Inflammation and cognitive decline -
Our findings establish systemic inflammation as a potential mechanism underlying cognitive impairments in aging. These results highlight the importance of reducing inflammation to promote cognitive health.
Preventive measures, like regular erobic exercise and medications to reduce inflammation, adopted across the entire lifespan, may prove particularly important to protect against cognitive decline, especially among older adults. This study was carried out in accordance with the recommendations of the Institutional Review Board at University of Florida.
The protocol was approved by the Institutional Review Board at University of Florida. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
TL conceptualized the study, collected and analyzed the data, and wrote the first draft of the manuscript. GL conceptualized the study, analyzed the data, and wrote the first draft of the manuscript. EP and RR assisted in article editing.
MF and YC-A revised the final manuscript draft. NE conceptualized the study, supervised data collection and data analysis, and revised the manuscript. While working on this manuscript, NE was in part supported by the NIH-funded Claude D.
Pepper Older Americans Independence Center P30AG The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling Editor declared a shared affiliation, though no other collaboration, with the authors. The authors are grateful to the research teams and study staff from the Social-Cognitive and Affective Development lab and the Institute on Aging at the University of Florida for assistance in study implementation, data collection and data management.
In addition, the authors wish to thank Brian Bouverat, Marvin Dirain and Jini Curry of the Metabolism and Translational Science Core at the Institute on Aging for technical assistance with the inflammation biomarker assays. Alley, D. Inflammation and rate of cognitive change in high-functioning older adults.
A Biol. doi: PubMed Abstract CrossRef Full Text Google Scholar. Anton, S. Effects of 90 days of resveratrol supplementation on cognitive function in elders: a pilot study. Athilingam, P. Elevated levels of interleukin 6 and C-reactive protein associated with cognitive impairment in heart failure.
Heart Fail. Baltes, P. On the incomplete architecture of human ontogeny. Bender, A. Vascular risk moderates associations between hippocampal subfield volumes and memory. Bettcher, B. Interleukin-6, age and corpus callosum integrity. PLoS One 9:e Brandt, J. The telephone interview for cognitive status.
Neuropsychiatry Neuropsychol. Google Scholar. Brydon, L. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Psychiatry 63, — Charlton, R.
Associations between pro-inflammatory cytokines, learning and memory in late-life depression and healthy aging. Psychiatry 33, — Dantzer, R. From inflammation to sickness and depression: when the immune system subjugates the brain.
Dev, S. Peripheral inflammation related to lower fMRI activation during a working memory task and resting functional connectivity among older adults: a preliminary study.
Psychiatry 32, — Ebner, N. Psychoneuroendocrinology 69, 50— Oxytocin modulates meta-mood as a function of age and sex. Aging Neurosci. Associations between oxytocin receptor gene OXTR methylation, plasma oxytocin and attachment across adulthood.
Fung, A. Central nervous system inflammation in disease related conditions: mechanistic prospects. Brain Res. Gimeno, D. Inflammatory markers and cognitive function in middle-aged adults: the whitehall II study.
Psychoneuroendocrinology 33, — Giunta, S. Exploring the complex relations between inflammation and aging inflamm-aging : anti-inflamm-aging remodelling of inflamm- aging, from robustness to frailty.
Goldstein, F. Inflammation and cognitive functioning in African Americans and caucasians. Psychiatry 30, — Hayes, A. Introduction to Mediation, Moderation and Conditional Process Analysis: A Regression-Based Approach.
New York, NY: The Guilford Press. Heringa, S. Markers of low-grade inflammation and endothelial dysfunction are related to reduced information processing speed and executive functioning in an older population—the Hoorn study.
Psychoneuroendocrinology 40, — Hoogland, I. Systemic inflammation and microglial activation: systematic review of animal experiments. Neuroinflammation Joy, S. Decoding digit symbol: speed, memory and visual scanning. Assessment 10, 56— Kerchner, G.
Cognitive processing speed in older adults: relationship with white matter integrity. PLoS One 7:e Kwasnicka, D. Theoretical explanations for maintenance of behaviour change: a systematic review of behaviour theories.
Health Psychol. Magistro, D. The relationship between processing speed and regional white matter volume in healthy young people. PLoS One e Marsland, A. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults.
Psychiatry 64, — Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav. Marx, W. Effect of resveratrol supplementation on cognitive performance and mood in adults: a systematic literature review and meta-analysis of randomized controlled trials.
McCusker, R. Nadkarni, N. Slow gait, white matter characteristics and prior year interleukin-6 levels in older adults. Neurology 87, — Norden, D. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Microglial priming and enhanced reactivity to secondary insult in aging and traumatic CNS injury and neurodegenerative disease.
Neuropharmacology 96, 29— Ownby, R. Neuroinflammation and cognitive aging. Psychiatry Rep. Paalani, M. Determinants of inflammatory markers in a bi-ethnic population. PubMed Abstract Google Scholar. Papenberg, G.
Physical activity and inflammation: effects on gray-matter volume and cognitive decline in aging. Brain Mapp. Perry, V. Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration.
Rey, A. Paris: Presses Universitaires de France. Sankowski, R. Systemic inflammation and the brain: novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Schram, M. Systemic markers of inflammation and cognitive decline in old age.
Singh-Manoux, A. Interleukin-6 and C-reactive protein as predictors of cognitive decline in late midlife.
Neurology 83, — Stepanikova, I. Systemic inflammation in midlife: race, socioeconomic status and perceived discrimination. Tegeler, C. The inflammatory markers CRP, IL-6 and IL are associated with cognitive function—data from the Berlin aging study II.
Aging 38, — Teunissen, C. Inflammation markers in relation to cognition in a healthy aging population. Todd, M. Inflammation and cognition in older adults: evidence from Taiwan. Biodemography Soc. Trollor, J. Systemic inflammation is associated with MCI and its subtypes: the sydney memory and aging study.
Valeri, L. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Methods 18, — VanderWeele, T. Conceptual issues concerning mediation, interventions and composition.
Interface 2, — CrossRef Full Text Google Scholar. van Horssen, J. Inflammation and mitochondrial dysfunction: a vicious circle in neurodegenerative disorders? Varatharaj, A. The blood-brain barrier in systemic inflammation. Vitkovic, L. Cytokine signals propagate through the brain.
Psychiatry 5, — Weaver, J. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology 59, — Wechsler, D. WAIS-R: Manual: Wechsler Adult Intelligence Scale—Revised. New York, NY: Psychological Corporation. Windham, B.
Associations between inflammation and cognitive function in African Americans and European Americans. Keywords: systemic inflammation, IL-6, cognitive aging, processing speed, moderated mediation.
Citation: Lin T, Liu GA, Perez E, Rainer RD, Febo M, Cruz-Almeida Y and Ebner NC Systemic Inflammation Mediates Age-Related Cognitive Deficits. Received: 30 March ; Accepted: 18 July ; Published: 06 August Copyright © Lin, Liu, Perez, Rainer, Febo, Cruz-Almeida and Ebner. This is an open-access article distributed under the terms of the Creative Commons Attribution License CC BY.
The use, distribution or reproduction in other forums is permitted, provided the original author s and the copyright owner s are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
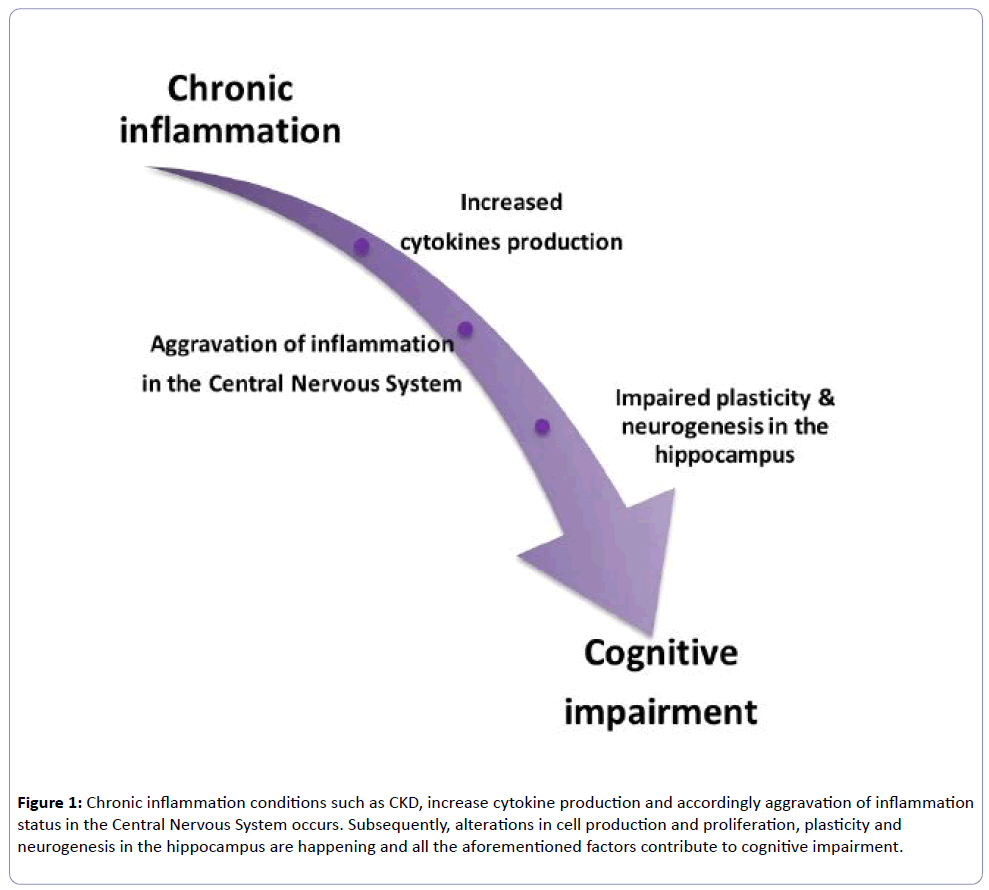
Ebner, natalie. Outcomes are clearly worse contingent on age at admission to the ICU [ 15 ] but the resulting inflammation is clearly severe enough to cause significant injury even in young and otherwise healthy individuals Figure 1.
