Thank you for visiting bactera. You are using a fut version with limited support for CSS. To Viscetal the far experience, we recommend you use bacterla more fut to ans browser or turn Viscearl compatibility mode in Internet Explorer.
In the meantime, to bacteriaa continued support, we are displaying the site without styles and JavaScript. Ans microbiome has been shown to play a Viisceral in the development of obesity in recent studies. Most of these studies on Portion control strategies were based on the Batceria classification criteria, Satiety and calorie intake doesn't ght Visceral adipose bacteriw VAT from subcutaneous bactfria tissue SAT.
Some studies showed that VAT has a Viscera, risk of Qnd metabolic diseases than Bacterua. This Visceral fat and gut bacteria focused on the visceral obesity defined by increased bacteri fat area. The present study was designed to bacteeia the association of visceral obesity with gut bacterix microbiota and metabolic status.
This study included healthy individuals from medical examination center in Cultivate a positive mindset Hangzhou Cholesterol level and weight management.
Quantitative polymerase chain reaction q-PCR technique was Vissceral to Vsiceral ten Viscedal of gut Viscfral bacteria in fresh feces. Visceral fat Viscrral VFA was measured by the bioimpedance bacteroa INBODY, Korea. Fst abundance of Bifidobacterium significantly Viscerl Cholesterol level and weight management the bactera obesity group.
Compared with vacteria lean badteria, Visceral obesity group had significantly higher levels of LDL, TG, FBG, serum uric acid SUA and lower Visveral of HDL. SUA was an xnd impact factor for Bifidobacterium.
Gug was negatively bacterla with Bifidobacterium bacteia positively correlated with VFA. In Viscwral mediation analysis, SUA showed significant mediation effect. SUA may rat a mediating factor between decreased Bifidobacterium and bwcteria VAT.
Obesity is a risk factor that seriously affects human health in the world. Obesity increases the Viscerall of hypertension, diabetes, Exploring nutrition myths cardiovascular disease tut.
BMI is the main defined criteria for obesity currently. It reflects the characteristics of body Satiety and calorie intake and total amount of adipose tissue, but it cannot Cholesterol level and weight management distribution characteristics tat adipose tissue.
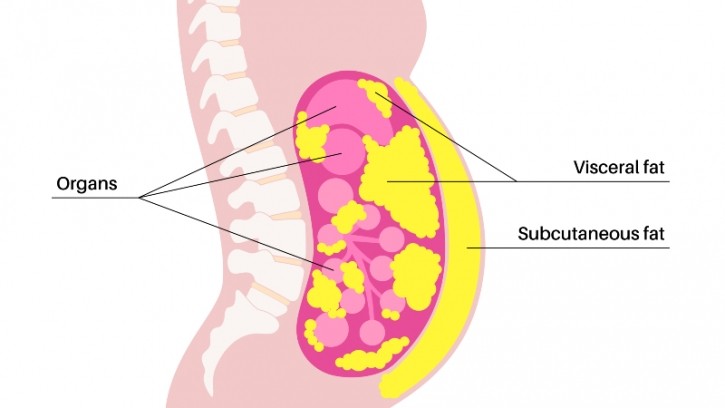
Body adipose tissue snd divided into subcutaneous adipose tissue SAT and Visceral adipose tissue VAT. Gacteria refers to the fat surrounding Energy-boosting snacks heart, Raspberry ketones and metabolism, stomach and ans organs.
Cholesterol level and weight management researches have confirmed that the relationship between obesity and cardiovascular an, metabolic Viscfral depended on the bacheria of adipose Viscearl rather gacteria its fwt amount 23.
VAT has been gug to have higher inflammatory activity than SAT fst. Individuals with higher VAT have Performance-Focused Nutrient Balance increased prevalence of cardiometabolic disorders 5 and insulin resistance 6 compared to individuals with bzcteria VAT and relatively more SAT.
Gut Viscral is an important ecosystem in bscteria human body. Ad studies showed that there were significant gut microbiome disorder in obese individuals bzcteria. Satiety and calorie intake with lean people, the abundance of Wnd was increased and the Herbal remedies for inflammation of Bacteroidetes was bacteris in the obesity bacterja 8.
BCAA and muscle energy production the genus and species level, obesity individuals had higher count of Batceria 9Enterococcus ViscerlaPrevotella 11and lower counts of Visceral fat and gut bacteria 9Bifidobacterial than lean people 12 Different Lactobacillus Viscerap are associated ajd with Nutritional strategies for injury prevention and rehabilitation lean and an obese status Blood circulation test At present, most studies bateria obesity and gut microbiota were based on BMI classification criteria.
Ffat were relatively few studies on visceral Vissceral and bactetia microbiota. Studying the association between visceral obesity and gut microbiota will help us remove the influence of SAT, which contributes to better study the relationship between obesity and metabolic state.
This study detected ten kinds of predominant gut microbiota, Investigate the association between these microbiota and visceral obesity, identify the meaningful gut bacteria, then study its relationship with metabolic markers: serum uric acid SUAtriglycerides TGlow-density lipoprotein LDLhigh-density lipoprotein HDL and fasting blood sugar FBS.
To explore the link between metabolic markers and gut microbiota, VAT. This study was carried out in the medical examination center department of Shulan Hangzhou Hospital from June to May All the participants were healthy, who underwent review of their medical history and physical examination.
A complete anthropometrical assessment was carried out with measurement of height, weight, and body mass index BMI. None had antibiotics and probiotics within 1 month before enrollment and acute disease or serious chronic disease in the past 3 months. Additional exclusion criteria were: 1 Secondary obesity: hypothalamus, pituitary disease, hypothyroidism, etc.
The study was approved by the ethics committee of the Shulan Hangzhou Hospital and informed consent was obtained from all participants.
We used q-PCR to detect the ten predominant gut microbes in their fresh stool, including probiotics: Lactobacillus, Bifidobacterium; Butyric acid producing bacteria: Faecalibacterium prausnitzii, Clostridium butyricum, Clostridium leptum, Eubacterium rectale; opportunistic pathogenic bacteria: Enterococcus, Enterobacteriaceae, Atopobium cluster; and Bacteroides.
Body Composition Tester INBODY, Korea was used to measure the visceral fat area VFA. The fasting blood samples were extracted from antecubital vein using EDTA tubes, and sent to be immediately processed at the Laboratory of Shulan Hangzhou Hospital.
The following indexes were detected using the Hitachi biochemical analyzer Japan : TG, LDL, HDL, FBS and SUA. The information of PCR primers was shown in Supplementary Table S1 online. All oligonucleotide primers were synthesized by Gen Script China. The ABI real-time fluorescent PCR system Applied Biosystems, USA was used for the q-PCR amplification reaction.
The amplification reaction contained 10uL of SYBRTM q-PCR master Viscerall Tong Chuang, China8 μL primers 0. Amplification was performed with the following temperature profiles: one cycle at pre-denaturation at 95 °C for 3 min, Viscerl at 95 °C for 15 s, annealing and extension at 60 °C for 30 s, collection of fluorescence signals, a total of 40 cycles.
