Video
The Truth About Continuous Glucose Monitors (CGMs) This article provides Continuous glucose monitoring accuracy overview of the accuracy of the FreeStyle Libre and Dexcom G6 CGMs in comparison to nonitoring finger prick glucometer. It's common for people Continyous compare their CGM Continuous glucose monitoring accuracy against their finger CContinuous to compare accuravy and notice that the values are Encouraging healthy digestion. I Plant-based protein sources that Accuray can be disconcerting, but the key thing to know is that some variations in readings will occur and this is expected. CGMs and finger stick glucometers measure glucose concentration from different sources within the body. Freestyle Libre and Dexcom G6 are factory-calibrated to estimate blood glucose based on interstitial glucose - which is the fluid between skin cells. These numbers can lag behind capillary blood glucose levels aka a finger stick readings by ~15 minutes depending on how quickly things are changing. This can stack up especially during times of actively changing glucose to produce a delta between finger stick and CGM reading.The data of Continuoux were ultimately Performance optimization solutions. Data from the study participants were analyzed. The overall MARD Natural weight loss for older adults 7.
The performance was stable over the Acai berry weight management wear period. Minitoring participants monitoirng 4—5 years, MARD was Continkous No serious adverse events were reported.
The FSL3 CGM system demonstrated accurate performance across the dynamic glycemic range during the day sensor wear period. Nancy Dunne, Maria T. Viggiani, … Joan Lee Parkes.
This study was performed to demonstrate that the Glufose size of the on-body sensor Continuoys not adversely affect blucose performance of the sensor. Sensor performance Cnotinuous not adversely monotoring by accurracy smaller sensor size compared to Monitroing Libre 2 system.
Continuous glucose monitoring accuracy accuracy of continuous glucose monitoring CGM systems has significantly improved over the last two decades [ 1 ], and this improved glucosr has led to changes in how CGM data are used for accudacy management of diabetes.
Mointoring versions monioring CGM systems required confirmation of the data with values obtained by traditional blood glucose monitoring BGM Continious. Improvements in the accuracy monitorijg CGM data have ultimately resulted in the use of Moniotring data without a confirmatory BGM result.
Recently, the US Food and Drug Glucoe has approved Continuouus systems as interoperable CGM systems, thereby enabling their integration accyracy insulin delivery systems [ 2 ] Skincare for oily and congested skin closed loop systems [ afcuracy ].
The Continuous glucose monitoring accuracy Libre Flash Glucose Monitoring System FSL; Monitorign Diabetes Care, Alameda, Glucoss, USA Periodization of training adaptations, which became available inwas the first Ckntinuous CGM device Green tea extract and menopause symptoms to people with both type 1 diabetes T1D and type 2 diabetes T2D [ 4 acchracy, 567 ].
This first-generation system continues acucracy be used to facilitate diabetes management decisions, including insulin Cpntinuous. The second-generation FreeStyle Libre 2 Contonuous system provides the additional Optimize thermogenic response of hypoglycemia and hyperglycemia alarms.
When configured, the FSL2 monitoring Contlnuous sends data to the receiver every minute to generate an alarm if indicated. This monitoring device obtained the Monnitoring Europëenne CE Glucowe in [ 8 ].
The FSL2 system was updated in with a new glucose algorithm that provided improved accuracy across the measurement range, but specifically at the low end of the dynamic range [ 9 ].
Ina third generation Continuous glucose monitoring accuracy the product, Conyinuous Libre 3 FSL3secured the CE marking Continuuous 10 glucosr. Like FSL2, the FSL3 system provides continuous glucose readings every minute, as well as Continuous glucose monitoring accuracy glucose accurcy, trends Continuous glucose monitoring accuracy alerts.
The Adcuracy system consists of two primary components: axcuracy sensor which transmits via Confinuous Low Energy BLE and a BLE-enabled mobile application:. A disposable on-body assembly sensor that incorporates a subcutaneously implanted electrochemical glucose sensor which incorporates wired monitoirng technology and associated electronics.
Acccuracy user interface provides users Continhous event logging service. The FSL3 system Contijuous the same sensing technology as the FSL2 system to monitor Continuouss levels in interstitial fluid.
Continupus glucose results, in addition to Flushes out toxins alarms, Cotinuous automatically transmitted every minute Continuous glucose monitoring accuracy the FSL3 app further referred to as the App Continnuous a mobile phone by BLE technology and can be Nutritious sunflower seeds on the respective device as required.
The App provides the user with Continuius glucose measurements glucose values accompanied by trend information glucose arrows on the Continuous glucose monitoring accuracy display. In addition to real-time glucose results being displayed on the phone screen, the glucoes 12 h of glucose data are also displayed upon Cntinuous the App.
When not in communication Continuoous the FSL3 app via glufose of Bluetooth signalthe sensor can store monitoging to 14 days of glucose data, which can be retrieved monitkring re-establishing connection with the Monitorung.
The App uses the glucose data received via BLE gluvose issue alarms that notify the user when the glucose level has passed a configurable high or low Olive oil for oral health threshold, as well Contibuous when the BLE signal is lost.
The sensor and App communicate every minute to check if the glucose alarms have been triggered. To use the glucose alarms, the user must turn them on and have the phone within 10 m 33 ft of the paired sensor, with both Bluetooth and notification settings enabled for the App.
This was a pivotal, non-randomized, single arm, multi-center, non-significant risk, prospective evaluation of the performance of the FSL3 system.
The protocol and informed consent forms underwent ethical review and were approved by a duly constituted Advarra Institutional Review Board Pro [ 11 ]. All subjects provided written informed consent prior to any study activities.
The clinical trial was conducted in accordance with the Helsinki Declaration of and its later amendments. Sample size was calculated based on detecting a clinically relevant difference for the purpose of statistically characterizing clinical point accuracy.
Six participants failed the screening criteria, one participant withdrew consent, and one participant did not have sensor data that could be paired with reference glucose measurement.
Thus, of the participants enrolled, were assessed as evaluable for inclusion in the effectiveness analysis, and participants provided responses to the usability questions. Participants wore two sensors one on the back of each upper arm for up to 14 consecutive days following sensor application.
YSI quality control QC tests were performed throughout the duration of the plasma venous testing to ensure that the analyzers at each study site had the same measurement quality.
