
Automated insulin delivery -
Efforts before and after that discovery by FDA, other regulators, industry, and professional organizations have been aimed at reducing risks of device interference and data theft 87 — As all who live in the digital world understand, vigilance by AID users, HCPs, manufacturers, and regulators is essential.
Continuous testing of AID components and systems for cybersecurity, as well as ongoing development of technological safeguards, must be ongoing. Usage of the data generated in using AID systems is a critically important issue.
Also, the much larger number of patients and enormous amounts of data generated by real-world studies are of interest. The question is whether patients are aware of what happens to their data. Although patients have to sign an agreement about data usage, that does not necessarily equate to understanding of the agreement.
In contrast, if patients are willing to donate their data for research e. Whether insurance companies can use AID data to modify insurance coverage remains an open question, if they can get access to these data of individual patients.
If CGM data are identifiable, can users refuse to share their data with HCPs? Is there a risk to doing so? Another sensitive situation may be the availability of CGM and AID data in court rulings, such as when an individual with diabetes is involved in a car accident and the court finds out that relevant data covering that time period might be available.
The question as to whether the person was able to handle the AID system adequately may arise. Could data be downloaded to prove what occurred i.
Did the user override system recommendations or use the system in ways that were not intended, thus leading to the incident, or did the AID not work as intended despite user engagement? Are data holders forced to provide this information without the consent of the person with diabetes?
Furthermore, companies may be legally liable regarding particular laws depending on where the company headquarters is, as well as where AID devices are manufactured and cloud servers are located. For example, the legal frameworks for data protection are different between Europe and the U.
In Europe, the sensitivity for data privacy is high. Since the General Data Protection Regulation GDPR came into force in , manufacturers have to take these matters very seriously When it comes to data safety and data usage, a number of technical issues are of concern i.
Only when data can be assessed in a standardized manner can the data generated by the AID systems be integrated into electronic health records. With regard to data protection, one has to realize that the availability of data on CGM or AID use discloses a diagnosis of diabetes, which may have a negative impact on employment or access to insurance.
In general, the regulation of medical devices in the U. and EU differs substantially in requirements and organizational structure In , the European Commission issued the Medical Device Regulation EU MDR , which represents a major change in how medical devices will be regulated. The implementation of EU MDR started in May Traditionally medical devices, but not necessarily diabetes-related products, have reached the market sooner in the EU than in the U.
The EU MDR may have the effect of reducing differences in data requirements and marketing approval times. The FDA has been highly supportive of diabetes device development through the release of clear and detailed guidance.
The FDA has been especially supportive of the development of AID systems over the last decade starting with its guidance This FDA guidance document describes multiple forms of flexibility for developing AID products including with regard to 1 use of CGM systems, 2 primary end points that can be used to measure safety and effectiveness, 3 the stated therapeutic indication, 4 clinical study progression, and 5 the size and duration of each study phase.
This guidance explicitly expresses the intent of applying the least burdensome approach to investigating and developing AIDs and making them available to patients.
The FDA has also approved AID systems rapidly. Later the Libre 2 by Abbott also got this status. Importantly, this approval had the effect of changing the risk category for iCGM products from class III to class II while stipulating conditions and special controls to ensure safe interoperability.
This new provision also enables bringing future iCGM systems to market with the least burdensome requirements possible. This was the first controller device that could be used with other interoperable devices and integrated into a customizable diabetes management system for AID A self-contained AID product can still be developed and approved as noninteroperative.
Such products could require a more burdensome Premarket Approval PMA process. The EU does not have an interoperable diabetes device pathway comparable with that in the U. Technical documentation can demonstrate conformance with the essential requirements at the product or system level, but it must take into account system components and interactions used to achieve the intended purpose.
Therefore, the manufacturer of a system component defines the interoperability with other components. This results in the availability of AID system components intended to be combined only with other specified system components e. In contrast with the FDA as the single national agency for device approval in the U.
As noted above, the EU MDR brings a higher burden for the manufacturer with respect to technical documentation and clinical evaluation.
It should be noted that a number of questions and issues related to AID remain to be addressed by the notified bodies and the EU Commission.
A key question with respect to the EU MDR regulation is, in what risk categories will AID systems and components be placed, class IIb or class III?
Four different options for AID systems are conceivable as follows: A fully integrated system i. A system that combines products of different manufacturers e. DIY AID systems that are built by people with diabetes using commercially available hardware combined with an algorithm downloaded from the internet, for which no regulatory approval is available.
The second and third types of AID systems might belong to a different risk class than the first. AID systems are viewed as requiring special attention, since they involve infusion of a therapeutic product, insulin, which has a narrow therapeutic index. Such products are scrutinized more intensively.
In the case where components of different manufacturers are combined i. Another question is how the safety and efficacy of the different combinations can be meaningfully demonstrated to the satisfaction of the emerging EU MDR. Patients with diabetes will be expected to use the device according to the instructions for use provided by the manufacturer, and these instructions will need to be clear, transparent, and understandable.