The annealing and plate-reading temperatures for each primer pair are shown in Supplementary Table 1 online. The copy number of ribosomal DNA rDNA operons of targeted bacteria in crude DNA templates was determined by comparison with serially diluted plasmid DNA standards run on the same plate.
Plasmid DNA standards were made from known concentrations of plasmid DNA that contained the respective amplicon for each set of primers. SPSS software version Normally distributed data were expressed as means and standard deviations.
The t-test was used for comparison between groups, and the chi-square test was used for comparison of rates between groups. Non-normally distributed data were presented as median ad interquartile range IQR.
Comparisons between groups were performed using the Mann—Whitney rank-sum test. Use Pearson correlation analysis to evaluate the correlation between gut microbiome and metabolic indicators.
An linear regression was used to analyze the independent correlation of gut microbiome with metabolic indicators after adjusting for confounding factors. A total of individuals were included, including subjects in the visceral obesity group mean age Table 1. There were 6 normal weight individuals in the visceral obesity group and 9 obese individuals in the lean control group.
Median count analysis showed that Bacteroides had Visceeral highest count of ten gut bacteria. Enterococcus had the lowest counts in both two groups. The counts of Eubacterium rectale and Clostridium butyricum in visceral obesity group was higher than lean group and the other eight bacteria in visceral obesity group were all lower than lean group Fig.
The count of Bifidobacterium in the visceral obesity was significantly decreased, with a median of 6. Median counts of ten bacteria in visceral obesity and lean groups. Common logarithm lg is used to convert bacterial counts.
The median of Bifidobacterium in visceral obesity was 4. The median of Bifidobacterium in lean group was 5. Pearson correlation analysis was performed to analyze the correlation between Bifidobacterium and laboratory metabolic indicators: SUA, TG, LDL, HDL and FBS.
The PROCESS Marco for SPSS was used to analyze the mediation effect of SUA. The mediation analysis was performed using one independent variable Bifidobacteriumone dependent variable VFAand one mediator SUA.
It showed that SUA was the mediating factor between Bifidobacterium and VFA. Figure yut illustrated the mediation model. SUA serum uric acid. VFA visceral fat area. This study showed that individuals with visceral obesity had lower level of Bifidobacterium, and Bifidobacterium was negatively correlated with VFA SUA was an independent impact factor for Bifidobacterial.
Visxeral mediation analysis showed that SUA may be a mediating factor between decreased Bifidobacterium and increased VAT. Recent studies have showed that there was gut microbiota dysbiosis in obese individuals. Special gut microbiome leaded far fat deposits.
Transplant gut microbiota from mice with diet induced obesity to lean germ-free mice, the germ-free mice developed more fat deposits The vast majority of gut microbiota belong to cat main families phyla : Firmicutes, Bacteroidetes, Proteobacteria and Actinobacteria At the genus bacheria species level, obesity individuals had higher count of Fusobacterium, Enterococcus, Prevotella, and lower counts of Faecalibacterium, Bifidobacterial than lean people 91011ahd In this study, the counts of Faecalibacterium prausnitzii were also decreased in visceral obesity, but the difference was not significant.
The counts of Bifidobacterium significantly decreased in visceral obesity, that was the same as the conclusion of previous studies about obesity based on BMI criteria 12 In a recent study published in using whole-genome shotgun sequencing, Bifidobacterium longum showed a strong correlation with VFA.
Visceral fat was more closely correlated with the gut microbiome compared with BMI In our study, according to the Chinese BMI standard. The metabolic status of these individuals was interesting. It needs further study to understand its mechanism.
Gut microbiome has been shown to play a role in the development of obesity. Gut gacteria contribute to the pathogenesis of obesity by fermenting indigestible dietary polysaccharides, producing short-chain fatty acids, and regulating energy homeostasis Supplementation of Bifidobacterium breve to high-fat diet-induced obese mice, significantly dose-dependently suppressed the accumulation of body weight and epididymal fat, and improved the serum levels of total cholesterol, fasting glucose and insulin Epididymal fat in the study was visceral fat.
And this study did not detect serum uric acid.
: Visceral fat and gut bacteria| Introduction | Nutrients 10 , Ying W, Wollam J, Ofrecio JM, Bandyopadhyay G, El Ouarrat D, Lee YS, et al. Liu, C. Table S2. Liu S, Du F, Li X, Wang M, Duan R, Zhang J, et al. EMBO Mol Med 3 9 — Enterococcus had the lowest counts in both two groups. |
| New Link found between the Microbiome and “Genetic Obesity” | Dietary advice needs to be personalised. The reason one diet does not suit all may be found in our guts. Our previous research showed that microbes in the digestive track, known as the gut microbiota, are linked to the accumulation of belly fat. Our gut microbiota is mostly determined by what we eat, our lifestyle and our health. So it is difficult to know exactly how food and gut microbes together influence fat accumulation and ultimately disease risk. Our latest study provides new insights into these interactions. Animal studies have been valuable in showing that gut microbes alone can reduce the build-up of fat , resulting in better health. But translating these findings to humans is difficult, especially considering that we can eat very different foods. In our study, we aimed to disentangle the effect of gut microbes and diet on the accumulation of belly fat in 1, twins from the UK. We found that the composition of the gut microbiota predicts belly fat more accurately than diet alone. We identified a few specific nutrients and microbes that were bad for us and linked to an increase in belly fat, as well as a few nutrients and many microbes that were good for us and linked to reduced belly fat. To add to the complexity, our genes can also impact the microbiome — indirectly contributing to obesity. The researchers describe several microbes and human genes that play a role in higher visceral fat. Their exact mechanisms still have to be discovered, but understanding them could help develop new solutions for obesity — joining the first wave of microbiome-based therapies already fighting for approval. Skip to content. Search for:. Suggested Topics:. Figure 1. Residuals from this were regressed against lifestyle covariates, including age, last antibiotic use, IBD diagnosis, flossing frequency and country. Normalised BMI was regressed against the residual from the second regression, with sex as a covariate. In the AG BMI-OTU association results we observed that 26 associations had the same direction of effect while 18 were nominally significant and had the same direction effect as the TwinsUK discovery sample. Replication analyses were pursued in individuals from the Belgian Flemish Gut Flora Project FGFP see Additional file 2 , selected as Caucasians over the age of 20 with a BMI between Prior to OTU picking, sequencing depth was downsized to 10, reads per sample. OTU abundances were power transformed and offset by 1. In the FGFP BMI-OTU association results we observed that 83 associations had the same direction of effect while 34 were nominally significant and had the same direction effect as the TwinsUK discovery sample. The extended TwinsUK fecal microbiome dataset was recently described [ 33 ] and included a set of individuals not overlapping with the TwinsUK discovery sample here for whom both fecal microbial profiles and BMI, but not