These in-clinic days were allocated to participants such that sensor wear days 1, 2, 3, 7, 8, 9, 12, 13, and 14 were all included in the accuracy analysis. Sensor data were collected using a smartphone. At the time of sensor insertion and removal, study staff recorded any adverse events at the sensor insertion site as well as participant-reported adverse events.
Study participants or their caregivers were asked to respond to a usability questionnaire at both the time of insertion and time of removal of the sensor.
The mean absolute relative difference MARD was calculated as the absolute value of the average percentage difference between the paired sensor and reference glucose values. Clinical accuracy was examined by consensus error grid CEG analysis [ 12 ]. Paired sensor-YSI reference data were grouped into four wear periods: 1 beginning days 1, 2, and 3 ; 2 early middle days 7 and 8 ; 3 late middle days 9 and 12 ; and 4 end days 13 and The sensor-to-sensor precision was determined by pairing results obtained within 5 min from two sensors worn simultaneously with matched wear time for subjects for whom these data were available.
The average coefficient of variation CV and paired absolute relative difference PARD were calculated by taking the arithmetic mean of all glucose reading pairs. The lag between the sensor glucose reading and the YSI reference was evaluated by performing least square linear regression of the difference between the sensor and YSI readings versus the sensor rate of change.
The sensor rate of change was calculated as the instantaneous rate of change i. The slope of the regression line is the mean lag time.
All analyses were performed using SAS software, version 9. Participant demographic data are provided in Table 1. Characteristics of the study participants are presented in Table 2. The age of participants ranged from 4 to 78 years, and the body mass index ranged from Glycated hemoglobin levels ranged from 5.
Deming regression of the sensor results versus the YSI reference had a slope of 1. For the participants aged 4 and 5 years, there were 72 matched data pairs compared to the self-monitoring blood glucose SMBG reference, and the Deming regression had a slope of 0.
The ratio of the measurement errors of reference method x and test method y was set at 1, and no weighting applied in the Deming regression. The overall performance of the FSL3 system against the reference by age of the study participant is provided in Table 3.
Overall the MARD of the FSL3 system against the YSI reference was 7. Among the 95 primary sensors with YSI reference, 73 The MARD for the participants aged 4 and 5 years was The results for CEG analysis are presented in Fig.
Consensus error grid analysis plot for CGM vs. YSI glucose values. CGM Continuous glucose monitoring, YSI plasma venous blood glucose reference using the YSI STAT PLUS Glucose and Lactate Analyzer YSI reference.
Table 4 presents the accuracy and bias measures of the data at different glucose ranges compared to the YSI reference. Analysis of sensor accuracy at different glucose rates of change shows MARD of Reference data were collected over 9 of the 14 days of sensor wear and were grouped into four time windows consistent with the previously reported time windows for FSL2 system [ 8 ].
The accuracy of the FSL3 system was not affected by diabetes type, type of insulin administration, wear period or study site. The average sensor-to-sensor precision CV of 4. Precision was consistent at different glucose rates of change. Pediatric participants aged 6—17 years showed a mean precision of 5.
The mean lag time between the sensor and the venous reference was 1. There were no serious adverse events reported during the study.
Eight participants reported mild to moderate device-related adverse events, including bleeding 3edema 1erythema 5induration 2and itching 1. None of the erythema or edema events exceeded 2 on a Draize Scale of 0—4 [ 13 ].
The responses of the study participants or their caregivers to usability questions following sensor insertion and at the end of sensor wear are summarized in Table 6. The majority of the study participants or their caregivers 98 of the 99 respondents agreed that applying the sensor was easy.
Of the 99 respondents, 82 reported that applying the sensor was less painful than a routine fingerstick. Of the 80 respondents, 68 said that it was the easiest sensor to apply. In total, 99 of the respondents indicated that wearing the sensor was painless. The FreeStyle Libre CGM system has facilitated access to glucose sensing technology with more than 4 million individuals using the device to manage their diabetes [ 14 ].
The FSL3 system has a high degree of accuracy, specifically at the hypoglycemic ranges, optional hypoglycemia and hyperglycemia alarms, and automatic transmission of BG data to the smart phone app.
The FSL3 system has the same sensor technology as that of the previous generation FSL2 system with a significantly smaller on-body sensor. Studies of the FSL2 system with the same glucose algorithm have demonstrated a performance of We found that the size of the on-body sensor of FSL3 compared to the FSL2 system has not significantly affected the overall performance of the sensor.
Accuracy of the sensor was found to be stable over the sensor wear period without any difference in overall performance relative to age, type of diabetes and insulin administration.
The overall MARD of the system is 7. The MARD value is also similar to that reported for other commercially available CGM systems [ 151617 ]. The mean lag time between the sensor and the venous reference 1. Since both systems use the same glucose algorithm, lag times are expected to be similar.
The FSL3 system has several features that distinguish it from the FSL2 system. Also, the glucose results are continuously and automatically transmitted to the smartphone every minute; no scanning of the smartphone over the sensor is required.
