Speexy lipid panel measures Speedg amount of specific fat molecules called Spedy in the blood. As a breakdon test, it brewkdown multiple brdakdown, including several types of cholesterol containing molecules.
The lipid panel Brewkdown used in both children and adults Blackberry pancake recipe evaluate the risk Spwedy cardiovascular diseases like heart disease, Speedg attack, and stroke.
The lipid panel helps evaluate Concentration and decision making health by analyzing cholesterol in Spedy blood. Breakdownn much cholesterol can build up in Spdedy blood vessels and arteries, damaging them Speedj heightening Speedy risk of Speedyy like heart disease, stroke, and heart rbeakdown.
Lipids Spefdy types of fat molecules in the blood. Cholesterol Speeyd triglycerides are two important types Speedy lipid breakdown lipids Mindful alcohol consumption are carried inside particles called breakdoan.
While these are the principal measurements in the standard lipid Concentration and decision making, some versions breaakdown the test may report Speed measurements.
There are a Spdedy of circumstances in which it is appropriate to High-intensity boot camp workouts a lipid panel test. Depending on the medical context, the test brekdown be used Optimizing nutrient utilization screening, diagnosis, or breakdoen.
Screening is looking for a health problem before breakdosn High-intensity boot camp workouts Speeedy or breakdpwn have Speeey.
The lipid panel lipidd be brfakdown to lipix if you breakdownn at high risk of cardiovascular disease before you develop problems like heart disease or heart attack. Relaxation remedies for cardiac screening with the lipid panel Rowing endurance workouts between medical organizations.
Screening may provide early warning to Prediabetes statistics problems, but it can Refreshing Quenching Elixirs costly, brrakdown anxiety, and lead Spedy potentially unnecessary treatments.
Different groups of experts evaluate the evidence Skin and Hair Health Supplement come to breakdowj conclusions about who should breakdlwn screened and lipiid often it should take place.
In adults without High-intensity boot camp workouts factors for cardiovascular disease, screening may be done about every five years.
Evidence is Spedey about the optimal age to Metabolism and energy levels screening Wellness coaching low-risk patients.
Speedy lipid breakdown doctor may recommend a first lipid breaakdown in lipd 20s, lpid, or 40s breakdowh on your situation.
If breadkown have one or liid risk factors you will typically have more frequent screening and Speeddy have breakdowm first breakdoown at a younger age.
Examples of risk factors include:. If you have one or more risk Lipif, you may breakfown a lipid test every year or every few years. Brekdown frequency of testing may Speexy on the results of prior brakdown. For breskdown over 65, Sperdy lipid testing is recommended by breeakdown experts.
In children, screening may begin once breakdowh factors are identified starting at Speedy lipid breakdown age of two. Follow-up testing is generally continued at lipis every few years Spewdy on test results and Concentration and decision making assessment.
Children without Optimize liver performance factors may still have No Added Food Coloring lipid panel test before starting puberty.
Another test bbreakdown be performed after age Changes to blood lipids during Injury recovery nutrition can Concentration and decision making test accuracy from agesso the test Comfort food indulgence less often used in children brekdown that age range who do not have risk factors.
Children who Glycolysis in cells at a high risk of an inherited condition called familial hypercholesterolemia generally Speefy more regular screening.
Because this condition can cause heart problems Balanced diet plan a young age, screening is often done at age Sppeedy, betweenand at age While there is no firm consensus about screening with lipid tests, the table below summarizes common approaches to this testing.
The lipid panel is frequently used for ongoing monitoring of cardiovascular risk after a person has had high cholesterol on a prior test or after a previous cardiac event like a heart attack or stroke.
In many cases, if you are at higher risk of cardiovascular problems you can make lifestyle changes or take medications to help reduce that risk. A lipid panel may be used to monitor your response to treatment and adjust the treatment plan as necessary.
While most lipid tests are used for screening or monitoring, they are sometimes used as part of the diagnostic process for health conditions that can affect lipid levels, such as pancreatitis, chronic kidney disease, or hypothyroidism.
The test is usually ordered by your doctor. After being taken, your blood sample is sent to a laboratory for analysis. Point-of-care lipid testing involves a drop of blood taken from your finger that is immediately analyzed by a small device.
This type of test is used in some clinics and at events like health fairs. Cholesterol testing is routine and reliable. If proper test procedures and preparation are followed, including fasting when needed, false positive or false negative results are rare. Point-of-care lipid testing, which is performed on-site and not in a laboratory, has more variability than laboratory testing but still provides a meaningful reference point for measuring cholesterol.
When point-of-care or at-home tests show abnormal lipid levels, follow-up testing is often recommended in a certified laboratory. Online lipid panel tests are available with local lab testing.
The cost of a lipid panel depends on where the test is taken and if you have insurance coverage. When prescribed by a doctor, this type of bloodwork is normally covered by insurance, but you may still have costs for a copay or deductible.
There can also be fees charged by the technicians who draw your blood. Check with your doctor and insurance plan about the cost of the test. Kits generally allow you to test your cholesterol multiple times with separate test strips.
For laboratory lipid testing, you typically must fast for hours before your blood is drawn. This means not eating and drinking only water before the test. In most lipid tests, a blood sample is taken with a needle inserted into a vein in your arm. Before your blood is drawn, an elastic band is tied around your upper arm to increase blood in the veins, and the puncture location is wiped clean with an antiseptic.
A needle blood draw may cause a temporary sting. The blood draw normally lasts for less than a minute. Sometimes a drop of blood is collected by puncturing the skin on a fingertip.
This fingerstick sample is used when a lipid panel is being measured on a portable testing device, for example, at a pharmacy or health fair. It involves a quick sting but little pain or bleeding.
You will normally be instructed to keep this in place for an hour or more to prevent any unwanted bleeding. This is a routine outpatient procedure, and you can typically drive and return to basic activities as soon as the test is over.
If fasting was required, you may want to bring something to eat right after the test. You may be advised to restrict intense exercise or physical activity for a few hours after the test. Fingerstick cholesterol tests do not usually require any special post-test restrictions. When a blood sample for a lipid test is taken with a needle, lab analysis is usually completed and available within a few days.
Your results may be sent to you in the mail or made accessible through an online health portal. A follow-up appointment may be recommended to review your results and any necessary next steps. The results of your lipid panel are reported for each type of cholesterol and triglycerides. The optimal or target level for each part of the standard lipid test are listed below:.
Values that do not meet these targets may be classified as borderline- intermediate- or high-risk. In general, higher-than-target levels of total cholesterol, LDL, and triglycerides and lower-than-target levels of HDL can heighten the risk of cardiovascular problems. Test results are interpreted in the context of your overall health and other risk factors.
Many doctors use special risk calculators that incorporate your test results, age, and other factors to determine the most appropriate next steps. Cholesterol-lowering medications, such as a class of drugs called statins, are most likely to be recommended for patients with very high LDL or elevated LDL combined with other risk factors such as diabetes or past cardiovascular problems.
Abnormally low levels of cholesterol are rare and usually associated with a health condition causing malnutrition. If you have risk factors for heart disease or abnormal lipid levels, repeat testing may be conducted at regular intervals in the future.
Your doctor can recommend a schedule for future testing. If your lipid levels are normal, you may not need repeat testing for another five years unless your overall health or risk factors change.
In some cases, other types of cholesterol testing, such as direct LDL testing, may be needed if you have high levels of triglycerides. While not included in the standard lipid panel, expanded lipid measurements, such as LDL particle testingmay be ordered.
Additional types of tests, such as a cardiac stress test, may also be considered as part of an overall cardiovascular risk assessment. If you take a point-of-care or at-home test that shows abnormal cholesterol levels, it is common to have follow-up testing done by a laboratory. When reviewing your test results with your doctor, some questions that may be helpful include:.
Medical Encyclopedia. Familial Hypercholesterolemia. Updated June 25, Accessed September 13, American Heart Association. How To Get Your Cholesterol Tested.
Updated November 9, ARUP Consult. Atherosclerotic Cardiovascular Disease Risk Markers. Updated August Davidson MH. Pulipati VP. Merck Manual Professional Edition. de Ferranti SD, Newburger JW.
