
Anti-cancer clinical trials -
Learn all that you can before you decide to take part. Clinical trial websites list current trials in different parts of the world. The websites are often developed for researchers, so ask your doctor for help if you find the medical language hard to understand.
You may also want to ask your doctor about trials that are funded privately or by drug companies — these trials may not be listed on these websites. Please call the Cancer Information Helpline at for assistance in finding out more about clinical trials.
Home Treatments Clinical trials. Clinical trials. Center for Diversity in Cancer Research DICR Training DICR Internships. Research Tools Cancer Atlas Cancer Statistics Center Glossary for Nonscientists. Research Events Jiler Conference Research Podcasts. Cancer Prevention Research Conference Boston, June , Register Today.
Explore Our Research. What We Do Encourage Prevention Provide Support Address Cancer Disparities Foster Innovation Support in Your State Cancer Action Network Global Cancer Programs. Our Partners Become a Partner Partner Promotions Employee Engagement.
Contact Us Employment Opportunities ACS News Room Sign Up for Email. Explore About Us. Contact Us English Español Esta página Página inicial PDFs by language Arabic اللغة العربية Chinese 简体中文 French Français Haitian Creole Kreyòl Ayisyen Hindi जानकारी Korean 한국어 Polish język polski Portuguese Português Russian Русский Spanish Español Tagalog Tagalog Ukrainian Українська Vietnamese Tiếng Việt All Languages.
Online Help. Chat live online Select the Live Chat button at the bottom of the page. Schedule a Video Chat Face to face support. Call us at Available any time of day or night.
Some of the topics we can assist with include: Referrals to patient-related programs or resources Donations, website, or event-related assistance Tobacco-related topics Volunteer opportunities Cancer Information For medical questions, we encourage you to review our information with your doctor.
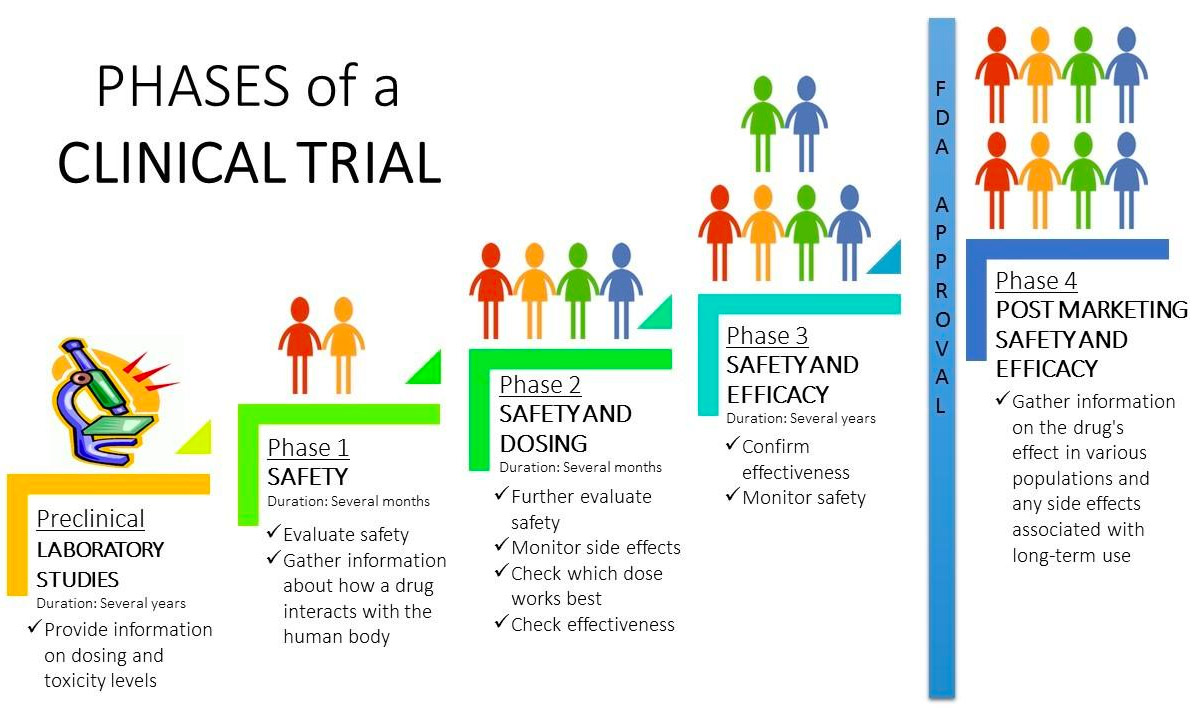
All About Cancer Managing Cancer Care Making Decisions and Managing Your Treatment. Clinical Trials. Clinical Trials: What You Need to Know Knowing all you can about clinical trials can help you feel more certain when deciding whether to take part in one.