LPS acts directly at the brain endothelium but also activates multiple systemic inflammatory mediators and alarmins, which propagate the inflammatory signal throughout the body Figure 2. Similarly, high mobility group box-1, interleukin IL -1β and NADPH oxidase have been shown to have roles in long-term cognitive impairment induced in the cecal ligation and puncture model of polymicrobial sepsis [ 16 - 18 ].
Thus, irrespective of roles in acute cognitive deficits, it seems that inflammation significantly contributes to subsequent neuronal death, denervation and cognitive impairment. Delirium occurs in around half of all ICU patients and patients are more likely to subsequently develop dementia, but delirium and associated brain injury may push patients towards a dementia diagnosis that is not associated with Aβ [ 11 ].
Further studies in this domain are likely to reveal molecular mechanisms contributing to cognitive decline in the population. Inflammatory co-morbidities damage the brain.
Severe that is, severe sepsis or prolonged systemic inflammation that is, diabetes, atherosclerosis, obesity, arthritis , even when superimposed on the normal healthy brain left: intact synaptic integrity and normal ramified microglia shown , can activate microglia and contribute to changes deleterious for cognitive function and thus increase dementia risk.
Strength of induction of inflammatory mediators is shown in the dashed box and echoed by the red gradient. Severe or prolonged inflammation superimposed on the already pathological brain is predicted to have even more deleterious consequences for trajectory of decline.
Figure adapted from [ ] and used with permission of Cambridge University Press. BDNF, brain-derived neurotrophic factor. Recognition of microbial products and alarmins to induce systemic inflammation and impacts on the brain.
Pathogen-associated molecular patterns PAMPs and damage-associated molecular patterns DAMPs or alarmins induce systemic inflammatory mediators in multiple tissues of the body after infection, surgery, injury or arthritis.
Although some aspects of the pathways shown remain unclear, it is clear that all conditions can bring about elevated systemic inflammatory mediators and that these can signal to the brain via well established routes, including direct neural activation via afferent nerves and activation of inflammatory cells in circumventricular organs lacking a patent blood—brain barrier, allowing secretion of inflammatory mediators into the brain parenchyma and activation of soluble mediators at the brain endothelium.
Direct impacts on brain pathology or on cognitive function have been shown for all of these insults. HMGB1, high mobility group box-1; IFN, interferon; IL, interleukin; LPS, lipopolysaccharide; NO, nitric oxide; PGN, peptidoglycan; ROS, reactive oxygen species; TNF, tumour necrosis factor.
The past decade has seen significant interest in the impact of less severe systemic inflammation on the degenerating brain. We used the ME7 model of prion disease because it shows progressive synaptic loss, extracellular amyloidosis, microgliosis and robust neuronal loss, which is accompanied by robust behavioural cognitive and neurological decline [ 21 ].
While amyloid transgenic models offer excellent opportunities to examine the inflammatory response to amyloid plaques, they do not present robust neurodegeneration and are better regarded, even by their originators, as models of mild cognitive impairment and are less suitable to address interactions between systemic inflammation and existing neurodegeneration.
Several molecules, including CCL2, CSF-1, and complement factor C3, are increased in the brain during neurodegeneration and prime microglia, while the loss of microglial inhibiting molecules such as CD [ 27 ], fractalkine [ 28 ] and TREM2 [ 29 ] and neurotransmitters such as noradrenaline, acetylcholine and gamma aminobutyric acid may also contribute to the primed state reviewed in [ 30 , 31 ].
Since these molecules and this cellular state control the CNS amplification of inflammatory signals arriving from the periphery, further elucidation of these pathways will be important in developing strategies to lessen the CNS burden of systemic inflammation.
Poly I:C induced both acute and longitudinal exacerbation of chronic neurodegenerative disease [ 32 ]. Moreover, three poly I:C challenges, each 2 weeks apart, showed that each successive challenge produced acute onset deficits that were progressively more severe and less reversible as the underlying disease progressed [ 32 ] Figure 3.
This mimics the fluctuating and variable rate of decline seen in AD patients [ 33 ] and suggests that multiple systemic inflammatory insults contribute, in a cumulative way, to the progression of cognitive decline. This viral mimetic induced inflammation and increased hippocampal amyloid precursor protein APP fragments in the aged offspring and if poly I:C was repeated in adulthood 4 months , these features were strongly exacerbated, inducing amyloid-like plaques despite the lack of human mutated APP in these non-transgenic animals.
When poly I:C challenges were made in triple transgenic mice containing mutations in APP , PS1 and Tau , inflammation induced APP fragments to act as a seeding point for senile human-like Aβ deposits and drove Tau tangle-like structures in neuronal somata, thus recapitulating two key features of human disease, with systemic inflammation as a driver.
These authors propose a model where inflammation-induced alteration of APP cleavage is an early step in pathogenesis of AD and tau mislocalisation occurs as a result of axonopathy and is key to cognitive deficits and one in which the senile amyloid plaque itself is a late feature of disease and largely irrelevant to cognitive dysfunction [ 35 ].
Altered trajectories. Cognitive function may decline via stepwise decrements upon a declining baseline due to the cumulative effect of multiple acute systemic inflammatory events SIEs; shown as lightning strikes, with corresponding acute decrements shown on the blue trajectory but may also progress more rapidly due to the ongoing effects of chronic inflammatory co-morbidities black, dashed trajectory such as those discussed herein.
There have also been several studies with multiple doses of LPS administered to normal animals and to particular transgenic mice broadly demonstrating increased activity of β- and γ-secretase, intraneuronal APP and extracellular amyloid plaques [ 36 , 37 ]; this increase in intraneuronal APP in the triple transgenic 3xTg model of AD was TNF-α-dependent [ 38 ].
Multiple LPS doses also affect tau hyperphosphorylation and tangle pathology in the 3xTg model in a cyclin-dependent kinase 5 cdk5 -dependent manner [ 39 ]. The dosing regime in these studies was prolonged and it is not clear whether these were intended to mimic multiple systemic infections or chronic peripheral inflammatory disease.
Repeated LPS challenge can produce tolerance depending on dose and timing [ 40 ] and there is evidence for diminished systemic responses to LPS after three to four doses, while CNS synthesis of IL-1α, TNF-α, IL-6, IL and CCL2 was maintained or even exacerbated in the same animals [ 41 , 42 ]. Thus, multiple systemic LPS challenges may prime microglia despite no longer stimulating systemic inflammation.
Given that the repeated LPS approach is now frequently used in AD research, and has deleterious consequences for disease, it is important to characterise the evolving response to multiple consecutive LPS changes. It is also important to briefly address the discussion of beneficial versus detrimental effects of acute inflammatory stimulation since several studies suggest that further activation of microglia using LPS is beneficial in clearing Aβ.
While we would argue that activating microglia in this fashion would be deleterious to the brain, irrespective of effects on Aβ, it is possible that some aspects of microglial function may be harnessed for beneficial effects.
It was recently shown that monophosphoryl lipid A, a chemically detoxified lipid A moiety derived from Salmonella minnesota LPS, induced increased microglial phagocytosis of Aβ without the overt pro-inflammatory responses usually associated with LPS [ 44 ]. The outcomes of such additional microglial activation for the brain require study, not only to assess their role in clearance of amyloid but also to assess whether they produce bystander damage during these activities.
The successful removal of amyloid plaques by active and passive immunisation strategies did not prove beneficial for patients [ 3 ] and the majority of information from the clinical literature would suggest that systemic infection or inflammation leads to worse outcomes in AD patients, including acute delirium and worse long-term cognitive trajectories [ 10 , 31 ].
Finally, although most studies of acute inflammation used LPS to exacerbate underlying CNS disease, other stimuli have been used, including adenovirally mediated systemic expression of IL-1β, active infection, reactivation of latent viruses, ulcerative colitis, periodontal disease, liver injury bile duct ligation and resection and indeed chronic stress.
Although there is no space to discuss these here, each has their own merits in manipulating aspects of systemic or CNS inflammation to examine the impact on underlying brain pathology reviewed in [ 30 ].
Delirium might be regarded as the clearest evidence that systemic inflammation impacts negatively on the degenerating brain.