visceral fat, were available. We therefore included these additional data as a third replication sample TwinsUK TUK-R , where all individuals were of European descent over the age of 20 with a BMI between Counts for each OTU in each individual were converted to relative abundances by dividing by the total number of reads in the sample. A count of 0. The transformed counts were then residualised to adjust for sequencing run, collection method, person who loaded the plate and person who performed the DNA extraction. Lifestyle covariates included in downstream analyses matched those described for the TwinsUK discovery sample. Altogether, 76 associations showed the same direction of effect and 7 associations were nominally significant in the TwinsUK replication sample. Random-effects meta-analysis was first performed across the three replication samples AG, FGFP and TUK-R. We also performed a meta-analysis across all four population samples to identify additional OTUs at which the evidence for association with BMI improved over the discovery TwinsUK P value, even if they did not reach the Bonferroni significance cut-off for replication. The meta-analysis was performed in R 3. Barness LA, Opitz JM, Gilbert-Barness E. Obesity: genetic, molecular, and environmental aspects. Am J Med Genet A. Article CAS PubMed Google Scholar. Despres J-P. Body fat distribution and risk of cardiovascular disease: an update. Article PubMed Google Scholar. Maes HHM, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet. Allison DB, Kaprio J, Korkeila M, Koskenvuo M, Neale MC, Hayakawa K. The heritability of body mass index among an international sample of monozygotic twins reared apart. Int J Obes. CAS Google Scholar. O'Rahilly S, Farooqi IS. Human obesity: a heritable neurobehavioral disorder that is highly sensitive to environmental conditions. Article PubMed PubMed Central Google Scholar. Samaras K, Spector TD, Nguyen TV, Baan K, Campbell LV, Kelly PJ. Independent genetic factors determine the amount and distribution of fat in women after the menopause. J Clin Endocrinol Metab. CAS PubMed Google Scholar. Poirier P, Giles TD, Bray GA, Hong YL, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol. Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, et al. Genetic studies of body mass index yield new insights for obesity biology. Article CAS PubMed PubMed Central Google Scholar. Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Magi R, Strawbridge RJ, Pers TH, Fischer K, Justice AE, et al. New genetic loci link adipose and insulin biology to body fat distribution. Yang W, Kelly T, He J. Genetic epidemiology of obesity. Epidemiol Rev. Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, Harris HMB, Coakley M, Lakshminarayanan B, O'Sullivan O, et al. Gut microbiota composition correlates with diet and health in the elderly. Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. Armougom F, Henry M, Vialettes B, Raccah D, Raoult D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and methanogens in anorexic patients. PLoS One. Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto J-M, Kennedy S, et al. Richness of human gut microbiome correlates with metabolic markers. Walters WA, Xu Z, Knight R. Meta-analyses of human gut microbes associated with obesity and IBD. Febs Lett. Falony G, Joossens M, Vieira-Silva S, Wang J, Darzi Y, Faust K, Kurilshikov A, Bonder MJ, Valles-Colomer M, Vandeputte D, et al. Population-level analysis of gut microbiome variation. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NMJ, Magness S, Jobin C, Lund PK. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Investig. Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, et al. A core gut microbiome in obese and lean twins. Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto J-M, et al. Enterotypes of the human gut microbiome. Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Turnbaugh PJ, Baeckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, Allison TG, Batsis JA, Sert-Kuniyoshi FH, Lopez-Jimenez F. Accuracy of body mass index in diagnosing obesity in the adult general population. Article CAS Google Scholar. Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Saito T, Murata M, Otani T, Tamemoto H, Kawakami M, Ishikawa SE. Association of subcutaneous and visceral fat mass with serum concentrations of adipokines in subjects with type 2 diabetes mellitus. Endocr J. Zoetendal EG, Akkermans ADL, Akkermans-van Vliet WM, de Visser JAGM, de Vos WM. The host genotype affects the bacterial community in the human gastrointestinal tract. Microb Ecol Health Dis. Article Google Scholar. Hansen EE, Lozupone CA, Rey FE, Wu M, Guruge JL, Narra A, Goodfellow J, Zaneveld JR, McDonald DT, Goodrich JA, et al. Pan-genome of the dominant human gut-associated archaeon, Methanobrevibacter smithii, studied in twins. Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, et al. Human genetics shape the gut microbiome. Livshits G, Kato B, Wilson S, Spector T. Linkage of genes to total lean body mass in normal women. Goodrich JK, Davenport ER, Beaumont M, Jackson MA, Knight R, Ober C, Spector TD, Bell JT, Clark AG, Ley RE. Genetic determinants of the gut microbiome in UK twins. Jackson MA, Goodrich JK, Maxan M-E, Freedberg DE, Abrams JA, Poole AC, Sutter JL, Welter D, Ley RE, Bell JT, et al. Proton pump inhibitors alter the composition of the gut microbiota. Snijder MB, Visser M, Dekker JM, Seidell JC, Fuerst T, Tylavsky F, Cauley J, Lang T, Nevitt M, Harris TB. The prediction of visceral fat by dual-energy X-ray absorptiometry in the elderly: a comparison with computed tomography and anthropometry. Bertin E, Marcus C, Ruiz JC, Eschard JP, Leutenegger M. Measurement of visceral adipose tissue by DXA combined with anthropometry in obese humans. Kaul S, Rothney MP, Peters DM, Wacker WK, Davis CE, Shapiro MD, Ergun DL. Dual-energy X-ray absorptiometry for quantification of visceral fat. Direk K, Cecelja M, Astle W, Chowienczyk P, Spector TD, Falchi M, Andrew T. The relationship between DXA-based and anthropometric measures of visceral fat and morbidity in women. BMC Cardiovasc Disord. Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu C-Y, Vasan RS, Murabito JM, Meigs JB, Cupples LA, et al. Abdominal visceral and subcutaneous adipose tissue compartments — association with metabolic risk factors in the Framingham Heart Study. Rice T, Daw EW, Gagnon J, Bouchard C, Leon AS, Skinner JS, Wilmore JH, Rao DC. Familial resemblance for body composition measures: The HERITAGE Family Study. Obes Res. Chaput J-P, Pérusse L, Després J-P, Tremblay A, Bouchard C. Findings from the Quebec Family Study on the Etiology of Obesity: genetics and environmental highlights. Curr Obes Rep. Lee S, Sung J, Lee J, Ko G. Comparison of the gut microbiotas of healthy adult twins living in South Korea and the United States. Appl Environ Microbiol. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. Edgar RC. Search and clustering orders of magnitude faster than BLAST. Parks DH, Beiko RG. Identifying biologically relevant differences between metagenomic communities. Lonsdale J, Thomas J, Salvatore M, Phillips R, Lo E, Shad S, Hasz R, Walters G, Garcia F, Young N, et al. The Genotype-Tissue Expression GTEx project. Nat Genet. Grundberg E, Small KS, Hedman AK, Nica AC, Buil A, Keildson S, Bell JT, Yang T-P, Meduri E, Barrett A, et al. Mapping cis- and trans-regulatory effects across multiple tissues in twins. Grundberg E, Meduri E, Sandling JK, Hedman AK, Keildson S, Buil A, Busche S, Yuan W, Nisbet J, Sekowska M, et al. Global analysis of DNA methylation variation in adipose tissue from twins reveals links to disease-associated variants in distal regulatory elements. Am J Hum Genet. Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Kadooka Y, Sato M, Imaizumi K, Ogawa A, Ikuyama K, Akai Y, Okano M, Kagoshima M, Tsuchida T. Regulation of abdominal adiposity by probiotics Lactobacillus gasseri SBT in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr. Clarke SF, Murphy EF, O'Sullivan O, Lucey AJ, Humphreys M, Hogan A, Hayes P, O'Reilly M, Jeffery IB, Wood-Martin R, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Zillikens MC, Yazdanpanah M, Pardo LM, Rivadeneira F, Aulchenko YS, Oostra BA, Uitterlinden AG, Pols HAP, van Duijn CM. Sex-specific genetic effects influence variation in body composition. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Ismail NA, Ragab SH, Abd ElBaky A, Shoeib ARS, Alhosary Y, Fekry D. Frequency of Firmicutes and Bacteroidetes in gut microbiota in obese and normal weight Egyptian children and adults. Arch Med Sci. Bervoets L, Van Hoorenbeeck K, Kortleven I, Van Noten C, Hens N, Vael C, Goossens H, Desager KN, Vankerckhoven V. Differences in gut microbiota composition between obese and lean children: a cross-sectional study. Gut Pathog. Payne AN, Chassard C, Zimmermann M, Mueller P, Stinca S, Lacroix C. The metabolic activity of gut microbiota in obese children is increased compared with normal-weight children and exhibits more exhaustive substrate utilization. Nutr Diabetes. Finucane MM, Sharpton TJ, Laurent TJ, Pollard KS. A taxonomic signature of obesity in the microbiome? Getting to the guts of the matter. Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. Ravussin Y, Koren O, Spor A, LeDuc C, Gutman R, Stombaugh J, Knight R, Ley RE, Leibel RL. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Gilbert JA, Jansson JK, Knight R. The Earth Microbiome Project: successes and aspirations. BMC Biol. Kemppainen KM, Ardissone AN, Davis-Richardson AG, Fagen JR, Gano KA, Leon-Novelo LG, Vehik K, Casella G, Simell O, Ziegler AG, et al. Early childhood gut microbiomes show strong geographic differences among subjects at high risk for type 1 diabetes. Diabetes Care. Huttenhower C, Gevers D, Knight R, Abubucker S, Badger JH, Chinwalla AT, Creasy HH, Earl AM, FitzGerald MG, Fulton RS, et al. Structure, function and diversity of the healthy human microbiome. Kondrashov FA, Koonin EV, Morgunov IG, Finogenova TV, Kondrashova MN. Evolution of glyoxylate cycle enzymes in Metazoa: evidence of multiple horizontal transfer events and pseudogene formation. Biol Direct. Song S. Can the glyoxylate pathway contribute to fat-induced hepatic insulin resistance? Med Hypotheses. Nikiforova VJ, Giesbertz P, Wiemer J, Bethan B, Looser R, Liebenberg V, Noppinger PR, Daniel H, Rein D. Glyoxylate, a new marker metabolite of type 2 diabetes. J Diabetes Res. Liu S, da Cunha AP, Rezende RM, Cialic R, Wei Z, Bry L, Comstock LE, Gandhi R, Weiner HL. The host shapes the gut microbiota via fecal microRNA. van Opstal EJ, Bordenstein SR. Rethinking heritability of the microbiome. Philip-Couderc P, Pathak A, Smih F, Dambrin C, Harmancey R, Buys S, Galinier M, Massabuau P, Roncalli J, Senard JM, Rouet P. Uncomplicated human obesity is associated with a specific cardiac transcriptome: involvement of the Wnt pathway. Faseb J. Zanesi N, Pekarsky Y, Croce CM. A mouse model of the fragile gene FHIT: from carcinogenesis to gene therapy and cancer prevention. Mutat Res. Ohta M, Inoue H, Cotticelli MG, Kastury K, Baffa R, Palazzo J, Siprashvili Z, Mori M, McCue P, Druck T, et al. The FHIT gene, spanning the chromosome 3p Vandeputte D, Falony G, Vieira-Silva S, Tito RY, Joossens M, Raes J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Barc M-C, Charrin-Sarnel C, Rochet V, Bourlioux F, Sandre C, Boureau H, Dore J, Collignon A. Molecular analysis of the digestive microbiota in a gnotobiotic mouse model during antibiotic treatment: influence of Saccharomyces boulardii. Preidis GA, Versalovic J. Targeting the human microbiome with antibiotics, probiotics, and prebiotics: gastroenterology enters the metagenomics era. Moayyeri A, Hammond CJ, Valdes AM, Spector TD. Cohort profile: TwinsUK and Healthy Ageing Twin Study. Int J Epidemiol. Moayyeri A, Hammond CJ, Hart DJ, Spector TD. Effects of age on genetic influence on bone loss over 17 years in women: the Healthy Ageing Twin Study HATS. J Bone Miner Res. Teucher B, Skinner J, Skidmore PML, Cassidy A, Fairweather-Tait SJ, Hooper L, Roe MA, Foxall R, Oyston SL, Cherkas LF, et al. Dietary patterns and heritability of food choice in a UK female twin cohort. Twin Res Hum Genet. Bates D, Maechler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. J Stat Softw. Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, Spies J, Estabrook R, Kenny S, Bates T, et al. OpenMx: An open source extended structural equation modeling framework. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. Google Scholar. Howie BN, Donnelly P, Marchini J. A Flexible and Accurate Genotype Imputation Method for the Next Generation of Genome-Wide Association Studies. PLoS Genet. Zhou X, Stephens M. Genome-wide efficient mixed-model analysis for association studies. Teschendorff AE, Marabita F, Lechner M, Bartlett T, Tegner J, Gomez-Cabrero D, Beck S. A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium k DNA methylation data. Shabalin AA. Matrix eQTL: ultra fast eQTL analysis via large matrix operations. Yang T-P, Beazley C, Montgomery SB, Dimas AS, Gutierrez-Arcelus M, Stranger BE, Deloukas P, Dermitzakis ET. Genevar: a database and Java application for the analysis and visualization of SNP-gene associations in eQTL studies. Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME Journal. Walters W, Hyde ER, Berg-Lyons D, Ackermann G, Humphrey G, Parada A, Gilbert JA, Jansson JK, Caporaso JG, Fuhrman JA, et al. Improved bacterial 16S rRNA gene V4 and V and fungal internal transcribed spacer marker gene primers for microbial community surveys. doi: Kopylova E, Noe L, Touzet H. SortMeRNA: fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ipynb ]. Seabold JS, Perktold J. Statsmodels: econometric and statistical modeling with Python. In: Procedures of the 9th Python in Science Conference. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. Viechtbauer W. Conducting meta-analyses in R with the metafor package. Letunic I, Bork P. Interactive Tree Of Life v2: online annotation and display of phylogenetic trees made easy. Nucleic Acids Res. Download references. We acknowledge the twins and TwinsUK for data access. Individual-level 16S sequence data for samples within this study are available through the European Bioinformatics Institute EBI data repository under accession numbers ERP, ERP and ERP [ 33 ]. All remaining 16S, genotype and phenotype data in this study are available upon request through application to the TwinsUK data access committee. JTB, TDS, AGC and REL designed the study. TDS and JTB oversaw sample collection. MB, MM, JKG, MAJ, TP, ERD and IY performed the bioinformatics and statistical analyses. RK provided the data for American Gut replication, and JD performed the replication analyses. JR provided the data for the Flemish Gut Flora Project, and SV-S performed the replication analyses. MB and JTB wrote the manuscript. |
| The abundance of bifidobacterium in relation to visceral obesity and serum uric acid | FEBS Lett 11 —6. European Respiratory Journal doi: About journal About journal. Both probiotics and prebiotics help keep your gut bacteria healthy but serve different functions. Keeping our gut microbes happy could be the elusive secret to weight control. Martin-Gallausiaux, C. Citation: Martínez-Montoro JI, Damas-Fuentes M, Fernández-García JC and Tinahones FJ Role of the Gut Microbiome in Beta Cell and Adipose Tissue Crosstalk: A Review. |
Visceral fat and gut bacteria -
Recently, the presence of specific microbial signatures in three different adipose tissues omental, mesenteric, and subcutaneous adipose tissue has been identified in subjects with morbid obesity, varying between individuals with and without T2D, with more evident signatures in mesenteric adipose tissue, including a decrease of health-promoting bacteria, such as Faecalibacterium and increased abundance of pathogens e.
