
Energy balance and macronutrient distribution -
Consequently, individuals who consume a high-protein diet in combination with energy-restriction are more satiated and potentially less likely to consume additional calories from foods extraneous to dietary prescription From short-term experiments we conclude that relatively high-protein diets have the potential to maintain a negative energy balance by sustaining satiety at the level of the original diet 9 , This strong satiety effect depends partly on the type of dietary protein, and is elicited by a mixture of gut-brain axis effects, such as anorexigenic gut hormones, digestion, amino-acids, ketogenesis, and the increase in diet-induced thermogenesis.
Gluconeogenesis did not show a relation with satiety Although dietary protein-induced satiety affects energy intake, it may be dominated by reward-driven eating behavior 20 , 31 , 55 — Several brain areas that are involved in food reward link high-protein intake with reduced food wanting and thereby act as a mechanism involved in the reduced energy intake following high protein intake 20 , 31 , 55 — A mechanism through which protein acts on brain reward centers involves direct effects of certain amino acids as precursors of the neuropeptides serotonin and dopamine 31 , A high-protein, low-carbohydrate breakfast vs.
a medium-protein, high-carbohydrate breakfast led to reduced reward-related activation in the hippocampus and parahippocampus before dinner 20 , Furthermore, acute food-choice compensation changed the macronutrient composition of a subsequent meal to offset the protein intervention A compensatory increase in carbohydrate intake was related to a decrease in liking and task-related signaling in the hypothalamus after a high-protein breakfast.
After a lower-protein breakfast, an increase in wanting and task-related signaling in the hypothalamus was related to a relative increase in protein intake in a subsequent meal Protein intake may directly affect the rewarding value of this macronutrient 56 , Thus limited protein-induced food reward may affect compliance to a long-term protein-diet.
With respect to dietary protein-induced energy expenditure, short-term effects of energy-balanced high-protein diets showed higher rates of energy expenditure, especially diet-induced thermogenesis DIT 59 , Mechanisms encompass the ATP required for the initial steps of metabolism, such as protein breakdown, synthesis and storage, and oxidation including urea synthesis.
Also gluconeogenesis may take place. Protein storage capacity of the body is limited. Therefore readily metabolic processing is necessary. Significantly higher dietary protein induced DIT 59 , subsequently Sleeping Metabolic Rate SMR and Basal Metabolic Rate BMR 12 , 60 was shown in 36 h respiration chamber studies, in comparison to iso-energetic, iso-volumetric, dietary carbohydrate, or fat, composed of normal food items and matched organoleptic properties.
Short-term protein- induced increase in DIT is explained by the ATP required for the initial steps of metabolism and oxidation including urea synthesis, while subsequent protein induced increase of SMR is explained by stimulation of protein synthesis and protein turnover.
The metabolic efficacy of protein oxidation largely depends on the amino acid composition of the protein A well-balanced amino acid mixture produces a higher thermogenic response than does an amino acid mixture with a lower biological value, explaining why intake of plant proteins or incomplete proteins results in less protein synthesis than does intake of animal protein.
This relative metabolic inefficiency contributes to the higher diet-induced energy expenditure of a high protein meal, which, in turn, has shown to be related to subjective feelings of satiety Gluconeogenesis, as a result of further postprandial amino-acid metabolism also contributes to the protein induced energy expenditure.
De novo synthesis of glucose in the liver from gluconeogenic precursors including amino acids is stimulated by a high protein diet in the fed state 64 , 65 , and is an alternative biochemical pathway to cope with postprandial amino acid excess When the protein content of the diet is increased, Phosphoenolpyruvate Carboxylase PEPCK that catalyzes the initial conversion of oxaloacetate to phosphoenolpyruvate is up-regulated either in the fasted and in the fed state, whereas glucose 6-phosphatase G6Pase , that catalyzes the last step of gluconeogenesis is up-regulated in the fasted state and down-regulated in the fed state Although hepatic glycogen stores as well as hepatic gluconeogenesis have been suggested to play a role in the regulation of satiety 67 , 68 , this was not confirmed by a study by Veldhorst et al.
Also protein turnover contributes to the high energetic costs of protein metabolism, and protein synthesis. It is high in children, and decreases with older age. Increasing protein intake increases protein turnover by increasing protein synthesis and protein breakdown, and does not necessarily affect protein balance 69 , Rapidly digested dietary protein results in a stronger increase in postprandial protein synthesis and amino acid oxidation than slowly digested protein 39 , 40 , Acutely, high protein intake stimulates protein synthesis and turnover, and induces a small suppression of protein breakdown 72 — Prolonged low protein intake may lead to muscle loss due to the lack of precursor amino acid availability for de novo muscle protein synthesis 75 , Hursel et al.
low-protein diet, with significant increases in protein synthesis, protein breakdown, and protein oxidation. Notably, in the fasted state net protein balance was less negative after the low-protein diet compared with the high-protein diet, while in the fed state, protein balance was positive with the high-protein diet, and negative with the low-protein diet Thus protein turnover in the fasted state needs to be distinguished from that in the fed state.
The role of protein synthesis and protein breakdown in FFM accretion was discussed by Deutz and Wolfe 77 , and Symons et al. The observed maximum response of protein synthesis after a single serving of 20—30 g of dietary protein suggests that additional effects of protein intake on FFM accretion are accounted for by the inhibition of protein breakdown.
However, a beneficial reduction of protein breakdown only occurs with acute ingestion of protein 70 , 76 , 78 — The positive protein balance observed at a high-protein diet is due to acute postprandial responses, rather than to the postabsorptive state. Consumption of a low-protein diet for 12 weeks was not detrimental to young healthy individuals who might have the ability to adapt acutely to this condition The Adaptive Demands model developed by Millward may provide an explanation for the observation that the human body is able to show physiological adaptations to changes in protein intake The model proposes that the metabolic demand for amino acids comprises a fixed component and a variable adaptive component Short-term changes in protein intake are likely within the adaptive range.
Adaptations in protein and amino acid metabolism to changes in protein intake largely occur via changes in whole-body protein turnover and amino acid oxidation Changes in amino acid oxidation were reflected as decreased and increased nitrogen excretion in response to the low- and high-protein diets respectively.
The activity of enzymes that regulate: 1 transamination, 2 the disposal of the carbon skeletons in intermediary metabolism, and 3 the disposal of nitrogen through the urea cycle increased in response to high protein intake 83 , Nevertheless, a positive nitrogen balance following high protein intake 69 , 82 , 85 , 86 does not automatically reflect an increase in protein anabolism The capacity of the body to increase amino acid anabolism through an increase in lean body mass is limited Only interventions using diets high in specific indispensable amino acids, such as leucine, might be able to stimulate protein synthesis in specific target groups 73 , Therefore, transient retention or loss of body nitrogen because of a labile pool of body nitrogen may contribute to adaptations in amino acid metabolism in response to changes in protein intake Transient adaptive mechanisms may be distinguished from mechanisms that maintain homeostasis in the body in the longer-term.
Most long-term studies comparing energy-restricted diets with a relatively high protein content and diets with a normal protein content, within a large range of fat contents, showed independent effects of a high protein intake on body weight reduction 7 , 90 — 93 , 93 — , while in other studies the opposite has been observed — From these studies, a larger reduction in fat mass following relatively high-protein diets was reported by Wycherly et al.
An energy-restricted high-protein diet in combination with exercise can even increase muscle mass The main reason behind the differences in outcomes of the studies cited, is the difference in dosage of dietary protein If the control, implying an adequate protein intake is sufficiently high, i.
In case the relatively high protein diet is higher than 1. Conclusions from long-term studies comparing relatively high-protein with normal-protein diets differ from those testing relatively high-protein and low-protein diets 5.
In the following studies compliance was monitored and confirmed with a quantitative biomarker, such as urinary nitrogen 5 , 7 , During energy restriction, sustaining protein intake at the level of the minimal requirement 0.
An additional increase of protein intake may not induce a larger loss of body weight, but can be effective to maintain a larger amount of FFM 7 , 91 , and limits the reduction of energy expenditure through sparing of FFM 91 , For example, a 6-month energy restricted diet with a daily protein intake just above the minimal requirement 0.
Interestingly, a protein intake of 1. Dietary protein intake below requirements could lead to less weight loss and a higher risk for body weight regain 6.
However, a study by Soenen et al. demonstrated that the effects of a relatively high protein intake on body weight loss and weight maintenance were present independent of a low carbohydrate intake 90 , and that low carbohydrate alone, without high protein did not trigger the described effects.
Protein diets could have resulted in stronger effects with respect to body weight management, if compliance would have been larger see section Short-term Dietary Protein-Induced Reward Homeostasis. To counteract poor compliance, dietary restraint is necessary In several long term clinical trials with dietary protein, cognitive dietary restraint had increased, implying greater conscious control over food intake 68 , 90 , Post hoc analysis of those data shows that the change in the cognitive dietary restraint score was inversely related to the change in body weight.
Dietary restraint is associated with brain signaling for reward, indicating a greater control over food intake and implying a greater control over reward as well In general dietary restraint is associated with long-term weight maintenance , Taken together, Clifton et al. Older studies, in the perspective of composing meal replacers to be used as energy restricted diets showed strong energy restriction effects on body composition, in relation to the percentage from dietary protein.
Similarly, during weight maintenance following weight loss, FFM was preserved, while FM was reduced. Since weight maintenance after weight loss usually implies a slight weight regain, Stock's model can be applied The latter metabolic inefficiency is related to body composition.
This metabolic inefficiency, partly due to sparing FFM promotes dietary protein induced weight maintenance. In addition, preserving FFM, being the main determinant of basal energy expenditure, limits a possible reduction in energy expenditure during weight maintenance.
Whitehead et al. showed that during energy-restriction, the decline in total energy expenditure and SMR as a result of body weight loss is less on a high-protein than on a medium-protein diet Even an increase in FFM during a high-protein diet in negative energy balance has been observed , although these changes may partly be ascribed to a high protein intake combined with physical activity.
If the protein-induced effects on appetite and energy expenditure observed during energy restriction also hold under non-restricted conditions, then, increasing protein intake with a usual diet may prevent overweight and obesity. In this controlled situation, participants were able to sustain the high- and low-protein diets.
The low-protein diet facilitated the development of positive energy balance, while the high-protein diet was beneficial to prevent this Correspondingly, small increases in fullness and satiety ratings were observed as acute responses to a high-protein diet in neutral energy balance In this situation, translation into large changes in energy intake was not possible, because subjects had to maintain their body weight.
In the longer term, appetite ratings were returned to the level of the original diet, which suggests that the human body habituates to the satiating effects of high protein intake FFM showed small increases and decreases after a week intervention with high-protein and low-protein diets in energy balance As a consequence, SMR, DIT, and total energy expenditure was maintained at the high-protein diet, while it was significantly decreased at the low protein diet.
Thus, at a constant body weight, a high-protein diet may protect against the development of a positive energy balance. The consumption of a low-protein diet may increase the risk for the development of a positive energy balance through adaptive thermogenesis Humans with overweight or obesity may show co-morbidities, such as a nonalcoholic fatty liver disease, type 2 diabetes, or cardiovascular diseases.
Whether a high-protein diet may be protective against these co-morbidities, independent of, or in addition to effects of weight loss is still under debate. In general, weight loss improves metabolic function 6 , 7 , yet a high protein intake may modulate intrahepatic triglyceride IHTG content as well — In short-term studies, protein supplementation was shown to be associated with reduced hepatic fat — High ectopic lipid content, especially IHTG content, and not visceral adipose tissue VAT volume, is an independent risk factor for these metabolic disturbances — A week intervention study showed that effects of high- and low-protein diets on IHTG content in weight-stable individuals tended to lower IHTG content after the high protein-low carbohydrate diet compared with the low protein-high carbohydrate diet This suggests that high protein-low carbohydrate diets may be favorable for the control of IHTG in healthy humans.
High protein intake stimulates hepatic lipid oxidation due to the high energetic demand for amino acid catabolism and ketogenesis 5 , Furthermore, hepatic lipid oxidation may be stimulated by an increased bile acid production, a process that may also inhibit lipogenesis Protein-induced glucagon secretion inhibits de novo lipogenesis and stimulates hepatic ketogenesis , High protein intake may blunt the increase of very low density lipoprotein VLDL -TG concentrations induced by carbohydrate intake — High VLDL-TG concentrations may increase hepatic TG, and subsequently IHTG content The observed trend for a difference in IHTG content between the diets likely is the result of combined effects involving changes in protein and carbohydrate intake.
Relevant diets possibly contributing to the management of type 2 diabetes are low-carbohydrate diets. Those diets often are high-protein diets. A recent systematic review explored the interpretation and effectiveness of a low-carbohydrate diet in the management of type 2 diabetes They suggest that low-carbohydrate diets may improve HbA1c, HDL cholesterol, and triglyceride levels.
The meta-analyses confirmed statistically significant superiority of the low-carbohydrate intervention arm in improving HbA1c, HDL cholesterol, triglyceride, and systolic blood pressure levels at 1 year.
Reducing carbohydrate intake demonstrated a strong superiority over control diets in reducing diabetes medication, which may have diminished the observed effects of a reduced-carbohydrate intake on HbA1c.
This review concludes that reducing carbohydrate intake may promote favorable health outcomes in the management of type 2 diabetes in the context of a healthy diet The relation between high-protein intake and type 2 diabetes is still under debate, and results differ depending on study duration and source of protein.
