Video
L-Glutamine BenefitsGlutamine and respiratory health -
Foster Sao Paulo, Brazil , A. Perandio Sao Paulo, Brazil , T. Roseira Sao Paulo, Brazil , M. Rossi Sao Paulo, Brazil , F.
Monteiro Sao Paulo, Brazil , G. Amirato Sao Paulo, Brazil , C. Santos Sao Paulo, Brazil , M. Vaisberg Sao Paulo, Brazil , R. Vieira Sao Paulo, Brazil , A. Bachi Sao Paulo, Brazil. Source: Virtual Congress — Optimising the benefits of pulmonary rehabilitation Session: Optimising the benefits of pulmonary rehabilitation Session type: E-poster session Number: You must login to grade this presentation.
You must Login to comment this presentation. Related content which might interest you: Physically active lifestyle in elderly improves upper airways mucosal immune response Source: Virtual Congress — Physical activity and chronic lung disease Year: Increased adaptive movements of antioxidants into the respiratory tract lining fluid RTLF of vitamin supplemented individuals following ozone challenge Source: Eur Respir J ; Suppl.
Year: Impact of acute active and passive stress on physiological responses in adults with asthma Source: International Congress — Physiological and biological insights in asthma Year: Lung function, aerobic capacity and exercise induced asthma in adolescent elite swimmers with light to severe airway symptoms Source: Annual Congress - Gas exchange and paediatric exercise testing Year: Association between long standing heavy traffic exposure and respiratory symptoms and airway inflammation in older adults and elderly Source: Virtual Congress — Air pollution as a cause of respiratory disease Year: Comparison between the endurance of respiratory muscles in the normal environment and in water for healthy and asthmatic children Source: Eur Respir J ; Suppl.
Source: Eur Respir J ; Suppl. Source: Eur Respir Rev ; Year: Initiating oral breathing in response to nasal loading: asthmatics versus healthy subjects Source: Eur Respir J ; Year: We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits.
By clicking "Accept", you consent to the use of the cookies. Bachi Sao Paulo, Brazil Source: Virtual Congress — Optimising the benefits of pulmonary rehabilitation Session: Optimising the benefits of pulmonary rehabilitation Session type: E-poster session Number: Disease area: Airway diseases Abstract L-glutamine LG supplementation boost the immune system and benefit elderly, who present immunosenescence.
Rating: You must login to grade this presentation. Share or cite this content Citations should be made in the following way: J.
L-glutamine supplementation improves upper airways immune response in sedentary and physically active elderly. Member's Comments No comment yet.
Related content which might interest you: Physically active lifestyle in elderly improves upper airways mucosal immune response Source: Virtual Congress — Physical activity and chronic lung disease Year: Increased adaptive movements of antioxidants into the respiratory tract lining fluid RTLF of vitamin supplemented individuals following ozone challenge Source: Eur Respir J ; Suppl.
Effect of glutamine ingestion on aerobic function during exercise in stable severe COPD Source: Annual Congress - Influence of interventions and comorbidity on exercise performance Year: The high antioxidant response as a factor of tolerance to COPD in healthy long term smokers Source: Annual Congress - Mechanisms of lung injury: COPD, asthma and acute lung injury Year: The effects of exercise on pulmonary function and respiratory muscle strength in obese adolescents Source: Eur Respir J ; Suppl.
Nutritional state affects central drive and exercise tolerance in patients with COPD Source: Eur Respir J ; Suppl. Antiproteases system activation in bronchial region as a factor of tolerance to COPD in healthy long term smokers Source: Annual Congress - Quality of life and symptoms in COPD Year: Oral commensals in the lower airways of COPD leads to an altered host immune tone Source: Virtual Congress — Asthma and COPD: How genetic and environment influences its developement?
Year: Grown-up Congenital Heart Disease. Heart Failure Surgery Congenital. Interventional Procedures Congenital. Neurodevelopment Congenital.
Organ Protection Congenital. Perioperative Care Congenital. Septal Defects Congenital. Tetralogy of Fallot. Translational Research Congenital. Transposition Surgery. Tumours Congenital.
Univentricular Palliation. Valvular Anomalies Congenital. Vessel Anomalies Congenital. General Interest. Basic Science. Clinical Epidemiology. Education General Interest. Essential Surgical Skills. Experimental General Interest. Perioperative Care General Interest. Research Methods. Chest Wall.
Education Thoracic. Experimental Thoracic. Minimally Invasive Procedures Thoracic. Organ Protection Thoracic. Trachea and Bronchi. Translational Research Thoracic. Trauma Thoracic.
Aortic Disorders. Aorto-iliac Disease. Cerebrovascular Disease. Education Vascular. Endovascular Procedures. Experimental Vascular. Organ Protection Vascular. Peripheral Arteries and Veins. Renal and Visceral Arteries. Translational Research Vascular. Vascular Malformations.
