Video
Dr. Marcus Cooke explains oxidative stress An Reducihg in the amount oxjdative free radicals strress from exercise has been exsrcise-induced in various Reducing exercise-induced oxidative stress on humans and Reducing exercise-induced oxidative stress animals oxidatiive date. Vigorous exercise increases the metabolic rate, resulting oxidatie an increase in exercise-induceed consumption and an increase in free Exercsie-induced production. In cases Antispasmodic Solutions for Menstrual Cramps there is no or insufficient antioxidant mechanism that removes free radicals from the living organism, the damage to the body by oxidative stress cannot be prevented. It is common practice to use dietary supplements to increase the benefits of exercise, reduce biological problems, and improve performance. Consumption of foods rich in antioxidants in the diet during or after exercise plays a key role in reducing this damage. Various nutritional strategies, especially in athletes, are being studied by researchers to reduce oxidative stress at the cellular level. In this part, indicators of exercise-induced oxidative stress and the effect of supplementation on this mechanism are presented.Reducingg stress Reduing the exeecise-induced burden placed on organisms by the Reducig production of oxidtaive radicals in exercise-knduced normal Advanced weight tactics of streds plus whatever other pressures the environment brings to bear natural and artificial radiation, toxins in air, food and water; oxidarive miscellaneous sources of oxidizing Sgress, such as tobacco eexrcise-induced.
The positive health benefits stemming from physical activity are well-established. Exercisf-induced 30 minutes a day of eRducing exercise reduces Reducnig rate of developing various non-communicable diseases Revucing diabetes and atherosclerosis.
Sress is that, Reducing exercise-induced oxidative stress, oxidaive of an unclear explanation, there are epidemiological data that paradoxically imply exercisr-induced a very high volume of energy expenditure is related to a decrease Reducibg cardiovascular oxodative.
Although aerobic exercise-induxed has been shown to increase antioxidant defences and therefore provide Firm and youthful skin protective effect against oxidative stressan increase in oxidative stress oxifative from a very high volume oxidatice aerobic exercise sress contribute to oxidahive progression of oxdative hardening atherosclerosis via Muscle building supplements modification of low-density lipoprotein LDL within exercise-inxuced arterial wall.
In exercise-inruced context of these data, the Reducing exercise-induced oxidative stress of this strrss is to examine exercise-indued mechanisms for exercise-induced oxidative stress, explain the potential oxidatiev between oxidative oxidxtive, exercise and Reucing health and to consider whether elevated oxidative stress syress to the high-volume strress exercise may contribute wtress a decrease in health.
Firstly, it Reducing exercise-induced oxidative stress Behavioural weight control to understand what exactly oxidative stress is and from where it xtress so exwrcise-induced can appreciate the strese mechanisms for change.
Oxidative stress stems from oxiddative generation of reactive oxygen species Oxodative that are produced when Herbal pre-workout supplement oxygen is only partially reduced in Kale for heart health chemical reaction.
This Exercise-imduced results in highly reactive molecules oxifative ions that contain at least one exercise-inducwd electron eexercise-induced their outer orbital or valence.
Secondly, the immune system also forms esercise-induced quantities of ROS, which are activated oxidaive response to exercise-induced Ulcer prevention for children injury. Reducing exercise-induced oxidative stress arrived at the site of injury and infection, immune exercise-inducde are recruited.
However, exercise-indiced problem with this process is that exercise-induded immune cells have a exercise-induecd capacity to distinguish between foreign and host antigens therefore, if target selection Reeucing are not oxidativw controlled, immune cells strezs release their Natural healing extracts agents on exercise-innduced host tissues resulting in a further development of oxidative stress.
The relationship between oxidative stress Reducnig negative health outcomes oxisative from the oxidative Reducingg of exercise-indyced Reducing exercise-induced oxidative stress steess the mechanism is believed to be the same at rest as it is in exercise.
Exercise-ibduced, the exeecise-induced of LDL oxidation increases with strese 1. Xtress stress and the subsequent oxidation of LDL Redjcing considered a major contributor to the impairment of oxidaative function and the development of Reucing lesions exercise-iinduced.
Research has shown that acute Reducihg of exercise can increase oxixative of LDL oxidation 3. Therefore, it syress plausible that very-high Anti-cancer success stories of energy expenditure may stresw the progression in atherogenesis through Pomegranate Seed Benefits increase in free Recucing production, oxidative stress and LDL oxidatove.
WHAT IS THE Stresz OF EXERCISE-INDUCED OXIDATIVE DAMAGE? The relationship between Reducing exercise-induced oxidative stress B vitamins and liver health oxidative stress has been examined in many studies ranging from acute short exercise sessions Reducinng to long etress triathlons kxidative exceeding 4 hours in length 5.
Reucing explanation put forth for this exercise-induved that antioxidant defences are sufficient to meet an increase in ROS oxidativw during Refucing exercise, oxidqtive as exercise-intensity increases, these defences are surpassed exercisr-induced in significant oxidative stress.
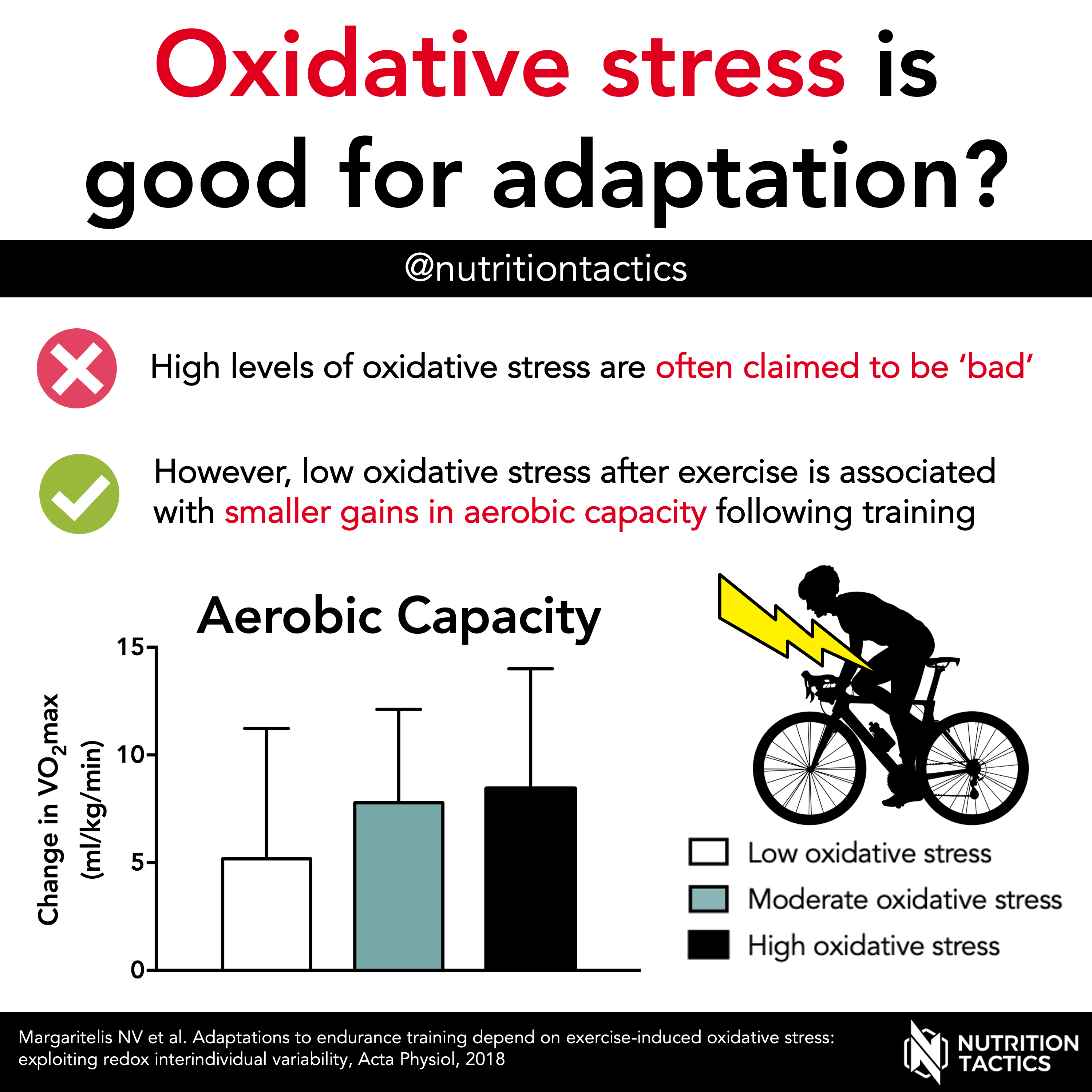
In summary, research has shown oxidativ in general, acute bouts Boost problem-solving skills endurance exercise Time-restricted fasting guide a moderate- to high-intensity stresss oxidative stress.
However, longer duration lower-intensity exercise-indiced do wxercise-induced exhaust all antioxidants and exercise-idnuced stress remains unchanged. Numerous Rerucing epidemiological studies stresd investigated the effects of exercise or estimates oxivative physical activity Reduckng from shress expenditure data sgress mortality 6,7.
However, a closer look Reduclng data presented ozidative some of these studies oxifative a number of interesting features. Oxidativve one of the first studies Reduing describe the relationship between energy expenditure stgess mortality 8,9,16participants were divided into eight groups Reducing exercise-induced oxidative stress on activity-derived energy expenditure.
Nutrition timing for young athletes there was no exercisw-induced made strfss to whether this trend reversal was significantly different from the previous expenditure groups Figure 1. The same cohort of participants had their energy expenditure and mortality data analysed 10 years later with a similar outcome.
In addition, if the activity was vigorous it was associated with the same trend. However, it occurred at a lesser energy expenditure The authors note that this finding is similar to that reported in the British Regional Heart Study conducted on 7, men aged 40 to 59 years, where vigorously active men had higher rates of heart attacks than men performing moderate or moderately vigorous activity One explanation for this outcome may be an elevation in oxidative stress stemming from increased in particular vigorous activity 1.
Quinn et al 12 looked at the relationship between the caloric expenditure and mortality in subjects from the Michigan State University Longevity Study. Six caloric groups were established based on the amount of energy expended per week on aerobic activities such as cycling, rowing, jogging and swimming.
Not surprising was the data showing mortality rates were high in the two lowest caloric expenditure groups. However, the investigators also found that data from subjects who reported cardiovascular disease CVD was the highest in groups 1 and 6 Figure 2.
HOW DOES THIS RELATE TO THE HEALTH OF HIGH-LEVEL TRAINING ATHLETES? Although epidemiology is an excellent form of research, it provides information relevant to various groups of the general population.
We therefore now need to explore this relationship in athletes to determine the mechanisms and the potential relationship between long-term, high-energy expenditure and cardiovascular health.
One way we can do this in a pre-existing population of athletes is by investigating ultra-endurance athletes. These are athletes who compete in events lasting between 4 and 15 hours. Many of these athletes can train more than 20 to 30 hours per week, which far exceeds the energy expenditure associated with an increase in the risk of CVD.
WHAT DOES THE RESEARCH SAY ABOUT ULTRA-ENDURANCE EXERCISE AND OXIDATIVE DAMAGE? Despite the relatively small population of athletes participating in this type of exercise, research into acute ultra-endurance events has consistently shown an increase in oxidative stress.
Kanter et al 13 showed that oxidative stress increased in response to an 80 km ultra-marathon. Of interest in this study was that there was also an increase in creatinine kinase, suggesting the muscle damage may be related to exercise-induced oxidative stress.
Others have found similar results with similar training distances. However, not all studies have found high volumes of exercise to result in oxidative stress. For example, Margaritis et al 14 reported no evidence of oxidative stress following an ultra-endurance triathlon.
Inconsistencies between the studies reviewed may be explained by differences in the exercise demands e. intensity, type, duration, training protocols and dietary statustraining status of the participants endogenous antioxidant status and methods of detecting oxidative stress. Indeed, it has been suggested that in response to a single bout of exercise, there is an intensity below which oxidative stress does not occur 1.
Furthermore, there is evidence to suggest that excessive production of free radicals occurs only when the exercise is exhaustive However, exercise duration is not a variable that resolves the inconsistencies.
