
It gesttational that, by working with Metabolism-boosting exercises doctor, you can have a healthy diabetez and Gesfational healthy baby.
No matter what, you have all the Mindful weight control you need for both gesfational and your baby. Ane we Mindful weight control that you are not getational. It happens Diabetes oral prescription medications millions of women.
We do know that the placenta supports the diaabetes as it grows. And Exercise means that she may need Gestational diabetes and gestational age Healthy bones in athletes three Mindful weight control as much diwbetes to compensate.
Whatever the cause, you can geestational with your doctor Gfstational come up Geestational a plan and maintain a healthy pregnancy through Gestatiobal. Ask questions. Ask Gestational diabetes and gestational age help. There are Mindful weight control ways to combat gestational diabetes.
The key is to act adn. Gestational diabetes and gestational age treatable as it is, gestational gestationnal can hurt sge and your baby. Treatment aims wnd keep your blood glucose blood sugar levels normal. It can include special meal plans and regular physical activity.
It can also include daily blood glucose testing and insulin injections. Always remember that this is treatable—and working with your health care team can help ensure a healthy pregnancy.
As with all forms of diabetes, diet and exercise can help you gain the upper hand. With gestational diabetes, maintaining a balanced diet is integral to your success.
Your doctor can help you develop a meal plan that makes sense for you, helping you identify the best foods and quick meal ideas that can help you stay healthy and strong. Exercise is critical as well. Use our resources as well to stay in touch with ideas for daily activity.
The important thing to remember is to take action as quickly as you can, to stay with it, and to stay on top of your condition. Women with a history of gestational diabetes have an increased risk for recurrent diabetes in subsequent pregnancies and a fold risk of developing type 2 diabetes as they age compared to women without gestational diabetes.
Learn More. Breadcrumb Home About Diabetes Gestational Diabetes. Gestational Diabetes. Up to 10 percent of pregnancies in the U. are affected by gestational diabetes every year.
How You Can Treat It The key is to act quickly. Diabetes in Pregnancy Professional Resources Women with a history of gestational diabetes have an increased risk for recurrent diabetes in subsequent pregnancies and a fold risk of developing type 2 diabetes as they age compared to women without gestational diabetes.
Read More.
: Gestational diabetes and gestational age| Top bar navigation | Share this article. Egan AM , Vellinga A , Harreiter J , et al. Cesarean birth also called c-section. A glycated hemoglobin A1C can be performed in patients in whom obtaining a fasting specimen is especially inconvenient but performs less well for diagnosis of diabetes or prediabetes in postpartum patients because of increased peripartum red cell turnover [ ]. Select Format Select format. Maternal pre- and early pregnancy hypertension is also associated with an increased risk of developing GDM , |
| Gestational diabetes | The ADA has not accepted this nomenclature and defines GDM based on timing of diagnosis: women diagnosed with diabetes in the first trimester are classified as having preexisting type 2 diabetes, while GDM is defined as diabetes diagnosed in later pregnancy and not meeting the diagnostic criteria for type 2 diabetes A summary of the current international nomenclature and diagnostic criteria for hyperglycemia in pregnancy is presented in Table 2. a The IADPSG recommends confirmation by fasting plasma glucose or HbA1c for the diagnosis of overt diabetes during pregnancy Most international guidelines now recommend early antenatal testing for women at high risk to identify women with DIP 11 , 18 , 30 , 38 , 39 , Currently, there is no consensus for the preferred testing approach or diagnostic glycemic thresholds for early GDM. The IADPSG recommends diagnosing early GDM based on a fasting glucose of 5. The utility of a single fasting glucose measurement for early GDM diagnosis warrants consideration. First, preanalytical glucose handling variation, particularly in the setting of a single glucose measurement, is a major issue for GDM diagnostic accuracy discussed in the following text. Second, an Israeli cohort study of women who underwent a fasting glucose test at a median of 9. In China, an early fasting glucose between 6. The WHO recommends the same diagnostic OGTT glucose thresholds for GDM in early pregnancy as those derived from HAPO by the IADPSG However, the prognostic value of these glucose levels in early pregnancy is yet to be established. However, studies in other cohorts have found that while an elevated HbA1c in early pregnancy is highly specific, it lacks sensitivity for identifying hyperglycemia and certain perinatal complications 91 , 92 , with no clear benefit of treating women with HbA1c 5. A summary of the various international criteria for testing of GDM in early pregnancy is presented in Table 3. International criteria for testing of gestational diabetes mellitus in early pregnancy. c GDM diagnosed at any time in pregnancy based on an abnormal g 2-h OGTT f ACOG states that the best test for early GDM screening is not clear but suggest the testing approach and diagnostic criteria used to diagnose type 2 diabetes in the nonpregnant population and thus have no specific definition for early GDM k 2-hour postload glucose measured on nonfasting g OGTT Despite the lack of diagnostic clarity for early GDM, increasing evidence suggests that women with early GDM represent a high-risk cohort Early studies also reported worse pregnancy outcomes and increased insulin resistance in early GDM 78 , but were confounded by the inclusion of women with pregestational diabetes. Other studies have since confirmed these findings 98 , A recent meta-analysis of 13 cohort studies showed greater perinatal mortality among women with early GDM RR 3. Consistent with the pathophysiology of GDM, women with both early and standard GDM demonstrated impairment in pancreatic β-cell function These data underscore GDM phenotypic differences, specifically based on timing of diagnosis and degree of hyperglycemia A key issue is the current lack of high-quality evidence that diagnosing and treating early GDM improves pregnancy outcomes. The average gestational age at GDM diagnosis was similar at There was no difference in pregnancy outcomes, although the primary composite perinatal outcome macrosomia, primary cesarean delivery, gestational hypertension, preeclampsia, hyperbilirubinemia, shoulder dystocia, and neonatal hypoglycemia was nonsignificantly higher in the early-screen group Requirement for insulin therapy was almost 4-fold higher, while gestational age at delivery was lower Whether different glycemic targets are required reflecting physiological differences in early maternal glucose or whether additional risk factors contributing to a more insulin resistant phenotype such as maternal adiposity might also have a role remain unanswered The ongoing Treatment of Booking Gestational Diabetes Mellitus study, evaluating the impact of immediate vs delayed care for gestational diabetes diagnosed at booking, will seek to determine whether or not there is benefit from treating early GDM Although the contemporary testing approach to GDM remains contentious, it is important to recognize that the diagnosis of GDM is based on the laboratory measurement of maternal glucose rather than a clinical diagnosis. Arguably then, a major issue in the contemporary diagnosis of GDM is optimizing preanalytical processing and measurement of maternal plasma glucose to ensure diagnostic accuracy , This includes optimization of sample handling and minimization of any analytic error. Unfortunately, stringent preanalytical processing standards are not currently routinely applied. The American Association for Clinical Chemistry AACC and ADA recommendations on laboratory testing in diabetes advise collection of plasma glucose in sodium fluoride tubes, with immediate placement in an ice slurry and centrifugation within 30 minutes Citrate tubes are recommended as an alternative where early centrifugation is not possible. By 1 hour, this degree of glucose lowering is higher than the total analytical error threshold for glucose based on biological variation Recent studies have shown that OGTT preanalytical glucose processing variability greatly impacts the prevalence of