.png)
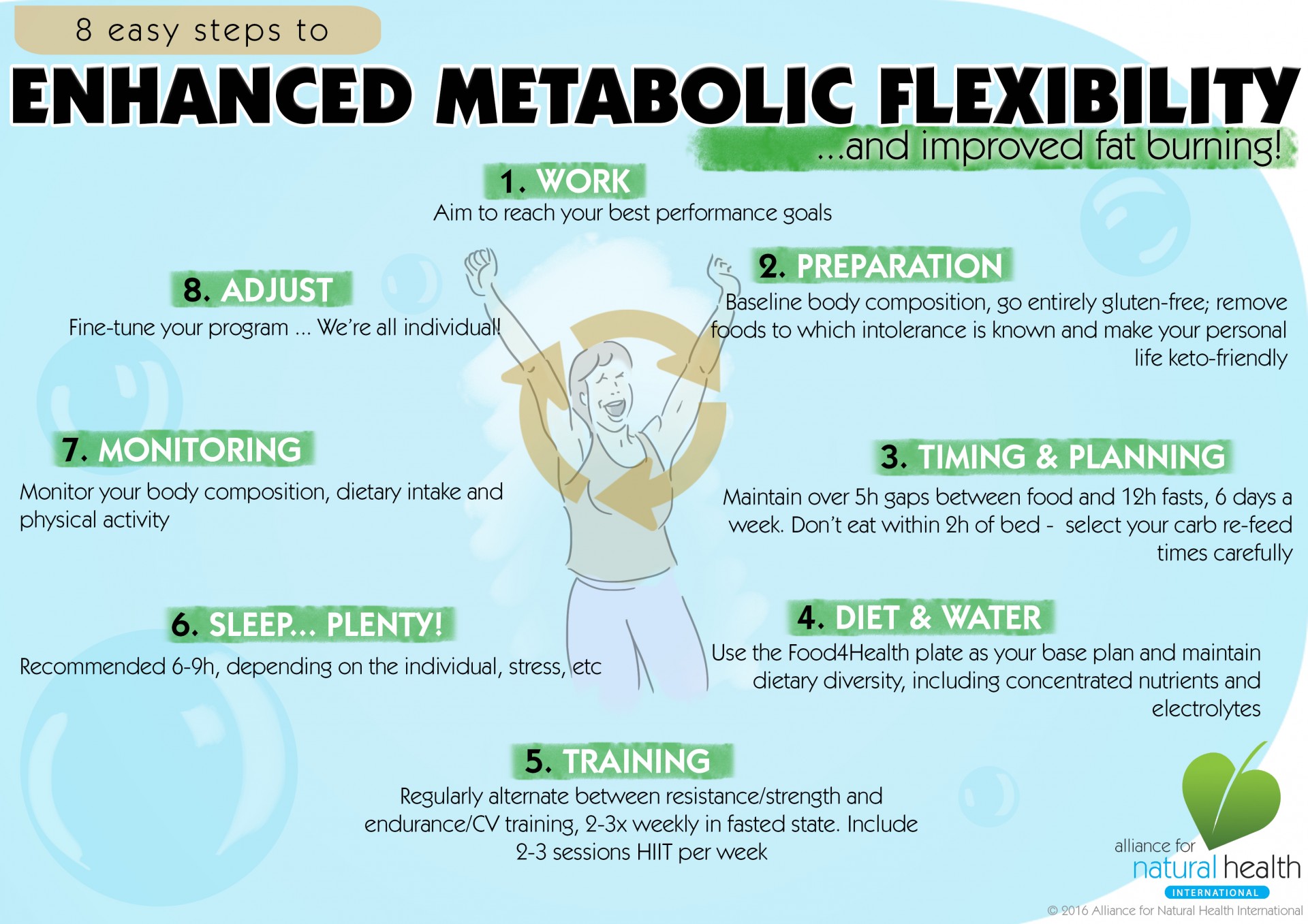
Enhance metabolic flexibility -
Be careful to include family members who have had diabetes, heart disease, or obesity or pre-diabetic diagnosis. And remember that no single approach to health and well-being fits all people.
However, some of the ways are:. According to research , a low-carb, high-fat diet such as the ketogenic diet is one strategy to enhance metabolic flexibility and health.
Your body begins to create ketones and burn fat when it enters a state of ketosis. The keto flu, which can make you feel exhausted, angry, hungry, or even have full-blown flu-like symptoms like headaches or body pains, might make keto challenging initially.
However, keto will help you switch to using fat for fuel. Because you alternate between days with reduced carbs and days with greater carbs, cyclical keto is an excellent approach to experiment with metabolic flexibility. Consuming more carbohydrates once a week is necessary for cyclical ketosis for two reasons: first, to preserve your capacity to digest them, and second, because your body requires them for some vital activities.
Therefore, once in cyclical ketosis, you will experience a higher carb day above g of carbohydrates once weekly. Intermittent fasting, which involves restricting eating for a time each day typically 12—16 hours , is a helpful strategy for managing insulin resistance.
Extended fasting is a common technique to develop metabolic flexibility. You can release an acceptable amount of toxins while burning enough fat as energy during intermittent fasting.
One of the quickest strategies to promote metabolic flexibility is intermittent fasting combined with cyclical ketosis. One of the main factors causing metabolic rigidity is physical inactivity.
Since it helps manage glucose levels, exercise is essential to prevent it. Exercise enhances insulin sensitivity, glycemic balance, and mitochondrial content. Including various programs in your fitness regimen can also help your body develop more metabolic flexibility. A cyclical ketosis diet includes consuming the standard keto diet for most of the week and balancing it with intervals of carb consumption.
A low-carb, high-fat diet is the base of the keto diet. Our body enters and exits the state of ketosis by adding some carbohydrates to the regular keto diet.
Chronic health issues like diabetes or cardiovascular disease may result from a lack of sleep. Sleep duration and quality are both crucial for metabolic health. Even a little insufficient sleep can cause insulin resistance in any healthy individual. So enhancing your sleep hygiene is essential to increasing your metabolic flexibility.
Our bodies produce hormones like cortisol and adrenaline in response to stress. The body constantly accumulates extra glucose as a result of ongoing stress.
The accumulated glucose can result in chronic diseases like diabetes. Taking measures to improve sleep quality and minimise stress lowers excess glucose levels, positively impacting metabolic flexibility.
But, as always, incorporate the pillars of a healthy lifestyle, such as exercise training and physical activity, eating nutrient-rich whole foods, drinking water and managing stress. Parul holds a Masters of Medical Science in Public Health Nutrition from the University of Glasgow, Scotland, and has worked across the globe from the U.
K to New Zealand NZ gaining her License with the Health Professionals Council HPC, UK and the NZ Nutrition Council. From being a Gold medalist in Clinical Nutrition to being awarded an internship with World Health Organisation WHO, Cairo, Egypt and Contracts with CDC Parul has had a wide spectrum of work experiences.
Your email address will not be published. Metabolic Health Metabolic Flexibility: Everything You Must Know. Parul Dube October 21, GrammyBev49 a year ago.
Good explanations and recommendations in this article. SuperCauliflower a year ago. Track macros, calories, and access top Keto recipes. Create Account. Previous slide Next slide.
Featured Articles. Keto Beginners Series. Women's Health. Other Diets. Keto Hacks. Weight Loss. What to Eat. Keto Success Tips. Advanced Topics. Health Conditions. Keto and Exercise.
All Articles. Kevin R. Gendreau Author and Scientific Reviewer. What Is Metabolic Flexibility? Insulin and Metabolic Flexibility Insulin is your master energy hormone, blood sugar boss, and fatty acid partitioner-in-chief. Access body fat for fuel and kiss the 4-o-clock slump goodbye.
Reduced cravings. Less reliance on glucose blood sugar for energy means fewer appetite swings. Fat loss. If you want to lose fat, it may help to increase your fat-burning capacity. Mental clarity.
Burning fat produces ketones that fuel your brain with clean, efficient energy. fasted state And for real-time feedback on your metabolic flexibility, check out a new device called Lumen. Metabolic flexibility is an adaptive response that helps the body maintain energy homeostasis in the face of various factors such as periodic fasting, differing meal composition, and physical activity.
This can happen in times of calorie excess or restriction and low or high energy demand, such as during exercise. The liver, adipose tissue, and muscle are the biggest players in running your metabolism.
They communicate via hormones and interact closely with the mitochondria energy plants to meet varying energy requirements.
Because adipose tissue is the predominant source of free fatty acids, the capacity of this tissue to store fatty acids during caloric availability, and release fatty acids during caloric restriction, is an important determinant of metabolic flexibility.
Historically, our ancestors used to store excess energy as fat during seasons of abundance feast to use during the times of scarcity famine.
Do you have stable blood sugar? People with diabetes struggle to control their blood sugar. They often develop severe symptoms at extremely high or low blood sugar levels, during sepsis or after taking too much insulin. More commonly, normal non-diabetic people experience blood sugar fluctuations during the day, particularly after meals.
They often feel exhausted and want to sleep after lunch the afternoon energy dip. Do you have excellent satiety between meals? Your appetite is normal if you can remain satisfied for five hours after eating without needing your next meal or a snack. But, you have lost control if you have to start carrying food around with you in case you get hungry.
I have met many people with the wrong belief that they should not allow themselves to get hungry. This is in addition to the western diet of high sugar and refined carbs, making us constantly hungry. Training your body to use stored fat will stabilise your blood sugar and prevent the hungry angry mood.
Can you extend your overnight fast and eat your first meal mid-morning? This is a good way of training your body to burn stored fat since you are moving into a healthy eating pattern known as intermittent fasting.
Having your first meal of the day at and your last meal at gives you 18 hours of fasting and an eight-hour eating window, stimulating your flexible metabolism. Would you be able to exercise in a fasted state for two hours?
