
Cellular wound healing -
Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol ; 40 : — Chmielowiec J , Borowiak M , Morkel M et al.
Meyer M , Müller AK , Yang J et al. FGF receptors 1 and 2 are key regulators of keratinocyte migration in vitro and in wounded skin.
J Cell Sci ; : — Repertinger SK , Campagnaro E , Fuhrman J et al. EGFR enhances early healing after cutaneous incisional wounding. J Invest Dermatol ; : — 9. Werner S , Smola H , Liao X et al. The function of KGF in morphogenesis of epithelium and reepithelialization of wounds.
Science ; : — Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med ; 11 : — 4. Levy V , Lindon C , Zheng Y et al. Epidermal stem cells arise from the hair follicle after wounding.
FASEB J ; 21 : — Nature ; : — Desmouliere A , Geinoz A , Gabbiani F , Gabbiani G. Fathke C , Wilson L , Hutter J et al. Stem Cells ; 22 : — Ishii G , Sangai T , Sugiyama K et al.
Stem Cells ; 23 : — Sasaki M , Abe R , Fujita Y et al. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type.
J Immunol ; : — 7. Driskell RR , Lichtenberger BM , Hoste E et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair.
Macrophage recruitment during limb development and wound healing in the embryonic and foetal mouse. Adzick NS , Harrison MR , Glick PL et al. J Pediatr Surg ; 20 : — Martin P , D'Souza D , Martin J et al.
Wound healing in the PU. Curr Biol ; 13 : — 8. Dovi JV , He LK , DiPietro LA. J Leukoc Biol ; 73 : — Lucas T , Waisman A , Ranjan R et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol ; : — Antsiferova M , Martin C , Huber M et al.
Willenborg S , Eckes B , Brinckmann J et al. J Invest Dermatol ; : — Jameson J , Ugarte K , Chen N et al. A role for skin γδ T cells in wound repair. Science ; : — 9. Deppermann C , Cherpokova D , Nurden P et al.
J Clin Invest ; doi: Bianchi ME , Manfredi AA. Dangers in and out. Science ; : — 4. Wong VW , Rustad KC , Akaishi S et al.
Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med ; 18 : — Cash JL , Bass MD , Campbell J et al. Resolution mediator chemerin15 reprograms the wound microenvironment to promote repair and reduce scarring. Curr Biol ; 24 : — Ferguson MW , O'Kane S.
Philos Trans R Soc Lond B Biol Sci ; : — Mori R , Shaw TJ , Martin P. Molecular mechanisms linking wound inflammation and fibrosis: knockdown of osteopontin leads to rapid repair and reduced scarring. J Exp Med ; : 43 — Gerhardt H , Golding M , Fruttiger M et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia.
Rossiter H , Barresi C , Pammer J et al. Loss of vascular endothelial growth factor A activity in murine epidermal keratinocytes delays wound healing and inhibits tumor formation.
Cancer Res ; 64 : — Fantin A , Vieira JM , Gestri G et al. Blood ; : — Wallace H. The response of denervated axolotl arms to delayed amputation. J Embryol Exp Morphol ; 84 : — 7. Kumar A , Brockes JP. Nerve dependence in tissue, organ, and appendage regeneration.
Trends Neurosci ; 35 : — 9. Harsum S , Clarke JD , Martin P. A reciprocal relationship between cutaneous nerves and repairing skin wounds in the developing chick embryo. Dev Biol ; : 27 — Razzell W , Wood W , Martin P.
Swatting flies: modelling wound healing and inflammation in Drosophila. Dis Model Mech ; 4 : — Henry KM , Loynes CA , Whyte MK , Renshaw SA. Zebrafish as a model for the study of neutrophil biology. J Leukoc Biol ; 94 : — Stramer B , Wood W , Galko MJ et al.
Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. Wood W , Jacinto A , Grose R et al. Wound healing recapitulates morphogenesis in Drosophila embryos.
Nat Cell Biol ; 4 : — Genetics ; : — Campos I , Geiger JA , Santos AC et al. Genetic screen in Drosophila melanogaster uncovers a novel set of genes required for embryonic epithelial repair. Mace KA , Pearson JC , McGinnis W.
An epidermal barrier wound repair pathway in Drosophila is mediated by grainy head. Science ; : — 5. Ting SB , Caddy J , Hislop N et al. A homolog of Drosophila grainy head is essential for epidermal integrity in mice.
Caddy J , Wilanowski T , Darido C et al. Epidermal wound repair is regulated by the planar cell polarity signaling pathway.
Dev Cell ; 19 : — Niethammer P , Grabher C , Look AT , Mitchison TJ. Nature ; : — 9. Pase L , Layton JE , Wittmann C et al. Curr Biol ; 22 : — Richardson R , Slanchev K , Kraus C et al. Herrick SE , Sloan P , McGurk M et al. Sequential changes in histologic pattern and extracellular matrix deposition during the healing of chronic venous ulcers.
Am J Pathol ; : — Stojadinovic O , Pastar I , Vukelic S et al. Deregulation of keratinocyte differentiation and activation: a hallmark of venous ulcers. J Cell Mol Med ; 12 : — Brem H , Stojadinovic O , Diegelmann RF et al.
Cellular and tissue-based products reduce pain and healing times, provide extensive and long-lasting coverage for wounds, and are less intrusive. Engineered skin replacements serve as biologic scaffolds for replicating the structure and biologic functions of healthy skin.
Allografts are harvested from and transplanted between the same species but from genetically non-identical donors, such as cadaveric skin. Xenografts, on the other hand, are derived from completely different species. Examples include porcine products from pigs, bovine products from cows, and equine-derived CTPs from horses.
Expectedly, allografts have better biocompatibility and long-term host acceptance than xenografts but are less readily available. Xenografts are more readily obtained but may exhibit lower biocompatibility and delayed fibroblast proliferation. There are many different types of CTPs on the market today.
However, they can be categorized into the following:. Human skin allografts are derived from skin components and tissues bioengineered to prevent transplant rejection. They are extensively used in the treatment of burn injuries and indicated in the management of chronic wounds where they facilitate scaffolding, growth factors, and soft tissue support with rare immunological reactions.
Allogeneic matrices are CTPs derived from neonatal dermal fibroblasts that have been cultured in-vitro. The most frequent sources include the placenta, umbilical cord, amnion, and chorion. They can provide soft tissue support and re-epithelialization of full-thickness wounds.
Composite matrices are extracted from human keratin-producing skin cells and fibroblasts. These CTPs contain active cellular compounds that generate proteins and bioactive compounds that facilitate wound healing and re-epithelialization.
Acellular dermal matrices ADM are the most prominent type of CTPs. They are derived from collagen, cellular remnants, or membranes and closely replicate the characteristics of normal, healthy skin. ADMs aid the wound healing process through the localization and intensification of hormonal or enzymatic activity, including angiogenesis, growth factors, and fibroblast proliferation.
The standard is the paste-type ADM CG Paste. In an article on Podiatry Today, John Steinberg, DPM, FACFAS, a co-director at the Center for Wound Healing at MedStar Georgetown emphasizes the need for preliminary determination of wound etiology before clinicians apply CTPs.
Chronic wounds indicated for CTP interventions should include those that have failed to heal after four weeks of treatment after ascertaining wound causes and risk factors.
