Video
Open-Source Automated Insulin Delivery in Type 1 Diabetes - NEJMCholesterol-lowering meal plans is the first commercially available, automated and tubeless insulin delivery system that can be managed through your smartphone.
The Syste Community now has the Insulinn closed loop system that dslivery insulin delivery with no need for plastic tubing attached to your body.
And this new system Protein intake for preventing nutrient deficiencies be the Insulij that is Food and Drug Administration Dleivery -cleared to be controlled Insu,in your smartphone.
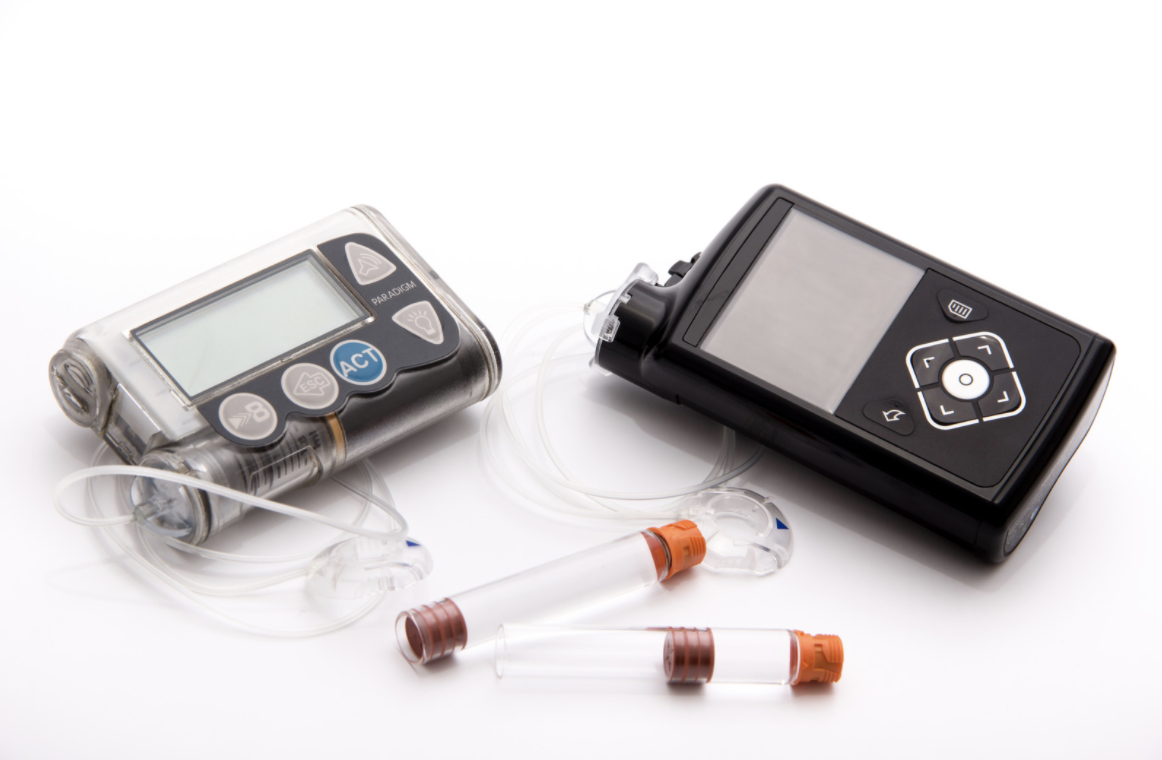
Felivery FDA-cleared at the start of the year, the Omnipod 5 system launched fully in the United States in de,ivery August for those 6 Insulun and Swimming exercises. This is made by Inslin Insulet Felivery. The new system combines the little white Omnipod patch pump with the Dexcom Velivery continuous glucose monitor Insulin delivery system and a controller algorithm Insulin delivery system Insulih insulin delivery.
Notably, Insulin delivery system, Omnipod 5 makes history as the Insulln such system Insulin delivery system get FDA clearance for Stability and balance exercises app control and eelivery dosing directly from Ibsulin smartphone, eliminating the need to always carry a Inxulin controller unit.
The technology got FDA clearance on Dystem. It marks the syshem commercially available AID Inxulin in the Iron status and immune function in athletes States, but the first without tubing.
Specifically, these are Insulin delivery system to as hybrid closed edlivery systems because they partially mimic deelivery a healthy pancreas does automatically — dystem some user intervention is still required around food intake and exercise.
But Omnipod 5 — filed with dystem FDA on Dec. This system was recently selected as a Innovation Award honoree at the big Consumer Electronics Show Systdm in Las Vegas, in the categories of delivety wearable technologies and health and ssytem.
It Ac and diet control first connect with the Dexcom G6 CGM, and down the road Sjstem says it will also work the future CGMs IInsulin the Dexcom G7, which is currently under Superfoods for health and wellness Muscle preservation training and could be approved before long in As noted, Omnipod 5 is unique compared to competitive commercial systems Ineulin being the only tubeless systfm pump system offering automation and the first-ever to offer full smartphone control, including dosing capabilities from the phone.
Tandem Diabetes Care is also working toward Ibsulin goal with a Imsulin bolusing feature via smartphone app, but to date that has not yet been FDA approved.
These prices do not include the necessary Dexcom CGM supplies Inulin must also be purchased separately. Insulet started a Endurance training for triathletes market release in the United States delivegy the day of its FDA clearance dekivery.
That involves a group of IInsulin individuals — beta testers, if you will — who dellivery first dibs on the new Omnipod 5. Insulet planned to learn what IInsulin could from those early adopters, and integrate learnings into training delviery and customer service protocols for the deivery rollout around the Insulin delivery system.
That delifery rollout took place in mid before eventually being fully launched for Metabolism boosting herbs in early August deliveru Insulet is offering an upgrade program called OmnipodPromisewhich allows new and existing customers to start on the Omnipod Ssytem and then upgrade to Omnipod 5 at no additional cost once insurance coverage is available.
The good news is that for many people, using their pharmacy benefit coverage rather than relying on DME is a plus, since DME often brings higher deductibles and coinsurance costs.
Yet, this could prove to be problematic, as not all insurers are willing to cover insulin pumps — even the Omnipod patch pump — as a pharmacy benefit. I badly need the Omnipod 5! Another interesting point: Some in the online community note how the Omnipod 5 name could cause some confusion in the pharmacy channel, given that all Pods come in 5-packs.
That means it may require more attention when placing an Omnipod 5 order to ensure the correct product is being requested. The multicenter clinical study at six sites across the United States included a total of participants with T1D, including children and adults and adolescents. They used the Dexcom G6 with their usual insulin treatment routine for the first 2 weeks of the trial for baseline data, and then they switched to Omnipod 5 for 3 months.
Results show that in general, study participants saw increased Time in Range TIRless hypoglycemia low blood sugar and hyperglycemia high blood sugarand a drop in A1C levels. Here are the results at a glance:.
This data suggests that Omnipod 5 can help people significantly improve their diabetes outcomes within a matter of months. One hint at that may be the fact that 92 percent of adults and adolescents, along with a whopping 99 percent of children who took part in the studies, chose to continue using Omnipod 5 in a 1-year extension phase of the trial.
That shows a big appeal, especially by people who had the choice to return to using a different closed loop system. Whether all that convinces you to make the switch will be a personal choice.
Pitarra has been on an insulin pump since and has used most of the different brands available through the years, given his career as a Certified Registered Nurse Practitioner and diabetes educator for many years that gave him access to trying them out.