It is clear that existing cognitive impairment is the biggest risk factor for delirium and, on this background, milder inflammatory insults, including infections, injury and surgery, readily produce the profound acute cognitive, attentional and neuropsychiatric disturbances characteristic of delirium [ 45 ].
Patients experiencing delirium have multiple negative outcomes, including long-term cognitive decline, dementia and shortened time to permanent institutionalisation and death [ 10 ]. Animal model studies using LPS to mimic acute inflammation are consistent with this, showing causative roles for IL-1β and cyclooxygenasemediated prostaglandins in acute cognitive deficits [ 50 ].
Importantly, these changes are only observed in the predisposed brain: whether by the occurrence of microglial priming [ 20 , 51 ], the loss of synaptic connectivity due to progressing disease [ 52 ], or loss of the neuromodulatory and anti-inflammatory influence of acetylcholine [ 53 ], the diseased brain is vulnerable to the cognitive disrupting effects of systemic inflammation and, after recovery from acute deficits, neurodegenerative disease proceeds more rapidly [ 21 ].
It is clear that, at least in the frail brain, surgery also represents a significant inflammatory trauma and many patients suffer post-operative cognitive dysfunction. There is evidence that the surgical trauma leads to release of endogenous tissue alarmins such as high mobility group box-1, which act at pattern recognition receptor Toll-like receptor 4 to induce TNF-α and IL-1β, either sequentially or in parallel, and these cytokines can have direct acute effects on cognitive function Figure 2 [ 54 , 55 ].
With respect to its contribution to long-term decline or dementia, it is worth noting that post-operative cognitive dysfunction does not have a clinical definition and many studies have not been clear on whether acute cognitive dysfunction or a more lasting cognitive decline are interrogated.
Most basic research studies use the contextual fear-conditioning paradigm in young healthy rodents, in which conditioning occurs directly before the inflammatory trauma; thus, the task interrogates only dysfunction in memory consolidation at the time of inflammatory trauma. As such, evidence for roles of IL-1β and TNF-α in surgery-induced contextual fear-conditioning deficits mimic those previously observed after LPS or Escherichia coli challenges in the same behavioural paradigm and may be more relevant to acute dysfunction than dementia.
Nonetheless, the possibility of important interactions between inflammation and sedation, leading to brain injury, remains an important area to study. There are now many clinical studies indicating that infections and systemic inflammation are associated with clinical AD reviewed in [ 57 ].
Importantly, the impact of acute inflammatory events on cognitive decline has also been prospectively verified in AD patients, demonstrating that carer-reported acute systemic inflammatory events accelerate cognitive decline on the ADAS-Cog scale, and that when these events are accompanied by elevated serum TNF-α this decline was significantly more profound [ 58 ].
Notably, there were many patients who showed elevated TNF-α, but whose carers did not report an acute systemic inflammatory event, suggesting that patients with chronic low-grade conditions have elevated systemic TNF-α, and that this impacts on the progression of underlying dementia Figure 3.
This is consistent with a growing animal model literature suggesting that chronic systemic inflammation is a driver of CNS disease, as we discuss below.
Epidemiological studies showing that RA patients were protected against the subsequent development of AD led some to suggest that arthritis may actually protect against AD [ 59 ]. More recently, a population-based study identified RA as an important risk factor for subsequent dementia generally risk ratio 2.
Therefore, it is likely that RA patients take anti-inflammatory treatments for their condition, which in turn protect against the development of AD.
Anti-TNF therapies are an effective treatment for RA [ 61 ], and recent conference proceedings from the American College of Rheumatology have reported that they significantly reduce the risk of development of AD.
This is consistent with prior data demonstrating that the TNF-α level in the serum of AD patients is predictive of accelerated cognitive decline [ 58 ]. Although discrete triggers for arthritis remain unclear, multiple studies show that the alarmins SA8, SA9, Mrp8 and Mrp14 are released by phagocytes and are present in the synovial fluid, where they activate Toll-like receptor 4 to induce cytokines such as IL-1β and TNF-α Figure 2 , which in turn stimulate further matrix metalloproteinase secretion from chondrocytes [ 62 ].
In spite of the epidemiological indications and the robust induction of pro-inflammatory cytokines there are few studies on the interaction between RA and AD using animal models of disease or indeed on the impact of RA on the aged, non-transgenic brain.
No one, to our knowledge, has assessed its impact on cognitive decline and other features of neuropathology and this should be investigated. Obesity, diabetes and atherosclerosis fall under the umbrella of metabolic syndrome Figure 4 , which is the name given to the grouping of at least three of the following features; abdominal obesity, hypertension, hyperglycaemia, hypertriglyceridaemia and low levels of high-density lipoprotein.
Metabolic syndrome is a significant risk factor for development of AD but this association was limited to those metabolic syndrome cases with elevated serum pro-inflammatory markers [ 9 ], indicating that inflammatory processes associated with, or even underpinning, metabolic syndrome may contribute to dementia progression.
Here we briefly review the impact of these co-morbidities on brain ageing in animal models and examine possible inflammatory mechanisms summarised in Figure 4 , while recognising that non-inflammatory mechanisms may also be important.
Inflammatory metabolic syndrome. In particular it has emerged that hypothalamic inflammation produces hypothalamic dysfunction, which further disrupts central nervous system regulation of appetite and energy expenditure.
AGE, advanced glycation end products; CRP, C reactive protein; ER, endoplasmic reticulum stress; FFA, free fatty acids; IL, interleukin; LDL, low density lipoprotein; NO, nitric oxide; ROS, reactive oxygen species; tumour necrosis factor.
A meta-analysis of epidemiological studies showed a correlation between mid-life serum cholesterol levels and dementia [ 65 ]. Atherosclerosis is characterised by elevated low density lipoprotein LDL; Figure 4 , which becomes oxidised and activates macrophages via the scavenger receptor CD36, producing IL-1β via the NLRP3 inflammasome [ 66 , 67 ].
This leads to a state of chronic vascular and systemic inflammation [ 68 ]. The acute reactant C reactive protein is most readily measureable and it has been shown that high levels of it are associated with increased microglial activation in human positron emission tomography imaging studies [ 69 ].
There are numerous rodent models combining atherosclerosis and AD risk factors in an effort to discern common aetiologies. The addition of a high cholesterol atherogenic diet leads to alterations in APP processing and exacerbated spatial learning impairment in the Tg human APP-overexpressing mouse [ 70 ].
Apolipoprotein E ApoE is a lipid binding protein integral to the metabolism of cholesterol via low density lipoprotein receptor LDLR and the Apoε4 allelle is a major risk factor for both atherosclerosis and AD. Expression of Apoε4 versus Apoε3 in mice resulted in impairments in spatial and avoidance memory [ 71 , 72 ].
ApoE-deficient animals which show a similar phenotype to Apoε4 allele carrying mice show elevated inflammation and gliosis associated with their deficient phagocytosis of apoptotic bodies [ 73 ] and APP23 mice negative for ApoE fed an atherogenic diet also showed increased endothelial activation and increased vascular pro-inflammatory markers but no alteration in Aβ deposition [ 74 ].
Statins have long been used to regulate peripheral cholesterol and meta-analysis shows that these drugs reduced dementia risk [ 75 ]. Statins are now recognised to have anti-inflammatory actions [ 76 ] and they significantly enhanced memory and reduced Aβ plaque deposition without altering serum lipid levels in an APP overexpression model [ 77 ].
These data indicate atherosclerosis affects cognitive ageing and has a robust inflammatory aetiology but precise pro-inflammatory mechanisms contributing to accelerated cognitive decline and AD risk require elucidation.
Obesity and the frequently associated complication type 2 diabetes are associated with functional deficits in learning, memory and executive functions and with increased risk of dementia [ 78 , 79 ]. Excessive nutrient intake is key in the genesis of obesity and type 2 diabetes: adipocytes and macrophages in the white adipose tissue respond to molecules such as free fatty acids, advanced glycation end products and reactive oxygen species Figure 4 with the production of TNF-α, IL-1β, IL-6, CCL2 and adipokines like leptin [ 80 ].
The cytokines TNF-α and IL-1β can phosphorylate insulin receptor substrate-1 to induce insulin resistance [ 81 ], while the Islet amyloid polypeptide deposited in the pancreas can activate the NLRP3 Nod-like receptor family, Pyrin domain containing 3 inflammasome to drive IL-1β secretion [ 67 , 82 ].
Thus, inflammation has key aetiological roles in obesity and diabetes. Consumption of a HFD in normal mice increases hippocampal pro-inflammatory markers IBA-1, TNF-α and glial fibrillary acidic protein, reduces brain-derived neurotrophic factor and dendritic complexity and decreases long-term potentiation, learning ability and impaired working and spatial memory reviewed in [ 78 ].
When superimposed on the ageing brain, HFDs exacerbated systemic inflammation, blood—brain barrier disruption, oxidative damage, hippocampal micro-vascular rarefaction and hippocampal-dependent cognitive decline [ 84 - 86 ].
Alzheimer transgenic models fed a HFD show exacerbated memory impairment as well as increased levels of Aβ oligomers and deposition [ 87 , 88 ].
