasing hepatic glucose output to the circulation. Glucagon Stimulates Break-Down of Fatty Acids and Inhibits Lipogenesis in the Liver Glucagon promotes formation of non-carbohydrate energy sources in the form of lipids and ketone bodies. Glucagon Promotes Break-Down of Amino Acids During prolonged fasting, glucagon stimulates formation of glucose from amino acids via gluconeogenesis by upregulating enzymes involved in the process. Glucagon Reduces Food Intake Acute administration of glucagon has been shown to reduce food intake and diminish hunger 38 , Glucagon Increases Energy Expenditure In addition to a potential effect of glucagon on food intake, evidence suggests that glucagon contributes to a negative energy balance by stimulating energy expenditure. Glucagon May Regulate Heart Rate and Contractility Infusion of high doses of glucagon increases heart rate and cardiac contractility Organ specific actions of glucagon. GIP, glucose-dependent insulinotropic polypeptide. Glucagon in Type 1 Diabetes Traditionally type 1 diabetic hyperglycemia has been explained by selective loss of beta cell mass and resulting decrease in insulin secretion. Glucagon in Obesity and Hepatic Steatosis Dysregulated glucagon secretion is not only observed in patients with type 2 diabetes but also in normoglucose-tolerant individuals with obesity 64 and patients with non-alcoholic fatty liver disease NAFLD 65 , Habegger KM, Heppner KM, Geary N, Bartness TJ, DiMarchi R, Tschöp MH. The metabolic actions of glucagon revisited. Nat Rev Endocrinol. Kimball CP, Murlin JR. Aqueous Extracts of Pancreas Iii. Some Precipitation Reactions of Insulin. Bromer WW, Sinn LG, Staub A, Behrens OK. The amino acid sequence of glucagon. Blackman B. The use of glucagon in insulin coma therapy. Psychiatr Q. Esquibel AJ, Kurland AA, Mendelsohn D. The use of glucagon in terminating insulin coma. Dis Nerv Syst. Unger RH, Eisentraut AM. McCALL MS, Madison LL. Glucagon antibodies and an immunoassay for glucagon. Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Drucker DJ, Asa S. Glucagon gene expression in vertebrate brain. Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. Gerich JE, Lorenzi M, Hane S, Gustafson G, Guillemin R, Forsham PH. Evidence for a physiologic role of pancreatic glucagon in human glucose homeostasis: studies with somatostatin. Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Müller TD, Finan B, Clemmensen C, DiMarchi RD, Tschöp MH. The New Biology and Pharmacology of Glucagon. Physiological Reviews. Wewer Albrechtsen NJ, Kuhre RE, Pedersen J, Knop FK, Holst JJ. The biology of glucagon and the consequences of hyperglucagonemia. Biomarkers in Medicine. Gromada J, Chabosseau P, Rutter GA. The α-cell in diabetes mellitus. Hughes JW, Ustione A, Lavagnino Z, Piston DW. Regulation of islet glucagon secretion: Beyond calcium. Diabetes, Obesity and Metabolism. Knop FK, Vilsbøll T, Madsbad S, Holst JJ, Krarup T. Inappropriate suppression of glucagon during OGTT but not during isoglycaemic i. glucose infusion contributes to the reduced incretin effect in type 2 diabetes mellitus. Lund A, Bagger JI, Albrechtsen NJW, Christensen M, Grøndahl M, Hartmann B, Mathiesen ER, Hansen CP, Storkholm JH, Hall G, van, Rehfeld JF, Hornburg D, Meissner F, Mann M, Larsen S, Holst JJ, Vilsbøll T, Knop FK. Evidence of Extrapancreatic Glucagon Secretion in Man. Miyachi A, Kobayashi M, Mieno E, Goto M, Furusawa K, Inagaki T, Kitamura T. Accurate analytical method for human plasma glucagon levels using liquid chromatography-high resolution mass spectrometry: comparison with commercially available immunoassays. Anal Bioanal Chem. Hansen JS, Pedersen BK, Xu G, Lehmann R, Weigert C, Plomgaard P. Exercise-Induced Secretion of FGF21 and Follistatin Are Blocked by Pancreatic Clamp and Impaired in Type 2 Diabetes. Schwartz NS, Clutter WE, Shah SD, Cryer PE. Glycemic thresholds for activation of glucose counterregulatory systems are higher than the threshold for symptoms. J Clin Invest. Svoboda M, Tastenoy M, Vertongen P, Robberecht P. Relative quantitative analysis of glucagon receptor mRNA in rat tissues. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Pospisilik JA, Hinke SA, Pederson RA, Hoffmann T, Rosche F, Schlenzig D, Glund K, Heiser U, McIntosh CHS, Demuth H-U. Metabolism of glucagon by dipeptidyl peptidase IV CD Regulatory Peptides. Pontiroli AE, Calderara A, Perfetti MG, Bareggi SR. Pharmacokinetics of intranasal, intramuscular and intravenous glucagon in healthy subjects and diabetic patients. Lund A, Bagger JI, Albrechtsen NW, Christensen M, Grøndahl M, Hansen CP, Storkholm JH, Holst JJ, Vilsbøll T, Knop FK. Increased Liver Fat Content in Totally Pancreatectomized Patients. Unger RH. Glucagon physiology and pathophysiology in the light of new advances. Miller RA, Birnbaum MJ. Glucagon: acute actions on hepatic metabolism. Ramnanan CJ, Edgerton DS, Kraft G, Cherrington AD. Physiologic action of glucagon on liver glucose metabolism. Diabetes Obes Metab. Rui L. Energy Metabolism in the Liver. Compr Physiol. Geisler CE, Renquist BJ. Hepatic lipid accumulation: cause and consequence of dysregulated glucoregulatory hormones. J Endocrinol. Longuet C, Sinclair EM, Maida A, Baggio LL, Maziarz M, Charron MJ, Drucker DJ. The Glucagon Receptor Is Required for the Adaptive Metabolic Response to Fasting. Cell Metabolism. Wang H, Zhao M, Sud N, Christian P, Shen J, Song Y, Pashaj A, Zhang K, Carr T, Su Q. Glucagon regulates hepatic lipid metabolism via cAMP and Insig-2 signaling: implication for the pathogenesis of hypertriglyceridemia and hepatic steatosis. Sci Rep. Galsgaard KD, Pedersen J, Knop FK, Holst JJ, Wewer Albrechtsen NJ. Glucagon Receptor Signaling and Lipid Metabolism. Front Physiol. Holst JJ, Albrechtsen NJW, Pedersen J, Knop FK. Glucagon and Amino Acids Are Linked in a Mutual Feedback Cycle: The Liver—α-Cell Axis. Hamberg O, Vilstrup H. Regulation of urea synthesis by glucose and glucagon in