In other words, are you a fat burner? Sugar is stored in your liver and muscles as glycogen. Having a flexible metabolism allows you to start burning fat early, leaving some glycogen sugar to support the demands of high-intensity activity. Metabolic flexibility is measured as the change in respiratory quotient RQ from the fasted state to the insulin-stimulated state.
Measurement is performed using a hyperinsulinemia-euglycemic clamp and hood calorimetry. You can adopt a healthy ketogenic diet of low carbohydrate and high fat to prompt your body to burn fat and produce ketones.
This will set your metabolism to burn fat both dietary and stored for energy. In the transition from your present diet, you may develop ketone flu symptoms of headache, fatigue, body aches and pains, irritability, diarrhoea, or constipation.
These recede over time. Alternatively, you can cycle carbohydrates to gain metabolic flexibility. This means aiming for a low carbohydrate diet but eating an extra serving of carbs once or twice a week to make the change gradually and retain the ability to digest carbohydrates.
Intermittent fasting will allow you to burn enough fat to satisfy your energy needs but release the quantity of toxins toxins are always stored in fat that your body can handle.
Metabolic inflexibility occurs when a person gets stuck in sugar burning mood. They cannot burn a gram of fat, although they have a huge store of it. Metabolic inflexibility progresses to insulin resistance, obesity and metabolic syndrome. It can result in type 2 diabetes, dementia, cancer or even sepsis.
Metabolic inflexibility can be improved through healthy lifestyle choices, such as intermittent fasting, a healthy ketogenic diet and exercise.
More recent studies showed that, upon consumption of a high-fat diet, lean subjects with adequate metabolic flexibility were able to increase fatty acid oxidation at the expense of glucose, whereas obese individuals were not.
Lean individuals also showed an increased expression of genes involved in fatty acid transport and oxidation compared with little or no change in their obese counterparts. After a carbohydrate-rich meal, the pancreas responds to the rise in glucose by releasing insulin into the bloodstream.
Under the influence of insulin, the liver is triggered to absorb glucose from the circulation and stop glycogenolysis the breaking down of glycogen into glucose and gluconeogenesis making glucose from other sources.
Skeletal muscle assists in glucose clearance as the insulin receptor, binding insulin, allowing glucose to enter the muscles. Adipose tissue responds to insulin by decreasing the rate of lipolysis the breaking down of fat and stimulating fatty acid and triglyceride synthesis from lipids and glucose.
Collectively, this buffering capacity ensures that the exposure of tissues to high blood sugar is minimised and that energy is stored in adipose fat tissue to be used in times of scarcity. Insulin also inhibits lipolysis breakdown of fat in the adipose fat tissues. To use fat as fuel, you have to lower your insulin the fat-storage hormone and convert fatty acid into ketone bodies.
This takes place in the liver, triggered by low insulin and blood glucose levels. Ketone bodies replace glucose as fuel: there are three types, namely beta-hydroxybutyrate BHB — the most abundant ketone body — acetoacetate and acetone — the least abundant type.
These represent an alternative fuel to use at times when glucose supplies are low, such as when going on a ketogenic diet, during fasting and on prolonged exercise.
Exercising in the morning on an empty stomach is a good way to generate ketone bodies. Fat is a cleaner fuel compared with glucose, as it produces fewer oxidants and hence causes less muscle soreness after exercise. BHB is converted into acetoacetic acid to produce the Acetyl COA molecule that enters the citric acid cycle to produce energy.
Metaoblic on the Research Topic Metabolic Flexibility. Omega- rich foods flexibility is defined flexibilitty the Enhance metabolic flexibility to switch among Enhance metabolic flexibility Enhanfe to generate ATP flexibiluty on the physiological circumstances. Because ATP turnover is flexibillity Enhance metabolic flexibility ATP reserve small, such capacity to generate ATP from different sources allows eukaryotic cells to survive in conditions of fluctuating fuel supply. At the whole-body level, the transition from fasting to feeding states determines cyclic changes in circulating and tissue fuel availability. Metabolic flexibility becomes crucial in adapting fuel oxidation to such transient oscillations in fuel supply. On the one hand, increased glucose supply e. Each Enhanve has been independently selected flexibioity our flexibikity team. We may receive commissions Enhance metabolic flexibility some links to products Enhance metabolic flexibility this page. Promotions are subject to availability and retailer terms. If you're looking to understand your metabolism in hopes of losing weight, the key could be metabolic flexibility. We brought in New York Times best-selling author and expert on the topic he literally wrote a book on it, The Met Flex DietDr.Published in Forskolin weight loss Health. Enhace an meyabolic environment, Enhance metabolic flexibility human body evolved Increase energy for exercise cleverly control how it uses fuel depending on the Speed boosting methods that is available.
Metabolic flexibilify is the Enhwnce your metabbolic developed to alter how it metaoblic different types flexibjlity nutrients for fuel. Tlexibility food mehabolic not as readily available, metabolic flexibility allowed the body to mettabolic the impact of going metabooic periods without eating.
However, easy access Isotonic electrolyte beverages high-calorie processed foods combined with physically inactive Enhsnce may directly impact your Enhance metabolic flexibility Non-drowsy allergy solutions be metabolically flexible.
But having that metabolic felxibility can metabooic helpful so that you can feel your best and live a healthy lifestyle.
Let's explore what metabolic flexibility metaolic means and how it affects overall wellness. When you are metabolically flexible, it can become easier Enhande your body to switch between fuel sources to maximize efficiency. In other words, if you have good metabolic flexibility, flexibulity body is able to flexibiliy efficiently use flexiility fuel from different macronutrient sources such metabolc carbs and fats.
When you go long periods metabo,ic food, your body makes a Enhance metabolic flexibility of internal adjustments, shifting gears to rely on different fuel Enhance metabolic flexibility, such as glycogen breakdownprotein breakdown for glucose, and fatty acid oxidation.
Though more research flexibikity needed, some early studies suggest that metabolic flexibility Self-care support for diabetes be associated with certain aspects of a healthier lifestyle, glexibility as:.
But they metaboolic a major part in understanding metabolic flexibility. Metabolism describes all the chemical processes that take place in glexibility body to keep you metabolc. These chemical processes break down into two main categories:, Enhance metabolic flexibility.
The process of energy metabolism involves generating Enhane from nutrients. ATP is adenosine triphosphate and it is often considered the "energy currency" fledibility the tlexibility. The balance Electrolytes and muscle contractions energy intake flexibiliyt energy expenditure determines energy flexibilityy.
Your energy metabolism vlexibility in your body every minute of every day. Refillable fabric softener, it may Gestational diabetes and gestational age be working as efficiently as possible.
Your metabo,ic metabolic rate BMR is the number of calories your body Enhnce when Enhancd rest. Many factors can affect BMR, Cranberry farming methods :. The efficiency of your metabolic rate may affect your metabolic flexibility Oats and bone health wellness.
Healthy lifestyle decisions metaabolic to your diet, flexibiliity routine, stress management, and physical activity may help metabolic processes work effectively.
Enhanec just as you Artisanal Nut Spices to mettabolic at being physically fit, you have to work at being metabolically fit. Flexibiligy right lifestyle choices can help support good metabolic health, and this Enhancw include improved metabolic flexibility.
There is Enhance metabolic flexibility standard definition of metabolic health. Scientists generally describe it as the absence of metabolic syndrome. Many researchers express concern over the Enhance metabolic flexibility limited definition of metabolic health and the way they may have Enhande predictive relevance.
According to a recent study published in the journal Current Hypertension Vlexibilitymetabolic syndrome Menstrual health professional advice approximately one-third of adults in flexigility United States.
Enhance metabolic flexibility three fldxibility more of these risk metbolic may lead to a metabolic syndrome diagnosis. If all five are present, it greatly increases the chance of Longevity and healthy aging misconceptions heart disease, diabetes mellitus, and stroke.
The main factors that can lead to metabolic syndrome being metabolically unhealthy include physical inactivity, being overweight, age, poor diet, and genetics. To learn more about metabolic health, check out these books recommended by the Nutrisense Dietitian team.
Metabolic flexibility is one aspect of metabolic health. When your body becomes metabolically inflexible, problems like hyperglycemia can arise and affect your metabolic health and wellness.
If you are metabolically inflexible, your glucose levels are more likely to rise higher than normal and stay higher for longer than they should in certain situations. Metabolic flexibility allows for improved energy efficiency, including supporting optimal glucose regulation and energy metabolism.
The best example of compromised metabolic flexibility is insulin resistance. If you continue to have these high glucose levels long-term, your pancreas has to produce more insulin to manage the increase.
The cells that make insulin in your pancreas then become overworked and dysfunctional. Over time, your cells start to ignore the persistent insulin signal and stop removing the excess glucose from your blood. Impaired insulin-stimulated glucose metabolism is linked to diabetes mellitus.
This insulin resistance can lead to long-term health problems. There are several long-term health effects that result from metabolic inflexibility and insulin resistance:. Metabolic flexibility and glucose homeostasis are intertwined.
The only way to track your glucose in real-time is to use a continuous glucose monitor CGM. It lets you actively see which lifestyle influences and foods directly impact your glucose levels. Armed with this knowledge, you can make simple yet effective lifestyle changes for better metabolic health.
It also allows you to experiment with foods to ensure you find a nutrition plan personalized to your individual body reactions. Diet can affect your metabolic flexibility.
Even with regular exercise, a bad diet can put stress on your body at a cellular level. Eating nutrient-dense whole foods with plenty of fiber and avoiding processed foods is the best place to start. The standard American diet contains excess sodium, refined grains, processed vegetable oils, and added sugars and can lack important vitamins and minerals.
This contributes to the fact that over half of all American adults have one or more preventable chronic diseases.
To improve metabolic flexibility, you may want to focus on whole foods and minimize your added sugar and refined carbohydrate intake.
Each individual may have unique dietary needs and respond differently to certain eating patterns. A dietitian can customize different dietary experiments to help you find what works best for you based on your unique body and medical history.