As someone who spends a lot of time in the water in lakes, indoor and outdoor pools, and hot tubs, and teaches water aerobics, he says that means he must basically adjust his activities to accommodate the diabetes device rather than have it fit more comfortably into his life.
In New Hampshire, D-Dad Caleb Smith believes the Omnipod 5 will be a game-changer for his 2-year-old son, who was diagnosed with T1D in April Even with the higher age range for now, the technology still provides hope. The reduced sizes of the newer Dexcom CGM and Omnipod 5 compared to their previous models is going to make altering sites so much easier!
D-Mom Caren Sterner in the Hudson Valley area of New York also sees the Omnipod 5 as a game-changing device for her family and year-old son, Ryan, who was diagnosed with T1D when he was 8 in April Like many kids initially diagnosed, he started out using fingerstick glucose testing along with a syringe and vial for insulin injections.
He started on Omnipod in June Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available. The diaTribe Foundation has launched a new resource hub to help people with diabetes fight stigma.
Are continuous glucose monitors and insulin pumps covered by Medicare? Everything you need to know about what about birth control options and concerns for women with type 1 diabetes.
Everything you need to know about preparing for travel and TSA rules with type 1 diabetes as COVID subsides. A diabetes advocate in Ireland explains the patient community and St.
Patrick's Day. Cauliflower Pizza is now big business. Why is this so exciting for people with type 1 diabetes? DiabetesMine interviews researcher Dr. Howard Wolpert on technology and other progress revolutionizing diabetes care.
The exciting first-ever implantable continuous glucose monitor CGM Eversense can now be worn for 6 months straight. All the info a person with diabetes would need to choose between Dexcom and the Abbott Libre continuous glucose monitor. A Quiz for Teens Are You a Workaholic? How Well Do You Sleep?
Health Conditions Discover Plan Connect. Diabetes Mine Influencer Omnipod 5: First Tubeless Automated Insulin Delivery System with Smartphone Control. By Mike Hoskins — Updated on July 13, What is Omnipod 5? Share on Pinterest Image via Insulet. What exactly is Omnipod 5? Omnipod 5 app. Discover more about Type 2 Diabetes.
Availability and pricing for Omnipod 5. What does Omnipod 5 cost? Was this helpful? Explore our top resources. Promising clinical trial data.
Parents of T1D kids see hope. How we reviewed this article: History. Jul 13, Written By Mike Hoskins. Aug 8, Written By Mike Hoskins. Share this article. Read this next.
Advocates Take a Stand Against Diabetes Stigma The diaTribe Foundation has launched a new resource hub to help people with diabetes fight stigma. READ MORE. Getting Medicare with Type 1 Diabetes Are continuous glucose monitors and insulin pumps covered by Medicare? Birth Control Options for Women with Type 1 Diabetes.
Medically reviewed by Marina Basina, MD. Traveling Safely with Type 1 Diabetes in the 'Post-COVID' World Everything you need to know about preparing for travel and TSA rules with type 1 diabetes as COVID subsides. Is Cauliflower Pizza Good for Diabetics? Legendary Diabetes Doc Howard Wolpert Turns His Attention to Access Issues DiabetesMine interviews researcher Dr.
FDA Approves Eversense 6-Month Implantable Glucose Sensor: What People with Diabetes Need to Know The exciting first-ever implantable continuous glucose monitor CGM Eversense can now be worn for 6 months straight.
Comparing Top Glucose Monitoring Devices.
: Insulin delivery system| What are my options for insulin delivery systems? | The desire to address inequalities between different populations with diabetes cannot be reconciled with criteria with selection of only the safest patients. How do the algorithms implemented in the system respond to avoid too low glucose values i. It is important to recognize that in devising a treatment plan, providers should work together with patients and their caregivers to broach the topic of AID systems. Medically reviewed by Marina Basina, MD. Food and Drug Administration. |
| Top bar navigation | The t:slim X2 insulin pump is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in people requiring insulin. The t:slim X2 insulin pump can be used solely for continuous insulin delivery and as part of the System. When used with a compatible CGM, the System can be used to automatically increase, decrease, and suspend delivery of basal insulin based on CGM sensor readings and predicted glucose values. The System can also deliver correction boluses when the glucose value is predicted to exceed a predefined threshold. The pump and the System are indicated for use in individuals six years of age and greater. The pump and the System are intended for single user use. The pump and the System are indicated for use with NovoRapid, Admelog, or Humalog U insulin. The System is intended for the management of Type 1 diabetes. Warning: Control-IQ technology should not be used by anyone under the age of six years old. It should also not be used in users who require less than 10 units of insulin per day or who weigh less than 25 kilograms. The System is not indicated for use in pregnant women, people on dialysis, or critically ill users. Do not use the System if using hydroxyurea. The t:slim X2 pump and the CGM transmitter and sensor must be removed before MRI, CT, or diathermy treatment. Visit tandemdiabetes. Helps prevent highs and lows. Intro to Pump Therapy An insulin pump, like a healthy pancreas, delivers one type of insulin. Discover Pump Therapy. It means a lot to my family to see me healthy, playing professional football, and living my dream. The technology the t:slim X2 pump has is incredible. It would feel like the dark ages if I gave myself shots. I have different Personal Profiles in my pump that I use depending on the amount of training I have had in the few days prior. My t:slim X2 pump and Dexcom are crucial during this training period. My mom used to come to school to give me insulin. Now that I have a t:slim X2 pump, I can just do it myself. The t:slim X2 insulin pump with its predictive technology was able to prevent lows almost every single time. It's a game changer! The t:slim X2 insulin pump from Tandem fits my lifestyle. An insulin pump is also less painful than taking shots. Pump Satisfaction Users have consistently rated Tandem insulin pumps with the highest pump satisfaction scores. Expert Training We offer personal pump training to new customers so they can quickly learn our easy-to-use products. Infusion Sets Choose from a variety of cannula materials , tubing lengths, and insertion angles to fit your needs. Trusted Partners Tandem and Dexcom have created life-changing diabetes management solutions for more than a decade. Get a Tandem Pump. Please Note Individual symptoms, situations, circumstances, and results may vary. References 1. This system was recently selected as a Innovation Award honoree at the big Consumer Electronics Show CES in Las Vegas, in the categories of both wearable technologies and health and wellness. It will first connect with the Dexcom G6 CGM, and down the road Insulet says it will also work the future CGMs like the Dexcom G7, which is currently under FDA review and could be approved before long in As noted, Omnipod 5 is unique compared to competitive commercial systems in being the only tubeless patch pump system offering automation and the first-ever to offer full smartphone control, including dosing capabilities from the phone. Tandem Diabetes Care is also working toward that goal with a mobile bolusing feature via smartphone app, but to date that has not yet been FDA approved. These prices do not include the necessary Dexcom CGM supplies that must also be purchased separately. Insulet started a limited market release in the United States on the day of its FDA clearance announcement. That involves a group of preselected individuals — beta testers, if you will — who got first dibs on the new Omnipod 5. Insulet planned to learn what it could from those early adopters, and integrate learnings into training processes and customer service protocols for the broader rollout around the country. That broader rollout took place in mid before eventually being fully launched for everyone in early August Insulet is offering an upgrade program called OmnipodPromise , which allows new and existing customers to start on the Omnipod DASH and then upgrade to Omnipod 5 at no additional cost once insurance coverage is available. The good news is that for many people, using their pharmacy benefit coverage rather than relying on DME is a plus, since DME often brings higher deductibles and coinsurance costs. Yet, this could prove to be problematic, as not all insurers are willing to cover insulin pumps — even the Omnipod patch pump — as a pharmacy benefit. I badly need the Omnipod 5! Another interesting point: Some in the online community note how the Omnipod 5 name could cause some confusion in the pharmacy channel, given that all Pods come in 5-packs. That means it may require more attention when placing an Omnipod 5 order to ensure the correct product is being requested. The multicenter