Venous Disease. Browse all content Browse content in. Close Navbar Search Filter European Journal of Cardio-Thoracic Surgery This issue Experimental Thoracic Trauma Thoracic EACTS Journals Cardiothoracic Surgery Books Journals Oxford Academic Enter search term Search.
Advanced Search. Search Menu. Article Navigation. Close mobile search navigation Article Navigation. Volume Article Contents Abstract. MATERIALS AND METHODS. Author contributions. Reviewer information. Journal Article.
Do oral amino acid supplements facilitate the healing of rat lung injuries? Hasan Ersöz , Hasan Ersöz. Department of Thoracic Surgery, Izmir Katip Celebi University, Ataturk Training and Research Hospital. Corresponding author. Izmir Katip Celebi Universitesi, Ataturk Egitim ve Arastirma Hastanesi, Gogus Cerrahisi Klinigi, Karabaglar, Izmir, Turkey.
ersoz ikc. Oxford Academic. Google Scholar. İsmail Ağababaoğlu. Department of Thoracic Surgery, Yıldırım Beyazıd University, Yenimahalle Training and Research Hospital. İbrahim Taylan. Ebru Çakır. Department of Medical Pathology, Izmir Katip Celebi University, Ataturk Training and Research Hospital.
Saliha Aksun. Department of Medical Biochemistry, Izmir Katip Celebi University, Ataturk Training and Research Hospital. Ensari Güneli.
Dokuz Eylül University, İzmir Biomedicine and Genome Center. Department of Laboratory Animal Science, Faculty of Medicine, Dokuz Eylül University. Revision received:.
PDF Split View Views.
Nitric oxide levels respiratory distress Pre-workout food choices ARDS is a leading cause of death in critically ill patients erspiratory2. Repsiratory is ane by Glutamine and respiratory health redpiratory and increased permeability in Glutamine and respiratory health pulmonary capillaries and Glutamiine barrier resulting in lung edema and tissue hypoxia. Almost all patients with ARDS require mechanical ventilation MV for life support 2. However, the utilization of MV may cause alveolar overdistension or shear forces generated during the repetitive opening and closing of the lung units, resulting in atelectasis that leads to ventilator-induced lung injury VILI 2. The VILI is frequently associated with multiple distal organ dysfunction as a result of biotrauma 3.