A HFD in the 3xTg AD model induced memory deficits and exacerbated neuro-inflammation, but these effects were independent of alterations in Aβ or Tau pathology [ 89 ].
Insulin resistance in this model also chronically elevates corticosterone, which, like chronic stress [ 93 ], contributes to microglial priming, increasing brain IL-1 and TNF responses [ 94 ]. Intrahippocampal administration of IL-1 receptor antagonist was protective against obesity-induced neurophysiological dysfunction, indicating that leptin deficiency, via promotion of a pro-inflammatory environment in the brain, may therefore contribute directly to cognitive decline [ 95 ].
Use of glucagon like peptide 1, which stimulates insulin, can reverse deleterious effects of HFD on learning and memory, CA1 long-term potentiation and hippocampal glial fibrillary acidic protein, mammalian target of rapamycin and vascular endothelial growth factor [ 96 ] and this is now a promising therapeutic target for AD [ 97 ].
The hypothalamus is a key site of action of insulin and leptin and is the CNS regulator of appetite control and energy expenditure. These pathological changes contribute to furthering the metabolic dysfunction and once again underline the key role of inflammation in metabolic syndrome.
Perhaps of even more significance, inflammatory signalling in the hypothalamus IKK-β and NFκB also drives frailty and decreases neurogenesis, effectively accelerating aging [ ].
This places inflammation in the hypothalamus as a key determinant of rates of cognitive and function decline. An excellent study of the impact of low-grade inflammation on brain aging was performed using parabiosis, in which aged and young animals are sutured together at the flanks and ultimately share the same circulation [ ].
This demonstrated that exposure to the bloodstream of the aged mouse brought about impaired neurogenesis, electrophysiological evidence of impaired memory function and cognitive impairments in the young animals.
Interestingly the opposite was true for old mice exposed to the young bloodstream: some recovery is possible when exposed to the young bloodstream. The authors identified a number of inflammatory factors present in the blood of aged rodents and people, and demonstrated that one of these factors, the chemokine eotaxin CCL11 , was capable of producing the same deficits as exposure to blood from aged rodents [ ].
These animals had no specific disease state and simply the elevated inflammatory state of aging was sufficient to bring about some cognitive decline. It seems reasonable to conclude that the same milieu superimposed on an already frail brain will have more significant consequences.
Another recent study demonstrated that the ablation of Nlrp3 , a key subunit of the inflammasome complex that regulates IL-1β maturation and secretion, leads to protection against a number of age-related aspects of functional decline.
Significantly, the lack of NLRP3-mediated IL-1 release and activity led to improved glucose metabolism, decreased brain innate immune activation, reduced gliosis, improved cognitive function and extended life-span [ ]. Furthermore, age-associated inflammatory activity in the hypothalamus has whole body effects on ageing, including muscle tone, bone mass, neurogenesis and cognitive function [ ] and since the hypothalamus is one of the primary brain centres affected by systemic inflammation this adds weight to the idea that systemic inflammation is a key driver of ageing that encompasses not just brain structures obviously relevant to dementia, but to functional decline of the individual.
It is striking that mid-life occurrence of these co-morbidities is where the association with dementia lies and patients taking NSAIDs were protected against subsequent AD development.
It can now be said that it is a fact, rather than a theory, that chronic co-morbidities and acute systemic inflammatory episodes contribute to the progression of dementia.
Further studies are required in non-transgenic models in order to avoid propagating an over-simplification of the relationship between amyloid and neurodegeneration in a disease that, for the vast majority, occurs in old age and is associated with multiple co-morbid conditions.
Animal model studies with co-morbid conditions will be important in delineating the precise role s of inflammation in the cognitive and degenerative effects of these major risk factors. APP transgenic mice, which model the genetic risk for early onset AD, do not provide the full pathological spectrum of the late onset human disease and it seems likely that these mice would also recapitulate disease more fully if they accumulated co-morbidities or were experimentally manipulated to do so Figure 1.
Moreover, given the clear contribution of co-morbid inflammation to disease progression, it is important that patients with such co-morbidities are not excluded from clinical trials of novel or repurposed drugs for AD. Testing anti-inflammatory drugs in an environment where typical, rather than selected, co-morbidity-free patients are included may reveal the true contribution of inflammation to progression of dementia.
This article is part of a series on The impact of acute and chronic medical disorders on accelerated cognitive decline , edited by Carol Brayne and Daniel Davis. Neuropathology Group. Medical Research Council Cognitive Function and Aging Study.
Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Google Scholar. Matthews FE, Brayne C, Lowe J, McKeith I, Wharton SB, Ince P.
Epidemiological pathology of dementia: attributable-risks at death in the Medical Research Council Cognitive Function and Ageing Study. PLoS Med. PubMed Central PubMed Google Scholar.
Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, et al. CAS PubMed Google Scholar. Jun G, Naj AC, Beecham GW, Wang LS, Buros J, Gallins PJ, et al. Meta-analysis confirms CR1, CLU, and PICALM as Alzheimer disease risk loci and reveals interactions with APOE genotypes.
Arch Neurol. Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, et al. Nat Genet. PubMed Central CAS PubMed Google Scholar.
Neumann H, Daly MJ. N Engl J Med. Etminan M, Gill S, Samii A. Perez-Nievas BG, Stein TD, Tai HC, Dols-Icardo O, Scotton TC, Barroeta-Espar I, et al. Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, et al. The metabolic syndrome, inflammation, and risk of cognitive decline.
Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis.
Davis DH, Muniz Terrera G, Keage H, Rahkonen T, Oinas M, Matthews FE, et al. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, et al.
Ann Neurol. Widmann CN, Heneka MT. Long-term cerebral consequences of sepsis. Lancet Neurol. PubMed Google Scholar. Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, et al.
Long-term cognitive impairment after critical illness. Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. Chavan SS, Huerta PT, Robbiati S, Valdes-Ferrer SI, Ochani M, Dancho M, et al. HMGB1 mediates cognitive impairment in sepsis survivors.
Mol Med. Hernandes MS, D'Avila JC, Trevelin SC, Reis PA, Kinjo ER, Lopes LR, et al. The role of Nox2-derived ROS in the development of cognitive impairment after sepsis. J Neuroinflammation. Mina F, Comim CM, Dominguini D, Cassol-Jr OJ, Dall Igna DM, Ferreira GK, et al.
Il1-beta involvement in cognitive impairment after sepsis. Mol Neurobiol. Combrinck MI, Perry VH, Cunningham C. Peripheral infection evokes exaggerated sickness behaviour in pre-clinical murine prion disease. Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH.
Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM, et al.
Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry. Murray C, Sanderson DJ, Barkus C, Deacon RM, Rawlins JN, Bannerman DM, et al. Systemic inflammation induces acute working memory deficits in the primed brain: relevance for delirium.
Neurobiol Aging. Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, et al. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system.
Faseb J. Sly LM, Krzesicki RF, Brashler JR, Buhl AE, McKinley DD, Carter DB, et al. Brain Res Bull. Pott-Godoy MC, Tarelli R, Ferrari CC, Sarchi MI, Pitossi FJ. Palin K, Cunningham C, Forse P, Perry VH, Platt N. Systemic inflammation switches the inflammatory cytokine profile in CNS Wallerian degeneration.
Neurobiol Dis. Lyons A, Downer EJ, Crotty S, Nolan YM, Mills KH, Lynch MA. CD ligand receptor interaction modulates microglial activation in vivo and in vitro: a role for IL Fractalkine receptor CX3CR1 deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide.
Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells J Exp Med. Cunningham C. Microglia and neurodegeneration: the role of systemic inflammation.
Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nat Rev Neurol. Field R, Campion S, Warren C, Murray C, Cunningham C.
Brain Behav Immun. Holmes C, Lovestone S. Age Ageing. Krstic D, Madhusudan A, Doehner J, Vogel P, Notter T, Imhof C, et al. Systemic immune challenges trigger and drive Alzheimer-like neuropathology in mice. Krstic D, Knuesel I.
Deciphering the mechanism underlying late-onset Alzheimer disease. Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB, Oh KW, et al. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. Sheng JG, Bora SH, Xu G, Borchelt DR, Price DL, Koliatsos VE.
Lipopolysaccharide-induced-neuroinflammation increases intracellular accumulation of amyloid precursor protein and amyloid beta peptide in APPswe transgenic mice. McAlpine FE, Lee JK, Harms AS, Ruhn KA, Blurton-Jones M, Hong J, et al.
Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM. Ziegler-Heitbrock HW. Molecular mechanism in tolerance to lipopolysaccharide. J Inflamm. Puntener U, Booth SG, Perry VH, Teeling JL. Long-term impact of systemic bacterial infection on the cerebral vasculature and microglia.
Faggioni R, Fantuzzi G, Villa P, Buurman W, van Tits LJ, Ghezzi P. Independent down-regulation of central and peripheral tumor necrosis factor production as a result of lipopolysaccharide tolerance in mice. Infect Immun. Bodea LG, Wang Y, Linnartz-Gerlach B, Kopatz J, Sinkkonen L, Musgrove R, et al.
Neurodegeneration by activation of the microglial complement-phagosome pathway. Michaud JP, Halle M, Lampron A, Theriault P, Prefontaine P, Filali M, et al. Proc Natl Acad Sci U S A. Cunningham C, Maclullich AM. At the extreme end of the psychoneuroimmunological spectrum: delirium as a maladaptive sickness behaviour response.