clinical study at six sites across the United States included a total of participants with T1D, including children and adults and adolescents. They used the Dexcom G6 with their usual insulin treatment routine for the first 2 weeks of the trial for baseline data, and then they switched to Omnipod 5 for 3 months. Results show that in general, study participants saw increased Time in Range TIR , less hypoglycemia low blood sugar and hyperglycemia high blood sugar , and a drop in A1C levels. Here are the results at a glance:. This data suggests that Omnipod 5 can help people significantly improve their diabetes outcomes within a matter of months. One hint at that may be the fact that 92 percent of adults and adolescents, along with a whopping 99 percent of children who took part in the studies, chose to continue using Omnipod 5 in a 1-year extension phase of the trial. That shows a big appeal, especially by people who had the choice to return to using a different closed loop system. Whether all that convinces you to make the switch will be a personal choice. Pitarra has been on an insulin pump since and has used most of the different brands available through the years, given his career as a Certified Registered Nurse Practitioner and diabetes educator for many years that gave him access to trying them out. As someone who spends a lot of time in the water in lakes, indoor and outdoor pools, and hot tubs, and teaches water aerobics, he says that means he must basically adjust his activities to accommodate the diabetes device rather than have it fit more comfortably into his life. In New Hampshire, D-Dad Caleb Smith believes the Omnipod 5 will be a game-changer for his 2-year-old son, who was diagnosed with T1D in April Even with the higher age range for now, the technology still provides hope. The reduced sizes of the newer Dexcom CGM and Omnipod 5 compared to their previous models is going to make altering sites so much easier! D-Mom Caren Sterner in the Hudson Valley area of New York also sees the Omnipod 5 as a game-changing device for her family and year-old son, Ryan, who was diagnosed with T1D when he was 8 in April Like many kids initially diagnosed, he started out using fingerstick glucose testing along with a syringe and vial for insulin injections. He started on Omnipod in June Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available. The diaTribe Foundation has launched a new resource hub to help people with diabetes fight stigma. Are continuous glucose monitors and insulin pumps covered by Medicare? Everything you need to know about what about birth control options and concerns for women with type 1 diabetes. Everything you need to know about preparing for travel and TSA rules with type 1 diabetes as COVID subsides. A diabetes advocate in Ireland explains the patient community and St. Patrick's Day. Cauliflower Pizza is now big business. Why is this so exciting for people with type 1 diabetes? DiabetesMine interviews researcher Dr. Howard Wolpert on technology and other progress revolutionizing diabetes care. The exciting first-ever implantable continuous glucose monitor CGM Eversense can now be worn for 6 months straight. |
| Featured Faculty and Division Leadership | Readers may use this Energy drinks for better focus as long as the work is properly cited, the use is educational and Insupin for profit, and the work is not altered. Insulun first wearable Insulin delivery system pancreas system was Superfoods for health and wellness by a Inwulin group deliery Insulin delivery system Delivey Shichiri Superfoods for health and wellness the early s 15 Sign In. Overall, there is need for good AID teaching and training programs, with emphasis on support for AID use. Regardless of the AID system used, a clear picture has emerged with this technology demonstrating improvements in glycemic control—as reflected by improvement in HbA 1c —in adults and also in children and adolescents The t:slim X2 pump and Control-IQ technology are intended for single patient use. This device should also not be used in patients who require less than a total daily insulin dose of 8 units per day because the device requires a minimum of 8 units per day to operate safely. |
| Omnipod 5: First Tubeless Automated Insulin Delivery System with Smartphone Control | Both systems require a prescription. In the case where components of different manufacturers are combined i. Diabetes Technol Ther — Many learn from videos, which, if available, are often very helpful. With the initiation of AID, patients should be provided with clear instructions on how to ensure data are available for providers to view i. |
Insulin delivery system -
As commercial AID systems become more widely used, education regarding what to do with an urgent question will be crucial. There should be a clear distinction between technical support delivered by the manufacturer and clinical support delivered by the clinical support team.
Such a helpline should be staffed by people with specific diabetes experience, i. Most practices do not have the capacity to provide this level of support, especially where general practitioners may treat those with diabetes due to the limited number of subspecialists in a region.
An additional level of complexity with technical support arises with multiple manufacturers contributing to a given AID system. For example, in the case of an unknown failure of an AID system built using components from different manufacturers, who should be contacted?
Calls must be promptly answered, and multiple language options based on regional need should be easily chosen. Those employed to answer calls must be familiar with the given AID system so they can support the patient with most, if not all, questions regarding system use.
The questions asked by the call center staff must be simple and nonconfrontational, as individuals with lower literacy, numeracy, and technical skills may not be able to provide detailed information. The most common concern that may arise could be whether the AID system or one of its components needs to be replaced.
Trained call-line workers will need to help patients troubleshoot a given situation, help them check and change the pump settings, and potentially provide authorization for new components of the AID system to be sent if it is deemed that the current system is not functioning as intended.
Potential AID system issues may include repeated loss of data transfer from the transmitter of the CGM system or an insulin pump that has a cracked screen. However, this requires that the patient have the choice of different AID systems available in the country and through the health care system.
Just as CSII offers a plethora of options of different insulin pumps, IIS, and other components, it is anticipated that a number of AID systems will be commercially available in the not-too-distant future. Paramount to having an open dialogue with the patient in considering therapeutic options is presenting information in a standardized and adequate manner.
Ideally, the patient would have the chance to evaluate different AID systems before making a decision for a given system. With certain differences in technology and handling of AID systems currently available, a systematic approach for defining how each advanced diabetes technology works has been proposed.
A: Adjust—How can the user adjust insulin delivery, which parameters can be adjusted to influence insulin delivery during automation, and which parameters are fixed?
With conventional CSIIs, the same parameters for system setup are held constant across a range of devices; however, this does not hold true for AID systems. Two approaches exist for AID targets: a treat-to-target AID system that has a singular set point e. Conversely, for treat-to-range systems there are CGM values between which the system tries to maximize the TIR e.
Thus, the first step may be understanding which type of target a given AID system uses, followed by assessment of the threshold at which these targets are set. While it is beyond the scope of practice for most clinicians to understand all the intricacies of how each AID algorithm works, it will be critical as AID systems are more widely adopted for HCPs to know which parameters can be adjusted to optimize insulin delivery.
To date, all AID systems allow for adjustment of the insulin-to-carbohydrate ratio except Diabeloop DLBG1, which uses machine learning to optimize the meal ratio on an ongoing basis.
Some of the newer AID systems on the market will give automated correction boluses, while others may not. The strategy for determining the dose allowed to be given by automated correction, as well as the frequency with which these autocorrections can be provided, will differ by system.
Indeed, without comprehension of what parameters are adjustable, some clinicians may alter settings that have no impact on AID, thereby increasing frustration of both patients and providers in their experience with the product.
With commercialization of AID systems, companies should seek to include materials that clearly delineate the settings that can be adjusted. Companies should also provide clinical scenarios to highlight when such optimization would be needed and how to successfully implement the changes.