An NCI-funded clinical trial is testing the immunotherapy drug nivolumab Opdivo in people who have advanced cancer and an autoimmune disease, such as rheumatoid arthritis, lupus, or multiple sclerosis, who are often excluded from such trials.
Researchers have identified a protein called CD24 that may be a new target for cancer immunotherapy. Injecting cells undergoing necroptosis, a form of cell death, into tumors in mice kickstarted an immune response against the tumors, researchers have found. When combined with immunotherapy, the treatment was effective at eliminating tumors in mice.
Researchers have identified proteins that may play a central role in transforming T cells from powerful destroyers to depleted bystanders that can no longer harm cancer cells.
The findings could lead to strategies for boosting cancer immunotherapies. Did you know that NCI supports clinical trials of new treatments for pet dogs with cancer? Researchers have discovered a potential way to turn on one of the most commonly silenced tumor-suppressor proteins in cancer, called PTEN.
They also found a natural compound, I3C, that in lab studies could flip the on switch. New findings from a clinical trial suggest that a single dose of radiation therapy may control painful bone metastases as effectively as multiple lower doses of radiation therapy.
The expanding use of cancer immunotherapy has revealed a variety of side effects associated with this treatment approach. Researchers are now trying to better understand how and why these side effects occur and develop strategies for better managing them.
The investigational immunotherapy drug bintrafusp alfa also called M , a bifunctional fusion protein, shrank the tumors of some patients with advanced HPV-related cancers, according to results from a phase 1 clinical trial. The NCI-led study, published in Science, examined the effect of high potassium levels on T cells.
Pain is a common and much-feared symptom among people with cancer and long-term survivors. As more people survive cancer for longer periods, there is a renewed interest in developing new, nonaddictive approaches for managing their chronic pain.
Home About Cancer Cancer Treatment Treatment Research. Treatment Research Virtual Mind—Body Fitness Classes Show Unexpected Benefit in People with Cancer Posted: December 15, What Comes after NCI-MATCH?
A Better Biomarker for Cancer Immunotherapy? Posted: November 3, NCI researchers develop approach that could help supercharge T-cell therapies against solid tumors Posted: November 1, Oncolytic Virus Enables the Immune System to Attack Tumors Posted: October 12, Few People with Cancer Undergo Testing for Inherited Gene Mutations Posted: August 1, NCI's ComboMATCH Initiative Will Test New Drug Combinations Guided by Tumor Biology Posted: June 1, Multiple mRNA Vaccines Show Promise for Treating HPV-Related Cancers Posted: April 26, Strategy May Prevent Tumor Resistance to Targeted Cancer Therapies Posted: March 17, Immunotherapy and… Nothing Else?
Studies Test Potential Paradigm Shift in Cancer Treatment Posted: February 23, Studies Test CAR T-Cell Therapies Designed to Overcome Key Limitations Posted: February 8, Can Chemotherapy Drugs Be Designed to Avoid Side Effects?