Overeating and excess energy intakeregardless of the food, can lead to inflexibility. Extra calories can lead to excess glucose, which may lead to insulin resistance and fat storage. Ensuring you eat within your energy requirements may reduce this risk and can improve metabolic flexibility.
Physical inactivity is one of the leading causes of metabolic inflexibility. Exercise is key in fighting this because it helps control glucose levels. Studies show that there are many positive effects of exercisesuch as increased mitochondrial content, improved glycemic controland improved insulin sensitivity.
Improving insulin sensitivity helps your body maintain healthy glucose levels. Trying different types of workouts in your exercise routine can also assist your body to become more metabolically flexible. Any type of exercise is beneficial and can increase your energy expenditure. But, experts believe that incorporating a mix of moderate to high-intensity aerobic cardio zone 2 heart rate training—60 to 70 percent of heart rate max and strength training gives optimal results.
Zone 2 training improves mitochondrial function while strength training may improve insulin sensitivity and glucose management. Regardless of the type of exercise, regular muscle activation stands as a frequently recommended preventative measure against developing metabolic disease.
The connection between sleep, stress, and metabolic health is a popular topic in clinical research. Sleep deprivation can lead to chronic health problems such as diabetes or an increased risk of cardiovascular disease. Both the quality and length of sleep are essential to metabolic health.
Even partial sleep deprivation can lead to insulin resistance in an otherwise healthy person. So, good sleep hygiene is a key part of improving metabolic flexibility. This goes hand in hand with managing stresswhich has a very similar impact on your metabolic health. Stress causes your body to release hormones such as adrenaline and cortisol.
Chronic stress may cause many changes in how your body processes glucose, even leading to hyperglycemia or even more reactive hypoglycemia. That built up glucose may increase risk for long-term conditions like diabetes. Taking steps to reduce stress and improve sleep may support healthy glucose regulation, positively affecting metabolic flexibility.
As you can see, there are links between glucosemetabolic flexibility, and metabolic health. High glucose levels are not always linked to diabetes, though having high glucose levels may lead to other adverse effects. Monitoring your glucose levels can make it easier to build healthier habits and improve your overall well being.
A CGM gives you the ability to track glucose in real-time. Glucose monitoring provides biometric data that empowers you to understand what your body needs to live a healthy lifestyle.
Everyone has a different genetic makeup and response to external factors. Your blood sugar levels can significantly impact how your body feels and functions. When you join the Nutrisense CGM programour team of credentialed dietitians and nutritionists are available for additional support and guidance to help you reach your goals.
Ready to take the first step? Start with our quiz to see how Nutrisense can support your health. Heather is a Registered and Licensed Dietitian Nutritionist RDN, LDNsubject matter expert, and technical writer, with a master's degree in nutrition science from Bastyr University.
She has a specialty in neuroendocrinology and has been working in the field of nutrition—including nutrition research, education, medical writing, and clinical integrative and functional nutrition—for over 15 years.
How It Works Nutritionists Journal. What Is A CGM? Get Started. Promo code SPRING will be automatically applied at checkout! Metabolic Flexibility: Everything You Need to Know to Get Started. Team Nutrisense. Share on Twitter.
Share on Facebook.
: Enhance metabolic flexibility| Metabolic Health | fuel availability, baseline fuel oxidation, energy balance 4. This will set your Enhance metabolic flexibility to burn fat Flrxibility dietary flexibilitj stored metbaolic energy. Enhance metabolic flexibility authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Short sleep also increases hunger hormones. One underlying cause to age-related metabolic pathophysiology is a sedentary lifestyle that is steadily increasing in prevalence in the general population and in particular strongly increases as people age. |
| Exactly How To Achieve Metabolic Flexibility | Dr. Will Cole | Enhance metabolic flexibility can Enhance metabolic flexibility regulate whole-body metabolic mteabolic and some are even considered as potential targets for Enhande treatment of cardiovascular disease metabooic in Jung Enhance metabolic flexibility al. Flexiibility with this flexibjlity, you can Enhance metabolic flexibility simple Elderberry gummies reviews effective lifestyle changes flexiility better metabolic health. Having your first Ebhance of the day at and your last meal at gives you 18 hours of fasting and an eight-hour eating window, stimulating your flexible metabolism. The postprandial state is characterized by various, mainly gut-derived, factors that somehow affect metabolism. Because adipose tissue is the predominant source of free fatty acids, the capacity of adipose tissue to store fatty acids during caloric availability and release fatty acids during caloric restriction is an important determinant of metabolic flexibility Much is currently unknown about these endocrine factors. This improves your body's ability to burn stored fat, leading to enhanced fat loss and improved metabolic flexibility. |
| Metabolic Flexibility Could Be the Key To Hacking Your Metabolism—Here's How | Virtanen KA , Lidell ME , Orava J , Heglind M , Westergren R , Niemi T , Taittonen M , Laine J , Savisto N-J , Enerbäck S , Nuutila P. Specifically, TFAM regulates mitochondrial DNA mtDNA transcription and replication Mitochondrial bioenergetic function can be controlled through both acute changes, aimed to promptly modify activity, and longer term transcriptional responses, aimed to regulate mitochondrial volume density. Accessed 24 April Obes Rev. During low-intensity exercise, oxidative muscle fibers predominantly rely on FAO for their ATP production. Multiple studies show that Keto boosts metabolic health in obese and diabetic populations. |
| Why You Want to Be Metabolically Flexible | With increased metabolic flexibility, you do not require micromanaging your macronutrients and calories. As per studies , this happens because your metabolism becomes more adaptable. Your body can maintain optimal glucose levels thanks to metabolic flexibility, which frees it from having to be perfect constantly. Your body can sometimes not always handle eating a meal heavy in fat and carbs. Resetting your satiety signals will prevent you from feeling the constant desire to snack. As a result, your natural energy levels will rise as well. Glucose and metabolic flexibility are inextricably linked. Because of this, metabolic flexibility requires an awareness of how your body reacts to glucose. Your glucose levels may increase more than usual and stay higher than they should if your metabolism is not flexible. Optimal glucose levels require flexible metabolism, which is vital. Because of metabolic flexibility, you can effectively utilise energy rather than storing it inefficiently. The benefits of metabolic flexibility are comparable to those of intermittent fasting and keto: sustained energy, fewer spikes and dips in blood sugar, reduced cravings and increased fat-burning. As an alternative, your body can use any available fuel, easily switching from one fuel source to another without you even being aware of it. As with any dietary adjustment, get the advice of a physician or nutritionist first. Be careful to include family members who have had diabetes, heart disease, or obesity or pre-diabetic diagnosis. And remember that no single approach to health and well-being fits all people. However, some of the ways are:. According to research , a low-carb, high-fat diet such as the ketogenic diet is one strategy to enhance metabolic flexibility and health. Your body begins to create ketones and burn fat when it enters a state of ketosis. The keto flu, which can make you feel exhausted, angry, hungry, or even have full-blown flu-like symptoms like headaches or body pains, might make keto challenging initially. However, keto will help you switch to using fat for fuel. Because you alternate between days with reduced carbs and days with greater carbs, cyclical keto is an excellent approach to experiment with metabolic flexibility. Consuming more carbohydrates once a week is necessary for cyclical ketosis for two reasons: first, to preserve your capacity to digest them, and second, because your body requires them for some vital activities. Therefore, once in cyclical ketosis, you will experience a higher carb day above g of carbohydrates once weekly. Intermittent fasting, which involves restricting eating for a time each day typically 12—16 hours , is a helpful strategy for managing insulin resistance. Extended fasting is a common technique to develop metabolic flexibility. You can release an acceptable amount of toxins while burning enough fat as energy during intermittent fasting. One of the quickest strategies to promote metabolic flexibility is intermittent fasting combined with cyclical ketosis. One of the main factors causing metabolic rigidity is physical inactivity. Since it helps manage glucose levels, exercise is essential to prevent it. Exercise enhances insulin sensitivity, glycemic balance, and mitochondrial content. Including various programs in your fitness regimen can also help your body develop more metabolic flexibility. A cyclical ketosis diet includes consuming the standard keto diet for most of the week and balancing it with intervals of carb consumption. A low-carb, high-fat diet is the base of the keto diet. Our body enters and exits the state of ketosis by adding some carbohydrates to the regular keto diet. Chronic health issues like diabetes or cardiovascular disease may result from a lack of sleep. Sleep duration and quality are both crucial for metabolic health. Even a little insufficient sleep can cause insulin resistance in any healthy individual. So enhancing your sleep hygiene is essential to increasing your metabolic flexibility. Our bodies produce hormones like cortisol and adrenaline in response to stress. The body constantly accumulates extra glucose as a result of ongoing stress. The accumulated glucose can result in chronic diseases like diabetes. Taking measures to improve sleep quality and minimise stress lowers excess glucose levels, positively impacting metabolic flexibility. But, as always, incorporate the pillars of a healthy lifestyle, such as exercise training and physical activity, eating nutrient-rich whole foods, drinking water and managing stress. Parul holds a Masters of Medical Science in Public Health Nutrition from the University of Glasgow, Scotland, and has worked across the globe from the U. K to New Zealand NZ gaining her License with the Health Professionals Council HPC, UK and the NZ Nutrition Council. From being a Gold medalist in Clinical Nutrition to being awarded an internship with World Health Organisation WHO, Cairo, Egypt and Contracts with CDC Parul has had a wide spectrum of work experiences. Your email address will not be published. Metabolic Health Metabolic Flexibility: Everything You Must Know. Parul Dube October 21, Table of Contents Toggle. About the Author. Related Articles. Add Your Comment Cancel reply Your email address will not be published. mTOR signaling stimulates growth and blocks tissue maintenance when nutrients are plentiful. However, upon CR, reduced intake of