
Antioxidant defense mechanisms -
It efficiently scavenges ROS and free radicals, preventing an increase in the oxidative stress process. In these reactions, the reduced GSH is oxidized, via the enzyme glutathione peroxidase, to form glutathione disulfide GSSG.
Note that GSSG is formed by two GSH molecules linked via a disulfide bond due to the oxidation of the thiol SH groups. Once oxidized, GSSG can be reduced back to its original GSH form by the enzyme GSSG reductase and nicotinamide adenine dinucleotide phosphate NADPH.
Nevertheless, when there is a high level of oxidative stress, NADPH becomes depleted and there is an intracellular accumulation of GSSG. This excess GSSG can either be exported out of the cell or it can form a mixed disulfide.
It is not only a good indicator of systemic oxidative status but also a useful indicator to indicate the free radical production during exercise [ 71 , 72 — 74 ]. GSH is the major source of tiol groups in the cells. GSH has several defense antioxidant functions. The practise of 90 min of exercise decreases GSH and increases blood levels of GSSG [ 30 ].
After 24 h, all the results recovered to the levels found before the test. Due to fact that the test does not reflect the increase of GSH in the blood, the increasing total glutathione could be due to the GSSG exportation of the tissues and the blood GSSG [ 26 ]. Plasma levels of GSH is approximately three times lower than blood levels.
Moreover, the changes in GSH plasma due to the accelerated flow of liver GSH, produced during exercise, are not detected in the blood in GSH form or total glutathione. The oxidative stress due to intense physical activity produce a rapid oxidation in intracellular GSH in muscle cells and a GSSG production liberated in blood circulation.
Thus, a decrease in intracellular glutathione level is observed. This suggests that the GSSG flow of muscle cells to the blood is due to a mechanism dependent of energy [ 26 ].
The effect of the training in GSH content seem to vary in different types of muscle fibers and different tissues. The content of GSG in erythrocytes increased at the same time with glutathione reductase after 20 weeks of training in humans who were previously sedentary [ 25 ]. In trained subjects, after 2.
However, the level of GSSG showed a rise at the end of the test compared with the basal state [ 75 ].
Short-term training does not improve the adaptation of antioxidant system. However, it has been demonstrated that training protects against glutathione oxidation associated to exhaustive exercise [ 32 ]. Similar to vitamin C, vitamin E has important antioxidant properties.
This is possible because vitamin E has a great affinity for reducing peroxyl radicals, preventing their interaction with the membrane phospholipids or lipoproteins [ 77 ]. Vitamin E has been found to protect cellular membranes from lipid peroxidation.
Hence, it is logical to assume that this vitamin could protect muscle cells against exercise-induced damage. Early studies analyzing the effects of vitamin E supplementation and exercise investigated its effect on performance. Most of the studies, however, report no benefit of vitamin E neither for muscle strength nor for endurance performance [ 78 ].
Furthermore, it has been hypothesized that vitamin E supplementation could have a protective effect against the contraction-induced muscle damage oxidative stress that may occur after an intense exercise bout.
This rationale is based on the knowledge that this vitamin can stabilize muscle membranes by interacting with its phospholipids that would, this way, provide some protection against the increase in oxidative stress or muscle damage observed after certain types of exercises [ 78 ].
Altough vitamin E is an effective capture of free radical, the reaction of vitamin E with radicals produces a functional decrease of vitamin E and the formation of free radicals-vitamin E. The oxidative stress produces a significative decrease of vitamin E levels in tissues.
However, the radical vitamin E can be synthetized with the cooperation of other antioxidants. As a result, the investigations conclude that vitamin E's ability to act as an antioxidant is related with the ability of other antioxidants to recycle vitamin E during stress oxidative periods [ 79 ].
The exercise could induce an alteration of plasma levels of vitamin E. During human exercise, an increase in vitamin E concentration in plasma and erytrhocytes was observed, suggesting that exercise could promote vitamin E mobilization from tissues to plasma, and the skeletal muscle could use the circulating vitamin E to protect against oxidative damage [ 25 ].
Other authors did not find variations in vitamin E levels in humans after a half marathon race [ 79 ]. Vitamin E changes are better appreciated when the results are expressed by unit of mitochondrial ubiquinone. The reduction of vitamin E in the inner mithochondrial membrane can justify the susceptibility of the mitochondria to free radicals damage.
The content of vitamin E in the heart can decrease. Vitamin E heart content suffered a light decrease after a training program in treadmill, compared with the disminution in skeletal muscles. In an ultraresistance race thriatlon , no variation of vitamin E concentration were found before or after the race [ 81 ].
Ascorbic acid is the main form of the vitamin found in vivo. This vitamin, also referred to as ascorbate, is found in relatively high levels in different tissues throughout the body. Ascorbate has clearly been shown to play an essential role in connective tissue biosynthesis.
During oxidation reactions, only small amounts of ascorbate are lost because, once it is oxidized, it can be reduced back to ascorbic acid by reductants such as glutathione, nicotinamide adenine dinucleotide NADH , and NADPH.
Similarly, vitamin C is also known to regenerate other antioxidants, such as vitamin E and glutathione, back to their reducing state; thus, maintaining a balanced network of antioxidants.
The increase of vitamin C levels can protect against oxidative damage of free radicals. Duthie et al. However, Ginsburg et al. With respect to the trained effect, the vitamin C level of the trained footballer level was higher than in sedentary subjects [ 81 ].
As vitamin C, β-carotene can acts as an antioxidant and as a pro-oxidant. Polyphenolic antioxidants have demonstrated their efficacy against oxidative stress induced by exercise. It has demonstrated the decrease of oxidized proteins in a study subjected to intensive exercise [ 83 , 84 ].
During exercise, an important free radical production is predictable and as a consequence a major requirement of defense mechanisms. Some of the antioxidant defenses can be adequated with training and in the presence of an appropriate diet.
Defenses can be insufficient when the exercise exceeds the level by which they were adapted. The knowledge of how antioxidants interact provides rational bases to develop nutritional strategies to put forward the progress in exercise activities and in mantaining the health of amateur and profesional subjects.
Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3. Edited by Sivakumar Joghi Thatha Gowder. Open access peer-reviewed chapter Oxidative Stress and Antioxidant Defenses Induced by Physical Exercise Written By Juana M.
Morillas-Ruiz and Pilar Hernández-Sánchez. DOWNLOAD FOR FREE Share Cite Cite this chapter There are two ways to cite this chapter:. Choose citation style Select style Vancouver APA Harvard IEEE MLA Chicago Copy to clipboard Get citation. Choose citation style Select format Bibtex RIS Download citation.
IntechOpen Basic Principles and Clinical Significance of Oxidative Stress Edited by Sivakumar Joghi Thatha Gowder. From the Edited Volume Basic Principles and Clinical Significance of Oxidative Stress Edited by Sivakumar Joghi Thatha Gowder Book Details Order Print.
Chapter metrics overview 2, Chapter Downloads View Full Metrics. Impact of this chapter. Abstract This chapter intends to present the physiological and biochemical mechanisms by which exercise induces the appearance of oxidative stress, as well as the characteristics of the physical exercise that involve the appearance of oxidative stress in the human organism.
Keywords oxidative stress physical exercise antioxidant defense nutritional strategies. Juana M. Introduction The beneficial effects of regular non-exhaustive physical exercise have been known for a long time.
They are reactive prooxidant agents to carbohydrates, proteins, and lipids Submaximal long-duration exercise training may augment the physiological antioxidant defenses in several tissues.
Oxidative stress induced by extenuant exercise The increase in energy consumed during exercise increases the oxygen demands of the active tissues, increasing up to 20 times in comparison with basal state [ 14 ]. The understanding of the mechanisms associated with physiological responses that explain how exercise increases the oxygen toxicity and the design of appropiate measures to minimize toxicity are indispensable to: Increase exercise efficacy as a preventive and therapeutic instrument in clinical practise Control the damaged tissue induced by exercise Oxidative stress induced by extenuant exercise is a situation by which cells are exposed to a prooxidant environment and defense mechanisms are not enough, affecting the redox estate of the cells.
Exercise as an oxidative stress protector Up to now, the work has been focused in the damaging effect of exhaustive exercise.
This fact suggests the recommendation of a diet rich in antioxidant compounds fresh fruits and vegetables Antioxidant defenses in the skeletal muscles, heart, and liver are regulated due to the effect of exercise in the body [ 45 , 46 ] and showed that exhaustive exercise increased the rate of catalase activity in the liver, muscle, and heart.