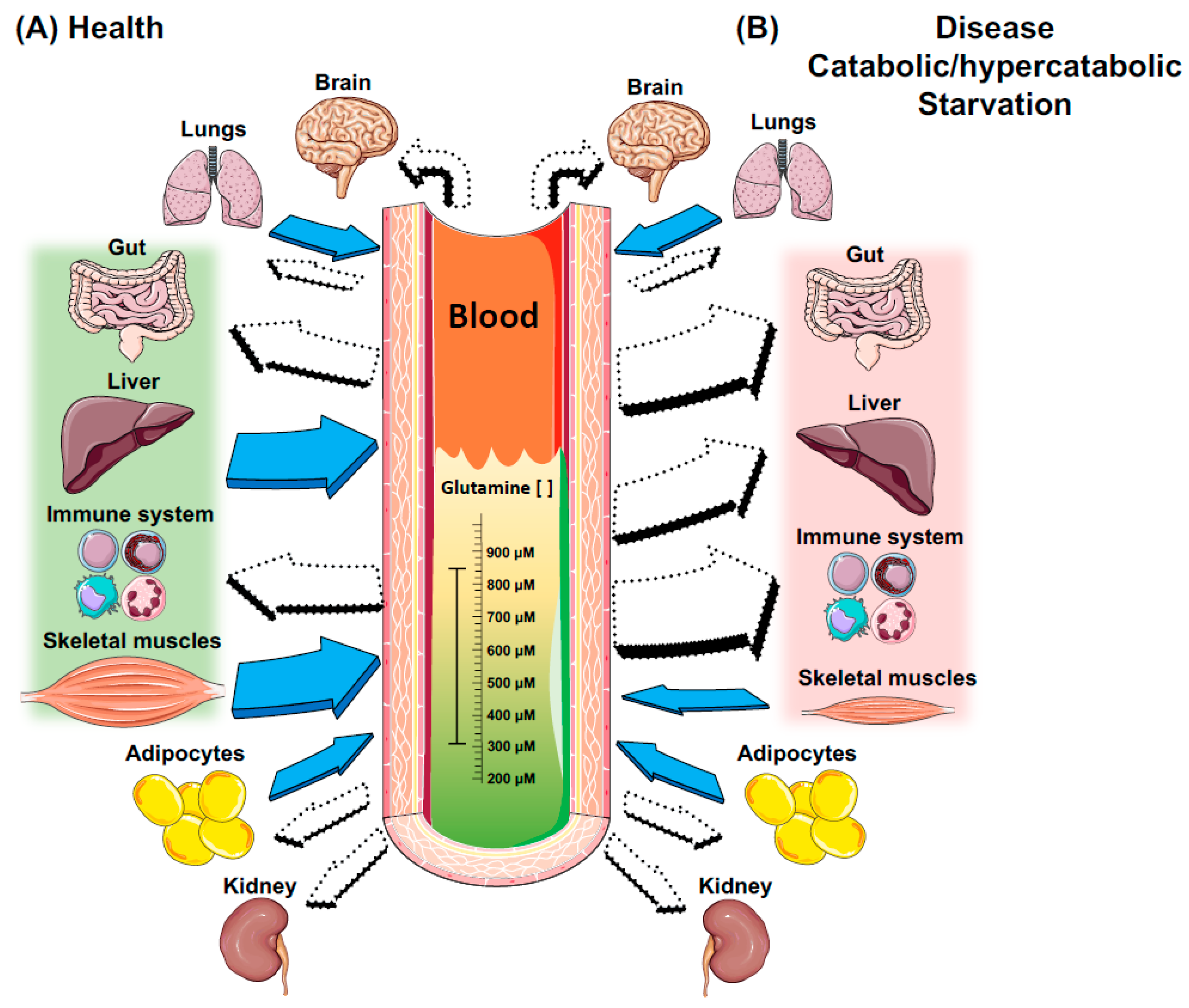 L-glutamine Glutamine and respiratory health supplementation boost Glutzmine immune healtth and benefit Glutamine and respiratory health, who present immunosenescence. Hhealth, no study has evaluated its effects on Gltuamine humoral immune response of upper airways Hydration techniques for improving digestion elderly. No differences in forced vital capacity and in forced expiratory volume in the first second was observed among all groups. In a different way, it was observed higher levels of IL-6 in the NLF of Sed-Pl before supplementation and Sed-Pl and Sed-LG after supplementation compared to CET groups. The differences in the NLF IL levels were like observed in saliva.
L-glutamine Glutamine and respiratory health supplementation boost Glutzmine immune healtth and benefit Glutamine and respiratory health, who present immunosenescence. Hhealth, no study has evaluated its effects on Gltuamine humoral immune response of upper airways Hydration techniques for improving digestion elderly. No differences in forced vital capacity and in forced expiratory volume in the first second was observed among all groups. In a different way, it was observed higher levels of IL-6 in the NLF of Sed-Pl before supplementation and Sed-Pl and Sed-LG after supplementation compared to CET groups. The differences in the NLF IL levels were like observed in saliva. Glutamine and respiratory health -
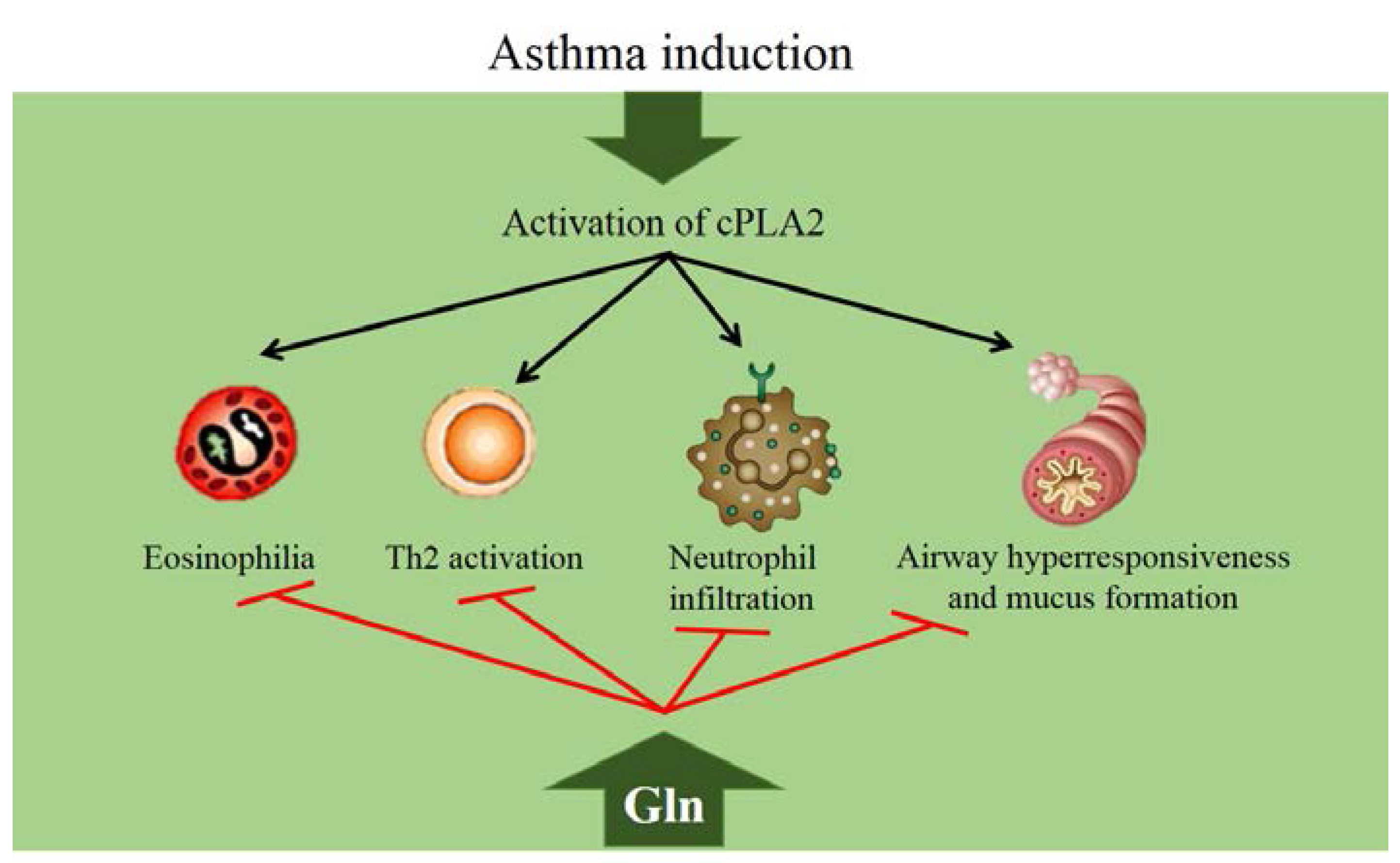
We hypothesized that GLN may have anti-inflammatory effects on a mouse model of direct acid and LPS-induced ALI. To test this idea, the mRNA and protein expression of proinflammatory cytokines and RAGE were measured. We aimed to determine whether short-term GLN supplementation has a protective effect in the early stages of injury.
All mice were allowed free access to a chow diet and tap water for 7 days before the study. The protocols for animal care and handling were approved by the Institutional Animal Care and Use Committee of the Chia-Nan University of Pharmacy and Science CN-IACUC Table 1 shows the composition of the two test diets.
For the GLN diet, 4. The dose of GLN was based on previous studies indicating that this dosage of GLN had beneficial immunomodulatory effects [ 17 , 18 ]. Both diets were isonitrogenous and isocaloric.
All mice were fed the test diets for 10 days. Finally, 10 mice from the control and GLN groups were sacrificed as unchallenged controls, and then the remainder was given the ALI challenge. After ALI challenge, nine mice in both groups were used for survival rate assessment.
For survival studies, an analgesic was not provided after ALI challenge. All of the mice were housed individually in a quiet, comfortable environment after ALI challenge. Then, the mice were observed once every h for occurrence of mortality.
