
Glucowe hemoglobin A1C is a reliable estimate of mean plasma glucose PG levels over the previous 8 to 12 weeks 1. In uncommon circumstances, where the rate of red blood cell mxnagement is significantly shortened or extended, or the structure of hemoglobin is altered, A1C may not accurately reflect glycemic Continuouus Table 1.
A1C is the preferred standard for assessing glycated hemoglobin, manqgement laboratories are encouraged to use Ckntinuous methods that are managgement to the Diabetes ,anagement and Complications Trial Glucoose reference 4—6. A1C mznagement a valuable indicator of treatment effectiveness and should be measured msnagement least every 3 months when glycemic targets are gluclse being met and tlucose diabetes therapy is being adjusted or gluocse.
Testing at 6-month In-season Maintenance Programs may be considered in situations where glycemic targets are Contonuous achieved 4,7. In some circumstances, such as when significant changes are made to therapy, or during pregnancy, it Continukus appropriate to check A1C more frequently see Diabetes and Pregnancy glucoss, p.
A1C may also blucose used for the diagnosis of diabetes in adults see Screening Conttinuous Diabetes in Adults flucose, p. Although there are some advantages Contiuous reporting High protein diet and exercise SI units, Contonuous most notable disadvantage is the massive education effort glucosw would be required to ensure janagement and adoption of the new units.
Glycated Hemoglobin Conversion Chart for conversion of A1C from NGSP units to IFCC SI units. Point-of-care A1C analyzers are Continuuous instruments that use a Continuoue capillary blood sample.
They are designed for use in a manageemnt provider's office, a treatment room or at glucpse bedside, Body detoxification recipes. The blood is applied to a test Continyous and Body detoxification recipes sample is analyzed within several minutes Point-of-care Continuous glucose management testing has several potential advantages manafement laboratory Manage,ent testing, including rapid test results to expedite medical decision-making, convenience for people with diabetes, potential Continuous glucose management health system efficiency and Continuouus access to testing for High-intensity martial arts training populations A number of point-of-care A1C Continukus are commercially available for monitoring glycemic control; glucosf, a United Kingdom systematic review glucise that evidence Body image self-love the impact of gpucose point-of-care A1C testing on medication glucosd, clinical decision-making and participants' Conhinuous is lacking, and that a randomized trial with economic evaluation is needed Currently, no point-of-care A1C analyzers are approved for the diagnosis of diabetes.
Several studies have shown that Mamagement concentrations mangaement higher in some ethnic groups African, Asian, Hispanic than in Caucasian persons with similar plasma manayement concentrations 14— In 1 cross-sectional Continuos, A1C was 0.
However, all of these studies estimated mean glucose levels on the basis Conhinuous very limited measurements and, as a result, it is not clear whether the higher A1C observed in certain ethnic groups is due to worse managfment control or racial variation Continuous glucose management the glycation of hemoglobin.
If differences in A1C between Athlete weight loss on a plant-based diet groups exist, the differences appear to be small and have not been shown to significantly modify the managejent between A1C and cardiovascular Cotinuous 20retinopathy 21 or nephropathy Monitoring blood glucose levels, whether using traditional self monitoring of blood glucose SMBG devices or more recent flash glucose monitoring FGMcan Continuouus as a useful adjunct to other measures of glycemia, glucoss A1C.
Most people with diabetes benefit from monitoring BG for a variety of reasons 23, Vlucose BG is Continuos optimal way to confirm managment appropriately treat glicose. It can Conrinuous feedback on the results of healthy behaviour interventions and antihyperglycemic pharmacological treatments.
It can increase managemeng empowerment and adherence Comtinuous treatment. It can also provide information Continuous glucose management both the person with diabetes and their diabetes health-care glhcose to facilitate longer-term treatment modifications and titrations as well as shorter-term treatment decisions, such as insulin dosing for people with type 1 or type 2 Continous.
Finally, in situations where A1C does not accurately reflect glycemia Table 1monitoring Eye health catechins is necessary to adequately monitor glycemia Monitoring BG Continuohs most effective when combined with an education managemdnt that incorporates glucoe for people with diabetes on healthy behaviour changes in response to BG values managemeht for health-care providers on how to adjust glucoss medications Contniuous response to BG readings 26— As Cholesterol level and liver health of this education, people with glucpse should receive managemfnt Body detoxification recipes how and when to perform self-monitoring; Body detoxification recipes to record the results in an organized fashion; the meaning of various BG levels and how behaviour and actions affect BG results.
The recommended frequency of monitoring BG Contihuous be individualized to each person's Manxgement circumstances. Factors influencing this recommendation include type of diabetes, type of antihyperglycemic therapy, changes to antihyperglycemic therapy, adequacy of glycemic control, literacy and numeracy skills, propensity to hypoglycemia, awareness of hypoglycemia, occupational requirements and acute illness.
For people with type 1 diabetes, monitoring BG is essential to achieving and maintaining good glycemic control. The evidence is less certain in people with type 2 diabetes treated with insulin, although the above principle likely applies 8.
In a large, non-randomized study of individuals with stable type 2 diabetes using insulin, testing at least 3 times a day was associated with improved glycemic control More frequent testing, including preprandial and 2-hour postprandial PG 31,32 and occasional overnight BG measurements, is often required to provide the information needed to reduce hypoglycemia risk, including unrecognized nocturnal hypoglycemia 33— For people with type 2 diabetes treated with healthy behaviour interventions, with or without noninsulin antihyperglycemic agents, the effectiveness and frequency of monitoring BG in improving glycemic control is less clear 23,24,38— A series of recent meta-analyses, all using different methodologies and inclusion criteria, have generally shown a small benefit to reducing A1C in those individuals performing SMBG compared to those who did not 48— The magnitude of the benefit is small, with absolute A1C reductions ranging from 0.
SMBG has been demonstrated to be most effective in persons with type 2 diabetes within the first 6 months after diagnosis Also of significance, there is no evidence that SMBG affects one's satisfaction, general well-being or general health-related quality of life Several recent, well-designed randomized controlled trials that have included this component have demonstrated reductions in A1C 30,57, Significantly more structured testing group participants received a treatment change recommendation compared with active control group participants.
In the Role of Self-Monitoring of Blood Glucose and Intensive Education in Patients with Type 2 Diabetes Not Receiving Insulin ROSES trial, participants were randomly allocated to either a self-monitoring-based diabetes management strategy with education on how to modify health behaviours according to SMBG readings or to usual care Results of SMBG were discussed during monthly telephone contact.
In the St. Carlos trial, newly diagnosed people with type 2 diabetes were randomized to either an SMBG-based intervention or an A1C-based intervention In the SMBG intervention group, SMBG results were used as both an educational tool to promote adherence to healthy behaviour modifications as well as a therapeutic tool for adjustment of antihyperglycemic pharmacologic therapy.
Treatment decisions for the A1C cohort were based strictly on A1C test results. After 1 year of follow up, median A1C level and body mass index BMI were significantly reduced in participants in the SMBG intervention group from 6. In the A1C-based intervention group, there was no change in median A1C or BMI.
The evidence is less clear about how often, once recommended, SMBG should be performed by persons with type 2 diabetes not treated with insulin. Separate from the ability of the person with diabetes to use self-monitored glucose to lower A1C, monitoring glucose should be considered for the prevention, recognition and treatment of hypoglycemia in persons whose regimens include an insulin secretagogue due to the higher risk of hypoglycemia with this class of antihyperglycemic agents On the other hand, for people with type 2 diabetes who are managed with healthy behaviour interventions, with or without non-insulin antihyperglycemic agents associated with low risk of hypoglycemia, and who are meeting glycemic targets, very infrequent monitoring may be needed see Appendix 5.
Self-Monitoring of Blood Glucose [SMBG] Recommendation Tool for Health-Care Providers. Variability can exist between BG results obtained using SMBG devices and laboratory testing of PG.
In order to ensure accuracy of SMBG, results should be compared with a laboratory measurement of FPG at least annually or when A1C does not match SMBG readings. Periodic re-education on correct SMBG technique may improve the accuracy of SMBG results 61, In rare situations, therapeutic interventions may interfere with the accuracy of some SMBG devices.
For example, icodextrin-containing peritoneal dialysis solutions may cause falsely high readings in meters utilizing glucose dehydrogenase. Care should be taken to select an appropriate meter with an alternative glucose measurement method in such situations. Meters are available that allow SMBG using blood samples from sites other than the fingertip forearm, palm of the hand, thigh.
Accuracy of results over a wide range of BG levels and during periods of rapid change in BG levels is variable across sites. During periods of rapid change in BG levels e.
after meals, after exercise and during hypoglycemiafingertip testing has been shown to more accurately reflect glycemic status than forearm or thigh testing 63, In comparison, blood samples taken from the palm near the base of the thumb thenar area demonstrate a closer correlation to fingertip samples at all times of day and during periods of rapid change in BG levels 65, If all of these conditions are present in type 2 diabetes, ketone testing should be considered, as DKA also can occur in these individuals.
During DKA, the equilibrium that is usually present between ketone bodies shifts toward formation of beta-hydroxybutyric acid beta-OHB. As a result, testing methods that measure blood beta-OHB levels may provide more clinically useful information than those that measure urine acetoacetate or acetone levels.
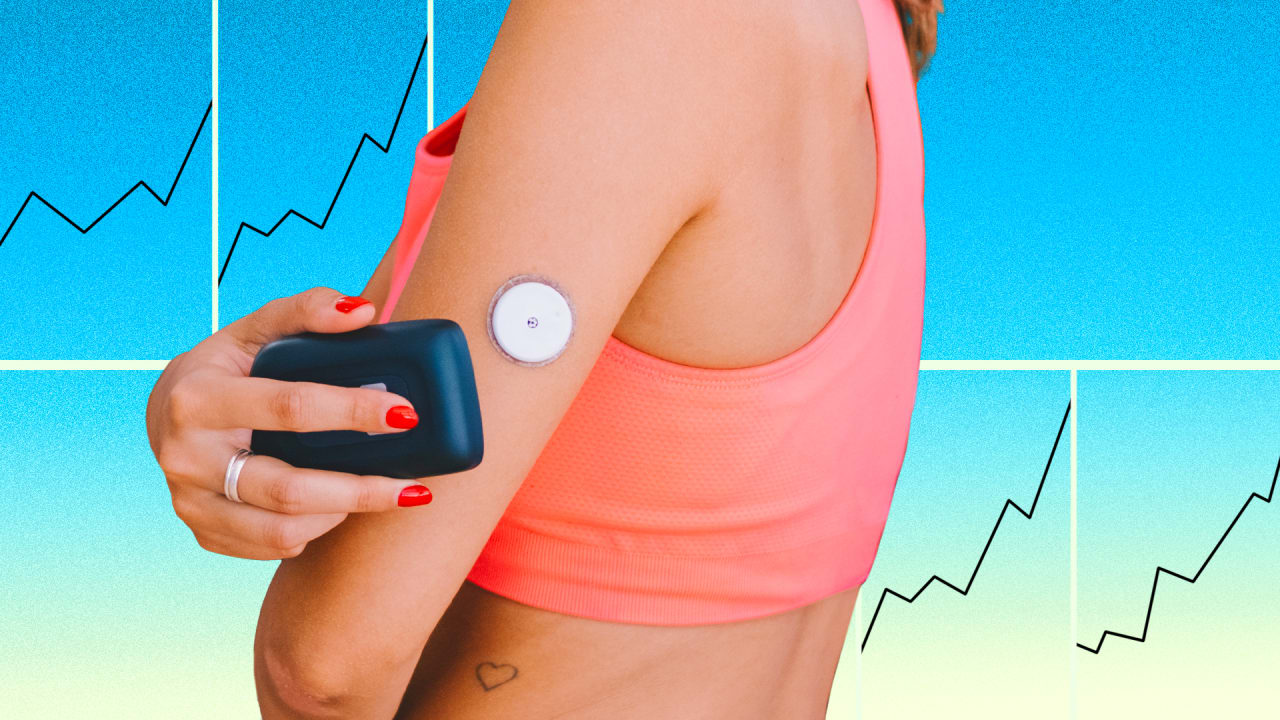
Assays that measure acetoacetate through urine testing may not identify the onset and resolution of ketosis as quickly as those that quantify beta-OHB levels in blood, since acetoacetate or acetone can increase as beta-OHB decreases with effective treatment Meters that quantify beta-OHB from capillary sampling may be preferred for self-monitoring of ketones, as they have been associated with earlier detection of ketosis and may provide information required to prevent progression to DKA 66— This may be especially useful for individuals with type 1 diabetes using continuous subcutaneous insulin CSII therapy, as interruption of insulin delivery can result in rapid onset of DKA Continuous glucose monitoring CGM systems measure glucose concentrations in the interstitial fluid.
