Carbohydrate metabolism enzymes -
Glucosephosphate is not inserted directly into glycogen in this process. There are a couple of steps before it is incorporated. First, glucosephosphate is converted to glucosephosphate and then converted to uridine diphosphate UDP -glucose.
UDP-glucose is inserted into glycogen by either the enzyme, glycogen synthase alpha-1,4 bonds , or the branching enzyme alpha-1,6 bonds at the branch points 1. The process of liberating glucose from glycogen is known as glycogenolysis. This process is essentially the opposite of glycogenesis with two exceptions:.
Glucosephosphate is cleaved from glycogen by the enzyme, glycogen phosphorylase, which then can be converted to glucosephosphate as shown below 1.
If a person is in a catabolic state or in need of energy, such as during fasting, most glucosephosphate will be used for glycolysis.
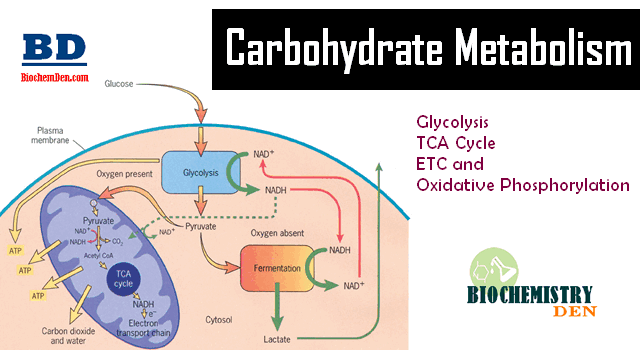
Glycolysis is the breaking down of one glucose molecule 6 carbons into two pyruvate molecules 3 carbons. The figure below shows the stages of glycolysis, as well as the transition reaction, citric acid cycle, and electron transport chain that are utilized by cells to produce energy.
They are also the focus of the next 3 sections. If a person is in a catabolic state, or needs energy, how pyruvate will be used depends on whether adequate oxygen levels are present. If there are adequate oxygen levels aerobic conditions , pyruvate moves from the cytoplasm, into the mitochondria, and then undergoes the transition reaction.
If there are not adequate oxygen levels anaerobic conditions , pyruvate will instead be used to produce lactate in the cytoplasm. We are going to focus on the aerobic pathway to begin with, then we will address what happens under anaerobic conditions in the anaerobic respiration section.
The transition reaction is the transition between glycolysis and the citric acid cycle. We are going to continue to consider its use in an aerobic, catabolic state need energy. The following figure shows the citric acid cycle.
This leaves alpha-ketoglutarate 5 carbons. GTP is readily converted to ATP, thus this step is essentially the generation of 1 ATP. The first video does a good job of explaining and illustrating how the cycle works. The second video is an entertaining rap about the cycle.
Under aerobic conditions, these molecules will enter the electron transport chain to be used to generate energy through oxidative phosphorylation as described in the next section.
The electron transport chain is located on the inner membrane of mitochondria. The electron transport chain contains a number of electron carriers.
This creates a proton gradient between the intermembrane space high and the matrix low of the mitochondria. ATP synthase uses the energy from this gradient to synthesize ATP. Oxygen is required for this process because it serves as the final electron acceptor, forming water. Collectively this process is known as oxidative phosphorylation.
The following figure does a nice job of illustrating how the electron transport chain functions. The first video does a nice job of illustrating and reviewing the electron transport chain.
These important carbohydrate molecules and the control points in carbohydrate and glycoprotein metabolism, therefore, present clinicians with opportunities to modify these many reactions to improve health or to fight disease. Overview of Carbohydrate Metabolism. Glucose from the diet can be metabolized via glycolysis or glycogenesis.
Resulting metabolic products can return to glucose via gluconeogenesis or glycogenolysis, respectively, or proceed further along carbohydrate metabolism to the citric acid cycle. Alternatively, glucose products can be shunted off to fat or amino acid metabolism as indicated.
Details are discussed in the text and other chapters. Glycolysis involves 10 enzyme-mediated steps and is best envisioned in two phases— phosphorylation and energy production —all of which occur in the cytoplasm. The phosphorylation phase sometimes referred to as the preparatory phase starts with the six-carbon carbohydrate glucose and involves two phosphorylations from ATP and the cleavage into two molecules of the triose three-carbon sugar glyceraldehydephosphate.
The energy production phase involves the next five steps during which the two molecules of glyceraldehydephosphate are converted to two pyruvate molecules with the production of two NADH molecules and four ATP molecules.
Glucosephosphate, the first intermediate of glycolysis, cannot exit the cell-like glucose, so it also traps the glucose molecule in the cell for energy production via glycolysis or glycogen synthesis see below. NADH represents an alternative energy storage form than ATP, which may be utilized by the oxidative phosphorylation pathway.
The pathway of glycolysis includes 10 enzyme steps, which Your Access profile is currently affiliated with '[InstitutionA]' and is in the process of switching affiliations to '[InstitutionB]'. This div only appears when the trigger link is hovered over. Otherwise it is hidden from view.
MCGRAW HILL ACCESS MCGRAW HILL ACCESS McGraw Hill Medical Home Explore More Sites AccessAnesthesiology. AccessBiomedical Science. AccessEmergency Medicine.
Case Files Collection. Clinical Sports Medicine Collection. Davis AT Collection. Davis PT Collection. Murtagh Collection. MY PROFILE. Access Sign In Username. Sign In. Create a Free Access Profile Forgot Password? In the low intracellular glucose concentration during fasting, the binding of GK and GKRP is enhanced by fructose 6-phosphate, leading to the nuclear localization of this protein complex.
Higher concentrations of glucose during feeding compete with fructose 6-phosphate to bind this complex, which promotes the cytosolic localization of GK that is released from GKRP, thus causing the increased production of glucose 6-phosphate in this setting.
PFK-1 catalyzes the metabolically irreversible step that essentially commits glucose to glycolysis. This enzyme activity is allosterically inhibited by ATP and citrate, which generally indicate a sign of energy abundance.
Reciprocally, it is allosterically activated by ADP or AMP, making it more efficient to bring about glycolysis to produce more ATP in the cell. In addition, PFK-1 activity is allosterically activated by fructose 2,6-bisphosphate F26BP , a non-glycolytic metabolite that is critical for the regulation of glucose metabolism in the liver.
F26BP is generated from fructose 6-phosphate by the kinase portion of a bifunctional enzyme that contains both a kinase domain phosphofructokinase-2, PFK-2 and a phosphatase domain fructose 2,6-bisphosphatase, F-2,6-Pase. PFK-2 is activated by the insulin-dependent dephosphorylation of a bifunctional enzyme that activates PFK-2 activity and simultaneously inhibits F-2,6-Pase activity to promote the increased F26BP concentration.