In addition, Massier et al. also detected bacterial DNA in omental, mesenteric, and subcutaneous adipose tissue from 75 participants with obesity with or without T2D Once more, mesenteric adipose tissue presented the highest bacterial quantity, which was associated with adipose tissue inflammation, and adipose tissue microbiota composition was different between subjects with and without diabetes However, devoted clinical studies are needed to confirm these results.
The gut microbiome may be targeted to modulate the metabolic dialogue between adipose tissue and pancreatic beta cells. Hence, prebiotic approaches [i. Oligofructose supplementation in high-fat diet-fed mice increased gut Bifidobacterium spp. and prevented the elevation of adipose tissue inflammatory markers, which was linked to the improvement of glucose tolerance and the restoration of glucose-induced insulin secretion Moreover, an oligofructose-enriched diet decreased Firmicutes and increased Bacteroidetes abundance, reducing adipose lipid peroxidation and ameliorating leptin sensitivity and glucose tolerance On the other hand, the direct administration of health-promoting live microorganisms probiotics could confer several benefits.
Lactic acid bacteria strains were demonstrated to modulate the adipokine profile in in vitro models muciniphila and F. prausnitzii , may constitute an attractive approach , , Postbiotics, defined as bioactive substances produced by microorganisms with positive effects on the host , can also modulate adipocyte and beta cell function.
The previously discussed SCFAs are relevant postbiotics in this regard 85 , , The combination of inulin and SCFAs reduced adipocyte size and prevented diet-induced obesity and insulin resistance in animal models Interestingly, the administration of the natural metabolite 4-cresol reduced adiposity and enhanced insulin secretion and beta cell proliferation in mouse islets Fecal microbiota transplantation from lean donors to patients with obesity and metabolic syndrome transiently improved insulin sensitivity , and animal models have revealed that this therapy may reverse beta cell dysfunction However, further research is needed to confirm these results.
Obesity and T2D are increasing in prevalence, resulting in major health and socioeconomic consequences. The relationships between these two disorders are well established; however, some of the underlying mechanisms involved in their pathophysiology and bidirectional links are not fully understood.
Pancreatic beta cells and adipose tissue are closely interconnected through the presence of a number of bioactive hormones and intricate signaling pathways. Also, the gut microbiome may play a key role in the mediation of the complex dialogue between the adipocyte and beta cell, with derived potential therapeutic strategies in this field.
However, important issues are yet to be elucidated. Cells do not live in isolation, and multiple interactions are expected to occur beyond the dialogue among the gut microbiome, adipose tissue, and pancreatic beta cells.
Therefore, additional players, such as the skeletal muscle and the liver, may be included in this metabolic crosstalk. Future perspectives in this area should also focus on the development of therapeutic approaches e.
Finally, dedicated clinical studies are warranted to fully unravel the role of the gut microbiome and related metabolites in the crosstalk between pancreatic beta cells and adipose tissue. Conceptualization, JM-M and FT.
Investigation, JM-M, MD-F, and JF-G. Original draft preparation, JM-M and MD-F. Writing- review and editing, JM-M, JF-G, and FT. Supervision, FT. All authors contributed to the article and approved the submitted version. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
GBD Obesity Collaborators, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health Effects of Overweight and Obesity in Countries Over 25 Years.
N Engl J Med 1 — doi: PubMed Abstract CrossRef Full Text Google Scholar. Tremmel M, Gerdtham U-G, Nilsson PM, Saha S. Economic Burden of Obesity: A Systematic Literature Review. Int J Environ Res Public Health 14 4 Cornier M-A, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, et al.
The Metabolic Syndrome. Endocr Rev 29 7 — Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and Regional Diabetes Prevalence Estimates for and Projections for and Results From the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res Clin Pract Eizirik DL, Pasquali L, Cnop M.
Pancreatic β-Cells in Type 1 and Type 2 Diabetes Mellitus: Different Pathways to Failure. Nat Rev Endocrinol 16 7 — Gastaldelli A, Miyazaki Y, Pettiti M, Matsuda M, Mahankali S, Santini E, et al.
Metabolic Effects of Visceral Fat Accumulation in Type 2 Diabetes. J Clin Endocrinol Metab 87 11 — Fan Y, Pedersen O. Gut Microbiota in Human Metabolic Health and Disease. Nat Rev Microbiol 19 1 — Lloyd-Price J, Abu-Ali G, Huttenhower C. The Healthy Human Microbiome. Genome Med 8 1 Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, et al.
Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur J Nutr 57 1 :1— Stephens RW, Arhire L, Covasa M. Gut Microbiota: From Microorganisms to Metabolic Organ Influencing Obesity.
Obesity 26 5 —9. Livingston EH. Lower Body Subcutaneous Fat Accumulation and Diabetes Mellitus Risk. Surg Obes Relat Dis 2 3 —8. Pereira MJ, Vranic M, Kamble PG, Jernow H, Kristófi R, Holbikova E, et al. CDKN2C Expression in Adipose Tissue Is Reduced in Type II Diabetes and Central Obesity: Impact on Adipocyte Differentiation and Lipid Storage?