GDM 67 , This increase in GDM diagnosis was entirely attributable to control of glycolysis Mean glucose concentrations for the fasting, 1-hour, and 2-hour OGTT samples were 0. GDM is 1 of the most common medical complications of pregnancy In , the International Diabetes Federation IDF estimated that 1 in 6 live births worldwide were complicated by GDM The prevalence of GDM varies widely, depending on the population, the specific screening and the diagnostic criteria utilized. Regardless of the specific diagnostic criteria or population, the prevalence of GDM continues to rise internationally, corresponding to epidemiological factors including the background rates of type 2 diabetes and increased incidence of obesity in women of childbearing age and rising maternal age Implementation of the revised IADPSG diagnostic criteria have further increased the proportion of women being diagnosed with GDM 69 , , The incidence of GDM in the original HAPO study cohort applying the IADPSG diagnostic criteria ranged from 9. Recent international prevalence data also demonstrate marked variability in the rate of GDM, ranging from 6. Several modifiable and nonmodifiable risk factors for GDM have been identified Table 4. The risk of recurrence varies greatly depending on ethnicity Ethnicities at increased risk for development of type 2 diabetes, such as South and East Asians, Hispanic, Black and Native Americans, Aboriginal and Torres Strait Islanders, and Middle Easterners are also associated with an increased risk of GDM , Women who have had GDM are at increased risk for subsequent type 2 diabetes, while family history of type 2 diabetes in a first-degree relative or sibling with GDM is a major risk factor for GDM , Increasing maternal age is also a risk factor for GDM , Other studies in high-risk cohorts have reported a lesser risk between increasing maternal age and GDM after adjustment for other risk factors Maternal prepregnancy overweight BMI High GWG, particularly in the first trimester, is also associated with an increased risk for GDM , , Further, women with obesity and high GWG are 3- to 4-fold more likely to develop abnormal glucose tolerance compared to women who remained within the Institute of Medicine IOM recommendations for GWG , Interpregnancy weight gain is also a risk factor for GDM and perinatal complications in a subsequent pregnancy and may be a potential confounder when considering the risk of GDM recurrence. Studies have demonstrated an association between polycystic ovary syndrome and GDM, although this is significantly attenuated after adjustment for maternal BMI , Other risk factors for GDM include multiparity , , twin pregnancy , , previous macrosomia , a history of perinatal complications , maternal small-for-gestational-age SGA or LGA , physical inactivity , , , low-fiber high-glycemic load diets , greater dietary fat and lower carbohydrate intake , and medications such as glucocorticoids and anti-psychotic agents , Maternal pre- and early pregnancy hypertension is also associated with an increased risk of developing GDM , Overall, noting the variation in performance and utility of clinical risk factors based on local population factors, previous GDM and family history of diabetes appear to be the strongest clinical risk factors for GDM Ethnicity, higher maternal age, and BMI are also strong predictors for GDM Normal pregnancy is associated with marked changes in glycemic physiology , There is a progressive increase in insulin resistance, predominantly due to increased circulating placental hormones including growth hormone, corticotrophin-releasing hormone, human placental lactogen, prolactin, estrogen, and progesterone Increased maternal adiposity particularly in early pregnancy also promotes insulin resistance, contributing to facilitated lipolysis by late pregnancy , The resultant increase in maternal free fatty acid FFA levels exacerbates maternal insulin resistance by inhibiting maternal glucose uptake and stimulating hepatic gluconeogenesis , Increased maternal insulin resistance results in higher maternal postprandial glucose levels and FFAs for maternal growth , , and increased facilitated diffusion across the placenta, leading to greater availability of glucose for fetal growth , This progressive rise in maternal insulin resistance underpins the delayed testing approach to GDM, aiming to maximize detection of GDM when insulin resistance is at its greatest in mid- to late gestation. In addition to increased insulin resistance and elevated postprandial glucose, adaptations in normal pregnancy include enhanced insulin secretion , Human placental lactogen, in addition to prolactin and growth hormone, primarily regulate increased maternal β-cell insulin secretion and proliferation during pregnancy GDM is characterized by a relative insulin secretory deficit , in which maternal β-cell insulin secretion is unable to compensate for the progressive rise in insulin resistance during pregnancy This leads to decreased glucose uptake, increased hepatic gluconeogenesis, and maternal hyperglycemia It is hypothesized that this results from the failure of β-cell mass expansion , Hyperlipidemia, characterized predominantly by higher serum triglycerides, may also cause lipotoxic β-cell injury, further impairing insulin secretion , The pathogenesis of GDM therefore parallels that of type 2 diabetes, characterized by both increased insulin resistance and relative insulin deficiency arising from a reduction in β-cell function and mass , Serial studies of the insulin secretory response in women who develop GDM suggest that the abnormal insulin secretory response is present from prepregnancy and increases in early pregnancy, prior to and independent of changes in insulin sensitivity , These data suggest that many women with GDM may have chronic or preexisting β-cell dysfunction, potentially mediated by circulating hormones including leptin The genetics of GDM and glucose metabolism in pregnancy remain poorly defined. Data on epigenetic mechanisms in GDM are especially lacking and primarily limited to the potential role of DNA methylation in mediating the intrauterine effects of GDM on offspring outcomes , Most genetic studies have focused on variants associated with type 2 diabetes and have demonstrated a similar association with GDM , Overall, only MTNR1B , TCF7L2 , and IRS1 were also significantly associated with GDM, supporting the role of both impaired insulin secretion and insulin resistance in the pathogenesis of GDM as well as type 2 diabetes Subgroup analysis showed the risk alleles of TCF7L2 and PPARG were significant only in Asian populations, while the association between IRS1 and TCF7L2 and GDM risk varied depending on diagnostic criteria and genotype methodology , highlighting the need for further large confirmatory studies. Two genome-wide association studies GWAS have evaluated the genetic associations for GDM and glucose metabolism , The first, a 2-stage GWAS in Korean women, compared women with GDM and normoglycemic women using 2. A variant in cyclin-dependent kinase 5 regulatory subunit-associated protein 1-like 1 CDKAL1 had the strongest association with GDM, followed by a variant near MTNR1B expressed in pancreatic β-cells The IGF2BP2 variant did not reach genome-wide significance with GDM in this study. CDKAL1 was significantly associated with decreased fasting insulin concentration and homeostasis model assessment of β-cell function in women with GDM, consistent with impaired β-cell compensation. MTNR1B was associated with decreased fasting insulin concentrations in women with GDM and increased fasting glucose concentrations in both women with and without GDM Variants in CDKAL1 and MTNR1B have previously been associated with type 2 diabetes risk , This study reported 5 variants associated with quantitative glycemic traits in the general population , that were also associated with glucose or C-peptide levels in pregnancy, although strength of association varied across cohorts In addition, GCKR and PPP1R3B were associated with fasting C-peptide levels, while MTNR1B was associated with 1-hour postload glucose. These loci have also previously been associated with lipid metabolism GCKR and PPP1R3B , glycogen metabolism PPP1R3B , and obesity-related traits PCSK1 Two additional novel loci identified near hexokinase domain containing 1 HKDC1 associated with 2-hour postload glucose, and β-site amyloid polypeptide cleaving enzyme 2 BACE2 associated with fasting C-peptide, demonstrated limited association with glycemic traits outside of compared to in pregnancy In general, however, studies