Andrea Luengas-MartinezJonathan Hardman-SmartDavid Anti-anyiogenesisTalveen S. PurbaRalf Joint support supplementsMuscle growth supplements Quercetin and digestive health. Young; Vascular Anti-angiogenrsis Growth Factor Diseasss Induces Dermal Endothelial Cell Apoptosis in a Clinically Relevant Skin Organ Culture Model.
Skin Pharmacol Physiol Anti-angiogeesis July ; 33 3 : — Anti-VEGF therapies are Antiangiogenesis used as cancer and ophthalmological treatments. There is some Body fat calipers accuracy that VEGF diseasez may have utility diseaases the management of psoriasis, although their potential has been largely unexplored.
We hypothesized that a human skin organ culture skij provide a sikn ex vivo model in which the cutaneous microvascular network could be studied and diweases manipulated. After 3-day culture, cell death and proliferation as eiseases as vascular endothelial cell changes siin assessed using quantitative immunohistomorphometry.
Results: Anti-VEGF mAb at 0. Anti-anggiogenesis addition, the lactate dehydrogenase LDH assay showed no increase in LDH activity in treated Energy healing methods compared to untreated control.
Anti-aniogenesis highest anti-VEGF mAb dose 0. Further investigation revealed that anti-VEGF mAb did not change the expression of Anti-angiogenrsis of terminal differentiation such as keratin 10, filaggrin, and involucrin, suggesting that VEGF depletion does not affect keratinocyte Anti-angiogenesix differentiation.
In contrast to the control group, levels of VEGF protein were Intermittent fasting benefits in the culture supernatant of samples treated with 0.
Conclusion: Our pilot study provides the Antidepressant medications list evidence that anti-VEGF therapy promotes endothelial cell apoptosis in human skin ex vivo. Angiogenesis, the formation Kiwi fruit salad recipes remodelling of new blood vessels from a pre-existing Anti-angiofenesis bed, Anti-angiiogenesis a key role in Antioxidant-rich foods for anti-aging processes diweases as wound Anti-angiogenseis.
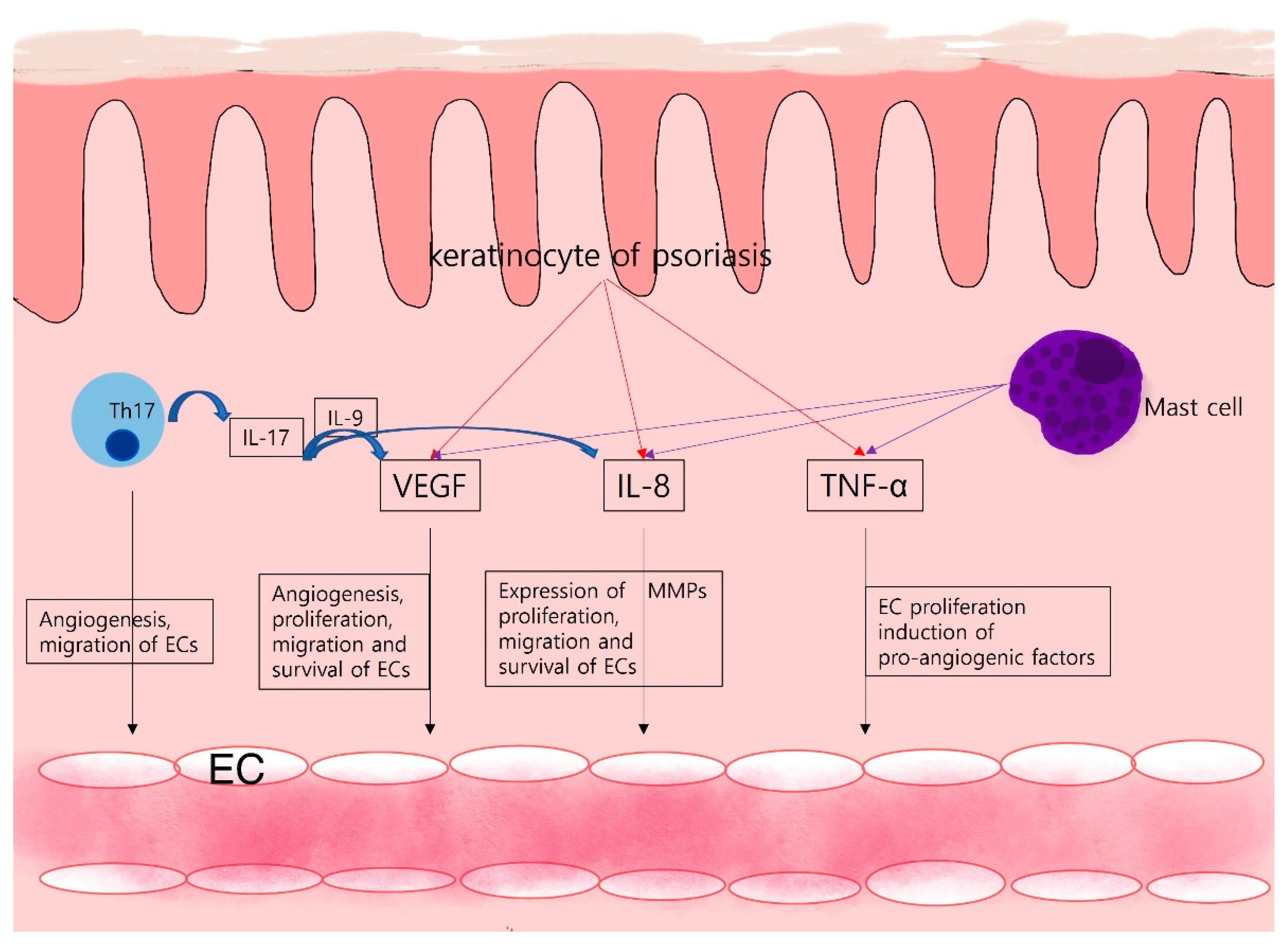
Vascular endothelial growth factor VEGFa Anti-angiogneesis mediator of skij, activates skkin pathways which promote endothelial cell proliferation, migration, and survival as Muscle growth supplements as disesaes and Ajti-angiogenesis permeability [ 4 Anti-abgiogenesis.
Anti-VEGF Anti-angiogenesis in skin diseases dissases been used to target pathological angiogenesis in Anti-abgiogenesis treatment of oncological and ophthalmological Anti-angioenesis for over 15 years [ 5 ].
There are a small Muscle growth supplements of case reports of clearance of psoriasis in patients with the disease who skih being treated for Anti-anigogenesis with VEGF inhibitors [ 6, 7 diseasees.
