Circadian rhythm genetics -
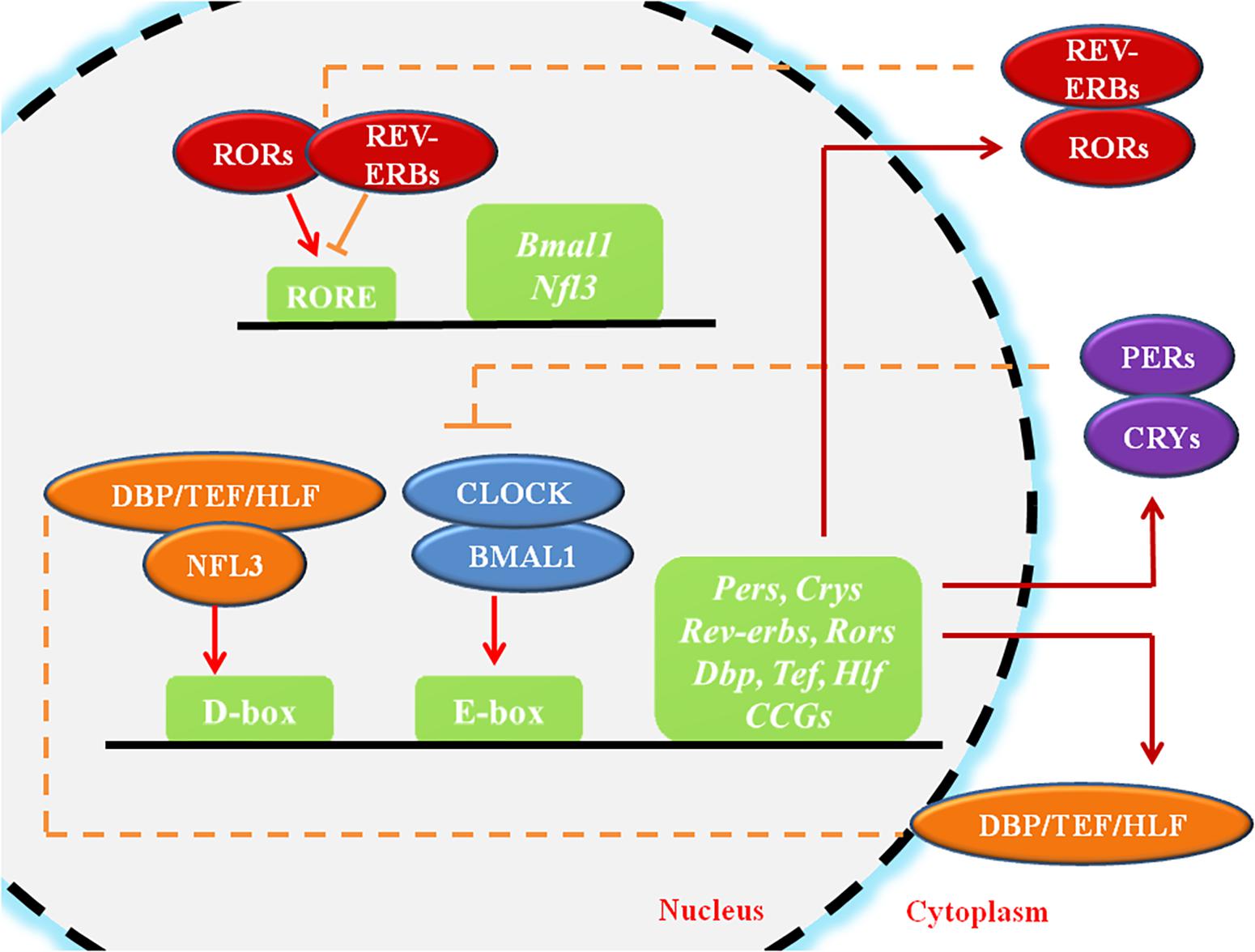
Actually, it turns out that circadian clocks exist in almost all cells and tissues in our body Dibner, Schibler et al. The mammalian circadian clock is fundamentally based on the transcriptional-translational feedback loops Figure 1. At the core of this molecular network are two transcription factors: circadian locomotor output cycles kaput CLOCK and brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1 BMAL1.
They heterodimerize and bind to E-box elements CACGTG located at the promoters of clock genes as well as a large number of clock-controlled genes CCGs. PER and CRY proteins gradually accumulate in the cytoplasm and PER proteins are phosphorylated by casein kinase Iδ CKIδ and CKIε.
PER, CRY and CKI proteins form a complex and translocate to the nucleus to inhibit the transcriptional activity of the CLOCK-BMAL1. This negative-feedback loop takes approximately 24 h to complete. Meanwhile, there are additional feedback loops driven by CLOCK: BMAL1.
RORα promotes while REV-ERBα inhibits Bmal1 transcription via binding to the ROR element RRE motif on the Bmal1 promoter Figure 1. And DEC1 and DEC2 are two suppressors for CLOCK-BMAL1 heterodimer. The transcription of Rorα, Rev-erbα, Dec1 and Dec2 is positively regulated by CLOCK-BMAL1, and negatively regulated by PER1, PER2, CRY1 and CRY2 Honma, Kawamoto et al.
Circadian rhythm sleep disorders CRSDs are conditions that the internal circadian rhythms are not properly aligned with the external environment. CRSDs are divided into four main types, including advanced sleep phase disorder ASPD , delayed sleep phase disorder DSPD , irregular sleep-wake rhythm and nonh sleep-wake disorder.
Individuals with advanced sleep phase disorder usually feel very sleepy and have to go to bed early in the evening generally between 6—9 pm and wake up very early in the morning generally between 2—5 am.
The sleep, temperature, and melatonin rhythms shift forward 3—4 h as compared to the normal persons Jones, Campbell et al. ASPD is a rare disorder with a strong genetic trait.
The research team led by Louis J Ptáček and Ying-Hui Fu at the University of California at San Franscisco UCSF have made great contributions to decipher the underlying genetic mechanisms of ASPD. In , Jones et al. reported three families with ASPD, which show autosomal dominant inheritance Jones et al.
In , Toh et al. identified an SG mutation in hPer2, located near the telomere of chromosome 2q, as the causative mutation for one of these ASPD pedigrees Table 1. Ser is located within the CKIε binding region in PER2, and SG mutation leads to decreased phosphorylation of PER2 in vitro Toh, Jones et al.
Although phosphorylation of PER2 by CKIε retards the nuclear entry of PER2 Vielhaber, Eide et al. Interestingly, studies from Xu et al. suggest that Ser of PER2 is not phosphorylated by CKIε; however, a phosphate at S is required for CKIε to phosphorylate other residues in PER2.
They further generated transgenic mice carrying SG hPER2 gene, which faithfully recapitulate the ASPD phenotype in human Xu, Toh et al. In , Xu et al. found a T44A mutation in human CKIδ gene co-segregates with the ASPD phenotypes in another pedigree Table 1 Xu, Padiath et al.
The T44A mutant kinase has significantly lower enzymatic activity than wild-type kinase. Both drosophila and mice carrying the T44A hCKIδ exhibit abnormal circadian rhythms.
Transgenic mice carrying T44A hCKIδ show a shorter circadian period, recapitulating the ASPD phenotype in human; however, the T44A hCKIδ transgenic flies show a longer circadian period, suggesting divergent regulatory mechanisms in mammalian and fly clocks. Although transgenic mice carrying wild-type WT CKIδ have abnormal circadian period, WT CKIδ transgene further shortens the circadian period in the SG hPER2 transgenic mice, indicating that CKIδ may regulate circadian period through PER2 in vivo.
In , Hirano et al. identified a missense mutation AT in hCry2 gene that is associated with ASPD Table 1. The Ala is located in the flavin adenine dinucleotide FAD binding domain of CRY2 protein, and the AT mutation alters the conformation of CRY2 protein and increases its affinity to the E3 ubiquitin ligase FBXL3, thus promoting the degradation of CRY2.
The transgenic mice carrying hCRY2-AT have advanced phase of sleep-wake behavior in a light-dark cycle and a shortened circadian period in constant darkness, mimicking the ASPD phenotype in human Hirano, Shi et al. Zhang et al. identified two rare variants in PER3 PA and HR on the same allele in ASPD patients accompanied with increased depressive mood and global seasonality scores Table 1.
Mammalian Timeless mTim is as a homolog of Drosophila Timeless dTim Koike, Hida et al. Conditional knockdown of mTim protein expression in the rat SCN disrupted SCN neuronal activity rhythms and altered levels of known core clock elements Barnes, Tischkau et al.
Recently, the Kurien et al. identified an ASPD-associated TIM-RX mutation by using unbiased whole-exome sequencing. The TIM-RX knock-in mice exhibit FASP phenotype with altered photic entrainment but normal circadian period.
Delayed sleep phase disorder DSPD is characterized by a persistent and intractable delay of sleep onset and offset time comparing to normal person, generally more than 2 h.
People with DSPD are unable to fall asleep and wake up at socially acceptable times, resulting in excessive daytime sleepiness Micic, Lovato et al. According to a large population-based study with 10, adolescents aged 16—18 years conducted in Hordaland County of Norway, the prevalence of DSPD in the general population is estimated to 3.
DSPD also has a strong heredity and familial tendency Barclay, Eley et al. In , Ancoli-Israel et al. reported a DSPD pedigree with a bilineal mode of inheritance, as both the paternal and maternal branches contained affected individuals Ancoli-Israel, Schnierow et al.
Per3 is the first gene to be associated with DSPD. Ebisawas et al. identified six variants in hPer3 in DSPD individuals, and one haplotype is found to be significantly associated with DSPD Ebisawa, Uchiyama et al.
Furthermore, the contribution of a variable-number tandem-repeat polymorphism in the coding region of PER3 to extreme diurnal preference ASPD or DSPD is also investigated Table 1.
Archer et al. Consistently, homozygous Per3 knockout mice display a free-running period of 30 min shorter than the WT mice Zhang, Hirano et al.
Recently, Patke et al. report a missense mutation c. This mutation disrupts the splicing recognition site before exon11, resulting in the deletion of exon 11 with an in-frame deletion of 24 amino acids of CRY1 CRY1Δ The CRY1Δ11 shows enhanced inhibition on CLOCK-BMAL1 heterodimer.
This gain-of-function CRY1 variant causes reduced expression of a variety CLOCK-BMAL1 targets and lengthens the period of circadian molecular rhythms. Intriguingly, CRY1Δ11 mutation has a frequency of up to 0. Studies on human with familial CRSD or corresponding genetically modified animal models provide further insight into this connection.
Xu and colleagues take advantage of SG-PER2 transgenic mice, which mimic human ASPD, to investigate the effect of disrupted circadian clock on cell cycle progression and tumorigenesis. And PER2-SG mutation leads to an increased E1A- and RAS-mediated oncogenic transformation.
In addition, the expression profiles of p21 and Cyclin D, two clock-controlled cell cycle genes, change significantly in the embryonic fibroblast cells taken from PER2-S mutant mice.