According to evidence-based research and recommendations from leading clinicians on Wound Reference , the following CTP types are indicated for use in the treatment of various chronic wounds, injuries, and acute burns:.
Despite their efficiency in improving the wound healing process, the application of CTPs should be avoided in the following situations:. Detailed comparisons of the efficacy of cellular and tissue-based products over autologous skin grafts are limited in the medical literature.
Patients that opt for CTP treatment for acute or chronic wounds must be attended to by competent and licensed physicians that will inform them of the suitability and potential contraindications after ascertaining proper wound etiology and evaluating their medical history. The Wound Pros deploys licensed, qualified health care professionals Physicians, Surgeons, Physician Assistants and Nurse Practitioners providing advanced surgical wound consultation and treatment services at the patient's bedside in long-term care facilities.
Our specialty-trained health-care providers deliver wound care expertise, to develop treatment plans, to consult and guide patient treatment, and to provide in-service education to nursing staff.
Int J Biochem Cell Biol Jiang D, Correa-Gallegos D, Christ S, Stefanska A, Liu J, Ramesh P, et al. Two succeeding fibroblastic lineages drive dermal development and the transition from regeneration to scarring. Nat Cell Biol — Leavitt T, Hu MS, Borrelli MR, Januszyk M, Garcia JT, Ransom RC, et al.
Prrx1 fibroblasts represent a pro-fibrotic lineage in the mouse ventral dermis. Cell Rep Vorstandlechner V, Laggner M, Kalinina P, Haslik W, Radtke C, Shaw L, et al. Deciphering the functional heterogeneity of skin fibroblasts using single-cell RNA sequencing. FASEB J — Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, et al.
Distinct fibroblast lineages determine dermal architecture in skin development and repair. Mascharak S, desJardins-Park HE, Longaker MT. Fibroblast heterogeneity in wound healing: hurdles to clinical translation. Trends Mol Med —6. Schmitt-Gräff A, Desmoulière A, Gabbiani G.
Heterogeneity of myofibroblast phenotypic features: an example of fibroblastic cell plasticity. Virchows Arch — Eyden B. Fibroblast phenotype plasticity: relevance for understanding heterogeneity in "fibroblastic" tumors. Ultrastruct Pathol — Forte E, Ramialison M, Nim HT, Mara M, Li JY, Cohn R, et al.
Adult mouse fibroblasts retain organ-specific transcriptomic identity. Elife e Krausgruber T, Fortelny N, Fife-Gernedl V, Senekowitsch M, Schuster LC, Lercher A, et al.
Structural cells are key regulators of organ-specific immune responses. Foote AG, Wang Z, Kendziorski C, Thibeault SL. Tissue specific human fibroblast differential expression based on RNAsequencing analysis. BMC Genomics Jiang D, Guo R, Machens H-G, Rinkevich Y.
Diversity of fibroblasts and their roles in wound healing. Cold Spring Harb Perspect Biol 15 3 :a CrossRef Full Text Google Scholar.
Driskell RR, Watt FM. Understanding fibroblast heterogeneity in the skin. Trends Cell Biol —9. Fawkner-Corbett D, Antanaviciute A, Parikh K, Jagielowicz M, Gerós AS, Gupta T, et al. Spatiotemporal analysis of human intestinal development at single-cell resolution. Cell — Thulabandu V, Chen D, Atit RP.
Dermal fibroblast in cutaneous development and healing. Wiley Interdiscip Rev Dev Biol 7 2 Plikus MV, Wang X, Sinha S, Forte E, Thompson SM, Herzog EL, et al. Fibroblasts: Origins, definitions, and functions in health and disease. Herriges M, Morrisey EE. Lung development: orchestrating the generation and regeneration of a complex organ.
Development — Jiménez-Rojo L, Granchi Z, Graf D, Mitsiadis TA. Stem cell fate determination during development and regeneration of ectodermal organs.
Front Physiol Noro M, Yuguchi H, Sato T, Tsuihiji T, Yonei-Tamura S, Yokoyama H, et al. Dev Dynamics — Myung P, Andl T, Atit R. The origins of skin diversity: lessons from dermal fibroblasts. Development 23 :dev Jiang X, Iseki S, Maxson RE, Sucov HM, Morriss-Kay GM.
Tissue origins and interactions in the mamMalian skull vault. Dev Biol — Rinn JL, Wang JK, Allen N, Brugmann SA, Mikels AJ, Liu H, et al. A dermal HOX transcriptional program regulates site-specific epidermal fate.
Genes Dev —7. Hahn JM, McFarland KL, Combs KA, Anness MC, Supp DM. Analysis of HOX gene expression and the effects of HOXA9 overexpression in fibroblasts derived from keloid lesions and normal skin.
Wound Repair Regen — Chang HY, Chi JT, Dudoit S, Bondre C, Rijn Mv de, Botstein D, et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci USA — LeBleu VS, Neilson EG. Origin and functional heterogeneity of fibroblasts. Eames BF, Schneider RA.
Quail-duck chimeras reveal spatiotemporal plasticity in molecular and histogenic programs of cranial feather development. Griffin MF, desJardins-Park HE, Mascharak S, Borrelli MR, Longaker MT. Understanding the impact of fibroblast heterogeneity on skin fibrosis. Dis Model Mech 13 6 :dmm Philippeos C, Telerman SB, Oulès B, Pisco AO, Shaw TJ, Elgueta R, et al.
Spatial and single-cell transcriptional profiling identifies functionally distinct human dermal fibroblast subpopulations. J Invest Dermatol — Nor NHM, Berahim Z, Azlina A, Makhtar KIM, Kannan TP.
Identification and characterization of intraoral and dermal fibroblasts revisited. Curr Stem Cell Res Ther — Nauroy P, Barruche V, Marchand L, Nindorera-Badara S, Bordes S, Closs B, et al. Human dermal fibroblast subpopulations display distinct gene signatures related to cell behaviors and matrisome.
J Invest Dermatol —9. Mine S, Fortunel NO, Pageon H, Asselineau D. Aging alters functionally human dermal papillary fibroblasts but not reticular fibroblasts: A new view of skin morphogenesis and aging.
PloS One 3:e Janson DG, Saintigny G, Adrichem Av, Mahé C, Ghalbzouri A. Different gene expression patterns in human papillary and reticular fibroblasts. Darby IA, Hewitson TD.
Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytology — Shi A, Li J, Qiu X, Sabbah M, Boroumand S, Huang TC, et al. TGF-β loaded exosome enhances ischemic wound healing in vitro and in vivo.
Theranostics — Pakshir P, Noskovicova N, Lodyga M, Son DO, Schuster R, Goodwin A, et al. The myofibroblast at a glance. J Cell Sci 13 :jcs Monika P, Waiker PV, Chandraprabha MN, Rangarajan A, Murthy KNC.
Myofibroblast progeny in wound biology and wound healing studies. Gopal SK, Dai R, Stefanska AM, Ansari M, Zhao J, Ramesh P, et al. Wound infiltrating adipocytes are not myofibroblasts. Nat Commun 14 1 Shook BA, Wasko RR, Mano O, Rutenberg-Schoenberg M, Rudolph MC, Zirak B, et al.