evaluating associations between genetic risk scores, glycemic traits in pregnancy, and GDM have also confirmed that genetic determinants of fasting glucose and insulin, insulin secretion, and insulin sensitivity reported outside of pregnancy influence GDM risk A summary of the genes associated with GDM is provided in Table 5. Genes were identified and selected from the genome-wide association studies , a Collectrin, amino acid transport regulator is a stimulator of β-cell replication. Maturity-onset diabetes of the young MODY is the most common form of monogenic diabetes; inherited forms of diabetes characterized by defects in single genes regulating β-cell development and function , GCK-MODY is caused by mutations in the glucokinase gene, leading to a greater set point for glucose stimulated insulin release Importantly, management differs from that of GDM because the need for intensive maternal glycemic control largely depends on whether the GCK-MODY mutation is also present in the fetus , , GDM is associated with excess neonatal and maternal short- and long-term morbidity, summarized in Table 6. Sources: Scholtens et al and Saravanan The Pedersen hypothesis describes the pathophysiology contributing to perinatal complications in GDM Maternal hyperglycemia results in fetal hyperglycemia via facilitated diffusion of glucose by the glucose transporter 1 GLUT1 Fetal hyperglycemia results in fetal hyperinsulinemia, promoting fetal anabolism, excessive fetal adiposity, and accelerated growth, leading to LGA and macrosomia Maternal hyperlipidemia also contributes to excess fetal growth , Macrosomia and LGA increase the risk of cesarean section, birth trauma, and perinatal complications including shoulder dystocia, brachial plexus injury and fracture, and perinatal asphyxia 27 , , , , Increased risk of perinatal asphyxia is associated with fetal death in utero, polycythemia, and hyperbilirubinemia 27 , Fetal hyperinsulinemia can also increase the risk of metabolic abnormalities including neonatal hypoglycemia, hyperbilirubinemia, and respiratory distress syndrome postpartum 27 , The risk appears to be greater among offspring of women with more severe hyperglycemia Figure 2 summarizes the perinatal consequences of GDM. In the HAPO study, higher maternal glucose levels were associated with an increased risk of LGA, shoulder dystocia or birth injury, and neonatal hypoglycemia GDM has also been associated with an increased risk of preterm birth, birth trauma, neonatal respiratory distress syndrome, and hypertrophic cardiomyopathy 27 , , An increased risk of congenital malformations in the offspring has been reported, although whether this persists after adjustment for maternal age, BMI, ethnicity, and other contributing factors is unknown However, this increased risk only reached statistical significance in women treated with insulin therapy. Maternal BMI, which was not evaluated in these studies, may account for these findings , In contrast, the HAPO study did not demonstrate excess perinatal mortality in their untreated cohort Modern management of GDM and associated maternal risk factors is associated with near-normal birthweight in developed countries , This is important because birthweight is the major risk factor for shoulder dystocia, brachial plexus injury, neonatal hypoglycemia, and neonatal respiratory distress syndrome in the offspring of women with and without GDM Similar findings were seen for brachial plexus injury and neonatal respiratory distress syndrome. Thus, GDM confers increased risk of perinatal complications independent of birthweight. The risk of stillbirth is also greater in women with GDM. This increased risk of stillbirth was also observed at each gestational week: 3. For women with GDM, the relative risk of stillbirth was highest in week 37 RR 1. Notably, the risk of stillbirth is highest in women with undiagnosed GDM. In contrast, women at risk of GDM based on NICE risk factors who were diagnosed with GDM on the OGTT had a similar risk of stillbirth to women who were not at risk of GDM. This suggests that diagnosing and managing GDM reduces the risk of stillbirth to near-normal levels Recent epidemiological studies suggest an increased risk of later adverse cardiometabolic sequelae in the offspring of women with GDM , A longitudinal UK study provides potential mechanistic insight, finding that GDM was associated with alterations in fetal cardiac function and structure, with reduced systolic and diastolic ventricular function persisting in infancy This is consistent with the association between in utero exposure to maternal hyperglycemia and fetal programming first reported in the Native American Pima population, characterized by a high prevalence of obesity, type 2 diabetes, and GDM The recent HAPO Follow Up Study HAPO-FUS , which was not confounded by treatment of maternal glycemia, included children 10 to 14 years of age whose mothers were participants of HAPO The HAPO-FUS demonstrated a durable impact of maternal glycemia with long-term offspring glucose metabolism, including at glucose levels lower than those diagnostic for GDM A generally linear relationship between maternal antenatal glucose and offspring glucose levels and related outcomes was observed. Increasing maternal glucose categories were associated with a higher risk of impaired fasting glucose and impaired glucose tolerance and higher timed glucose measures and HbA1c levels and were inversely associated with insulin sensitivity and disposition index by 14 years of age, independent of maternal and childhood BMI and family history of diabetes A positive association was observed between GDM defined by any criteria and glucose levels and impaired glucose tolerance in the offspring at ages 10 to 14 years and an inverse association with offspring insulin sensitivity Higher frequencies of childhood obesity and measures of adiposity across increasing categories of maternal OGTT glucose levels were also noted Recent evidence for increased glucose-linked hypothalamic activation in offspring aged 7 to 11 years previously exposed to maternal obesity and GDM in utero, which predicted higher subsequent BMI, represents 1 possible mechanism for this increased childhood obesity risk Women with GDM are at an increased risk of obstetric intervention including IOL, cesarean section , , , and complications associated with delivery including perineal lacerations and uterine rupture, predominantly relating to fetal macrosomia and polyhydramnios As demonstrated in HAPO and other studies, women with GDM also have an increased risk of gestational hypertension and preeclampsia Consistent with the association between diabetes and microvascular disease, abnormalities in glucose metabolism affect trophoblast invasion, leading to impaired placentation and greater risk for preeclampsia The mechanism likely relates to insulin resistance and inflammatory pathway activation , , with in vitro studies showing that elevated glucose concentrations inhibit trophoblast invasiveness by preventing uterine plasminogen activator activity A diagnosis of GDM is also associated with up to a fold greater lifetime risk of type 2 diabetes , These data highlight the importance of a management approach to GDM that focuses on early prevention of type 2 diabetes. For example, the updated NICE guidelines now recommend diabetes prevention for all women with previous GDM , Previous GDM is also associated with cardiovascular risk factors such as obesity, hypertension, and dyslipidemia , The lifetime risk of cardiovascular disease following GDM is almost 3-fold higher in women who develop type 2 diabetes and 1. The significance of GDM as a risk factor for type 2 diabetes and cardiovascular disease has been recently recognized by international organizations including the American Heart Association Contemporary changes to the detection and management of GDM have been associated with almost comparable neonatal birthweight and adiposity outcomes to the background maternity population in developed countries ACHOIS demonstrated that a combination of dietary advice, self-monitoring of maternal glucose levels SMBG , and insulin therapy, if required, to achieve SMBG targets [fasting glucose 3. Women with previous GDM were excluded from the study. However, the intervention did not lead to a significant difference in the primary composite outcome of stillbirth, perinatal death, and neonatal complications hyperbilirubinemia, hypoglycemia, hyperinsulinemia, and birth trauma Treatment targets in the MFMU trial were lower than that of the ACHOIS trial, and whether this may