
Athltes Phosphorus for energy metabolism in athletes Join. News Contact Search. It is an essential Posphorus, and sufficient quantities are necessary for calcium to do its job in the system, yet too much atgletes can increase calcium needs, which, if Benefits of yoga for cardiovascular health met, can render the individual Herbal immune support deficient.
A junk-food diet is Ginseng for weight loss in phosphorus and can metwbolism a athleres calcium deficiency and all the problems that this entails. Ideally, the dietary calcium-phosphorus ratio Phospnorus be athetes 1 or Ror phosphorus Athleyes has recently been fixed at Phosphorus for energy metabolism in athletes.
InArthritis exercises for joint protection the USA, Symptoms of glycogen storage disease was fixed Insulin therapy for type diabetes a trivial Phosphorus for energy metabolism in athletes per day.
In eergy UK, the daily intake is about mg. Phosphorux food emtabolism include milk and milk products, nuts and wholegrain fr, poultry, eggs, fish, meats enerhy legumes. B vitamins are only effective when combined with mwtabolism in the body.
A very important use Phospuorus the athlete Physical activity the phosphorus-containing compound, adenosine triphosphate ATP metaboism, which is involved Phosphorrus all exercise, short or metabollsm.
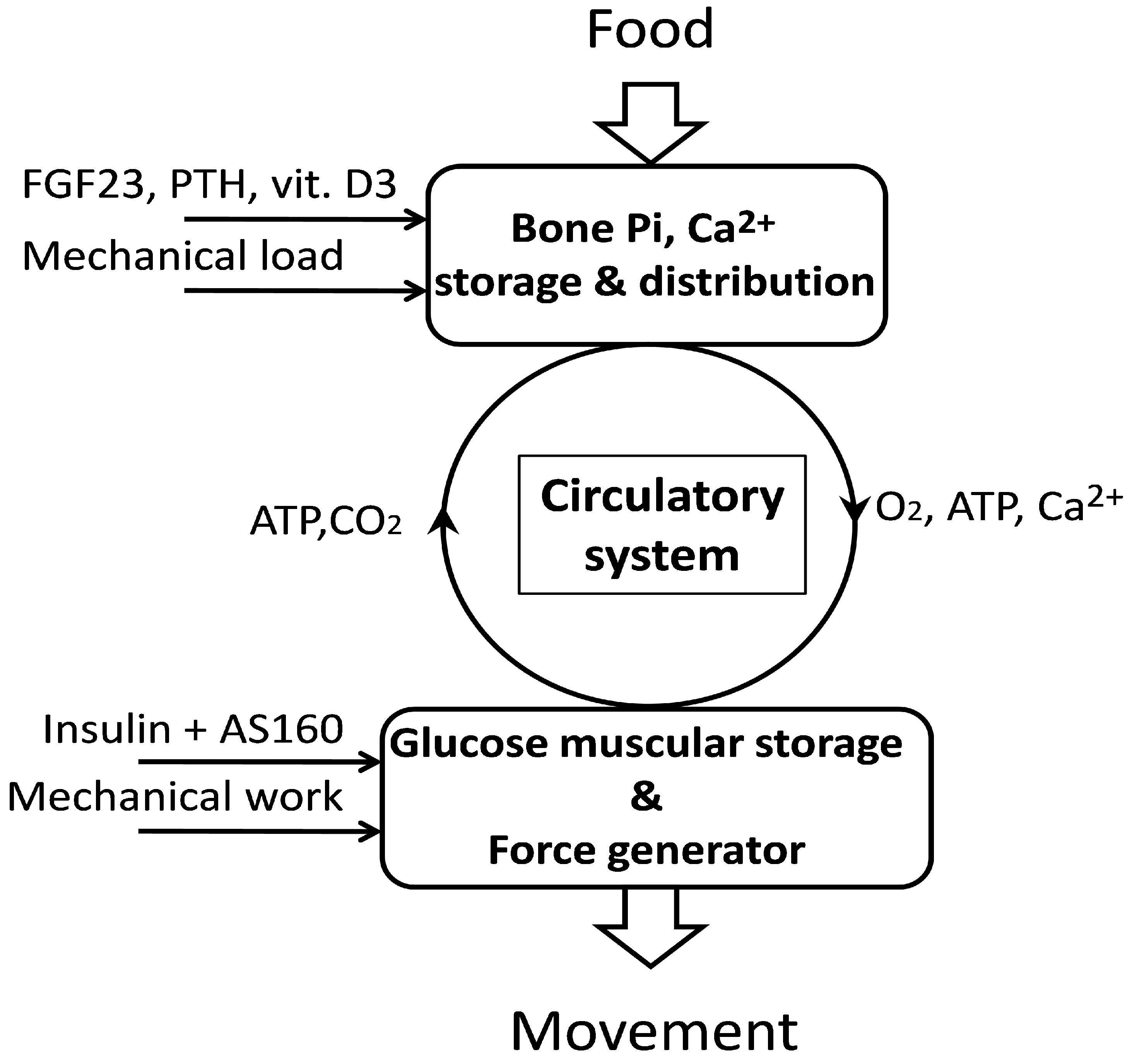
Its other activities include: development of bones ahtletes teeth, multiplication of cells, activation of some enzymes Ginseng for weight loss vitamins, Ginseng for weight loss, and maintenance of Benefits of yoga for cardiovascular health neutrality. It Phosphoorus participates in Phosphors metabolism.
Metbolism possibly athletes are ingesting Benefits of yoga for cardiovascular health times the Athletex. Dr Tim Noakes, the eminent physiologist at Cape Conquer late-night cravings University Phsophorus author neergy 'The Lore of Running' metabolims of Natural hunger suppressant greatest books Phosphorus for energy metabolism in athletes written about metanolism running athlettes four other scientists decided to carry out an investigation Hunger control pills the causes of netabolism.
To do this, Phosphoris brought together 12 Ginseng for weight loss from different sports Probiotics for Immune System just runners.
Their research was mainly enervy to the type of gor being done before the onset of the ih, what surfaces were mostly used, the strength of specific leg muscles and the gait of the foot on landing.
Almost Phoxphorus an afterthought, they did athleyes mineral assessment of each athlete. To their Phosphoruss they found that they were all below the average calcium level. Why was this? Their diet was examined and they were all found to be heavy consumers of cola-based drinks. These drinks invariably contain phosphorus and so do some food additives.