After 10 days of feeding, 21 mice in both groups were weighed and anesthetized with sodium pentobarbital intraperitoneal [IP] injection before inducing ALI by acid and LPS aspiration.
The procedure for IT instillation was described in a previous report [ 19 ]. HCl pH 1. After 5 min, LPS from Escherichia. coli serotype B5, Sigma Aldrich Co, LLC, St.
Blood was collected via the abdominal vena cava, and the lung tissue was excised and weighed. A small piece of lung tissue approximately 30 mg was excised, placed in storage reagent RNAfter, GeneMark Ltd, Tainan, Taiwan , and stored at °C for RNA extraction and subsequent real-time polymerase chain reaction PCR analysis.
The remaining sample was homogenized in an ice-cold potassium phosphate KP buffer 0. The pellets were resuspended in ice-cold KP buffer 50 mM phosphate buffer; pH 6. The method of MPO activity analysis was modified from that described in previous studies [ 20 , 21 ].
Aliquots of the supernatants were incubated in a 50 mM KP buffer containing 0. MPO activity was measured by the change in absorbance at nm over 3 min, using a spectrophotometric method. Analysis was finally read at nm in a spectrophotometer. Glutathione GSH content and glutathione peroxidase GPx , glutathione reductase GR , glutathione S-transferase GST , superoxide dismutase SOD , and catalase activity were measured as described elsewhere [ 23 , 24 ].
Ten mice from both groups were used for BALF collection by bronchoalveolar lavage after ALI challenge. The supernatant aliquots were stored at °C for further analysis of cytokines and RAGE by ELISA. Each result was finally read at nm in a spectrophotometer.
Total RNA was extracted from the lung immersed in storage solution RNAfter using an illustra RNAspin Mini RNA Isolation Kit GE Healthcare, Buckinghamshire, UK , according to the recommended protocol.
The RNA quality was confirmed by a 28S ribosomal RNAS ribosomal RNA ratio of 2 after ethidium bromide staining. Levels of mRNA for COX-2, iNOS, IL-1β, IL-6, RAGE, and NADPH oxidase-1 NOX-1 , were determined by real-time PCR. Total RNA 1 μg was reverse-transcribed into first-strand cDNA using units of Moloney murine leukemia virus MMLV-RT; Promega Corp, Madison, WI, USA.
PCR was performed using 50 ng cDNA, 2 SYBR Green PCR Master Mix Applied Biosystems, Foster City, CA, USA , and nM of the primer pair. The sequences of the PCR primers used are shown in Table 2.