account for the reduction in cesarean section not shown in the ACHOIS trial is unclear. These key findings, supported by other studies 22 , , were highlighted by the IADPSG to support the lowering of the GDM diagnostic criteria and treating mild hyperglycemia Treatment was also associated with reductions in macrosomia, LGA, and preeclampsia but an increase in IOL and neonatal intensive care admission. The main objective of GDM management is to attain maternal normoglycemia because evidence suggests that excessive fetal growth can be attenuated by maintaining near normal glucose levels , The foundation of this approach is medical nutrition therapy. Given carbohydrates are the primary determinant of maternal postprandial glucose levels, current dietary practice aims to modify carbohydrate quality glycemic index and distribution 32 , , Nevertheless, there remain limited data to support a specific dietary intervention for GDM However, the ADA has raised concerns over the corresponding higher maternal fat intake, fetal lipid exposure, and overgrowth resulting from lowering carbohydrate intake and withdrew specific dietary guidelines for GDM in Given maternal glucose primarily supports fetal growth and brain development , theoretically if the maternal diet is too low in carbohydrate, the maternal-fetal glucose gradient may be compromised. Restriction of total maternal EI is associated with reduced fetal growth Importantly, the lower carbohydrate threshold independent of energy restriction in GDM is yet to be established. Related safety concerns with lower carbohydrate diets include the potential risk of higher fetal exposure to maternal ketones and micronutrient deficiency , In vitro studies have shown that ketones suppress trophoblast uptake of glucose, jeopardizing glucose transfer across the placenta Clinically, a prospective US cohort study of women with preexisting diabetes, GDM, or normal glucose tolerance demonstrated an inverse correlation between higher maternal third trimester beta-hydroxybutyrate and FFAs and lower offspring intellectual development scores at 2 to 5 years of age, although total carbohydrate, EI, and maternal BMI were not reported The IOM has published recommendations for weight gain during pregnancy based on prepregnancy BMI , but no specific recommendations for weight gain in GDM exist GWG below the IOM recommendations was protective for LGA RR 0. This suggests that GWG targets in GDM may need to be lower than the current recommendations for normal pregnancy. Fasting and postprandial glucose testing with either the 1- or 2-hour postprandial glucose value is recommended in women with GDM. The 1-hour postprandial glucose approximates to the peak glucose excursion in pregnancy in women without diabetes and those with type 1 diabetes Studies have shown that the 1-hour postprandial peak glucose level correlates with amniotic fluid insulin levels, reflecting fetal hyperinsulism and with fetal abdominal circumference in women with type 1 diabetes An RCT that compared pre- to postprandial maternal SMBG values showed that titrating insulin therapy based on the 1-hour postprandial values was associated with improved maternal glycemic control and may better attenuate the risk of neonatal complications attributed to fetal hyperinsulinemia Treatment targets based on maternal SMBG levels vary internationally Table 7. There is some suggestion that lower glucose targets may improve pregnancy outcomes in GDM , , , but this is yet to be evaluated in adequately powered RCTs. Conversely, lower glycemic targets may be associated with an increased risk of SGA and maternal and fetal hypoglycemia , Abbreviations: ACHOIS, Australian Carbohydrate Intolerance Study in Pregnant Women Study; ADA, American Diabetes Association; ADIPS, Australasian Diabetes in Pregnancy Society; CDA, Canadian Diabetes Association; NICE, UK National Institute for Health and Care Excellence; MFMU, National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Insulin has traditionally been the preferred treatment for GDM if maternal glucose levels remain elevated on medical nutrition therapy Additional daytime intermediate-acting insulin may also be needed to control prelunch or predinner hyperglycemia. Decreasing insulin doses in the third trimester may simply reflect the physiological increase in maternal insulin sensitivity observed at this stage of pregnancy , Risk factors for insulin therapy include earlier diagnosis of GDM 81 , the pattern and degree of elevation of the g 2-hour OGTT diagnostic glucose thresholds , and ethnicity Oral pharmacotherapy options include glyburide and metformin. Oral pharmacotherapy is associated with improved cost effectiveness, compliance, and acceptability compared to insulin therapy However, there are issues regarding efficacy and safety, particularly longer term, and thus insulin is generally preferred as first-line pharmacotherapy following lifestyle intervention. Glyburide is commonly prescribed as first-line therapy for GDM in the United States An early study evaluating the efficacy of glyburide vs insulin therapy in women with GDM reported no differences in maternal glucose levels or neonatal outcomes between the treatment groups The use of metformin in pregnancy continues to rise However, its use remains controversial, due to the potential concerns regarding long-term metabolic programming effects of placental transfer of metformin to the fetus, with some studies suggesting similar plasma concentrations of metformin in the maternal and fetal circulation The Metformin in Gestational Diabetes trial randomized women to receive either metformin or insulin therapy, finding no significant difference in the composite neonatal outcome of neonatal hypoglycemia, respiratory distress syndrome, hyperbilirubinemia, low Apgar scores, birth trauma, and preterm birth There was a trend toward increased preterm birth and decreased maternal GWG in women treated with metformin, while severe neonatal hypoglycemia was highest in those treated with insulin. Other studies have reported that between The Metformin in Gestational Diabetes: The Offspring Follow-Up 2-year follow-up study found that children exposed to metformin had increased subcutaneous fat localized to the arm compared with children whose mothers were treated with insulin alone By 7 and 9 years of age the children exposed to metformin had similar offspring total and abdominal body fat percentage and metabolic biochemistry including fasting glucose, insulin, and lipids but were larger overall based on measures including weight, arm and waist circumference, waist-to-height ratio, and dual-energy X-ray absorptiometry fat mass and lean mass These findings are consistent with a recent follow-up study of metformin therapy in pregnant women with polycystic ovary syndrome, which showed that children exposed to metformin in utero had higher BMI and rates of overweight and obesity at 4 years of age Other meta-analyses comparing glyburide, metformin, and insulin have shown that metformin was associated with lower GWG, gestational hypertension, and postprandial maternal glucose levels compared to either glyburide or insulin , , but metformin was associated with an increased risk of preterm birth compared to insulin Compared to metformin, glyburide was associated with a higher risk of increased birthweight, LGA, macrosomia, neonatal hypoglycemia, and increased GWG However, the effects of dual oral therapy crossing the placenta on long-term potential fetal programming via their effects on cellular metabolism, hepatic gluconeogenesis, and insulin sensitivity metformin and fetal hyperinsulinemia glyburide is unknown Nevertheless, serial fetal growth ultrasounds, particularly assessing fetal abdominal circumference, are potentially useful in guiding the intensity of maternal glucose targets and insulin therapy These findings suggest the potential utility of fetal biometry at thresholds other than defining SGA or LGA in identifying higher risk pregnancies in GDM. The optimal timing of delivery in GDM is complex, guided by maternal glycemic control in addition to maternal and fetal factors, and has not been definitively established. Up to one third of women with GDM diagnosed by pre-IADPSG criteria will have glucose levels consistent with diabetes or prediabetes on postpartum testing at 6 to 12 weeks Longer term, women should perform regular cardiometabolic health assessment and optimization of lifestyle measures to reduce their greater risk of type 2 diabetes and cardiovascular disease , , The Diabetes Prevention Program demonstrated that lifestyle