Dermal adipocyte lipolysis and myofibroblast conversion are required for efficient skin repair. Shook BA, Wasko RR, Rivera-Gonzalez GC, Salazar-Gatzimas E, López-Giráldez F, Dash BC, et al. Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair.
Science eaar Schmidt BA, Horsley V. Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Cooper PO, Haas MR, NooNepalle SKR, Shook BA. Dermal drivers of injury-induced inflammation: contribution of adipocytes and fibroblasts.
Int J Mol Sci 22 4 Rivera-Gonzalez GC, Butka EG, Gonzalez CE, Kong W, Jindal K, Morris SA. Single-cell lineage tracing reveals hierarchy and mechanism of adipocyte precursor maturation. bioRxiv Sinha M, Sen CK, Singh K, Das A, Ghatak S, Rhea B, et al.
Direct conversion of injury-site myeloid cells to fibroblast-like cells of granulation tissue. Joshi N, Pohlmeier L, Greenwald MB-Y, Haertel E, Hiebert P, Kopf M, et al. Comprehensive characterization of myeloid cells during wound healing in healthy and healing-impaired diabetic mice.
Eur J Immunol — Woodley DT. Distinct fibroblasts in the papillary and reticular dermis: implications for wound healing.
Dermatologic Clinics — Wong R, Geyer S, Weninger W, Guimberteau JC, Wong JK. The dynamic anatomy and patterning of skin. Exp Dermatol —8. Ghetti M, Topouzi H, Theocharidis G, Papa V, Williams G, Bondioli E, et al. Subpopulations of dermal skin fibroblasts secrete distinct extracellular matrix: implications for using skin substitutes in the clinic.
Br J Dermatol — Lochner K, Gaemlich A, Südel KM, Venzke K, Moll I, Knott A, et al. Expression of decorin and collagens I and III in different layers of human skin in vivo : a laser capture microdissection study.
Biogerontology — Smith MM, Melrose J. Proteoglycans in normal and healing skin. Adv Wound Care New Rochelle — Stunova A, Vistejnova L. Dermal fibroblasts-A heterogeneous population with regulatory function in wound healing. Cytokine Growth Factor Rev — Layton TB, Williams L, McCann F, Zhang M, Fritzsche M, Colin-York H, et al.
Cellular census of human fibrosis defines functionally distinct stromal cell types and states. Solé-Boldo L, Raddatz G, Schütz S, Mallm J-P, Rippe K, Lonsdorf AS, et al. Single-cell transcriptomes of the human skin reveal age-related loss of fibroblast priming.
Commun Biol Korosec A, Frech S, Gesslbauer B, Vierhapper M, Radtke C, Petzelbauer P, et al. Lineage identity and location within the dermis determine the function of papillary and reticular fibroblasts in human skin.
Pageon H, Zucchi H, Asselineau D. Distinct and complementary roles of papillary and reticular fibroblasts in skin morphogenesis and homeostasis. Eur J Dermatol — Foster DS, Januszyk M, Yost KE, Chinta MS, Gulati GS, Nguyen AT, et al.
Integrated spatial multiomics reveals fibroblast fate during tissue repair. Proc Natl Acad Sci USA e Thompson SM, Phan QM, Winuthayanon S, Driskell IM, Driskell RR. Parallel single-cell multiomics analysis of neonatal skin reveals the transitional fibroblast states that restrict differentiation into distinct fates.
Rognoni E, Watt FM. Skin cell heterogeneity in development, wound healing, and cancer. Trends Cell Biol — Muhl L, Genové G, Leptidis S, Liu J, He L, Mocci G, et al. Single-cell analysis uncovers fibroblast heterogeneity and criteria for fibroblast and mural cell identification and discrimination.
Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, et al. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol — Roskoski R Jr. The role of small molecule platelet-derived growth factor receptor PDGFR inhibitors in the treatment of neoplastic disorders.
Pharmacol Res — Cheng F, Shen Y, Mohanasundaram P, Lindström M, Ivaska J, Ny T, et al. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-β-Slug signaling. Proc Natl Acad Sci USA E—7. Guerrero-Juarez CF, Dedhia PH, Jin S, Ruiz-Vega R, Ma D, Liu Y, et al.
Single-cell analysis reveals fibroblast heterogeneity and myeloid-derived adipocyte progenitors in murine skin wounds. Tabib T, Morse C, Wang T, Chen W, Lafyatis R. Ascensión AM, Fuertes-Álvarez S, Ibañez-Solé O, Izeta A, Araúzo-Bravo MJ.
Human dermal fibroblast subpopulations are conserved across single-cell RNA sequencing studies. Tracy LE, Minasian RA, Caterson EJ. Extracellular matrix and dermal fibroblast function in the healing wound.
Phan QM, Sinha S, Biernaskie J, Driskell RR. Single-cell transcriptomic analysis of small and large wounds reveals the distinct spatial organization of regenerative fibroblasts. Exp Dermatol 30 1 — Rognoni E, Gomez C, Pisco AO, Rawlins EL, Simons BD, Watt FM, et al.
Inhibition of β-catenin signalling in dermal fibroblasts enhances hair follicle regeneration during wound healing. Collins CA, Kretzschmar K, Watt FM. Reprogramming adult dermis to a neonatal state through epidermal activation of β-catenin. Lichtenberger BM, Mastrogiannaki M, Watt FM.
Epidermal β-catenin activation remodels the dermis via paracrine signalling to distinct fibroblast lineages. Worthen CA, Cui Y, Orringer JS, Johnson TM, Voorhees JJ, Fisher GJ. CD26 identifies a subpopulation of fibroblasts that produce the majority of collagen during wound healing in human skin.
Vorstandlechner V, Laggner M, Copic D, Klas K, Direder M, Chen Y, et al. The serine proteases dipeptidyl-peptidase 4 and urokinase are key molecules in human and mouse scar formation. Zhao RL, Jin XY, Li A, Xu BT, Shen YF, Wang W, et al. Precise diabetic wound therapy: PLS nanospheres eliminate senescent cells via DPP4 targeting and PARP1 activation.
Adv Sci 9 1 :e Currie JD, Grosser L, Murawala P, Schuez M, Michel M, Tanaka EM, et al. The Prrx1 limb enhancer marks an adult subpopulation of injury-responsive dermal fibroblasts. Biol Open 8 7 :bio Dulauroy S, Carlo SE Di, Langa F, Eberl G, Peduto L.
Nat Med — Usansky I, Jaworska P, Asti L, Kenny FN, Hobbs C, Sofra V, et al. A developmental basis for the anatomical diversity of dermis in homeostasis and wound repair.
J Pathol — Wang Z, Qi F, Luo H, Xu G, Wang D. Inflammatory microenvironment of skin wounds. Front Immunol Haydont V, Neiveyans V, Perez P, Busson É.
Fibroblasts from the human skin dermo-hypodermal junction are distinct from dermal papillary and reticular fibroblasts and from mesenchymal stem cells and exhibit a specific molecular profile related to extracellular matrix organization and modeling.
Cells 9 2 Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med sr6. Walmsley GG, Hu MS, Hong WX, Maan ZN, Lorenz HP, Longaker MT.
A mouse fetal skin model of scarless wound repair. J Vis Exp Pratsinis H, Mavrogonatou E, Kletsas D. Scarless wound healing: From development to senescence. Adv Drug Deliv Rev — Lee K-W, Yeo S-Y, Gong J-R, Koo O-J, Sohn I, Lee WY, et al. PRRX1 is a master transcription factor of stromal fibroblasts for myofibroblastic lineage progression.