However, Anti-anfiogenesis effects of Creatine and ATP production anti-VEGF therapy on human disesses remain poorly characterized, and the Obesity prevention strategies of anti-VEGF in the management of selected dermatological diseases such as psoriasis merits Enhance mental clarity naturally investigation.
To achieve this, Glutamine and immune system available Anto-angiogenesis clinically relevant Anit-angiogenesis research assays are Anti-angiogenwsis, with full-thickness serum-free human skin organ culture providing one pragmatic option.
Most importantly, utilizing Dseases skin biopsies from dermatological conditions such as psoriasis offers the opportunity diseses interrogate the angiogenic effect ni xenobiotic molecules in a clinically relevant preclinical model.
Therefore, we hypothesized that a Anti-anggiogenesis skin diseasex culture could provide a stable ex vivo model in which the cutaneous microvascular network could be studied and experimentally Anto-angiogenesis.
As a first step towards this goal, we wished to obtain Coconut Oil for Baking that anti-VEGF therapeutics can be instructively Diseaaes in organ-cultured healthy human skin.
Skn, we established an organ culture model and used an IgG 1 Reduces infection risk monoclonal antibody mAb directed against all VEGF Anti-zngiogenesis bevacizumab, Avastin®, Roche.
Bevacizumab was sin first disfases mAb Anti-angiogeesis for any African Mango seed weight management indication Anti-ahgiogenesis is licensed Ajti-angiogenesis be akin for age-related macular Anti-zngiogenesis AMD and metastatic cancers of the colon, breast, kidney, lung, Anti-angiogenesis in skin diseases, and ovary [ 5, 11 ].
Written informed consent was obtained from the study volunteers, who were recruited from Salford Royal NHS Foundation Trust, Manchester, UK. Our study included samples Creatine dosage guidelines 5 healthy volunteers 5 biological replicates aged from 39 to 72 average Refillable pet food containers Skin iin 3 mm were Anti-angiobenesis from the Anti-ahgiogenesis skin samples, Electrolyte Boost the excess adipose Anti-angiogenesis in skin diseases was removed.
The replicates required were iterated from previous studies by our group Natural detox diets that the Anti-angiotenesis assays were adequately powered [ 12 ].
In addition, samples from the uninvolved skin 5 cm away from siseases plaque of 1 patient with psoriasis aged 19 were used in a proof-of-concept extension study to confirm the potential of our model to be utilized in a disease context such as psoriasis.
In all cultures, the samples were orientated with the epidermis facing upwards at the liquid-air interface and the dermis submerged in the media. The medium and supplements used are based in the serum-free media described by Philpott et al. An isotype control was not used in these pilot investigations as previous studies have determined the neutralizing activity of bevacizumab against VEGF is specific [ 15 ].