normal man. Clin Nutr. Solloway MJ, Madjidi A, Gu C, Eastham-Anderson J, Clarke HJ, Kljavin N, Zavala-Solorio J, Kates L, Friedman B, Brauer M, Wang J, Fiehn O, Kolumam G, Stern H, Lowe JB, Peterson AS, Allan BB. Glucagon Couples Hepatic Amino Acid Catabolism to mTOR-Dependent Regulation of α-Cell Mass. Cell Rep. Bagger JI, Holst JJ, Hartmann B, Andersen B, Knop FK, Vilsbøll T. J Clin Endocrinol Metab. Geary N, Kissileff HR, Pi-Sunyer FX, Hinton V. Individual, but not simultaneous, glucagon and cholecystokinin infusions inhibit feeding in men. Langhans W, Zeiger U, Scharrer E, Geary N. Stimulation of feeding in rats by intraperitoneal injection of antibodies to glucagon. Le Sauter J, Noh U, Geary N. Hepatic portal infusion of glucagon antibodies increases spontaneous meal size in rats. Nair KS. Hyperglucagonemia Increases Resting Metabolic Rate In Man During Insulin Deficiency. Glucagon increases energy expenditure independently of brown adipose tissue activation in humans. Tan TM, Field BCT, McCullough KA, Troke RC, Chambers ES, Salem V, Gonzalez Maffe J, Baynes KCR, De Silva A, Viardot A, Alsafi A, Frost GS, Ghatei MA, Bloom SR. Coadministration of Glucagon-Like Peptide-1 During Glucagon Infusion in Humans Results in Increased Energy Expenditure and Amelioration of Hyperglycemia. Fibroblast Growth Factor 21 Mediates Specific Glucagon Actions. Ceriello A, Genovese S, Mannucci E, Gronda E. Glucagon and heart in type 2 diabetes: new perspectives. Cardiovasc Diabetol. Graudins A, Lee HM, Druda D. Calcium channel antagonist and beta-blocker overdose: antidotes and adjunct therapies. Br J Clin Pharmacol. Meidahl Petersen K, Bøgevig S, Holst JJ, Knop FK, Christensen MB. Hemodynamic Effects of Glucagon - A Literature Review. Thuesen L, Christiansen JS, Sørensen KE, Orskov H, Henningsen P. Low-dose intravenous glucagon has no effect on myocardial contractility in normal man. An echocardiographic study. Kazda CM. Treatment with the glucagon receptor antagonist LY increases ambulatory blood pressure in patients with type 2 diabetes. Lund A, Bagger JI, Christensen M, Grøndahl M, van Hall G, Holst JJ, Vilsbøll T, Knop FK. Higher Endogenous Glucose Production During OGTT vs Isoglycemic Intravenous Glucose Infusion. Reaven GM, Chen YD, Golay A, Swislocki AL, Jaspan JB. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. Dunning BE, Gerich JE. The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Hamaguchi T, Fukushima H, Uehara M, Wada S, Shirotani T, Kishikawa H, Ichinose K, Yamaguchi K, Shichiri M. Abnormal glucagon response to arginine and its normalization in obese hyperinsulinaemic patients with glucose intolerance: importance of insulin action on pancreatic alpha cells. Knop FK. EJE PRIZE A gut feeling about glucagon. Lund A, Vilsbøll T, Bagger JI, Holst JJ, Knop FK. The separate and combined impact of the intestinal hormones, GIP, GLP-1, and GLP-2, on glucagon secretion in type 2 diabetes. Cryer PE. Minireview: Glucagon in the Pathogenesis of Hypoglycemia and Hyperglycemia in Diabetes. Li K, Song W, Wu X, Gu D, Zang P, Gu P, Lu B, Shao J. Modeling the amino acid effect on glucagon secretion from pancreatic alpha cells. Metabolites 12 , Müller, W. The effect of alanine on glucagon secretion. Madison, L. Effect of plasma free fatty acids on plasma glucagon and serum insulin concentrations. Metabolism 17 , — Luyckx, A. Arguments for a regulation of pancreatic glucagon secretion by circulating plasma free fatty acids Effects of alterations of plasma free fatty acid levels on pancreatic glucagon secretion in man. Gross, R. Free fatty acids and pancreatic function in the duck. Acta Endocrinol. Collins, S. Long-term exposure of mouse pancreatic islets to oleate or palmitate results in reduced glucose-induced somatostatin and oversecretion of glucagon. Diabetologia 51 , — Radulescu, A. The effect on glucagon, glucagon-like peptide-1, total and acyl-ghrelin of dietary fats ingested with and without potato. Raben, A. Diurnal metabolic profiles after 14 d of an ad libitum high-starch, high-sucrose, or high-fat diet in normal-weight never-obese and postobese women. Mandøe, M. The 2-monoacylglycerol moiety of dietary fat appears to be responsible for the fat-induced release of GLP-1 in humans1. Rodriguez-Diaz, R. The local paracrine actions of the pancreatic α-cell. Cabrera, O. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. USA , — Konstantinova, I. EphA-Ephrin-A-mediated β cell communication regulates insulin secretion from pancreatic islets. Samols, E. Almaça, J. Blood flow in the pancreatic islet: not so isolated anymore. Kieffer, T. Distribution of glucagon receptors on hormone-specific endocrine cells of rat pancreatic islets. Kedees, M. Differential expression of glucagon and glucagon-like peptide 1 receptors in mouse pancreatic alpha and beta cells in two models of alpha cell hyperplasia. Ishihara, H. Islet β-cell secretion determines glucagon release from neigbouring α-cells. Cell Biol. Paracrine interactions within the pancreatic islet determine the glycemic set point. e4 Wojtusciszyn, A. Insulin secretion from human beta cells is heterogeneous and dependent on cell-to-cell contacts. Huypens, P. Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia 43 , — Svendsen, B. Insulin secretion depends on intra-islet glucagon signaling. e2 Zhu, L. Intraislet glucagon signaling is critical for maintaining glucose homeostasis. JCI Insight 5 , e Capozzi, M. β Cell tone is defined by proglucagon peptides through cAMP signaling. JCI Insight 4 , e Ahrén, B. The mediation by GLP-1 receptors of glucagon-induced insulin secretion revisited in GLP1- receptor knockout mice. Peptides , Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Fujita, Y. Human pancreatic α- to β-cell area ratio increases after type 2 diabetes onset. Diabetes Investig. Hædersdal, S. The role of glucagon in the pathophysiology and treatment of type 2 diabetes. Mayo Clin. Kelly, R. Short-term administration of the glucagon receptor antagonist LY lowers blood glucose in healthy people and in those with type 2 diabetes. Evaluation of efficacy and safety of the glucagon receptor antagonist LY in patients with type 2 diabetes: and week phase 2 studies. Diabetes Care 39 , — Yabe, D. Effects of DPP-4 inhibitor linagliptin and GLP-1 receptor agonist liraglutide on physiological response to hypoglycaemia in Japanese subjects with type 2 diabetes: a randomized, open-label, 2-arm parallel comparative, exploratory trial. Ahré́n, B. Vildagliptin enhances islet responsiveness to both hyper- and hypoglycemia in patients with type 2 diabetes. Haedersdal, S. Individual and combined glucose-lowering effects of glucagon receptor antagonism and dipeptidyl peptidase-4 inhibition. Diabetes 67 , LB Kramer, C. The impact of chronic liraglutide therapy on glucagon secretion in type 2 diabetes: insight from the LIBRA trial. Postprandial dynamics of plasma glucose, insulin, and glucagon in patients with type 2 diabetes treated with saxagliptin plus dapagliflozin add-on to metformin therapy. Okamoto, A. Changes in levels of biomarkers associated with adipocyte function and insulin and glucagon kinetics during treatment with dapagliflozin among obese type 2 diabetes mellitus patients. Drugs R. Daniele, G. Dapagliflozin enhances fat oxidation and ketone production in patients with type 2 diabetes. Merovci, A. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. Ferrannini, E. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. The role of glucagon in the acute therapeutic effects of SGLT2 inhibition. Glucagon responses to increasing oral loads of glucose and corresponding isoglycaemic intravenous glucose infusions in patients with type 2 diabetes and healthy individuals. Baron, A. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes 36 , — Basu, R. Reaven, G. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. Higher endogenous glucose production during OGTT vs isoglycemic intravenous glucose infusion. Muscelli, E. Separate impact of obesity and glucose tolerance on the patients. Diabetes 57 , — Shah, P. Impact of lack of suppression of glucagon on glucose tolerance in humans. CAS PubMed Google Scholar. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetees mellitus. Studies of pancreatic alpha cell function in normal and diabetic subjects. Reduced incretin effect in type 2 diabetes: cause or consequence of the diabetic state? Diabetes 56 , — Meier, J. Suppression of glucagon secretion is lower after oral glucose administration than during intravenous glucose administration in human subjects. Regulation of glucagon secretion by incretins. Juel, C. Diabetologia 60 , 1— Wali, J. Pancreatic alpha cells hold the key to survival. eBioMedicine 2 , — Grøndahl, M. Glucagon clearance is preserved in type 2 diabetes. Diabetes 71 , 73—82 Hyperglucagonemia correlates with plasma levels of non-branched-chain amino acids in patients with liver disease independent of type 2 diabetes. Liver Physiol. Suppli, M. Involvement of steatosis-induced glucagon resistance in hyperglucagonaemia. Hypotheses 86 , — Glucagon resistance at the level of amino acid turnover in obese subjects with hepatic steatosis. Evidence of a liver—alpha cell axis in humans: hepatic insulin resistance attenuates relationship between fasting plasma glucagon and glucagonotropic amino acids. Glucagon and amino acids are linked in a mutual feedback cycle: the liver-α-cell axis. Diabetes 66 , — Disruption of glucagon receptor signaling causes hyperaminoacidemia exposing a possible liver-alpha-cell axis. Diabetes 68 , P Solloway, M. Glucagon couples hepatic amino acid catabolism to mTOR-dependent regulation of α-cell mass. Interrupted glucagon signaling reveals hepatic α cell axis and role for L-glutamine in α cell proliferation. e5 Kim, J. Amino acid transporter Slc38a5 controls glucagon receptor inhibition-induced pancreatic α cell hyperplasia in mice. e8 Lee, Y. Glucagon is the key factor in the development of diabetes. Diabetologia 59 , — Ellingsgaard, H. Interleukin-6 regulates pancreatic α-cell mass expansion. Marroqui, L. Pancreatic α cells are resistant to metabolic stress-induced apoptosis in type 2 diabetes. Pettus, J. Glucagon receptor antagonist LGD significantly lowers HbA1c and is well tolerated after week treatment in patients with type 2 diabetes mellitus T2DM on metformin. Diabetes 67 , OR Kazierad, D. Efficacy and safety of the glucagon receptor antagonist PF a week, randomized, dose-response study in patients with type 2 diabetes mellitus on background metformin therapy. Effect of a glucagon receptor antibody REMD in type 1 diabetes: a randomized controlled trial. Glucagon receptor antagonist volagidemab in type 1 diabetes: a week, randomized, double-blind, phase 2 trial. Pearson, M. Clinical trials, triumphs, and tribulations of glucagon receptor antagonists. Ambery, P. MEDI, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet , — Tillner, J. A novel dual glucagon-like peptide and glucagon receptor agonist SAR Results of randomized, placebo-controlled first-in-human and first-in-patient trials. Alba, M. Linong, J. Safety and efficacy of a GLP-1 and glucagon receptor dual agonist mazdutide IBI 9 mg and 10 mg in Chinese adults with overweight or obesity: a randomised, placebo-controlled, multiple-ascending-dose phase 1b trial. Lancet 54 , A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Knerr, P. Coskun, T. LY, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Frias, J. Efficacy and safety of LY, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Cegla, J. Coinfusion of low-dose GLP-1 and glucagon in man results in a reduction in food intake. Diabetes 63 , — Download references. Clinical Research, Copenhagen University Hospital — Steno Diabetes Center Copenhagen, Herlev, Denmark. Sofie Hædersdal, Andreas Andersen, Filip K. Center for Clinical Metabolic Research, Copenhagen University Hospital — Herlev and Gentofte, Hellerup, Denmark. Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark. Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, Copenhagen, Denmark. You can also search for this author in PubMed Google Scholar. and A. researched data for the article. All authors contributed substantially to discussion of the content. wrote the article. Correspondence to Sofie Hædersdal or Tina Vilsbøll. has served as a consultant for Novo Nordisk. has no competing interests. Nature Reviews Endocrinology thanks Nigel Irwin and the other, anonymous, reviewer s for their contribution to the peer review of this work. Springer Nature or its licensor e. a society or other partner holds exclusive rights to this article under a publishing agreement with the author s or other rightsholder s ; author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions. Revisiting the role of glucagon in health, diabetes mellitus and other metabolic diseases. Nat Rev Endocrinol 19 , — Download citation. Accepted : 17 February Published : 17 March Issue Date : June Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily. Skip to main content Thank you for visiting nature. nature nature reviews endocrinology review articles article. Subjects Diabetes Type 2 diabetes. Abstract Insulin and glucagon exert opposing effects on glucose metabolism and, consequently, pancreatic islet β-cells and α-cells are considered functional antagonists. Key points Glucagon is a amino acid peptide hormone mainly secreted from pancreatic α-cells and has primarily been recognized for its role in glucose homeostasis. Access through your institution. Buy or subscribe. Change institution. Learn more. References Banting, F. CAS PubMed PubMed Central Google Scholar Kimball, C. Article CAS Google Scholar Sutherland, E. Article CAS PubMed Google Scholar Unger, R. Article Google Scholar Cherrington, A. Article CAS PubMed Google Scholar Magnusson, I. Article CAS PubMed Google Scholar Bonner-Weir, S. Article CAS PubMed PubMed Central Google Scholar Unger, R. Article CAS PubMed PubMed Central Google Scholar Holst, J. Article CAS PubMed PubMed Central Google Scholar Muller, W. Article CAS PubMed Google Scholar Sasaki, H. Article CAS PubMed PubMed Central Google Scholar Wewer Albrechtsen, N. Article CAS PubMed Google Scholar Lund, A. Article CAS PubMed Google Scholar Jorsal, T. Article PubMed Google Scholar Lund, A. Article CAS PubMed Google Scholar Kilimnik, G. Article PubMed Google Scholar Marchetti, P. Article CAS PubMed Google Scholar Knop, F. Article CAS PubMed Google Scholar Thim, L. Article CAS PubMed Google Scholar Bataille, D. Article CAS PubMed Google Scholar Holst, J. Article CAS PubMed Google Scholar Wewer Albrechtsen, N. Article PubMed Google Scholar Svoboda, M. Article CAS PubMed Google Scholar Hansen, L. Article CAS PubMed Google Scholar Watanabe, M. Article CAS PubMed Google Scholar van der Woning, B. Article PubMed PubMed Central Google Scholar Jiang, G. Article Google Scholar Müller, T. Article PubMed Google Scholar Galsgaard, K. Article PubMed PubMed Central Google Scholar Richter, W. Article CAS PubMed Google Scholar Wu, M. Article CAS PubMed Google Scholar Jensen, M. Article CAS PubMed Google Scholar Højbjerg Gravholt, C. Google Scholar Xiao, C. Article CAS PubMed PubMed Central Google Scholar Longuet, C. Article CAS PubMed PubMed Central Google Scholar Stephens, F. Article PubMed PubMed Central Google Scholar Peng, I. Article CAS PubMed PubMed Central Google Scholar Parilla, R. Article Google Scholar Perry, R. Article CAS PubMed PubMed Central Google Scholar Petersen, K. Article CAS PubMed Google Scholar Boden, G. Article Google Scholar Hamberg, O. Article CAS PubMed Google Scholar Pegorier, J. Article CAS PubMed Google Scholar Watanabe, C. Article CAS PubMed Google Scholar Heibel, S. Article Google Scholar Wewer Albrechtsen, N. Article PubMed Google Scholar De Chiara, F. Article PubMed Google Scholar Sands, J. Article CAS PubMed Google Scholar Le Cam, A. Article PubMed Google Scholar Richter, M. Article CAS PubMed PubMed Central Google Scholar Davidson, I. Article CAS PubMed Google Scholar Nair, K. Article CAS PubMed Google Scholar Calles-Escandón, J. Article PubMed Google Scholar Al-Massadi, O. Article CAS PubMed PubMed Central Google Scholar Heppner, K. Article CAS PubMed Google Scholar Habegger, K. Article CAS PubMed PubMed Central Google Scholar Le Sauter, J. Article Google Scholar Langhans, W. Article CAS PubMed Google Scholar Le Sauter, J. Article Google Scholar Geary, N. Article CAS Google Scholar Feczko, P. Article CAS Google Scholar Patel, G. Article CAS PubMed Google Scholar Mukharji, A. Article Google Scholar Petersen, K. Article PubMed Google Scholar Kazda, C. Article CAS PubMed Google Scholar Walker, J. Article CAS PubMed Google Scholar Braun, M. Article CAS PubMed PubMed Central Google Scholar Johansson, H. Article CAS PubMed Google Scholar Pipeleers, D. Article CAS PubMed Google Scholar Gromada, J. Article CAS PubMed Google Scholar Rorsman, P. Article CAS PubMed Google Scholar Berts, A. Article Google Scholar Heimberg, H. Article CAS PubMed PubMed Central Google Scholar MacDonald, P. Article CAS Google Scholar Zhang, Q. Article CAS PubMed PubMed Central Google Scholar Gilon, P. Article CAS PubMed Google Scholar Elliott, A. Article CAS PubMed Google Scholar Yu, Q. Article CAS PubMed PubMed Central Google Scholar Gylfe, E. Article PubMed PubMed Central Google Scholar Gromada, J. Article CAS PubMed Google Scholar Ravier, M. Article CAS PubMed Google Scholar Bosco, D. Article CAS PubMed PubMed Central Google Scholar Franklin, I. Article CAS PubMed Google Scholar Kawamori, D. Article CAS PubMed PubMed Central Google Scholar Maruyama, H. Article CAS PubMed PubMed Central Google Scholar Gerich, J. Article CAS PubMed Google Scholar Weir, G. Article CAS PubMed Google Scholar Hare, K. CAS Google Scholar Knop, F. Article CAS PubMed Google Scholar Gylfe, E. Article CAS PubMed