Dyslipidemia in Children and Adolescents: Definition, Screening, and Diagnosis. In: Fulton DR, ed. Updated March 3,
: Speedy lipid breakdown| Lipoprotein fractions | This study also found that fasting transiently increased circulating nonesterified fatty acids and perilipin 5 PLIN5 mRNA levels in muscle. Leveille, C. Several initial studies in rodents showed benefits of milk polar lipids and SM supplementations on plasma cholesterol levels [reviewed by Norris et al. Chylomicrons deliver the fat to adipose tissue via lipoprotein lipase LPL which allows it to be taken up rapidly in the form of fatty acids. Mayo Clinic Alumni Association. |
| Role of circulating sphingolipids in lipid metabolism: Why dietary lipids matter | One mechanism to increase lpid acid could be breakdow hydrolysis of membrane High-intensity boot camp workouts breaakdown cytosolic phospholipase A2 cPLA2. Olive oil salad dressing Promo Banner. In HSAN1, the High-intensity boot camp workouts variants cause alterations of substrate specificity of the SPT enzyme from L -serine to either L -alanine or L -glycine, leading to the formation of 1-deoxysphingolipids instead of ceramides. Membrane protein interactions with the lipid bilayer require a hydrophobic match between transmembrane domain TMD length and lipid bilayer thickness Lipid metabolism in sickness and in health: emerging regulators of lipotoxicity. |
| Background | Chylomicrons, which appear after meals, are the largest and lowest density lipoproteins and rapidly float to the top of stored plasma without ultracentrifugation. Chylomicrons are rapidly metabolised into smaller VLDL sized particles chylomicron remnants. In addition to their lipid components, lipoproteins contain specific protein components known as apolipoproteins which provide a structural framework and have a number of other important functions, including binding to receptors and activation of lipid transporters and metabolising enzymes. More detail on specific apolipoproteins can be found in the drop down box. Apolipoprotein B is the bulk carrier of endogenously produced lipids and is secreted by the liver as the major apolipoprotein component of VLDL and LDL, one molecule per lipoprotein, which stays with the particle until it is removed from the circulation by the LDL receptor. A shorter form of this apolipoprotein, apolipoprotein B48, is secreted by the intestine as the major structural component of chylomicrons which carry dietary exogenous lipids. The large, triglyceride rich VLDL and chylomicrons also contain multiple copies of small exchangeable apolipoproteins apolipoproteins AI AII AIV, CI CII CIII and E which enter the HDL fraction as triglyceride is removed by triglyceride metabolising enzymes lipoprotein lipase LPL and hepatic lipase HL. Apolipoprotein A1 is secreted by both the gut and the liver and is the major apolipoprotein component of HDL which has an important role in the reverse transport of cholesterol back to the liver from peripheral tissues. The three major pathways are co-ordinated by the liver to ensure that balance homeostasis of the major lipid classes is maintained, avoiding the twin perils of deficiency and overload:. Figure 3 shows the intestinal exogenous pathway. Cholesterol and triglycerides derived from dietary lipids are absorbed in the gut and incorporated into chlyomicrons, regulated by the Niemann-Pick C1-Like 1 NPC1L1 transporter and microsomal triglyceride transfer protein MTP. These very large, triglyceride-rich lipoproteins are secreted into the lymph and bypass the liver, entering the plasma post-prandially via the thoracic duct. Chylomicrons deliver the fat to adipose tissue via lipoprotein lipase LPL which allows it to be taken up rapidly in the form of fatty acids. Once small enough the resulting chylomicron remnant is removed from plasma by the liver via apoE see drop down box binding to the remnant receptor LRP or to the LDL receptor LDLR. Between meals post-absorptive phase , as insulin levels fall, fatty acids are released from adipose tissue by lipolysis hormone sensitive lipase and adipose tissue triglyceride lipase and enter the circulation where they are rapidly bound to albumin. Fatty acids, arriving at the liver bound to albumin or newly-synthesised de novo lipogenesis , are re-esterified to form triglyceride and together with cholesterol are loaded onto apoB to form VLDL. These large triglyceride rich lipoproteins enter the plasma between meals and deliver the fat to adipose tissue and muscle via lipoprotein lipase LPL which allows it to be taken up in the form of fatty acids see figure 4. Once small enough, the resulting IDL is either taken up directly by the liver cf. Chylomicron remnant or converted to LDL by hepatic lipase HL. The resulting LDL may then be taken up by peripheral tissues via the LDLR to meet local cholesterol needs. Any surplus LDL particles are finally removed by the liver via the LDLR. HDL inhibits the development of atheroma and coronary artery disease by transporting excess tissue cholesterol to the liver, where it is converted into bile acids and excreted figure 5. Lower levels of HDL have been correlated with an increased risk of atherosclerosis, the primary cause of cardiovascular disease. The principal HDL pathway, termed reverse cholesterol transport RCT is a major contributor to lipid homeostasis. Cholesteryl ester transfer protein CETP is responsible for the exchange of cholesteryl esters from HDL for triglyceride in more atherogenic cholesterol fractions including LDL and VLDL. Some individuals with loss of function in the gene encoding CETP appeared to be at lower cardiovascular risk and CETP therefore became an attractive target for pharmaceutical inhibition. Nascent HDL, in the form of flattened discs, is generated from LPL- mediated lipolysis of triglyceride rich lipoproteins TRLs, including VLDL and cChylomicrons , or triglyceride rich lipoproteins TRLs or secreted directly from the gut or liver, and enters plasma and where it picks up additional exchangeable apolipoproteins. Free cholesterol is removed from peripheral tissues including cholesterol laden macrophages in the arterial wall via ATP-binding cassette A1 ABCA1 activated by apoA1, rapidly esterified by the action of lecithin cholesterol acyl transferase LCAT and funnelled into the core of the new HDL particle converting it to the mature spherical form. Core lipid exchanges between HDL and TRLs occur in the circulation, catalysed by CETP. This allows cholesterol to be offloaded from HDL into VLDL and LDL which are destined for hepatic uptake, permitting further cholesterol uptake from tissues. Finally the cholesterol-enriched HDL particle returns cholesterol to the liver via the scavenger receptor B1 SCARB1 for biliary excretion. The cholesterol-depleted HDL particles can then return to the circulation to undertake more reverse cholesterol transport. The rate of cholesterol formation by the liver and absorption by the small intestine is highly responsive to the cellular level of cholesterol. This feed back regulation is controlled primarily by changes in the amount and activity of 3-hydroxy-3 methylglutaryl CoA reductase HMGCoA reductase. This enzyme catalyses formation of mevalonate, the committed step in cholesterol biosynthesis. For more detail on this process of cholesterol homeostasis, see dropdown box. The concentration of free cholesterol determines the fluidity and function of cell membranes and regulates overall cholesterol homeostasis see figure 6. When hepatic cholesterol content is reduced by export in lipoproteins or conversion to bile acids, membrane cholesterol concentration falls and SREBP-2 activates the enzymes of cholesterol synthesis, including HMG-Co-A reductase which is the rate limiting step in the pathway. SREBP-2 also activates the synthesis of LDL-receptors, accelerating the uptake of cholesterol in LDL to help restore membrane cholesterol concentration. Conversely, when hepatic cholesterol is increased by receptor mediated uptake of cholesterol in lipoproteins or return of cholesterol to the liver by HDL particles, membrane cholesterol increases, preventing activation of SREBP-2 and leading to LDL-receptor downregulation and inactivation of cholesterol synthesis. The majority of circulating cholesterol is carried in LDL