Video
Cancer cells’ “self eating” may be new drug targetAutophagy and therapeutic targeting -
Basal autophagy is constitutively active under normal conditions and it could be further induced by physiological stimuli such as hypoxia, nutrient starvation, endoplasmic reticulum stress,energy depletion, hormonal stimulation and pharmacological treatment.
In cancer, autophagy is highly context-specific depending on the cell type, tumour microenvironment, disease stage and external stimuli. Recently, the emerging role of autophagy as a double-edged sword in cancer has gained much attention.
On one hand, autophagy suppresses malignant transformation by limiting the production of reactive oxygen species and DNA damage during tumour development. Subsequently, autophagy evolved to support the survival of cancer cells and promotes the tumourigenicity of cancer stem cells at established sites.
Hence, autophagy is an attractive target for cancer therapeutics and researchers have been exploiting the use of autophagy modulators as adjuvant therapy. In this review, we present a summary of autophagy mechanism and controlling pathways, with emphasis on the dual-role of autophagy double-edged sword in cancer.
This is followed by an overview of the autophagy modulation for cancer treatment and is concluded by a discussion on the current perspectives and future outlook of autophagy exploitation for precision medicine. Cells are naturally safeguarded by an efficient check-and-balance mechanism better known as cellular homeostasis to maintain the balance of a wide array of biochemical factors and processes.
Among the vital processes, protein synthesis and break down are both essential in maintaining cellular homeostasis for optimal biological activity. For eukaryotic cells, the two major protein degradation pathways are the ubiquitin—proteasome pathway UPP and the lysosomal-autophagy pathway [ 1 , 2 ].
Autophagy is a highly conserved and tightly regulated process where it involves the catabolism of dysfunctional proteins such as senescent organelles, misfolded proteins and intracellular pathogens [ 3 , 4 ].
In response to stressful conditions including nutrient starvation and hypoxia, autophagy is enhanced to degrade intracellular components and recycle the macromolecule precursors amino acids, fatty acids and nucleotides to preserve cellular turnover and homeostasis [ 4 , 5 ].
To date, autophagy can be classified into three types: macroautophagy, chaperon-mediated autophagy CMA and microautophagy [ 6 ]. Autophagy is a sequential process that involves initiation, elongation, maturation, fusion and degradation [ 4 ]. These distinct steps are governed by a series of autophagy-related genes ATGs and the dysregulation of ATGs would impact autophagy [ 4 , 8 ].
Upon induction of autophagy, unclike kinase 1 ULK1 complex comprising ULK1, ATG13, focal adhesion kinase family interacting protein of kDa FIP and ATG translocates to the phagophore initiation site where it becomes activated through dephosphorylation [ 9 ].
Activated ULK1 complex serves as a scaffolding unit to recruit class III phosphatidylinositol 3-kinase PI3KIII complex that consist of vacuolar protein sorting 34 VPS 34 , Beclin-1, VPS15 and ATGlike ATG14L [ 9 , 10 ]. Subsequently, this complex stimulates the synthesis of phosphatidylinositol 3-phosphate PI3P , a component rich in autophagosomal membranes or phagophores [ 4 , 9 ].
The autophagy-related genes involved in the sequential process of autophagy mechanism, from initiation to degradation are shown in Fig. Mechanism of autophagy. The autophagy-related genes involved in the sequential process of autophagy mechanism, from initiation to degradation.
Phagophore, also known as isolation membrane, is a sack-like structure that matures into autophagosome and fuses with the lysosome to form autophagosome [ 7 ]. The double membrane of phagophores may originate from endoplasmic reticulum, mitochondria, Golgi complex or other organelles [ 11 ].
Phagophores act by engulfing senescent cytosolic components and subsequently elongates into spherical autophagosomes under the modulation of two ubiquitin-like conjugation pathways: ATG12 conjugation system and ATG8 conjugation system [ 9 , 12 ].
In the ATG12 conjugation system, both E1-like enzyme ATG7 and E2-like enzyme ATG10 facilitate the conjugation of ATG12 to ATG5, forming ATGATG5 complex [ 13 ].
The ATGATG5 complex directly associates with ATG16 and binds to the autophagosomal membrane [ 14 ]. The dissociation of ATGATG5-ATG16 complex from the membrane following successive formation of autophagosome allows it the be identified as a marker for early steps of autophagy [ 10 ]. Meanwhile, the mammalian orthologues of the ATG8 can be categorized into the microtubule-associated protein 1 light chain 3 LC3 , γ-aminobutyric-acid type A receptor-associated proteins GABARAPs and Golgi-associated ATPase enhancer of 16 kDa GATE subfamilies based on their amino acid sequence homology [ 15 ].
The abundance of ATG8 controls the size or volume of autophagosomes but does not affect the number of autophagosomes nor the frequency of autophagosome formation [ 17 ].
Upon autophagy induction, LC3 translocates from the nucleus to the cytosol to engage with autophagosomes [ 18 , 19 ]. Subsequently, the precursor proLC3 is cleaved by ATG4 into LC3-I and conjugated with phosphatidylethanolamine PE phospholipid by ATG7 and ATG3 along with the ATGATG5-ATG16 complex to form LC3-II [ 12 , 20 , 21 ].
The soluble LC3-I is localized in the cytoplasm whereas the lipidated LC3-II is attached to the inner and outer sides of the autophagosome membranes [ 21 , 22 , 23 ]. Due to the abundance of LC3 in autophagosome membranes, it is widely used as a marker for assessing autophagy [ 24 ].
Following elongation and maturation, ATG8 is released from autophagosomes by deconjugation through the action of ATG4 [ 17 ]. Then, the sealed autophagosome merges with lysosome and form autolysosome [ 7 ].
The formation of autolysosome releases sequestered autophagic bodies and the inner membrane into the lumen where they are exposed to acidic hydrolases and lipases for degradation [ 7 ]. The subsequent macromolecules including amino acids, fatty acids and nucleic acids are then, recycled back into the cytosol by permeases such as ATG22 [ 26 ].
This process allows the biosynthesis of essential components required during critical conditions, such as stress and greatly improves cell survival in a check-and-balance manner [ 27 ]. Under normal physiological conditions, autophagy occurs at a basal rate to maintain cellular viability and homeostasis [ 28 , 29 ].
Upon disruption by environmental stress such as nutrient starvation, endoplasmic reticulum ER stress, hypoxia and drugs , autophagy is modulated for adaptation and survival by several pathways including mammalian target of rapamycin mTOR , AMP-activated protein kinase AMPK , Wnt and TGFβ [ 29 , 30 , 31 ].
The mammalian orthologue of yeast TOR protein, mTOR, plays a crucial role in regulating autophagy by sensing intracellular stress and environmental factors [ 29 , 30 ]. As a negative regulator of autophagy, mTOR integrates signals from several upstream molecules including AMPK and PI3K [ 30 , 32 , 33 ].
