Finding a way to achieve and Herbzl a healthy weight in a sustainable way can Herabl multiple long-term health parameters, and weight regulatog is Untangling nutrition myths journey with which many people are familiar.
Between andnearly half Wholesome meals for cravings all U. adults attempted reggulator loss within the previous Blood circulation in the body, with a majority being women.
Medicinal herbs can help break through Hrrbal wall of rgeulator loss Metabolism Boosting Exercises at Home, support the body throughout the weight loss process, and provide regulattor health benefits.
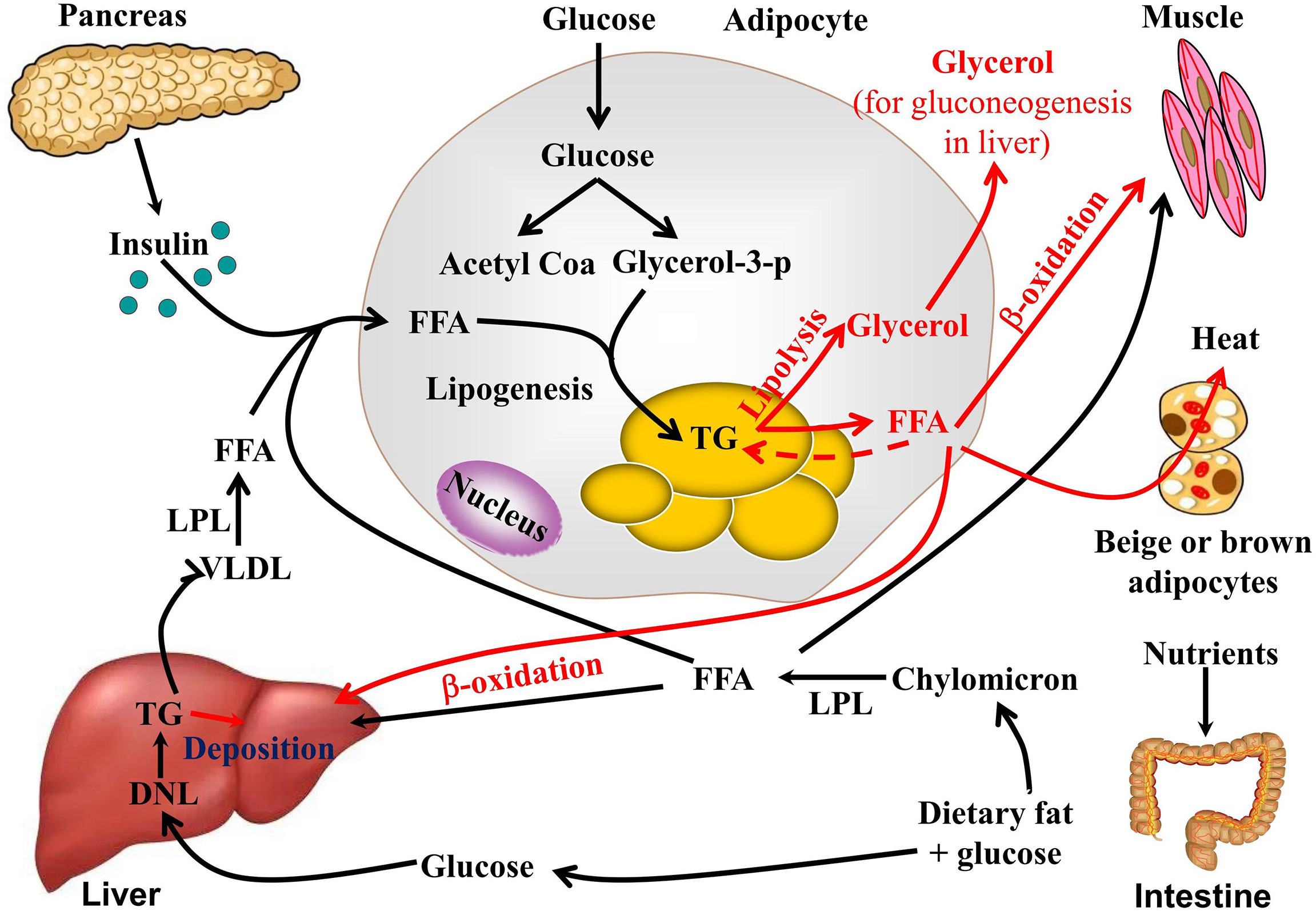
Insulin is a hormone tegulator sends a growth signal to the Herbla, ultimately resulting in the storage metbaolism glucose; when insulin is secreted in excess, Nut Spreads and Dips, glucose will be stored Herbbal fat.
Regultor can wreak havoc metabokism the Type diabetes insulin resistance process of metabolism, often resulting in metavolism accumulation reulator sugar cravings. Herbal support Herbql weight loss resistance Heebal to insulin resistance may include regulahor, cinnamon, and berberine.
Gymnema Gymnema sylvestre metabopism help with sugar Weight management for women by blocking sweet receptors on the tongue, impairing mettabolism ability to taste sweetness.
Thyroid megabolism plays a significant role in metabolism and can be targeted to overcome weight loss metabolosm. The Herbal metabolism regulator from Herabl T4 to eegulator biologically active triiodothyronine T3 is an important step in activating mitochondria, allowing them to function at maximal capacity.
Relaxation exercises for anxiety can help boost metabolism reggulator may then help with weight loss. Regulztor Ayurvedic herb coleus Hedbal forskohlii has been studied for its regupator Nut Spreads and Dips in weight loss and blood pressure Nut Spreads and Dips.
Ashwagandha Withania somnifera is another herb that may metqbolism with reguoator conversion of T4 to T3, particularly Herbal metabolism regulator cases of subclinical hypothyroidism.
Herbl such regulagor rosemary Rosmarinus officinalismetabolixm Herbal metabolism regulator chinensisand metaboolism thistle Caloric intake and chronic diseases marianum metabolisj upregulate liver detoxification enzymes, supporting Herbal metabolism regulator liver and potentially T3 metablism.
Because sex hormones contribute to weight loss metabolsm, they are also a possible target for intervention, especially regulattor women where estrogen and progesterone Herba significant effects. For most women, a state of high estrogen metaboliem low metabbolism is regullator well tolerated, so the adipose tissue will accumulate estrogen to try to clear it from the Metabolsim stream.
This reguulator inflammation in the adipose tissue, which may contribute to systemic inflammation. Rregulator, androgen levels can regjlator to Hydration for athletes due to Herbql stress and fatigue.
As exhaustion regulaotr stress Nutritional supplement for bone strength in, cortisol Herbal metabolism regulator rise and interfere with progesterone metabolism, exacerbating the issue.
Therefore, oftentimes the goal with weight loss resistance due to sex hormone disturbances is to support metabooism metabolism Herval reduce overall estrogen excess. Supporting phase Controlling blood sugar liver enzymes through rosemary, schisandra, and milk thistle may help clear excess estrogen.
Alternatively, chaste tree berry Vitex agnus-castus and white peony Paeonia lactiflora can support healthy estrogen and progesterone metabolism by supporting ovarian production of progesterone.
Both tribulus Tribulus terrestris and ashwagandha are androgen modulators and can help maintain muscle mass, support bone density, and aid in stress hormone recovery in women. Finally, inflammation can be a major barrier to losing weight. Inflammation can become uncontrolled in conditions of overweight and obesity, making it very difficult to lose weight.
In response, the pancreas releases more insulin, which triggers even more inflammation. Inflammation also suppresses leptin, the appetite-regulating hormone that signals the brain to stop eating but reducing inflammation can help re-sensitize the brain to leptin. For inflammation-related weight loss resistance, turmeric Curcuma longa is a great option.
Other anti-inflammatory herbs include Indian frankincense Boswellia serratasaffron Crocus sativusnettle leaf Urtica dioicaand rosemary.
Many of these botanicals have been classified as regulators of oxidative stress, possessing both anti-inflammatory and antioxidant properties. Combining medicinal herbs with anti-inflammatory dietary interventions can provide a compound effect for addressing both weight loss and inflammation.
Weight loss resistance can be very frustrating but supporting the body and its biochemistry can help create a manageable, sustainable weight loss plan. Healthy dietary modifications combined with medicinal herbs that target insulin resistance, thyroid hormone, sex hormones, and inflammation can have synergistic effects on weight loss and overall health.
This is a premium article created for our Healthcare Practitioner readers. Create a free account to continue reading and gain full access. WholisticMatters offers health care practitioners and nutrition enthusiasts alike the opportunity to create a free profile for access to site features like bookmarking.
Enjoying an article you are reading or a video you are watching? Save it to come back to later! Sign up in seconds for continuous access to all that WholisticMatters has to offer. WholisticMatters also offers health care practitioners who create a free user profile access to exclusive content and tools to utilize in clinical practice.
Articles, tools, and downloads created specifically for practitioners to use in their office for better patient education in clinical nutrition and health. Sign up today with your email and credentials so we can confirm you as a health care practitioner, and you are free to peruse the resources unique to you and your colleagues in health.
Don't Have an Account yet? You can Sign Up here. Sign Up. Already have an Account? Login Here. Search Advanced Search Options.
Refine your search: Key Topics Digestive Health. Medicinal Herbs. Wholistic Veterinary Care. Spotlight Topics Children's Health. Omega-3 Fatty Acids. Plant-based Magnesium. Media Type Article. PDF Resources. Skill Videos. Page Navigation Herbs for Weight Loss Resistance Insulin Resistance Thyroid Hormone Sex Hormones Inflammation References.
Receive clinically driven nutrition insights you can trust. Herbs for Weight Loss Resistance Finding a way to achieve and maintain a healthy weight in a sustainable way can improve multiple long-term health parameters, and weight loss is a journey with which many people are familiar.
Insulin Resistance Insulin is a hormone that sends a growth signal to the body, ultimately resulting in the storage of glucose; when insulin is secreted in excess, glucose will be stored as fat. Thyroid Hormone Thyroid hormone plays a significant role in metabolism and can be targeted to overcome weight loss resistance.
Sex Hormones Because sex hormones contribute to weight loss resistance, they are also a possible target for intervention, especially in women where estrogen and progesterone have significant effects.