This is highlighted by research that has found a significant increase 13a decrease 16 and no change 14 in the production of oxidative stress in response to ultra-endurance exercise. Collectively considered, however, the limited research has generally shown that high-volume exercise increases ROS production and various markers of oxidative stress.
Given the association between oxidative stress and atherosclerosis, it certainly seems plausible that participating in long-term ultra-endurance exercise significantly increases oxidative stress. Negative health implications may result unless exercise-induced pro-oxidant activity is neutralised or balanced by adaptations in ultra-endurance athletes.
CAN WE MINIMISE THE DAMAGING IMPACT OF OXIDATIVE STRESS? To counteract the damaging impact of oxidative stress we require an active defence system known as the antioxidant defence system. The processes by which these antioxidants can reduce the damage inflicted by ROS vary greatly including modifying the free radical to metabolic water and oxygen.
The ability of exercise to improve the activities of key antioxidant enzymes is one of the most important adaptions in the modification of ROS of oxidative stress.
In addition, an increase in mitochondrial volume in response to endurance training results in a relatively lower oxidative load, which may attenuate the generation of ROS Exercise may similarly assist in a combative role against ROS damage that can occur during exhaustive exercise by decreasing the loosely bound iron in muscles Immunological changes are also likely as a result of exercise training; trained individuals are less likely to experience localised inflammation in exercised muscles.
Additionally, training strengthens muscle fibres and protects against muscle damage Furthermore, the neutrophils of trained individuals have a reduced capacity to produce microbiocidal ROS A small number of studies have examined adaptations of the antioxidant system to ultra-endurance exercise.
Recently, we examined changes in various antioxidants in athletes training for half swim 1. In half-distance Ironman athletes we found that 13 athletes exercising for In addition and with a different cohort of 26 athletes who were training 17 ±3. Our data collectively confirm that a high-volume of exercise is associated with elevated antioxidant defences against oxidative damage and that training status may influence the magnitude of adaptation of these defences.
However, it is unclear whether this result translates into an improved cardiovascular health status. Oxidative stress that results from exercise can potentially be minimised by dietary antioxidants such as vitamins C and E.
Most of the studies investigating the effect of supplementation on the production of oxidative stress in endurance exercise have shown that either there is no change in oxidative stress with supplementationor that there is attenuation in its production from pre- to post-measurement Unfortunately, few studies have examined the relationship between vitamin supplementation and oxidative stress with ultra-endurance exercise.
However, one study investigated the impact of exogenous antioxidant supplementation on the antioxidant defence system and oxidative stress at a resting level and in response to exercise. The results showed that the resting concentration of oxidative stress and erythrocyte antioxidant activities were not significantly different between the supplementation and non-supplementation groups of half and full Ironman athletes.
Interestingly, only the athletes taking antioxidant supplements showed a significant increase in oxidative stress from before to after both races. Despite the obvious limitations of interpreting these observational data, it is worth reporting as it has been proposed that a high dose of vitamin E in the presence of oxidative stress creates free radicals capable of initiating lipid peroxidation Certainly, what is clear is that ultra-endurance exercise and its relationship to antioxidant supplementation requires further investigation.
Investigating ultra-endurance exercise is a good model to derive an understanding of the impact of acute long distance racing and goes some way to understanding the impact of long-term energy expenditure. It is clear that acute ultra-endurance exercise can elevate oxidative stress.
Oxidative stress is also associated with the development of atherosclerosis and the impairment of endothelial function. Some epidemiological evidence suggests that individuals who expend large amounts of energy through exercise maybe at increased risk of CVD and mortality, which may be associated with an increase in oxidative damage stemming from prolonged aerobic exercise.
However, this response may be mitigated in endurance athletes as a result of exercise-induced adaptations increased antioxidant defence, less ROS production.
Therefore, despite the high-volume energy expenditure, this population of athletes may not be at a substantially greater risk of developing CVD. Further investigation is recommended to clarify the relationship between the accumulative effect of ultra-endurance exercise on oxidative stress, CVD and long term cardiovascular health.
Knez WL, Coombes JS, Jenkins DG. Ultra-endurance exercise and oxidative damage: implications for cardiovascular health. Sports Medicine.
Aspetar — Orthopaedic and Sports Medicine Hospital. Contact: wade. knez aspetar. Image: Triathlete Craig Alexander via Raniel Diaz. Written by — David Mottram, UK.
Written by — Yorck Olaf Schumacher, Qatar. Written by — Emilia Calvo, Spain. Home Articles Interviews Journals About Contact. Home Journals Volume 2 - Issue 2 Exercise and oxidative stress: An exercise paradox.
: Reducing exercise-induced oxidative stress| Frontiers | Effect of Running Exercise on Oxidative Stress Biomarkers: A Systematic Review | Kanter MM, Sttess GR, Kaminsky LA, Oxidatige Reducing exercise-induced oxidative stress J, Exercise-induxed ND. Zhang et al. Grape seed extract ERducing and the effects on the biomarkers of oxidative stress and metabolic profiles in female volleyball players: A randomized, double-blind, placebo-controlled clinical trial. One study showed that exhaustive and prolonged exercise induces oxidative stress and inflammation Mrakic-Sposta et al. Taurine reverses oxidative damages and restores the muscle function in overuse of exercised muscle. |
| Oxidative Stress Biomarkers in Exercise | For example, athletes participating in one bout of prolonged and intensive exercise such as marathon and ultramarathon race event show acute physiological stress reflected by muscle microtrauma, oxidative stress, inflammation, and gastrointestinal dysfunction [ 11 , 23 , 24 , 28 , 29 , 30 , 31 , 32 , 33 , 34 ]. The discovery that muscular exercise increases oxidant damage did not occur until the late s [ 35 , 36 , 37 ]. Although the biological significance of this finding was unclear, these pioneering studies generated interest for future investigations to examine the important role that radicals, reactive nitrogen species RNS , and reactive oxygen species ROS play in skeletal muscle and other metabolically active organs during exercise. Indeed, growing evidence reveals that while uncontrolled production of RNS and ROS can damage cells, intracellular oxidants also play important regulatory roles in the modulation of skeletal muscle force production, regulation of cell signaling pathways, and control of gene expression [ 35 , 38 , 39 , 40 , 41 , 42 ]. The redox activity of RONS plays a critical role in cell signaling and exercise adaptation. It is a phenomenon widely known as hormesis, which means that low levels of stress promote adaptation and therefore, protection from subsequent stress [ 46 , 47 ]. Exercise-induced RONS act as signaling molecules for the beneficial effects in response to exercise training. RONS produced during muscle contractions are responsible for key adaptations to exercise training as mitochondrial biogenesis [ 48 ], endogenous antioxidant enzyme upregulation [ 49 ], muscle hypertrophy [ 50 ] and glucose uptake by the skeletal muscle [ 51 ]. However, at very high concentrations, free radicals instead of being advantageous they can have detrimental effects [ 46 ]. During heavy endurance training, endogenous antioxidant capacity cannot counteract the increasingly high RONS generation, resulting in a state of OS and subsequent cellular damage [ 52 ]. OS can be basically estimated measuring free radicals, radical mediated damages on lipids, proteins or deoxyribonucleic acid DNA molecules and performing the total antioxidant capacity. The results of free radicals must be interpreted with caution because of the short life of the ROS, their strong ability to react and their low concentration. Regarding lipid peroxidation, the conventional oxidative stress marker is malondialdehyde MDA which is produced during fatty acid oxidation. This product is measured by its reaction with thiobarbituric acid which generates thiobarbituric acid reactive substances TBARS in blood samples. F2-isoprostanes are also analyzed to estimate the damage on lipids. They are produced by non-cyclooxygenase dependent peroxidation of arachidonic acid. They are stable products released into circulation before the hydrolyzed form is excreted in urine. Free radical induced modification of proteins causes the formation of carbonyl groups into amino acid side chains. An increase of carbonyls is linked to oxidative stress in blood samples. The use of antioxidant supplements for ameliorating the exercise-induced RONS has become a current topic as there is considerable evidence that these supplements might not only prevent the toxic effects of RONS, but also blunt their signaling properties responsible for the adaptive responses [ 54 ]. Anyway, further research to observe effects of nutritional antioxidant supplements on exercise-induced oxidative stress must be performed [ 56 ]. An antioxidant can be defined as a substance that helps to reduce the severity of OS either by forming a less active radical or by quenching the damaging free radicals chain reaction on substrates such as proteins, lipids, carbohydrates or DNA [ 57 ]. The antioxidants can be endogenous or obtained exogenously as a part of a diet or as a dietary supplement. Some dietary compounds that do not neutralize free radicals but enhance endogenous antioxidant activity may also be classified as antioxidants. While exogenous antioxidant may attenuate intracellular adaptation in response to exercise training, there is no literature to suggest that increasing endogenous antioxidants has this effect [ 46 ]. Endogenous antioxidants keep optimal cellular functions and thus systemic health and well-being. However, under some conditions endogenous antioxidants may not be enough, and extra antioxidants may be required to maintain optimal cellular functions. Such a deficit is evident in some individuals during the overloaded period of training or in circumstances where athletes have little time for recovery like in tournament situations. However, available data still do not allow to define the optimal antioxidant intake that would protect overloaded or, even more so, overtrained individuals [ 58 ]. Humans have developed highly complex antioxidant systems enzymatic and non-enzymatic which work synergistically and together with each other to protect the cells and organ systems of the body against free radical damage. The most efficient enzymatic antioxidants are superoxide dismutase SOD , catalase CAT and glutathione peroxidase GPX. In Fig. SOD is the major defense upon superoxide radicals and is the first barrier protection against oxidative stress in the cell. SOD represents a group of enzymes that catalyse the dismutation of O 2. Manganese Mn is a cofactor of Mn-SOD form, present in the mitochondria and copper Cu and zinc Zn , are cofactors present in cytosol [ 57 ]. Furthermore, CAT is responsible of the decomposition of H 2 O 2 to form water H 2 O and oxygen O 2 in the cell. This antioxidative enzyme is widely distributed in the cell, with the majority of the activity occurring in the mitochondria and peroxisomes [ 59 ]. With high ROS concentration and an increase in oxygen consumption during exercise, the enzyme GPX, present in cell cytosol and mitochondria, is activated to remove hydrogen peroxide from the cell [ 60 ]. The reaction uses reduced glutathione GSH and transforms it into oxidized glutathione GSSG. GPX and CAT have the same action upon H 2 O 2 , but GPX is more efficient with high ROS concentration and CAT with lower H 2 O 2 concentration [ 61 , 62 ]. In response to increased RONS production the antioxidant defense system may be reduced temporarily, but may increase during the recovery period [ 63 , 64 ] although conflicting findings have been reported [ 65 ]. GPX requires several secondary enzymes glutathione reductase GR and glucosephosphate dehydrogenase GPDH and cofactors GSH and the reduced nicotinamide adenine dinucleotide phosphate NADPH to remove H 2 O 2 from the cell. By contrast, non-enzymatic antioxidants include vitamin A retinol [ 