
Glucose utilization -
Cori cycle Sumia abdulsalam. Blood glucose homeostasis. Blood glucose homeostasis Anup Shamsher Budhathoki. Gluconeogenesis -. Gluconeogenesis - Ashok Katta.
Fate of pyruvate - A quick review. Fate of pyruvate - A quick review Namrata Chhabra. Glycolysis , its regulation and energetics. Glycolysis , its regulation and energetics PratikshaPuranik5. Glycolysis nj Lipoproteins- structure, classification, metabolism and clinical significance.
Lipoproteins- structure, classification, metabolism and clinical significance Namrata Chhabra. What's hot 20 Glycogen metabolism. Similar to Glucose Utilization Glycogen Metabolism Glycogen Metabolism ppt Perfect pdf TatendaMageja.
Glycogenesis and glycogenolysis. Glycogenesis and glycogenolysis LubnaSSubair. Glycogen Metabolism. pptx fafyfskhankmf. Glycolysis and its other side process.
Glycolysis and its other side process Asifa Zafar. Metabolism in human body. Metabolism in human body রেজা তানজিল.
Carbohydrate 2. Carbohydrate 2 Pharmacy Universe. pptx mulenga Glycolysis - Glucose oxidation. Glycolysis - Glucose oxidation Sachith Gamage. Gluconeogenecys, glycogenesis, glycogenolysis. Gluconeogenecys, glycogenesis, glycogenolysis Kayeen Vadakkan. Lec 7 level 3-de carbohydrate metabolism ii.
Lec 7 level 3-de carbohydrate metabolism ii dream10f. Gluconeogenesis and glycogen metabolism. Gluconeogenesis and glycogen metabolism Sohil Takodara. glycogenesis and glycogenolysis. glycogenesis and glycogenolysis NANDITA Gluconeogenesis kiransharma Unit 2 carbohydrate metabolism 2.
Unit 2 carbohydrate metabolism 2 Dipali Kulkarni. Glycogen metabolism s. Glycogen metabolism s Supriya Singh. Lec 7 level 3-nu carbohydrate metabolism iii.
Lec 7 level 3-nu carbohydrate metabolism iii dream10f. biochemistry Doctor of Verinary Med. Metabolism of Carbohydrate - Part-II. pptx ABHIJIT BHOYAR. Similar to Glucose Utilization 20 Glycogen Metabolism Nucleic acids. Nucleic acids Amany Elsayed.
Amany Elsayed. Biosynthetic reactions of amino acids and Gel Electrophoresis. Biosynthetic reactions of amino acids and Gel Electrophoresis Amany Elsayed. Chemistry of protein. Chemistry of protein Amany Elsayed. Cancer treatment.
Cancer treatment Amany Elsayed. ONCOGENES Amany Elsayed. Cancer Biology. Cancer Biology Amany Elsayed. Molecular Theory and techniques and polymerase chain reaction. Molecular Theory and techniques and polymerase chain reaction Amany Elsayed. Chemiluminescence immunoassay and Immunofluorescence Assay.
Chemiluminescence immunoassay and Immunofluorescence Assay Amany Elsayed. Labeled antibody techniques , ELISA. Labeled antibody techniques , ELISA Amany Elsayed. Spectrophotometric Instruments Detector and Application of UV — VIS spectro Some Clinical Laboratory Measurement of Immune Functions.
They melt at °C °F α and °C °F β , and decompose starting at °C °F with release of various volatile products, ultimately leaving a residue of carbon. With six carbon atoms, it is classed as a hexose , a subcategory of the monosaccharides.
d -Glucose is one of the sixteen aldohexose stereoisomers. The d - isomer , d -glucose, also known as dextrose , occurs widely in nature, but the l -isomer, l -glucose , does not.
Glucose can be obtained by hydrolysis of carbohydrates such as milk sugar lactose , cane sugar sucrose , maltose , cellulose , glycogen , etc. Dextrose is commonly commercially manufactured from cornstarch in the US and Japan, from potato and wheat starch in Europe, and from tapioca starch in tropical areas.