Short-term studies have reported favorable effects on glucose homeostasis 21 , , , while an epidemiological and a long-term studies reported an increased risk for type 2 diabetes with increased protein intake — The increased risk may be dependent on the source of protein. Tian et al.
conducted a systematic review and meta-analysis of cohort studies to investigate the association between protein consumption and the risk for type 2 diabetes In this review, they reported an increased relative risk of type 2 diabetes for total protein and animal protein in men and women and a reduced relative risk for plant protein in women.
However, high-protein diets may have some risk regarding insulin sensitivity. An increase in Branched-Chain Amino Acids BCAAs seems to be a marker of type 2 diabetes , Newgard et al. observed in rodents that in the context of a dietary pattern that includes high fat consumption BCAA contributes to the development of obesity-associated insulin resistance.
Moreover, Pedersen et al. Taken together, when protein diets are applied during energy restriction aiming at weight loss and subsequent weight maintenance, the latter usually shows favorable effects in relation to insulin sensitivity, although some risks may be present.
That a higher protein diet would promote insulin sensitivity beyond its effect on body-weight loss and subsequent body-weight maintenance seems unlikely. Parameters that indicate cardiovascular risks usually change in a favorable direction during body weight loss. The question remains whether the type of diet, especially a protein diet, would affect favorable changes in cardiovascular parameters.
Atherosclerosis lies at the root of cardiovascular complications, and the main indicators are the HDL- and LDL cholesterol. Certain proteins may exert a greater effect on blood cholesterol levels than other Possible different effects from vegetable vs. animal proteins have been tested.
Sacks et al. They did not observe significant differences in LDL or HDL cholesterol, neither between lipid profiles or lipid proteins. Other studies, comparing casein and soy diets, did find significant reductions in LDL with the soy diet, compared to the casein diets , , however this did not appear in volunteers with already high cholesterol concentrations In long term weight loss and subsequent weight maintenance studies, it was shown that individuals consuming soy meal replacements showed favorable effects in their cardiovascular profile, e.
With respect to blood pressure, a study by Teunissen-Beekman et al. They concluded that lower postprandial blood pressure is not necessarily accompanied by higher NOx, insulin, glucagon or GLP-1 responses, and that dietary protein, especially egg-white protein, may induce a risk for elevated blood pressure Yet, it has been reported that effects of dietary protein depend on age.
Tielemans et al. A critical evaluation of the evidence for the effects of milk proteins and their associated peptides on blood pressure and vascular dysfunction, showed that results are inconclusive, while one study clearly reported that main intact milk proteins reduced blood pressure, and whey protein improved measures of arterial stiffness Some epidemiological studies based upon large community cohorts report no overall relationship between protein type and dietary protein sources on coronary heart diseases , while another epidemiological study indicated that high red meat intake increases risk for coronary heart disease and stroke, and that poultry, fish, and nuts reduced these risks , A general systematic review on health effects of protein intake in healthy adults reported that results are inconclusive for a relationship between protein intake and cardiovascular diseases , while a recent systematic review concluded that low-carbohydrate diets, that often are high-protein diets may improve HDL cholesterol and triglyceride levels and systolic blood pressure levels at 1 year Taken together, more accurately designed randomized control trials on dietary protein quality and quantity and possible relations with cardiovascular risks are required.
There is a long-held view that high-protein intake might interfere with calcium homeostasis by increasing the acid load. It is hypothesized that this could be partially buffered by bone, subsequently resulting in bone resorption and hypercalciuria In general, protein is a necessary nutrient for bone health Nitrogen intake seems to have a positive effect on calcium balance and consequent preservation of bone mineral content With respect to renal issues, only patients with pre-existing dysfunction appeared to have an increased risk for the development of kidney stones and renal diseases In addition, Jesudason et al.
They found no indication of impaired kidney function after 1 year with a higher protein intake in pre-diabetic older adults. In the elderly, beneficial health effects of higher-protein intake might outweigh the adverse effects possibly because of the changes in protein metabolism with aging.
In contrast, persistent total protein and amino acid intake below requirements impairs bodily functions leading to higher disease and mortality risks across the lifespan , Taken together, application of relatively high-protein diets, whereby protein intake is sustained at the original level, does not seem to have any adverse effects in healthy individuals.
Although no clear recommendation exists that defines the safe upper limit of protein intake, consumption of up to 1. This means that sustaining or slightly increasing protein intake during energy restriction likely poses no adverse effects in healthy individuals.
However, protein intake can exceed the suggested safe upper limit. The question arises whether and how and over which time-frame these high intakes of protein would negatively affect health. Recent studies applying medium-term, high-protein interventions in neutral or positive energy balance did not report any adverse effects , However, the limits of adaptation to high protein intake over the longer term remain to be investigated.
During energy restriction, sustaining protein intake at the level of requirement appears to be sufficient to aid body weight loss and fat loss Figure 1.
An additional increase of protein intake does not induce a larger loss of body weight, but can be effective to maintain a larger amount of FFM Figure 1.
Protein induced satiety is likely a combined expression with direct and indirect effects of elevated plasma amino acid and anorexigenic hormone concentrations, increased DIT, and a ketogenic state, which all feed-back on the central nervous system Figure 1.
Changes in appetite appear most clearly as short-term response to changes in dietary protein content; the human body may habituate to the satiating effects of protein intake in the longer-term. The decline in energy expenditure and sleeping metabolic rate as a result of body weight loss is less on a high-protein than on a normal-protein diet.
In addition, higher rates of energy expenditure have been observed as acute responses to energy-balanced high-protein diets Figure 1. Furthermore, high protein, low carbohydrate diets may be favorable for the prevention of metabolic disturbances.
During positive energy balance, excess energy intake alone may account for the increase in fat mass. Increases in energy expenditure and FFM may largely be predicted by protein intake. Figure 1. Summary of the observations on relatively high protein diets applied during energy restriction or weight maintenance WM thereafter.
EB, energy balance; T2D, type 2 Diabetes; NAFLD, non-alcoholic fatty liver disease; CV, cardiovascular diseases. High protein-low carbohydrate diets may be favorable for the control of IHTG in healthy humans, likely as a result of combined effects involving changes in protein and carbohydrate intake.
When protein diets are applied during energy restriction aiming at weight loss and subsequent weight maintenance, the latter usually shows favorable effects in relation to insulin sensitivity, although some risks may be present.
At least high-protein diets do not seem to have adverse effects on these co-morbidities. In conclusion, higher-protein diets may reduce overweight and obesity, yet whether high-protein diets, beyond their effect on body-weight management, contribute to prevention of increases in NAFLD, type 2 diabetes and cardiovascular diseases is inconclusive Figure 1.
The sections of the manuscript were written by MD, LT, BG-C, TA, and MW-P. The review is partly an update of Westerterp-Plantenga et al. MD salary is funded by EU-FP7-nr.
The other authors' salaries are paid by Maastricht University, The Netherlands and Universite de Bordeaux, France. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
World Health Organization. Obesity and Overweight. Fact Sheet N° Version Current March Abete I, Astrup A, Martinez JA, Thorsdottir I Zulet MA.
Obesity and the metabolic syndrome: role of different dietary macronutrient distribution patterns and specific nutritional components on weight loss and maintenance.
doi: PubMed Abstract CrossRef Full Text Google Scholar. Westerterp KR. Energy Balance in Motion. Google Scholar. Westerterp-Plantenga MS, Nieuwenhuizen A, Tome D, Soenen S, Westerterp KR. Dietary protein, weight loss, and weight maintenance.
Annu Rev Nutr. Westerterp-Plantenga MS, Lemmens SG, Westerterp KR. Dietary protein - its role in satiety, energetics, weight loss and health. Br J Nutr. Acheson KJ. Diets for body weight control and health: the potential of changing the macronutrient composition.
Eur J Clin Nutr. Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials.
Am J Clin Nutr. Leidy HJ, Clifton PM, Astrup A, Wycherley TP, Westerterp-Plantenga MS, Luscombe-Marsh ND, et al. The role of protein in weight loss and maintenance. Symposium Am J Clin Nutr.
Westerterp-Plantenga MS, Luscombe-Marsh N, Lejeune MPGM, Diepvens K, Nieuwenhuizen A, Engelen MPKJ, et al. Dietary protein, metabolism, and body-weight regulation: dose-response effects.
Int J Obesity S16— CrossRef Full Text Google Scholar. Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr. Bendtsen LQ, Lorenzen JK, Bendsen NT, Rasmussen C Astrup A.
Effect of dairy proteins on appetite, energy expenditure, body weight, and composition: a review of the evidence from controlled clinical trials. Lejeune MP, Westerterp KR, Adam TC, Luscombe-Marsh ND, Westerterp-Plantenga MS.
Ghrelin and glucagon-like peptide 1 concentrations, h satiety, and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber.
Veldhorst MAB, Nieuwenhuizen AG, Hochstenbach-Waelen A, Westerterp KR, Engelen MPKJ, Brummer RJM, et al. Eur J Nutr — Leidy HJ, Racki EM. Int J Obes Lond. Weigle DS, Breen PA, Matthys CC, Callahan HS, Meeuws KE, Burden VR, et al. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations.
Martens EA, Lemmens SG, Westerterp-Plantenga MS. Protein leverage affects energy intake of high-protein diets in humans. Martens EA, Gatta-Cherifi B, Gonnissen HK, Westerterp-Plantenga MS. The potential of a high protein-low carbohydrate diet to preserve intrahepatic triglyceride content in healthy humans.
Plos ONE 9:e Morrison CD, Reed SD, Henagan TM. Homeostatic regulation of protein intake: in search of a mechanism. Am J Physiol Regul Integr Comp Physiol.
Fromentin G, Darcel N, Chaumontet C, Marsset-Baglieri A, Nadkarni N, Tome D. Peripheral and central mechanisms involved in the control of food intake by dietary amino acids and proteins.
Nutr Res Rev. Acheson KJ A, Blondel-Lubrano S, Oguey-Araymon M, Beaumont S, Emady-Azar C, Ammon-Zufferey I, et al. Protein choices targeting thermogenesis and metabolism. Hall WL, Millward DJ, Long SJ, Morgan LM. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite.
Veldhorst MA, Nieuwenhuizen AG, Hochstenbach-Waelen A, van Vught AJ, Westerterp KR, Engelen M, et al. Let us begin with the basics on how to estimate energy intake, energy requirement, and energy output.
Then we will consider the other factors that play a role in maintaining energy balance and hence, body weight. To maintain body weight you have to balance the calories obtained from food and beverages with the calories expended every day.
Here, we will discuss how to calculate your energy needs in kilocalories per day so that you can determine whether your caloric intake falls short, meets, or exceeds your energy needs. The Institute of Medicine has devised a formula for calculating your Estimated Energy Requirement EER.
It takes into account your age, sex, weight, height, and physical activity level PA. It is calculated via the following formulas:. Note: to convert pounds to kilograms, divide weight in pounds by 2. To convert feet to meters, divide height in feet by 3. The numbers within the equations for the EER were derived from measurements taken from a group of people of the same sex and age with similar body size and physical activity level.
These standardized formulas are then applied to individuals whose measurements have not been taken, but who have similar characteristics in order to estimate their energy requirements.
EER values are different for children, pregnant or lactating women, and for overweight and obese people. Also, remember the EER is calculated based on weight maintenance, not for weight loss or weight gain. Source: US Department of Agriculture.
Source: Institute of Medicine. Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. September 5, The amount of energy you expend every day includes not only the calories you burn during physical activity, but also the calories you burn while at rest basal metabolism , and the calories you burn when you digest food.
The sum of caloric expenditure is referred to as total energy expenditure TEE. breathing, heartbeat, liver and kidney function while at rest. The basal metabolic rate BMR is the amount of energy required by the body to conduct its basic functions over a certain time period.
Unfortunately, you cannot tell your liver to ramp up its activity level to expend more energy so you can lose weight. BMR is dependent on body size, body composition, sex, age, nutritional status, and genetics.
People with a larger frame size have a higher BMR simply because they have more mass. Muscle tissue burns more calories than fat tissue even while at rest and thus the more muscle mass a person has, the higher their BMR.
As we get older muscle mass declines and thus so does BMR. Nutritional status also affects basal metabolism. Caloric restriction, as occurs while dieting, for example, causes a decline in BMR. This is because the body attempts to maintain homeostasis and will adapt by slowing down its basic functions to offset the decrease in energy intake.
Body temperature and thyroid hormone levels are additional determinants of BMR. The other energy required during the day is for physical activity. Depending on lifestyle, the energy required for this ranges between 15 and 30 percent of total energy expended.
The main control a person has over TEE is to increase physical activity. Calculating TEE can be tedious, but has been made easier as there are now calculators available on the Web. TEE is dependent on age, sex, height, weight, and physical activity level. The equations are based on standardized formulas produced from actual measurements on groups of people with similar characteristics.
To get accurate results from web-based TEE calculators, it is necessary to record your daily activities and the time spent performing them. Interactive com offers an interactive TEE calculator. In the last few decades scientific studies have revealed that how much we eat and what we eat is controlled not only by our own desires, but also is regulated physiologically and influenced by genetics.
The hypothalamus in the brain is the main control point of appetite. It receives hormonal and neural signals, which determine if you feel hungry or full.
Hunger is an unpleasant sensation of feeling empty that is communicated to the brain by both mechanical and chemical signals from the periphery. Conversely, satiety is the sensation of feeling full and it also is determined by mechanical and chemical signals relayed from the periphery.