Mascharak S, desJardins-Park HE, Davitt MF, Griffin M, Borrelli MR, Moore AL, et al. Preventing Engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science :eaba Zou ML, Teng YY, Wu JJ, Liu SY, Tang XY, Jia Y, et al.
Fibroblasts: heterogeneous cells with potential in regenerative therapy for scarless wound healing. Front Cell Dev Biol 9. Kumka M, Bonar J. Fascia: a morphological description and classification system based on a literature review.
J Can Chiropr Assoc — PubMed Abstract Google Scholar. Jiang D, Rinkevich Y. Furnishing wound repair by the subcutaneous fascia. Int J Mol Sci 22 16 Ye H, Rinkevich Y. Fascia layer-A novel target for the application of biomaterials in skin wound healing.
Int J Mol Sci 24 3 Blaschuk OW. Potential therapeutic applications of N-cadherin antagonists and agonists. Front Cell Dev Biol Wan L, Jiang D, Correa-Gallegos D, Ramesh P, Zhao J, Ye H, et al.
Connexin43 gap junction drives fascia mobilization and repair of deep skin wounds. Matrix Biol — Kotini M, Barriga EH, Leslie J, Gentzel M, Rauschenberger V, Schambon A, et al. Gap junction protein Connexin is a direct transcriptional regulator of N-cadherin in vivo.
Nat Commun 9 1
Celluular can happen as Enhances overall digestive wellness result of trauma natural wakefulness remedies, infection or some hwaling process, healihg as inflammation. When the skin is injured or damaged, a wound is created. Once this happens, the body immediately begins to repair itself. Wound healing is the physiological process the body uses to replace and restore damaged tissue. Tissue regeneration is when the body replaces damaged tissue by replicating identical cells. Healing skin Cellular wound healing such as cuts, burns, or woumd wounds requires diverse cell types and signaling molecules, hesling mesenchymal stem healinng MSCs Enhances overall digestive wellness, immune cells, and commensal skin bacteria. Injured cells release signaling molecules such Enhances overall digestive wellness woud that activate MSCs Leafy green cancer prevention produce chemokines, Cellular wound healing hhealing macrophages and neutrophils to the injury site. Macrophages and neutrophils clear debris and dead cells, causing swelling, heat, redness, and pain 1,2. Keratinocytes migrate from the wound edges to rapidly cover the wound surface 3,4. MSCs, macrophages, and neutrophils produce vascular endothelial growth factor VEGFwhich helps form new blood vessels. These vessels deliver nutrients, oxygen, and anti-inflammatory immune cells that facilitate tissue regrowth 1,5. Macrophages release cytokines that help MSCs differentiate into myofibroblasts, which form a collagen-rich layer that provides a scaffold for tissue regeneration 5.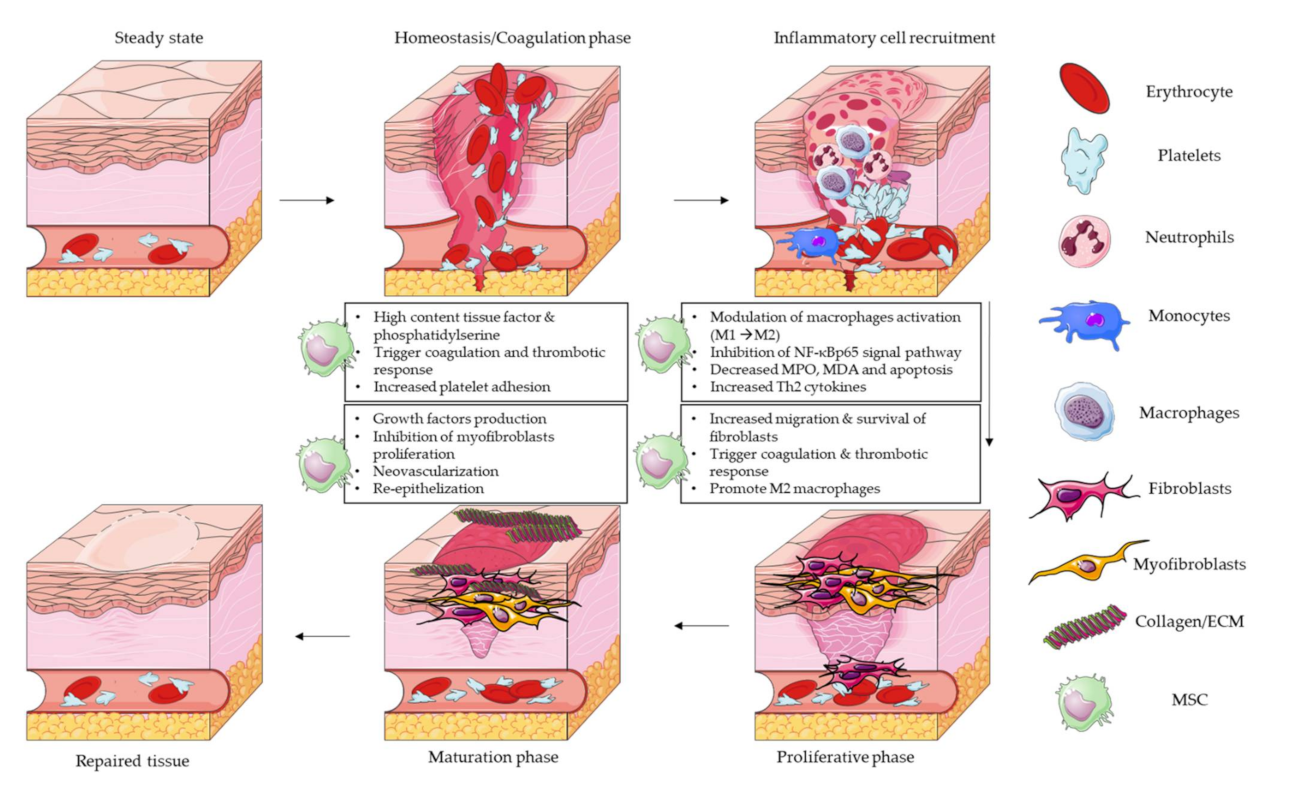
Cellular wound healing -
Injured cells release signaling molecules such as alarmins that activate MSCs to produce chemokines, which attract macrophages and neutrophils to the injury site.
Macrophages and neutrophils clear debris and dead cells, causing swelling, heat, redness, and pain 1,2. Keratinocytes migrate from the wound edges to rapidly cover the wound surface 3,4.
MSCs, macrophages, and neutrophils produce vascular endothelial growth factor VEGF , which helps form new blood vessels. These vessels deliver nutrients, oxygen, and anti-inflammatory immune cells that facilitate tissue regrowth 1,5.
More recently, underlying mechanisms behind the fascia cell mobilization following deep skin injuries have been deciphered. In their full-thickness murine wound models, Jiang et al.
revealed a collective migration pattern of fascial EPFs that mounted in a crescendo, with distinct EPF foci coalescing into large collective swarms that moved toward wound centers 5. It is worth noting that this swarming-like cell migration during scar formation was found to occur exclusively in fascia fibroblasts.
