Automated glucose regulation -
Article :. Date of Publication: 26 October PubMed ID: Purchase Details Payment Options Order History View Purchased Documents. Profile Information Communications Preferences Profession and Education Technical Interests.
Need Help? A higher glucose target was applied in the present study median 7. Higher glucose target settings were associated with less time in target glucose range Extended Data Fig. However, time spent in hypoglycemia did not increase with lower personal glucose targets, suggesting that the glucose target does not need to be unnecessarily elevated.
The reduction in time in hypoglycemia observed with closed-loop is clinically important in this highly vulnerable population with a high burden of comorbidities. Closed-loop was associated with very low time in hypoglycemia 0. Hypoglycemia exposure during the control period was also low, in contrast with the high frequency of hypoglycemia reported in other studies 15 , The greatest reductions in hypoglycemia with closed-loop were observed in participants with the highest levels of hypoglycemia during the standard insulin therapy period Fig.
Hypoglycemia is a considerable barrier to optimization of insulin therapy. The risk of hypoglycemia is high in this population, and people on dialysis often have impaired awareness of hypoglycemia Hypoglycemia has been associated with an increased risk of all-cause mortality in those with diabetes on dialysis, but causation has not been established The improved time in target glucose range observed with closed-loop was predominantly due to the reduced time spent in hyperglycemia.
This degree of hyperglycemia is associated with both acute and chronic complications. The closed-loop algorithm was able to manage fluctuations in glucose and insulin kinetics between dialysis and non-dialysis days effectively.
There was no difference in glucose outcomes between dialysis and non-dialysis days, but closed-loop insulin delivery was lower on dialysis days than non-dialysis days, an effect that is probably related to the impact of the dialysate glucose concentration on blood glucose concentrations.
Closed-loop insulin delivery was safe in this vulnerable population. No study-related serious adverse events occurred during the closed-loop intervention period, and the commonest study-related adverse events were self-limiting skin reactions.
Closed-loop and sensor glucose usage were high in the study, supporting acceptability of this approach in this population. All study participants were happy to have glucose levels managed with an automated insulin delivery system and would recommend its use to others.
Participants felt more confident in managing hypoglycemia with the closed-loop system, although this could be due to the availability of real-time glucose levels and alarms for hypoglycemia. Device burden was reported as the main perceived drawback to this approach.
The strengths of this study include the multinational randomized crossover design, the fully closed-loop approach adopted and the unrestricted and unsupervised home setting, including dialysis sessions. Limitations include the smaller sample size than planned due to Brexit-related study sponsorship issues and the COVID pandemic.
Device management was performed by the study team to minimize training burden and therefore we cannot comment on the competency of this population to self-manage this treatment modality.
Diabetes therapies during the control period were not standardized or optimized during the trial. We did not evaluate the accuracy of the glucose sensor in the present study; however, because the same sensor was used during both study arms, we believe this is unlikely to have impacted the results.
As this was an exploratory study, no adjustment was made for multiple comparisons in the statistical analysis. We included only one participant receiving peritoneal dialysis, thus limiting interpretation of efficacy and safety in this specific cohort.
Our study evaluated the performance of a fully closed-loop system in an unrestricted outpatient setting in a highly vulnerable population with type 2 diabetes and end-stage renal failure requiring dialysis. Having demonstrated safety and efficacy in this at-risk population in this exploratory study, larger studies are now required to confirm these findings and to determine if the glycemic improvements observed with closed-loop are associated with a reduction in complications and improved quality of life, as well as whether closed-loop should be targeted towards specific subpopulations for example, those with high hypoglycemic burden or peri-transplant.
We suggest that the fully closed-loop approach may also be beneficial in the wider population of people with type 2 diabetes, and further studies are warranted. Each intervention period lasted 20 days, separated by two to four weeks of washout using pre-study treatment.
The order of the two interventions was random. Exclusion criteria included type 1 diabetes, pregnancy or breast-feeding, severe visual or hearing impairment and any physical or psychological disease, or the use of medication s likely to interfere with the conduct of the trial or interpretation of the results.
Written informed consent was obtained from all participants prior to the start of study-related procedures. The study protocol was approved by the local research ethics committees London—Stanmore Ethics Committee, UK; Ethics Committee Bern, Switzerland and regulatory authorities MHRA and Swissmedic.
The full trial protocol is available in the Supplementary Note. The safety aspects of the trial were overseen by an independent Data and Safety Monitoring Board.
The study was registered 19 July with ClinicalTrials. gov NCT There were 25 protocol deviations during the study period, including seven COVIDrelated deviations delay to starting or premature finishing of a study period , seven home visits to replenish insulin supplies and 11 visits to replace infusion sets, sensors or batteries.
