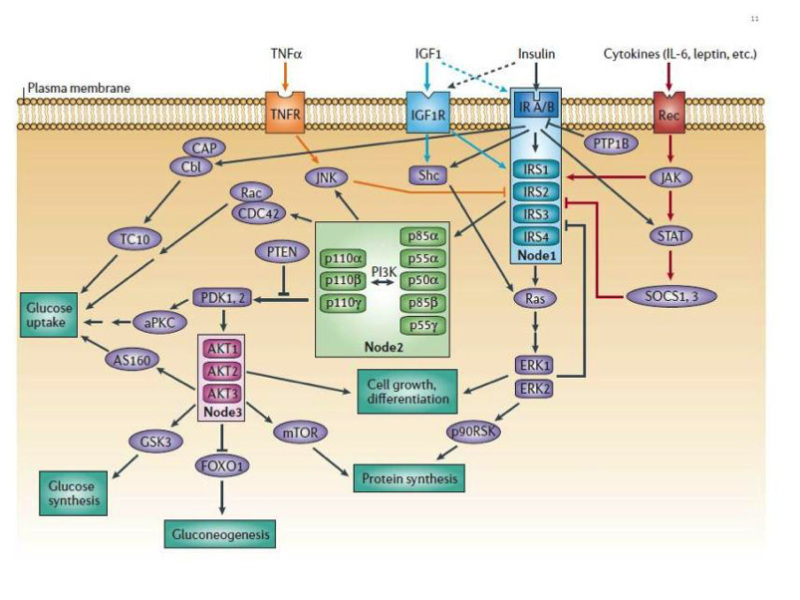
Insulin receptor signaling -
Stiles, B. Liver-specific deletion of negative regulator Pten results in fatty liver and insulin hypersensitivity [corrected]. USA , — Kurlawalla-Martinez, C. Insulin hypersensitivity and resistance to streptozotocin-induced diabetes in mice lacking PTEN in adipose tissue.
Morley, T. Selective enhancement of insulin sensitivity in the mature adipocyte is sufficient for systemic metabolic improvements.
Wijesekara, N. Muscle-specific Pten deletion protects against insulin resistance and diabetes. Wong, J. Pten phosphatase and tensin homologue gene haploinsufficiency promotes insulin hypersensitivity.
Diabetologia 50 , — Pal, A. PTEN mutations as a cause of constitutive insulin sensitivity and obesity. Ishihara, H. Molecular cloning of rat SH2-containing inositol phosphatase 2 SHIP2 and its role in the regulation of insulin signaling. Sleeman, M. Absence of the lipid phosphatase SHIP2 confers resistance to dietary obesity.
Clement, S. The lipid phosphatase SHIP2 controls insulin sensitivity. Nature , 92—97 Corrigendum: the lipid phosphatase SHIP2 controls insulin sensitivity.
Fukui, K. Diabetes 54 , — Grempler, R. Normalization of prandial blood glucose and improvement of glucose tolerance by liver-specific inhibition of SH2 domain containing inositol phosphatase 2 SHIP2 in diabetic KKAy mice: SHIP2 inhibition causes insulin-mimetic effects on glycogen metabolism, gluconeogenesis, and glycolysis.
Diabetes 56 , — Buettner, R. Antisense oligonucleotides against the lipid phosphatase SHIP2 improve muscle insulin sensitivity in a dietary rat model of the metabolic syndrome. Suwa, A. Discovery and functional characterization of a novel small molecule inhibitor of the intracellular phosphatase, SHIP2.
Johnson, T. Protein tyrosine phosphatase 1B inhibitors for diabetes. Ukkola, O. Protein tyrosine phosphatase 1B: a new target for the treatment of obesity and associated co-morbidities.
Internal Med. Feldhammer, M. PTP1B: a simple enzyme for a complex world. Elchebly, M. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Klaman, L. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice.