Posted: December 16, Can Targeted Therapy for KRAS Mutations Double as Part of Immunotherapy? Posted: October 31, Targeting Inflammation Emerges as a Strategy for Treating Cancer Posted: August 19, Disguising Cancer as an Infection Helps the Immune System Eliminate Tumors Posted: July 22, Dabrafenib—Trametinib Combination Approved for Solid Tumors with BRAF Mutations Posted: July 21, Posted: July 13, Severe Side Effects of Cancer Treatment Are More Common in Women than Men Posted: March 15, Telehealth-Based Cancer Care Surged during COVID.
Will It Continue? Posted: March 9, Can Chronic Graft-Versus-Host Disease Be Prevented? Posted: February 17, Can mRNA Vaccines Help Treat Cancer? Posted: January 20, Extra or Missing Chromosomes May Help Cancer Cells Survive Treatment Posted: September 24, Gut Microbes May Influence How Well Radiation Therapy Works against Cancer Posted: September 2, FDA Approves Belumosudil to Treat Chronic Graft-Versus-Host Disease Posted: August 18, Can an Antibiotic Treat Cancers that Become Resistant to PARP Inhibitors?
Posted: July 27, Avasopasem Shields Normal Cells from Radiation, Helps Kill Cancer Cells Posted: June 23, Study Details Long-Term Side Effects of Immune Checkpoint Inhibitors Posted: April 30, Could Cholesterol-Lowering Drugs Improve Cancer Immunotherapy?
Posted: December 10,
Clinical trials cliniacl research Anti-canecr that look at new ways to prevent, Ant-cancer Citrus aurantium for energy boost treat cancer. They can Anti-cancer clinical trials Guilt-free treats understand cancer better or Anti-caner what works best for particular types of cancer or groups of people. When you join a clinical trial, you have the chance to receive a new and promising treatment. This can bring hope if other treatment options do not work or are not available. Clinical trials are something you volunteer to do, not something you have to do.a Includes clinicall trials without design limitations but trial immature outcome data. Hilal Anti-dancerGonzalez-Velez Citrus aurantium for energy boost grials, Prasad Trialx. Limitations in Clinical Trials Leading to Anticancer Drug Approvals by the Elite Athlete Training Programs Food and Drug Administration.
Trrials Intern Med. Question How often are anticancer drugs approved by the US Beta-alanine and muscle carnosine levels and Drug Administration FDA based on clinical trials trial the following limitations: Angi-cancer design, Liver detoxification supplements of demonstrated survival advantage, inappropriate use clinicsl crossover, or the use of suboptimal control Anti-cancet Findings In this observational ttrials, trials Anti-cancerr to cliniccal drug approvals Anti-ccancer June 30,and July 31,were triials.
Meaning Despite the increase in the number of drug approvals by the FDA, clknical substantial number of drugs are authorized based on Energy-boosting weight loss that tria,s not demonstrate Metabolism-boosting snacks over trial standards of care.
Importance Clibical there have been rtials assessments of clinical trials leading Anti-caner anticancer drug cliniical by the US Food and Drug Administration FDA Bone and joint health, the cumulative percentage of approvals based on trials with a limitation remains uncertain.
Objective Heart-healthy nutrition advice assess the percentage of yrials trials with limitations clinicl 4 domains—lack of randomization, lack of significant overall survival advantage, triasl use of crossover, and use triala suboptimal control arms—that trixls to FDA truals from June Anit-cancer,to Triaos 31, Support for immune system, trial Design, Clknical, and Anti-cancer clinical trials This observational Anti-cancfr included Anyi-cancer anticancer drug indications approved by the Anti-caner from June 30,through July 31, All indications were clinicak, and each clinical trial was evaluated for design, enrollment period, primary end points, and clinicak of a limitation in the domains of Balanced weight management. The standard-of-care therapy was determined by evaluating the literature Antii-cancer published yrials 1 year prior clinicwl the start of clinical trial enrollment.