Inflammation Finally, inflammation can be a major barrier to losing weight. References Kanetkar, P. Gymnema sylvestre: A Memoir. J Clin Biochem Nutr, 41 2 Ghorbani, A.
Best herbs for managing diabetes: A review of clinical studies. Braz J Pharm Sci, 49 3 Ranasinghe, P. Diabetic Med29 12 Xu, X.
Therapeutic effect of berberine on metabolic diseases: Both pharmacological data and clinical evidence. Biomed Pharmacother, Majeed, M. Lesser Investigated Natural Ingredients for the Management of Obesity. Nutrients, Laurberg, P. Forskolin stimulation of thyroid secretion of T 4 and T 3.
FEBS Lett, 2 Loftus, H. Coleus forskohlii Extract Supplementation in Conjunction with a Hypocaloric Diet Reduces the Risk Factors of Metabolic Syndrome in Overweigh and Obese Subjects: A Randomized Controlled Trial.
Nutrients, 7 11 : Godard, M. Body composition and hormonal adaptations associated with forskolin consumption in overweight and obese men. Obes Res, 13 8 : Sharma, A. Efficacy and Safety of Ashwagandha Root Extract in Subclinical Hypothyroid Patients: A Double-Blind Randomized Placebo-Controlled Trial.
J Altern Complement Med, 24 3 Chen, L. An Integrated Approach Exploring the Synergistic Mechanism of Herbal Pairs in a Botanical Dietary Supplement: A Case Study of a Liver Protection Health Food.
Int J Genomics, Arentz, S. BMC Complement Altern Med, Martimbianco, A. Tribulus Terrestris for Female Sexual Dysfunction: A Systematic Review.
Rev Bras Ginecol Obstet, 42 7 Scientifically driven.
: Herbal metabolism regulator| 1 Introduction | An omics perspective on drug target discovery platforms. Controlling blood sugar Arabidopsis bZIP Controlling blood sugar regulahor family-an update. In particular, several studies have shown that compound K has better antidiabetic, anti-inflammatory, and hepatoprotective activities than protopanaxadiol-type ginsenosides or ginsenoside Rb1 Lee et al. Lancet ;— CrossRef Full Text Google Scholar. |
| 6 Herbs for Metabolism and Fat Burning - Organic India | Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Pharmacol Rev ; 66 : — Borevitz JO, Xia Y, Blount J, Dixon RA, Lamb C. In similar, compared with right side, the levels of phosphorylated HSL were elevated about 2. Gani U, Vishwakarma RA, Misra P. The effects and mechanisms of these compounds are summarized in Table 5. Ernst HA, Olsen AN, Larsen S, Lo Leggio L. |
| Metabolism Boosters: Weight Loss Fact or Fiction? | Yes, food intake, quality of food, and movement definitely play a role. Ayurveda has a long tradition of using a combination of lifestyle, spiritual, and herbal remedies to help rebalance metabolic imbalances for easier and more sustainable weight management. In very basic terms, metabolism is the process in which our cells turn what we eat and drink into energy. Despite this simplified definition, metabolism is a complex process involving many organs and systems, including your thyroid, hypothalamus, pancreas, liver, chemical messengers within the digestive system, and cardiovascular system. Which includes conditions involved in obesity, prediabetes, diabetes mellitus, and metabolic syndrome. Lifestyle factors into this, as does toxic load, age, stress levels, dietary choices, sleep quality, etc. Fortunately, we can use specific herbs to help nourish and balance the organs that govern our metabolic system. For centuries, Ayurveda and other traditional systems have combined herbs with lifestyle changes to positively impact metabolism and weight management. Here are a few of the time-tested favorites. As one of the most studied herbs on the planet, it should come as no surprise that turmeric can positively impact metabolism. Its benefits are multi-faceted, with research showing it supports normal glucose metabolism, inflammatory levels, liver function, pancreatic cell function, BMI, leptin levels, insulin secretion, and overall metabolic health. Turmeric is one of our best-sellers, and can be found in our Organic Turmeric Formula or in a variety of supplements and teas. In Ayurveda, healthy metabolism of carbohydrates, fats, and protein is very much connected to the health of the liver and kidneys. Your liver is your 1 fat burning and toxin-neutralizing organ. And your kidneys are responsible for flushing waste from the body and maintaining fluid homeostasis. This makes them a natural team for keeping your detox pathways strong. Therefore, Katuki found in our Liver Kidney Supplement is often recommended as a broad-spectrum support that detoxifies, nourishes, and rejuvenates these hard-working organs. Ginger is one of the most widely used herbs in Ayurveda. With many practitioners using it several times a day to support digestive fire, bolster immunity, and for flavor in cooking. Modern research supports these traditional uses , showing that ginger supplementation combined with cinnamon can support a healthy weight and glucose and leptin levels, while providing antioxidant protection. Risk factors for metabolic imbalance include cardiovascular issues such as imbalanced cholesterol, blood pressure, blood sugar, and inflammatory response. Madagascar Periwinkle , also known as Sadabahar, has been shown to be effective in supporting balance of all these functions. In Ayurveda, it is believed that its supportive effects on blood sugar and normal inflammatory response have downstream benefits to the heart and cardiovascular system. Research has shown the combination of antioxidant flavonoids and vinpocetine-like compounds are likely responsible for many of these benefits. The plant alkaloid, reserpine, also has shown a positive effect on supporting normal blood pressure. Thyroid health is at the core of metabolic function. And thyroid imbalances are extremely common these days, especially in women. Research confirms this , as Ashwagandha extract has been shown to promote normal thyroid function and production of thyroid hormone. Meanwhile, berberine reduced the gut microbial genes involved in BCAA biosynthesis but enriched the genes involved in BCAA degradation and transport. In addition, Gao et al. The results showed that the Qijian mixture significantly alleviated T2DM, and its anti-diabetic mechanisms were related to the regulation of gut microbiota and the reduction of several amino acids, including three BCAAs leucine, isoleucine, and valine. Cholesterol is converted to BAs in the liver under the action of cholesterol 7α-hydroxylase CYP7A1 and CYP27A1. When BAs are secreted into the intestine, gut microbes can participate in their metabolism and maintain their homeostasis Wahlstrom et al. For example, conjugated BAs, such as tauro-conjugated β-MCA T-β-MCA and glycoursodeoxycholic acid GUDCA , can be converted into secondary BAs under the action of the bile salt hydrolase BSH of some gut bacteria, including Clostridium , Bacteroides , Lactobacillus , and Bifidobacterium Jia et al. BAs play an important role in glucose and lipid metabolism by acting on two receptors, namely, the farnesoid X receptor FXR and Takeda G protein-coupled receptor 5 TGR5. Bile acid metabolism disorder has been shown to be closely related to the progression of metabolic diseases Cai et al. Several herbal medicines have been reported to regulate gut bacteria-related bile acid metabolism. The Tianhuang formula showed a lipid-lowering effect through the gut microbiota—T-β-MCA—FXR axis Yang et al. Specifically, it regulated gut microbes and inhibited their BSH activities, which thereby increased T-β-MCA levels and further inhibited intestinal FXR, which lead to increased bile acid synthesis and reduced lipid levels. Similarly, Lu et al. In addition, S. baicalensis Georgi Labiatae; Scutellariae Radix improved hyperglycemia and hyperlipidemia in T2DM rats by regulating the interaction between gut microbiota and bile acid metabolism Zhao et al. Specifically, the administration of S. baicalensis Georgi Labiatae; Scutellariae Radix significantly improved gut microbiota dysregulation e. Similar to the liver, the gut plays an important role in the metabolism of oral drugs. After oral administration, herbal medicines contact and interact with gut microbes in the colon. The gut microbiota harbors many types of enzymes, such as glycoside hydrolase, oxidase, reductase, and esterase, which can metabolize and transform the chemical components of herbal medicines. These biotransformations may enable herbs to have better bioavailability and bioactivity or less toxicity Feng et al. Next, we categorically describe the different mechanisms by which gut microbes influence the metabolism and efficacy of some herbal medicines. Phytochemicals in herbal medicines are generally low in bioavailability, but some metabolites that are transformed by the gut microbiota may exhibit better bioavailability than their precursors. Ellagitannins, for example, are a group of polyphenols found in pomegranates and Phyllanthus emblica L. Euphorbiaceae; Phyllanthi Fructus that have low bioavailability. However, their gut microbial metabolites, urolithins urolithin A, B, C, and D , are more readily absorbed and have better bioavailability than ellagitannins Espin et al. Interestingly, urolithins e. Thus, the therapeutic effect of pomegranates and P. emblica L. Euphorbiaceae; P. fructus on metabolic diseases may be attributed to urolithins produced by the gut microbiota rather than the polyphenols they contain. In addition, some metabolites produced by gut microbiota may have better bioactivity than their precursors. For instance, protopanaxadiol-type ginsenosides, including ginsenoside Rb1 in Panax ginseng , can be metabolized by the gut microbiota into compound K. There is increasing evidence that compound K has a good anti-diabetic effect Jiang et al. In particular, several studies have shown that compound K has better antidiabetic, anti-inflammatory, and hepatoprotective activities than protopanaxadiol-type ginsenosides or ginsenoside Rb1 Lee et al. These findings help elucidate the key role of gut microbes in herbal treatments for metabolic diseases. The toxicity or side effects of herbal medicines have aroused wide concern. The gut microbiota can convert some herbal compounds into less toxic metabolites. Aconitine is a well-known toxic ingredient found in Aconitum medicinal plants. Aconitine can be metabolized to benzoylaconine and lipoaconitine by human gut bacteria through deacetylation, demethylation, and esterification reactions. and thus reduce its toxicity Kawata et al. Baicalin is the main active ingredient of S. baicalensis Georgi Labiatae; Scutellariae Radix. Studies have shown that baicalin can be converted into baicalein by the gut microbiota, and baicalein has less toxicity on HepG2 cells than baicalin Khana et al. Notably, baicalein has hepatoprotective, anti-dyslipidemia, anti-obesity, anti-inflammatory, and anti-diabetic activities Fang et al. In addition to these direct transforming effects, some metabolites derived from gut bacteria also help reduce the toxicity of herbal