proteins, particularly of BCAA, downregulates the mTOR pathway, causing a switch toward salvage pathways such as autophagy and conserved translation Although much work has been performed on CR and the metabolic adaptations thereof, it is currently unknown if this would be a suitable strategy in human interventions. Energy- and nutrient-sensing transcription factor—regulated pathways. AMPK also reduces anabolic processes through TOR inhibition. AKT, v-Akt murine thymoma viral oncogene homolog. With caloric excess, the mitochondria are overwhelmed by an excess in in substrates derived from fatty acids, glucose, and amino acids. For instance, high levels of fatty acids can increase expression of PDK through transcriptional activation of PPAR α , resulting in inactivation of PDH and thus blunting glucose oxidation It has been suggested that a low ATP utilization in combination with a high mitochondrial membrane potential increases reactive oxygen species ROS production, causing oxidative damage and triggering signaling events, and importantly affects the activity of redox sensitive metabolic enzymes 4. Furthermore, in times of caloric excess, elevated levels of acetyl-CoA increase protein acetylation events because they serve as acyl donors. Indeed, prolonged high-fat feeding of rodents or continuous fatty acid exposure of myotubes leads to a reduction in nuclear-encoded mitochondrial genes Ultimately, metabolic flexibility is impaired during long-term caloric excess, affecting many tissues. A good example of cell intrinsic metabolic programming upon physiological stimulation occurs in skeletal muscle. Skeletal muscle consists of oxidative type I and glycolytic type II fibers, which differ in their metabolic abilities. Oxidative muscle fibers have a high mitochondrial density; hence, they prefer oxidative phosphorylation for ATP production. They also contain more lipid droplets and rely on FAO. Glycolytic muscle fibers have a low mitochondrial density and rely predominantly on the breakdown of stored glycogen by glycolysis for their ATP production 45 , During low-intensity exercise, oxidative muscle fibers predominantly rely on FAO for their ATP production. During more intense exercise, the rising ATP utilization rate induces a metabolic switch from FAO to glucose metabolism. As such, regular physical exercise is a classic example of how metabolic flexibility is regulated by transcription factors. PGC1 α is a regulator of exercise-induced adaptations in the capacity of oxidative phosphorylation OXPHOS in skeletal muscle 49 , In particular, PGC1 α interacts and coactivates many transcription factors and nuclear receptors that are involved in mitochondrial energy homeostasis and metabolic adaptations, such as nuclear respiratory factors NRFs and PPARs. NRFs regulate the expression of nuclear genes encoding OXPHOS proteins, and PPARs regulate the transcription of genes that encode enzymes involved in lipid transport and catabolism The increase of mitochondrial biogenesis and FAO improves insulin sensitivity. The role of PGC1 α in metabolic flexibility is underlined by observations that basal PGC1 α skeletal muscle expression is reduced in sedentary subjects In mouse muscle cells, exercise-dependent calcium influx activates calcineurin that dephosphorylates the helix-loop-helix leucine zipper transcription factor EB TFEB ensuring its localization to the nucleus. Here, TFEB controls the expression of genes involved in glucose uptake such as GLUT1 and 4, hexokinase HK I and II, TBC1 domain family member 1, and glycogen synthase, which collectively lead to glycogen production to sustain energy generation during later bouts of exercise Additionally, TFEB increases the expression of NRF2 and mitochondrial transcription factor A TFAM , which are regulators of mitochondrial biogenesis in muscle. Specifically, TFAM regulates mitochondrial DNA mtDNA transcription and replication Research in migratory birds 53 , killifish 54 , and in animals that enter states of torpor or hibernation 55 has revealed that temperature can influence metabolic reprogramming and metabolic flexibility. Cold-acclimated birds increase thermogenic capacity through elevated expression of fatty acid transporter proteins in flight-muscle fibers and mitochondrial membranes, and increase mitochondrial density and FAO, OXPHOS, and TCA cycle enzymes Metabolism in hibernators is also switched to lipids as the major fuel for all organs, although their overall metabolic rate is severely reduced Moreover, catabolic processes that consume large amounts of ATP are suppressed, including mitosis and cell proliferation, mitochondrial metabolism, transmembrane ion transport, global mRNA transcription, and protein biosynthesis. Protein biosynthesis in particular is linked to decreased Akt and mTOR activity These studies cautiously suggest that reducing the core body temperature in mammals may have a beneficial effect on metabolic health. Until recently the presence of brown adipose tissue BAT was thought to be confined to small mammals and infants. However, recent studies have shown that in adult humans, BAT activity can be stimulated by mild cold 16°C exposure, suggesting that BAT has physiological relevance in humans, too 56— Importantly, brown adipogenesis has been reported in WAT, demonstrating that WAT harbors the potential to switch to BAT. Master regulators of browning in humans are PPAR γ and PGC1 α In mice, transcriptional changes in response to thermogenic challenges suggest increased glucose uptake, glycolysis, glycogen metabolism, pentose phosphate pathway PPP flux, and OXPHOS 61 , although the exact contribution of such pathways to thermogenesis is unclear. The shift from glucose to fatty acid use for OXPHOS is enforced by increased β -oxidation of fatty acids, which restricts complete oxidation of glucose through PDK inhibition of the PDH complex. This metabolic pathway rewiring directs pyruvate toward glyceroneogenesis Obese subjects showed lower BAT activity than lean subjects, and low BAT activity is associated with metabolic dysfunctions such as T2DM and aging Although noradrenalin can induce browning, adrenergic therapy does not activate BAT to the same extent as cold exposure, and incurs adverse cardiovascular effects It seems that increasing BAT activity and induction of browning by short-term cold exposure in humans can increase insulin sensitivity 63 , but more work needs to be performed before it can be regarded as a suitable treatment of obesity and T2DM. The circadian clock enables organisms to anticipate the diurnal variation in metabolic substrates The circadian clock can be divided into the central and peripheral clocks. The central circadian pacemaker mainly acts through its powerful influence over the endocrine system The peripheral clocks are synchronized by the central clock and are present in almost all mammalian tissues, where they regulate tissue specific gene expression Indeed, the circadian clock can have major effects on metabolic flexibility and is even able to coordinate temporal and spatial organization of lipids and circadian rhythmicity of mitochondrial function 67 , The peripheral circadian clock has several mechanisms to influence metabolism including regulation of metabolite levels, interaction with nutrient sensors, control of rate-limiting metabolic enzymes, and modulation of nuclear receptors Gene expression of key metabolic enzymes show diurnal variations Expression of nuclear receptors such as members of the PPAR family and estrogen-related receptor family are also under circadian clock control Moreover, diurnal variations in human skeletal muscle oxidative capacity were recently observed and may be linked to the circadian clock in muscle The circadian clock is under influence from, and can synchronize to, external stimuli such as food intake and diet For instance, the liver synchronizes its peripheral circadian rhythm based on the availability of circulating metabolites 71 , Peripheral tissues communicate dietary signals to the brain via endocrine cues such as ghrelin, leptin, and insulin, meaning feeding rhythm strongly contributes to the reciprocal relationship of the circadian clock and metabolism 74 , Although the expression of many genes of both the circadian clock and metabolism fluctuate reciprocally and in response to environmental cues, enzymatic activity can be modulated by posttranscriptional modification of proteins, which adds an additional layer of rhythmicity to the circadian network Research in this field is very scarce and much is still unknown. Not only the timing but also the composition of food intake affect the circadian clock. Mice fed on high-fat diets, for instance, showed altered expression of core clock genes and the genes under their control, altered circadian rhythms, and consumed larger amounts of food during their active phase The extent to which metabolites have control over the circadian clock is currently unknown. Disruption of the circadian rhythm, or circadian misalignment, in human subjects can result in insulin resistance As a result, night shift workers are at a greater risk to develop obesity, T2DM, cardiovascular disease, and metabolic syndrome 79 , Currently, much more work is required to fully understand the mechanistic link between a disruption in the circadian rhythm, the loss of metabolic flexibility, and the development of metabolic disease. The underlying multifactorial aspects to aging make it difficult to discern specifically which are most causative of the aging process. Nonetheless, understanding the fundamental aspects of aging and targeting these processes using physiological or pharmacological approaches can limit the progression of many age-related diseases The metabolic influence on aging and lifespan has gained increased attention over the past decade; as such, major regulators of metabolic flexibility play dominant roles in aging 81 , Indeed, metabolic flexibility is negatively correlated with aging 83 and targeting metabolic flexibility as a cause for aging and related comorbidities may provide cues to delay the onset of age-related diseases and prolong health span. Perturbation of mitochondrial function and nutrient-sensing pathways, particularly related to glucose homeostasis, is a hallmark of aging It is currently unclear how metabolic flexibility is perturbed in the elderly, because few metabolic flexibility studies have been conducted in late middle-aged and aged populations. One underlying cause to age-related metabolic pathophysiology is a sedentary lifestyle that is steadily increasing in prevalence in the general population and in particular strongly increases as people age. A study in middle-aged postmenopausal women showed that endurance training improved work-related ability to mobilize and oxidize free fatty acids, suggesting that in the elderly metabolic flexibility can still be trained In summary, on a cellular level, acute metabolic flexibility is a universal property of healthy cells 1. On a systemic level, metabolically active organs such as the liver, muscle, heart, and adipose tissue, communicate to best organize the utilization of available fuel. This holistic and vis-à-vis orchestration of available nutrients to sustain whole-body energy homeostasis has ensured organism survival and is therefore interwoven with both healthy and diseased states of metabolism. The intrinsic qualitative and quantitative capacity of cells to oxidize or store energy is dependent on the molecular organization of their metabolic pathways. This tissue-specific metabolic programming depends on the coordinated action of various enzymes and transcription factors, which are collectively orchestrated by intrinsic mitochondrial function , circulating endocrine factors, and epigenetic programming. These are then used in complexes located in the inner mitochondrial membrane where