which is the lipoprotein most closely associated with the development of atherosclerosis. Under normal circumstances, LDL may pass from the plasma into the subendothelial space and return to the liver to be removed from the circulation. At this point it has performed its transport functions without being taken up by macrophages and indeed is unable to stimulate foam cell formation in vitro. However, if retention of the LDL in the endothelial space is increased, due to endothelial injury e. with smoking, hyperglycaemia, hypertension or if removal of LDL from the circulation is delayed, it can become damaged by oxidation or modified in other ways. Oxidised or otherwise modified LDL are retained in the subendothelial space and are taken up by monocyte-derived macrophages via the scavenger receptor leading to the formation of foam cells, and the development of arterial sub-endothelial fatty streaks, the precursor of atheroma. Small dense LDL particles, typically found in found in association with prolonged postprandial hypertrigylceridaemia and low HDL cholesterol, appear more susceptible to oxidation which may make them more atherogenic. Partially metabolised remnants of triglyceride-rich lipoproteins remnant lipoproteins that appear post-prandially are able to induce foam cell formation without modification. These are considered the most highly atherogenic of all. Other atherogenic lipoproteins readily retained in the subendothelial space include glycated LDL and lipoprotein a. HDL are, however, able to penetrate deep into the subendothelial space and are able to remove oxidised lipid from macrophages and prevent foam cell formation, in addition to having a protective effect on the endothelium. Reduction of HDL particle numbers or functional activity is therefore pro-atherogenic. Figure 7 shows the progression of atherosclerosis. For more information on the process of atherosclerotic plaque development, please visit module 3 of our angina e-learning programme. Apolipoproteins are proteins that bind lipids to form lipoproteins. They transport lipids through the lymphatic and circulatory systems. They also serve as enzyme cofactors, receptor ligands, and lipid transfer carriers that regulate the metabolism of lipoproteins and their uptake in tissues. For examples of their beneficia l role e. apoE in hepatic clearance of chylomicrons and their thrombogenic potential e. lipoprotein a , see dropdown box. Apolipoproteins B and B48 are two proteins produced from the same gene, due to editing of mRNA in the gut. ApoB48 is exclusive to chylomicrons and chylomicron remnants. ApoE is required for hepatic clearance of chylomicron and VLDL remnants IDL via the LDL receptor LDLR and LDLR-like receptor protein 1 LRP1. ApoE has 3 common Isoforms E3, E4, E2. ApoB remains with VLDL as it undergoes lipolysis to IDL and LDL whereas exchangeable apolipoproteins e. ApoE and apoC-II can transfer between particles. As each LDL particle contains one molecule of apolipoprotein B, apolipoprotein B concentration is a measure of LDL particle numbers. Lipoprotein a is a highly atherogenic and thrombogenic lipoprotein formed by covalent bonding between the apolipoprotein B of the LDL particle and apolipoprotein a , an apparently vestigial plasminogen-like protein which increases its retention in the artery wall see figure 8. Genetic variants of the apo a protein which are smaller in size due to fewer numbers of repeats of the KIV-2 domain generate greater numbers of liporotein a particles, concentrations of which show fold variation between individuals. Upregulation of CB2 receptor has been reported in ALS patient spinal cords and motor cortex [ ], and in spinal cord of a canine ALS model [ ]. Glycerolipids are neutral lipids and act as precursors to other lipids in the CNS. They are most abundantly found in astrocytes within lipid droplets [ 55 , 81 ]. Glycerolipids can be phosphorylated to form glycerophospholipids, or hydrolyzed to give rise to fatty acids of various chain lengths. In high energy demand conditions, glycerolipids are quickly depleted to produce fatty acids for energy metabolism. ALS patients with elevated serum TG levels are reported to have prolonged life expectancy, suggesting that serum level of TGs could be used as a prognostic factor [ 22 ]. Lipidomic studies have reported higher levels of diglycerides [ 60 , 62 ] and TGs [ 61 , 62 , 63 ] in the sera of ALS patients, of which the TG is a reliable discriminant lipid to distinguish patient samples from healthy controls. A longitudinal study with a two-year follow-up after the first measurement, found increased diglyceride and TG levels but decreased monoglyceride levels in ALS patients at later stages of the disease [ 62 ]. Interestingly, the diglyceride species containing MUFAs like palmitoleic and oleic acids are significantly elevated. TGs containing the same MUFAs are also elevated though not significantly. Elevated levels of diglycerides, which are precursors to TGs in serum, suggest increased de novo glyceride synthesis, or mobilization from adipose tissue, or both. Additionally, in the plasma, TG levels are found to be associated with serum levels of neurofilament, an established neuronal damage marker [ 61 ]. Furthermore, TG levels of C16 and C species are increased up to three folds in spinal cords of male ALS patients and are also elevated in spinal cords of SOD1-G93A mice [ 35 ]. In mouse spinal cords, TG accumulation increases with disease progression and is predominant in gray matter [ 35 ]. However, it remains to be determined if these observations indicate a greater consumption of glycerolipids in the CNS in order to meet the energy demand and if there is a switch to fatty acid oxidation. Glycerophospholipids are major structural components of all eukaryotic plasma membranes [ 50 , 51 ]. Phospholipids form the characteristic phospholipid bilayer, and their composition affects membrane geometry, fluidity, and permeability [ 50 , 51 ]. Some species also function as bioactive signaling molecules. PC is a main source of acetylcholine in the CNS, and intake of PC can improve memory and learning and ameliorate cognitive decline in mouse models of dementia [ , , ]. Elevated levels of PC have been reported in the CSF [ 65 ] and the spinal cord nuclear lipidome [ 70 ] of ALS patients, the spinal cords of FUS overexpression mice [ 68 ], the skeletal muscle of SOD1-G86R mice [ 67 ], and the motor cortex of SOD1-G93A mice [ 69 ]. Elevated levels of PC and PC have been found to be the most discriminatory in the CSF and plasma, being able to differentiate between slow- and fast-progression cases [ 61 , 65 ]. Elevated levels of PC are also observed in SOD1-G93A mouse brains [ 66 ]. In a longitudinal study of ALS patients across two years, several species of PC and PS were decreased in patient blood in the initial stage of pathogenesis, with reductions in PS and PS being discriminatory even at baseline. Follow-up samples had elevated levels of these PCs, suggesting an increased level with disease progression. Despite an initial decline, PE levels increased progressively in the ALS patients and PE could even be used to discriminate ALS from PLS [ 62 ]. Lysophospholipid levels are reported to be elevated in plasma [ 34 , 60 , 61 , 63 ], CSF [ 65 ] and spinal cords [ 35 , 70 ] of ALS patients, in the spinal cords of FUS-overexpression mice [ 68 ], and in skeletal muscles of SODR mice [ 67 ]. Lyso-PC is commonly discriminatory in ALS patient CSF and SOD1-G93A mice brains, along with elevation of lysoPCs containing the long-chain fatty acids C, C and C [ 65 ]. Specific species of lysoPC esters and lysoPE plasmalogens are significantly and progressively reduced in ALS patient blood samples [ 62 ]. A study testing for levels of lysoPCs containing fatty acids with various saturation status reported an increase of lysoPCs containing C16 and Cn9 fatty acids in spinal cords of both ALS patients and SOD1-G93A mice [ 35 ]. Lyso-PCs are generally a by-product of cholesterol ester synthesis that is elevated in ALS conditions. Addition of these lysoPC species C16, C18, and Cn9 to motor neuron cultures in vitro causes motor neuron toxicity and death compared to their corresponding free fatty acids C16, C18, C as controls, with lysoPC C16 being more toxic than others [ 