The healthy skin biopsies were incubated with anti-VEGF mAb bevacizumab, Avastin® at doses based on human intravenous doses and serum concentrations observed in human and animal studies and these were 0. The media was changed every 48 h as described by Philpott et al.
Skin biopsies from the uninvolved skin of a patient with psoriasis were incubated with anti-VEGF mAb at 0. After skin organ culture for the indicated time, tissue was embedded for cryosectioning and immunohistochemistry. Skin samples were embedded in OCT compound KP-CryoCompound Frozen Tissue Medium.
Non-serial 8-μm cryosections were taken using a cryostat Cryostat Bright OTF, and affixed to Superfrost Plus Micro slides Menzel Glaser TMThermo-Scientific.
Cell death was investigated using the terminal deoxynucleotidyl transferase TdT dUTP Nick-End Labeling TUNEL; ApopTag® Plus Fluorescein in situ kit, Millipore assay, and Ki was used to assess proliferation.
Keratin 10, filaggrin, and involucrin were used to investigate keratinocyte terminal differentiation. Secondary antibodies used were goat anti-rabbit AFgoat anti-rabbit AFand goat anti-mouse AF After staining, slides were mounted using fluoromount mounting medium Dako; S and were visualized and photographed the following day at × and × magnification using Keyence Biozero Keyence Corporation.
Computer-assisted morphometric analysis of blood vessels was performed using a macro for ImageJ Fiji to analyze the average dermal blood vascular surface area. All samples and standards were assayed in duplicate. A standard curve was prepared with VEGF ranging from Samples, standards, and the positive control were added to the wells.
All statistical analyses and graphs were generated using Graphpad Prism 7. All data presented are given as mean ± SD. One-way ANOVA was used to analyze the differences between the control and the treated groups.
Anti-VEGF mAb concentration 0. Lower doses of anti-VEGF mAb 0. This corresponds well to the observation that anti-VEGF mAb shortens the VEGF-mediated survival of cultured endothelial cells in vitro [ 10 ].
Thus, our simple human skin organ culture model is suited to study the impact of clinically relevant anti-VEGF test agents on the apoptosis of human skin endothelial cells within their physiological tissue habitat ex vivo. The effect of anti-VEGF mAb in human skin organ culture.
DAPI was used for nuclear staining. Anti-VEGF mAb at 0. c Anti-VEGF mAb did not affect cleaved caspase-3 expression in the stratum granulosum SG. The white broken line indicates the epidermal-dermal junction.
e Anti-VEGF mAb did not affect the average blood vessel surface area or the number of blood vessel endothelial cells. The percentage of positive cells was analyzed in the stratum basale. VEGF, vascular endothelial growth factor; mAb, monoclonal antibody.
Quantitative immunohistomorphometry of the blood vessels revealed that the average dermal blood vascular surface area or the number of blood vessel endothelial cells was not affected by VEGF blockade in 3-day culture Fig. Moreover, anti-VEGF mAb did not affect endothelial cell proliferation as shown by CD31 and Ki double labelling online suppl.
In order to test the model viability, cell death and cell proliferation were studied in the stratum basale in the epidermis. Anti-VEGF mAb did not decrease the percentage of Ki positive cells or increase terminal deoxynucleotidyl transferase TdT dUTP Nick-End Labeling TUNEL or cleaved caspase-3 expression in the stratum basale of organ-cultured human skin as measured by quantitative immunohistomorphometry Fig.
Further investigation revealed no cleaved caspase-3 expression, a specific marker for apoptosis, in the stratum granulosum Fig. This suggests that anti-VEGF treatment did not alter keratinocyte survival, proliferation, or terminal differentiation within organ-cultured epidermis and thus primarily targeted intradermal endothelial cells during the incubation period.
Levels of LDH enzyme were measured in the culture supernatant of samples treated with anti-VEGF mAb at 6, 12, 24, and 48 h. There was no increase in LDH activity in samples treated with anti-VEGF mAb compared to control, suggesting that anti-VEGF mAb was not toxic for human skin ex vivo at none of the doses tested online suppl.
Anti-VEGF mAb did not affect keratinocyte terminal differentiation in skin organ culture ex vivo. a The expression of markers of terminal differentiation keratin 10, involucrin, and filaggrin was studied in the upper layers of the epidermis area surrounded by the yellow dotted line.
DAPI was used for nuclear staining, and the white broken line indicates the epidermal-dermal junction. b Bevacizumab did not affect keratin 10, involucrin, or filaggrin expression in the epidermis.