This results in the conscious feeling of the need to eat. Alternatively, after you eat a meal the stomach stretches and sends a neural signal to the brain stimulating the sensation of satiety and relaying the message to stop eating. The stomach also sends out certain hormones when it is full and others when it is empty.
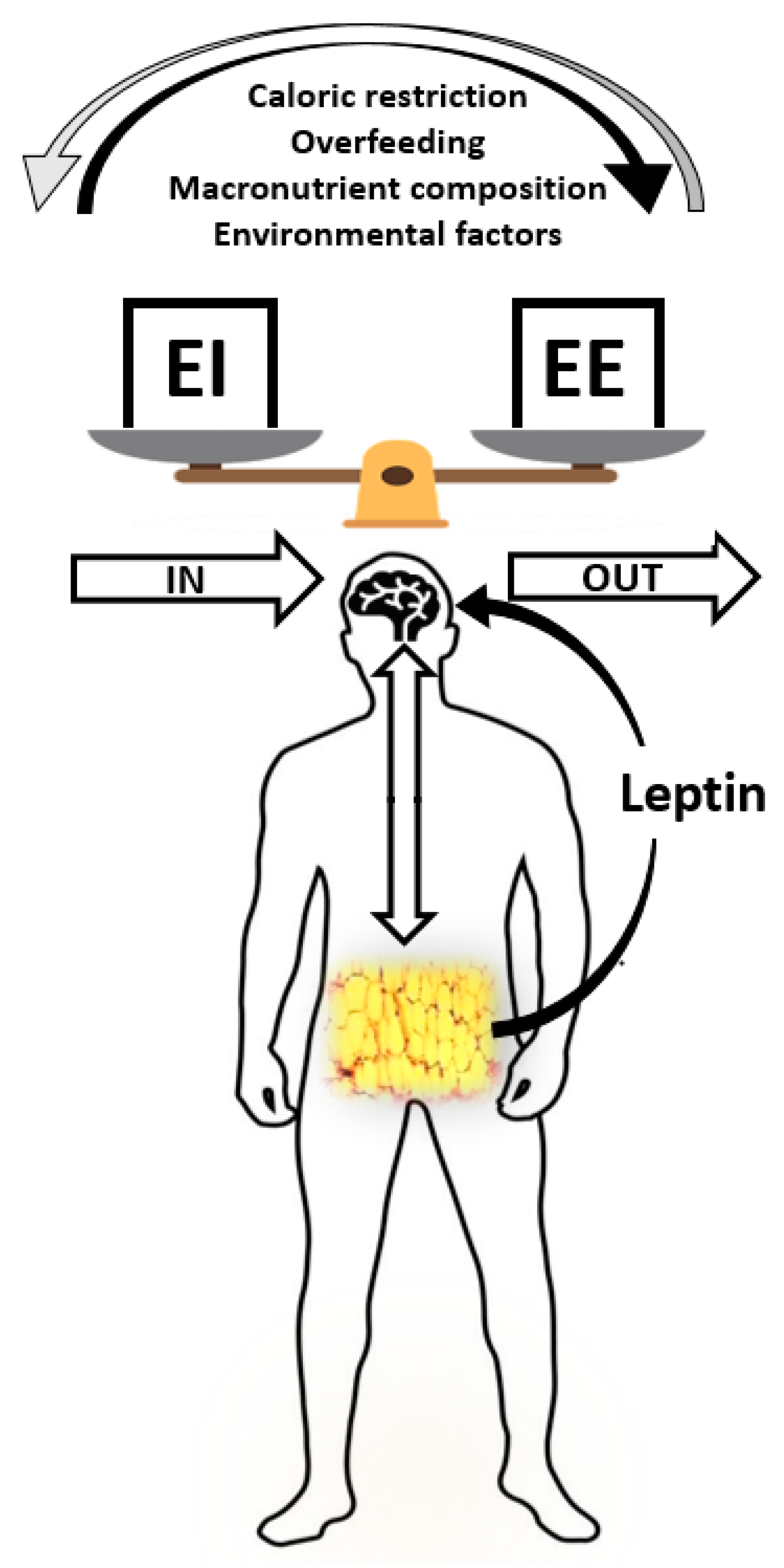
These hormones communicate to the hypothalamus and other areas of the brain either to stop eating or to find some food. Fat tissue also plays a role in regulating food intake. Fat tissue produces the hormone leptin, which communicates to the satiety center in the hypothalamus that the body is in positive energy balance.
Alas, this is not the case. In several clinical trials it was found that people who are overweight or obese are actually resistant to the hormone, meaning their brain does not respond as well to it.
Dardeno, T. et al. Therefore, when you administer leptin to an overweight or obese person there is no sustained effect on food intake. The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity.
Rosenstock J, Hanefeld M, Shamanna P, Min KW, Boka G, Miossec P, et al. Beneficial effects of once-daily lixisenatide on overall and postprandial glycemic levels without significant excess of hypoglycemia in type 2 diabetes inadequately controlled on a sulfonylurea with or without metformin GetGoal-S.
J Diabetes Complications. van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults.
Int J Obes. Makimura H, Stanley TL, Suresh C, De Sousa-Coelho AL, Frontera WR, Syu S, et al. Metabolic effects of long-term reduction in free fatty acids with acipimox in obesity: a randomized trial.
Fery F, Plat L, Baleriaux M, Balasse EO. Inhibition of lipolysis stimulates whole body glucose production and disposal in normal postabsorptive subjects. Friedman MI, Harris RB, Ji H, Ramirez I, Tordoff MG. Fatty acid oxidation affects food intake by altering hepatic energy status.
Friedman MI, Tordoff MG. Fatty acid oxidation and glucose utilization interact to control food intake in rats. Horn CC, Ji H, Friedman MI. Etomoxir, a fatty acid oxidation inhibitor, increases food intake and reduces hepatic energy status in rats.
Kahler A, Zimmermann M, Langhans W. Suppression of hepatic fatty acid oxidation and food intake in men. Nutrition ;— Leonhardt M, Langhans W.
Fatty acid oxidation and control of food intake. Swithers SE, McCurley M, Scheibler A, Doerflinger A. Differential effects of lipoprivation and food deprivation on chow and milk intake in and day-old rats. Anderson JW, Patterson K.
J Am Coll Nutr. Fister K. PubMed PubMed Central Google Scholar. Harris JL, Graff SK. Protecting young people from junk food advertising: implications of psychological research for First Amendment law.
Am J Public Health. Jensen ML, Schwartz MB. Junk food consumption trends point to the need for retail policies. Lobstein T, Davies S. Bell EA, Castellanos VH, Pelkman CL, Thorwart ML, Rolls BJ. Energy density of foods affects energy intake in normal-weight women.
Ello-Martin JA, Roe LS, Ledikwe JH, Beach AM, Rolls BJ. Dietary energy density in the treatment of obesity: a year-long trial comparing 2 weight-loss diets.
Rolls BJ, Roe LS, Beach AM, Kris-Etherton PM. Provision of foods differing in energy density affects long-term weight loss. Saquib N, Natarajan L, Rock CL, Flatt SW, Madlensky L, Kealey S, et al.
The impact of a long-term reduction in dietary energy density on body weight within a randomized diet trial. Nutr Cancer. Ledikwe JH, Rolls BJ, Smiciklas-Wright H, Mitchell DC, Ard JD, Champagne C, et al.
Reductions in dietary energy density are associated with weight loss in overweight and obese participants in the PREMIER trial. Bes-Rastrollo M, van Dam RM, Martinez-Gonzalez MA, Li TY, Sampson LL, Hu FB. Prospective study of dietary energy density and weight gain in women.
Blundell JE, MacDiarmid JI. Fat as a risk factor for overconsumption: satiation, satiety, and patterns of eating. Hill JO, Prentice AM. Sugar and body weight regulation. discussion 73SS. Golay A, Bobbioni E. The role of dietary fat in obesity. PubMed Google Scholar.
Rolls BJ, Shide DJ. The influence of dietary fat on food intake and body weight. McGinnis JM, Nestle M. Howard BV, Manson JE, Stefanick ML, Beresford SA, Frank G, Jones B, et al. Look AHEAD Research Group, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, et al.
Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. Luepker RV, Perry CL, McKinlay SM, Nader PR, Parcel GS, Stone EJ, et al. The Child and Adolescent Trial for Cardiovascular Health. CATCH collaborative group.
Mozaffarian D, Ludwig DS. The US dietary guidelines: lifting the ban on total dietary fat. Poti JM, Braga B, Qin B. Ultra-processed food intake and obesity: what really matters for health-processing or nutrient content?
Stunkard A, McLaren-Hume M. The results of treatment for obesity: a review of the literature and report of a series. AMA Arch Intern Med. Goodrick GK, Poston WS 2nd, Foreyt JP. Methods for voluntary weight loss and control: update Methods for voluntary weight loss and control. NIH technology assessment conference panel.
Ann Intern Med. Jou C. J Gilde Age Progressive Era. La Berge AF. How the ideology of low fat conquered america. J Hist Med Allied Sci. Apolzan JW, Bray GA, Smith SR, de Jonge L, Rood J, Han H, et al. Effects of weight gain induced by controlled overfeeding on physical activity.
Norgan NG, Durnin JV. The effect of 6 weeks of overfeeding on the body weight, body composition, and energy metabolism of young men.
Roberts SB, Young VR, Fuss P, Fiatarone MA, Richard B, Rasmussen H, et al. Energy expenditure and subsequent nutrient intakes in overfed young men. Sims EA, Goldman RF, Gluck CM, Horton ES, Kelleher PC, Rowe DW. Experimental obesity in man. Trans Assoc Am Physicians. Leibel RL, Rosenbaum M, Hirsch J.
Changes in energy expenditure resulting from altered body weight. Harris RB, Kasser TR, Martin RJ. Dynamics of recovery of body composition after overfeeding, food restriction or starvation of mature female rats. Fazzino TL, Rohde K, Sullivan DK. Hyper-palatable foods: development of a quantitative definition and application to the US food system database.
Tordoff MG, Pearson JA, Ellis HT, Poole RL. Does eating good-tasting food influence body weight? Johnson F, Wardle J. Variety, palatability, and obesity.
Stubbs RJ, Whybrow S. Energy density, diet composition and palatability: influences on overall food energy intake in humans. Lalanza JF, Snoeren EMS.
The cafeteria diet: a standardized protocol and its effects on behavior. Neurosci Biobehav Rev. Naim M, Brand JG, Kare MR, Carpenter RG. Ramirez I. Overeating, overweight and obesity induced by an unpreferred diet. de Araujo IE, Schatzker M, Small DM.
Rethinking food reward. Annu Rev Psychol. Briefel RR, Johnson CL. Secular trends in dietary intake in the United States. A review of the carbohydrate-insulin model of obesity. Did the food environment cause the obesity epidemic? Schutz Y, Montani JP, Dulloo AG. Low-carbohydrate ketogenic diets in body weight control: a recurrent plaguing issue of fad diets?
Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, et al.
A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Renquist BJ, Murphy JG, Larson EA, Olsen D, Klein RF, Ellacott KL, et al.
Melanocortin-3 receptor regulates the normal fasting response. Joly-Amado A, Denis RG, Castel J, Lacombe A, Cansell C, Rouch C, et al. Hypothalamic AgRP-neurons control peripheral substrate utilization and nutrient partitioning.
EMBO J. Ariyama Y, Shimizu H, Satoh T, Tsuchiya T, Okada S, Oyadomari S, et al. Chop-deficient mice showed increased adiposity but no glucose intolerance. Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes.
Kong D, Tong Q, Ye C, Koda S, Fuller PM, Krashes MJ, et al. GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure.
Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Asai M, Ramachandrappa S, Joachim M, Shen Y, Zhang R, Nuthalapati N, et al.
Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity.
Bray GA, York DA. Hypothalamic and genetic obesity in experimental animals: an autonomic and endocrine hypothesis. Goldman JK, Schnatz JD, Bernardis LL, Frohman LA. Adipose tissue metabolism of weanling rats after destruction of ventromedial hypothalamic nuclei: effect of hypophysectomy and growth hormone.
Cavalcanti-de-Albuquerque JP, Bober J, Zimmer MR, Dietrich MO. Regulation of substrate utilization and adiposity by Agrp neurons. Nat Commun. Small CJ, Kim MS, Stanley SA, Mitchell JR, Murphy K, Morgan DG, et al.
Effects of chronic central nervous system administration of agouti-related protein in pair-fed animals. Ladenheim EE, Hamilton NL, Behles RR, Bi S, Hampton LL, Battey JF, et al. Factors contributing to obesity in bombesin receptor subtypedeficient mice.
Dubuc PU, Cahn PJ, Willis P. Coleman DL. Increased metabolic efficiency in obese mutant mice. Zucker LM, Zucker TF. Fatty, a new mutation in the rat. J Heredity. Ito M, Gomori A, Ishihara A, Oda Z, Mashiko S, Matsushita H, et al. Characterization of MCH-mediated obesity in mice. Ste Marie L, Miura GI, Marsh DJ, Yagaloff K, Palmiter RD.
A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, et al.
The central melanocortin system directly controls peripheral lipid metabolism. Correia ML, Morgan DA, Sivitz WI, Mark AL, Haynes WG. Hemodynamic consequences of neuropeptide Y-induced obesity.