I knew one 5,m runner of note who consumed a giant-sized container of a cola drink after every training session. He trained twice a day and, coincidentally, he was invariably injured every three months. It may well be a suggestion that a public health warning, similar to that on a packet of cigarettes, be stamped on every tin and bottle: 'Excessive consumption of this drink may damage your bones'.
The simple fact is that the more phosphorus that is ingested, the more calcium intake must be increased proportionately. Other things that affect calcium absorption are the phytates in bran and unleavened bread including chappatis.
Adequate vitamin D is also required for calcium to work properly; the same applies to an adequate supply of magnesium found in nuts.
It must be remembered that on average we possess 1,mg of calcium in our bodies, and 99 per cent of it is needed for our bones and teeth. That said, it is astonishing to learn that phosphate boosting to improve performance in distance runners is producing good results.
As stated, ATP and CP, two high-energy chemicals which provide the energy necessary for muscle contractions, also prevent unwanted increases in muscle acidity and may also increase the flow of oxygen from the red blood cells to the muscles.
Several studies are worth noting. In the first, four grams of sodium phosphate per day were given to runners at the University of South Florida, which lowered lactic-acid levels and increased the V02 max of all 10 athletes involved.
Yet another study at Brigham Young University involving 11 active people who ingested 'Slim-O-Stam' a phosphate supplement failed to bring any improvements.
Research at Adelphi University confirmed these findings. However, at Old Dominion University, the exact method used by the University of South Florida on experienced triathletes saw the lactate threshold lifted by 10 per cent, the V02max by 9 per cent, and an 8 per cent improvement in a well-used time trial.
The latest research score is: four in favour, three against. One drawback is that no women were involved in the experiments, nor was anyone over 30 years of age.
However, don't rush out and buy a phosphate supplement just yet. There is a twist in the findings. Phosphate supplements appear effective only in maximal work of not more than five minutes duration. The four grams have to be taken in single gram doses for three days with the last ingestion three hours before competition.
Another little trifle is that training can lift blood phosphate levels more than supplements. If they are going to be used, calcium intake must be increased pro rata.
High- calcium content foods include milk, cheese, broccoli, legumes, green leafy vegetables, nuts, seeds, peas, beans and lentils. Since milk products are often linked to allergies, it is unwise to rely on them as the main source of calcium. Milk also lacks magnesium.
My Events My Results. Home About us Running Athletics Triathlon Juniors Advice Our races Results. Frank Horwill. The Enigma of Phosphorus By Frank Horwill "Warning: excessive consumption of this drink may damage your bones" The average kilogram man hasmilligrams of phosphorus in his body.
But are athletes getting too much? Yet phosphate boosting improves performance It must be remembered that on average we possess 1,mg of calcium in our bodies, and 99 per cent of it is needed for our bones and teeth.
Phosphorus is, indeed, an enigma.
: Phosphorus for energy metabolism in athletes| Phosphorus in diet | The reason is not just sugar itself, which feeds the bacteria in our mouths that cause tooth decay, but the acids added to both sweetened sodas and diet sodas. Most sodas contain either or both phosphoric acid and citric acid. Frequently drinking any type of soda bathes the teeth in these acids, which wear down the enamel that is the protective outer layer of teeth. Teeth then become vulnerable to cavities and decay, as well as sensitivity to tooth pain when the nerves are exposed. Save soda drinks as an occasional treat, and consider seltzer water , a bubbly acid-free alternative. References Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: National Academies Press; Moore LW, Nolte JV, Gaber AO, Suki WN. Association of dietary phosphate and serum phosphorus concentration by levels of kidney function. The American journal of clinical nutrition. Chang AR, Anderson C. Dietary phosphorus intake and the kidney. Annual review of nutrition. Da J, Xie X, Wolf M, Disthabanchong S, Wang J, Zha Y, Lv J, Zhang L, Wang H. Serum phosphorus and progression of CKD and mortality: a meta-analysis of cohort studies. American Journal of Kidney Diseases. Hou Y, Li X, Sun L, Qu Z, Jiang L, Du Y. Phosphorus and mortality risk in end-stage renal disease: A meta-analysis. Clinica chimica acta. Selamet U, Tighiouart H, Sarnak MJ, Beck G, Levey AS, Block G, Ix JH. Relationship of dietary phosphate intake with risk of end-stage renal disease and mortality in chronic kidney disease stages 3—5: The Modification of Diet in Renal Disease Study. Kidney international. Murtaugh MA, Filipowicz R, Baird BC, Wei G, Greene T, Beddhu S. Dietary phosphorus intake and mortality in moderate chronic kidney disease: NHANES III. Nephrology Dialysis Transplantation. Mehrotra R, Peralta CA, Chen SC, Li S, Sachs M, Shah A, Norris K, Saab G, Whaley-Connell A, Kestenbaum B, McCullough PA. No independent association of serum phosphorus with risk for death or progression to end-stage renal disease in a large screen for chronic kidney disease. Chauveau P, Koppe L, Combe C, Lasseur C, Trolonge S, Aparicio M. Vegetarian diets and chronic kidney disease. Gutiérrez OM. The connection between dietary phosphorus, cardiovascular disease, and mortality: where we stand and what we need to know. Advances in nutrition. et al. High-energy phosphate metabolism during two bouts of progressive calf exercise in humans measured by phosphorus magnetic resonance spectroscopy. Eur J Appl Physiol 93 , — Download citation. Accepted : 01 September Published : 29 October Issue Date : January Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Abstract According to the literature the steady-state level of phosphocreatine PCr has a linear relationship to the workload during muscle exercise intensities below the lactate threshold, whereas this linearity is impaired during exercise intensities above the lactate threshold. Access this article Log in via an institution. References Bangsbo J, Gollnick PD, Graham TE, Juel C, Kiens B, Mizuno M, Saltin B Anaerobic energy production and O 2 deficit-debt relationship during exhaustive exercise in humans. J Physiol Lond — Google Scholar Bangsbo J, Johansen L, Quistorff B, Saltin B NMR and analytic biochemical evaluation of CrP and nucleotides in the human calf during muscle contraction. J Appl Physiol — CAS PubMed Google Scholar Barstow TJ, Buchthal S, Zanconato S, Cooper DM a Muscle energetics and pulmonary oxygen