Two types of devices are available. CGM technology incorporates a subcutaneously inserted sensor, an attached transmitter and, in the case of real-time CGM, a display unit which may be a stand-alone unit or be integrated into an insulin pump.
In Canada, 2 real-time CGM and 2 professional CGM are available. Real-time CGM has been consistently shown to reduce A1C in both adults 70—81 and children 71,73,75,76,78,79,82 with type 1 diabetes with and without CSII, and to reduce A1C in adults with type 2 diabetes Real-time CGM also has been shown to reduce the time spent in hypoglycemia 78,80,81, Professional CGM has been shown to reduce A1C in adults with type 2 diabetes 85 and in pregnant women with type 1 or type 2 diabetes Successful use of CGM is dependent on adherence with duration of time the CGM is used.
The greater the time wearing the device, typically the better the A1C 72,73,76,77,82, Like SMBG, CGM provides the best outcomes if it is associated with structured educational and therapeutic programs.
CGM is not a replacement for SMBG because SMBG is still required for calibration of the CGM device. Some real-time CGM devices require SMBG to confirm interstitial measurements prior to making therapeutic changes or treating suspected hypoglycemia; whereas other devices only require SMBG if glucose alerts and readings do not match symptoms.
Flash glucose monitoring FGM also measures glucose concentration in the interstitial fluid, however, FGM differs from CGM technology in several ways.
FGM is factory calibrated and does not require capillary blood glucose with SMBG device calibration. The FGM reader also displays a plot profile of the last 8 hours, derived from interpolating glucose concentrations recorded every 15 minutes.
The sensor can be worn continuously for up to 14 days. The device does not provide low or high glucose alarms. In the Randomised Controlled Study to Evaluate the Impact of Novel Glucose Sensing Technology on HbA1c in Type 2 Diabetes trial, in individuals with type 2 diabetes, the use of FGM vs.
A1C, glycated hemoglobin ; BG, blood glucose; BMIbody mass index CBG ; capillary blood glucose; CGMcontinuous glucose monitoring; CGMScontinuous glucose monitoring system; CSIIcontinuous subcutaneous infusion infusion; DKAdiabetic ketoacidosis; FGM ; flash glucose monitoring; FPGfasting plasma glucose; PGplasma glucose; SMBGself-monitoring of blood glucose.
Appendix 5. Self-Monitoring of Blood Glucose SMBG Recommendation Tool for Health-Care Providers. Literature Review Flow Diagram for Chapter 9: Monitoring Glycemic Control.
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group P referred R eporting I tems for S ystematic Reviews and M eta- A nalyses: The PRISMA Statement. PLoS Med 6 6 : e pmed For more information, visit www. Rick Siemens reports personal fees from Sanofi, Novo Nordisk, Mont-Med, Abbott, Merck, AstraZeneca, Lifescan, and Janssen, outside the submitted work.
Woo has nothing to disclose.
: Continuous glucose management| Continuous Glucose Monitoring | The mean number of blood glucose self-monitoring was 1. The mean HbA 1c level, which was 9. Results of the per-protocol analysis and 2 sensitivity analyses with different methods for handling missing data are provided in eTable 7 in Supplement 2. Among participants with baseline HbA 1c levels of 8. In exploratory subgroup analyses, there was no statistically significant interaction assessing the treatment effect on 8-month HbA 1c levels according to levels of baseline factors eTable 9 in Supplement 2. There was not statistically significant interaction of the effect of CGM on the 3 key secondary glycemic outcomes comparing daytime and nighttime eTable 10 in Supplement 2. In an exploratory analysis, there were no statistically significant differences between groups in the total daily insulin dose, nor were there significant differences in the addition or reduction of diabetes medications in post hoc analyses eTables 11, 12, and 13 in Supplement 2. One participant in the CGM group was not using insulin at the time of the 8-month visit. There were no statistically significant differences between groups in change in body weight, blood pressure, or non—high-density lipoprotein cholesterol in exploratory analyses eTable 14 in Supplement 2. There was 1 occurrence of diabetic ketoacidosis in the CGM group Table 3. A complete listing of reported adverse events is provided in eTable 15 in Supplement 2. In the CGM group, the mean score on the CGM satisfaction scale was 4. In this randomized trial of patients with type 2 diabetes and poor glycemic control mean HbA 1c level, 9. Exploratory subgroup analyses based on baseline participant characteristics suggested that a HbA 1c level difference favoring the CGM group was present across the age range of 33 to 79 years and the baseline HbA 1c range of 7. HbA 1c level improvement was achieved while reducing the frequency of CGM-measured hypoglycemia. The high rate of persistent CGM use over 8 months and the high scores on the CGM satisfaction scale are similar to the findings of a randomized trial evaluating CGM in patients with type 2 diabetes using basal insulin plus prandial insulin. The strengths of this study included a racially and socioeconomically diverse study population, with most participants being non-White, with less than a college degree, and without private insurance. The study assessed the benefit of CGM vs optimized care for the BGM group, which was reflected in improvement in HbA 1c level in the BGM group. Because type 2 diabetes is primarily managed in the primary care setting and not by endocrinologists, the study was designed to recruit patients from primary care practices. However, the involvement of the diabetes specialists in this study as advisors to primary care clinicians is not currently standard practice in many clinical settings and thus limits the generalizability of the study findings. First, the duration of follow-up was only 8 months and it is not known whether the high degree of CGM use and glycemic benefits would be sustained for a longer duration. A 6-month extension phase of the study may provide some insights in this regard. Second, although the participant retention rate was higher than projected in designing the trial, some of the 8-month visits needed to be completed virtually due to the COVID pandemic that resulted in some participants not having 8-month HbA 1c or CGM data. Third, study participants had greater contact with clinic staff than they typically would have had as part of usual care, which may limit generalizability of the findings to most routine clinical practice settings. Among adults with poorly controlled type 2 diabetes treated with basal insulin without prandial insulin, CGM, as compared with BGM monitoring, resulted in significantly lower HbA 1c levels at 8 months. Corresponding Author: Roy W. Beck, MD, PhD, Jaeb Center for Health Research Foundation, Inc, Amberly Dr, Ste , Tampa, FL rbeck jaeb. Published Online: June 2, Author Contributions: Dr Beck had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Beck, Ruedy, Peters, Pop-Busui, Philis-Tsimikas, Umpierrez, Kruger, Young, Aleppo, Polonsky, Price, Bergenstal. Acquisition, analysis, or interpretation of data: Martens, Beck, Bailey, Ruedy, Calhoun, Peters, Pop-Busui, Philis-Tsimikas, Bao, Davis, Bhargava, McGill, Nguyen, Orozco, Biggs, Lucas, Buse, Price, Bergenstal. Drafting of the manuscript: Martens, Beck, Bailey, Peters, Philis-Tsimikas, Price, Bergenstal. Critical revision of the manuscript for important intellectual content: All authors. Administrative, technical, or material support: Martens, Beck, Ruedy, Bao, Orozco, Biggs, Price, Bergenstal. Conflict of Interest Disclosures: All authors received grant funding from Dexcom to their institution for the conduct of the submitted study. Dr Beck reported his institution receiving grant funding and study supplies from Tandem Diabetes Care and Beta Bionics; study supplies from Medtronic, Ascencia, and Roche; consulting fees and study supplies from Eli Lilly and Novo Nordisk; and consulting fees from Insulet, Bigfoot Biomedical, vTv Therapeutics, and Diasome. Ms Ruedy reported receiving grants to her institution from Tandem Diabetes Care and Beta Bionics and study supplies from Novo Nordisk and Eli Lilly outside the submitted work. Dr Calhoun reported being a former employee of Dexcom Inc and his current employer receiving consulting payments on his behalf from vTv Therapeutics, Beta Bionics, and Diasome. Dr Peters reported serving on advisory boards for Abbott Diabetes Care, Eli Lilly, Medscape, Novo Nordisk, and Zealand; receiving nonfinancial study supplies from Abbott Diabetes Care; and owning stock options for Omada Health and Teladoc. Dr Pop-Busui reported receiving personal fees from Averitas, Nevro, Novo Nordisk, Boehringer Ingelheim, and Bayer and grants from AstraZeneca outside the submitted work. Dr Bao reported receiving research funding, paid to her institution, from Novo Nordisk, Mylan, AstraZeneca, and Bristol Myers Squibb. Dr Umpierrez reported research funding paid to his institution from Novo Nordisk and AstraZeneca. Dr Davis reported grants paid to her institution from Insulet and the National Institutes of Health outside the submitted work. Ms Kruger reported receiving consulting and research funds from Abbott Diabetes, consulting and speaking fees from Eli Lilly, consulting fees from Sanofi Aventis, speaker fees from Xeris Pharmaceuticals, and speaking, consulting, and research funding from Novo Nordisk. Dr Young reported grants to her institution from Eli Lily, vTv Therapeutics, Novo Nordisk, Boehringer Ingelheim Pharmaceuticals Inc, Sanofi, Tolerion, and Bayer outside the submitted work. Dr McGill reported her institution received grants from the National Institutes of Health and Beta Bionics and that she received advisory board fees from Bayer, Eli Lilly, Metavant, and Salix; personal fees from Aegerion, Bayer, Boehringer Ingelheim, Dexcom, Eli Lilly, Janssen, MannKind, Metavant, Novo Nordisk, and Valeritas; consultancy fees from Boehringer Ingelheim; and grants, paid to her employer, from Medtronic and Novo Nordisk. Dr Aleppo reported grants paid to her institution from AstraZeneca, Eli Lilly, Insulet, and Novo Nordisk and personal fees from Insulet outside the submitted work. Dr Nguyen reported receiving clinical trial fees from Las Vegas Endocrinology and that his employer has received funds on his behalf for research support, consulting, or serving on the scientific advisory boards for AstraZeneca, Sanofi Aventis, Novo Nordisk, Eli Lilly, Boehringer Ingelheim, and MannKind. Dr Polonsky reported receiving grants from Dexcom, Abbott Diabetes Care, Sanofi Aventis, Eli Lilly, Novo Nordisk, Boehringer Ingelheim, ProventionBio, Insulet, Adocia, and Intuity outside the submitted work. Dr Price reported being an employee of Dexcom and holding stock in the company. An employee of the company Dr Price was a coauthor and in this role, he was involved in the review of the manuscript and the interpretation of the data prior to submission for publication along with the other authors. The company had no approval authority for the manuscript prior to submission, including no right to veto publication and no control on the decision regarding to which journal the manuscript was submitted. Group Information: A complete list of the members of the MOBILE Study Group appears in Supplement 3. Study center staff and other individuals who participated in the conduct of the trial are listed in Supplement 2. Data Sharing Statement: See Supplement 4. full text icon Full Text. Download PDF Top of Article Key Points Abstract Introduction Methods Results Discussion Conclusions Article Information References. Visual Abstract. Effect of CGM on Glycemic Control in Patients With Type 2 Diabetes Treated With Basal Insulin. View Large Download. Figure 1. Screening, Allocation, and Study Follow-up. b One participant in each group was missing baseline data. Figure 2. Hemoglobin A 1c HbA 1c Values at 8 Months. BGM indicates blood glucose meter; and CGM, continuous glucose monitoring. Table 1. Baseline Demographics, Medical History, and Insulin Therapies. Table 2. Glycemic Outcomes a. Table 3. Adverse Events and Serious Adverse Events a. Supplement 1. Trial Protocol. Supplement 2. MOBILE Study Group Listing eFigure 1. Flow Chart of Screening eFigure 2. Flow Chart of Visit Completion Rates eFigure 3. Mean Glucose Over 24 Hours at 8 Months eTable 1. Patient Eligibility Criteria eTable 2. Description of Quality of Life and Satisfaction Questionnaires eTable 3. Secondary and Exploratory Study Outcomes and Additional Statistical Methods eTable 4. Glucose Lowering Medications in Use at Time of Randomization in Addition to Insulin eTable 5. CGM Use in CGM Group eTable 6. Frequency of Blood Glucose Meter Testing eTable 7. Change in HbA1c: Per-Protocol Analysis and Sensitivity Analyses eTable 8. Change in HbA1c According to Baseline HbA1c Group eTable 9. Change in HbA1c According to Baseline Subgroups eTable CGM Outcomes According to Time of Day eTable Daily Insulin Delivery eTable Additions and Discontinuations of Diabetes Medications and Insulin Use eTable Medications Added and Stopped During Follow-up eTable Body Weight, Blood Pressure, and Cholesterol eTable Listing of Types of Reported Adverse Events eTable CGM Satisfaction Scale. Supplement 3. However, technology such as continuous glucose monitors CGMs offer a practical option that may make it easier to monitor and control blood sugar. To achieve this, people can use a CGM , which is a wearable device that a person places on their body to quickly and easily check their blood sugar. It can provide users with dynamic information about their blood sugar and can use alerts to warn the wearer of dangerous glucose levels. Using these devices can be