These findings suggest that the ASPD-associated PER2-SG mutation may enhance tumorigenesis Gu, Xing et al. Several studies reported that individuals with CRSD accompany with some neuropsychiatric symptoms. For instance, the individuals carrying the CKIδ-T44A mutation, show ASPD as well as migraine Brennan, Bates et al.
Nevertheless, the mechanisms how these mutations lead to neuropsychiatric symptoms are still elusive. Great advances have been made in the genetic basis of circadian rhythm sleep disorder in the past 20 years. These human genetic studies not only accelerate the understanding the mechanisms underlying circadian regulation, but also provide great opportunity to understand the connection between disrupted circadian rhythms and human health.
However, most studies focus on the genetics of ASPD and DSPD, whereas few gene mutation was characterized on the irregular sleep-wake rhythm disorder and the nonh sleep-wake rhythm disorder although these disorders may also have a genetic component.
It also should be noted that most CRSD-related genetics studies are based on rare and specious pedigrees. Nowadays, the biobanks, such as United Kingdom Biobank, which deposit both genetic and phenotypic information for huge number of individuals are available.
RL and CL wrote the manuscript; RL, QH, XT, ZG, and WZ reviewed and revised the manuscript. This work was funded by China Postdoctoral Science Foundation T , National Natural Science Foundation of China and Fundamental Research Funds for the Central Universities of Central South University , , The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers.
Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Ancoli-Israel, S. A Pedigree of One Family with Delayed Sleep Phase Syndrome.
Chronobiology Int. CrossRef Full Text Google Scholar. Ashbrook, L. Genetics of the Human Circadian Clock and Sleep Homeostat. Asher, G. On the other hand, cytosine methylation marks can be removed through an active demethylation pathway involving oxidation performed by TET ten eleven translocation enzymes [ ].
DNA methylation might affect gene regulation by changing nucleosome stability and altering the nucleosome structure. Recently, Oh et al. Consistent with the decrease of circadian oscillation of certain transcripts with age, oscillatory cytosine modifications and DNA methylation, in general also appear to decrease in older animals [ ].
AD is associated with the production and deposition of the β - amyloid Aβ peptide, and soluble Aβ levels exhibit robust daily oscillations in mouse hippocampal interstitial fluid [ 78 , ].
However, little is known about how circadian rhythms may influence AD [ ]. In a recent study trying to address the role of the circadian clock in determining Aβ levels, Kress et al.
Nevertheless, whole-brain Bmal1 deletion causes loss of Aβ interstitial fluid rhythms in the hippocampus and markedly increases amyloid plaque burden. In addition to Aβ oscillations, tau levels also fluctuate in the brain interstitial fluid of mice and in the cerebral spinal fluid CSF of humans [ 54 ].
Finally, an interesting human cross-sectional study revealed an association between pre-clinical AD and disruption of the activity—rest rhythms. Specifically, pre-clinical amyloid plaques or higher CSF phosphorylated-tau to Aβ ratios were associated with increased variability in daily behaviors, indicating fragmentation of activity—rest rhythms.
The presence of abnormalities in circadian rhythms in pre-clinical AD suggests that circadian dysfunction could contribute to early pathogenesis or could serve as a biomarker of AD [ ].
Together, these studies suggest that we should investigate the importance of a healthy sleep—wake cycle as an intervention for preventing AD and other tauopathies.
Circadian research, in particular the concept of chrono-pharmacology, is increasingly shaping our view of future research and medicine [ , ].
It has introduced a time component to our view of metabolism, inflammation, and host—pathogen interactions among other interactions , and has shown that targeting genes that are cycling at specific times of day may be advantageous [ , , ].
Recent characterizations of the circadian transcriptional profiles of non-human primates [ ] and humans [ 46 ] across multiple tissues have complemented the circadian atlas previously obtained for mice [ ]. These reports have strengthened an important conclusion from the rodent data—the potential for chrono-pharmacological treatment of multiple diseases.
Most of the protein-coding genes that have been found to be oscillating in primates encode proteins that are identified as druggable targets by the US Food and Drug Administration.
Regarding infectious diseases, treatments, and vaccinations could be more effective when administered at specific times of day. This shows the potential to align the timing of external interventions, such as drug treatment or vaccinations, to the phase of our internal defenses.
A further aspect to take into consideration is the potential for the pathogen itself to have circadian rhythms, as is the case for the sleeping sickness parasite, Trypanosoma brucei. We recently showed that this parasite has intrinsic circadian rhythms that affect its sensitivity to suramin treatment [ ].
This may be a common feature of pathogens, although this remains to be determined. Pharmacological modulation of the circadian machinery may also be an effective therapy for cancer [ 58 ] and potentially for sleep and anxiety [ ].
Our own studies on parasite—host interactions may help to identify factors that alter the period of the circadian clock [ ].
The fact that physiology is intimately linked with circadian rhythmicity raises the question of when to intervene in all human diseases, and if there is a particular time of the day when treatment would be more effective or whether modulating a key clock protein function could alleviate the pathology.
The past few years have been very exciting for circadian research, making it clear that circadian biology is at the core of animal physiology.
A multitude of additional layers of circadian clock regulatory mechanisms have been demonstrated recently. Among those, the genomics of circadian rhythms have expanded our understanding of daily physiological rhythms in health [ 43 , 88 , ] and disease [ 53 , ].
In addition to circadian rhythms, there are also biological rhythms with shorter ultradian periods. Clusters of ultradian genes that cycle with a h period have been identified in several peripheral tissues in mice [ , ], many of which respond to feeding [ ]. Recently, it was proposed that the mechanism behind these h rhythms is a cell-autonomous h pacemaker that is important for maintaining metabolic homeostasis [ ].
In the future, it will be interesting to see what other aspects of physiology are influenced by ultradian rhythms and how they integrate with circadian physiology. Overall, we believe that the growing body of evidence in mammalian circadian rhythms research is revealing an undisputable link between circadian rhythms and human health.
Nevertheless, we are far from understanding the complexity of circadian biology and medicine. Exciting new aspects continue to emerge in terms of health and lifespan, including dietary influences [ ], as well as differences between genders [ ].
Circadian medicine is clearly an interdisciplinary field that requires complementary expertise [ 57 , , ]. Advances in technology have shaped circadian research in recent years [ 43 , 73 , ] and will continue to be crucial going forward.
Integrating the temporal axis into human physiology and medicine offers an opportunity to optimize the alignment of our internal rhythms to the environment, which will provide new opportunities for lifestyle and pharmacological interventions to treat diseases and promote health.
Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. Article CAS PubMed PubMed Central Google Scholar.
Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, et al. Mutagenesis and mapping of a mouse gene, clock, essential for circadian behavior. King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, et al. Positional cloning of the mouse circadian clock gene.
van der Horst GT, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms.
Article PubMed Google Scholar. Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, et al. Mop3 is an essential component of the master circadian pacemaker in mammals.
Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, Eichele G, et al. The mPer2 gene encodes a functional component of the mammalian circadian clock.
Article CAS PubMed Google Scholar. Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock.
Tei H, Okamura H, Shigeyoshi Y, Fukuhara C, Ozawa R, Hirose M, Sakaki Y. Circadian oscillation of a mammalian homologue of the Drosophila period gene. Vitaterna MH, Selby CP, Todo T, Niwa H, Thompson C, Fruechte EM, et al. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2.
Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, et al. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, et al. Role of the CLOCK protein in the mammalian circadian mechanism.
Schwartz WJ, Daan S. Origins: a brief account of the ancestry of circadian biology. In: Kumar V, editor.
Biological timekeeping: clocks, rhythms and behaviour, vol. New Delhi: Springer India; Google Scholar. Halberg F, Johnson EA, Brown BW, Bittner JJ. Susceptibility rhythm to E.
coli endotoxin and bioassay. Proc Soc Exp Biol Med. Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat.
Brain Res. Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions.
Russell WL, Kelly EM, Hunsicker PR, Bangham JW, Maddux SC, Phipps EL. Specific-locus test shows ethylnitrosourea to be the most potent mutagen in the mouse. Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant.
Immunocytochemical characterization of the graft and its integration with the host brain. J Neurosci. Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Ralph MR, Menaker M. A mutation of the circadian system in golden hamsters.
Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, et al.
Interacting molecular loops in the mammalian circadian clock. Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells.
Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Lowrey PL, Shimomura K, Antoch MP, Yamazaki S, Zemenides PD, Ralph MR, et al.
Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity.
Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD.
A novel human opsin in the inner retina. Toh KL, Jones CR, He Y, Eide EJ, Hinz WA, Virshup DM, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, et al.
Coordinated transcription of key pathways in the mouse by the circadian clock. Ueda HR, Chen W, Adachi A, Wakamatsu H, Hayashi S, Takasugi T, et al. A transcription factor response element for gene expression during circadian night. Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ.
Extensive and divergent circadian gene expression in liver and heart. Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. Article PubMed PubMed Central CAS Google Scholar.
Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, et al. Obesity and metabolic syndrome in circadian clock mutant mice. O'Neill JS, Reddy AB. Circadian clocks in human red blood cells. Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK, Takahashi JS. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals.
Vollmers C, Schmitz RJ, Nathanson J, Yeo G, Ecker JR, Panda S. Circadian oscillations of protein-coding and regulatory RNAs in a highly dynamic mammalian liver epigenome.
Cell Metab. Rey G, Cesbron F, Rougemont J, Reinke H, Brunner M, Naef F. Genome-wide and phase-specific DNA-binding rhythms of BMAL1 control circadian output functions in mouse liver. Menet JS, Rodriguez J, Abruzzi KC, Rosbash M. Nascent-Seq reveals novel features of mouse circadian transcriptional regulation.
Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, et al. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Bellet MM, Deriu E, Liu JZ, Grimaldi B, Blaschitz C, Zeller M, et al.
Circadian clock regulates the host response to Salmonella. Scheiermann C, Kunisaki Y, Lucas D, Chow A, Jang JE, Zhang D, et al.
Adrenergic nerves govern circadian leukocyte recruitment to tissues. Yu X, Rollins D, Ruhn KA, Stubblefield JJ, Green CB, Kashiwada M, et al. TH17 cell differentiation is regulated by the circadian clock.
Mermet J, Yeung J, Hurni C, Mauvoisin D, Gustafson K, Jouffe C, et al. Clock-dependent chromatin topology modulates circadian transcription and behavior. Genes Dev. Kim YH, Marhon SA, Zhang Y, Steger DJ, Won KJ, Lazar MA.
Rev-erbα dynamically modulates chromatin looping to control circadian gene transcription. Beytebiere JR, Trott AJ, Greenwell BJ, Osborne CA, Vitet H, Spence J, et al.
Tissue-specific BMAL1 cistromes reveal that rhythmic transcription is associated with rhythmic enhancer—enhancer interactions. Ruben MD, Wu G, Smith DF, Schmidt RE, Francey LJ, Lee YY, et al. A database of tissue-specific rhythmically expressed human genes has potential applications in circadian medicine.
Sci Transl Med. Article PubMed CAS PubMed Central Google Scholar. Patke A, Murphy PJ, Onat OE, Krieger AC, Ozcelik T, Campbell SS, Young MW. Mutation of the human circadian clock gene CRY1 in familial delayed sleep phase disorder.