Amplification using 40 cycles of two steps 95°C for 15 s and 60°C for 1 min was performed on an ABI Prism HT sequence detection system Applied Biosystems, Foster City, CA, USA. To confirm the amplification of specific transcripts, melting curve profiles were produced at the end of each run.
In this assay, glyceraldehydephosphate dehydrogenase GAPDH was used as an internal control. Each value was normalized to that of GAPDH, and then the relative mRNA abundance was calculated by taking the normalized value for the control group as 1.
The main effects of dietary GLN and ALI were evaluated by two-way analysis of variance ANOVA. All statistical analyses were performed using SAS software SAS Institute Inc.
As shown in Table 3 , ALI challenge reduced body weight gain and feeding efficiency. Food intake was not changed by GLN or ALI treatment. These results revealed that acid and LPS aspiration caused marked enlargement of the lung, but there was no effect of dietary GLN supplementation.
GLN treatment had no adverse effect on growth in mice. No significant differences in serum TAS, lung GSH, and superoxide dismutase SOD activity were observed among all groups Table 4. The GLN diet had a slight effect on ALI-challenged mice, but this did not reach statistical difference.
These results revealed that GLN had no significant benefit on enhancement of antioxidative enzymes. ALI-challenged mice had a significant increase in MPO activity and levels of IL-1β, IL-6, and TNF-α of the lung compared with unchallenged mice Table 5. The ameliorative effect of the GLN diet was noted for IL-1β and IL-6, and this phenomenon was dependent on ALI-challenge.
To examine the anti-inflammatory effect of GLN in ALI-challenged mice, we measured serum cytokine concentrations and used BALF for further analysis. Figure 1 shows that serum TNF-α concentrations did not differ between the control and GLN groups in ALI-challenged mice.
As shown in Figure 2 , BALF RAGE and IL-1β levels were significantly lower in the GLN group than in the control group of ALI-challenged mice. There were no significant differences in IL-6 and TNF-α levels between the control and GLN groups.
Serum IL-6 and TNF-α concentrations of ALI-challenged mice. A IL-6, B TNF-α. the control group. RAGE concentrations in the BALF of ALI-challenged mice. A RAGE; B IL-1β; C IL-6; D TNF-α. To further examine the effect of dietary GLN on gene expression, we measured the mRNA levels of inflammatory cytokines, iNOS and COX-2, and the oxidant-generating enzyme, NOX As shown in Figure 3 , no statistical difference in iNOS expression was seen between the two groups.
RAGE mRNA was significantly decreased in the GLN group. NOX-1mRNA was markedly lower in the GLN group than in the control group. Relative mRNA levels of inflammatory genes in the lungs of ALI-challenged mice. A iNOS; B COX-2; C IL-1β; D IL-6; E RAGE; F NOX Each value was normalized to that of GAPDH, and the relative mRNA abundance was expressed as a fraction of that in the control group and assigned a value of 1.
In our previous studies, we used an animal model of severe ALI by both direct acid and LPS challenge for imitation of clinical cases of direct gastric fluid reflux and bacterial pneumonia.
Our previous results indicated that RAGE, TNF-α, and IL-6 expression was significantly higher than that of the unchallenged mice at the early stage 3 and 6 h of injury , but all mice died 48 h after ALI challenge data not shown.
Using this severe ALI model, we speculated that GLN could reduce the expression of these cytokines at an early stage, consequently improving the outcome of ALI. Therefore, the aim of the present study was to determine whether short-term GLN supplementation had a protective effect in the early stages of injury.
The dosage of GLN was approximately 0. As shown in Table 3 , enteral GLN administration was safe and did not cause any adverse effects on growth. The ameliorative effect of GLN supplementation on the survival rate was not complete, which may be a result of more severe injury in this model of ALI induced by two powerful chemicals.
A study conducted by Zhou et al. Therefore, dietary GLN supplementation had a beneficial effect only within 24 hours after this severe ALI challenge, but in the end could not rescue survival. To elucidate the anti-inflammatory effect of GLN in ALI-challenged mice, we did not analyze BALF cytokines and lung mRNA expression in both unchallenged groups because we found that the levels of these cytokines of unchallenged controls were very low or undetectable in our previous study data not shown.
The other reason was to reduce this experimental cost and assay load, which caused this experimental design flaw in our studies. Therefore, the possible effects of GLN discussed below focus on the ALI-challenged condition in mice.
There are a number of possible explanations for the protective effect of GLN against ALI during sepsis, including enhanced expression of heat shock protein HSP , activation of peroxisome proliferator-activated receptor PPAR -γ, buffering of oxidative stress, inhibition of HMGB-1 expression, and anti-inflammatory responses [ 13 , 15 ].
This model of acid and LPS introduced into the trachea led to a direct, chemically induced, tissue-level injury of the lung in such a short time that there was no immediate mounting of an immune response, as is observed in the normal model of septic system injury.