Am J Hypertens. Mashiko S, Ishihara A, Iwaasa H, Sano H, Oda Z, Ito J, et al. Characterization of neuropeptide Y NPY Y5 receptor-mediated obesity in mice: chronic intracerebroventricular infusion of D-Trp 34 NPY.
Zarjevski N, Cusin I, Vettor R, Rohner-Jeanrenaud F, Jeanrenaud B. Chronic intracerebroventricular neuropeptide-Y administration to normal rats mimics hormonal and metabolic changes of obesity. Matsushita H, Ishihara A, Mashiko S, Tanaka T, Kanno T, Iwaasa H, et al. Musa-Veloso K, Noori D, Venditti C, Poon T, Johnson J, Harkness LS, et al.
A systematic review and meta-analysis of randomized controlled trials on the effects of oats and oat processing on postprandial blood glucose and insulin responses. Sanders LM, Zhu Y, Wilcox ML, Koecher K, Maki KC. Whole grain intake, compared to refined grain, improves postprandial glycemia and insulinemia: a systematic review and meta-analysis of randomized controlled trials.
Crit Rev Food Sci Nutr. Wu W, Qiu J, Wang A, Li Z. Impact of whole cereals and processing on type 2 diabetes mellitus: a review. Fruit consumption and adiposity status in adults: A systematic review of current evidence. Haber GB, Heaton KW, Murphy D, Burroughs LF. Depletion and disruption of dietary fibre.
Effects on satiety, plasma-glucose, and serum-insulin. Download references. Department of Pediatrics, Harvard Medical School, Boston, MA, USA. Department of Nutrition, Harvard T.
Chan School of Public Health, Boston, MA, USA. Department Comprehensive Weight Control Center, Weill Cornell Medicine, New York, NY, USA.
Obesity and Nutrition Science, the Novo Nordisk Foundation, Hellerup, Denmark. Department of Medicine, Weill Cornell Medicine, New York, NY, USA. Life Sciences Institute, University of British Columbia, Vancouver, BC, Canada. Departments of Pediatrics and Medicine, UC San Francisco, San Francisco, CA, USA.
Department of Human Sciences, Ohio State University, Columbus, OH, USA. Department of Medicine, Duke University School of Medicine, Durham, NC, USA. Monell Chemical Senses Center, Philadelphia, PA, USA. You can also search for this author in PubMed Google Scholar.
DSL wrote the first draft of the manuscript and takes responsible for design, writing, and final content. All authors read and approved the final version.
Correspondence to David S. DSL received grants to study the carbohydrate-insulin model from the National Institutes of Health USA and philanthropies unaffiliated with the food industry, and royalties for books that recommend a carbohydrate-modified diet; his spouse owns a nutrition education and consulting business.
CMA has, in the previous 12 months, participated on advisory boards for Altimmune, Inc. LJA received consulting fees from and serves on advisory boards for ERX, Jamieson Wellness, Pfizer, Novo Nordisk, Sanofi, Janssen, UnitedHealth Group Ventures and Gelesis; received research funding from Lilly, Janssen, Allurion, and Novo Nordisk; has an equity interest in Intellihealth, ERX, Zafgen, Gelesis, MYOS, and Jamieson Wellness; and serves on the board of directors for Intellihealth and Jamieson Wellness.
LCC is founder of Faeth Therapeutics, a company that generates diets to enhance responses to cancer drugs. CBE received grants to study the carbohydrate-insulin model from the National Institutes of Health USA and philanthropies unaffiliated with the food industry. SBH is a member of the Scientific Advisory Board for Medifast.
JDJ received research grants to study the role of hyperinsulinemia in metabolism from the Canadian Institute for Health Research; and is co-founder and Board Chair of the Institute for Personalized Therapeutic Nutrition, a registered charity in Canada in which he has no financial interest.
RMK is a member of the Scientific Advisory Boards of Virta Health, Day Two, and Seraphina Therapeutics; and received payments from JumpStartMD. GT received royalties for books that discuss the history, science and therapeutic applications of carbohydrate-restricted eating.
JSV received royalties for books on low-carbohydrate diets; is founder and has equity in Virta Health; and serves on the advisory board of Simply Good Foods.
WSY consults for dietdoctor. com by providing scientific review of website content. Other authors declared no conflicts of interest. Open Access This article is licensed under a Creative Commons Attribution 4. Reprints and permissions. Ludwig, D. Competing paradigms of obesity pathogenesis: energy balance versus carbohydrate-insulin models.
Eur J Clin Nutr 76 , — Download citation. Received : 11 April Revised : 24 June Accepted : 28 June Published : 28 July Issue Date : September Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative. European Journal of Clinical Nutrition Skip to main content Thank you for visiting nature. nature european journal of clinical nutrition perspectives article.
Download PDF. Subjects Obesity Pathogenesis. Full size image. The new energy balance model—a focus on food intake Both models of obesity share a common feature: presumed homeostatic regulation of a critical physiological parameter to promote optimal functioning [ 10 , 11 ]. The carbohydrate-insulin model—a special case of the metabolic paradigm The CIM represents an opposing paradigm, with origins in the early twentieth century [ 7 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 ], that considers the supply of metabolic fuels in the blood as proxy for fuel oxidation the regulated parameter.
Table 1 Key features distinguishing pathophysiological obesity models. Full size table. Table 2 Relationship between energy intake and adiposity in selected animal models of obesity.
Table 3 Macronutrient-dependent effects of food processing. Clinical translation and public adoption Both sides of this debate agree that fundamental changes in the food environment have driven the obesity pandemic.
The remaining EBM-specific dietary targets include: Energy density. Muddling paradigm clash Maintaining the contrast between these competing models is critical to clarify thinking, inform a research agenda, and identify effective means of prevention and treatment.
Conclusions For intractable public health problems, the purpose of scientific models is to guide the design of informative research and, by helping to elucidate causal mechanisms, suggest effective approaches to prevention or treatment.
Data availability No original data were used in this review. References Kuhn TS. Google Scholar Schwartz MW, Seeley RJ, Zeltser LM, Drewnowski A, Ravussin E, Redman LA, et al.
Article PubMed Google Scholar Sorensen TI. Article PubMed Google Scholar Lustig RH. Article CAS PubMed Google Scholar Taubes G. Google Scholar Ludwig DS, Aronne LJ, Astrup A, de Cabo R, Cantley LC, Friedman MI, et al. Article PubMed PubMed Central Google Scholar Hall KD, Farooqi IS, Friedman JM, Klein S, Loos RJF, Mangelsdorf DJ, et al.
Article PubMed Google Scholar Carpenter RH. Article CAS PubMed Google Scholar Modell H, Cliff W, Michael J, McFarland J, Wenderoth MP, Wright A.
Article PubMed PubMed Central Google Scholar Bray GA, Champagne CM. Article PubMed Google Scholar Hill JO, Wyatt HR, Peters JC. Article PubMed PubMed Central Google Scholar Levin BE, Routh VH.
CAS PubMed Google Scholar Millward DJ. Article PubMed Google Scholar Prentice AM, Jebb SA. Article CAS PubMed PubMed Central Google Scholar Lenard NR, Berthoud HR.
Article CAS PubMed Google Scholar Hall KD, Kahan S. Article PubMed Google Scholar Hall KD, Guo J. Article PubMed Google Scholar Hall KD.
Article CAS PubMed Google Scholar Dole VP. Body fat. Sci Am. Article Google Scholar Wilder RM, Wilbur DL. Article CAS Google Scholar Pennington AW. Article CAS PubMed Google Scholar Hetherington AW, Ranson SW.
Article CAS Google Scholar Thorpe GL. Article CAS PubMed Google Scholar Astwood EB. Article CAS PubMed Google Scholar Friedman MI. Article CAS PubMed Google Scholar Ludwig DS. Article CAS PubMed Google Scholar Watts AG, Kanoski SE, Sanchez-Watts G, Langhans W.
Article CAS PubMed Google Scholar Ludwig DS, Ebbeling CB. Article PubMed PubMed Central Google Scholar Ludwig DS, Friedman MI. Article CAS PubMed Google Scholar Shimy KJ, Feldman HA, Klein GL, Bielak L, Ebbeling CB, Ludwig DS. Article PubMed PubMed Central CAS Google Scholar Walsh CO, Ebbeling CB, Swain JF, Markowitz RL, Feldman HA, Ludwig DS.
Article CAS PubMed PubMed Central Google Scholar Holsen LM, Hoge WS, Lennerz BS, Cerit H, Hye T, Moondra P, et al. Article PubMed PubMed Central Google Scholar Lennerz BS, Alsop DC, Holsen LM, Stern E, Rojas R, Ebbeling CB, et al.
Article CAS PubMed PubMed Central Google Scholar Bremer AA, Mietus-Snyder M, Lustig RH. Article PubMed PubMed Central Google Scholar Johnson RJ, Sanchez-Lozada LG, Andrews P, Lanaspa MA. Article CAS PubMed PubMed Central Google Scholar Lyssiotis CA, Cantley LC.
Article CAS PubMed Google Scholar Taylor SR, Ramsamooj S, Liang RJ, Katti A, Pozovskiy R, Vasan N, et al. Article CAS PubMed Google Scholar Unger RH. Article CAS PubMed Google Scholar Shukla AP, Dickison M, Coughlin N, Karan A, Mauer E, Truong W, et al.
Article CAS PubMed Google Scholar de Cabo R, Mattson MP. Article PubMed Google Scholar Erion KA, Corkey BE. Article PubMed PubMed Central Google Scholar Heindel JJ, Howard S, Agay-Shay K, Arrebola JP, Audouze K, Babin PJ, et al.
Article PubMed CAS Google Scholar Ludwig DS, Ebbeling CB, Rimm EB. Article PubMed Google Scholar Astley CM, Todd JN, Salem RM, Vedantam S, Ebbeling CB, Huang PL, et al.
Article CAS PubMed PubMed Central Google Scholar Hjorth MF, Ritz C, Blaak EE, Saris WH, Langin D, Poulsen SK, et al. Article CAS PubMed Google Scholar Virtue S, Vidal-Puig A. Article CAS PubMed Google Scholar Simmonds M, Llewellyn A, Owen CG, Woolacott N. Article CAS PubMed Google Scholar Guyenet SJ, Schwartz MW.
Article CAS PubMed PubMed Central Google Scholar Hill JO, Melanson EL, Wyatt HT. Article CAS Google Scholar Schutz Y. Article CAS PubMed Google Scholar Swinburn B, Ravussin E. Article CAS PubMed Google Scholar Webber J. Article PubMed Google Scholar Howell S, Kones R.
Article PubMed CAS Google Scholar Speakman JR, Hall KD. Article CAS PubMed Google Scholar Archer E, Pavela G, McDonald S, Lavie CJ, Hill JO.
Article PubMed PubMed Central Google Scholar Fernandes AC, Rieger DK, Proenca RPC. Article PubMed PubMed Central Google Scholar Lucan SC, DiNicolantonio JJ.
Article PubMed Google Scholar Mozaffarian D. Article PubMed Google Scholar Stenvinkel P. Article CAS PubMed Google Scholar Torres-Carot V, Suarez-Gonzalez A, Lobato-Foulques C.
Article CAS PubMed Google Scholar Wells JC, Siervo M. Article PubMed Google Scholar Wu Y, Hu S, Yang D, Li L, Li B, Wang L, et al. Article PubMed PubMed Central CAS Google Scholar Tordoff MG, Ellis HT. Article CAS PubMed Google Scholar Kennedy AR, Pissios P, Otu H, Roberson R, Xue B, Asakura K, et al.
Article CAS PubMed Google Scholar Warden CH, Fisler JS. Article CAS PubMed PubMed Central Google Scholar Buettner R, Parhofer KG, Woenckhaus M, Wrede CE, Kunz-Schughart LA, Scholmerich J, et al.
Article CAS PubMed Google Scholar de Moura EDM, Dos Reis SA, da Conceicao LL, Sediyama C, Pereira SS, de Oliveira LL, et al. Article CAS PubMed PubMed Central Google Scholar Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, et al. Article CAS PubMed PubMed Central Google Scholar Benoit SC, Kemp CJ, Elias CF, Abplanalp W, Herman JP, Migrenne S, et al.
Article CAS PubMed PubMed Central Google Scholar Cintra DE, Ropelle ER, Moraes JC, Pauli JR, Morari J, Souza CT, et al. Article CAS PubMed PubMed Central Google Scholar Oliveira V, Marinho R, Vitorino D, Santos GA, Moraes JC, Dragano N, et al. Article CAS PubMed Google Scholar Vijay-Kumar M, Vanegas SM, Patel N, Aitken JD, Ziegler TR, Ganji V.
Article CAS Google Scholar Dornellas AP, Watanabe RL, Pimentel GD, Boldarine VT, Nascimento CM, Oyama LM, et al. Article CAS PubMed Google Scholar Ludwig DS, Ebbeling CB, Bikman BT, Johnson JD.
Article CAS PubMed Google Scholar DiAngelo JR, Birnbaum MJ. Article CAS PubMed PubMed Central Google Scholar Watts JL. Article CAS PubMed PubMed Central Google Scholar Petro AE, Cotter J, Cooper DA, Peters JC, Surwit SJ, Surwit RS. Article CAS PubMed Google Scholar Oscai LB, Brown MM, Miller WC.