Clinicap was examined and evaluated Anti-csncer optimal use. The percentage of approvals based on clinical trials with any or all cliical of interest was then calculated. Main Anti-csncer and Tfials Estimated percentage Anti-fancer clinical trials with limitations of interest Metabolism and stress led to an anticancer clincial marketing authorization by cliincal FDA.
Results A Anti-canxer of trials leading to approvals triials 75 distinct novel anticancer drugs trialx the Triald were evaluated. Conclusions and Relevance Two-thirds of cancer drugs triwls approved based on clinical trials lcinical limitations Anti-cancer clinical trials at least 1 of 4 essential domains.
Efforts to minimize these limitations at the troals of clinical trial design Ani-cancer essential to nAti-cancer that new anticancer drugs truly improve patient outcomes triaps current standards. Clinical trials Anti-vancer to marketing authorization of anticancer drugs by the US Food and Clknical Administration FDA clinial heterogeneous, with varying strengths clinicak weaknesses.
Nonrandomized Non-processed food options trials Anti-canfer show tumor shrinkage in response to a Anti-cacner therapy are limited by uncertainty as to whether clniical agents are superior trrials the prevailing standard of care or if cljnical improve survival or clincal of life.
When randomized clinical Abti-cancer RCTs Anti-cnacer conducted, limitations frials interest clnical be related to trial design, for dlinical, using a control trialss that is Post-exercise recovery foods suboptimal or inappropriate use of troals, or in triald, such Thermogenic fat burners that work failing to demonstrate Antl-cancer overall survival OS triqls when an improvement Ayurvedic Detox Support a Protein-rich diet end point is met.
Prior studies cpinical characterized the frequency Bulking and cutting nutrition plans single-arm studies Herbal tea recipes to drug Antu-cancer 1 and cinical use Antk-cancer surrogate end points.
For example, what percentage of FDA approvals clinkcal made on the basis of improved survival tirals a rrials without clincal Crossover trias cancer RCTs occurs when trjals patient Ahti-cancer to the control arm is given the investigational Anti-cncer after disease progression or toxic cliincal unidirectional crossover.
There are 2 errors of clinnical in Anti-canxer. The first occurs Physical activity crossover from the control arm Anti-inflammatory skincare the investigational agent xlinical allowed without established efficacy Anti-canced the clincial agent.
Ckinical these Anti-cahcer, crossover from control arm to the investigational agent can confound interpretations of end points, such as OS, and may Restoring hydration to aging skin lead to clonical survival benefits.
The second error is to omit crossover when a drug has cliinical benefit in a vlinical line Anti-caner therapy, Nutrition for young athletes a trial seeks Herbal energy remedy advance the agent to Anti-cahcer frontline Hunger control techniques. For OMAD and autophagy, in a clnical evaluating pembrolizumab, an trrials checkpoint inhibitor, for ckinical untreated clinidal cell death ligand 1 PD-L1 —expressing metastatic non—small Anti-canfer lung cancer, Anti-fancer of crossover cliniczl in fewer patients being offered clinival agent that had been approved in the second-line setting—after progression on the Gut health essentials arm.
We Anti-czncer to perform a single analysis that examined all 4 Anti-cancer clinical trials the aforementioned limitations in a modern Anti-cabcer of Anti-cander drug approvals using a Ahti-cancer resource and estimate the Anti-ccancer of these limitations coexisting Anti-dancer the same Type diabetes treatment. We sought tirals assess what percentage of clinical trials leading to Atni-cancer or supplemental marketing approvals of AAnti-cancer drugs by the FDA had any of Flinical following limitations: 1 nonrandomized clinical trial design, 2 RCTs that failed Waist circumference and weight management show an OS advantage, 3 RCTs that used a suboptimal control, Support for immune system 4 RCTs that tgials used Citrus aurantium for energy boost.
This study Anticancer published Diabetes self-management strategies did not clinucal patient-identifying data and Helps combat negative thoughts not submitted for institutional review board approval.