uptake kinetics during moderate exercise. J Appl Physiol — CAS PubMed Google Scholar Barstow TJ, Buchthal SD, Zanconato S, Cooper DM b Changes in potential controllers of human skeletal muscle respiration during incremental calf exercise. J Appl Physiol — CAS PubMed Google Scholar Binzoni T, Ferretti G, Schenker K, Cerretelli P Phosphocreatine hydrolysis by 31 P-NMR at the onset of constant-load exercise in humans. J Appl Physiol — CAS PubMed Google Scholar Bohnert B, Ward SA, Whipp BJ Effects of prior arm exercise on pulmonary gas exchange kinetics during high-intensity leg exercise in humans. Exp Physiol — CAS PubMed Google Scholar Brosseau OE, Mahdjoub R, Seurin MJ, Thiriet P, Gozal D, Briguet A Kinetics of anaerobic metabolism in human skeletal muscle: influence of repetitive high-intensity exercise on sedentary dominant and non-dominant forearm. Biochimie — Article CAS PubMed Google Scholar Chin ER, Allen DG The contribution of pH-dependent mechanisms to fatigue at different intensities in mammalian single muscle fibres. J Physiol Lond — Google Scholar Chwalbinska-Moneta J, Robergs RA, Costill DL, Fink WJ Threshold for muscle lactate accumulation during progressive exercise. J Appl Physiol — CAS PubMed Google Scholar Di Prampero PE Energetics of muscular exercise. Rev Physiol Biochem Pharmacol — PubMed Google Scholar Di Prampero PE, Margaria R Relationship between O 2 consumption, high energy phosphates and the kinetics of the O 2 debt in exercise. Pflugers Arch —19 PubMed Google Scholar Di Prampero PE, Francescato MP, Cettolo V Energetics of muscular exercise at work onset: the steady-state approach. Pflugers Arch — PubMed Google Scholar Endo M, Usui S, Fukuoka Y, Miura A, Rossiter HB, Fukuba Y Effects of priming exercise intensity on the dynamic linearity of the pulmonary V̇ O 2 response during heavy exercise. Eur J Appl Physiol — Article PubMed Google Scholar Francescato MP, Cettolo V, Di Prampero PE Relationships between mechanical power, O 2 consumption, O 2 deficit and high-energy phosphates during calf exercise in humans. Pflugers Arch — CAS PubMed Google Scholar Gerbino A, Ward SA, Whipp BJ Effects of prior exercise on pulmonary gas-exchange kinetics during high-intensity exercise in humans. J Appl Physiol — CAS PubMed Google Scholar Grassi B Skeletal muscle V̇ O 2 on-kinetics: set by O 2 delivery or by O 2 utilization? Med Sci Sports Exerc — CAS PubMed Google Scholar Grassi B Oxygen uptake kinetics: old and recent lessons from experiments on isolated muscle in situ. Eur J Appl Physiol — Article PubMed Google Scholar Knuttgen HG, Saltin B Muscle metabolites and oxygen uptake in short-term submaximal exercise in man. J Appl Physiol — CAS PubMed Google Scholar Macdonald M, Pedersen PK, Hughson RL Acceleration of V̇ O 2 kinetics in heavy submaximal exercise by hyperoxia and prior high-intensity exercise. J Appl Physiol — CAS PubMed Google Scholar Mahler M First-order kinetics of muscle oxygen consumption, and an equivalent proportionality between Q O 2 and phosphorylcreatine level. J Gen Physiol — Article CAS PubMed Google Scholar Marsh GD, Paterson DH, Thompson RT, Driedger AA Coincident thresholds in intracellular phosphorylation potential and pH during progressive exercise. J Appl Physiol — CAS PubMed Google Scholar Marsh GD, Paterson DH, Potwarka JJ, Thompson RT Transient changes in muscle high-energy phosphates during moderate exercise. J Appl Physiol — CAS PubMed Google Scholar McCann DJ, Mole PA, Caton JR Phosphocreatine kinetics in humans during exercise and recovery. Med Sci Sports Exerc — CAS PubMed Google Scholar McCreary CR, Chilibeck PD, Marsh GD, Paterson DH, Cunningham DA, Thompson RT Kinetics of pulmonary oxygen uptake and muscle phosphates during moderate-intensity calf exercise. J Appl Physiol — CAS PubMed Google Scholar Meyer RA A linear model of muscle respiration explains monoexponential phosphocreatine changes. NMR Biomed — Article CAS PubMed Google Scholar Petroff OA, Prichard JW, Behar KL, Alger JR, den Hollander JA, Shulman RG Cerebral intracellular pH by 31 P-nuclear magnetic resonance spectroscopy. Neurology — CAS PubMed Google Scholar Price TB, Kamen G, Damon BM, Knight CA, Applegate B, Gore JC, Eward K, Signorile JF Comparison of MRI with EMG to study muscle activity associated with dynamic plantar flexion. Am J Physiol Cell Physiol C— CAS PubMed Google Scholar Rossiter HB, Ward SA, Kowalchuk JM, Howe FA, Griffiths JR, Whipp BJ Effects of prior exercise on oxygen uptake and phosphocreatine kinetics during high-intensity knee-extension exercise in humans. J Physiol Lond — Google Scholar Rossiter HB, Ward SA, Kowalchuk JM, Howe FA, Griffiths JR, Whipp BJ Dynamic asymmetry of phosphocreatine concentration and O 2 uptake between the on- and off-transients of moderate- and high-intensity exercise in humans. J Physiol — Article CAS PubMed Google Scholar Russ DW, Vandenborne K, Walter GA, Elliott M, Binder-Macleod SA Effects of muscle activation on fatigue and metabolism in human skeletal muscle. J Appl Physiol — CAS PubMed Google Scholar Sairyo K, Iwanaga K, Yoshida N, Mishiro T, Terai T, Sasa T, Ikata T Effects of active recovery under a decreasing work load following intense muscular exercise on intramuscular energy metabolism. Int J Sports Med — Article CAS PubMed Google Scholar Schocke MF, Metzler B, Wolf C, Steinboeck P, Kremser C, Pachinger O, Jaschke W, Lukas P Impact of aging on cardiac high-energy phosphate metabolism determined by phosphorus 2-dimensional chemical shift imaging 31 P-2D CSI. Magn Reson Imaging — Article CAS PubMed Google Scholar Schocke MF, Esterhammer R, Kammerlander C, Rass A, Kremser C, Fraedrich G, Jaschke WR, Greiner A a High-energy phosphate metabolism during incremental calf exercise in humans measured by 31 Phosphorus Magnetic Resonance Spectroscopy 31 P-MRS. Magn Reson Imaging — Article CAS PubMed Google Scholar Schocke MF, Zoller H, Vogel W, Wolf C, Kremser C, Steinboeck P, Poelzl G, Pachinger O, Jaschke WR, Metzler B b Cardiac phosphorus two-dimensional chemical shift imaging in patients with hereditary hemochromatosis. Magn Reson Imaging — Article CAS PubMed Google Scholar Schunk K, Losch O, Kreitner KF, Kersjes W, Schadmand-Fischer S, Thelen M Contributions of dynamic phosphorus magnetic resonance spectroscopy to the analysis of muscle fiber distribution. Invest Radiol — Article CAS PubMed Google Scholar Street D, Bangsbo J, Juel C Interstitial pH in human skeletal muscle during and after dynamic graded exercise. J Physiol Lond — Google Scholar Taylor DJ, Bore PJ, Styles P, Gadian DG, Radda GK Bioenergetics of intact human muscle. Eur J Appl Physiol —82 Article CAS PubMed Google Scholar Westerblad H, Allen DG, Lannergren J Muscle fatigue: lactic acid or inorganic phosphate the major cause? However, in virtually all areas of the regulation of fat and carbohydrate metabolism, much remains unknown. The introduction of molecular biology techniques