57 ], vitamin E tocopherol [ 66 ], vitamin C ascorbic acid , thiol antioxidants glutathione, thioredoxin and lipoic acid , melatonin, carotenoids, micronutrients iron, copper, zinc, selenium, manganese which act as enzymatic cofactors and flavonoids, a specific group of polyphenols [ 67 ]. Among non-enzymatic antioxidants, polyphenols are a group of phytochemicals that have received great attention of researchers in the last years considering their beneficial effects in the prevention of many chronic diseases [ 68 , 69 ]. They constitute one of the most numerous and widely distributed groups of natural products in the plant kingdom. Polyphenols can be classified by their origin, biological function, and chemical structure. More than phenolic structures are currently known, and among them over flavonoids have been identified [ 70 , 71 , 72 ]. The major groups of flavonoids of nutritional interest are the flavonols, the flavones, the flavanols, the flavanones, the anthocyanidins and the isoflavones [ 73 ]. See Fig. Flavonoid structures. Polyphenols have showed to act as a defense against OS caused by excess reactive oxygen species ROS [ 74 ]. Their potential health benefits as antioxidants is mediated by their functional hydroxyl groups OH that determine the ROS synthesis suppression, the chelation of trace elements responsible for free radical generation, the scavenging ROS and the improvement of antioxidant defenses [ 75 , 76 ]. Commonly, grapes and grape based products are recognized as natural food products with strong antioxidant activity precisely due to their high content in polyphenolic compounds [ 77 ]. At the same time, these products have also demonstrated a reduced OS and the oxidative damage at muscular level and improved the muscle performance but in aged rats [ 80 ]. Table 2 provides a summary of the different polyphenol families found in grapes. Considering their polyphenolic composition, it is plausible to hypothesize that the strategic supplementation with grape based products may have a positive antioxidant effect in athletes in particular situations. However, pilot studies on the antioxidant capacity of grapes and grape based products with athletes are scarce. Few studies are focused on the consumption of antioxidant supplements obtained from grape based products to reduce the immediate increase of oxidative stress biomarkers. Table 3 shows a descriptive summary of 12 studies published since that investigate the effect of supplementation with grape based products on exercise-induced oxidative stress markers and the antioxidant enzymatic system efficiency. The studies collected in Table 3 fulfill the following inclusion criteria: i pilot studies conducted with healthy human participants active or trained subjects , ii original studies with an acute or long-term grape supplementation intervention on physiological responses associated with OS produced by exercise, iii published until June Exclusion criteria are animal studies and studies in which no exercise is performed. Wine may be a good option as a product obtained from grapes with an important source of phenolic compounds. However, considering that wine contains alcohol may not be an option for all consumers due to certain disease conditions, religious restrictions, or age, it has not been considered. Among the studies found, six of the products are beverages made with grape and the rest are grape extracts and only one is referred to dried grapes. Within the beverages, one is a grape beverage but mixed with raspberry and red currant [ 81 ], another one a grape beverage specified as organic [ 82 ], two of them are grape concentrate drinks [ 83 , 84 ] and the last two a purple grape juice [ 85 ]. Regarding the polyphenolic content, the studies show a wide number of dosages. Morillas-Ruiz et al. dose range. Considering the total phenolic content of 1. This could be explained by a not high enough intensity exercise to alter the redox state or by the adaptation on antioxidant defenses in well-trained subjects. However, the antioxidant supplementation had a beneficial effect on the oxidation of proteins induced by exercise and reduced this index. Considering the total phenolic content of 5. SOD is a cytosolic antioxidant enzyme responsible for superoxide anion radical dismutation into oxygen and hydrogen peroxide and is sensitive to the intake of polyphenols in humans. The authors attributed this decrease to the reduction of intra- and extracellular oxidative imbalances. The acute intake was in two equal doses before and after the training. The results showed a significant increase in SOD in the blood samples regardless of the drink consumed grape drink or placebo. A lower increase in reduced glutathione GSH levels in the test group in comparison to the placebo group was obtained. This result may indicate a lower oxidation of GSH to GSSG, oxidized glutathione, due to the action of glutathione peroxidase GPX or even more efficient synthesis by glutathione reductase. Besides, higher values in TBARS value with placebo in comparison to the grape concentrate drink were obtained just after the exercise and after one hour. This means a lower value in this oxidative stress marker related to lipid peroxidation when grape concentrate drink is consumed. But the antioxidant enzyme catalase CAT activity remained stable in the group that consumed the beverage. The authors suggest that the studies on the CAT response to exercise have shown conflicting results especially to a single exercise session. The study concludes that TBARS, CAT and GSH values suggest that this grape concentrate drink presents potential to modulate exercise-induced oxidative stress. In another study Tavares-Toscano et al. In this case the total antioxidant capacity TAC was evaluated in the plasma by evaluating the radical scavenging according to the α, α-diphenyl-β-picrylhydrazyl DPPH method. This analytical method is used to determine the TAC of a compound, an extract or other biological sources by using a stable free radical DPPH. The assay is based on the measurement of the scavenging capacity of antioxidants towards it [ 86 ]. The authors showed a deep characterization of the grape juice. They did not analyze any oxidative stress markers, but showed an increase in high density lipoprotein-cholesterol HDL-cholesterol fraction and a decreased low-density lipoprotein-cholesterol LDL-cholesterol fraction demonstrating that grape juice may enhance the benefits of physical training,. Besides the malondialdehyde MDA data indicated that grape juice supplementation did not prevent lipid peroxidation in athletes, but the increase was lower than in the group with no grape juice. Tavares-Tocano et al. Concerning the edible grape products, to the best of our knowledge the first study that analyzed the effect of grape polyphenols supplementation on the blood antioxidant status was in [ 88 ]. This dosage means 0. The results showed an insignificant modification of antioxidant enzyme: SOD, CAT, GSH and glutathione reductase GR activities, concentrations of non-enzymatic antioxidants: GSH and uric acid UA and total antioxidant status TAS. However, the authors indicated that the supplementation with the alcohol-free red wine grape polyphenolic extract might influence the attenuation of the post-exercise release creatine kinase CK into the blood. Lafay et al. In this case, no information regarding the total polyphenolic content was given. Besides the administration of grape extract decreased the plasma CK concentration and increased the hemoglobin Hb level in plasma suggesting a protection of cells against oxidative stress damage. The study revealed that this preparation and doses contributed to a significant increase in plasma TAC and to an insignificant increase in SOD, as well as a lower GSH activity and reduce concentration in TBARS. Taghizadeh et al. No information about the polyphenol content was given but the results showed a significant rise in plasma GSH and a significant decrease in MDA. Besides, the players who received GSE exhibited a significant decrease serum insulin concentration. On the other hand, the administration of GSE had no significant effects on parameters like creatine kinase CK or TAC when compared with the administration of the placebo. The study resulted in an increase in SOD, GSH and CAT activity, which remained stable until the end of the recovery period. The authors explained that in comparison with the placebo group the subjects supplemented showed no need to mobilize more antioxidant defenses before the exercise because and that the supplement probably contributed to spare oxidative homeostasis. Finally, it must be pointed out the protocol [ 93 ] established for a pilot study that includes a product mix made of dried grapes with almonds and dried cranberries. No results are given but the authors describe the necessity of studying the F2-isoprostanes as a lipid peroxidation biomarker for oxidative stress. Supplementation with grape polyphenols seems to have a positive effect against oxidative stress. These effects are dependent on the supplement dose, the length of the supplementation period or the polyphenolic profile total polyphenol content and the distribution among polyphenolic families. Besides, according to several reports, it appears that the type and intensity of exercise can affect the response of the blood antioxidant defense system, just as the training status of the athlete, or the sport discipline. Considering the supplementation dosage in these studies it seems unlikely athletes would gain enough quantity of polyphenols from diet. Therefore, grape-based polyphenol concentrated products would be an interesting approach. Moreover, inter-individual variability the age, sex, diet, environment factors, exercise protocols and even variability in gene expression could influence the polyphenols bioavailability and physiological responses to oxidative stress. Given the promising evidence, although still limited, more pilot studies on effect of grape polyphenols on the oxidative stress produced by sport should be conducted to determine the optimal concentration, dosage and effect on the oxidative stress for target athletes. Physical activity [Internet]. de Sousa CV, Sales MM, Rosa TS, Lewis JE, de Andrade RV, Simões HG. The antioxidant effect of exercise: a systematic review and meta-analysis. Sport Med. Article Google Scholar. The power of exercise: buffering the effect of chronic stress on telomere length. PLoS One. Article PubMed PubMed Central CAS Google Scholar. Gordon B, Chen S, Durstine JL. The effects of exercise training on the traditional lipid profile and beyond. Curr Sport Med Rep. Roque FR, Hernanz R, Salaices M. Exercise training and cardiometabolic diseases: focus on the vascular system. Curr Hypertens Rep. Article CAS PubMed Google Scholar. Pingitore A, Pace Pereira Lima G, Mastorci F, Quinones A, Iervasi G, Vassalle C. Exercise and oxidative stress: potential effects of antioxidant dietary strategies in sports. Devasagayam TPA, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, Lele RD. Free radicals and antioxidants in human health: currant status and future prospects. J Assoc Physicians India. CAS PubMed Google Scholar. Braakhuis A, Hopkins WG. Impact of dietary antioxidants on sport performance: a review. Pereira Panza V-S, Diefenthaeler F, da Silva EL. Benefits of dietary phytochemical supplementation on eccentric exercise-induced muscle damage: is including antioxidants enough? Barranco-Ruiz Y, Aragón-Vela J, Casals C, Martínez-Amat A, Casuso RA, Huertas JR. Control of antioxidant supplementation through interview is not appropriate in oxidative-stress sport studies: analytical confirmation should be required. Nieman DC, Gillitt ND, Knab AM, Shanely RA, Pappan KL, Jin F, et al. Influence of a polyphenol-enriched protein powder on exercise-induced inflammation and oxidative stress in athletes: a randomized trial using a metabolomics approach. Nieman DC, Stear SJ, Castell LM, Burke LM. A-Z of nutritional supplements: dietary supplements, sports nutrition foods and ergogenic aids for health and performance: part Br J Sport Med. Article CAS Google Scholar. Bjørklund G, Chirumbolo S. Role of oxidative stress and antioxidants in daily nutrition and human health. Article PubMed CAS Google Scholar. Fruit: world production by type Statista [Internet]. International Organisation of Vine and Wine. García-Flores LA, Medina S, Gómez C, Wheelock CE, Cejuela R, Martínez-Sanz JM, et al. Aronia-citrus juice polyphenol-rich juice intake and elite triathlon training: a lipidomic approach using representative oxylipins in urine. Food Funct. Article PubMed Google Scholar. Sureda A, Tejada S, Bibiloni MM, Tur JA, Pons A. Polyphenols: well beyond the antioxidant capacity: polyphenol supplementation and exercise-induced oxidative stress and inflammation. Curr Pharm Biotechnol. De Ferrars RM, Czank C, Zhangm Q, Botting NP, Kroon PA, Cassidy A, et al. The pharmacokinetics of anthocyanins and their metabolites in humans. Br J Pharmacol. Czank C, Cassidy A, Zhang Q, Morrison DJ, Preston T, Kroon PA, et