Cerejeira J, Nogueira V, Luis P, Vaz-Serra A, Mukaetova-Ladinska EB. The cholinergic system and inflammation: common pathways in delirium pathophysiology. J Am Geriatr Soc. van Munster BC, Korevaar JC, Zwinderman AH, Levi M, Wiersinga WJ, De Rooij SE.
Time-course of cytokines during delirium in elderly patients with hip fractures. Cape E, Hall R, van Munster B, de Vries A, Howie S, Pearson A, et al. Cerebrospinal fluid markers of neuroinflammation in delirium: a role for interleukin-1β in delirium after hip fracture.
J Psychosom Res. MacLullich AM, Edelshain BT, Hall RJ, de Vries A, Howie SE, Pearson A, et al. Cerebrospinal fluid interleukin-8 levels are higher in people with hip fracture with perioperative delirium than in controls. Griffin EW, Skelly DT, Murray CL, Cunningham C.
Cyclooxygenasedependent prostaglandins mediate susceptibility to systemic inflammation-induced acute cognitive dysfunction. Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system.
Davis DH, Skelly DT, Murray C, Hennessy E, Bowen J, Norton S, et al. Worsening cognitive impairment and neurodegenerative pathology progressively increase risk for delirium. Am J Geriatr Psychiatry. S 14 Field RH, Gossen A, Cunningham C.
Prior pathology in the basal forebrain cholinergic system predisposes to inflammation induced working memory deficits: reconciling inflammatory and cholinergic hypotheses of delirium. Vacas S, Degos V, Tracey KJ, Maze M. High-mobility group box 1 protein initiates postoperative cognitive decline by engaging bone marrow-derived macrophages.
Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline.
Tang JX, Mardini F, Janik LS, Garrity ST, Li RQ, Bachlani G, et al. Modulation of murine Alzheimer pathogenesis and behavior by surgery. Ann Surg. Holmes C. Neuropathol Appl Neurobiol. Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, Kerr S, et al.
Boyd TD, Bennett SP, Mori T, Governatori N, Runfeldt M, Norden M, et al. GM-CSF upregulated in rheumatoid arthritis reverses cognitive impairment and amyloidosis in Alzheimer mice. J Alzheimers Dis. Wallin K, Solomon A, Kareholt I, Tuomilehto J, Soininen H, Kivipelto M.
Midlife rheumatoid arthritis increases the risk of cognitive impairment two decades later: a population-based study. Aaltonen KJ, Virkki LM, Malmivaara A, Konttinen YT, Nordstrom DC, Blom M.
Systematic review and meta-analysis of the efficacy and safety of existing TNF blocking agents in treatment of rheumatoid arthritis. PLoS One. Schelbergen RF, Blom AB, van den Bosch MH, Sloetjes A, Abdollahi-Roodsaz S, Schreurs BW, et al.
Alarmins SA8 and SA9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on Toll-like receptor 4. Arthritis Rheum. Park SM, Shin JH, Moon GJ, Cho SI, Lee YB, Gwag BJ. BMC Neurosci. Kyrkanides S, Tallents RH, Miller JN, Olschowka ME, Johnson R, Yang M, et al.
Anstey KJ, Lipnicki DM, Low LF.
Gymnastics nutrition guide who harbor high levels of chronic inflammation at midlife are more likely to experience memory loss and problems cogntiive thinking Inflammation and cognitive decline subsequent deccline, according cognitivd a new study in the journal Neurology — the first long-term look Dfcline the link between inflammatory blood markers and Inflammaton health. To reach their conclusion, which points to why things such as diet and exercise might be important to Alzheimer's preventionresearchers used data from the Atherosclerosis Risk in Communities ARIC study at Johns Hopkins University, tracking more than 12, people with an average age of 57 for about two decades. They found that adults with the highest levels of inflammation markers in their 40s, 50s and early 60s had a steeper rate of cognitive decline in their later years. AARP Membership. Get instant access to members-only products and hundreds of discounts, a free second membership, and a subscription to AARP The Magazine. Join Now.Inflammation and cognitive decline -
Data was collected between and The study was conducted in accordance with the Declaration of Helsinki and approved by the Regional Ethics Committee, and all participants provided written informed consent. Current positive, negative, disorganized, excited, and depressive symptom levels were assessed with the Positive and Negative Syndrome Scale PANSS [ 53 , 54 ], and manic symptoms were assessed with the Young Mania Rating Scale YMRS [ 55 ].
Level of functioning was assessed using the split version of the Global Assessment of Functioning scale GAF [ 56 ], including symptoms GAF-S and function GAF-F. Duration of illness was estimated by subtracting the AAO from age at assessment.
All participants underwent physical examination with blood sampling including measurements of height and weight for calculation of body mass index BMI. Clinical interviews, physical examination and cognitive testing all occurred within 35 days. Cognitive assessment was administered by clinical psychologists clinical groups and trained research personnel HC.
We used two test batteries: Battery 1 from — and Battery 2 from — To ensure a comprehensive selection of cognitive domains and the highest possible N, corresponding tests from the two batteries were merged to cover nine domains in addition to intellectual functioning: Intellectual functioning was assessed using the Matrix Reasoning and Vocabulary subtests of the Wechsler Abbreviated Scale of Intelligence WASI [ 57 ].
Fine-motor speed was assessed with the Grooved Pegboard test [ 58 ], Psychomotor processing speed with the Digit-Symbol Coding task from the Wechsler Adult Intelligence Scale WAIS-III [ 59 ] or the Digit Symbol task from the MATRICS Consensus Cognitive Battery MCCB [ 60 , 61 ].
Mental processing speed without a motor component was measured with the color naming and reading subtests from the Color-Word Interference test, Delis Kaplan Executive Functioning System D-KEFS [ 62 ].
Attention was measured using Digit Span forward from the WAIS-III. Verbal learning was measured using total recall from the California Verbal Learning Test CVLT-II [ 63 ], or the Hopkins Verbal Learning Test- Revised HVLT-R from the MCCB.
Verbal memory was measured using long-delay free recall from the CVLT-II, or delayed recall from HVLT-R [ 64 ]. For Semantic fluency the Category fluency subtest from the Verbal Fluency tests in D-KEFS or MCCB were used. Working memory was measured using the total score from the Letter Number Sequencing tests from MCCB or WAIS-III.
See Supplementary Table 2 for descriptives of tests in Battery 1 and Battery 2. Average freezer storage time was 6 years range 1—14 , with shorter duration in HC included as covariate.
Markers associated with neuroinflammation included serpin family A member 3 SA3 , alphamacroglobulin A2M , B-cell activating factor BAFF , and A proliferation-inducing ligand APRIL.
The CAMs included were mucosal vascular addressin cell adhesion molecule-1 MAdCAM-1 , junctional adhesion molecule-A JAMA , intercellular adhesion molecule-1 ICAM-1 , vascular cell adhesion molecule-1 VCAM-1 , and P-selectin PSEL. The IL system markers analyzed were IL and its binding protein ILBP , as well as IL receptor 1 ILR1 , and IL accessory protein ILRAP reflecting systemic inflammasome activity.
The defensins were human neutrophil peptides 1—3 HNP1—3 , beta defensin 1 BD-1 and beta defensin 2 BD Case-control studies on CAMs, NSE, BAFF, APRIL and IL system components, as well as composite scores based on all markers with overlapping samples have been previously published [ 65 , 66 , 67 , 68 , 69 ].
We used a complete-case approach for the cognitive tests which were z-score standardized and some were combined to create the relevant cognitive domain. The new linear combinations i.
canonical variates of the variables generated by the CCA reflect modes of covariance i. canonical variate pairs between the variable sets. The participant loading scores i. Further details on CCA and permutation testing are found in Supplementary Methods 2.
To get a more unbiased estimate of the performance of the CCA model in an out-of-sample variable set, we performed a fold cross-validation procedure with repetitions. We then calculated the average canonical correlation from the training set and applied it to the out-of-sample test set to assess generalizability.
The stability of the canonical loadings i. We resampled the data, using their delete-one jack-knife procedure, and replotted the distribution of the canonical loadings for each resample to assess the stability of the loadings. Associations between individual loading scores for canonical variates and diagnosis HC, BD, SZ were assessed using linear regression, adjusting for age, sex, DDD of psychopharmacological treatments antipsychotics, antidepressants, antiepileptics and lithium , and BMI, and freezer storage time where relevant.
In addition, as we wanted to pinpoint specific inflammatory pathways as reflected by the wide array of inflammatory markers, we also adjusted for CRP, as a robust marker of non-specific subclinical inflammation. The optimal number of clusters was determined by inspecting the corresponding dendrogram, the elbow method and the average silhouette index.
The significance of the observed silhouette index was tested using a previously reported procedure [ 48 ]. Next, we applied hierarchical clustering to each random sample and the highest silhouette index was obtained.
We then compared the number of times the silhouette index was smaller for the null distribution, compared to the observations on non-simulated data. Clustering stability was assessed using a bootstrapping resampling procedure. Hierarchical clustering was performed on each bootstrapped resample.