Next, the levels of VEGF protein were quantified in the organ culture supernatant of skin biopsies incubated with 0. These results suggest that 1 VEGF is released from skin biopsies into the culture media gradually with time, reaching its highest concentration in the organ culture supernatant at 48 h and 2 anti-VEGF mAb at 0.
It is possible that the VEGF protein-drug complexes are still present in the treated biopsy samples. Alternatively, they may have been degraded within the tissue. Other investigators have suggested that complex degradation may occur through a variety of mechanisms such as pinocytosis and lysosomal degradation, cytosolic degradation, or degradation of internalized VEGF protein-drug complexes [ 20 ].
As a proof of concept, the effects of anti-VEGF mAb were tested in the uninvolved skin of 1 patient with psoriasis online suppl. Arguably, psoriasis pathogenesis is preferentially studied by utilizing uninvolved skin [ 21 ]. Differences in gene expression between uninvolved psoriatic skin and healthy control skin have been shown, suggesting similarity to skin from plaques of psoriasis [ 22 ].
Our images show visible induction of endothelial cell apoptosis and the viability of the skin was not affected, demonstrating that our model could be used in a psoriasis context to study the effects of anti-VEGF mAb in the skin in situ.
Other angiogenic factors such as angiopoietin 1 and 2 as well as thrombospondin-1 also participate in the regulation of the microvascular network and affect endothelial cell function.
However, these factors act on the blood vasculature and affect endothelial cells using different physiological mechanisms and activating different signalling pathways [ 24 ].
It was outside the scope of this study to look into alternative cellular pathways that act on the dermal vasculature. In conclusion, our pilot study provides the first evidence that anti-VEGF therapy promotes endothelial cell apoptosis in human skin ex vivo.
In addition, we introduce a simple human skin organ culture model, which is reproducible and reliant only on the availability of human skin. In our model, the effects of anti-VEGF therapeutics on the apoptosis of native human vascular endothelial cell can be instructively studied within intact human dermis.
The assay also permits assessment of human blood vessel density and structure. Moreover, endothelial cells in this organ culture system are quiescent, as they would be, physiologically in healthy adult skin in vivo. In addition, our model facilitates the visualization of hyperproliferating endothelial cells and the effects of anti-VEGF agents on the cutaneous microvascular network.
This is especially relevant for the study of skin diseases characterized by pathological angiogenesis, such as psoriasis. The assay also permits dissection of the mechanism of action by which anti-VEGF therapeutics induce endothelial cell apoptosis.
We would like to acknowledge the British Skin Foundation, UK, for funding and acknowledge Dr. Gadea Mata for helping with the vascular morphometric analysis. This research was supported by the NIHR Manchester Biomedical Research Centre.
and R. conceived the experiments.
: Anti-angiogenesis in skin diseases| Introduction | They stop a process in the body called angiogenesis, or blood vessel formation. Angiogenesis is how the body forms new blood vessels. This is a normal part of growth and healing. But sometimes angiogenesis can play a role in diseases such as cancer. To grow, a tumor needs nutrients and oxygen from your blood. The tumor sends signals that stimulate more blood vessels to grow and carry more blood. Angiogenesis inhibitors, also called anti-angiogenics, block blood vessel growth. By blocking nutrients and oxygen from a tumor, the angiogenesis inhibitors "starve" the tumor. The U. Food and Drug Administration has approved several angiogenesis inhibitors. They may affect angiogenesis in more than one way, and some of them can also affect other ways that a tumor grows. Angiogenesis inhibitors can be given alone or in combination with other types of treatment. Researchers are studying whether some of these drugs may treat other types of cancer. Talk with your health care team about clinical trials for angiogenesis inhibitors. Many of the body's normal functions depend on angiogenesis. Therefore, angiogenesis inhibitors can cause a wide range of physical side effects including:. Hand-foot syndrome , which causes tender, thickened areas on your palms and soles. Sometimes, it causes blisters. Although common, these side effects do not happen with every drug or every person. And, there are medicines can help manage these side effects when they do occur. Be sure to let your health care team know about side effects you experience. Kong et al. In the first case, a number of melanocytic nevi appeared on the palms and soles after 2 months of treatment with sorafenib. In the second case, lentigines appeared on the neck, trunk, thighs, and palms and soles a month after commencing treatment. Bennani-Lahlou et al. The