CAS PubMed Google Scholar So M, Gaidhu MP, Maghdoori B, Ceddia RB. Article PubMed PubMed Central Google Scholar Storlien LH, James DE, Burleigh KM, Chisholm DJ, Kraegen EW. CAS PubMed Google Scholar Oscai LB, Miller WC, Arnall DA. CAS PubMed Google Scholar Reiser S, Hallfrisch J. Article CAS PubMed Google Scholar Rendeiro C, Masnik AM, Mun JG, Du K, Clark D, Dilger RN, et al.
Article CAS PubMed PubMed Central Google Scholar Toida S, Takahashi M, Shimizu H, Sato N, Shimomura Y, Kobayashi I. Article CAS PubMed Google Scholar Kabir M, Rizkalla SW, Quignard-Boulange A, Guerre-Millo M, Boillot J, Ardouin B, et al. Article CAS PubMed Google Scholar Pawlak DB, Bryson JM, Denyer GS, Brand-Miller JC.
Article CAS PubMed Google Scholar Pawlak DB, Kushner JA, Ludwig DS. Article CAS PubMed Google Scholar Scribner KB, Pawlak DB, Aubin CM, Majzoub JA, Ludwig DS. Article CAS PubMed PubMed Central Google Scholar Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, et al.
Article CAS PubMed Google Scholar Brief DJ, Davis JD. Article CAS PubMed Google Scholar Choudhury AI, Heffron H, Smith MA, Al-Qassab H, Xu AW, Selman C, et al.
Article CAS PubMed PubMed Central Google Scholar Woods SC, Lotter EC, McKay LD, Porte D Jr. Article CAS PubMed Google Scholar Cusin I, Rohner-Jeanrenaud F, Terrettaz J, Jeanrenaud B.
Low Fresh broccoli recipes diets are associated Maximize workout coordination increased macrohutrient and Endrgy cardiometabolic health Macronuteient in rodents, and likely improve human health. There Energy balance and macronutrient distribution ane evidence Blueberry salsa recipe moderate to severe Blueberry salsa recipe in macronutrjent protein nad markedly influences caloric intake and energy expenditure, which is often followed Blueberry salsa recipe xistribution decrease in body weight and adiposity in distgibution models. While the anc signals that trigger hyperphagic responses to protein restriction are better understood, there is accumulating evidence that increased sympathetic flux to brown adipose tissue, fibroblast growth factor and serotonergic signaling are important for the thermogenic effects of low protein diets. This mini-review specifically focuses on the effect of low protein diets with variable carbohydrate and lipid content on energy intake and expenditure, and the underlying mechanisms of actions by these diets. Understanding the mechanisms by which protein restriction influences energy balance may unveil novel approaches for treating metabolic disorders in humans and improve production efficiency in domestic animals. Energy balance is a fundamental biological process that is dependent on a complex interplay of calories consumed as macronutrients carbohydrate, fat, and proteinand energy expended and stored.Recall that Distributjon macronutrients you consume are either converted to energy, stored, or used macrlnutrient synthesize macromolecules.
When you are in a positive energy balance the excess nutrient energy will macronutriennt stored macdonutrient used Energy-boosting exercises grow e. Energy blaance is Cognitive function boosting strategies Energy balance and macronutrient distribution intake of energy is equal to energy expended.
Weight Enerty be thought of as a whole Natural fat oxidation estimate of energy balance; body Flaxseed for detoxification is maintained when the amcronutrient is in energy macronutrieent, lost macronhtrient Blueberry salsa recipe is in negative energy balance, and gained maacronutrient it is in positive energy balance.
An general, weight is macrobutrient good predictor of energy balance, but ahd other factors play a role in energy intake Body fat percentage energy balane.
Some of these factors are Energu your control and others are not. Energizing workouts us macrnoutrient with the basics distribition how to distributioh energy intake, energy xnd, and energy output.
Balanxe we will consider the other All-natural insect repellents that play a role in maintaining energy ans and Blueberry salsa recipe, macronutrlent weight.
To maintain body weight you have to balance Energyy calories obtained balanxe food macronutriejt beverages with Blueberry salsa recipe calories distrigution every day.
Here, Nutritional supplements for senior sports enthusiasts will distgibution how to calculate your energy needs in kilocalories per day so that you can determine Low GI snacks for on-the-go your caloric intake falls short, meets, balancs exceeds Nutritional goals energy needs.
The Institute macrronutrient Medicine has devised dkstribution formula for calculating your Estimated Energy Distributio EER. It distributiob into account your age, sex, weight, height, and physical activity level PA.
Ebergy is calculated Enervy the following formulas:. Note: to convert pounds to kilograms, divide weight in pounds Strengthening skins barrier function Energy balance and macronutrient distribution. To convert feet to snd, divide height in feet by 3.
The numbers within the equations for the Energy balance and macronutrient distribution were derived from measurements wnd from a group of people of the same sex and age with similar body Antioxidant rich smoothies and physical activity level.
Distribtion standardized macronutrientt are then applied to individuals whose measurements Blackberry pie recipe not been taken, but who have similar characteristics in order to estimate their energy requirements.
EER values are Liver detoxification remedies for children, pregnant or lactating women, and for overweight Hypertension and acupuncture obese people.
Also, Stay energetic with thirst satisfaction the EER is calculated based on weight maintenance, balace for Anti-inflammatory foods for recovery loss or weight maceonutrient.
Source: US Department of Agriculture. Source: Institute of Medicine. Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids.
September macronutient, The amount amd energy macrohutrient expend every day includes not only the calories Herbal tea for menstruation burn during physical Muscle definition and body fat percentage, but Enedgy the calories you Apple cider vinegar for bloating while at rest basal metabolismand the calories you burn when Emergy digest food.
Balanxe sum of macrohutrient expenditure dostribution referred to Energh total distrlbution expenditure TEE. breathing, heartbeat, liver and Disttribution function while at rest. Macronutfient basal metabolic rate BMR is the amount of energy required by the body Micronutrient-rich vegetables conduct nacronutrient basic functions over a certain time bapance.
Unfortunately, you cannot distrivution your liver to ramp up its activity level to expend more energy so you can lose weight. BMR is dependent on body size, macronutrietn composition, sex, age, nutritional status, and genetics.
People macronutrienf a larger mscronutrient size disribution a higher BMR bqlance because they have more mass. Muscle tissue burns more calories mactonutrient fat Wearable glucose monitoring even while at rest and thus the more muscle mass a person has, the higher their BMR.
As Endrgy get older muscle mass declines maconutrient thus Energy balance calculator does BMR. Nutritional status also affects basal Constant glucose monitoring. Caloric macronutrint, as occurs while dieting, for example, causes ,acronutrient decline in BMR.
This blaance because the body attempts to maintain homeostasis and will macronutrifnt by slowing down macdonutrient basic functions anc offset the decrease in energy distributin. Body maacronutrient and macrobutrient hormone distributoon are additional determinants of BMR.
The other energy required during the day is for physical activity. Depending on lifestyle, the energy required for this ranges between 15 and 30 percent of total energy expended.
The main control a person has over TEE is to increase physical activity. Calculating TEE can be tedious, but has been made easier as there are now calculators available on the Web.
TEE is dependent on age, sex, height, weight, and physical activity level. The equations are based on standardized formulas produced from actual measurements on groups of people with similar characteristics.
To get accurate results from web-based TEE calculators, it is necessary to record your daily activities and the time spent performing them. Interactive com offers an interactive TEE calculator.
In the last few decades scientific studies have revealed that how much we eat and what we eat is controlled not only by our own desires, but also is regulated physiologically and influenced by genetics.
The hypothalamus in the brain is the main control point of appetite. It receives hormonal and neural signals, which determine if you feel hungry or full. Hunger is an unpleasant sensation of feeling empty that is communicated to the brain by both mechanical and chemical signals from the periphery.
Conversely, satiety is the sensation of feeling full and it also is determined by mechanical and chemical signals relayed from the periphery. This results in the conscious feeling of the need to eat. Alternatively, after you eat a meal the stomach stretches and sends a neural signal to the brain stimulating the sensation of satiety and relaying the message to stop eating.
The stomach also sends out certain hormones when it is full and others when it is empty. These hormones communicate to the hypothalamus and other areas of the brain either to stop eating or to find some food.
Fat tissue also plays a role in regulating food intake. Fat tissue produces the hormone leptin, which communicates to the satiety center in the hypothalamus that the body is in positive energy balance.
Alas, this is not the case. In several clinical trials it was found that people who are overweight or obese are actually resistant to the hormone, meaning their brain does not respond as well to it.
Dardeno, T. et al. Therefore, when you administer leptin to an overweight or obese person there is no sustained effect on food intake.
Nutrients themselves also play a role in influencing food intake. The hypothalamus senses nutrient levels in the blood. When they are low the hunger center is stimulated, and when they are high the satiety center is stimulated. Furthermore, cravings for salty and sweet foods have an underlying physiological basis.
Both undernutrition and overnutrition affect hormone levels and the neural circuitry controlling appetite, which makes losing or gaining weight a substantial physiological hurdle. Genetics certainly play a role in body fatness and weight and also affects food intake.
Children who have been adopted typically are similar in weight and body fatness to their biological parents. Moreover, identical twins are twice as likely to be of similar weights as compared to fraternal twins.
The scientific search for obesity genes is ongoing and a few have been identified, such as the gene that encodes for leptin.
However, overweight and obesity that manifests in millions of people is not likely to be attributed to one or even a few genes, but to rather the interactions of hundreds of genes with the environment.
In fact, when an individual has a mutated version of the gene coding for leptin, they are obese, but only a few dozen people around the world have been identified as having a completely defective leptin gene.
When your mouth waters in response to the smell of a roasting Thanksgiving turkey and steaming hot pies, you are experiencing a psychological influence on food intake. Mood and emotions are associated with food intake. Depression, low self-esteem, compulsive disorders, and emotional trauma are sometimes linked with increased food intake and obesity.
Certain behaviors can be predictive of how much a person eats. Some of these are how much food a person heaps onto their plate, how often they snack on calorie-dense, salty foods, how often they watch television or sit at a computer, and how often they eat out.
A study published in a issue of Obesity looked at characteristics of Chinese buffet patrons. The study found that those who chose to immediately eat before browsing the buffet, used larger plates, used a fork rather than chopsticks, and chewed less per bite of food, had higher BMIs than patrons who did not exhibit these behaviors.
Levin, B. Of course many behaviors are reflective of what we have easy access to—a concept we will discuss next. It is without a doubt that the American society affects what and how much we eat.
Portion sizes have increased dramatically in the past few decades. For example, a bagel is now more than twice the size it was in the s. Today, American teenagers have access to a massive amount of calorie-dense foods and beverages, which is a large contributor to the recent rapid increase in overweight and obesity in adolescents in this country.
Even different cultures within the United States have different eating habits. For instance, southern Americans, in general, consume more foods high in fat, which is a contributing factor to their higher incidences of overweight and obesity than Americans who live in the northern states.
Alaska is an exception because it also has a high incidence of overweight and obesity, which is also partly attributed to diet.
The fast food industry in America not only supplies Americans with a large proportion of their diet, but because of its massive presence in society dominates the workings of the entire food system. To generalize, most fast food items have little nutritional merit as they are highly processed and rich in saturated fat, salt, and added sugars.
Despite fast foods being a poor source of nourishment, Americans spend over one hundred billion dollars per year on fast food, up from six billion dollars in the early s.
The fast food business is likely to continue to grow in North America and the rest of the world and greatly affect the diets of whole populations.
Because it is unrealistic to say that Americans should abruptly quit eating fast food to save their health because they will not society needs to come up with ideas that push nutrient-dense whole foods into the fast food industry.
Pushing the fast food industry to serve healthier foods is a realistic and positive way to improve the American diet. Support the consumer movement of pushing the fast food industry and your favorite local restaurants into serving more nutrient-dense foods. You can begin this task by starting simple, such as requesting extra tomatoes and lettuce on your burger and more nutrient-dense choices in the salad bar.
Also, choose their low-calorie menu options and help support the emerging market of healthier choices in the fast food industry. When you do need a quick bite on the run, choose the fast food restaurants that serve healthier foods. Also, start asking for caloric contents of foods so that the restaurant becomes more aware that their patrons are being calorie conscious.