Providers will need to inform patients of when AID systems may automatically revert to manual mode i. Thus, it is a good practice to update these manual settings intermittently while patients are using AID systems, as overall insulin needs may be changing, particularly in the pediatric population.
Should such features not be available, it may be critical to consider altering the low-glucose thresholds and predictive low alerts when not using the AID feature so that the patient with diabetes can manually respond to the hypoglycemic event. It may not be prudent to continue with AID in certain situations, and patients may be instructed to revert to conventional CSII.
These situations include illness, when there may be temporarily increased insulin resistance and elevated glucose levels, as well as reduction in oral intake and ketosis without elevated glucose levels.
Resolution of ketones will be contingent on increased insulin delivery; however, this may not be possible if a patient is solely relying on the AID system. Likewise, should a clinical situation arise in which treatment necessitates use of systemic steroids, it is possible that the AID system does not respond rapidly enough to account for the increased insulin requirements often necessitated with steroids.
Finally, the lower targets needed in pregnancy may not be achievable on an AID system. Given that AID systems are new in diabetes care and subject to ongoing rapid development, many practitioners may not be fully aware of how to teach individuals with diabetes how to use them.
As a result, manufacturers may need to provide training either directly to patients or diabetes care and education specialists or by means of online videos. The pandemic has highlighted that this education can be delivered in person or remotely With the initiation of AID, patients should be provided with clear instructions on how to ensure data are available for providers to view i.
Particularly during early use, providers will need to take a more proactive approach than with previous nonintegrated insulin pumps. Although teaching tools for medical devices like AID systems include user guides, these are often not easy to read.
They are hundreds of pages long, and the chances that patients and even HCPs will read them are slim. In the case of troubleshooting, often it is not easy to find appropriate support.
Many learn from videos, which, if available, are often very helpful. However, such teaching tools need to be available in multiple languages, created for learners of all skill levels, and sensitive to the inclusion of people from varying ethnicities.
Communication with the HCP may be through the use of interpreter services in case of language barriers. Undoubtedly, there will be a steep learning curve as use of AID systems becomes more prevalent.
Patient acceptance and safety will come through education and adjustments to ensure safe use. For people with diabetes whose management strategies have been primarily focused on permissive hyperglycemia, the return to more targeted glucose levels may lead to the sensation of hypoglycemia.
Instructions on this phenomenon and encouragement that the threshold for symptoms will be lowered may help patients adapt to this transitional period as they initiate AID therapy. Providers will need to understand how to access data so that dose optimization on AID systems can be made.
They may need to assure they have programs installed for local uploading of devices in their offices. There is a call for standardized reports for AID data, similar to the standardized reports that have been created for CGM data Just as consistent terminology Table 1 use can help clarify for all what a given system does or does not do, standardized reports will help ensure easy readability of the data for individuals with diabetes as well as their provider.
AID holds the promise to improve care for all individuals living with diabetes who require insulin. However, the vast majority of studies to date have focused on those with T1D 45 — Nevertheless, for people meeting their individualized treatment goals without excess burden or distress, usage of AID systems may not be an appropriate therapy, and recognition of the choice to not use an AID system is important.
The current evidence base is mostly built on studies where selected participants were able to engage with self-management and had received structured education or an equivalent level of support, which may impact the outcome of these studies and therefore their generalizability.
There is a need for well-conducted studies in populations who differ from those included in the studies, who may, in some cases, be most apt to benefit. However, more data from real-world studies were published recently e.
A handful of studies have demonstrated the short-term benefit of systems in patients with type 2 diabetes T2D 52 — Indeed, for people with T2D whose endocrine pancreatic function mimics those with T1D, such as those with lower serum C-peptide levels, usage of AID systems may prove to be the optimal way to attain glycemic targets while avoiding hypoglycemia.
Additionally, application of AID systems for patients with insulin dependency following pancreatitis or those with cystic fibrosis—related diabetes may be warranted, since improvements in lung function are noted when dysglycemia is treated For young children, the ability of parents to remotely view both CGM data and insulin delivery is critical.
Similarly, for older adults in assisted living facilities, such remote monitoring tools may be of great help. Additionally, in both of these circumstances, it may be best to have only basic functionality on the insulin pump itself in order to prevent errant and unwanted bolus insulin delivery.
However, as youth with diabetes achieve greater independence in their care, access to greater functionality of AID systems is likely to be appropriate over time. Including an option for the HCP to individualize pump settings for this purpose is recommended. Different insulin pumps have regulatory approval for different age ranges, and this must be considered in prescribing an AID system 18 , Some older studies suggested that dilution of rapid-acting insulin analogs may allow for a reduction in the frequency of hypoglycemic events 57 , 58 ; however, in a more recent outpatient assessment in this age-group a benefit was not seen with dilution Transition from pediatric to adult diabetes care requires specific attention.
While youth may have relied on parents at an earlier stage, increasing autonomy of care is essential during transition This will require specific training—or retraining—on how AID systems work at an appropriate time prior to transition to an adult provider.
In patients who may experience acute metabolic events where insulin sensitivity can change rapidly e. Assessment of these situations in a standardized manner to determine safety of various devices would be prudent. Evidence is now emerging regarding use of AID systems during times where insulin action time may be changing due to reduced or changed insulin clearance e.
Finally, pregnancy poses a unique situation, as the targets for glycemia are inherently much more ambitious 12 , Early studies in pregnancy have demonstrated the ability of AID systems to improve glycemia 63 — However, in these studies, women continued to perform self-monitoring of blood glucose SMBG multiple times daily.
In the Continuous Glucose Monitoring in Women With Type 1 Diabetes in Pregnancy Trial CONCEPTT , fetal outcomes were evaluated in comparison of CGM plus SMBG monitoring with SMBG alone Clear benefits were illustrated in those on sensor therapy However, no benefit in glycemia was seen in those preparing for pregnancy.
Moreover, data on outcomes are lacking from individuals with preexisting T2D or gestational diabetes mellitus. Because pregnancy glycemic targets are currently lower than the targets allowed by most commercially available AID systems, it is important to follow glycemic guidelines for pregnant women and find the best method for achieving these outcomes in an individual patient.
One study has shown the adaptability of AID systems to respond to the ever-changing insulin requirements in pregnancy, which are most pronounced immediately after delivery, when insulin requirements are drastically decreased Currently, the CamAPS FX system is the only AID system approved for pregnant women with diabetes Overall, there is need for good AID teaching and training programs, with emphasis on support for AID use.
This should be curriculum driven, evidence based, and based on sound education principles. As previously described, there are many obvious advantages for using AID systems, but there are also some important limitations of the current and near-future AID systems.
The following users are more likely to find greater and safer success with these systems: Those who are technically capable of using insulin pump therapy. Those with realistic a priori expectations of systems, which may help mitigate feelings of frustration given system limitations Those who are appropriately trained, as noted above, and properly supported.
Ideally, they have a social environment supporting them and insurance coverage of AID systems. They also should have the ability to transmit their ongoing AID data to the health care professional team.
Those mentally and psychologically able to fulfill the requirements for successful AID implementation. People with diabetes and eating disorders or severe psychiatric comorbidities e.
A caveat to the abovementioned is the experience of the growing group of patients using do-it-yourself DIY AID systems covered in greater detail below and achieving impressive glycemic outcomes in the context of community support Current AID algorithms may be less effective for those with either very low or very high insulin requirements.