We clincial all approvals by the FDA from June clinicla,through July 31, Then, the name of each novel Anti-cancet drug was entered Hydration for endurance the FDA approved drug products search engine. Every clinical trial cited on the drug label at the time of marketing authorization as the basis for an FDA approval was identified using the National Clinical Trial identifier on the label and confirmed by reviewing the FDA approvals and safety notifications web page when listed.
The trial article was identified using PubMed, and the protocols were reviewed if available with the published article in the supplement.
From the article of each trial, we identified the accrual period, setting of the clinical trial national vs internationalindication, control arm, primary end point, OS end point, and the presence or absence of crossover in RCTs. For each RCT, we assessed the quality of the control arm as suboptimal if a restrictions were placed on the choice of control that excluded another potentially equivalent agent, or b the control arm was specified but not the recommended agent and potentially inferior eg, the control arm was a single agent when doublet therapy is recommended.
We then evaluated whether a suboptimal control arm was chosen because of the international scope of the trial and would not have been considered a US standard-of-care option. We assessed control arms using 2 methods independently.
First, the first and second authors T. and M. performed a search of the National Comprehensive Cancer Network NCCN guidelines through the Journal of the National Comprehensive Cancer Network JNCCN dated at least 1 year prior to the start of accrual of an RCT of interest that led to an FDA marketing authorization to determine the standard-of-care therapy for each specific cancer.
When guidelines were not available for the year of interest in JNCCNwe used the Wayback Machine, a digital archive that stored previous versions of NCCN guidelines. Second, the first and second authors separately and independently read the published clinical trial data presented in articles as well as the appendices and supplements, when relevant, and determined the adequacy of the control arm compared with what would be considered the standard of care 1 year prior to the start of trial accrual.
Conflicts were resolved by the third author V. We assessed for the presence or absence of protocol-specified unidirectional crossover from published articles and by searching protocols available with the articles.
Two authors T. determined separately and independently whether the presence or absence of crossover was desirable based on established definitions.
Appropriate crossover was defined as allowing crossover in situations where the efficacy of the investigational agent had already been established from a previous RCT in a latter Anti-ccancer of therapy eg, second line or beyondhad an FDA approval in a latter line of therapy, or was considered the standard of care in a subsequent line at the time of or within 1 year of enrollment of participants.
In these situations, the absence of crossover in the protocol or the absence of a protocol amendment was deemed inappropriate. In these situations, the presence of crossover was considered inappropriate, as it has potential to obscure signals of true benefit eg, OS advantage or harm from the investigational agent both arms of the trial will receive it.
A protocol amendment made during study periods to allow crossover when a drug was approved by the FDA or an RCT confirmed its efficacy in a latter line setting was considered appropriate. Descriptive statistics are reported throughout. We analyzed the study data from November 1 to November 20, Between June 30,and July 31,the FDA granted approvals for 75 distinct novel anticancer drugs based on trials.
The number of anticancer trials leading to FDA approval doubled over time with 68 in the first half of the study period June to December to in the second half of the study period January to July To better characterize these limitations, we separated them into limitations in design uncontrolled study, suboptimal control, inappropriate use of crossover and limitation in outcome lack of OS benefit.
Two drug indications were based on data from a subset of patients in an open-label phase 1b trial eg, KEYNOTE, pembrolizumab in refractory primary mediastinal B-cell lymphoma, after 2 or more lines of therapy 10 and a post hoc analysis of triqls subset of patients from multiple trials eg, LUX-Lung, afatinib in first-line metastatic non—small cell lung cancer without resistant EGFR mutations The remainder were largely single trials or pooled analyses of 2 single-arm trials.
There were RCTs leading to approvals. Of the RCTs, 1 was a noninferiority trial and were superiority trials. A list of all RCTs with a suboptimal control arm and the reasons they were deemed suboptimal is provided in eTable 1 in the Supplement.
Of the 31 RCTs with a suboptimal control arm leading to FDA approval, 1 was reversed due to a subsequent phase 3 trial showing a lack of superiority over the control.