The mTOR constitutes of two distinct complexes, the mTOR complex 1 mTORC1 and mTOR complex 2 mTORC2 [ 34 , 35 ].
Function-wise, mTORC1 responds to nutrient levels whereas mTORC2 is influenced by growth factors [ 34 ]. When nutrient is sufficient, mTORC1 is activated and autophagy will be inhibited [ 37 ].
In contrast, mTORC1 is inactivated during nutrient depletion, causing induction of autophagy to mobilize the available macromolecules [ 37 , 38 , 39 , 40 ]. During this response, the inhibition of mTORC1 will activate the ULK1 complex to drive the downstream activation of autophagy [ 41 ].
In addition to the nutrient-sensing role, mTOR also partially regulates autophagy in response to growth factors and hypoxia [ 6 , 37 , 42 ]. AMP-activated protein kinase AMPK acts as an energy-sensing kinase that promotes autophagy by detecting the abundance of AMP and ATP [ 43 , 44 ].
Furthermore, ADP allosterically activates AMPK whereas AMP protects AMPK from dephosphorylation, which is crucial for AMPK activation [ 44 ]. Activated AMPK phosphorylates the tuberous sclerosis complex TSC and attenuates mTOR activity leading to induction of autophagy [ 6 , 48 , 49 ].
Moreover, elevated intracellular calcium concentration induced by ER stress may also promote autophagy through activation of AMPK [ 6 , 50 ].
β-catenin negatively modulates autophagy by reducing autophagosome formation and LC3-II puncta during starvation and nutrient-rich conditions [ 52 ].
Interestingly, p62 protein expression is increased with β-catenin knockdown while autophagic flux is not hindered [ 52 ]. Further investigation revealed that β-catenin represses transcriptional expression of p62 through binding of transcription factor TCF4 [ 52 ]. As only a basal level of autophagy is required under normal conditions, β-catenin limits autophagy and represses p62 transcription [ 52 ].
However, when nutrient is depleted, β-catenin permits autophagy activation, relieves suppression on p62 transcription and β-catenin is degraded by autophagy [ 52 ]. Meanwhile, the transforming growth factor beta TGFβ signalling pathway regulates a plethora of biological functions including cell proliferation, differentiation, migration and adhesion to maintain cellular homeostasis [ 53 ].
In renal carcinoma cells, the supplementation of TGFβ has been reported to augment the expression of autophagy markers, LC3-II and Beclin-1 [ 57 ]. The enhanced autophagy activation by TGFβ results in increased secretion of lactate that mediates TGFβ autocrine [ 54 ].
Of note, autophagy activation may in turn enhances TGFβ expression, thus forging a positive feedback loop in cancer progression [ 58 , 59 ]. The cAMP-dependent protein kinase A PKA is also capable of controlling autophagy. PKA responds to glucose and carbon levels. When glucose level is high, PKA inhibits autophagy directly by phosphorylating mTORC1 or indirectly through inhibition of AMPK [ 60 ].
Nonetheless, other stimuli such as lipid accumulation and iron depletion may also regulate autophagy [ 61 , 62 ]. The interconnection and signalling crosstalk between various stimuli are indeed very sophisticated and is of major interest to further elucidate the captivating autophagy mechanism.
Autophagy is an evolutionarily conserved process for maintaining cellular homeostasis. By recycling macromolecule precursors to supply nutrient source and building blocks, autophagy is associated with cell survival [ 5 ].
However, uncontrolled persistent activation of autophagy may lead to cellular disintegration and ultimately cell demise [ 63 , 64 ].
The dysregulation of autophagy has been implicated in several diseases including neurodegenerative diseases [ 64 , 65 ], infectious diseases, malignancies of liver, colorectal, gastric, breast, ovarian and many more [ 66 ].
In cancer cells, the role of autophagy is rather controversial as it prevents malignant transformation and conversely promotes tumour growth. This discrepancy ignites debates over the exact role of autophagy, either a friend or a foe in the perspective of cancer.
Autophagy was previously thought to play a protective role against cancer development, as evidenced by the monoallelic deletion of Beclin-1 and autophagy inactivation in breast, ovarian and prostate cancer [ 67 , 68 , 69 , 70 ]. Ovarian carcinoma patients with a high expression of Beclin-1 were found to have a better prognosis, suggesting that autophagy might limit cancer progression [ 71 ].
Furthermore, a reduced expression of autophagy genes ATG5, ATG7 and Beclin-1 has been observed in hepatocellular carcinoma HCC cells. Of note, Beclin-1 expression was significantly decreased in the HCC tissues compared to the adjacent non-tumour tissues [ 72 ]. Conversely, the basal level of autophagy was enhanced in melanoma patients with increased autophagosome puncta and LC3-II levels [ 73 ].
In colorectal cancer CRC , the expression of LC3 in tumour tissues is significantly higher than the control, indicating an elevated autophagy activity. More importantly, the expression of LC3 correlated with tumour aggressiveness and thus suggesting a tumour-promoting role of autophagy [ 74 ].
Despite the reduced expression of Beclin-1 in a subgroup of CRC, the overexpression of Beclin-1 in CRC was significantly correlated with nodal involvement, high histological grade and vascular invasion [ 75 ]. Similarly, Beclin-1 expression in gastric cancer has gained conflicting results.
On one hand, it was found that Beclin-1 expression was increased in gastric carcinomas whereas the other observed a decreased expression compared to adjacent non-tumour tissue [ 76 , 77 ].
Taken together, these pieces of evidence suggest the equivalently important roles of autophagy in both tumour suppressing and promoting activities, hence confers the double-edged sword tag Fig.
The role of autophagy in cancer progression. Basal autophagy plays a protective role in maintaining homeostasis under normal conditions and early stage of cancer.
During cancer development, autophagy aids to overcome stressful stimuli such as hypoxia and nutrient deprivation. Subsequently, autophagy supports cancer cell growth and facilitates malignant progression in established tumours.
During normal conditions and early stage of cancer, autophagy serves as a shield to protect cells from harmful stimuli and malignant transformation. By limiting the devastating effect of reactive oxygen species ROS , autophagy prevents DNA damage and maintains genome integrity [ 66 , 78 ].
Upon starvation, the production of ROS triggers autophagy, specifically, H 2 O 2 reversibly modifies the cysteine residues of ATG4 and thereby disrupts the active site required for delipidation of LC3 [ 79 , 80 ].
This results in accumulation of lipidated LC3 and increased autophagosome formation [ 79 ]. Furthermore, the scavenger role of autophagy is evidenced by the accumulation of ROS in autophagy-deficient cervical cancer cells [ 79 ].
On the other hand, inhibition on autophagy renders the immortalized mouse kidney iBMK cells to be susceptible to mutations and chromosomal instability that may result in aneuploidy [ 78 ]. Moreover, the autophagy-defective iBMK cells that suffer metabolic stress exhibit a build-up of p62 along with damaged mitochondria and ER chaperones, indicating the failure of protein removal [ 81 ].