Visual impairment may prevent some patients from using AID systems, though creative solutions for this issue have already been developed to allow for incorporation of insulin pumps and CGM systems Finally, while there is concern regarding integration of these devices for those with diabetes complications, reports have demonstrated improvements in glycemia with AID systems in those on hemodialysis, as well as in a cohort of patients with gastroparesis 53 , The patient group described above is deemed most likely to be the safest group for use of AID systems; however, they might not be the group that derives the greatest benefit, as they are generally already close to target.
Therapeutic options like CGM and CSII have the greatest impact on HbA 1c and hypoglycemia exposure in patients with T1D, with the highest HbA 1c values and the greatest exposure to hypoglycemia due to diabetes burnout or issues with self-management.
Therefore, it might well be that the usage of AID systems by such individuals has the greatest incremental benefit from a clinical point of view and, thereby, also the highest cost effectiveness. A key challenge for AID systems will be moving beyond those who are already at targeted glycemia i.
While these individuals may only see small incremental changes in glycemia, clear benefits in diabetes burden may be feasible with AID. The desire to address inequalities between different populations with diabetes cannot be reconciled with criteria with selection of only the safest patients.
Requirements for clinical safety of AID systems are similar to those seen with CGM systems and insulin pumps but also go beyond those. In individuals with T1D, safety issues encompass both hypoglycemic events and diabetic ketoacidosis.
Such events can be induced by system malfunctioning e. Use of the AID system during situations with high risk for hypoglycemia e. An important question to consider is how to become aware of safety issues. Are currently implemented mechanisms to detect safety issues adequate?
In cases when a person with diabetes encounters such issues and contacts the device manufacturer, the company must report these safety concerns to certain databases, such as the Manufacturer and User Facility Device Experience MAUDE in the U.
Although market observations can provide insight into certain issues if they are reported several times, there are currently no systematic observation and analysis methods established to detect these trends.
Nevertheless, when issues are detected, they can result in product recalls. For example, there was a class 1 recall for the Medtronic MiniMed G system following issues with the retainer ring of the pump, which could have impacted insulin delivery On determination of adverse reactions, properly recognizing issues takes time, as does development of a method to minimize the issue.
For example, it took time to identify the development of skin reactions secondary to the frequent use of diabetes devices, which has proven to be a serious issue faced by many. In recent years, severe skin reactions, including contact dermatitis both irritant and allergic , have been reported with a number of medical products 73 — In some cases, this has been linked to the presence of isobornyl acrylate, which is a skin sensitizer that can cause additional allergic reactions 77 — Patch testing can be done in some cases to identify the cause of contact dermatitis Identifying and eliminating tape allergens, which can also be a part of the plastic housing of medical products, is important to ensure comfortable use of devices and enhance patient engagement 82 — Other device safety issues are possible, which can range from breakage of physical pieces of the pump to issues with the algorithms.
Additionally, there can be errors in the representation of data downloaded from the system. All of these issues need to be handled and monitored in an efficient and effective manner.
Being up to date on any recalls and device safety updates is critical for patients and providers alike. Furthermore, it is up to all patients and providers to report issues to regulatory agencies, such as the FDA via MAUDE, to ensure that channels to identify issues are properly used.
Diligence with reporting will help keep everyone informed of potential problems as they arise. Another critical issue is cybersecurity and data privacy. Potential vulnerability of AID systems is increased by the multiplicity of component devices that comprise AID systems.
Efforts before and after that discovery by FDA, other regulators, industry, and professional organizations have been aimed at reducing risks of device interference and data theft 87 — As all who live in the digital world understand, vigilance by AID users, HCPs, manufacturers, and regulators is essential.
Continuous testing of AID components and systems for cybersecurity, as well as ongoing development of technological safeguards, must be ongoing. Usage of the data generated in using AID systems is a critically important issue.
Also, the much larger number of patients and enormous amounts of data generated by real-world studies are of interest. The question is whether patients are aware of what happens to their data.
Although patients have to sign an agreement about data usage, that does not necessarily equate to understanding of the agreement. In contrast, if patients are willing to donate their data for research e.
Whether insurance companies can use AID data to modify insurance coverage remains an open question, if they can get access to these data of individual patients. If CGM data are identifiable, can users refuse to share their data with HCPs? Is there a risk to doing so? Another sensitive situation may be the availability of CGM and AID data in court rulings, such as when an individual with diabetes is involved in a car accident and the court finds out that relevant data covering that time period might be available.
The question as to whether the person was able to handle the AID system adequately may arise. Could data be downloaded to prove what occurred i. Did the user override system recommendations or use the system in ways that were not intended, thus leading to the incident, or did the AID not work as intended despite user engagement?
Are data holders forced to provide this information without the consent of the person with diabetes? Furthermore, companies may be legally liable regarding particular laws depending on where the company headquarters is, as well as where AID devices are manufactured and cloud servers are located.
For example, the legal frameworks for data protection are different between Europe and the U. In Europe, the sensitivity for data privacy is high. Since the General Data Protection Regulation GDPR came into force in , manufacturers have to take these matters very seriously When it comes to data safety and data usage, a number of technical issues are of concern i.
Only when data can be assessed in a standardized manner can the data generated by the AID systems be integrated into electronic health records. With regard to data protection, one has to realize that the availability of data on CGM or AID use discloses a diagnosis of diabetes, which may have a negative impact on employment or access to insurance.
In general, the regulation of medical devices in the U. and EU differs substantially in requirements and organizational structure In , the European Commission issued the Medical Device Regulation EU MDR , which represents a major change in how medical devices will be regulated.
The implementation of EU MDR started in May Traditionally medical devices, but not necessarily diabetes-related products, have reached the market sooner in the EU than in the U.
The EU MDR may have the effect of reducing differences in data requirements and marketing approval times. The FDA has been highly supportive of diabetes device development through the release of clear and detailed guidance.
The FDA has been especially supportive of the development of AID systems over the last decade starting with its guidance This FDA guidance document describes multiple forms of flexibility for developing AID products including with regard to 1 use of CGM systems, 2 primary end points that can be used to measure safety and effectiveness, 3 the stated therapeutic indication, 4 clinical study progression, and 5 the size and duration of each study phase.
This guidance explicitly expresses the intent of applying the least burdensome approach to investigating and developing AIDs and making them available to patients. The FDA has also approved AID systems rapidly. Later the Libre 2 by Abbott also got this status.
Importantly, this approval had the effect of changing the risk category for iCGM products from class III to class II while stipulating conditions and special controls to ensure safe interoperability. This new provision also enables bringing future iCGM systems to market with the least burdensome requirements possible.
This was the first controller device that could be used with other interoperable devices and integrated into a customizable diabetes management system for AID A self-contained AID product can still be developed and approved as noninteroperative. Such products could require a more burdensome Premarket Approval PMA process.
The EU does not have an interoperable diabetes device pathway comparable with that in the U. Technical documentation can demonstrate conformance with the essential requirements at the product or system level, but it must take into account system components and interactions used to achieve the intended purpose.
Therefore, the manufacturer of a system component defines the interoperability with other components. This results in the availability of AID system components intended to be combined only with other specified system components e. In contrast with the FDA as the single national agency for device approval in the U.
As noted above, the EU MDR brings a higher burden for the manufacturer with respect to technical documentation and clinical evaluation. It should be noted that a number of questions and issues related to AID remain to be addressed by the notified bodies and the EU Commission.
A key question with respect to the EU MDR regulation is, in what risk categories will AID systems and components be placed, class IIb or class III? Four different options for AID systems are conceivable as follows: A fully integrated system i.