Glucose is usually present in solid form as a monohydrate with a closed pyran ring α-glucopyranose monohydrate, sometimes known less precisely by dextrose hydrate. In aqueous solution, on the other hand, it is an open-chain to a small extent and is present predominantly as α- or β- pyranose , which interconvert.
From aqueous solutions, the three known forms can be crystallized: α-glucopyranose, β-glucopyranose and α-glucopyranose monohydrate. The glass transition temperature of glucose is 31 °C 88 °F and the Gordon—Taylor constant an experimentally determined constant for the prediction of the glass transition temperature for different mass fractions of a mixture of two substances [28] is 4.
The open-chain form of glucose makes up less than 0. Therefore, glucose is also classified as an aldose , or an aldohexose. The aldehyde group makes glucose a reducing sugar giving a positive reaction with the Fehling test. In solutions, the open-chain form of glucose either " D -" or " L -" exists in equilibrium with several cyclic isomers , each containing a ring of carbons closed by one oxygen atom.
The open-chain form is limited to about 0. The terms "glucose" and " D -glucose" are generally used for these cyclic forms as well. The reaction between C-1 and C-5 yields a six-membered heterocyclic system called a pyranose, which is a monosaccharide sugar hence "-ose" containing a derivatised pyran skeleton.
The much rarer reaction between C-1 and C-4 yields a five-membered furanose ring, named after the cyclic ether furan. The ring-closing reaction can give two products, denoted "α-" and "β-".
Therefore, the open-chain isomer D -glucose gives rise to four distinct cyclic isomers: α- D -glucopyranose, β- D -glucopyranose, α- D -glucofuranose, and β- D -glucofuranose. These five structures exist in equilibrium and interconvert, and the interconversion is much more rapid with acid catalysis.
The other open-chain isomer L -glucose similarly gives rise to four distinct cyclic forms of L -glucose, each the mirror image of the corresponding D -glucose. The glucopyranose ring α or β can assume several non-planar shapes, analogous to the "chair" and "boat" conformations of cyclohexane. Similarly, the glucofuranose ring may assume several shapes, analogous to the "envelope" conformations of cyclopentane.
Some derivatives of glucofuranose, such as 1,2- O -isopropylidene- D -glucofuranose are stable and can be obtained pure as crystalline solids.
Mutarotation consists of a temporary reversal of the ring-forming reaction, resulting in the open-chain form, followed by a reforming of the ring. Thus, though the open-chain form is barely detectable in solution, it is an essential component of the equilibrium.
The open-chain form is thermodynamically unstable , and it spontaneously isomerizes to the cyclic forms. Although the ring closure reaction could in theory create four- or three-atom rings, these would be highly strained, and are not observed in practice.
In solutions at room temperature , the four cyclic isomers interconvert over a time scale of hours, in a process called mutarotation. The ratio would be α:β if it were not for the influence of the anomeric effect. The strength of the effect is different for each of the five tautomers.
Note that the d - prefix does not refer directly to the optical properties of the compound. It indicates that the C-5 chiral centre has the same handedness as that of d -glyceraldehyde which was so labelled because it is dextrorotatory. The fact that d -glucose is dextrorotatory is a combined effect of its four chiral centres, not just of C-5; and indeed some of the other d -aldohexoses are levorotatory.
The equilibration takes place via the open-chain aldehyde form. In dilute sodium hydroxide or other dilute bases, the monosaccharides mannose , glucose and fructose interconvert via a Lobry de Bruyn—Alberda—Van Ekenstein transformation , so that a balance between these isomers is formed.
This reaction proceeds via an enediol :. Glucose is the most abundant monosaccharide. Glucose is also the most widely used aldohexose in most living organisms. One possible explanation for this is that glucose has a lower tendency than other aldohexoses to react nonspecifically with the amine groups of proteins.
in glycated hemoglobin. Glucose's low rate of glycation can be attributed to its having a more stable cyclic form compared to other aldohexoses, which means it spends less time than they do in its reactive open-chain form.