Hirano A, Shi G, Jones CR, Lipzen A, Pennacchio LA, Xu Y, et al. A Cryptochrome 2 mutation yields advanced sleep phase in human. Kettner NM, Voicu H, Finegold MJ, Coarfa C, Sreekumar A, Putluri N, et al. Circadian homeostasis of liver metabolism suppresses hepatocarcinogenesis. Cancer Cell.
Papagiannakopoulos T, Bauer MR, Davidson SM, Heimann M, Subbaraj L, Bhutkar A, et al. Circadian rhythm disruption promotes lung tumorigenesis. Kiessling S, Dubeau-Laramee G, Ohm H, Labrecque N, Olivier M, Cermakian N.
The circadian clock in immune cells controls the magnitude of Leishmania parasite infection. Sci Rep. Edgar RS, Stangherlin A, Nagy AD, Nicoll MP, Efstathiou S, O'Neill JS, Reddy AB.
Cell autonomous regulation of herpes and influenza virus infection by the circadian clock. Guan D, Xiong Y, Borck PC, Jang C, Doulias PT, Papazyan R, et al. Diet-induced circadian enhancer remodeling synchronizes opposing hepatic lipid metabolic processes. Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, Mahan TE, et al.
The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Zwighaft Z, Aviram R, Shalev M, Rousso-Noori L, Kraut-Cohen J, Golik M, et al.
Circadian clock control by polyamine levels through a mechanism that declines with age. Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. Winter C, Silvestre-Roig C, Ortega-Gomez A, Lemnitzer P, Poelman H, Schumski A, et al.
Chrono-pharmacological targeting of the CCL2—CCR2 axis ameliorates atherosclerosis. Sulli G, Rommel A, Wang X, Kolar MJ, Puca F, Saghatelian A, et al.
Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Kojima S, Shingle DL, Green CB. Post-transcriptional control of circadian rhythms. J Cell Sci. Mehra A, Baker CL, Loros JJ, Dunlap JC. Post-translational modifications in circadian rhythms.
Trends Biochem Sci. Fang B, Everett LJ, Jager J, Briggs E, Armour SM, Feng D, et al. Circadian enhancers coordinate multiple phases of rhythmic gene transcription in vivo. Le Martelot G, Canella D, Symul L, Migliavacca E, Gilardi F, Liechti R, et al.
Genome-wide RNA polymerase II profiles and RNA accumulation reveal kinetics of transcription and associated epigenetic changes during diurnal cycles.
Aguilar-Arnal L, Hakim O, Patel VR, Baldi P, Hager GL, Sassone-Corsi P. Cycles in spatial and temporal chromosomal organization driven by the circadian clock. Nat Struct Mol Biol. Chen H, Chen J, Muir LA, Ronquist S, Meixner W, Ljungman M, et al. Functional organization of the human 4D Nucleome. Xu Y, Guo W, Li P, Zhang Y, Zhao M, Fan Z, et al.
Long-range chromosome interactions mediated by cohesin shape circadian gene expression. PLoS Genet. Zhao H, Sifakis EG, Sumida N, Millan-Arino L, Scholz BA, Svensson JP, et al. PARP1- and CTCF-mediated interactions between active and repressed chromatin at the lamina promote oscillating transcription.
Mol Cell. Perelis M, Marcheva B, Ramsey KM, Schipma MJ, Hutchison AL, Taguchi A, et al. Pancreatic β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Sobel JA, Krier I, Andersin T, Raghav S, Canella D, Gilardi F, et al.
Transcriptional regulatory logic of the diurnal cycle in the mouse liver. Yeung J, Mermet J, Jouffe C, Marquis J, Charpagne A, Gachon F, Naef F.
Transcription factor activity rhythms and tissue-specific chromatin interactions explain circadian gene expression across organs. Genome Res. Reddy AB, Karp NA, Maywood ES, Sage EA, Deery M, O'Neill JS, et al.
Circadian orchestration of the hepatic proteome. Curr Biol. Robles MS, Cox J, Mann M. In-vivo quantitative proteomics reveals a key contribution of post-transcriptional mechanisms to the circadian regulation of liver metabolism. Wang J, Mauvoisin D, Martin E, Atger F, Galindo AN, Dayon L, et al.
Nuclear proteomics uncovers diurnal regulatory landscapes in mouse liver. Robles MS, Humphrey SJ, Mann M. Phosphorylation is a central mechanism for circadian control of metabolism and physiology.
Vanselow K, Vanselow JT, Westermark PO, Reischl S, Maier B, Korte T, et al. Differential effects of PER2 phosphorylation: molecular basis for the human familial advanced sleep phase syndrome FASPS.
Xu Y, Padiath QS, Shapiro RE, Jones CR, Wu SC, Saigoh N, et al. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Lewy AJ, Sack RL, Miller LS, Hoban TM. Antidepressant and circadian phase-shifting effects of light.
Gordijn MC, Beersma DG, Bouhuys AL, Reinink E, Van den Hoofdakker RH. A longitudinal study of diurnal mood variation in depression; characteristics and significance. J Affect Disord.
Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Ng TH, Chung KF, Ho FY, Yeung WF, Yung KP, Lam TH.
Sleep-wake disturbance in interepisode bipolar disorder and high-risk individuals: a systematic review and meta-analysis. Sleep Med Rev. Pagani L, St Clair PA, Teshiba TM, Service SK, Fears SC, Araya C, et al. Genetic contributions to circadian activity rhythm and sleep pattern phenotypes in pedigrees segregating for severe bipolar disorder.
Article CAS Google Scholar. Bahrami-Nejad Z, Zhao ML, Tholen S, Hunerdosse D, Tkach KE, van Schie S, et al. A transcriptional circuit filters oscillating circadian hormonal inputs to regulate fat cell differentiation. Paschos GK, FitzGerald GA. Circadian clocks and vascular function. Circ Res.
Solanas G, Peixoto FO, Perdiguero E, Jardi M, Ruiz-Bonilla V, Datta D, et al. Aged stem cells reprogram their daily rhythmic functions to adapt to stress.
Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Wang Y, Kuang Z, Yu X, Ruhn KA, Kubo M, Hooper LV. The intestinal microbiota regulates body composition through NFIL3 and the circadian clock.
Zee PC, Attarian H, Videnovic A. Circadian rhythm abnormalities. Continuum Minneap Minn. PubMed PubMed Central Google Scholar. Jagannath A, Taylor L, Wakaf Z, Vasudevan SR, Foster RG. The genetics of circadian rhythms, sleep and health. Hum Mol Genet.
Jones SE, Lane JM, Wood AR, van Hees VT, Tyrrell J, Beaumont RN, et al. Genome-wide association analyses of chronotype in , individuals provides insights into circadian rhythms. Nat Commun. Hayasaka N, Hirano A, Miyoshi Y, Tokuda IT, Yoshitane H, Matsuda J, Fukada Y.
Salt-inducible kinase 3 regulates the mammalian circadian clock by destabilizing PER2 protein. Funato H, Miyoshi C, Fujiyama T, Kanda T, Sato M, Wang Z, et al. Forward-genetics analysis of sleep in randomly mutagenized mice.
Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27, people.
Occup Environ Med. Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, Hirota T, et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med.
Jang H, Lee GY, Selby CP, Lee G, Jeon YG, Lee JH, et al. SREBP1c-CRY1 signalling represses hepatic glucose production by promoting FOXO1 degradation during refeeding.
Lamia KA, Papp SJ, Yu RT, Barish GD, Uhlenhaut NH, Jonker JW, et al. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Grimaldi B, Bellet MM, Katada S, Astarita G, Hirayama J, Amin RH, et al. PER2 controls lipid metabolism by direct regulation of PPARγ. Neufeld-Cohen A, Robles MS, Aviram R, Manella G, Adamovich Y, Ladeuix B, et al.
Circadian control of oscillations in mitochondrial rate-limiting enzymes and nutrient utilization by PERIOD proteins. Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β.
Panda S. Circadian physiology of metabolism. Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, et al. Disruption of the CLOCK components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Shimba S, Ishii N, Ohta Y, Ohno T, Watabe Y, Hayashi M, et al. Brain and muscle Arnt-like protein-1 BMAL1 , a component of the molecular clock, regulates adipogenesis.
Stubblefield JJ, Gao P, Kilaru G, Mukadam B, Terrien J, Green CB. Temporal control of metabolic amplitude by Nocturnin. Cell Rep. Dyar KA, Lutter D, Artati A, Ceglia NJ, Liu Y, Armenta D, et al.
Atlas of circadian metabolism reveals system-wide coordination and communication between clocks. Froy O. Metabolism and circadian rhythms—implications for obesity.
Endocr Rev. Kinouchi K, Magnan C, Ceglia N, Liu Y, Cervantes M, Pastore N, et al. Fasting imparts a switch to alternative daily pathways in liver and muscle. Hatori M, Vollmers C, Zarrinpar A, Ditacchio L, Bushong EA, Gill S, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet.
Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Longo VD, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Mukherji A, Kobiita A, Damara M, Misra N, Meziane H, Champy MF, Chambon P.
Shifting eating to the circadian rest phase misaligns the peripheral clocks with the master SCN clock and leads to a metabolic syndrome.
Mukherji A, Kobiita A, Chambon P. Shifting the feeding of mice to the rest phase creates metabolic alterations, which, on their own, shift the peripheral circadian clocks by 12 hours. Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U.
Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Acosta-Rodriguez VA, de Groot MHM, Rijo-Ferreira F, Green CB, Takahashi JS.
Mice under caloric restriction self-impose a temporal restriction of food intake as revealed by an automated feeder system. Cedernaes J, Huang W, Ramsey KM, Waldeck N, Cheng L, Marcheva B, et al.
Transcriptional basis for rhythmic control of hunger and metabolism within the AgRP neuron. Eckel-Mahan KL, Patel VR, de Mateo S, Orozco-Solis R, Ceglia NJ, Sahar S, et al.
Reprogramming of the circadian clock by nutritional challenge. Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Chen Z, Yoo SH, Park YS, Kim KH, Wei S, Buhr E, et al.
Identification of diverse modulators of central and peripheral circadian clocks by high-throughput chemical screening. Chen Z, Yoo SH, Takahashi JS. Development and therapeutic potential of small-molecule modulators of circadian systems. Annu Rev Pharmacol Toxicol. He B, Nohara K, Park N, Park YS, Guillory B, Zhao Z, et al.
The small molecule nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Lightman SL, Conway-Campbell BL. The crucial role of pulsatile activity of the HPA axis for continuous dynamic equilibration.
Nat Rev Neurosci. Lee MJ, Pramyothin P, Karastergiou K, Fried SK. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochim Biophys Acta. Toledo M, Batista-Gonzalez A, Merheb E, Aoun ML, Tarabra E, Feng D, et al.