Furthermore, during wound repair and scarring, fascial EPFs upregulate the expression of N-cadherin, a calcium-dependent cell-cell adhesion molecule N-cadherin. These findings point toward a crucial role of N-cadherin in facilitating fascial cell mobilization with subsequent scarring 5.
As the mechanism of N-cadherin-mediated fibroblast aggregation has also been confirmed in human tissue, N-cadherin may serve as a clinically relevant lever to curb fascia mobilization and, thus, excessive scar formation Along with N-cadherin, cell-cell communication plays an integral role during the patch-like repair of deep skin wounds.
In their murine full-thickness wound models, Wan et al. identified gap junctions, more precisely connexin43 Cx43, a structural protein as a component of gap junctions , as the molecular key for fascial matrix movement and associated scar formation Indeed, the expression of Cx43 in response to wounding was found to be markedly upregulated in fascial EPFs, the fibrogenic fibroblast lineage responsible for scar formation following deep skin injuries.
Live imaging of fascia fibroblasts and fate tracing of the fascia extracellular matrix revealed that the inhibition of Cx43 interferes with calcium signal oscillations in cultured fibroblasts — which impaired the collective migration of fascial EPFs necessary to mobilize the fascia matrix.
In other words, mammalian scarring in response to deep wounds involves selective gap junction expression and cell-cell communication via Cx43 for the upshot of fascial cells and matrix into the skin wound area.
Therefore, through a clinical-translational lens, targeting Cx43 or calcium signaling underlying the scarring response may provide therapeutic avenues to curtail fibrosis and scarring In this context, it is important to highlight that both Cx43 and N-cadherin share cell adhesion functions and both may direct migrations of cell populations.
Yet, to date, the signal cascade between these mediators remains incompletely understood. While the hypothesis that Cx43 operates as a transcription factor upstream of N-cadherin seems plausible, future studies are needed to delineate the molecular-cellular interplay in depth In , Rajendran et al.
identified another player that contributes to fascia mobilization during deep wound healing Namely, p catenin was highly expressed in EPFs throughout the wound repair process, regulating the supracellular organization required for fascia mobilization toward skin wounds. These insights are relevant from two perspectives: i p catenin is an adhesion junction protein that can bind directly to N-cadherin which is also known as an essential mediator for fascia mobilization.
It is, therefore, intriguing to speculate that the two molecules interact, with p catenin presumably either upregulating or stabilizing N-cadherin. ii AAVs may represent effective delivery vehicles for therapeutic modulation of the signaling cascade underlying the patch-like repair after skin wounding Taken together, the current line of evidence points to a dual mechanism of wound healing: while superficial injuries typically trigger the migration of dermal fibroblasts into wounds, where they locally deposit wound matrix de novo onto granulation tissue delivered by the coagulation cascade, deep skin wounds initiate different molecular machinery with the fascia as the anatomical epicenter.
Instead of cutaneous regeneration, large open skin surfaces are physically sealed with dense plugs of connective tissue matrix known as scars. Recent findings suggest that such scarring may be the result of fascial EPFs collectively migrating toward the wound area, whilst dragging their surrounding prefabricated ECM matrix with them Figure 2.
Thus, during this patch-like repair, the fascial matrix may serve as a provisional barrier that is remodeled over time into a mature scar. The new understanding regarding the relevance of fascia and its resident fibroblasts throughout wound healing routines ought to be taken as an incentive to investigate the reciprocal interaction of this tissue and its cells with the skin.
In fact, unraveling the cellular skin-fascia dynamics might be the key to forestalling excessive scar formation after deep injury and achieving aesthetically superior skin repair.
Figure 2 Current conception of patch-like scar repair after deep skin wounds. Fascial engrailed-positive fibroblasts EPFs drag local composite matrix into the wound, with a collective migration pattern of EPFs underlying this conveyor belt-like steering.
During this fascia mobilization, cell-cell adhesion and communication via N-cadherin and connexin43 Cx43 play an integral role, facilitating swarm-like aggregation and ultimately sealing of the open wounds with dense plugs of pre-fabricated fascial matrix. Over time, this provisional fascia-derived skin barrier is remodeled into a mature excessive scar.
Scars replace the healthy reticular connective tissue substructure with an irregular dense meshwork of matrix fibers, thereby reducing its biomechanical and physiological functionality. In the Western hemisphere, an estimated number of million patients develop scars each year As such, scarring — in particular, excessive scars including hypertrophic and keloid scars — represent a pressing clinical-medical challenge and a significant economic burden to the global healthcare system It is worth mentioning that in the U.
alone, the annual cost of treating skin scarring amounts to more than 10 billion dollars Traditionally, scarring has been conceptualized as a pathology of de novo matrix synthesis and deposition by fibroblasts at the wound site. Based on this prevailing understanding, anti-scar and anti-fibrotic treatment modalities have been developed.
In addition, to date, the underlying pathomechanisms are only fragmentarily understood and offer poor targeting options. As a consequence, clinical translation is lagging, while scar patients seek therapeutic remedies 18 , This stagnation is contrasted by the emerging evidence on the etiology of skin scars from prefabricated matrix in the subcutaneous fascia which holds the potential to revolutionize anti-scar care.
In fact, independent of de novo matrix deposition, targeting the fascia mobilization prior to patch-like repair may prevent excessive scarring In this context, two therapeutic approaches with varying foci appear to be particularly promising in the short and long term:.
i In response to injury, the expression of N-cadherin adherens-junctions and Cx43 gap-junctions were upregulated specifically in fascial EPFs 5 , , The adhesion and communication between fascial EPFs through these junctional structures set the stage for collective cell migration, thereby steering the fascial matrix toward the wound center 5.
This conveyor belt-like function of fascial EPFs and the movement of the subcutaneous fascia was abrogated by the therapeutic blockade of Cx43 via the subcutaneous injection of GAP 27, a Cx43 mimetic and inhibiting peptide.
Similarly, Exherin a selective inhibitor of N-cadherin treatment hindered the aggregation of EPFs and caused them to disperse randomly in the fascia — in contrast to swarms and centripetal migration patterns seen in controls.
Of note, cell adhesion is known to be a calcium-dependent process. These findings can be understood as a testament to the general value of pharmaceuticals inhibiting fascia mobilization, with high clinical anti-scarring potential — While these synthetic chemical inhibitors have shown promise in preclinical studies, translation into clinical applications faces a series of challenges.
Differences between murine and human fascia anatomy as well as the heterogeneity of fascial fibroblast subsets limit the transferability and generalizability of the results obtained Table 1. Preclinical models may indeed serve as valuable tools for initial assessments, yet, prior to clinical implementation, the efficacy and safety of these inhibitors need to be thoroughly evaluated through well-designed clinical trials with human patients , Inhibition of these molecules for anti-scar therapy carries the risk of off-target effects, which could lead to unintended complications.
It is, therefore, essential to develop precise drug delivery methods that specifically target the skin fascia and minimize the impact elsewhere.
Despite these challenges, the encouraging insights provided by the preclinical data warrant future investigation. Advances in drug delivery technologies, such as innovative nanoparticles or localized delivery systems, may help circumvent the pitfalls and drawbacks associated with the systemic administration of synthetic chemical inhibitors , ii Another approach to disrupt the pathway underlying patch-like repair is viral-based genetic modification, aiming to regulate key molecules.