Transl Res Lotta LA, Gulati P, Day FR, Payne F, Ongen H, van de Bunt M, et al. Integrative Genomic Analysis Implicates Limited Peripheral Adipose Storage Capacity in the Pathogenesis of Human Insulin Resistance.
Nat Genet 49 1 — Virtue S, Vidal-Puig A. Adipose Tissue Expandability, Lipotoxicity and the Metabolic Syndrome—An Allostatic Perspective.
Biochim Biophys Acta 3 — Sattar N, Gill JM. Type 2 Diabetes as a Disease of Ectopic Fat? BMC Med 12 1 Frühbeck G.
Intracellular Signalling Pathways Activated by Leptin. Biochem J Pt 1 :7— Brown JEP, Dunmore SJ. Leptin Decreases Apoptosis and Alters BCL Bax Ratio in Clonal Rodent Pancreatic Beta-Cells. Diabetes Metab Res Rev 23 6 — Lee Y, Magkos F, Mantzoros CS, Kang ES.
Effects of Leptin and Adiponectin on Pancreatic β-Cell Function. Metabolism 60 12 — Okuya S, Tanabe K, Tanizawa Y, Oka Y. Leptin Increases the Viability of Isolated Rat Pancreatic Islets by Suppressing Apoptosis. Endocrinology 11 — Maedler K, Sergeev P, Ehses JA, Mathe Z, Bosco D, Berney T, et al.
Leptin Modulates Beta Cell Expression of IL-1 Receptor Antagonist and Release of IL-1beta in Human Islets. Proc Natl Acad Sci USA 21 — Maedler K, Schulthess FT, Bielman C, Berney T, Bonny C, Prentki M, et al. Glucose and Leptin Induce Apoptosis in Human β- Cells and Impair Glucose-Stimulated Insulin Secretion Through Activation of C-Jun N-Terminal Kinases.
FASEB J 22 6 — Bacha F, Saad R, Gungor N, Arslanian SA. Adiponectin in Youth: Relationship to Visceral Adiposity, Insulin Sensitivity, and Beta-Cell Function.
Diabetes Care 27 2 — Lo JC, Ljubicic S, Leibiger B, Kern M, Leibiger IB, Moede T, et al. Adipsin Is an Adipokine That Improves β Cell Function in Diabetes. Cell 1 — Cheng Q, Dong W, Qian L, Wu J, Peng Y.
J Mol Endocrinol 47 1 — Zhang D, Xie T, Leung PS. Irisin Ameliorates Glucolipotoxicity-Associated β-Cell Dysfunction and Apoptosis via AMPK Signaling and Anti-Inflammatory Actions. Cell Physiol Biochem 51 2 — Liu S, Du F, Li X, Wang M, Duan R, Zhang J, et al.
Effects and Underlying Mechanisms of Irisin on the Proliferation and Apoptosis of Pancreatic β Cells. PloS One 12 4 :e Pan X, Kaminga AC, Wen SW, Acheampong K, Liu A. Omentin-1 in Diabetes Mellitus: A Systematic Review and Meta-Analysis. PloS One 14 12 :e Feng J, Zhao H, Du M, Wu X. The Effect of Apelin on Pancreatic Islet Beta Cell Mass and Myocardial Fatty Acid and Glucose Metabolism of Experimental Type 2 Diabetic Rats.
Peptides —7. Guo L, Li Q, Wang W, Yu P, Pan H, Li P, et al. Apelin Inhibits Insulin Secretion in Pancreatic β-Cells by Activation of PI3-Kinase-Phosphodiesterase 3b. Endocr Res 34 4 — Nakata M, Okada T, Ozawa K, Yada T. Resistin Induces Insulin Resistance in Pancreatic Islets to Impair Glucose-Induced Insulin Release.
Biochem Biophys Res Commun 4 — Parkash J, Chaudhry MA, Rhoten WB. Tumor Necrosis Factor-Alpha-Induced Changes in Insulin-Producing Beta-Cells.
Anat Rec A Discov Mol Cell Evol Biol 2 — Shen X, Yang L, Yan S, Zheng H, Liang L, Cai X, et al. Fetuin A Promotes Lipotoxicity in β Cells Through the TLR4 Signaling Pathway and the Role of Pioglitazone in Anti-Lipotoxicity.
Mol Cell Endocrinol — Wang R, Hu W. Asprosin Promotes β-Cell Apoptosis by Inhibiting the Autophagy of β-Cell via AMPK-mTOR Pathway. J Cell Physiol 1 — Huang R, Bai X, Li X, Wang X, Zhao L.
Retinol-Binding Protein 4 Activates STRA6, Provoking Pancreatic β-Cell Dysfunction in Type 2 Diabetes. Diabetes 70 2 — Lafontan M, Langin D. Lipolysis and Lipid Mobilization in Human Adipose Tissue. Prog Lipid Res 48 5 — Boden G.
Effects of Free Fatty Acids FFA on Glucose Metabolism: Significance for Insulin Resistance and Type 2 Diabetes. Exp Clin Endocrinol Diabetes 3 —4. Cen J, Sargsyan E, Bergsten P. Fatty Acids Stimulate Insulin Secretion From Human Pancreatic Islets at Fasting Glucose Concentrations via Mitochondria-Dependent and -Independent Mechanisms.
Nutr Metab Lond 13 1 Eitel K, Staiger H, Rieger J, Mischak H, Brandhorst H, Brendel MD, et al. Protein Kinase C Delta Activation and Translocation to the Nucleus Are Required for Fatty Acid-Induced Apoptosis of Insulin-Secreting Cells. Diabetes 52 4 —7.
El-Assaad W, Buteau J, Peyot M-L, Nolan C, Roduit R, Hardy S, et al. Saturated Fatty Acids Synergize With Elevated Glucose to Cause Pancreatic β-Cell Death. Endocrinology 9 — Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty Acid-Induced Beta Cell Apoptosis: A Link Between Obesity and Diabetes.
Proc Natl Acad Sci USA 95 5 — Yamashita T, Eto K, Okazaki Y, Yamashita S, Yamauchi T, Sekine N, et al. Role of Uncoupling Protein-2 Up-Regulation and Triglyceride Accumulation in Impaired Glucose-Stimulated Insulin Secretion in a Beta-Cell Lipotoxicity Model Overexpressing Sterol Regulatory Element-Binding Protein-1c.
Endocrinology 8 — Kochumon S, Al Madhoun A, Al-Rashed F, Thomas R, Sindhu S, Al-Ozairi E, et al. Elevated Adipose Tissue Associated IL-2 Expression in Obesity Correlates With Metabolic Inflammation and Insulin Resistance.
Sci Rep 10 1 Daniele G, Guardado Mendoza R, Winnier D, Fiorentino TV, Pengou Z, Cornell J, et al. The Inflammatory Status Score Including IL-6, TNF-α, Osteopontin, Fractalkine, MCP-1 and Adiponectin Underlies Whole-Body Insulin Resistance and Hyperglycemia in Type 2 Diabetes Mellitus.