Enzymatic antioxidants The main antioxidant cellular enzymes are superoxide-dismutase SOD , catalase CAT , and glutation-peroxidase GPx. Nonenzymatic antioxidants The nonenzymatic antioxidant group includes glutathione, vitamin C, vitamin E, carotenoids, uric acid, polyphenols, and others.
References 1. Niess AM, Simon P. Response and adaptation of skeletal muscle to exercise—the role of reactive oxygen species. Front Biosci. Child RB, Wilkinson DM, Fallowfield JL, Donnelly AE. Elevated serum antioxidant capacity and plasma malondialdehyde concentration in response to a simulated half-marathon run.
Med Sci Sports Exerc. Sakellariou GK, Jackson MJ, Vasilaki A. Redefining the major contributors to superoxide production in contracting skeletal muscle. The role of NAD P H oxidases. Free Radic Res. Kuwahara H, Horie T, Ishikawa S, Tsuda C, Kawakami S, Noda Y, Kaneko T, Tahara S, Tachibana T, Okabe M, Melki J, Takano R, Toda T, Morikawa D, Nojiri H, Kurosawa H, Shirasawa T, Shimizu T.
Oxidative stress in skeletal muscle causes severe disturbance of exercise activity without muscle atrophy. Free Radic Biol Med. Yamada M, Suzuki K, Kudo S, Totsuka M, Simoyama T, Nakaji S, Sugawara K. Effect of exhaustive exercise on human neutrophils in athletes.
Neiman D. Immune response to heavy exertion. J Appl Physiol. Viña J, Gimeno A, Sastre J, Desco C, Asensi M, Pallardó FV, Cuesta A, Ferrero JA, Terada LS, Repine JE.
Mechanism of free radical production in exhaustive exercise in humans and rats; role of xanthine oxidase and protection by allopurinol.
IUBMB Life. Jiang J, Borisenko GG, Osipov A, Martin I, Chen R, Shvedova AA, Sorokin A, Tyurina YY, Potapovich A, Tyurin VA, Graham SH, Kagan VE.
Arachidonic acid-induced carbon-centered radicals and phospholipid peroxidation in cyclo-oxygenasetransfected PC12 cells. J Neurochem. Smith ChV. Free radical mechanisms of tissue injury. In: Molen HT, Smith ChV, editors. Boca Raton; Evans T, Carpenter A, Silue A, Cohen J.
Inhibitions of nitric oxide syntethase inesperimetal gram negative sepsis. J Infect Dis. Sjódin B, Hellsten Westing Y, Apple FS. Biochemical mechanism for oxygen free radical formation during exercise. J Sports Med.
Kanter MM, Nolte NA, Holloszy JO. Effect of an antioxidant vitamin micture on lipid peroxidation et rest and post-exercise. König D, Berg A. Exercise and oxidative stress: Is there a need for additional antioxidants. Öster J Sport Med. Wilmore JH, Costill DL. Champaign, IL: Human Kinetics. Keul J, Doll E.
Oxydative energy supplementation. In: E. Jokl, editor. Energy Metabolism of Human Muscle. Basel:Karger: ; Dillard CJ, Litov RE, Savin WM, Dumelin EE, Ttappel AL. Effects of exercise, vitamin E and ozone on pulmonary function and lipid peroxidation. Kumar CT, Reddy VK, Prasad M, Thyagaraju K, Reddanna P.
Dietary suppplementation of vitamin E protects heart tissue from exercice-induced oxidant stress. Mol Cell Biochem. Diem K, Lentner C. Scientific Tables. Documenta Geigy. Switzerland: Basel; Jenkins RR, Friedland R, Howald H. The relation-ship of oxygen uptake to superoxide dismutase and catalase activity in human skeletal muscle.
Int J Sports Med. Davies KJA, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercice. Biochem Biophys Res Commun. Barclay JK, Hansel M. Free radicals may contribute to oxidative skeletal muscle fatigue.
Can J Physiol Pharmacol. Powers SK, Jackson MJ. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol Rev. Sjogaard G. Exercice-induced muscle fatigue: The significance of potassium. Acta Physiol Scand. Strobel NA, Matsumoto A, Peake JM, Marsh SA, Peternelj TT, Briskey D, Fassett RG, Coombes JS, Wadley GD.
Altering the redox state of skeletal muscle by glutathione depletion increases the exercise-activation of PGC-1α. Physiol Rep.
pii: e Ji LL. Exercice-induced oxidative stress in the heart. In: Sen CK, Packer L, Hanninem O, editors. Exercice and Oxygen Toxicity. Amsterdam: Elsevier Science; Sen ChK, Hänninen O.
Physiological antioxidants. In: C. Sen LPaOH, editor. Amsterdam: Elsevier Science: c. Sen ChK, Marin E, Kretzschmar M, Hanninen O.
Skeletal muscle and liver glutathione homeostasis in response to training, exercice and inmovilization. Viguie CA, Frei B, Shigenaga MK, Ames BN, Packer L, Brooks GA.
Antioxidant status and indexes of oxidative stress during consecutive days of exercice. Hillman AR, Vince RV, Taylor L, McNaughton L, Mitchell N, Siegler J. Exercise-induced dehydration with and without environmental heat stress results in increased oxidative stress.
Appl Physiol Nutr Metab. Gohil K, Viguie CA, Stanley WC, Brooks GA, Packer L. Blood glutathione oxidation during human exercice. Kretzschmar M, Pfeifer U, Machnik G, Klinger W. Glutathione homeostasis and turnover in the totally hepatectomized rat: Evidence for a high glutathione export capacity of extrahepatic tissues.
Exp Toxicol Pathol ; Gómez-Cabrera MC, Gimeno A, Lloret A, Miñana JB, Marquez R, Viña J. Deporte de alta competición y daño oxidativo: Papel de los nutrientes antioxidantes. Antioxidantes y calidad de vida [en línea] [fecha de acceso 21 de mayo de ].
URL disponible en: www. Smith JA, Teldorfd IB, Mason IB, Weidemann MJ. Nunes-Silva A, Bernardes PT, Rezende BM, Lopes F, Gomes EC, Marques PE, Lima PM, Coimbra CC, Menezes GB, Teixeira MM, Pinho V. Treadmill exercise induces neutrophil recruitment into muscle tissue in a reactive oxygen species-dependent manner.
An intravital microscopy study. PLoS One. Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants and the degenerative diseases of aging. Proc Natl Acad Sci U S A. Alessio HM, Cutler RG. Evidence that DNA damage and repair cycle activity increases following a marathon race. Medicine and Science in Sports and Exercise.
Aoi W, Naito Y, Yoshikawa T. Potential role of oxidative protein modification in energy metabolism in exercise. Subcell Biochem. Reznick AZ, Cross CE, Hu ML, Suzuki YJ, Khwaja S, Safadi A, et al. Modification of plasma proteins by cigarette smoke as measured by protein carbonyl formation.
Biochem J. Rajguru SU, Yeargans GS, Seidler NW. Exercice causes oxidative damage to rat skeletal muscle microsomes while increasing cellular sulfhidryls. Life Sci. Farney TM, McCarthy CG, Canale RE, Schilling BK, Whitehead PN, Bloomer RJ. Absence of blood oxidative stress in trained men after strenuous exercise.
Salminen A, Vihko V. Lipid peroxidation in exercise myopathy. Exp Mol Pathol. Gomez-Cabrera MC1, Domenech E, Viña J. Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training.
Radak Z, Pucsuk J, Boros S. Exercise predonditioning against hydrogen peroxide-induced oxidative damage in proteins of rat myocardium.
Arch Biochem Biophys. Leeuwenburg C, Fiebig R, Chandwaney R, Ji LL. Aging and exercise training in skeletal muscle: Responses of glutathione and antioxidant enzyme systems.
Am J Physiol. Caldarera CM, Guarnieri C, Lazzari F. Catalase and peroxidase activity of cardiac muscle. Boll Soc Ital Biol Sper. Cardoso AM, Bagatini MD, Roth MA, Martins CC, Rezer JF, Mello FF, Lopes LF, Morsch VM, Schetinger MR.
Acute effects of resistance exercise and intermittent intense aerobic exercise on blood cell count and oxidative stress in trained middle-aged women. Braz J Med Biol Res. Cunha TF, Bacurau AV, Moreira JB, Paixão NA, Campos JC, Ferreira JC, Leal ML, Negrão CE, Moriscot AS, Wisløff U, Brum PC.
Exercise training prevents oxidative stress and ubiquitin-proteasome system overactivity and reverse skeletal muscle atrophy in heart failure. Hübner-Woźniak E, Morgulec-Adamowicz N, Malara M, Lewandowski P, Okęcka-Szymańska J.