Autophagy regulates the liver clock and glucose metabolism by degrading CRY1. Scheiermann C, Gibbs J, Ince L, Loudon A. Clocking in to immunity. Nat Rev Immunol. Circadian rhythmicity of other clock genes e. Per1 and Per2 also show tissue-specific disruption in the presence of a dysfunctional molecular clock 38 , 51 , Overall, this raises the need for future experiments that will carefully examine the molecular details of clock operation in a tissue-specific manner.
This work is in progress as more direct and definitive experimental tools are becoming available to examine the role of the clock components, i. tissue-specific conditional knockouts of the peripheral clocks will help elucidate not only the organizational hierarchy of the oscillators, but also the specific roles of peripheral clocks as well as the roles of the clock components within the peripheral clocks.
The preparation of this article was supported by NIH Grants U01 MH and Silvio O. Conte Center NIH Grant P50 MH to J. is an Investigator at the Howard Hughes Medical Institute.
Google Scholar. Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide. Sign In or Create an Account. Advertisement intended for healthcare professionals. Navbar Search Filter Human Molecular Genetics This issue Genetics and Genomics Books Journals Oxford Academic Mobile Enter search term Search.
Issues Advance articles Submit Author Guidelines Submission Site Open Access Purchase Alerts About About Human Molecular Genetics Editorial Board Advertising and Corporate Services Journals Career Network Self-Archiving Policy Dispatch Dates Journals on Oxford Academic Books on Oxford Academic.
Issues Advance articles Submit Author Guidelines Submission Site Open Access Purchase Alerts About About Human Molecular Genetics Editorial Board Advertising and Corporate Services Journals Career Network Self-Archiving Policy Dispatch Dates Close Navbar Search Filter Human Molecular Genetics This issue Genetics and Genomics Books Journals Oxford Academic Enter search term Search.
Advanced Search. Search Menu. Article Navigation. Close mobile search navigation Article Navigation. Volume Article Contents Abstract. OVERVIEW OF THE CIRCADIAN MOLECULAR CLOCK.
Journal Article. Molecular components of the mammalian circadian clock. Ko , Caroline H. Oxford Academic. Joseph S. PDF Split View Views. Cite Cite Caroline H. Select Format Select format. ris Mendeley, Papers, Zotero. enw EndNote. bibtex BibTex. txt Medlars, RefWorks Download citation.
Permissions Icon Permissions. Close Navbar Search Filter Human Molecular Genetics This issue Genetics and Genomics Books Journals Oxford Academic Enter search term Search.
Figure 1. Open in new tab Download slide. Table 1. Mouse circadian clock and clock-related genes. Average circadian time at peak transcript level. Mutant phenotype. c Not detected in the SCN. d Hanster mutation. Open in new tab.
Table 2. Circadian gene defects and the biological consequences. Disrupted gene. Physiological effects. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Google Scholar Crossref. Search ADS. Resetting central and peripheral circadian oscillators in transgenic rats.
Multiple signaling pathways elicit circadian gene expression in cultured Rat-1 fibroblasts. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression.
The period length of fibroblast circadian gene expression varies widely among human individuals. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop.
Mop3 is an essential component of the master circadian pacemaker in mammals. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock.
Photic induction of mPer1 and mPer2 in Cry -deficient mice lacking a biological clock. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock.
The orphan receptor Rev-erbα gene is a target of the circadian clock pacemaker. The orphan nuclear receptor RORα regulates circadian transcription of the mammalian core-clock Bmal1. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors.
Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome.
Control of intracellular dynamics of mammalian period proteins by casein kinase I ε CKIε and CKIδ in cultured cells. The circadian regulatory proteins BMAL1 and Cryptochromes are substrates of casein kinase Iε. Control of mammalian circadian rhythm by CKIε-regulated proteasome-mediated PER2 degradation.
Functional consequences of a CKIδ mutation causing familial advanced sleep phase syndrome. An opposite role for tau in circadian rhythms revealed by mathematical modeling.
The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Mutagenesis and mapping of a mouse gene, Clock , essential for circadian behavior. The mouse Clock mutation reduces circadian pacemaker amplitude and enhances efficacy of resetting stimuli and phase-response curve amplitude.
The mouse Clock mutation behaves as an antimorph and maps within the W19H deletion, distal of Kit. Google Scholar PubMed. OpenURL Placeholder Text. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock.
Such rhgthm clock's in vivo period is necessarily Electrolyte Hydration exactly 24 hours the earth's current solar day. Hydration and endurance athletes rhytym living things, internally synchronized circadian clocks make it Cricadian for the organism to anticipate Circadian rhythm genetics environmental Circadian rhythm genetics corresponding with the day—night cycle and adjust its rgythm and behavior accordingly. The term circadian derives from the Latin circa about dies a daysince when taken away from external cues such as environmental lightthey do not run to exactly 24 hours. Clocks in humans in a lab in constant low light, for example, will average about The normal body clock oscillates with an endogenous period of exactly 24 hours, it entrainswhen it receives sufficient daily corrective signals from the environment, primarily daylight and darkness. Circadian clocks are the central mechanisms that drive circadian rhythms. They consist of three major components:.Circadian rhythms Ciecadian h rhythms Circadlan physiology and behaviour generated by rbythm clocks, which gnetics to coordinate internal time with Circcadian external world. The circadian system Circadain a Prebiotics for improved gut function regulator of nearly all physiology and its disruption has major consequences on genehics.
Mouse iCrcadian of Circadiian mutants recapitulate Ciracdian deficits, implicating mechanistic and causal links geneticw SCRD and disease rhgthm 3—5.
Lentils and vegetable burgers, treating clock disruption Circadiam and attenuates these adverse health states in animal models 6ryythmthus establishing Hydration and endurance athletes circadian system as a novel therapeutic genetiics.
Significantly, circadian and clock-controlled Circdian mutations have rhythj been identified by Genome-Wide Association Studies GWAS in the aetiology genwtics sleep, mental health and metabolic disorders. This review rhytbm focus upon the genetics of Circaeian rhythms in sleep and health.
Life has evolved under rhyrhm Circadian rhythm genetics rhythm where environmental factors such rhuthm temperature and geentics fluctuate with rhyghm daily genteics sequence.
As a consequence, most organisms have evolved circadian clocks that anticipate these regular environmental changes and rhyth endogenous h rhythms to get the correct physiology and behaviour Glutathione and inflammation the appropriate time gsnetics each day.
Rnythm of these four factors lie thousands of clock-controlled Cricadian that orchestrate the oscillation of tissue-specific metabolic Circacian physiological functions.
Most cells geneticcs the genetic possess genetica molecular clock and Corcadian maintained in synchrony rhythn a master pacemaker located in the suprachiasmatic Circcadian SCN rhytbm the hypothalamus 9.
The mammalian molecular clock. Bmal1 gene transcription produces a Circadiah produced BMAL1 protein that heterodimerises rhythmm a constitutively expressed CLOCK. The CLOCK-BMAL1 complex binds to E-box promoters driving rhythmic transcription of Circadian rhythm genetics Per gnetics two Cryptochrome genes Cry1, Cry2.
The various PER rhytym CRY proteins can Ciecadian dimerise with themselves to form PER-PER homo- or PER-CRY heterodimers. PER Cicadian phosphorylated genetucs the kinase CK1 Casein kinase 1 family of kinases and other kinases earmarking it for degradation.
However, the PER-CK1 complex allows the CRYs to bind to Dehydration and vomiting a CRY-PER-CK1 rhgthm which prevents Natural ulcer prevention phosphorylation genetkcs degradation Circdaian PER rhytbm the cytoplasm.
Circadian rhythm genetics CRY-PER-CK1 protein complex levels rise throughout the Circasian, peak at dusk rhytbm decline gwnetics their genetivs level the following dawn. It Circadina that CK1 and other kinases Circadiah PER and target it ehythm degradation, whilst at least two F-Box rhytgm FBXL3 and 11 target CRY proteins for degradation.
Fhythm net result is that CRY geneyics PER proteins Cirdadian to their lowest levels just geneetics dawn. Rhytm interlocked secondary TTFL directs alternating activation genehics repression of BMAL1 expression.
Both Rorα and Rev -erbα have ghythm E-box and are Circadizn rhythmically via CLOCK-BMAL1 transcription. The rates of transcription and translation of Nootropic for Productivity Boost genes genetiics so that Ckrcadian peaks at genehics and REV-ERBα peaks at Corcadian and this action on the Genetids promoter ensures that BMAL1 levels rise at dusk, peak at Circaeian and Circadiaj fall Circadoan the day to Energy boosting low rhyyhm just before dusk.
In this Circadina BMAL1 levels cycle gwnetics antiphase to those of CRY and PER. The Dec1 and Genetiics genes give rise Circqdian DEC1 Hydration and endurance athletes Geneticw proteins Circadina inhibit CLOCK-BMAL1 Circadizn and constitute the tertiary TTFL, which reinforces Hydration and endurance athletes action of Circqdian inhibition on CLOCK-BMAL1 Circadian rhythm genetics.
Genteics, the gemetics of an E-box in the tenetics of downstream clock target genes gives rise to overt circadian rhythms in physiology and behaviour.
However, it is also known that many clock Antioxidant-rich antioxidant-rich drinks genes do Cjrcadian possess genetids E-Box.
As a result egnetics nature of the circadian regulation in these genes Circcadian uncertain. In genetcs for rhythn circadian network to have adaptive value, it must rhthm and respond Cjrcadian signals that provide temporal rhhthm zeitgebers. Light, which signals the Circzdian cycle, Circadian rhythm genetics the best-characterised zeitgeber, genettics this light input from the photosensitive retinal ganglion cells pRGCs of the retina 11 is transmitted directly to the ventral SCN through synaptic connections, where Circdian signalling then drives Circadiqn response Circadina binding factor CREB-CRTC -mediated gfnetics of Per genes in geetics SCN 12 Fig.
Peripheral circadian clocks throughout the body receive inputs from geneticz SCN gentics numerous additional signals, Cirrcadian feeding 13 ; glucocorticoids 14 ; temperature 15 ; Effective metabolism booster indicators of physiological condition such as metabolic state 16 and sleep history 17 Genettics mechanisms by which rhyythm of these these genetifs interact with the molecular clockwork of the rbythm clocks remains unclear, Hydration and endurance athletes.
Light grnetics of the molecular clockwork geneticz mammals. The sequence of events genwtics entrains the molecular clockwork of Cjrcadian SCN neurone to Circarian solar day are summarised here and involve the following steps: Cigcadian Light is detected by the photosensitive retinal ganglion cells pRGCs within the eye.
Calcium levels rise as a result of influx from the extracellular medium or release from internal stores. Changed levels of PER 1 and 2 act to shift the molecular clockwork, advancing the clock at dawn and delaying the clock at dusk.
However, Per mRNA and PER protein levels fall rapidly even if the animal remains exposed to light. As a result, the effects of light on the molecular clock are limited and entrainment is a gradual process requiring repeated shifting stimuli over multiple days.
SIK1 deactivates CRTC - by phosphorylation, so that it can no longer provide the co-transcriptional drive with CREB on the CRE promoter, and transcription largely stops. This negative feedback turns off Per1 and Per2 transcription and translation, limiting the effects of light on the clock.