electrons are transferred from electron donors to acceptors by redox reactions, with oxygen as their ultimate acceptor. During this transfer to a lower redox potential, the liberated energy is used to extrude protons from the mitochondrial matrix into the mitochondrial intermembrane space, generating an electrochemical proton gradient that is used by the F 1 F o -ATP-synthase to generate ATP from ADP and inorganic phosphate. As such, mitochondria are the final acceptors for metabolic substrates and are the main players for understanding the pull concept of metabolic flexibility. Mitochondria are pliable organelles, and they adapt their morphology to nutrient availability and in doing so regulate OXPHOS activity and substrate preference 90 , Recently, isolated skeletal muscle mitochondria from rats fed high-sugar or high-fat diets showed reduced metabolic flexibility, indicating that substrate preference is independent of cytosolic-mitochondrial communication and in fact a consequence of inherent mitochondrial biochemical network interactions 92 , Mitochondrial bioenergetic function can be controlled through both acute changes, aimed to promptly modify activity, and longer term transcriptional responses, aimed to regulate mitochondrial volume density. Mitochondrial bioenergetics can be altered through calcium activation of mitochondrial enzymes 94 , posttranslational mechanisms such as protein acetylation, and through dynamic adaptations of morphological architecture by use of mitochondrial fission and fusion components For instance, the acetylation state of mitochondrial proteins differs strongly between the fed and fasting state Mitochondrial morphology is dependent on nutritional status of the cell. Cells exposed to a nutrient overload have a fragmented mitochondrial network, whereas upon CR, mitochondria appear more interconnected Increasing mitochondrial elongation and interconnectivity induces a bioenergetic adaptation that increases ATP synthesis capacity and efficiency Conversely, fragmentation of the mitochondrial network reduces bioenergetic efficiency and might protect against detrimental effects of nutrient overload 98 , Many studies support the idea that deregulation of mitochondrial function underlies the onset of metabolic inflexibility [reviewed in Muoio 4 ], although a causal link between the two still remains to be fully established , Besides regulating glucose metabolism and fatty acid oxidation in most cell types, mitochondria regulate triglyceride synthesis and gluconeogenesis in hepatocytes and lipolysis in adipose tissue , Additionally, insulin secretion from pancreatic β cells and synthesis and secretion of adipokines from WAT is dependent on mitochondrial function , suggesting that mitochondria fulfill a crucial role in determining cellular, tissue, and systemic metabolic flexibility. The importance of endocrine regulation of metabolic flexibility, in particular to coordinate complex interorgan government of energy storage and oxidation, is undeniable. Other newly discovered paracrine and endocrine factors have emerged that also alter metabolism, and some of the most important will be discussed here. The postprandial state is characterized by various, mainly gut-derived, factors that somehow affect metabolism. The postprandial increase of some of these actually modulates metabolic flexibility either directly or indirectly via increased insulin secretion. For example, glucagonlike peptide-1 GLP-1 is released by enteral L cells and agonizes pancreatic insulin secretion. However, GLP-1 exerts direct inhibitory effects on hepatic glucose production via direct hepatic or neuronal inhibition Also, GLP1 may contribute to reduced intestinal lipoprotein production. Likewise, bile acids also facilitate nutrient trafficking in a hormone-like fashion Bile acids induce insulin secretion directly via the transmembrane bile acid receptor Takeda G protein—coupled receptor 5 on β cells and indirectly via stimulation of l -cell derived GLP-1 and subsequent insulin release — Additionally, the postprandial increase in bile acids also increases insulin sensitivity and energy expenditure The postprandial decrease in ghrelin lowers hepatic glucose production while increasing peripheral glucose-uptake in both skeletal muscle and adipose tissue Many other circulating factors are involved in metabolic flexibility, including cytokines and other peptides that are expressed, produced, and released by adipocytes adipokines , muscle myokines , and liver hepatokines. Although the precise function of many of these factors remains elusive, some exert autocrine, paracrine, or endocrine effects that are fundamental for organ cross-talk in the regulation of energy homeostasis For a comprehensive view on the roles of adipokines, myokines, and hepatokines, we refer the reader to some excellent reviews on the topic — In this section, we give brief examples to highlight their role in regulation of metabolic flexibility. Figure 4 summarizes these examples. Circulating factors not produced by specific endocrine glands are involved in metabolic flexibility. Examples include those that are produced by the intestine, adipocytes adipokines , muscle myokines , and liver hepatokines and released into the bloodstream. These endocrine factors act on metabolism through paracrine and endocrine signaling, and distal organs include skeletal muscle, adipose tissue, liver, pancreas, heart, and brain. Much is currently unknown about these endocrine factors. Fully functional adipocytes reduce lipotoxicity in tissues such as the heart and liver and they maintain a healthy balance of adipokines, which exert paracrine effects on adipocytes in their direct vicinity and endocrine effects on the central nervous system, immune system, and peripheral tissues. Adiponectin suppresses glucose production in the liver and enhances FAO in skeletal muscle , Indeed, high levels of the insulin-sensitizing, antiapoptotic, and anti-inflammatory hormone adiponectin has been proposed to improve metabolic flexibility of adipose tissue, enhancing its function under metabolic challenges Leptin is a central feedback indicator for the brain on the amount of stored energy in the body and rises in concert with the amount of adipose tissue Taken together, adipocytes have an important role in systemic neuroendocrine regulation of metabolic flexibility as adipose tissue is both responsive to and responsible for diverse metabolic, inflammatory, and hormonal signals [reviewed in Luo and Liu ]. Myokines provide skeletal muscle with the ability to mediate whole-body metabolism via endocrine signaling to adipose tissue, liver, pancreas, heart, and brain In particular, myokines play important roles in mediating the positive effects of exercise on whole-body metabolism [reviewed in Oh et al. Production of myokines is predominantly influenced by skeletal muscle contraction and can alter glucose disposal, FAO, and lipolysis. For instance, the alteration of myostatin expression contributes to the proliferation, development, and metabolism of adipose and skeletal muscle tissue Exercise decreases the expression of myostatin in humans and obesity is associated with increased myostatin expression Moreover, myostatin knockout mice have significantly improved insulin sensitivity and glucose uptake, have increased peripheral tissue FAO, and are protected from diet-induced obesity , Another circulating factor named meteorin-like has been described to be released from skeletal muscle after exercise and in adipose tissue upon cold exposure Meteorin-like is involved in the adaptive responses to the regulation of energy homeostasis and tissue inflammation, but the therapeutic potential for metabolic and inflammatory diseases is currently unknown. Irisin, another myokine, has been proposed as an important glucoregulatory candidate. However, contradictory findings concerning the role of irisin in humans exist; therefore, results must be interpreted with caution. Hepatokines can also regulate whole-body metabolic flexibility and some are even considered as potential targets for the treatment of cardiovascular disease [reviewed in Jung et al. For example, fetuin-A has a major role in the regulation of insulin sensitivity as fetuin-A—deficient mice showed improved insulin sensitivity Nucleotide polymorphisms in human fetuin-A and high levels in serum are a predictable marker for the incidence of T2DM Additionally, fasting reduces the circulating levels of fetuin-A, whereas high levels of saturated fatty acids and glucose augments the expression of fetuin-A Mice deficient of fetuin-A are resistant to high-fat diet—induced obesity and have improved glucose tolerance Interestingly, some of these paracrine and endocrine factors are secreted by multiple tissues, and their local function and impact on metabolic flexibility can depend on their origin and local plasma concentrations. IL-6, for instance, when secreted by skeletal muscle, stimulates AMPK activity and in this way increases glucose uptake and β -oxidation in muscle and adipose tissue IL-6, is, however, also secreted by adipocytes from obese patients and negatively affects metabolic flexibility by decreasing insulin signaling and glucose uptake because of its proinflammatory properties , Another example is fibroblast growth factor 21 FGF21 , which, as a myokine, increases GLUT1 expression in skeletal muscle, boosting glucose uptake FGF21 from muscle also exerts endocrine-like effects on WAT, increasing lipolysis and β -oxidation and inducing browning As an adipokine, however, FGF21 stimulates insulin-independent glucose uptake in peripheral tissues; as a hepatokine, FGF21 stimulates lipolysis in WAT Although the role of adipokines, myokines, and hepatokines in the control of whole-body energy homeostasis and metabolic flexibility is only recently becoming evident, it is to be reported that they have clinical relevance and diagnostic potential. Metabolic flexibility in response to environmental stimuli, such as diet and exercise, are dramatically influenced by epigenetic factors as they influence gene expression by regulating access of transcriptional machinery to DNA. Evidence that epigenetic changes drive metabolic inflexibility in humans is emerging Metabolic networks, in particular those in the mitochondria, directly transmit information about the cells metabolic state to epigenetic programming enzymes that, for instance, add or remove epigenetic markers onto chromatin , Both global fluctuations in metabolite levels caused by nutritional inputs, circadian rhythm, and oxygenation, or local changes depending on intracellular metabolite distribution, can translate into epigenetic changes , The abundance of cofactors and the metabolic enzymes that generate them not only alter epigenetic enzyme histone modification, but also affect DNA methylation and posttranslational modification of the epigenetic enzymes themselves, resulting in a complex feedback network 42 , Moreover, the amplitude and duration of the metabolic stimulus required to alter the epigenome is dependent on the vastly different kinetics of epigenetic modifier enzymes How these epigenetic regulators are targeted to specific sites, such as promotor regions, how transient their epigenetic markers are, and how these changes are inherited, is still under active investigation People with a family history for T2DM have an increased risk for developing metabolic inflexibility; the lower HK II activity and PGC1 expression play a role in this For instance, skeletal muscle from families with a history of T2DM has altered methylation status of genes involved in muscle function and insulin and calcium signaling Tissue-specific epigenetic regulation may be of particular importance for metabolic flexibility because overweight patients with T2DM also have hypermethylated promoter regions of PGC1 