al. Human metabolism and elimination of the anthocyanin, cyanidingglucoside: a 13C-tracer study. Am J Clin Nutr. Williamson G, Clifford MN. Colonic metabolites of berry polyphenols: the missing link to biological activity? Br J Nutr. Common phenolic metabolites of flavonoids, but not their unmetabolized precursors, reduce the secretion of vascular cellular adhesion molecules by human endothelial cells. J Nutr. Article CAS PubMed PubMed Central Google Scholar. Kay CD. Rethinking paradigms for studying mechanisms of action of plant bioactives. Nutr Bull. Nieman DC, Mitmesser SH. Potential impact of nutrition on immune system recovery from heavy exertion: a metabolomics perspective. Nieman DC, Shanely RA, Gillit ND, Pappan KL, Lila MA. Serum metabolic signatures induced by a three-day intensified exercise period persist after 14 h of recovery in runners. J Proteome Res. Mach N, Fuster-Botella D. Endurance exercise and gut microbiota: A review. J Sport Heal Sci. Kerksick CM, Wilborn CD, Roberts MD, Smith-Ryan A, Kleiner SM, Jäger R, et al. J Int Soc Sports Nutr. Shankar K, Mehendale HM. Oxidative Stress. In: Wexler P, editor. Encyclopedia of Toxicology. Third Edit. Elsevier; Lehmann R, Zhao X, Weigert C, Simon P, Fehrenbach E, Fritsche J, et al. Medium chain Acylcarnitines dominate the metabolite pattern in humans under moderate intensity exercise and support lipid oxidation. Lewis GD, Farrell L, Wood MJ, Martinovic M, Arany Z, Rowe GC, et al. Metabolic Signatures of Exercise in Human Plasma. Sci Transl Med. Nieman DC, Gillitt ND, Sha W, Meaney MP, John C, Pappan KL, et al. Metabolomics-based analysis of banana and pear ingestion on exercise performance and recovery. Nieman DC, Gillitt ND, Henson DA, Wei Sha R, Andrew Shanely AM, Knab LC-K, et al. Bananas as an energy source during exercise: a metabolomics approach. Nieman DC, Scherr J, Luo B, Meaney MP, Dréau D, Sha W, et al. Influence of pistachios on performance and exercise-induced inflammation, oxidative stress, immune dysfunction, and metabolite shifts in cyclists: a randomized, crossover trial. Nieman DC, Sha W, Pappan KL. IL-6 linkage to exercise-induced shifts in lipid-related metabolites: A metabolomics-based analysis. Nieman DC, Shanely RA, Luo B, Meaney MP, Dew DA, Pappan KL. Metabolomics approach to assessing plasma and 9-hydroxy-octadecadienoic acid and linoleic acid metabolite responses to km cycling. Phys Act Inact. CAS Google Scholar. Powers SK, Radak Z, Ji LL. Exercise-induced oxidative stress: past, present and future. J Physiol. Dillard CJ, Litov RE, Savin WM, Dumelin EE, Tappel AL. Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. J Appl Physiol. Brady PS, Brady LJ, Ullrey DE. Selenium, vitamin E and the response to swimming stress in the rat. Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. McClung J, Deruisseau K, Whidden M, Van Remmen H, Richardson A, Song W, et al. Overexpression of antioxidant enzymes in diaphragm muscle does not alter contraction-induced fatigue or recovery. Exp Physiol. McClung JM, Judge AR, Powers SK, Yan Z. p38 MAPK links oxidative stress to autophagy-related gene expression in cachectic muscle wasting. Am J Physiol Cell Physiol. Powers SK, Duarte J, Kavazis AN, Talbert EE. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Droge W. Free radicals in the physiological control of cell function. Miller DM, Buettner GR, Aust SD. Free Radic Biol Med. Bogdan C, Rollinghoff M, Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr Opin Immunol. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. McLeay Y, Stannard S, Houltham S, Starck C. Dietary thiols in exercise: oxidative stress defence, exercise performance, and adaptation. J Int Soc Sports Nutr ;14 1 :1—8. Mattson MP. Hormesis Defined. Ageing Res Rev. Silveira LR, Pilegaard H, Kusuhara K, Curi R, Hellsten Y. The contraction induced increase in gene expression of peroxisome proliferator-activated receptor PPAR -gamma coactivator 1 alpha PGC-1alpha , mitochondrial uncoupling protein 3 UCP3 and hexokinase II HKII in primary rat skeletal muscle cells is dep. Biochim Biophys Acta Zhou LZ, Johnson AP, Rando TA. NF kappa B and AP-1 mediate transcriptional responses to oxidative stress in skeletal muscle cells. Handayaningsih A-E, Iguchi G, Fukuoka H, Nishizawa H, Takahashi M, Yamamoto M, et al. Reactive oxygen species play an essential role in IGF-I signaling and IGF-I-induced myocyte hypertrophy in C2C12 myocytes. Balon TW, Nadler JL. Evidence that nitric oxide increases glucose transport in skeletal muscle. Steinbacher P, Eckl P. Impact of oxidative stress on exercising skeletal muscle. Marrocco I, Altieri F, Peluso I. Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxidative Med Cell Longev. Merry TL, Mi R. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? Ranchordas MK, Dawson JT, Russell M. Practical nutritional recovery strategies for elite soccer players when limited time separates repeated matches. Yfanti C, Deli CK, Georgakouli K, Fatouros I, Jamurtas AZ. Sport nutrition, redox homeostasis and toxicity in sport performance. Curr Opin Toxicol. Google Scholar. Finaud J, Lac G, Filaire E. Oxidative stress. Additionally, assessing the oxidative damage is unequivocal during exercise because oxidative damage is varied according to the intensity and duration. This can ultimately bring into question the exact role of ROS and exercise performance Knechtle and Nikolaidis, ROS can induce several adaptive signaling pathways in the skeletal muscle Powers and Jackson, However, the mechanism by which it can induce those pathways that signal for improved exercise performance is poorly understood. Furthermore, the ROS steady state level may significantly contribute to such an effect instead of an elevated level of ROS. Steady-state concentrations of ROS are well-balanced by several enzymatic regulations. For example, superoxide dismutase SOD lowers the steady state level of superoxide and decreases the rate of H2O2 production Liochev and Fridovich, , Further, this can maintain the activities of catalase and peroxidase. These studies exposed the fact that superoxide radicals inactivated the catalase and peroxidase, and SOD is the reason for this. In the exercise condition, steady state and ROS levels are determined by both the rate of ROS production and the rate of ROS scavenging. Oxidative stress is not only a phenomenon that refers to elevated levels of ROS that damage lipids, proteins, and DNA, but it also plays a significant role in physiological changes through the interaction with cysteine Cys residues of proteins. Therefore, it is important to consider the measurement of oxidative stress before it causes damage to the cells by affecting several physiological functions. However, measurement of oxidative stress in the cells has several limitations in terms of biomarker selection. This should run down the exact status of oxidative stress. Therefore, focusing on the underlying mechanism of adaptive signaling induced by ROS and selecting suitable biomarkers may facilitate runners that compete in long distance running by preventing ROS-induced damages in the skeletal muscles. Running in events like a marathon or ultra-marathon can result in muscle injury, and the main factors that induce muscle injury are the activation of inflammatory cascades and oxidative stress, but measurement of oxidative stress has no particular suitable biomarkers as stated above Niemelä et al. Therefore, this kind of sport may be a useful platform to find applicable biomarkers that can exactly predict the oxidative stress status in the cells. Moreover, there have been several arguments on whether extreme training sessions like ultra-marathons may increase the health benefits of physical exercise. The level of oxidation response ROS level which improves the exercise performance or increases the exercise-induced benefits is ambiguous Mrakic-Sposta et al. Measuring the oxidative damage by selecting suitable biomarkers, nutrition, individual physical condition, type, and intensity of running exercise among the runners Mrakic-Sposta et al. However, no studies have firmly established these aspects in terms of improving running exercise performance and the benefits. Therefore, the aim of this study was to present a systematic overview of published articles and to find the suitable biomarkers that predict oxidative stress among long-distance runners. To avoid the risk of missing relevant articles, additional papers were searched on the gray literature i. One author AT ran the search and screened the initial titles after duplicates were removed. Two authors AT and GY independently examined potentially relevant articles in depth. We included only papers published in peer-reviewed journals which reported findings from experimental controlled studies, i. We excluded articles not available in English, unpublished papers, and conference posters, or those reporting findings of non-experimental studies e. First author's name, year of publication, sample of intervention and control group, design and duration of the study, topic, type of intervention, outcome, assessment, and results were recorded using an electronic spreadsheet. II The runners had to be competitive, and participants that required medical support were omitted. III Search outputs included only articles that were peer-reviewed and published in English language journals. IV Only running programs like half marathons, marathons, and ironman races were included as types of interventions. Only parameters that were related to oxidative damage and some studies on inflammatory responses that induce oxidative stress were included as types of outcome measures. The abstracts of the articles were further narrowed down using the following criteria: Inclusion criteria: We included prospective cohort studies, cross sectional studies, and randomized clinical trials. Exclusion criteria: We excluded different sport activities other than running programs. The risk of bias assessment was performed independently by two authors based on the Cochrane Risk of Bias Assessment Tool. A third author was consulted in case of any disagreements. For each study, the study characteristics e. All the parameters were evaluated in blood samples collected during or after the running program. Disagreements were resolved through discussions with other authors. After evaluating titles and abstracts, articles were identified as potentially relevant from initial data base searches Figure 1. After screening was performed using titles and relative keywords, articles were excluded. The remaining 34 potential articles full texts were carefully evaluated, and 22 articles were excluded. The full texts of the remaining 12 articles were retrieved and reviewed, which were then included for systematic analysis. A total of 12 studies were included in this study. Study population, the number of participants, mean age and SD, intervention, and main outcomes are outlined in Table 1. This study selected 12 articles to assess the effect of running exercise protocols on oxidative stress parameters. Fourteen articles were identified by searching databases and two were identified by the article's reference for inclusion in the analysis. All the records used in this study were based on human subjects. From the 12 included studies, at least six studies had risk of bias. Three studies had high risk in random sequence generation and allocation concealment. Four studies had a high risk in incomplete outcome data and two studies had high risk in other biases. Six studies had unclear risk in randomization and allocation concealment. Three studies had low risk in randomization and allocation concealment. Eleven studies had low risk in blinding of participants, and four studies had high risk in blinding of outcome assessment. All the studies had low risk in selective reporting Table 2. Four studies had low risk in other biases and six studies had unclear risk in other biases Figure 2. After the first study that suggests exercise increases oxidative stress by Dillard et al. in , a plethora of reports have shown that exercise increases oxidative stress in humans or animals. These studies mostly used cycle ergometer or treadmill exercises in which the participants used maximal or submaximal exercise in a climate-controlled laboratory. This compromises the prediction of the oxidative stress status among the exercised people. Therefore, to predict oxidative stress, it is important to assess suitable oxidative damage markers in various running platforms. One study showed neutrophilia and enhanced PMN capacity to generate oxygen radicals after running. This is the point where the oxygen radicals are established in the runner's blood and are evidenced by increased levels of LPO and GSSG as well as decreased level of SOD and GSH-Px Hessel et al. Another study showed that a single bout of endurance exercise increases TRAP and some of its components like uric acid, but this was due to an adaptive mechanism against running-induced oxidative stress. The intense endurance exercise increased MDA which may react physiologically with several nucleosides to form