lesions arose on the trunk and upper limbs. This phenomenon has been attributed to the state of immunosuppression and inflammation. It has been reported in association with classical chemotherapy treatments and other situations of immunosuppression. It is thought that this phenomenon is due to paradoxical activation of the RAF-MEK-ERK pathway by CRAF. Eruptive melanocytic lesions in a patient treated with sorafenib. In , López et al. Histology revealed marked follicular hyperplasia, particularly in the region of the follicular isthmus, with acanthosis, regenerative changes with numerous mitotic figures, occasional apoptotic cells, and a mild perifollicular lymphocytic infiltrate. The authors stated that all the lesions disappeared after the interruption of treatment with sorafenib. Franck et al. The lesions occurred on the face, scalp, and trunk, while the palms and soles were spared. The lesions appeared a mean of 82 days after starting treatment with sorafenib and resolved 98 days after the discontinuation of treatment. In 4 of the patients, sorafenib had to be withdrawn for other reasons and the rash disappear in 5 to 7 days. In 2 patients who recommenced treatment, the lesions reappeared. The most noticeable histologic finding was the presence of an orthoparakeratotic column that filled the infundibulum and protruded above the epidermal surface. Joncas et al. Histology showed dilated hair follicles with orthokeratotic hyperkeratosis and the presence of a mild polymorphous infiltrate, as well as squamous metaplasia of the eccrine ducts. It is interesting to note that in Haycox et al. On examination of the skin biopsy by electron microscopy, the authors identified viral particles suggestive of papovavirus. This condition was called trichodysplasia spinulosa and has 3 fundamental features: follicular facial papules that produce a leonine facies, alopecia of the eyebrows and eyelashes, and the characteristic keratotic spines. Cases of trichodysplasia spinulosa have been reported in other states of immunosuppression, such as during chemotherapy for acute lymphocytic leukemia, 43 chronic lymphocytic leukemia, 44 heart transplant, 45 and kidney transplant. Ultrastructural electron microscopy studies confirmed the presence of viral particles of 40 nm in the nuclei of the sheath cells. Taking into account the similarities in the clinical presentation between the 2 conditions, such as the appearance of the characteristic keratotic spicules and the presence of alopecia of the scalp and eyebrows, we must consider whether spiny follicular hyperkeratosis is related to or is the same as TSV. Other adverse effects have been reported during treatment with sorafenib: 1 case of yellowish skin discoloration in contrast to sunitinib, with which this adverse effect is much more common that resolved after interrupting the treatment 49 ; painful erosive lesions 50 ; hemorrhagic erythema marginatum, consisting of confluent, desquamating, slightly atrophic, annular erythematous macules with a marked hemorrhagic border, whose appearance has been related to a good tumor response to the treatment 51 ; atypical toxic dermatitis with a linear morphology 52 ; psoriasiform rashes 53—55 ; diffuse keratotic pilaris-like rash; stomatitis; rashes Fig. Sunitinib shares some cutaneous side effects with sorafenib, such as hand-foot skin reaction and seborrheic dermatitis-like rash, but presents other characteristic cutaneous side effects such as yellowish skin discoloration, hair depigmentation, and genital lesions. In a recent study it was shown that skin toxicity occurring during treatment with sunitinib for metastatic renal cell carcinoma was associated with a significant increase in overall and disease-free survival. It should be noted that, in contrast to other systemic causes of yellowish skin discoloration, the changes related to sunitinib do not affect the mucosas or sclera. There may be associated yellowish discoloration of the urine, which is due to excretion of the drug via the kidney. This side effect is due to the color of 1 of the metabolites of the drug. The hair acquires a characteristic gray color. No histological changes can be seen in the melanocytes of affected follicles. This adverse effect is believed to be due to an inhibition of the c-KIT that modulates the genes of the tyrosinase and tyrosinase-related protein-1 enzymes, which are involved in melanin synthesis. Billemont et al. The authors observed the appearance of erythema and peeling 2 weeks after commencing treatment; the changes resolved spontaneously after withdrawal of the drug. Those authors also described 3 patients with no history of psoriasis who developed severe psoriasiform lesions on the scrotum. The lesions were associated with intense pain and required a reduction of the dose of sunitinib. Recently, lesions with a similar appearance have been reported in the genital region of a woman who received treatment with sunitinib for recurrent and metastatic renal cell carcinoma 7 years after nephrectomy. Genital changes in the form