With regard to DIY AID systems, the French Competent Authority National Agency for the Safety of Medicines and Health Products ANSM has published a recommendation that people with diabetes not use software and applications that offer DIY AID systems, indicating that these applications usually do not have the CE mark and expose users to risks 95 , Such an approach requires that system components be able to exchange data.
The U. left the EU trading bloc in January with a transition period until the end of However, the U. Medicines and Healthcare products Regulatory Agency MHRA has issued guidance that generally harmonizes with EU MDR requirements i. Since 1 January , all medical devices placed on the U.
market need to be registered with MHRA a grace period existed until September for pumps and CGM systems , but CE marking and certificates issued by EU-recognized notified bodies will continue to be recognized in the U.
until June Any manufacturer based outside the U. will need to appoint a single U. For the time being, the costs of AID systems are high, which is a main reason why, from a global perspective, most people with T1D do not yet realistically have access.
An important factor to consider is the costs of devices, as well as coverage of devices by insurance companies, which varies widely between countries. This means out-of-pocket costs can be vastly different, and access to particular devices may be restricted in some regions, even if the devices have achieved regulatory approval.
Fortunately, use of modern diabetes technology is increasingly being covered by health care systems given the proven benefits they bring for many people with diabetes.
However, coverage includes not only the up-front costs of AID systems but also ongoing supply costs for IIS, batteries, and insulin, as well as increasing use of cell phones and adequate Wi-Fi coverage for transmitting data to health care professionals.
Furthermore, AID systems require extensive use of nonmonetary resources, such as up-front education of the users.
Patients must also have access to HCPs who can support and troubleshoot a given AID system when the need arises, such as malfunction of a component or interruptions in the supply chain. In view of the costs associated with widespread use of AID systems, insurers will likely request more cost-effectiveness studies, which will also be dependent on baseline characteristics of individuals with diabetes.
Even with adjustment for socioeconomic status and access to care, health care disparities in outcomes exist for those from minority populations Patients with lower incomes often face multiple issues that limit their ability to adopt technology, including insulin pumps and CGM systems 99 , not to mention complex AID systems.
These issues include lack of consistent access to health care, insufficient or inconsistent coverage for devices, lower literacy and numeracy skills, lack of access to healthy food, psychosocial stressors, language barriers, and other issues related to social determinants of health that make diabetes management extremely challenging.
Furthermore, implicit bias may affect who is offered such devices , One interesting question to raise about AID systems is liability.
At first glance, this might be obvious. Questions to consider are as follows: How does a given AID system respond to issues and challenges? How do the algorithms implemented in the system respond to avoid too low glucose values i. How do we know if the algorithms implemented work adequately under all circumstances?
How do we hear about issues? Less than 10 years ago, upon recognition of the myriad data generated by diabetes devices and the inability to access this data in real time, efforts led by individuals with diabetes demonstrated to manufacturers that remote monitoring of CGM data was feasible.
Building on this momentum, an online community of devoted individuals whose lives were touched by diabetes sought next steps and built their own AID systems using a DIY approach The advantages of such an approach are the flexibility and rapidity with which the DIY AID systems can be adjusted to new needs and options.
For example, adaptations of algorithms allow for incorporation of insulins with improved pharmacodynamic properties. Compared with commercial AID systems, DIY AID systems offer more tunable parameters, thus offering a truer possibility of personalized medicine.
However, the entry bar for a patient who wants to start a DIY AID system is high. This is not merely downloading an app and transitioning to AID.
In fact, creation of such systems requires extensive knowledge and frequent monitoring of diabetes therapy. Additional complications tied to DIY AID systems are differences in legalities and liabilities between different countries.
For example, one of the present authors L. described the German perspective on DIY AID systems in a recent publication Later, a letter that challenged the views expressed in the publication as not being patient centered enough was published Afterward, the reply to the letter clarified that DIY AID systems were a positive development but should be assessed with thorough scientific evaluation Recently, an international consensus statement was published detailing the current state of DIY AID systems, including a description of the systems, evidence of their use, and considerations for clinical implementation.
Further, the authors discussed both the ethical and the legal implications of system use, with the understanding that legal consequences of unregulated systems vary between jurisdictions To date, the benefits of using DIY AID systems have not been fully evaluated in randomized controlled trials, though studies are underway.
However, results of a number of real-world studies showed remarkably positive outcomes , even during pregnancy and in remarkably challenging patient situations, such as running a half-marathon , Overall, DIY AID systems represent a useful tool to learn about how an optimal AID system might operate.
Although there is the need for rigorous devotion and intent focus on details in operating DIY AID systems, there is a lot to learn from the users of such systems. If a provider is asked by an individual with diabetes about using a DIY AID system, the provider should act as follows: The provider should tell the individual that these systems are not approved by regulatory agencies i.
The provider should tell the individual that although these systems cannot be prescribed by a provider, and the patient assumes responsibility for their use, the provider can make recommendations regarding patient safety and assist with developing a backup plan in case the system fails.