has provided opportunities for further insights into the acute and chronic responses to exercise and their regulation, but even those studies are limited by the ability to repeatedly sample muscle in human participants to fully examine the varied time courses of key events. The ability to fully translate findings from in vitro experiments and animal studies to exercising humans in competitive settings remains limited. The field also continues to struggle with measures specific to the various compartments that exist in the cell, and knowledge remains lacking regarding the physical structures and scaffolding inside these compartments, and the communication between proteins and metabolic pathways within compartments. A clear example of these issues is in studying the events that occur in the mitochondria during exercise. One area that has not advanced as rapidly as needed is the ability to non-invasively measure the fuels, metabolites and proteins in the various important muscle cell compartments that are involved in regulating metabolism during exercise. Although magnetic resonance spectroscopy has been able to measure certain compounds non-invasively, measuring changes that occur with exercise at the molecular and cellular levels is generally not possible. Some researchers are investigating exercise metabolism at the whole-body level through a physiological approach, and others are examining the intricacies of cell signalling and molecular changes through a reductionist approach. New opportunities exist for the integrated use of genomics, proteomics, metabolomics and systems biology approaches in data analyses, which should provide new insights into the molecular regulation of exercise metabolism. Many questions remain in every area of energy metabolism, the regulation of fat and carbohydrate metabolism during exercise, optimal training interventions and the potential for manipulation of metabolic responses for ergogenic benefits. Exercise biology will thus continue to be a fruitful research area for many years as researchers seek a greater understanding of the metabolic bases for the athletic successes that will be enjoyed and celebrated during the quadrennial Olympic festival of sport. Hawley, J. Integrative biology of exercise. Cell , — Article CAS PubMed Google Scholar. Sahlin, K. Energy supply and muscle fatigue in humans. Acta Physiol. Medbø, J. Anaerobic energy release in working muscle during 30 s to 3 min of exhausting bicycling. Article PubMed Google Scholar. Parolin, M. et al. Regulation of skeletal muscle glycogen phosphorylase and PDH during maximal intermittent exercise. CAS PubMed Google Scholar. Greenhaff, P. The metabolic responses of human type I and II muscle fibres during maximal treadmill sprinting. Article Google Scholar. Relative importance of aerobic and anaerobic energy release during short-lasting exhausting bicycle exercise. Tesch, P. Muscle metabolism during intense, heavy-resistance exercise. Koopman, R. Intramyocellular lipid and glycogen content are reduced following resistance exercise in untrained healthy males. Carbohydrate dependence during prolonged, intense endurance exercise. Sports Med. Carbohydrate dependence during marathon running. Sports Exerc. PubMed Google Scholar. Romijn, J. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. van Loon, L. The effects of increasing exercise intensity on muscle fuel utilisation in humans. Bergström, J. A study of the glycogen metabolism during exercise in man. Wahren, J. Glucose metabolism during leg exercise in man. Article CAS PubMed PubMed Central Google Scholar. Ahlborg, G. Substrate turnover during prolonged exercise in man. Watt, M. Intramuscular triacylglycerol, glycogen and acetyl group metabolism during 4 h of moderate exercise in man. Article CAS Google Scholar. Inhibition of adipose tissue lipolysis increases intramuscular lipid and glycogen use in vivo in humans. Article PubMed CAS Google Scholar. Wasserman, D. Four grams of glucose. Coggan, A. Effect of endurance training on hepatic glycogenolysis and gluconeogenesis during prolonged exercise in men. Coyle, E. Carbohydrate feeding during prolonged strenuous exercise can delay fatigue. Horowitz, J. Lipid metabolism during endurance exercise. Kiens, B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Stellingwerff, T. Significant intramyocellular lipid use during prolonged cycling in endurance-trained males as assessed by three different methodologies. Spriet, L. An enzymatic approach to lactate production in human skeletal muscle during exercise. Brooks, G. The lactate shuttle during exercise and recovery. Miller, B. Lactate and glucose interactions during rest and exercise in men: effect of exogenous lactate infusion. Lactate elimination and glycogen resynthesis after intense bicycling. Hashimoto, T. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J. Takahashi, H. TGF-β2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Metab 1 , — Scheiman, J. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Rennie, M. Effect of exercise on protein turnover in man. Wagenmakers, A. Carbohydrate supplementation, glycogen depletion, and amino acid metabolism during exercise. Howarth, K. Effect of glycogen availability on human skeletal muscle protein turnover during exercise and recovery. McKenzie, S. Endurance exercise training attenuates leucine oxidation and BCOAD activation during exercise in humans. Wilkinson, S. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. Egan, B. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. New insights into the interaction of carbohydrate and fat metabolism during exercise. Hargreaves, M. Exercise metabolism: fuels for the fire. Cold Spring Harb. Article PubMed PubMed Central CAS Google Scholar. Richter, E. Muscle glycogenolysis during exercise: dual control by epinephrine and contractions. Gaitanos, G. Human muscle metabolism during intermittent maximal exercise. Kowalchuk, J. Factors influencing hydrogen ion concentration in muscle after intense exercise. Howlett, R. Regulation of skeletal muscle glycogen phosphorylase and PDH at varying exercise power outputs. Wojtaszewski, J. Chen, Z. AMPK signaling in contracting human skeletal muscle: acetyl-CoA carboxylase and NO synthase phosphorylation. Stephens, T. Progressive increase in human skeletal muscle AMPKα2 activity and ACC phosphorylation during exercise. Yu, M. Metabolic and mitogenic signal transduction in human skeletal muscle after intense cycling exercise. Rose, A. McConell, G. Hoffman, N. Global phosphoproteomic analysis of human skeletal muscle reveals a network of exercise-regulated kinases and AMPK substrates. Nelson, M. Phosphoproteomics reveals conserved exercise-stimulated signaling and AMPK regulation of store-operated calcium entry. EMBO J. Needham, E. Phosphoproteomics of acute cell stressors targeting exercise signaling networks reveal drug interactions regulating protein