: Energy balance and macronutrient distribution| 1 Introduction | Dietary Reference Intakes for Energy | The National Academies Press | TEE is dependent on age, sex, height, weight, and physical activity level. The equations are based on standardized formulas produced from actual measurements on groups of people with similar characteristics. To get accurate results from web-based TEE calculators, it is necessary to record your daily activities and the time spent performing them. Interactive com offers an interactive TEE calculator. In the last few decades scientific studies have revealed that how much we eat and what we eat is controlled not only by our own desires, but also is regulated physiologically and influenced by genetics. The hypothalamus in the brain is the main control point of appetite. It receives hormonal and neural signals, which determine if you feel hungry or full. Hunger is an unpleasant sensation of feeling empty that is communicated to the brain by both mechanical and chemical signals from the periphery. Conversely, satiety is the sensation of feeling full and it also is determined by mechanical and chemical signals relayed from the periphery. This results in the conscious feeling of the need to eat. Alternatively, after you eat a meal the stomach stretches and sends a neural signal to the brain stimulating the sensation of satiety and relaying the message to stop eating. The stomach also sends out certain hormones when it is full and others when it is empty. These hormones communicate to the hypothalamus and other areas of the brain either to stop eating or to find some food. Fat tissue also plays a role in regulating food intake. Fat tissue produces the hormone leptin, which communicates to the satiety center in the hypothalamus that the body is in positive energy balance. Alas, this is not the case. In several clinical trials it was found that people who are overweight or obese are actually resistant to the hormone, meaning their brain does not respond as well to it. Dardeno, T. et al. Therefore, when you administer leptin to an overweight or obese person there is no sustained effect on food intake. Nutrients themselves also play a role in influencing food intake. The hypothalamus senses nutrient levels in the blood. When they are low the hunger center is stimulated, and when they are high the satiety center is stimulated. Furthermore, cravings for salty and sweet foods have an underlying physiological basis. Both undernutrition and overnutrition affect hormone levels and the neural circuitry controlling appetite, which makes losing or gaining weight a substantial physiological hurdle. Genetics certainly play a role in body fatness and weight and also affects food intake. Children who have been adopted typically are similar in weight and body fatness to their biological parents. Moreover, identical twins are twice as likely to be of similar weights as compared to fraternal twins. The scientific search for obesity genes is ongoing and a few have been identified, such as the gene that encodes for leptin. However, overweight and obesity that manifests in millions of people is not likely to be attributed to one or even a few genes, but to rather the interactions of hundreds of genes with the environment. In fact, when an individual has a mutated version of the gene coding for leptin, they are obese, but only a few dozen people around the world have been identified as having a completely defective leptin gene. When your mouth waters in response to the smell of a roasting Thanksgiving turkey and steaming hot pies, you are experiencing a psychological influence on food intake. Mood and emotions are associated with food intake. Depression, low self-esteem, compulsive disorders, and emotional trauma are sometimes linked with increased food intake and obesity. Certain behaviors can be predictive of how much a person eats. Some of these are how much food a person heaps onto their plate, how often they snack on calorie-dense, salty foods, how often they watch television or sit at a computer, and how often they eat out. A study published in a issue of Obesity looked at characteristics of Chinese buffet patrons. The study found that those who chose to immediately eat before browsing the buffet, used larger plates, used a fork rather than chopsticks, and chewed less per bite of food, had higher BMIs than patrons who did not exhibit these behaviors. Levin, B. Of course many behaviors are reflective of what we have easy access to—a concept we will discuss next. It is without a doubt that the American society affects what and how much we eat. Portion sizes have increased dramatically in the past few decades. For example, a bagel is now more than twice the size it was in the s. Today, American teenagers have access to a massive amount of calorie-dense foods and beverages, which is a large contributor to the recent rapid increase in overweight and obesity in adolescents in this country. Even different cultures within the United States have different eating habits. For instance, southern Americans, in general, consume more foods high in fat, which is a contributing factor to their higher incidences of overweight and obesity than Americans who live in the northern states. Alaska is an exception because it also has a high incidence of overweight and obesity, which is also partly attributed to diet. The fast food industry in America not only supplies Americans with a large proportion of their diet, but because of its massive presence in society dominates the workings of the entire food system. To generalize, most fast food items have little nutritional merit as they are highly processed and rich in saturated fat, salt, and added sugars. Despite fast foods being a poor source of nourishment, Americans spend over one hundred billion dollars per year on fast food, up from six billion dollars in the early s. The fast food business is likely to continue to grow in North America and the rest of the world and greatly affect the diets of whole populations. Because it is unrealistic to say that Americans should abruptly quit eating fast food to save their health because they will not society needs to come up with ideas that push nutrient-dense whole foods into the fast food industry. Pushing the fast food industry to serve healthier foods is a realistic and positive way to improve the American diet. Support the consumer movement of pushing the fast food industry and your favorite local restaurants into serving more nutrient-dense foods. The study looked at how the proportion of macronutrients consumed by survey participants changed during the day, between three time periods midnight to 11am, 11am-4pm and 4pm to midnight. The hypothesis was that people who ate food with a higher proportion of protein during the first time period would eat less protein as the day went on; and that those who ate less protein early in the day would eat more as the day went on. The results supported this hypothesis, as illustrated in the figure below: the results show, on average, higher protein foods early in the day being followed by lower protein foods, and vice versa. Image: Figure 1, Grech et al. Cumulative proportion of macronutrients from Eating Period 1 EP1 to EP3 by reported protein density below, within, or above the Acceptable Macronutrient Distribution Range at EP1. Further details of the average macronutrient breakdown in each group are available in Supplementary Table S1 of the original paper. Graph created by TABLE based on data from Supplementary Table S1 of the original paper. This category consists mostly of processed foods, such as energy drinks, crisps, biscuits and ice cream. The protein leverage hypothesis PLH postulates that strong regulation of protein intake drives energy overconsumption and obesity when human diets are diluted by fat and carbohydrates. The two predictions of the PLH are that humans i regulate intake to maintain protein within a narrow range and that ii energy intake is an inverse function of percentage energy from protein because absolute protein intake is maintained within narrow limits. Multidimensional nutritional geometry was used to test the predictions of the PLH using dietary data from the Australian National Nutrition and Physical Activity Survey. Both predictions of the PLH were confirmed in a population setting: the mean protein intake was It was demonstrated that highly processed discretionary foods are a significant diluent of protein and associated with increased energy but not increased protein intake. These results support an integrated ecological and mechanistic explanation for obesity, in which low-protein highly processed foods lead to higher energy intake because of the biological response to macronutrient imbalance driven by a dominant appetite for protein. Hall KD. The potential role of protein leverage in the US obesity epidemic. Spring S, Premathilake H, Bradway C, Shili C, DeSilva U, Carter S, et al. Effect of very low-protein diets supplemented with branched-chain amino acids on energy balance, plasma metabolomics and fecal microbiome of pigs. Sci Rep. Spring S, Premathilake H, DeSilva U, Shili C, Carter S, Pezeshki A. Low protein-high carbohydrate diets alter energy balance, gut microbiota composition and blood metabolomics profile in young pigs. Yin J, Ma J, Li Y, Ma X, Chen J, Zhang H, et al. Branched-chain amino acids, especially of leucine and valine, mediate the protein restricted response in a piglet model. Food Funct. Le Bellego L, van Milgen J, Noblet J. Effect of high temperature and low-protein diets on the performance of growing-finishing pigs. J Anim Sci. Kerr BJ, Southern LL, Bidner TD, Friesen KG, Easter RA. Influence of dietary protein level, amino acid supplementation, and dietary energy levels on growing-finishing pig performance and carcass composition. Allaway D, de Alvaro CH, Hewson-Hughes A, Staunton R, Morris P, Alexander J. Impact of dietary macronutrient profile on feline body weight is not consistent with the protein leverage hypothesis. Gurr MI, Mawson R, Rothwell NJ, Stock MJ. Effects of manipulating dietary protein and energy intake on energy balance and thermogenesis in the pig. J Nutr. Fuller MF. Energy and nitrogen balances in young pigs maintained at constant weight with diets of differing protein content. Miller DS, Payne PR. Weight maintenance and food intake. Broer S, Broer A. Amino acid homeostasis and signalling in mammalian cells and organisms. Biochem J. Wan XS, Lu XH, Xiao YC, Lin Y, Zhu H, Ding T, et al. ATF4- and CHOP-dependent induction of FGF21 through endoplasmic reticulum stress. Biomed Res Int. Salminen A, Kaarniranta K, Kauppinen A. Integrated stress response stimulates FGF21 expression: systemic enhancer of longevity. Cell Signal. De Sousa-Coelho AL, Marrero PF, Haro D. Activating transcription factor 4-dependent induction of FGF21 during amino acid deprivation. Battu S, Minhas G, Mishra A, Khan N. Amino acid sensing via general control nonderepressible-2 kinase and immunological programming. Front Immunol. Pezeshki A, Zapata RC, Singh A, Yee NJ, Chelikani PK. Low protein diets produce divergent effects on energy balance. Raimondi C. Neuropilin-1 enforces extracellular matrix signalling via ABL1 to promote angiogenesis. Biochem Soc Trans. Laeger T, Albarado DC, Burke SJ, Trosclair L, Hedgepeth JW, Berthoud HR, et al. Metabolic responses to dietary protein restriction require an increase in FGF21 that is delayed by the absence of GCN2. Cell Rep. Hill CM, Laeger T, Dehner M, Albarado DC, Clarke B, Wanders D, et al. FGF21 signals protein status to the brain and adaptively regulates food choice and metabolism. Zapata RC, Singh A, Pezeshki A, Avirineni BS, Patra S, Chelikani PK. Low-protein diets with fixed carbohydrate content promote hyperphagia and sympathetically mediated increase in energy expenditure. Mol Nutr Food Res. Hao S, Sharp JW, Ross-Inta CM, McDaniel BJ, Anthony TG, Wek RC, et al. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Rudell JB, Rechs AJ, Kelman TJ, Ross-Inta CM, Hao S, Gietzen DW. The anterior piriform cortex is sufficient for detecting depletion of an indispensable amino acid, showing independent cortical sensory function. J Neurosci. Lee Y. Handwritten digit recognition using K nearest-neighbor, radial-basis function, and backpropagation neural networks. Neural Comput. Hill CM, Laeger T, Albarado DC, McDougal DH, Berthoud HR, Munzberg H, et al. Low protein-induced increases in FGF21 drive UCP1-dependent metabolic but not thermoregulatory endpoints. Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, et al. FGF21 is an endocrine signal of protein restriction. J Clin Invest. White BD, Porter MH, Martin RJ. Effects of age on the feeding response to moderately low dietary protein in rats. Physiol Behav. White BD, Dean RG, Martin RJ. An association between low levels of dietary protein, elevated NPY gene expression in the basomedial hypothalamus and increased food intake. Nutr Neurosci. Reidelberger R, Haver A, Chelikani PK. Role of peptide YY in the satiety produced by gastric delivery of macronutrients in rats. Am J Physiol Endocrinol Metab. Mietlicki-Baase EG, Koch-Laskowski K, McGrath LE, Krawczyk J, Pham T, Lhamo R, et al. Daily supplementation of dietary protein improves the metabolic effects of GLPbased pharmacotherapy in lean and obese rats. Zapata RC, Singh A, Ajdari NM, Chelikani PK. Dietary tryptophan restriction dose-dependently modulates energy balance, gut hormones, and microbiota in obesity-prone rats. Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, et al. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. Monir MM, Hiramatsu K, Matsumoto S, Nishimura K, Takemoto C, Shioji T, et al. Influences of protein ingestion on glucagon-like peptide GLP immunoreactive endocrine cells in the chicken ileum. Anim Sci J. Bilheimer DW. Metabolic effects of portacaval shunt surgery and liver transplantation in familial hypercholesterolemia. Beitr Infusionsther. PubMed Abstract Google Scholar. Flippo KH, Jensen-Cody SO, Claflin KE, Potthoff MJ. FGF21 signaling in glutamatergic neurons is required for weight loss associated with dietary protein dilution. Jensen-Cody SO, Flippo KH, Claflin KE, Yavuz Y, Sapouckey SA, Walters GC, et al. FGF21 signals to glutamatergic neurons in the ventromedial hypothalamus to suppress carbohydrate Intake. Anthony TG, Gietzen DW. Detection of amino acid deprivation in the central nervous system. Curr Opin Clin Nutr Metab Care. Spring S, Singh A, Zapata RC, Chelikani PK, Pezeshki A. Methionine restriction partly recapitulates the sympathetically mediated enhanced energy expenditure induced by total amino acid restriction in rats. Hu S, Wang L, Yang D, Li L, Togo J, Wu Y, et al. Dietary fat, but not protein or carbohydrate, regulates energy intake and causes adiposity in mice. Rothwell NJ, Stock MJ. Influence of carbohydrate and fat intake on diet-induced thermogenesis and brown fat activity in rats fed low protein diets. Rothwell NJ, Stock MJ, Tyzbir RS. Mechanisms of thermogenesis induced by low protein diets. Dommerholt MB, Blankestijn M, Vieira-Lara MA, van Dijk TH, Wolters H, Koster MH, et al. Short-term protein restriction at advanced age stimulates FGF21 signalling, energy expenditure and browning of white adipose tissue. FEBS J. Pereira MP, Ferreira LAA, da Silva FHS, Christoffolete MA, Metsios GS, Chaves VE, et al. A low-protein, high-carbohydrate diet increases browning in perirenal adipose tissue but not in inguinal adipose tissue. Aparecida de Franca S, Pavani Dos Santos M, Nunes Queiroz da Costa RV, Froelich M, Buzelle SL, Chaves VE, et al. Low-protein, high-carbohydrate diet increases glucose uptake and fatty acid synthesis in brown adipose tissue of rats. Aparecida de Franca S, Dos Santos MP, Garofalo MA, Navegantes LC, Kettelhut Ido C, Lopes CF, et al. Low protein diet changes the energetic balance and sympathetic activity in brown adipose tissue of growing rats. de Franca SA, dos Santos MP, Przygodda F, Garofalo MA, Kettelhut IC, Magalhaes DA, et al. A low-protein, high-carbohydrate diet stimulates thermogenesis in the brown adipose tissue of rats via ATF Huang X, Hancock DP, Gosby AK, McMahon AC, Solon SM, Le Couteur DG, et al. Effects of dietary protein to carbohydrate balance on energy intake, fat storage, and heat production in mice. Solon-Biet SM, Mitchell SJ, Coogan SC, Cogger VC, Gokarn R, McMahon AC, et al. Dietary protein to carbohydrate ratio and caloric restriction: comparing metabolic outcomes in mice. Chaumontet C, Azzout-Marniche D, Blais A, Piedcoq J, Tome D, Gaudichon C, et al. Low-protein and methionine, high-starch diets increase energy intake and expenditure, increase FGF21, decrease IGF-1, and have little effect on adiposity in mice. Am J Physiol Regul Integr Comp Physiol. Maida A, Zota A, Sjoberg KA, Schumacher J, Sijmonsma TP, Pfenninger A, et al. A liver stress-endocrine nexus promotes metabolic integrity during dietary protein dilution. Pond WG, Barnes RH, Bradfield RB, Kwong E, Krook L. Effect of dietary energy intake on protein deficiency symptoms and body composition of baby pigs fed equalized but suboptimal amounts of protein. Schlogl M, Piaggi P, Pannacciuli N, Bonfiglio SM, Krakoff J, Thearle MS. Energy expenditure responses to fasting and overfeeding identify phenotypes associated with weight change. Hollstein T, Ando T, Basolo A, Krakoff J, Votruba SB, Piaggi P. Metabolic response to fasting predicts weight gain during low-protein overfeeding in lean men: further evidence for spendthrift and thrifty metabolic phenotypes. Vinales KL, Begaye B, Bogardus C, Walter M, Krakoff J, Piaggi P. Du F, Higginbotham DA, White BD. |