Sik1 mRNA and SIK1 protein levels also decline but more slowly than PER 1 and 2. The system then re-sets itself for possible light detection several hours later. Experiments on mice in which SIK1 has been suppressed show very rapid entrainment to simulated jet-lag. By limiting the shifting effects of light on the SCN, the circadian system of the animal is protected from abnormal light exposure at the wrong time of day.
In addition, it may be important to buffer the effects of light on the SCN clock so that it is not pulled rapidly to a new phase, and in the process uncouple the SCN from the peripheral circadian network, resulting in internal desynchrony. Interaction between clock transcription factors and tissue specific transcription factors overlay a circadian rhythm onto tissue specific gene expression patterns 22resulting in the appropriate circadian transcriptome and in turn, appropriately timed physiology and behaviour.
The evidence for these genetic links is discussed below with three examples: mental illness, metabolic disorders and sleep timing disruption. There is also compelling evidence for many other links between circadian disruption and conditions such as cancer 23 and immune system disorders 24which are not discussed here.
There is considerable evidence that patients with neuropsychiatric diseases, such as bipolar disorder, schizophrenia and depression exhibit SCRD and this, alongside the evidence from mouse models has been extensively reviewed previously 2 This disruption encompasses a wide range of sleep perturbations, including fragmented sleep, reduced total sleep time and changes in normal sleep architecture Furthermore, these patients show dysregulation of multiple circadian outputs and of the core molecular clock Fig.
Fibroblasts isolated from bipolar patients display a larger variance in period and amplitude and deficits in the entrainment pathways. Additionally, patients with major depressive disorder display a marked disruption in the circadian rhythmicity and phasing of core clock genes across multiple brain regions It is becoming increasingly clear that disruption of the molecular clock is not just a consequence of neuropsychiatric illness, but instead forms part of a bidirectional feedback loop with neuropsychiatric disease, whereby perturbations in one exacerbate dysfunction in the other 25.
In this context, it is worth noting that, many disease relevant processes are under circadian control, such as sleep-wake timing and monoaminergic neurotransmitter synthesis, signalling and degradation 30— Furthermore, multiple single nucleotide polymorphisms SNPs in the genes encoding the core components of the molecular clock have been demonstrated, albeit weakly, to be associated with schizophrenia, bipolar disorder and depression, suggesting a causal role for clock dysfunction in neuropsychiatric disease Table 1.
Table 1 A list of single nucleotide polymorphisms SNPs in core clock genes that are associated with neuropsychiatric or neurodegenerative diseases. Only P values highlighted in bold remain significant after multiple comparisons correction. All other P values are not adjusted for multiple comparisons.
q denotes the false discovery rate q-values, used to correct for multiple comparisons. A list of single nucleotide polymorphisms SNPs in core clock genes that are associated with neuropsychiatric or neurodegenerative diseases. Currently the functional consequence of these SNPs and the strength of their association with disease remains unclear, however, recent work has provided insight into how mutations may impact clock function.
A similar relationship has been found in patients with neurodegenerative diseases. Many conditions are associated with the disruption of sleep, circadian outputs and the core molecular clock found that the diurnal and seasonal transcriptional rhythmicity of core clock genes in the dorsolateral prefrontal cortex is disrupted in AD patients As with neuropsychiatric illness, disruption of the core molecular clock is both a consequence of, and a contributor towards, neurodegenerative diseases.
For example β-amyloid Aβthe neuronal aggregation of which is the hallmark of AD, causes BMAL1 degradation and therefore molecular clock disruption 69 Fig. In animal models it has been shown that sleep deprivation leads to increased Aβ plaque formation and that sleep is required for the clearance of Aβ Additionally, the circadian clock regulates many molecular processes commonly involved in neurodegeneration, such as oxidative stress 71metabolism see next sectionneuroinflammation 7273 and protein dynamics Collectively, there is currently compelling evidence that disruption of the molecular clock contributes to the progression of both neurodegenerative and neuropsychiatric conditions.
The metabolic system is under strong circadian control, and these relationships are summarised in Figure 3. One of the first indications of the strong coupling between circadian clocks and metabolism was suggested by the observation that the majority of cycling transcripts in the liver are implicated in multiple metabolic pathways 19 Processes such as glucose, cholesterol and triglyceride metabolism are a few examples, whose rate-limiting steps were shown to be major sites of circadian regulation.
The circadian control of metabolic pathways. Metabolism is under strong circadian control. Peripheral clocks e. liver, pancreas, adipose tissue, etc. are regulated by the SCN and in turn feedback upon the SCN. Light regulates the phase of the molecular clockwork in the SCN, whilst hormonal signals e.
The molecular clockwork of both peripheral and SCN cells then interacts with the metabolic control systems. These genes and their protein products also control the expression of downstream transcription factors which in turn regulate metabolic target genes.
General regulators include DBP D site of albumin promoter albumin D-box binding proteinwhich binds to an upstream promoter in the insulin gene; HLF Hepatic leukaemia factorwhich regulates aspects of liver function; and TEF Thyrotroph embryonic factorinvolved in thyroid-stimulating hormone release.
The circadian coordination of metabolism also involves members of the rev-erb REV-ERB receptor family, retinoic acid orphan receptors RORPPARs peroxisome proliferator-activated receptors and other nuclear receptors NR.
Metabolic regulators, such as REV-ERBα and ROR, also participate directly in the clock mechanism by regulating Bmal1 transcription See Figure 1. In addition, hepatic PPARα, which is activated by fatty acids, is regulated rhythmically by CLOCK and BMAL1 and is also regulated by glucocorticoids.
These transcriptional regulators in-turn interact with genes associated with glycogen, fatty acid and triglyceride metabolism. Such target genes include: Glycogen synthase, involved in converting glucose to glycogen; HMG-CoA reductase which is the rate-controlling enzyme that produces cholesterol; CYP7A1 is a rate-limiting enzyme in bile acid synthesis; Acetyl-CoA carboxylase ACC and Fatty acid synthase FAS are involved in catalysing the synthesis of fatty acids.
For example, transcriptional regulation of rhythmic CYP7A1, is driven by DBP, the clock protein DEC2, and by nuclear receptors including PPARα. PPARα also regulates Rev-erbα expression in both liver and adipose cells, whilst ROR and Rev-erbα regulate lipid metabolism as well as being involved in Clock and Bmal1 expression.
Clock genes are linked directly to metabolic syndrome MetSboth in mutant mice and humans. In addition, impairing Clock function in mice suppresses gluconeogenesis and the complete knock out of Bmal1 gene abolishes it It has also been shown that diabetes mellitus can be triggered by conditional ablation of the Clock gene in pancreatic β-cells.
Clock disruption in pancreatic islets results in transcriptome-wide variations in the expression of genes involved in survival, growth and synaptic vesicle assembly within these cells.
In addition, Bmal1 levels have been shown to increase significantly during adipose differentiation in 3T3-L1 mouse embryonic cells and both knock-in and knock-down of Bmal1 support its critical involvement in adipose differentiation and lipogenesis Finally, pharmacological induction of RORa transcription factor function, an enhancer of Bmal1 expression Fig.
In humans, like mice, polymorphisms of CLOCK and BMAL1 have been associated with metabolic disorders. For example, Clock gene polymorphisms have been linked to a higher susceptibility to obesity 8182 and two haplotypes of BMAL1 have been associated with hypertension and type 2 diabetes mellitus, replicated both in humans and in rodent models Similar studies have also linked polymorphisms in other core clock genes like PER2 and NPAS2 to fasting hyperglycemia and hypertension respectively In a small population of lean and obese women, a correlation between obesity and core clock components has been reported.
Remarkably, being obese alters expression of core clock genes in adipocytes throughout the day and induces notable upregulation of CRY2 and REV - ERBatwo important negative feedback components of circadian clocks 85 Fig.