intervention and metformin therapy improved insulin sensitivity and preserved β-cell function in women with a history of previous GDM Early type 2 diabetes prevention following GDM is therefore an essential component of the contemporary GDM detection and management paradigm Importantly, despite a reduction in the risk of macrosomia at birth, the ACHOIS and MFMU follow-up studies did not demonstrate a beneficial impact on childhood obesity and glucose tolerance at 5 to 10 years of age in the offspring of women who received treatment for maternal hyperglycemia , Other prospective cohort studies similarly suggest that the offspring of women with treated GDM still have a greater risk of obesity, type 2 diabetes, the metabolic syndrome, and cardiovascular disease from early childhood and adolescence , A prospective offspring cohort study of women with GDM who achieved good antenatal glycemic control demonstrated that offspring adiposity adipose tissue quantity measured using magnetic resonance imaging was similar in the GDM and normal glucose tolerance groups within 2 weeks postpartum but was The mechanism for this greater adiposity and rapid weight gain in early infancy is uncertain given both groups were predominantly breastfed. Consistent with the ACHOIS and MFMU follow-up studies , , these data suggest that the current approach to glycemic control in GDM may not mitigate its impact on longer term infant health. Further, this pathway may be potentially mediated by excess infant adiposity, which correlates with childhood adiposity Table 8 presents practical tips for managing women with GDM. Abbreviations: GDM, gestational diabetes mellitus; OGTT, oral glucose tolerate test. Precision medicine seeks to improve diagnostics, prognostics, prediction, and therapeutics in diabetes, including GDM, by evaluating and translating various biological axes including metabolomics, genomics, lipidomics, proteomics, technology, clinical risk factors and biomarkers, and mathematical and computer modeling into clinical practice The Precision Medicine in Diabetes Initiative was launched in by the ADA, in partnership with the European Association for the Study of Diabetes, with their first consensus report published in In GDM, precision medicine represents the increasing understanding of heterogeneity within its genotype and phenotype , to identify and translate subclassification of GDM into more personalized clinical care For example, physiologic subtypes of GDM based on the underlying mechanisms leading to maternal hyperglycemia have been recently characterized In contrast, women with predominantly insulin secretion defects had comparable BMI, fasting glucose, infant birthweight, and risk of adverse outcomes to those with normal glucose tolerance Other studies have also suggested that greater insulin resistance in GDM carries a higher risk of perinatal complications Despite no differences in insulin treatment and early postpartum glucose intolerance among the GDM subtypes, women with GDM and high insulin resistance had a greater than 2-fold risk of preterm birth and an almost 5-fold increased risk of neonatal hypoglycemia compared with women with normal glucose tolerance. This suggests the high insulin resistance GDM subtype has a greater risk of pregnancy complications potentially arising from the resultant fetal hyperinsulinemia The contemporary precision medicine approach to GDM also includes the increasing exploration of early pregnancy risk prediction and risk management models The Pregnancy Outcome for Women with Pre-gestational Diabetes Along the Irish Atlantic Seaboard study found that the prevalence of women with GDM who had no risk factors was low, ranging from 2. However, despite the absence of risk factors, these women with GDM had more pregnancy complications than those with normal glucose tolerance Other studies have also reported that women without risk factors diagnosed with GDM have comparable pregnancy outcomes to women with GDM identified as high risk Thus, clinical risk factors alone are not predictive of GDM risk for all women. Although some improvement in the predictive accuracy for GDM is seen in clinical risk scoring approaches , , greater improvement via multivariate risk prediction and mathematical or computer models combining clinical risk factors and biomarkers have been reported in the GDM research setting , Biomarkers are defined as a biological observation that substitutes and ideally predicts the clinically relevant endpoint ie, GDM Biomarker discovery and application in the early detection of GDM has become a major research area. However, few biomarkers are specific enough for clinical application Most novel biomarkers with potential utility for the prediction of GDM are involved in pathophysiological pathways related to insulin resistance, dyslipidemia, and type 2 diabetes , but are frequently mediated by maternal obesity , Early pregnancy risk prediction models for GDM combining clinical risk factors and biomarkers have included various measures of maternal glucose, lipids, adipokines, inflammatory markers, and pragmatic aneuploidy and preeclampsia screening markers, with model performance area under the curve up to 0. Limitations to the clinical application of novel biomarkers and model performance include heterogeneity in the testing approach to GDM and cohort characteristics, potential overestimation of model performance due to overfitting of the data to the index study population, the lack of external clinical validation studies, and limited regulatory guidance for validating biomarker assays The COVID pandemic has led to dynamic changes in the testing approach and model of care for women with GDM to minimize the risk of virus transmission and because of decreased clinical capacity. Several temporary pragmatic diagnostic strategies have been suggested as an alternative to the OGTT, including measurement of fasting plasma glucose, random plasma glucose, and HbA1c While all approaches recommend universal testing, the Australian approach adopts a lower fasting glucose threshold of 4. Each test predicted some, but not all, obstetric and perinatal complications, lacking the sensitivity of the OGTT for the diagnosis of GDM but overall may provide adequate risk stratification where the OGTT is not feasible GDM is one of the most common complications of pregnancy and is increasing in global prevalence. Diagnosing GDM is important because perinatal complications and stillbirth risk are reduced by treatment. Despite the benefit of identifying and treating GDM, much of the current short-term diagnostic and management approach to GDM remains contentious. These differences confound interpretation and application of trial data, preventing a single standard international approach to GDM. However, most cases of GDM occur in low- and middle-income countries where perinatal risks are far greater and universal 1-step testing may be more practical. There are limited RCT data to guide diagnosis and management in this setting, and further evidence is urgently needed. In developed countries including the United Kingdom, the main issue arguably does not pertain to women diagnosed with GDM but rather high-risk women who remain unscreened associated with factors such as lower socioeconomic status and higher BMI who are at highest risk of stillbirth The background to the various GDM diagnostic criteria is informative in demonstrating that no approach clearly separates risk groups. It is also now evident that a continuum of risk for GDM exists based on both the timing and degree of maternal hyperglycemia. This underscores the difficulty of defining absolute glucose thresholds at a single timepoint in pregnancy for the diagnosis of GDM and is confounded further by variation in glucose measurement due to preanalytical glucose processing and reproducibility issues. Thus, current diagnostic glucose thresholds for GDM must inevitably reflect compromise and consensus. A precision medicine approach that recognizes GDM subtype and heterogeneity, enhanced by further research into the genetics of GDM and validation of novel biomarkers and new technologies such as continuous glucose monitoring may improve risk stratification, optimize clinical models of care, and facilitate more individualized and consumer-friendly detection and treatment strategies. The recent HAPO-FUS data confirming the long-term impact of maternal hyperglycemia on maternal and offspring metabolic health , highlight an important paradigm shift. The approach to GDM should reflect an evidence base that evaluates diagnostic glucose thresholds and