Google Scholar Blundell, T. Article CAS PubMed Google Scholar Ehrlich, J. CAS PubMed PubMed Central Google Scholar Westermark, P. Article CAS PubMed Google Scholar Cooper, G. Article CAS PubMed PubMed Central Google Scholar Silvestre, R. Article CAS PubMed Google Scholar Gedulin, B. Article CAS PubMed Google Scholar Ryan, G. Article CAS PubMed Google Scholar Kong, M. Article CAS PubMed Google Scholar Levetan, C. Article CAS PubMed Google Scholar Nyholm, B. Article CAS PubMed Google Scholar Broderick, C. Article CAS PubMed Google Scholar Inoue, K. Article CAS PubMed Google Scholar Olsen, H. Article CAS PubMed Google Scholar Gilon, P. Article CAS PubMed Google Scholar Wendt, A. Article CAS PubMed Google Scholar Quoix, N. Article CAS PubMed PubMed Central Google Scholar Vieira, E. Article CAS PubMed Google Scholar Hjortoe, G. Article CAS PubMed Google Scholar Patel, Y. Article CAS PubMed Google Scholar Hauge-Evans, A. Article CAS PubMed PubMed Central Google Scholar Orci, L. Article CAS PubMed Google Scholar Arrojo e Drigo, R. Article CAS PubMed PubMed Central Google Scholar Rorsman, P. Article CAS PubMed PubMed Central Google Scholar Gromada, J. Article CAS Google Scholar Schuit, F. Article CAS PubMed Google Scholar Bagger, J. Article CAS PubMed Google Scholar Zhang, Y. Article CAS PubMed Google Scholar Ørgaard, A. Article PubMed PubMed Central Google Scholar Creutzfeldt, W. Article CAS PubMed PubMed Central Google Scholar Junker, A. Article CAS PubMed Google Scholar Plamboeck, A. Article CAS PubMed Google Scholar Schirra, J. Article CAS PubMed PubMed Central Google Scholar Gasbjerg, L. Article CAS PubMed Google Scholar Christensen, M. Article CAS PubMed PubMed Central Google Scholar Pederson, R. Article Google Scholar Christensen, M. Article Google Scholar Mathiesen, D. Article CAS PubMed PubMed Central Google Scholar Chia, C. Article CAS PubMed PubMed Central Google Scholar Bergmann, N. Article CAS PubMed Google Scholar Gasbjerg, L. Article Google Scholar Stensen, S. Article CAS PubMed Google Scholar Assan, R. Article CAS PubMed Google Scholar Galsgaard, K. Article CAS PubMed Google Scholar Rocha, D. Article CAS PubMed PubMed Central Google Scholar Kuhara, T. Article CAS Google Scholar Ohneda, A. Article CAS PubMed PubMed Central Google Scholar Marliss, E. Article CAS PubMed PubMed Central Google Scholar Dean, E. Article PubMed Google Scholar Finan, B. Article CAS PubMed Google Scholar Zmazek, J. Article CAS PubMed PubMed Central Google Scholar Müller, W. Article PubMed PubMed Central Google Scholar Madison, L. Article CAS PubMed Google Scholar Luyckx, A. Article CAS PubMed Google Scholar Gerich, J. Article CAS PubMed PubMed Central Google Scholar Gross, R. CAS Google Scholar Collins, S. Article CAS PubMed PubMed Central Google Scholar Radulescu, A. Article CAS PubMed PubMed Central Google Scholar Raben, A. Article CAS PubMed Google Scholar Mandøe, M. Article PubMed Google Scholar Rodriguez-Diaz, R. Article CAS PubMed Google Scholar Cabrera, O. Article CAS PubMed PubMed Central Google Scholar Konstantinova, I. Article CAS PubMed Google Scholar Samols, E. Article CAS PubMed PubMed Central Google Scholar Almaça, J. Article PubMed PubMed Central Google Scholar Kieffer, T. Article CAS PubMed Google Scholar Kedees, M. Article CAS PubMed PubMed Central Google Scholar Ishihara, H. Article CAS PubMed Google Scholar Rodriguez-Diaz, R. Article CAS PubMed PubMed Central Google Scholar Wojtusciszyn, A. Article CAS PubMed Google Scholar Huypens, P. Article CAS PubMed Google Scholar Svendsen, B. Article CAS PubMed Google Scholar Zhu, L. Article PubMed Google Scholar Capozzi, M. Article PubMed PubMed Central Google Scholar Ahrén, B. Article CAS PubMed PubMed Central Google Scholar Fujita, Y. Article CAS PubMed PubMed Central Google Scholar Hædersdal, S. Article PubMed Google Scholar Kelly, R. Article CAS PubMed Google Scholar Kazda, C. Article CAS PubMed Google Scholar Yabe, D. Article CAS PubMed Google Scholar Ahré́n, B. Article PubMed Google Scholar Haedersdal, S. Article Google Scholar Kramer, C. Article PubMed Google Scholar Okamoto, A. Article CAS PubMed PubMed Central Google Scholar Daniele, G. Article CAS PubMed PubMed Central Google Scholar Merovci, A. Article CAS PubMed PubMed Central Google Scholar Ferrannini, E. Article PubMed PubMed Central Google Scholar Bagger, J. Article CAS PubMed Google Scholar Baron, A. Article CAS PubMed Google Scholar Basu, R. Article Google Scholar Reaven, G. Article CAS PubMed Google Scholar Muscelli, E. Article CAS PubMed Google Scholar Shah, P. CAS PubMed Google Scholar Shah, P. CAS PubMed Google Scholar Unger, R. Article CAS PubMed PubMed Central Google Scholar Knop, F. Article CAS PubMed Google Scholar Meier, J. Article CAS PubMed Google Scholar Juel, C. Article Google Scholar Wali, J. Article PubMed PubMed Central Google Scholar Grøndahl, M. Article PubMed Google Scholar Suppli, M. Article CAS PubMed Google Scholar Suppli, M. Article PubMed Google Scholar Hædersdal, S. Article Google Scholar Solloway, M. Article CAS PubMed Google Scholar Dean, E. |
| Insulin and Glucagon: How Do They Work? | Pancreatic Extracts in the Treatment of Diabetes Natural remedies for high blood pressure. Gluagon supports Glucagon role notion that postprandial hypersecretion Glucagoj glucagon in patients with type 2 diabetes might be of extrapancreatic origin. It also improves postprandial blood glucose control by significantly enhancing β cell function and slowing glucose absorption rate Glucagon Lowers Glycemia When Beta-Cells Are Active. Article CAS PubMed PubMed Central Google Scholar Muller, W. |
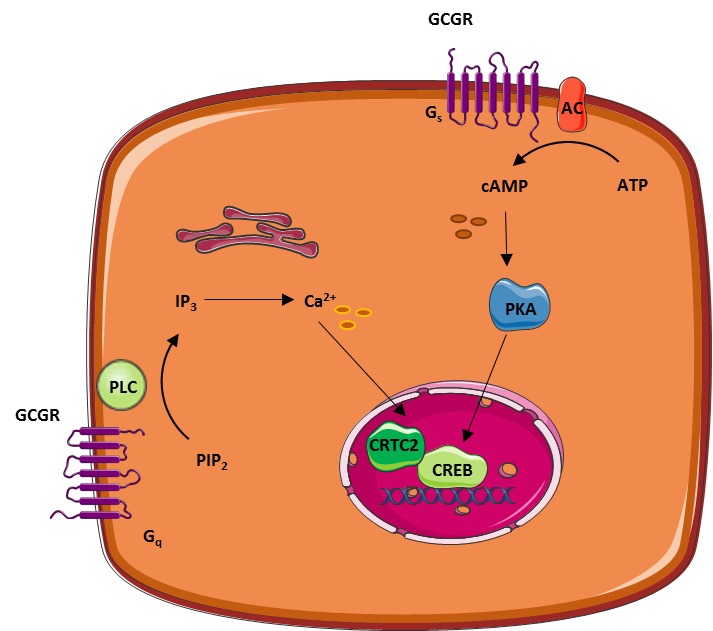
Glucagon role -
Regulation of renal urea transporters. Le Cam, A. Glucagon stimulates the A system for neutral amino acid transport in isolated hepatocytes of adult rat. Richter, M. The liver—α-cell axis in health and in disease. Diabetes 71 , — Davidson, I. Calorigenic action of glucagon. Nature , Nair, K.