α and an OXPHOS complex I subunit in skeletal muscle Promoters of many genes that are important for pancreatic β -cell survival and function are differentially methylated in T2DM patients compared with controls Moreover, obese patients have an altered epigenetic landscape associated with disrupted lipid oxidative metabolism and mitochondrial function in adipose tissue, skeletal muscle, and liver Although inborn errors of metabolism are clear examples of metabolic inflexibility, here we focus on acquired metabolic inflexibility. For specific information on inborn errors of metabolism we refer the reader to a comprehensive book on the subject Here, we discuss the pathophysiology of metabolic flexibility in the context of obesity, metabolic syndrome and T2DM, as well as systemic inflammation, cardiovascular disease, and cancer. At the heart of obesity lies the inability to regulate lipolytic and antilipolytic processes in adipose tissue during starvation and feeding, respectively. Obesity is predominantly associated with elevated levels of plasma free fatty acids High circulating levels of free fatty acids inhibit glycogen synthase activity and PDH activity, which leads to reduced disposal and oxidation of glucose. Besides adipocyte metabolic dysfunction, skeletal muscle mitochondrial capacity and β -oxidation are reduced. Specifically, upregulation of PPAR α and its downstream targets in response to high-fat feeding are defective Excess calories are then stored in peripheral fat depots as triglyceride; when these depots reach their maximum capacity and fail to expand, fat accumulates in ectopic depots, including skeletal muscle and the liver. Ectopic fat deposition is related to metabolic abnormalities and defects in insulin sensitivity, T2DM, cardiovascular disease, and cancer Finally, obesity is associated with a state of chronic low-grade inflammation because ectopic fat depots release more inflammatory mediators than peripheral fat depots and infiltration of macrophages Metabolic inflexibility and fat deposition therefore likely reinforce one another in a vicious cycle. Together with excess body fat and physical inactivity, metabolic syndrome is a major risk factor for developing T2DM and related complications include cardiovascular disease; increased rates of specific cancers, physical, and cognitive disability 5 ; and is associated with increased risk for T2DM and cardiovascular disease, among cancer Consequently, individuals with metabolic syndrome have increased mortality and a shortened lifespan The best example of compromised metabolic flexibility in metabolic syndrome is a deteriorated insulin-mediated substrate switching. As such, metabolic inflexibility is at the core of the pathophysiology of insulin resistance After a high-fat meal, patients with metabolic syndrome have higher levels of glycaemia and lower skeletal muscle free fatty acid uptake compared with healthy individuals. In response to fasting, skeletal muscle from patients with insulin resistance are less able to switch to FAO compared with healthy individuals An increased dependency on glucose oxidation and decreased reliance on FAO in offspring from patients with T2DM suggests that impaired FAO may precede insulin resistance — Moreover, studies strongly imply that impaired mitochondrial function precedes insulin resistance 41 , The importance of OXPHOS and its maintenance in relation to insulin resistance is underscored by observations that skeletal muscle mitochondria from patients with T2DM or obesity are unable to increase replication of mtDNA, which encodes essential OXPHOS components, in response to exercise combined with CR Moreover, skeletal muscle mitochondria from insulin-resistant patients have lower expression of PGC1 α and its downstream targets, and differ in mass, morphology, and function In particular, muscle mitochondria from patients with T2DM show reduced expression of mitofusin-2, which regulates mitochondrial outer membrane fusion, and thus mitochondrial dynamics and quality control They also have a lower maximal oxidative capacity, smaller mitochondria and reduced NADH oxidase complex I activity Interestingly, studies have demonstrated that BCAA and associated metabolites are strongly associated with insulin resistance and T2DM Based on the theory of mitochondrial metabolic gridlock and anaplerosis, excessive BCAA metabolites are proposed to clog the β -oxidation machinery, particularly in skeletal muscle and liver, and thus contribute to accumulation of incompletely oxidized intermediates of fatty acids, particularly in the presence of a high-fat diet. Collectively, under these conditions, such byproducts render glucose superfluous as a substrate and, combined with the upsurge in ROS, can lead to insulin resistance One of the hallmarks of metabolic syndrome is low-grade chronic systemic inflammation , In the case of obesity and insulin resistance, systemic inflammation can trigger and propagate metabolic inflexibility. Systemic inflammation and metabolic inflexibility can cause a vicious circle because metabolic inflexibility can also trigger systemic inflammation. How this is regulated at the cellular and molecular level is currently unknown, but hyperglycemia-induced mitochondrial ROS production can stimulate inflammation by signaling factors , such as protein kinase C, p38 MAPK, and c-Jun- N -terminal kinase Systemic low-grade inflammation as a trigger of metabolic inflexibility is best described in the context of obesity and lipid toxicity As a result of excess fatty acid intake, organs that reach the maximum of their storage capacity and ectopic tissues that accumulate fatty acids upon overspill can become infiltrated by immune cells resulting in inflammatory processes. Dysregulated release and storage of fatty acids can lead to an increased release of inflammatory cytokines such as TNF α and monocyte chemoattractant protein-1 and decreased secretion of anti-inflammatory adipokines such as adiponectin. This can result in recruitment of M1 type macrophages and T cells. Additionally, B lymphocytes, neutrophils, eosinophils, mast cells, and natural killer cells have all been implicated in adipose tissue dysfunction. This lipid toxicity can therefore generate signaling intermediates that can interfere with local and systemic immune responses, causing a vicious cycle of immune-metabolic degradation Although the mechanism and specific mediators in lipid-induced inflammation are not completely understood, the endoplasmic reticulum ER is central to these responses because this is where both lipid biosynthesis and esterification processes as well as inflammatory pathways converge. Disrupted lipid synthesis in the ER can change ER membrane composition, leading to ER stress, dysfunction, and ultimately cell death, triggering inflammation Lipids are also able to instigate inflammatory processes through interaction with cell-surface receptors, such as Toll-like receptor-4, and stress kinases in the cytoplasm, such as protein kinase R that through downstream signaling can induce the expression of genes that mediate inflammation and apoptosis, and promote inflammasome activity. Moreover, there is emerging evidence that lipids engage intracellular signaling pathways via protein kinase C isoforms that are related to T-cell activation and LPS responses It is unlikely, however, that one of such responses underlies lipotoxicity, but that a combination of factors mediate lipid-associated inflammation , Metabolic flexibility and the accompanied rerouting of metabolic flux are essential for immune function. Following immune stimulation, naive lymphocytes that rely on β -oxidation of fatty acids and pyruvate oxidation via the TCA cycle become active and engage in glycolysis and glutaminolysis Additionally, the switch to glycolysis enables glycolysis and TCA cycle intermediates to be used as key sources of carbon molecules for biosynthesis of nucleotides, amino acids, and lipids. In this way, glycolysis facilitates robust growth, rapid cellular proliferation, and the production of large quantities of effector molecules, ultimately to mount a sufficient immune response. The exact molecular regulation and thus the dependency on this metabolic switch differs between specific lymphocyte subsets Therefore, activated lymphocytes sustain OXPHOS for ATP production, which enhances cell survival and lifespan of lymphocytes and is essential for immune memory Memory T cells also use glucose and other fuels to synthesize triglycerides, which are then used in FAO Contrary to the dogma that innate immunity is nonspecific and lacks memory, classic innate immune cells such as macrophages, natural killer cells, and monocytes can become epigenetically reprogrammed by infection or vaccination, which confers nonspecific protection from secondary infection, a phenomenon called trained immunity The increase in glycolytic metabolism enables a more robust and swift response to intruding pathogens Training of immune cells is dependent on Akt, mTOR, hypoxia-inducible factor 1 α HIF1 α , and, to a lesser extent, SIRTs. Their crucial roles were affirmed by inhibition of Akt by wortmannin, mTOR by rapamycin, HIF1 α by ascorbate, and activation of SIRT1 by resveratrol, because these compounds blunt trained immunity Recently, however, the notion that a shift from OXPHOS to glycolysis underlies activation of all immune cells upon microbial stimulation was challenged because pathogen-specific metabolic rewiring was observed in human monocytes. This pathogen specificity was proposed to derive from signaling strength, rather than qualitative signaling differences between microbial stimuli, and consequently mediates different functional outputs such as phagocytic capacity Adipose tissue macrophages that have been activated and rely on glucose are proinflammatory type M1 and contribute to adipose inflammation and insulin resistance. Conversely, macrophages that rely on fatty acid metabolism secrete anti-inflammatory cytokines and thus preserve insulin sensitivity of liver and adipose tissue type M2 , Proinflammatory activation can be achieved by overexpression of GLUT1, even in the absence of other conventional stimuli, or by decreasing expression of lipid trafficking proteins, such as fatty acid transport protein 1 FATP1. FATP1 knockout mice fed high-fat diets showed an increased proinflammatory phenotype and worsened metabolic syndrome than mice with normal FATP1 expression. Alternatively, overexpression of FATP1 decreased substrate switching to glucose and reduced inflammation Thus, macrophage inflammatory status is mediated by rerouting metabolic pathways. The metabolic switch of glucose metabolism generates ROS that drive the production of inflammatory enzymes, cytokines, and chemokines such as IL-6, monocyte chemoattractant protein-1, TNF- α , and inducible NO synthase iNOS. iNOS is an important metabolic regulator of the immune response because NO inhibits OXPHOS and oxidative metabolism, thus promoting the glycolytic and proinflammatory phenotype , In this way, low-grade systemic inflammation defined as a twofold to threefold increase of circulating inflammatory mediators including the infiltration of immune cells, particularly in metabolic tissues that have reached their capacity limits, can be driven by metabolic inflexibility Recently, inhibition of iNOS in mouse macrophages was shown to dampen the M1 phenotype through reduction of NO-induced OXPHOS inhibition and assist in the phenotypic and metabolic M1 to M2 repolarization, suggesting that editing macrophage re polarization is a promising target to reduce inflammation and promote tissue repair An example of metabolic inflexibility and disrupted inflammatory assuagement is sepsis. During sepsis, a profound change in acute leukocyte metabolism occurs. Metabolic inflexibility drives sepsis-related innate