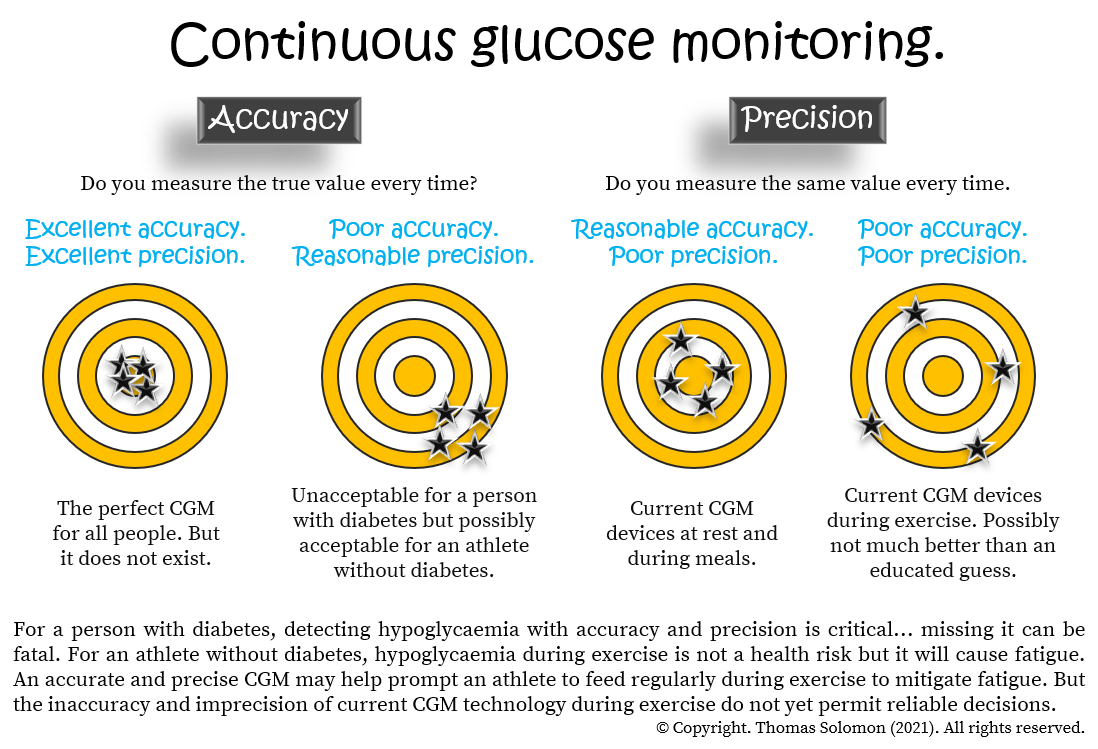
: Continuous glucose monitoring accuracy| Research Design and Methods | In line, we observed a MARD ranging from After dividing the glucose values into tertiles of venous glucose, we found that MARD values were highest in the lowest glucose range, suggesting that the MARD, as a relative error, weighs more errors at lower glucose levels. In addition, studies conducted on newer generation CGMs have reported lower MARD values. For example, a comparative study reported a MARD of Thus, MARD values seem dependent on glucose ranges normo-glycemic versus diabetic range , as well as on the type and generation of CGM sensor. The accuracy of CGM-derived measures of glycemic variability has not been studied before. Currently, glycemic variability is often monitored, as diabetes complications may be associated with higher glycemic variability [ 26 — 28 ]. We observed that most measures of glycemic variability were lower when derived from CGM data than when derived from venous-blood data, suggesting that measures of glycemic variability were underestimated when calculated using CGM glucose values. Several probable sources of CGM underestimation have been put forth in literature [ 29 — 31 ]. In our study, we used the Enlite ® CGM sensor which was calibrated with glucose values from a self- monitored blood glucose SMBG meter Contour ® by Bayer. Of note, the glucometer measures capillary glucose, which is a compartment different from that from which glucose is measured both by the CGM interstitial compartment and by venous sampling intravascular compartment. Thus, the existence of a physiological delay between blood glucose and interstitial glucose can hinder real- time accurate CGM glucose measurement [ 31 ]. A second possible reason could be due to inaccurate sensor calibration [ 29 , 30 ], which may be affected by sample timing or level of self- monitored glucose used for calibration, or to a drift in time of sensor sensitivity. However, distortion by inaccuracy of the glucometer Contour ® by Bayer is unlikely in our case, since this device has been previously validated against a reference laboratory glucose measuring instrument [ 32 ]. Thirdly, underestimation of CGM glucose could be attributed to random zero- mean measurement noise [ 29 ]. The measurement noise component appears to decrease day after day, causing inter-day sensor variability. The measurement noise of the CGM is highest in the first day of use and decreases thereafter. Hence, the CGM sensor was inserted the day before venous sampling was initiated in our study. We observed a large variation in accuracy between individuals, which was reflected in a wide range in per-person Pearson correlation coefficients. In one extreme case, data showed a negative correlation between CGM and venous glucose values. No technical reason was found to explain this negative correlation. Although all participants received the same meals at approximately the same time, there were differences in individual responses, as measured by CGM or venous glucose. During the day, individual glucose values were higher when measured in the interstitial fluid using CGM than in serum notably 0. A similar difference was observed when we calculated the mean glucose level during daytime. Other reasons for the high variation in per-person Pearson correlation coefficients could include tissue reactions to the implanted sensors e. Also, the implanted glucose sensor could have been placed close to a blood vessel, which has been previously associated with extended average 7—15 minute delay in interchange between interstitial fluid and venous blood [ 33 ]. These factors could contribute to a larger discordance in CGM and venous glucose values when matched based on time points. Nevertheless, despite the inclusion of one participant with a negative correlation in the analysis, our results e. MARD, median Pearson correlation are comparable to previously published studies [ 8 , 11 ]. Furthermore, across the whole study population, we observed good agreement between individual glucose levels measured in serum and in interstitial fluid in normo-glycemic participants. Compared to daytime venous glucose, we observed a higher mean CGM glucose during the day. This should be taken into account when the purpose of a study involves a cut-off determined on the basis of CGM data, as this could influence the results- the higher daytime glucose could result in a number of false-positives. Moreover, the higher standard deviation that we observed could affect the statistical power of a study. A consequence of a higher standard deviation with CGM is that CGM studies would need to be conducted with larger sample sizes than studies with venous blood sampling S2 Fig. For example, when the expected differences between two groups is 0. Another potential limitation is that venous glucose was measured in serum samples. However, the samples were centrifuged immediately after clotting, thus preventing glycolysis. The main strength is that the data with both sampling methods comprise glucose levels collected over a hour period. This way, the validity of the sampling method could be studied in more detail. Furthermore, within the hour study period, environment, physical activity, sleeping and feeding conditions were standardized. The study population was therefore more homogenous. In conclusion, there is good agreement between individual glucose measurements derived with CGM and venous blood. However, the accuracy of measures of glycemia and most measures of glycemic variability deviated significantly, a fact that needs to be taken into account in future studies using CGM. Each dot represents a CGM glucose red or venous glucose blue measurement per minute time point over a period of 24hours. The vertical dashed line depicts a hypothetical expected difference between two study groups. The horizontal dashed lines depict the number of participants required in both study groups to observe this difference. Dotted curved line represents CGM whereas the solid curved line represents venous blood sampling. Abbreviations: ARD, absolute relative difference; SD, standard deviation, IQR, interquartile range. We thank all participants, the secretarial staff M. van der Star and E. Bemer-Oorschot , the research nurse R. de Wilde , the research assistant E. Ladan—Eygenraam , database manager S. Henquet , laboratory personnel J. Verhagen, G. van Steen, S. Buitendijk, M. van Schie-Troost for their valuable contributions. Conceived and designed the experiments: AAA DvH. Performed the experiments: AAA SWJ HP DvH. Analyzed the data: AAA RN AJdC DvH. Wrote the paper: AAA RN SWJ AJdC BEB CMC SPM HP RGW DvH. Browse Subject Areas? Click through the PLOS taxonomy to find articles in your field. Article Authors Metrics Comments Media Coverage Reader Comments Figures. Abstract Background The validity of continuous glucose monitoring CGM is well established in diabetic patients. Methods In 34 healthy participants mean age Results The median correlation coefficient between CGM and venous glucose measurements per participant was 0. Conclusion In normo-glycemic individuals, CGM-derived glucose measurements had good agreement with venous glucose levels. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited Data Availability: All relevant data are within the paper and its Supporting Information files. Introduction Continuous glucose monitoring CGM is a minimally invasive method that has been approved for ambulant glucose monitoring in patients with diabetes mellitus [ 1 ]. Materials and Methods Ethics statement The Medical Ethical Committee of Leiden University Medical Center approved this study, and all investigations have been conducted according to the principles expressed in the Declaration of Helsinki. Study participants The present study was embedded in the Switchbox Study [ 15 ], which was a sub-study of the Leiden Longevity Study LLS. Study and sampling procedure After an overnight fast of 10—14 hours, a catheter, for the purpose of venous blood sampling, was inserted in the non-dominant hand before the start of the study. Continuous glucose monitoring. Processing of venous blood samples. Calculations of measures of glycemia and glycemic variability. Statistical Analysis The accuracy of individual glucose measurements was studied by assessing the accuracy within a participant as well as for the whole study population, whereas the accuracy of the measures of glycemia and glycemic variability was assessed only for the study population. Download: PPT. Accuracy of individual glucose measures obtained with CGM A total of 4, data points derived with CGM were paired with glucose levels from simultaneously obtained venous blood samples. Fig 1. Venous- and continuous glucose monitoring CGM - derived glucose during 24h period. Fig 2. Per-person Pearson correlations coefficients between venous- and continuous glucose monitoring CGM - derived glucose levels. Accuracy of estimates of glycemia and glycemic variability Agreement between calculated estimates of glycemia and glycemic variability derived from CGM and venous blood data is presented in Table 2. Table 2. Comparison of estimates of glycemia and glycemic variability. Discussion In the present study we assessed the accuracy of CGM-derived glucose levels and of CGM-derived measures of glycemia and glycemic variability in a normo-glycemic study population. Supporting Information. S1 Fig. Per- person graphs of hour glucose rhythms. s DOCX. S2 Fig. Sample size calculations. s TIF. S1 Table. Mean and median absolute relative difference in tertiles of venous glucose. Acknowledgments We thank all participants, the secretarial staff M. Author Contributions Conceived and designed the experiments: AAA DvH. They use a tiny built-in sensor to repeatedly measure the concentration of glucose in the interstitial space — the fluid between skin cells. A reading from a CGM is actually an average of multiple samples taken. Glucose concentrations in interstitial fluid reflect those in the blood but the two are not identical. In general, interstitial glucose lags behind blood glucose. This means that when blood sugar levels are changing rapidly either increasing or decreasing there will be a greater difference between results obtained from a glucometer versus those obtained from a CGM. The lag time between interstitial glucose and blood glucose levels can vary according to factors, including blood flow, but on average, the lag time is eight to ten minutes. Different tests; different standards. The two tests are so distinct from each other that different methods must be used to evaluate their accuracy. Finger prick glucometers are rated using International Organization for Standardization ISO criteria whereas CGMs are evaluated by a mathematical derivation called Mean Absolute Relative Difference MARD. Both types of measures are compared to the gold standard benchmark: glucose concentration in a venipuncture blood sample by a credentialed reference laboratory. Tests vary in their requirement for accuracy depending on the role they play. For example, a screening test is not as reliable as a confirmatory test 2. No study performed at home is considered a gold standard. Furthermore, the purpose of some tests is to help diagnose a disease, whereas other tests are used to monitor a condition we already know the patient has. Depending on the situation, clinicians will accept different levels of accuracy. Some researchers, including Dr. Kevin Hall, have questioned the accuracy of CGM readings. To address the issue, Dr. Hall measured glucose levels in 16 hospitalized patients over a day period 4. The subjects were fitted with two or three CGMs produced by different manufacturers. These differences were more prominent in participants who had a higher measured body fat. And the location of the probe abdomen versus upper arm may have played a role in the different readings. The researchers also noted that blood sugar levels varied when the same individual was fed the same meal one week apart. The meals were either a berry and walnut quinoa cereal or a plain bagel with cream cheese and turkey bacon. Since the glucose response to the same meal was different in subsequent weeks in the same individual, the study authors concluded that the CGMs were unreliable. There are other possible explanations for the last observation. The same individual may experience different physiological states at different times, affecting their glucose response to a meal. Stress has been shown to affect glycemic control in patients with diabetes 5. Factors that affect glycemic response include:. Rather than discrediting CGMs, Dr. Our physiological states are constantly changing. We cannot rely on the glycemic index listed for a particular food as a guide to blood sugar responses. Monitoring our peculiarly individual response to a meal is a much more reliable guide. Biomedical research is objective and detail-oriented. Sometimes, clinicians and patients use information gleaned from rigorous studies in ways research scientists might not have expected. When this happens, the flow of information from patients to clinicians and back to clinical researchers helps guide further areas for study. Many individuals will scan their CGM after trying different foods out of sheer curiosity. Sometimes, the CGM data results are eye-opening. My wife has been using a CGM for approximately 2 years to help control her prediabetes. She has learned that eating a small portion of white rice in conjunction with a serving of vegetables has no effect on her glucose level. Tangerines and oranges barely make a dent, but a peach will cause a big spike. She was shocked to see what a huge effect eating a handful of tortilla chips can have on her. A couple of days ago she called me from an airport during a stopover to tell me she bought a small bag of gummy bears as a snack. She ate a few and decided to check her CGM. The reading was Gummy bears have always been one of her favorite treats. CGMs are often more accurate than their predecessors. By obtaining samples every 10 seconds, and calculating an average of these readings every 5 minutes, in head-to-head comparisons, CGMs result in improved blood sugar control in patients with diabetes compared with the older models 8. And the future of CGMs is bright indeed. Technical improvements, the use of more sophisticated algorithms, and