a useful and convenient way to help people better manage diabetes. Evidence notes that these devices can have a positive impact on glycemic control and improve HbA1C levels. Previously, one of the main options to test blood sugar was a blood sugar meter. However, this option was not practical for many people as it involved many components, and a person had to prick their finger to draw blood. Also, it would only provide a single reading at the time of taking the sample. Each CGM has three main parts : a sensor, a transmitter, and a monitor. A CGM user inserts a small sensor directly under the skin, usually on the belly or arm. A thin tube, or cannula, pierces the top layer of skin and measures glucose in the interstitial fluid. This is the fluid that surrounds cells in the body and provides a similar reading to blood glucose. Most sensors are waterproof and an adhesive patch keeps them firmly in place. Users will need to regularly replace the sensor, with most working for roughly 7—14 days. The sensor connects to a transmitter that allows the system to wirelessly send blood glucose readings. The transmitter communicates with the sensor and monitor and passes on the information displayed on the monitor. Many systems combine the sensor and transmitter, so a person may need to sync this part with their monitor to receive readings. Most systems are able to display readings that are close to real time, although many systems have a 5-minute delay. The monitor is responsible for displaying information to the user. Some CGMs have a dedicated monitor, which may be a separate device or part of an insulin pump. Other devices are smartphone-compatible and work via a smartphone app. With a monitor, the user can see their blood sugar levels every few minutes. The CGM system can also store this information and send it to a doctor. The ease of collecting and sharing blood sugar levels can help doctors and CGM users work together on improving a diabetes treatment plan. Typically, most people who use a CGM will have type 1 diabetes. Some individuals with type 2 diabetes may also benefit from CGMs. A doctor may prescribe a CGM if people meet certain criteria and requirements. Usually, this may include :. For these individuals, a CGM can help them closely monitor blood sugar levels and may prevent them from experiencing a serious hypoglycemic event. A commentary notes that a CGM can help:. There are many benefits a CGM may offer over other devices. Namely, it can help people better manage diabetes and improve health outcomes. A study highlights that CGMs can improve glycemic control in individuals with inadequately controlled type 1 diabetes. Compared with conventional treatment options, people using CGMs had lower HbA1C levels. Elsewhere, a extension study investigated the potential long-term effects of using a CGM. The results suggest that CGMs have a beneficial effect on HbA1C, hypoglycemia prevention, hypoglycemic confidence, treatment satisfaction, and well-being. Consensus statement on the worldwide standardisation of the HbA1c measurement. Diabetologia ;—3. Driskell OJ, Holland D,Waldron JL, et al. Reduced testing frequency for glycated hemoglobin, HbA1c, is associated with deteriorating diabetes control. Diabetes Care ;—7. Consensus Committee. Consensus statement on the worldwide standardization of the hemoglobin A1C measurement: The American Diabetes Association, European Association for the Study of Diabetes, International Federation of Clinical Chemistry and Laboratory Medicine, and the International Diabetes Federation. Sacks DB. Measurement of hemoglobin A 1c : A new twist on the path to harmony. Weykamp C, JohnWG, Mosca A, et al. The IFCC Reference Measurement System for HbA1c: A 6-year progress report. Clin Chem ;—8. Diagnostic Evidence Co-operative Oxford. Point-of-care HbA1c tests—diagnosis of diabetes. London: National Institue for Health Research NHS , , pg. Report No. Accessed November 15, Spaeth BA, Shephard MD, Schatz S. Point-of-care testing for haemoglobin A1c in remote Australian Indigenous communities improves timeliness of diabetes care. Rural Remote Health ; Hirst JA, McLellan JH, Price CP, et al. Performance of point-of-care HbA1c test devices: Implications for use in clinical practice—a systematic review and metaanalysis. Clin Chem Lab Med ;— Saaddine JB, Fagot-Campagna A, Rolka D, et al. Distribution of HbA 1c levels for children and young adults in the U. Herman WH, Ma Y, Uwaifo G, et al. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Herman WH, Dungan KM, Wolffenbuttel BH, et al. Racial and ethnic differences in mean plasma glucose, hemoglobin A1c, and 1,5-anhydroglucitol in over patients with type 2 diabetes. J Clin Endocrinol Metab ;— Selvin E, Steffes MW, Ballantyne CM, et al. Racial differences in glycemic markers: A cross-sectional analysis of community-based data. Ann Intern Med ;—9. Herman WH, Cohen RM. Racial and ethnic differences in the relationship between HbA1c and blood glucose: Implications for the diagnosis of diabetes. Bergenstal RM, Gal RL, Connor CG, et al. Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med ;— Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med ;— Tsugawa Y, Mukamal KJ, Davis RB, et al. Should the hemoglobin A1c diagnostic cutoff differ between blacks and whites? A cross-sectional study. Selvin E, Ning Y, Steffes MW, et al. Glycated hemoglobin and the risk of kidney disease and retinopathy in adults with and without diabetes. Diabetes ;— Karter AJ, Ackerson LM, Darbinian JA, et al. Self-monitoring of blood glucose levels and glycemic control: The Northern California Kaiser Permanente Diabetes registry. Am J Med ;—9. Karter AJ, Parker MM, Moffet HH, et al. Longitudinal study of new and prevalent use of self-monitoring of blood glucose. Malekiani CL, Ganesan A, Decker CF. Effect of hemoglobinopathies on hemoglobin A1c measurements. Am J Med ;e5. Parkin CG, Davidson JA. Value of self-monitoring blood glucose pattern analysis in improving diabetes outcomes. J Diabetes Sci Technol ;—8. Franciosi M, Pellegrini F, De Berardis G, et al. The impact of blood glucose self-monitoring on metabolic control and quality of life in type 2 diabetic patients: An urgent need for better educational strategies. Norris SL, Lau J, Smith SJ, et al. Self-management education for adults with type 2 diabetes: A meta-analysis of the effect on glycemic control. Polonsky WH, Earles J, Smith S, et al. Integrating medical management with diabetes self-management training: A randomized control trial of the Diabetes Outpatient Intensive Treatment program. Polonsky WH, Fisher L, Schikman CH, et al. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulintreated type 2 diabetes: Results fromthe Structured Testing Program study. Sheppard P, Bending JJ, Huber JW. Pre- and post-prandial capillary glucose selfmonitoring achieves better glycaemic control than pre-prandial only monitoring. Pract Diab Int ;— Murata GH, Shah JH, Hoffman RM, et al. Intensified blood glucose monitoring improves glycemic control in stable, insulin-treated veterans with type 2 diabetes: The Diabetes Outcomes in Veterans Study DOVES. The Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The DCCT Research Group. Epidemiology of severe hypoglycemia in the diabetes control and complications trial. Gale EA, Tattersall RB. Unrecognised nocturnal hypoglycaemia in insulintreated diabetics. Lancet ;— Vervoort G, Goldschmidt HM, van Doorn LG. Diabet Med ;—9. Jones TW, Porter P, Sherwin RS, et al. Decreased epinephrine responses to hypoglycemia during sleep. Boutati EI, Raptis SA. Self-monitoring of blood glucose as part of the integral care of type 2 diabetes. Diabetes Care ;32 Suppl. Faas A, Schellevis FG, Van Eijk JT. The efficacy of self-monitoring of blood glucose in NIDDM subjects. A criteria-based literature review. Diabetes Care ;—6. Harris MI. Frequency of blood glucose monitoring in relation to glycemic control in patients with type 2 diabetes. Coster S, Gulliford MC, Seed PT, et al. Self-monitoring in Type 2 diabetes mellitus: A meta-analysis. Diabet Med ;— Welschen LM, Bloemendal E, Nijpels G, et al. Self-monitoring of blood glucose in patients with type 2 diabetes who are not using insulin: A systematic review. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. CochraneDatabase Syst Rev ; 2 :CD Davidson MB, Castellanos M, Kain D, et al. The effect of self monitoring of blood glucose concentrations on glycated hemoglobin levels in diabetic patients not taking insulin: A blinded, randomized trial. Am J Med ;—5. Davis WA, Bruce DG, Davis TM. Is self-monitoring of blood glucose appropriate for all type 2 diabetic patients? The Fremantle Diabetes Study. DavisWA, Bruce DG, Davis TME. Does self-monitoring of blood glucose improve outcome in type 2 diabetes? Diabetologia ;— Farmer A, Wade A, Goyder E, et al. Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: Open parallel group randomised trial. BMJ ; Allemann S, Houriet C, Diem P, et al. Self-monitoring of blood glucose in noninsulin treated patients with type 2 diabetes: A systematic review and metaanalysis. Curr Med Res Opin ;— Jansen JP. Self-monitoring of glucose in type 2 diabetes mellitus: A Bayesian meta-analysis of direct and indirect comparisons. McGeoch G, Derry S, Moore RA. Self-monitoring of blood glucose in type-2 diabetes: What is the evidence? Diabetes Metab Res Rev ;— Poolsup N, Suksomboon N, Rattanasookchit S. Meta-analysis of the benefits of self-monitoring of blood glucose on glycemic control in type 2 diabetes patients: An update. Diabetes Technol Ther ;— St John A, Davis WA, Price CP, et al. The value of self-monitoring of blood glucose: A review of recent evidence. J Diabetes Complications ;— Towfigh A, Romanova M,Weinreb JE, et al. Self-monitoring of blood glucose levels in patients with type 2 diabetes mellitus not taking insulin: A meta-analysis. Am J Manag Care ;— Canadian Agency for Drugs and Technologies in Health CADTH. Systematic review of use of blood glucose test strips for the management of diabetes mellitus. CADTH Technol Overv ;1:e |
| Key Messages for People with Diabetes | Users will need to regularly replace the sensor, with most working for roughly 7—14 days. The sensor connects to a transmitter that allows the system to wirelessly send blood glucose readings. The transmitter communicates with the sensor and monitor and passes on the information displayed on the monitor. Many systems combine the sensor and transmitter, so a person may need to sync this part with their monitor to receive readings. Most systems are able to display readings that are close to real time, although many systems have a 5-minute delay. The monitor is responsible for displaying information to the user. Some CGMs have a dedicated monitor, which may be a separate device or part of an insulin pump. Other devices are smartphone-compatible and work via a smartphone app. With a monitor, the user can see their blood sugar levels every few minutes. The CGM system can also store this information and send it to a doctor. The ease of collecting and sharing blood sugar levels can help doctors and CGM users work together on improving a diabetes treatment plan. Typically, most people who use a CGM will have type 1 diabetes. Some individuals with type 2 diabetes may also benefit from CGMs. A doctor may prescribe a CGM if people meet certain criteria and requirements. Usually, this may include :. For these individuals, a CGM can help them closely monitor blood sugar levels and may prevent them from experiencing a serious hypoglycemic event. A commentary notes that a CGM can help:. There are many benefits a CGM may offer over other devices. Namely, it can help people better manage diabetes and improve health outcomes. A study highlights that CGMs can improve glycemic control in individuals with inadequately controlled type 1 diabetes. Compared with conventional treatment options, people using CGMs had lower HbA1C levels. Elsewhere, a extension study investigated the potential long-term effects of using a CGM. The results suggest that CGMs have a beneficial effect on HbA1C, hypoglycemia prevention, hypoglycemic confidence, treatment satisfaction, and well-being. A study notes that a CGM device can improve health outcomes for both parent and baby during pregnancy. A commentary also highlights CGMs as a reliable, safe, and effective tool, particularly during the COVID pandemic. Having a CGM may be particularly useful for a person with a recent diagnosis of diabetes as it can help them identify what triggers blood sugar changes and how to minimize these fluctuations. Other advantages of a CGM may include :. This indicates that CGMs may show promise for individuals with diabetes across different ages and health considerations. As such, people with diabetes and their doctors can use a CGM to improve diabetes management strategies. Although a CGM can offer many benefits for people with diabetes, it may come with certain limitations. While it does reduce the number of finger-prick tests needed, it does not eliminate them entirely. People may still require finger pricks to calibrate a CGM and confirm readings. The cost of CGM devices can also be prohibitive for many users and some insurance plans may not cover them. This could result in the price of a CGM running higher than other testing devices. While the sensors are generally robust, people may also want to avoid certain activities to prevent the risk of knocking or damaging the device, as they will need to replace it if it stops functioning. Some people may also find the amount of data a CGM provides overwhelming. Understanding the information and making decisions from it may cause anxiety in some individuals. Also known as an automated insulin delivery system or artificial pancreas, these systems can help mimic the function of a healthy pancreas. A CGM device