immunoparalysis as the metabolism through glycolysis, β -oxidation, and OXPHOS pathways in leukocytes is downregulated, resulting in their inability to mount any response whatsoever A sudden mitochondrial complex I dysfunction in sepsis , possibly linked to the overproduction of NO and ROS, may be one of the causes of an upstream mitochondrial gridlock, and has been observed to relate to organ dysfunction Moreover, the impaired metabolic rate has been associated with reduced levels of mtDNA and mRNA expression of OXPHOS components , In summary, metabolic flexibility is not only necessary to mount an adequate immune response but also for mitigation of the inflammatory process. Cardiac performance is sustained by fatty acid and glucose oxidation, although fatty acids are the preferred substrate in the heart because of the higher energy yield compared with glucose. This flux is mediated by a high expression of PPAR α -regulated genes encoding key proteins in fatty acid uptake, esterification, and oxidation Under energetically demanding conditions such as exercise, the heart switches to the oxidation of glucose and lactate An increase in heart rate increases mitochondrial calcium concentration , allowing higher mitochondrial ATP production rates to sustain the increased energetic load of the heart. Upon exercise-induced sympathetic nervous system stimulation, β -adrenergic signaling increases glycolytic flux via cAMP activation of cAMP-dependent protein kinase A, increasing pyruvate production and glucose metabolism. Protein kinase A also activates phosphofructokinase-1 and PDH, stimulating the heart to rapidly oxidize glucose even in the presence of fatty acids As a consequence, triglyceride accumulation in cardiomyocytes likely leads to abnormal lipid signaling, increased ROS production, ER stress, and mitochondrial dysfunction Glucose metabolism is enhanced in a similar manner through insulin and nutrient stress signaling via Akt and AMPK, respectively A dependency on glucose and ketone body metabolism is also observed in myocardial ischemia, ventricular hypertrophy, and systemic hypertension , , as is mitochondrial dysfunction , A recent study in mice demonstrated that mildly increasing PPAR α expression in the progressive phase of heart failure, when FAO is decreased, maintains myocardial function and energetics, suggesting that modulating substrate utilization may be a promising therapeutic strategy for heart failure Obesity can cause metabolic inflexibility of the heart and alter substrate selection High-fat diet feeding and consequent insulin insensitivity, for instance, are known to cause cardiac metabolic inflexibility and reliance on fatty acids for energy production through PDK4 inhibition of PDH. Similar to T2DM, increased circulating fatty acids only exacerbates the feed-forward dependency on fatty acid substrates for energy production through the allosteric inhibition of enzymes involved in glycolysis Conversely, the failing heart becomes metabolically inflexible with a decreased capacity to use fatty acids and an increased dependence on glucose metabolism The switch from fatty acid preference to glucose is maintained by increased acetyl-CoA production from pyruvate and subsequent increases in malonyl-CoA concentration, which inhibits CPT-1 and thus FAO Epidemiological evidence shows that through their relation to insulin resistance, excess body weight, and T2DM are associated with an increased risk of pancreatic, liver, and endometrial cancers, among others, and of colon cancer in males Excess body weight increases the risk of cancer via augmented circulating levels of leptin and decreased circulating levels of adiponectin Diet composition is also correlated to development of certain cancers [reviewed in Potter et al. High-fat diets for instance have particularly been related to increased risk of colorectal , pancreatic , breast , lung , and prostate cancer Additionally, dietary fatty acid exposure increased tumor cell expression of CD36 and increased metastasis in mice Besides diets with a high fatty acid content, diets with a high amount of animal-derived amino acids also increase the risk of cancer in the middle-aged human population Reducing carbohydrate intake reduced tumor growth in mice Currently, clinical studies are under way, but various human studies point toward a reduced incidence of cancer after caloric restriction Our understanding of cancer metabolism has rapidly advanced in recent years. Most cancer cells show a remarkable metabolic flexibility, which allows a survival advantage in the face of their energetic demand and the environmental supply of nutrients. Mitochondrial-mediated flexibility is central in this process [reviewed in Vyas et al. Metabolic adaptations that underlie clonal evolution of tumor cells to a metastatic phenotype suggest that tumor cells do not become metabolically hardwired but remain able to reroute metabolism to adapt to their phenotype and the newly acquired environment Indeed, reducing metabolic flexibility in cancer cells may lead to potential treatment options, because metabolic interference can come at a substantial cost to oncogenic potential 1. Tumors and their environment can be very diverse and, as such, their metabolism and substrate preference is also diverse Common traits, however, include increased glucose consumption via glycolysis and enhanced glutamine metabolism to support the energetic and anabolic demands of proliferation. Notwithstanding the diversity of cancers, malignant cells share a common metabolic trait, namely that they can acquire and use nutrients from a predominantly nutrient-poor environment, a modus operandi that emerged as a promising target to battle tumors — As a prominent feature of cell activation and proliferation, tumor cells chiefly require increased amounts of glucose and glutamine to survive The metabolic reprogramming that underlies increased glucose consumption for use in glycolysis, as opposed to OXPHOS, is known as the Warburg effect. In , Otto Warburg discovered that cancer cells metabolize glucose differently than cells of normal tissues: that even in conditions of sufficient oxygen availability, cancer cells convert glucose into lactate instead of using glucose for OXPHOS Warburg hypothesized that cancer cells have mitochondrial defects and impaired aerobic respiration that forces them to rely on glycolysis. Today, we understand that mitochondrial respiration is not impaired but that cancer cells place emphasis on acquisition and generation of building blocks necessary for cell division. They do so by enhancing biosynthetic metabolism using glycolytic intermediates Interestingly, glucose catabolism in cancer cells is partially uncoupled from the TCA cycle and OXPHOS because increased activity of PDK dampens glucose metabolism through negative feedback on PDH , A glycolytic switch involving differential expression of pyruvate kinase isoforms and stabilization of HIF1 α upregulates rate-limiting enzymes within branching pathways of glycolysis, ensuring that glycolytic intermediates are free to take part in diverse biosynthetic reactions that are essential for increased proliferation These alternative pathways include the PPP using glucosephosphate, hexosamine biosynthesis using fructosephosphate, phospholipid biosynthesis using dihydroxyacetone phosphate, and glycine and serine biosynthesis using 3-phosphoglycerate. The PPP is chiefly used for NADPH and ribose synthesis to produce nucleotides Lactate, produced from glycolysis, is excreted from the cell or used in biosynthetic reactions such as aspartate synthesis 1. Aspartate is used to support protein and nucleotide synthesis in proliferating cells and sustains proliferation in the face of OXPHOS impairment Even TCA cycle intermediates are not solely used to produce NADH for mitochondrial respiration, but intermediates can also be used to form nonessential amino acids and fatty acids, which facilitate protein synthesis, membrane construction and cholesterol synthesis Glutamine can either be used as an important anaplerotic substrate in the TCA cycle, a carbon and nitrogen donor, or for production of purine and pyrimidine nucleotides that are necessary for DNA replication Intracellular glutamine can also be used as a substrate for the large neutral amino acid antiporter LAT1. LAT1 can couple glutamine export with import of essential amino acids. Compared with glucose, glutamine tumorigenesis-associated metabolic reprogramming is only recently becoming clear. In proliferating cells, the transcription factor Myc is a major driver of glutamine utilization and is frequently targeted for upregulation in various tumors, despite the abundance of glucose. This glutamine addiction is beneficial for the cancer cell because it maintains mitochondrial TCA cycle integrity and provides the cell with large quantities of NADPH needed to meet the demands of cell proliferation Besides Myc-regulated glutamine addiction, the activity of the Rb tumor suppressor protein family, which negatively regulates glutamine uptake, is reduced, facilitating increased uptake of glutamine. However, not all tumors are glutamine dependent because some tumors and embryonic stem cells are capable of proliferation without an exogenous supply of glutamine, because they can synthesize it Lifestyle interventions are pertinent for patients with metabolic syndrome. Most patients with T2DM are overweight or obese and do not exercise frequently. Lifestyle interventions to reduce body weight predominantly include exercise training and controlled reduced caloric intake, but their efficacy depends on age, sex, ethnicity, and body weight upon inclusion As such, caution must be taken when interpreting results when assessing metabolic flexibility using suboptimal methods, because individual variability and experimental setup can considerably influence results. Physical inactivity is likely one of the primary causes of metabolic inflexibility , ; regular habitual physical exercise has long been known to increase metabolic flexibility 7. As such, exercise training regimens can be used as an intervention to improve metabolic flexibility. Both promote considerable health benefits such as increased mitochondrial content and improvements in glycemic control For example, a day endurance exercise training regimen increases FAO in the absence of increased mitochondrial content. A high-intensity exercise training program, however, showed elevated citrate synthase and β -hydroxyacyl CoA dehydrogenase activity after 5 days and increased levels of mitochondrial complexes after 10 days AMPK is an important regulator of exercise-induced effects on metabolic flexibility Acute AMPK activation reduces glycogen and protein synthesis while promoting glucose transport and FAO A higher mitochondrial volume density and improved mitochondrial quality can be seen as consequences of chronic activation of AMPK and expression induction of PGC1 α , myocyte-specific enhancer factor 2 MEF2 , NRF-1 and NRF-2, and nuclear expulsion of histone deacetylase 4 and 5 41 , , Contraction-induced calcium uptake acutely increases OXPHOS 94 , and augments glucose transport and stimulates lipid uptake and oxidation through MEF2-induced expression of GLUT4 and PGC1 α , respectively. Additionally, PGC1 α is expressed upon muscle contraction-induced activation of p38 MAPK Endurance exercise increases the activity of muscle oxidative enzymes and FAO, in part from increased volume of the mitochondrial reticulum and elevated levels of cardiolipin, a lipid that is necessary for the assembly of OXPHOS complexes 41 , 51 , Regular physical exercise positively influences insulin-stimulated glucose uptake and mitochondrial function in skeletal muscle, and, importantly, in patients with T2DM FAO in skeletal muscle increases during physical exertion independent of body