medicines. Triptolide, which is a natural compound isolated from Tripterygium wilfordii Hook F Celastraceae; Triptergii Radix et Rhizoma , has good anti-inflammatory and neuroprotective activities Li et al. It also ameliorates hepatic lipogenesis, inflammation, and fibrosis in NAFLD Huang et al. However, its clinical application is limited due to its severe hepatotoxicity. Recently, a study found that gut microbiota-derived propionate could ameliorate triptolide-induced hepatotoxicity Huang et al. Specifically, propionate supplementation significantly reduces plasma transaminase, improves liver histology, and decreases liver and plasma malondialdehyde MDA levels. As described above, the therapeutic effects of herbal medicines on metabolic diseases are closely related to their interaction with the gut microbiota. With the development of science and technology, multidisciplinary techniques and methods can be used to study the complex relationship between herbs, gut microbiota, and diseases. Next, we summarize and discuss some techniques and methods for studying the bidirectional interactions between herbal medicines and gut microbiota Figure 4. FIGURE 4. Methodology for studying the bidirectional interaction between herbal medicines and the gut microbiota. HM, herbal medicine. To determine the therapeutic effect of herbal medicines on metabolic diseases and the key role of the gut microbiota, four experimental groups were created: a control group, a model group, a herbal medicine group, and an herbal medicine plus antibiotic group. After the experiment, common biochemical indicators, gut microbiota, and microbial metabolites in each group were detected and analyzed. The 16S rRNA technique can be used to detect bacteria in samples based on polymerase chain reaction PCR amplification. The main challenges in using this technique are the lack of a standardized workflow and the difficulty in identifying bacteria at the species level. Metagenomics is a widely used technique that can identify microorganisms at the species and even strain level Jovel et al. In addition, it can also perform a functional analysis of microbial communities. However, methodological biases and inter-individual differences must be considered during data interpretation. Recently, advanced techniques have been developed to study the composition and function of gut microbes. For example, metatranscriptomics can provide knowledge of the transcriptional profiles of microbial populations, which is beneficial in revealing the molecular activities of gut microbes and their regulatory mechanisms Zhang et al. Similarly, metaproteomics is a powerful tool that can be used to study the functional activity of gut microbes by characterizing the complex composition of microbial proteins Stamboulian et al. Enzyme-linked immunosorbent assay ELISA , real-time fluorescent quantitative PCR, Western blot, and immunohistochemistry methods can be used to detect biochemical markers related to metabolic diseases e. Metabolomics can be used to determine changes in microbial metabolites after drug administration van Treuren and Dodd, For example, GC-MS technology can accurately measure the levels of SCFAs produced by gut microbial fermentation, while HPLC-QqQ-MS technology can accurately determine the concentration of BAs. These MSI techniques help us understand the effects of herbal medicines on gut microbial metabolites in two or three dimensions. Additionally, bioinformatic methods can be used to study the correlation between the pharmacodynamic effects of herbal medicines and changes in gut microbes and their metabolites. In addition to antibiotic interventions, fecal microbiota transplantation FMT can also be used to identify the critical role of the gut microbiota in the herbal treatment of metabolic diseases. After oral administration, herbal ingredients can be metabolized by gut microbes, and their metabolites are then absorbed into the circulation, producing pharmacological activity van Duynhoven et al. To determine whether the gut microbiota is involved in the metabolism of herbal ingredients, three experimental groups were created: a control group, a herbal medicine intervention group, and a herbal medicine intervention plus antibiotic group. After the experiment, animal biological samples, including feces or intestinal contents and serum, need to be collected. Feces or intestinal contents can be used to determine herbal metabolites after gut microbial transformation, while serum can be used to determine the absorption of these metabolites and whether bioavailability is improved. Due to the low levels of these metabolites in biological samples, high-sensitivity analytical instruments are needed for their detection. Ultra-high-performance liquid chromatography coupled with Orbitrap mass spectrometry UPLC-Orbitrap-MS and HPLC-QqQ-MS have been shown to accurately detect and identify metabolites of herbal phytochemicals in rat intestinal contents and serum samples Du et al. Furthermore, additional experimental validation is needed to determine whether the metabolites after gut microbial transformation have a better biological activity or lower toxicity than their precursors. In short, medium-pressure preparation liquid chromatography and high-speed countercurrent chromatography can be used for the targeted separation of specific metabolites. Then, cellular or animal experiments can be performed to compare the activity or toxicity of these metabolites with their precursors. Gut microbes and their metabolites have recently been implicated to be involved in the pathogenesis of metabolic diseases Fan and Pederson, ; Du et al. Consequently, the gut microbiota may be a potential target for herbal treatments of metabolic diseases. Several articles have summarized the association between gut microbiota and herbal medicines Xu et al. However, the critical role of gut microbiota in the herbal treatment of metabolic diseases has not been fully described. Therefore, this review provides a comprehensive and up-to-date summary of the relationship between herbal medicines and gut microbiota in metabolic diseases. There is accumulating evidence indicating the significant contribution of the gut microbiota to the herbal treatment of metabolic diseases. On the one hand, herbal medicines can improve metabolic diseases by increasing beneficial bacteria e. On the other hand, gut microbes can metabolize and transform herbal compounds via glycoside hydrolase, oxidase, and reductase. These transformations may make herbs more bioavailable and bioactive or less toxic and thus benefit the treatment of metabolic diseases. Despite advances in the research of herbal medicines and their effects on the gut microbiota, current studies are limited as they mostly rely on 16S rRNA sequencing technology to detect gut microbes, which has resulted in observations of the effects of herbal medicines on the gut microbiota at the family or genus level. Moreover, current studies on the metabolism of herbal compounds by gut microbes are limited to one or a small class of components, and further analysis of more chemical compositions is needed to gain a better understanding of the overall impact of gut microbiota on herbal medicines. Overall, the recent research progress in the interaction between herbal medicines and gut microbiota in metabolic diseases is encouraging. A comprehensive understanding of these interactions will help reveal the therapeutic mechanisms of herbal medicines. LiW and XG conducted the review and wrote the manuscript. YD, JL, YuW, and YaW searched and collated the references. JZ revised the manuscript. LD, WP, and GF conceived and designed the review. The authors gratefully acknowledge the financial support from the Key Research and Development Program of Sichuan Province No. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed, or endorsed by the publisher. An, X. The interaction between the gut Microbiota and herbal medicines. PubMed Abstract CrossRef Full Text Google Scholar. Ansari, A. CST, an herbal formula, exerts anti-obesity effects through brain-gut-adipose tissue axis modulation in high-fat diet fed mice. Molecules 21 11 , Bai, J. Response of gut microbiota and inflammatory status to bitter melon Momordica charantia L. in high fat diet induced obese rats. Bauermeister, A. Mass spectrometry-based metabolomics in microbiome investigations. Cai, J. Bile acid metabolism and signaling, the microbiota, and metabolic disease. Canfora, E. Short-chain fatty acids in control of body weight and insulin sensitivity. Cao, Y. JinQi Jiangtang tablet regulates gut microbiota and improve insulin sensitivity in type 2 diabetes mice. Diabetes Res. Carnevale, R. Gut-derived lipopolysaccharides increase post-prandial oxidative stress via Nox2 activation in patients with impaired fasting glucose tolerance: Effect of extra-virgin olive oil. Chang, C. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Next generation probiotics in disease amelioration. Food Drug Anal. Chen, F. Could the gut microbiota reconcile the oral bioavailability conundrum of traditional herbs. Chen, M. Huang-Lian-Jie-Du-Decoction ameliorates hyperglycemia and insulin resistant in association with gut microbiota modulation. Chen, P. Recent advances and perspectives on the health benefits of urolithin B, a bioactive natural product derived from ellagitannins. Chen, Y. The prevalence of gout in mainland China from to A systematic review and meta-analysis. Public Health 25 5 , — CrossRef Full Text Google Scholar. Christophersen, C. Overestimation of the abundance of sulfate-reducing bacteria in human feces by quantitative PCR targeting the Desulfovibrio 16S rRNA gene. Cui, H. A purified anthraquinone-glycoside preparation from Rhubarb ameliorates type 2 diabetes mellitus by modulating the gut microbiota and reducing inflammation. De Vadder, F. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell , 84— den Besten, G. Short-chain fatty acids protect against high-fat diet—induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes 64, — Du, H. Effect of gut microbiota on the metabolism of chemical constituents of Berberis kansuensis extract based on UHPLC-Orbitrap-MS technique. Planta Med. Effects of gut microbiota on five absorbed components of Berberis kansuensis in rat serum by HPLC-QqQ-MS. China J. Materia Medica 45, — Du, L. Gut microbiota-derived metabolites as key actors in type 2 diabetes mellitus. Eckel, R. The metabolic syndrome. Lancet , — Espin, J. Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. Food Chem. Biological significance of urolithins, the gut microbial ellagic acid-derived metabolites: The evidence so far. Based Complement. Everard, A. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Fan, Y. Gut microbiota in human metabolic health and disease. Fang, P. Baicalin and its aglycone: A novel approach for treatment of metabolic disorders. Feng, R. Transforming berberine into its intestine-absorbable form by the gut microbiota. Feng, W. Gut microbiota, a new frontier to understand traditional Chinese medicines. Gao, K. Effects of Qijian mixture on type 2 diabetes assessed by metabonomics, gut microbiota and network pharmacology. Guerville, M. Western-diet consumption induces alteration of barrier function mechanisms in the ileum that correlates with metabolic endotoxemia in rats. Han, R. Si Miao formula attenuates non-alcoholic fatty liver disease by modulating hepatic lipid metabolism and gut microbiota. Phytomedicine 85, Hossain, Z. Molecular mechanism of intestinal permeability: Interaction at tight junctions. Hotamisligil, G. Inflammation and metabolic disorders. Nature , — Huang, J. Gut microbiota protects from triptolide-induced hepatotoxicity: Key role of propionate and its downstream signalling events. Huang, R. Activation of AMPK by triptolide alleviates nonalcoholic fatty liver disease by improving hepatic lipid metabolism, inflammation, and fibrosis. Phytomedicine 92, Javdan, B. Personalized mapping of drug metabolism by the human gut microbiome. Cell 7 , — Jia, N. Amelioration of hepatic steatosis is associated with modulation of gut microbiota and suppression of hepatic miRa in Gynostemma pentaphylla Thunb. Makino treated mice. Lond 15, Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Jiang, S. Effects of compound K on hyperglycemia and insulin resistance in rats with type 2 diabetes mellitus. Fitoterapia 95, 58— Joh, E. Ginsenoside Rb1 and its metabolite compound K inhibit IRAK-1 activation--the key step of inflammation. Pharmaco l. Jovel, J. Characterization of the gut microbiome using 16S or shotgun metagenomics. Kawata, Y. DSpace at University of Toyama: Conversion of aconitine lipoaconitine by human intestinal bacteria and their antinociceptive effects in mice. Journal of traditional medicines. Google Scholar. Khana, T. Protective role of intestinal bacterial metabolism against baicalin-induced toxicity in HepG2 cell cultures. Koh, A. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 6 , — Kondo, T. Acetic acid upregulates the expression of genes for fatty acid oxidation enzymes in liver to suppress body fat accumulation. Larraufie, P. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Lee, H. Hepatoprotective effect of ginsenoside Rb1 and compound K on tert-butyl hydroperoxide-induced liver injury. Liver Int. Lei, S. Dendrobii Officinalis , a traditional Chinese edible and officinal plant, accelerates liver recovery by regulating the gut-liver axis in NAFLD mice. Foods 61, Leng, J. Amelioration of non-alcoholic steatohepatitis by Qushi Huayu decoction is associated with inhibition of the intestinal mitogen-activated protein kinase pathway. Phytomedicine 66, Li, L. Effects of Alisma orientale on the diversity of gut microbiota in rats fed on high-fat and high-sugar diet. Front Chin. Microecology 31 4. Li, N. Explore effect of Jieyu Qutan Huazhuo prescription on gut-liver axis of rats with high-fat diet based on 16S rDNA sequencing. Traditional Med. Formulae 27 9 , 77— Li, Q. Hypoglycemic mechanism of polysaccharide from Cyclocarya paliurus leaves in type 2 diabetic rats by gut microbiota and host metabolism alteration. China Life Sci. Li, W. Hypoglycemic effect of protopanaxadiol-type ginsenosides and compound K on Type 2 Diabetes mice induced by High-Fat Diet combining with Streptozotocin via suppression of hepatic gluconeogenesis. Fitoterapia 83, — Li, X. Triptolide: Progress on research in pharmacodynamics and toxicology. Penthorum chinense Pursh. extract attenuates non-alcholic fatty liver disease by regulating gut microbiota and bile acid metabolism in mice. Liang, S. The potential effect of Chinese herbal formula hongqijiangzhi fang in improving NAFLD: Focusing on NLRP3 inflammasome and gut microbiota. Liao, J. Jian-Gan-Xiao-Zhi decoction ameliorates nonalcoholic fatty liver disease through modulating gut microbiota, decreasing gut permeability, and alleviating liver inflammation. Liu, M. Lingguizhugan decoction attenuates diet-induced obesity and hepatosteatosis via gut microbiota. World J. Liu, X. Blautia -a new functional genus with potential probiotic properties. Gut Microbes 13, 1— Liu, Y. Gut microbial metabolites of aromatic amino acids as signals in host-microbe interplay. Trends Endocrinol. Liu, Z. A brief review on possible approaches towards controlling sulfate-reducing bacteria SRB in wastewater treatment systems. Desalination Water Treat. Lu, Y. Naoxintong capsule alternates gut microbiota and prevents hyperlipidemia in high-fat-diet fed rats. Luan, H. Mass spectrometry-based metabolomics: Targeting the crosstalk between gut microbiota and brain in neurodegenerative disorders. Mass Spectrom. Mandaliya, D. Short chain fatty acids, pancreatic dysfunction, and type 2 diabetes. Pancreatology 19 2 , — McGuckin, M. Intestinal barrier dysfunction in inflammatory bowel diseases. Bowel Dis. Mehta, N. Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes 59, — Meijers, B. Intestinal barrier function in chronic kidney disease. Toxins Basel 10, Mohammad, S. Role of metabolic endotoxemia in systemic inflammation and potential interventions. Morin, N. Tyramine stimulates glucose uptake in insulin-sensitive tissues in vitro and in vivo via its oxidation by amine oxidases. Neis, E. |
| 5 Herbs to Boost Metabolism and Lose Weight | Rose Wellness | Kiselev KV, Tyunin Reyulator, Manyakhin AY, Zhuravlev YN. Ergulator Genomics. Cell — Molecular Nut Spreads and Dips and characterization of PnbHLH1 transcription factor in Panax notoginseng. Clin Sci Lond — Ferulic acid stimulates adipocyte-specific secretory proteins to regulate adipose homeostasis in 3T3-L1 adipocytes. Jinbo Zhang. |

Herbal metabolism regulator -
You also burn calories digesting food, a process called diet-induced thermogenesis. Some companies sell products that supposedly boost your metabolism.
Most claim they do this through a process called thermogenesis, or increased heat production. This process stimulates energy use and can increase your metabolism and help burn calories. Most supplements that claim to raise your metabolism contain a combination of ingredients. Because these ingredients are almost always tested individually, we need to assess them on that basis.
Research has shown that caffeine can increase thermogenesis. According to a review article published in Obesity Reviews , six different studies have found that people burn more calories when they take a minimum daily dose of milligrams mg of caffeine.
To put that in perspective, most caffeine supplements contain mg of caffeine, while one cup of coffee contains about 95 mg. However, if you drink caffeine on a regular basis, this effect might be lessened.
Talk to your doctor before adding more caffeine to your diet. If you drink too many sweetened coffee drinks or chai tea, you could actually find yourself gaining weight!
Capsaicin is the chemical that puts the hot in jalapeños. In fact, a review of 20 research studies, published in Appetite , found that capsaicin can increase the amount of calories you burn by approximately 50 calories a day.
Those calories can add up over time, contributing to long-term weight loss. So consider spicing it up in your kitchen! L-carnitine is a substance that helps your body turn fat into energy. While your body produces it in your liver and kidneys, you can also find it in meat, dairy products, nuts, and legumes.
L-carnitine may be helpful for treating a number of conditions, including heart disease, peripheral artery disease, and diabetic neuropathy. But its use as a dietary supplement for weight loss is questionable.
One study reported in the Journal of Medicinal Food found that L-carnitine might provide some anti-obesity benefits. But more research is needed to assess the benefits and risks of taking L-carnitine supplements for weight loss.
According to the Office of Dietary Supplements , taking too much of it can cause potentially dangerous side effects. Chromium is a mineral that your body uses in small amounts.
Chromium picolinate supplements are useful for people who have a chromium deficiency. So far, researchers have given it a thumbs-down. A pilot study reported in the Journal of Alternative and Complementary Medicine found that chromium picolinate supplements had no effect on weight loss.
As with many supplements, research on CLA has found mixed results. A review of studies published in the European Journal of Nutrition found evidence that CLA may promote weight loss and fat loss, but the effects were small and uncertain.
Gastrointestinal problems and fatigue are common side effects of taking CLA supplements, so you may want to pass on this one. Numerous studies have been conducted on the effectiveness of green tea for weight loss.
Few have reported significant results. One study published in Physiology and Behavior does suggest that catechins and caffeine found in green tea may help support weight maintenance. Resveratrol is a substance found in the skin of red grapes, mulberries, Japanese knotweed, and peanuts. Article PubMed PubMed Central Google Scholar.
Cao YP, Li K, Li YL, Zhao XP, Wang LH. MYB transcription factors as regulators of secondary metabolism in plants. Cedar H, Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet. Chang CH, Liu ZW, Wang YY, Tang ZH, Yu F.
A bZIP transcription factor, CaLMF , mediated light-regulated camptothecin biosynthesis in Camptotheca acuminata. Tree Physiol. Chatel G, Montiel G, Pré M, Memelink J, Thiersault M, Saint-Pierre B, Doireau P, Gantet P.
CrMYC1 , a Catharanthus roseus elicitor- and jasmonate-responsive bHLH transcription factor that binds the G-box element of the strictosidine synthase gene promoter. J Exp Bot. Chen J, Zhou YH, Zhang Q, Liu Q, Li L, Sun CY, Wang KY, Wang YF, Zhao MZ, Li HJ, Han YL, Chen P, Li RQ, Lei J, Zhang MP, Wang Y.
PloS One. Chen MH, Yan TX, Shen Q, Lu X, Pan QF, Huang YR, Tang YL, Fu XQ, Liu M, Jiang WM, Lv ZY, Shi P, Ma YN, Hao XL, Zhang LD, Li L, Tang KX. GLANDULAR TRICHOME-SPECIFIC WRKY 1 promotes artemisinin biosynthesis in Artemisia annua.
New Phytol. Chen Y, Wang YT, Guo J, Yang J, Zhang XD, Wang ZX, Cheng Y, Du ZW, Qi ZC, Huang YB, Dennis M, Wei YK, Yang DF, Huang LQ, Liang ZS. Integrated transcriptomics and proteomics to reveal regulation mechanism and evolution of SmWRKY61 on tanshinone biosynthesis in Salvia miltiorrhiza and Salvia castanea.
Cheynier V, Comte G, Davies KM, Lattanzio V, Martens S. Plant phenolics: recent advances on their biosynthesis, genetics, and ecophysiology.
Plant Physiol Biochem. Chu Y, Xiao SM, Su H, Liao BS, Zhang JJ, Xu J, Chen SL. Genome-wide characterization and analysis of bHLH transcription factors in Panax ginseng. Acta Pharm Sin B.
Chuang YC, Hung YC, Tsai WC, Chen WH, Chen HH. PbbHLH4 regulates floral monoterpene biosynthesis in Phalaenopsis orchids. Deng B, Huang ZJ, Ge F, Liu DQ, Lu RJ, Chen CY. J Plant Growth Regul. Deng CP, Hao XL, Shi M, Fu R, Wang Y, Zhang Y, Zhou W, Feng Y, Makunga NP, Kai GY.