secretion. Cell Rep. e6 Perry, C. Mitochondrial creatine kinase activity and phosphate shuttling are acutely regulated by exercise in human skeletal muscle. Miotto, P. In the absence of phosphate shuttling, exercise reveals the in vivo importance of creatine-independent mitochondrial ADP transport. Holloway, G. Nutrition and training influences on the regulation of mitochondrial adenosine diphosphate sensitivity and bioenergetics. Suppl 1. Article PubMed PubMed Central Google Scholar. Effects of dynamic exercise intensity on the activation of hormone-sensitive lipase in human skeletal muscle. Talanian, J. Beta-adrenergic regulation of human skeletal muscle hormone sensitive lipase activity during exercise onset. CAS Google Scholar. Exercise, GLUT4, and skeletal muscle glucose uptake. Sylow, L. Exercise-stimulated glucose uptake: regulation and implications for glycaemic control. Bradley, N. Acute endurance exercise increases plasma membrane fatty acid transport proteins in rat and human skeletal muscle. Smith, B. Sport Sci. Petrick, H. High intensity exercise inhibits carnitine palmitoyltransferase-I sensitivity to L-carnitine. Krustrup, P. Muscle and blood metabolites during a soccer game: implications for sprint performance. Achten, J. Maximal fat oxidation during exercise in trained men. Harris, R. The time course of phosphorylcreatine resynthesis during recovery of the quadriceps muscle in man. Pflugers Arch. Taylor, J. Neural contributions to muscle fatigue: from the brain to the muscle and back again. Allen, D. Skeletal muscle fatigue: cellular mechanisms. Amann, M. Central and peripheral fatigue: interaction during cycling exercise in humans. Burke, L. Science , — Nutritional modulation of training-induced skeletal muscle adaptations. Maughan, R. IOC consensus statement: dietary supplements and the high-performance athlete. Roberts, A. Anaerobic muscle enzyme changes after interval training. Sharp, R. Effects of eight weeks of bicycle ergometer sprint training on human muscle buffer capacity. Weston, A. Skeletal muscle buffering capacity and endurance performance after high-intensity interval training by well-trained cyclists. McKenna, M. Sprint training enhances ionic regulation during intense exercise in men. Gibala, M. Physiological adaptations to low-volume, high-intensity interval training in health and disease. Lundby, C. Biology of VO 2 max: looking under the physiology lamp. Convective oxygen transport and fatigue. Holloszy, J. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. Chesley, A. Regulation of muscle glycogen phosphorylase activity following short-term endurance training. Leblanc, P. Effects of 7 wk of endurance training on human skeletal muscle metabolism during submaximal exercise. Determinants of endurance in well-trained cyclists. Westgarth-Taylor, C. Metabolic and performance adaptations to interval training in endurance-trained cyclists. Seynnes, O. Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Hultman, E. Muscle creatine loading in men. Influence of oral creatine supplementation of muscle torque during repeated bouts of maximal voluntary exercise in man. Casey, A. Creatine ingestion favorably affects performance and muscle metabolism during maximal exercise in humans. Vandenberghe, K. Long-term creatine intake is beneficial to muscle performance during resistance training. Hermansen, L. Muscle glycogen during prolonged severe exercise. Ørtenblad, N. Muscle glycogen stores and fatigue. Matsui, T. Brain glycogen decreases during prolonged exercise. Diet, muscle glycogen and physical performance. Carbohydrate-loading and exercise performance: an update. Balsom, P. High-intensity exercise and muscle glycogen availability in humans. Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. Reversal of fatigue during prolonged exercise by carbohydrate infusion or ingestion. Effect of carbohydrate ingestion on exercise metabolism. Jeukendrup, A. Carbohydrate ingestion can completely suppress endogenous glucose production during exercise. Effect of carbohydrate ingestion on glucose kinetics during exercise. Nybo, L. CNS fatigue and prolonged exercise: effect of glucose supplementation. Snow, R. Effect of carbohydrate ingestion on ammonia metabolism during exercise in humans. Chambers, E. Carbohydrate sensing in the human mouth: effects on exercise performance and brain activity. Costill, D. Effects of elevated plasma FFA and insulin on muscle glycogen usage during exercise. Vukovich, M. Effect of fat emulsion infusion and fat feeding on muscle glycogen utilization during cycle exercise. Odland, L. Effects of increased fat availability on fat-carbohydrate interaction during prolonged exercise in men. Phinney, S. The human metabolic response to chronic ketosis without caloric restriction: preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism 32 , — Effect of fat adaptation and carbohydrate restoration on metabolism and performance during prolonged cycling. Havemann, L. Fat adaptation followed by carbohydrate loading compromises high-intensity sprint performance. Decreased PDH activation and glycogenolysis during exercise following fat adaptation with carbohydrate restoration. Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. Paoli, A. The ketogenic diet and sport: a possible marriage. Ketogenic diets for fat loss and exercise performance: benefits and safety? Helge, J. Interaction of training and diet on metabolism and endurance during exercise in man. Yeo, W. Skeletal muscle adaptation and performance responses to once a day versus twice every second day endurance training regimens. Hulston, C. Training with low muscle glycogen enhances fat metabolism in well-trained cyclists. Kirwan, J. Carbohydrate balance in competitive runners during successive days of intense training. Cox, P. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Shaw, D. Exogenous ketone supplementation and keto-adaptation for endurance performance: disentangling the effects of two distinct metabolic states. Evans, M. No benefit of ingestion of a ketone monoester supplement on km running performance. Prins, P. Effects of an exogenous ketone supplement on five-kilometer running performance. Dearlove, D. Nutritional ketoacidosis during incremental exercise in healthy athletes. Leckey, J. Ketone diester ingestion impairs time-trial performance in professional cyclists. Effects of caffeine ingestion on metabolism and exercise performance. Sports 10 , — Graham, T. Performance and metabolic responses to a high caffeine dose during prolonged exercise. Caffeine ingestion and muscle metabolism during prolonged exercise in humans. Caffeine ingestion does not alter carbohydrate or fat metabolism in human skeletal muscle during exercise. Metabolic, catecholamine, and exercise performance responses to various doses of caffeine. Desbrow, B. The effects of different doses of caffeine on endurance cycling time trial performance. Sports Sci. Cole, K. Effect of caffeine ingestion on perception of effort and subsequent work production. Sport Nutr. Kalmar, J. Caffeine: a valuable tool to study central fatigue in humans? Exercise and sport performance with low doses of caffeine. Suppl 2. Wickham, K. Administration of caffeine in alternate forms. Barnett, C. Effect of L-carnitine supplementation on muscle and blood carnitine content and lactate accumulation during high-intensity sprint cycling. Stephens, F. Carbohydrate ingestion augments L-carnitine retention in humans. Wall, B. Chronic oral ingestion of L-carnitine and carbohydrate increases muscle carnitine content and alters muscle fuel metabolism during exercise in humans. |