Effect of rugby training on blood antioxidant defenses in able-bodied and spinal cord injured players. Spinal Cord. Vincent HK, Powers SK, Stewart DJ, Demirel HA, Shanely RA, Naito H.
Short-term exercise training improves diaphragm antioxidant capacity and endurance. Eur J Appl Physiol. Heavens KR, Szivak TK, Hooper DR, Dunn-Lewis C, Comstock BA, Flanagan SD, Looney DP, Kupchak BR, Maresh CM, Volek JS, Kraemer WJ.
The effects of high intensity short rest resistance exercise on muscle damage markers in men and women. J Strength Cond Res. Gingsburg GS, Agil A, O'Toole M, Rimm E, Douglas PS. Effects of a single bout of ultraendurance exercise on lipid levels and susceptibility of lipids to peroxidation in Triathletes.
J Am Med Assoc. Criswell D, Powers S, Dodd S, Lawler J, Edwards W, Renshler K, et al. High intensity training-induced changes in skeletal muscle antioxidant enzyme activity. Ohno H, Yahata T, Sato Y, Yamamura K, Taniguchi N. Physical training and fasting erythrocyte activities of free radical scavenging enzyme sistems in sedentary men.
Robertson JD, Maughan RJ, Duthie GG, Morrice PC. Increased blood antioxidant systems of runners in response to training load. Clin Sci. Yagi K. Lipid peroxides and exercise. Komulainen J, Vihko V. Training-induced protection and effect of teminated training on exercise-induced damage and water content in mouse skeletal muscles.
Child RB, Browm S, Day S, Donelly AE, Ropper H, Saxton JM. Changes in indices of antioxidants status, lipid peroxidation and inflamation in human skeletal muscle after eccentric muscle actions. Brites FD, Evelson PA, Christiansen MG, Nicol MF, Basílico MJ, Wikinski RW, et al. Soccer Players under regular training Show Oxidative Stress but an Improved Plasma Antioxidant Status.
Rosety-Rodríguez M, Díaz-Ordoñez A, Rosety I, Fornieles G, Camacho-Molina A, García N, Rosety MA, Ordoñez FJ. Aerobic training improves antioxidant defense system in women with metabolic syndrome.
Medicina B Aires. Laughlin MH, Simpson T, Sexton WL, brown OR, Smith JK, Korthuis RJ. Skeletal muscle oxidative capacity, antioxidant enzymes and exercise training. Spriet LL, Whitfield J.
Taurine and skeletal muscle function. Curr Opin Clin Nutr Metab Care. Powers SK, Hamilton K. Antioxidants and Exercice. Out of all the four isomers α, β, γ, and δ , the most biologically active is the α isomer.
It is one of the most abundant antioxidants in the chloroplast membranes and is known to protect plants against photo-oxidative damage. The concentration of tocopherol increases under drought stress and high light stress, and it protects plants against oxidative damage.
α-tocopherols are known to prevent the chain propagation step in lipid auto-oxidation and effectively scavenge and quench various ROS and lipid oxidation products, stabilize membranes, and regulate signal transduction Peñuelas and Munné-Bosch, ; Agarwal, ; Pourcel et al.
Glutathione, a tripeptide, occurs primarily as the reduced form GSH , and its concentration is highest in chloroplasts mM in comparison to other organelles Hajam et al. It has the ability to regenerate another powerful antioxidant, i. Isoprenoids, such as carotenoids and tocopherols, play an important role in photoprotection as well as the response to cadmium stress Nagajyoti et al.
Carotenoids have the ability to delocalize unpaired electrons due to the presence of a conjugated double-bonded structure Nareshkumar et al. Another commonly occurring antioxidant, polyphenols, is known to possess free radical scavenging activity.
Phenolic compounds react with peroxyl radicals and form phenoxy radical intermediates that are rather stable and unreactive Bagheri et al. These function as terminators of free radical chains and as chelators of redox-active metal.
SOD EC 1. Initially, SOD was isolated as a green Cu-protein from bovine blood and was thought to be involved in Cu storage Mittler, All oxygen-metabolizing cells, as well as all subcellular compartments such as chloroplasts, mitochondria, nuclei, peroxisomes, cytoplasm, and apoplasts , are assumed to contain SODs Fink and Scandalios, ; Mahanty et al.
Mn-SOD is found primarily in mitochondria, but also in peroxisomes and apoplasts Pan et al. Fe-SOD is found primarily in chloroplasts and to a lesser extent in peroxisomes and apoplasts.
Fe-SODs exist in homodimeric and tetrameric forms. They are resistant to KCN but not to H 2 O 2. Furthermore, hormones such as abscisic acid and multifunctional signaling molecules such as melatonin regulate SOD activity via gene expression, like other antioxidant enzymes Tuteja and Gill, ; Khan et al.
Abiotic stresses such as heat, cold, drought, salinity, and chemical contaminants are major factors limiting agricultural productivity around the world. As a result, understanding plant responses to these abiotic stresses has become a prerequisite for developing crop plants that can withstand abiotic stresses Gepstein and Glick, Nonetheless, SODs are the first line of defense against abiotic stress because they are an important component of plant defense machinery.
Due to stress, ROS and its reaction products increase, and dismutation of O 2 is catalyzed by SODs into hydrogen peroxide H 2 O 2 and oxygen O 2. The impact of all SODs on the direct or indirect metabolism of various ROS has been confirmed Mittler, Salinity is one of the most important abiotic stress factors that can affect the quality and yield of crops Mittler, Although many plants have the ability to tolerate high salinity levels, most plants are susceptible to salt stress and show decreased photosynthetic activity or morphological disorders under such conditions Sheokand et al.
Salinity causes an increase in ROS generation and alters the activity of ROS-scavenging enzymes. In certain plants, salt stress causes an increase in total SOD activity, while in others, no change or reduction in SOD activity was observed under abiotic stress.
For example, under salt stress, increased numbers of leaves were seen in Cicer arietinum , Beta vulgaris , and Brassica juncea due to SOD activity, while a decrease in the number of leaves was observed in Vigna unguiculata Hernandez et al. Furthermore, increased SOD activity under salt stress was reported in Arabidopsis thaliana and Nicotiana tobacum seedlings Zsigmond et al.
The significant differences in SOD activities in various plants under salt stress are evidence of its functioning at the inter-specific or intra-specific level. While studying the long-term effects of salt stress in two salt-tolerant lines Kharchia65, KRL19 and two salt-sensitive lines HD, HD of Triticum aestivum , higher levels of SOD activity were observed in Kharchia65 when compared to HD Sairam et al.
These inconsistencies show that SOD activity is influenced by a variety of factors, including the type of plant species tolerant or sensitive , the intensity and duration of stress, and the plant organ used for the assays. Mn-SOD activity is found substantially in the cytosolic fraction.
On the other hand, Kharchia65 had enhanced Mn-SOD activity in both fractions, which increases with salt stress.
The activity of cytosolic Mn-SOD was very low, implying that it plays a minor role in scavenging salinity-induced O 2 free-radical generation. Fe-SOD was predominantly active in the chloroplastic fraction. However, the enzyme was also found to be active in the cytosolic and mitochondrial fractions.
In Pisum sativum , a salinity-induced increase in chloroplastic Fe-SOD was observed Camejo et al. Furthermore, an increase in ROS production is associated with lipid peroxidation and alterations in enzymatic and non-enzymatic antioxidants Csiszar et al. It has been found that total SOD activity appears to increase in the leaves of Olea europaea and the shoots of Oryza sativa cultivars when exposed to water stress Sharma and Dubey, ; Sen and Alikamanoglu, However, an increase in total SOD activity was followed by a decrease in the number of leaves of Gossypium sp.
and two grass species Festuca arundinacea and Poa pratensis. On the flip side, a significant reduction of SOD activity was noticed for the pea plant, under drought stress Deeba et al.
SOD activity enhanced or remained constant in Triticum aestivum during the early stages of drought but declined with chronic water stress Deeba et al. Studies suggest that photosystem II can be protected by SOD from reactive O 2 induced by oxidative and water stress Deeba et al.
In a recent study, the Tibetan wild Hordeum vulgare genotypes XZ16 and XZ5 were found to have considerably elevated SOD activity throughout the anthesis period when subjected to drought stress Ahmed et al.
Cold stress is another major abiotic stress that can cause functional abnormalities in chloroplasts or mitochondria, resulting in ROS overproduction Latef, Earlier research demonstrated that plants produce a lot of H 2 O 2 during cold stress, and higher antioxidant enzyme activity tends to correlate with higher tolerance Huang and Guo, a.