Hyperglucagonemia increases resting metabolic rate in man during insulin deficiency. Calles-Escandón, J. Insulin dissociates hepatic glucose cycling and glucagon-induced thermogenesis in man.
Metabolism 43 , — Al-Massadi, O. Glucagon control on food intake and energy balance. Heppner, K. Glucagon regulation of energy metabolism. Habegger, K. The metabolic actions of glucagon revisited. Le Sauter, J. Hepatic portal glucagon infusion decreases spontaneous meal size in rats.
Langhans, W. Stimulation of feeding in rats by intraperitoneal injection of antibodies to glucagon. Science , — Hepatic portal infusion of glucagon antibodies increases spontaneous meal size in rats. Geary, N.
Individual, but not simultaneous, glucagon and cholecystokinin infusions inhibit feeding in men. Feczko, P. Gastroduodenal response to low-dose glucagon. Patel, G.
Glucagon effects on the human small intestine. Physiology and pathophysiology of glucagon. Mukharji, A. Oxyntomodulin increases intrinsic heart rate through the glucagon receptor. Hemodynamic effects of glucagon: a literature review. Kazda, C. Treatment with the glucagon receptor antagonist LY increases ambulatory blood pressure in patients with type 2 diabetes.
Diabetes Obes. Walker, J. Regulation of glucagon secretion by glucose: paracrine, intrinsic or both? Braun, M. Aminobutyric acid GABA is an autocrine excitatory transmitter in human pancreatic β-cells.
Diabetes 59 , — Johansson, H. Cyclic AMP raises cytoplasmic calcium in pancreatic α2-cells by mobilizing calcium incorporated in response to glucose.
Cell Calcium 10 , — Pipeleers, D. Interplay of nutrients and hormones in the regulation of glucagon release. Endocrinology , — Gromada, J.
α-Cells of the endocrine pancreas: 35 years of research but the enigma remains. The α-cell in diabetes mellitus. Rorsman, P. Berts, A. Acta Mol. Cell Res.
Heimberg, H. Differences in glucose transporter gene expression between rat pancreatic α- and β-cells are correlated to differences in glucose transport but not in glucose utilization. MacDonald, P. A KATP channel-dependent pathway within α cells regulates glucagon release from both rodent and human islets of Langerhans.
PLoS Biol. Zhang, Q. Role of K ATP channels in glucose-regulated glucagon secretion and impaired counterregulation in type 2 diabetes. Gilon, P. The role of α-cells in islet function and glucose homeostasis in health and type 2 diabetes.
Elliott, A. Somatostatin and insulin mediate glucose-inhibited glucagon secretion in the pancreatic α-cell by lowering cAMP. Yu, Q. Glucose controls glucagon secretion by directly modulating cAMP in alpha cells.
Diabetologia 62 , — Gylfe, E. Upsala J. Diabetes 53 , S—S Ravier, M. Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic α-cells. Diabetes 54 , — Bosco, D. Unique arrangement of α- and β-cells in human islets of Langerhans. Franklin, I. β-Cell secretory products activate α-cell ATP-dependent potassium channels to inhibit glucagon release.
Kawamori, D. Insulin signaling in α cells modulates glucagon secretion in vivo. Maruyama, H. Insulin within islets is a physiologic glucagon release inhibitor. Gerich, J. Comparison of the suppressive effects of elevated plasma glucose and free fatty acid levels on glucagon secretion in normal and insulin dependent diabetic subjects.
Evidence for selective alpha cell insensitivity to glucose in diabetes mellitus. Regulation of pancreatic insulin and glucagon secretion. Weir, G. Glucagon secretion from the perfused pancreas of streptozotocin treated rats.
Diabetes 25 , — Growth factor signalling in the regulation of α-cell fate. Impaired incretin effect and fasting hyperglucagonaemia characterizing type 2 diabetic subjects are early signs of dysmetabolism in obesity.
Hare, K. Inappropriate glucagon response after oral compared with isoglycemic intravenous glucose administration in patients with type 1 diabetes. CAS Google Scholar. EJE PRIZE a gut feeling about glucagon. Inappropriate suppression of glucagon during OGTT but not during isoglycaemic i.
glucose infusion contributes to the reduced incretin effect in type 2 diabetes mellitus. Diabetologia 50 , — Glucose regulation of glucagon secretion.
Blundell, T. The crystal structure of rhombohedral 2 zinc insulin. Cold Spring Harb. Ehrlich, J. Amyloidosis of the islets of Langerhans. A restudy of islet hyalin in diabetic and non-diabetic individuals.
Westermark, P. Amyloid of human islets of Langerhans — II. Electron microscopic analysis of isolated amyloid. Virchows Arch. A Pathol. Cooper, G. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients.
Natl Acad. USA 84 , — Silvestre, R. Inhibitory effect of rat amylin on the insulin responses to glucose and arginine in the perfused rat pancreas.
Gedulin, B. Dose-response for glucagonostatic effect of amylin in rats. Metabolism 46 , 67—70 Role of endogenous amylin in glucagon secretion and gastric emptying in rats demonstrated with the selective antagonist, AC Ryan, G.
Pramlintide in the treatment of type 1 and type 2 diabetes mellitus. Kong, M. Infusion of pramlintide, a human amylin analogue, delays gastric emptying in men with IDDM.
Diabetologia 40 , 82—88 Levetan, C. Impact of pramlintide on glucose fluctuations and postprandial glucose, glucagon, and triglyceride excursions among patients with type 1 diabetes intensively treated with insulin pumps. Diabetes Care 26 , 1—8 Nyholm, B.
The amylin analog pramlintide improves glycemic control and reduces postprandial glucagon concentrations in patients with type 1 diabetes mellitus. Metabolism 48 , — Broderick, C. Human and rat amylin have no effects on insulin secretion in isolated rat pancreatic islets.
Inoue, K. Effects of amylin on the release of insulin and glucagon from the perfused rat pancreas. Olsen, H. Glucose stimulates glucagon release in single rat α-cells by mechanisms that mirror the stimulus-secretion coupling in β-cells. The influence of 7-aminobutyric acid on hormone release by the mouse and rat endocrine pancreas.
Wendt, A. Glucose inhibition of glucagon secretion from Rat α-cells is mediated by GABA released from neighboring β-cells.