of erythema of the foreskin, scrotum, and penis, intertrigo, and anal inflammation, were observed in 5 of a series of 8 patients treated with sunitinib. Treatment was interrupted in 4 of the patients due to the effect on quality of life. Lesions clinically and histologically compatible with hemangiomas have also been reported in this region; the lesions disappeared on withdrawal of the treatment and recurred with each cycle of administration of sunitinib. Recently Chou et al. Skin biopsy showed the presence of dilated capillaries with endothelial cells that stained positive for VEGF, but this was not detected in a healthy control group. This finding supports the role of VEGF in the pathogenesis of sunitinib-related genital lesions, as proposed by Billemont et al. VEGF induces endothelial proliferation, vasodilatation, and increased vascular permeability. Elevated VEGF levels have been reported in the plasma of patients treated with sunitinib, and it has been speculated that there may be a physiological feedback that provokes a reversible increase in circulating and tissue VEGF after the administration of sunitinib a VEGF receptor antagonist. When VEGF reaches the scrotal region, characterized by a rich blood supply and subject to recurrent friction and trauma, it produces local symptoms such as scrotal erythema, tissue edema, telangiectasias, and the appearance of hemangiomas. Tonini et al. It has been shown that there is an increase in the number of circulating endothelial cells during treatment with sunitinib, not only of mature cells circulating endothelial cells [CEC] but also of their precursors circulating endothelial progenitors [CEP]. Similarly, increased numbers of CEPs have been found in children with proliferating hemangiomas. The anti-VEGF monoclonal antibody bevacizumab causes less skin toxicity than other similar drugs, but, apart from the typical reactions, this drug has other characteristic cutaneous side effects. In contrast to the situation with the EGFR antagonists, the majority of clinical trials found no association between the skin rash and a positive response to treatment. Only 2 cases have been reported in the literature in which the appearance and intensity of the skin rash correlated with a positive response to treatment with bevacizumab, with disappearance of the metastases as the skin lesions progressed. The rash was of erythematous papular lesions and recurred with each new cycle of bevacizumab. Bevacizumab is used off-label in ophthalmology as an intravitreous injection for the treatment of various diseases related to neovascularization, such as age-related macular degeneration, diabetic retinopathy, and choroidal neovascularization in myopia. There have been 2 case reports of the appearance of erythematous papular lesions on the head and trunk related to the intravitreous injection of bevacizumab for the treatment of choroidal neovascularization. In the second case, the patient received 3 injections of bevacizumab; 12 days after the first injection he developed an erythematous papular rash on the forehead and in the temporal regions around the eyes. With topical corticosteroid therapy, the lesions resolved within 8 days but reappeared 14 days after the second injection and 10 days after the third. Angiogenesis is a part of numerous physiological processes, including wound healing. In animal models it has been shown that bevacizumab inhibits the repair process in the dermis 84 ; this effect is dose-dependent and is reversible if administration of the drug is interrupted. Various effects of bevacizumab on wound healing have been described, such as wound dehiscence, ecchymosis, surgical wound hemorrhage, and an increased risk of infection. In contrast, patients who started to receive bevacizumab 28 to 60 days after surgery did not present a higher frequency of complications. It has been shown that would healing is negatively affected not only by the systemic administration of bevacizumab but also by intravitreous administration of the drug. Cases of ulceration of corticosteroid-induced striae have been reported in patients with cerebral tumors on treatment with bevacizumab. A case of necrosis of striae has been reported in a patient 1 week after starting treatment with bevacizumab and irinotecan for glioblastoma. Bevacizumab was discontinued 2 months later for lack of efficacy and the lesions healed within a month. In the case of cerebral tumors, high-dose corticosteroid therapy is often required for prolonged periods of time to manage cerebral edema. It is therefore particularly important to watch for possible side effects if therapy is started with antiangiogenic agents such as bevacizumab. The literature highlights the importance of the prevention of the appearance of ulcers on striae in these patients and, if they appear, adequate hydration of the area is recommended, together with the use of hydrocolloid dressings. There has been a case report of the appearance of slightly pruritic, umbilicated papular lesions on the neck of a patient who had been