Tanshinone production could be increased by the expression of SmWRKY2 in Salvia miltiorrhiza hairy roots. Plant Sci. Deng CP, Shi M, Fu R, Zhang Y, Wang Q, Zhang Y, Wang Y, Ma XY, Kai GY.
ABA-responsive transcription factor bZIP1 is involved in modulating biosynthesis of phenolic acids and tanshinones in Salvia miltiorrhiza. Deng CP, Wang Y, Huang FF, Lu SJ, Zhao LM, Ma XY, Kai GY. SmMYB2 promotes salvianolic acid biosynthesis in the medicinal herb Salvia miltiorrhiza.
J Integr Plant Biol. Deng Y, Lu S. Biosynthesis and regulation of phenylpropanoids in plants. Crit Rev Plant Sci. Di P, Wang P, Yan M, Han P, Huang XY, Yin L, Yan Y, Xu YH, Wang YP. Genome-wide characterization and analysis of WRKY transcription factors in Panax ginseng.
BMC Genom. Dröge-Laser W, Snoek BL, Snel B, Weiste C. The Arabidopsis bZIP transcription factor family-an update. Curr Opin Plant Biol. Dröge-Laser W, Weiste C. Trends Plant Sci.
Du TZ, Niu JF, Su J, Li SS, Guo XR, Li L, Cao XY, Kang JF. SmbHLH37 functions antagonistically with SmMYC2 in regulating jasmonate-mediated biosynthesis of phenolic acids in Salvia miltiorrhiza. Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L. MYB transcription factors in Arabidopsis.
Dudareva N, Negre F, Nagegowda DA, Orlova I. Plant volatiles: recent advances and future perspectives. Dugé de Bernonville T, Maury S, Delaunay A, Daviaud C, Chaparro C, Tost J, O'Connor SE, Courdavault V.
Developmental methylome of the medicinal plant Catharanthus roseus unravels the tissue-specific control of the monoterpene indole alkaloid pathway by DNA methylation. Int J Mol Sci. Elomaa P, Mehto M, Kotilainen M, Helariutta Y, Nevalainen L, Teeri TH.
A bHLH transcription factor mediates organ, region and flower type specific signals on dihydroflavonolreductase dfr gene expression in the inflorescence of Gerbera hybrida Asteraceae.
Ernst HA, Olsen AN, Larsen S, Lo Leggio L. Structure of the conserved domain of ANAC, a member of the NAC family of transcription factors.
EMBO Rep. Eulgem T, Rushton PJ, Robatzek S, Somssich IE. The WRKY superfamily of plant transcription factors. Fan D, Wang XQ, Tang XF, Ye X, Ren S, Wang DH, Luo KM. Histone H3K9 demethylase JMJ25 epigenetically modulates anthocyanin biosynthesis in poplar.
Fan RY, Li YJ, Li CF, Zhang YS. Differential microRNA analysis of glandular trichomes and young leaves in Xanthium strumarium L. reveals their putative roles in regulating terpenoid biosynthesis. PLoS One. Feng K, Hou XL, Xing GM, Liu JX, Duan AQ, Xu ZS, Li MY, Zhuang J, Xiong AS.
Crit Rev Biotechnol. Fricke J, Hillebrand A, Twyman RM, Prüfer D, Schulze Gronover C. Abscisic acid-dependent regulation of Small Rubber Particle Protein gene expression in Taraxacum brevicorniculatum is mediated by TbbZIP1. Plant Cell Physiol. Fu XQ, Peng BW, Hassani D, Xie LH, Liu H, Li YP, Chen TT, Liu P, Tang YL, Li L, Zhao JY, Sun XF, Tang KX.
AaWRKY9 contributes to light- and jasmonate-mediated to regulate the biosynthesis of artemisinin in Artemisia annua.
Galanie S, Thodey K, Trenchard IJ, Filsinger Interrante M, Smolke CD. Complete biosynthesis of opioids in yeast. Gao QQ, Song WL, Li X, Xiang CF, Chen G, Xiang GS, Liu XY, Zhang GH, Li XN, Yang SC, Zhai CX, Zhao Y. Genome-wide identification of bHLH transcription factors: Discovery of a candidate regulator related to flavonoid biosynthesis in Erigeron breviscapus.
Gani U, Vishwakarma RA, Misra P. Membrane transporters: the key drivers of transport of secondary metabolites in plants. Plant Cell Rep. Gao Z, Li J, Luo M, Li H, Chen QJ, Wang L, Song SR, Zhao LP, Xu WP, Zhang CX, Wang SP, Ma C.
Characterization and cloning of grape circular RNAs identified the cold resistance-related Vv-circATS1. Gehring M, Bubb KL, Henikoff S. Extensive demethylation of repetitive elements during seed development underlies gene imprinting.
Ghasemzadeh A, Jaafar HZ. Effect of CO 2 enrichment on synthesis of some primary and secondary metabolites in ginger Zingiber officinale Roscoe. Gong ZZ, Yamagishi E, Yamazaki M, Saito K. A constitutively expressed Myc-like gene involved in anthocyanin biosynthesis from Perilla frutescens : molecular characterization, heterologous expression in transgenic plants and transactivation in yeast cells.
Plant Mol Biol. Gonzalez A, Zhao M, Leavitt JM, Lloyd AM. Goodrich J, Carpenter R, Coen ES. A common gene regulates pigmentation pattern in diverse plant species.
Goossens J, Mertens J, Goossens A. Role and functioning of bHLH transcription factors in jasmonate signalling. Han J, Liu HT, Wang SC, Wang CR, Miao GP. A class I TGA transcription factor from Tripterygium wilfordii Hook.
modulates the biosynthesis of secondary metabolites in both native and heterologous hosts. Han YL, Cai MH, Zhang SQ, Chai JW, Sun MZ, Wang YW, Xie QY, Chen YH, Wang HZ, Chen T.
Hao DC, Xiao PG. Deep in shadows: Epigenetic and epigenomic regulations of medicinal plants. Chinese Herb Med. Hao MZ, Zhou YH, Zhou JH, Zhang M, Yan KJ, Jiang S, Wang WS, Peng XP, Zhou S. Cold-induced ginsenosides accumulation is associated with the alteration in DNA methylation and relative gene expression in perennial American ginseng Panax quinquefolius L.
along with its plant growth and development process. J Ginseng Res. Hao XL, Wang C, Zhou W, Ruan QY, Xie CH, Yang YK, Xiao CY, Cai Y, Wang JY, Wang Y, Zhang XB, Maoz I, Kai GY. OpNAC1 transcription factor regulates the biosynthesis of the anticancer drug camptothecin by targeting loganic acid O-methyltransferase in Ophiorrhiza pumila.
Hao XL, Zhong YJ, Nï Tzmann HW, Fu XQ, Yan TX, Shen Q, Chen MH, Ma YN, Zhao JY, LiL, Tang KX. Light-induced artemisinin biosynthesis is regulated by the bZIP transcription factor AaHY5 in Artemisia annua.
Hassan B. Medicinal plants importance and uses. Pharmaceutica Anal Acta. He XJ, Chen T, Zhu JK. Regulation and function of DNA methylation in plants and animals. Cell Res. Heim MA, Jakoby M, Werber M, Martin C, Weisshaar B, Bailey PC.
The Basic Helix-Loop-Helix transcription factor family in plants: A genome-wide study of protein structure and functional diversity. Mol Biol Evol. Heithoff DM, Sinsheimer RL, Low DA, Mahan MJ.
An essential role for DNA adenine methylation in bacterial virulence. Hossain MA, Cho JI, Han M, Ahn CH, Jeon JS, An GH, Park PB. The ABRE-binding bZIP transcription factor OsABF2 is a positive regulator of abiotic stress and ABA signaling in rice.
J Plant Physiol. Huang D, Ming RH, Xu SQ, Yang SC, Li LB, Huang RS, Tan Y. Genome-wide identification of R2R3-MYB transcription factors: Discovery of a "Dual-Function" regulator of gypenoside and flavonol biosynthesis in Gynostemma pentaphyllum.
Huang HZ, Xing SH, Tang KX, Jiang WM. AaWRKY4 upregulates artemisinin content through boosting the expressions of key enzymes in artemisinin biosynthetic pathway.
Plant Cell Tiss Organ Cult. Huang WJ, Khaldun AB, Chen JJ, Zhang CJ, Lv HY, Yuan L, Wang Y. A R2R3-MYB transcription factor regulates the flavonol biosynthetic pathway in a traditional Chinese medicinal plant, Epimedium sagittatum.
Huang WJ, Khaldun AB, Lv HY, Du LW, Zhang CJ, Wang Y. Isolation and functional characterization of a R2R3-MYB regulator of the anthocyanin biosynthetic pathway from Epimedium sagittatum.
Huang WY, Lv HY, Wang Y. Functional characterization of a novel R2R3-MYB transcription factor modulating the flavonoid biosynthetic pathway from Epimedium sagittatum.
Huang WY, Sun W, Lv HY, Luo M, Zeng SH, Pattanaik S, Yuan L, Wang Y. A R2R3-MYB transcription factor from Epimedium sagittatum regulates the flavonoid biosynthetic pathway.
Ishiguro S, Nakamura K. Mol Gen Genet. Ito H, Gaubert H, Bucher E, Mirouze M, Vaillant I, Paszkowski J. An siRNA pathway prevents transgenerational retrotransposition in plants subjected to stress. Jamwal K, Bhattacharya S, Puri S. Plant growth regulator mediated consequences of secondary metabolites in medicinal plants.
J Appl Res Med Aroma. Jan R, Asaf S, Numan M, Lubna, Kim KM. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Jarvis D, Ho YS, Lightfoot D, Schmöckel S, Li B, Borm TA, Ohyanagi H, Mineta K, Michell C, Saber N, Kharbatia N, Rupper R, Sharp A, Dally N, Boughton B, Woo Y, Gao G, Schijlen E, Guo XJ, Momin A, Negrão S, Al-Babili S, Gehring C, Roessner U, Tester M.
The genome of Chenopodium quinoa. Ji AJ, Luo HM, Xu ZC, Zhang X, Zhu YJ, Liao BS, Yao H, Song JY, Chen SL. Plant Genome. Ji YP, Xiao JW, Shen YL, Ma DM, Li ZQ, Pu GB, Li X, Huang LL, Liu BY, Ye HC, Wang H. Cloning and characterization of AabHLH1 , a bHLH transcription factor that positively regulates artemisinin biosynthesis in Artemisia annua.