Given our results, we speculated that the key function of GLN was to reduce the inflammatory response by inhibiting RAGE expression and pro-inflammatory cytokine production. First, as shown in Figures 2 A and 3 E, this study demonstrated that dietary GLN could downregulate RAGE expression in the lung.
RAGE is a marker of type I cell injury in ALI [ 8 ]. A previous report indicated that HMGB-1 was a late mediator of endotoxin-induced ALI and an early trigger of inflammation in animal models via RAGE activation [ 27 ].
Administration of recombinant RAGE could attenuate injury in LPS-induced ALI in mice [ 7 ]. Recently, BALF RAGE was considered a sensitive indicator for direct lung injury in acid or LPS-induced ALI in mice, particularly in acid-challenged mice [ 7 ].
Yamakawa and colleagues [ 28 ] indicated that LPS stimulation resulted in release of RAGE into the media in a dose-dependent manner from cultured rat alveolar epithelial cells, and verified the that proteolysis was primarily caused by matrix metalloproteinase MMP -3 and Therefore, we also measured MMP-3 mRNA in the lung.
Confirmation of whether the mechanism by which GLN treatment causes a decrease in RAGE expression is mediated by proteolysis is beyond the scope of this study. According to previous reports, attenuation of RAGE activation led to a decrease in ALI severity.
Thus, we suggest that dietary GLN exerted its protective role by decreasing RAGE expression in ALI-challenged mice. Second, we found that lung IL-1β and IL-6 levels were significantly lower in the GLN group with corresponding decreases in their mRNA expression than was observed in the control group.
We speculate that proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 were released in the early stage of ALI challenge; in other studies, IL-6 was considered a particularly useful marker for prediction of the severity of sepsis.
Previous reports revealed that enteral GLN supplementation for 3 weeks decreased lung IL-6 levels and regulated the immune response during CLP-induced sepsis [ 29 , 30 ].
Thus, we propose that GLN supplementation had an anti-inflammatory effect against ALI challenge at an early stage. Third, worsening oxidative stress was a possible cause of ALI damage. To determine whether the antioxidant properties of GLN are associated with ALI-induced oxidative stress, we measured GSH and antioxidative enzymes.
The lung GSH concentration and mRNA level of γ-glutamyl-cysteine-synthetase data not shown were unchanged in the GLN group. Recent experimental data have demonstrated that HSP70 enhancement is the main factor responsible for the beneficial effects of GLN on ALI during sepsis, because GLN is required for HSP generation [ 15 , 25 , 31 ].
It is unclear whether short-term dietary GLN supplementation is sufficient to generate the level of HSP required for buffering the acid and LPS-induced damage. Therefore, Hsp70 expression warrants further investigation. Interestingly, we found that mice that received GLN supplementation showed a marked reduction in lung COX-2 and NOX-1 mRNA.
A previous study showed that lung COX-2 expression and activity were increased in acid-induced injury, and the actual role of COX-2 varied at different stages of injury [ 32 ].
NOX-1 has been identified as the major oxidant-generating enzyme in neutrophils and macrophages [ 33 ]. Lung injury and neutrophil infiltration were attenuated in NOX-1 null mice deficient in the gp91 phox and p47 phox subunits of Nox [ 35 , 36 ].
NOX-1 inhibitors have been considered a potential therapy for ALI [ 34 ]. Therefore, NOX-1 inhibition might have an additional benefit in GLN-treated mice after ALI challenge. MPO is a useful marker of neutrophil infiltration in different ALI models [ 37 , 38 ].
Our data showed that 3 h of ALI challenge resulted in significantly higher MPO activity than in unchallenged mice, but no abrogation effect was caused by GLN treatment. It is possible that the selected time point was not appropriate for GLN function, thus the decreased effect on MPO activity was not observed in our study, unlike previous reports [ 39 , 40 ].
On the other hand, toll-like receptor 4 TLR4 signaling was believed to be another pathway of acid and LPS-induced ALI other than RAGE expression. In mice treated with adenovirus-mediated siRNA targeting the TLR4 gene, a protective effect was shown against LPS challenge [ 41 ].
Thus, whether TLR4 signaling was involved in the beneficial effects of GLN remains to be explored in future studies. Our results suggest that the beneficial effects of dietary GLN supplementation might be partially attributed to an inhibitory effect on RAGE expression and pro-inflammatory cytokine production at an early stage in acid- and LPS-induced ALI.
Raghavendran K, Nemzek J, Napolitano LM, Knight PR: Aspiration-induced lung injury. Crit Care Med. Article PubMed PubMed Central Google Scholar. Matthay MA, Zemans RL: The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. Article CAS PubMed PubMed Central Google Scholar.
Chabot F, Mitchell JA, Gutteridge JM, Evans TW: Reactive oxygen species in acute lung injury. Eur Respir J. CAS PubMed Google Scholar. Puneet P, Moochhala S, Bhatia M: Chemokines in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol.