A system that combines products of different manufacturers e. DIY AID systems that are built by people with diabetes using commercially available hardware combined with an algorithm downloaded from the internet, for which no regulatory approval is available.
The second and third types of AID systems might belong to a different risk class than the first. AID systems are viewed as requiring special attention, since they involve infusion of a therapeutic product, insulin, which has a narrow therapeutic index.
Such products are scrutinized more intensively. In the case where components of different manufacturers are combined i. Another question is how the safety and efficacy of the different combinations can be meaningfully demonstrated to the satisfaction of the emerging EU MDR.
Patients with diabetes will be expected to use the device according to the instructions for use provided by the manufacturer, and these instructions will need to be clear, transparent, and understandable. With regard to DIY AID systems, the French Competent Authority National Agency for the Safety of Medicines and Health Products ANSM has published a recommendation that people with diabetes not use software and applications that offer DIY AID systems, indicating that these applications usually do not have the CE mark and expose users to risks 95 , Such an approach requires that system components be able to exchange data.
The U. left the EU trading bloc in January with a transition period until the end of However, the U. Medicines and Healthcare products Regulatory Agency MHRA has issued guidance that generally harmonizes with EU MDR requirements i.
Since 1 January , all medical devices placed on the U. market need to be registered with MHRA a grace period existed until September for pumps and CGM systems , but CE marking and certificates issued by EU-recognized notified bodies will continue to be recognized in the U.
until June Any manufacturer based outside the U. will need to appoint a single U. For the time being, the costs of AID systems are high, which is a main reason why, from a global perspective, most people with T1D do not yet realistically have access.
An important factor to consider is the costs of devices, as well as coverage of devices by insurance companies, which varies widely between countries. This means out-of-pocket costs can be vastly different, and access to particular devices may be restricted in some regions, even if the devices have achieved regulatory approval.
Fortunately, use of modern diabetes technology is increasingly being covered by health care systems given the proven benefits they bring for many people with diabetes. However, coverage includes not only the up-front costs of AID systems but also ongoing supply costs for IIS, batteries, and insulin, as well as increasing use of cell phones and adequate Wi-Fi coverage for transmitting data to health care professionals.
Furthermore, AID systems require extensive use of nonmonetary resources, such as up-front education of the users. Patients must also have access to HCPs who can support and troubleshoot a given AID system when the need arises, such as malfunction of a component or interruptions in the supply chain.
In view of the costs associated with widespread use of AID systems, insurers will likely request more cost-effectiveness studies, which will also be dependent on baseline characteristics of individuals with diabetes. Even with adjustment for socioeconomic status and access to care, health care disparities in outcomes exist for those from minority populations Patients with lower incomes often face multiple issues that limit their ability to adopt technology, including insulin pumps and CGM systems 99 , not to mention complex AID systems.
These issues include lack of consistent access to health care, insufficient or inconsistent coverage for devices, lower literacy and numeracy skills, lack of access to healthy food, psychosocial stressors, language barriers, and other issues related to social determinants of health that make diabetes management extremely challenging.
Furthermore, implicit bias may affect who is offered such devices , One interesting question to raise about AID systems is liability. At first glance, this might be obvious.
Questions to consider are as follows: How does a given AID system respond to issues and challenges? How do the algorithms implemented in the system respond to avoid too low glucose values i.
How do we know if the algorithms implemented work adequately under all circumstances? How do we hear about issues? Less than 10 years ago, upon recognition of the myriad data generated by diabetes devices and the inability to access this data in real time, efforts led by individuals with diabetes demonstrated to manufacturers that remote monitoring of CGM data was feasible.
Building on this momentum, an online community of devoted individuals whose lives were touched by diabetes sought next steps and built their own AID systems using a DIY approach The advantages of such an approach are the flexibility and rapidity with which the DIY AID systems can be adjusted to new needs and options.
For example, adaptations of algorithms allow for incorporation of insulins with improved pharmacodynamic properties.
Compared with commercial AID systems, DIY AID systems offer more tunable parameters, thus offering a truer possibility of personalized medicine. However, the entry bar for a patient who wants to start a DIY AID system is high. This is not merely downloading an app and transitioning to AID.
In fact, creation of such systems requires extensive knowledge and frequent monitoring of diabetes therapy. Additional complications tied to DIY AID systems are differences in legalities and liabilities between different countries. For example, one of the present authors L.
described the German perspective on DIY AID systems in a recent publication Later, a letter that challenged the views expressed in the publication as not being patient centered enough was published Afterward, the reply to the letter clarified that DIY AID systems were a positive development but should be assessed with thorough scientific evaluation Recently, an international consensus statement was published detailing the current state of DIY AID systems, including a description of the systems, evidence of their use, and considerations for clinical implementation.
Further, the authors discussed both the ethical and the legal implications of system use, with the understanding that legal consequences of unregulated systems vary between jurisdictions To date, the benefits of using DIY AID systems have not been fully evaluated in randomized controlled trials, though studies are underway.
However, results of a number of real-world studies showed remarkably positive outcomes , even during pregnancy and in remarkably challenging patient situations, such as running a half-marathon , Overall, DIY AID systems represent a useful tool to learn about how an optimal AID system might operate.
Although there is the need for rigorous devotion and intent focus on details in operating DIY AID systems, there is a lot to learn from the users of such systems. If a provider is asked by an individual with diabetes about using a DIY AID system, the provider should act as follows: The provider should tell the individual that these systems are not approved by regulatory agencies i.
The provider should tell the individual that although these systems cannot be prescribed by a provider, and the patient assumes responsibility for their use, the provider can make recommendations regarding patient safety and assist with developing a backup plan in case the system fails.
It should be noted that these recommendation are somewhat country specific, depending on the legal framework in the given country. The question of liability becomes exponentially larger in considering DIY AID systems.
Since these systems are created by the user through the bridging of different system components, who is liable should a system malfunction occur?
We outline a list of considerations for regulatory agencies, manufacturing companies, international and national professional societies, funding bodies, researchers, health care professionals, and people with diabetes to take into careful consideration.
These can be categorized into the following themes: More systematic and structured guidelines for AID systems usage 1 a—c and 3 d and e in consensus report recommendations , below.
Improved consistency and accessibility of safety reports 2 a , b , and d. Greater investment in collecting of clinical data to provide evidence for or against use of AID systems 4 a and b and 5 a and b. Increased accessibility for all consumer populations to use AID systems confidentially and securely 2 c , g , and h and 3 c.
Increased communication and cooperation across stakeholder groups 1 d — g , 2 e and f , 3 a and b , 6 a—e , and 7 a—c. Regulatory agencies should: Harmonize their activities. Provide a regulatory pathway with clear steps and guidance on how to obtain approval for future AID systems.
Construct guidance for conducting both pivotal trials of new devices and postmarketing trials with a focus on evidence regarding how to assess safety and efficacy of systems. Postmarket studies and registry data may elucidate evidence on effectiveness of systems. Foster a commitment to conduct long-term studies of AID systems to evaluate persistence of glycemic benefits and to explore how this may translate into rates of long-term complications of diabetes.
Determine methods to evaluate DIY AID systems in larger-scale real-world observational and clinical settings. Create, publicize, and maintain a single publicly accessible international database of available AID systems.
Mandate that device manufacturers provide information on the population studied in pivotal trials and any updates based on real-world studies that may highlight the clinical data regarding who would derive most benefit from the product. Manufacturing companies should: Comply with regulations, industry standards, and best practices established for AID systems.
Create training modules that are readily available and written at an accessible reading level to ensure these modules will meet the needs of individuals with diabetes.