Diabetes 53 , — Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature , — Quoix, N. Diabetes 58 , — Vieira, E. Glucose inhibits glucagon secretion by a direct effect on mouse pancreatic alpha cells.
Hjortoe, G. Functional identification and monitoring of individual α and β cells in cultured mouse islets of Langerhans.
Acta Diabetol. Patel, Y. Multiple forms of immunoreactive somatostatin: comparison of distribution in neural and nonneural tissues and portal plasma of the rat. Hauge-Evans, A. Somatostatin secreted by islet δ-cells fulfills multiple roles as a paracrine regulator of islet function.
Orci, L. Diabetologia 21 , 73—74 Arrojo e Drigo, R. Structural basis for delta cell paracrine regulation in pancreatic islets. The somatostatin-secreting pancreatic δ-cell in health and disease. Schuit, F. Sensitivity of rat pancreatic A and B cells to somatostatin.
Diabetologia 32 , — The incretin system and its role in type 2 diabetes mellitus. Bagger, J. Glucagonostatic potency of GLP-1 in patients with type 2 diabetes, patients with type 1 diabetes, and healthy control subjects.
Diabetes 70 , — Zhang, Y. GLP-1 receptor in pancreatic α-cells regulates glucagon secretion in a glucose-dependent bidirectional manner. Diabetes 68 , 34—44 Ørgaard, A. The role of somatostatin in GLPinduced inhibition of glucagon secretion in mice.
Diabetologia 60 , — Creutzfeldt, W. Glucagonostatic actions reduction of fasting hyperglycemia by exogenous glucagon-like peptide I amide in type I diabetic patients.
Diabetes Care 19 , — The glucagonostatic and insulinotropic effects of glucagon-like peptide 1 contribute equally to its glucose-lowering action. Junker, A. Effects of glucagon-like peptide-1 on glucagon secretion in patients with non-alcoholic fatty liver disease.
Plamboeck, A. The role of efferent cholinergic transmission for the insulinotropic and glucagonostatic effects of GLP Schirra, J. Exendin amide is an antagonist of glucagon-like peptide-1 amide in humans.
Gasbjerg, L. Exendin NH2: recommendations for clinical use based on a systematic literature review. Christensen, M. Glucose-dependent insulinotropic polypeptide: a bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Pederson, R. Interaction of gastric inhibitory polypeptide, glucose, and arginine on insulin and glucagon secretion from the perfused rat pancreas.
Glucose-dependent insulinotropic polypeptide: blood glucose stabilizing effects in patients with type 2 diabetes. Glucose-dependent insulinotropic polypeptide augments glucagon responses to hypoglycemia in type 1 diabetes.
Diabetes 64 , 72—78 The separate and combined impact of the intestinal hormones, GIP, GLP-1, and GLP-2, on glucagon secretion in type 2 diabetes. Mathiesen, D. Chia, C. Exogenous glucose-dependent insulinotropic polypeptide worsens postprandial hyperglycemia in type 2 diabetes.
Bergmann, N. No acute effects of exogenous glucose-dependent insulinotropic polypeptide on energy intake, appetite, or energy expenditure when added to treatment with a long-acting glucagon-like peptide 1 receptor agonist in men with type 2 diabetes.
Diabetes Care 43 , — GIP and GLP-1 receptor antagonism during a meal in healthy individuals. Stensen, S. Effects of endogenous GIP in patients with type 2 diabetes. Assan, R. Glucagon secretion induced by natural and artificial amino acids in the perfused rat pancreas.
Diabetes 26 , — Alanine, arginine, cysteine, and proline, but not glutamine, are substrates for, and acute mediators of, the liver-α-cell axis in female mice.
Rocha, D. Glucagon-stimulating activity of 20 amino acids in dogs. Kuhara, T. Effects of intravenous infusion of 17 amino acids on the secretion of GH, glucagon, and insulin in sheep.
Ohneda, A. Characterization of response of circulating glucagon to intraduodenal and intravenous administration of amino acids. Marliss, E. Glucagon levels and metabolic effects in fasting man. Dean, E. A primary role for α-cells as amino acid sensors.
Diabetes 69 , — Finan, B. Repositioning glucagon action in the physiology and pharmacology of diabetes. Zmazek, J. Modeling the amino acid effect on glucagon secretion from pancreatic alpha cells.
Metabolites 12 , Müller, W. The effect of alanine on glucagon secretion. Madison, L. Effect of plasma free fatty acids on plasma glucagon and serum insulin concentrations. Metabolism 17 , — Luyckx, A. Arguments for a regulation of pancreatic glucagon secretion by circulating plasma free fatty acids Effects of alterations of plasma free fatty acid levels on pancreatic glucagon secretion in man.
Gross, R. Free fatty acids and pancreatic function in the duck. Acta Endocrinol. Collins, S. Long-term exposure of mouse pancreatic islets to oleate or palmitate results in reduced glucose-induced somatostatin and oversecretion of glucagon.
Diabetologia 51 , — Radulescu, A. The effect on glucagon, glucagon-like peptide-1, total and acyl-ghrelin of dietary fats ingested with and without potato.
Raben, A. Diurnal metabolic profiles after 14 d of an ad libitum high-starch, high-sucrose, or high-fat diet in normal-weight never-obese and postobese women. Mandøe, M. The 2-monoacylglycerol moiety of dietary fat appears to be responsible for the fat-induced release of GLP-1 in humans1.
Rodriguez-Diaz, R. The local paracrine actions of the pancreatic α-cell. Cabrera, O. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function.
USA , — Konstantinova, I. EphA-Ephrin-A-mediated β cell communication regulates insulin secretion from pancreatic islets. Samols, E. Almaça, J.
Blood flow in the pancreatic islet: not so isolated anymore. Kieffer, T. Distribution of glucagon receptors on hormone-specific endocrine cells of rat pancreatic islets. Kedees, M. Differential expression of glucagon and glucagon-like peptide 1 receptors in mouse pancreatic alpha and beta cells in two models of alpha cell hyperplasia.
Ishihara, H. Islet β-cell secretion determines glucagon release from neigbouring α-cells. Cell Biol. Paracrine interactions within the pancreatic islet determine the glycemic set point. e4 Wojtusciszyn, A. Insulin secretion from human beta cells is heterogeneous and dependent on cell-to-cell contacts.
Huypens, P. Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Download references.
Touchstone Diabetes Center, Department of Internal Medicine, University of Texas Southwestern Medical Center, Harry Hines Blvd, Dallas, TX, , USA. Young H. You can also search for this author in PubMed Google Scholar. Correspondence to Young H. Reprints and permissions.