is an important piece of a hybrid closed-loop system. These systems typically consist of three different components:. In this system, the CGM keeps track of the blood sugar at regular intervals. It sends information about blood sugar levels to the control algorithm. The control algorithm analyzes this information and then sends instructions to the insulin pump. This way, the pump can deliver an appropriate dose of insulin when necessary. Many systems may only be compatible with basal, or slow-acting, insulin. In these cases, people will still need to calculate and manually administer bolus, or rapid-acting, insulin at certain times, such as with meals. However, other systems, such as the Omnipod , can calculate and suggest a bolus dose using an algorithm and the CGM reading. These systems can take the guesswork out of insulin injections during the day. Many users find them helpful for simplifying the process of blood sugar regulation. Management of diabetes involves strict control of blood sugar levels. A CGM can help facilitate this by providing users with a quick and convenient way to monitor blood glucose. Evidence notes that these devices can aid glycemic control, prevent hypos, and improve overall health and well-being. Individuals interested in using a CGM can consult with a medical professional about their suitability and how it may help with their health. Experts say more adults who develop type 1 diabetes are being misdiagnosed as having type 2 diabetes. That, they say, can lead to ineffective…. We do not capture any email address. Skip to main content. Open Access. Stewart B. Harris , Basel Bari and Jeremy Gilbert. Continuous glucose monitoring CGM in diabetes improves outcomes and enhances patient self-management Compared with traditional fingerstick testing, CGM improves glycemic control and quality of life, and is now recommended for people with type 1 and type 2 diabetes using basal—bolus insulin. Continuous glucose monitoring overcomes the limitations of glycated hemoglobin HbA 1c Unlike HbA 1c , CGM can guide immediate decisions on blood glucose management and provides important metrics, including time in range Appendix 1, available at www. There are 2 types of CGM systems — real-time and intermittently scanned Real-time CGM automatically collects and displays glucose data, while intermittently scanned CGM requires manual scanning at least every 8 hours. Interpretation of CGM results is straightforward Reports can be easily accessed by smartphone, receiver or CGM-specific software Appendix 1. Potential challenges should be considered Challenges may include body image concerns, sensor adhesion issues, skin irritation and alert fatigue. Footnotes Competing interests: Stewart B. This article has been peer reviewed. Diabetes Canada Clinical Practice Guidelines Steering Committee. Blood glucose monitoring in adults and children with diabetes: update Can J Diabetes ; 45 : — 7. Martens T , Beck RW , Bailey R , et al. MOBILE Study Group. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA ; : — OpenUrl CrossRef PubMed. Edelman SV , Cavaiola TS , Boeder S , et al. Utilizing continuous glucose monitoring in primary care practice: what the numbers mean. Prim Care Diabetes ; 15 : — Yapanis M , James S , Craig ME , et al. Complications of diabetes and metrics of glycemic management derived from continuous glucose monitoring. J Clin Endocrinol Metab ; : e — Czupryniak L , Dzida G , Fichna P , et al. Ambulatory glucose profile AGP report in daily care of patients with diabetes: practical tips and recommendations. Diabetes Ther ; 13 : — Previous Next. Back to top. In this issue. Table of Contents Index by author. Article tools Respond to this article. Download PDF. Article Alerts. To sign up for email alerts or to access your current email alerts, enter your email address below:. Email Article. Thank you for your interest in spreading the word on CMAJ. |
| Continuous glucose monitoring (CGM) | Medtronic | You can explain that it will take more time and steps than a new prescription for a medication. Reinforce your patient conversations with this postcard. Patient education videos are also available on familydoctor. org : Patient Experience with CGM, FAQ and Demo, and Understanding Your CGM Data. What Type of Coverage Does the Patient Have? Medicare Coverage and Ordering for Personal CGM Medicare has eligibility requirements that must be met in order to provide coverage for personal CGM. Seen for diabetes management in past 6 months. For continuing eligibility , all of the above must continue to be met. Gather Information for Ordering and Insurance Authorization A Prescription is Required: Many EHRs support directly prescribing CGM as you would any medication. Choose a Supplier: Medicare covers CGM through the durable medical equipment DME benefit, not through the pharmacy benefit. Chart Notes: Some order forms also ask about frequency of glucose checks. Important Step: Schedule a telehealth or in-person diabetes follow up within 6 months of the last visit for continuing eligibility. How to Order CGM by Brand. Abbott Freestyle Libre 2 System. Medtronic CGM. Medicaid Coverage and Ordering for Personal CGM Eligibility Medicaid coverage for for Personal CGM varies from state to state. What information is needed as part of Prior Authorization? What other coverage criteria exist? Minimum number of insulin doses per day? Gather Information for Ordering and Insurance Authorization Gather the documentation required. This usually includes: A Prescription: Many EHRs support directly prescribing them as you would any medication. Chart Notes Eligibility and ordering guidelines for personal CGM vary state by state. Colorado Medicaid Eligibility Requirements for personal CGM: To be eligible for Colorado Medicaid coverage of personal CGM, the following requirements must be met: The patient self-monitors glucose at least 3 times daily e. For continuing eligibility , all of the above must be met and Colorado Medicaid requires recertification every 6 months; be sure to schedule diabetes follow up for within 6 months of the last visit. How to Order CGM by Brand These specific instructions follow the state of Colorado example. Freestyle Libre 2 System. Step 2: To order a Freestyle Libre 2 if patient has a compatible smartphone: Freestyle Libre 2 Sensors — change sensor every 14 days, dispense 2 sensors day supply , 11 refills If patient does not have a compatible smartphone then also order: Freestyle Libre 2 Reader - dispense 1 reader reader life is approx. Step 2: Complete the Dexcom New Patient Letter-Byers. Private Insurance Coverage and Ordering Commercial or private insurance plans likely have certain eligibility requirements that must be met in order to provide coverage for personal CGM. Eligibility Eligibility requirements may vary widely from one payer to another, or even from one plan to another for any particular payer, so specific guidance is very difficult to obtain with certainty. Commercial payers generally do not require a Certificate of Medical Necessity or specific order form to prescribe to a pharmacy. The italicized text are examples to guide your chart notes for patients you feel would benefit from CGM. This patient has a diagnosis of diabetes; is treated with 3 or more daily administrations of insulin or continuous insulin infusion via pump; requires frequent adjustment of the insulin treatment regimen based on glucose results; and has been personally seen to evaluate their diabetes treatment within the past 6 months. This patient is treated with insulin and would benefit from use of a continuous glucose monitor CGM , as recommended in the American Diabetes Association Standards of Medical Care in Diabetes— Abridged for Primary Care Providers Clin Diabetes ;40 1 : Recommendation 7. For Recommendations 7. Gather Information for Ordering and Insurance Authorization A Prescription Will Be Required: Many EHRs support directly prescribing them as you would any medication. Choose a Supplier: Some commercial insurance plans cover CGM through the pharmacy benefit, some cover it through the durable medical equipment DME benefit, and some will allow either. To order a Freestyle Libre if patient has a compatible smartphone: Freestyle Libre 2 Sensors — change sensor every 14 days, dispense 2 sensors day supply , 11 refills If patient does not have a compatible smartphone, then also order: Freestyle Libre 2 Reader - dispense 1 reader reader life is approx. To order Dexcom if patient has a compatible smartphone: Dexcom G6 transmitter - dispense 1 per 90 days, 3 refills Dexcom G6 sensors - dispense 3 boxes 9 sensors per 90 days, change sensor every 10 days, 3 refills If patient does not have a compatible smartphone then also order: Dexcom G6 receiver- dispense 1, no refills Send an electronic prescription from your EHR to ASPN Pharmacies. ASPN Pharmacy phone: , fax: Patients Without Coverage for Personal CGM Patients who do not meet their payer's eligibility criteria for personal CGM or those who cannot afford the full cost of obtainings a personal CGM may still be able to get and benefit from CGM. Professional CGM — used by practices on a one-time or occasional basis for a given patient. It involves applying the CGM in the office setting and using equipment belonging to the practice. Insurance authorization is rarely required for this. Many practices find this a particularly successful path. Self-pay for Personal CGM — patient may be willing to pay out of pocket for device and sensors. Consider cost assistance programs for eligible patients. This may be a good option for patients who have insurance, but do not meet thier payer's eligibility criteria for personal CGM. Sample or Trial of Personal CGM — Manufacturers may have voucher, sample, or trial programs for eligible patients. This may be a good option for patients who do not have insurance or have high deductible insurance plans. Ensure You Have Proper Documentation The information in your chart notes should reflect why you feel that CGM is appropriate for your patient. Gather Information for Ordering A Prescription Will Be Required: Many EHRs support directly prescribing them as you would any medication. Forms required for cost assistance or patient assistance if applicable. Download PDF. Article Alerts. To sign up for email alerts or to access your current email alerts, enter your email address below:. Email Article. Thank you for your interest in spreading the word on CMAJ. You are going to email the following Continuous glucose monitoring. Message Subject Your Name has sent you a message from CMAJ. Message Body Your Name thought you would like to see the CMAJ web site. Your Personal Message. This question is for testing whether or not you are a human visitor and to prevent automated spam submissions. Citation Tools. Continuous glucose monitoring. Harris , Basel Bari , Jeremy Gilbert. CMAJ Nov , 44 E; DOI: Citation Manager Formats BibTeX Bookends EasyBib EndNote tagged EndNote 8 xml Medlars Mendeley Papers RefWorks Tagged Ref Manager RIS Zotero. Share This Article: Copy. Tweet Widget Facebook Like. Jump to section Article Continuous glucose monitoring CGM in diabetes improves outcomes and enhances patient self-management Continuous glucose monitoring overcomes the limitations of glycated hemoglobin HbA 1c There are 2 types of CGM systems — real-time and intermittently scanned Interpretation of CGM results is straightforward Potential challenges should be considered Footnotes References. Related Articles PubMed Google Scholar. No citing articles found. Google Scholar. Papulonecrotic tuberculid. Postinfectious cough in adults. Melioidosis with septic arthritis in a returning traveller. Show more Practice. Collections Article Types Five Things to Know About. Learn more about how you can improve the lives of people with diabetes by supporting increased access to CGMs. Watch the videos below to hear patient and practitioner perspectives on how CGMs are shaping the future of diabetes care. Has your life been changed by wearing a Continuous Glucose Monitor? If so we want to hear from you! CGMs are the new standard in diabetes care, and should be accessible to every person with diabetes. CGMs provide significant, potentially life changing benefits for diabetes management. CGMs are recommended for several reasons because they:. People with type 1 and type 2 diabetes who use a CGM have fewer instances of hypoglycemia and a lower A1C. One obstacle with CGMs is the cost of access to diabetes technology. Many people with diabetes who have put off getting an insulin pump or CGM, do so because they are too expensive. Another major obstacle is due to strict Medicaid coverage policies they are not accessible for people who need them. In fact, people with diabetes on Medicaid, especially in minority communities who use Medicaid, are the least likely to use a CGM. This is concerning since people with diabetes are more than twice as likely to receive their health care from Medicaid as those without diabetes. Individuals who meet the coverage criteria listed in the FAQs below for a CGM and want to learn more about them should talk to their health care provider to ensure it is the right tool for the management of their diabetes. The American Diabetes Association ® ADA released a new study looking at pharmacy and medical benefit claims for CGMs across commercial insurance plans, Medicare and Medicaid and data on age, race, geography, and diabetes prevalence. The findings show people of lower income and older people of color who live in states with the highest rates of diabetes prevalence and mortality are the least likely to get access to a CGM. ADA is quite concerned about these findings, given the effect of the COVID pandemic on this population and the importance of tools like CGMs in diabetes management. Learn more by viewing the study PDF. |