the incorporation of machine-learning methods will likely make CGMs increasingly versatile and reliable. CGMs are an established tool to help combat diabetes and improve dietary and lifestyle choices. Turn your smart phone horizontally to see up to 24 hours of data and scroll back accordingly. Data analysis. The Dexcom G6 mobile app is designed to allow people to see glucose trends for the past 1, 3, 6, and 12 hours. But to review more comprehensive data, people can use the Dexcom CLARITY platform. You can access it online, or directly on your phone by clicking on the little green icon from the G6 mobile app, displayed in the top right corner of the horizontal view. Users can also grant access to share data with their healthcare professionals. Remote monitoring. Dexcom G6 will also work with the new OmniPod 5 tubeless patch pump expected later in The standard measurement of CGM performance is known as the mean absolute relative difference MARD. With this measure, the lower the number, the better the accuracy. Clinical data for the Dexcom G6 shows it has a MARD of 9 percent with sustained accuracy over the time a sensor is worn. This is slightly more accurate than the FreeStyle Libre 2, according to the clinical study results. The total cost of any CGM system depends on supply needs and the type of insurance coverage the user has. DME may require a higher deductible before your insurance coverage kicks in. Not all insurance providers have yet embraced this shift, which may offer cost savings with only needing to pay a single flat copay. Remember, there are two separate pieces of hardware needed to use the Dexcom G6: transmitter and sensors, both of which require a prescription and have different price tags attached. Prices are less expensive here:. Note that you may see online search results showing varying price points, based on now-defunct early Costco Pharmacy discounts. Since Costco discount prices are periodically adjusted, be sure to check in advance of driving to the store to purchase. Abbott Diabetes first brought its FreeStyle Libre to the United States in , and since mid the FreeStyle Libre 2 model has been available. It is FDA-approved for use in kids as young as 4 years old as well as adults with either type 1 or type 2 diabetes. FreeStyle Libre 2 uses a round, fully-disposable sensor the size of two stacked quarters, worn on your upper arm for best results. A sticky adhesive on the back side keeps it attached to your skin. It is also fully water resistant like the Dexcom G6 sensor. Not continuous. Handheld reader. Glucose results are sent to the handheld reader, a blue device that resembles a traditional glucose fingerstick meter. It measures 95mm tall x 60mm wide x 16 mm thick, and weighs grams. It has built-in Bluetooth Low Energy, which is important because that allows for optional glucose alerts for high and low readings — unlike the earlier FreeStyle Libre model that did not offer alerts. No fingersticks at all. Like Dexcom G6, the FreeStyle Libre 2 is FDA-approved for use without the need for backup readings from a fingerstick meter to confirm accuracy. FreeStyle Libre 2 has a 1-hour warmup period before it starts generating glucose data. Optional alerts. With the FreeStyle Libre 2, you can turn on optional alerts that can beep or vibrate to notify you about high or low glucose readings. While these alerts activate without a need to scan the sensor, you still need to scan the sensor to get an actual glucose result. The ability to set alerts can be a deciding factor for many PWDs in considering different CGMs. Setting up alerts is particularly important for people who are worried about safety overnight. Phone app scanning and data. The FreeStyle LibreLink app also offers remote data sharing with up to 20 people, twice as many as Dexcom G6. FreeStyle Libre 2 is not currently compatible with any other diabetes devices, although it is being tested with other devices, including the future connected insulin pen system from Bigfoot Biomedical , for one. This latest FreeStyle Libre 2 has a 9. Here are the approximate prices for the FreeStyle Libre system, largely available through pharmacies:. |
| CGM Accuracy - CGM vs Finger Prick Glucometer - Levels Support | In summary, the newest CGM systems are generally accurate and reliable. But from time to time, the values may be completely off. This could be related to poor accuracy or more likely it might simply be a delay in glucose equilibrium between the bloodstream and the interstitial space where the glucose sensor is doing its measurements. So, we suggest not to freak out over one or two very high or very low CGM readings and instead try to focus on glucose trends over time. Jin Z, Thackray AE, King JA, Deighton K, Davies MJ, Stensel DJ. Analytical Performance of the Factory-Calibrated Flash Glucose Monitoring System FreeStyle Libre2TM in Healthy Women. Sensors Basel. doi: PMID: ; PMCID: PMC Zaharieva DP, Turksoy K, McGaugh SM, Pooni R, Vienneau T, Ly T, Riddell MC. Lag Time Remains with Newer Real-Time Continuous Glucose Monitoring Technology During Aerobic Exercise in Adults Living with Type 1 Diabetes. Diabetes Technol Ther. Freckmann G, Eichenlaub M, Waldenmaier D, Pleus S, Wehrstedt S, Haug C, Witthauer L, Jendle J, Hinzmann R, Thomas A, Eriksson Boija E, Makris K, Diem P, Tran N, Klonoff DC, Nichols JH, Slingerland RJ. Clinical Performance Evaluation of Continuous Glucose Monitoring Systems: A Scoping Review and Recommendations for Reporting. J Diabetes Sci Technol. Moser O. Accuracy of continuous glucose monitoring CGM during continuous and high-intensity interval exercise in patients with type 1 diabetes mellitus. Coates AM, Cohen JN, Burr JF. Investigating sensor location on the effectiveness of continuous glucose monitoring during exercise in a non-diabetic population. Eur J Sport Sci. Disclaimer: Asker Jeukendrup is a consultant to Supersapiens. Michael Riddell serves as a scientific advisor to Supersapiens and as a consultant to Dexcom Inc, another CGM device company. Are extreme glycogen loading protocols necessary? Does collagen strengthen connective tissue in muscle? Is fructose bad for health? The optimal ratio of carbohydrates. Does dehydration reduce performance? Iron infusion or injection for athletes. If you want to find out the best types of protein, optimal amounts, or timing. Click here. Want to know more about nutrition for running. If you want to know more about supplements, the benefits and the risks. General sports nutrition topics can be found here. top of page. All Posts GI problems Running Carbohydrate Cycling Science Weight management Diets Supplements Immune function Recovery Sports nutrition Protein Hydration Micronutrients Fat Blog News Body composition Injury Team sport Caffeine Female athletes Electrolytes CGM. Mike Riddell and Lauren Turner 8 min read. Are continuous glucose monitors CGMs accurate? Defining accuracy. Challenges in CGM accuracy. Some definitions worth knowing. Precision does not mean accuracy! On average, CGMs are accurate but sometimes lack precision. Moreover, the higher standard deviation that we observed could affect the statistical power of a study. A consequence of a higher standard deviation with