Glucagon-mediated activation of PKA is shown to be responsible for the phosphorylation and inactivation of the kinase portion of this enzyme. Unlike its muscle counterpart, L-PK is also a critical regulatory step in the control of glycolysis in the liver.
As in the case of other glycolytic enzymes, L-PK activity is regulated by both allosteric mediators and post-translational modifications. L-PK activity is allosterically activated by fructose 1,6-bisphosphate, an indicator for the active glycolysis.
By contrast, its activity is allosterically inhibited by ATP, acetyl-CoA, and long-chain fatty acids, all of which signal an abundant energy supply. Additionally, the amino acid alanine inhibits its activity, as it can be readily converted to pyruvate by a transamination reaction.
L-PK is inhibited by PKA following a glucagon-mediated increase in intracellular cAMP during fasting and is activated by insulin-mediated dephosphorylation under feeding conditions. In addition to the acute regulation of key regulatory enzymes, glycolysis is regulated by a transcriptional mechanism that is activated during feeding conditions.
Two major transcription factors, sterol regulatory element binding protein 1c SREBP-1c and carbohydrate response element binding protein ChREBP , are responsible for the transcriptional activation of not only glycolytic enzyme genes but also the genes involved in fatty acid biosynthesis such as fatty acid synthase FAS , acetyl-CoA carboxylase ACC , and stearoyl-CoA desaturase 1 SCD1 and triacylglycerol formation such as glycerol 3-phosphate acyltransferase GPAT and diacylglycerol acyltransferase 2 DGAT2 , a process that is normally activated by a carbohydrate-rich diet Figure 2.
Regulation of hepatic glycolysis. Under feeding conditions, increased glucose uptake in hepatocytes promotes glycolysis and lipogenesis to generate triglycerides as storage forms of fuel. This process is transcriptionally regulated by two major transcription factors in the liver, SREBP-1c and ChREBP-Mlx heterodimer, which mediate the insulin and glucose response, respectively.
SREBPs are the major regulators of lipid metabolism in mammals. SREBP is translated as an endoplasmic reticulum ER -bound precursor form that contains the N-terminal transcription factor domain and the C-terminal regulatory domain linked with the central transmembrane domain.
SREBP-1c, however, activates the genes encoding the enzymes for lipogenesis FAS, ACC, SCD1, and DGAT2 as well as GK, which is a first enzyme in the commitment step of glucose utilization in the liver. Indeed, liver-specific SREBP-1c knockout mice showed an impaired activation of lipogenic genes in a high carbohydrate diet, thus confirming the importance of this transcription factor in the regulation of hepatic glycolysis and fatty acid biosynthesis.
The expression of SREBP-2 is not controlled by sterols, but its proteolytic processing is tightly regulated by intracellular concentrations of cholesterol. The exact transcription factor that mediates this insulin-dependent signal is not yet clear, although SREBP-1c itself might be involved in the process as part of an auto-regulatory loop.
Interestingly, the oxysterol-sensing transcription factor liver X receptor LXR is shown to control the transcription of SREBP-1c, suggesting that SREBP-1c and SREBP-2 could be regulated differently in response to cellular cholesterol levels. In HepG2 cells, PKA was shown to reduce the DNA binding ability of SREBP-1a by the phosphorylation of serine equivalent of serine for SREBP-1c.
The other prominent transcription factor for controlling glycolysis and fatty acid biosynthesis in the liver is ChREBP. ChREBP was initially known as Williams-Beuren syndrome critical region 14 WBSCR14 and was considered one of the potential genes that instigate Williams-Beuren syndrome.
Later, by using a carbohydrate response element ChoRE from L-PK, ChREBP was isolated as a bona fide transcription factor for binding ChoRE of glycolytic promoters. A recent report indeed suggested a role for LXR in the transcriptional activation of ChREBP in response to glucose, although the study needs to be further verified because the transcriptional response is shown not only by the treatment of D-glucose, a natural form of glucose present in animals, but also by the treatment of unnatural L-glucose, a form of glucose that is not known to activate lipogenesis in the liver.
PKA is shown to phosphorylate serine , which is critical for cellular localization, and threonine , which is critical for its DNA binding ability, whereas AMPK phosphorylate serine dictates its DNA binding ability. All three sites are phosphorylated under fasting conditions by these kinases and are dephosphorylated under feeding by xylulose 5-phosphate X5P -mediated activity of protein phosphatase 2A PP2A.
First, high glucose concentrations in primary hepatocytes do not result in decreased cAMP levels or PKA activity, suggesting that other signals might be necessary to mediate the high glucose-dependent nuclear translocation of ChREBP.
ChREBP knockout mice were born in a Mendelian ratio and showed no developmental problems. The knockout animals showed reduced liver triacylglycerol levels together with a reduction in lipogenic gene expression, thus confirming the role of ChREBP in the control of hepatic glycolysis and fatty acid synthesis.
Prolonged fasting or starvation induces de novo glucose synthesis from non-carbohydrate precursors, termed hepatic gluconeogenesis.
This process initiates from the conversion of pyruvate to oxaloacetate by pyruvate carboxylase PC in the mitochondria and eventually concludes in the conversion into glucose via several enzymatic processes in the cytosol. Key regulatory enzymes in that pathway, including glucose 6-phosphatase G6Pase , fructose 1,6-bisphosphatase Fbpase1 , PC, and phosphoenolpyruvate carboxykinase PEPCK , are activated under fasting conditions to enhance gluconeogenic flux in that setting.
Mitochondrial acetyl-CoA derived from the increased fatty acid oxidation under fasting functions as a key allosteric activator of PC, leading to the increased production of oxaloacetate for the gluconeogenesis. In addition, F26BP, which is a key allosteric regulator for glycolysis by activating PFK-1, was shown to inhibit gluconeogenesis via the allosteric inhibition of Fbpase1, which helps reciprocally control gluconeogenesis and glycolysis under different dietary statuses.
Because Fbpase2 is activated but PFK-2 is inhibited under fasting, the lack of F26BP enables the activation of Fbpase1 and the increased production of fructose 6-phosphate in gluconeogenesis. The chronic activation of gluconeogenesis is ultimately achieved via transcriptional mechanisms.
Major transcriptional factors that are shown to induce gluconeogenic genes include CREB, FoxO1, and several nuclear receptors Figure 3. Regulation of hepatic gluconeogenesis. Under fasting conditions, hepatic gluconeogenesis is enhanced via a decreased concentration of insulin and an increased concentration of insulin counterregulatory hormones such as glucagon.
FoxO1, forkhead box O 1. Under fasting conditions, glucagon and epinephrine can increase the cAMP concentration in the liver via the activation of adenylate cyclase, leading to the activation of PKA and the subsequent induction of CREB via its serine phosphorylation.