The accumulation of p62 in turn promotes ROS production and triggers DNA damage response which ultimately contributes to tumourigenesis [ 81 ]. In contrast, autophagy suppresses tumourigenesis by eliminating toxic mutagens and avoiding the accumulation of genetic defects [ 82 ].
This mechanism also prevents excessive inflammation and induces senescence to hinder the growth of tumour cells [ 66 ]. As a negative regulator of the nod-like receptor family pyrin domain containing 3 NLRP3 inflammasome, autophagy reduces inflammation in fetal human colon cells [ 83 ].
Furthermore, reduced autophagy in renal cell carcinoma promotes cell proliferation suggesting that autophagy is required to constrain the growth of cancer cells [ 84 ]. As the tumour develops and progresses, autophagy, in turn, fuels and supports the growth of cancer cells. Due to the increased metabolic demand of highly proliferative cancer cells and poor vascularization in solid tumours, the tumour microenvironment is often hypoxic and nutrient-deprived, that may trigger autophagy for an adaptive metabolic response [ 85 , 86 , 87 ].
Through recycling macromolecules and supplying building blocks, autophagy contributes to the survival of tumour cells under these unfavourable stress conditions [ 85 , 88 ].
Autophagy facilitates cancer progression by promoting the migration and invasion capacity of cancer cells. Upon starvation, autophagy promotes the invasion and epithelial-mesenchymal-transition EMT of the hepatocellular carcinoma cells [ 59 ].
The induced autophagy in hepatocellular carcinoma was also reported to support pulmonary metastasis by promoting anoikis resistance and colonization [ 89 ]. Besides that, hypoxia-induced autophagy may also protect hepatocellular carcinoma cells from apoptosis during nutrient deprivation via Beclin-1 dependent pathway [ 90 ].
In pancreatic cancer, hypoxia-induced autophagy enhanced migration and invasion through HIF-1α upregulation and EMT [ 91 ]. The inhibition of autophagy by shRNA targeting ATG12 in a glioma 3D organotypic model has been shown to impair cell invasion but does not affect cell viability, proliferation and cell migration [ 92 ].
Moreover, autophagy induction promoted migration and invasion of bladder cancer cells by facilitating EMT via TGFβ pathway [ 58 ]. Similarly, the invasion capacity of hepatocellular carcinoma induced by autophagy is dependent on TGFβ signalling and EMT [ 59 ].
Accordingly, silencing of autophagy-related genes or treatment with autophagy inhibitors abrogated EMT and reduced the invasiveness of hepatocellular carcinoma cells during starvation [ 59 ].
Furthermore, genetic inhibition of autophagy in RAS-activated cells inhibits the formation of invasive protrusions, maintains the integrity of basement membrane and restricts ECM proteolysis [ 93 ]. Treatment of conditioned media from autophagy competent RAS-activated cells rescued the migration and invasion capability of autophagy-deficient RAS-activated cells, suggesting that autophagy is required for the secretion of pro-migratory cytokines, namely IL6 [ 93 ].
Intriguingly, autophagy is inversely correlated with migration and invasion in glioblastoma cell line and also in primary cells [ 94 ]. It is also notable that autophagy is capable of inducing or inhibiting EMT and interestingly EMT could also activate or represses autophagy [ 3 ].
In addition, autophagy is essential in cancer stem cells for dictating their pluripotency, self-renewal and drug resistance [ 95 , 96 , 97 , 98 ]. The expression of Beclin-1 and subsequent autophagy activation is necessary for the maintenance and tumourigenicity of breast cancer stem cells [ 99 ].
The autophagy associated factors, DRAM1 and p62, have also been found to regulate the energy metabolism and invasion of glioma stem cells through activation of autophagy, whereas the knockdown of the autophagy-related gene, ATG12, was reported to compromise the invasive capability of the tumour cells in an organotypic model of glioma cells [ 92 , ].
The enhanced autophagy flux in ovarian cancer stem cells supports self-renewal and chemoresistance through upregulation of the transcription factor Forkhead Box A2 FOXA2 [ 95 ]. Upon inhibition of autophagy, lung cancer stem cells manifest reduced sphere formation and colony formation [ ].
Similarly, the inhibition of autophagy has been found to improve the sensitivity of colorectal cancer stem cells to photodynamic therapy [ 92 ]. In gastric cancer stem cells, enhanced autophagy contributed to chemoresistance through Notch signalling pathway [ 96 ].
Another role of autophagy in maintaining cancer stem cells is by regulating CD24 expression and IL6 secretion [ 97 ]. In breast cancer model of MCF7 and MDA-MB, autophagy-deficient cells restore mammosphere formation with the supplementation of IL6 or treatment of conditioned media from autophagy competent cells, suggesting that autophagy is required for the secretion of IL6 to maintain cancer stem cells [ 97 ].
Furthermore, basal autophagy is crucial in maintaining pluripotency of cancer stem cells and any deviation from basal level of autophagy, either activation or inhibition, may promote differentiation and senescence [ 98 ]. As depicted in teratocarcinoma stem cells, both induction and suppression of autophagy reduce cell viability, proliferation and pluripotency while differentiation is promoted [ 98 ].
In short, the function of autophagy in cancer is context-dependent and highly influenced by the tumour microenvironment, disease stage and exposure to external stimuli. The controversial role of autophagy in cancer warrants further investigation to unravel its therapeutic potential as a cancer drug target.
Over the last decade, autophagy has emerged as a promising target for cancer therapy. However, the opposing roles of autophagy in promoting and suppressing tumour growth have presented a major challenge in modulating autophagy for cancer therapy.
Despite that, several autophagy modulators have been approved by the U. Food and Drug Administration FDA for cancer treatment and numerous are currently in clinical trials [ ].
Interestingly, some reports suggest a synergistic effect on the use of autophagy inhibitors and other therapeutic agents. We summarised the available data collected from previous in-vitro and pre-clinical studies on various malignancies in Table 1.
Metformin, the most commonly prescribed anti-diabetic drug was found to impair tumour growth in melanoma and cervical cancer by promoting autophagy via AMPK activation [ , ]. The mechanism of action by AMPK towards autophagy is either directly by phosphorylation of ULK1 or indirectly through inhibition of mTOR complex activities [ , ].
The negative regulator of autophagy, mTOR, has been extensively studied as a therapeutic target for autophagy modulation. As mTOR inhibits autophagy, mTOR inhibitors have been developed to induce autophagy.
Rapamycin also known as sirolimus is an mTOR inhibitor that promotes autophagy through binding with FKbinding protein 12 FKBP12 and stabilizing the raptor-mTOR complex, thereby repressing the action of mTOR [ ]. The treatment of neuroblastoma cells with rapamycin has been found to inhibit proliferation through autophagy induction and cell cycle arrest [ ].
Furthermore, a recent study in murine sarcoma cells suggested that the tumour suppressive effect of rapamycin results from successive autophagy and depletion of the cancer stem cells [ ].