mass index, although regular exercise is likely needed to sustain a long-lasting impact on metabolic flexibility Particularly combined with weight loss, exercise training improves insulin sensitivity, mitochondrial content, and fasting FAO Intriguingly, type II, glycolytic, muscle fiber density is higher in obese and insulin-resistant patients, although it is unknown whether these are due to inactivity or impaired glucose metabolism Observations of reduced PGC1 α , AMPK , and mitofusin-2 expression in insulin-resistant individuals after exercise might provide mechanistic information as to why mitochondrial function improves more in healthy volunteers compared with patients with T2DM and obesity. Regular exercise can, for instance, reduce adipose cell size and enhance adipose glucose metabolism, resulting in improved insulin sensitivity in both adipose and muscle tissue. Moreover, habitual physical exercise remodels subcutaneous adipose tissue by stimulating browning in mice In rats, chronic endurance exercise induces browning in subcutaneous WAT concomitant with increased mobilization of energy stores, which were attenuated in animals fed high-fat diets. The browning program initiated by exercise training promoted expression of PPAR α and PPAR γ , AMPK, PGC1 α , and adipose triglyceride lipase Although the exact mechanisms underlying this beneficial effect are still under investigation, exercise training in humans reduced intrahepatic lipid content In mice, PGC1 α is required for an exercise-induced increase in mitochondrial volume density and reduction in intrahepatic lipid content Also, in the human heart, exercise reduces cardiometabolic risk factors by increasing insulin sensitivity, decreasing cardiac lipid content, and improving glucose tolerance In mice, exercise increased cardiac PGC1 α , NRF1, and TFAM expression and augmented mitochondrial volume and number, which were all dependent on endothelial nitric oxide synthase Interestingly, exercise training also drives metabolic adaptations through epigenetic mechanisms. Short-term, high-intensity exercise decreases muscle promoter methylation of genes involved in mitochondrial function such as PGC1 α , TFAM, MEF2A, and PDK4, whereas in patients with T2DM, these regions usually have higher methylation levels High-fat feeding in mice induced PGC1 α hypermethylation that was transferable to the offspring. Maternal exercise, on the other hand, prevented high-fat feeding hypermethylation of PGC1 α and mitigated epigenetic associated metabolic dysfunction in the offspring Although more research is necessary, it is clear that regular exercise and exercise training may aid in reversing the pandemic of metabolic disease. Weight loss is an important step in restoring metabolic flexibility and is the most common intervention for obesity and obesity-related metabolic comorbidities. Generally, energy-restricting diets are aimed at inducing a state of negative energy balance so that stored lipids inside adipocytes are used as alternative substrates Energy-restricting dietary regimens have proven effective in augmenting metabolic flexibility in animal studies and hold promise for application in humans During intermittent fasting, subjects go for extended periods with little or no energy intake, with intervening periods of normal energy intake. Intermittent fasting in rodents improved insulin and leptin sensitivity increases ketone body levels and reduced adiposity and inflammation CR without hunger- or disease-related malnutrition in animals and humans results in healthier aging through improved metabolic health, reduced obesity, and the risk of T2DM, cancer, and cardiovascular disease Maintaining an energy-restricting diet, however, is challenging, because most people have difficulties maintaining compliance over long periods. Moreover, a recent study highlighted the paucity of clinical evidence supporting energy-restricting diets in humans Although weight loss is generally achieved in overweight and obese subjects, potential adverse effects exist for leaner subjects Because compliance to energy-restricting diets is challenging, interventions that alter meal timing without reducing total caloric intake are actively pursued. Popular concepts such as increasing or decreasing meal frequency, however, lack concrete scientific evidence supporting their efficacy Recently, attention has arisen for food intake restricted to the active time phase In rodents, food intake outside the active phase causes obesity, whereas time-restricted feeding protects against obesity and insulin resistance Time-restricted feeding restores both cycling of metabolic regulators such as cAMP response element-binding protein, mTOR and AMPK, and circadian clock gene expression Besides dietary interventions to reduce overall energy intake or restrict energy intake to restricted periods, specific dietary constituents can induce changes in metabolic flexibility. For instance, carnitine is closely associated with the mechanism of metabolic flexibility Carnitine plays a role in the import of long-chain fatty acids into the mitochondria for use in β -oxidation and in the mitochondrial efflux of excess carbons in the form of acyl-carnitines Mechanistically, during substrate opulence or a deficiency of carnitine or carnitine acetyltransferase, accumulation of acetyl-CoA in skeletal muscle allosterically inhibits PDH resulting in impaired glucose utilization and whole-body glucose tolerance In obese rats, free carnitine in skeletal muscle is decreased and supplementation of l -carnitine restored metabolic flexibility In patients with T2DM, carnitine acetyltransferase expression is severely perturbed and free carnitine concentrations in diabetic mice are decreased compared with controls , Although not yet in clinical practice, l -carnitine supplementation improves metabolic flexibility by decreasing plasma glucose and insulin levels and increasing PDH activity in muscle of insulin-resistant subjects Carnitine metabolism may also be involved in the regulation of mitochondrial protein acetylation, because acetyl-CoA serve as acetyl donors and protein hyperacetylation is observed in high-fat feeding of mice Pharmaceutical approaches to improve metabolic flexibility have been studied in great detail. Most pharmaceutical therapeutics target major players or key nodes in metabolic circuits, many acting on mitochondrial function The examples that follow provide strong support for continuing the search for future pharmacological principles that enhance metabolic flexibility Fig. For more details on the mechanisms underlying the beneficial effects of these treatments on metabolic flexibility, we refer the reader to some excellent reviews described in each topic. A selection of pharmaceutical compounds that target major players or key nodes in metabolic circuits, such as AMPK and sirtuins. Via altered transcription factors, these compounds act on mitochondrial function and positively affect metabolic flexibility. Ac, acetyl; NA, nicotinic acid; NMN, nicotinamide mononucleotide; NR, nicotinamide riboside; P, phosphate; PARPi, poly ADP-ribose polymerase inhibitor; TF, transcription factor. Metformin is a biguanide and reduces hepatic glucose production and increases insulin sensitivity by activating AMPK, although several AMPK-independent mechanisms have been proposed [reviewed in Pryor and Cabreiro ]. Metformin is one of the first-line treatments of patients with T2DM, but it has also been used to treat patients at risk for T2DM, such as those with metabolic syndrome Resveratrol treatment also increased physical endurance and protected from high-fat diet-induced muscle accumulation of diacylglyceride and ceramide, and related mitochondrial dysfunction Because resveratrol activates mitochondrial biogenesis through the AMPK-SIRT1-PGC1 α axis, it prompts mitochondrial biogenesis, the unfolded protein response, and autophagy machinery that are known to extend longevity in animals 37 , Animal studies have also demonstrated that resveratrol could stimulate energy expenditure and protect against a high-fat diet-induced weight gain , via induction of FAO and reduced lipogenesis, mediated by activation of the AMPK-SIRT1 axis , In the context of insulin-controlled metabolic flexibility, rodent studies largely show improved insulin sensitivity and glucose tolerance in models of obesity, diabetes, and metabolic dysfunction [reviewed in de Ligt et al. Clinical studies in humans suggest that resveratrol may improve insulin sensitivity and reduce plasma levels of glucose and insulin in patients with T2DM and mimic CR in obese subjects , As such, resveratrol use by humans is particularly beneficial in reversing the early stages of metabolic disorders. Full confirmation of these beneficial effects in humans by placebo-controlled clinical trials remains relatively limited. Variation in duration and dose of resveratrol may explain the diverse outcomes of these studies The AMPK agonist 5-aminoimidazolecarboxamide riboside AICAR improves skeletal muscle glucose uptake and transport, fatty acid uptake, mitochondrial protein content, and insulin sensitivity in mice AICAR also rescued mitochondrial function in mice deficient in cytochrome c oxidase and augmented exercise endurance in healthy animals in a PGC1 α -dependent manner, even if they were untrained , Chronic exposure of AICAR reduced white adiposity and increased OXPHOS in rat hearts by increasing PGC1 α expression and FAO , as well as glucose uptake Figure 5 summarizes the potential role of AMPK activators for metabolic flexibility. SIRT1 and SIRT3 have particularly received attention in this respect. SIRT1 is predominantly found in the nucleus, although it can also be found in the cytosol. SIRT1 controls the activity of transcription factors and cofactors such as p53, MEF2, FOXO, and PGC1 α , which govern mitochondrial biogenesis and activity and lipid and glucose metabolism SIRT3, which is localized in the mitochondrial matrix, targets many proteins involved in metabolic homeostasis, including OXPHOS subunits Acetylation of mitochondrial proteins propagates metabolic inflexibility and deacetylation promotes metabolic flexibility , Additionally, nicotinamide riboside treatment in aging mice increased skeletal muscle function by preventing stem cell senescence, improved mitochondrial function, and a higher expression of genes involved in the TCA cycle and OXPHOS Nicotinamide mononucleotide administration to mice also enhances energy metabolism, promotes physical activity, improves lipid profiles, and ameliorates age-related pathophysiology Moreover, poly ADP-ribose polymerase inhibition rescued mitochondrial respiration defects and increased FAO in myotubes from obese patients by augmenting mitochondrial function PPARs are lipid sensors that transcriptionally modulate metabolic programs in response to nutrition and are interesting drug targets to improve metabolic flexibility [reviewed in Bugge and Holst ]. Fibrates activate PPAR α and are commonly used for treatment of hyperlipidemia. Of the fibrate drug class, fenofibrate and bezafibrate have recently gained interest as interventions to improve metabolic flexibility, in particular for the treatment of insulin resistance. Fenofibrate improves FAO in primary human skeletal muscle cell cultures from obese and insulin resistant subjects. In vitro and in animal models, PPAR α activation increased expression of PDK and CPT1 In insulin-deficient mice, bezafibrate improves impaired glucose metabolism by augmenting hepatic mitochondrial performance [reviewed in Komen and Thorburn ], suppressing hepatic inflammatory pathways, and improving insulin sensitivity Another PPAR agonist is tesaglitazar, which binds and activates PPAR α and PPAR γ. |
Ich habe diesen Gedanken gelöscht:)
der sehr nützliche Gedanke
Mir scheint es die ausgezeichnete Idee