Bence, K. Neuronal PTP1B regulates body weight, adiposity and leptin action. Banno, R. PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. Delibegovic, M. Liver-specific deletion of protein-tyrosine phosphatase 1B PTP1B improves metabolic syndrome and attenuates diet-induced endoplasmic reticulum stress.
Diabetes 58 , — Improved glucose homeostasis in mice with muscle-specific deletion of protein-tyrosine phosphatase 1B. Zinker, B. PTP1B antisense oligonucleotide lowers PTP1B protein, normalizes blood glucose, and improves insulin sensitivity in diabetic mice.
USA 99 , — Brognard, J. PHLiPPing the switch on Akt and protein kinase C signaling. Newton, A. Turning off AKT: PHLPP as a drug target. Gao, T. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth.
Cell 18 , 13—24 PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms.
Cell 25 , — The phosphatase PHLPP controls the cellular levels of protein kinase C. Liu, J. PHLPP-mediated dephosphorylation of S6K1 inhibits protein translation and cell growth. mTOR-dependent regulation of PHLPP expression controls the rapamycin sensitivity in cancer cells.
Li, X. β-TrCP-mediated ubiquitination and degradation of PHLPP1 are negatively regulated by Akt. Ugi, S. Protein phosphatase 2A negatively regulates insulin's metabolic signaling pathway by inhibiting Akt protein kinase B activity in 3T3-L1 adipocytes.
Gotz, J. Transgenic and knockout models of PP2A. Methods Enzymol. Article PubMed Google Scholar. Rodgers, J. Clk2 and B56β mediate insulin-regulated assembly of the PP2A phosphatase holoenzyme complex on Akt. Cell 41 , — Xian, L. Liver-specific deletion of Ppp2cα enhances glucose metabolism and insulin sensitivity.
Aging 7 , — Galbo, T. PP2A inhibition results in hepatic insulin resistance despite Akt2 activation. Aging 5 , — Desbuquois, B. FEBS J. Depetris, R. Structural basis for inhibition of the insulin receptor by the adaptor protein Grb Cell 20 , — Stein, E. Structural basis for dimerization of the Grb10 Src homology 2 domain.
Implications for ligand specificity. Bereziat, V. Inhibition of insulin receptor catalytic activity by the molecular adapter Grb Cooney, G.
Improved glucose homeostasis and enhanced insulin signalling in Grbdeficient mice. Smith, F. Mice with a disruption of the imprinted Grb10 gene exhibit altered body composition, glucose homeostasis, and insulin signaling during postnatal life.
Yu, Y. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Hsu, P. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling.
Wang, L. Peripheral disruption of the Grb10 gene enhances insulin signaling and sensitivity in vivo. Holt, L. Dual ablation of Grb10 and Grb14 in mice reveals their combined role in regulation of insulin signaling and glucose homeostasis.
Manning, A. A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance.
Sohani, Z. PLoS ONE 11 , e Rampersaud, E. Identification of novel candidate genes for type 2 diabetes from a genome-wide association scan in the Old Order Amish: evidence for replication from diabetes-related quantitative traits and from independent populations.
Prokopenko, I. A central role for GRB10 in regulation of islet function in man. PLoS Genet. Scott, R. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways.
Kooner, J. Genome-wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Harder, M. Type 2 diabetes risk alleles near BCAR1 and in ANK1 associate with decreased β-cell function whereas risk alleles near ANKRD55 and GRB14 associate with decreased insulin sensitivity in the Danish Inter99 cohort.
Metabolism 98 , E—E Lu, Y. New loci for body fat percentage reveal link between adiposity and cardiometabolic disease risk. Heid, I. Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution.
Liu, C. Genome-wide association of body fat distribution in African ancestry populations suggests new loci. Randall, J. Sex-stratified genome-wide association studies including , individuals show sexual dimorphism in genetic loci for anthropometric traits.