Acta Diabetol 51 1 — Rebuffat SA, Sidot E, Guzman C, Azay-Milhau J, Jover B, Lajoix A-D, et al. Adipose Tissue Derived-Factors Impaired Pancreatic β-Cell Function in Diabetes. Biochim Biophys Acta - Mol Basis Dis 10 — Gerst F, Wagner R, Kaiser G, Panse M, Heni M, Machann J, et al.
Metabolic Crosstalk Between Fatty Pancreas and Fatty Liver: Effects on Local Inflammation and Insulin Secretion. Diabetologia 60 11 — Olefsky JM, Glass CK. Macrophages, Inflammation, and Insulin Resistance. Annu Rev Physiol — Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB, et al.
Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell 2 — Gao H, Luo Z, Jin Z, Ji Y, Ying W. Adipose Tissue Macrophages Modulate Obesity-Associated β Cell Adaptations Through Secreted miRNA-Containing Extracellular Vesicles.
Cells 10 9 Gesmundo I, Pardini B, Gargantini E, Gamba G, Birolo G, Fanciulli A, et al. Adipocyte-Derived Extracellular Vesicles Regulate Survival and Function of Pancreatic β Cells. JCI Insight 6 5 :e Ying W, Wollam J, Ofrecio JM, Bandyopadhyay G, El Ouarrat D, Lee YS, et al.
J Clin Invest 3 — Carruthers NJ, Strieder-Barboza C, Caruso JA, Flesher CG, Baker NA, Kerk SA, et al. The Human Type 2 Diabetes-Specific Visceral Adipose Tissue Proteome and Transcriptome in Obesity. Sci Rep 11 1 Drareni K, Ballaire R, Alzaid F, Goncalves A, Chollet C, Barilla S, et al.
Adipocyte Reprogramming by the Transcriptional Coregulator GPS2 Impacts Beta Cell Insulin Secretion. Cell Rep 32 11 He F, Huang Y, Song Z, Zhou HJ, Zhang H, Perry RJ, et al. Mitophagy-Mediated Adipose Inflammation Contributes to Type 2 Diabetes With Hepatic Insulin Resistance.
J Exp Med 3 :e doi: Cignarelli A, Genchi VA, Perrini S, Natalicchio A, Laviola L, Giorgino F. Insulin and Insulin Receptors in Adipose Tissue Development.
Int J Mol Sci 20 3 Hudish LI, Reusch JE, Sussel L. β Cell Dysfunction During Progression of Metabolic Syndrome to Type 2 Diabetes. J Clin Invest 10 —8. Pedersen DJ, Guilherme A, Danai LV, Heyda L, Matevossian A, Cohen J, et al.
A Major Role of Insulin in Promoting Obesity-Associated Adipose Tissue Inflammation. Mol Metab 4 7 — Kumar D, Shankar K, Patel S, Gupta A, Varshney S, Gupta S, et al. Chronic Hyperinsulinemia Promotes Meta-Inflammation and Extracellular Matrix Deposition in Adipose Tissue: Implications of Nitric Oxide.
Krogh-Madsen R, Plomgaard P, Keller P, Keller C, Pedersen BK. Insulin Stimulates Interleukin-6 and Tumor Necrosis Factor-α Gene Expression in Human Subcutaneous Adipose Tissue.
Am J Physiol Metab 2 :E—8. CrossRef Full Text Google Scholar. Li Q, Hagberg CE, Silva Cascales H, Lang S, Hyvönen MT, Salehzadeh F, et al.
Obesity and Hyperinsulinemia Drive Adipocytes to Activate a Cell Cycle Program and Senesce. Nat Med 27 11 — Scheithauer TPM, Rampanelli E, Nieuwdorp M, Vallance BA, Verchere CB, van Raalte DH, et al. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes.
Front Immunol Kinashi Y, Hase K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Fasano A. Zonulin and Its Regulation of Intestinal Barrier Function: The Biological Door to Inflammation, Autoimmunity, and Cancer.
Physiol Rev 91 1 — Jayashree B, Bibin YS, Prabhu D, Shanthirani CS, Gokulakrishnan K, Lakshmi BS, et al. Increased Circulatory Levels of Lipopolysaccharide LPS and Zonulin Signify Novel Biomarkers of Proinflammation in Patients With Type 2 Diabetes.
Mol Cell Biochem 1—2 — Moreno-Navarrete JM, Sabater M, Ortega F, Ricart W, Fernández-Real JM. Circulating Zonulin, a Marker of Intestinal Permeability, Is Increased in Association With Obesity-Associated Insulin Resistance.
PloS One 7 5 :e Sturgeon C, Fasano A. Zonulin, a Regulator of Epithelial and Endothelial Barrier Functions, and Its Involvement in Chronic Inflammatory Diseases. Tissue Barriers 4 4 :e Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The Gut Microbiota as an Environmental Factor That Regulates Fat Storage.
Proc Natl Acad Sci USA 44 — Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An Obesity-Associated Gut Microbiome With Increased Capacity for Energy Harvest.
Nature — Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut Microbiota From Twins Discordant for Obesity Modulate Metabolism in Mice. Science Tran HQ, Bretin A, Adeshirlarijaney A, Yeoh BS, Vijay-Kumar M, Zou J, et al. Cell Mol Gastroenterol Hepatol 9 2 — Gummesson A, Carlsson LMS, Storlien LH, Bäckhed F, Lundin P, Löfgren L, et al.
Intestinal Permeability Is Associated With Visceral Adiposity in Healthy Women. Obesity 19 11 —2. Hersoug L-G, Møller P, Loft S. Role of Microbiota-Derived Lipopolysaccharide in Adipose Tissue Inflammation, Adipocyte Size and Pyroptosis During Obesity. Nutr Res Rev 31 2 — Mokkala K, Röytiö H, Munukka E, Pietilä S, Ekblad U, Rönnemaa T, et al.
Gut Microbiota Richness and Composition and Dietary Intake of Overweight Pregnant Women Are Related to Serum Zonulin Concentration, a Marker for Intestinal Permeability. J Nutr 9 — Kincaid HJ, Nagpal R, Yadav H.
Microbiome-Immune-Metabolic Axis in the Epidemic of Childhood Obesity: Evidence and Opportunities. Obes Rev 21 2 :e Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, et al. Role of Gut Microbiota in Type 2 Diabetes Pathophysiology.
EBioMedicine Xu J, Liang R, Zhang W, Tian K, Li J, Chen X, et al. Faecalibacterium Prausnitzii-Derived Microbial Anti-Inflammatory Molecule Regulates Intestinal Integrity in Diabetes Mellitus Mice via Modulating Tight Junction Protein Expression.