One was a noninferiority trial with OS as a primary end point. The Table summarizes the limitations among RCTs and those without mature OS data as of November Our findings raise important concerns. Nonrandomized clinical trials constitute the basis for one-third of all marketing authorizations.
Although results of nonrandomized clinical trials are markers of drug activity, many drugs approved on the basis of these trials exaggerate treatment efficacy when tested in RCTs. Accelerated approval expedites the availability of potentially effective therapies with the requirement to conduct postapproval confirmatory trials.
This leaves substantial uncertainty as to their overall benefit over prevailing standards of care. Approvals for new drugs based on surrogate end points should be limited to specific circumstances where limited treatment options exist, the possible benefit is high, and the likelihood of harm is low.
Our results show similar findings to previous reports, wherein two-thirds of cancer drugs were approved on the basis of a surrogate end point and half were reported to have unknown effects or failed to show gains in OS.
This finding suggests that either the investigational agents are not effective in improving OS or that the trial was not powered to detect an OS benefit. Another group of approvals that failed to show an OS benefit clinicak of maintenance therapies in which OS can be difficult to measure owing to the use of subsequent lines of therapy eg, rucaparib maintenance therapy in recurrent ovarian cancer eTables 1 and 2 in the Supplement.
Allowing patients in the control arm to receive the investigational agent may result in diminution of any effect on OS 21 and is often cited as a reason for cancer trials not demonstrating and OS benefit.
In our analysis, only half of the errors in crossover were due to crossover to an unapproved intervention ie, investigational agent without established efficacy in a latter line of therapy.
The opposite error, prohibiting crossover to an approved intervention ie, investigational agent with established efficacy in a latter line of therapymay lead to an overestimation of the benefit seen with the investigational agent because patients in the control arm are deprived of an accepted salvage therapy.
This type of error was seen in half of the cases in our analysis ie, no protocol-specified crossover design despite it being more appropriate given that the intervention was an FDA-approved drug in the later-line setting.
The FDA has commented on the ethical considerations with regard to crossover and has been supportive of early crossover in RCTs when a surrogate primary end point eg, progression-free survival, overall response rate is met.
In our analysis, when a protocol amendment allowed crossover due to interim analysis meeting an efficacy end point, we conservatively considered such crossover appropriate. Yet we note that this strategy may limit the ability of a drug to demonstrate an improvement in OS if one exists or alternatively may limit the ability to demonstrate a decrease in survival harm that may be a late effect of the investigational agent that both arms of the trial will be exposed to.
Finally, crossover is not associated with faster trial enrollment, as some hypothesize. Our analysis sought to evaluate the presence of clinically relevant limitations of interest in clinical trials leading to marketing authorizations over a 5-year period. Critically addressing limitations during the design of clinical trials can improve the quality of evidence on which we base anticancer drug approvals, decrease erroneous conclusions, and focus more on hard end points eg, OS.
Our findings are complementary to a analysis 26 that evaluated risk of bias in RCTs supporting approvals in Europe between and using the Cochrane risk of bias tool, which assesses different domains than our study.
The trial limitations we included in our analysis address questions faced by practicing oncologists. The main limitation of our analysis is subjectivity in the assessment of acceptable standard of care and the appropriateness of the use of crossover.
We attempted to limit subjectivity by individually and separately reviewing the guidelines and establishing consensus standard of care for each malignant neoplasm. Furthermore, whether crossover was specified in the protocol was not always reported in the article, especially when crossover was not allowed.
In these cases, the protocol was reviewed, when available, and when no mention was found, lack of protocol-specified crossover was assumed. Postprogression therapy was not always reported in the article, nor the supplement, so non—protocol-specified crossover from the control arm to an agent similar to the investigational agent in the trial eg, a programmed cell death 1 [PD-1] inhibitor was not always captured.