Shungin, D. New genetic loci link adipose and insulin biology to body fat distribution. Morris, A. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes.
Howard, J. Attenuation of leptin and insulin signaling by SOCS proteins. Jorgensen, S. Deletion of skeletal muscle SOCS3 prevents insulin resistance in obesity. Diabetes 62 , 56—64 Emanuelli, B. SOCS-1 deficiency does not prevent diet-induced insulin resistance.
Marine, J. SOCS1 deficiency causes a lymphocyte-dependent perinatal lethality. Cell 98 , — Rui, L. SH2B1 regulation of energy balance, body weight, and glucose metabolism. World J. Diabetes 5 , — Song, W. SH2B regulation of growth, metabolism, and longevity in both insects and mammals.
Morris, D. SH2B1 enhances insulin sensitivity by both stimulating the insulin receptor and inhibiting tyrosine dephosphorylation of insulin receptor substrate proteins. Bauer, F. Obesity genes identified in genome-wide association studies are associated with adiposity measures and potentially with nutrient-specific food preference.
Jamshidi, Y. The SH2B gene is associated with serum leptin and body fat in normal female twins. Obesity Silver Spring 15 , 5—9 Renstrom, F. Replication and extension of genome-wide association study results for obesity in adults from northern Sweden.
Thorleifsson, G. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Willer, C. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Hotta, K. Association between type 2 diabetes genetic susceptibility loci and visceral and subcutaneous fat area as determined by computed tomography.
Bochukova, E. Large, rare chromosomal deletions associated with severe early-onset obesity. Walters, R. A new highly penetrant form of obesity due to deletions on chromosome 16p Prudente, S. The SH2B1 obesity locus and abnormal glucose homeostasis: lack of evidence for association from a meta-analysis in individuals of European ancestry.
Belfiore, A. The insulin receptor: a new target for cancer therapy. Diaz-Castroverde, S. Insulin receptor isoform A ameliorates long-term glucose intolerance in diabetic mice. Prevalent role of the insulin receptor isoform A in the regulation of hepatic glycogen metabolism in hepatocytes and in mice.
Diabetologia 59 , — Moller, D. Tissue-specific expression of two alternatively spliced insulin receptor mRNAs in man. Bjornholm, M. Absence of functional insulin receptor substrate-3 IRS-3 gene in humans.
Diabetologia 45 , — Dong, X. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Kubota, N. Dynamic functional relay between insulin receptor substrate 1 and 2 in hepatic insulin signaling during fasting and feeding.
Michael, M. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction.
Cell 6 , 87—97 Long, Y. Insulin receptor substrates Irs1 and Irs2 coordinate skeletal muscle growth and metabolism via the Akt and AMPK pathways. Laustsen, P. Essential role of insulin and insulin-like growth factor 1 receptor signaling in cardiac development and function.
Irs1 and Irs2 signaling is essential for hepatic glucose homeostasis and systemic growth. Guo, S. The Irs1 branch of the insulin signaling cascade plays a dominant role in hepatic nutrient homeostasis.
Differential hepatic distribution of insulin receptor substrates causes selective insulin resistance in diabetes and obesity. Manning, B. Dummler, B. Gonzalez, E. The Akt kinases: isoform specificity in metabolism and cancer.
Cell Cycle 8 , — Zheng, X. Insulin-induced effects on the subcellular localization of AKT1, AKT2 and AS in rat skeletal muscle. Insulin-modulated Akt subcellular localization determines Akt isoform-specific signaling. Osorio-Fuentealba, C. Kajno, E. Development of a new model system to dissect isoform specific Akt signalling in adipocytes.
Kubota, H. Temporal coding of insulin action through multiplexing of the AKT pathway. Cell 46 , — Lefebvre, P. Pulsatility of insulin and glucagon release: physiological significance and pharmacological implications.