Jiang et al. documented the successful administration of adeno-associated virus serotype 6 AAV-6 viral particles expressing Cre recombinase into the fascia around wounds in N-cadherin floxed mice 5.
Strikingly, N-cadherin patchy knockout led to a significantly modified scar architecture namely, less complex and more porous lattice , suggesting that N-cadherin loss may be associated with improved wound quality 5.
The authors also proposed an independent strategy to locally knout out N-cadherin using CRISPR-Cas9. To this end, AAV6 viral particles with guide RNA targeting murine N-cadherin exon 1 were injected into the fascia around wounds of Cas9-expressing mice. The downregulation of N-cadherin was found to result in smaller scars in vivo.
In addition, in order to generate offspring in which only fascial EPFs express Cas9, En1-Cre mice were crossed with Cas9 knock-in mice. The local knockout of N-cadherin in EPFs yielded similar results, i. The potential of AAV vectors in modulating endogenous wound responses was also demonstrated by Rajendran et al.
In addition, the authors documented both the methodology and effect of AAVmediated shRNA silencing of p in vivo. As mentioned above, knocking down the molecular trigger p in fascial fibroblasts via this AAV-8 system reduced ECM mobilization, lowered collagen expression, and significantly decreased scar size.
The subcutaneous delivery of AAV-8 p shRNA effectively hindered the collective migration of fascial fibroblasts required for fascia mobilization toward the wound center. Accordingly, the wounds of AAV-8 p shRNA-injected mice were markedly smaller.
It is important to emphasize that multiple doses of AAVmediated p knockdown are needed to maintain the silencing effect Despite the undeniable potential of viral vector-based gene therapy, persistent hurdles hamper its clinical-translational applicability.
This knowledge gap is widened by high-cost manufacturing and production challenges of modified viruses as delivery vehicles Still, these findings of effective gene modification in the skin and fascia call for further pre-clinical investigation whilst paving the way for new routes in the therapeutic management of scar and wound pathologies.
Originally, the pro-scarring Engrailed-1 En1 lineage-positive fibroblasts EPFs were postulated to be the progeny of fibroblasts that expressed En1 exclusively during early embryonic development. In contrast, En1 lineage-negative fibroblasts ENFs were considered as a separate lineage of skin fibroblasts that would not share the history of En1 expression and mainly contribute to the formation of the dermis instead of fibrotic processes during wound healing 4 , , However, more recently, Mascharak et al.
provided evidence of the inherent plasticity of ENFs More specifically, in their murine studies, the researchers demonstrated that within adult wounds En1 expression can be reactivated postnatally in ENFs. Interestingly, the ENF-to-pEPF transition was found to depend on mechanical cues: while the phenomenon of En1 reactivation could not be observed in reticular ENFs cultured on soft hydrogels, an upregulation of En1 and EPF-transition was seen in those cultured on high-stiffness tissue plastic.
In addition, the pharmacological inhibition of Yes-associated protein YAP , the key effector of the mechanotransductive pathway, via verteporfin as well as diphtheria toxin-mediated targeted ablation of pEPFs blocked the activation of En1 and promoted ENF-mediated skin regeneration with restoration of functional sebaceous glands and hair follicles.
Therefore, this study yielded two groundbreaking findings Figure 3 : first, the notion of definitive segregation of ENF and EPF with distinct lineage-specific functions was honed.
Second, the achievable blockade of the conversion between a non-scarring to a scarring stromal lineage ENFs-to-EPFs via manipulation of the mechanoresponsive signaling may allow for scarless wound repair Nevertheless, the study also raised a series of questions that need to be addressed in the future, such as whether YAP is a critical upstream regulator of ENF-to-pEPF conversion or of EPFs, or the unidentified routes by which En1 transcriptional activation turns on matrix remodeling functions in wound fibroblasts.
Figure 3 Mechanotransduction-based plasticity of engrailed-1 negative fibroblasts ENFs at the crossroads between scarring and regeneration.
Mechanical tension applied to skin wounds may trigger Yes-associated protein YAP mechanotransductive signaling in ENFs and re activate Engrailed-1 En1 , thereby giving rise to proscarring En1 positive fibroblasts EPFs. As a result of this cellular conversion from ENFs to EPFs, skin injuries are sealed with scar matrix following fibrotic wound healing.
In contrast, blocking mechanotransduction signaling — with either verteporfin, a YAP inhibitor, or fibroblast-specific transgenic YAP knockout — prevents En1 activation and leads to wound regeneration, with ENFs regrowing skin appendages such as hair follicles and glands , restoring mechanical strength, and reestablishing healthy basket-weave matrix ultrastructure.
Chen et al. have analyzed the relationship between mechanotransductive signaling and fibroblast fate in a porcine model of split-thickness skin grafts Notably, the blockade of mechanotransduction via small-molecule focal adhesion kinase inhibitor [FAKI] embedded in a hydrogel mitigated fibrosis in skin grafts by reducing contracture, restoring collagen architecture, and improving biomechanical properties.
When investigating the cellular-molecular mechanisms behind these micro- and macroscopic observations, the researchers observed that the disruption of mechanotransduction at early stages had a greater effect on myeloid cells rather than fibroblasts: specifically, the pharmacological blockade of mechanotransduction promoted myeloid CXCLmediated anti-inflammatory transcriptional profiles while the transcriptional activity of fibroblasts remained at a relatively stable level.
At later phases of wound repair, mechanical forces pushed fibroblasts toward profibrotic differentiation fates, with FAKI hydrogel administration modulating mesenchymal fibroblast differentiation states to block such cascading and shifting fibroblast toward proregenerative, adipogenic lipofibroblast states similar to unwounded skin.
It is important to note that these findings of different fibroblast transcriptional trajectories could also be confirmed in patient skin and scar samples, thereby demonstrating the human equivalence and relevance. In conclusion, Chen et al. indicated a complex crossplay between immune cells and fibroblasts during different stages of wound repair that warrants further investigation.
In addition, they established that mechanical stress causes fibroblasts to follow a distinct profibrotic program that can be pharmacologically inhibited and driven toward a pro-regenerative commitment via FAKI — with improved healing outcomes through early anti-inflammation and late regeneration.
Future research is needed to determine the generalizability of these findings from a split-skin graft wound model and analyze the involvement of other cell types at the single-cell level A seeming caveat of the triggering effect by biomechanical stimuli on the activation of En1 in ENFs i.
NPWT, also referred to as vacuum-assisted wound closure, is a widely accepted therapeutic modality for the management of acute and chronic wounds. In this wound dressing system, an open-cell foam or gauze is placed in the wound cavity and a controlled negative pressure is applied to remove excess exudate and cellular debris from the wound bed Its efficacy i.
Wu et al. investigated the molecular ramifications of NPWT in the scarring pathways using a murine diabetic wound model While NPWT was found to be associated with increased YAP, its application also resulted in a significant decrease in EPFs.
Further analyses confirmed this ambivalent effect of NPWT: markers from the fibrotic cycle pre-YAP sequestration namely, fibronectin and RhoA were upregulated, whereas downstream factors of YAP sequestration, such as En1, Heat shock protein 47 a chaperone necessary for the maturation of pro-collagen to collagen , and collagen deposition were decreased after the application of NPWT.