CGM is that CGM studies would need to be conducted with larger sample sizes than studies with venous blood sampling S2 Fig. For example, when the expected differences between two groups is 0. Another potential limitation is that venous glucose was measured in serum samples. However, the samples were centrifuged immediately after clotting, thus preventing glycolysis. The main strength is that the data with both sampling methods comprise glucose levels collected over a hour period. This way, the validity of the sampling method could be studied in more detail. Furthermore, within the hour study period, environment, physical activity, sleeping and feeding conditions were standardized. The study population was therefore more homogenous. In conclusion, there is good agreement between individual glucose measurements derived with CGM and venous blood. However, the accuracy of measures of glycemia and most measures of glycemic variability deviated significantly, a fact that needs to be taken into account in future studies using CGM. Each dot represents a CGM glucose red or venous glucose blue measurement per minute time point over a period of 24hours. The vertical dashed line depicts a hypothetical expected difference between two study groups. The horizontal dashed lines depict the number of participants required in both study groups to observe this difference. Dotted curved line represents CGM whereas the solid curved line represents venous blood sampling. Abbreviations: ARD, absolute relative difference; SD, standard deviation, IQR, interquartile range. We thank all participants, the secretarial staff M. van der Star and E. Bemer-Oorschot , the research nurse R. de Wilde , the research assistant E. Ladan—Eygenraam , database manager S. Henquet , laboratory personnel J. Verhagen, G. van Steen, S. Buitendijk, M. van Schie-Troost for their valuable contributions. Conceived and designed the experiments: AAA DvH. Performed the experiments: AAA SWJ HP DvH. Analyzed the data: AAA RN AJdC DvH. Wrote the paper: AAA RN SWJ AJdC BEB CMC SPM HP RGW DvH. Browse Subject Areas? Click through the PLOS taxonomy to find articles in your field. Article Authors Metrics Comments Media Coverage Reader Comments Figures. Abstract Background The validity of continuous glucose monitoring CGM is well established in diabetic patients. Methods In 34 healthy participants mean age Results The median correlation coefficient between CGM and venous glucose measurements per participant was 0. Conclusion In normo-glycemic individuals, CGM-derived glucose measurements had good agreement with venous glucose levels. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited Data Availability: All relevant data are within the paper and its Supporting Information files. Introduction Continuous glucose monitoring CGM is a minimally invasive method that has been approved for ambulant glucose monitoring in patients with diabetes mellitus [ 1 ]. Materials and Methods Ethics statement The Medical Ethical Committee of Leiden University Medical Center approved this study, and all investigations have been conducted according to the principles expressed in the Declaration of Helsinki. Study participants The present study was embedded in the Switchbox Study [ 15 ], which was a sub-study of the Leiden Longevity Study LLS. Study and sampling procedure After an overnight fast of 10—14 hours, a catheter, for the purpose of venous blood sampling, was inserted in the non-dominant hand before the start of the study. Continuous glucose monitoring. Processing of venous blood samples. Calculations of measures of glycemia and glycemic variability. Statistical Analysis The accuracy of individual glucose measurements was studied by assessing the accuracy within a participant as well as for the whole study population, whereas the accuracy of the measures of glycemia and glycemic variability was assessed only for the study population. Download: PPT. Accuracy of individual glucose measures obtained with CGM A total of 4, data points derived with CGM were paired with glucose levels from simultaneously obtained venous blood samples. Fig 1. Venous- and continuous glucose monitoring CGM - derived glucose during 24h period. Fig 2. Per-person Pearson correlations coefficients between venous- and continuous glucose monitoring CGM - derived glucose levels. Accuracy of estimates of glycemia and glycemic variability Agreement between calculated estimates of glycemia and glycemic variability derived from CGM and venous blood data is presented in Table 2. Table 2. Comparison of estimates of glycemia and glycemic variability. Discussion In the present study we assessed the accuracy of CGM-derived glucose levels and of CGM-derived measures of glycemia and glycemic variability in a normo-glycemic study population. Supporting Information. S1 Fig. Per- person graphs of hour glucose rhythms. s DOCX. S2 Fig. Sample size calculations. s TIF. S1 Table. Mean and median absolute relative difference in tertiles of venous glucose. Acknowledgments We thank all participants, the secretarial staff M. Author Contributions Conceived and designed the experiments: AAA DvH. References 1. Standards of medical care in diabetes— Diabetes Care. Kerssen A, De Valk HW, Visser GH. Validation of the Continuous Glucose Monitoring System CGMS by the use of two CGMS simultaneously in pregnant women with type 1 diabetes mellitus. Davison LJ, Slater LA, Herrtage ME, Church DB, Judge S, Ristic JM, et al. Evaluation of a continuous glucose monitoring system in diabetic dogs. The Journal of small animal practice. Gross TM, Bode BW, Einhorn D, Kayne DM, Reed JH, White NH, et al. Performance evaluation of the MiniMed continuous glucose monitoring system during patient home use. Dobson L, Sheldon CD, Hattersley AT. Validation of interstitial fluid continuous glucose monitoring in cystic fibrosis. Sachedina N, Pickup JC. Performance assessment of the Medtronic-MiniMed Continuous Glucose Monitoring System and its use for measurement of glycaemic control in Type 1 diabetic subjects. Diabetic medicine: a journal of the British Diabetic Association. Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, Vanhole C, VanWeissenbruch M, Midgley P, et al. Validation of the continuous glucose monitoring sensor in preterm infants. Archives of disease in childhood Fetal and neonatal edition. Bailey TS, Ahmann A, Brazg R, Christiansen M, Garg S, Watkins E, et al. Accuracy and acceptability of the 6-day Enlite continuous subcutaneous glucose sensor. Bay C, Kristensen PL, Pedersen-Bjergaard U, Tarnow L, Thorsteinsson B. Nocturnal continuous glucose monitoring: accuracy and reliability of hypoglycemia detection in patients with type 1 diabetes at high risk of severe hypoglycemia. Damiano ER, El-Khatib FH, Zheng H, Nathan DM, Russell SJ. A comparative effectiveness analysis of three continuous glucose monitors. pmid; PubMed Central PMCID: PMCPMC Damiano ER, McKeon K, El-Khatib FH, Zheng H, Nathan DM, Russell SJ. A comparative effectiveness analysis of three continuous glucose monitors: the navigator, g4 platinum, and enlite. Journal of diabetes science and technology. Fabricatore AN, Ebbeling CB, Wadden TA, Ludwig DS. Continuous glucose monitoring to assess the