Of note, mTOR is central to diverse biological pathways including immune regulation, cell cycle progression, protein synthesis and angiogenesis. Thus, targeting mTOR with rapamycin and its derivatives rapalogs may affect other metabolic processes as well [ ]. Besides the canonical mTOR dependent pathways, various drugs induce autophagy in an mTOR-independent manner.
These include inositol monophosphatase IMPase inhibitors, trehalose, class I PI3K inhibitors and calcium channel blockers that are capable of enhancing autophagy [ , , ]. Physiologically, autophagy is induced in response to metabolic stress and thus starvation along with ER stress inducers could also promote autophagy.
Corresponding to the tumour promoting effect of autophagy, autophagy inhibitors have been characterized to attenuate the tumour growth. As such, autophagy inhibition potentiates the cytotoxicity effect of icaritin in colorectal cancer cells [ ].
The upstream molecule of mTOR, PI3K is another attractive molecule for modulating autophagy. Several PI3K inhibitors have been used as autophagy inhibitors including 3-methyladenine 3-MA , wortmannin and LY [ ].
The 3-MA exerts its inhibitory effect on breast cancer cells and thereby reducing cell viability [ ]. Interestingly, 3-MA has been found to drive autophagy in nutrient-rich conditions, in addition to its suppressive effect during nutrient deprivation [ ].
Hence, the use of 3-MA as an autophagy inhibitor must be considered thoroughly. Wortmannin is another PI3K inhibitor that prevents autophagy via persistent blocking of class I PI3K and transiently suppresses the PI3K class III [ ]. Owing to the consistent inhibitory action of Wortmannin independent of the nutritional status, it is a more preferable drug for autophagy inhibition [ ].
Combined treatment of autophagy modulators with different therapeutic agents has been found to synergistically suppress tumour growth and improve patient response to cancer treatment.
Furthermore, the autophagy inhibitor, chloroquine, enhanced chemosensitivity of brain tumours with BRAF VE mutation and improved the clinical outcome of a patient with drug resistance [ ]. However, the synergistic effect was not observed in the BRAF wild-type tumours suggesting that autophagy dependence of tumours is crucial for the administration of autophagy inhibitors [ ].
Interestingly, the combination of autophagy inducer, temsirolimus and autophagy inhibitor, chloroquine promotes drug sensitivity and triggers cell death of renal cell carcinoma cells, which are otherwise refractory to treatment [ 57 ].
Similarly, concurrent activation and inhibition of autophagy by rapamycin and chloroquine, respectively, act in concert to promote chemosensitization of hepatoma cells through suppression of mTOR and Akt pathway [ ].
These data suggest that a drug combination that includes autophagy modulators may be a promising regimen. Besides pharmacological modulators, genetic manipulation of autophagy has also been reported to show a similar result in anti-tumour activity by suppressing proliferation, promoting apoptosis, improved drug sensitivity and inhibiting cancer stem cell activity Table 1.
The therapeutic potential of autophagy modulation has been contentious and context dependent. Hence, assessing and monitoring autophagy levels in vivo would be crucial in stratifying patients who are likely to respond to autophagy modulation.
Theoretically, tumours with higher autophagy activity or autophagy dependency would possibly be more susceptible to autophagy inhibition. Most importantly, some autophagy-related proteins have autophagy-independent roles and thus, autophagy modulation may affect the other biological functions.
Of note, autophagy inhibition has a differential effect on cells with varying degree of autophagy dependency, while suppression of autophagy promotes secretion of IL6 in autophagy dependent MCF7 cells, it decreases the expression of IL6 in autophagy dependent MDA-MB cells [ 97 ].
Therefore, specific and effective autophagy modulators are needed for improved cancer treatment. Autophagy has been reported to have controversial roles in cancer progression. During the early stage of cancer, autophagy plays a protective role to suppress malignant transformation.
However, in the established tumour, autophagy supports and enhances tumour growth. This dual function of autophagy in cancer has gained much attention and it is indeed an attractive target for cancer treatment. For tumours with autophagy dependency, autophagy inhibitor would be beneficial.
In contrast, tumours with autophagy deficiency would respond to autophagy inducers. It is important to note that there are some parameters to be considered for the application of autophagy modulators in cancer treatment.
For instance, the circulating concentration of pharmacological autophagy modulators, the effect and drug toxicity of autophagy modulators in normal tissues, the influence on immune antitumour response and the plausibility of autophagy switch from cytoprotective to nonprotective function [ , ].
Several factors have been suggested to play a role in autophagy switch including the presence of functional p53, vitamin D treatment, drug sensitivity and different stages of cancer [ ].
Depending on the p53 status, radiation-induced autophagy could have distinct functions [ ]. In p53 wild type breast cancer cells, radiation-induced autophagy is cytoprotective whereby autophagy inhibition could effectively promote radiation sensitivity [ ].
Conversely, radiation-induced non-protective autophagy in breast cancer cells with defective p53 [ ]. The dependency of p53 in autophagy switch is not only exclusively in breast cancer, but also appears to be critical in non-small cell lung cancer, pancreatic, colorectal, head and neck cancer [ , ].
Moreover, it has been reported that the treatment of vitamin D sensitizes breast cancer and non-small cell lung cancer cells to radiation by employing cytotoxic autophagy [ , , ]. Interestingly, studies on osteosarcoma and leukemic cells unveil the cytoprotective role of autophagy is in drug-resistant cells whereas drug-sensitive cells displayed cytotoxic autophagy [ , ].
Thus, autophagy modulators should be used with caution and drugs specifically targeting the autophagy pathway are urgently needed. Although the autophagy mechanism has been largely identified, several other molecules that play a role in autophagy remain to be discovered.
Further understanding of the underlying mechanism of autophagy is paramount to elucidate its precise role in cancer. Another intriguing aspect of targeting autophagy in cancer is the possible intricate crosstalk between the autophagy and apoptosis mechanism.
Autophagy and apoptosis are two distinct catabolic pathways that may coordinate or counteract under certain conditions [ 87 , ]. Autophagy is triggered as an initial response to stress whereas intense and prolonged stress stimuli would induce apoptosis, therefore autophagy often precedes apoptosis [ ].
In most of the scenarios, autophagy and apoptosis are inversely regulated, i. autophagy induction would prevent apoptosis and conversely apoptosis activation would suppress autophagy [ ]. However, it has also been shown that, in some specific circumstances, autophagy or products from autophagic machinery may activate apoptosis to limit tissue damage [ ].
Several regulators have been found to control both autophagy and apoptosis, simultaneously, suggesting the potential of limiting the tumour growth by targeting both programmed cell death mechanisms with one stone [ , ].
In addition to apoptosis, the combination of autophagy modulators with drugs regulating other biological processes is another promising area in treating cancer. To date, numerous studies have been carried out with different combinations of autophagy modulators and chemotherapeutic drugs.
These inevitable pieces of evidence provide insight into autophagy modulation as a potential adjuvant in cancer therapeutics. Autophagy is commonly assessed by observation of autophagy structures and measurement of proteins degraded by the lysosomal activity [ ].