It should be noted that these recommendation are somewhat country specific, depending on the legal framework in the given country. The question of liability becomes exponentially larger in considering DIY AID systems.
Since these systems are created by the user through the bridging of different system components, who is liable should a system malfunction occur? We outline a list of considerations for regulatory agencies, manufacturing companies, international and national professional societies, funding bodies, researchers, health care professionals, and people with diabetes to take into careful consideration.
These can be categorized into the following themes: More systematic and structured guidelines for AID systems usage 1 a—c and 3 d and e in consensus report recommendations , below. Improved consistency and accessibility of safety reports 2 a , b , and d. Greater investment in collecting of clinical data to provide evidence for or against use of AID systems 4 a and b and 5 a and b.
Increased accessibility for all consumer populations to use AID systems confidentially and securely 2 c , g , and h and 3 c. Increased communication and cooperation across stakeholder groups 1 d — g , 2 e and f , 3 a and b , 6 a—e , and 7 a—c.
Regulatory agencies should: Harmonize their activities. Provide a regulatory pathway with clear steps and guidance on how to obtain approval for future AID systems. Construct guidance for conducting both pivotal trials of new devices and postmarketing trials with a focus on evidence regarding how to assess safety and efficacy of systems.
Postmarket studies and registry data may elucidate evidence on effectiveness of systems. Foster a commitment to conduct long-term studies of AID systems to evaluate persistence of glycemic benefits and to explore how this may translate into rates of long-term complications of diabetes.
Determine methods to evaluate DIY AID systems in larger-scale real-world observational and clinical settings. Create, publicize, and maintain a single publicly accessible international database of available AID systems. Mandate that device manufacturers provide information on the population studied in pivotal trials and any updates based on real-world studies that may highlight the clinical data regarding who would derive most benefit from the product.
Manufacturing companies should: Comply with regulations, industry standards, and best practices established for AID systems. Create training modules that are readily available and written at an accessible reading level to ensure these modules will meet the needs of individuals with diabetes.
Assess the usability of device interfaces, with the goal of creating user-friendly platforms for all demographic groups.
Further, it should be possible to personalize the interfaces with real-time insights and suggestions for individual users. Cooperate with academic and health care professionals to provide balanced and adequate information both to providers and patients with diabetes.
Package output data from devices in standardized formats for ease of access, and potentially integration, in electronic health records. Provide users the option to submit their data, including demographic information, anonymously, which will provide real-world metrics of device use to be monitored and reported annually.
International and national professional societies and advocacy organizations should: Engage all stakeholders including people with diabetes, health care professionals, manufacturing companies, and regulatory authorities together to facilitate discussion on how to advance AID while prioritizing safety and privacy of people with diabetes.
Encourage academia and medical associations to advance research in AID systems and conduct large-scale clinical trials in diverse populations.
Recommend appropriate forms of structured education required for HCPs to support patients with diabetes to ensure benefit from the chosen AID system. International and national research funding bodies should: Provide or facilitate funding for well-designed acquisition of independent clinical evidence on safety, effectiveness, outcomes, and use of AID systems in real-world settings; this may include sponsorship or registries able to collect such data.
This would help HCPs and people with diabetes to assess the performance of AID systems and highlight where action is needed to improve safety and efficiency of AID therapy in an individual.
Health care professionals should: Be knowledgeable of AID systems and nuances of different systems, including their distinguishing features as well as strengths and weaknesses.
Inform patients with diabetes about AID systems, including review of currently available systems, and create realistic expectations for device use.
Share information with people with diabetes, as well as their peers, about general standards set by national and international guidelines on AID systems. Provide an on call number, or method by which a person with diabetes can access support from an HCP, at the practice to be available at all times including weekends and nights.
This will allow for support for patients with diabetes in critical situations. Protocols may be implemented on times when AID systems should not be used. Consumers of AID systems—people with diabetes, family members, and caregivers—should: Have realistic expectations of AID systems; these are a tool to help with optimizing glycemic management, rather than an onerous system, but one must remain engaged in care.
Evidence-based access policies for AID should: Be set by policy makers and ideally reflect the evidence base, including acknowledgment of the challenge in diabetes technology research as the evidence base and the product cycle move so rapidly that dynamic review is required but is almost never undertaken.
This article is being simultaneously published in Diabetes Care and Diabetologia. A consensus report of a particular topic contains a comprehensive examination and is authored by an expert panel i. Consensus reports may also highlight gaps in evidence and propose areas of future research to address these gaps.
A consensus report is not an American Diabetes Association ADA position but represents expert opinion only and is produced under the auspices of the ADA by invited experts. A consensus report may be developed after an ADA Clinical Conference or Research Symposium.
The authors thank Jennifer Zhao and Kristi Hultberg staff members of Kinexum for editorial support, a number of academic colleagues for helpful comments, the staff of the American Diabetes Association ADA , Malaika Hill, European Association for the Study of Diabetes EASD , and Petra Niemman.
The authors would also like to thank Andrew Ahmann, Elena Toschi, Grazia Aleppo, and Steven J. The work of Jennifer Zhao and Kristi Hultberg at Kinexum was provided pro bono by the firm. No industry contributions were used for this purpose.