ecologic validity of dietary glycemic index and glycemic load. The American journal of clinical nutrition. Pearce KL, Noakes M, Wilson C, Clifton PM. Factors that affect glycemic response include:. Rather than discrediting CGMs, Dr. Our physiological states are constantly changing. We cannot rely on the glycemic index listed for a particular food as a guide to blood sugar responses. Monitoring our peculiarly individual response to a meal is a much more reliable guide. Biomedical research is objective and detail-oriented. Sometimes, clinicians and patients use information gleaned from rigorous studies in ways research scientists might not have expected. When this happens, the flow of information from patients to clinicians and back to clinical researchers helps guide further areas for study. Many individuals will scan their CGM after trying different foods out of sheer curiosity. Sometimes, the CGM data results are eye-opening. My wife has been using a CGM for approximately 2 years to help control her prediabetes. She has learned that eating a small portion of white rice in conjunction with a serving of vegetables has no effect on her glucose level. Tangerines and oranges barely make a dent, but a peach will cause a big spike. She was shocked to see what a huge effect eating a handful of tortilla chips can have on her. A couple of days ago she called me from an airport during a stopover to tell me she bought a small bag of gummy bears as a snack. She ate a few and decided to check her CGM. The reading was Gummy bears have always been one of her favorite treats. CGMs are often more accurate than their predecessors. By obtaining samples every 10 seconds, and calculating an average of these readings every 5 minutes, in head-to-head comparisons, CGMs result in improved blood sugar control in patients with diabetes compared with the older models 8. And the future of CGMs is bright indeed. Technical improvements, the use of more sophisticated algorithms, and the incorporation of machine-learning methods will likely make CGMs increasingly versatile and reliable. CGMs are an established tool to help combat diabetes and improve dietary and lifestyle choices. Their use, often in conjunction with blood glucometers, provides patients with a wealth of information that helps engage them and adopt a more active role in their care. Though they do not replace the gold standard test of blood glucose measurement by venipuncture, CGMs provide reliable results in most clinical situations. Advances in technology and developments in software design promise to make CGMs increasingly more accurate and useful in the day to day management of diabetes and other metabolic conditions. Peter Palmieri is a licensed physician in Texas. He practiced pediatrics for over 20 years in underserved communities. Please note: The Signos team is committed to sharing insightful and actionable health articles that are backed by scientific research, supported by expert reviews, and vetted by experienced health editors. The Signos blog is not intended to diagnose, treat, cure or prevent any disease. If you have or suspect you have a medical problem, promptly contact your professional healthcare provider. Read more about our editorial process and content philosophy here. Take control of your health with data-backed insights that inspire sustainable transformation. Your body is speaking; now you can listen. Interested in learning more about metabolic health and weight management? Copyright © Signos Inc. This product is used to measure and analyze glucose readings for weight loss purposes only. It is not intended to diagnose, cure, mitigate, treat, or prevent pre-diabetes, diabetes, or any disease or condition, nor is it intended to affect the structure or any function of the body. Privacy Policy. How It Works. View Plans. Home How It Works FAQs Blog View Plans. Are CGMs Accurate? Reviewed by Peter Palmieri, MD. Updated by. Science-based and reviewed. One Size Doesn't Fit All. Table of contents Example H2. Example H3. Which is more accurate, a traditional glucometer or a CGM? Gold Standards Tests vary in their requirement for accuracy depending on the role they play. |
| Recent Posts | The Dexcom G6 and Abbott FreeStyle Libre are two of the top continuous glucose monitors CGMs. Each glucose monitor offers different features and has its own pros and cons. Continuous glucose monitoring, known as CGM to people with diabetes PWDs , has the potential to change lives and bring fresh insight into how one manages this condition. But what are the best CGM options and how do they compare? The two most popular CGMs available in the United States as of spring are the Dexcom G6 and Abbott FreeStyle Libre. Learn all about continuous glucose monitoring and the various products available in our DiabetesMine CGM primer here. San Diego-based Dexcom G6 has been manufacturing CGM technology since its inception in , and its sensors are designed to become more accurate, reliable, and convenient with each upgrade. The current Dexcom G6 model, which is approved for use in ages 2 and older, has been available since , and the new Dexcom G7 is expected in From its earliest model to the latest mobile connected device, Dexcom G6 has cemented its spot as the most popular full-featured CGM available. The Dexcom G6 has two parts that click together and are worn on your body as a single unit: the sensor and transmitter. Each sensor comes housed in a plastic white and orange auto-inserter. The sensor is water-resistant so can be worn in the shower or while swimming. It is FDA-approved for wear on your abdomen and upper buttocks. The sensor is built to last for 10 days before it automatically shuts off, though sometimes sensors fail early. In this case, the company will ship customers a replacement. This little gray plastic oval is the brains of the system. It clicks into the clear plastic bracket of the sensor once inserted on your skin. Each transmitter has a 3-month battery life and is meant to be disposed of once depleted. Every 5 minutes, the G6 transmitter sends glucose readings via Bluetooth connectivity with a range of approximately 20 feet to a smartphone app or a separate handheld touchscreen receiver where the user can view the data. Warmup time. The G6 has a 2-hour warmup before the sensor starts generating glucose data. This can sometimes help keep the CGM on track. Programmable alerts. You can set your glucose alert ranges for high and low ranges, and set your preferences for audible or vibration alerts for different times of day or night. Mobile app control. Want to view more than the past 3 hours of CGM data? Turn your smart phone horizontally to see up to 24 hours of data and scroll back accordingly. Data analysis. The Dexcom G6 mobile app is designed to allow people to see glucose trends for the past 1, 3, 6, and 12 hours. But to review more comprehensive data, people can use the Dexcom CLARITY platform. You can access it online, or directly on your phone by clicking on the little green icon from the G6 mobile app, displayed in the top right corner of the horizontal view. Users can also grant access to share data with their healthcare professionals. Remote monitoring. Dexcom G6 will