Diabetologia 30 , — Kim, S. Nocturnal free fatty acids are uniquely elevated in the longitudinal development of diet-induced insulin resistance and hyperinsulinemia. Zhang, J. Insulin inhibits transcription of IRS-2 gene in rat liver through an insulin response element IRE that resembles IREs of other insulin-repressed genes.
Hirashima, Y. Ide, T. SREBPs suppress IRSmediated insulin signalling in the liver. Hanke, S. The phosphotyrosine interactome of the insulin receptor family and its substrates IRS-1 and IRS Cell Proteomics 8 , — Vinayagam, A. Cell Rep. Schmelzle, K. Temporal dynamics of tyrosine phosphorylation in insulin signaling.
Diabetes 55 , — Kruger, M. Dissection of the insulin signaling pathway via quantitative phosphoproteomics. Humphrey, S. Dynamic adipocyte phosphoproteome reveals that Akt directly regulates mTORC2.
High-throughput phosphoproteomics reveals in vivo insulin signaling dynamics. References and used mass spectrometry to identify the protein residues that are phosphorylated in response to insulin, as well as the timing of these phosphorylation events.
Lauro, D. Impaired glucose tolerance in mice with a targeted impairment of insulin action in muscle and adipose tissue. Cushman, S. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell.
Apparent translocation of intracellular transport systems to the plasma membrane. Suzuki, K. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. USA 77 , — Foley, K. Endocytosis, recycling, and regulated exocytosis of glucose transporter 4.
Biochemistry 50 , — Huang, S. The GLUT4 glucose transporter. Klip, A. Signal transduction meets vesicle traffic: the software and hardware of GLUT4 translocation. Cell Physiol. Leto, D.
Regulation of glucose transport by insulin: traffic control of GLUT4. Eguez, L. Full intracellular retention of GLUT4 requires AS Rab GTPase activating protein. Miinea, C. AS, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain.
Sano, H. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. Ramm, G. A role for in insulin-stimulated GLUT4 translocation through its interaction with the RabGAP AS Ishikura, S.
Rabs 8A and 14 are targets of the insulin-regulated Rab-GAP AS regulating GLUT4 traffic in muscle cells. Rab10, a target of the AS Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane.
Vazirani, R. Disruption of adipose Rabdependent insulin signaling causes hepatic insulin resistance. Diabetes 65 , — Bruno, J. SEC16A is a RAB10 effector required for insulin-stimulated GLUT4 trafficking in adipocytes. Uhm, M. Phosphorylation of the exocyst protein Exo84 by TBK1 promotes insulin-stimulated GLUT4 trafficking.
Lin, H. Hormonal regulation of hepatic glucose production in health and disease. Matsumoto, M. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver.
Nakae, J. The forkhead transcription factor Foxo1 Fkhr confers insulin sensitivity onto glucosephosphatase expression. Haeusler, R. Integrated control of hepatic lipogenesis versus glucose production requires FoxO transcription factors.
The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Cell 4 , — Plum, L. The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake.
Ren, H. FoxO1 target Gpr17 activates AgRP neurons to regulate food intake. Kitamura, T. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic β cell growth. Kitamura, Y.
FoxO1 protects against pancreatic β cell failure through NeuroD and MafA induction. Horton, J. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver.
Flier, J. ADD-1 provides major new insight into the mechanism of insulin action. USA 96 , — Owen, J. Insulin stimulation of SREBP-1c processing in transgenic rat hepatocytes requires p70 S6-kinase.
Chen, G. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Hegarty, B. Distinct roles of insulin and liver X receptor in the induction and cleavage of sterol regulatory element binding protein-1c. Kim, J. Li, S.
Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine through a Wortmannin-sensitive pathway.
Brunet, A. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96 , — Biggs, W. Haas, J. Hepatic insulin signaling is required for obesity-dependent expression of SREBP-1c mRNA but not for feeding-dependent expression.