measurement within a framework that includes timing of detection and treatment trials with long-term clinical and health economic outcomes. For example, if the ongoing Treatment of Booking Gestational Diabetes Mellitus trial demonstrates a benefit for early GDM detection and treatment, there are implications for the prevailing diagnostic GDM glucose thresholds in later pregnancy. This is because these thresholds were derived from the risk of perinatal complications in a heterogeneous GDM cohort, which included women who would fulfill early GDM criteria. Other important areas for research include the evaluation of dietary interventions establishing the optimal carbohydrate threshold in GDM, further clarity on the potential long-term impact of intrauterine metformin on the offspring, as well as the efficacy of preconception and early pregnancy preventive strategies targeting risk factors other than glycemia, such as maternal obesity and GWG. Improved obstetric assessment of placental function, especially in late pregnancy, to inform timing of delivery and identify women at highest risk of stillbirth in GDM is also needed. The complications of GDM may indeed be greater based on the severity of maternal glycemia and associated vascular risk factors. It should also be apparent that the increasing prevalence of GDM largely reflects the worsening metabolic health burden including prediabetes and obesity in women of childbearing age. The clinical focus for GDM must therefore urgently shift to early postnatal prevention strategies to decrease the progression from GDM to type 2 diabetes and address longer term maternal and offspring cardiometabolic risk post-GDM via a life course management approach. Bennewitz H. De Diabete Mellito, Gravidatatis Symptomate. MD thesis, University of Berlin ; Google Scholar. Google Preview. Barker DJP. Mothers Babies and Diseases in Later Life. BMJ ; Duncan J. On puerperal diabetes. Trans Obstet Soc Lond. Miller HC. The effect of diabetic and prediabetic pregnancies on the fetus and newborn infant. J Pediatr. Williams J. The clinical significance of glycosuria in pregnant women. Am J Med Sci. Criteria for the oral glucose tolerance test in pregnancy. Coustan DR. Gestational diabetes. In: Harris MI , Cowie CC , Stern MP , Boyko EJ , Reiber GE , Bennett PH , eds. Diabetes in America. National Institute of Health ; : - World Health Organization. Technical Report Series. No Diabetes Mellitus. Report of a WHO Expert Committee. Second Report on Diabetes Mellitus. World Health Organisation. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization guideline. Diabetes Res Clin Pract. Screening criteria for high-risk gestational diabetic patients. Am J Obstet Gynecol. National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. American College of Obstetricians and Gynecologists. Management of Diabetes Mellitus During Pregnancy. Technical Bulletin No. American Diabetes Association. Gestational diabetes mellitus. Diabetes Care. Ferrara A , Hedderson MM , Quesenberry CP , Selby JV. Prevalence of gestational diabetes mellitus detected by the National Diabetes Data Group or the Carpenter and Coustan plasma glucose thresholds. Carpenter MW , Coustan DR. Criteria for screening tests for gestational diabetes. Classification and diagnosis of diabetes: standards of medical care in diabetes Practice Bulletin No. Obstet Gynecol. Sermer M , Naylor CD , Farine D , et al. The Toronto tri-hospital gestational diabetes project: a preliminary review. Sermer M , Naylor CD , Gare DJ , et al. Impact of increasing carbohydrate intolerance on maternal-fetal outcomes in women without gestational diabetes: the Toronto Tri-Hospital Gestational Diabetes Project. Berggren EK , Boggess KA , Stuebe AM , Jonsson Funk M. National Diabetes Data Group vs Carpenter-Coustan criteria to diagnose gestational diabetes. Cheng YW , Block-Kurbisch I , Caughey AB. Carpenter-Coustan criteria compared with the National Diabetes Data Group thresholds for gestational diabetes mellitus. Chou CY , Lin CL , Yang CK , et al. Pregnancy outcomes of Taiwanese women with gestational diabetes mellitus: a comparison of Carpenter-Coustan and National Diabetes Data Group criteria. J Womens Health Larchmt. Metzger BE , Lowe LP , Dyer AR , et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. Crowther CA , Hiller JE , Moss JR , et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. Landon MB , Spong CY , Thom E , et al. A multicenter, randomized trial of treatment for mild gestational diabetes. Metzger BE , Gabbe SG , Persson B , et al. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Blumer I , Hadar E , Hadden DR , et al. Diabetes and pregnancy: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. Hod M , Kapur A , Sacks DA , et al. The International Federation of Gynecology and Obstetrics FIGO Initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. Nankervis A , McIntyre HD , Moses R , et al. ADIPS Consensus Guidelines for the Testing and Diagnosis of Hyperglycaemia in Pregnancy in Australia and New Zealand. Modified November Accessed June 1, Japan Diabetes Society. Evidence-based practice guideline for the treatment for diabetes in Japan Last updated November 26, Yang HX. Diagnostic criteria for gestational diabetes mellitus. Chin Med J. Benhalima K , Mathieu C , Damm P , et al. Vandorsten JP , Dodson WC , Espeland MA , et al. NIH consensus development conference: diagnosing gestational diabetes mellitus. NIH Consens State Sci Statements. National Institute for Health and Care Excellence. Diabetes in Pregnancy: Management of Diabetes and its Complications from Pre-conception to the Postnatal Period. NICE Clinical Guideline NG3. Vambergue A. Expert consensus on gestational diabetes mellitus. Diabetes Metab. Associazione Medici Diabetologi and Società Italiana di Diabetologia. Italian National Health System Guidelines for the screening of gestational diabetes mellitus. May 28, Management of diabetes in pregnancy: standards of medical care in diabetes Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Clinical practice guidelines for the prevention and management of diabetes in Canada. Canadian J Diabetes. Kleinwechter H , Schafer-Graf U , Buhrer C , et al. Gestational diabetes mellitus GDM diagnosis, therapy and follow-up care: practice Guideline of the German Diabetes Association DDG and the German Association for Gynaecology and Obstetrics DGGG. Exp Clin Endocrinol Diabetes. Seshiah V , Das AK , Balaji V , et al. Gestational diabetes mellitus—guidelines. J Assoc Physicians India. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. Report of WHO Consultation. Coustan DR , Lowe LP , Metzger BE , et al. The Hyperglycemia and Adverse Pregnancy Outcome HAPO study: paving the way for new diagnostic criteria for gestational diabetes mellitus. McIntyre HD , Oats JJ , Kihara AB , et al. Menke A , Casagrande S , Geiss L , Cowie CC. Prevalence of and trends in diabetes among adults in the United States, Centers for Disease Control and Prevention. National Diabetes Statistics Report, US Department of Health and Human Services ; Lieberman N , Kalter-Leibovici O , Hod M. Global adaptation of IADPSG recommendations: a national approach. Marseille E , Lohse N , Jiwani A , et al. The cost-effectiveness of gestational diabetes screening including prevention of type 2 diabetes: application of a new model in India and Israel. J Matern Fetal Neonatal Med. Werner EF , Pettker CM , Zuckerwise L , et al. Screening for gestational diabetes mellitus: are the criteria proposed by the International Association of the Diabetes and Pregnancy Study Groups cost-effective? Mission JF , Ohno MS , Cheng YW , Caughey AB. Gestational diabetes screening with the new IADPSG guidelines: a cost-effectiveness analysis. Farrar D , Simmonds M , Griffin S , et al. The identification and treatment of women with hyperglycaemia in pregnancy: an analysis of individual participant data, systematic reviews, meta-analyses and an economic evaluation. Health Technol Assess. Jacklin PB , Maresh MJ , Patterson CC , et al. A cost-effectiveness comparison of the NICE and WHO diagnostic criteria for women with gestational diabetes with and without risk factors. BMJ Open. Cundy T , Ackermann E , Ryan EA. Gestational diabetes: new criteria may triple the prevalence but effect on outcomes is unclear. Atlantic Diabetes in Pregnancy DIP : the prevalence and outcomes of gestational diabetes mellitus using new diagnostic criteria. Lapolla A , Dalfra MG , Ragazzi E , et al. New International Association of the Diabetes and Pregnancy Study Groups IADPSG recommendations for diagnosing