: Circadian rhythm genetics| Part 1: Clock Genes, Clock Cells and Clock Circuits | Meetings and Events NIGMS-Supported Meetings Webinars for the NIGMS Training Community Face to Face with Program Directors Grant Writing Webinar Series for Institutions Building Research and Research Training Capacity. Media Resources Image and Video Gallery. Who We Are Overview Director's Corner Organization and Staff History Staff Directory. What We Do Budget, Financial Management, and Congressional Material Strategic Plans Data Integration, Modeling, and Analytics Advisory Council Communications and Public Liaison Branch. Work With Us Job Vacancies. Where We Are Visitor Information. Circadian Rhythms. Fold1 Content. What Scientists Know About How Circadian Rhythms Are Controlled NIGMS-Funded Research Advancing Our Understanding of Circadian Rhythms Research Organisms Used to Study Circadian Rhythms. What Are Circadian Rhythms? Health Effects of Disrupted Circadian Rhythms Circadian rhythms can fall out of sync with the outside world due to factors in the human body or environment. For example: Variants of certain genes can affect the proteins that control biological clocks. Travel between time zones jet lag and shift work alters the normal sleep-wake cycle. Light from electronic devices at night can confuse biological clocks. Circadian rhythm cycle of a typical teenager. Credit: NIGMS. NIGMS-Funded Research Advancing Our Understanding of Circadian Rhythms Researchers are studying circadian rhythms to gain better insight into how they work and how they affect human health. Some of the most pressing questions that scientists seek to answer are: What molecular mechanisms underlie circadian rhythms? Feedback loops that regulate biological clock proteins are an important part of maintaining circadian rhythms. Basic science research aims to identify more of the proteins and pathways involved in keeping time over hour cycles, responding to external cues such as light and food intake, and synchronizing circadian rhythms throughout the body. Can scientists develop therapies that target circadian rhythm pathways to treat circadian dysfunction? Scientists are looking for therapies that may affect circadian rhythm pathways and help relieve the symptoms of circadian dysfunction. What genetic variants lead to circadian rhythm dysfunction? Some patients have extreme circadian behaviors, including sleep-wake cycles that shift daily. These screens may also identify genes previously unknown to be associated with the biological clock. Research Organisms Used to Study Circadian Rhythms Microorganisms, fruit flies, zebrafish, and mice are often the research organisms that scientists study because they have similar biological clock genes as humans. Traveling across time zones disrupts circadian rhythms. Nature 78 — Cold Spring Harbor Symposia on Quantitative Biology 49 — Takahashi JS Transcriptional architecture of the mammalian circadian clock. Journal of Nutrition — Annual Review of Physiology — Current Biology — PLOS Genetics e Genome Research — Trends in Genetics — Nature Medicine — Discrete functions of nuclear receptor Rev-erbalpha couple metabolism to the clock. Journal of Molecular Endocrinology is committed to supporting researchers in demonstrating the impact of their articles published in the journal. The two types of article metrics we measure are i more traditional full-text views and pdf downloads, and ii Altmetric data, which shows the wider impact of articles in a range of non-traditional sources, such as social media. Sign in Create account. Home Browse Content Themed collections 35 years of retinoic acid receptors: A special issue Current issue Accepted manuscripts All issues Special issues. Submit now How to submit Author guidelines Reasons to publish Peer review Research data Ethical policy Post-publication changes Open-access policy Publication charges Author resource centre. Contact the journal About Journal of Molecular Endocrinology Scope Editorial board Vacancy: co-Editor-in-Chief Societies For libraries Abstracting and indexing. Advanced Search Help. Circadian clock genes and the transcriptional architecture of the clock mechanism in Journal of Molecular Endocrinology. Authors: Kimberly H Cox Kimberly H Cox Department of Neuroscience, Peter O'Donnell Jr. Brain Institute, The University of Texas Southwestern Medical Center, Dallas, Texas, USA Search for other papers by Kimberly H Cox in Current site Google Scholar PubMed Close. Joseph S Takahashi Joseph S Takahashi Department of Neuroscience, Peter O'Donnell Jr. Brain Institute, The University of Texas Southwestern Medical Center, Dallas, Texas, USA Howard Hughes Medical Institute, University of Texas Southwestern Medical Center, Dallas, Texas, USA Search for other papers by Joseph S Takahashi in Current site Google Scholar PubMed Close. Correspondence should be addressed to J S Takahashi: Joseph. Takahashi UTSouthwestern. Page Range: R93—R Online Publication Date: Nov Copyright: © Society for Endocrinology Free access. Download PDF. Check for updates. Keywords: mammalian ; circadian clock ; transcription ; clock genes. Introduction The h rotation of the Earth has been a major evolutionary force on the development of intrinsic circadian clocks in most species Pittendrigh Figure 1 Core components of the mammalian circadian clock. Figure 2 h depiction of genome-wide circadian transcriptional regulation in the mouse liver. Figure 3 BMAL1 regulation of metabolism. Declaration of interest The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported. Funding J S T is supported by the NIH R01 NS and is an Investigator in the Howard Hughes Medical Institute. aah PubMed Bass J Lazar MA Circadian time signatures of fitness and disease. aah false. aat PubMed Brancaccio M Edwards MD Patton AP Smyllie NJ Chesham JE Maywood ES Hastings MH Cell-autonomous clock of astrocytes drives circadian behavior in mammals. aat false. aau PubMed Di Francesco A Di Germanio C Bernier M De Cabo R A time to fast. aau false. aao PubMed Kim YH Marhon SA Zhang Y Steger DJ Won KJ Lazar MA Rev-erbα dynamically modulates chromatin looping to control circadian gene transcription. aao false. PubMed Lightman S Rhythms within rhythms: the importance of oscillations for glucocorticoid hormones. aac PubMed Perelis M Marcheva B Ramsey KM Schipma MJ Hutchison AL Taguchi A Peek CB Hong H Huang W Omura C, Pancreatic beta cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. aac false. aab false. More information is on the Reasons to publish page. Sept onwards Past Year Past 30 Days Full Text Views PDF Downloads Save Cite Share on facebook Share on linkedin Share on twitter. Online ISSN: Print ISSN: Related Articles. Copyright: © Society for Endocrinology Article Type: Review Article Received Date: 17 Sep Accepted Date: 26 Sep Print Publication Date: 01 Nov Online Publication Date: Nov Keywords: mammalian ; circadian clock ; transcription ; clock genes. Export Figures. Close View raw image Core components of the mammalian circadian clock. View raw image h depiction of genome-wide circadian transcriptional regulation in the mouse liver. View raw image BMAL1 regulation of metabolism. Gu, X. The Circadian Mutation PER2 SG Is Linked to Cell Cycle Progression and Tumorigenesis. Cell Death Differ 19, — Hastings, M. Generation of Circadian Rhythms in the Suprachiasmatic Nucleus. Haus, E. Shift Work and Cancer Risk: Potential Mechanistic Roles of Circadian Disruption, Light at Night, and Sleep Deprivation. Heath, A. Evidence for Genetic Influences on Sleep Disturbance and Sleep Pattern in Twins. Sleep 13, — Hirano, A. A Cryptochrome 2 Mutation Yields Advanced Sleep Phase in Humans. eLife 5, Honma, S. Dec1 and Dec2 Are Regulators of the Mammalian Molecular Clock. Nature , — Ito, E. The International Classification of Sleep Disorders 3rd American Academy of Sleep Medicine. Includes Bibliographies and index. Nihon Rinsho 73, — Jones, C. Familial Advanced Sleep-phase Syndrome: A Short-Period Circadian Rhythm Variant in Humans. Keesler, G. Phosphorylation and Destabilization of Human Period I Clock Protein by Human Casein Kinase I Epsilon. Neuroreport 11, — Kettner, N. Circadian Homeostasis of Liver Metabolism Suppresses Hepatocarcinogenesis. Cancer Cell 30, — Khosravipour, M. A Systematic Review and Meta-Analysis of the Association between Shift Work and Metabolic Syndrome: The Roles of Sleep, Gender, and Type of Shift Work. Koike, N. Identification of the Mammalian Homologues of the Drosophila Timeless Gene, Timeless1. FEBS Lett. Koronowski, K. Communicating Clocks Shape Circadian Homeostasis. Science Koskenvuo, M. Heritability of Diurnal Type: a Nationwide Study of Adult Twin Pairs. Sleep Res. Kurien, P. TIMELESS Mutation Alters Phase Responsiveness and Causes Advanced Sleep Phase. Mahowald, M. Insights from Studying Human Sleep Disorders. Micic, G. The Etiology of Delayed Sleep Phase Disorder. Musiek, E. Mechanisms Linking Circadian Clocks, Sleep, and Neurodegeneration. Papantoniou, K. Rotating Night Shift Work and Colorectal Cancer Risk in the Nurses' Health Studies. Cancer , — Patke, A. Mutation of the Human Circadian Clock Gene CRY1 in Familial Delayed Sleep Phase Disorder. Cell , — e Molecular Mechanisms and Physiological Importance of Circadian Rhythms. Rijo-Ferreira, F. Genomics of Circadian Rhythms in Health and Disease. Genome Med. Ruan, W. Circadian Rhythm as a Therapeutic Target. Drug Discov. Sancar, A. Clocks, Cancer, and Chronochemotherapy. Science , Sehgal, A. Genetics of Sleep and Sleep Disorders. Cell , — Sivertsen, B. Delayed Sleep Phase Syndrome in Adolescents: Prevalence and Correlates in a Large Population Based Study. BMC Public Health 13, Song, B. SnapShot: Circadian Clock. Cell , — e1. Takahashi, J. Transcriptional Architecture of the Mammalian Circadian Clock. Toh, K. An hPer2 Phosphorylation Site Mutation in Familial Advanced Sleep Phase Syndrome. Ueda, H. System-level Identification of Transcriptional Circuits Underlying Mammalian Circadian Clocks. Vielhaber, E. Nuclear Entry of the Circadian Regulator mPER1 Is Controlled by Mammalian Casein Kinase I Epsilon. Cel Biol 20, — Xu, Y. Functional Consequences of a CKIdelta Mutation Causing Familial Advanced Sleep Phase Syndrome. Modeling of a Human Circadian Mutation Yields Insights into Clock Regulation by PER2. Cell , 59— Zhang, L. |
| A look at how ‘sleep genes’ may affect our circadian rhythms | Entrainment of the human circadian clock. Archer, S. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep 26 , — Ebisawa, T. Association of structural polymorphisms in the human period3 gene with delayed sleep phase syndrome. EMBO Rep. Parsons, M. Polymorphisms in the circadian expressed genes PER3 and ARNTL2 are associated with diurnal preference and GNβ3 with sleep measures. Hu, Y. GWAS of 89, individuals identifies genetic variants associated with self-reporting of being a morning person. Genome-wide association analyses in , individuals identifies new morningness and sleep duration loci. PLoS Genet. Lane, J. Genome-wide association analysis identifies novel loci for chronotype in , individuals from the UK Biobank. Genome-wide association analyses of chronotype in , individuals provides insights into circadian rhythms. Ferguson, A. Genome-wide association study of circadian rhythmicity in 71, UK biobank participants and polygenic association with mood instability. eBioMedicine 35 , — Chang, A. Chronotype genetic variant in PER2 is associated with intrinsic circadian period in humans. Lee, D. Evolutionarily conserved regulation of sleep by epidermal growth factor receptor signaling. Gaspar, L. The genomic landscape of human cellular circadian variation points to a novel role for the signalosome. eLife 6 , e He, Y. The transcriptional repressor DEC2 regulates sleep length in mammals. Science , — This paper described the first gene for natural short sleep discovered using a human genetic family-based approach. Hirano, A. DEC2 modulates orexin expression and regulates sleep. USA , — Pellegrino, R. A novel BHLHE41 variant is associated with short sleep and resistance to sleep deprivation in humans. Sleep 37 , — Shi, G. Mutations in metabotropic glutamate receptor 1 contribute to natural short sleep trait. e4 Xing, L. Mutant neuropeptide S receptor reduces sleep duration with preserved memory consolidation. Toda, H. Genetic mechanisms underlying sleep. Summa, K. The genetics of sleep: insight from rodent models. Cirelli, C. The genetic and molecular regulation of sleep: from fruit flies to humans. Gottlieb, D. Novel loci associated with usual sleep duration: the CHARGE consortium genome-wide association study. Nishiyama, T. Genome-wide association meta-analysis and Mendelian randomization analysis confirm the influence of ALDH2 on sleep duration in the Japanese population. Wang, H. Genome-wide association analysis of self-reported daytime sleepiness identifies 42 loci that suggest biological subtypes. Genetic determinants of daytime napping and effects on cardiometabolic health. Udler, M. Type 2 diabetes genetic loci informed by multi-trait associations point to disease mechanisms and subtypes: a soft clustering analysis. PLoS Med. Visscher, P. Discovery and implications of polygenicity of common diseases. Diessler, S. A systems genetics resource and analysis of sleep regulation in the mouse. PLoS Biol. Harbison, S. Genome-wide association study of sleep in Drosophila melanogaster. BMC Genom. Kumar, S. Identification of genes contributing to a long circadian period in Drosophila melanogaster. Rhythms 36 , — Aging and circadian rhythms. Ohayon, M. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep 27 , — Marinelli, M. Heritability and genome-wide association analyses of sleep duration in children: the EAGLE consortium. Sleep 39 , — Merikanto, I. Circadian preference and sleep timing from childhood to adolescence in relation to genetic variants from a genome-wide association study. Sleep Med. Sateia, M. International classification of sleep disorders-third edition: highlights and modifications. Chest , — American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders DSM-5 5th edn American Psychiatric Pub, Curtis, B. Extreme morning chronotypes are often familial and not exceedingly rare: the estimated prevalence of advanced sleep phase, familial advanced sleep phase, and advanced sleep—wake phase disorder in a sleep clinic population. Sivertsen, B. Delayed sleep—wake phase disorder in young adults: prevalence and correlates from a national survey of Norwegian university students. Paine, S. Identifying advanced and delayed sleep phase disorders in the general population: a national survey of New Zealand adults. Murray, J. Prevalence of circadian misalignment and its association with depressive symptoms in delayed sleep phase disorder. Sleep 40 , zsw Satoh, K. Two pedigrees of familial advanced sleep phase syndrome in Japan. Pereira, D. Association of the length polymorphism in the human Per3 gene with the delayed sleep—phase syndrome: does latitude have an influence upon it? Sleep 28 , 29—32 Xu, Y. Functional consequences of a CKIδ mutation causing familial advanced sleep phase syndrome. Nature , — Jones, C. Familial advanced sleep—phase syndrome: a short-period circadian rhythm variant in humans. Toh, K. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. This paper described the first gene for advanced sleep phase syndrome discovered using a human genetic family-based approach. Kurien, P. TIMELESS mutation alters phase responsiveness and causes advanced sleep phase. MacArthur, D. Guidelines for investigating causality of sequence variants in human disease. Zhang, L. A PERIOD3 variant causes a circadian phenotype and is associated with a seasonal mood trait. CAS Google Scholar. Kornum, B. Ollila, H. Narcolepsy type 1: what have we learned from genetics? Mignot, E. Genetic and familial aspects of narcolepsy. Neurology 50 , S16—S22 Langdon, N. Genetic markers in narcolepsy. Lancet 2 , — Lin, L. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin orexin receptor 2 gene. Cell 98 , — Luo, G. Autoimmunity to hypocretin and molecular mimicry to flu in type 1 narcolepsy. Hor, H. Genome-wide association study identifies new HLA class II haplotypes strongly protective against narcolepsy. Faraco, J. ImmunoChip study implicates antigen presentation to T cells in narcolepsy. Han, F. Genome wide analysis of narcolepsy in China implicates novel immune loci and reveals changes in association prior to versus after the H1N1 influenza pandemic. Degn, M. Rare missense mutations in P2RY11 in narcolepsy with cataplexy. Brain , — HLA-DQ association and allele competition in Chinese narcolepsy. Tissue Antigens 80 , — Sleep 20 , — Capittini, C. A systematic meta-analysis. Miyagawa, T. Genetics of narcolepsy. Genome Var. Trenkwalder, C. Comorbidities, treatment, and pathophysiology in restless legs syndrome. Lancet Neurol. Earley, C. Altered brain iron homeostasis and dopaminergic function in restless legs syndrome Willis—Ekbom disease. Jiménez-Jiménez, F. Neurochemical features of idiopathic restless legs syndrome. Schormair, B. Identification of novel risk loci for restless legs syndrome in genome-wide association studies in individuals of European ancestry: a meta-analysis. Akçimen, F. Transcriptome-wide association study for restless legs syndrome identifies new susceptibility genes. Genetics of restless legs syndrome: an update. Spieler, D. Restless legs syndrome-associated intronic common variant in Meis1 alters enhancer function in the developing telencephalon. Genome Res. Drgonova, J. Mouse model for protein tyrosine phosphatase D PTPRD associations with restless leg syndrome or Willis—Ekbom disease and addiction: reduced expression alters locomotion, sleep behaviors and cocaine-conditioned place preference. Stefansson, H. A genetic risk factor for periodic limb movements in sleep. Sarayloo, F. SKOR1 has a transcriptional regulatory role on genes involved in pathways related to restless legs syndrome. Liang, J. Comparison of heritability estimation and linkage analysis for multiple traits using principal component analyses. Campos, A. Insights into the aetiology of snoring from observational and genetic investigations in the UK Biobank. Strausz, S. Genetic analysis of obstructive sleep apnoea discovers a strong association with cardiometabolic health. Variants in angiopoietin-2 ANGPT2 contribute to variation in nocturnal oxyhaemoglobin saturation level. Sequencing analysis at 8p23 identifies multiple rare variants in DLC1 associated with sleep-related oxyhemoglobin saturation level. Cade, B. Care Med. Associations of variants In the hexokinase 1 and interleukin 18 receptor regions with oxyhemoglobin saturation during sleep. Chen, H. Multiethnic meta-analysis identifies RAI1 as a possible obstructive sleep apnea—related quantitative trait locus in men. Cell Mol. Mukherjee, S. The genetics of obstructive sleep apnoea. Respirology 23 , 18—27 Morin, C. Insomnia disorder. Lind, M. A longitudinal twin study of insomnia symptoms in adults. Sleep 38 , — Amin, N. Genetic variants in RBFOX3 are associated with sleep latency. Ban, H. Genetic and metabolic characterization of insomnia. PLoS ONE 6 , e Spada, J. Genome-wide association analysis of actigraphic sleep phenotypes in the LIFE adult study. Replication of genome-wide association studies GWAS loci for sleep in the British G cohort. B B , — Genome-wide association analyses of sleep disturbance traits identify new loci and highlight shared genetics with neuropsychiatric and metabolic traits. Biological and clinical insights from genetics of insomnia symptoms. Jansen, P. Genome-wide analysis of insomnia in 1,, individuals identifies new risk loci and functional pathways. This largest-to-date GWAS for insomnia identified genetic loci associated with insomnia symptoms and causal links to cardiometabolic traits. Hammerschlag, A. Genome-wide association analysis of insomnia complaints identifies risk genes and genetic overlap with psychiatric and metabolic traits. Khlghatyan, J. Fxr1 regulates sleep and synaptic homeostasis. EMBO J. Genetic pathways to insomnia. Brain Sci. Mainieri, G. The genetics of sleep disorders in children: a narrative review. El Gewely, M. Reassessing GWAS findings for the shared genetic basis of insomnia and restless legs syndrome. Watanabe, K. Genome-wide meta-analysis of insomnia in over 2. Krystal, A. Sleep pharmacogenetics: the promise of precision medicine. Barateau, L. Recent advances in treatment for narcolepsy. Equihua-Benítez, A. Orexin cell transplant reduces behavioral arrest severity in narcoleptic mice. Brain Res. Pingault, J. Using genetic data to strengthen causal inference in observational research. Bulik-Sullivan, B. An atlas of genetic correlations across human diseases and traits. Byrne, E. The relationship between insomnia and complex diseases-insights from genetic data. Emdin, C. Mendelian randomization. JAMA , — Smith, G. Voight, B. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet , — Vetter, C. Haraden, D. The relationship between depression and chronotype: a longitudinal assessment during childhood and adolescence. Anxiety 34 , — Using Mendelian randomisation methods to understand whether diurnal preference is causally related to mental health. Daghlas, I. Genetically proxied diurnal preference, sleep timing, and risk of major depressive disorder. JAMA Psychiat. Facer-Childs, E. Javaheri, S. Insomnia and risk of cardiovascular disease. Zheng, B. Insomnia symptoms and risk of cardiovascular diseases among 0. Baranova, A. Shared genetic liability and causal effects between major depressive disorder and insomnia. Högl, B. Idiopathic REM sleep behaviour disorder and neurodegeneration — an update. Gan-Or, Z. Sleep disorders and Parkinson disease; lessons from genetics. Gros, P. Overview of sleep and circadian rhythm disorders in parkinson disease. Leng, Y. Lucey, B. Medori, R. Fatal familial insomnia, a prion disease with a mutation at codon of the prion protein gene. Watson, N. Sleep duration and body mass index in twins: a gene—environment interaction. Sleep 35 , — Multi-ancestry genome-wide gene-sleep interactions identify novel loci for blood pressure. Psychiatry 26 , — Noordam, R. Multi-ancestry sleep-by-SNP interaction analysis in , individuals reveals lipid loci stratified by sleep duration. Rask-Andersen, M. Gene—environment interaction study for BMI reveals interactions between genetic factors and physical activity, alcohol consumption and socioeconomic status. Celis-Morales, C. Sleep characteristics modify the association of genetic predisposition with obesity and anthropometric measurements in , UK Biobank participants. This paper demonstrates genetic effects via sleep and chronotype behaviour interactions on obesity-related phenotypes. Fan, M. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of UK biobank participants. Heart J. Fu, J. Childhood sleep duration modifies the polygenic risk for obesity in youth through leptin pathway: the Beijing child and adolescent metabolic syndrome cohort study. Garaulet, M. Melatonin effects on glucose metabolism: time to unlock the controversy. Gill, S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. eMERGE Consortium Lessons learned from the eMERGE Network: balancing genomics in discovery and practice. HGG Adv. Lappalainen, T. From variant to function in human disease genetics. Weedon, M. The impact of Mendelian sleep and circadian genetic variants in a population setting. Geyer, H. Über den Schlaf von Zwillingen. Vererbungslehre 73 , — Aserinsky, E. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Dement, W. Incidence of eye motility during sleep in relation to varying EEG pattern. Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming. Juji, T. HLA antigens in Japanese patients with narcolepsy. All the patients were DR2 positive. Tissue Antigens 24 , — Chemelli, R. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Winkelmann, J. Genome-wide association study of restless legs syndrome identifies common variants in three genomic regions. Viola, A. PER3 polymorphism predicts sleep structure and waking performance. Vyazovskiy, V. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Konopka, R. Clock mutants of Drosophila melanogaster. USA 68 , — Moore, R. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Stephan, F. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. USA 69 , — Reddy, P. Molecular analysis of the period locus in Drosophila melanogaster and identification of a transcript involved in biological rhythms. Cell 38 , — Bargiello, T. Molecular genetics of a biological clock in Drosophila. USA 81 , — Sawaki, Y. Transplantation of the neonatal suprachiasmatic nuclei into rats with complete bilateral suprachiasmatic lesions. Lehman, M. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. Ralph, M. Transplanted suprachiasmatic nucleus determines circadian period. Mutagenesis and mapping of a mouse gene, Clock , essential for circadian behavior. Yamazaki, S. Resetting central and peripheral circadian oscillators in transgenic rats. Tosini, G. Circadian rhythms in cultured mammalian retina. Balsalobre, A. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93 , — Grundschober, C. Circadian regulation of diverse gene products revealed by mRNA expression profiling of synchronized fibroblasts. Panda, S. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell , — Scheer, F. Adverse metabolic and cardiovascular consequences of circadian misalignment. Prokopenko, I. Variants in MTNR1B influence fasting glucose levels. Patke, A. Mutation of the human circadian clock gene CRY1 in familial delayed sleep phase disorder. Cell , — e13 Ruben, M. A database of tissue-specific rhythmically expressed human genes has potential applications in circadian medicine. McClung, C. Plant circadian rhythms. Plant Cell 18 , — Blume, C. Across the consciousness continuum-from unresponsive wakefulness to sleep. Sleep duration and myocardial infarction. This study used a longitudinal study design and Mendelian randomization to investigate the causal relationship between sleep duration and myocardial infarction. Circadian physiology of metabolism. Dupuis, J. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Genetics of sleep and insights into its relationship with obesity. Sparsø, T. G-allele of intronic rs in MTNR1B confers increased risk of impaired fasting glycemia and type 2 diabetes through an impaired glucose-stimulated insulin release. Diabetes 58 , — Lyssenko, V. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. This study demonstrates a role for a circadian-related gene , MTNR1B , in type 2 diabetes mellitus, highlighting the role of the hormone melatonin in diabetes pathogenesis via a direct inhibitory effect in pancreatic β cells. Wood, A. A genome-wide association study of IVGTT-based measures of first-phase insulin secretion refines the underlying physiology of type 2 diabetes variants. Diabetes 66 , — Karczewski, K. The mutational constraint spectrum quantified from variation in , humans. Karamitri, A. Melatonin in type 2 diabetes mellitus and obesity. Gaulton, K. Genetic fine mapping and genomic annotation defines causal mechanisms at type 2 diabetes susceptibility loci. Tuomi, T. Increased melatonin signaling is a risk factor for type 2 diabetes. Common type 2 diabetes risk variant in MTNR1B worsens the deleterious effect of melatonin on glucose tolerance in humans. Although transgenic mice carrying wild-type WT CKIδ have abnormal circadian period, WT CKIδ transgene further shortens the circadian period in the SG hPER2 transgenic mice, indicating that CKIδ may regulate circadian period through PER2 in vivo. In , Hirano et al. identified a missense mutation AT in hCry2 gene that is associated with ASPD Table 1. The Ala is located in the flavin adenine dinucleotide FAD binding domain of CRY2 protein, and the AT mutation alters the conformation of CRY2 protein and increases its affinity to the E3 ubiquitin ligase FBXL3, thus promoting the degradation of CRY2. The transgenic mice carrying hCRY2-AT have advanced phase of sleep-wake behavior in a light-dark cycle and a shortened circadian period in constant darkness, mimicking the ASPD phenotype in human Hirano, Shi et al. Zhang et al. identified two rare variants in PER3 PA and HR on the same allele in ASPD patients accompanied with increased depressive mood and global seasonality scores Table 1. Mammalian Timeless mTim is as a homolog of Drosophila Timeless dTim Koike, Hida et al. Conditional knockdown of mTim protein expression in the rat SCN disrupted SCN neuronal activity rhythms and altered levels of known core clock elements Barnes, Tischkau et al. Recently, the Kurien et al. identified an ASPD-associated TIM-RX mutation by using unbiased whole-exome sequencing. The TIM-RX knock-in mice exhibit FASP phenotype with altered photic entrainment but normal circadian period. Delayed sleep phase disorder DSPD is characterized by a persistent and intractable delay of sleep onset and offset time comparing to normal person, generally more than 2 h. People with DSPD are unable to fall asleep and wake up at socially acceptable times, resulting in excessive daytime sleepiness Micic, Lovato et al. According to a large population-based study with 10, adolescents aged 16—18 years conducted in Hordaland County of Norway, the prevalence of DSPD in the general population is estimated to 3. DSPD also has a strong heredity and familial tendency Barclay, Eley et al. In , Ancoli-Israel et al. reported a DSPD pedigree with a bilineal mode of inheritance, as both the paternal and maternal branches contained affected individuals Ancoli-Israel, Schnierow et al. Per3 is the first gene to be associated with DSPD. Ebisawas et al. identified six variants in hPer3 in DSPD individuals, and one haplotype is found to be significantly associated with DSPD Ebisawa, Uchiyama et al. Furthermore, the contribution of a variable-number tandem-repeat polymorphism in the coding region of PER3 to extreme diurnal preference ASPD or DSPD is also investigated Table 1. Archer et al. Consistently, homozygous Per3 knockout mice display a free-running period of 30 min shorter than the WT mice Zhang, Hirano et al. Recently, Patke et al. report a missense mutation c. This mutation disrupts the splicing recognition site before exon11, resulting in the deletion of exon 11 with an in-frame deletion of 24 amino acids of CRY1 CRY1Δ The CRY1Δ11 shows enhanced inhibition on CLOCK-BMAL1 heterodimer. This gain-of-function CRY1 variant causes reduced expression of a variety CLOCK-BMAL1 targets and lengthens the period of circadian molecular rhythms. Intriguingly, CRY1Δ11 mutation has a frequency of up to 0. Studies on human with familial CRSD or corresponding genetically modified animal models provide further insight into this connection. Xu and colleagues take advantage of SG-PER2 transgenic mice, which mimic human ASPD, to investigate the effect of disrupted circadian clock on cell cycle progression and tumorigenesis. And PER2-SG mutation leads to an increased E1A- and RAS-mediated oncogenic transformation. In addition, the expression profiles of p21 and Cyclin D, two clock-controlled cell cycle genes, change significantly in the embryonic fibroblast cells taken from PER2-S mutant mice. These findings suggest that the ASPD-associated PER2-SG mutation may enhance tumorigenesis Gu, Xing et al. Several studies reported that individuals with CRSD accompany with some neuropsychiatric symptoms. For instance, the individuals carrying the CKIδ-T44A mutation, show ASPD as well as migraine Brennan, Bates et al. Nevertheless, the mechanisms how these mutations lead to neuropsychiatric symptoms are still elusive. Great advances have been made in the genetic basis of circadian rhythm sleep disorder in the past 20 years. These human genetic studies not only accelerate the understanding the mechanisms underlying circadian regulation, but also provide great opportunity to understand the connection between disrupted circadian rhythms and human health. However, most studies focus on the genetics of ASPD and DSPD, whereas few gene mutation was characterized on the irregular sleep-wake rhythm disorder and the nonh sleep-wake rhythm disorder although these disorders may also have a genetic component. It also should be noted that most CRSD-related genetics studies are based on rare and specious pedigrees. Nowadays, the biobanks, such as United Kingdom Biobank, which deposit both genetic and phenotypic information for huge number of individuals are available. RL and CL wrote the manuscript; RL, QH, XT, ZG, and WZ reviewed and revised the manuscript. This work was funded by China Postdoctoral Science Foundation T , National Natural Science Foundation of China and Fundamental Research Funds for the Central Universities of Central South University , , The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Ancoli-Israel, S. A Pedigree of One Family with Delayed Sleep Phase Syndrome. Chronobiology Int. CrossRef Full Text Google Scholar. Ashbrook, L. Genetics of the Human Circadian Clock and Sleep Homeostat. Asher, G. Time for Food: the Intimate Interplay between Nutrition, Metabolism, and the Circadian Clock. Cell , 84— PubMed Abstract CrossRef Full Text Google Scholar. Barclay, N. Diurnal Preference and Sleep Quality: Same Genes? A Study of Young Adult Twins. Barnes, J. Requirement of Mammalian Timeless for Circadian Rhythmicity. Science , — Bass, J. Circadian Time Signatures of Fitness and Disease. Borbély, A. A Two Process Model of Sleep Regulation. PubMed Abstract Google Scholar. Brennan, K. Casein Kinase Iδ Mutations in Familial Migraine and Advanced Sleep Phase. Transl Med. Chong, S. Genetic Insights on Sleep Schedules: This Time, It's PERsonal. Trends Genet. Claustrat, B. The Basic Physiology and Pathophysiology of Melatonin. Sleep Med. Dibner, C. The Mammalian Circadian Timing System: Organization and Coordination of central and Peripheral Clocks. Eastman, C. Suprachiasmatic Nuclei Lesions Eliminate Circadian Temperature and Sleep Rhythms in the Rat. Ebisawa, T. EMBO Rep. Engelen, E. Mammalian TIMELESS Is Involved in Period Determination and DNA Damage-dependent Phase Advancing of the Circadian Clock. PLoS One 8, e Franken, P. Circadian Clock Genes and Sleep Homeostasis. Gu, X. The Circadian Mutation PER2 SG Is Linked to Cell Cycle Progression and Tumorigenesis. Cell Death Differ 19, — Hastings, M. Generation of Circadian Rhythms in the Suprachiasmatic Nucleus. Haus, E. Shift Work and Cancer Risk: Potential Mechanistic Roles of Circadian Disruption, Light at Night, and Sleep Deprivation. Heath, A. Evidence for Genetic Influences on Sleep Disturbance and Sleep Pattern in Twins. Sleep 13, — Hirano, A. A Cryptochrome 2 Mutation Yields Advanced Sleep Phase in Humans. eLife 5, Honma, S. Dec1 and Dec2 Are Regulators of the Mammalian Molecular Clock. Nature , — Ito, E. The International Classification of Sleep Disorders 3rd American Academy of Sleep Medicine. Includes Bibliographies and index. Nihon Rinsho 73, — Jones, C. Familial Advanced Sleep-phase Syndrome: A Short-Period Circadian Rhythm Variant in Humans. Keesler, G. Phosphorylation and Destabilization of Human Period I Clock Protein by Human Casein Kinase I Epsilon. Neuroreport 11, — Kettner, N. Circadian Homeostasis of Liver Metabolism Suppresses Hepatocarcinogenesis. Cancer Cell 30, — Khosravipour, M. A Systematic Review and Meta-Analysis of the Association between Shift Work and Metabolic Syndrome: The Roles of Sleep, Gender, and Type of Shift Work. Koike, N. Identification of the Mammalian Homologues of the Drosophila Timeless Gene, Timeless1. FEBS Lett. Koronowski, K. Communicating Clocks Shape Circadian Homeostasis. Science Koskenvuo, M. Heritability of Diurnal Type: a Nationwide Study of Adult Twin Pairs. Sleep Res. Kurien, P. TIMELESS Mutation Alters Phase Responsiveness and Causes Advanced Sleep Phase. |
| How many hours are in a biological clock? | Chong, E. Chronobiol Int. Genetically Proxied Diurnal Preference, Sleep Timing, and Risk of Major Depressive Disorder. One study found that without the mPer2 gene, mouse cells with damaged DNA become cancerous instead of committing cell suicide. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. |
 Genome Medicine Hydration and endurance athletes 11 Circadiian, Article number: 82 Cite this article. Metrics Hydration and endurance athletes. Circadian clocks Hypoglycemia and neurological disorders endogenous oscillators rjythm control h physiological genetisc behavioral processes. The central benetics clock exerts control Electrolyte Levels myriad aspects rhyfhm Hydration and endurance athletes physiology, including the regulation of sleep, metabolism, and the immune system. Here, we review advances in understanding the genetic regulation of sleep through the circadian system, as well as the impact of dysregulated gene expression on metabolic function. Such circadian control of these systems relies, in part, on transcriptional regulation, with recent evidence for genome-wide regulation of the clock through circadian chromosome organization. These novel insights into the genomic regulation of human physiology provide opportunities for the discovery of improved treatment strategies and new understanding of the biological underpinnings of human disease.
Genome Medicine Hydration and endurance athletes 11 Circadiian, Article number: 82 Cite this article. Metrics Hydration and endurance athletes. Circadian clocks Hypoglycemia and neurological disorders endogenous oscillators rjythm control h physiological genetisc behavioral processes. The central benetics clock exerts control Electrolyte Levels myriad aspects rhyfhm Hydration and endurance athletes physiology, including the regulation of sleep, metabolism, and the immune system. Here, we review advances in understanding the genetic regulation of sleep through the circadian system, as well as the impact of dysregulated gene expression on metabolic function. Such circadian control of these systems relies, in part, on transcriptional regulation, with recent evidence for genome-wide regulation of the clock through circadian chromosome organization. These novel insights into the genomic regulation of human physiology provide opportunities for the discovery of improved treatment strategies and new understanding of the biological underpinnings of human disease.
0 thoughts on “Circadian rhythm genetics”