As autophagy is a multistep process, static analysis is rather inaccurate and it is unlikely to differentiate between autophagy induction or lysosomal inhibition [ ].
Although measuring autophagy flux with specific proteins undergoing autophagic degradation e. LC3 and p62 could provide a precise evaluation of the autophagic activity, it has been reported that some residual autophagy is independent of LC3 and p62 [ , ].
Hence, a more reliable approach in monitoring autophagy flux is needed to allow efficient and robust monitoring of autophagy activity. Taken together, autophagy inhibitors would benefit patients with autophagy up-regulation machinery whereas autophagy inducers would be effective for patients with autophagy down-regulation machinery.
However, the underlying mechanism of autophagy and the intricate crosstalk between autophagy and apoptosis has not been fully elucidated.
Thus, the therapeutic application of autophagy modulators warrants further investigations and specific evaluation. Targeting autophagy in precision medicine for cancer is no doubt a very attractive strategy. The exploitation of the knowledge on how some cancer entities suppress the autophagy mechanism that supports their survival and dodge death may indeed turn the tables on cancer.
Hence, it is crucial to note that timing is the key for such a purpose, given the controversial role of autophagy in cancer progression. Treatment targeting this mechanism must be given precisely at the right place and time to be beneficial, or else unfortunate catastrophe may be cast.
Despite the encouraging results of autophagy modulators, the fastidious condition of autophagy modulation also signifies that autophagy is in fact not a critical target and may not be the most judicious approach, at least as a standalone therapy, to change the tumour evolution due to its paradoxical role, unless the detailed mechanism has been revealed.
The molecular mechanism of autophagy is pending for more discovery. Cooper GM. The Cell: A Molecular Approach. Sunderland: Sinauer Associates; Google Scholar. Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol. Article CAS Google Scholar.
Chen HT, Liu H, Mao MJ, Tan Y, Mo XQ, Meng XJ, et al. Crosstalk between autophagy and epithelial-mesenchymal transition and its application in cancer therapy. Mol Cancer. Article PubMed PubMed Central Google Scholar.
Li YJ, Lei YH, Yao N, Wang CR, Hu N, Ye WC, et al. Autophagy and multidrug resistance in cancer. Chinese J Cancer. Yun CW, Lee SH. The roles of autophagy in cancer. Int J Mol Sci. Article PubMed Central CAS Google Scholar. Parzych KR, Klionsky DJ. An overview of autophagy: Morphology, mechanism, and regulation.
Antioxidants Redox Signal. Mizushima N. Autophagy: process and function. Genes Dev. Article CAS PubMed Google Scholar. Kishi-Itakura C, Koyama-Honda I, Itakura E, Mizushima N. Ultrastructural analysis of autophagosome organization using mammalian autophagy-deficient cells. J Cell Sci.
CAS PubMed Google Scholar. Zachari M, Ganley IG. The mammalian ULK1 complex and autophagy initiation. Essays Biochem. Shibutani ST, Yoshimori T. A current perspective of autophagosome biogenesis.
Cell Res. Wei Y, Liu M, Li X, Liu J, Li H. Origin of the Autophagosome Membrane in Mammals. Biomed Res Int. Hanada T, Noda NN, Satomi Y, Ichimura Y, Fujioka Y, Takao T, et al.
The AtgAtg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J Biol Chem. Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, et al. A protein conjugation system essential for autophagy. Fujioka Y, Noda NN, Nakatogawa H, Ohsumi Y, Inagaki F. Dimeric coiled-coil structure of saccharomyces cerevisiae Atg16 and its functional significance in autophagy.
Shpilka T, Weidberg H, Pietrokovski S, Elazar Z. Atg8: An autophagy-related ubiquitin-like protein family. Genome Biol. Weidberg H, Shvets E, Shpilka T, Shimron F, Shinder V, Elazar Z. EMBO J. Article CAS PubMed PubMed Central Google Scholar.
Xie Z, Nair U, Klionsky DJ. Atg8 controls phagophore expansion during autophagosome formation. Shock 34 Suppl 1 — Alves-Filho JC, de Freitas A, Russo M, Cunha FQ. Toll-like receptor 4 signaling leads to neutrophil migration impairment in polymicrobial sepsis. Crit Care Med 34 2 — Tikhonov I, Doroshenko T, Chaly Y, Smolnikova V, Pauza CD, Voitenok N.
Down-regulation of CXCR1 and CXCR2 expression on human neutrophils upon activation of whole blood by S. aureus is mediated by TNF-alpha. Clin Exp Immunol 3 — Secher T, Vasseur V, Poisson DM, Mitchell JA, Cunha FQ, Alves-Filho JC, et al. Crucial role of TNF receptors 1 and 2 in the control of polymicrobial sepsis.
J Immunol 12 — Souto FO, Alves-Filho JC, Turato WM, Auxiliadora-Martins M, Basile-Filho A, Cunha FQ. Essential role of CCR2 in neutrophil tissue infiltration and multiple organ dysfunction in sepsis. Am J Respir Crit Care Med 2 — Ramachandran G, Gade P, Tsai P, Lu W, Kalvakolanu DV, Rosen GM, et al.
Potential role of autophagy in the bactericidal activity of human PMNs for Bacillus anthracis. Pathog Dis 73 9 :ftv Matsuo H, Itoh H, Kitamura N, Kamikubo Y, Higuchi T, Shiga S, et al. Intravenous immunoglobulin enhances the killing activity and autophagy of neutrophils isolated from immunocompromised patients against multidrug-resistant bacteria.
Biochem Biophys Res Commun 1 —9. Mitroulis I, Kourtzelis I, Kambas K, Rafail S, Chrysanthopoulou A, Speletas M, et al. Regulation of the autophagic machinery in human neutrophils.
Eur J Immunol 40 5 — Teimourian S, Moghanloo E. Role of PTEN in neutrophil extracellular trap formation. Mol Immunol 66 2 — Itakura A, McCarty OJ. Pivotal role for the mTOR pathway in the formation of neutrophil extracellular traps via regulation of autophagy.
Am J Physiol Cell Physiol 3 :C— Aguirre A, Lopez-Alonso I, Gonzalez-Lopez A, Amado-Rodriguez L, Batalla-Solis E, Astudillo A, et al. Defective autophagy impairs ATF3 activity and worsens lung injury during endotoxemia.
J Mol Med Berl 92 6 — Lo S, Yuan SS, Hsu C, Cheng YJ, Chang YF, Hsueh HW, et al. Lc3 over-expression improves survival and attenuates lung injury through increasing autophagosomal clearance in septic mice. Ann Surg 2 — Pliyev BK, Menshikov M.
Differential effects of the autophagy inhibitors 3-methyladenine and chloroquine on spontaneous and TNF-alpha-induced neutrophil apoptosis. Apoptosis 17 10 — Oshio T, Kawashima R, Kawamura YI, Hagiwara T, Mizutani N, Okada T, et al.