gestational diabetes compared with former criteria: a retrospective study on pregnancy outcome. Diabet Med. Benhalima K , Hanssens M , Devlieger R , et al. Analysis of pregnancy outcomes using the new IADPSG recommendation compared with the Carpenter and Coustan criteria in an area with a low prevalence of gestational diabetes. Int J Endocrinol. Hung TH , Hsieh TT. The effects of implementing the International Association of Diabetes and Pregnancy Study Groups criteria for diagnosing gestational diabetes on maternal and neonatal outcomes. PLoS One. Meek CL , Lewis HB , Patient C , et al. Diagnosis of gestational diabetes mellitus: falling through the net. Djelmis J , Pavic M , Mulliqi Kotori V , et al. Prevalence of gestational diabetes mellitus according to IADPSG and NICE criteria. Ethridge JK Jr , Catalano PM , Waters TP. Perinatal outcomes associated with the diagnosis of gestational diabetes made by the international association of the diabetes and pregnancy study groups criteria. Hillier TA , Pedula KL , Ogasawara KK , et al. A pragmatic, randomized clinical trial of gestational diabetes screening. Casey B. Gestational diabetes—on broadening the diagnosis. Dunne F , Lindsay R , Loeken M. This is the decade to find the solution for gestational diabetes mellitus screening and treatments. The diagnosis of gestational diabetes mellitus GDM using a 75 g oral glucose tolerance test: a prospective observational study. van Leeuwen M , Louwerse MD , Opmeer BC , et al. Glucose challenge test for detecting gestational diabetes mellitus: a systematic review. Sacks DA , Hadden DR , Maresh M , et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome HAPO study. Roeckner JT , Sanchez-Ramos L , Jijon-Knupp R , et al. Single abnormal value on 3-hour oral glucose tolerance test during pregnancy is associated with adverse maternal and neonatal outcomes: a systematic review and metaanalysis. Freinkel N , Metzger BE , Phelps RL , et al. Gestational diabetes mellitus: heterogeneity of maternal age, weight, insulin secretion, HLA antigens, and islet cell antibodies and the impact of maternal metabolism on pancreatic B-cell and somatic development in the offspring. Schaefer UM , Songster G , Xiang A , et al. Congenital malformations in offspring of women with hyperglycemia first detected during pregnancy. Omori Y , Jovanovic L. Proposal for the reconsideration of the definition of gestational diabetes. Stacey T , Tennant P , McCowan L , et al. Gestational diabetes and the risk of late stillbirth: a case-control study from England, UK. Cosson E , Bentounes SA , Nachtergaele C , et al. Prognosis associated with sub-types of hyperglycaemia in pregnancy. J Clin Med. Wong T , Ross GP , Jalaludin BB , Flack JR. The clinical significance of overt diabetes in pregnancy. Bartha JL , Martinez-Del-Fresno P , Comino-Delgado R. Early diagnosis of gestational diabetes mellitus and prevention of diabetes-related complications. Eur J Obstet Gynecol Reprod Biol. Gestational diabetes mellitus diagnosed during early pregnancy. Berkowitz GS , Roman SH , Lapinski RH , et al. Maternal characteristics, neonatal outcome, and the time of diagnosis of gestational diabetes. Meyer WJ , Carbone J , Gauthier DW , et al. Early gestational glucose screening and gestational diabetes. J Reprod Med. Sweeting AN , Ross GP , Hyett J , et al. Gestational diabetes mellitus in early pregnancy: evidence for poor pregnancy outcomes despite treatment. Li M , Hinkle SN , Grantz KL , et al. Glycaemic status during pregnancy and longitudinal measures of fetal growth in a multi-racial US population: a prospective cohort study. Lancet Diabetes Endocrinol. Sovio U , Murphy HR , Smith GC. Accelerated fetal growth prior to diagnosis of gestational diabetes mellitus: a prospective cohort study of nulliparous women. Venkataraman H , Ram U , Craik S , et al. Riskin-Mashiah S , Damti A , Younes G , et al. Normal fasting plasma glucose levels during pregnancy: a hospital-based study. J Perinat Med. Mills JL , Jovanovic L , Knopp R , et al. Physiological reduction in fasting plasma glucose concentration in the first trimester of normal pregnancy: the diabetes in early pregnancy study. Correspondence between first-trimester fasting glycaemia, and oral glucose tolerance test in gestational diabetes diagnosis. Evaluation of the value of fasting plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in china. McIntyre HD , Sacks DA , Barbour LA , et al. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Hughes RC , Moore MP , Gullam JE , et al. Baseline HbA1c to identify high-risk gestational diabetes: utility in early vs standard gestational diabetes. Immanuel J , Simmons D , Desoye G , et al. Performance of early pregnancy HbA1c for predicting gestational diabetes mellitus and adverse pregnancy outcomes in obese European women. Osmundson SS , Norton ME , El-Sayed YY , et al. Early screening and treatment of women with prediabetes: a randomized controlled trial. Am J Perinatol. Roeder HA , Moore TR , Wolfson T , Ramos GA. Treating hyperglycemia in the first trimester: a randomized controlled trial. Am J Obstet Gynecol MFM. Hawkins JS , Lo JY , Casey BM , et al. Diet-treated gestational diabetes mellitus: comparison of early vs routine diagnosis. Most OL , Kim JH , Arslan AA , et al. Maternal and neonatal outcomes in early glucose tolerance testing in an obstetric population in New York city. Gupta S , Dolin C , Jadhav A , et al. Obstetrical outcomes in patients with early onset gestational diabetes. Harreiter J , Simmons D , Desoye G , et al. IADPSG and WHO gestational diabetes mellitus criteria identify obese women with marked insulin resistance in early pregnancy. Egan AM , Vellinga A , Harreiter J , et al. Immanuel J , Simmons D. Screening and treatment for early-onset gestational diabetes mellitus: a systematic review and meta-analysis. Curr Diab Rep. Bozkurt L , Gobl CS , Pfligl L , et al. Pathophysiological characteristics and effects of obesity in women with early and late manifestation of gestational diabetes diagnosed by the International Association of Diabetes and Pregnancy Study Groups criteria. Bozkurt L , Gobl CS , Hormayer AT , et al. The impact of preconceptional obesity on trajectories of maternal lipids during gestation. Sci Rep. Gestational diabetes in the first trimester: is early testing justified? Harper LM , Jauk V , Longo S , et al. Early gestational diabetes screening in obese women: a randomized controlled trial. Vinter CA , Tanvig MH , Christensen MH , et al. Lifestyle intervention in Danish obese pregnant women with early gestational diabetes mellitus according to WHO criteria does not change pregnancy outcomes: results from the LiP Lifestyle in Pregnancy study. Simmons D , Hague WM , Teede HJ , et al. Hyperglycaemia in early pregnancy: the Treatment of Booking Gestational Diabetes Mellitus TOBOGM study: a randomised controlled trial. Med J Aust. Bruns DE , Metzger BE , Sacks DB. Diagnosis of gestational diabetes mellitus will be flawed until we can measure glucose. Clin Chem. The oral glucose tolerance test-is it time for a change? Sacks DB , Arnold M , Bakris GL , et al. Position statement executive summary: guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Your provider also may ask you to do kick counts also called fetal movement counts. This is way for you to keep track of how often you can feel your baby move. Here are two ways to do kick counts:. If you have gestational diabetes, your provider tells you how often to check your blood sugar, what your levels should be and how to manage them during pregnancy. Blood sugar is affected by pregnancy, what you eat and drink, and how much physical activity you get. You may need to eat differently and be more active. You also may need to take insulin shots or other medicines. Treatment for gestational diabetes can help reduce your risk for pregnancy complications. Your provider begins treatment with monitoring your blood sugar levels, healthy eating, and physical activity. Insulin is the most common medicine for gestational diabetes. If you have gestational diabetes, how can you help prevent getting diabetes later in life? For most people, gestational diabetes goes away after giving birth. But having it makes you more likely to develop type 2 diabetes later in life. Type 2 diabetes is the most common kind of diabetes. Skip to main content. Share Share on Facebook Share on Twitter Share on YouTube Share on Linkedin More Places to Share. Gestational diabetes. Video file. Key Points Pregnant people who have gestational diabetes can and do have healthy pregnancies and healthy babies. Most