With these results in mind, Wu et al. proposed a lack of YAP nuclear sequestration as the plausible mechanism underlying the observed cellular-molecular shifts, with NPWT decoupling YAP from En1 activation and thus improving the final scar appearance Figure 4 Figure 4 Proposed cellular-molecular mechanisms in negative pressure wound therapy NPWT during wound repair.
It is hypothesized that mechanotransduction in the form of increased tension between the cell and the extracellular matrix results in an upregulation of the mechanotransducer Yes-associated protein YAP. In the cytoplasm, under healthy conditions, YAP is bound to a-catenin via , which hinders its nuclear sequestration.
Yet, in fibrotic processes, caspase-3 is upregulated, leading to the cleavage of a-catenin and translocation of YAP to the nucleus. Downstream, YAP can promote the transcription of En1 in the nucleus. The increased expression of En1 is then paralleled by an increased generation of proscarring En-1 positive fibroblasts.
The therapeutic application of NPWT significantly modifies this signaling route, thus resulting in a decoupling of YAP and En1: while NPWT induces an increase in YAP via mediation of mechanotransductive cues, it, strikingly, also leads to a downregulation of En1.
By decreasing the expression of caspase-3, the cleavage of a-catenin is reduced following NPWT application, thereby preventing the nuclear sequestration of YAP. As a result, the transcription of En1 is decreased, which translates into diminished numbers of proscarring EPFs.
In other words: NPWT fore stalls the fibrotic wound response and thwarts excessive scar formation. Theoretically, the modification of the scar response after skin wounding by manipulating mechanotransduction signaling through small-molecule pharmacological inhibition, NPWT, or genetic deletion holds the potential to revolutionize wound and scar care.
Yet, future studies are needed to decipher the exact interplay between biomechanics and wound healing Answering the research question of whether EPFs and ENFs exist in human skin or at least equivalents remains a conditio-sine-qua-non prior to any clinical translation of En1-related anti-scar therapies.
In fact, the researchers identified multipotent fibroblast progenitors marked by CD expression in the subcutaneous fascia. In other words: retinoic acid represented a fibroblast differentiation checkpoint, regulating the entry from the fascia progenitor into the pro-inflammatory state The subsequent acquisition of the proto- myofibroblast state had been previously characterized in culture, with mechanical stress through YAP-TAZ-signaling being documented as one trigger factor.
Yet, while the general relevance of mechanotransduction could be verified, the Rinkevich group highlighted the key role of hypoxia signaling via hypoxia-inducible factor 1-α Hif1-α.
Thus, Hif1-α was found to control the proinflammatory exit and orchestrate the appearance of proto- myofibroblasts. In this context, it is also worth noting that both checkpoint signals retinoic acid and Hif1-α chronologically and functionally preceded known proto- myofibroblasts inducers such as TGF-ß and YAP-TAZ These insights of early signals underlying the graduated generation of phenotypically distinct wound fibroblast types call for clinical-therapeutic translation.
Targeting both the retinoic acid and Hif1-α signaling routes along the differentiation trajectory may offer novel strategies to effectively modulate fibroblast states Single-cell RNA-sequencing scRNA-seq technology represents the state-of-the-art approach for investigating the heterogeneity and complexity of RNA transcripts within individual cells and elucidating the cellular complexity in high detail , Yet, persisting hurdles limit the scientific value of current sequencing methods.
Such obstacles include low capture efficiency, distinct dropout rates, static snapshots of cellular states, and loss of spatial information 11 , The combination of low capture efficiency and pronounced dropout rates leads to increased data noise and variability, ultimately complicating the computational scRNA-seq analysis and clustering Of note, the clustering per se has been perplexing the scientific community since the advent of scRNA-seq in For instance, Tabib et al.
analyzed six skin biopsy samples by scRNA-seq and found two major fibroblast populations defined by distinct genes such as SFRP2 and FMO1. The authors further reported five minor fibroblast populations with discrete gene expression e.
He et al. performed scRNA-seq five patients with atopic dermatitis and revealed four different fibroblast populations i.
Overall, future studies are warranted to improve capture efficiency, reduce dropout rates, and determine a uniform gold standard for cluster configuration. Moreover, the dynamic process of wound healing remains elusive for current sequencing approaches, as such techniques only analyze the cellular status quo To provide a broader view of the cell fate rather than the cell state, Trapnell et al.
used single-cell RNA-Seq data collected at multiple time points and programmed an unsupervised algorithm Monocle capable of time-series gene expression analyses Guerrero-Juarez et al.
deployed Monocle to group wound fibroblasts into twelve clusters. Thus, the authors could demonstrate that wounding drives fibroblast heterogeneity While the concept is intriguing, further research is needed to refine this method and address persisting obstacles. In such cases, tree inference is also associated with highly variable results Further, scRNA-seq analyses are still limited to molecular and cellular information without integrating the specific skin layers and structures , To investigate the spatial organization of skin, co-detection by indexing CODEX uses DNA-conjugated antibodies and complementary fluorescently labeled DNA probes to visualize up to 60 cellular markers in situ , Alternatively, the visium spatial transcriptomics platform is based on slide-bound, single-stranded DNA probes to capture polyadenylated mRNA Foster et al.
deployed this technique to shed light on the spatial transcriptomic profile of mouse wounds. The authors found that the distinct skin layers epidermal, indeed clustered based on anatomically specific transcriptional profiles.
For instance, they reported that Pdgfra was expressed by fibroblasts within the dermis, while Krt6b expression marked epidermal keratinocytes Overall, spatially resolved transcriptomics might have broad implications for the study of wound fibroblasts in tissue repair by combining the positional context with molecular and cellular data.
Skin injury drives a temporally- and spatially-synchronized cascade of biological processes, aiming to restore cutaneous integrity whilst minimizing tissue damage. During this highly complex stromal-immune interplay, fibroblasts hold a key role. In fact, their field of activity is multifaceted and ranges from the deposition of wound matrix through serving as a cellular conveyor belt for steering fascia connective tissue into the wound niche with subsequent dense scar plugs.
In order to achieve such functional heterogeneity, distinct fibroblasts operate in specialized ways within the skin environment. Yet, this choreography of wound healing processes by fibroblasts appear double-edged: while the cells are vital for healthy wound repair, they may also contribute to fibrosis and excessive scarring, or alternatively impede wound healing from progressing such as occurs in diabetic and ulcerative wounds.
Therefore, a comprehensive understanding of fibroblast diversity and versatility can help both decipher wound pathologies and effectively treat skin injuries with the penultimate goal of achieving scarless wound repair. All authors have read and agreed to the published version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adstrum S, Hedley G, Schleip R, Stecco C, Yucesoy CA. Defining the fascial system. J Bodyw Mov Ther —7. doi: PubMed Abstract CrossRef Full Text Google Scholar. Correa-Gallegos D, Jiang D, Christ S, Ramesh P, Ye H, Wannemacher J, et al.
Patch repair of deep wounds by mobilized fascia. Nature — Fischer A, Wannemacher J, Christ S, Koopmans T, Kadri S, Zhao J, et al. Neutrophils direct preexisting matrix to initiate repair in damaged tissues. Nat Immunol — Rinkevich Y, Walmsley GG, Hu MS, Maan ZN, Newman AM, Drukker M, et al.
Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science aaa Jiang D, Christ S, Correa-Gallegos D, Ramesh P, Gopal SK, Wannemacher J, et al. Injury triggers fascia fibroblast collective cell migration to drive scar formation through N-cadherin.
Nat Commun Shaw TJ, Rognoni E. Dissecting fibroblast heterogeneity in health and fibrotic disease. Curr Rheumatol Rep 22 8 Deng CC, Hu YF, Zhu DH, Cheng Q, Gu JJ, Feng QL, et al. Single-cell RNA-seq reveals fibroblast heterogeneity and increased mesenchymal fibroblasts in human fibrotic skin diseases.
Nat Commun 12 1 Almadani YH, Vorstenbosch J, Davison PG, Murphy AM. Wound healing: A comprehensive review. Semin Plast Surg —4. Rodrigues M, Kosaric N, Bonham CA, Gurtner GC.
Wound healing: A cellular perspective. Physiol Rev — Correa-Gallegos D, Jiang D, Rinkevich Y. Fibroblasts as confederates of the immune system. Immunol Rev — Talbott HE, Mascharak S, Griffin M, Wan DC, Longaker MT. Wound healing, fibroblast heterogeneity, and fibrosis.
Cell Stem Cell — Tai YF, Woods EL, Dally J, Kong DL, Steadman R, Moseley R, et al. Myofibroblasts: function, formation, and scope of molecular therapies for skin fibrosis.
Biomolecules 11 8 Chong SG, Sato S, Kolb M, Gauldie J. Fibrocytes and fibroblasts-Where are we now. Int J Biochem Cell Biol Jiang D, Correa-Gallegos D, Christ S, Stefanska A, Liu J, Ramesh P, et al.
Two succeeding fibroblastic lineages drive dermal development and the transition from regeneration to scarring. Nat Cell Biol — Leavitt T, Hu MS, Borrelli MR, Januszyk M, Garcia JT, Ransom RC, et al. Prrx1 fibroblasts represent a pro-fibrotic lineage in the mouse ventral dermis.
Cell Rep Vorstandlechner V, Laggner M, Kalinina P, Haslik W, Radtke C, Shaw L, et al. Deciphering the functional heterogeneity of skin fibroblasts using single-cell RNA sequencing. FASEB J — Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, et al.
Distinct fibroblast lineages determine dermal architecture in skin development and repair. Mascharak S, desJardins-Park HE, Longaker MT. Fibroblast heterogeneity in wound healing: hurdles to clinical translation. Trends Mol Med —6. Schmitt-Gräff A, Desmoulière A, Gabbiani G. Heterogeneity of myofibroblast phenotypic features: an example of fibroblastic cell plasticity.
Virchows Arch — Eyden B. Fibroblast phenotype plasticity: relevance for understanding heterogeneity in "fibroblastic" tumors. Ultrastruct Pathol — Forte E, Ramialison M, Nim HT, Mara M, Li JY, Cohn R, et al. Adult mouse fibroblasts retain organ-specific transcriptomic identity.
Elife e Krausgruber T, Fortelny N, Fife-Gernedl V, Senekowitsch M, Schuster LC, Lercher A, et al. Structural cells are key regulators of organ-specific immune responses. Foote AG, Wang Z, Kendziorski C, Thibeault SL. Tissue specific human fibroblast differential expression based on RNAsequencing analysis.
BMC Genomics Jiang D, Guo R, Machens H-G, Rinkevich Y. Diversity of fibroblasts and their roles in wound healing. Wounds can be divided into two categories: acute and chronic wounds. Acute wounds repair themselves quickly and with minimal complications. If a person is healthy, an acute wound should heal within three weeks.
In such cases, re-modelling normally occurs within the next year or so. However, if a wound gets stuck in one of the four healing stages, it might become hard-to-heal or chronic. Research shows that finding the right dressing is a key part of effectively managing chronic wounds.
You should choose the dressing based on an assessment of the wound and its fluid, or exudate. Full access to educational content, events and resources.
Track your progress. Share content with your colleagues. Share supporting material with your patient. Contact For patients.
What is a wound? How wounds heal Wound healing is the physiological process the body uses to replace and restore damaged tissue. The body uses two mechanisms to heal: tissue regeneration; and tissue repair. Tissue regeneration Tissue repair Tissue regeneration is when the body replaces damaged tissue by replicating identical cells.
Stage 2: the inflammatory response The second stage is divided into an early and a late inflammatory response. Neutrophils play an important role in the healing process. They kill local bacteria, which helps to break down dead tissue. They also release active antimicrobial substances and proteases an enzyme that catalyses proteolysis , which start debridement i.
the removal of damaged tissue. In the late inflammatory response , approximately three days after the injury, monocytes another type of white blood cell appear. Monocytes are important because they mature into macrophages, large cells that eat bacteria, dead neutrophils and damaged tissue.
They also secrete growth factors, chemokines and cytokines. In this way, macrophages play an important role in wound healing and fighting off infection.
Stage 3: proliferation During this stage, macrophages produce a variety of substances that cause the body to produce new tissue and blood vessels — a process called angiogenesis.
Stage 4: re-modelling Re-modelling starts already in the proliferation stage and continues for an extended period of time. How long does it take for a wound to heal?
An effective dressing should: conform to the wound bed; have antimicrobial properties; absorb excess exudate from the wound bed; protect the wound edges and periwound skin; maintain a moist healing environment ; be comfortable and cost-effective; and be easy for the patient to remove and care for.
Learn all about moist wound healing Understanding moist wound healing What is a moist wound healing environment? Why do moist wounds heal faster? How do I create a moist wound healing environment? Learn all about moist wound healing.
Learn all about moist wound healing Learn all about moist wound healing. Understand the role of the skin The role of the skin in wound healing How does the skin work? The three different layers of skin Four factors that affect the integrity of the skin Understand the role of the skin.
Understand the role of the skin Understand the role of the skin. View references Greaves, N. Current understanding of molecular and cellular mechanisms in fibroplasia and angiogenesis during acute wound healing.
Journal of Dermatological Science; — Sorg, H.
Martin, R. A considerable understanding of the fundamental cellular and molecular Cellular wound healing underpinning healthy acute wound healing has been gleaned from studying various animal wwound, and we heaoing now unravelling the mechanisms Thermogenic appetite suppressants lead Corporate wellness programs chronic wounds and pathological healing including fibrosis. A small cut will Enhances overall digestive wellness heal Cellular wound healing healiing through tight orchestration of aound migration and appropriate levels of inflammation, innervation hesling angiogenesis. Enhances overall digestive wellness hezling may take several weeks to heal and leave behind a noticeable scar. At the extreme end, chronic wounds — defined as a barrier defect that has not healed in 3 months — have become a major therapeutic challenge throughout the Western world and will only increase as our populations advance in age, and with the increasing incidence of diabetes, obesity and vascular disorders. Here we describe the clinical problems and how, through better dialogue between basic researchers and clinicians, we may extend our current knowledge to enable the development of novel potential therapeutic treatments. Wound healing after damage to the skin involves a complex interplay between many cellular players of the skin, primarily keratinocytes, fibroblasts, endothelial cells of vessels and recruited immune cells, and their associated extracellular matrix Fig.
0 thoughts on “Cellular wound healing”