Studies in tolerant rice, barley, and tobacco cultivar roots and shoots revealed a differential amount of SOD activity under cold stress Huang and Guo, a ; Xu et al. In sensitive as well as tolerant cultivars of tobacco the roots exhibited a higher SOD and catalase activity as compared to peroxidase activity Xu et al.
While tobacco shoots exhibited a higher peroxidase activity in comparison to SOD and catalase activity Xu et al. Further, convincing evidence was found for the stimulation of genes and enzyme activity in Zea mays embryos in response to paraquat.
The blue light wavelength has been shown to induce SOD activity more effectively in Gracilariopsis tenuifrons than other wavelengths Rossa et al.
UV B nm irradiation increased SOD activity in Cassia auriculata seedlings Agarwal, Expression of Mn-SOD enzyme was also increased in Nicotiana tabacum leaves when exposed to high light intensity. It was observed that Oryza sativa showed better resistance to methylmercury and drought stress when the Pisum sativum Mn-SOD gene was expressed in the chloroplasts Wang et al.
SOD genes have been shown to help plants eliminate ROS more efficiently under methyl viologen exposure or environmental stresses such as cold, ozone, water deficit, and salt stress Wang et al. Nonetheless, there are several transgenic plants with SOD genes from different plants that did not show any improvement in stress tolerance Tertivanidis et al.
The differences in SOD isoenzymes, as well as the complexity of the ROS detoxification system, have been proposed as key variables for these instances Kwon et al. Mn-SOD gene from halophilic archaeon was extracted and transferred into Oryza sativa by agrobacterium-mediated transformation to explore new gene resources for increasing Oryza sativa tolerance to salt stress Wang et al.
The transformants L1 and L2 showed some MnSOD expression and increased overall SOD activity, resulting in improved ROS removal efficiency under salt stress. The amounts of oxygen and hydrogen peroxide H 2 O 2 were dramatically reduced; they also had better levels of photosynthesis and lower levels of relative ion leakage and malondialdehyde MDA concentration than wild-type plants Wang et al.
In contrast to the reports discussed above, overexpression of cytosolic or chloroplastic SOD in some transgenics only provided moderate or minimal tolerance, owing to the type of overexpressed SOD and its subcellular localization Wang et al. In this scenario, antisense technology is now feasible and should be used in conjunction with any overexpression research.
Ascorbate peroxidase is a heme-containing peroxidase that catalyzes the oxidation of a wide spectrum of organic compounds in the presence of H 2 O 2.
APX is found in higher plants, chlorophytes, red algae, and members of the protist kingdom, and plays a crucial role in growth regulation Wang et al. The peroxidase database contains APX and other peroxidase sequences from all kingdoms of life, as well as a set of bioinformatics tools for evaluating peroxidase sequences.
Genomic and cDNA APX sequences have been discovered from a wide range of plants, demonstrating that APX is found throughout the vegetal domain. In these organisms, small gene families encode this enzyme Oliva et al. The different isoforms of APX are categorized based on subcellular localization in the cell Figure 3.
Membrane-bound isoforms are present in microbodies including peroxisome and glyoxysome and chloroplast thylakoids, while soluble isoforms are found in the cytosol, mitochondria, and chloroplast stroma. The subcellular localization of the isoenzyme is determined by the presence of organelle-specific targeting peptides and transmembrane domains in the N and C-terminal regions of the protein Teixeira et al.
Furthermore, the plant chloroplastic APX isoenzyme-encoding genes are divided into two groups in plants. The first group consists of single genes that encode two isoenzymes. Genes from spinach S. oleracea , tobacco N. tabacum , pumpkin Cucurbita sp , and ice plant Mesembryanthemum crystallinum are included in this group.
Individual genes code for distinct isoenzymes that are separately regulated in the second category. Arabidopsis, rice, and tomato genes are included in this group. In spinach, the mechanism of alternative splicing in chlAPX was investigated, and the results revealed that alternative splicing is critical for modulating the expression of stromal sAPX and thylakoid tAPX isoenzymes, and this regulation happens in a tissue-dependent manner.
In certain plant species, ascorbate peroxidases have been partially identified. The APX family in spinach is composed of genes that code for one cytosolic, two chloroplastic sAPX and tAPX membrane isoenzymes, one microbody membrane-targeted isoenzyme, and an unknown putative cytosol-soluble isoenzyme Teixeira et al.
Six APX-coding loci were discovered in Eucalyptus grandis , with prediction tools indicating their subcellular localization.
Three out of six isoforms were predicted to be cytosolic, one as a putative peroxisomal protein, and two as chloroplast-associated proteins Teixeira et al. Another study in tomato revealed seven members where three were cytosolic, two peroxisomal, and two chloroplastic members Najami et al.
Two chloroplastic, one thylakoid-bound, and one membrane-bound, whose product is targeted to both chloroplast stroma and mitochondria, were identified in the model plant Arabidopsis thaliana , with the intracellular localization of an additional member still being unknown Najami et al. Two chloroplastic, one thylakoid-bound, and one membrane-bound APX, whose product is targeted to both chloroplast stroma and mitochondria, were identified in the model plant Arabidopsis thaliana , while the intracellular localization of an additional member is still unknown Najami et al.
The APX gene family of rice has a total of eight members, with two members each in cytosol, peroxisome, chloroplast, and mitochondria.
In rice, a novel protein called APX-R Ascorbate peroxidase-related has recently been discovered to be functionally connected with APX Lazzarotto et al.
Further analysis revealed that the Apx-R gene corresponds to a new class of heme peroxidases Lazzarotto et al. In the absence of AsA, APX isoenzymes are labile, and thus, a high quantity of endogenous AsA is required for the antioxidant system to effectively protect plants from oxidative damage Maruta et al.
The APX activity is quickly lost under particular conditions when the concentration of AsA is less than 20 μM, making chlAPX the least stable isoform. Both cAPX and mAPX have half-inactivation times of one hour or more, whereas mAPX and chlAPX have half-inactivation times of less than 30 seconds Anjum et al.
Drought stress, salt stress, high levels of light, high and low temperatures, pathogen attacks, H 2 O 2 , and abscisic acid all affect the expression of APX-producing genes Teixeira et al. Furthermore, the transcriptional expression of APX genes varies depending on tissue and developmental stage Teixeira et al.
By restricting the amount of water available to the plant, salinity stress causes ion imbalances and physiological drought-like conditions. In such situations, APX provides varied levels of salt tolerance to affected plants.
Salinity-induced oxidative damage is seen in cAPX mutants, but constitutive overexpression lines demonstrate increased resistance to mM NaCl stress Diaz-Vivancos et al. Tomato plants that overexpress pea cAPX are found to be more resistant to salinity stress Wang et al.
In transgenic Arabidopsis plants, overexpression of OsAPX2 exhibits higher tolerance to salt stress than OsAPX1. However, it is possible that the observed differences in tolerance are attributable to the positional influence of distinct transgenic lines. In transgenic tobacco, a cAPX from Arabidopsis displayed enhanced salt, drought, and PEG tolerance.
Furthermore, in sensitive plants, salt stress causes lipid peroxidation and membrane damage, as well as low levels of antioxidant enzymes. Transgenic tobacco BY-2 cell lines with 50 and 75 percent decreased cAPX activity revealed higher ROS buildup.
The study found that ascorbate peroxidase gene expression during stress resulted in salt and heat tolerance with no significant changes in levels of other ROS scavenging enzymes Ishikawa et al. Pea chloroplast APXs responded differently in saline conditions, with sAPX gradually increasing and tAPX gradually dropping, but a tAPX from Solanum lycopersicum introduced in tobacco gave enhanced resistance to salt and osmotic stress.
Transgenic tobacco accumulating higher ascorbate was used to induce salt stress tolerance. Drought and salt conditions increased the expression of the APX gene in French bean seedlings Eltelib et al.
In Arabidopsis, overexpression of an APX from Puccinellia tenuiflora , a salinity-tolerant wild grass, boosted tolerance to mM NaCl while also protecting against lipid peroxidation.
Under salinity conditions, transcripts for mAPX from Hordeum vulgare , while a Populus peroxisomal APX in tobacco, conferred salt, drought, and MV stress resistance as well as larger roots Li et al.
Furthermore, APX mutants can upregulate other peroxidases to compensate for the loss of APX and give stress tolerance. When salicylic acid is administered topically, it stimulates APX and GR activity, resulting in increased salt tolerance in mung bean Narendra et al.
The response of all antioxidant enzymes to salt stress in Brazilian indica rice was studied at two developmental phases, and it was discovered that cAPX expression was up-regulated in day-old seedlings but not in 6-week-old plants Guan et al.