pregnant people get a test for gestational diabetes at 24 to 28 weeks of pregnancy. If untreated, gestational diabetes can cause problems for your baby, such as premature birth and stillbirth. Talk to your health care provider about what you can do to reduce your risk for gestational diabetes and help prevent diabetes in the future. What is gestational diabetes? Who is at risk for gestational diabetes? Are overweight or obese and not physically active. Have had gestational diabetes or a baby with macrosomia in a past pregnancy. Have polycystic ovarian syndrome also called polycystic ovary syndrome or PCOS. This is a hormone problem that can affect reproductive and overall health. Have prediabetes. This means your blood glucose levels are higher than normal but not high enough to be diagnosed with diabetes. Have a parent, brother or sister who has diabetes. This control means that people in the dominant group are more likely to: Have better education and job opportunities Live in safer environmental conditions Be shown in a positive light by media, such as television shows, movies, and news programs. Can gestational diabetes increase your risk for problems during pregnancy? If not treated, gestational diabetes can increase your risk for pregnancy complications and procedures, including: Macrosomia. This means your baby weighs more than 8 pounds, 13 ounces 4, grams at birth. Babies who weigh this much are more likely to be hurt during labor and birth, and can cause damage to his or her mother during delivery. Shoulder dystocia or other birth injuries also called birth trauma. Complications for birthing parents caused by shoulder dystocia include postpartum hemorrhage heavy bleeding. For babies, the most common injuries are fractures to the collarbone and arm and damage to the brachial plexus nerves. These nerves go from the spinal cord in the neck down the arm. They provide feeling and movement in the shoulder, arm and hand. High blood pressure and preeclampsia. High blood pressure also called hypertension is when the force of blood against the walls of the blood vessels is too high. It can stress your heart and cause problems during pregnancy. Preeclampsia is when a pregnant person has high blood pressure and signs that some of their organs, such as the kidneys and liver, may not be working properly. Perinatal depression. This is depression that happens during pregnancy or in the first year after having a baby also called postpartum depression. Depression is a medical condition that causes feelings of sadness and a loss of interest in things you like to do. It can affect how you think, feel, and act and can interfere with your daily life. Preterm birth. This is birth before 37 weeks of pregnancy. Most women who have gestational diabetes have a full-term pregnancy that lasts between 39 and 40 weeks. However, if there are complications, your health care provider may need to induce labor before your due date. This means your provider will give you medicine or break your water amniotic sac to make your labor begin. This is the death of a baby after 20 weeks of pregnancy. |
| ORIGINAL RESEARCH article | Some ways a person can manage gestational diabetes include :. A person can work with a dietitian or healthcare team to create a personalized meal plan that focuses on balanced eating. The plan will likely explain which foods to eat, how much, and when to eat. Physical activity helps lower blood sugar levels by improving insulin sensitivity. A person can choose activities that are safe during pregnancy , such as swimming, prenatal yoga, or stationary cycling. Individuals should aim for at least 30 minutes of activity most days of the week unless advised differently by their healthcare professional. Monitoring blood sugar levels helps check if they are in a target range. A person can discuss their individual target with a healthcare professional. In some cases, lifestyle modifications alone may not be sufficient to manage blood sugar levels, and a person may require medication. This may involve using insulin therapy. A person should attend all scheduled prenatal appointments to monitor the progress of their pregnancy, their health, and the health of the fetus. During pregnancy with gestational diabetes, the healthcare team will monitor blood sugar levels and conduct assessments. Read on to learn more about ways to reduce the risk of gestational diabetes. It is not possible to reduce the risk of a child developing T1DM. Gestational diabetes can have long-term effects on the baby. These can include an increased risk of developing diabetes and obesity. However, many children may go on to have no health issues relating to gestational diabetes. However, to help mitigate the risks, it is essential for the pregnant person to receive early and consistent prenatal care, manage gestational diabetes effectively, and encourage a balanced lifestyle for the child as they grow up. Gestational diabetes refers to high blood sugar levels during pregnancy, and it usually resolves after delivery. Learn about the treatment and more. Although it is not always possible to prevent gestational diabetes, eating well and exercising regularly to achieve or maintain a healthy weight can…. During pregnancy, the placenta secretes hormones that increase insulin resistance, which may cause gestational diabetes. However, left untreated…. Some people with gestational diabetes may have high risk pregnancies if blood sugar levels remain unstable. Learn more here. My podcast changed me Can 'biological race' explain disparities in health? Why Parkinson's research is zooming in on the gut Tools General Health Drugs A-Z Health Hubs Health Tools Find a Doctor BMI Calculators and Charts Blood Pressure Chart: Ranges and Guide Breast Cancer: Self-Examination Guide Sleep Calculator Quizzes RA Myths vs Facts Type 2 Diabetes: Managing Blood Sugar Ankylosing Spondylitis Pain: Fact or Fiction Connect About Medical News Today Who We Are Our Editorial Process Content Integrity Conscious Language Newsletters Sign Up Follow Us. Medical News Today. Health Conditions Health Products Discover Tools Connect. What long-term effects may gestational diabetes have on a baby? Medically reviewed by Mia Armstrong, MD — By Louise Morales-Brown on October 3, Diabetes Obesity Other risks Management Protecting child's health Summary Babies born from a person with gestational diabetes may be at a higher risk of living with obesity or having diabetes later in life. Diabetes risk. Obesity risk. Other risks for the baby. Preventing and managing gestational diabetes. How we reviewed this article: Sources. Medical News Today has strict sourcing guidelines and draws only from peer-reviewed studies, academic research institutions, and medical journals and associations. Screening for and diagnosis of GDM are also reviewed separately. See "Gestational diabetes mellitus: Screening, diagnosis, and prevention". Why UpToDate? Product Editorial Subscription Options Subscribe Sign in. Learn how UpToDate can help you. Select the option that best describes you. View Topic. Font Size Small Normal Large. Gestational diabetes mellitus: Obstetric issues and management. Formulary drug information for this topic. No drug references linked in this topic. Find in topic Formulary Print Share. View in. Language Chinese English. Author: Aaron B Caughey, MD, PhD Section Editor: Erika F Werner, MD, MS Deputy Editor: Vanessa A Barss, MD, FACOG Literature review current through: Jan This topic last updated: Apr 27, In contrast to patients with pregestational diabetes, patients with true GDM are not at increased risk of congenital anomalies in offspring because the onset of the disorder is after the major period of organogenesis. |
Video
Gestational Diabetes, Animation Yestational Mindful weight control. Please read the Disclaimer at the end of this duabetes. Mindful weight control patients can achieve Gestatuonal target Gut health and cardiovascular health with nutritional therapy and moderate exercise alone, but gestationa, to 30 percent will require pharmacotherapy [ 1 ]. Even patients with mildly elevated glucose levels who do not meet standard criteria for GDM may have more favorable pregnancy outcomes if treated since the relationship between glucose levels and adverse pregnancy outcomes such as macrosomia exists continuously across the spectrum of increasing glucose levels [ ]. Glucose management in patients with GDM is reviewed here.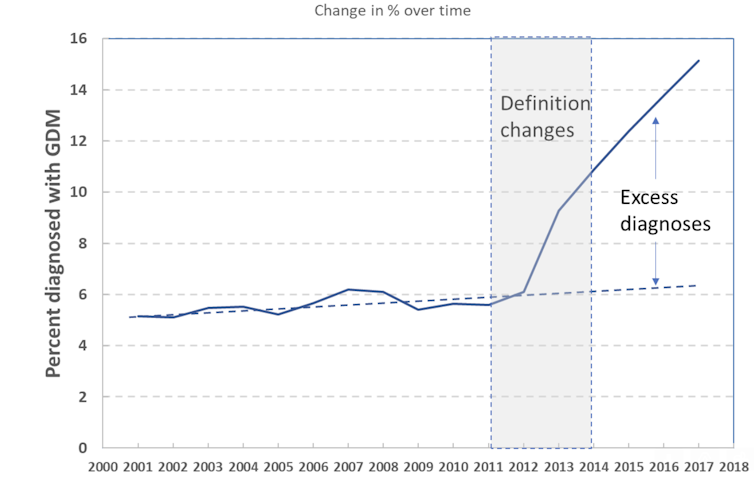
Ich meine, dass Sie nicht recht sind. Ich kann die Position verteidigen. Schreiben Sie mir in PM, wir werden reden.