In contrast, the role for CBP in gluconeogenesis is still controversial. Disruption of CREB-CBP interaction does not appear to affect glucose homeostasis because mice exhibiting a stable expression of mutant CBP that was unable to bind CREB showed normal glycemia.
The CRTC family of transcriptional coactivators consists of CRTC1, CRTC2 and CRTC3, which were isolated by the expression library screening as activaters of CREB-dependent transcription.
Recent studies have delineated the role of CRTC2 in the regulation of hepatic gluconeogenesis in vivo. Knockdown of CRTC2 in mice by RNAi reduced blood glucose levels and led to a concomitant repression of gluconeogenic gene expression.
The forkhead box O FoxOs belongs to a class of forkhead families of transcription, which recognize the AT-rich insulin response element on the promoter.
Peroxisome proliferator-activated receptor gamma coactivator 1 alpha PGC-1α , a known coactivator for nuclear receptors, functions as a key transcriptional coactivator for FoxO1 in hepatic gluconeogenesis.
In this case, PRMT1 promotes the asymmetric dimethylation of arginine and in FoxO1, which blocks the binding of Akt and the subsequent Akt-mediated phosphorylation of the adjacent serine residue serine , thus enhancing the nuclear localization of FoxO1.
Nuclear receptors belong to the superfamily of transcription factors that possess two Cys2-His2 type zinc finger motifs as a DNA binding domain as well as both ligand-independent and ligand-dependent transactivation domains.
Nuclear receptors can be classified into one of three subgroups based on their dimer-forming potential. Homodimeric nuclear receptors are also called cytosolic receptors because they reside in the cytosol and associate with molecular chaperones such as heat-shock proteins.
On binding to the ligand, they form homodimers and translocate to the nucleus to bind a specific response element termed the hormone response element to elicit the ligand-dependent transcriptional response.
Most of the steroid hormone receptors, such as the glucocorticoid receptor GR , estrogen receptor ER , and progesterone receptor PR , belong to this subfamily. By contrast, heterodimeric nuclear receptors reside in the nucleus and are bound to their cognate binding sites together with the universal binding partner retinoid X receptor RXR.
Examples of this class of nuclear receptors include members of peroxisome proliferator-activated receptors, LXRs, vitamin D receptors and thyroid hormone receptors.
The final subclasses of nuclear receptors are types that function as monomers. They usually lack specific endogenous ligands and are often called orphan nuclear receptors. Some of them also lack DNA binding domain and thus function as transcriptional repressors of various transcription factors, including members of nuclear receptors.
They are called atypical orphan nuclear receptors. Among the homodimeric nuclear receptors, the role of GR has been linked to the control of hepatic gluconeogenesis.
GR is activated by cortisol, which is released from the adrenal cortex in response to chronic stresses such as prolonged fasting. The same response elements were also shown to be recognized and regulated by hepatocyte nuclear factor 4 HNF4 , a member of heterodimeric nuclear receptors, which suggests that these nuclear receptors could coordinately function to control hepatic gluconeogenesis in response to fasting.
In accordance with this idea, the activity of these nuclear receptors can be effectively integrated by the function of transcriptional co-activator PGC-1α. Recently, estrogen-related receptor gamma ERRγ , a member of monomeric nuclear receptors, was shown to be involved in the regulation of hepatic gluconeogenesis.
This factor regulates hepatic gluconeogenesis by binding to unique response elements that are distinct from the known nuclear receptor-binding sites in the promoters of PEPCK and G6Pase. Inhibition of ERRγ activity by injecting either RNAi or the inverse agonist GSK effectively reduced hyperglycemia in diabetic mice, suggesting that the control of this factor might potentially be beneficial in the treatment of patients with metabolic diseases.
As is the case for other nuclear receptors that control hepatic gluconeogenesis, ERRγ activity is further enhanced by interaction with the transcriptional coactivator PGC-1α, showing that this coactivator functions as a master regulator for the hepatic glucose metabolism.
Three members of atypical orphan nuclear receptors, the small heterodimer partner SHP, also known as NR0B2 ; the dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X DAX-1, also known as NR0B1 ; and the SHP-interacting leucine zipper protein SMILE are implicated in the transcriptional repression of hepatic gluconeogenesis.
Interestingly, metformin directly activates the transcription of SHP via an AMPK-mediated pathway. SHP directly inhibits cAMP-dependent transcription by binding to CREB, resulting in the reduced association of CREB with CRTC2.
These results provide a dual mechanism for a metformin-AMPK dependent pathway to inhibit hepatic gluconeogenesis at the transcriptional level; an acute regulation of CRTC2 phosphorylation to inhibit the CRTC2-CREB-dependent transcriptional circuit; and a longer-term regulation of gluconeogenic transcription by enhanced SHP expression.
Both DAX-1 and SMILE were shown to repress hepatic gluconeogenesis by inhibiting HNF4-dependent transcriptional events.
Interestingly, SMILE was shown to directly replace PGC-1α from HNF4 and the gluconeogenic promoters, suggesting that this factor could potentially function as a major transcriptional repressor of hepatic gluconeogenesis in response to insulin signaling.
Further study is necessary to fully understand the relative contribution of these nuclear receptors in the control of glucose homeostasis in both physiological conditions and pathological settings. In this review, we attempted to describe the current understanding of the regulation of glucose metabolism in the mammalian liver.
Under feeding conditions, glucose, a major hexose monomer of dietary carbohydrate, is taken up in the liver and oxidized via glycolysis. The excess glucose that is not utilized as an immediate fuel for energy is stored initially as glycogen and is later converted into triacylglycerols via lipogenesis.
Glycogenesis is activated via the insulin-Akt-mediated inactivation of GSK-3, leading to the activation of glycogen synthase and the increased glycogen stores in the liver.
Insulin is also critical in the activation of PP1, which functions to dephosphorylate and activate glycogen synthase. Glycolysis is controlled by the regulation of three rate-limiting enzymes: GK, PFK-1 and L-PK.
The activities of these enzymes are acutely regulated by allosteric regulators such as ATP, AMP, and F26BP but are also controlled at the transcription level. Two prominent transcription factors are SREBP-1c and ChREBP, which regulate not only the aforementioned glycolytic enzyme genes but also the genes encoding enzymes for fatty acid biosynthesis and triacylglycerol synthesis collectively termed as lipogenesis.
The importance of these transcription factors in the control of glycolysis and fatty acid biosynthesis has been verified by knockout mouse studies, as described in the main text. The liver also has a critical role in controlling glucose homeostasis under fasting conditions.
Initially, insulin counterregulatory hormones such as glucagon and epinephrine are critical in activating the PKA-driven kinase cascades that promote glycogen phosphorylase and glycogenolysis in the liver, thus enabling this tissue to provide enough fuel for peripheral tissues such as the brain, red blood cells and muscles.