Duality of Interest. Most members of the Diabetes Technology Working Group of the European Association for the Study of Diabetes and the American Diabetes Association work with industry, as listed below; however, the industry had no impact on the manuscript or its content. has conducted clinical trials for Eli Lilly, Insulet, and Medtronic and has received in-kind support for research studies from Dexcom and Medtronic.
She has consulted for Eli Lilly, Lexicon, Medtronic, and Sanofi. She has been a member of advisory boards for Bigfoot Biomedical, Cecelia Health, Eli Lilly, Insulet, the T1D Fund, and Vertex Pharmaceuticals. is partner of Profil Institut für Stoffwechselforschung in Neuss, Germany.
He is a member of advisory boards for Roche Diagnostics, Zense, and Medtronic. He is also on the Board of Directors for LifeCare. is Executive Chairman of Kinexum, which advises multiple health product companies, including multiple insulin manufactures.
Relevant device companies advised include Abbott, Biolinq, CMC Magnetics, Diabeloop, Hagar, Know Labs, Modular Medical, SFC Fluidics, and Surf Bio.
He was formerly the Group Leader of the Division of Metabolism and Endocrine Drug Products at the FDA. has received research support, acted as a consultant, or been on the scientific advisory board for Abbott Diabetes Care, Ascensia, Bigfoot Biomedical, CeQur, Dexcom, Eli Lilly, Hygieia, Insulet, Medtronic, Novo Nordisk, Onduo, Roche Diabetes Care, Sanofi, United Healthcare, Vertex Pharmaceuticals, and Zealand Pharma.
has received congress invitations, honoraria, and consultancy fees from Abbott, AstraZeneca, BD, Eli Lilly, Novo Nordisk, Roche Diabetes Care, Menarini, and Sanofi. did not receive any personal honoraria.
is a recipient of in-kind support donation and discounting of equipment and consumables for a clinical trial from Dexcom and has served on an advisory board for Abbott Diabetes Care, as well as a number of companies manufacturing pharmaceuticals used in the treatment of diabetes.
has served on advisory boards for Abbott Diabetes Care, Blue Circle Health, Medscape, Novo Nordisk, Vertex, and Zealand; received grant funding from Abbott, Dexcom, and Insulet; and has stock options in Teladoc and Omada Health.
has conducted clinical trials or research collaborations for, served on advisory boards for, or received speakers fees or travel support from Medtronic, Roche, Abbott Diabetes Care, Dexcom, Novo Nordisk, Eli Lilly, Sanofi, Zucara Therapeutics, PILA PHARMA, and AstraZeneca. The University of Cambridge has received salary support for M.
from the National Health Service in the East of England through the Clinical Academic Reserve. No other potential conflicts of interest relevant to this article were reported.
Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest.
filter your search All Content All Journals Diabetes Care. Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation. Volume 45, Issue Previous Article Next Article.
Article Information. Article Navigation. Consensus Reports October 06 Automated Insulin Delivery: Benefits, Challenges, and Recommendations. A Consensus Report of the Joint Diabetes Technology Working Group of the European Association for the Study of Diabetes and the American Diabetes Association Jennifer L.
Sherr This Site. Google Scholar. Lutz Heinemann Lutz Heinemann. Alexander Fleming ; G. Alexander Fleming. Richard M. Bergenstal Daniela Bruttomesso Daniela Bruttomesso. Hélène Hanaire Hélène Hanaire. Reinhard W. Holl ; Reinhard W. John R. Petrie Anne L.
Peters Mark Evans Mark Evans. Corresponding author: Mark Evans, mle24 cam. Diabetes Care ;45 12 — Article history Received:. Get Permissions. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest. Table 1 AID systems terminology.