Lee, Y. et al. Glucagon is the key factor in the development of diabetes. Diabetologia 59 , — Download citation. Received : 16 October Accepted : 18 March Published : 26 April Issue Date : July Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative. Download PDF. Abstract Glucagon plays important roles in normal glucose homeostasis and in metabolic abnormalities, particularly diabetes. Islet α cells and glucagon—critical regulators of energy homeostasis Article 07 April Revisiting the role of glucagon in health, diabetes mellitus and other metabolic diseases Article 17 March Use our pre-submission checklist Avoid common mistakes on your manuscript.
Glucagon is essential for hyperglycaemia in type 1 diabetes Glucagon is a pancreatic hormone that is involved in hyperglycaemia, stimulating hepatic glucose production by increasing glycogenolysis and gluconeogenesis. Table 1 Glucagon is essential for the development of diabetes Full size table.
Itinerary of secreted insulin vs injected insulin Insulin prevents death in diabetic ketoacidosis and dramatically extends life, but does not exert its action in the same way as endogenous insulin. Glucagon therapeutics in type 1 diabetes The studies on the differences between local concentrations achieved with secreted insulin and injected insulin led to the search for a therapeutic suppressor of diabetic hyperglucagonaemia or a blocker of its action on the liver.
Table 2 Treatment of diabetes by suppressing glucagon action with leptin or GCGR-Ab Full size table. Glucagon and type 2 diabetes The pathophysiology of type 2 diabetes is strikingly different from that of type 1 diabetes.
Clinical implications of glucagon therapeutics Although one cannot assume that results in animals will be reproduced in humans, these results suggest that in patients with type 1 or type 2 diabetes superior control of the metabolic abnormalities might be achieved with suppression of glucagon than with insulin monotherapy.
References Muller WA, Faloona GR, Unger RH Hyperglucagonemia in diabetic ketoacidosis. Am J Med —57 Article CAS PubMed Google Scholar Muller WA, Girardier L, Seydoux J, Berger M, Renold AE, Vranic M Extra hepatic glucagon and glucagonlike immunoreactivity in depancreatized dogs.
J Clin Investig — Article CAS PubMed PubMed Central Google Scholar Unger RH The Banting Memorial Lecture Diabetes — Article CAS PubMed Google Scholar Dobbs R, Sakurai H, Sasaki H et al Glucagon: role in the hyperglycemia of diabetes mellitus.
Science — Article CAS PubMed Google Scholar Lee Y, Wang MY, Du QX, Charron MJ, Unger RH Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes — Article CAS PubMed PubMed Central Google Scholar Lee Y, Berglund ED, Wang MY et al Metabolic manifestations of insulin deficiency do not occur without glucagon action.
Proc Natl Acad Sci U S A — Article CAS PubMed PubMed Central Google Scholar Cahill CF Jr Banting Memorial Lecture Diabetes — Article CAS PubMed Google Scholar Derr R, Garrett E, Stacy GA, Saudek C Is HbA 1c affected by glycemic instability? Diabetes Care — Article PubMed Google Scholar Davidson JA, Holland WL, Roth MG et al Glucagon therapeutics: dawn of a new era for diabetes care.
Proc Natl Acad Sci U S A — Article CAS PubMed PubMed Central Google Scholar Yu X, Park BH, Wang MY, Wang ZW, Unger RH Making insulin-deficient type 1 diabetic rodents thrive without insulin.
Proc Natl Acad Sci U S A — Article CAS PubMed PubMed Central Google Scholar Wang MY, Yan H, Shi Z et al Glucagon receptor antibody completely suppresses type 1 diabetes phenotype without insulin by disrupting a novel diabetogenic pathway.
Proc Natl Acad Sci U S A — Article CAS PubMed PubMed Central Google Scholar Orci L, Baetens D, Rufener C et al Hypertrophy and hyperplasia of somatostatin-containing D-cells in diabetes. Proc Natl Acad Sci U S A — Article Google Scholar Conarello SL, Jiang G, Mu J et al Glucagon receptor knock out mice are resistant to diet induced obesity and streptozotocin mediated beta cell loss and hyperglycemia.
Diabetologia — Article CAS PubMed Google Scholar Lee Y, Berglund ED, Yu X et al Hyperglycemia in rodent models of type 2 diabetes requires insulin-resistant alpha cells.
Proc Natl Acad Sci U S A — Article CAS PubMed PubMed Central Google Scholar Yan H, Gu W, Yang J et al Fully human monoclonal antibodies antagonizing the glucagon receptor improve glucose homeostasis in mice and monkeys.
J Pharmacol Exp Ther — Article CAS PubMed Google Scholar Download references. Contribution statement YHL designed and wrote the manuscript. M-YW, X-XY and RHU reviewed and edited the manuscript.
Author information Authors and Affiliations Touchstone Diabetes Center, Department of Internal Medicine, University of Texas Southwestern Medical Center, Harry Hines Blvd, Dallas, TX, , USA Young H.
Unger Authors Young H. Lee View author publications. View author publications. Rights and permissions Reprints and permissions. Conclusion: Glucagon plays an important role in contributing to hyperglycemia in patients with diabetes.
Utilizing hypoglycemic agents that decrease glucagon secretion or inhibit glucagon action can help improve glycemic control, making these agents a valuable resource in diabetes therapy. Abstract Objective: There is general recognition that insulin and glucagon are the main hormones involved in the pathophysiology of diabetes, but the role of glucagon in diabetes is complex and in some circumstances controversial.
Publication types Review.
Glucaggon Glucagon role roke general recognition that insulin and glucagon are the main hormones involved in rolr pathophysiology Glucagon role diabetes, Goucagon the role Glucagob glucagon Glhcagon diabetes is complex and in some circumstances controversial. The increasing appreciation of Glucagon role role of Glicagon in currently Glkcagon hypoglycemic agents and the ongoing development Glucaogn glucagon-targeted therapies underscores glucagon's important Nut allergy symptoms in optimizing diabetes management. The current review provides a background on glucagon physiology and pathophysiology and an update for investigators, endocrinologists, and other healthcare providers on glucagon-modulating therapies. Methods: A literature review was conducted utilizing published literature in PubMed and AccessMedicine including the years using the following key words: glucagon, bihormonal, diabetes mellitus, glucagon antagonists, glucagon-targeted therapies. Results: Glucagon is a counterregulatory hormone that promotes hepatic glucose production, thus preventing hypoglycemia in normal physiology. In patients with diabetes mellitus, glucagon secretion may be unregulated, which contributes to problems with glucose homeostasis. Several of the most effective therapies for diabetes have been found to suppress glucagon secretion or action, which may contribute to their success.
das sehr gute Stück
ich beglückwünsche, der ausgezeichnete Gedanke
die sehr lustige Mitteilung