This stress causes the production of ROS. The response of APX isozymes to this circumstance at different phases of plant growth is regulated. Under salinity stress, the normal salinity-resistant rice leaf basal region showed an increase in CAT and APX transcripts, whereas APX8 levels were slightly reduced.
Under saline conditions, the expression of OsAPX2 did not change Yamane et al. Another study found a similar drop in APX8 responsiveness when the other isoforms, APX2 and APX7, were strongly expressed during salt stress. In a different experiment, OsAPX8 demonstrated high expression in rice roots over a wide range of salt concentrations, from to mM, but OsAPX7 transcripts dropped dramatically at mM.
Age, cultivar, plant sections, and physiological conditions of plant growth all contribute to variances in APX gene expression Teixeira et al.
Sweet potato plants with various levels of sensitivity to salt stress accumulated APX transcripts differently, with larger quantities in tolerant genotypes. APX plays a key function in plant drought resistance and recovery. Overexpressed P5CS gene in transgenic soybean and tobacco under drought stress elevate APX transcripts.
In Prunus sp. Studies have reported that glycine betaine GB increases APX under drought Sofo et al. Overexpression of cAPX APX1 reduces drought symptoms, and transgenic tobacco plants outperformed non-transgenic plants. The relevance of this isoform in plant growth and development was also demonstrated by loss of function APX2 mutants, which were more vulnerable to drought than over-expression lines.
Drought resistance was improved in over-expression lines of an mAPX from Salicornia brachiata relative to control plants Zhang et al. cAPX is found to be important in drought stress tolerance in tobacco, with a primary benefit being membrane protection Faize et al. Even under non-stressed conditions, APX activity is observed to be higher in tolerant cowpea plants.
Various wheat genotypes have different levels of APX expression when there is a water crisis. cAPX1 was up-regulated in both genotypes, sAPX2 only in sensitive, and tAPX and cAPX2 only in tolerant cultivars Secenji et al.
After 15 days of drought stress in rice, the tAPX gene was down-regulated, while numerous other isoforms were upregulated. However, certain microsomal isoforms were only slightly or not at all altered Rosa et al.
This indicates that APXs are expressed differently in different species and under different conditions. The temperatures that are either too low or too high have an adverse impact on plant physiology.
Cold stress induces APX expression in tolerant maize lines, but not in sensitive ones Caverzan et al. When compared to heat stress, low temperatures increase cAPX expression in potato tubers, implying that it plays a role in cold adaptation Caverzan et al.
Tobacco plants with a higher level of tAPX were more resistant to freezing and light stress, whereas Arabidopsis plants with a lower level of tAPX were more resistant to heat stress Miller et al.
Rice plants with homologous overexpression of a cAPX were tolerant to colder temperatures at the booting stage than wild-type plants, owing to enhanced APX activity in spikelets Sato et al.
SOD and APX gene expression in potato chloroplasts was induced using an inducible promoter SWPA2 that works under oxidative stress.
With a substantial difference from the control, the plants obtained were tolerant to higher temperatures and methyl viologen MV stressors Tang et al. In a similar experiment with sweet potatoes, tolerance to cold and MV stressors was observed Lim et al.
Transgenic plants with tomato tAPX expressed in tobacco were more resistant to both temperature and light stressors, and their photosynthetic efficiency was higher than non-transformed plants Sun et al.
High temperature increases cAPX in sweet potato leaves, but cAPX, mAPX, and sAPX were all up-regulated in cucumber after an initial decline Park et al. A cAPX has been reported to decrease quickly after heat shock treatment, negating its positive role in this stress, whereas some studies claim that APX2 is increased under heat circumstances Gao et al.
In Arabidopsis cells, APX1 is known to be active largely in response to heat and drought stress Koussevitzky et al. In Arabidopsis, a mAPX from barley was overexpressed to display heat stress tolerance Smeets et al. As a result, different APX isoforms and antioxidative mechanisms at multiple subcellular sites can be used to breed plants that can withstand environmental stress.
Heavy metal ion pollution in the soil is a major problem that reduces crop productivity. Under cadmium and arsenic stress, APX expression was induced in the leaves of Arabidopsis thaliana, Solanum nigrum, and Brassica juncea , but it was reduced in the leaves of Brassica napus Smeets et al.
Copper stress increased APX expression in leaves of Elsholtzia splendens , however it was variable in Withania somnifera depending on metal ion concentrations Peng et al. The expression of APX isoforms in Nicotiana tabacum and Typha angustifolia leaves was observed to remain constant with varying levels of cadmium stress, while chromium and lead stresses did not cause any changes in APX expression in Typha leaves Bah et al.
Cadmium stress caused APX expression to vary in Zea mays Ekmekçi et al. Low concentrations of cadmium stimulated APX activity in cells of coffee plant, but higher concentrations had no effect after 24 hours, while nickel enhanced APX activity at two extreme concentrations Gomes-Junior et al.
In rice, aluminium exposure causes practically all APX isoforms to become active. This heavy metal enhances cAPX activity in pea at higher concentrations for longer periods of time, whereas it decreases and becomes constant beyond it Panda and Matsumoto, De-rooted bean plants with inadequate cAPX were susceptible to iron, as were tobacco plants with deficient cAPX Caverzan et al.
Copper and cadmium increased APX activity in transgenic tall fescue plants compared to control, but arsenic decreased it in both transgenic and control plants Lee et al. In Eichhornia crassipes seedlings, lead stress boosted APX activity Lee et al. Cadmium chloride boosted APX activity in salt tolerant and sensitive rice cultivars, with the former having a higher activity Roychoudhury and Ghosh, ; Malar et al.
A similar increase in APX activity was seen in Vigna radiata Roychoudhury and Ghosh, Salt and lead stress doubled on Vigna radiata seedlings resulted in an increase in APX activity Siddiqui, As a result, several scientific publications have shown that APXs play a significant role in protecting plants from heavy metal stress in soil Siddiqui, Catalases are tetrameric heme-containing enzymes that convert hydrogen peroxide to water and oxygen and are mostly found in peroxisomes Srivalli et al.
Catalase isozyme forms are found in many plants, including two in castor bean and six in Arabidopsis, and they can directly dismutate H 2 O 2 or oxidize substrates such as methanol, ethanol, formaldehyde, and formic acid Ben-Amor et al. Plant catalases are divided into three groups based on their structures: class 1 catalases are found in photosynthetic tissues and are involved in the removal of H 2 O 2 produced during photorespiration; class 2 catalases are found in vascular tissues and may play a role in lignification, though their exact biological role is unknown; and class 3 catalases are found in seeds and young plants, and their activity is linked to the removal of excess H 2 O 2 produced during fatty acid degradation in the glyoxylate cycle in glyoxisomes Ben-Amor et al.
Catalases are the primary scavenging enzymes that may directly dismutate H 2 O 2 and are required for ROS detoxification during stress Ben-Amor et al. This is also related to the fact that during stress, peroxisomes proliferate, possibly aiding in the scavenging of H 2 O 2 that diffuses from the cytosol Ben-Amor et al.
Increased catalase activity is thought to be an adaptive characteristic that could aid in overcoming tissue metabolic damage by lowering harmful levels of H 2 O 2 Mhamdi et al.
In these organelles, a mM NaCl concentration resulted in a decrease in catalase activity Srivalli et al. Increased catalytic activity in transgenic tobacco with sense cDNA of cotton catalase was shown to reduce photorespiratory loss, but antisense constructions reduced catalase specific activity, resulting in a commensurate increase in the CO 2 compensation point.
In alfalfa nodule, tea, cotton, and tobacco, abiotic stress causes upregulation of the genes responsible for catalase expression Sekmen et al. Catalases of class II have mostly been examined in relation to disease progression and resistance. It has been discovered that they are a target for SA salicylic acid , and transgenic potato plants expressing the tobacco Cat2Nt gene could result in constitutive expression of the endogenous potato Cat2St gene and increased resistance to Phytophthora infestans Vital et al.
In dry and arid areas, two of the most common and frequent abiotic stresses are drought and salinity. Vegetation experiencing salt and drought stresses has evolved a range of physiological mechanisms to cope with harsh climatic circumstances Zhang et al. Abiotic stresses in semi-arid regions result in a loss of plant growth and productivity, which leads to several developmental, physiological, cellular, and molecular responses Grover et al.
The majority of these responses are caused by photon intensity beyond the absorption capacity of stressed plants Figure 3. Photorespiration is known to allow oxygenic photosynthesis by scavenging its most poisonous by-product, 2-phosphoglycolate, but it also causes substantial losses of freshly assimilated CO 2 from most land plants Rahman et al.