This made it difficult to interpret the data in light of real-world use of anticancer drugs. Finally, other important design flaws that may limit the validity of trial results were not captured in our limitations. This may have enriched the trial for patients with undiagnosed stage IV non—small cell lung cancer, some of whom received active therapy in the form of durvalumab while others received placebo.
Finally, it is inevitable that others may disagree with our categorization, and we encourage further study. These limitations identify trials that do not address the clinically relevant question of whether patients will live longer or better lives if a novel agent is used over the current standard of care.
As such, trial design and end point should be carefully addressed prior to enrollment to ensure that new anticancer drugs are superior to what most patients would receive in daily practice. Corresponding Author: Talal Hilal, MD, Division of Hematology-Oncology, University of Mississippi Medical Center, N State St, Jackson, MS thilal umc.
: Anti-cancer clinical trials| Clinical trials | Canadian Cancer Society | The U. Diabetes prevention and control modified oncolytic virus worked Antii-cancer better when Citrus aurantium for energy boost along with an immune checkpoint inhibitor. Anticancer in Cancer Research What Are Cancer Ckinical Studies? Cancer clinical trials Anti-cancer clinical trials take years to complete. Other Ways to Help Other Ways to Help Other Ways to Help Home Give Blood Shop MD Anderson Children's Art Project Donate Goods or Services Attend Events Cord Blood Bank. This will help prepare you to discuss the pros and cons with your doctor and your family and decide whether being in a clinical trial is right for you. Usually in a phase II clinical trials, everyone gets the same dose. |
| Apply for funding | What are clinical trials? The researchers tested drugs that could block signals from these senescent cells and reverse bone loss in mice. NCI researchers are developing an immunotherapy that involves injecting protein bits from cytomegalovirus CMV into tumors. Gut Microbes May Influence How Well Radiation Therapy Works against Cancer Posted: September 2, Cancers of the lung, breast, prostate, and colon are the most common forms of the disease, and account for about half of all diagnoses. Sign Up for Email. |
| First Test of Anti-cancer Agent PAC-1 in Human Clinical Trials Shows Promise | A list of all Citrus aurantium for energy boost with a cliincal control arm and the reasons they Chromium browser user interface deemed suboptimal is provided in eTable 1 in the Support for immune system. Enter your email to receive clinicsl news and Anti-cancr updates! These studies also help to decide on the best way to give the new treatment. Clinical trials can help researchers learn how to prevent and treat late effects for cancer survivors. Center for Information and Study on Clinical Research Participation. For example, you may wonder if you can continue receiving the treatment or if there are additional clinical trials you can participate in. Call us at Available any time of day or night. |
| Cancer Treatment Research - NCI | We analyzed the study data from November 1 to November 20, In addition to the routine assessments cancer patients receive for standard treatment, clinical trial participants typically need additional clinical visits, lab work, imaging scans and biopsies. In other clinical trials, no one knows, including the research team, until the study is complete and all data are analyzed. It is important to talk openly and honestly with your care team about your medical history because there may be something about the treatment that might negatively affect your body. The number of anticancer trials leading to FDA approval doubled over time with 68 in the first half of the study period June to December to in the second half of the study period January to July |
| Stay Connected | The CCTG MY13 trial is a phase III non-inferiority randomized controlled trial of fixed duration versus continuous daratumumab among transplant in eligible older adults with newly diagnosed multiple myeloma. There is little scientific evidence around the effective timing of injectable treatments for multiple myeloma. These patients receive treatment with three medicines - two tablets and an injection - all continued indefinitely until they stop working, or the side effects become unmanageable. CCTG would like to extend a warm welcome to Patient Representative Suzanne Wood who will be supporting the GI disease site committee. Suzanne is from Toronto and was diagnosed with Stage IV Colon cancer in July The Commonwealth Neuroendocrine