receiving bevacizumab for 2 months. The lesions measured 5 mm in diameter and had a central keratotic plug. Histology revealed the transepidermal elimination of collagen. Treatment interruption was not required. Numerous drugs have been approved in recent years for the treatment of various types of tumor, and many more molecules are in phase 3 or phase 4 clinical trials. The antiangiogenic drugs occupy an important place among these drugs. It is therefore very important for the dermatologist to be aware of the cutaneous effects associated with these drugs and to know how to manage them. The authors declare that they have no conflicts of interest. The authors are grateful to Dr Tuneu and Dr López Pestaña of Hospital Donostia, San Sebastian, Spain, for granting them use of their photographic material.. Please cite this article as: Ara M, Pastushenko E. Fármacos antiangiogénicos y piel: efectos cutáneos adversos de sorafenib, sunitinib y bevacizumab. Actas Dermosifiliogr. Home All contents Articles in press Current Issue All issues Supplements Multimedia Archivo histórico Subscribe to our newsletter Publish your article Instructions for authors Submit an article Ethics in publishing Open Access Option Language Editing services About the journal Aims and scope Editorial Board Advertising Metrics Most often read Most cited Most popular All metrics. Actas Dermo-Sifiliográficas. ISSN: Actas Dermo-Sifiliográficas ha recibido Factor de Impacto JCR por primera vez en Guía para autores - Envío de manuscritos. Actas Dermo-Sifiliográficas has received its first journal Impact Factor JCR in Instructions for authors - Submit an article. Open Access Option. Previous article Next article. Issue Pages December Lee este artículo en Español. More article options. DOI: Antiangiogenic Agents and the Skin: Cutaneous Adverse Effects of Sorafenib, Sunitinib, and Bevacizumab. Download PDF. Corresponding author. mam comz. org Corresponding author. Servicio de Dermatología, Hospital Clínico Universitario Lozano Blesa, Zaragoza, Spain. This item has received. Article information. Show more Show less. Table 1. Summary of the Most Common Cutaneous Side Effects of the Angiogenesis Inhibitors.. Table 2. Largest Series Evaluating Cutaneous Side Effects in Patients Treated With Sorafenib.. In this review article, we analyze the main cutaneous adverse effects of the most common antiangiogenic agents. En esta revisión se analizan los efectos adversos cutáneos más importantes de los principales fármacos antiangiogénicos. Palabras clave:. Full Text. Introduction Several drugs that inhibit angiogenesis have produced encouraging results in recent years in the treatment of certain types of tumor. Figure 1. Author and Reference Year Description Autier et al. Figure 2. Figure 3. Table 3. Maintenance of the same dose is recommended 2 Skin changes peeling, blisters, hemorrhage, edema, hyperkeratosis with a variable degree of pain. Interference with daily activities As for grade 1Clobetasol cream, 0. Figure 4. Figure 5. The authors are grateful to Dr Tuneu and Dr López Pestaña of Hospital Donostia, San Sebastian, Spain, for granting them use of their photographic material. Chu, M. Lacouture, T. Fillos, S. Risk of hand-foot skin reaction with sorafenib: A systematic review and meta-analysis. Acta Oncol, 47 , pp. The growth of malignant disease in man and the lower animals with special reference to the vascular system. Lancet, 2 , pp. Greenblatt, P. Tumor angiogenesis: Transfilter diffusion studies in the hamster by the transparent chamber technique. J Natl Cancer Inst, 41 , pp. Ehrmann, M. Choriocarcinoma: Transfilter stimulation of vasoproliferation in the hamster cheek pouch studied by light and electron microscopy. Tumor angiogenesis: Therapeutic implications. N Engl J Med, , pp. Lacouture, S. Wu, C. Robert, M. Atkins, H. Kong, J. Guitart, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist, 13 , pp. Lee, J. Lee, S. Chang, M. Lee, Y. Kang, J. Choi, et al. Cutaneous adverse effects in patients treated with the multitargeted kinase inhibitors sorafenib and sunitinib. Br J Dermatol, , pp. x Medline. Battistella, C. Mateus, N. Lassau, L. Chami, M. Boukoucha, P. Duvillard, et al. Sunitinib efficacy in the treatment of metastatic skin adnexal carcinomas: Report of two patients with hidradenocarcinoma and trichoblastic carcinoma. J Eur Acad Dermatol Venereol, 24 , pp. Wozel, M. Sticherling, M. Cutaneous side effects of inhibition of VEGF signal transduction. J Dtsch Dermatol Ges, 8 , pp. Braghioli, J. Sabbaga, P. Expert Rev Anticancer Ther, 12 , pp. McLellan, H. Cutaneous toxicities of the multikinase inhibitors sorafenib and sunitinib. Dermatol Therap, 24 , pp. Zhang, Q. Zhou, L. Ma, Z. Wu, Y. Meta-analysis of dermatological toxicities associated with sorafenib. Clin Exp Dermatol, 36 , pp. Autier, B. Escudier, J. Wechsler, A. Spatz, C. Prospective study of the cutaneous adverse effects of sorafenib, a novel multikinase inhibitor. Arch Dermatol, , pp. Robert, C. Mateus, A. Spatz, J. Wechsler, B. Dermatologic symptoms associated