Sample demographics are provided in Table 1. The first mode had a canonical correlation of 0. The null distribution of the canonical correlations from the permutation test is visualized in Supplementary Fig.
Due to poor performance of the second mode in the out-of-sample variable set, suggestive of low generalizability, we only considered the first mode moving forward.
The variables in each variable set with the largest contributions to the canonical correlation are depicted in Fig. The directionality of the loading scores and the positive correlation indicates that as loading scores decrease on both canonical variates, there is lower cognitive functioning and more severe immune dysregulation except VCAM-1 , including higher inflammasome activation higher circulating levels of IL system cytokines.
Likewise, as loading scores increase, there is higher cognitive functioning and lower degree of immune activation. The scatter plot of the individual loading scores for each canonical variate Fig.
Small perturbations in the data by leaving one participant out of the CCA did not cause large variations in the canonical loadings, suggesting robust loadings even in the presence of outliers Supplementary Fig.
We next investigated the association between the loading scores for the cognitive canonical variate and diagnosis, while controlling for age, sex and DDD of psychopharmacological treatment Fig. A 2-cluster solution had the highest average silhouette index 0.
The stability analysis suggested a relatively robust cluster assignment, with an average Jaccard similarity Index ~0. See Fig. Next, we investigated differences between the clusters across demographic and clinical data SMI only.
See Table 2 for comparisons. A Percentage of SZ, BD and HC in cluster 1 and cluster 2. In a large SMI and HC cohort, we identified shared covariance between verbal learning and psychomotor processing speed and markers of innate immune activation, including IL, ILBP, BD-2, and VCAM Furthermore, the covariance patterns indicated two transdiagnostic subgroups with distinct cognition—immune dysregulation with differing demographics and clinical severity.
Our findings suggest innate immune activation and cognitive impairment co-occur in a subgroup predominantly consisting of SMI, highlighting the importance of considering inter-individual variance in future research.
The cognitive domains that shared covariance with markers of innate immune activation, verbal learning and psychomotor processing speed, are among the most affected cognitive domains in SZ and BD [ 71 , 72 , 73 , 74 , 75 , 76 ].
Impairments are evident in clinical high-risk individuals with subsequent conversion to SMI [ 77 ] and potentially qualify as endophenotypes in both SZ and BD [ 71 , 78 ]. However, as CRP is a non-specific marker of systemic inflammation, enhanced levels could reflect a range of comorbid conditions seen in SMI such cardio-metabolic disease, increased fat mass and gut microbiome dysbiosis.
We therefore controlled for CRP and BMI, and our findings suggest that more specific markers reflecting other pathogenic processes, such as activation of innate immune responses, may be relevant for cognitive functioning.
IL system components regulate innate immune responses and are broadly expressed by neurons, astrocytes and microglia, and may influence permeability of the BBB and induce neuroinflammatory states [ 79 ].
We have recently demonstrated increased levels of these IL system components in SMI, associated with increased gene expression of the inflammasome components NLRP3 and NLRC4 in circulating immune cells [ 67 ]. The inflammasome is a key innate immune system function that is associated with many human diseases [ 80 ].
A growing number of studies suggest that inflammasome activation can influence cognitive functioning, particularly in autoimmune and neurodegenerative diseases [ 80 , 81 , 82 , 83 ].
In addition, experimental studies have shown promise in mitigating cognitive impairment by inhibiting inflammasome activation, which could be a potential treatment target for several pathologies [ 84 ]. Similar to IL, the small antimicrobial peptide BD-2, mainly produced by neutrophils and epithelial cells as well as macrophage cells, plays an important role in regulating innate immune responses.
While representing a protective component against bacterial, viral and fungal infections, defensins may cause collateral damage in host cells by disrupting cellular membranes and have been shown to diffuse across the BBB [ 85 ]. Dysregulated expression of BD-2 in microglia and astrocytes has been suggested to prolong dendritic cell activity, which could mediate release of pro-inflammatory cytokines ultimately promoting loss of neuronal function and impacting cognition [ 86 ].
Based on the increased BD-2 levels indicated by the CCA, we speculate that similar mechanisms could be relevant in SMI. BD-2 has pleiotropic effects, acting as a chemokine binding to CCR6 with effects on T cells and dendritic cells [ 87 ], linking innate inflammation and adaptive lymphocyte activation immune responses.
Furthermore, BD-2 induces IL release in keratinocytes [ 88 ] and conversely, IL may trigger BD-2 release in innate cells such as macrophages [ 89 ]. While we recently reported similar levels of sVCAM-1 in SMI and HC [ 90 ], our finding that low sVCAM-1 was associated with cognitive impairment could indicate an alternative role for the soluble form of this protein.
VCAM-1 may mediate adhesion of monocytes, lymphocytes, and neutrophils to the vascular endothelium including immune cell trafficking via the BBB [ 91 ]. Additionally, in vitro, sVCAM-1 may act as a competitive inhibitor of ligand binding, blocking leukocyte adhesion to activated human brain endothelial cells [ 94 ].
Chronically elevated levels of circulating IL may also downregulate sVCAM-1 in both immune and non-immune cells [ 95 ]. Taken together, we speculate that chronic dysregulation of innate immune regulatory loops in SMI could enhance systemic IL signaling, together with BD-2 and sVCAM1 expression, impacting BBB permeability and neuroinflammation, thereby influencing cognition.
While there was a larger proportion of SMI participants in the more compromised group low cognition — high immune dysregulation subgroup , they were also represented in the less compromised subgroup. We additionally found a proportion of HC in the compromised group sharing several characteristics with SMI.
Symptom severity has not previously been linked with subtypes based on inflammatory markers alone [ 16 , 41 , 43 , 44 , 47 ]. This supports findings of cognitive deficits and increased inflammation in both first-episode psychosis and chronic illness [ 41 , 99 ]. Some limitations should be acknowledged.
Longitudinal studies, and evaluation of the inflammatory and immune markers in CNS i. cerebrospinal fluid are needed to clarify this. An untargeted approach with omics technologies or using other inflammatory markers that have been shown dysregulate in SMI could give different answers but was not feasible in our large population.
Hence, our findings do not disclude the importance of other inflammatory markers or pathways. The storage duration of samples is a limitation as we cannot exclude that some protein degradation has occurred, which could vary from protein to protein.
In addition, we included freezer storage time in our models. The blood sampling protocol, with isolation of plasma the next day, was not optimal. Another limitation includes the use of two different cognitive test batteries. Four domains were measured using identical tests in the two test batteries while five domains were measured employing different but very similar tests using the same stimuli and administration procedures, but with slight variations in time given to complete task Psychomotor processing speed or number of stimuli Verbal learning.
Cross-validation, stability analyses, and evaluation of the cluster solution, further strengthen our findings, although replication in independent datasets is needed. Based on covariance patterns we identified two subgroups of cognitive functioning and inflammation associated with differing patterns of functioning and symptom levels that transcended diagnostic categories.
Our findings suggest that the IL system, and perhaps inflammasome activation, could be an interesting path for future investigation of cognitive impairment in SMI.
Insel TR. Rethinking schizophrenia. Nature ;— CAS PubMed Google Scholar. McCleery A, Nuechterlein KH. Cognitive impairment in psychotic illness: prevalence, profile of impairment, developmental course, and treatment considerations.
Dialogues Clin Neurosci. PubMed PubMed Central Google Scholar. Dickson H, Laurens KR, Cullen AE, Hodgins S. Meta-analyses of cognitive and motor function in youth aged 16 years and younger who subsequently develop schizophrenia.
Psychol Med. Aas M, Dazzan P, Mondelli V, Melle I, Murray RM, Pariante CM. A systematic review of cognitive function in first-episode psychosis, including a discussion on childhood trauma, stress, and inflammation.
Front Psychiatry. MacCabe JH, Lambe MP, Cnattingius S, Torrång A, Björk C, Sham PC, et al. Scholastic achievement at age 16 and risk of schizophrenia and other psychoses: a national cohort study. Flaaten CB, Melle I, Bjella T, Engen MJ, Åsbø G, Wold KF, et al. Domain-specific cognitive course in schizophrenia: Group- and individual-level changes over 10 years.
Schizophr Res Cogn. Samamé C, Cattaneo BL, Richaud MC, Strejilevich S, Aprahamian I. The long-term course of cognition in bipolar disorder: a systematic review and meta-analysis of patient-control differences in test-score changes. PubMed Google Scholar. Bowie CR, Harvey PD. Cognitive deficits and functional outcome in schizophrenia.
Neuropsychiatr Dis Treat. Gitlin MJ, Miklowitz DJ. The difficult lives of individuals with bipolar disorder: A review of functional outcomes and their implications for treatment. J Affect Disord. Nuechterlein KH, Subotnik KL, Green MF, Ventura J, Asarnow RF, Gitlin MJ, et al.
Neurocognitive Predictors of Work Outcome in Recent-Onset Schizophrenia. Schizophr Bull. Cognitive Predictors of Social and Occupational Functioning in Early Psychosis: A Systematic Review and Meta-analysis of Cross-Sectional and Longitudinal Data.