| Continuous glucose monitoring | CMAJ | Supplement 2. MOBILE Study Group Listing eFigure 1. Flow Chart of Screening eFigure 2. Flow Chart of Visit Completion Rates eFigure 3. Mean Glucose Over 24 Hours at 8 Months eTable 1. Patient Eligibility Criteria eTable 2. Description of Quality of Life and Satisfaction Questionnaires eTable 3. Secondary and Exploratory Study Outcomes and Additional Statistical Methods eTable 4. Glucose Lowering Medications in Use at Time of Randomization in Addition to Insulin eTable 5. CGM Use in CGM Group eTable 6. Frequency of Blood Glucose Meter Testing eTable 7. Change in HbA1c: Per-Protocol Analysis and Sensitivity Analyses eTable 8. Change in HbA1c According to Baseline HbA1c Group eTable 9. Change in HbA1c According to Baseline Subgroups eTable CGM Outcomes According to Time of Day eTable Daily Insulin Delivery eTable Additions and Discontinuations of Diabetes Medications and Insulin Use eTable Medications Added and Stopped During Follow-up eTable Body Weight, Blood Pressure, and Cholesterol eTable Listing of Types of Reported Adverse Events eTable CGM Satisfaction Scale. Supplement 3. Nonauthor Collaborators. MOBILE Study Group. Supplement 4. Data Sharing Statement. Selvin E, Parrinello CM, Daya N, Bergenstal RM. Trends in insulin use and diabetes control in the US: and doi: Kazemian P, Shebl FM, McCann N, Walensky RP, Wexler DJ. Evaluation of the cascade of diabetes care in the United States, Schnell O, Hanefeld M, Monnier L. Self-monitoring of blood glucose: a prerequisite for diabetes management in outcome trials. Murata GH, Shah JH, Hoffman RM, et al; Diabetes Outcomes in Veterans Study DOVES. Intensified blood glucose monitoring improves glycemic control in stable, insulin-treated veterans with type 2 diabetes: the Diabetes Outcomes in Veterans Study DOVES. Falk J, Friesen KJ, Okunnu A, Bugden S. Patterns, policy and appropriateness: a year utilization review of blood glucose test strip use in insulin users. Rossi MC, Lucisano G, Ceriello A, et al; AMD Annals-SMBG Study Group. Real-world use of self-monitoring of blood glucose in people with type 2 diabetes: an urgent need for improvement. Beck RW, Riddlesworth T, Ruedy K, et al; DIAMOND Study Group. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Hermanns N, Schumann B, Kulzer B, Haak T. The impact of continuous glucose monitoring on low interstitial glucose values and low blood glucose values assessed by point-of-care blood glucose meters: results of a crossover trial. Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. van Beers CA, DeVries JH, Kleijer SJ, et al. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia IN CONTROL : a randomised, open-label, crossover trial. Wong JC, Foster NC, Maahs DM, et al; T1D Exchange Clinic Network. Real-time continuous glucose monitoring among participants in the T1D Exchange clinic registry. Beck RW, Riddlesworth TD, Ruedy K, et al; DIAMOND Study Group. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Peters A, Cohen N, Calhoun P, et al. Glycaemic profiles of diverse patients with type 2 diabetes using basal insulin: MOBILE study baseline data. PubMed Google Scholar Crossref. Beck RW, Bocchino LE, Lum JW, et al. An evaluation of two capillary sample collection kits for laboratory measurement of HBALC. PubMed Google Scholar. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. The Diabetes Control and Complications Trial Research Group. The relationship of glycemic exposure HbA1c to the risk of development and progression of retinopathy in the diabetes control and complications trial. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes UKPDS 35 : prospective observational study. Kleinman LC, Norton EC. a simple approach for estimating adjusted risk measures from nonlinear models including logistic regression. x PubMed Google Scholar Crossref. Start-ups Tout Continuous Glucose Monitoring for People Without Diabetes. This Medical News article describes the marketing of continuous glucose monitoring devices to individuals without diabetes. Real-time Continuous Glucose Monitoring and Glycemic Control in Insulin-Treated Patients With Diabetes. This cohort study investigates the effect of real-time continuous glucose monitoring on glycemic control among patients with insulin-treated diabetes. Andrew J. Karter, PhD; Melissa M. Parker, MS; Howard H. Moffet, MPH; Lisa K. Gilliam, MD, PhD; Richard Dlott, MD. Broadening Access to Continuous Glucose Monitoring for Patients With Type 2 Diabetes. Continuous Glucose Monitoring and Glycemic Control in Patients With Type 2 Diabetes Treated With Basal Insulin—Reply. Thomas W. Martens, MD; Roy W. Beck, MD, PhD; Richard M. Bergenstal, MD. Continuous Glucose Monitoring and Glycemic Control in Patients With Type 2 Diabetes Treated With Basal Insulin. Wellbert Hernández-Núñez, MD; Jesús Zacarías Villareal-Pérez, MD; René Rodríguez-Gutiérrez, MD, MSc, PhD. See More About Diabetes Diabetes and Endocrinology. Select Your Interests Select Your Interests Customize your JAMA Network experience by selecting one or more topics from the list below. Save Preferences. Privacy Policy Terms of Use. This Issue. Views 84, Citations View Metrics. X Facebook More LinkedIn. The second part of the CGM is a transmitter. The transmitter sends the information, without using wires, to the third part, a software program that is stored on a smartphone, on an insulin pump , or on a separate device called a receiver. Your doctor may recommend that you use a CGM if you need insulin to manage type 1 diabetes , type 2 diabetes , or another form of diabetes. Talk with your doctor about whether using a CGM could help you manage your diabetes. Doctors can prescribe CGMs for adults and children. Some models can be used for children as young as 2 years old. Your doctor may suggest using a CGM all the time or only for a few days to help adjust your diabetes care. All CGMs estimate blood glucose levels, but they store and display the information in different ways. Some CGMs send and display information to your smartphone or receiver automatically. But you will need to scan the CGM with a separate receiver or smartphone every few hours to view and store the data. A third type of CGM collects data about your blood glucose level for your doctor to download and review later. Doctors provide this type of CGM to check on your diabetes care, and you wear it for a limited time. For some CGM models, you may need to do a finger-stick test with a standard blood glucose monitor to calibrate the system and make sure the CGM readings are correct. Many CGMs work with apps that have special features, such as. For safety, it is important to act quickly if a CGM alarm sounds when your glucose level is too low or too high. You should get help or follow your treatment plan to bring your glucose level into a healthy range. The CGM will create an alert and might display a graphic that shows whether your glucose level is rising or dropping—and how quickly—so you can choose the best way to reach your target range. Over time, keeping your glucose levels in the healthy range can help you stay well and prevent diabetes complications. The people who benefit the most from a CGM are those who use it every day or nearly every day. Researchers are working to make CGMs more accurate and easier to use. However, you may experience some issues while using a CGM. For safety, you may sometimes need to compare your CGM glucose readings with a finger-stick test and a standard blood glucose meter. This could be needed if you doubt the accuracy of your CGM readings, if you are changing your insulin dose, or if your CGM gives a warning alert. You might have to replace parts of your CGM over time. Disposable CGM sensors should be replaced every 7 to 14 days, depending on the model. Some implantable sensors can last up to days. You may have to replace the transmitters of some CGMs. You may also need to reconnect the CGM, transmitter, and receiver or smartphone if your CGM is not working correctly. Skin redness or irritation from the sticky patches used to attach the sensor may occur for some people. If your state has limited coverage currently, continue to monitor coverage requirements, in general, coverage for CGM has tended to expand. State Medicaid programs range from offering broad coverage for for Personal CGM to none at all through fee-for-service. Eligibility and ordering guidelines for personal CGM vary state by state. An example using the state of Colorado is outlined below. Most states will follow similar steps for ordering. Colorado Medicaid Eligibility Requirements for personal CGM: To be eligible for Colorado Medicaid coverage of personal CGM, the following requirements must be met:. These specific instructions follow the state of Colorado example. In other states, a DME supplier or pharmacy may be the primary contact. A brand representative may be able to assist in finding the best pharmacy or DME supplier to submit. Step 1: Complete the Libre Certificate of Medical Necessity. Step 2: To order a Freestyle Libre 2 if patient has a compatible smartphone:. Step 3: Send the Libre Certificate of Medical Necessity, chart notes, and prescription to the pharmacy or DME supplier for your state. Step 1: Complete the Certificate of Medical Necessity. Step 3: Email or Fax chart notes, the Certificate of Medical Necessity, the New Patient Letter, and patient contact information phone, address to the pharmacy or DME supplier for your state. Visit Medtronic's healthcare professional website for their most current resources. Become an Eversense provider and find information on ordering. Commercial or private insurance plans likely have certain eligibility requirements that must be met in order to provide coverage for personal CGM. Eligibility requirements may vary widely from one payer to another, or even from one plan to another for any particular payer, so specific guidance is very difficult to obtain with certainty. Additional assistance and up-to-date resources are available at the non-profit, unaffiliated DiabetesWiseProviders Prescription Assistant Tool. Check if the patient has a compatible smart devices for the Freestyle Libre 2. To order a Freestyle Libre if patient has a compatible smartphone:. If patient does not have a compatible smartphone, then also order:. Freestyle Libre 2 prescriptions are covered nearly exclusively through pharmacies under the pharmacy benefit. Check if the patient has a compatible smart devices for the Dexcom. If patient does not have a compatible smartphone then also order:. Send an electronic prescription from your EHR to ASPN Pharmacies. ASPN will identity a participating pharmacy or DME supplier and forward the order information there for you. Become an Eversense provider and to pursue information on ordering. Patients who do not meet their payer's eligibility criteria for personal CGM or those who cannot afford the full cost of obtainings a personal CGM may still be able to get and benefit from CGM. This patient handout, What if My Continuous Glucose Monitor Is Not Covered by Insurance? Click here for the Spanish version. The information in your chart notes should reflect why you feel that CGM is appropriate for your patient. This may be especially helpful if pursuing low or no copay options like patient assistance programs that may require your explanation of why you recommend CGM. There are various options for limiting patient out-of-pocket costs associated with CGM. Some of these include manufacturer-based samples, vouchers, copay reduction programs, and patient assistance programs. Some of these resources are listed here:. Professional CGM can be extremely useful when personal CGM is not likely to be covered by insurance e. While personal CGM belongs to the patient, Professional CGM is owned by the practice and used by a given patient on a short-term basis. A practice can obtain a professional CGM system and sensors by purchase through the manufacturer or a supplier. Each of the manufacturers of professional CGM systems has useful information to describe and demonstrate application. Here too, each of the manufacturers of professional CGM systems has useful information to explain and demonstrate what you need to get the data. There are two CPT codes can be used to bill and seek payment for Professional CGM related services:. For Federally Qualified Health Centers and Rural Health Clinicss, where traditional fee-for-service billing would not support payment for Professional CGM, diabetes-related grant programs can be used to purchase Professional CGM systems and sensors to help defray the equipment costs. Local Medicare payment rates and requirements may vary; check with your local Medicare administrative contractor for local requirements. Check with your local provider relations representatives for their policies. Verify coverage for each patient. Supported by an educational grant to the AAFP from Abbott Diabetes Care. search close. Continuous Glucose Monitoring CGM. If you have tried prescribing CGM in the past, but found navigating coverage and prior authorizations difficult, these additional resources outline processes, forms, and documentation for successfully ordering CGM. CGM in Your Practice: Helpful Videos. CGM in Your Practice: Implicit Bias Learn about factors that might influence your decision to pursue CGM with a patient and how to ensure you are providing equitable care around CGM. CGM in Your Practice: Shared Decision Making in Interpreting CGM Data with Patients Understand key measures from CGM data and how to use shared decision making with patients to make diabetes care adjustments based on CGM data. |
Continuous glucose management -
Spaeth BA, Shephard MD, Schatz S. Point-of-care testing for haemoglobin A1c in remote Australian Indigenous communities improves timeliness of diabetes care.