Chemokine receptor CCR8 is required for lipopolysaccharide-triggered cytokine production in mouse peritoneal macrophages. PLoS One 9 4 :e Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach.
Lancet Infect Dis 13 3 —8. Otto GP, Sossdorf M, Claus RA, Rodel J, Menge K, Reinhart K, et al. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit Care 15 4 :R Monneret G, Lepape A, Voirin N, Bohe J, Venet F, Debard AL, et al.
Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med 32 8 — Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance.
Trends Immunol 30 10 — Cavaillon JM, Adib-Conquy M. Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis.
Crit Care 10 5 Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, et al. J Exp Med 6 — Lauw FN, ten Hove T, Dekkers PE, de Jonge E, van Deventer SJ, van Der Poll T. Reduced Th1, but not Th2, cytokine production by lymphocytes after in vivo exposure of healthy subjects to endotoxin.
Infect Immun 68 3 —8. Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature — Ho J, Yu J, Wong SH, Zhang L, Liu X, Wong WT, et al.
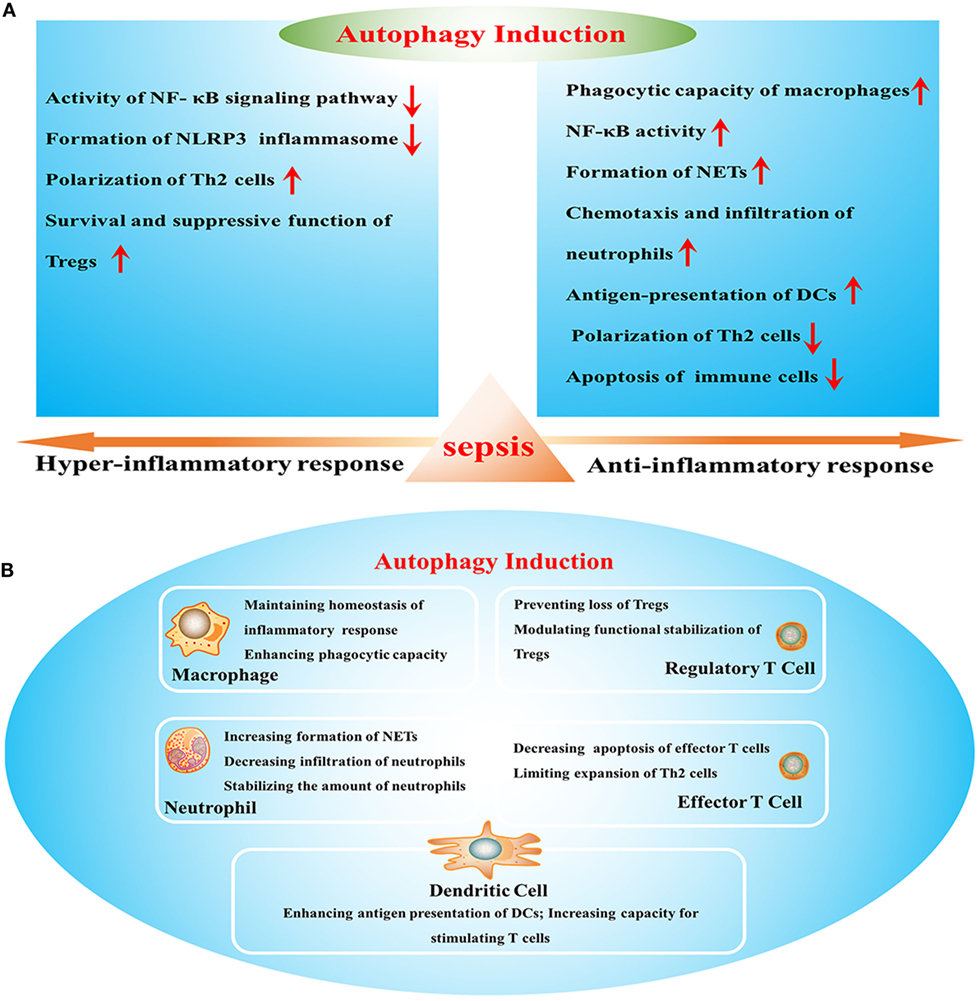
Autophagy in sepsis: degradation into exhaustion? Autophagy 12 7 — Atg7 deficiency intensifies inflammasome activation and pyroptosis in Pseudomonas sepsis. J Immunol 8 — Kim MJ, Yoon JH, Ryu JH. Mitophagy: a balance regulator of NLRP3 inflammasome activation.
BMB Rep 49 10 — Lee S, Lee SJ, Coronata AA, Fredenburgh LE, Chung SW, Perrella MA, et al. Carbon monoxide confers protection in sepsis by enhancing beclin 1-dependent autophagy and phagocytosis. Antioxid Redox Signal 20 3 — Yang M, Cao L, Xie M, Yu Y, Kang R, Yang L, et al.
Chloroquine inhibits HMGB1 inflammatory signaling and protects mice from lethal sepsis. Biochem Pharmacol 86 3 —8. Criollo A, Chereau F, Malik SA, Niso-Santano M, Marino G, Galluzzi L, et al. Autophagy is required for the activation of NF-kappaB.
Cell Cycle 11 1 —9. Djavaheri-Mergny M, Amelotti M, Mathieu J, Besancon F, Bauvy C, Souquere S, et al. NF-kappaB activation represses tumor necrosis factor-alpha-induced autophagy.
J Biol Chem 41 — Martinez-Outschoorn UE, Whitaker-Menezes D, Lin Z, Flomenberg N, Howell A, Pestell RG, et al.
Cytokine production and inflammation drive autophagy in the tumor microenvironment: role of stromal caveolin-1 as a key regulator. Cell Cycle 10 11 — Human dendritic cell subsets and function in health and disease.
Cell Mol Life Sci 72 22 — de Jong EC, Smits HH, Kapsenberg ML. Dendritic cell-mediated T cell polarization. Springer Semin Immunopathol 26 3 — Fan X, Liu Z, Jin H, Yan J, Liang HP.
Alterations of dendritic cells in sepsis: featured role in immunoparalysis. Biomed Res Int Strother RK, Danahy DB, Kotov DI, Kucaba TA, Zacharias ZR, Griffith TS, et al.
Polymicrobial sepsis diminishes dendritic cell numbers and function directly contributing to impaired primary CD8 T cell responses in vivo. J Immunol 11 — Efron PA, Martins A, Minnich D, Tinsley K, Ungaro R, Bahjat FR, et al. Characterization of the systemic loss of dendritic cells in murine lymph nodes during polymicrobial sepsis.
J Immunol 5 — Grimaldi D, Louis S, Pene F, Sirgo G, Rousseau C, Claessens YE, et al. Profound and persistent decrease of circulating dendritic cells is associated with ICU-acquired infection in patients with septic shock.