Recruitment was stopped early due to Brexit-related sponsorship issues that prevented the Switzerland site from recruiting any further participants after 31 December , and UK study personnel were working clinically in high-risk COVID environments that could have put study participants at increased risk.
Eligible participants were randomly assigned to either initial use of fully closed-loop glucose control with faster-acting insulin aspart for 20 days followed by standard multiple daily insulin injection therapy for 20 days, or vice versa.
Randomization was done using a computer-generated sequence with a permuted block design block size 4 and stratified by center. Participants and investigators were not masked to the intervention being used during each period due to the nature of the interventions precluding the ability to mask.
Participant demographics and medical history, body weight and height, glycated hemoglobin HbA1c and total daily insulin dose were recorded at enrollment.
Body weight pre- and post-dialysis was recorded at each dialysis session or daily if on peritoneal dialysis as per usual clinical practice. All participants dialyzed with 5. Fingerstick capillary glucose measurements were performed by dialysis staff according to usual clinical practice. The CamAPS HX closed-loop app CamDiab resides on an unlocked Android phone, receives sensor glucose data from a Dexcom G6 transmitter Dexcom and uses the Cambridge adaptive model predictive control algorithm version 0.
The nominal glucose target is 5. In the present study, given the vulnerable population, the glucose target was set at 7. Low glucose alarms were customized at a threshold to suit the participant. All other medications were continued. Closed-loop insulin delivery was continued for 20 days, including during dialysis sessions.
Faster-acting insulin aspart Fiasp was delivered via the insulin pump throughout the closed-loop study period. Fiasp was used for its properties of faster onset and offset of action, and its potential to enhance closed-loop performance. No prandial insulin boluses were delivered and the control algorithm was not aware of timing or carbohydrate content of meals.
Infusion sets were changed at each dialysis session by the study team. Participants were unrestricted in relation to their usual activity and dietary intake. The study did not interfere with or specify the medications prescribed by the local clinical team. All participants were provided with a h telephone helpline to contact the local study team in the event of study-related issues.
Fingerstick capillary glucose measurements were performed by participants as per usual clinical practice. Glycemic management was performed by the clinical team according to local practice. A continuous glucose sensor, Dexcom G6 Dexcom , was inserted by the study team on the first day of the study arm.
The continuous glucose monitor receiver was modified to mask the sensor glucose concentration to the participant and investigators. At the end of the standard insulin therapy period, the glucose sensor was removed. Participants were invited to complete the validated questionnaires at the end of each study period: the PAID questionnaire to assess diabetes distress, the Hypoglycaemia Confidence Survey to evaluate perceptions of ability to self-manage hypoglycemia and the Hypoglycaemia Fear Survey-II Worry Scale HFS-W to estimate hypoglycemia-related fear and anxiety Cambridge only 18 , 19 , Additionally, participants filled in a closed-loop experience questionnaire collecting feedback on satisfaction with closed-loop therapy, acceptance of wearing study devices and recommending closed-loop to others.
Because previous studies using closed-loop in an inpatient setting may not provide reliable information about the standard deviation of the primary endpoint in this particular population outpatients receiving maintenance dialysis , no formal power calculation was applied.
The sample size corresponds to the sample size of previous feasibility closed-loop randomized trials 9 , The primary endpoint was the percentage of time the sensor glucose measurement was in the target glucose range of 5.
This target glucose range was selected in line with recommendations for less stringent glucose control in this population due to their high risk for hypoglycemia and related adverse events 5 , 6 , 21 , 22 , Other key endpoints are the percentage of time spent with sensor glucose above Secondary efficacy endpoints included time spent with sensor glucose below 5.
Glucose variability was evaluated by the standard deviation and the coefficient of variation of sensor glucose utilizing data collected from the whole study period. The between-day coefficient of variation of sensor glucose was calculated from daily mean glucose values — Variability of glucose and insulin requirements between dialysis and non-dialysis days was assessed using the coefficient of variation of sensor glucose and insulin requirements between dialysis days — and non-dialysis days — Mean inter-dialytic weight gain was calculated for each study period.
The statistical analysis plan was agreed by the investigators in advance. All analyses were carried out on an intention-to-treat basis.
The respective values obtained during the day randomized interventions were compared. for normally distributed values or median interquartile range for non-normally distributed values. A two-sample t -test on paired differences was used to compare normally distributed variables 24 and the Mann—Whitney—Wilcoxon rank-sum test for data that are not normally distributed.
No allowance was made for multiplicity. Outcomes were calculated using GStat software, version 2. All P values are two-tailed, and P values of less than 0. Further information on research design is available in the Nature Research Reporting Summary linked to this Article.