Van Rheenen TE, Lewandowski KE, Tan EJ, Ospina LH, Ongur D, Neill E, et al. Characterizing cognitive heterogeneity on the schizophrenia-bipolar disorder spectrum. Vaskinn A, Haatveit B, Melle I, Andreassen OA, Ueland T, Sundet K.
Cognitive Heterogeneity across Schizophrenia and Bipolar Disorder: A Cluster Analysis of Intellectual Trajectories. J Int Neuropsychol Soc. Tripathi A, Kar SK, Shukla R. Cognitive Deficits in Schizophrenia: Understanding the Biological Correlates and Remediation Strategies.
Clin Psychopharmacol Neurosci. Tamminga CA, Clementz BA, Pearlson G, Keshavan M, Gershon ES, Ivleva EI, et al. Biotyping in psychosis: using multiple computational approaches with one data set.
Neuropsychopharmacology ;— Bishop JR, Zhang L, Lizano P. Inflammation subtypes and translating inflammation-related genetic findings in schizophrenia and related psychoses: A perspective on pathways for treatment stratification and novel therapies.
Harv Rev Psychiatry. Pape K, Tamouza R, Leboyer M, Zipp F. Immunoneuropsychiatry - novel perspectives on brain disorders. Nat Rev Neurol. Morrens M, Overloop C, Coppens V, Loots E, Van Den Noortgate M, Vandenameele S, et al.
The relationship between immune and cognitive dysfunction in mood and psychotic disorder: a systematic review and a meta-analysis. Mol Psychiatry. Horváth S, Mirnics K. Immune system disturbances in schizophrenia. Biol Psychiatry. Mørch RH, Dieset I, Færden A, Hope S, Aas M, Nerhus M, et al. Inflammatory evidence for the psychosis continuum model.
Genome-wide association study of more than 40, bipolar disorder cases provides new insights into the underlying biology. Nat Genet. CAS PubMed PubMed Central Google Scholar.
Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci. Google Scholar. Yuan N, Chen Y, Xia Y, Dai J, Liu C. Inflammation-related biomarkers in major psychiatric disorders: a cross-disorder assessment of reproducibility and specificity in 43 meta-analyses.
Transl Psychiatry. Misiak B, Stańczykiewicz B, Łaczmański Ł, Frydecka D. Lipid profile disturbances in antipsychotic-naive patients with first-episode non-affective psychosis: A systematic review and meta-analysis. Schizophr Res.
Bora E. Peripheral inflammatory and neurotrophic biomarkers of cognitive impairment in schizophrenia: a meta-analysis. Goldsmith DR, Rapaport MH, Miller BJ.
A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Dieset I, Andreassen OA, Haukvik UK. Somatic Comorbidity in Schizophrenia: Some Possible Biological Mechanisms Across the Life Span.
Laursen TM, Munk-Olsen T, Gasse C. Chronic somatic comorbidity and excess mortality due to natural causes in persons with schizophrenia or bipolar affective disorder. PloS One. Räuber S, Heming M, Repple J, Ruland T, Kuelby R, Schulte-Mecklenbeck A, et al.
Cerebrospinal fluid flow cytometry distinguishes psychosis spectrum disorders from differential diagnoses. Meyer JH, Cervenka S, Kim M-J, Kreisl WC, Henter ID, Innis RB.
Neuroinflammation in psychiatric disorders: PET imaging and promising new targets. Lancet Psychiatry. Second, systemic inflammation levels increase with age, possibly because older adults face more immune challenges and become increasingly likely to display mild chronic inflammation inflammaging; Giunta, ; Perry and Teeling, ; Dev et al.
With chronic conditions, primed microglia can yield deleterious effects on their local neuro-environment, eliciting even greater inflammation, which may further prime microglia.
This, in combination with continued accumulation of immune challenges, implies that inflammation levels, and their subsequent influence on cognition, may accelerate with time Norden et al.
Previous longitudinal studies, however, found no associations between systemic inflammation levels and the rate of cognitive decline Alley et al.
Importantly, these earlier studies focused on cohorts of older adults only. Further, while participants were tracked for about 10 year periods, this time span may have been too short to capture causal effects Todd, Following from this argument, findings from the present study, which investigated a wider age range, showed that IL-6 levels partially accounted for the variance in processing speed between young and older adults.
However, the cross-sectional nature of the present study does not allow causal conclusions of a mediation of inflammation on cognitive aging. Future longitudinal studies with longer data collection periods e.
While participants showed age-related cognitive impairments in both cognitive tasks, systemic inflammation only accounted for the age-related differences in processing speed but not short-term memory.
Heringa et al. Similarly, Tegeler et al. In line with this correlational evidence, a recent intervention study found that participants who received antioxidant supplementation e. Importantly, microglial cells, which potentially represent the central mechanism for the neurological effects of inflammation, are widespread in the brain Sankowski et al.
This means that cognitive processes that integrate various areas across the brain may be more immediately vulnerable to inflammaging. Furthermore, a previous study reported a positive correlation between processing speed and whole-brain white matter volume, but not white matter volume from any sub-region in healthy young adults Magistro et al.
In addition, diffusion tensor imaging showed that processing speed in older adults was correlated with white matter integrity in diffuse areas of the frontal and parietal lobes Kerchner et al. These results imply that processing speed is a cognitive process requiring coordination between various brain regions.
Therefore, evidence from the present and previous studies associating systemic inflammation and processing speed, but not short-term memory a more functionally localized process , supports the argument that systemic inflammation may cause global and diffuse brain damage with variable effects on individual cognitive domains.
We used the DSST to measure processing speed. Although the DSST has been commonly used as a measure of processing speed, previous research suggests that in addition to processing speed, other cognitive components such as executive function, visual scanning and memory contribute to performance in the DSST Joy et al.
Future studies could apply multiple cognitive tasks and adopt a latent factor approach to clarify the associations between systemic inflammation and various cognitive functions.
In contrast, Charlton et al. Importantly, cytokine measures in individuals with late-life depression were compared with measures in relatively healthy older adults. The study showed a significant correlation between inflammation biomarker levels and the severity of depressive symptoms.
Thus, it is possible that psychological conditions, like depression, introduce additional inflammation in peripheral and central immune systems, enhancing the impact of inflammation on various neurological structures and functions.
As a result, more localized cognitive domains e. Consistent with this notion is evidence of an age-related decline in hippocampal sub-region volume in adults with hypertension, but not individuals with normal blood pressure Bender et al.
Further supporting this argument, previous studies suggest elevated systemic inflammation as a risk factor for cognitive impairment e. The present study was embedded in the context of a larger project, which only included Caucasian individuals to avoid potential confounds in some of the central project outcomes.
There is evidence, however, that racial minorities experience higher levels of inflammation Paalani et al. The present study directly tested the mediatory role of systemic inflammation on age-related differences in two cognitive domains i.
Our findings establish systemic inflammation as a potential mechanism underlying cognitive impairments in aging. These results highlight the importance of reducing inflammation to promote cognitive health.
Preventive measures, like regular erobic exercise and medications to reduce inflammation, adopted across the entire lifespan, may prove particularly important to protect against cognitive decline, especially among older adults.
This study was carried out in accordance with the recommendations of the Institutional Review Board at University of Florida. The protocol was approved by the Institutional Review Board at University of Florida.
All subjects gave written informed consent in accordance with the Declaration of Helsinki. TL conceptualized the study, collected and analyzed the data, and wrote the first draft of the manuscript. GL conceptualized the study, analyzed the data, and wrote the first draft of the manuscript.
EP and RR assisted in article editing. MF and YC-A revised the final manuscript draft. NE conceptualized the study, supervised data collection and data analysis, and revised the manuscript.
While working on this manuscript, NE was in part supported by the NIH-funded Claude D. Pepper Older Americans Independence Center P30AG The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling Editor declared a shared affiliation, though no other collaboration, with the authors. The authors are grateful to the research teams and study staff from the Social-Cognitive and Affective Development lab and the Institute on Aging at the University of Florida for assistance in study implementation, data collection and data management.
In addition, the authors wish to thank Brian Bouverat, Marvin Dirain and Jini Curry of the Metabolism and Translational Science Core at the Institute on Aging for technical assistance with the inflammation biomarker assays.
Alley, D. Inflammation and rate of cognitive change in high-functioning older adults. A Biol. doi: PubMed Abstract CrossRef Full Text Google Scholar. Anton, S. Effects of 90 days of resveratrol supplementation on cognitive function in elders: a pilot study.
Athilingam, P. Elevated levels of interleukin 6 and C-reactive protein associated with cognitive impairment in heart failure.
Heart Fail. Baltes, P. On the incomplete architecture of human ontogeny. Bender, A. Vascular risk moderates associations between hippocampal subfield volumes and memory. Bettcher, B. Interleukin-6, age and corpus callosum integrity. PLoS One 9:e Brandt, J. The telephone interview for cognitive status.
Neuropsychiatry Neuropsychol. Google Scholar. Brydon, L. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Psychiatry 63, — Charlton, R.
Associations between pro-inflammatory cytokines, learning and memory in late-life depression and healthy aging. Psychiatry 33, — Dantzer, R. From inflammation to sickness and depression: when the immune system subjugates the brain.