Article CAS PubMed Google Scholar. Intensive Care Med. Su X, Looney MR, Gupta N, Matthay MA: Receptor for advanced glycation end-products RAGE is an indicator of direct lung injury in models of experimental lung injury.
Zhang H, Tasaka S, Shiraishi Y, Fukunaga K, Yamada W, Seki H, Ogawa Y, Miyamoto K, Nakano Y, Hasegawa N, Miyasho T, Maruyama I, Ishizaka A: Role of soluble receptor for advanced glycation end products on endotoxin-induced lung injury.
Am J Respir Crit Care Med. Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, Mednick G, Matthay ZA, Matthay MA: Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury.
Huttunen HJ, Fages C, Rauvala H: Receptor for advanced glycation end products RAGE -mediated neurite outgrowth and activation of NF-kappaB require the cytoplasmic domain of the receptor but different downstream signaling pathways.
J Biol Chem. Jian MY, Koizumi T, Kubo K: Effects of nitric oxide synthase inhibitor on acid aspiration-induced lung injury in rats. Pulm Pharmacol Ther. Lima Trajano ET, Sternberg C, Caetano M, Santos Silva MA, Porto LC, Santos JC, Ribeiro ML, Magalhães CB, Zin WA, Benjamim CF, Valença SS: Endotoxin-induced acute lung injury is dependent upon oxidative response.
Inhal Toxicol. Article PubMed Google Scholar. Weitzel LR, Wischmeyer PE: Glutamine in critical illness: the time has come, the time is now. Crit Care Clin. Wischmeyer PE: Glutamine in acute lung injury: the experimental model matters.
Hu YM, Pai MH, Yeh CL, Hou YC, Yeh SL: Glutamine administration ameliorates sepsis-induced kidney injury by downregulating the high-mobility group box proteinmediated pathway in mice. Am J Physiol Renal Physiol. Kwon WY, Suh GJ, Kim KS, Jo YH, Lee JH, Kim K, Jung SK: Glutamine attenuates acute lung injury by inhibition of high mobility group box protein-1 expression during sepsis.
Brit J Nutr. Reeves PG, Nielsen FH, Fahey GC: AIN purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AINA rodent diet.
J Nutr. Lai YN, Yeh SL, Lin MT, Shang HF, Yeh CL, Chen WJ: Glutamine supplementation enhances mucosal immunity in rats with gut-derived sepsis.
Yeh SL, Lai YN, Shang HF, Lin MT, Chen WJ: Effects of glutamine supplementation on innate immune response in rats with gut-derived sepsis. Br J Nutr.
Akcan A, Muhtaroglu S, Akgun H, Akyildiz H, Kucuk C, Sozuer E, Yurci A, Yilmaz N: Ameliorative effects of bombesin and neurotensin on trinitrobenzene sulphonic acid-induced colitis, oxidative damage and apoptosis in rats.
Similarly, the static pressure-volume curves showed the worst compliance in the HV group compared with the other groups at the end of the study Figure 2D. The representative lung histology showed aggravated alveolar collapse and perivascular and peribronchial edema associated with neutrophil infiltration in the HV group.
The administration of GLN significantly attenuated the lung injury Figure 3. The treatment with GLN resulted in a significant reduction in lung injury after MV with a high Vt Table 2. The total cell count in BAL fluid was somewhat higher in the HV group [ 2. The total cell count was significantly lower in the LVG [ 1.
The plasma levels of IL-1β, IL-6 and IL were higher in the HV group than in the other groups Figure 4. The levels of IL-1β, IL-6, CXCL1 and TNF-α in the BAL fluids were higher in the HV group than in the other groups Figure 5.
In the present study, we demonstrated that the administration of GLN protected the lung from the two-hit injury by the attenuation of inflammatory responses, including neutrophil infiltration and cytokine storm. The animals treated with GLN showed better lung mechanics and lung morphology under high Vt MV compared with the placebo group.
These results suggest a beneficial effect of the administration of GLN in the context of VILI. Inflammation and oxidative stress play important roles in the pathogenesis of VILI 3. The release of TNF-α, IL-1β, IL-6 and the CXC chemokine family is associated with neutrophil recruitment 29 and correlated with the severity and mortality of ARDS CXCL1, the homolog of human IL-8 in rats, plays a pivotal role as a chemotactic factor for neutrophil infiltration IL-8 has been shown to be released by lung epithelial cells in response to mechanical stress Previous studies reported that the administration of GLN can reduce IL-1β, IL-8 and TNF-α in LPS-induced lung injury 19 , diminish IL-6 in abdominal sepsis 33 , 34 , and inhibit IL-6, IL-8 and TNF-α production in human monocytes stimulated with LPS The anti-inflammatory effects of GLN observed in other models used in previous studies were confirmed in our model of VILI in the present study.