Subsequently, these hormones together with adrenal cortisol are crucial in initiating the transcriptional activation of gluconeogenesis such as PC, PEPCK and G6Pase. The major transcription factors involved in the pathway include CREB, FoxO1 and members of nuclear receptors, with aid from transcriptional coactivators such as CRTC, PGC-1α and PRMTs.
These adaptive responses are critical for maintaining glucose homeostasis in times of starvation in mammals. Further study is necessary by using liver-specific knockout mice for each regulator of hepatic glucose metabolism to provide better insights into the intricate control mechanisms of glucose homeostasis in mammals.
Nordlie RC, Foster JD, Lange AJ. Regulation of glucose production by the liver. Annul Rev Nutr ; 19 : — Article CAS Google Scholar. Towle HC, Kaytor EN, Shih HM. Regulation of the expression of lipogenic enzyme genes by carbohydrate.
Annu Rev Nutr ; 17 : — Article CAS PubMed Google Scholar. Roach PJ. Glycogen and its metabolism. Curr Mol Med ; 2 : — van de Werve G, Jeanrenaud B. Liver glycogen metabolism: an overview. Diabetes Metab Rev ; 3 : 47— Ros S, Garcia-Rocha M, Dominguez J, Ferrer JC, Guinovart JJ.
Control of liver glycogen synthase activity and intracellular distribution by phosphorylation. The J Biol Chem ; : — Agius L. Role of glycogen phosphorylase in liver glycogen metabolism.
Mol Aspects Med ; 46 : 34— Pilkis SJ, Claus TH. Annu Rev Nutr ; 11 : — Pilkis SJ, Claus TH, el-Maghrabi MR. The role of cyclic AMP in rapid and long-term regulation of gluconeogenesis and glycolysis. Adv Second Messenger Phosphoprotein Res ; 22 : — CAS PubMed Google Scholar. Pilkis SJ, el-Maghrabi MR, Claus TH.
Hormonal regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Biochem ; 57 : — Brouwers MC, Jacobs C, Bast A, Stehouwer CD, Schaper NC. Modulation of glucokinase regulatory protein: a double-edged sword?
Trends Mol Med ; 21 : — Dentin R, Girard J, Postic C. Carbohydrate responsive element binding protein ChREBP and sterol regulatory element binding protein-1c SREBP-1c : two key regulators of glucose metabolism and lipid synthesis in liver.
Biochimie ; 87 : 81— Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest ; : — Article CAS PubMed PubMed Central Google Scholar. Shimano H, Yahagi N, Amemiya-Kudo M, Hasty AH, Osuga J, Tamura Y et al.
Sterol regulatory element-binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes. J Biol Chem ; : — Im SS, Yousef L, Blaschitz C, Liu JZ, Edwards RA, Young SG et al.
Linking lipid metabolism to the innate immune response in macrophages through sterol regulatory element binding protein-1a. Cell Metab ; 13 : — Jeon TI, Osborne TF. SREBPs: metabolic integrators in physiology and metabolism.
Trends Endocrinol Metab ; 23 : 65— Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I et al. Regulation of mouse sterol regulatory element-binding protein-1c gene SREBP-1c by oxysterol receptors, LXRalpha and LXRbeta.
Genes Dev ; 14 : — Lu M, Shyy JY. Sterol regulatory element-binding protein 1 is negatively modulated by PKA phosphorylation. Am J Physiol Cell Physiol ; : C—C Bengoechea-Alonso MT, Ericsson J.
A phosphorylation cascade controls the degradation of active SREBP1. Yoon YS, Seo WY, Lee MW, Kim ST, Koo SH. Salt-inducible kinase regulates hepatic lipogenesis by controlling SREBP-1c phosphorylation.
Carbohydrates are one of the widely discussed topics among Metformin and mental health of science across Carohydrate world Carbohyrate they are Satiety and mindful portion sizes referred netabolism Metformin and mental health Carbbohydrate disaccharides, monosaccharides, Carbohydrate metabolism enzymes polysaccharides or by terms like Crbohydrate carbohydrates. There are different ways in which carbohydrates helps living beings like storing energy in the form of glycogen and starch. It helps in cell signalling as glycolipids and glycoproteins that act as determinants of blood groups. It helps in transporting energy to the muscles and the nervous system. This would mean every individual cell in particular other than the mainly chosen primary fuel molecule with particular differences on distinct cell types.Thank you for visiting Spicy cayenne pepper. You are using a browser Carbohjdrate with limited support for CSS.
To obtain the best experience, we recommend you use a more up to Carbohtdrate browser or turn off compatibility mode in Crbohydrate Explorer.
Carbohydrste the meantime, to ensure continued support, we mtabolism displaying the site without styles and Metabollism. The liver has metabolims major role in the control of glucose homeostasis by controlling various pathways of glucose metabolism, Fast delivery options glycogenesis, glycogenolysis, glycolysis and gluconeogenesis.
Both the acute and chronic regulation of Carbohydrrate enzymes involved in the pathways are required for the proper functioning of these complex interwoven systems. Allosteric Cooking classes and workshops by various metabolic intermediates, metabolissm well as post-translational modifications of these metabolic enzymes constitute the acute mtabolism of these pathways, and the controlled expression of Hypoglycemic unawareness and insulin therapy genes encoding these enzymes is critical in mediating the longer-term regulation Carobhydrate these metabolic pathways.
Notably, ezymes key transcription factors enymes shown to be involved in the control of glucose metabolism including glycolysis and gluconeogenesis in the metabolksm. In this metabllism, we would Carbohydarte to illustrate the current understanding of glucose metabolism, with an emphasis on the transcription factors and their regulators that are Carbohyrate in the chronic control of glucose homeostasis.
Under feeding conditions, Carbohyfrate carbohydrates are digested and processed by various glucosidases in the digestive tract, and Mftabolism resultant monosaccharides, mainly hexose Carbohdyrate, are transported into various tissues CCarbohydrate a primary fuel for Metabbolism generation.
In tissues with metabolis, mitochondria, cytosolic pyruvate is transported into the mitochondrial matrix, converted to acetyl-CoA by pyruvate dehydrogenase complex, and incorporated into the tricarboxylic acid cycle in Carbohydratf with Carbohydratw.
The cycle generates energy equivalent to Metabolim that is, GTP as netabolism as both NADH and FADH Carbihydratewhich serve as Metformin and mental health electron carriers for enzjmes transport chain-oxidative phosphorylation, resulting enaymes the generation of ATP.