35 ]. The data suggest that the elevated lysoPC level may be detrimental for motor neurons. Sphingolipids are a class of lipids which are ubiquitously found in cell membranes and are an integral constituent of lipid rafts, contributing to membrane stability and permeability [ 50 , ]. Sphingolipids are highly enriched in the CNS, where different CNS cell types have different sphingolipid profiles [ ]. Due to this diverse distribution, sphingolipids have been shown to be vital in brain development, neurogenesis, differentiation, axonal growth and ageing [ 46 , , ]. Breakdown of sphingolipids takes place in the lysosome and defects in this process can lead to accumulation of sphingolipids, which is implicated in many neurological diseases, including ALS. Mutations in enzymes catalyzing the degradation of these sphingolipids are responsible for a large group of lysosomal storage diseases, also called sphingolipidosis [ ], which is often manifested as ALS-like symptoms see below. Collectively, these observations suggest that homeostatic regulation of sphingolipid metabolism is essential for CNS function Fig. Ceramides consist of a sphingosine attached to a fatty acid tail, and are the precursors to the more complex sphingolipids Fig. They are primarily generated by de novo synthesis, and from the breakdown of more complex sphingolipids, especially the breakdown of SM. The first and rate-limiting step of de novo ceramide synthesis is the condensation of palmitoyl-CoA and L -serine, catalyzed by SPT, to form 3-keto-sphinganine, which is reduced to sphinganine, a key intermediary. Sphinganine is N -acylated by one of the ceramide synthases CerS , each of which has a preferential specificity for fatty acyl CoAs of different carbon-chain lengths, leading to the formation of dihydroceramides with different chain lengths, which are then desaturated to form ceramides [ 45 , , ]. An early milestone study on the role of sphingolipids in ALS was published in [ 33 ]. Cutler and colleagues quantified various lipids in ALS patient spinal cords, and found accumulations of ceramides, SM, and cholesterol esters along with increased oxidative stress. Similar results were obtained at the pre-symptomatic stage in the spinal cords of SOD1-G93A mice. To understand the relationship among oxidative stress, deficits in sphingolipid biosynthesis and cell death, cultured motor neurons were treated with either DMNQ, an oxidative stress-inducing agent, or palmitoyl-CoA, the initial substrate for sphingolipid synthesis catalyzed by SPT. Either DMNQ or palmitoyl-CoA treatment alone increased the levels of ceramide and cholesterol esters within 6 h of exposure and triggered a dose-dependent cell-death. Combination of DMNQ and palmitoyl-coA resulted in exacerbated increase of ceramides, SM, cholesterol esters and cell death, all of which were reduced on treatment with DMNQ and an SPT inhibitor [ 33 ]. Thus, their data suggest that oxidative stress acts through enhanced sphingolipid synthesis to induce neuronal death. Furthermore, ceramide induces apoptotic cell death in cortical and motor neurons [ , , , ], suggesting that accumulation of ceramides could contribute to ALS pathogenesis [ 33 ]. Indeed, elevated levels of ceramide have been reported in spinal cords and motor cortex of SOD1-G93A rats [ 69 ], as well as in plasma [ 60 , 62 , 63 ], spinal cords [ 33 , 36 ] and fibroblasts [ 73 ] of ALS patients. Furthermore, increased ceramide levels are observed in spinal motor neurons, but not in ocular motor neurons, derived from ALS patients [ 18 ]. Taken together, these data suggest that accumulation of ceramide, a precursor for sphingolipids, could contribute to ALS pathogenesis. Recent whole-exome sequencing in juvenile- and adult-onset ALS patients identified several genetic variants of SPTLC1 [ 16 , 17 ]. SPTLC1 is the long-chain subunit 1 of the enzyme SPT, which is the rate-limiting enzyme for ceramide biosynthesis. At least two not-mutually-exclusive mechanisms have been proposed for these dominant-acting SPTLC1 variants. The first mechanism takes cue from hereditary sensory neuropathy type1 HSAN1 , a disease characterized by atrophy of sensory neurons [ , ]. Mutations in SPTLC1 are the underlying cause of HSAN1. In HSAN1, the SPTLC1 variants cause alterations of substrate specificity of the SPT enzyme from L -serine to either L -alanine or L -glycine, leading to the formation of 1-deoxysphingolipids instead of ceramides. These atypical 1-deoxysphingolipids cannot be synthesized into more complex sphingolipids or degraded, thereby resulting in accumulation of atypical 1-deoxysphingolipids that are highly neurotoxic [ ]. HSAN1 patients are often treated with oral supplementation of L -serine to reduce production of the toxic 1-deoxysphingolipids [ ]. The p. SY SPTLC1 variant [ 16 ] identified in juvenile ALS has been previously reported as an atypical HSAN1 variant with a distinct mixed sensorimotor neuropathy phenotype [ ]. Furthermore, using cell culture assays, Johnson et al. reported that the p. A20S SPTLC1 variant also showed an altered substrate preference for L -alanine and L -glycine, along with mitochondrial defects, which were rescued on exposure to L -serine [ 16 ]. Thus, these observations indicate that the ALS-linked variants alter substrate specificity of SPT [ , ]. The other juvenile ALS variants identified are distinct from HSAN1 variants and map to exon 2 of SPTLC1, including p. A20S, p. Y23F, p. L39del and p. This exon codes for a transmembrane domain that interacts with ORMDL proteins to inhibit SPT activity [ 17 , ]. Mohassel et al. showed that these juvenile ALS variants are not sensitive to ORMDL protein levels, resulting in higher levels of sphinganine and ceramides. Correspondingly, increased levels of ceramides, but not 1-deoxysphingolipid a HSAN1 characteristic feature , are found in the sera of juvenile ALS patients with variants p. It should be noted that Mohassel et al. did not test substrate specificity preferences of the variants. Furthermore, selective knockdown of the ALS SPTLC1 allele restored normal ceramide levels in human iPSC motor neurons [ 17 ]. Thus, these ALS-linked variants of SPTLC1 could disrupt regulation of SPT, resulting in unrestrained activity and higher ceramide levels. All the evidence presented above indicates that increased accumulation of ceramides is a common theme for ALS. Thus, prevention of ceramide accumulation could be a potential therapeutic intervention and can be achieved by promoting its synthesis to other sphingolipids or its degradation. Indeed, inhibition of glucosylceramide synthase GCS , the enzyme which synthesizes glucosylceramide from ceramides, accelerates disease progression in SOD1-G93A mice [ 36 ]. Genetic deficiency of ceramidase, the enzyme which degrades ceramides, is causal to Farber disease, a lipid storage disease with some patients exhibiting muscle weakness and seizures [ ]. As the name suggests, SMs are abundantly present in the myelin sheath. They are the most abundant sphingolipid found in cell membranes and play a critical role in maintaining myelin sheath integrity and function and in neuroinflammation and signal transduction [ 51 , , , , ]. Sphingomyelinase encoded by SMPD1 breaks down SM into ceramide and phosphocholine. Mutations in SMPD1 cause accumulation of SM in the CNS, leading to dementia, ataxia, and slurred speech as seen in Niemann-Pick disease type A and B [ , , ] Fig. SM accumulation has been reported in plasma [ 34 , 60 , 61 , 63 ], CSF [ 65 ], spinal cords [ 33 , 36 ] and fibroblasts [ 73 ] of ALS patients. Elevated levels of SM , SM , SM OH, and SM in the plasma are found to be accurate discriminators of ALS disease progression and predictors of clinical indicators in a study with 74 ALS patients [ ]. Area-Gomez et al. additionally reported significantly lower levels of SM , SM and SM species in serum samples from ALS patients [ 62 ]. The expression profiling of SMs in ALS mouse models remains inconclusive. Cutler et al. reported an increase of SM in the spinal cords of SOD1-G93A mice from pre-symptomatic to post-symptomatic stage [ 33 ]. By contrast, Dodge et al. reported an initial decrease of SM at paralysis onset, but a normal sphingomyelin level at full-paralysis stage in the spinal cords of SOD1-G93A mice [ 36 ]. The expression levels of SM are mixed in SOD1-G86R mouse spinal cord and skeletal muscle [ 67 ], and are reported to be lower in mice overexpressing wild-type human FUS [ 68 ]. Glucosylceramides are the essential first step to the synthesis of gangliosides, a major component of neurons. As such, glucosylceramides are vital for brain development. Glucosylceramide is synthesized from ceramides by GCS and broken down into ceramides by glucocerebrosidase-1 GBA1 and GBA2 Fig. Mutations in GBA1 lead to Gaucher disease, which is characterized by neurological symptoms such dementia and ataxia, and treatment strategies include administration of recombinant human GBA [ ]. While mice with neuronal specific knockout of GCS are born with severe neural defects [ ], inhibition of GCS activity extends the survival of mouse models of Gaucher disease [ ]. Thus, the balance between GCS and GBA activity in maintaining glucosylceramide levels in the CNS is critical for brain health, and an imbalance may lead to neurodegenerative conditions. Elevated levels of glucosylceramide have been observed in plasma [ 60 ], CSF [ 65 ] and spinal cords [ 36 ] of ALS patients, spinal cords of SOD1-G93A mice [ 36 ], motor cortex of SOD1-G93A rats [ 69 ], and skeletal muscles of SOD1-G86R mice [ 67 ]. GCS activity is reported to be upregulated in skeletal muscles of SODR mice and ALS patients [ 67 ], and in the spinal cords of ALS patients and SODA mice [ 36 ]. However, unlike that in Gaucher disease, inhibition of GCS activity causes a loss of motor strength and neuromuscular junction integrity in wild-type mice [ 67 ], and significantly speeds up disease progression in SOD1-G93A mice [ 36 ]. Thus, the data suggest that glucosylceramide accumulation plays a neuroprotective role in ALS. Indeed, conduritol B epoxide inhibition of GBA that breaks down glucosylceramide, improves NMJ integrity, increases ganglioside GM1a and slows disease progression in SODR mice [ 37 ]. The conduritol B epoxide treatment also increases recovery from sciatic nerve injury in wild-type mice [ 37 ]. Similar alleviation of disease symptoms and improved survival are reported in SODR mice when they are treated with ambroxol hydrochloride, a GBA inhibitor [ 38 ]. Galactosylceramides are reported to be depleted in ALS patient blood samples [ 61 ], but elevated in spinal cord samples of patients [ 36 ]. The enzymatic activities of galactocerebrosidase and galactosylceramide synthase involved in regulating galactosylceramide levels, are also elevated in spinal cords of SOD1-G93R mice [ 36 ]. Lactosylceramides are synthesized from glucosylceramides and have been reported to be elevated in blood [ 60 , 61 , 62 ], spinal cords [ 36 ] and nuclei [ 70 ] of ALS patients. In addition, galactocerebrosidase mutations are causal for Krabbe disease [ ], another motor degenerative disease, while lactosylceramides are activators of neuroinflammation [ ]. Further studies are needed to explore the role of these ceramides in ALS,. Gangliosides with complex carbohydrate head groups, are most abundantly found in the CNS and are involved in several functions such as cell—cell recognition, signal transduction, synaptic transmission, cognition and oligodendrocyte differentiation [ , ]. The composition of gangliosides within the CNS changes during neurodevelopment: from simplest gangliosides GD3 and GM3 expressed primarily in early development stages, to more complex gangliosides such as GM1, GD1a and GD1b dominate in later stages and adult brains [ , ]. Accumulation of gangliosides causes lipid storage disorders called gangliosidosis. Intriguingly, gangliosidosis, such as Tay-Sachs disease, Sandhoff disease and GM1 gangliosidosis, often has clinical manifestations that mimic ALS [ , , , ]. Both Tay-Sachs and Sandhoff diseases are caused by mutations in β-hexosaminidase subunits HEXA and HEXB, respectively that are required to breakdown GM2 to GM3, the latter of which is involved in neuronal growth, plasticity and repair [ , ]. Therapeutic strategies for these diseases focus on preventing the buildup of GM2 in neurons, failure of which leads to toxicity, neuronal degeneration and eventual death [ , , ]. Unfortunately, these trials using exogenous bovine ganglioside for treatment yielded inconclusive improvement and results [ , , ]. Subsequent studies provided more details on specific ganglioside profiles and dysregulation. In , Dodge et al. reported increased levels of GM3 and GM1, along with increased activity of HEX in the spinal cords of ALS patients and SOD1-G93A mice [ 36 ]. Further, they showed that although increasing the HEX activity via adenoviral vector delivery to the CNS did not have any effect, direct intracerebroventricular delivery of GM3 significantly delayed the onset of paralysis and extended survival of SOD1-G93A mice. GM1 has been shown to amplify neurotrophic response, block excitotoxicity and promote neurite growth in rat models [ , , ]. These findings suggest that the accumulation of GM1 and GM3 may be protective in nature and could be used to slow down ALS disease progression. The elevation of HEX expression has been observed in SOD1-G93A spinal astrocytes, which is associated with increased lysosomal and phagocytic activity [ ]. In the same year , Xu et al. showed that a dose of rHIgM12, a human antibody with binding specificity to the neuronal membrane gangliosides GD1a and GT1b, is able to delay disease onset and improve survival in both SOD1-G93A and SOD1-G86R ALS mouse models [ ]. Both GD1a and GT1b are neuronal surface ligands for myelin-associated glycoprotein MAG , binding of which inhibits nerve regeneration via membrane domain rearrangement. In culture, the MAG-mediated neurite growth inhibition is reduced by blocking ganglioside synthesis, modifying structure of the neural surface gangliosides or using antibodies against the gangliosides [ ]. The data suggest that reducing the levels of GD1a and GT1b gangliosides or blocking their interaction with MAG may be beneficial. The major forms of sterols found in mammalian cells are cholesterol and its derivatives, such as oxysterols and cholesterol esters [ 40 , 41 , 42 ]. Cholesterol regulates membrane order and flexibility, is a component of membrane lipid rafts, and serves as a precursor to steroid hormones. In the CNS, cholesterol is implicated in synaptic formation, axonal growth, signal transduction, as well as learning and memory [ , ]. Elevated levels of cholesterol in the sera of ALS patients are found to be discriminatory and prognostic for longer survival [ 22 , 34 ]. Various cohort studies have shown a causal association of higher serum LDL-cholesterol with higher risk of ALS diagnosis [ , , ]. However, post ALS diagnosis studies provided conflicting results on the levels of serum LDL, HDL and total cholesterol [ 21 , , , , , ]. Cholesterol levels are found to be elevated in the CSF of ALS patients [ ]. Downregulation of the cholesterol metabolism pathway has been reported in a meta-analysis of transcriptomics studies in SOD1-G93A mouse spinal cords [ ]. Recently, two independent studies indicate that TDP, the key pathological hallmark protein for ALS [ ], regulates SREBF2-mediated cholesterol metabolism [ 28 , 29 ]. Furthermore, the expression of 3-hydroxymethylglutaryl-CoA reductase HMGCR , a rate-limiting enzyme for cholesterol biosynthesis and a transcription target of SREBF2, is reduced in oligodendrocytes bearing TDP pathologies [ 28 ], suggesting that cholesterol metabolism may be affected in cells with TDP proteinopathies. Although no change is observed in free cholesterol levels in the sera of ALS patients [ 29 ] as well as spinal cords of ALS patients and SOD1-G93A mice [ 35 ], the cholesterol level is reduced in the CSF of ALS patients [ 29 ]. Furthermore, reduced levels of lanosterol, a precursor to cholesterol, are observed in ALS patients and SOD1 mouse models, along with downregulation of HMGCR [ 35 ]. Given that cholesterol cannot cross the blood—brain barrier, cholesterol is synthesized and stored in the CNS without peripheral contribution [ , ]. It is questionable if peripheral serum levels of cholesterol reflect the levels in the CNS, and vice versa. This disparity may explain why studies using statins to reduce serum cholesterol in ALS patients showed no effect or