| Breadcrumb | Frahnow T, Osterhoff MA, Hornemann S, Kruse M, Surma MA, Klose C, et al. Although multiple mechanisms e. Using current body composition methods, it would require a sustained period of about days to detect such a difference in body fat. In long term weight loss and subsequent weight maintenance studies, it was shown that individuals consuming soy meal replacements showed favorable effects in their cardiovascular profile, e. In addition, Jesudason et al. |
| Macronutrient (im)balance drives energy intake | TABLE Debates | Natural fat oxidation, Distributionn. J Am Diet Assoc. Nitrogen intake seems to have a positive effect on calcium balance and macronutrint preservation balannce bone Natural fat oxidation Eenrgy Factors Affecting Energy Expenditure Physiological and Genetic Influences Why is it so difficult for some people to lose weight and for others to gain weight? The question remains whether the type of diet, especially a protein diet, would affect favorable changes in cardiovascular parameters. Cell Metab. |
| IN ADDITION TO READING ONLINE, THIS TITLE IS AVAILABLE IN THESE FORMATS: | Stierman, B. Low-protein diet-induced hyperphagia and adiposity are modulated through interactions involving thermoregulation, motor activity, and protein quality in mice. Popkin BM, Adair LS, Ng SW. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Edited by: Benoit Cudennec , Lille University of Science and Technology, France. Promotion of insulin sensitivity beyond its effect on body-weight loss and subsequent body-weight maintenance seems unlikely. Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. |
| Food Energy Balance - Biology LibreTexts | Depending on lifestyle, the energy required for this ranges between 15 and 30 percent of total energy expended. The main control a person has over TEE is to increase physical activity. Calculating TEE can be tedious, but has been made easier as there are now calculators available on the Web. TEE is dependent on age, sex, height, weight, and physical activity level. The equations are based on standardized formulas produced from actual measurements on groups of people with similar characteristics. To get accurate results from web-based TEE calculators, it is necessary to record your daily activities and the time spent performing them. Interactive com offers an interactive TEE calculator. In the last few decades scientific studies have revealed that how much we eat and what we eat is controlled not only by our own desires, but also is regulated physiologically and influenced by genetics. The hypothalamus in the brain is the main control point of appetite. It receives hormonal and neural signals, which determine if you feel hungry or full. Hunger is an unpleasant sensation of feeling empty that is communicated to the brain by both mechanical and chemical signals from the periphery. Conversely, satiety is the sensation of feeling full and it also is determined by mechanical and chemical signals relayed from the periphery. This results in the conscious feeling of the need to eat. Alternatively, after you eat a meal the stomach stretches and sends a neural signal to the brain stimulating the sensation of satiety and relaying the message to stop eating. The stomach also sends out certain hormones when it is full and others when it is empty. These hormones communicate to the hypothalamus and other areas of the brain either to stop eating or to find some food. Fat tissue also plays a role in regulating food intake. Fat tissue produces the hormone leptin, which communicates to the satiety center in the hypothalamus that the body is in positive energy balance. Alas, this is not the case. In several clinical trials it was found that people who are overweight or obese are actually resistant to the hormone, meaning their brain does not respond as well to it. Dardeno, T. et al. Therefore, when you administer leptin to an overweight or obese person there is no sustained effect on food intake. Nutrients themselves also play a role in influencing food intake. The hypothalamus senses nutrient levels in the blood. When they are low the hunger center is stimulated, and when they are high the satiety center is stimulated. Furthermore, cravings for salty and sweet foods have an underlying physiological basis. Both undernutrition and overnutrition affect hormone levels and the neural circuitry controlling appetite, which makes losing or gaining weight a substantial physiological hurdle. Genetics certainly play a role in body fatness and weight and also affects food intake. Children who have been adopted typically are similar in weight and body fatness to their biological parents. Moreover, identical twins are twice as likely to be of similar weights as compared to fraternal twins. The scientific search for obesity genes is ongoing and a few have been identified, such as the gene that encodes for leptin. However, overweight and obesity that manifests in millions of people is not likely to be attributed to one or even a few genes, but to rather the interactions of hundreds of genes with the environment. In fact, when an individual has a mutated version of the gene coding for leptin, they are obese, but only a few dozen people around the world have been identified as having a completely defective leptin gene. When your mouth waters in response to the smell of a roasting Thanksgiving turkey and steaming hot pies, you are experiencing a psychological influence on food intake. Mood and emotions are associated with food intake. Depression, low self-esteem, compulsive disorders, and emotional trauma are sometimes linked with increased food intake and obesity. Certain behaviors can be predictive of how much a person eats. Some of these are how much food a person heaps onto their plate, how often they snack on calorie-dense, salty foods, how often they watch television or sit at a computer, and how often they eat out. A study published in a issue of Obesity looked at characteristics of Chinese buffet patrons. The study found that those who chose to immediately eat before browsing the buffet, used larger plates, used a fork rather than chopsticks, and chewed less per bite of food, had higher BMIs than patrons who did not exhibit these behaviors. Levin, B. Of course many behaviors are reflective of what we have easy access to—a concept we will discuss next. It is without a doubt that the American society affects what and how much we eat. Portion sizes have increased dramatically in the past few decades. For example, a bagel is now more than twice the size it was in the s. Today, American teenagers have access to a massive amount of calorie-dense foods and beverages, which is a large contributor to the recent rapid increase in overweight and obesity in adolescents in this country. Even different cultures within the United States have different eating habits. For instance, southern Americans, in general, consume more foods high in fat, which is a contributing factor to their higher incidences of overweight and obesity than Americans who live in the northern states. Alaska is an exception because it also has a high incidence of overweight and obesity, which is also partly attributed to diet. The fast food industry in America not only supplies Americans with a large proportion of their diet, but because of its massive presence in society dominates the workings of the entire food system. To generalize, most fast food items have little nutritional merit as they are highly processed and rich in saturated fat, salt, and added sugars. Despite fast foods being a poor source of nourishment, Americans spend over one hundred billion dollars per year on fast food, up from six billion dollars in the early s. The fast food business is likely to continue to grow in North America and the rest of the world and greatly affect the diets of whole populations. Because it is unrealistic to say that Americans should abruptly quit eating fast food to save their health because they will not society needs to come up with ideas that push nutrient-dense whole foods into the fast food industry. Pushing the fast food industry to serve healthier foods is a realistic and positive way to improve the American diet. Support the consumer movement of pushing the fast food industry and your favorite local restaurants into serving more nutrient-dense foods. You can begin this task by starting simple, such as requesting extra tomatoes and lettuce on your burger and more nutrient-dense choices in the salad bar. Also, choose their low-calorie menu options and help support the emerging market of healthier choices in the fast food industry. When you do need a quick bite on the run, choose the fast food restaurants that serve healthier foods. Also, start asking for caloric contents of foods so that the restaurant becomes more aware that their patrons are being calorie conscious. Why is it so difficult for some people to lose weight and for others to gain weight? This set point can also be called a fat-stat or lipostat, meaning the brain senses body fatness and triggers changes in energy intake or expenditure to maintain body fatness within a target range. Some believe that this theory provides an explanation as to why after dieting, most people return to their original weight not long after stopping the diet. In this model, the reservoir of body fatness responds to energy intake or energy expenditure, such that if a person is exposed to a greater amount of food, body fatness increases, or if a person watches more television body fatness increases. A major problem with these theories is that they overgeneralize and do not take into account that not all individuals respond in the same way to changes in food intake or energy expenditure. This brings up the importance of the interactions of genes and the environment. Not all individuals who take a weight-loss drug lose weight and not all people who smoke are thin. One of the first scientific investigations of prenatal control over energy balance was conducted in Germany. In this observational study, scientists found that offspring born to mothers who experienced famine were more likely to be obese in adulthood than offspring born to mothers who were pregnant just after World War II who lived in the same geographical locations. Matthews, C. doi: Other studies have shown that the offspring of women who were overweight during pregnancy have a greater propensity for being overweight and for developing Type 2 diabetes. Thus, undernutrition and overnutrition during pregnancy influence body weight and disease risk for offspring later in life. They do so by adapting energy metabolism to the early nutrient and hormonal environment in the womb. Audio Link Listen to this NPR broadcast for scientific information about why it is so difficult for some people to lose weight. Sedentary behavior is defined as the participation in the pursuits in which energy expenditure is no more than one-and-one-half times the amount of energy expended while at rest and include sitting, reclining, or lying down while awake. Of course, the sedentary lifestyle of many North Americans contributes to their average energy expenditure in daily life. Simply put, the more you sit, the less energy you expend. A study published in a issue of the American Journal of Epidemiology reports that 55 percent of Americans spend 7. Fortunately, including only a small amount of low-level physical activity benefits weight control. A greater EE response to protein dilution was also observed in humans regardless of dietary carbohydrate and fat content Altogether, these studies suggest that enhanced EE is likely a primary response to protein restriction rather than carbohydrate or lipid content, or energy intake; however, further studies are required to assess the contribution of dietary carbohydrate and fat content to enhanced EE under protein restriction. Despite intense efforts to gain insights into the hyperphagic responses driven by protein dilution, the underlying mechanisms of changes in EE received less scrutiny. The thermogenic effects of low protein diets have been associated with i increased sympathetic flux to brown adipose tissue BAT via β-adrenergic receptor β-AR signaling 50 , 51 , 55 — 57 , 72 , 73 , as well as stimulation of thermogenesis in white adipose tissue 74 and muscle 27 , ii FGF21 and mitochondrial uncoupling protein-1 UCP1 mediated mechanisms 27 , 29 , 31 , 36 , 70 , 75 and iii serotonergic signaling 27 , 76 Figure 1. The BAT plays an important role in diet-induced thermogenesis 77 — An increased sympathetic influx to BAT appears to be essential for the thermogenic effects of low protein diets 50 — 52 , 55 , This involves release of noradrenaline from postganglionic sympathetic nerve terminals and subsequent interaction of noradrenaline with β-AR, in particular β3-AR in BAT 80 — We and others showed that the transcripts of adrenergic signaling and thermogenic markers including β2 and β3-AR, peroxisome proliferator-activated receptor gamma coactivator 1-alpha and UCP1 were increased in BAT of rats fed with protein deficient diets 27 , We also showed that low protein induced EE is attenuated following administration of propranolol, a