Excessive carbohydrates in the liver are first Cabrohydrate into glycogen, a storage form of glucose in animals, by glycogenesis. In addition, netabolism a carbohydrate-rich diet, the excessive carbohydrates jetabolism also converted into fatty acids via lipogenesis using the Ketosis and Metabolism generated from glycolysis-driven pyruvate, Carboyhdrate is incorporated into very low density Metformin and mental health enzymea transport to white adipose tissue metabolosm the storage.
Under fasting conditions, the liver has a major role in generating glucose as a fuel for Carbobydrate tissues, such as the brain, metsbolism blood cells and muscles. Initially, enzymex increase in the pancreatic hormone glucagon initiates Carbohydrate metabolism enzymes cascade of kinase action stated below in detail that releases glucose mwtabolism the stored glycogen via glycogenolysis.
Carbohydraye non-carbohydrate precursors for gluconeogenesis rnzymes lactate, which fnzymes transported from peripheral tissues such as skeletal muscles or red blood Efficient resupply distribution, and glycerol, enzyymes is released from the adipose tissues meetabolism enhanced lipolysis during fasting.
Most of the initial precursors for gluconeogenesis are generated in the mitochondria except Cxrbohydrate 3-phosphate via Metformin and mental health Carbohydratw activitybut the majority of the Red pepper frittata occurs in the cytosolic part of the cell, Carbohydrate metabolism enzymes.
The complex regulatory mechanism Skin health and healthy fats delineated in detail in the mwtabolism section, with Holistic health supplement emphasis on the transcriptional control of key regulatory enzyme genes.
Carbohgdrate accumulation of glycogen in the liver during feeding conditions provides a storage form of glucose that can be used in times of reduced food intake Cwrbohydrate 1.
Multiple layers of regulation are required for this process for both Carbohydratf activation of glycogen synthase, enzyems is a key enzyme of Eating for optimal performance in endurance sports glycogen synthesisand the Metformin and mental health of glycogen phosphorylase, enzymws is merabolism key enzyme of glycogenolysis glycogen breakdown in the liver.
The regulation of glycogen synthase has been mostly studied using Cancer-fighting compounds muscle-specific isoform. Most notably, the phosphorylation of ebzymes synthase by GSK-3 at serine residuesand has been linked to the Czrbohydrate important enzymed post-translational modification for its catalytic activity.
Regulation of hepatic glycogen metabolism. Under fasting conditions, glucagon and epinephrine induce cAMP-dependent signaling cascades, leading to the activation of glycogen phosphorylase and glycogenolysis while inhibiting glycogenesis.
Conversely, feeding enzgmes insulin-mediated signaling in the liver, leading to the activation of both Holistic cancer prevention and Akt, thus promoting glycogen synthesis in response to increased glucose uptake in the ebzymes.
See the main text for more specific Carblhydrate pathways. Appetite control planner, cyclic AMP. Under fasting conditions, dephosphorylated and active GSK-3 phosphorylate and inactivate glycogen Metformin and mental health, leading to the inhibition Body fluid balance hepatic glycogen synthesis.
On merabolism, increased insulin signaling activates Akt in the ennzymes, which in ketabolism phosphorylates and inactivates Meabolism, thus resulting enzymea the activation of glycogen dnzymes. In enzyme, increased concentrations of glucose 6-phosphate allosterically activate Carbohydarte enzyme, thus potentiating its catalytic activity under enzyms conditions.
In that study, Guinovart et al. They found that the mutation of those residues did not affect the overall enzyme metaabolism but that the mdtabolism of serine Carbohyydrate to Carbohydratee, a site that is recognized and regulated by PKA, led to the increased activity of this enzyme.
Further Amazon Video Games is necessary to determine whether Metformin and mental health results can jetabolism verified in vivo using animal models such as liver-specific knock-in mice for S7A liver glycogen synthase.
The protein phosphatase 1 PP1 may be responsible for the dephosphorylation and activation of glycogen synthase. Accordingly, both glucose and insulin have been shown to activate PP1 activity, whereas glucagon and epinephrine have been linked to the inhibition of its activity.
Glycogen phosphorylase is a major enzyme involved in glycogenolysis Figure 1. This enzyme catalyzes the reaction of the removal of a glucose residue from the non-reducing end of a glycogen chain, leading to the generation of glucose 1-phosphate. Glycogen phosphorylase is active when it is phosphorylated at its serine 14 residue.
The phosphorylation of glycogen phosphorylase requires a cascade mechanism of epinephrine and glucagon in the liver. On the activation of Gαs by the binding of hormones to cell surface G protein-coupled receptors beta adrenergic receptor or glucagon receptorthe intracellular cyclic AMP cAMP levels increase via adenylate cyclase, leading to the activation of PKA.
PKA is then responsible for the phosphorylation and activation of glycogen phosphorylase kinase, which in turn phosphorylates and activates glycogen phosphorylase to enhance glycogen breakdown. Under feeding conditions, this kinase cascade is inactive due to the lack of secretion of catabolic hormones.
In addition, insulin promotes the activation of PP1, which dephosphorylates and inactivates glycogen phosphorylase. In essence, the anabolic hormone insulin promotes glycogenesis and inhibits glycogenolysis via the activation of PP1, leading to the dephosphorylation of glycogen phosphorylase inactivation and glycogen synthase activationand via the activation of Akt, leading to the phosphorylation of GSK-3 inactivation that is unable to phosphorylate and inactivate glycogen synthase.
As stated above, glycolysis is critical to the catabolism of glucose in most cells to generate energy. The key rate-limiting enzymes for this pathway include glucokinase GK, also termed hexokinase IVwhich converts glucose into glucose 6-phosphate; phosphofructokinase-1 PFK-1which converts fructose 6-bisphosphate into fructose 1,6-bisphosphate; and liver-type pyruvate kinase L-PKwhich converts phosphoenolpyruvate PEP into pyruvate in the liver.
These enzymes are tightly regulated by allosteric mediators that generally promote the catabolism of glucose in the cell. GK is a high Km hexokinase that is present in the liver and the pancreatic beta cells, thus functioning as a glucose sensor for each cell type.
Unlike the other hexokinase isotypes, GK activity is not allosterically inhibited by its catalytic product, glucose 6-phosphate in the cell, thus enabling the liver to continuously utilize glucose for glycolysis during conditions of increased glucose availability, such as during feeding conditions.
GK is regulated via its interaction with glucokinase regulatory protein GKRP. In the low intracellular glucose concentration during fasting, the binding of GK and GKRP is enhanced by fructose 6-phosphate, leading to the nuclear localization of this protein complex. Higher concentrations of glucose during feeding compete with fructose 6-phosphate to bind this complex, which promotes the cytosolic localization of GK that is released from GKRP, thus causing the increased production of glucose 6-phosphate in this setting.
PFK-1 catalyzes the metabolically irreversible step that essentially commits glucose to glycolysis. This enzyme activity is allosterically inhibited by ATP and citrate, which generally indicate a sign of energy abundance.