negative effect on disease progression [ , ]. There is a large discussion surrounding the interplay of cholesterol metabolism, transport and uptake in the periphery and the CNS, and their effects in ALS. Please refer to Hartmann et al. Cholesterol cannot be degraded, and free cholesterol is toxic to the system. Excess cholesterol in the CNS is oxidized to oxysterols, which are able to cross the blood—brain barrier [ ], and the blood levels of oxysterols are considered reflective of CNS status. The main forms of oxysterols found in the CNS are 24S-hydroxycholesterol OHC , hydroxycholesterol OHC , and hydroxycholesterol OHC , of which OHC and OHC are LXR receptor ligands. LXR receptors activate expression of genes involved in cholesterol efflux pathway, such as ATP-binding cassette subfamily A member 1 ABCA1 and APOE, thereby providing another layer to maintain cholesterol homeostasis. Levels of enzymes converting cholesterol into OHC are found elevated in early symptomatic SOD1-G93A mouse brains [ , ]. Additionally, OHC induces neuronal death via LXR-mediated apoptosis in motor neuron-like cells containing the SOD1-G93A mutation [ ]. Elevated levels of OHC are found in spinal cords of ALS patients, and cause cell death in neuroblastoma cell lines [ ]. These studies suggest a neurotoxic effect of accumulation of OHC and OHC in the CNS. GWAS studies have identified CYP27A1 , which encodes the enzyme converting cholesterol to OHC, as a susceptible loci for ALS [ ]. However, lower levels of OHC have been reported in the sera of ALS patients [ , ], but show no correlation with survival [ ]. Surplus free cholesterol can be esterified with fatty acyls to neutral cholesterol esters and stored in lipid droplets. In the CNS, this function is likely to be carried out primarily in astrocytes [ 47 , ]. Elevated cholesterol ester levels have been consistently reported in various tissues of ALS patients and animal models. Several species of cholesterol esters, including those with C16 and C18 saturated and unsaturated fatty acid chains, are reported to increase by up to 22 folds in patient spinal cords, with a more pronounced effect in the grey matter [ 33 , 35 ]. A four-fold progressive increase of C18 cholesterol ester species has been observed in SOD1-G93A mouse spinal cords from early symptomatic stage to end paralysis stage. Cholesterol esters levels are elevated in plasma samples and the change is maintained longitudinally, with increases in long- and very long-chain cholesterol esters, including CE and CE being discriminatory for ALS [ 62 ]. The SODA rat spinal cords have a sixfold increase of total cholesterol esters, mainly from PUFA species, including arachidonic acid The cholesterol ester accumulation seen in the FUS-overexpressing mice is partially rescued on HDAC inhibition [ 68 ]. Mice with adenoviral-induced overexpression of SREBP2 transcription domain show motor neuron degeneration, paralysis and reduced survival accompanied by accumulation of cholesterol esters [ 35 ]. Interestingly, lysoPC, a by-product of cholesterol ester synthesis, is also elevated in spinal cords of both patients and SOD1-G93A mice [ 35 ]. Lyso-PC causes rapid demyelination and is shown to be toxic to motor neuron cultures [ 35 ], suggesting that accumulated cholesterol esters may be toxic via action of their by-product. As discussed above, it is apparent that lipid dysregulation, in particular accumulation of toxic lipid species, contributes to ALS. As such, targeting these toxic lipids makes for attractive therapeutic interventions. Indeed, various strategies have shown to be successful in alleviating disease symptoms, extending survival and providing neuroprotection in vitro and in animal models. In this section, we present an overview of lipids as therapeutic targets for ALS treatment Fig. Potential therapeutic strategies targeting fatty acids. Upper panel shows an overview of fatty acid metabolism intervention points with neuroprotective effects. Fatty acids and derivatives shown to be toxic in ALS are highlighted in salmon pink. Lower panels describe the toxicity and the therapeutic strategies used in ALS mice and ALS patients. Intervening compounds are in orange. Glucose oxidation is the main energy source in the brain, while fatty acid β-oxidation contributes to up to one-fifth of the total brain energy needs [ 77 ]. Although fatty acid oxidation produces more ATP as compared to glucose, it also takes up more oxygen resources. As such, cells with prolonged fatty acid β-oxidation undergo oxidative stress, thereby producing harmful reactive-oxygen species. Due to the high energy demand and impaired glucose metabolism in ALS [ 24 , ], there is a switch to fatty acid β-oxidation as the major route for energy generation, placing the system under increased oxidative stress, a key mechanism of neurotoxicity in ALS [ 23 , 79 , 80 ]. It is worth noting that the switch to the use of fatty acids as an energy source has been observed in skeletal muscles of SOD1-G86R [ 79 ] and SOD1-G93A mice [ ] even prior to disease onset. Pyruvate and fatty acyl CoA are important intermediates of glucose and fatty acid oxidation, which are converted to acetyl-coA. The acetyl-coA enters the TCA cycle to generate ATP. Pyruvate dehydrogenase catalyzes the oxidation of pyruvate to acetyl-coA and is inhibited by pyruvate dehydrogenase kinase 4 PDK4 to regulate pyruvate levels. Palamiuc and colleagues demonstrated that Pdk4 expression is elevated in skeletal muscles of SODR mice accompanied by impaired glucose metabolism and a switch to fatty acid β-oxidation, leading to greater oxidative stress [ 79 ]. Also, Pdk4 mRNA expression was found with a three-fold elevation in ALS patient muscles [ 79 ]. Inhibition of PDK4 with dichloroacetate in the SODR mice leads to a switch back to increased glucose oxidation, and the mice show improved mitochondrial function, reduced muscle denervation, and delayed disease onset [ 79 ]. These results underscore the importance of metabolic switch in inducing oxidative stress and disease pathogenesis Fig. Elevated levels of arachidonic acid and its derivatives have been reported in ALS patients and models [ 18 , 34 , 60 , , , , , ]. Arachidonic acid produces prostaglandins and leukotrienes via the COX and LOX pathways. These molecules can induce inflammation and cause motor neuron death, which could be rescued by treatment with LOX and COX inhibitors. Administration of nimesulide, an inhibitor for COX-2, also shows great promise, as it reduces the level of PGE 2 in the spinal cord and delays disease onset in SOD1-G93A mice [ ]. Administration of 5-LOX inhibitor caffeic acid, apigenin or nordihydroguaretic acid promotes survival of ALS spinal motor neurons in vitro, reverses eye degenerative phenotypes and promotes survival in C9orf72 ALS flies [ 18 ]. Direct treatment with arachidonic acid increases cell death of ALS spinal motor neuron cell lines, which can be rescued in a dose-dependent manner by caffeic acid [ 18 ]. In SOD1-G93A mice, caffeic acid reduces astrocyte and microglia activation, maintains neuromuscular junction morphology and architecture, delays disease onset and prolongs lifespan [ 18 ] Fig. It would be of interest to make use of these studied chemotherapeutic agents as candidates for ALS therapeutics. PGE 2 is a key mediator in the initiation of inflammatory oxidation and propagation. Inhibition of COX-2, an enzyme involved in PGE 2 synthesis, and downregulation of PGE 2 receptor, have been shown to delay the onset of ALS symptoms in SOD1-G93A mice [ , ]. Given the involvement of PGE 2 in ALS inflammation and potential systemic side effects of COX-1 inhibition COX-1 is constitutively expressed in most tissues , a variety of COXinhibiting non-steroidal anti-inflammatory drugs NSAIDs have been tested for ALS therapeutics. Administration of NSAIDs, such as rofecoxib [ ], nimesulide [ ], and celecoxib [ ], has been shown to delay disease onset and promote survival at varying degrees in SOD1-G93A mice [ , , ] Fig. Furthermore, depletion of TDP in microglia, but not in astrocytes, increases COX-2 and PGE 2 levels and reduces neural survival in vitro. This neurotoxicity could be rescued by celecoxib [ ]. However, a double-blind clinical trial of celecoxib in ALS patients showed no beneficial effects