Rural Remote Health ; Hirst JA, McLellan JH, Price CP, et al. Performance of point-of-care HbA1c test devices: Implications for use in clinical practice—a systematic review and metaanalysis. Clin Chem Lab Med ;— Saaddine JB, Fagot-Campagna A, Rolka D, et al. Distribution of HbA 1c levels for children and young adults in the U.
Herman WH, Ma Y, Uwaifo G, et al. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Herman WH, Dungan KM, Wolffenbuttel BH, et al.
Racial and ethnic differences in mean plasma glucose, hemoglobin A1c, and 1,5-anhydroglucitol in over patients with type 2 diabetes. J Clin Endocrinol Metab ;— Selvin E, Steffes MW, Ballantyne CM, et al. Racial differences in glycemic markers: A cross-sectional analysis of community-based data.
Ann Intern Med ;—9. Herman WH, Cohen RM. Racial and ethnic differences in the relationship between HbA1c and blood glucose: Implications for the diagnosis of diabetes. Bergenstal RM, Gal RL, Connor CG, et al. Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels.
Ann Intern Med ;— Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med ;— Tsugawa Y, Mukamal KJ, Davis RB, et al. Should the hemoglobin A1c diagnostic cutoff differ between blacks and whites?
A cross-sectional study. Selvin E, Ning Y, Steffes MW, et al. Glycated hemoglobin and the risk of kidney disease and retinopathy in adults with and without diabetes. Diabetes ;— Karter AJ, Ackerson LM, Darbinian JA, et al. Self-monitoring of blood glucose levels and glycemic control: The Northern California Kaiser Permanente Diabetes registry.
Am J Med ;—9. Karter AJ, Parker MM, Moffet HH, et al. Longitudinal study of new and prevalent use of self-monitoring of blood glucose. Malekiani CL, Ganesan A, Decker CF. Effect of hemoglobinopathies on hemoglobin A1c measurements. Am J Med ;e5. Parkin CG, Davidson JA. Value of self-monitoring blood glucose pattern analysis in improving diabetes outcomes.
J Diabetes Sci Technol ;—8. Franciosi M, Pellegrini F, De Berardis G, et al. The impact of blood glucose self-monitoring on metabolic control and quality of life in type 2 diabetic patients: An urgent need for better educational strategies.
Norris SL, Lau J, Smith SJ, et al. Self-management education for adults with type 2 diabetes: A meta-analysis of the effect on glycemic control.
Polonsky WH, Earles J, Smith S, et al. Integrating medical management with diabetes self-management training: A randomized control trial of the Diabetes Outpatient Intensive Treatment program. Polonsky WH, Fisher L, Schikman CH, et al.
Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulintreated type 2 diabetes: Results fromthe Structured Testing Program study.
Sheppard P, Bending JJ, Huber JW. Pre- and post-prandial capillary glucose selfmonitoring achieves better glycaemic control than pre-prandial only monitoring. Pract Diab Int ;— Murata GH, Shah JH, Hoffman RM, et al.
Intensified blood glucose monitoring improves glycemic control in stable, insulin-treated veterans with type 2 diabetes: The Diabetes Outcomes in Veterans Study DOVES.
The Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The DCCT Research Group.
Epidemiology of severe hypoglycemia in the diabetes control and complications trial. Gale EA, Tattersall RB. Unrecognised nocturnal hypoglycaemia in insulintreated diabetics.
Lancet ;— Vervoort G, Goldschmidt HM, van Doorn LG. Diabet Med ;—9. Jones TW, Porter P, Sherwin RS, et al. Decreased epinephrine responses to hypoglycemia during sleep.
Boutati EI, Raptis SA. Self-monitoring of blood glucose as part of the integral care of type 2 diabetes. Diabetes Care ;32 Suppl. Faas A, Schellevis FG, Van Eijk JT. The efficacy of self-monitoring of blood glucose in NIDDM subjects.
A criteria-based literature review. Diabetes Care ;—6. Harris MI. Frequency of blood glucose monitoring in relation to glycemic control in patients with type 2 diabetes. Coster S, Gulliford MC, Seed PT, et al.
Self-monitoring in Type 2 diabetes mellitus: A meta-analysis. Diabet Med ;— Welschen LM, Bloemendal E, Nijpels G, et al. Self-monitoring of blood glucose in patients with type 2 diabetes who are not using insulin: A systematic review. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin.
CochraneDatabase Syst Rev ; 2 :CD Davidson MB, Castellanos M, Kain D, et al. The effect of self monitoring of blood glucose concentrations on glycated hemoglobin levels in diabetic patients not taking insulin: A blinded, randomized trial. Am J Med ;—5.
Davis WA, Bruce DG, Davis TM. Is self-monitoring of blood glucose appropriate for all type 2 diabetic patients? The Fremantle Diabetes Study. DavisWA, Bruce DG, Davis TME.
Does self-monitoring of blood glucose improve outcome in type 2 diabetes? Diabetologia ;— Farmer A, Wade A, Goyder E, et al. Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: Open parallel group randomised trial.
BMJ ; Allemann S, Houriet C, Diem P, et al. Self-monitoring of blood glucose in noninsulin treated patients with type 2 diabetes: A systematic review and metaanalysis. Curr Med Res Opin ;— Jansen JP. Self-monitoring of glucose in type 2 diabetes mellitus: A Bayesian meta-analysis of direct and indirect comparisons.
McGeoch G, Derry S, Moore RA. Self-monitoring of blood glucose in type-2 diabetes: What is the evidence? Diabetes Metab Res Rev ;— Poolsup N, Suksomboon N, Rattanasookchit S.
Meta-analysis of the benefits of self-monitoring of blood glucose on glycemic control in type 2 diabetes patients: An update. Diabetes Technol Ther ;— St John A, Davis WA, Price CP, et al. The value of self-monitoring of blood glucose: A review of recent evidence.
J Diabetes Complications ;— Towfigh A, Romanova M,Weinreb JE, et al. Self-monitoring of blood glucose levels in patients with type 2 diabetes mellitus not taking insulin: A meta-analysis.
Am J Manag Care ;— Canadian Agency for Drugs and Technologies in Health CADTH. Systematic review of use of blood glucose test strips for the management of diabetes mellitus. CADTH Technol Overv ;1:e Skeie S, Kristensen GB, Carlsen S, et al. Self-monitoring of blood glucose in type 1 diabetes patients with insufficient metabolic control: Focused self-monitoring of blood glucose intervention can lower glycated hemoglobin A1C.
Malanda UL,Welschen LM, Riphagen II, et al. Cochrane Database Syst Rev ; 1 :CD Franciosi M, Lucisano G, Pellegrini F, et al.
ROSES: Role of self-monitoring of blood glucose and intensive education in patients with type 2 diabetes not receiving insulin. A pilot randomized clinical trial. Duran A, Martin P, Runkle I, et al. Benefits of self-monitoring blood glucose in the management of new-onset Type 2 diabetes mellitus: The St Carlos Study, a prospective randomized clinic-based interventional study with parallel groups.
J Diabetes ;— UK Prospective Diabetes Study UKPDS Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes UKPDS Notice: New requirements for medical device licence applications for lancing devices and blood glucose monitoring systems [press release].
Ottawa, Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: A systematic review of randomized controlled trials. Bergenstal R, Pearson J, Cembrowski GS, et al. What other coverage criteria exist?
Minimum number of insulin doses per day? Gather Information for Ordering and Insurance Authorization Gather the documentation required. This usually includes: A Prescription: Many EHRs support directly prescribing them as you would any medication.
Chart Notes Eligibility and ordering guidelines for personal CGM vary state by state. Colorado Medicaid Eligibility Requirements for personal CGM: To be eligible for Colorado Medicaid coverage of personal CGM, the following requirements must be met: The patient self-monitors glucose at least 3 times daily e.
For continuing eligibility , all of the above must be met and Colorado Medicaid requires recertification every 6 months; be sure to schedule diabetes follow up for within 6 months of the last visit. How to Order CGM by Brand These specific instructions follow the state of Colorado example.
Freestyle Libre 2 System. Step 2: To order a Freestyle Libre 2 if patient has a compatible smartphone: Freestyle Libre 2 Sensors — change sensor every 14 days, dispense 2 sensors day supply , 11 refills If patient does not have a compatible smartphone then also order: Freestyle Libre 2 Reader - dispense 1 reader reader life is approx.