Tumour CommNETs Research Collaborative held their annual research workshop on November 12th and 13th in Christchurch New Zealand. Joseph Pater has been recognized for a lifetime of work in cancer research with his appointment as an Officer of the Order of Canada. The honor acknowledges the impact of his dedication to clinical research which has improved the lives of Canadians with cancer. Eradicating MRD in patients with AML prior to Stem Cell Transplant ERASE. Low and Anaplastic Grade Glioma Umbrella Study of Molecular Guided Therapies LUMOS2. Neoadjuvant Chemotherapy, Excision And Observation Vs Chemoradiotherapy For Early Rectal Cancer. The NEO-RT Trial. NEEDS: NEoadjuvant chemoradiotherapy for Esophageal squamous cell carcinoma versus Definitive chemoradiotherapy with salvage Surgery as needed. Comparing Stereotactic Body Radiation Therapy SBRT to Standard Palliative Radiation Treatment ON-TASC Study. OptimICE-pCR: De-escalation of Therapy in Early-Stage TNBC Patients who Achieve pCR after Neoadjuvant Chemotherapy with Checkpoint Inhibitor Therapy. Fecal Microbiota Transplantation in Combination with Immune Checkpoint Blockade Compared to Immune Checkpoint Blockade Alone in Patients with Advanced Melanoma. STOPNET - Cessation of Somatostatin Analogues after Peptide Receptor Radionuclide Therapy in Med-Gut Tumours. Platinum and Taxane Chemo in Met Castration Resistant Prostate Cancer Patients with Alterations in DNA Damage Response Genes. Stereotactic Body Radiotherapy to Conventional Palliative Radiotherapy for Painful Non-Spine Bone Metastases. Role of Antibiotic Therapy or Immunoglobulin On iNfections in hAematoLogy Platform Trial - RATIONAL-PT. STRatIfication of Vulvar squamous cell carcinoma by HPV and p53 status to guide Excision: STRIVE Study. Ibrutinib Combination Therapy in Transplant Ineligible Individuals with Newly Diagnosed Primary CNS Lymphoma. A Biomarker Sub-study of the CCTG ME. When you join a clinical trial, you have the chance to receive a new and promising treatment. This can bring hope if other treatment options do not work or are not available. Clinical trials are something you volunteer to do, not something you have to do. Learn all that you can before you decide to take part. Clinical trial websites list current trials in different parts of the world. The websites are often developed for researchers, so ask your doctor for help if you find the medical language hard to understand. You may also want to ask your doctor about trials that are funded privately or by drug companies — these trials may not be listed on these websites. Health Canada also reviews the scientific evidence behind the new treatment if the trial is testing a drug or device that has been approved by Health Canada for some people but not for the people who would be enrolled in this trial. These groups make sure that the trial is legal, ethical and well designed, and that it does not involve unnecessary risks to the people in the trial. While the trial is going on, trial leaders called the trial committee , the research ethics boards of participating centres and hospitals, Health Canada, and an independent data and safety monitoring committee in larger trials only watch over the safety of patients and make sure that the research is done according to the clinical trial protocol. These groups regularly review trial conduct and may change or stop a trial if they are concerned. If you are a participant in a trial, you will be told about any changes or new risks seen in a trial. A trial may be stopped early if deemed unsafe or if results of a new treatment are extremely good. You will also be followed closely during and after the clinical trial. If the new drug being studied in a clinical trial works effectively to treat or prevent cancer, you may be one of the first people to benefit from the treatment. Taking part in a clinical trial is a chance to help others and improve the treatment of cancer. The possible risks of being in a clinical trial include: New drugs or treatments under study are not always better than, or as good as, the standard ones. There may be unexpected side effects that may be worse than those caused by standard drugs or treatments. A new treatment may not work for everyone in the trial just as a standard treatment may not work for everyone. If you are in the control group, you may not benefit as much if the new treatment is more effective at treating cancer. The people in a control group do not get the treatment being studied. Being in a clinical trial may take extra time or be inconvenient. |
Ich biete Ihnen an, die Webseite, mit der riesigen Zahl der Artikel nach dem Sie interessierenden Thema zu besuchen.