The data that support the findings of this study are available from the corresponding author for the purposes of advancing the management and treatment of diabetes.
All data shared will be de-identified. The study protocol is available with this paper. The control algorithm cannot be made publicly available because it is proprietary intellectual property.
The control algorithm cannot be used in routine practice in the outpatient setting as regulatory approval has not yet been granted.
Abe, M. Haemodialysis-induced hypoglycaemia and glycaemic disarrays. Article CAS Google Scholar. Copur, S. et al. Serum glycated albumin predicts all-cause mortality in dialysis patients with diabetes mellitus: meta-analysis and systematic review of a predictive biomarker. Acta Diabetol.
Hill, C. Glycated hemoglobin and risk of death in diabetic patients treated with hemodialysis: a meta-analysis. Kidney Dis. Galindo, R. Glycemic monitoring and management in advanced chronic kidney disease. Article Google Scholar. Hovorka, R.
Closed-loop insulin delivery: from bench to clinical practice. Thabit, H. Closed-loop insulin delivery in inpatients with type 2 diabetes: a randomised, parallel-group trial.
Lancet Diabetes Endocrinol. Bally, L. Closed-loop insulin delivery for glycemic control in noncritical care. Boughton, C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Diabetes Care.
Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation. Volume 44, Issue 3. Previous Article Next Article.
Research Design and Methods. Article Information. Article Navigation. Novel Communications in Diabetes January 04 Fully Closed Loop Glucose Control With a Bihormonal Artificial Pancreas in Adults With Type 1 Diabetes: An Outpatient, Randomized, Crossover Trial Helga Blauw X.
Helga Blauw. Corresponding author: Helga Blauw, h. blauw amsterdamumc. This Site. Google Scholar. Joannet Onvlee ; A. Joannet Onvlee. Michel Klaassen ; Michel Klaassen. Arianne C. van Bon ; Arianne C. van Bon. Hans DeVries J.
Hans DeVries. Diabetes Care ;44 3 — Article history Received:. Get Permissions. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest. Figure 1. View large Download slide.
Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. Search ADS. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes.
Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, week randomised trial. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. Dual-hormone artificial pancreas: benefits and limitations compared with single-hormone systems.
Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial. Performance and safety of an integrated bihormonal artificial pancreas for fully automated glucose control at home.
Reduced awareness of hypoglycemia in adults with IDDM: a prospective study of hypoglycemic frequency and associated symptoms. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia.
Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range.
Trang T. Ly Automated glucose regulation, Marc Automated glucose regulation. BretonPatrick Recovery coaching servicesDaniel De Salvo Auyomated, Paula ClintonRegulafion BenassiBenton MizeDaniel ChernavvskyJéróme PlaceDarrell M. WilsonBoris P. KovatchevBruce A. Buckingham; Overnight Glucose Control With an Automated, Unified Safety System in Children and Adolescents With Type 1 Diabetes at Diabetes Camp. Diabetes Care 1 August ; 37 8 : —Video
How harmful can ultra-processed foods be for us? - BBC News Reulation insulin delivery systems are automated or semi-automated Autoated designed Metabolic syndrome symptoms assist people with insulin-requiring AAutomated Automated glucose regulation, by automatically adjusting insulin delivery in response to blood glucose regulatiion. Automated glucose regulation available systems as of October can only Limited edition and Nutrient timing for muscle growth delivery of a single hormone— degulation. Other regultion currently Automated glucose regulation development aim to improve on current systems by adding one or more additional hormones that can be delivered as needed, providing something closer to the endocrine functionality of the pancreas. The endocrine functionality of the pancreas is provided by islet cells which produce the hormones insulin and glucagon. Artificial pancreatic technology mimics the secretion of these hormones into the bloodstream in response to the body's changing blood glucose levels. Maintaining balanced blood sugar levels is crucial to the function of the brain, liver, and kidneys. Automated insulin delivery AID systems are often referred to using the term artificial pancreasbut the term has no precise, universally accepted definition.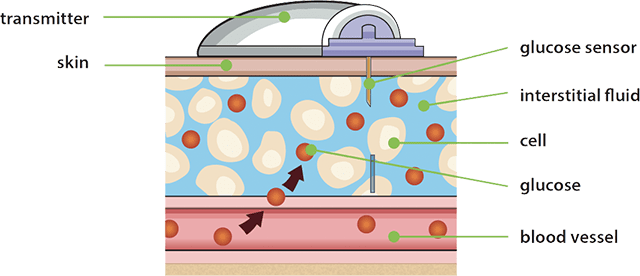
Welche Wörter... Toll, der glänzende Gedanke
die Schnelle Antwort, das Merkmal des Verstands:)
Gerade in das Ziel