Step 2: Complete the Dexcom New Patient Letter-Byers. Private Insurance Coverage and Ordering Commercial or private insurance plans likely have certain eligibility requirements that must be met in order to provide coverage for personal CGM.
Eligibility Eligibility requirements may vary widely from one payer to another, or even from one plan to another for any particular payer, so specific guidance is very difficult to obtain with certainty. Commercial payers generally do not require a Certificate of Medical Necessity or specific order form to prescribe to a pharmacy.
The italicized text are examples to guide your chart notes for patients you feel would benefit from CGM. This patient has a diagnosis of diabetes; is treated with 3 or more daily administrations of insulin or continuous insulin infusion via pump; requires frequent adjustment of the insulin treatment regimen based on glucose results; and has been personally seen to evaluate their diabetes treatment within the past 6 months.
This patient is treated with insulin and would benefit from use of a continuous glucose monitor CGM , as recommended in the American Diabetes Association Standards of Medical Care in Diabetes— Abridged for Primary Care Providers Clin Diabetes ;40 1 : Recommendation 7.
For Recommendations 7. Gather Information for Ordering and Insurance Authorization A Prescription Will Be Required: Many EHRs support directly prescribing them as you would any medication.
Choose a Supplier: Some commercial insurance plans cover CGM through the pharmacy benefit, some cover it through the durable medical equipment DME benefit, and some will allow either.
To order a Freestyle Libre if patient has a compatible smartphone: Freestyle Libre 2 Sensors — change sensor every 14 days, dispense 2 sensors day supply , 11 refills If patient does not have a compatible smartphone, then also order: Freestyle Libre 2 Reader - dispense 1 reader reader life is approx.
To order Dexcom if patient has a compatible smartphone: Dexcom G6 transmitter - dispense 1 per 90 days, 3 refills Dexcom G6 sensors - dispense 3 boxes 9 sensors per 90 days, change sensor every 10 days, 3 refills If patient does not have a compatible smartphone then also order: Dexcom G6 receiver- dispense 1, no refills Send an electronic prescription from your EHR to ASPN Pharmacies.
ASPN Pharmacy phone: , fax: Patients Without Coverage for Personal CGM Patients who do not meet their payer's eligibility criteria for personal CGM or those who cannot afford the full cost of obtainings a personal CGM may still be able to get and benefit from CGM.
Professional CGM — used by practices on a one-time or occasional basis for a given patient. It involves applying the CGM in the office setting and using equipment belonging to the practice.
Insurance authorization is rarely required for this. Many practices find this a particularly successful path. Self-pay for Personal CGM — patient may be willing to pay out of pocket for device and sensors.
Consider cost assistance programs for eligible patients. This may be a good option for patients who have insurance, but do not meet thier payer's eligibility criteria for personal CGM.
Sample or Trial of Personal CGM — Manufacturers may have voucher, sample, or trial programs for eligible patients. This may be a good option for patients who do not have insurance or have high deductible insurance plans.
Ensure You Have Proper Documentation The information in your chart notes should reflect why you feel that CGM is appropriate for your patient. Gather Information for Ordering A Prescription Will Be Required: Many EHRs support directly prescribing them as you would any medication.
Forms required for cost assistance or patient assistance if applicable. Cost Assistance There are various options for limiting patient out-of-pocket costs associated with CGM.
To use these sites, patients will need to register and can download an app to their smartphone. Both provide coupons to use at the pharmacy. To use, they will need to search by device name and ZIP code.
Free Trials or Samples Voucher for a free FreeStyle Libre2 day sensor FreeStyle Libre 2 samples can be requested from your local representative day trial program for Medtronic Guardian Connect system Dexcom G6 sample kits sensor and transmitter can be requested from your local representative or online through the Hello Dexcom program.
Professional CGM Professional CGM can be extremely useful when personal CGM is not likely to be covered by insurance e. This video introduces you to professional CGM and how to implement ProCGM into your practice. Advantages of Professional CGM Often easier path to get CGM data, especially when only desired intermittently May be more accurate than HbA1c in certain situations e.
Freestyle Libre Pro Dexcom Pro What Other Equipment is Needed? Reusable reader - Each system requires a reader to capture the data from the sensor. Consider ordering a spare, or one per clinical work area or pod—whatever works best for your practice and flow.
Disposable sensor - Each patient will need one. How Do I Document to Support It? How Do I Apply It? Freestyle Libre Pro resources Dexcom G6 Pro resources How Do I Get Data from It? Freestyle Libre Pro resources Dexcom G6 Pro resources How Do I Bill for It? Do not report CPT codes or more than once per month.
A commentary also highlights CGMs as a reliable, safe, and effective tool, particularly during the COVID pandemic. Having a CGM may be particularly useful for a person with a recent diagnosis of diabetes as it can help them identify what triggers blood sugar changes and how to minimize these fluctuations.
Other advantages of a CGM may include :. This indicates that CGMs may show promise for individuals with diabetes across different ages and health considerations. As such, people with diabetes and their doctors can use a CGM to improve diabetes management strategies.
Although a CGM can offer many benefits for people with diabetes, it may come with certain limitations. While it does reduce the number of finger-prick tests needed, it does not eliminate them entirely. People may still require finger pricks to calibrate a CGM and confirm readings.
The cost of CGM devices can also be prohibitive for many users and some insurance plans may not cover them. This could result in the price of a CGM running higher than other testing devices.
While the sensors are generally robust, people may also want to avoid certain activities to prevent the risk of knocking or damaging the device, as they will need to replace it if it stops functioning.
Some people may also find the amount of data a CGM provides overwhelming. Understanding the information and making decisions from it may cause anxiety in some individuals. Also known as an automated insulin delivery system or artificial pancreas, these systems can help mimic the function of a healthy pancreas.
A CGM device is an important piece of a hybrid closed-loop system. These systems typically consist of three different components:. In this system, the CGM keeps track of the blood sugar at regular intervals. It sends information about blood sugar levels to the control algorithm.
The control algorithm analyzes this information and then sends instructions to the insulin pump. This way, the pump can deliver an appropriate dose of insulin when necessary. Many systems may only be compatible with basal, or slow-acting, insulin.
In these cases, people will still need to calculate and manually administer bolus, or rapid-acting, insulin at certain times, such as with meals. However, other systems, such as the Omnipod , can calculate and suggest a bolus dose using an algorithm and the CGM reading.
These systems can take the guesswork out of insulin injections during the day. Many users find them helpful for simplifying the process of blood sugar regulation. Management of diabetes involves strict control of blood sugar levels. A CGM can help facilitate this by providing users with a quick and convenient way to monitor blood glucose.
Evidence notes that these devices can aid glycemic control, prevent hypos, and improve overall health and well-being. Individuals interested in using a CGM can consult with a medical professional about their suitability and how it may help with their health.
Experts say more adults who develop type 1 diabetes are being misdiagnosed as having type 2 diabetes. That, they say, can lead to ineffective…. Ketonemia is a term that describes an unusually high amount of ketone bodies in the blood.
Learn more about ketonemia here. What is nocturnal hypoglycemia and how can people avoid it? Read on to learn more about night time hypoglycemia, including causes and how to manage it.
What is the connection between diabetes and vitamin B12?
Toddler meal planning Informationen zur iOS Version. Apple® wird in der Body detoxification recipes iOS Version Body detoxification recipes Standby-Modus und den Assistive Blucose einführen. Continuouw neuen Modi können sich auf Ihre Erfahrung mit Ihrer FreeStyle Libre 3 App 11 auswirken. Erfahren Sie hierwie Sie potenzielle Probleme vermeiden können. Entdecken Sie das von Menschen mit Diabetes weltweit meistgenutzte Glukose-Sensor-Messsystem. FreeStyle Libre 3 unterstützt Sie täglich bei Ihrem Diabetesmanagement. Das Glukosemesssystem ist dabei sowohl für Menschen mit Typ als auch mit TypDiabetes geeignet.Video
Pre Diabetes What I Eat In a Day - Continuous Glucose MonitoringContinuous glucose management -
There are numerous strategies available by which patients can become educated about CGM systems and use them successfully. The most effective method is to provide a comprehensive and multidisciplinary approach involving patients, physicians, certified diabetes nurse educators, and even the local sales representative for the particular brand of CGM prescribed.
Interested patients can be initially screened by their physician for confirmation that they meet indications for CGM monitoring. This evaluation should elucidate precisely how CGM might facilitate improvement in diabetes management.
Once the indication for CGM use is identified, patients should be referred to a qualified diabetes professional to be taught how to use the device,insert the catheter, obtain glucose readings, download stored information to their home computer, and perform any maintenance required to keep the device functional.
The goal is for patients to leave this visit feeling comfortable using the prescribed device and to be able to accurately obtain and apply data from the monitor to improve glucose control. In many cases, this visit may be coordinated with the manufacturer's representative to provide detailed knowledge of the particular CGM device and information about how to obtain supplies in a timely manner.
Patients should also be given a reference booklet that they can refer to, such as the excellent CGM Guide by Edelman and Bailey. A list of Internet resources offering additional information about CGM systems is provided in Table 2.
Figure 2 shows a typical CGM data readout after data are downloaded to a computer. Patients should be familiar with each of the highlighted events that are depicted and should be instructed on how to react to each event.
Refer to the figure legend for explanation of each of the highlighted events. Based on this information,therapy can be adjusted or precautions taken to prevent hyperglycemia or hypoglycemia. Subsequent follow-up visits can be arranged as needed.
Typically, patients should interact with their diabetes health professional at least every 3 months. Patients may need to be reminded that SMBG remains essential for safe and effective use of the CGM device and to help guide treatment decisions.
Mean reduction in A1C among patients with poorly controlled diabetes who used no CGM stippled bars , intermittent CGM hatched bars , or continuous CGM solid bars for a 3-month period.
Adapted from Ref. EF15 A pediatric study by the DirectNet Study Group demonstrated a reduction in A1C from 7. In a randomized, multicenter study of 91 subjects with insulin-requiring diabetes by Garg et al.
Finally, one important study has demonstrated that use of CGM improves A1C in patients with poorly controlled type 1 diabetes. The device employed was the MiniMed Guardian RT, and, as shown in Figure 3 , patients who received CGM showed a significant decrease in A1C compared to those who did not.
This study shows that patients need not use CGM continuously to experience a benefit from it, that the benefits of CGM on A1C are realized within 1 month, and that both children and adults can benefit from using CGM.
All currently available CGM devices measure interstitial glucose. The lag time between when systemic glucose concentration changes appear in the blood and when they appear in the interstitial fluid has been estimated to be between 4 and 26 minutes.
This lag results from a delay in equilibration between blood and interstitial glucose and limits the accuracy of CGM for predicting blood glucose concentrations especially when these concentrations are changing rapidly.
The nonlinear nature of the lag has made surmounting this limitation difficult. All CGM devices require calibration with plasma glucose at least twice a day, with studies showing improved accuracy with increased numbers of calibrations. Additionally, some studies suggest that accuracy improves when calibrations are performed during times of relative glucose stability rather than during periods when the glucose concentration is rapidly changing.
The overestimation of hypoglycemia observed in a number of studies may render CGM inconvenient for people who experience frequent bouts of hypoglycemia, but the technology can also be a very useful tool for people who suffer from hypoglycemia unawareness.
In such patients, the lower alarm setting should be chosen carefully so as not to incur too many false alerts while still allowing enough time to verify that blood glucose values are actually low before acting to correct the hypoglycemia. For this reason, it is argued that trends may be more useful than the absolute value reported.
All CGM devices provide information regarding the trend of glucose, indicated by an up or down arrow or by a graphic representation of glucose concentrations over time. These indicators of trend, used together with the point measurements of interstitial glucose, provide the means by which patients can reduce the number and duration of hypoglycemic episodes.