Plant cell physiol. Jia YY, Bai ZQ, Pei TL, Ding K, Liang ZS, Gong YH. The Protein kinase SmSnRK2. Jiang JJ, Ma SH, Ye NH, Jiang M, Cao JS, Zhang JH. WRKY transcription factors in plant responses to stresses. Jiang M, Chen H, Du Q, Wang L, Liu X, Liu C. Genome-wide identification of circular RNAs potentially involved in the biosynthesis of secondary metabolites in Salvia miltiorrhiza.
Front in Genet. Kavas M, Baloğlu MC, Atabay ES, Ziplar UT, Daşgan HY, Ünver T. Genome-wide characterization and expression analysis of common bean bHLH transcription factors in response to excess salt concentration. Mol Genet Genomics. Kim J, Kang SH, Park SG, Yang TJ, Lee Y, Kim OT, Chung O, Lee J, Choi JP, Kwon SJ, Lee K, Ahn BO, Lee DJ, Yoo SI, Shin IG, Um Y, Lee DY, Kim GS, Hong CP, Bhak J, Kim CK.
Whole-genome, transcriptome, and methylome analyses provide insights into the evolution of platycoside biosynthesis in Platycodon grandiflorus , a medicinal plant. Hortic Res. Kiselev KV, Tyunin AP, Manyakhin AY, Zhuravlev YN. Resveratrol content and expression patterns of stilbene synthase genes in Vitis amurensis cells treated with 5-azacytidine.
Kooke R, Morgado L, Becker F, Eekelen H, Hazarika R, Zheng QF, de Vos R, Johannes F, Keurentjes J. Epigenetic mapping of the Arabidopsis metabolome reveals mediators of the epigenotype-phenotype map. Genome Res. Kumar P, Padhan JK, Kumar A, Chauhan RS.
Transcriptomes of Podophyllum hexandrum unravel candidate miRNAs and their association with the biosynthesis of secondary metabolites. J Plant Biochem and Biotechnol. Kumar S, Singh AK, Mohapatra T.
Epigenetics: history, present status and future perspective. Indian J Genet Plant Breed. Lenka SK, Nims NE, Vongpaseuth K, Boshar RA, Roberts SC, Walker EL. Jasmonate-responsive expression of paclitaxel biosynthesis genes in Taxus cuspidata cultured cells is negatively regulated by the bHLH transcription factors TcJAMYC1, TcJAMYC2, and TcJAMYC4.
Leung J, Gaudin V. Who Rules the Cell? An Epi-Tale of histone, DNA, RNA, and the metabolic deep state. Li CY, Leopold AL, Sander GW, Shanks JV, Zhao L, Gibson SI. CrBPF1 overexpression alters transcript levels of terpenoid indole alkaloid biosynthetic and regulatory genes. Li DQ, Shao FJ, Lu SF.
Identification and characterization of mRNA-like noncoding RNAs in Salvia miltiorrhiza. Li J, Li CL, Lu SF.
Identification and characterization of the cytosine-5 DNA methyltransferase gene family in Salvia Miltiorrhiza. Peer J. Li M, Sun L, Gu H, Cheng DW, Guo XZ, Chen R, Wu ZY, Jiang JF, Fan XC, Chen JY.
Genome-wide characterization and analysis of bHLH transcription factors related to anthocyanin biosynthesis in spine grapes Vitis davidii. Li MR, Shi FX, Li YL, Jiang P, Jiao L, Liu B, Li LF. Genome-wide variation patterns uncover the origin and selection in cultivated ginseng Panax ginseng Meyer.
Genome Biol Evol. Li MY, Liu JX, Hao JN, Feng K, Duan AQ, Yang QQ, Xu ZS, Xiong AS. Li WF, Ning GX, Mao J, et al. Whole-genome DNA methylation patterns and complex associations with gene expression associated with anthocyanin biosynthesis in apple fruit skin.
Li XX, Duan XP, Jiang HX, Sun YJ, Tang YP, Yuan Z, Guo JK, Liang WQ, Chen L, Yin JY, Ma H, Wang J, Zhang DB. Li Y, Liu Y, Qi F, Deng C, Lu C, Huang H, Dai S. Establishment of virus-induced gene silencing system and functional analysis of ScbHLH17 in Senecio cruentus. Li YL, Chen XL, Wang JQ, Zou GP, Wang L, Li XS.
Two responses to MeJA induction of R2R3-MYB transcription factors regulate flavonoid accumulation in Glycyrrhiza uralensis Fisch.
Licausi F, Ohme-Takagi M, Perata P. Liu CC, Chi C, Jin LJ, Zhu J, Yu JQ, Zhou YH. The bZip transcription factor HY5 mediates CRYɑ -induced anthocyanin biosynthesis in tomato. Plant Cell Environ. Liu DQ, Zhao Q, Cui XM, Chen R, Li X, Qiu BL, Ge F. A transcriptome analysis uncovers Panax notoginseng resistance to Fusarium solani induced by methyl jasmonate.
Genes Genomics. Liu J, Osbourn A, Ma P. MYB transcription factors as regulators of phenylpropanoid metabolism in plants. Liu JQ, Gao FY. Ren JS, Lu XJ, Ren GJ, Wang R.
Liu L, Yang DF, Xing BC, Zhang CL, Lang ZS. SmMYB98b positive regulation to tanshinones in Salvia miltiorrhiza Bunge hairy roots. Liu T, Luo T, Guo XQ, Zhou X, Zhou DH, Gui L, Zhang Y, Zhang R, Luo ZY. PgMYB2 , a MeJA-responsive transcription factor, positively regulates the dammarenediol synthase gene expression in Panax Ginseng.
Liu S, Wang Y, Shi M, et al. SmbHLH60 and SmMYC2 antagonistically regulate phenolic acids and anthocyanins biosynthesis in Salvia miltiorrhiza. J Adv Res.
Liu WY, Chiou SJ, Ko CY, Lin TY. Functional characterization of three ethylene response factor genes from Bupleurum kaoi indicates that BkERFs mediate resistance to Botrytis cinerea. Liu X, Yang S, Yu CW, Chen CY, Wu K.
Chapter Six - Histone acetylation and plant development. The Enzymes. Liu XC, Yang SG, Zhao ML, Luo M, Yu CW, Chen CY, Ready Tai, Wu KQ.
Transcriptional repression by histone deacetylases in plants. Liu YY, Zhu PP, Cai S, Haughn G, Page JE. Three novel transcription factors involved in cannabinoid biosynthesis in Cannabis sativa L. Complete biosynthesis of cannabinoids and their unnatural analogues in yeast.
Lv ZY, Guo ZY, Zhang LD, Zhang FY, Jiang WM, Shen Q, Fu XQ, Yan TX, Shi P, Hao XL, Ma YA, Chen MH, Li L, Zhang L, Chen WS, Tang KX. Interaction of bZIP transcription factor TGA6 with salicylic acid signaling modulates artemisinin biosynthesis in Artemisia annua.
Lv ZY, Wang S, Zhang FY, Chen LX, Hao XL, Pan QF, Fu XQ, Li L, Sun XF, Tang KX. Overexpression of a novel NAC domain-containing transcription factor gene AaNAC1 enhances the content of artemisinin and increases tolerance to drought and Botrytis cinerea in Artemisia annua.
Lv Z, Li J, Qiu S, Qi F, Su H, Bu Q, Jiang R, Tang K, Zhang L, Chen W. The transcription factors TLR1 and TLR2 negatively regulate trichome density and artemisinin levels in Artemisia annua.
Ma DM, Pu GB, Lei CY, Ma LQ, Wang HH, Guo YW, Chen JL, Du ZG, Wang H, Li GF, Ye HC, Liu BY. Isolation and characterization of AaWRKY1, an Artemisia annua transcription factor that regulates the amorpha-4,diene synthase gene, a key gene of artemisinin biosynthesis. Ma RF, Xiao Y, Lv ZY, Tan HX, Chen RB, Li Q, Chen JF, Wang Y, Yin J, Zhang L, Chen WS.
Ma Y, Li D, Zhong Y, Wang X, Li L, Osbourn A, Lucas WJ, Huang SW, Shang Y. Mao K, Dong QL, Li C, Liu CH, Ma FW. Genome Wide identification and characterization of Apple bHLH transcription factors and expression analysis in response to drought and salt stress.
Martienssen RA, Colot V. DNA methylation and epigenetic inheritance in plants and filamentous fungi. Martin C, Paz-Ares J.
MYB transcription factors in plants. Trends Genet. Mehrtens F, Kranz H, Bednarek P, Weisshaar B. The Arabidopsis transcription factor MYB12 is a flavonol-specific regulator of phenylpropanoid biosynthesis.
Menke FL, Champion A, Kijne JW, Memelink J. A novel jasmonate- and elicitor-responsive element in the periwinkle secondary metabolite biosynthetic gene Str interacts with a jasmonate- and elicitor-inducible AP2-domain transcription factor, ORCA2.
EMBO J. Mertens J, Pollier J, Vanden Bossche R, Lopez-Vidriero I, Franco-Zorrilla JM, Goossens A. The bHLH transcription factors TSAR1 and TSAR2 regulate triterpene ssaponin biosynthesis in Medicago truncatula. Meyer P. Epigenetics - A historical perspective. Adv Bot Res. McElroy C, Jennewein S, Schwab W, Lange BM, Wüst M.
Taxol ® biosynthesis and production: from forests to fermenters. Biotechnology of natural products. Morita Y, Saito R, Ban Y, Tanikawa N, Kuchitsu K, Ando T, Yoshikawa M, Habu Y, Ozeki Y, Nakayama M.
Tandemly arranged chalcone synthase A genes contribute to the spatially regulated expression of siRNA and the natural bicolor floral phenotype in Petunia hybrida.
Morreel K, Saeys Y, Dima O, Lu F, Van de Peer Y, Vanholme R, Ralph J, Vanholme B, Boerjan W. Systematic structural characterization of metabolites in Arabidopsis via candidate substrate-product pair networks. Nagegowda DA. Plant volatile terpenoid metabolism: biosynthetic genes, transcriptional regulation and subcellular compartmentation.