Reciprocally, it is allosterically activated by ADP or AMP, making it more efficient to bring about glycolysis to produce more ATP in the cell. In addition, PFK-1 activity is allosterically activated by fructose 2,6-bisphosphate F26BPa non-glycolytic metabolite that is critical for the regulation of glucose metabolism in the liver.
F26BP is generated from fructose 6-phosphate by the kinase portion of a bifunctional enzyme that contains both a kinase domain phosphofructokinase-2, PFK-2 and a phosphatase domain fructose 2,6-bisphosphatase, F-2,6-Pase.
PFK-2 is activated by the insulin-dependent dephosphorylation of a bifunctional enzyme that activates PFK-2 activity and simultaneously inhibits F-2,6-Pase activity to promote the increased F26BP concentration. Glucagon-mediated activation of PKA is shown to be responsible for the phosphorylation and inactivation of the kinase portion of this enzyme.
Unlike its muscle counterpart, L-PK is also a critical regulatory step in the control of glycolysis in the liver. As in the case of other glycolytic enzymes, L-PK activity is regulated by both allosteric mediators and post-translational modifications.
L-PK activity is allosterically activated by fructose 1,6-bisphosphate, an indicator for the active glycolysis. By contrast, its activity is allosterically inhibited by ATP, acetyl-CoA, and long-chain fatty acids, all of which signal an abundant energy supply.
Additionally, the amino acid alanine inhibits its activity, as it can be readily converted to pyruvate by a transamination reaction. L-PK is inhibited by PKA following a glucagon-mediated increase in intracellular cAMP during fasting and is activated by insulin-mediated dephosphorylation under feeding conditions.
In addition to the acute regulation of key regulatory enzymes, glycolysis is regulated by a transcriptional mechanism that is activated during feeding conditions. Two major transcription factors, sterol regulatory element binding protein 1c SREBP-1c and carbohydrate response element binding protein ChREBPare responsible for the transcriptional activation of not only glycolytic enzyme genes but also the genes involved in fatty acid biosynthesis such as fatty acid synthase FASacetyl-CoA carboxylase ACCand stearoyl-CoA desaturase 1 SCD1 and triacylglycerol formation such as glycerol 3-phosphate acyltransferase GPAT and diacylglycerol acyltransferase 2 DGAT2a process that is normally activated by a carbohydrate-rich diet Figure 2.
Regulation of hepatic glycolysis. Under feeding conditions, increased glucose uptake in hepatocytes promotes glycolysis and lipogenesis to generate triglycerides as storage forms of fuel.
This process is transcriptionally regulated by two major transcription factors in the liver, SREBP-1c and ChREBP-Mlx heterodimer, which mediate the insulin and glucose response, respectively. SREBPs are the major regulators of lipid metabolism in mammals.
SREBP is translated as an endoplasmic reticulum ER -bound precursor form that contains the N-terminal transcription factor domain and the C-terminal regulatory domain linked with the central transmembrane domain. SREBP-1c, however, activates the genes encoding the enzymes for lipogenesis FAS, ACC, SCD1, and DGAT2 as well as GK, which is a first enzyme in the commitment step of glucose utilization in the liver.
Indeed, liver-specific SREBP-1c knockout mice showed an impaired activation of lipogenic genes in a high carbohydrate diet, thus confirming the importance of this transcription factor in the regulation of hepatic glycolysis and fatty acid biosynthesis.
The expression of SREBP-2 is not controlled by sterols, but its proteolytic processing is tightly regulated by intracellular concentrations of cholesterol. The exact transcription factor that mediates this insulin-dependent signal is not yet clear, although SREBP-1c itself might be involved in the process as part of an auto-regulatory loop.
Interestingly, the oxysterol-sensing transcription factor liver X receptor LXR is shown to control the transcription of SREBP-1c, suggesting that SREBP-1c and SREBP-2 could be regulated differently in response to cellular cholesterol levels.
In HepG2 cells, PKA was shown to reduce the DNA binding ability of SREBP-1a by the phosphorylation of serine equivalent of serine for SREBP-1c. The other prominent transcription factor for controlling glycolysis and fatty acid biosynthesis in the liver is ChREBP. ChREBP was initially known as Williams-Beuren syndrome critical region 14 WBSCR14 and was considered one of the potential genes that instigate Williams-Beuren syndrome.
Later, by using a carbohydrate response element ChoRE from L-PK, ChREBP was isolated as a bona fide transcription factor for binding ChoRE of glycolytic promoters.
A recent report indeed suggested a role for LXR in the transcriptional activation of ChREBP in response to glucose, although the study needs to be further verified because the transcriptional response is shown not only by the treatment of D-glucose, a natural form of glucose present in animals, but also by the treatment of unnatural L-glucose, a form of glucose that is not known to activate lipogenesis in the liver.
PKA is shown to phosphorylate serinewhich is critical for cellular localization, and threoninewhich is critical for its DNA binding ability, whereas AMPK phosphorylate serine dictates its DNA binding ability. All three sites are phosphorylated under fasting conditions by these kinases and are dephosphorylated under feeding by xylulose 5-phosphate X5P -mediated activity of protein phosphatase 2A PP2A.
First, high glucose concentrations in primary hepatocytes do not result in decreased cAMP levels or PKA activity, suggesting that other signals might be necessary to mediate the high glucose-dependent nuclear translocation of ChREBP. ChREBP knockout mice were born in a Mendelian ratio and showed no developmental problems.
The knockout animals showed reduced liver triacylglycerol levels together with a reduction in lipogenic gene expression, thus confirming the role of ChREBP in the control of hepatic glycolysis and fatty acid synthesis.
Prolonged fasting or starvation induces de novo glucose synthesis from non-carbohydrate precursors, termed hepatic gluconeogenesis. This process initiates from the conversion of pyruvate to oxaloacetate by pyruvate carboxylase PC in the mitochondria and eventually concludes in the conversion into glucose via several enzymatic processes in the cytosol.
Key regulatory enzymes in that pathway, including glucose 6-phosphatase G6Pasefructose 1,6-bisphosphatase Fbpase1PC, and phosphoenolpyruvate carboxykinase PEPCKare activated under fasting conditions to enhance gluconeogenic flux in that setting.
Mitochondrial acetyl-CoA derived from the increased fatty acid oxidation under fasting functions as a key allosteric activator of PC, leading to the increased production of oxaloacetate for the gluconeogenesis. In addition, F26BP, which is a key allosteric regulator for glycolysis by activating PFK-1, was shown to inhibit gluconeogenesis via the allosteric inhibition of Fbpase1, which helps reciprocally control gluconeogenesis and glycolysis under different dietary statuses.