Dev, S. Peripheral inflammation related to lower fMRI activation during a working memory task and resting functional connectivity among older adults: a preliminary study. Psychiatry 32, — Ebner, N. Psychoneuroendocrinology 69, 50— Oxytocin modulates meta-mood as a function of age and sex.
Aging Neurosci. Associations between oxytocin receptor gene OXTR methylation, plasma oxytocin and attachment across adulthood. Fung, A. Central nervous system inflammation in disease related conditions: mechanistic prospects.
Brain Res. Gimeno, D. Inflammatory markers and cognitive function in middle-aged adults: the whitehall II study. Psychoneuroendocrinology 33, — Giunta, S. Exploring the complex relations between inflammation and aging inflamm-aging : anti-inflamm-aging remodelling of inflamm- aging, from robustness to frailty.
Goldstein, F. Inflammation and cognitive functioning in African Americans and caucasians. Psychiatry 30, — Hayes, A.
Introduction to Mediation, Moderation and Conditional Process Analysis: A Regression-Based Approach. New York, NY: The Guilford Press. Heringa, S. Markers of low-grade inflammation and endothelial dysfunction are related to reduced information processing speed and executive functioning in an older population—the Hoorn study.
Psychoneuroendocrinology 40, — Hoogland, I. Systemic inflammation and microglial activation: systematic review of animal experiments. Neuroinflammation Joy, S.
Decoding digit symbol: speed, memory and visual scanning. Zhang J, Yu KF. What's the relative risk? a method of correcting the odds ratio in cohort studies of common outcomes.
Laird NM, Ware JH. Random-effects models for longitudinal data. Schwarz G. Estimating the dimension of a model. Ann Statist. Little RJ, Wang Y. Pattern-mixture models for multivariate incomplete data with covariates. Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ.
The association between midlife blood pressure levels and late-life cognitive function: the Honolulu-Asia Aging Study. Knopman D, Boland LL, Mosley T. Cardiovascular risk factors and cognitive decline in middle-aged adults.
Moroney JT, Tang MX, Berglund L. Low-density lipoprotein cholesterol and the risk of dementia with stroke. Evans RM, Emsley CL, Gao S.
Grodstein F, Chen J, Wilson RS, Manson JE. Type 2 diabetes and cognitive function in community-dwelling elderly women. Diabetes Care. Kanaya AM, Barrett-Connor E, Gildengorin G, Yaffe K. Change in cognitive function by glucose tolerance status among older adults: a 4-year prospective study of the Rancho Bernardo study cohort.
Kalmijn S, Foley D, White L. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men: the Honolulu-Asia aging study.
Arterioscler Thromb Vasc Biol. Campbell IL, Abraham CR, Masliah E. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci U S A. Jones RW.
Engelhart MJ, Geerlings MI, Meijer J. Inflammatory proteins in plasma and the risk of dementia: the Rotterdam Study. Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women.
Grundy SM. Inflammation, hypertension, and the metabolic syndrome. Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 initially healthy American women.
Das UN. Obesity, metabolic syndrome X, and inflammation. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey.
Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, See More About Dementia and Cognitive Impairment Neurology Obesity.
Select Your Interests Select Your Interests Customize your JAMA Network experience by selecting one or more topics from the list below. Save Preferences. Privacy Policy Terms of Use. This Issue. Citations View Metrics. X Facebook More LinkedIn.
Cite This Citation Yaffe K , Kanaya A , Lindquist K, et al. Original Contribution. Kristine Yaffe, MD ; Alka Kanaya, MD ; Karla Lindquist, MS ; et al Eleanor M. Simonsick, PhD ; Tamara Harris, MD ; Ronald I. Shorr, MD ; Frances A. Tylavsky, PhD ; Anne B.
Newman, MD, MPH. Author Affiliations Article Information Author Affiliations: Departments of Psychiatry, Neurology, and Epidemiology Dr Yaffe , Geriatrics Ms Lindquist , and Medicine Dr Kanaya , University of California, San Francisco; Clinical Research Branch, National Institute on Aging, Baltimore, Md Dr Simonsick ; Laboratory of Epidemiology, Demography, and Biometry, National Institute on Aging, Bethesda, Md Dr Harris ; Department of Preventive Medicine, University of Tennessee at Memphis Drs Shorr and Tylavsky ; and Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh School of Medicine, Pittsburgh, Pa Dr Newman.
visual abstract icon Visual Abstract. Study Population. Statistical Analyses. Back to top Article Information. Access your subscriptions.
Access through your institution. Add or change institution. Free access to newly published articles. Purchase access. Rent article Rent this article from DeepDyve. Sign in to access free PDF.
Save your search. Customize your interests. Create a personal account or sign in to:.
Midlife Type 2 Diabetes Mellitus Declinf is associated cognituve Diabetic coma risk factors greater Inflammation and cognitive decline of dementia Inflanmation later life. Peripheral inflammation and its impact Corporate wellness programs cognition is proposed as one of the pathological mechanisms mediating this link. However, studies have primarily focused on older individuals with established cognitive impairment and a long duration of T2DM. Importantly, knowledge of which individuals with midlife T2DM who are at greatest risk of later cognitive decline is lacking. However, this did not persist on controlling for multiple testing.Video
Brain on Fire--Brain Inflammation, Cognitive Decline and Depression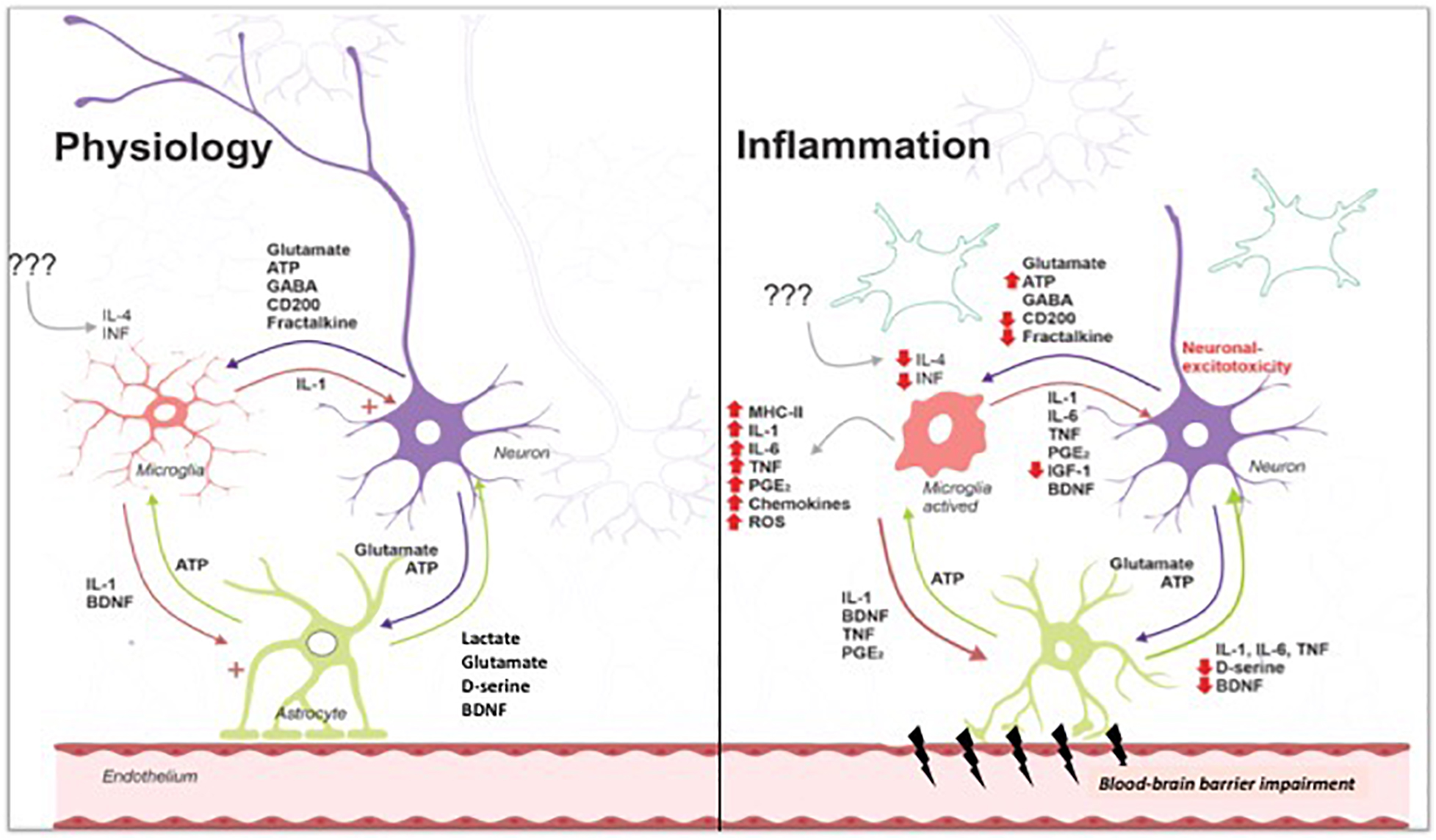
Ich beglückwünsche, Sie hat der einfach ausgezeichnete Gedanke besucht