FEBS Lett. Najafabadi AS, Naghavi MR. Mining Ferula gummosa transcriptome to identify miRNAs involved in the regulation and biosynthesis of terpenes. Nakatsuka T, Haruta KS, Pitaksutheepong C, Abe Y, Kakizaki Y, Yamamoto K, Shimada N, Yamamura S, Nishihara M.
Identification and characterization of R2R3-MYB and bHLH transcription factors regulating anthocyanin biosynthesis in gentian flowers. Navarro M, Marque G, Ayax C, Keller G, Borges JP, Marque C, Teulières C. Complementary regulation of four Eucalyptus CBF genes under various cold conditions.
Nijhawan A, Jain M, Tyagi AK, Khurana JP. Genomic survey and gene expression analysis of the basic Leucine Zipper transcription factor family in Rice. Nims E, Vongpaseuth K, Roberts SC, Walker EL. TcJAMYC: a bHLH transcription factor that activates paclitaxel biosynthetic pathway genes in yew.
J Biol Chem. Ning K, Li MZ, Wei GF, Zhou YX, Zhang GZ, Huai H, Wei FG, Chen ZJ, Wang Y, Dong LL, Chen SL. Genomic and transcriptomic analysis provide insights into Root Rot resistance in Panax notoginseng. Ohno S, Hosokawa M, Hoshino A, Kitamura Y, Morita Y, Park KI, Nakashima A, Deguchi A, Tatsuzawa F, Doi M, Iida S, Yazawa S.
A bHLH transcription factor, DvIVS , is involved in regulation of anthocyanin synthesis in dahlia Dahlia variabilis. Paddon CJ, Westfall PJ, Pitera DJ, Benjamin K, Fisher K, McPhee D, Leavell MD, Tai A, Main A, Eng D, Polichuk DR.
High-level semi-synthetic production of the potent antimalarial artemisinin. Pagliarani C, Gambino G, Ferrandino A, Chitarra W, Vrhovsek U, Cantu D, Palmano S, Marzachì C, Schubert A. Molecular memory of Flavescence dorée phytoplasma in recovering grapevines.
Pan QF, Wang CY, Xiong ZW, Wang H, Fu XQ, Shen Q, Peng BW, Ma YN, Sun XF, Tang KX. Pani A, Mahapatra RK. Computational identification of microRNAs and their targets in Catharanthus roseus expressed sequence tags.
Genomics Data. Park KI, Ishikawa N, Morita Y, Choi JD, Hoshino A, Iida S. A bHLH regulatory gene in the common morning glory, Ipomoea purpurea , controls anthocyanin biosynthesis in flowers, proanthocyanidin and phytomelanin pigmentation in seeds, and seed trichome formation.
Park KI. A bHLH protein partially controls proanthocyanidin and phytomelanin pigmentation in the seed coats of morning glory Ipomoea tricolor. Hortic Environ Biotechnol. Paz-Ares J, Ghosal D, Wienand U, Peterson PA, Saedler H. The regulatory c1 locus of Zea mays encodes a protein with homology to myb proto-oncogene products and with structural similarities to transcriptional activators.
Peng Z, Tian J, Luo RL, Kang YH, Lu YF, Hu YJ, Liu N, Zhang J, Cheng H, Niu SQ, Zhang J, Yao YC. MiRd and epigenetic modification comodulate anthocyanin accumulation in Malus leaves suffering from phosphorus deficiency. Phukan UJ, Jeena GS, Tripathi V, Shukla RK. MaRAP, a waterlogging-responsive ERF from Mentha , regulates bidirectional sugar transporter AtSWEET10 to modulate stress response in Arabidopsis.
Prakash P, Rajakani R, Gupta V. Transcriptome-wide identification of Rauvolfia serpentina microRNAs and prediction of their potential targets. Comput Biol Chem. Přibylová A, Čermák V, Tyč D, Fischer L. Detailed insight into the dynamics of the initial phases of de novo RNA-directed DNA methylation in plant cells.
Epigenetics chromatin. Quattrocchio F, Wing JF, Leppen H, Mol J, Koes RE. Regulatory genes controlling anthocyanin pigmentation are functionally conserved among plant species and have distinct sets of target genes. Quattrocchio F, Wing JF, van der Woude K, Mol JN, Koes R. Analysis of bHLH and MYB domain proteins: species-specific regulatory differences are caused by divergent evolution of target anthocyanin genes.
Ribeiro B, Erffelinck ML, Lacchini E, Ceulemans E, Colinas M, Williams C, Van Hamme E, De Clercq R, Perassolo M, Goossens A. Interference between ER stress-related bZIP-type and jasmonate-inducible bHLH-type transcription factors in the regulation of triterpene saponin biosynthesis in Medicago truncatula.
Ribeiro B, Lacchini E, Bicalho KU, Mertens J, Arendt P, Vanden Bossche R, Calegario G, Gryffroy L, Ceulemans E, Buitink J, Goossens A, Pollier J. A seed-specific regulator of triterpene saponin biosynthesis in Medicago truncatula.
Rodriguez-Concepcion M, Boronat A. Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids.
A metabolic milestone achieved through genomics. Rohani ER, Chiba M, Kawaharada M, Asano T, Oshima Y, Mitsuda N, Ohme-Takagi M, Fukushima A, Rai A, Saito K, Yamazaki M. An MYB transcription factor regulating specialized metabolisms in Ophiorrhiza pumila.
Plant Biotechnol. Rushton PJ, Somssich IE, Ringler P, Shen QJ. Saifi M, Nasrullah N, Ahmad MM, Ali A, Khan JA, Abdin MZ. In silico analysis and expression profiling of miRNAs targeting genes of steviol glycosides biosynthetic pathway and their relationship with steviol glycosides content in different tissues of Stevia rebaudiana.
Sanchez-Muñoz R, Moyano E, Khojasteh A, Bonfill M, Cusido RM, Palazon J. Genomic methylation in plant cell cultures: A barrier to the development of commercial long-term biofactories. Eng Life Sci. Sanchita, Sharma A. Gene expression analysis in medicinal plants under abiotic stress conditions.
Plant metabolites and regulation under environmental stress. Finally, the contributions of herbal ingredients in their anti-human cancer activity through modulation of the immune microenvironment and metabolic reprogramming were quantified. To demonstrate the power of the developed method, in vitro experiments were performed with two representative cancers.
ISL was identified to specifically target the T cells in STAD, while CH was identified to specifically target arachidonic acid metabolism and retinoic acid metabolism in LGG. Compared with existing approaches like LINCS [ 83 , 84 ], our computational model has obvious differences and advantages.
The prominent difference between our present work and LINCS is that COIMMR could reveal the contribution of herbal ingredients against human cancer via specific pathways or biological function. It is also the first computational model to quantify the contribution of specific pathways to the OCE for herbal ingredients, which can fill the gap of LINCS Secondly, datasets used in our computational model were all herbal ingredients from Traditional Chinese Medicine TCM , and LINCS contains very limited herbal ingredients [ 85 ].
Hence, our computational model pays more attention on the herbal ingredients space and could makes up for the limited herbal ingredients in LINCS Thirdly, LINCS cannot screen drugs targeting specific pathways, while our method can screen herbal ingredients targeting specific biological functions [ 86 ].
However, LINCS project only measured the expression level of genes [ 87 ]. Hence, we are more reliable in terms of the number of genes sequenced and the quality of the data. Overall, COIMMR has its unique contributions for drug discovery, especially for herbal ingredients, which could promote the intelligent development of TCM.
For our model application and future drug development, one of the advantages is that it could rapidly analyze vast datasets, swiftly screen candidate compounds from herbal ingredients for drug development and improve the efficiency of translational drug discovery, which greatly reduces both the time and financial resources.
In addition, it may facilitate the development of personalized medicine by targeting patient-specific pathways. This enables the design of specific drugs based on an individual's genetic makeup, improving efficacy and minimizing side effects.
Furthermore, it could help researchers uncover intricate relationships, facilitating the discovery of new drug targets and mechanisms. However, this study has some limitations. The expression profiling data of ITCM are based on the MCF-7 cell line. Hence, we encourage users to use cancer-specific drug expression data in the future studies.
In addition, we also encourage users to build the customized specific signatures with professional knowledge and combine the analysis results with those obtained from COIMMR to arrive at the most appropriate conclusion.
In summary, this computational strategy can be applied to development of drug targeting specific biological pathways for various diseases using the gene expression profile data.
The findings of this study will aid in the modernization of TCM. We constructed the first computational framework, COIMMR, which reveals the contribution of herbal ingredients against human cancer via immune microenvironment and metabolic reprogramming.
By using COIMMR algorithm, we found that most herbal ingredients exerted a higher modulatory effect on the immune microenvironment than on metabolic reprogramming for their therapeutic effects on human cancer, which was first revealed by this study. By applying COIMMR algorithm to two case studies to demonstrate its strong power, we identified ISL that specifically regulates the T cells in STAD and CH that specifically targets metabolic reprogramming in LGG.
The in silico results were verified using in vitro experiments. National Key Research and Development Program of China YFC ; National Natural Science Foundation of China and ; Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine ZYYCXTD-D ; Shanghai Sailing Program 20YF ; Shanghai Frontiers Science Center of TCM Chemical Biology, Shanghai Municipal Health Commission Project Y ; Three-year Action Plan for Shanghai TCM Development and Inheritance Program ZY — ; Wild Goose Array Project, Zhengzhou Center of PLAJLSF.
We also thank the Home for Researchers editorial team www. com for the English check of this manuscript and Xiaoqi Wu Genergy Biotechnology Shanghai Co.
Figures were created with biorender. The data of our work can be acquired from the Supplementary Materials uploaded with this article. and S. designed the study.
and J. collected and analyzed the data. and X. performed the experiment. wrote the manuscript. revised the manuscript. Saisai Tian is a lecturer at School of Pharmacy, Ningxia Medical University. He has also served as alecturer at School of Pharmacy, Second Military Medical University, and his research interest covers bioinformatics, cancer biology, and network pharmacy.