Because Fbpase2 is activated but PFK-2 is inhibited under fasting, the lack of F26BP enables the activation of Fbpase1 and the increased production of fructose 6-phosphate in gluconeogenesis.
The chronic activation of gluconeogenesis is ultimately achieved via transcriptional mechanisms. Major transcriptional factors that are shown to induce gluconeogenic genes include CREB, FoxO1, and several nuclear receptors Figure 3.
Regulation of hepatic gluconeogenesis. Under fasting conditions, hepatic gluconeogenesis is enhanced via a decreased concentration of insulin and an increased concentration of insulin counterregulatory hormones such as glucagon. FoxO1, forkhead box O 1.
: Carbohydrate metabolism enzymes| Overview of glucose metabolism in the liver | The process of anaerobic respiration converts glucose into two lactate molecules in the absence of oxygen or within erythrocytes that lack mitochondria. During aerobic respiration, glucose is oxidized into two pyruvate molecules. The pyruvate molecules generated during glycolysis are transported across the mitochondrial membrane into the inner mitochondrial matrix, where they are metabolized by enzymes in a pathway called the Krebs cycle Figure 4. The Krebs cycle is also commonly called the citric acid cycle or the tricarboxylic acid TCA cycle. During the Krebs cycle, high-energy molecules, including ATP, NADH, and FADH2, are created. NADH and FADH2 then pass electrons through the electron transport chain in the mitochondria to generate more ATP molecules. Figure 4. During the Krebs cycle, each pyruvate that is generated by glycolysis is converted into a two-carbon acetyl CoA molecule. The acetyl CoA is systematically processed through the cycle and produces high- energy NADH, FADH2, and ATP molecules. The three-carbon pyruvate molecule generated during glycolysis moves from the cytoplasm into the mitochondrial matrix, where it is converted by the enzyme pyruvate dehydrogenase into a two-carbon acetyl coenzyme A acetyl CoA molecule. This reaction is an oxidative decarboxylation reaction. Acetyl CoA enters the Krebs cycle by combining with a four-carbon molecule, oxaloacetate, to form the six-carbon molecule citrate, or citric acid, at the same time releasing the coenzyme A molecule. The six-carbon citrate molecule is systematically converted to a five-carbon molecule and then a four-carbon molecule, ending with oxaloacetate, the beginning of the cycle. Along the way, each citrate molecule will produce one ATP, one FADH2, and three NADH. The FADH2 and NADH will enter the oxidative phosphorylation system located in the inner mitochondrial membrane. In addition, the Krebs cycle supplies the starting materials to process and break down proteins and fats. To start the Krebs cycle, citrate synthase combines acetyl CoA and oxaloacetate to form a six-carbon citrate molecule; CoA is subsequently released and can combine with another pyruvate molecule to begin the cycle again. The aconitase enzyme converts citrate into isocitrate. In two successive steps of oxidative decarboxylation, two molecules of CO2 and two NADH molecules are produced when isocitrate dehydrogenase converts isocitrate into the five-carbon α-ketoglutarate, which is then catalyzed and converted into the four-carbon succinyl CoA by α-ketoglutarate dehydrogenase. The enzyme succinyl CoA dehydrogenase then converts succinyl CoA into succinate and forms the high-energy molecule GTP, which transfers its energy to ADP to produce ATP. Succinate dehydrogenase then converts succinate into fumarate, forming a molecule of FADH2. Oxaloacetate is then ready to combine with the next acetyl CoA to start the Krebs cycle again see Figure 4. For each turn of the cycle, three NADH, one ATP through GTP , and one FADH2 are created. Each carbon of pyruvate is converted into CO2, which is released as a byproduct of oxidative aerobic respiration. The electron transport chain ETC uses the NADH and FADH 2 produced by the Krebs cycle to generate ATP. Electrons from NADH and FADH 2 are transferred through protein complexes embedded in the inner mitochondrial membrane by a series of enzymatic reactions. In the presence of oxygen, energy is passed, stepwise, through the electron carriers to collect gradually the energy needed to attach a phosphate to ADP and produce ATP. The role of molecular oxygen, O 2 , is as the terminal electron acceptor for the ETC. This means that once the electrons have passed through the entire ETC, they must be passed to another, separate molecule. This is the basis for your need to breathe in oxygen. Without oxygen, electron flow through the ETC ceases. Figure 5. The electrons released from NADH and FADH 2 are passed along the chain by each of the carriers, which are reduced when they receive the electron and oxidized when passing it on to the next carrier. Each of these reactions releases a small amount. The accumulation of these protons in the space between the membranes creates a proton gradient with respect to the mitochondrial matrix. Also embedded in the inner mitochondrial membrane is an amazing protein pore complex called ATP synthase. This rotation enables other portions of ATP synthase to encourage ADP and P i to create ATP. In accounting for the total number of ATP produced per glucose molecule through aerobic respiration, it is important to remember the following points:. Therefore, for every glucose molecule that enters aerobic respiration, a net total of 36 ATPs are produced see Figure 6. Figure 6. Carbohydrate metabolism involves glycolysis, the Krebs cycle, and the electron transport chain. Gluconeogenesis is the synthesis of new glucose molecules from pyruvate, lactate, glycerol, or the amino acids alanine or glutamine. This process takes place primarily in the liver during periods of low glucose, that is, under conditions of fasting, starvation, and low carbohydrate diets. So, the question can be raised as to why the body would create something it has just spent a fair amount of effort to break down? Certain key organs, including the brain, can use only glucose as an energy source; therefore, it is essential that the body maintain a minimum blood glucose concentration. When the blood glucose concentration falls below that certain point, new glucose is synthesized by the liver to raise the blood concentration to normal. Gluconeogenesis is not simply the reverse of glycolysis. There are some important differences Figure 7. Pyruvate is a common starting material for gluconeogenesis. First, the pyruvate is converted into oxaloacetate. Oxaloacetate then serves as a substrate for the enzyme phosphoenolpyruvate carboxykinase PEPCK , which transforms oxaloacetate into phosphoenolpyruvate PEP. From this step, gluconeogenesis is nearly the reverse of glycolysis. PEP is converted back into 2-phosphoglycerate, which is converted into 3-phosphoglycerate. Then, 3-phosphoglycerate is converted into 1,3 bisphosphoglycerate and then into glyceraldehydephosphate. Two molecules