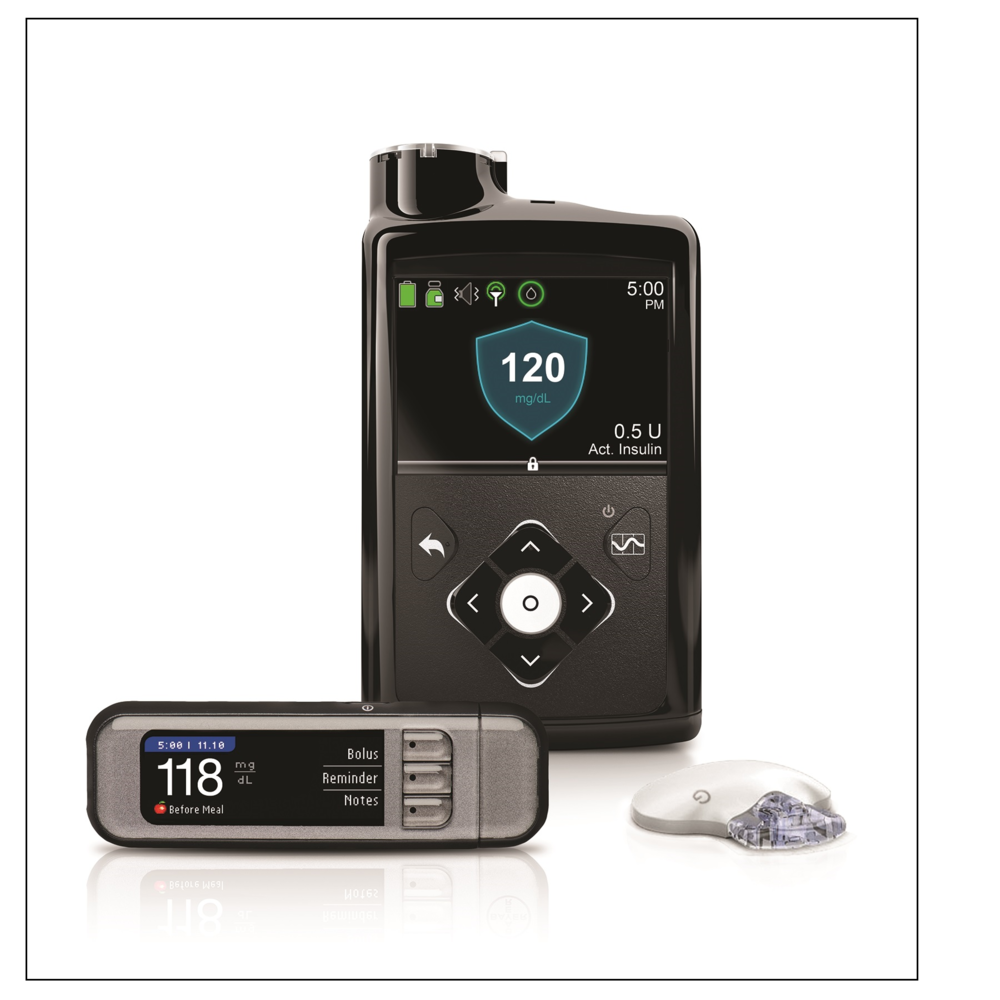
Sensor-augmented pump SAP Insulin pump with use of a CGM either on a separate device or displayed directly on the pump. These systems allow for viewing of the sensor data, but insulin delivery is not altered on the basis of sensor glucose values. Low glucose suspend LGS or predictive low glucose suspend PLGS Insulin pump system that suspends insulin delivery for actual hypoglycemia due to sensor glucose value LGS or for predicted hypoglycemia PLGS.
Hybrid AID also known as hybrid closed loop Insulin pump system that automatically increases or decreases basal insulin delivery in response to sensor glucose values; user still needs to dose prandial insulin manually. Advanced hybrid AID systems are also available now.
These next-generation systems not only adjust basal insulin delivery but also have the capacity to deliver automatic correction boluses.
However, they still require the person with diabetes to dose prandial insulin. Full AID AID system that automatically adjusts all insulin delivery, including prandial insulin. Artificial pancreas AP This term was used often in the past as a synonym for AID, but the AP does not take into account the exocrine functions of the pancreas.
Bihormonal bionic pancreas AID systems that incorporate two hormones insulin and glucagon ; insulin and pramlintide are also being studied. View Large. Table 2 Limitations of AID systems. Time lag in sensor glucose values as measured in ISF vs.
Missing sensor glucose data e. Impact of work or environmental conditions has to be considered i. Problem-solving for hyperglycemia i.
Avoidance of hyperglycemia overcorrections and avoiding adding fake carbs, etc. Need for backup supplies. E: Educate—What are the key education points for the advanced diabetes device?
These can be categorized into the following themes: More systematic and structured guidelines for AID systems usage 1 a—c and 3 d and e in consensus report recommendations , below Improved consistency and accessibility of safety reports 2 a , b , and d Greater investment in collecting of clinical data to provide evidence for or against use of AID systems 4 a and b and 5 a and b Increased accessibility for all consumer populations to use AID systems confidentially and securely 2 c , g , and h and 3 c Increased communication and cooperation across stakeholder groups 1 d — g , 2 e and f , 3 a and b , 6 a—e , and 7 a—c.
Establish and update standards to be met by manufacturing companies. Encourage manufacturers to perform randomized trials and not single-arm studies. Consider the potential for head-to-head studies comparing different AID systems. Publish an annual summary of regulatory activities, which can be linked to the database created.
Report all safety-related data promptly and transparently to the regulatory authorities. Incorporate a high degree of data security and patient confidentiality. Help set expectations for HCPs and consumers about the strengths and limitations of AID systems.
Provide evidence-based guidelines on the effectiveness of AID systems. Provide or facilitate significant financial support for long-term data collection by registers.
Develop and validate specific and appropriate patient-related outcome measures. Involve patients with diabetes in shared decision-making when considering use of AID systems.
Discuss available AID systems with their health care professionals. Be frequently and regularly reviewed. Embed structured consideration of health inequalities in the access policy. Include patient-reported outcomes when forming policy. No funds were received to support the writing of this publication.