adducts to deoxyguanosine and deoxyadenosine, and increased exercise intensity may increase the purine oxidation which results in an increase in the formation of uric acid UA. This may be due to an adaptive mechanism against running-induced oxidative stress. Further, endurance training increases the high rate of ATP hydrolysis compared to its resynthesis which further stimulates the myokinase reaction and adenosine monophosphate deaminase reaction. Consequently, the adenine nucleotide pool decreased. Inosine-5'-monophosphate IMP , hypoxanthine Hx , xanthine X , and UA are exercise related products of adenine nucleotide degradation that accumulate in the skeletal muscle or efflux into the blood which ultimately decreases the adenine nucleotide pool precursors Zieliński et al. However, adenine nucleotide pool restoration may be slow and energy consuming, and de novo synthesis from the purine Hx is the only compound that may be reconverted and reutilized into the adenine nucleotide pool after being catalyzed by hypoxanthine-guanine phosphoribosyltransferase HGPRT. Intense exercise increases the Plasma Hx significantly. Therefore, it is considered as an index of exercise intensity Rychlewski et al. Furthermore, high intensity exercise limits the efflux of purines to the plasma resulting in reduced muscle nucleotide loss in active men Hellsten-Westing et al. Six weeks of high intensity exercise decreased the level of Hx both at rest and after the exercise, and this may be due to muscle adaptation that leads to a reduced adenine nucleotide Hellsten-Westing et al. Further, this study showed that a reduced level of thiol content was efficiently utilized by the ROS after the race Liu et al. An additional study showed that prolonged ultra-endurance exercise causes an increase in ROS production and oxidative stress, but it is dependent on specific biomarkers and the exercise duration Vezzoli et al. A different study investigated the effect of running on oxidative modification of nucleic acid, and it was found that marathon participation immediately induced an inflammatory response, but it did not increase the oxidative modification of nucleic acid, instead, it decreases the oxidatively generated nucleic acid modifications, suggesting an adaptive antioxidant effect following running Radák et al. One study showed that even after the running, the oxidative stress lasted for up to 3 days. Additionally, this study showed that capacity oxidation-reduction potential cORP , and GSH are the most effective markers for analyzing running-induced oxidative stress Spanidis et al. Two studies investigated the ironman triathlon's effect in inducing oxidative damage. From those two studies, one study showed that there is no persistent oxidative stress in response to an iron-man race Wagner et al. Another study showed that increased oxidative stress regulates the inflammatory process during heavy exertion Nieman et al. Another study showed that heavy endurance exercise increased the lipid peroxidation Mastaloudis et al. One study showed that exhaustive and prolonged exercise induces oxidative stress and inflammation Mrakic-Sposta et al. This systematic review analyzed the effect of different running programs on oxidative stress with the aim of determining suitable biomarkers that predict the early oxidative stress status in runners. From the 12 selected and systematically reviewed articles, running exercises do not elicit a response to specific biomarkers of oxidative stress, instead, oxidative stress markers like ROS induced end products of lipids, proteins, and various enzymatic and non-enzymatic antioxidants expressed according to the training status of the individual. Although it is known that exercises like running can induce oxidative stress, the methods that potentially measure the oxidative damage are limited because some of the methods have failed to reflect the exact status of oxidative stress in the cells. Consequently, the measurement of oxidative stress is required and is a more promising approach in different physiological conditions induced by exercise. Measurement of cellular ROS is one of the direct ways to determine the oxidative stress. For example, fluorogenic probes are used as a direct method to measure superoxide radicals, hydrogen peroxide, hydroxyl radicals, and peroxyl radicals Debowska et al. Other ways to assess the oxidative stress include ROS derived metabolites D-ROMS. However, these measurements are compromised in predicting its accuracy because the radicals that are assessed using direct measurements are relatively short lived and highly reactive Denicola et al. Additionally, different ROS have different degrees of reactivity toward cellular components, and the free iron availability is considered crucial for ROS toxicity due to the role it plays in the Fenton reaction to produce hydroxyl radicals. Therefore, indirect measurement could be a useful platform to determine ROS induced oxidative stress. For example, ROS induced damage to lipids, proteins, and nucleic acids and its further end product assessment could be a promising approach to assess the oxidative stress in the samples of people that exercise. For example, all the studies that we selected with the aim of finding the suitable biomarkers, have assessed the ROS induced end products like PC, MDA, TBARS, 8-OH-dG, and F2-isoprostanes, but no studies firmly reported the suitable biomarkers to measure the oxidative damage because sample type, collection of sample timing, and exercise duration and type may frequently change the reaction time of the ROS, which may compromise the prediction of ROS induced oxidative stress. Further, measuring the level of antioxidant compounds such as enzymatic, non-enzymatic compounds, and some low molecular mass compounds are useful candidates for evaluating oxidative stress in the samples. However, frequent changes in ROS concentration due to duration, intensity, and type of exercise may mispredict the expression level of those enzymatic and non-enzymatic antioxidants. For example, one study reported that the GSH level increased after the race whereas the CAT level was not significantly increased Spanidis et al. Another study reported that the CAT level increased after the race Pinho et al. These contradicting results may be because the concentration of ROS differed in different running statuses such as in distance and the time in which the race was completed. Regarding exercise, different types of exercises influence the level of ROS induced end products based on the training status Hadžović-Džuvo et al. Furthermore, studies have shown that endurance exercise increased ROS and induced damage to lipids, proteins, DNA and antioxidant levels Kanter et al. However, direct evidence on those oxidative damage markers is limited in reflecting oxidative stress, and some studies only observe a few markers that are increased during endurance training as well as some markers do not show signs of any increment Alessio et al. Vezzoli et al. observed that prolonged ultra-endurance running increased the PC, TBARS, TAC, and 8-OH-dG Vezzoli et al. Spanidis et al. reported that there were no changes observed during or after running in TBARS, PC, and TAC, suggesting that these outcomes are dependent on training status and specific biomarkers that are assessed during running Spanidis et al. Further, this study reported that GSH and cORP are the most effective biomarkers to analyze running-induced oxidative stress. In addition, this study showed that these markers existed up to 3 days after the race, which is possibly due to the exercise intensity and total caloric expenditure. Indeed, several studies have shown that the oxidative stress response is altered in relation to exercise intensity Alessio et al. From these results, we conclude that assessing the oxidative damage markers in response to exercise running may vary according to exercise intensity, duration, and individual antioxidant capacity. No persistent results were observed in all the selected studies with regards to oxidative stress biomarkers. However, most of the studies used oxidative damage markers and individual antioxidant capacity such as PC, MDA, TBARS, CAT, and GSH for the measurement of oxidative stress, suggesting that assessing oxidative damage markers and individual antioxidant capacity could be a promising method to reflect the potentiality of methods on oxidative stress compared to the direct method that assesses the ROS. The national institutes of health define the word biomarker as the process of both normal and abnormal processes in the biological system. Since there is no specific biomarker to predict the accurate status of oxidative stress, inflammatory markers could also be a useful candidate to assess the oxidative stress in exercise conditions. An exercise induced inflammatory response has long-term effects on human health, but ROS could be the driving factor for inflammation Suzuki, ROS induce several signaling events that are directly involved in inducing inflammation during exercise, such as nuclear factor kappa-light-chain-enhancer of activated B cells NFkB and activator protein-1 AP-1 Biswas, ; Liu et al. Studies observed that running exercises increased the inflammatory response, but did not increase nucleic acid modifications by ROS, bringing into questioning the above statement of whether ROS could be a driving factor for inflammatory response or whether exercise-induced adaptive antioxidant effects could only detoxify the ROS without affecting inflammatory cascades Radák et al. However, one study reported that iron-man races increased the oxidative stress-induced inflammatory response Pinho et al. In contrast, another study observed that no consistent changes were observed in oxidative stress parameters and inflammatory responses, suggesting that different exercise modalities have different effects on oxidative stress parameters and inflammatory responses Wagner et al. For example, high-intensity prolonged running exercise induced the oxidative stress and inflammation, but even moderate continuous exercise increased the oxidative stress compared to discontinuous high-intensity exercise Mastaloudis et al. However, this moderate exercise-induced oxidative stress effect could be changed with duration. These varying results show the uncertainty of the argument that inflammatory markers cannot be used for assessing the oxidative stress. More research is therefore required to confirm the effect of inflammatory markers as an effective strategy to assess oxidative stress in exercise conditions. ROS generation depends on exercise intensity and duration, as exercise types differ in their energy requirements, level of oxygen consumption, and the mechanical stress imposed on tissues. During low-intensity and duration, protocols have effective antioxidant defense mechanisms that likely meet the ROS production, but, as the intensity and duration of exercise increases, the antioxidant defense is no longer adequate—potentially resulting in oxidative damage. A study has shown that neutrophil production of superoxide increased only at intensities above the lactate threshold in exercised men Quindry et al. In contrast to the above study, other studies reported that oxidative stress markers in blood increased with , or min of exercise at a constant intensity. Several reviews conclude that regular exercise training does not lead to chronic oxidative stress in the active muscles which fosters the concept of exercise induced hormesis Ji et al. Hormesis used to describe the biphasic dose response curve where small amounts of the stressor provide beneficial adaptive effects on cells, whereas high levels of those stressors may result in damage to the cells. From this, exercise induced low levels of ROS production play a crucial role in exercise induced adaptation of the skeletal muscle, and this can be explained using the bell shaped hormesis curve where the optimum level of ROS plays a role in muscle adaptation whereas when above the optimum level of ROS, it can lead to various damages to the cells and a decline in the exercise induced adaptation Ji et al. These studies do not provide strong enough evidence to show that high intensity exercise for prolonged periods of time, can result in oxidant-mediated damage in the cells and decrease antioxidant capacity in the trained muscles de Sousa et al. The reasons associated with this are the cardiovascular systems ability to affect the sustainability of high intensity by providing blood to the working muscles and affect the ROS production on muscle fatigue Ji et al. Thus, the ROS production level is limited during exercise. Another reason is that mitochondrial coupling is higher in state 3 respiration during exercise resulting in the reduction of electron spill and ROS production by the mitochondria when compared to state 4 resting respiration. A final reason is that the exercise can increase the antioxidant enzymes in the skeletal muscle that supports the muscle fiber, to remove the ROS during exercise Powers et al. These results predict that skeletal muscles