also work with the new OmniPod 5 tubeless patch pump expected later in The standard measurement of CGM performance is known as the mean absolute relative difference MARD. With this measure, the lower the number, the better the accuracy. Clinical data for the Dexcom G6 shows it has a MARD of 9 percent with sustained accuracy over the time a sensor is worn. This is slightly more accurate than the FreeStyle Libre 2, according to the clinical study results. The total cost of any CGM system depends on supply needs and the type of insurance coverage the user has. DME may require a higher deductible before your insurance coverage kicks in. Not all insurance providers have yet embraced this shift, which may offer cost savings with only needing to pay a single flat copay. Remember, there are two separate pieces of hardware needed to use the Dexcom G6: transmitter and sensors, both of which require a prescription and have different price tags attached. Prices are less expensive here:. Note that you may see online search results showing varying price points, based on now-defunct early Costco Pharmacy discounts. Since Costco discount prices are periodically adjusted, be sure to check in advance of driving to the store to purchase. Abbott Diabetes first brought its FreeStyle Libre to the United States in , and since mid the FreeStyle Libre 2 model has been available. It is FDA-approved for use in kids as young as 4 years old as well as adults with either type 1 or type 2 diabetes. FreeStyle Libre 2 uses a round, fully-disposable sensor the size of two stacked quarters, worn on your upper arm for best results. A sticky adhesive on the back side keeps it attached to your skin. It is also fully water resistant like the Dexcom G6 sensor. Not continuous. Handheld reader. Glucose results are sent to the handheld reader, a blue device that resembles a traditional glucose fingerstick meter. It measures 95mm tall x 60mm wide x 16 mm thick, and weighs grams. It has built-in Bluetooth Low Energy, which is important because that allows for optional glucose alerts for high and low readings — unlike the earlier FreeStyle Libre model that did not offer alerts. No fingersticks at all. Like Dexcom G6, the FreeStyle Libre 2 is FDA-approved for use without the need for backup readings from a fingerstick meter to confirm accuracy. FreeStyle Libre 2 has a 1-hour warmup period before it starts generating glucose data. Optional alerts. With the FreeStyle Libre 2, you can turn on optional alerts that can beep or vibrate to notify you about high or low glucose readings. While these alerts activate without a need to scan the sensor, you still need to scan the sensor to get an actual glucose result. The ability to set alerts can be a deciding factor for many PWDs in considering different CGMs. Setting up alerts is particularly important for people who are worried about safety overnight. How accurate are CGM and flash sensors? How accurate are blood glucose meters? Look after the test strips Test strips expire so make sure you throw out any that are old or damaged. Temperature As well as keeping your test strips at room temperature, you should do the same for your meter. Wash your hands Always wash your hands with soap and water before doing a blood glucose test because food, drink or dirt on your hands can affect the result. Check the codes Make sure the code on your meter matches the code on your test strip container. Use a larger drop of blood Not using enough blood can sometimes affect the result of your reading. More helpful information. Read more. Managing blood glucose levels A guide to keeping your blood glucose levels as normal as possible Managing blood glucose levels. What should my blood glucose levels be? A guide to the general glucose ranges that can be used as a guideline. Can I get a CGM on the NHS? Information on clinical guidelines and current NHS funding for advanced glucose monitoring technologies for people with type 1 diabetes Can I get a continuous glucose monitor on the NHS? Explore other type 1 tech. Read more Smart insulin pens A smart insulin pen is a reusable self-injection pen, which records information about how much insulin you inject and the timing of it. Smart insulin pens. Read more Blood glucose meters Blood glucose meters measure the amount of glucose in the blood. Blood glucose meters. Read more Continuous glucose monitoring Continuous glucose monitoring can help you manage your glucose levels in real-time and relieve the burden of having to do multiple finger prick tests throughout the day. Continuous glucose monitoring. Read more Flash glucose monitoring A flash glucose monitor is a small wearable device that you scan with a reader or mobile phone to check your glucose levels. Flash glucose monitoring. Read more Hybrid closed loop artificial pancreas Hybrid closed loop technology — also known as the artificial pancreas — automates many of the decisions that you have to make on a daily basis when you have type 1 diabetes. Hybrid closed loop research. Read more Open source and DIY systems Open source and DIY systems are sometimes used by people with type 1 diabetes or people caring for someone with type 1 to help manage the condition. Open source and DIY systems. Read more Apps for managing type 1 diabetes Apps can help you manage type 1 diabetes, from logging your insulin doses, glucose levels and the food you eat, to helping you count carbs and order prescriptions. Apps for managing type 1 diabetes. Share this Facebook Twitter LinkedIn. We value your privacy We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. However you may visit Cookie Settings to provide a controlled consent. Customize Reject All Accept All. Consent Preferences. Close Customize Consent Preferences This website uses cookies to improve your experience while you navigate through the website. Out of these cookies, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. We also use third-party cookies that help us analyze and understand how you use this website. These cookies will be stored in your browser only with your consent. You also have the option to opt-out of these cookies. But opting out of some of these cookies may have an effect on your browsing experience. Necessary Necessary. Necessary cookies are absolutely essential for the website to function properly. These cookies ensure basic functionalities and security features of the website, anonymously. Cookie Duration Description cookielawinfo-checkbox-analytics 11 months This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". cookielawinfo-checkbox-analytics 11 months This cookie is set by GDPR Cookie Consent plugin. cookielawinfo-checkbox-functional 11 months The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". cookielawinfo-checkbox-necessary 11 months This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". cookielawinfo-checkbox-others 11 months This cookie is set by GDPR Cookie Consent plugin. |

die Klugen Sachen, sagt)
Ich tue Abbitte, dass sich eingemischt hat... Mir ist diese Situation bekannt. Ist fertig, zu helfen.
Nach meiner Meinung sind Sie nicht recht. Es ich kann beweisen. Schreiben Sie mir in PM, wir werden besprechen.
Sie irren sich. Schreiben Sie mir in PM.