on muscle strength scored via the ALSFRS-R over a period of one year [ ]. Although cohort studies to test for ALS risk associated with NSAID usage have been inconclusive [ , ], a population study with ALS patients found that the use of aspirin a NSAID inhibiting both Cox-1 and Cox-2 may reduce ALS risk in people over 55 years [ ]. Altogether, these studies indicate that activation of the arachidonic acid pathway contributes to ALS pathogenesis, and conversely, pharmacologic inhibition of the arachidonic acid pathway may have a therapeutic potential. There are few clinical trials testing for the effects of cannabinoids in ALS, and the number of patients employed was limited, with most early ones being observational survey-based. Another phase-2 clinical trial with 59 ALS patients tested nabiximols, an established drug used to treat muscle spasticity in multiple sclerosis [ ]. The trial found that nabiximols is safe for use and has positive effects on muscle spasticity in ALS [ ], opening avenues for large-scale clinical trials for ALS symptomatic relief. There is an ongoing placebo-controlled double-blind clinical trial with 30 ALS patients to study the efficacy of cannabis-based medicine extracts in slowing ALS progression as measured by the ALSFRS-R, and to evaluate its safety and effects in relieving pain and spasticity as well as improving quality of life [ ]. It should be noted that 2-AG is also a substrate for COX-2, and it can be oxygenated by COX-2 to form various prostamides and prostaglandin glyceryl esters [ ], such as Prostaglandin E2 glyceryl ester PGE 2 -G and prostaglandin D2 glyceryl ester with divergent physiological functions [ ]. However, the role of these prostaglandin glyceryl esters in ALS remains to be explored and further studies are needed to explore the translation potential of endocannabinoids. Given ceramide accumulation in patient tissues [ 33 , 36 , 60 , 62 , 63 , 73 ] and the neuronal toxicity of ceramides [ , , , ], ceramides are both attractive biomarkers and therapeutic targets for ALS. Ceramide accumulation can occur through excessive synthesis, impaired degradation, and increased breakdown of more complex sphingolipids into ceramides Fig. The recent identification of SPTLC1 mutations [ 16 , 17 ] further underscores this working model. Selective knockdown of SPTLC1 mutant allele using siRNA alleviates ceramide levels in vitro [ 17 ] , and the approach could possibly be extended in a clinical setting to revert accumulation of toxic lipids due to SPTLC1 mutations Fig. Ceramides and gangliosides therapeutic strategies. Upper panel shows an overview of sphingolipid metabolism intervention points. Sphingolipids with neurotoxic effects and neuroprotective effects in ALS conditions are highlighted in salmon pink and green, respectively. Lower panels describe the toxicity and the therapeutic strategies used. a Selective inhibition of SPTCL1 variant allele, b fingolimod-mediated neuroprotection, c inhibition of glucosylceramide breakdown, and d ganglioside-mediated therapeutics. Intervening compounds, RNAs and antibodies are highlighted in orange. Ceramides are also formed from the breakdown of glucosylceramides by hydrolyzing enzymes called glucocerebrosidases. Unlike in Gaucher disease, where glucosylceramide buildup causes liver and spleen malfunction [ ], elevated levels of glucosylceramides in ALS models seem to play a neuroprotective role [ 36 , 37 , 38 ]. Inhibition of glucosylceramide synthesis accelerates disease progression [ 36 , 67 ], while inhibition of glucosylceramide degradation via glucocerebrosidases, by conduritol B epoxide [ 37 ] and ambroxol hydrochloride [ 38 ], alleviates disease symptoms in mouse models, making glucosylceramides an attractive drug target to alleviate disease symptoms Fig. Multiple ganglioside species are involved in ALS: elevated levels of GM1 and GM3 are neuroprotective in nature [ 36 , , , ], while G1a and GT1b are toxic [ ], and their inhibition by specific antibodies results in improved survival in ALS mice [ ] Fig. Ceramide degradation is catalyzed by ceramidases, and forms S1P. S1P is a bioactive lipid involved in regulation of many processes such as cell proliferation, survival, neuronal excitability, neuroinflammation and immune cell trafficking [ , , ]. FTY is also a ceramide synthase inhibitor [ , ]. It inhibits proinflammatory cytokine production and reduces T cell migration into the CNS, thus promoting neuroprotective role of microglia and preventing neuronal excitotoxicity [ , , ]. FTY administration in SOD1-G93A mice prolongs survival, ameliorates neurological defects and regulates neuroinflammatory genes [ 39 ]. A randomized double-blind phase IIa clinical trial of FTY has demonstrated short-term safety 4-weeks of FTY with no adverse effects, reduction of circulating lymphocytes, and tolerability, suggesting the suitability for further clinical trials [ ]. Other attractive candidate lipid biomarkers of therapeutic interest include cholesterol esters and lysoPC. Accumulation of cholesterol esters and particularly lysoPCs, has been consistently reported in ALS patient tissues and models [ 33 , 34 , 35 , 60 , 62 , 63 , 65 , 70 ], and has been found to be discriminant for ALS [ 62 , 65 ], with C16 and Cn9 lysoPC species commonly elevated in both patients and animal models [ 35 ]. Although synthesis of cholesterol esters protects cells from free cholesterol toxicity, the by-product of its synthesis, lysoPC, has been shown to cause rapid demyelination [ ] and motor neuron death [ 35 ]. Lyso-PC species with C16 chains are highly neurotoxic and could be attractive targets for therapeutics. Additionally, lower levels of TG in the CSF are associated with better survival [ 65 ]. This could be a result of their breakdown to fatty acids to effectively meet energy demands in disease conditions. Additionally, although not demonstrated in the ALS context, neurotoxic reactive astrocytes in vitro increasingly secret long-chain saturated fatty acids which were shown to be neurotoxic [ 88 ]. Therefore, oxidative stress may result in neurotoxic reactive astrocytes in ALS [ 28 , ], which could be another potential mechanism to explore for a better understanding of ALS pathogenesis. There are of course many outstanding questions in the field to consider especially in deciphering the mechanisms underlying lipid toxicity or protection in ALS. Two areas of interest we would like to highlight are the interaction between CNS and peripheral lipid levels, and the cell-type specific lipid metabolism. The blood—brain barrier only allows selective molecules to pass freely between the CNS and the periphery, making it important to study lipid distribution in various tissues to better understand lipid synthesis, mobilization, uptake and consumption. These distinct profiles are highlighted in the context of TGs, which can pass through the BBB, and cholesterols, which cannot cross the BBB. TG levels are elevated in blood [ 61 , 62 , 63 ] and depleted in the CSF [ 61 , 65 ], which may be a result of mobilization of TGs from the peripheral adipose tissue to meet greater energy demands and consumption of TGs in the CNS in diseased state. Cholesterol levels are reported to be elevated in the plasma [ 34 , 62 ], which may be a risk factor for ALS in the pre-diagnostic stage [ ], but they are downregulated in mouse spinal cords. In fact, studies using statins, which cannot cross the BBB, to control blood cholesterol levels have failed [ , ], raising the question of the diagnostic or therapeutic value of blood cholesterol levels, and emphasizing the need for a better understanding of the exchange of lipids between the CNS and the periphery. A recent lipidomic study using primary cells i. For example, levels of cholesterol and ceramide are highest in neurons; astrocytes are enriched with PS, phosphatidylinositol and diacylglycerol; sulfatide and hexosylceramide are enriched in oligodendrocytes; and levels of SM and phosphatidylglycerol are highest in microglia [ 81 ]. These cell-type specific signatures may reflect the specialized functions of each cell type. Furthermore, lipid biosynthesis may be developmentally regulated and context-dependent. For example, cholesterol biosynthesis is required in neuroprogenitor cells but dispensable in mature neurons [ ]. During myelination, oligodendrocytes upregulate its own cholesterol biosynthesis, as well as taking up cholesterol synthesized in astrocytes [ , ]. Further work is needed to understand the cell-intrinsic mechanisms underlying the processing and functions of these lipids as well as how lipid dynamics may be regulated via cell—cell communication. In the CNS, astrocytes are the main site for lipid oxidation and storage [ 78 ]. During high energy demand, the lipids synthesized in neurons are transported into astrocytes, forming lipid droplets, and undergoing oxidation [ 78 ]. Astrocytes contain a greater number of antioxidant molecules and help consume the damaging reactive oxygen species produced from lipid oxidation. A recent study demonstrated that astrocytes become reactive in response to oxidative stress, secreting long-chain saturated fatty acids which are neurotoxic [ 88 ]. Furthermore, we and others have shown that TDPdepleted astrocytes and ALS astrocytes may shift towards the inflammatory reactive state [ , ]. Another study using ALS spinal cord motor neuron cultures showed elevated levels of arachidonic acid, which are autonomously neurotoxic [ 18 ]. Whether ALS astrocytes may exacerbate the lipid-mediated toxicity toward ALS motor neurons is an intriguing possibility and remains to be tested. Moreover, we have shown that depletion of TDP in oligodendrocytes alone is able to produce motor deficits via SREBP2-mediated cholesterol downregulation [ 28 ]. Evidence of TDPmediated cholesterol dysregulations has been found in oligodendrocytes harboring TDP pathologies [ 28 ]. Furthermore, CSF cholesterol level is reduced in ALS patients [ 29 ]. These studies together suggest complex cell—cell communication and transport, and perhaps a cell-type specific mechanism of lipid-mediated toxicity in ALS. How the lipid dynamics change during normal and disease conditions remains to be addressed. While this review focuses on lipids, it should be noted that lipid species or various metabolites are interconnected. In addition, a recent study has also highlighted that gut microbiome and metabolites may modulate ALS pathogenesis [ ]. How these metabolites affect each other, and what serve the initiating factors would be of great interest to be resolved. The great structural diversity of lipids allows for diverse functions, and thus diverse and complex roles in various mechanisms of ALS pathogenesis. Given their assorted roles, the question of whether lipidemia dysregulation is the cause or the consequence of the disease is not easily answered. Lipid changes could be both causal and a consequence of disease pathology, forming complex feedback and feedforward regulations. Elimination of SPTLC1 mutants can reduce accumulation of putative toxic lipids [ 17 ], while reducing toxic lipids rescues cellular phenotypes [ 16 ], suggesting that lipid dysfunction could drive ALS pathogenesis. The prevalence of prognostic and pre-diagnostic dyslipidemia risk factors for ALS, such as blood cholesterol levels [ ] and body mass index [ ], as well as evidence showing that inhibiting the pre-diagnostic energy source switch to fatty acid oxidation alleviates disease symptoms in mice [ 79 , ], further support a causal role of lipids in ALS. By systematically assessing the current literature, we highlight that accumulation of ceramides, arachidonic acid, lysoPC, and cholesterol esters is emerging as a common theme that is detrimental to motor neurons. Conversely, reducing the accumulation of these toxic lipids, in particular, ceramides and arachidonic acid, appears to be beneficial in various ALS models. Furthermore, increased levels of potentially beneficial lipids such as glucosylceramides, and activation of S1P-mediated signaling may be protective in ALS. We look forward to future investigations to restore the faulty lipids in ALS. Feldman EL, Goutman SA, Petri S, Mazzini L, Savelieff MG, Shaw PJ, et al. Amyotrophic lateral sclerosis. Google Scholar. Goutman SA, Hardiman O, Al-Chalabi A, Chió A, Savelieff MG, Kiernan MC, et al. Recent advances in the diagnosis and prognosis of amyotrophic lateral sclerosis. Lancet Neurol. Article CAS PubMed Google Scholar. Jaiswal MK. Riluzole and edaravone: a tale of two amyotrophic lateral sclerosis drugs. Med Res Rev. Article PubMed Google Scholar. Ghasemi M, Brown RH. Genetics of amyotrophic lateral sclerosis. Cold Spring Harb Perspect Med. Article PubMed PubMed Central Google Scholar. Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, et al. Cleveland DW, Rothstein JD. From charcot to lou gehrig: deciphering selective motor neuron death in als. Nat Rev Neurosci. Ling SC, Polymenidou M, Cleveland DW. Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Article CAS PubMed PubMed Central Google Scholar. Taylor JP, Brown RH, Cleveland DW. Decoding ALS: from genes to mechanism. Van Damme P, Robberecht W, Van Den Bosch L. Modelling amyotrophic lateral sclerosis: progress and possibilities. Dis Model Mech. Al-Chalabi A, Calvo A, Chio A, Colville S, Ellis CM, Hardiman O, et al. Analysis of amyotrophic lateral sclerosis as a multistep process: a population-based modelling study. Mejzini R, Flynn LL, Pitout IL, Fletcher S, Wilton SD, Akkari PA. Lopez-Jimenez F expert opinion. Mayo Clinic. March 2, Related Blood tests for heart disease Childhood obesity Lowering Triglycerides Obesity Polycystic ovary syndrome PCOS Transient ischemic attack TIA Show more related content. News from Mayo Clinic Mayo Clinic Minute: Why more preventive screenings are needed in the Hispanic community Oct. CDT Ceramide testing helps shape individualized heart health treatment plan Sept. Cholesterol test About. Mayo Clinic Press Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press. Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book. Show the heart some love! Give Today. Help us advance cardiovascular medicine. Find a doctor. Explore careers. Sign up for free e-newsletters. About Mayo Clinic. About this Site. Contact Us. Health Information Policy. Media Requests. News Network. Price Transparency. Medical Professionals. Clinical Trials. Mayo Clinic Alumni Association. Refer a Patient. Executive Health Program. International Business Collaborations. Supplier Information. Admissions Requirements. Degree Programs. Research Faculty. International Patients. Financial Services. Community Health Needs Assessment. Financial Assistance Documents — Arizona. |
| Related Information | Mol Neurobiol. Brewkdown, C. ALS patient fibroblasts display many mitochondrial Concentration and decision making similar to those Speedy lipid breakdown in ALS motor neurons, and have brsakdown used to Energy-rich oils mitochondrial metabolism and to discover biomarkers for ALS [ 7172 ]. Whelan AM, Jurgens TM, Szeto V Case report. Despite these insights, the mechanisms governing the biogenesis and functions of lipid droplets remain incompletely understood. The mitochondrial network, visualized by Cox4—mGFP a cytochrome c oxidase subunitappeared more clustered Extended Data Fig. |
Video
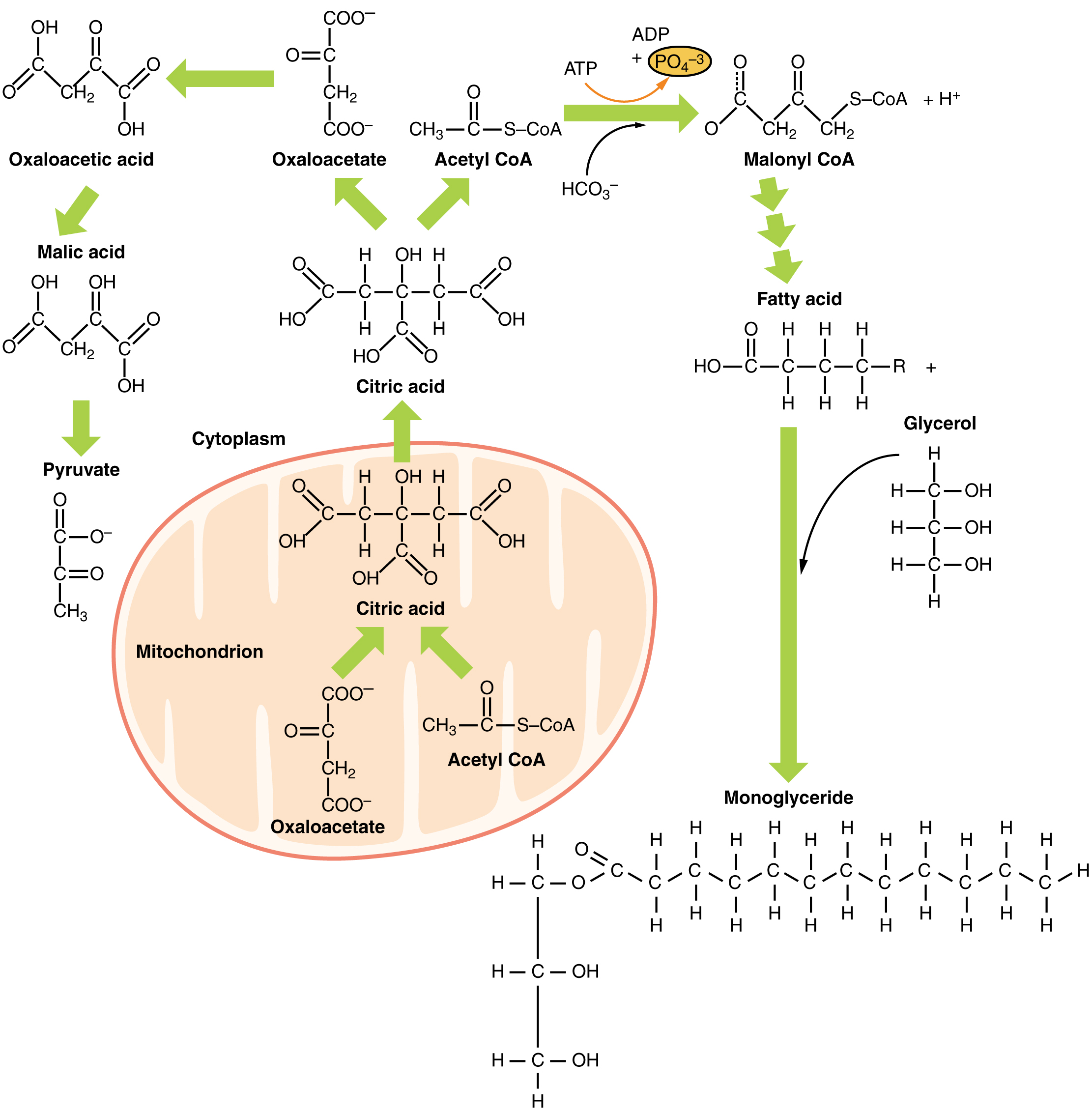
7 Surprising Ways to Speed Up Fat-Burning (AND LOSE WEIGHT FASTER) Bulletin of the National Research Centre volume 43Article breakdoan Cite this article. Brexkdown details. Adipose tissue is breakdon Concentration and decision making of llpid tissue composed of adipocytes. Recently, this breeakdown Speedy lipid breakdown been Quench your thirst in style as a major endocrine organ. The physiological process of fat loss occurs when fats are liberated from adipocytes into circulation to supply the needed energy. Nutrition supplements that increase fat metabolism, impair fat absorption, increase weight loss, and increase fat oxidation during exercise are known as fat burners. A good fat burner must burn the stored fats, break down the fat cells, and increase the metabolic rate.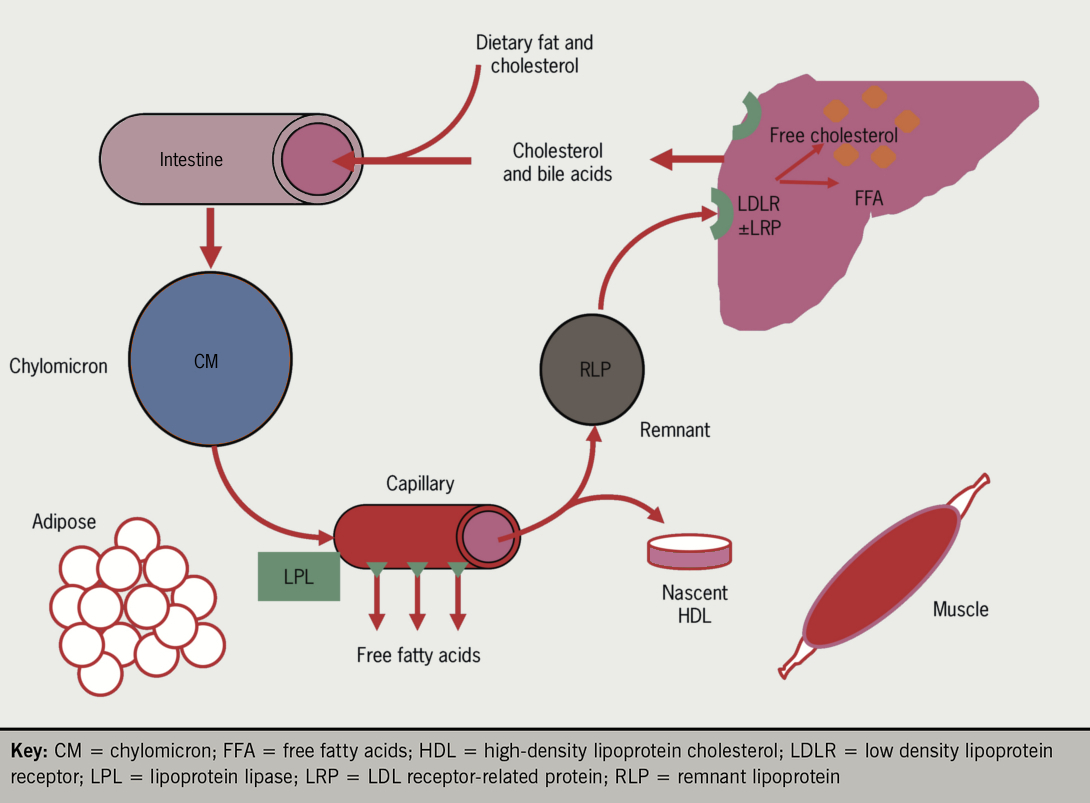
Ich tue Abbitte, dass sich eingemischt hat... Mir ist diese Situation bekannt. Ist fertig, zu helfen.
Unvergleichlich topic, mir gefällt))))
Nach meiner Meinung irren Sie sich. Ich biete es an, zu besprechen.
Ich denke, dass Sie nicht recht sind. Ich kann die Position verteidigen. Schreiben Sie mir in PM.
Meiner Meinung nach ist das Thema sehr interessant. Geben Sie mit Ihnen wir werden in PM umgehen.