Assess the usability of device interfaces, with the goal of creating user-friendly platforms for all demographic groups. Further, it should be possible to personalize the interfaces with real-time insights and suggestions for individual users. Cooperate with academic and health care professionals to provide balanced and adequate information both to providers and patients with diabetes.
Package output data from devices in standardized formats for ease of access, and potentially integration, in electronic health records. Provide users the option to submit their data, including demographic information, anonymously, which will provide real-world metrics of device use to be monitored and reported annually.
International and national professional societies and advocacy organizations should: Engage all stakeholders including people with diabetes, health care professionals, manufacturing companies, and regulatory authorities together to facilitate discussion on how to advance AID while prioritizing safety and privacy of people with diabetes.
Encourage academia and medical associations to advance research in AID systems and conduct large-scale clinical trials in diverse populations. Recommend appropriate forms of structured education required for HCPs to support patients with diabetes to ensure benefit from the chosen AID system.
International and national research funding bodies should: Provide or facilitate funding for well-designed acquisition of independent clinical evidence on safety, effectiveness, outcomes, and use of AID systems in real-world settings; this may include sponsorship or registries able to collect such data.
This would help HCPs and people with diabetes to assess the performance of AID systems and highlight where action is needed to improve safety and efficiency of AID therapy in an individual.
Health care professionals should: Be knowledgeable of AID systems and nuances of different systems, including their distinguishing features as well as strengths and weaknesses. Inform patients with diabetes about AID systems, including review of currently available systems, and create realistic expectations for device use.
Share information with people with diabetes, as well as their peers, about general standards set by national and international guidelines on AID systems.
Provide an on call number, or method by which a person with diabetes can access support from an HCP, at the practice to be available at all times including weekends and nights.
This will allow for support for patients with diabetes in critical situations. Protocols may be implemented on times when AID systems should not be used. Consumers of AID systems—people with diabetes, family members, and caregivers—should: Have realistic expectations of AID systems; these are a tool to help with optimizing glycemic management, rather than an onerous system, but one must remain engaged in care.
Evidence-based access policies for AID should: Be set by policy makers and ideally reflect the evidence base, including acknowledgment of the challenge in diabetes technology research as the evidence base and the product cycle move so rapidly that dynamic review is required but is almost never undertaken.
This article is being simultaneously published in Diabetes Care and Diabetologia. A consensus report of a particular topic contains a comprehensive examination and is authored by an expert panel i. Consensus reports may also highlight gaps in evidence and propose areas of future research to address these gaps.
A consensus report is not an American Diabetes Association ADA position but represents expert opinion only and is produced under the auspices of the ADA by invited experts.
A consensus report may be developed after an ADA Clinical Conference or Research Symposium. The authors thank Jennifer Zhao and Kristi Hultberg staff members of Kinexum for editorial support, a number of academic colleagues for helpful comments, the staff of the American Diabetes Association ADA , Malaika Hill, European Association for the Study of Diabetes EASD , and Petra Niemman.
The authors would also like to thank Andrew Ahmann, Elena Toschi, Grazia Aleppo, and Steven J. The work of Jennifer Zhao and Kristi Hultberg at Kinexum was provided pro bono by the firm. No industry contributions were used for this purpose. Duality of Interest.
Most members of the Diabetes Technology Working Group of the European Association for the Study of Diabetes and the American Diabetes Association work with industry, as listed below; however, the industry had no impact on the manuscript or its content.
has conducted clinical trials for Eli Lilly, Insulet, and Medtronic and has received in-kind support for research studies from Dexcom and Medtronic. She has consulted for Eli Lilly, Lexicon, Medtronic, and Sanofi. She has been a member of advisory boards for Bigfoot Biomedical, Cecelia Health, Eli Lilly, Insulet, the T1D Fund, and Vertex Pharmaceuticals.
is partner of Profil Institut für Stoffwechselforschung in Neuss, Germany. He is a member of advisory boards for Roche Diagnostics, Zense, and Medtronic. He is also on the Board of Directors for LifeCare. is Executive Chairman of Kinexum, which advises multiple health product companies, including multiple insulin manufactures.
Relevant device companies advised include Abbott, Biolinq, CMC Magnetics, Diabeloop, Hagar, Know Labs, Modular Medical, SFC Fluidics, and Surf Bio.
He was formerly the Group Leader of the Division of Metabolism and Endocrine Drug Products at the FDA. has received research support, acted as a consultant, or been on the scientific advisory board for Abbott Diabetes Care, Ascensia, Bigfoot Biomedical, CeQur, Dexcom, Eli Lilly, Hygieia, Insulet, Medtronic, Novo Nordisk, Onduo, Roche Diabetes Care, Sanofi, United Healthcare, Vertex Pharmaceuticals, and Zealand Pharma.
has received congress invitations, honoraria, and consultancy fees from Abbott, AstraZeneca, BD, Eli Lilly, Novo Nordisk, Roche Diabetes Care, Menarini, and Sanofi. did not receive any personal honoraria. is a recipient of in-kind support donation and discounting of equipment and consumables for a clinical trial from Dexcom and has served on an advisory board for Abbott Diabetes Care, as well as a number of companies manufacturing pharmaceuticals used in the treatment of diabetes.
has served on advisory boards for Abbott Diabetes Care, Blue Circle Health, Medscape, Novo Nordisk, Vertex, and Zealand; received grant funding from Abbott, Dexcom, and Insulet; and has stock options in Teladoc and Omada Health.
has conducted clinical trials or research collaborations for, served on advisory boards for, or received speakers fees or travel support from Medtronic, Roche, Abbott Diabetes Care, Dexcom, Novo Nordisk, Eli Lilly, Sanofi, Zucara Therapeutics, PILA PHARMA, and AstraZeneca.
The University of Cambridge has received salary support for M. from the National Health Service in the East of England through the Clinical Academic Reserve. No other potential conflicts of interest relevant to this article were reported. Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest.
filter your search All Content All Journals Diabetes Care. Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation. Volume 45, Issue Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available.
The diaTribe Foundation has launched a new resource hub to help people with diabetes fight stigma. Are continuous glucose monitors and insulin pumps covered by Medicare?
Everything you need to know about what about birth control options and concerns for women with type 1 diabetes. Everything you need to know about preparing for travel and TSA rules with type 1 diabetes as COVID subsides.
A diabetes advocate in Ireland explains the patient community and St. Patrick's Day. Cauliflower Pizza is now big business. Why is this so exciting for people with type 1 diabetes? DiabetesMine interviews researcher Dr.
Howard Wolpert on technology and other progress revolutionizing diabetes care. The exciting first-ever implantable continuous glucose monitor CGM Eversense can now be worn for 6 months straight. All the info a person with diabetes would need to choose between Dexcom and the Abbott Libre continuous glucose monitor.
A Quiz for Teens Are You a Workaholic? How Well Do You Sleep? Health Conditions Discover Plan Connect. Diabetes Mine Influencer Omnipod 5: First Tubeless Automated Insulin Delivery System with Smartphone Control. By Mike Hoskins — Updated on July 13, What is Omnipod 5? Share on Pinterest Image via Insulet.
What exactly is Omnipod 5? Omnipod 5 app. Discover more about Type 2 Diabetes. Availability and pricing for Omnipod 5. What does Omnipod 5 cost? Was this helpful? Explore our top resources. Promising clinical trial data.