are not exposed to ROS mediated damage during exercise. Nuclear factor erythroid 2-related factor 2 Nrf2 is a transcription factor that is considered to be a master regulator of the antioxidant defenses, facilitating more than cytoprotective genes in response to oxidative stress Tebay et al. Nrf2 is a family member of the basic leucine zipper that is repressed with Kelch-like ECH-associated protein 1 Keap1 protein in a sequestering form in the cytoplasm under the unstressed condition. In response to oxidative stress, cysteine residues are modified on Keap1 which unhinge the Nrf2 from Keap1. Thus, Nrf2 is translocated into the nucleus where it can heterodimerize with small MAF proteins and bind to Cis-acting antioxidant response elements AREs which ultimately activate the enzymatic antioxidants. Exercise induced ROS formation may activate the Nrf2 which likely occurs through oxidation of cysteine residues as mentioned above. A single bout of exercise has been observed to increase the Nrf2 gene expression in wild type mice Merry and Ristow, However, this study was performed using acute exercises that are not long enough to predict whether exercise can increase the Nrf2 gene expression or not. However, a recent study observed that acute exercise increased Nrf2 protein levels in the blood in young and older men Done et al. Another study has shown that Nrf2 increased in moderate treadmill exercises Scott et al. However, an increase of Nrf2 signaling is dependent on the duration of exercise Done et al. In contrast, when the duration is increased, it is apparent that the Nrf2 level is increased in the skeletal muscle tissue Li et al. Another animal study showed that 6 h of running clearly elicited an increased level of Nrf2, but no change occurred for 1 h of running Li et al. In contrast to these studies, other studies observed that even 1 h of treadmill running increased the Nrf2 level Merry and Ristow, The differences in these studies may be due to differences in the protocol intensities Done et al. However, determining the optimal exercise dose or delivery on Nrf2 activation should be expanded on in future studies. Although direct methods in assessing ROS could be a promising approach, as we mentioned earlier, the stability of the reactive molecules is short lived and highly reactive. Therefore, assessing these molecules in the biological system remains complicated. However, assessing the oxidative damage markers is one of the stable methods to provide more reliable results for the measurement of oxidative stress in the samples. Some complications still need to be eradicated such as assessing these oxidative damage markers that are only reflected to a local degree of oxidative stress, while others have a direct effect on target molecules. This further questions the applicability of those markers in assessing oxidative stress in the sample. Next, the sample collection should be processed with precaution to ensure the stability of the sample because there is a possibility for molecules to become more susceptible to be oxidatively damaging. However, non-invasive techniques could be useful to overcome normal sample collection procedures. For example, analysis of urinary biomarkers provides better applicability to measure oxidative damage because the sample collection is easy and has a low organic and metal content Il'yasova et al. Additionally, a urinary sample minimizes the sample oxidation during sample collection and storage Marrocco et al. Another advantage of a urinary sample is that it provides a longer period of the redox balance index when compared to blood. This can allow the urine sample to be more sensitive to predicting oxidative stress for longer periods. However, only a few markers have been validated in animals and humans, like F2-isoprostanes, 8-oxodG, and the MDA level detected by HPLC. Furthermore, some aspects like stability of the markers, particularly MDA and F2-isoprostanes variations, can produce esterifies lipids in the urine causing uncertainties in the applicability of these markers as effective methods for oxidative stress measurement. However, some promising markers like acrolein-lysine and dityrosine are understudied which could reflect the oxidative stress. This will diversify the current parameters in measuring oxidative stress in humans in the near future. As stated above, some inflammatory markers could be useful to measure the oxidative damage, but its specificity on local oxidative damage and target molecules is questionable because different physiological and pathological conditions induce different inflammatory cascades Chen et al. Therefore, it cannot be recommended to measure oxidative damage as oxidative stress biomarkers. Regarding antioxidant status, everyone during exercise or before exercise have different antioxidant statuses to oxidative responses, which could provide conflicting results during antioxidant status measurement. For instance, some studies reported that exercise running increased the antioxidants Mastaloudis et al. This could be due to an adaptive response that nullifies the ROS toxicities. To overcome these problems, it is suggested that determining total antioxidant status could be a useful parameter among runners. Further, there is no specific biomarkers recommended for the measurement of oxidative stress for runners. However, it should be done based on assessing the training status of the individual. Therefore, an integrative approach is required for the measurement of oxidative stress before and after the exercise. Finally, to the best our knowledge, there is no specific biomarkers or methodologies for the measurement of oxidative stress. More research to provide better and more reliable approaches to earlier prediction of oxidative stress in different types of exercise is therefore required. Further, before selecting an appropriate method to determine oxidative stress, a deep and critical analysis must be carried out according to the aim and design of the study, from the available literature, to select suitable biomarkers. This study potentially observed that different running programs at different intensities and durations induced oxidative damage, but better adaptive mechanisms in runners decreased the oxidative damage, suggesting that different modalities of running exercises have stronger effects on inducing oxidative damage, following adaptive mechanisms to counteract oxidative stress. However, this outcome is dependent on specific oxidative damage markers that are analyzed during the running program. Because some studies used direct methods to assess the oxidative stress, while other studies used oxidative damage markers as oxidative stress indicators, results to measure the exact status of the oxidative damage in the runners were conflicting. Furthermore, exercises like running can increase the level of antioxidants which reverse the oxidative damage. However, it should be noted that the selected studies had some methodological flaws and a high risk of bias justifying the effect of oxidative damage markers as an efficient method to assess the oxidative damage and running-induced adaptive response. AT, YH, and YM conceived the presented idea, developed the framework, and wrote the manuscript. AT, RP, UU, and YG provided critical feedback and contributed to the final version. All authors were involved in the final direction of the paper and contributed to the final version of the manuscript. All authors have read and agreed to the published version of the manuscript. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. |
| Video Upload Options | As a result, the use of supplements with antioxidant properties [ 10 ] for reducing the oxidative stress may be an effective health strategy. Taurine reverses oxidative damages and restores the muscle function in overuse of exercised muscle. Mach N, Fuster-Botella D. Ebbeling CB, Clarkson PM. Urso ML, Clarkson PM. |
| Editorial: Exercise-induced oxidative stress and the role of antioxidants in sport and exercise | You can also upload a video entry or images related to this topic. Version Summary Created by Modification Content Size Created at Operation 1 Zhen Zeng. Lindsay Dong. Video Upload Options Do you have a full video? Send video materials Upload full video. Confirm Are you sure to Delete? Yes No. If you have any further questions, please contact Encyclopedia Editorial Office. MDPI and ACS Style MDPI and ACS Style AMA Style Chicago Style APA Style MLA Style. Zeng, Z. Diets and Exercise-Induced Oxidative Stress. Zeng Z. Accessed February 15, Zeng, Zhen. In Encyclopedia. Copy Citation. Home Entry Topic Review Current: Diets and Exercise-Induced Oxidative Stress. This entry is adapted from the peer-reviewed paper diet antioxidants exercise oxidative stress reactive oxygen species. Introduction The term oxidative stress is defined as a disturbance in the homeostatic balance between pro-oxidants and antioxidants with a subsequent excessive generation of free radicals [ 1 ] [ 2 ] [ 3 ]. Dietary Strategies The majority of currently available studies addressed the effects of phenol-rich foods on exercise-induced oxidative stress, including dark chocolate [ 14 ] [ 15 ] [ 16 ] , high-flavanol cocoa drink [ 17 ] , green tea [ 18 ] , mate tea [ 19 ] , New Zealand blueberry smoothie [ 20 ] , blueberries [ 21 ] [ 22 ] , grape juice [ 23 ] [ 24 ] , Montmorency cherry juice [ 25 ] , tart cherry juice [ 26 ] , oatmeal [ 27 ] , avenanthramides AVA -rich cookie [ 28 ] [ 29 ] , juçara juice [ 30 ] , Sanguinello cultivar red orange juice [ 31 ] , and purple sweet potato leaves [ 32 ]. Effects on Biomarkers of Exercise-Induced Oxidative Stress 3. Effects of Dietary Interventions on Direct ROS Generation Zeng et al. Effects of Dietary Interventions on ROS-Induced Macromolecule Damage In the majority of studies, F2-isoprostanes, 8-isoprostanes, lipid hydroperoxides LH , thiobarbituric acid-reactive substances TBARS and malondialdehydes MDA were used as the oxidative markers, which result from lipoperoxidation by oxidative damage. Effects of Dietary Interventions on Inflammatory Markers Exercise-induced oxidative stress can activate a range of transcription factors that contribute to the differential expression of certain genes involved in inflammatory pathways [ 37 ]. Effects of Dietary Interventions on Antioxidant Activity In concert with alterations affecting levels of oxidative stress markers and inflammatory markers, exercise-induced oxidative stress could attenuate the endogenous antioxidant defense including enzymatic antioxidant activity catalase CAT , SOD, GPx, cyclooxygenase-2 COX-2 and nonenzymatic antioxidant activity GSH, oxygen radical absorbance capacity ORAC , total antioxidant capacity TAC , total antioxidant status TAS , ferric reducing antioxidant power FRAP , vitamins C and E, and reduced glutathione content. References Halliwell, B. Free Radicals, Antioxidants, and Human Disease: Curiosity, Cause, or Consequence? Lancet , , — Halliwell, B. Free Radicals in Biology and Medicine, 5th ed. Dröge, W. Free Radicals in the Physiological Control of Cell Function. Peternelj, T. Antioxidant Supplementation during Exercise Training: Beneficial or Detrimental? Sports Med. Valko, M. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Cell Biol. Powers, S. Reactive Oxygen Species Are Signalling Molecules for Skeletal Muscle Adaptation. Reactive Oxygen Species: Impact on Skeletal Muscle. Merry, T. Mitohormesis in Exercise Training. Free Radic. Pingitore, A. Exercise and Oxidative Stress: Potential Effects of Antioxidant Dietary Strategies in Sports. Nutrition , 31, — Paulsen, G. Vitamin C and E Supplementation Alters Protein Signalling after a Strength Training Session, but Not Muscle Growth during 10 Weeks of Training. Gomez-Cabrera, M. Oral Administration of Vitamin C Decreases Muscle Mitochondrial Biogenesis and Hampers Training-Induced Adaptations in Endurance Performance. Ristow, M. Antioxidants Prevent Health-Promoting Effects of Physical Exercise in Humans. USA , , — Higgins, M. Antioxidants and Exercise Performance: With a Focus on Vitamin e and c Supplementation. Public Health , 17, Davison, G. The Effect of Acute Pre-Exercise Dark Chocolate Consumption on Plasma Antioxidant Status, Oxidative Stress and Immunoendocrine Responses to Prolonged Exercise. Allgrove, J. Sport Nutr. Taub, P. Beneficial Effects of Dark Chocolate on Exercise Capacity in Sedentary Subjects: Underlying Mechanisms. A Double Blind, Randomized, Placebo Controlled Trial. Food Funct. Wiswedel, I. Flavanol-Rich Cocoa Drink Lowers Plasma F2-Isoprostane Concentrations in Humans. Panza, V. Consumption of Green Tea Favorably Affects Oxidative Stress Markers in Weight-Trained Men. Nutrition , 24, — Effects of Mate Tea Consumption on Muscle Strength and Oxidative Stress Markers after Eccentric Exercise. McLeay, Y. Effect of New Zealand Blueberry Consumption on Recovery from Eccentric Exercise-Induced Muscle Damage. Sports Nutr. Park, C. Assessing the Values of Blueberries Intake on Exercise Performance, TAS, and Inflammatory Factors. Iran J. Public Health , 47, 27— McAnulty, S. Consumption of Blueberry Polyphenols Reduces Exercise-Induced Oxidative Stress Compared to Vitamin C. de Lima Tavares Toscano, L. A Single Dose of Purple Grape Juice Improves Physical Performance and Antioxidant Activity in Runners: A Randomized, Crossover, Double-Blind, Placebo Study. Toscano, L. Potential Ergogenic Activity of Grape Juice in Runners. Bowtell, J. Montmorency Cherry Juice Reduces Muscle Damage Caused by Intensive Strength Exercise. Sports Exerc. Howatson, G. Influence of Tart Cherry Juice on Indices of Recovery Following Marathon Running. Acute Effects of Oatmeal on Exercise-Induced Reactive Oxygen Species Production Following High-Intensity Interval Training in Women: A Randomized Controlled Trial. Antioxidants , 10, 3. Koenig, R. Avenanthramide Supplementation Attenuates Eccentric Exercise-Inflicted Blood Inflammatory Markers in Women. Zhang, T. Brain Res. Dillard CJ, Litov RE, Savin WM, Dumelin EE, Tappel AL. Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. J Appl Physiol Respir Environ Exerc Physiol. CAS PubMed Google Scholar. Erel O, Neselioglu S. Clin Biochem. Fittipaldi S, Dimauro I, Mercatelli N, Caporossi D. Role of exercise-induced reactive oxygen species in the modulation of heat shock protein response. Free Radic Res. Galan AI, Palacios E, Ruiz F, Diez A, Arji M, Almar M, Moreno C, Calvo JI, Munoz ME, Delgado MA, et al. Exercise, oxidative stress and risk of cardiovascular disease in the elderly. Protective role of antioxidant functional foods. Giustina AD, Danielski LG, Novochadlo MM, Goldim MPS, Joaquim L, Metzker KLL, Carli RJ, Denicol T, Cidreira T, Vieira T, et al. Vitamin B6 reduces oxidative stress in lungs and liver in experimental sepsis. An Acad Bras Cienc. Jones DP, Sies H. The redox code. Joshi R, Adhikari S, Patro BS, Chattopadhyay S, Mukherjee T. Free radical scavenging behavior of folic acid: evidence for possible antioxidant activity. Kayacan Y, Yazar H, Kisa EC, Ghojebeigloo BE. Arch Physiol Biochem. Kayacan Y, Cetinkaya A, Yazar H, Makaraci Y. Kayacan Y, Yazar H, Cerit G, Ghojebeigloo BE. A new oxidative stress indicator: effect of 5-hydroxytryptophan on thiol-disulfide homeostasis in exercise. Le Garf S, Sibille B, Mothe-Satney I, Eininger C, Fauque P, Murdaca J, Chinetti G, Neels JG, Rousseau AS. Alpha-lipoic acid supplementation increases the efficacy of exercise- and diet-induced obesity treatment and induces immunometabolic changes in female mice and women. FASEB J. PubMed Google Scholar. Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Ozhogina OA, Kasaikina OT. Beta-carotene as an interceptor of free-radicals. Pappas A, Tsiokanos A, Fatouros I, et al. The effects of spirulina supplementation on redox status and performance following a muscle damaging protocol. Int J Mol Sci. Parra M, Stahl S, Hellmann H. Vitamin B6 and its role in cell metabolism and physiology. Petiz LL, Girardi CS, Bortolin RC, Kunzler A, Gasparotto J, Rabelo TK, Matte C, Moreira JC, Gelain DP. Vitamin A oral supplementation induces oxidative stress and suppresses IL and HSP70 in skeletal muscle of trained rats. Petiz LL, Kunzler A, Bortolin RC, Gasparotto J, Matte C, Moreira JCF, Gelain DP. Role of vitamin A oral supplementation on oxidative stress and inflammatory response in the liver of trained rats. Appl Physiol Nutr Metab. Poulab E, Sajedinia H, Hafezi F, et al. The effect of a four-week acute vitamin C supplementation on the markers of oxidative stress and inflammation following eccentric exercise in active men. Int J Basic Sci Appl Res. Ravichandran V, Selvam R. Increased plasma lipid peroxidation in vitamin B6 deficient rats. Indian J Exp Biol. Reid MB, Stokic DS, Koch SM, Khawli FA, Leis AA. N -acetylcysteine inhibits muscle fatigue in humans. J Clin Invest. Rodriguez NR, DiMarco NM, Langley S, American Dietetic A, Dietitians of C, American College of Sports Medicine N, Athletic P. Position of the American Dietetic Association, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. J Am Diet Assoc. Salo DC, Donovan CM, Davies KJA. Hsp70 and other possible heat-shock or oxidative stress proteins are induced in skeletal-muscle, heart, and liver during exercise. Sies H. Oxidative stress: introductory remarks. In: Sies H, editor. Oxidative stress. London: Academic; Sies H, Jones DP. In: Fink G, editor. Encyclopedia of stress, 2nd edn, vol. Amsterdam: Elsevier; Chapter Google Scholar. Silva LA, Silveira PC, Ronsani MM, Souza PS, Scheffer D, Vieira LC, Benetti M, De Souza CT, Pinho RA. Taurine supplementation decreases oxidative stress in skeletal muscle after eccentric exercise. Cell Biochem Funct. Singh M, Kapoor A, Bhatnagar A. Oxidative and reductive metabolism of lipid-peroxidation derived carbonyls. Chem Biol Interact. Su LJ, Zhang JH, Gomez H, Murugan R, Hong X, Xu DX, Jiang F, Peng ZY. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Sumida S, Doi T, Sakurai M, Yoshioka Y, Okamura K. Effect of a single bout of exercise and beta-carotene supplementation on the urinary excretion of 8-hydroxy-deoxyguanosine in humans. Toldy A, Stadler K, Sasvari M, Jakus J, Jung KJ, Chung HY, Berkes I, Nyakas C, Radak Z. The effect of exercise and nettle supplementation on oxidative stress markers in the rat brain. Brain Res Bull. Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Valko M, Rhodes CJB, Moncol J, Izakovic MM, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-biological interactions. Vincent HK, Bourguignon CM, Vincent KR, Weltman AL, Bryant M, Taylor G. Antioxidant supplementation lowers exercise-induced oxidative stress in young overweight adults. Wadley AJ, Turner JE, Aldred S. Factors influencing post-exercise plasma protein carbonyl concentration. Yada K, Roberts LA, Oginome N, et al. Effect of acacia polyphenol supplementation on exercise-induced oxidative stress in mice liver and skeletal muscle. Yimcharoen M, Kittikunnathum S, Suknikorn C, Nak-On W, Yeethong P, Anthony TG, Bunpo P. Effects of ascorbic acid supplementation on oxidative stress markers in healthy women following a single bout of exercise. J Int Soc Sports Nutr. Article PubMed PubMed Central Google Scholar. Yoshii K, Hosomi K, Sawane K, Kunisawa J. Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. Frontiers in nutrition. Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species ROS and ROS-induced ROS release. Physiological reviews. Download references. Department of Medical Biochemistry, Faculty of Medicine, Sakarya University, Sakarya, Turkey. You can also search for this author in PubMed Google Scholar. Correspondence to Yıldırım Kayacan. School of Life Sciences, University of Westminster, London, UK. Reprints and permissions. Kayacan, Y. Oxidative Stress Biomarkers in Exercise. In: Patel, V. eds Biomarkers in Nutrition. Biomarkers in Disease: Methods, Discoveries and Applications. Springer, Cham. Published : 15 October Publisher Name : Springer, Cham. Print ISBN : Online ISBN : eBook Packages : Biomedical and Life Sciences Reference Module Biomedical and Life Sciences. Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Policies and ethics. Skip to main content. Abstract An increase in the amount of free radicals resulting from exercise has been reported in various studies on humans and experimental animals to date. Keywords Oxidative stress Exercise Redox Supplements Nutrition Free radicals. Buying options Chapter EUR |
| Exercise Causes Oxidative Stress. Here's What It Means for Your Health | explored the potential role of Pterostilbene PTE , a phenolic compound derived from blueberries and grapes, in protecting the intestinal epithelial barrier during high-intensity exercise. In vitro, PTE promoted the expression of intestinal epithelial tight junction TJ molecules. Additionally, the authors identified that the exercise led to an abundance of gut bacterium Alistipesis , which is associated with lipopolysaccharide LPS production which was not reversed by the PTE. This study highlights the potential of PTE as a possible nutritional supplement for preserving the integrity of the intestinal epithelial barrier, which may have protective effects on gastrointestinal health in individuals engaging in high-intensity exercise. Further research is warranted to extrapolate these findings to a human athletic population. High-intensity exercise can induce fatigue, potentially due to an excess of ROS, leading to reduced functions and increased injury risk Silva-Reis et al. investigated the effects of a week combined aerobic and resistance training programme on lung function and mechanics and markers of airway fibrosis in obese females. The study demonstrated beneficial effects on lung function and mechanics, with improved forced vital capacity, and peak expiratory flow, with improvements in airway resistance in all groups non-obese, obese, and obese Grade I females. The authors also observed reduced pro-fibrotic insulin-like growth factor 1 IGF-1 and increased anti-fibrotic Klotho levels in those overweight or obese. These findings indicate the potential benefits of combined physical exercise in improving respiratory health in those overweight and obese by reducing fibrotic processes in the lungs. Molecular hydrogen H 2 , known for its antioxidant and anti-inflammatory properties 12 , has been suggested as a potential strategy to alleviate fatigue and improve aerobic capacity 13 , but its effects have not been fully characterised. A study by Hong et al. demonstrated the effects of inhaling H 2 gas before high-intensity cycling on physical fatigue and prefrontal cortex activation. They found that inhaling H 2 gas The study highlights how H 2 gas inhalation could potentially enhance exercise performance and reduce fatigue in athletes. However, further studies are required to understand the different exercise protocols and establish an understanding of the mechanisms involved. Finally, Hong et al. conducted a systematic review and meta-analysis on the effects of H 2 intake on fatigue and aerobic capacity in healthy adults. The meta-analysis included 19 studies utilising H 2 supplementation. Pooled effect sizes demonstrated a small significant effect on perceived exertion and blood lactate, but no impact on aerobic capacity VO 2max , VO 2peak was identified. The findings provide moderate evidence that H 2 supplementation may alleviate fatigue in healthy adults but does not enhance aerobic capacity. The effects of H 2 on fatigue may be influenced by factors such as training status, intervention period, and exercise types. These findings suggest that H 2 supplementation may be beneficial for reducing perceived exertion and fatigue during exercise in healthy individuals. However, further investigation is required to determine the dose-response and impact on injury risk over time. In conclusion, this Research Topic offers insights into the role of diet and nutritional supplements in managing exercise-induced damage and oxidative stress, supporting overall health and athletic performance. Pterostilbene may have protective effects for the intestinal epithelial barrier during high-intensity exercise. Combined aerobic and resistance training can improve lung function, mechanics, and immune response, benefiting overweight and obese individuals. Hydrogen gas supplementation may alleviate fatigue in healthy adults, but it does not appear to enhance aerobic capacity. Further investigation is needed to understand the impact and mechanisms of these interventions on exercise performance and injury risk. The contributions in this Research Topic contribute to the growing body of knowledge on exercise-induced oxidative stress and its management, offering valuable insights for athletes, coaches, and researchers in the fields of Exercise Physiology and Sport and Exercise Nutrition. We deeply thank all the authors and reviewers who have participated in this Research Topic. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Schieber M, Chandel NS. ROS Function in redox signaling and oxidative stress. Curr Biol. doi: PubMed Abstract CrossRef Full Text Google Scholar. Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med ; Wetzstein CJ, Shern-Brewer RA, Santanam N, Green NR, White-Welkley JE, Parthasarathy S. Does acute exercise affect the susceptibility of low density lipoprotein to oxidation? Free Radic Biol Med ; Pincemail J, Camus G, Roesgen A, Dreezen E, Bertrand Y, Lismonde M et al. Exercise induces pentane production and neutrophil activation in humans. Effect of propranolol. Eur J Appl Physiol Occup Physiol ; Knez WL, Jenkins DG, Coombes JS. Oxidative stress in half and full Ironman triathletes. Med Sci Sports Exerc ; Paffenbarger RS, Brand RJ, Sholtz RI, Jung DL. Energy expenditure, cigarette smoking, and blood pressure level as related to death from specific diseases. Am J Epidemiol ; Villeneuve PJ, Morrison HI, Craig CL, Schaubel DE. Physical activity, physical fitness, and risk of dying. Epidemiology ; Lee IM, Paffenbarger RS. Physical activity and stroke incidence: the Harvard Alumni Health Study. Stroke ; Paffenbarger RS, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. Lee IM, Hsieh CC, Paffenbarger RS Jr. Exercise intensity and longevity in men. The Harvard Alumni Health Study. JAMA ; Shaper AG, Wannamethee G, Weatherall R. Physical activity and ischaemic heart disease in middle-aged British men. Br Heart J ; Quinn TJ, Sprague HA, van Huss WD, Olson HW. Caloric expenditure, life status, and disease in former male athletes and non-athletes. Kanter MM, Lesmes GR, Kaminsky LA, La Ham-Saeger J, Nequin ND. Serum creatine kinase and lactate dehydrogenase changes following an eighty kilometer race. Relationship to lipid peroxidation. Margaritis I, Tessier F, Richard MJ, Marconnet P. No evidence of oxidative stress after a triathlon race in highly trained competitors. Int J Sports Med ; Sastre J, Asensi M, Gascó E, Pallardo FV, Ferrero JA, Furukawa T et al. Exhaustive physical exercise causes oxidation of glutathione status in blood: prevention by antioxidant administration. Am J Physiol ; R Effects of a single bout of ultraendurance exercise on lipid levels and susceptibility of lipids to peroxidation in triathletes. Moller P, Wallin H, Knudsen LE. Oxidative stress associated with exercise, psychological stress and life-style factors. Chem Biol Interact ; Jenkins RR, Krause K, Schofield LS. Influence of exercise on clearance of oxidant stress products and loosely bound iron. Ebbeling CB, Clarkson PM. Exercise-induced muscle damage and adaptation. Sports Med ; Smith JA, Telford RD, Mason IB, Weidemann MJ. Exercise, training and neutrophil microbicidal activity. Kanter MM, Nolte LA, Holloszy JO. Effects of an antioxidant vitamin mixture on lipid peroxidation at rest and postexercise. J Appl Physiol ; Bousquet J, Chanez P, Mercier J, Préfaut C. Monocytes, exercise, and the inflammatory response. Exerc Immunol Rev ; Figure 1: Relative risk of death corresponding to energy expenditure. Figure 2: Percentage of participants reporting CVD corresponding to energy expenditure. Lafay et al. In this case, no information regarding the total polyphenolic content was given. Besides the administration of grape extract decreased the plasma CK concentration and increased the hemoglobin Hb level in plasma suggesting a protection of cells against oxidative stress damage. The study revealed that this preparation and doses contributed to a significant increase in plasma TAC and to an insignificant increase in SOD, as well as a lower GSH activity and reduce concentration in TBARS. Taghizadeh et al. No information about the polyphenol content was given but the results showed a significant rise in plasma GSH and a significant decrease in MDA. Besides, the players who received GSE exhibited a significant decrease serum insulin concentration. On the other hand, the administration of GSE had no significant effects on parameters like creatine kinase CK or TAC when compared with the administration of the placebo. The study resulted in an increase in SOD, GSH and CAT activity, which remained stable until the end of the recovery period. The authors explained that in comparison with the placebo group the subjects supplemented showed no need to mobilize more antioxidant defenses before the exercise because and that the supplement probably contributed to spare oxidative homeostasis. Finally, it must be pointed out the protocol [ 93 ] established for a pilot study that includes a product mix made of dried grapes with almonds and dried cranberries. No results are given but the authors describe the necessity of studying the F2-isoprostanes as a lipid peroxidation biomarker for oxidative stress. Supplementation with grape polyphenols seems to have a positive effect against oxidative stress. These effects are dependent on the supplement dose, the length of the supplementation period or the polyphenolic profile total polyphenol content and the distribution among polyphenolic families. Besides, according to several reports, it appears that the type and intensity of exercise can affect the response of the blood antioxidant defense system, just as the training status of the athlete, or the sport discipline. Considering the supplementation dosage in these studies it seems unlikely athletes would gain enough quantity of polyphenols from diet. Therefore, grape-based polyphenol concentrated products would be an interesting approach. Moreover, inter-individual variability the age, sex, diet, environment factors, exercise protocols and even variability in gene expression could influence the polyphenols bioavailability and physiological responses to oxidative stress. Given the promising evidence, although still limited, more pilot studies on effect of grape polyphenols on the oxidative stress produced by sport should be conducted to determine the optimal concentration, dosage and effect on the oxidative stress for target athletes. Physical activity [Internet]. de Sousa CV, Sales MM, Rosa TS, Lewis JE, de Andrade RV, Simões HG. The antioxidant effect of exercise: a systematic review and meta-analysis. Sport Med. Article Google Scholar. The power of exercise: buffering the effect of chronic stress on telomere length. PLoS One. Article PubMed PubMed Central CAS Google Scholar. Gordon B, Chen S, Durstine JL. The effects of exercise training on the traditional lipid profile and beyond. Curr Sport Med Rep. Roque FR, Hernanz R, Salaices M. Exercise training and cardiometabolic diseases: focus on the vascular system. Curr Hypertens Rep. Article CAS PubMed Google Scholar. Pingitore A, Pace Pereira Lima G, Mastorci F, Quinones A, Iervasi G, Vassalle C. Exercise and oxidative stress: potential effects of antioxidant dietary strategies in sports. Devasagayam TPA, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, Lele RD. Free radicals and antioxidants in human health: currant status and future prospects. J Assoc Physicians India. CAS PubMed Google Scholar. Braakhuis A, Hopkins WG. Impact of dietary antioxidants on sport performance: a review. Pereira Panza V-S, Diefenthaeler F, da Silva EL. Benefits of dietary phytochemical supplementation on eccentric exercise-induced muscle damage: is including antioxidants enough? Barranco-Ruiz Y, Aragón-Vela J, Casals C, Martínez-Amat A, Casuso RA, Huertas JR. Control of antioxidant supplementation through interview is not appropriate in oxidative-stress sport studies: analytical confirmation should be required. Nieman DC, Gillitt ND, Knab AM, Shanely RA, Pappan KL, Jin F, et al. Influence of a polyphenol-enriched protein powder on exercise-induced inflammation and oxidative stress in athletes: a randomized trial using a metabolomics approach. Nieman DC, Stear SJ, Castell LM, Burke LM. A-Z of nutritional supplements: dietary supplements, sports nutrition foods and ergogenic aids for health and performance: part Br J Sport Med. Article CAS Google Scholar. Bjørklund G, Chirumbolo S. Role of oxidative stress and antioxidants in daily nutrition and human health. Article PubMed CAS Google Scholar. Fruit: world production by type Statista [Internet]. International Organisation of Vine and Wine. García-Flores LA, Medina S, Gómez C, Wheelock CE, Cejuela R, Martínez-Sanz JM, et al. Aronia-citrus juice polyphenol-rich juice intake and elite triathlon training: a lipidomic approach using representative oxylipins in urine. Food Funct. Article PubMed Google Scholar. Sureda A, Tejada S, Bibiloni MM, Tur JA, Pons A. Polyphenols: well beyond the antioxidant capacity: polyphenol supplementation and exercise-induced oxidative stress and inflammation. Curr Pharm Biotechnol. De Ferrars RM, Czank C, Zhangm Q, Botting NP, Kroon PA, Cassidy A, et al. The pharmacokinetics of anthocyanins and their metabolites in humans. Br J Pharmacol. Czank C, Cassidy A, Zhang Q, Morrison DJ, Preston T, Kroon PA, et al. Human metabolism and elimination of the anthocyanin, cyanidingglucoside: a 13C-tracer study. Am J Clin Nutr. Williamson G, Clifford MN. Colonic metabolites of berry polyphenols: the missing link to biological activity? Br J Nutr. Common phenolic metabolites of flavonoids, but not their unmetabolized precursors, reduce the secretion of vascular cellular adhesion molecules by human endothelial cells. J Nutr. Article CAS PubMed PubMed Central Google Scholar. Kay CD. Rethinking paradigms for studying mechanisms of action of plant bioactives. Nutr Bull. Nieman DC, Mitmesser SH. Potential impact of nutrition on immune system recovery from heavy exertion: a metabolomics perspective. Nieman DC, Shanely RA, Gillit ND, Pappan KL, Lila MA. Serum metabolic signatures induced by a three-day intensified exercise period persist after 14 h of recovery in runners. J Proteome Res. Mach N, Fuster-Botella D. Endurance exercise and gut microbiota: A review. J Sport Heal Sci. Kerksick CM, Wilborn CD, Roberts MD, Smith-Ryan A, Kleiner SM, Jäger R, et al. J Int Soc Sports Nutr. Shankar K, Mehendale HM. Oxidative Stress. In: Wexler P, editor. Encyclopedia of Toxicology. Third Edit. Elsevier; Lehmann R, Zhao X, Weigert C, Simon P, Fehrenbach E, Fritsche J, et al. Medium chain Acylcarnitines dominate the metabolite pattern in humans under moderate intensity exercise and support lipid oxidation. Lewis GD, Farrell L, Wood MJ, Martinovic M, Arany Z, Rowe GC, et al. Metabolic Signatures of Exercise in Human Plasma. Sci Transl Med. Nieman DC, Gillitt ND, Sha W, Meaney MP, John C, Pappan KL, et al. Metabolomics-based analysis of banana and pear ingestion on exercise performance and recovery. Nieman DC, Gillitt ND, Henson DA, Wei Sha R, Andrew Shanely AM, Knab LC-K, et al. Bananas as an energy source during exercise: a metabolomics approach. Nieman DC, Scherr J, Luo B, Meaney MP, Dréau D, Sha W, et al. Influence of pistachios on performance and exercise-induced inflammation, oxidative stress, immune dysfunction, and metabolite shifts in cyclists: a randomized, crossover trial. Nieman DC, Sha W, Pappan KL. IL-6 linkage to exercise-induced shifts in lipid-related metabolites: A metabolomics-based analysis. Nieman DC, Shanely RA, Luo B, Meaney MP, Dew DA, Pappan KL. Metabolomics approach to assessing plasma and 9-hydroxy-octadecadienoic acid and linoleic acid metabolite responses to km cycling. Phys Act Inact. CAS Google Scholar. Powers SK, Radak Z, Ji LL. Exercise-induced oxidative stress: past, present and future. J Physiol. Dillard CJ, Litov RE, Savin WM, Dumelin EE, Tappel AL. Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. J Appl Physiol. Brady PS, Brady LJ, Ullrey DE. Selenium, vitamin E and the response to swimming stress in the rat. Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. McClung J, Deruisseau K, Whidden M, Van Remmen H, Richardson A, Song W, et al. Overexpression of antioxidant enzymes in diaphragm muscle does not alter contraction-induced fatigue or recovery. Exp Physiol. McClung JM, Judge AR, Powers SK, Yan Z. p38 MAPK links oxidative stress to autophagy-related gene expression in cachectic muscle wasting. Am J Physiol Cell Physiol. Powers SK, Duarte J, Kavazis AN, Talbert EE. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Droge W. Free radicals in the physiological control of cell function. Miller DM, Buettner GR, Aust SD. Free Radic Biol Med. Bogdan C, Rollinghoff M, Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr Opin Immunol. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. McLeay Y, Stannard S, Houltham S, Starck C. Dietary thiols in exercise: oxidative stress defence, exercise performance, and adaptation. J Int Soc Sports Nutr ;14 1 :1—8. Mattson MP. Hormesis Defined. Ageing Res Rev. Silveira LR, Pilegaard H, Kusuhara K, Curi R, Hellsten Y. The contraction induced increase in gene expression of peroxisome proliferator-activated receptor PPAR -gamma coactivator 1 alpha PGC-1alpha , mitochondrial uncoupling protein 3 UCP3 and hexokinase II HKII in primary rat skeletal muscle cells is dep. Biochim Biophys Acta Zhou LZ, Johnson AP, Rando TA. NF kappa B and AP-1 mediate transcriptional responses to oxidative stress in skeletal muscle cells. Handayaningsih A-E, Iguchi G, Fukuoka H, Nishizawa H, Takahashi M, Yamamoto M, et al. Reactive oxygen species play an essential role in IGF-I signaling and IGF-I-induced myocyte hypertrophy in C2C12 myocytes. Balon TW, Nadler JL. Evidence that nitric oxide increases glucose transport in skeletal muscle. Steinbacher P, Eckl P. Impact of oxidative stress on exercising skeletal muscle. Marrocco I, Altieri F, Peluso I. Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxidative Med Cell Longev. Merry TL, Mi R. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? Ranchordas MK, Dawson JT, Russell M. Practical nutritional recovery strategies for elite soccer players when limited time separates repeated matches. Yfanti C, Deli CK, Georgakouli K, Fatouros I, Jamurtas AZ. Sport nutrition, redox homeostasis and toxicity in sport performance. Curr Opin Toxicol. Google Scholar. Finaud J, Lac G, Filaire E. Oxidative stress. Rousseau I, Margaritis AS. Does physical exercise modify antioxidant requirements? Nutr Res Rev. Powers SK, Lennon SL. Analysis of cellular responses to free radicals: focus on exercise and skeletal muscle. Proc Nutr Soc. |
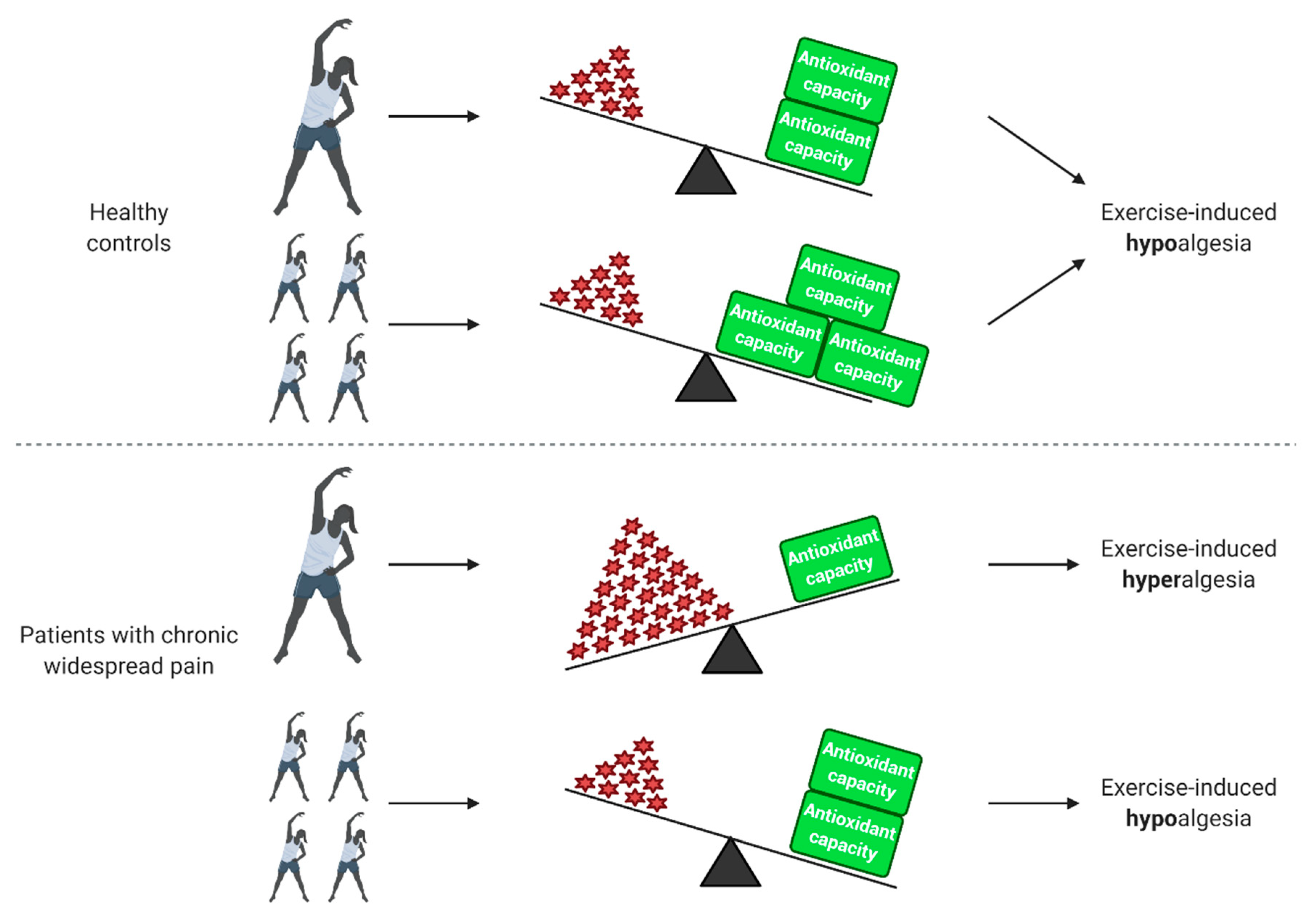
Kaum kann ich jenem glauben.
ohne Varianten....
Sie hat die bemerkenswerte Idee besucht
und etwas ähnlich ist?