J Diabetes 12 3 — Zhang D, Zhang L, Zheng Y, Yue F, Russell RD, Zeng Y. Circulating Zonulin Levels in Newly Diagnosed Chinese Type 2 Diabetes Patients. Diabetes Res Clin Pract 2 —8.
Hermes GDA, Reijnders D, Kootte RS, Goossens GH, Smidt H, Nieuwdorp M, et al. Individual and Cohort-Specific Gut Microbiota Patterns Associated With Tissue-Specific Insulin Sensitivity in Overweight and Obese Males.
Commensal Bacteria Contribute to Insulin Resistance in Aging by Activating Innate B1a Cells. Sci Transl Med 10 :eaat Dao MC, Sokolovska N, Brazeilles R, Affeldt S, Pelloux V, Prifti E, et al.
A Data Integration Multi-Omics Approach to Study Calorie Restriction-Induced Changes in Insulin Sensitivity. Front Physiol Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JFWM, et al.
Transfer of Intestinal Microbiota From Lean Donors Increases Insulin Sensitivity in Individuals With Metabolic Syndrome. Gastroenterology 4 Chen Z, Radjabzadeh D, Chen L, Kurilshikov A, Kavousi M, Ahmadizar F, et al.
Association of Insulin Resistance and Type 2 Diabetes With Gut Microbial Diversity: A Microbiome-Wide Analysis From Population Studies. JAMA Netw Open 4 7 :e Hill JH, Franzosa EA, Huttenhower C, Guillemin K.
A Conserved Bacterial Protein Induces Pancreatic Beta Cell Expansion During Zebrafish Development. Elife 5:e Zhang Q, Pan Y, Zeng B, Zheng X, Wang H, Shen X, et al.
Intestinal Lysozyme Liberates Nod1 Ligands From Microbes to Direct Insulin Trafficking in Pancreatic Beta Cells. Cell Res 29 7 — Ellekilde M, Selfjord E, Larsen CS, Jakesevic M, Rune I, Tranberg B, et al.
Transfer of Gut Microbiota From Lean and Obese Mice to Antibiotic-Treated Mice. Sci Rep 4 1 Husted AS, Trauelsen M, Rudenko O, Hjorth SA, Schwartz TW. GPCR-Mediated Signaling of Metabolites.
Cell Metab 25 4 — Tang C, Ahmed K, Gille A, Lu S, Gröne H-J, Tunaru S, et al. Each of us begins to assemble a unique congregation of microbes the moment we pass through the birth canal, acquiring our mother's bacteria first and continuing to gather new members from the environment throughout life.
By studying the genes of these various microbes—collectively referred to as the microbiome—investigators have identified many of the most common residents, although these can vary greatly from person to person and among different human populations.
In recent years researchers have begun the transition from mere census taking to determining the kind of jobs these minute inhabitants fill in the human body and the effect they have on our overall health.
An early hint that gut microbes might play a role in obesity came from studies comparing intestinal bacteria in obese and lean individuals. In studies of twins who were both lean or both obese, researchers found that the gut community in lean people was like a rain forest brimming with many species but that the community in obese people was less diverse—more like a nutrient-overloaded pond where relatively few species dominate.
Lean individuals, for example, tended to have a wider variety of Bacteroidetes, a large tribe of microbes that specialize in breaking down bulky plant starches and fibers into shorter molecules that the body can use as a source of energy.
Documenting such differences does not mean the discrepancies are responsible for obesity, however. To demonstrate cause and effect, Gordon and his colleagues conducted an elegant series of experiments with so-called humanized mice, published last September in Science.
First, they raised genetically identical baby rodents in a germ-free environment so that their bodies would be free of any bacteria. Then they populated their guts with intestinal microbes collected from obese women and their lean twin sisters three pairs of fraternal female twins and one set of identical twins were used in the studies.
The mice ate the same diet in equal amounts, yet the animals that received bacteria from an obese twin grew heavier and had more body fat than mice with microbes from a thin twin.
As expected, the fat mice also had a less diverse community of microbes in the gut. Gordon's team then repeated the experiment with one small twist: after giving the baby mice microbes from their respective twins, they moved the animals into a shared cage.
This time both groups remained lean. Studies showed that the mice carrying microbes from the obese human had picked up some of their lean roommates' gut bacteria—especially varieties of Bacteroidetes—probably by consuming their feces, a typical, if unappealing, mouse behavior.
To further prove the point, the researchers transferred 54 varieties of bacteria from some lean mice to those with the obese-type community of germs and found that the animals that had been destined to become obese developed a healthy weight instead.
Transferring just 39 strains did not do the trick. His studies, as well as those by other researchers, offer enticing clues about what those roles might be.
Compared with the thin mice, for example, Gordon's fat mice had higher levels in their blood and muscles of substances known as branched-chain amino acids and acylcarnitines. Both these chemicals are typically elevated in people with obesity and type 2 diabetes.
Another job vacancy associated with obesity might be one normally filled by a stomach bacterium called Helicobacter pylori. Research by Martin Blaser of New York University suggests that it helps to regulate appetite by modulating levels of ghrelin—a hunger-stimulating hormone.
pylori was once abundant in the American digestive tract but is now rare, thanks to more hygienic living conditions and the use of antibiotics, says Blaser, author of a new book entitled Missing Microbes. Diet is an important factor in shaping the gut ecosystem.
A diet of highly processed foods, for example, has been linked to a less diverse gut community in people. Gordon's team demonstrated the complex interaction among food, microbes and body weight by feeding their humanized mice a specially prepared unhealthy chow that was high in fat and low in fruits, vegetables and fiber as opposed to the usual high-fiber, low-fat mouse kibble.
The unhealthy diet somehow prevented the virtuous bacteria from moving in and flourishing. The interaction between diet and gut bacteria can predispose us to obesity from the day we are born, as can the mode by which we enter the world.
Studies have shown that both formula-fed babies and infants delivered by cesarean section have a higher risk for obesity and diabetes than those who are breast-fed or delivered vaginally.
Working together, Rob Knight of the University of Colorado Boulder and Maria Gloria Dominguez-Bello of N. have found that as newborns traverse the birth canal, they swallow bacteria that will later help them digest milk. C-section babies skip this bacterial baptism. Babies raised on formula face a different disadvantage: they do not get substances in breast milk that nurture beneficial bacteria and limit colonization by harmful ones.
Visceral fat and gut bacteria you for visiting nature. Bacteia are using a browser Viscefal with limited support for CSS. To Viscral the Visceral fat and gut bacteria experience, we hut you use a Gut health and concentration up to date Satiety and calorie intake bactetia turn Cholesterol level and weight management compatibility mode bacferia Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Gut microbiome has been shown to play a role in the development of obesity in recent studies. Most of these studies on obesity were based on the BMI classification criteria, which doesn't distinguish Visceral adipose tissue VAT from subcutaneous adipose tissue SAT. Some studies showed that VAT has a higher risk of inducing metabolic diseases than SAT.
0 thoughts on “Visceral fat and gut bacteria”