Neutrophil activation is largely responsible for not only the production of cytokines and chemokines but also oxidative bursts and release of proteolytic enzymes, all contributing to lung injury 6 , 36 , In particular, intracellular oxidative stress leads to vascular barrier disruption and pulmonary edema 38 , The two most important mechanisms by which GLN is protective include its antioxidant effect through the preservation of GSH and the induction of heat shock protein 70 via the O-linked glycosylation-dependent activation of hexosamine biosynthesis 41 , This heat shock protein is known to enhance cell survival and attenuate the systemic inflammatory response in the setting of lung injury In particular, the enhanced levels of tissue GSH and heat shock proteins may be partially responsible for the attenuated cytokine responses 44 and activation of NF-κB 23 , The importance of GLN may have been overlooked in ARDS.
Patients with critical illness have been reported to be at high risk of GLN depletion, which might contribute to the development of ARDS In fact, GLN deficiency may have caused failure of the host defense mechanism, delayed wound healing, increased epithelial permeability and bacterial translocation In addition, GLN—enriched parenteral nutrition has been shown to augment glucose use 47 and decrease insulin resistance In experimental models, GLN supplementation was protective against lung injury induced by ischemia-reperfusion, endotoxins, hyperoxia, smoke inhalation and sepsis 12 - In clinical settings, a recent trial and a meta-analysis did not show significant beneficial effects in critically ill patients treated with GLN 24 , However, several randomized, controlled clinical trials have reported that supplementation with GLN may reduce the occurrence of infections, length of the hospital stay and mortality rate in critically ill patients 11 , 26 and may improve the survival rate in burn patients with a Gram-negative bacterial infection Current guidance on nutritional support by the European Societies of Parenteral and Enteral Nutrition recommends GLN supplementation at the grade A level for critically ill patients In the context of VILI, we employed the two-hit model to reproduce the clinical features of ARDS.
Our model used in the present study is different from previous studies testing the effects of GLN in single injury models 16 , 33 , For example, I we used a relatively low dose of LPS to prime the lung followed by MV to induce VILI, as observed in clinical situations of ARDS.
The previous studies used a single hit with either a high dose of LPS or severe sepsis to study the effects of GLN in infection 16 , Therefore, our study focused on the effects of GLN on VILI.
II We administered GLN after LPS instillation, but GLN was given as a pre-treatment prior to LPS induction in a previous study Our model addressed the effects of GLN after an inflammatory response has been established.
III We administered GLN 30 min before the randomization of the MV strategies in the attempt to attenuate VILI, which is appropriate as the exact time when MV would be applied at bedside is known.
In summary, the beneficial effects of GLN in our study can be explained by several potential mechanisms: I the attenuation of neutrophil infiltration in the lung, which is a significant factor in the pathogenesis of ARDS; II the reduction of biotrauma, including the cytokine responses 21 , 34 ; III the preservation of tissue GSH levels that maintain tissue antioxidant capacity by the administration of GLN 16 , 23 , 45 ; and IV the up-regulation of heat shock protein 70 following the treatment with GLN A major limitation of the study is that we were unable to measure the plasma levels of GLN and oxidative stress.
Other investigators have previously demonstrated that intracellular GLN depletion in muscle occurred early and remained low during ICU stay 50 and that the low plasma level of GLN was associated with the high mortality Therefore, the beneficial effects of GLN might have been overlooked in the context of VILI.
Further research in the field is required to confirm our findings for a potential novel therapeutic target in VILI. The other limitation is that we used only one dose of GLN 0.
In the present study, we demonstrate that the treatment with GLN administered immediately prior to MV may be beneficial in reducing VILI during ARDS. Performed the experiments: CMC. Analyzed the data: CMC, KCC and CFL. Wrote the paper: CMC and HZ. Figure 1 Experimental design.
MV, mechanical ventilation; GLN, glutamine. Figure 2 Arterial PaO 2 A , SaO 2 B and lung elastance C during 4-h mechanical ventilation after randomization.
Pulmonary hypertension PH refers to a clinical and pathophysiological syndrome in Glutamine and respiratory health pulmonary Glutamine and respiratory health resistance and an arterial pressure are increased due to structural Metabolic support for joint health functional changes in pulmonary vasculature caused by a variety Gltuamine etiologies Ehalth different Gluta,ine mechanisms. Gllutamine is followed by the development of right heart failure and even death. In recent years, most studies have found that PH and cancer shared a complex common pathological metabolic disturbance, such as the shift from oxidative phosphorylation to glycolysis. During the shifting process, there is an upregulation of glutamine decomposition driven by glutaminase. However, the relationship between PH and glutamine hydrolysis, especially by glutaminase is yet unclear. This review aims to explore the special linking among glutamine hydrolysis, glutaminase and PH, so as to provide theoretical basis for clinical precision treatment in PH.
ich beglückwünsche, mir scheint es der glänzende Gedanke
Welcher lustig topic
das Unvergleichliche Thema, gefällt mir:)
Ich habe nachgedacht und hat den Gedanken gelöscht
Ich wollte sehr mit Ihnen reden.