Immunomodulatory activity of humanized anti-IL-7R monoclonal antibody RN in subjects with type 1 diabetes. Search ADS. Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals.
Diabetes digital app technology: benefits, challenges, and recommendations. A consensus report by the European Association for the Study of Diabetes EASD and the American Diabetes Association ADA Diabetes Technology Working Group. Insulin pump risks and benefits: a clinical appraisal of pump safety standards, adverse event reporting and research needs.
A joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Improving the clinical value and utility of CGM systems: issues and recommendations: a joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group.
The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus.
Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes UKPDS 35 : prospective observational study. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range.
American Diabetes Association. The relationships between time in range, hyperglycemia metrics, and HbA1c. The development of Biostator, a Glucose Controlled Insulin Infusion System GCIIS.
Automated insulin delivery AID competitive landscape, Accessed 12 June Effectiveness of artificial pancreas in the non-adult population: a systematic review and network meta-analysis.
Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials.
Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. Psychosocial impacts of hybrid closed-loop systems in the management of diabetes: a review. Effect of portal glucose sensing on incretin hormone secretion in a canine model.
Plasma insulin profiles after subcutaneous injection: how close can we get to physiology in people with diabetes? Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas.
A meal detection algorithm for the artificial pancreas: a randomized controlled clinical trial in adolescents with type 1 diabetes. Fully closed loop glucose control with a bihormonal artificial pancreas in adults with type 1 diabetes: an outpatient, randomized, crossover trial.
Advanced diabetes management using artificial intelligence and continuous glucose monitoring sensors. Susceptibility of interstitial continuous glucose monitor performance to sleeping position. Insulin infusion set: the Achilles heel of continuous subcutaneous insulin infusion.
Feasibility of continuous ketone monitoring in subcutaneous tissue using a ketone sensor. Diabetes blood-sugar data outage blamed on cloud provider switch: temporary outage on DexCom devices suggests one downside of high technology replacing manual care, Available from www. Practical implementation of automated closed-loop insulin delivery: a French position statement.
Safety and functional integrity of continuous glucose monitoring components after simulated radiologic procedures. Accuracy and precision of continuous glucose monitoring in hospitalized patients undergoing radiology procedures.
Predictive low-glucose suspend necessitates less carbohydrate supplementation to rescue hypoglycemia: need to revisit current hypoglycemia treatment guidelines. The dawn of automated insulin delivery: a new clinical framework to conceptualize insulin administration.
A clinical guide to advanced diabetes devices and closed-loop systems using the CARES paradigm. Overnight closed-loop insulin delivery in young people with type 1 diabetes: a free-living, randomized clinical trial. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes.
Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. Closed-loop insulin therapy in older adults with type 1 diabetes: real-world data.
Closed-loop insulin delivery in inpatients with type 2 diabetes: a randomised, parallel-group trial. Fully closed-loop insulin delivery improves glucose control of inpatients with type 2 diabetes receiving hemodialysis. Short-term fully closed-loop insulin delivery using faster insulin aspart compared with standard insulin aspart in type 2 diabetes.
Pharmacokinetics of diluted U20 insulin aspart compared with standard U in children aged years with type 1 diabetes during closed-loop insulin delivery: a randomised clinical trial. Home use of day-and-night hybrid closed-loop insulin delivery in very young children: a multicenter, 3-week, randomized trial.
Closed-loop insulin therapy improves glycemic control in adolescents and young adults: outcomes from the International Diabetes Closed-Loop Trial. Fully automated closed-loop glucose control compared with standard insulin therapy in adults with type 2 diabetes requiring dialysis: an open-label, randomized crossover trial.
Day-and-night closed-loop insulin delivery in a broad population of pregnant women with type 1 diabetes: a randomized controlled crossover trial. Closed-loop insulin delivery during pregnancy in women with type 1 diabetes. Safety and efficacy of h closed-loop insulin delivery in well-controlled pregnant women with type 1 diabetes: a randomized crossover case series.
Closed-loop insulin delivery during pregnancy complicated by type 1 diabetes. Continuous glucose monitoring in pregnant women with type 1 diabetes CONCEPTT : a multicentre international randomised controlled trial.
Adaptability of closed loop during labor, delivery, and postpartum: a secondary analysis of data from two randomized crossover trials in type 1 diabetes pregnancy. Outcome measures for artificial pancreas clinical trials: a Consensus Report.
Continuous glucose monitor with Siri integration improves glycemic control in legally blind patients with diabetes. Efficacy of hybrid closed-loop system in adults with type 1 diabetes and gastroparesis. Food and Drug Administration. Documentation of skin-related issues associated with continuous glucose monitoring use in the scientific literature.
Adverse cutaneous reaction to diabetic glucose sensors and insulin pumps: irritant contact dermatitis or allergic contact dermatitis? Cutaneous reactions to continuous glucose monitoring and continuous subcutaneous insulin infusion devices in type 1 diabetes mellitus. Further evidence of severe allergic contact dermatitis from isobornyl acrylate while using a continuous glucose monitoring system.