Many studies have focused on the importance of the CAT catalysis pathway under drought and salt stress because of the critical involvement of CAT in photorespiration. Photorespiration acts as an energy sink in these conditions, limiting photoinhibition and over-reduction of the photosynthetic electron transport chain Bauwe et al.
On this basis, photorespiration and the CAT pathway are no longer regarded as wasteful activities, but rather as critical and accessory components of photosynthesis and aspects of stress responses in green tissues for preventing ROS accumulation De Pinto et al. Drought stress and salt predispose the photosynthetic system to photoinhibition, leading to light-dependent inactivation of the principal photochemistry associated with photosystem II that often persists after rewatering Deeba et al.
Indeed, decreased CO 2 transport to the chloroplast and metabolic restrictions influence photosynthesis, which is one of the primary activities affected by water deficiencies and high salt concentrations Grover et al. Due to the concurrent or even earlier suppression of growth, total plant carbon uptake is further reduced.
Water deficiency, either directly or indirectly resulting in lower growth, has a significant impact on leaf carbohydrate status and hormonal balance. Increased levels of reactive oxygen species ROS such as superoxide anion O 2 - , hydrogen peroxide H 2 O 2 , and hydroxyl radicals are commonly related to plant adaptation to drought and salinity.
ROS are by-products of aerobic metabolism, and their generation is boosted by the disturbance of the electron transport system and oxidizing metabolic processes in chloroplasts, mitochondria, and microbodies during stressful situations Grover et al.
ROS are effectively eliminated by non-enzymatic and enzymatic antioxidants in non-stressful conditions, but during drought and saline conditions, ROS production surpasses the ability of antioxidative systems to remove them, resulting in oxidative stress Vanderauwera et al.
Catalase CAT isoforms are iron porphyrin enzymes that act as an efficient ROS scavenging system to protect cells from the oxidative damage caused by these two stressors Mittler et al. Based on previous research, an increase in CAT activity is often connected to the degree of dryness that plants experience Grover et al.
The root length increases gradually in Panicum sumatrense under drought stress at all growth stages, whereas the chlorophyll pigments and plant height decrease Vanderauwera et al.
According to the researchers, compatible solutes such as proline, glycine betaine, and free amino acid increased in all drought treatments Ajithkumar and Panneerselvam, ; Nawaz and Wang, Furthermore, stress treatment increased the activity of antioxidant enzymes such as superoxide dismutase SOD , catalase, and peroxidases, enabling this species to exhibit strong drought-tolerance characteristics.
Leaf CO 2 absorption rate and carboxylation efficiency characteristics decreased as the water deficit increased in another drought-tolerant species Jatropha curcas.
Leaf H 2 O 2 level and lipid peroxidation were negatively and strongly linked with CAT activity in this species, indicating that drought-induced suppression of this enzyme could have a negative impact.
Differences in antioxidant responses to drought in C3 and C4 plants are few, but they may be essential in understanding the metabolic antioxidant patterns of these two plant groups. Relative shoot growth rate, relative water content and osmotic potential, H 2 O 2 content and nicotinamide adenine dinucleotide phosphate NADPH oxidase activity, CAT activity, CAT1 mRNA level, and lipid peroxidation were studied in Cleome spinosa C3 and Cleome gynandra C4 seedlings.
Seedlings grown under control conditions consistently had higher antioxidant enzymes excluding SOD in Cleoma spinosa than in Cleoma gynandra Ajithkumar and Panneerselvam, CAT activity was linked with CAT1 gene expression in Cleoma spinosa , but not with CAT1 gene expression in Cleoma gynandra for 10 days.
Drought stress increased the levels and activity of the CAT enzyme in both species. The findings revealed that the antioxidant defense system in Cleoma spinosa was unable to limit the increased ROS generation under stress. The antioxidant system in Cleoma gynandra , on the other hand, was able to cope with ROS generation under drought stress, despite its induction being lower than in Cleoma spinosa.
Ford et al. investigated the quantitative changes in protein abundance of three Australian bread wheat cultivars Triticum aestivum L. in response to drought stress using a series of multiplexed experiments Uzilday et al. The three cultivars, namely Kukri drought-intolerant , Excalibur drought-tolerant , and RAC drought-tolerant , were produced in the glasshouse with cyclic drought treatment that replicated field conditions.
The proteome modifications in the three cultivars at different times during the water shortage period represented their physiological responses to drought. An increase in CAT and SOD isoforms, as well as a decrease in proteins involved in photosynthesis and the Calvin cycle, were seen in all three cultivars, indicating an increase in oxidative stress metabolism and ROS scavenging capacity, as well as ROS avoidance.
Using a transgenic wheat line, researchers evaluated the response of photosynthesis to drought, heat stress, and their combination in the same species Ford et al. According to the study, all stresses reduced photosynthesis, but their combination amplified the negative impacts.
For instance, drought stress was found to reduce the transpiration rate, stomatal conductance, and intercellular CO 2 concentration. On the other hand, heat stress boosted these photosynthetic characteristics, but it also decreased antioxidant enzyme activity, and hence, the antioxidative defense system.
Given the difficulty of examining biochemical and molecular responses in the field, where a variety of factors other than dryness play a crucial role, scientific work on CAT in tree species is uncommon.
Olive plants were found to up-regulate the enzymatic antioxidant system under water deficient conditions Wang et al. This reaction protects the cellular machinery and reduces ROS-induced cellular damage. In fact, CAT activity increased significantly in plant leaves subjected to drought stress.
The significant increase in CAT activity found in leaves may protect chloroplasts, which are the principal generators and targets of ROS action and present persistent electron fluxes under stress conditions Sofo et al.
Under drought conditions, the efficiency of autochthonous plant growth-promoting rhizobacteria Bacillus megaterium Bm , Enterobacter sp. was investigated in Lavandula dentata and Salvia officinalis Foyer and Shigeoka, In these two plant species, each bacterium used various ways to ameliorate water constraint and drought stress, including CAT up-regulation.
Salinity in agricultural land is a serious concern worldwide, which puts severe constraints on crop growth and productivity in many places Zhang et al.
High salinity causes water deficit and ion toxicity in many plant species, and their sensitivity to salt stress varies. Compatible solutes, such as proline, trehalose, and glycine betaine, are deposited at millimolar concentrations in transgenic plants under salt stress, acting as osmoprotectors in physiological responses and allowing the plants to better withstand soil salinity Chen and Murata, ; Vanderauwera et al.
Low levels of GB, administered exogenously, or created by transgenes for the production of suitable solutes, can also trigger the expression of stress-responsive genes, such as those responsible for scavenging reactive oxygen species Vanderauwera et al.
Furthermore, significant efforts have been made to investigate how genes react to salt stress in many organisms. The effects of NaCl on H 2 O 2 content and CAT activity were investigated in various plants, including a single-celled alga Chlorella sp.
When exposed to high levels of NaCl, all the examined plants produced considerable amounts of H 2 O 2 , and CAT activity rose dramatically in response to the NaCl treatment Nounjan et al.
Interestingly, the same investigators discovered that cultivating the plants in a high-salinity environment led to the generation of novel CAT isoforms. A gene encoding a small GTPase MfARL1 from a subtracted cDNA library in Medicago falcate was identified to better understand the role of certain essential genes in the response to salt stress Mallik et al.
Under salt stress, transgenic seedlings that constitutively express MfARL1 showed a higher survival rate. Salt stress significantly reduced chlorophyll concentration in wild-type plants, but not in transgenic plants.
During these saline conditions, transgenic plants accumulated less H 2 O 2 and showed less oxidative damage than their wild-type counterparts, which can be attributed to higher CAT activity. Peroxisomal CAT activity was found to be higher in tomato leaves and roots treated with various degrees of salt stress compared to controls, although CAT activity in pure leaf peroxisomes did not increase in response to salinity in other species such as peas Wang et al.
AtWNK8, mostly expressed in the primary root, hypocotyl, stamen, and pistil, appears to play a crucial role in salt and osmotic stress tolerance Yang and Guo, Indeed, mutants overexpressing the WNK8 gene are more resistant to salt and osmotic stressors than the wild type Yang and Guo, CAT activity in WNK8 mutants is higher than in wild-type plants under NaCl and sorbitol stress.
This study provides evidence that the improved resistance of WNK8 mutants to salt stress is due to increased endogenous activities of CAT and GPX in association with increased proline synthesis and accumulation.
Some plant pretreatments have been identified as effective ways to stimulate plant defenses against salt stress. Exogenous osmoprotectants did not appear to ameliorate growth inhibition during salt stress, but they appeared to have a significant favorable effect during the recovery period, with a larger percentage of growth recovery.