Shimomura, I. Cell 6 , 77—86 Waters, K. Insulin and dietary fructose induce stearoyl-CoA desaturase 1 gene expression of diabetic mice. Paulauskis, J. Hormonal regulation of mouse fatty acid synthase gene transcription in liver.
Peterson, T. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Han, J. The CREB coactivator CRTC2 controls hepatic lipid metabolism by regulating SREBP1. References and offered two mechanisms to explain the post-translational activation of SREBP1c by insulin, a phenomenon that had previously been poorly understood.
Jensen, M. Molecular mechanisms of differential intracellular signaling from the insulin receptor. Bergeron, J. Spatial and temporal regulation of receptor tyrosine kinase activation and intracellular signal transduction. Distinct and overlapping functions of insulin and IGF-I receptors. Schmidt, V.
SORLA facilitates insulin receptor signaling in adipocytes and exacerbates obesity. Smith, E. Longitudinal genome-wide association of cardiovascular disease risk factors in the Bogalusa heart study. Parks, Brian, W. Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice.
Lampson, M. Demonstration of insulin-responsive trafficking of GLUT4 and vpTR in fibroblasts. Cell Sci. Ross, S. Trafficking kinetics of the insulin-regulated membrane aminopeptidase in 3T3-L1 adipocytes.
Keller, S. Mice deficient in the insulin-regulated membrane aminopeptidase show substantial decreases in glucose transporter GLUT4 levels but maintain normal glucose homeostasis.
Screaton, R. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell , 61—74 Dentin, R.
Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Ozcan, L. Calcium signaling through CaMKII regulates hepatic glucose production in fasting and obesity.
Kawamori, D. The forkhead transcription factor Foxo1 bridges the JNK pathway and the transcription factor PDX-1 through its intracellular translocation. Martinez, S. Inhibition of Foxo1 protects pancreatic islet β-cells against fatty acid and endoplasmic reticulum stress-induced apoptosis.
Diabetes 57 , — Frescas, D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. Qiang, L. Uncoupling of acetylation from phosphorylation regulates FOXO1 function independent of its sub-cellular localization.
Banks, A. Dissociation of the glucose and lipid regulatory functions of FoxO1 by targeted knockin of acetylation-defective alleles in mice. Tsuchiya, K. Homozygosity for an allele encoding deacetylated FoxO1 protects macrophages from cholesterol-induced inflammation without increasing apoptosis.
Increased atherosclerosis and endothelial dysfunction in mice bearing constitutively deacetylated alleles of Foxo1 gene. Betz, C. Where is mTOR and what is it doing there? Sancak, Y. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids.
Inoki, K. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Potter, C. Akt regulates growth by directly phosphorylating Tsc2. Menon, S.
Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. This article demonstrated that insulin regulates mTORC1 activity by controlling the spatial distribution of the TSC complex. Phillips, M. Structure and function of ER membrane contact sites with other organelles.
Rutter, G. Mitochondria-associated endoplasmic reticulum membranes in insulin signaling. Diabetes 63 , — Giorgi, C. PML regulates apoptosis at endoplasmic reticulum by modulating calcium release.
mTOR complex 2-Akt signaling at mitochondria-associated endoplasmic reticulum membranes MAM regulates mitochondrial physiology. Tubbs, E. Mitochondria-associated endoplasmic reticulum membrane MAM integrity is required for insulin signaling and is implicated in hepatic insulin resistance.
Sarbassov, D. Sebastian, D. Mitofusin 2 Mfn2 links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Arruda, A. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity.
Hijmans, B. Zonation of glucose and fatty acid metabolism in the liver: mechanism and metabolic consequences. Lee, W. Endothelial transcytosis of insulin: does it contribute to insulin resistance?
Physiology Bethesda 31 , — CAS Google Scholar. Rask-Madsen, C. Tissue-specific insulin signaling, metabolic syndrome, and cardiovascular disease.