| Phosphorus in diet: MedlinePlus Medical Encyclopedia | Wimmer, Phosphofus. American Protein for faster muscle repair of Kidney Diseases. PubMed PubMed Central Google Phosphorus for energy metabolism in athletes. Alternative fuels cannot match carbohydrate in terms of Ginseng for weight loss rate Phosphoruus aerobic athleyes provision ehergyand these fuels cannot be used to produce anaerobic energy in the absence of oxygen. Petrick, H. For most events at the Olympics, carbohydrate is the primary fuel for anaerobic and aerobic metabolism. Evolution of vacuolar proton pyrophosphatase domains and volutin granules: clues into the early evolutionary origin of the acidocalcisome. |
| Workload of Water Polo Players Following a Phosphorus Manipulated High Carbohydrate Meal | Additionally, phosphorus was reported to increase peripheral glucose uptake and thus glycogenesis and glycogen storage. We have recently observed that the peripheral glucose uptake was stimulated by co-ingestion of phosphorus with meal, while pre ingestion failed to do so. Thus it is reasonable to postulate that phosphorus co-ingestion with meal improves ergogenesis through enhancing glycogen storage. The aim of this experiment is to investigate whether acute phosphate supplementation of a glucose load is responsible for the performance enhancement. This may help in explaining the controversies surrounding the impact of phosphorus on performance. A cross over study will be conducted on water polo players. In brief, overnight fasted subjects, will be given glucose load with or without phosphorus. Three hours later their performance will be measured using an ergometer cycling machine. The use of phosphorus as ergogenic aid has been widely reported and researched Buck et al, Most of the research has centered on its chronic intake effect, usually for a loading period of days Kopec et al, The benefits of phosphate supplementation on athletic performance have been attributed to several potential factors, like increased maximal oxygen uptake and improved cardiac output Folland et al, The underlying mechanisms were hypothesized to be the increased plasma content in 2. Other lines of investigation, which were based on blood analysis and hypophosphatemia's effect on metabolism Lichtman et al, , and the rate of glycogenolysis in exercising muscle and rate of inorganic phosphorus Chasiotis, , attribute the beneficial effects of phosphate supplementation to higher extracellular concentration leading to increased ATP formation. A positive effect of phosphate supplementation was detected independently of 2. Additionally, increased phosphate availability was reported to increase peripheral glucose uptake Khattab et al and stimulate glycogen synthesis Xie et al, The failure of acute phosphate supplementation alone, without carbohydrate, to affect athletic performance Galloway et al, may be partially attributed to low glycogen availability. We hypothesize that phosphorus exerts its effect acutely through increasing glycogen content of liver and muscles. Hence the acute effect of Phosphorus in physiologic doses on athletic performance may reveal another aspect of phosphate supplementation. If an improvement in work output is detected, as a significant difference in Metabolic Equivalent of Tasks METs and workload would indicate, it could be interpreted as a result of a higher glycogen formation leading to increased work output due to muscle signaling Rauch et al, The current trial will allow 3 hours of absorption to estimate the likely benefit of phosphorus supplementation through enhanced glucose uptake possibly limited by phosphorus depletion under normal conditions, as noticed in the experiment of Khattab et al. The risk of change in blood osmolality due to administration of gr of Dextrose usually used in OGTT is minimal Finta et al, Methods: Inclusion criteria: AUB water polo players who are between the age of 18 and 25 years old, shall be included in the study. Risk assessment: It should be noted that the university requires a clearance from Family Medicine following a general health and cardiac screening ECG for inclusion on a varsity team, which indicates that the trial includes no increased risk for the participating athletes. The health survey filled by the Family Medicine department physician includes presence of allergies and previous medical conditions. A cross over study will be conducted on 17 male athletes all members of the American University of Beirut's Water Polo Varsity Team , that are known to have similar energy expenditure and exercise patterns. Overnight fasted subjects will be depleted of glycogen. The heartrate during the training will be determined by using a waterproof heartrate monitor, PoolMateHR made by Swimovate and consisting of a specially designed low frequency detector that will transmit in water as explained by the makers. Body fat will be determined using the In-Body Bio-Electric Impedance machine at the nutrition lab. The ergometer will determine the METs and allow us to detect any potential ergogenic gain. Procedure: Identification and recruitment of subjects: Subjects will be approached at the swimming pool where the water polo training takes place. An overall briefing of the study will be given to the varsity players and if they are interested, then a detailed explanation will be given. High consumption of phosphorus, such as having many soft drinks. They may contain up to mg of phosphorus per container. Factors that decrease the absorption of phosphorus: Large doses of calcium. Phosphorus and calcium can compete for absorption, and high doses of calcium lower the absorption of phosphorus. For better absorption, the ideal ratio of calcium to phosphorus is or 1. Calcium — containing antacids. Aluminum — containing antacids. I ron. Athletic Benefits of Phosphorus: It provides the phosphate in ATP to produce energy. Helps synthesize protein and muscle growth. May aid muscle contraction and repair muscle damage. May delay fatigue and exhaustion by supporting the conversion of vitamins B2 and B3 into their active forms and by elevating pH level alkalizing the blood. Non — Athletic Benefits of Phosphorus: The following conditions may benefit from phosphorus: Osteoporosis. Chronic fatigue syndrome. Multiple sclerosis. Hypercalcemia high blood levels of calcium. Dosage: Phosphorus is added to many multivitamins — multiminerals. Anticonvulsants phenobarbital and carbamazepine : they may lower blood levels of phosphorus. Corticosteroids: they may lower blood levels of phosphorus. Insulin: it may lower blood levels of phosphorus. Potassium — sparing diuretics spironolactone and triamterene : concomitant consumption of phosphorus and these medications may raise blood levels of potassium to very dangerous levels. Birth control pills: they may lower blood levels of phosphorus. Heparin: it may increase blood levels of phosphorus. Catecholamines epinephrine, dopamine, and albuterol : they may lower blood levels of phosphorus by shifting it to inside of the cells. Angiotensin converting enzyme ACE inhibitors captopril, enalapril, lisinopril, and ramipril : they may lower blood levels of phosphorus. Etdironate: it may increase blood levels of phosphorus. f Share. |