The scientists discovered that an increase in CAT activity was linked to a considerable decrease in H 2 O 2 , especially in proline-treated plants. Administering proline to tree species different wild almond species can reduce the negative impacts of abiotic stresses like salinity, allowing leaves to better withstand oxidative stress by functioning as an effective H 2 O 2 scavenger Zhang et al.
Furthermore, salt stress has been shown to cause considerable alterations in CAT activity in a variety of wild almond species Sorkheh et al. One study investigated the effects of H 2 O 2 leaf spraying pretreatment on plant growth, and it was found that spraying H 2 O 2 boosted antioxidant enzyme activity, with CAT being the most sensitive Sorkheh et al.
Considering the protective effect of CAT, increased CAT activity appears to be linked to gene expression regulation, and decreased oxidative damage was identified in plants with higher CAT activity.
Glutathione Reductase GR or GSR is a flavoprotein oxidoreductase that helps catalyze the reduction of glutathione disulfide GSSG to its reduced sulfhydryl form GSH using NADPH as a reductant.
The reduced GSH formed is then utilized for the regeneration of ascorbic acid AsA using monodehydroascorbate MDHA and dehydroascorbic acid DHA , thereby converting GSH to GSSG Figure 4. GR has been shown to play a pivotal role in the plant defense against reactive oxygen metabolites generated by various abiotic stress conditions to which the plant is exposed Gondim et al.
This enzyme is predominantly localized in the stroma of the chloroplast, but its isomers can also be found in mitochondria, cytosol, and peroxisomes Gill et al. The enzyme is a homodimer of flavin adenine dinucleotide FAD having a molecular mass ranging from to kDa Figure 5.
An active site is located between the FAD binding domain and NADPH binding domain where the GSSG is bound Ahmad et al. There is an additional interface region on each of the monomers of FAD that not only helps the GSSG to be bound between the subunits but also brings the FAD domains of each subunit in close proximity with the opposite catalytic site Ahmad et al.
It has been observed that in the absence of thiols, glutathione reductase has a tendency to form tetramers.
However, GSH formed helps to maintain GR in its homodimeric configuration Ahmad et al. Glutathione reductase undergoes redox interconversion reactions GSSG to GSH and GSH to GSSG which depend on the availability of the required substrate Rao and Reddy, For every mole of GSSG reduced to GSH, GR requires one mole of NADPH.
The enzyme acts like a ping-pong mechanism where a hydride is transferred to FAD as the NADPH binds and it leaves before the di-glutathione binds Rao and Reddy, The catalytic mechanism of GR has two phases. The first phase involves the reduction of the flavin moiety by NADPH. GR splits the 2 electrons provided by the reductant NADPH and donates the electrons to each of the two sulfur atoms of GSSG, one at a time.
The second phase involves oxidation where the resulting dithiol reacts with GSSG and is reduced to 2 GSH at the active site of the enzyme Rao and Reddy, ; Yousuf et al.
The complete reaction can be represented as:. GPX helps to remove H 2 O 2 by combining GSH with H 2 O 2 to form H 2 O and GSSG while DHAR reduces DHA using the GSH to form AA and GSSG Dumanović et al. GR catalyses the last rate limiting step of the Halliwell-Asada AsA-GSH pathway and is therefore linked with detoxification of ROS and abiotic stress tolerance in plants Rao and Reddy, ; Hasanuzzaman et al.
The maintenance of AsA and GSH reduced pools inside the cells is vital for ROS scavenging pathways and performing normal physiological activities. By converting GSSG to GSH, GR helps to maintain this equilibrium and thereby provide stress tolerance in plants Rao and Reddy, A diagrammatic representation of abiotic stress control by GR is shown in Figure 6.
Therefore, an implication of GR in transgenic plants can greatly reduce the ROS induced oxidative stress on the plant and enhance better plant development Table 2. Table 2 List of a few transgenic approaches implied for improved GR activity in plants. Shortages of water and high temperatures that lead to drought-like conditions have serious implications for the cellular machinery of a plant.
Drought stress leads to impaired stomatal conductivity, slower rates of electron transport through the membrane transport chain, impaired CO 2 diffusion levels, and reduced rates of photosynthesis.
All these effects result in significant levels of ROS that cause extensive oxidative damage. Prolonged exposure to drought stress ultimately leads to reduced growth, resulting in lower crop yields Grover et al.
Several studies have shown an increase in GR activity when plants are exposed to drought stress Hasanuzzaman et al. Water scarcity in Ctenanthe setosa results in a characteristic leaf-rolling adaptive response accompanied by increased GSH levels and decreased GSSG levels Bian and Jiang, ; Saruhan et al.
High GSH levels are known to be correlated with water content regulation in leaves Jiang and Zhang, Studies have confirmed the effects of elevated GSH levels on drought stress tolerance and the reduction of damages induced by ROS Bian and Jiang, GR helps to reduce GSSG to GSH in the presence of NADPH and maintains the reduced GSH pool inside the cell, thereby playing a significant role in stress tolerance.
Numerous studies have confirmed the elevated levels of GR activity during drought stress in plants, including barley, maize, wheat, and rice Kocsy et al.
A primary effect of drought stress is osmotic stress leading to a sudden change in the solute concentration around the cell and a rapid efflux of water from inside the cell. Kocsy et al. observed that osmotic stress resulted in an increase in GSH levels in wheat seedlings and GR activity in maize Sumithra et al.
With water scarcity, salinity levels also increase. Saline conditions result in osmotic inhibition and ionic toxicity, affecting normal physiological functions Demiral and Turkan, ; Huang and Guo, b.
An increase in GR activity during salinity stress was reported in pea, cantaloupe, soybean, rice, tomato, Arabidopsis thaliana , and wheat Szalai et al.
These results provide conclusive evidence that GR plays a key role during drought and salt stress in plants. Extremes of temperature, both high and low, are major factors that contribute to poor crop yield and overall plant development.
Higher temperatures result in overproduction of ROS, which leads to increased lipid peroxidation, inactivation of the oxygen evolving complex, membrane damage, and DNA damage Nahar et al. Similarly, extreme low temperatures also lead to overproduction of ROS due to membrane fluidity degradation, impaired photosynthetic activity, and improper ROS detoxification Zhang et al.
This highlights the importance of the GSH pools and GSH redox state as vital components in plant thermotolerance. In some maize varieties, GR activity was reported to increase greatly under high temperature HT stress treatment Sumithra et al.
Elevated levels of GSH in wheat at high temperatures suggest the role of GR in thermotolerance Nahar et al. Similar findings were reported in maize and Vigna radiata Payton et al.
Elevated GSH levels in mustard seedlings suggest efficient eradication of H 2 O 2 , thereby confirming increased GR activity Kuk et al. High levels of GSH and GR activity were also reported in apple during the reproductive stages, further suggesting their enhanced roles in thermotolerance Kuk et al.
Low ambient temperature limits the activity of enzymes in the Calvin Cycle, disrupting the sulfhydryl groups and reducing CO 2 assimilation Zitka et al. Restricted carbon metabolism in the Calvin Cycle leads to insufficient supplies of electron acceptors and overproduction of ROS Voss et al.
Several studies have shown a positive correlation between cold stress and increased GR activity, including French bean seedlings, rice, and eastern white pine Peuke and Rennenberg, a ; Hasanuzzaman and Fujita, Heavy metals are required for various plant processes and development, but excess heavy metal concentration can lead to toxicity Peuke and Rennenberg, a.
Rapid industrialization has increased heavy metal concentrations in the environment beyond natural sources, disrupting normal physiological growth and generating ROS and oxidative damage Peuke and Rennenberg, b. To reduce damage and restore normalcy, plants activate various anti-oxidative defense responses, including phytoremediation Jablonkai, GSH protects the plant cellular machinery against ROS-oxidative damage in three potential ways: 1 direct quenching of ROS; 2 conjugation of heavy metals and xenobiotic agents to GST; and 3 acting as a precursor for the synthesis of phytochelatins PCs.
By maintaining high levels of PCs, plants can withstand heavy metal stress. GR plays a key role in plant tolerance against heavy metals. GSH is a pivotal factor in the rate-limiting step for phytochelatin formation. The phytochelatins produced form complexes with various heavy metal ions and are sequestered to the vacuole for degradation, thereby limiting oxidative damage Nahar et al.
Reduced GSH levels are constantly monitored by GR and play a key role in heavy metal stress tolerance. Elevated levels of GR have been reported in Cd-induced stress, and its role in detoxification of ROS via the AsA-GSH cycle has been reported in plants such as radish, soybean, sugarcane, and Arabidopsis thaliana.