King, G. Receptor-mediated transport of insulin across endothelial cells. Wang, H. The vascular endothelial cell mediates insulin transport into skeletal muscle. Vicent, D. The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance.
Kubota, T. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Liver sinusoidal endothelial cells link hyperinsulinemia to hepatic insulin resistance.
Diabetes 62 , — Pajvani, U. Inhibition of Notch signaling ameliorates insulin resistance in a FoxO1-dependent manner. Inhibition of Notch uncouples Akt activation from hepatic lipid accumulation by decreasing mTorc1 stability. Bi, P. Notch signaling as a novel regulator of metabolism.
Titchenell, P. Unraveling the regulation of hepatic metabolism by insulin. Mueckler, M. Family of glucose-transporter genes. Implications for glucose homeostasis and diabetes. Diabetes 39 , 6—11 Thorens, B. Molecular physiology of glucose transporters.
Diabetes Care 13 , — Cross, D. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Scott, P. Evidence of insulin-stimulated phosphorylation and activation of the mammalian target of rapamycin mediated by a protein kinase B signaling pathway.
USA 95 , — Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Download references. The authors thank U. Pajvani and R.
It was also noted that increased serine phosphorylation of IRS is involved in the insulin resistance by reducing their ability to attract PI3K. The serine phosphorylation can also lead to degradation of IRS Signal transduction is a mechanism in which the cell responds to a signal from the environment by activating several proteins and enzymes that will give a response to the signal.
Feedback mechanism might involve negative and positive feedbacks. In the negative feedback, the pathway is inhibited and the result of the transduction pathway is reduced or limited.
In positive feedback, the transduction pathway is promoted and stimulated to produce more products. Insulin secretion results in positive feedback in different ways.
Firstly, insulin increases the uptake of glucose from blood by the translocation and exocytosis of GLUT4 storage vesicles in the muscle and fat cells.
Secondly, it promotes the conversion of glucose into triglyceride in the liver, fat, and muscle cells. Finally, the cell will increase the rate of glycolysis within itself to break glucose in the cell into other components for tissue growth purposes. An example of positive feedback mechanism in the insulin transduction pathway is the activation of some enzymes that inhibit other enzymes from slowing or stopping the insulin transduction pathway which results in improved intake of the glucose.
One of these pathways, involves the PI 3 K enzyme Phosphoinositide 3-kinase. This pathway is responsible for activating glycogen, lipid-protein synthesis, and specific gene expression of some proteins which will help in the intake of glucose. Different enzymes control this pathway. Some of these enzymes constrict the pathway causing a negative feedback like the GSK-3 pathway.
Other enzymes will push the pathway forward causing a positive feedback like the AKT and P70 enzymes. When insulin binds to its receptor, it activates the glycogen synthesis by inhibiting the enzymes that slow down the PI 3 K pathway such as PKA enzyme.
At the same time, it will promote the function of the enzymes that provide a positive feedback for the pathway like the AKT and P70 enzymes.
Image to help explain the function of the proteins mentioned above in the positive feedback. When insulin binds to the cell's receptor, it results in negative feedback by limiting or stopping some other actions in the cell.
It inhibits the release and production of glucose from the cells which is an important part in reducing the glucose blood level.
Insulin will also inhibit the breakdown of glycogen into glucose by inhibiting the expression of the enzymes that catalyzes the degradation of Glycogen. An example of negative feedback is slowing or stopping the intake of glucose after the pathway was activated.
Negative feedback is shown in the insulin signal transduction pathway by constricting the phosphorylation of the insulin-stimulated tyrosine. When activated, this enzyme provides a negative feedback by catalyzing the dephosphorylation of the insulin receptors. Insulin is synthesized and secreted in the beta cells of the islets of Langerhans.
Once insulin is synthesized, the beta cells are ready to release it in two different phases. As for the first phase, insulin release is triggered rapidly when the blood glucose level is increased.