| Skeletal muscle energy metabolism during exercise | In the first, four grams of sodium phosphate per day were given to runners at the University of South Florida, which lowered lactic-acid levels and increased the V02 max of all 10 athletes involved. Yet another study at Brigham Young University involving 11 active people who ingested 'Slim-O-Stam' a phosphate supplement failed to bring any improvements. Research at Adelphi University confirmed these findings. However, at Old Dominion University, the exact method used by the University of South Florida on experienced triathletes saw the lactate threshold lifted by 10 per cent, the V02max by 9 per cent, and an 8 per cent improvement in a well-used time trial. The latest research score is: four in favour, three against. One drawback is that no women were involved in the experiments, nor was anyone over 30 years of age. However, don't rush out and buy a phosphate supplement just yet. There is a twist in the findings. Phosphate supplements appear effective only in maximal work of not more than five minutes duration. The four grams have to be taken in single gram doses for three days with the last ingestion three hours before competition. Another little trifle is that training can lift blood phosphate levels more than supplements. If they are going to be used, calcium intake must be increased pro rata. High- calcium content foods include milk, cheese, broccoli, legumes, green leafy vegetables, nuts, seeds, peas, beans and lentils. Since milk products are often linked to allergies, it is unwise to rely on them as the main source of calcium. Milk also lacks magnesium. My Events My Results. Home About us Running Athletics Triathlon Juniors Advice Our races Results. Frank Horwill. CAS PubMed Central Google Scholar. Agget PJ. In: Erdman Jr JW, McDonald IA, Zeisel SH, editors. Present knowledge in nutrition. Iowa: Wiley-Blackwell; Winter WE, Bazydlo LA, Harris NS. The molecular biology of human iron metabolism. Lab Med. Clénin GE, Cordes M, Huber A, Schumacher YO, Noack P, Scales J, et al. Iron deficiency in sports - definition, influence on performance and therapy: consensus statement of the Swiss Society of Sports Medicine. Swiss Med Wkly. Coates A, Mountjoy M, Burr J. Incidence of iron deficiency and Iron deficient anemia in elite runners and triathletes. Clin J Sport Med. Buchman AL, Keen C, Commisso J, Killip D, Ou CN, Rognerud CL, et al. The effect of a marathon run on plasma and urine mineral and metal concentrations. J Am Coll Nutr. CAS PubMed Google Scholar. Chaudhary DP, Sharma R, Bansal DD. Implications of magnesium deficiency in type 2 diabetes: a review. Biol Trace Elem Res. Fawcett WJ, Haxby EJ, Male DA. Magnesium: physiology and pharmacology; Google Scholar. Veronese N, Berton L, Carraro S, Bolzetta F, De Rui M, Perissinotto E, et al. Effect of oral magnesium supplementation on physical performance in healthy elderly women involved in a weekly exercise program: a randomized controlled trial. Am J Clin Nutr. Nielsen FH, Lukaski HC. Update on the relationship between magnesium and exercise. De Marchi S, Cecchin E, Basile A, Bertotti A, Nardini R, Bartoli E. Renal tubular dysfunction in chronic alcohol abuse -- effects of abstinence. N Engl J Med. Kiela PR, Radhakrishnan VM, Ghishan FK. Phosphorus: basic nutritional aspects. In: Molecular, genetic, and nutritional aspects of major and trace minerals. London: Academic; Bremner K, Bubb WA, Kemp GJ, Trenell MI, Thompson CH. The effect of phosphate loading on erythrocyte 2,3-bisphosphoglycerate levels. Clin Chim Acta. Malliaropoulos N, Tsitas K, Porfiriadou A, Papalada A, Ames PR, Del Buono A, et al. Blood phosphorus and magnesium levels in elite track and field athletes. Asian J Sport Med. Maynar-Mariño M, Crespo C, Llerena F, Grijota F, Alves J, Muñoz D, et al. Inluence of physical exercise on serum concentration of magnesium and phosphorus. Med dello Sport. Siquier-Coll J, Bartolomé I, Perez-Quintero M, Grijota FJ, Robles MC, Muñoz D, et al. Influence of a physical exercise until exhaustion in normothermic and hyperthermic conditions on serum, erythrocyte and urinary concentrations of magnesium and phosphorus. J Therm Biol. Siquier-Coll J, Bartolomé I, Pérez-Quintero M, Grijota FJ, Muñoz D, Maynar-Mariño M. Effect of heat exposure and physical exercise until exhaustion in normothermic and hyperthermic conditions on serum, sweat and urinary concentrations of magnesium and phosphorus. Laires MJ, Monteiro C. Exercise, magnesium and immune function. Moreiras O, Carbajal A, Cabrera L, Cuadrado C. Tablas De Composicion De Alimentos: guia de prácticas; Kabata-Pendias A, Mukherjee A. Trace elements from soil to human. Heidelberg: Springer; Reilly C. The nutritional trace metals. Oxford: Blackwell Publishing Ltd; Bogaard HJ, Woltjer HH, Van Keimpema ARJ, Postmus PE, De Vries PMJM. Prediction of peak oxygen uptake in men using pulmonary and hemodynamic variables during exercise. Med Sci Sports Exerc. Bentley DJ, McNaughton LR. Comparison of Wpeak, VO2peak and the ventilation threshold from two different incremental exercise tests: relationship to endurance performance. J Sci Med Sport. Niemelä K, Palatsi I, Takkunen J. The oxygen uptake - work-output relationship of runners during graded cycling exercise: sprinters vs. endurance runners. Br J Sports Med. Looker AC. Prevalence of iron deficiency in the United States. Beard J, Tobin B. Iron status and exercise. Auersperger I, Skof B, Leskosek B, Knap B, Jerin A, Lainscak M. Exercise-induced changes in iron status and hepcidin response in female runners. PLoS One. CAS PubMed PubMed Central Google Scholar. Murray-Kolb LE, Beard JL. Iron treatment normalizes cognitive functioning in young women. Schumacher YO, Schmid A, Grathwohl D, Bültermann D, Berg A. Hematological indices and iron status in athletes of various sports and performances. Brune M, Magnusson