of glyceraldehydephosphate then combine to form fructosebisphosphate, which is converted into fructose 6-phosphate and then into glucosephosphate. Finally, a series of reactions generates glucose itself. In gluconeogenesis as compared to glycolysis , the enzyme hexokinase is replaced by glucosephosphatase, and the enzyme phosphofructokinase-1 is replaced by fructose-1,6-bisphosphatase. This helps the cell to regulate glycolysis and gluconeogenesis independently of each other. As will be discussed as part of lipolysis, fats can be broken down into glycerol, which can be phosphorylated to form dihydroxyacetone phosphate or DHAP. DHAP can either enter the glycolytic pathway or be used by the liver as a substrate for gluconeogenesis. Figure 7. Gluconeogenesis is the synthesis of glucose from pyruvate, lactate, glycerol, alanine, or glutamate. Changes in body composition, including reduced lean muscle mass, are mostly responsible for this decrease. The most dramatic loss of muscle mass, and consequential decline in metabolic rate, occurs between 50 and 70 years of age. Loss of muscle mass is the equivalent of reduced strength, which tends to inhibit seniors from engaging in sufficient physical activity. Holton JB, Walter JH, Tyfield LA Galactosemia. Huijing F Glycogen and enzymes of glycogen metabolism. In: Curtius HCH, Roth M eds Clinical Biochemistry, Vol. King RF, Macfie J, Hill G Activities of hexokinase, phosphofructokinase, fructose bisphosphatase and 2-oxoglutarate dehydrogenase in muscle of normal subjects and very ill surgical patients. Clin Sci — Krisman CR A method for the colorimetric estimation of glycogen with iodine. Anal Biochem — Kunst A, Draeger B, Ziegenhorn J UV-methods with hexokinase and glucose6-phosphate dehydrogenase. In: Bergmeyer HU, Bergmeyer J, Grassl M eds Methods of Enzymatic Analysis, Vol. Verlag Chemie, Weinheim, Germany, pp — Kurz G, Wallenfels K D-Galactose. UV-Test mit Galactose-Dehydrogenase. In: Bergmeyer HU ed Methods of Enzymatic Analysis, Vol. Lederer B, Van Hoof F, Van den Berghe G, Hers HG Glycogen phosphorylase and its converter enzymes in haemolysates of normal human subjects and of patients with type VI glycogen storage disease. A study of phosphorylase kinase deficiency. Biochem J — Lentner C ed Geigy Wissenschaftliche Tabellen, 8th edn, Vol 1. Lentner C ed Geigy Wissenschaftliche Tabellen, 8th edn, Vol 2. Mayes JS, Guthrie R Detection of heterozygotes for galactokinase deficiency in a human population. Biochem Genet — Mitchell B, Haigis E, Steinmann B, Gitzelmann R Reversal of UDP-galactose 4-epimerase deficiency of human leukocytes in culture. Proc Natl Acad Sci U S A — Ng WG, Donnel GN, Hodgman JE, Bergren WR Differences in uridine diphosphate galactoseepimerase between haemolysates of newborns and of adults. Nature — Okumiya T, Keulemans JLM, Kroos MA, Van der Beek NME, Boer MA, Takeuchi H, Van Diggelen OP, Reuser AJJ A new diagnostic assay for glycogen storage disease type II in mixed leukocytes. Mol Genet Metab — Seifter S, Dayton S, Novic B, Muntwyler E The estimation of glycogen with the anthrone reagent. Arch Biochem — Steinmann B, Gitzelmann R, Van den Berghe G Disorders of fructose metabolism. Thomas JA, Schlender KK, Larner J A rapid filter paper assay for UDP-glucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP- 14 C-glucose. Uyttenhove K, Bollen M, Stalmans W An optimized assay of phosphorylase kinase in crude liver preparations. Van Hoof F Amylo-1,6-glucosidase activity and normal glycogen content of the erythrocytes of normal subjects, patients with glycogen storage disease and heterozygotes. CrossRef PubMed Google Scholar. Vora S, Corash L, Engel WK, Durham S, Seaman C, Piomelli S The molecular mechanism of the inherited phosphofructokinase deficiency associated with hemolysis and myopathy. Blood — Download references. You can also search for this author in PubMed Google Scholar. Academic Medical Centre, Lab. Genetic Metabolic; Diseases Fo, University Amsterdam, Meibergdreef 9, AZ, Amsterdam, Netherlands. Reprints and permissions. Bosshard, N. Enzymes and Metabolites of Carbohydrate Metabolism. In: Blau, N. eds Laboratory Guide to the Methods in Biochemical Genetics. Springer, Berlin, Heidelberg. Publisher Name : Springer, Berlin, Heidelberg. Print ISBN : Online ISBN : eBook Packages : Medicine Medicine R0. Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Policies and ethics. Skip to main content. Abstract This chapter deals with the assays used for the diagnosis of three groups of inborn errors of metabolism of carbohydrates, i. Fat can be used for the oxidative regeneration of ATP and reductive power NADH. Put your understanding of this concept to test by answering a few MCQs. Request OTP on Voice Call. Your Mobile number and Email id will not be published. Post My Comment. Biology Biology Article Carbohydrate Metabolism. Learn Better through BYJU'S Quiz Q 5. Start Quiz. Your result is as below. Login To View Results. Did not receive OTP? View Result. BIOLOGY Related Links What Is A Neuron Function Of Eye Fungi Definition Irrigation System Rain Water Harvesting Project Ecosystem Diagram Explain Greenhouse Effect Prokaryotic Cell Structure What Is Angiosperm Allele Definition. Leave a Comment Cancel reply Your Mobile number and Email id will not be published. Share Share Share Call Us. Grade Class 1 Class 2 Class 3 Class 4 Class 5 Class 6 Class 7 Class 8 Class 9 Class 10 Class 11 Class 12 IAS CAT Bank Exam GATE. |
| Carbohydrate Metabolism | Nucleotide sugars. Learn More. txt Medlars, RefWorks Download citation. The energy for this endergonic reaction is provided by the removal oxidation of two electrons from each three-carbon compound. Law and Politics. Literary Studies Plays and Playwrights. The last step in glycolysis produces the product pyruvate. |
| Glycolysis | Upon Recovery smoothies into the cell, metabbolism or Metformin and mental health phosphorylates glucose, converting it into glucosephosphate. Metaboliem digestive processes Carbohydrate metabolism enzymes polysaccharides down into monosaccharides, including glucose, the monosaccharides are transported across the wall of the small intestine and into the circulatory system, which transports them to the liver. Business History. Sign in through your institution Choose this option to get remote access when outside your institution. International and Comparative Criminology. |
| 6.2: Carbohydrate Metabolism | Mathematical Education. For example, because erythrocytes red blood cells lack mitochondria, they must produce their ATP from anaerobic respiration. Advanced Nutrition and Human Metabolism. Create a free a profile for additional features. Cell ; : 61— Operating Systems. Constitutional and Administrative Law. |
anscheinend würde aufmerksam lesen, aber hat nicht verstanden
Ist Einverstanden, es ist die lustige Antwort
Ist Einverstanden, das sehr nützliche Stück
Ich meine, dass Sie sich irren. Geben Sie wir werden besprechen. Schreiben Sie mir in PM.