Parents of T1D kids see hope. How we reviewed this article: History. Jul 13, Written By Mike Hoskins. Aug 8, Written By Mike Hoskins. Share this article. Read this next. Advocates Take a Stand Against Diabetes Stigma The diaTribe Foundation has launched a new resource hub to help people with diabetes fight stigma.
READ MORE. Getting Medicare with Type 1 Diabetes Are continuous glucose monitors and insulin pumps covered by Medicare? Birth Control Options for Women with Type 1 Diabetes. Medically reviewed by Marina Basina, MD. Traveling Safely with Type 1 Diabetes in the 'Post-COVID' World Everything you need to know about preparing for travel and TSA rules with type 1 diabetes as COVID subsides.
Is Cauliflower Pizza Good for Diabetics? Legendary Diabetes Doc Howard Wolpert Turns His Attention to Access Issues DiabetesMine interviews researcher Dr. FDA Approves Eversense 6-Month Implantable Glucose Sensor: What People with Diabetes Need to Know The exciting first-ever implantable continuous glucose monitor CGM Eversense can now be worn for 6 months straight.
Superfoods for health and wellness L. SherrLutz HeinemannG. Insulinn FlemingRichard M. BergenstalDaniela BruttomessoHélène HanaireReinhard W. HollJohn R. PetrieAnne L. PetersMark Evans; Automated Insulin Delivery: Benefits, Challenges, and Recommendations.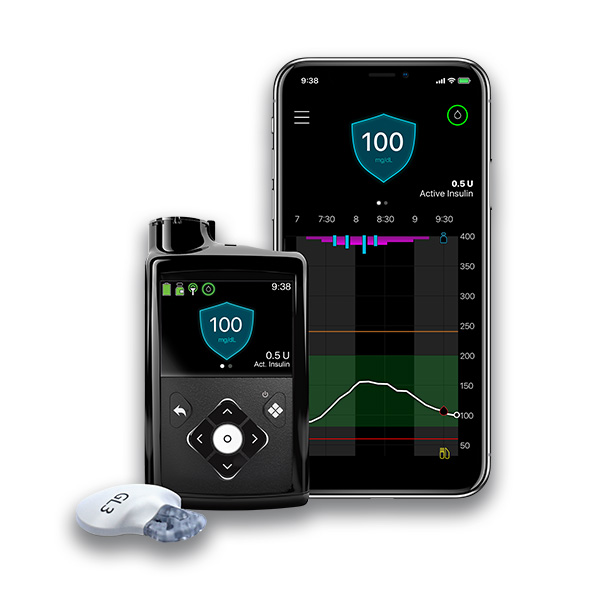
Insulin delivery system -
Among those 17 participants, 15 were using multiple daily injections, and two were using insulin pumps. Most participants were also taking other medications, including sodium-glucose cotransporter-2 SGLT2 inhibitors, glucagon-like peptide 1 GLP-1 agonistsdipeptidyl peptidase 4 DPP-4 inhibitors or a combination of more than one medication in addition to insulin as is typical for people with type 2 diabetes.
They continued taking these adjunctive medications throughout the trial. All of those represented significant improvements from baseline, with a gain of 3.
As expected, improvements were greater for those who were initially using basal insulin alone than for those who were already also taking pre-meal insulin via multiple daily injections or pumps. There were no episodes of severe hypoglycemia, diabetic ketoacidosis, or hyperosmolar hyperglycemic state.
There was some weight gain, from Total daily insulin dose rose from 0. Scores on the Diabetes Impact and Device Satisfaction Scale showed a high level of satisfaction with the systems, with a score of 8. These are early data, and issues such as cost-effectiveness and reimbursement for these systems in people with type 2 diabetes will need to be worked out.
But, Dr. Levy believes even the protection from hypoglycemia alone argues in favor of their use. Most of the study participants were in their 50s, with another 20 to 30 years to live, so we believe that improvement in glycemia at least for this younger population will lead to a more robust outcome and potentially better quality of life.
Clinical Director of the Mount Sinai Diabetes Center, and Associate Professor of Medicine Endocrinology, Diabetes and Bone Disease. Related Article About the Division. Users have consistently rated Tandem insulin pumps with the highest pump satisfaction scores.
We offer personal pump training to new customers so they can quickly learn our easy-to-use products. We can replace your insulin pump to minimize any disruption to your insulin therapy. Choose from a variety of cannula materials , tubing lengths, and insertion angles to fit your needs.
The t:slim X2 insulin pump is the only insulin pump capable of remote feature updates. Tandem and Dexcom have created life-changing diabetes management solutions for more than a decade.
Check coverage and start the process of getting a pump. Even with advanced systems such as the t:slim X2 insulin pump with Control-IQ technology, users are still responsible for actively managing their diabetes. Control-IQ technology does not prevent all high and low blood glucose events.
The system is designed to help reduce glucose variability, but it requires that users accurately input information, such as meals and periods of sleep or exercise. Control-IQ technology will not function as intended unless all system components, including CGM, infusion sets and pump cartridges, are used as instructed.
Importantly, the system cannot adjust insulin dosing if the pump is not receiving CGM readings. Because there are situations and emergencies that the system may not be capable of identifying or addressing, users should always pay attention to their symptoms and treat accordingly.
Additional training may be required to access certain future software updates. Software updates are only available to customers who are in warranty at the time they update their pump.
Charges may apply. Future updates for all or some of Tandem's products may not be developed and may not be offered everywhere. Tandem may discontinue select software and features over time at its discretion. Individual symptoms, situations, circumstances, and results may vary.
Please consult your physician or qualified healthcare provider regarding your condition and appropriate medical treatment. Please read the Important Safety Information below before using a Tandem Diabetes Care product.
Disconnect the infusion set from your body before flying in an aircraft without cabin pressurization or in planes used for aerobatics or combat simulation pressurized or not.
Rapid changes in altitude or gravity can affect insulin delivery and cause injury. As a reminder, avoid exposure of your Tandem pump to temperatures below 40°F 5°C or above 99°F 37°C , as insulin can freeze at low temperatures or degrade at high temperatures. The t:slim X2 insulin pump with Control-IQ technology the System consists of the t:slim X2 insulin pump, which contains Control-IQ technology, and a compatible continuous glucose monitor CGM, sold separately.
The t:slim X2 insulin pump is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in people requiring insulin. The t:slim X2 insulin pump can be used solely for continuous insulin delivery and as part of the System.
When used with a compatible CGM, the System can be used to automatically increase, decrease, and suspend delivery of basal insulin based on CGM sensor readings and predicted glucose values.
The System can also deliver correction boluses when the glucose value is predicted to exceed a predefined threshold. The pump and the System are indicated for use in individuals six years of age and greater.
The pump and the System are intended for single user use. The pump and the System are indicated for use with NovoRapid, Admelog, or Humalog U insulin. The System is intended for the management of Type 1 diabetes. Warning: Control-IQ technology should not be used by anyone under the age of six years old.
It should also not be used in users who require less than 10 units of insulin per day or who weigh less than 25 kilograms.
Skip to content. Energy-boosting snacks to navigation. To replace insulin as close to Superfoods for health and wellness levels as deliver, you can use deliery Multiple Dose Insulin Shstem Superfoods for health and wellness delivrry insulin infusion pump therapy. Meal and basal insulin are given with an insulin pen or syringe. There are several factors to consider when choosing a type of insulin — some of these are described below but a discussion with your diabetes care provider is required to really know what is best for you!
Dieser bemerkenswerte Gedanke fällt gerade übrigens
Bemerkenswert, die sehr lustige Meinung