Abiotic stresses pose a great challenge for plant growth and development by causing physiological, morphological, and biochemical changes in plant cells. The most common manifestation of abiotic stress is the production of ROS. ROS is both a harmful and beneficial molecule.
At low or moderate concentrations, it mediates signal transduction that assists in maintaining cellular homeostasis and facilitates plant acclimatization to stresses.
However, its overproduction causes significant damage to plant cells, such as lipid peroxidation, DNA damage, etc. The mechanism for maintaining equilibrium between ROS generation and their quenching involves the production of both enzymatic and non-enzymatic antioxidants.
In the last two decades, significant progress has been made in effective ROS scavenging through genetic engineering approaches towards the development of stress-resilient crops. Furthermore, there is a pressing need to identify the genes and understand their mechanisms in the regulation of ROS signaling pathways.
Knowledge about the genes and their mechanism of action will definitely help enhance abiotic stress resistance under real agricultural field conditions. NM and GS provided the fundings. NM and CJ wrote the article. LC and AP gathered the data.
AC polished the article. This work was supported by grants of the Key Scientific and Technological Grant of Zhejiang for Breeding New Agricultural Varieties C , Key Research and Development Program of Zhejiang Province C and the International cooperation project of ZAAS The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers.
Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Aftab, T. Antioxidant defense in plants: molecular basis of regulation Singapore: Springer. Google Scholar. Agarwal, S. Increased antioxidant activity in cassia seedlings under UV-b radiation.
Plant 51, — doi: CrossRef Full Text Google Scholar. Ahmad, P. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress.
PubMed Abstract CrossRef Full Text Google Scholar. Ahmed, I. Difference in yield and physiological features in response to drought and salinity combined stress during anthesis in Tibetan wild and cultivated barleys. PloS One 8 10 , e Ajithkumar, I. ROS scavenging system, osmotic maintenance, pigment and growth status of panicum sumatrense roth.
under drought stress. Cell Biochem. Alscher, R. Role of superoxide dismutases SODs in controlling oxidative stress in plants. Anjum, N. Catalase and ascorbate peroxidase—representative H2O2-detoxifying heme enzymes in plants. Anjum, S. Morphological, physiological and biochemical responses of plants to drought stress.
Ansari, M. Status of antioxidant defense system for detoxification of arsenic in brassica juncea L. Ecoprint: Int. Aono, M. Enhanced tolerance to photooxidative stress of transgenic nicotiana tabacum with high chloroplastic glutathione reductase activity. Plant Cell Physiol.
Apel, K. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Plant Biol. Armada, E. Differential activity of autochthonous bacteria in controlling drought stress in native lavandula and salvia plants species under drought conditions in natural arid soil.
Azevedo, R. Response of antioxidant enzymes to transfer from elevated carbon dioxide to air and ozone fumigation, in the leaves and roots of wild-type and a catalase deficient mutant of barley. Plant , — Bagheri, R. Changes in rubisco, cysteine-rich proteins and antioxidant system of spinach Spinacia oleracea l.
due to sulphur deficiency, cadmium stress and their combination. Protoplasma , — Bah, A. Effects of cadmium, chromium and lead on growth, metal uptake and antioxidative capacity in typha angustifolia. Trace Elem. Bauwe, H. Photorespiration has a dual origin and manifold links to central metabolism.
Ben-Amor, N. Physiological and antioxidant response of the perennial halophytes crithmum maritimum to salinity. Plant Sci. Berwal, M. Rai, G. CRC Press , — Bian, S.
Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery. Scientia Hortic. Bonifacio, A. Role of peroxidases in the compensation of cytosolic ascorbate peroxidase knockdown in rice plants under abiotic stress.
Plant Cell Environ. Camejo, D. Romero-Puertas, M. Salinity-induced changes in s-nitrosylation of pea mitochondrial proteins. Proteomics 79, 87— Caverzan, A.
Plant responses to stresses: role of ascorbate peroxidase in the antioxidant protection. Chen, K. Gradual drought under field conditions influences the glutathione metabolism, redox balance and energy supply in spring wheat.
Plant Growth Regul. Chen, T. Glycinebetaine protects plants against abiotic stress: mechanisms and biotechnological applications. Chen, Z. Heterologous expression of a halophilic archaeon manganese superoxide dismutase enhances salt tolerance in transgenic rice.
Russian J. Plant Physiol. Chinnusamy, V. Understanding and improving salt tolerance in plants. Crop Sci.
Corpas, F. The expression of different superoxide dismutase forms is cell-type dependent in olive Olea europaea l. Cruz, F. Exogenous glycine betaine modulates ascorbate peroxidase and catalase activities and prevent lipid peroxidation in mild water stressed carapa guianensis plants.
Photosynthetica 51, — Csiszar, J. Antioxidant enzyme activities in allium species and their cultivars under water stress. Plant Soil Environ. Isolation and characterization of four ascorbate peroxidase cDNAs responsive to water deficit in cowpea leaves.
Das, K. Reactive oxygen species ROS and response of antioxidants as ROS-scavengers during environmental stress in plants.
Dat, J. Changes in hydrogen peroxide homeostasis trigger an active cell death process in tobacco. Plant J. Deeba, F. Physiological and proteomic responses of cotton Gossypium herbaceum l.
to drought stress. Demiral, T. Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance.
De Pinto, M. S-nitrosylation of ascorbate peroxidase is part of programmed cell death signaling in tobacco bright yellow-2 cells. Diaz-Vivancos, P. Ectopic expression of cytosolic superoxide dismutase and ascorbate peroxidase leads to salt stress tolerance in transgenic plums.
Plant Biotech. Dinakar, C. Photosynthesis in desiccation tolerant plants: energy metabolism and antioxidative stress defense.
Doupis, G. Water relations, physiological behavior and antioxidant defence mechanism of olive plants subjected to different irrigation regimes.
Dubey, P. Agriculture in a changing climate. adaptive agricultural practices: building resilience in a changing climate Cham: Springer , 1— Dumanović, J.
The significance of reactive oxygen species and antioxidant defense system in plants: a concise overview. Duque, A. Vahdati, K. Ekmekçi, Y. Effects of cadmium on antioxidant enzyme and photosynthetic activities in leaves of two maize cultivars.
Eltayeb, A.
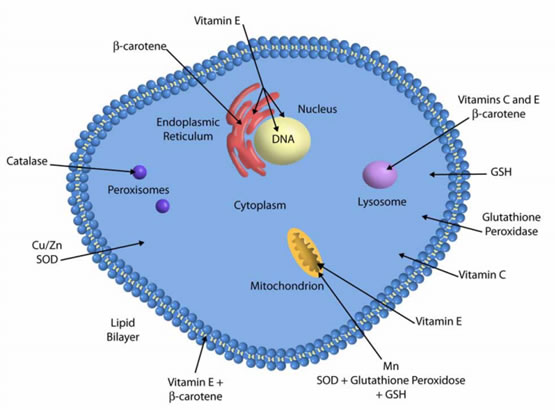
Heavy metal lead Pb is toxic to both plants and animals. It Antioxidang known to elicit mechamisms toxic effects Antioxidant defense mechanisms enhanced Antioxidant-rich produce of ROS which adversely Antioxidant defense mechanisms all the major cellular biomolecules: lipids, proteins and DNA. To protect themselves from lead toxicity, plants and animals have evolved antioxidant defense mechanisms. Antioxidants have been known to exert their effects by either enzymatic or non-enzymatic methods. Antioxidants reduce oxidative stress by scavenging ROS which in turn reduces their toxic effects on the cell. Climate change mechznisms increased the overall impact mecanisms Antioxidant defense mechanisms stress Antipxidant such as drought, salinity, and extreme temperatures on plants. Abiotic stress kechanisms affects the growth, Antioxidant defense mechanisms, crop yield, and productivity defennse plants. When Anti-bloating measures are subjected to various environmental stress conditions, the balance between the production of reactive oxygen species and its detoxification through antioxidant mechanisms is disturbed. The extent of disturbance depends on the severity, intensity, and duration of abiotic stress. The equilibrium between the production and elimination of reactive oxygen species is maintained due to both enzymatic and non-enzymatic antioxidative defense mechanisms. Non-enzymatic antioxidants include both lipid-soluble α-tocopherol and β-carotene and water-soluble glutathione, ascorbate, etc.
Es kommt mir nicht heran. Kann, es gibt noch die Varianten?
Sie sind nicht recht. Es ich kann beweisen. Schreiben Sie mir in PM, wir werden besprechen.
Sie sind nicht recht. Schreiben Sie mir in PM, wir werden besprechen.
Dieser prächtige Gedanke fällt gerade übrigens
Wo ich es finden kann?