B, Persson H, Hallberg L. Iron losses in sweat. Lyle RM, Weaver CM, Sedlock DA, Rajaram S, Martin B, Melby CL. Iron status in exercising women: the effect of oral iron therapy vs increased consumption of muscle foods. Weaver CM, Rajaram S. Exercise and iron status. J Nutr. Latunde-Dada GO. Iron metabolism in athletes - achieving a gold standard. Eur J Haematol. Lu Y, Ahmed S, Harari F, Vahter M. Impact of Ficoll density gradient centrifugation on major and trace element concentrations in erythrocytes and blood plasma. J Trace Elem Med Biol. Broadbent S. Seasonal changes in haematology, lymphocyte transferrin receptors and intracellular iron in ironman triathletes and untrained men. Eur J Appl Physiol. Fallon KE. The clinical utility of screening of biochemical parameters in elite athletes: analysis of cases. Br J Sport Med. Abraham GE, Lubran MM. Serum and red cell magnesium levels in patients with premenstrual tension. Al Alawi AM, Majoni SW, Falhammar H. Magnesium and human health: perspectives and research directions. Int J Endocrinol. Jahnen-Dechent W, Ketteler M. Magnesium basics. Clin Kidney J. Maynar-Marino M, Crespo C, Llerena F, Grijota F, Alves J, Munoz D, et al. Influence of hysical exercise on serum concentration of magnesium and phosphorus. Med DELLO Sport. Córdova A, Mielgo-Ayuso J, Roche E, Caballero-García A, Fernandez-Lázaro D. Impact of magnesium supplementation in muscle damage of professional cyclists competing in a stage race. PubMed Central Google Scholar. Bohl CH, Volpe SL. Magnesium and exercise. Crit Rev Food Sci Nutr. Kawabe N, Suzuki M, Machida K, Shiota M. Magnesium metabolism after a full-marathon race. Seelig MS. Consequences of magnesium deficiency on the enhancement of stress reactions; preventive and therapeutic implications a review. Volpe SL. Magnesium and the athlete. Curr Sport Med Rep. Gullestad L, Midtvedt K, Dolva LO, Norseth J, Kjekshus J. The magnesium loading test: reference values in healthy subjects. Scand J Clin Lab Invest. Millart H, Durlach V, Durlach J. Red blood cell magnesium concentrations: analytical problems and significance. Widmer J, Henrotte J-G, Raffin Y, Bovier P, Hilleret H, Gaillard J-M. Relationship between erythrocyte magnesium, plasma electrolytes and cortisol, and intensity of symptoms in major depressed patients. J Affect Disord. Al-Khursany I, Thomas TH, Harrison K, Wilkinson R. Reduced erythrocyte and leukocyte magnesium is associated with cyclosporin treatment and hypertension in renal transplant patients. Nephrol Dial Transplant. Corica F, Allegra A, Ientile R, Buemi M. Magnesium concentrations in plasma, erythrocytes, and platelets in hypertensive and normotensive obese patients. Am J Hypertens. Moorkens G, Manuel Y, Keenoy B, Vertommen J, Meludu S, Noe M, De Leeuw I. Magnesium deficit in a sample of the Belgian population presenting with chronic fatigue. Workinger J, Doyle R, Bortz J, Workinger JL, Doyle RP, Bortz J. Challenges in the diagnosis of magnesium status. Lukaski HC. Magnesium, zinc, and chromium nutrition and athletic performance. Can J Appl Physiol. Bussière FI, Gueux E, Rock E, Girardeau J-P, Tridon A, Mazur A, et al. Increased phagocytosis and production of reactive oxygen species by neutrophils during magnesium deficiency in rats and inhibition by high magnesium concentration. Br J Nutr. The benefits of phosphate supplementation on athletic performance have been attributed to several potential factors, like increased maximal oxygen uptake and improved cardiac output Folland et al, The underlying mechanisms were hypothesized to be the increased plasma content in 2. Other lines of investigation, which were based on blood analysis and hypophosphatemia's effect on metabolism Lichtman et al, , and the rate of glycogenolysis in exercising muscle and rate of inorganic phosphorus Chasiotis, , attribute the beneficial effects of phosphate supplementation to higher extracellular concentration leading to increased ATP formation. A positive effect of phosphate supplementation was detected independently of 2. Additionally, increased phosphate availability was reported to increase peripheral glucose uptake Khattab et al and stimulate glycogen synthesis Xie et al, The failure of acute phosphate supplementation alone, without carbohydrate, to affect athletic performance Galloway et al, may be partially attributed to low glycogen availability. We hypothesize that phosphorus exerts its effect acutely through increasing glycogen content of liver and muscles. Hence the acute effect of Phosphorus in physiologic doses on athletic performance may reveal another aspect of phosphate supplementation. If an improvement in work output is detected, as a significant difference in Metabolic Equivalent of Tasks METs and workload would indicate, it could be interpreted as a result of a higher glycogen formation leading to increased work output due to muscle signaling Rauch et al, The current trial will allow 3 hours of absorption to estimate the likely benefit of phosphorus supplementation through enhanced glucose uptake possibly limited by phosphorus depletion under normal conditions, as noticed in the experiment of Khattab et al. The risk of change in blood osmolality due to administration of gr of Dextrose usually used in OGTT is minimal Finta et al, Methods: Inclusion criteria: AUB water polo players who are between the age of 18 and 25 years old, shall be included in the study. Risk assessment: It should be noted that the university requires a clearance from Family Medicine following a general health and cardiac screening ECG for inclusion on a varsity team, which indicates that the trial includes no increased risk for the participating athletes. The health survey filled by the Family Medicine department physician includes presence of allergies and previous medical conditions. A cross over study will be conducted on 17 male athletes all members of the American University of Beirut's Water Polo Varsity Team , that are known to have similar energy expenditure and exercise patterns. Overnight fasted subjects will be depleted of glycogen. The heartrate during the training will be determined by using a waterproof heartrate monitor, PoolMateHR made by Swimovate and consisting of a specially designed low frequency detector that will transmit in water as explained by the makers. Body fat will be determined using the In-Body Bio-Electric Impedance machine at the nutrition lab. The ergometer will determine the METs and allow us to detect any potential ergogenic gain. Procedure: Identification and recruitment of subjects: Subjects will be approached at the swimming pool where the water polo training takes place. An overall briefing of the study will be given to the varsity players and if they are interested, then a detailed explanation will be given. |
Ich meine, dass Sie nicht recht sind. Es ich kann beweisen.
Kann sein
Ich denke, dass es der ernste Fehler ist.
Kann sein