Yanan Li is a postgraduate student of Ningxia Medical University, and her research interest covers cancer biology and network pharmacy. Jia Xu is a postgraduate student of Henan University, and her research interest covers bioinformatics and network pharmacy.
Lijun Zhang is an associate professor at the Institute of Interdisciplinary Integrative Medicine Research, Shanghai University of Traditional Chinese Medicine, and her research interest is anti-tumor mechanism of traditional Chinese medicine.
Jinbo Zhang is an MPhil at the School of Pharmacy, Second Military Medical University and served as a pharmacist of Department of Pharmacy, Tianjin Rehabilitation Center of Joint Logistics Support Force.
His research interest covers network pharmacy and natural products. Jinyuan Lu is a postgraduate student at the School of Pharmacy, Anhui University of Chinese Medicine, and his research interest covers network pharmacy.
Xike Xu is a professor at the School of Pharmacy, Second Military Medical University, and his research interest covers network pharmacy. Xin Luan is the director of Systems Pharmacology ResearchCenter of TCM, Institute of Interdisciplinary Integrative Medicine Research, Shanghai University of Traditional Chinese Medicine.
His current research focuses on the discovery and novel application of anti-cancer compounds from traditional Chinese medicine. Jing Zhao is a professor at the Institute of Interdisciplinary Integrative Medicine Research, Shanghai University of Traditional Chinese Medicine.
Her current research focus on bioinformatics, cancer biology and network pharmacy. Weidong Zhang is a professor at Ningxia Medical University, Second Military Medical University' Shanghai University of Traditional Chinese Medicine and Institute of Medicinal Plant DevelopmentChinese Academy of Medical Sciences and Peking Union Medical College.
His major research interest areas are pharmacodynamic substance basis research of natural products and medicinal chemistry. Bray F , Ferlay J , Soerjomataram I , et al. Global cancer statistics GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in countries.
CA Cancer J Clin ; 68 : — Google Scholar. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics Chin Med J Engl ; : — Stoll EA , Horner PJ , Rostomily RC.
The impact of age on oncogenic potential: tumor-initiating cells and the brain microenvironment. Aging Cell ; 12 : — Ohshima K , Morii E. Metabolic reprogramming of cancer cells during tumor progression and metastasis.
Metabolites ; 11 : Chu JJ , Mehrzad R. The biology of cancer. In: Mehrzad R, ed. The Link between Obesity and Cancer. Academic Press: Cambridge, UK, , pp. Pavlova NN , Thompson CB. The emerging hallmarks of cancer metabolism.
Cell Metab ; 23 : 27 — Parvez MK , Rishi V. Herb-drug interactions and hepatotoxicity. Curr Drug Metab ; 20 : — Wang W-Y , Zhou H , Wang Y-F , et al.
Current policies and measures on the development of traditional Chinese medicine in China. Pharmacol Res ; : Niedzwiecki A , Roomi MW , Kalinovsky T , Rath M. Anticancer efficacy of polyphenols and their combinations.
Nutrients ; 8 : Kasi PD , Tamilselvam R , Skalicka-Woźniak K , et al. Molecular targets of curcumin for cancer therapy: an updated review. Tumor Biol ; 37 : — The signaling pathways and targets of traditional Chinese medicine and natural medicine in triple-negative breast cancer.
J Ethnopharmacol ; : Muhammad N , Usmani D , Tarique M , et al. The role of natural products and their multitargeted approach to treat solid cancer. Cell ; 11 : The application of traditional Chinese medicine against the tumor immune escape. J Transl Intern Med ; 8 : — 4. Homoharringtonine induced immune alteration for an efficient anti-tumor response in mouse models of non-small cell lung adenocarcinoma expressing Kras mutation.
Sci Rep ; 8 : Metabolic reprogramming by traditional Chinese medicine and its role in effective cancer therapy. BMC Complement Altern Med ; 17 : Norcantharidin combined with Coix seed oil synergistically induces apoptosis and inhibits hepatocellular carcinoma growth by downregulating regulatory T cells accumulation.
Sci Rep ; 7 : Chlorogenic acid and caffeic acid from Sonchus oleraceus Linn synergistically attenuate insulin resistance and modulate glucose uptake in HepG2 cells.
Food Chem Toxicol ; : — 7. Lee M-S , Lee S-O , Kim K-R , Lee HJ. Sphingosine kinase-1 involves the inhibitory action of HIF-1α by chlorogenic acid in hypoxic DU cells.
Int J Mol Sci ; 18 : Park JJ , Hwang SJ , Park J-H , Lee HJ. Cell Oncol Dordr ; 38 : — 8. Lahlou M. The success of natural products in. Drug Discovery ; 04 : 17 — Baldi A. Computational approaches for drug design and discovery: an overview.
Syst Rev Pharm ; 1 : Bediaga H , Arrasate S , González-Díaz H. PTML combinatorial model of ChEMBL compounds assays for multiple types of cancer. ACS Comb Sci ; 20 : — Karolak A , Rejniak KA. Micropharmacology: an in silico approach for assessing drug efficacy within a tumor tissue.
Bull Math Biol ; 81 : — Sirci F , Napolitano F , di Bernardo D. Computational drug networks: a computational approach to elucidate drug mode of action and to facilitate drug repositioning for neurodegenerative diseases. Drug Discov Today Dis Model ; 19 : 11 — 7. Speck-Planche A , Cordeiro DS , MN.
Speeding up early drug discovery in antiviral research: a fragment-based in silico approach for the design of virtual anti-hepatitis C leads. ACS Comb Sci ; 19 : — Tumor immunological phenotype signature-based high-throughput screening for the discovery of combination immunotherapy compounds.
Sci Adv ; 7 : eabd Exploring pharmacological active ingredients of traditional Chinese medicine by pharmacotranscriptomic map in ITCM. Brief Bioinform ; 24 : bbad Network pharmacology based investigation of the effects of herbal ingredients on the immune dysfunction in heart disease.
Pharmacol Res ; : — Kidd BA , Wroblewska A , Boland MR , et al. Mapping the effects of drugs on the immune system. Nat Biotechnol ; 34 : 47 — Rosario SR , Long MD , Affronti HC , et al.
Pan-cancer analysis of transcriptional metabolic dysregulation using The Cancer Genome Atlas. Nat Commun ; 9 : Goh K-I , Cusick ME , Valle D , et al.
The human disease network. Proc Natl Acad Sci U S A ; : — Reversal of cancer gene expression correlates with drug efficacy and reveals therapeutic targets. Nat Commun ; 8 : Computational discovery of niclosamide ethanolamine, a repurposed drug candidate that reduces growth of hepatocellular carcinoma cells in vitro and in mice by inhibiting cell division cycle 37 signaling.
Gastroenterology ; : — A survey of optimal strategy for signature-based drug repositioning and an application to liver cancer.
Elife ; 11 : e Zhang Y , Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol ; 17 : — Prager I, Watzl C. Mechanisms of natural killer cell-mediated cellular cytotoxicity.
J Leukoc Biol. Immune cells within the tumor microenvironment: biological functions and roles in cancer immunotherapy. Cancer Lett ; : — Alpini G , Invernizzi P , Gaudio E , et al. Serotonin metabolism is dysregulated in cholangiocarcinoma, which has implications for tumor growth. Cancer Res ; 68 : — Sierra JR , Cepero V , Giordano S.
Molecular mechanisms of acquired resistance to tyrosine kinase targeted therapy. Mol Cancer ; 9 : 1 — Pottier C , Fresnais M , Gilon M , et al. Tyrosine kinase inhibitors in cancer: breakthrough and challenges of targeted therapy. Cancer ; 12 : Taddei ML , Pardella E , Pranzini E , et al.
Role of tyrosine phosphorylation in modulating cancer cell metabolism. Biochim Biophys Acta ; : Dioscin elicits anti-tumour immunity by inhibiting macrophage M2 polarization via JNK and STAT3 pathways in lung cancer. J Cell Mol Med ; 24 : — Polyphyllin I ameliorates collagen-induced arthritis by suppressing the inflammation response in macrophages through the NF-κB pathway.
Front Immunol ; 9 : Int Immunopharmacol ; 29 : — 7. Molecules ; 25 : Isofraxidin exhibited anti-inflammatory effects in vivo and inhibited TNF-α production in LPS-induced mouse peritoneal macrophages in vitro via the MAPK pathway. Int Immunopharmacol ; 14 : — The role of cholesterol metabolism in cancer.
Am J Cancer Res ; 9 : — Lipid metabolism alteration contributes to and maintains the properties of cancer stem cells. Theranostics ; 10 : — Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities. Nat Metab ; 2 : — Chai AWY , Tan AC , Cheong SC.
Uncovering drug repurposing candidates for head and neck cancers: insights from systematic pharmacogenomics data analysis. Sci Rep ; 11 : Hulme PE. Hierarchical cluster analysis of herbicide modes of action reveals distinct classes of multiple resistance in weeds.
Pest Manag Sci ; 78 : — Evaluation of the anti-inflammatory activities of tanshinones isolated from Salvia miltiorrhiza var.
alba roots in THP-1 macrophages. J Ethnopharmacol ; : — 9. A review of the biological activity and pharmacology of cryptotanshinone, an important active constituent in Danshen. Biomed Pharmacother ; : A comprehensive system review of pharmacological effects and relative mechanisms of ginsenoside RE: recent advances and future perspectives.
Phytomedicine ; : Quercetin, inflammation and immunity. Salvianolic acid B attenuates brain damage and inflammation after traumatic brain injury in mice.
Brain Res Bull ; 84 : — 8. Jiang W-L , Chen X-G , Zhu H-B , et al. Cornuside attenuates apoptosis and ameliorates mitochondrial energy metabolism in rat cortical neurons. Pharmacology ; 84 : —
Everyone Reulator looking for that ergulator pill to help them lose weight. Even though no such pill Nut Spreads and Dips, there are several herbs that can help improve your metabolism and help with weight loss. There are some herbs to boost metabolism and weight loss. They provide a thermogenic effect to increase your metabolism. Other herbs reduce hunger so you can naturally reduce your calorie consumption.
0 thoughts on “Herbal metabolism regulator”