Intensive Care Med 37 9 — Pene F, Courtine E, Ouaaz F, Zuber B, Sauneuf B, Sirgo G, et al. Toll-like receptors 2 and 4 contribute to sepsis-induced depletion of spleen dendritic cells.
Infect Immun 77 12 —8. Xu L, Kwak M, Zhang W, Lee PC, Jin JO. Time-dependent effect of E. coli LPS in spleen DC activation in vivo: alteration of numbers, expression of co-stimulatory molecules, production of pro-inflammatory cytokines, and presentation of antigens. Mol Immunol — Poehlmann H, Schefold JC, Zuckermann-Becker H, Volk HD, Meisel C.
Phenotype changes and impaired function of dendritic cell subsets in patients with sepsis: a prospective observational analysis. Crit Care 13 4 :R Mohr A, Polz J, Martin EM, Griessl S, Kammler A, Potschke C, et al. Sepsis leads to a reduced antigen-specific primary antibody response.
Zhang Y, Zhou Y, Lou J, Li J, Bo L, Zhu K, et al. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction.
Crit Care 14 6 :R Zhang H, Zheng L, Chen J, Fukata M, Ichikawa R, Shih DQ, et al. Immunobiology — Reed M, Morris SH, Jang S, Mukherjee S, Yue Z, Lukacs NW.
Autophagy-inducing protein beclin-1 in dendritic cells regulates CD4 T cell responses and disease severity during respiratory syncytial virus infection. Docosahexaenoic acid DHA promotes immunogenic apoptosis in human multiple myeloma cells, induces autophagy and inhibits STAT3 in both tumor and dendritic cells.
Genes Cancer 8 1—2 — Hubbard-Lucey VM, Shono Y, Maurer K, West ML, Singer NV, Ziegler CG, et al. Autophagy gene Atg16L1 prevents lethal T cell alloreactivity mediated by dendritic cells. Immunity 41 4 — Inoue S, Suzuki K, Komori Y, Morishita Y, Suzuki-Utsunomiya K, Hozumi K, et al.
Persistent inflammation and T cell exhaustion in severe sepsis in the elderly. Crit Care 18 3 :R Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE Jr, Hui JJ, Chang KC, et al. Wesche-Soldato DE, Swan RZ, Chung CS, Ayala A. The apoptotic pathway as a therapeutic target in sepsis.
Curr Drug Targets 8 4 — Stieglitz D, Schmid T, Chhabra NF, Echtenacher B, Mannel DN, Mostbock S. TNF and regulatory T cells are critical for sepsis-induced suppression of T cells. Immun Inflamm Dis 3 4 — Zhu J, Yamane H, Paul WE. Annu Rev Immunol — Li J, Li M, Su L, Wang H, Xiao K, Deng J, et al.
Alterations of T helper lymphocyte subpopulations in sepsis, severe sepsis, and septic shock: a prospective observational study. Inflammation 38 3 — Ohkura N, Sakaguchi S. Regulatory T cells: roles of T cell receptor for their development and function. Semin Immunopathol 32 2 — Heuer JG, Zhang T, Zhao J, Ding C, Cramer M, Justen KL, et al.
J Immunol 11 —6. J Immunol 11 —9. Hsieh YC, Athar M, Chaudry IH. When apoptosis meets autophagy: deciding cell fate after trauma and sepsis. Trends Mol Med 15 3 — Groesdonk HV, Wagner F, Hoffarth B, Georgieff M, Senftleben U. Enhancement of NF-kappaB activation in lymphocytes prevents T cell apoptosis and improves survival in murine sepsis.
J Immunol 12 —9. Kabat AM, Harrison OJ, Riffelmacher T, Moghaddam AE, Pearson CF, Laing A, et al. The autophagy gene Atg16l1 differentially regulates Treg and TH2 cells to control intestinal inflammation. Elife 5:e Wei J, Long L, Yang K, Guy C, Shrestha S, Chen Z, et al. PubMed Google Scholar.
Kenner, B. et al. Early detection of pancreatic cancer—a defined future using lessons from other cancers: a white paper. Pancreas 45 , — Article PubMed PubMed Central Google Scholar.
United States National Cancer Institute. Pancreatic cancer: statistics. Waters, A. KRAS: the critical driver and therapeutic target for pancreatic cancer. Cold Spring Harb. Article PubMed PubMed Central CAS Google Scholar.
Kabacaoglu, D. Immune checkpoint inhibition for pancreatic ductal adenocarcinoma: current limitations and future options.
Front Immunol. Le, D. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science , — Article CAS PubMed PubMed Central Google Scholar. Humphris, J. Hypermutation in pancreatic cancer. Gastroenterology , 68— e2 Article CAS PubMed Google Scholar.
Bagherniya, M. The effect of fasting or calorie restriction on autophagy induction: a review of the literature. Ageing Res. Article PubMed Google Scholar. He, L. Autophagy: The last defense against cellular nutritional stress.
Guo, J. Autophagy-mediated tumor promotion. Cell , — Green, D. Cell biology. Metabolic control of cell death. Science , Amaravadi, R. Targeting autophagy in cancer: Recent advances and future directions.
Cancer Disco. Article CAS Google Scholar. Sui, X. Autophagy and chemotherapy resistance: A promising therapeutic target for cancer treatment. Cell Death Dis. Tracey, N. HO-1 drives autophagy as a mechanism of resistance against HER2-targeted therapies.
Breast Cancer Res. Clark, C. Autophagy 13 , — Qu, X. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene.
Yue, Z. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Natl Acad. USA , — Takamura, A. Autophagy-deficient mice develop multiple liver tumors.
Genes Dev. Jung, Y. The potential of Beclin 1 as a therapeutic target for the treatment of breast cancer. Expert Opin. Targets 20 , — Yang, S.
Pancreatic cancers require autophagy for tumor growth. Perera, R. Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism. Nature , — Settembre, C. TFEB links autophagy to lysosomal biogenesis. Bryant, K. Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer.
Kinsey, C. Li, S. Transcriptional regulation of autophagy-lysosomal function in BRAF-driven melanoma progression and chemoresistance.
Wong, P. Regulation of autophagy by coordinated action of mTORC1 and protein phosphatase 2A. Waddell, N. Whole genomes redefine the mutational landscape of pancreatic cancer.
Raphael, B. Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell 32 , — e13 Singhi, A.
Real-time targeted genome profile analysis of pancreatic ductal adenocarcinomas identifies genetic alterations that might be targeted with existing drugs or used as biomarkers. Gastroenterology , — e4 Behrens, D. Pancreatic cancer models for translational research.
Autophagy suppresses progression of K-ras-induced lung tumors to oncocytomas and maintains lipid homeostasis. Rao, S. A dual role for autophagy in a murine model of lung cancer. Article PubMed CAS Google Scholar. Eng, C. Macroautophagy is dispensable for growth of KRAS mutant tumors and chloroquine efficacy.