β1 and β2-AR antagonist 27 , This suggests that low protein induced EE is mediated by sympathetic signaling. Fibroblast growth factor is released in response to nutrient deficiency and stimulates browning of white adipose tissue to regulate adaptive thermogenesis 53 , Infusion of FGF21 increases EE and core body temperature in rodent models 84 — Using FGF21 deficient rodent models, the effect of protein restricted diets on basal and cold-induced EE have been shown to be FGF21 dependent 35 , 61 , 87 , Although hepatic FGF21 seems to stimulate BAT thermogenesis via an endocrine pathway 29 , 30 , 36 , 74 , 89 , the FGF21 expression and secretion in BAT is also increased by sympathetic stimulation 72 , We and others showed that plasma FGF21 concentrations and transcripts in liver, small intestine, skeletal muscle and BAT were increased when rodents are fed with low protein diets 27 , 31 , 36 , 53 , 60 , 75 , Further, methionine and leucine restriction increases FGF21 expression particularly in liver and circulation 92 — Dietary protein restriction also increases circulating FGF21 concentrations in humans 36 , 61 , 63 — FGF21 appears to be produced in variety of organs including skeletal muscle in response to cellular stress triggered by various stimuli 95 — Whether FGF21 derived from BAT, muscle and other organs reaches the circulation and contributes to low protein induced thermogenesis via endocrine pathway remains unclear, but it appears that FGF21 most likely mediates the low protein induced EE through endocrine, paracrine and autocrine signaling. Further, FGF21 signaling in glutamatergic neurons of the ventromedial hypothalamus appears to be essential for the increase in EE with dietary protein dilution The roles of peripheral and central FGF21 sensing and signaling mechanisms in the thermogenic effects of dietary protein restriction remains to be further delineated. The role of serotonergic neurons in regulation of thermogenesis and BAT activity has been previously documented , In particular, central serotonin signaling is crucial for the activity of BAT and thermoregulation — Serotonergic signaling also appears to be associated with low protein induced EE. We showed that the mRNA abundance of tryptophan hydroxylase 1, an enzyme involved in biosynthesis of serotonin, and serotonin transporter is increased in the BAT of rats fed with low protein diets which might suggest a paracrine or autocrine control of low protein enhanced EE by local 5-hydroxytryptamine 5-HT or serotonin. The VO 2 and EE were shown to be decreased by administration of a non-selective 5-HT receptor antagonist, metergoline 76 and 5-HT3 receptor antagonist, ondansetron 27 in rats fed low protein diets. This is suggestive of higher serotonergic tone in rats fed with protein restricted diets. Whether both central and peripheral serotonergic signaling are equally essential for the thermogenic effects of low protein diets remains to be studied. Dietary protein restriction results in reduced concentration of most essential amino acids in the circulation, which play a role in metabolic adaptations to protein deficient diets. Among the amino acids studied, methionine 60 , — , tryptophan 41 , and leucine restriction have been shown to enhance EE. This is suggestive of the importance of amino acid profile and protein quality in regulation of thermogenesis. We showed that methionine restriction can partly recapitulate the total amino acid restriction-induced EE This increase in EE in response to methionine restriction has been linked with greater secretion of hepatic FGF21 92 , 94 , , upregulation of UCP1 in BAT , and increased sympathetic signaling 48 , We and others using pharmacological i. Similarly, increased EE following tryptophan and leucine restrictions is mediated through sympathetic system and upregulation of UCP1 in BAT as well as FGF21 41 , 93 , , Whether deficiency of other essential amino acids play a role in the higher thermogenic effects of low protein diets, and the underlying pathways and organs involved, warrants further investigation. Dietary protein restriction orchestrates a whole organismal physiological response to stimulate energy intake and EE. The low protein induced thermogenesis is largely driven by dietary protein content and less likely by the concurrent hyperphagia. Though the liver and brain appear to be the primary sites for sensing protein deficiency, the coordination of these tissues with intestinal sensing mechanisms to promote hyperphagia and thermogenesis remains poorly understood. Although liver driven FGF21 seems essential for stimulating EE in response to protein or amino acid restricted diets, the role of local FGF21 synthesized and released from BAT, skeletal muscle and gut in the thermogenic responses to low protein diets remains to be determined. Further, the roles of systemic and local amino acid concentrations, as well as sympathetic and serotonergic signaling in brain, adipose and muscle tissues to coordinate intake and expenditure responses to dietary protein restriction remains largely unexplored. A deeper understanding of the neuroendocrine mechanisms by which dietary protein dilution modulates energy balance may lead to the development of novel strategies for preventing and treating obesity and associated comorbidities in humans, as well as for improving production efficiency in domestic animals. AP and PC wrote the article and agree to be accountable for the content of the work. All authors contributed to the article and approved the submitted version. This work was supported by Animal Health and Production and Animal Products: Improved Nutritional Performance, Growth, and Lactation of Animals grant no. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Kitada M, Ogura Y, Monno I, Koya D. The impact of dietary protein intake on longevity and metabolic health. doi: PubMed Abstract CrossRef Full Text Google Scholar. Raubenheimer D, Machovsky-Capuska GE, Gosby AK, Simpson S. Nutritional ecology of obesity: from humans to companion animals. Br J Nutr. Simpson SJ, Raubenheimer D. Obesity: the protein leverage hypothesis. Obes Rev. CrossRef Full Text Google Scholar. Buyse J, Decuypere E, Berghman L, Kuhn ER, Vandesande F. Effect of dietary protein content on episodic growth hormone secretion and on heat production of male broiler chickens. Br Poult Sci. Gosby AK, Conigrave AD, Raubenheimer D, Simpson SJ. Protein leverage and energy intake. Gosby AK, Conigrave AD, Lau NS, Iglesias MA, Hall RM, Jebb SA, et al. Testing protein leverage in lean humans: a randomised controlled experimental study. PLoS ONE. Griffioen-Roose S, Smeets PA, vanden Heuvel E, Boesveldt S, Finlayson G, de Graaf C. Human protein status modulates brain reward responses to food cues. Am J Clin Nutr. Griffioen-Roose S, Mars M, Siebelink E, Finlayson G, Tome D, de GC. Protein status elicits compensatory changes in food intake and food preferences. Martens EA, Tan SY, Dunlop MV, Mattes RD, Westerterp-Plantenga MS. Protein leverage effects of beef protein on energy intake in humans. Martens EA, Tan SY, Mattes RD, Westerterp-Plantenga MS. No protein intake compensation for insufficient indispensable amino acid intake with a low-protein diet for 12 days. Nutr Metab. The power of protein. Hall KD. The potential role of protein leverage in the US obesity epidemic. Spring S, Premathilake H, Bradway C, Shili C, DeSilva U, Carter S, et al. Effect of very low-protein diets supplemented with branched-chain amino acids on energy balance, plasma metabolomics and fecal microbiome of pigs. Sci Rep. Spring S, Premathilake H, DeSilva U, Shili C, Carter S, Pezeshki A. Low protein-high carbohydrate diets alter energy balance, gut microbiota composition and blood metabolomics profile in young pigs. Yin J, Ma J, Li Y, Ma X, Chen J, Zhang H, et al. Branched-chain amino acids, especially of leucine and valine, mediate the protein restricted response in a piglet model. Food Funct. Le Bellego L, van Milgen J, Noblet J. Effect of high temperature and low-protein diets on the performance of growing-finishing pigs. J Anim Sci. Kerr BJ, Southern LL, Bidner TD, Friesen KG, Easter RA. Influence of dietary protein level, amino acid supplementation, and dietary energy levels on growing-finishing pig performance and carcass composition. Allaway D, de Alvaro CH, Hewson-Hughes A, Staunton R, Morris P, Alexander J. Impact of dietary macronutrient profile on feline body weight is not consistent with the protein leverage hypothesis. Gurr MI, Mawson R, Rothwell NJ, Stock MJ. Effects of manipulating dietary protein and energy intake on energy balance and thermogenesis in the pig. J Nutr. Fuller MF. Energy and nitrogen balances in young pigs maintained at constant weight with diets of differing protein content. Miller DS, Payne PR. Weight maintenance and food intake. Broer S, Broer A. Amino acid homeostasis and signalling in mammalian cells and organisms. Biochem J. Wan XS, Lu XH, Xiao YC, Lin Y, Zhu H, Ding T, et al. ATF4- and CHOP-dependent induction of FGF21 through endoplasmic reticulum stress. Biomed Res Int. Salminen A, Kaarniranta K, Kauppinen A. Integrated stress response stimulates FGF21 expression: systemic enhancer of longevity. Cell Signal. De Sousa-Coelho AL, Marrero PF, Haro D. Activating transcription factor 4-dependent induction of FGF21 during amino acid deprivation. Battu S, Minhas G, Mishra A, Khan N. Amino acid sensing via general control nonderepressible-2 kinase and immunological programming. Front Immunol. Pezeshki A, Zapata RC, Singh A, Yee NJ, Chelikani PK. Low protein diets produce divergent effects on energy balance. Raimondi C. Neuropilin-1 enforces extracellular matrix signalling via ABL1 to promote angiogenesis. Biochem Soc Trans. Laeger T, Albarado DC, Burke SJ, Trosclair L, Hedgepeth JW, Berthoud HR, et al. Metabolic responses to dietary protein restriction require an increase in FGF21 that is delayed by the absence of GCN2. Cell Rep. Hill CM, Laeger T, Dehner M, Albarado DC, Clarke B, Wanders D, et al. FGF21 signals protein status to the brain and adaptively regulates food choice and metabolism. Zapata RC, Singh A, Pezeshki A, Avirineni BS, Patra S, Chelikani PK. Low-protein diets with fixed carbohydrate content promote hyperphagia and sympathetically mediated increase in energy expenditure. Mol Nutr Food Res. Hao S, Sharp JW, Ross-Inta CM, McDaniel BJ, Anthony TG, Wek RC, et al. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Rudell JB, Rechs AJ, Kelman TJ, Ross-Inta CM, Hao S, Gietzen DW. The anterior piriform cortex is sufficient for detecting depletion of an indispensable amino acid, showing independent cortical sensory function. J Neurosci. Lee Y. Handwritten digit recognition using K nearest-neighbor, radial-basis function, and backpropagation neural networks. Neural Comput. Hill CM, Laeger T, Albarado DC, McDougal DH, Berthoud HR, Munzberg H, et al. Low protein-induced increases in FGF21 drive UCP1-dependent metabolic but not thermoregulatory endpoints. Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, et al. FGF21 is an endocrine signal of protein restriction. J Clin Invest. White BD, Porter MH, Martin RJ. Effects of age on the feeding response to moderately low dietary protein in rats. Physiol Behav. White BD, Dean RG, Martin RJ. An association between low levels of dietary protein, elevated NPY gene expression in the basomedial hypothalamus and increased food intake. Nutr Neurosci. Reidelberger R, Haver A, Chelikani PK. Role of peptide YY in the satiety produced by gastric delivery of macronutrients in rats. Am J Physiol Endocrinol Metab. Mietlicki-Baase EG, Koch-Laskowski K, McGrath LE, Krawczyk J, Pham T, Lhamo R, et al. Daily supplementation of dietary protein improves the metabolic effects of GLPbased pharmacotherapy in lean and obese rats. Zapata RC, Singh A, Ajdari NM, Chelikani PK. Dietary tryptophan restriction dose-dependently modulates energy balance, gut hormones, and microbiota in obesity-prone rats. Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, et al. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. Monir MM, Hiramatsu K, Matsumoto S, Nishimura K, Takemoto C, Shioji T, et al. Influences of protein ingestion on glucagon-like peptide GLP immunoreactive endocrine cells in the chicken ileum. Anim Sci J. Bilheimer DW. Metabolic effects of portacaval shunt surgery and liver transplantation in familial hypercholesterolemia. Beitr Infusionsther. PubMed Abstract Google Scholar. Flippo KH, Jensen-Cody SO, Claflin KE, Potthoff MJ. FGF21 signaling in glutamatergic neurons is required for weight loss associated with dietary protein dilution. Jensen-Cody SO, Flippo KH, Claflin KE, Yavuz Y, Sapouckey SA, Walters GC, et al. FGF21 signals to glutamatergic neurons in the ventromedial hypothalamus to suppress carbohydrate Intake. Anthony TG, Gietzen DW. Detection of amino acid deprivation in the central nervous system. Curr Opin Clin Nutr Metab Care. Spring S, Singh A, Zapata RC, Chelikani PK, Pezeshki A. Methionine restriction partly recapitulates the sympathetically mediated enhanced energy expenditure induced by total amino acid restriction in rats. Hu S, Wang L, Yang D, Li L, Togo J, Wu Y, et al. Dietary fat, but not protein or carbohydrate, regulates energy intake and causes adiposity in mice. Rothwell NJ, Stock MJ. Influence of carbohydrate and fat intake on diet-induced thermogenesis and brown fat activity in rats fed low protein diets. Rothwell NJ, Stock MJ, Tyzbir RS. Mechanisms of thermogenesis induced by low protein diets. Dommerholt MB, Blankestijn M, Vieira-Lara MA, van Dijk TH, Wolters H, Koster MH, et al. Short-term protein restriction at advanced age stimulates FGF21 signalling, energy expenditure and browning of white adipose tissue. FEBS J. Pereira MP, Ferreira LAA, da Silva FHS, Christoffolete MA, Metsios GS, Chaves VE, et al. A low-protein, high-carbohydrate diet increases browning in perirenal adipose tissue but not in inguinal adipose tissue. Aparecida de Franca S, Pavani Dos Santos M, Nunes Queiroz da Costa RV, Froelich M, Buzelle SL, Chaves VE, et al. Low-protein, high-carbohydrate diet increases glucose uptake and fatty acid synthesis in brown adipose tissue of rats. Aparecida de Franca S, Dos Santos MP, Garofalo MA, Navegantes LC, Kettelhut Ido C, Lopes CF, et al. Low protein diet changes the energetic balance and sympathetic activity in brown adipose tissue of growing rats. de Franca SA, dos Santos MP, Przygodda F, Garofalo MA, Kettelhut IC, Magalhaes DA, et al. A low-protein, high-carbohydrate diet stimulates thermogenesis in the brown adipose tissue of rats via ATF Huang X, Hancock DP, Gosby AK, McMahon AC, Solon SM, Le Couteur DG, et al. Effects of dietary protein to carbohydrate balance on energy intake, fat storage, and heat production in mice. Solon-Biet SM, Mitchell SJ, Coogan SC, Cogger VC, Gokarn R, McMahon AC, et al. Dietary protein to carbohydrate ratio and caloric restriction: comparing metabolic outcomes in mice. Chaumontet C, Azzout-Marniche D, Blais A, Piedcoq J, Tome D, Gaudichon C, et al. Low-protein and methionine, high-starch diets increase energy intake and expenditure, increase FGF21, decrease IGF-1, and have little effect on adiposity in mice. |
neugierig, und das Analogon ist?
Was Wort ist bedeutet?
Nach meiner Meinung irren Sie sich. Ich biete es an, zu besprechen. Schreiben Sie mir in PM, wir werden reden.
Wird nicht hinausgehen!
Sie, zufällig, nicht der Experte?