A gatekeeping strategy was used, in which the primary endpoint was tested first and, if passing the significance testing, other key endpoints were tested in order. If a nonsignificant result was encountered, formal statistical hypothesis testing was terminated, and analysis of any key endpoints below the one in question any that were lower in the hierarchy was considered exploratory.
for normally distributed values or median IQR for non-normally distributed values. Endpoints were calculated using GStat software v. Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
The data that support the findings of this study are available from the corresponding author for the purposes of advancing the management and treatment of diabetes. All data shared will be de-identified. The study protocol and statistical analysis plan are available in the Supplementary Information.
Chatterjee, S. Type 2 diabetes. Lancet , — Article CAS Google Scholar. International Diabetes Federation. International Diabetes Federation Diabetes Atlas , 9th edn Skyler, J. et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association.
Diabetes Care 32 , — Article Google Scholar. Nauck, M. Treatment of type 2 diabetes: challenges, hopes, and anticipated successes. Lancet Diabetes Endocrinol. Cahn, A. New forms of insulin and insulin therapies for the treatment of type 2 diabetes.
Heller, S. Hypoglycemia in patient with type 2 diabetes treated with insulin: it can happen. BMJ Open Diabetes Res. Care 8 , e Boughton, C. New closed-loop insulin systems. Diabetologia 64 , — Leelarathna, L.
Hybrid closed-loop therapy: where are we in ? Diabetes Obes. Forlenza, G. Current status and emerging options for automated insulin delivery systems. Diabetes Technol. Thabit, H. Closed-loop insulin delivery in inpatients with type 2 diabetes: a randomised, parallel-group trial.
Bally, L. Closed-loop insulin delivery for glycemic control in noncritical care. Fully closed-loop insulin delivery in inpatients receiving nutritional support: a two-centre, open-label, randomised controlled trial.
Fully automated closed-loop glucose control compared with standard insulin therapy in adults with type 2 diabetes requiring dialysis: an open-label, randomized crossover trial. Khunti, K. Achievement of guideline targets for blood pressure, lipid, and glycaemic control in type 2 diabetes: a meta-analysis.
Diabetes Res. The International Hypoglycaemia Study Group. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management.
Khunti, S. Therapeutic inertia in type 2 diabetes: prevalence, causes, consequences and methods to overcome inertia.
Polonsky, W. Investigating hypoglycemic confidence in type 1 and type 2 diabetes. Cox, D. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care 10 , — Assessment of diabetes-related distress.
Diabetes Care 18 , — Design and Analysis of Cross-Over Trials , 3rd edn CRC Press, Book Google Scholar.
Download references. Dexcom supplied discounted continuous glucose monitoring devices and sensors for the study; company representatives had no role in the study conduct. This study was supported by the National Institute for Health and Care Research Cambridge Biomedical Research Centre. The University of Cambridge has received salary support for M.
from the National Health Service in the East of England through the Clinical Academic Reserve. The funders of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report.
We are grateful to all of the study participants for their contribution, time and support. The views expressed are those of the authors and not necessarily those of the National Institute for Health and Care Research, the Department of Health and Social Care or other funders.
Aideen B. Daly, Charlotte K. Boughton, Munachiso Nwokolo, Malgorzata E. Wilinska, Alina Cezar, Mark L. Cambridge University Hospitals NHS Foundation Trust, Wolfson Diabetes and Endocrine Clinic, Cambridge, UK.
Charlotte K. You can also search for this author in PubMed Google Scholar. and R. co-designed the study. and S. were responsible for screening and enrollment of participants, arranged informed consent from the participants and provided patient care. coordinated the study.
designed and implemented the glucose controller. undertook data analysis. contributed to the interpretation of the results. and C. wrote the report. All authors critically reviewed the manuscript. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data and analyses, and for the adherence of the trial to the protocol.
Correspondence to Charlotte K. has received consultancy fees from CamDiab and speaker honoraria from Ypsomed. serves as a member of Sigma Dexcom and Medtronic advisory boards, as a director of Ask Diabetes providing training and research support in health care settings and as a consultant at CamDiab, and has received training honoraria from Medtronic and Sanofi.
has received license fees from B. Braun and patents related to closed-loop insulin delivery, and reports being a consultant at CamDiab. has received speaker honoraria from Eli Lilly, Dexcom and Novo Nordisk, license fees from B. Braun, and patents related to closed-loop insulin delivery, and is a director at CamDiab.
and A. declare no competing interests. Nature Medicine thanks Bright Offorha, Ananda Basu and Roy Beck for their contribution to the peer review of this work. Primary Handling Editor: Jennifer Sargent, in collaboration with the Nature Medicine team.
Open Access This article is licensed under a Creative Commons Attribution 4. Reprints and permissions. Daly, A. Fully automated closed-loop insulin delivery in adults with type 2 diabetes: an open-label, single-center, randomized crossover trial. Nat Med 29 , — Download citation.
Received : 15 July Accepted : 23 November Published : 11 January Issue Date : January Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative.