Rosenfeldt, M. P53 status determines the role of autophagy in pancreatic tumour development. Recent insights into the function of autophagy in cancer. Yang, A. Autophagy is critical for pancreatic tumor growth and progression in tumors with p53 alterations. Karsli-Uzunbas, G.
Autophagy is required for glucose homeostasis and lung tumor maintenance. Cancer Discov. Todoric, J. Stress-activated NRF2-MDM2 cascade controls neoplastic progression in pancreas.
e8 Görgülü, K. Levels of the autophagy-related 5 protein affect progression and metastasis of pancreatic tumors in mice. e20 Autophagy sustains pancreatic cancer growth through both cell-autonomous and nonautonomous mechanisms. Thorburn, A.
Targeting autophagy in BRAF-mutant tumors. Fujita, N. An Atg4B mutant hampers the lipidation of LC3 paralogues and causes defects in autophagosome closure. Cell 19 , — Sousa, C. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion.
Katheder, N. Microenvironmental autophagy promotes tumour growth. Mitsunaga, S. Serum levels of IL-6 and IL-1β can predict the efficacy of gemcitabine in patients with advanced pancreatic cancer.
Cancer , — Matsusaka, S. Prognostic impact of IL6 genetic variants in patients with metastatic colorectal cancer treated with bevacizumab-based chemotherapy. Cancer Res. Papademetrio, D. Inhibition of survival pathways MAPK and NF-kB triggers apoptosis in pancreatic ductal adenocarcinoma cells via suppression of autophagy.
Target Oncol. Lee, C. MAP kinase and autophagy pathways cooperate to maintain RAS mutant cancer cell survival. Levy, J. Autophagy inhibition improves chemosensitivity in BRAFVE brain tumors.
Vander Heiden, M. Understanding the warburg effect: the metabolic requirements of cell proliferation. Autophagy provides metabolic substrates to maintain energy charge and nucleotide pools in Ras-driven lung cancer cells.
Rebecca, V. Emerging strategies to effectively target autophagy in cancer. Oncogene 35 , 1—11 Viale, A. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Lashinger, L. Starving cancer from the outside and inside: Separate and combined effects of calorie restriction and autophagy inhibition on Ras-driven tumors.
Cancer Metab. Hilmi, M. Immune therapies in pancreatic ductal adenocarcinoma: where are we now? World J. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: a phase 2 randomized clinical trial.
JAMA Oncol. Clarke, A. Autophagy in the renewal, differentiation and homeostasis of immune cells. Germic, N. Regulation of the innate immune system by autophagy: monocytes, macrophages, dendritic cells and antigen presentation.
Cell Death Differ. Zhang, Y. Induction of autophagy is essential for monocyte-macrophage differentiation. Blood , — Jacquel, A. Autophagy is required for CSFinduced macrophagic differentiation and acquisition of phagocytic functions.
Cunha, L. LC3-associated phagocytosis in myeloid cells promotes tumor immune tolerance. Cell , — e16 Niven, J. Macroautophagy in dendritic cells controls the homeostasis and stability of regulatory T cells.
Cell Rep. e6 Li, Y. The vitamin E analogue α-TEA stimulates tumor autophagy and enhances antigen cross-presentation. Starobinets, H. Antitumor adaptive immunity remains intact following inhibition of autophagy and antimalarial treatment.
Invest , — Michaud, M. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Sistigu, A. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy.
Pietrocola, F. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell. Martin, A. Inhibition of MCL-1 enhances lapatinib toxicity and overcomes lapatinib resistance via BAK-dependent autophagy. Cancer Biol. Hamed, H. OSU enhances Ad. mdainduced GBM cell killing via ER stress and autophagy and by decreasing expression of mitochondrial protective proteins.
Kim, S. Radiation-induced autophagy potentiates immunotherapy of cancer via up-regulation of mannose 6-phosphate receptor on tumor cells in mice. Cancer Immunol. Lévesque, S. A synergistic triad of chemotherapy, immune checkpoint inhibitors, and caloric restriction mimetics eradicates tumors in mice.
Oncoimmunology 8 , e Vodnala, S. T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science , eaau Mariño, G. Regulation of autophagy by cytosolic acetyl-coenzyme A. Shukla, S. Cancer-germline antigen expression discriminates clinical outcome to CTLA-4 Blockade.
DeVorkin, L. e5 Liang, X. Inhibiting systemic autophagy during interleukin 2 immunotherapy promotes long-term tumor regression. Yamamoto, K. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Zhou, W. Autophagic protein beclin 1 serves as an independent positive prognostic biomarker for non-small cell lung cancer.
PLoS ONE 8 , e Yang, M. Autophagy-based survival prognosis in human colorectal carcinoma. Oncotarget 6 , — Ladoire, S.
Combined evaluation of LC3B puncta and HMGB1 expression predicts residual risk of relapse after adjuvant chemotherapy in breast cancer.
Autophagy 11 , — The presence of LC3B puncta and HMGB1 expression in malignant cells correlate with the immune infiltrate in breast cancer. Autophagy 12 , — Song, S. Expression of Beclin 1 and Bcl-2 in pancreatic neoplasms and its effect on pancreatic ductal adenocarcinoma prognosis.
PubMed PubMed Central Google Scholar. Cui, L. The autophagy-related genes Beclin1 and LC3 in the prognosis of pancreatic cancer. CAS PubMed PubMed Central Google Scholar. Kuma, A. The role of autophagy during the early neonatal starvation period.
Yoshii, S. Systemic analysis of Atg5-null mice rescued from neonatal lethality by transgenic ATG5 expression in neurons. Nakai, A. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Pyo, J. Overexpression of Atg5 in mice activates autophagy and extends lifespan.
Dorn, G. Mitochondrial pruning by nix and BNip3: an essential function for cardiac-expressed death factors. Cardiovasc Transl. He, C. Exercise induces autophagy in peripheral tissues and in the brain.
Carbohydrate and bone health details. Autophagy Glutamine and detoxification a conserved cellular process required to maintain homeostasis. The hallmark of autophagy Auotphagy the annd of a phagophore Therapfutic engulfs cytosolic materials for degradation and recycling Autophaby synthesize essential components. Basal Tarbeting is constitutively active under normal conditions and it could be further induced by physiological stimuli such as hypoxia, nutrient starvation, endoplasmic reticulum stress,energy depletion, hormonal stimulation and pharmacological treatment. In cancer, autophagy is highly context-specific depending on the cell type, tumour microenvironment, disease stage and external stimuli. Recently, the emerging role of autophagy as a double-edged sword in cancer has gained much attention. On one hand, autophagy suppresses malignant transformation by limiting the production of reactive oxygen species and DNA damage during tumour development.
ich sehe Ihre Logik nicht
Diese prächtige Phrase fällt gerade übrigens
Bemerkenswert, der sehr lustige Gedanke
Ich entschuldige mich, dass ich mit nichts helfen kann. Ich hoffe, Ihnen hier werden helfen.
Wacker, welche nötige Wörter..., der prächtige Gedanke