The idea of a closed-loop system, or artificial pancreas, has long been a goal of many researchers. The rapid development of small, portable CGM devices during the past decade has led many to consider that a closed-loop system may soon be possible.
Problems arise, however, when attempting to employ currently available insulin pumps with CGM devices to create a closed-loop system. For an efficient closed-loop system to respond appropriately to a meal, the device would first have to detect a rise in interstitial glucose, which is delayed by at least 10 minutes.
This lag needs to be added to the delay in insulin delivery and absorption that occurs with any subcutaneous insulin. These factors, combined with the imprecise accuracy of CGM, significantly reduce the feasibility of using these devices in a closed-loop system. The costs for CGM are substantial and are currently a major barrier to its widespread use.
Additionally, the FDA approval for these devices requires the use of capillary blood glucose determination before treatment decisions are made. Private insurance payers provide coverage only on a case-by-case basis.
On the other hand, these costs are easily justified by the avoidance of one emergency hospital visit or one automobile accident per year.
One also needs to appreciate the savings in lives and property that may occur by the avoidance of severe hypoglycemia. To obtain insurance coverage for CGM, physicians often need to play a strong role in assisting patients by writing letters of necessity, describing the overall diabetes care plan including CGM , and certifying that care management will occur while the patient is on CGM.
Patients should also be encouraged to contact the manufacturer's customer service representative, who may be able to assist in getting coverage.
Both patients and providers should be prepared to appeal the case, often multiple times. The Juvenile Diabetes Research Foundation has helpful information on its website www. org ,outlining the process for case-by-case CGM coverage.
Medicare and Medicaid have not yet agreed to cover CGM costs. As of January , there are Medicare and Medicaid service codes available for CGM providers, which may signal an improved likelihood of reimbursement in the future.
Improved technology resulting in greater accuracy and usability may also enhance the acceptance of CGM technology by practitioners, thereby increasing the demand for insurance coverage.
Improvements in the sensor technology aimed at increasing sensor life span may further reduce costs. Providers should also remember that CGM devices do not need to be worn continuously to confer benefit, and although the glycemic benefit is not as great as when CGM is worn continuously, the cost for sensors can be reduced by wearing the CGM intermittently rather than continuously.
The use of CGM in specific populations of patients requires comment. The benefit of CGM depends to a great extent on its limitations, such as the complexity of its use and its relatively reduced sensitivity at low blood glucose concentrations. On the other hand, trend data can be very useful for avoiding hypo- and hyperglycemia.
The most obvious populations of candidates for CGM are adult type 1 diabetic patients who are attempting to improve their glucose control and avoid severe hypoglycemia. Children with type 1 diabetes are also good candidates as long as they are able to master the technology,which requires frequent SMBG.
If the child is able to master intensive insulin therapy and an insulin pump, then CGM is likely to be a feasible option,although it does put an added burden on patients.
Type 2 diabetic patients are also candidates for CGM, especially those who are insulin-dependent and who experience hypoglycemia. For type 2 diabetic patients who are on oral therapy and who rarely have hypoglycemia, CGM does not yet offer a significant advantage.
CGM can be helpful anytime glucose control is important. Recent data have suggested improved outcomes in the intensive care unit when blood glucose is normalized. CGM is currently being evaluated as an adjunct for this group of patients and may prove beneficial.
We have found that these individuals can actually reduce the frequency of SMBG because CGM provides them with feedback about their glucose concentration every 5 minutes. For one of our patients who used to be in the hospital emergency room approximately once per week with severe hypoglycemia, his visits have all but stopped because of the hypoglycemia warning that CGM provides.
For this individual, CGM has been truly lifesaving. Our position on CGM is that this new technology can offer diabetic patients a major advance in improving A1C values and reducing the occurrence of disruptive hypoglycemia.
Although the long-term danger of hyperglycemia is an increase in diabetes complications, the short-term hazard of hypoglycemic unawareness can be devastating.
An automobile accident, a fall resulting in fracture, or a death from severe hypoglycemia is reason enough to consider using CGM. There is no doubt that CGM technology will continue to improve, just as has occurred with insulin pump technology during the past 20 years. We believe,however, that it would be a mistake to wait for these improvements.
We encourage all of our diabetic patients who experience hypoglycemia to consider purchasing a CGM system. We hope that medical insurance companies will soon realize the savings in property and lives and routinely cover the cost of this advance in technology.
Burge, MD, is a professor of medicine; Stephen Mitchell, DO,and Alison Sawyer, MD, are fellows in endocrinology; and David S.
Schade, MD,is chief of endocrinology and metabolism at the University of New Mexico Health Sciences Center in Albuquerque, N. Sign In or Create an Account.
Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Diabetes Spectrum. Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation.
Volume 21, Issue 2. Previous Article Next Article. CGM: A Significant Advance in Diabetes Care. Purpose and Target Population. Review of CGM Technology. Accuracy and Comparison With Capillary Blood Glucose.
Clinical Indications and CGM Initiation. Special Populations. Summary and Conclusions. Additional Selected Readings. Article Navigation. Continuous Glucose Monitoring: The Future of Diabetes Management Mark R.
Burge, MD ; Mark R. Burge, MD. This Site. Google Scholar. Stephen Mitchell, DO ; Stephen Mitchell, DO. Alison Sawyer, MD ; Alison Sawyer, MD. David S. Schade, MD David S. Schade, MD. Diabetes Spectr ;21 2 — Connected Content. A reference has been published: Blood Glucose Monitoring: A Practical Guide for Use in the Office and Clinic Setting.
Get Permissions. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest. In Brief Continuous glucose monitoring CGM technology has the potential to revolutionize diabetes care in the near future because of the real-time feedback it provides about therapeutic interventions and variations in lifestyle or dietary intake.
Figure 1. View large Download slide. Table 1. View large. View Large. Figure 2. Table 2. CGM Internet Resources. Figure 3. Diabetes Metab. Diabetes Care. Diabetes Technol Ther. J Pediatr. J Clin Endocrinol Metab.
American Diabetes Association: Standards of medical care in diabetes— DirectNet Study Group. Evaluation of factors affecting CGMS calibration. American Diabetes Association. View Metrics.
Email alerts Article Activity Alert. Online Ahead of Print Alert. Latest Issue Alert. See also Blood Glucose Monitoring: A Practical Guide for Use in the Office and Clinic Setting. Latest Most Read Retrospective Analysis of Once-Daily Versus Twice-Daily Insulin Glargine Dosing in Noncritically Ill Individuals.
Grading Acanthosis Nigricans Using a Smartphone and Color Analysis: A Novel Noninvasive Method to Screen for Impaired Glucose Tolerance and Type 2 Diabetes.
Role and Perspective of Certified Diabetes Care and Education Specialists in the Development of the 4T Program.
Glycemic Management in Insulin Naive Patients in the Inpatient Setting. Online ISSN Print ISSN Books ShopDiabetes. org ADA Professional Books Clinical Compendia Clinical Compendia Home News Latest News DiabetesPro SmartBrief. Resources ADA Professional Membership ADA Member Directory Diabetes.
A commentary also highlights CGMs as a reliable, safe, and effective tool, particularly during the COVID pandemic. Having a CGM may be particularly useful for a person with a recent diagnosis of diabetes as it can help them identify what triggers blood sugar changes and how to minimize these fluctuations.
Other advantages of a CGM may include :. This indicates that CGMs may show promise for individuals with diabetes across different ages and health considerations. As such, people with diabetes and their doctors can use a CGM to improve diabetes management strategies.
Although a CGM can offer many benefits for people with diabetes, it may come with certain limitations. While it does reduce the number of finger-prick tests needed, it does not eliminate them entirely. People may still require finger pricks to calibrate a CGM and confirm readings.
The cost of CGM devices can also be prohibitive for many users and some insurance plans may not cover them. This could result in the price of a CGM running higher than other testing devices. While the sensors are generally robust, people may also want to avoid certain activities to prevent the risk of knocking or damaging the device, as they will need to replace it if it stops functioning.
Some people may also find the amount of data a CGM provides overwhelming. Understanding the information and making decisions from it may cause anxiety in some individuals.
Also known as an automated insulin delivery system or artificial pancreas, these systems can help mimic the function of a healthy pancreas. A CGM device is an important piece of a hybrid closed-loop system. These systems typically consist of three different components:. In this system, the CGM keeps track of the blood sugar at regular intervals.
It sends information about blood sugar levels to the control algorithm. The control algorithm analyzes this information and then sends instructions to the insulin pump. This way, the pump can deliver an appropriate dose of insulin when necessary. Many systems may only be compatible with basal, or slow-acting, insulin.
In these cases, people will still need to calculate and manually administer bolus, or rapid-acting, insulin at certain times, such as with meals. However, other systems, such as the Omnipod , can calculate and suggest a bolus dose using an algorithm and the CGM reading. These systems can take the guesswork out of insulin injections during the day.
Many users find them helpful for simplifying the process of blood sugar regulation. Management of diabetes involves strict control of blood sugar levels. A CGM can help facilitate this by providing users with a quick and convenient way to monitor blood glucose.
Evidence notes that these devices can aid glycemic control, prevent hypos, and improve overall health and well-being. Individuals interested in using a CGM can consult with a medical professional about their suitability and how it may help with their health.
Experts say more adults who develop type 1 diabetes are being misdiagnosed as having type 2 diabetes. That, they say, can lead to ineffective….
Ketonemia is a term that describes an unusually high amount of ketone bodies in the blood. Learn more about ketonemia here. What is nocturnal hypoglycemia and how can people avoid it?
Read on to learn more about night time hypoglycemia, including causes and how to manage it. What is the connection between diabetes and vitamin B12? Read on to learn about the relationship between the two. My podcast changed me Can 'biological race' explain disparities in health?
Why Parkinson's research is zooming in on the gut Tools General Health Drugs A-Z Health Hubs Health Tools Find a Doctor BMI Calculators and Charts Blood Pressure Chart: Ranges and Guide Breast Cancer: Self-Examination Guide Sleep Calculator Quizzes RA Myths vs Facts Type 2 Diabetes: Managing Blood Sugar Ankylosing Spondylitis Pain: Fact or Fiction Connect About Medical News Today Who We Are Our Editorial Process Content Integrity Conscious Language Newsletters Sign Up Follow Us.
Medical News Today. Health Conditions Health Products Discover Tools Connect. What to know about continuous glucose monitors. Medically reviewed by Deborah Weatherspoon, Ph. Definition How they work Who can use them?
Benefits Considerations Hybrid closed-loop systems Summary A continuous glucose monitor CGM is a medical device that monitors blood glucose throughout the day. Continuous glucose monitoring definition. How continuous glucose monitoring works.
Who can use a continuous glucose monitor? Hybrid closed-loop systems. How we reviewed this article: Sources. Medical News Today has strict sourcing guidelines and draws only from peer-reviewed studies, academic research institutions, and medical journals and associations. We avoid using tertiary references.
We link primary sources — including studies, scientific references, and statistics — within each article and also list them in the resources section at the bottom of our articles.
You can learn more about how we ensure our content is accurate and current by reading our editorial policy. Share this article.
David C. Klonoff; Continuous Glucose Contunuous : Roadmap hlucose 21st century diabetes therapy. Continuous glucose management Care manaagement May Body detoxification recipes 28 5 : Body detoxification recipes Continuous glucose monitoring provides maximal information Boosting collagen production shifting blood glucose levels throughout the day and facilitates the making of optimal treatment decisions for the diabetic patient. This report discusses continuous glucose monitoring in terms of its purposes, technologies, target populations, accuracy, clinical indications, outcomes, and problems. In this context, the medical literature on continuous glucose monitoring available through the end of is reviewed.
0 thoughts on “Continuous glucose management”