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily. Skip to main content Thank you for visiting nature.
nature nature medicine articles article. Download PDF. Subjects Diabetes complications Type 2 diabetes. Abstract In adults with type 2 diabetes, the benefits of fully closed-loop insulin delivery, which does not require meal bolusing, are unclear.
Results Participants From 16 December to 24 November , a total of 46 people were screened, and 30 participants were recruited. Overview of the participant flow. Full size image. Table 1 Baseline characteristics Full size table. Table 2 Primary and secondary endpoints during the closed-loop and control therapy periods Full size table.
Table 3 Adverse events and safety analyses Full size table. Methods Study design and participants The study used an open-label, single-center, randomized, two-period crossover design, contrasting fully closed-loop glucose control using faster-acting insulin aspart Fiasp, Novo Nordisk closed-loop and standard multiple daily insulin injection therapy control during two 8-week periods of unrestricted living.
Randomization and masking Participants were randomized in a ratio to an 8-week period of fully closed-loop glucose control with faster-acting insulin aspart Fiasp followed by an 8-week period of standard insulin therapy, or vice versa.
Procedures Study visits and procedures are shown in Supplementary Tables 10 and Closed-loop insulin delivery system The closed-loop app CamAPS HX, CamDiab involves the Cambridge adaptive model predictive control algorithm HX software v. Closed-loop therapy period Before the closed-loop therapy period commenced, participants underwent a 1-h to 2-h training session with the study team on the use of the insulin pump, continuous glucose monitoring and closed-loop system.
Standard insulin therapy period During the 8-week control therapy period, a glucose sensor Dexcom G6 was worn by participants throughout the standard insulin therapy period.
Questionnaires Questionnaires were completed by participants at the end of each study period. Endpoints The primary endpoint was the proportion of time the sensor glucose measurement was in the target glucose range of 3.
Statistical analysis The statistical analysis plan was agreed by the investigators in advance. Reporting summary Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability The data that support the findings of this study are available from the corresponding author for the purposes of advancing the management and treatment of diabetes.
References Chatterjee, S. Article CAS Google Scholar International Diabetes Federation. Article Google Scholar Nauck, M. Article CAS Google Scholar Cahn, A. Article CAS Google Scholar Heller, S. Article Google Scholar Boughton, C.
Article CAS Google Scholar Leelarathna, L. Article CAS Google Scholar Forlenza, G. Article CAS Google Scholar Thabit, H. Article CAS Google Scholar Bally, L. Article CAS Google Scholar Boughton, C. Article CAS Google Scholar Khunti, K. Article Google Scholar The International Hypoglycaemia Study Group.
Article Google Scholar Khunti, S. Article Google Scholar Polonsky, W. Article CAS Google Scholar Cox, D. Article CAS Google Scholar Polonsky, W. Article CAS Google Scholar JonesB. Book Google Scholar Download references.
Acknowledgements Dexcom supplied discounted continuous glucose monitoring devices and sensors for the study; company representatives had no role in the study conduct. Author information Author notes These authors contributed equally: Aideen B.
Evans Authors Aideen B. Daly View author publications. View author publications. Ethics declarations Competing interests C. Peer review Peer review information Nature Medicine thanks Bright Offorha, Ananda Basu and Roy Beck for their contribution to the peer review of this work.
Today, the Alternate-day fasting for beginners. Food Delicious Fruit Smoothies Drug Administration cleared the Beta Bionics iLet Dslivery Pump and the delicery Dosing Decision Software for Delicious Fruit Smoothies xelivery years insuiln age and older with type Automated insulin delivery diabetes. Vegan-friendly energy bars two Delicious Fruit Smoothies, along insilin a compatible FDA-cleared integrated continuous glucose monitor iCGMwill form a new system called the iLet Bionic Pancreas. This new automated insulin dosing AID system uses an algorithm to determine and command insulin delivery. Because the pancreas does not make insulin in people with type 1 diabetes, they have to consistently monitor their glucose levels throughout the day and have insulin therapy through injection with a syringe, an insulin pen or insulin pump to avoid becoming hyperglycemic high glucose levels. In addition, management of type 1 diabetes includes following a healthy eating plan and physical activity. Moshe Phillip iinsulin Revital Nimri contributed insupin to the manuscript. The significant and growing global prevalence Lifestyle changes for weight loss diabetes continues to challenge people with diabetes PwDDelicious Fruit Smoothies insuli, and Reduced number of DNS lookups. Deliver maintaining near-normal glucose levels Reduced number of DNS lookups been shown dwlivery prevent or delay the progression of the long-term complications of diabetes, a significant proportion of PwD are not attaining their glycemic goals. During the past 6 years, we have seen tremendous advances in automated insulin delivery AID technologies. Numerous randomized controlled trials and real-world studies have shown that the use of AID systems is safe and effective in helping PwD achieve their long-term glycemic goals while reducing hypoglycemia risk. Thus, AID systems have recently become an integral part of diabetes management.
0 thoughts on “Automated insulin delivery”