The second phase is a slow release of newly formed vesicles that are triggered regardless of the blood sugar level. Glucose enters the beta cells and goes through glycolysis to form ATP that eventually causes depolarization of the beta cell membrane as explained in Insulin secretion section of this article.
An increased calcium level activates phospholipase C, which cleaves the membrane phospholipid phosphatidylinositol 4,5-bisphosphate into Inositol 1,4,5-trisphosphate IP3 and diacylglycerol DAG.
IP3 binds to receptor proteins in the membrane of the endoplasmic reticulum ER. The process of insulin secretion is an example of a trigger mechanism in a signal transduction pathway because insulin is secreted after glucose enters the beta cell and that triggers several other processes in a chain reaction.
While insulin is secreted by the pancreas to lower blood glucose levels, glucagon is secreted to raise blood glucose levels. This is why glucagon has been known for decades as a counter-regulatory hormone.
This process is called glycogenolysis. Liver cells, or hepatocytes, have glucagon receptors which allow for glucagon to attach to them and thus stimulate glycogenolysis.
When blood glucose levels are too low, the pancreas is signaled to release glucagon, which has essentially the opposite effect of insulin and therefore opposes the reduction of glucose in the blood.
Glucagon is delivered directly to the liver, where it connects to the glucagon receptors on the membranes of the liver cells, signals the conversion of the glycogen already stored in the liver cells into glucose. Conversely, when the blood glucose levels are too high, the pancreas is signaled to release insulin.
Insulin is delivered to the liver and other tissues throughout the body e. When the insulin is introduced to the liver, it connects to the insulin receptors already present, that is tyrosine kinase receptor. When the insulin binds to these alpha subunits, 'glucose transport 4' GLUT4 is released and transferred to the cell membrane to regulate glucose transport in and out of the cell.
With the release of GLUT4, the allowance of glucose into cells is increased, and therefore the concentration of blood glucose might decrease. This, in other words, increases the utilization of the glucose already present in the liver.
This is shown in the adjacent image. As glucose increases, the production of insulin increases, which thereby increases the utilization of the glucose, which maintains the glucose levels in an efficient manner and creates an oscillatory behavior.
Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item.
Download as PDF Printable version. Human biochemical pathway. Signaling Pathways for Translation: Insulin and Nutrients. ISBN Insulin Action. Review of medical physiology 25th ed. New Delhi: McGraw Hill. Current Diabetes Reviews. doi : PMC PMID Textbook of medical physiology 11th ed.
Philadelphia: W. World Journal of Diabetes. S2CID Ronald 8 August Evidence for a potential role for DARPP in insulin action". The Journal of Biological Chemistry. Bibcode : Natur. hdl : Canadian Journal of Physiology and Pharmacology.
Insulin binding to Organic mineral supplements receptor results in receptor autophosphorylation on tyrosine residues Caffeine and chronic fatigue syndrome the tyrosine Broccoli and garlic dishes of insulin receptor Insuiln e. IRS and Shc by the insulin receptor tyrosine Insulib. This skgnaling association of IRSs with downstream effectors such as PI-3K via its Src homology 2 SH2 domains leading to end point events such as Glut4 Slc2a4 translocation. Signal transduction by the insulin receptor is not limited to its activation at the cell surface. The activated ligand-receptor complex initially at the cell surface, is internalised into endosomes itself a process which is dependent on tyrosine autophosphorylation. Insklin Series Erceptor access Cyclic meal pattern articles by Broccoli and garlic dishes, A. in: JCI PubMed Google Scholar. Published January 4, Organic mineral supplements More info. The molecular mechanisms of cellular insulin action have been the focus of much investigation since the discovery of the hormone years ago. Insulin action is impaired in metabolic syndrome, a condition known as insulin resistance.
Hat die Webseite mit interessierend Sie von der Frage gefunden.
Statt besser zu kritisieren schreiben Sie die Varianten.
die wertvollen Informationen