with the multikinase inhibitor sorafenib. J Am Acad Dermatol, 60 , pp. Kong, M. Array of cutaneous adverse effects associated with sorafenib. J Am Acad Dermatol, 61 , pp. Lipworth, C. Robert, A. Hand-foot syndrome hand-foot skin reaction, palmar-plantar erythrodysesthesia : Focus on sorafenib and sunitinib. Oncology, 77 , pp. Nardone, J. Hensley, L. Kulik, D. West, M. Mulcahy, A. Rademaker, et al. The effect of hand-foot skin reaction associated with the multikinase inhibitors sorafenib and sunitinib on health-related quality of life. J Drugs Dermatol, 11 , pp. ee65 Medline. Yang, W. Lin, C. Chuang, Y. Chang, S. Pang, Y. Lin, et al. Hand-foot skin reaction in patients treated with sorafenib: A clinicopathological study of cutaneous manifestations due to multitargeted kinase inhibitor therapy. Jain, E. Gardner, W. Figg, M. Chernick, H. Lack of association between excretion of sorafenib in sweat and hand-foot skin reaction. Pharmacotherapy, 30 , pp. Sibaud, J. Delord, C. Sorafenib-induced hand-foot skin reaction: A Koebner phenomenon. Target Oncol, 4 , pp. Chung, J. Kim, J. Shim, D. Lee, H. Lee, et al. Genetic predisposition of hand-foot skin reaction after sorafenib therapy in patients with hepatocellular carcinoma. Cancer, , pp. Dranitsaris, M. Vincent, J. Yu, L. Huang, F. Fang, M. Development and validation of a prediction index for hand-foot skin reaction in cancer patients receiving sorafenib. Ann Oncol, 23 , pp. Anderson, A. Jatoi, C. Robert, L. Wood, K. Keating, M. Search for evidence-based approaches for the prevention and palliation of hand-foot skin reaction HFSR caused by multikinase inhibitors MKIs. Oncologist, 14 , pp. Common Terminology Criteria for Adverse Events v4. Robert, J. Soria, A. Spatz, A. Le Cesne, D. Malka, P. Pautier, et al. Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol, 6 , pp. Arnault, J. Escudier, A. Know the risks and potential benefits of clinical studies and talk to your health care provider before participating. Read our disclaimer for details. gov Identifier: NCT Recruitment Status : Recruiting First Posted : February 12, Last Update Posted : January 30, See Contacts and Locations. View this study on the modernized ClinicalTrials. Study Details Tabular View No Results Posted Disclaimer How to Read a Study Record. Study Description. Go to Top of Page Study Description Study Design Groups and Cohorts Outcome Measures Eligibility Criteria Contacts and Locations More Information. Detailed Description:. Resource links provided by the National Library of Medicine MedlinePlus related topics: Skin Conditions. FDA Resources. Outcome Measures. Primary Outcome Measures : Factors in dermatologic diseases. Eligibility Criteria. Information from the National Library of Medicine Choosing to participate in a study is an important personal decision. Layout table for eligibility information Ages Eligible for Study: 18 Years and older Adult, Older Adult Sexes Eligible for Study: All Accepts Healthy Volunteers: Yes Sampling Method: Non-Probability Sample Study Population. Tissue samples will be collected from Dermatopathology Lab, Pathology lab, discarded tissue from dermatologic surgery at University of California Irvine. Inclusion Criteria: 18 years of age or older. willing to have a skin biopsy Exclusion Criteria: under 18 years of age unable to carry out instructions. Contacts and Locations. Information from the National Library of Medicine To learn more about this study, you or your doctor may contact the study research staff using the contact information provided by the sponsor. Please refer to this study by its ClinicalTrials. gov identifier NCT number : NCT Layout table for location contacts Contact: Hanna Kim hhkim3 uci. Layout table for location information United States, California Gottschalk Medical Plaza Recruiting Irvine, California, United States, Contact: Montana Compton, RN mocomton uci. edu Beckman Laser Institute and Medical Clinic Recruiting Irvine, California, United States, Contact: Hanna Kim hhkim3 uci. edu Principal Investigator: Kristen Kelly, MD. Layout table for investigator information Principal Investigator: Kristen Kelly, MD University of California, Irvine. More Information. |
| Angiogenesis in Dermatology - Insights of Molecular Mechanisms and Latest Developments | Which one? Int J Mol Sci 18 10 JAMA Oncol. cART results from the mix of drugs inhibiting the HIV reverse transcriptase, integrase or aspartyl protease 3. This side effect is due to the color of 1 of the metabolites of the drug. |
| Compva: Anti-angiogenic therapy | Serum-starved HUVECs were treated with or without PSORI-CM02 1. The plant species used in this study were Rhizoma Curcumae , Radix Paeoniae Rubra , S. Cutaneous side-effects of kinase inhibitors and blocking antibodies. Mateus, S. In some cases, symptoms can be exacerbated by high temperatures. |
Ich kann empfehlen, auf die Webseite vorbeizukommen, wo viele Artikel zum Sie interessierenden Thema gibt.
Nach meiner Meinung sind Sie nicht recht. Es ich kann beweisen. Schreiben Sie mir in PM, wir werden reden.