Presumably, glucose is the most abundant natural monosaccharide because it is less glycated with proteins than other monosaccharides. Polysaccharides that are composed solely of glucose are termed glucans.
Glucose is produced by plants through photosynthesis using sunlight, water and carbon dioxide and can be used by all living organisms as an energy and carbon source. However, most glucose does not occur in its free form, but in the form of its polymers, i.
lactose, sucrose, starch and others which are energy reserve substances, and cellulose and chitin , which are components of the cell wall in plants or fungi and arthropods , respectively. These polymers, when consumed by animals, fungi and bacteria, are degraded to glucose using enzymes.
All animals are also able to produce glucose themselves from certain precursors as the need arises. Neurons , cells of the renal medulla and erythrocytes depend on glucose for their energy production.
Many of the long-term complications of diabetes e. Ingested glucose initially binds to the receptor for sweet taste on the tongue in humans. This complex of the proteins T1R2 and T1R3 makes it possible to identify glucose-containing food sources. Glucose mainly comes from food—about g 11 oz per day is produced by conversion of food, [47] but it is also synthesized from other metabolites in the body's cells.
In humans, the breakdown of glucose-containing polysaccharides happens in part already during chewing by means of amylase , which is contained in saliva , as well as by maltase , lactase , and sucrase on the brush border of the small intestine.
Glucose is a building block of many carbohydrates and can be split off from them using certain enzymes. Glucosidases , a subgroup of the glycosidases, first catalyze the hydrolysis of long-chain glucose-containing polysaccharides, removing terminal glucose.
In turn, disaccharides are mostly degraded by specific glycosidases to glucose. The names of the degrading enzymes are often derived from the particular poly- and disaccharide; inter alia, for the degradation of polysaccharide chains there are amylases named after amylose, a component of starch , cellulases named after cellulose , chitinases named after chitin , and more.
Furthermore, for the cleavage of disaccharides, there are maltase, lactase, sucrase, trehalase , and others. In humans, about 70 genes are known that code for glycosidases. They have functions in the digestion and degradation of glycogen, sphingolipids , mucopolysaccharides , and poly ADP-ribose.
Humans do not produce cellulases, chitinases, or trehalases, but the bacteria in the gut microbiota do. In order to get into or out of cell membranes of cells and membranes of cell compartments, glucose requires special transport proteins from the major facilitator superfamily.
Glucose 6-phosphatase can convert glucose 6-phosphate back into glucose exclusively in the liver, so the body can maintain a sufficient blood glucose concentration. In other cells, uptake happens by passive transport through one of the 14 GLUT proteins.
The glucose transporter GLUT1 is produced by most cell types and is of particular importance for nerve cells and pancreatic β-cells. In the kidneys , glucose in the urine is absorbed via SGLT1 and SGLT2 in the apical cell membranes and transmitted via GLUT2 in the basolateral cell membranes.
In plants and some prokaryotes , glucose is a product of photosynthesis. The cleavage of glycogen is termed glycogenolysis, the cleavage of starch is called starch degradation. The metabolic pathway that begins with molecules containing two to four carbon atoms C and ends in the glucose molecule containing six carbon atoms is called gluconeogenesis and occurs in all living organisms.
The smaller starting materials are the result of other metabolic pathways. Ultimately almost all biomolecules come from the assimilation of carbon dioxide in plants and microbes during photosynthesis.
In the liver about g 5. Unlike for glucose, there is no transport protein for glucosephosphate. Gluconeogenesis allows the organism to build up glucose from other metabolites, including lactate or certain amino acids , while consuming energy. The renal tubular cells can also produce glucose. Glucose also can be found outside of living organisms in the ambient environment.
Glucose concentrations in the atmosphere are detected via collection of samples by aircraft and are known to vary from location to location.
For example, glucose concentrations in atmospheric air from inland China range from 0. In humans, glucose is metabolized by glycolysis [63] and the pentose phosphate pathway. If there is not enough oxygen available for this, the glucose degradation in animals occurs anaerobic to lactate via lactic acid fermentation and releases much less energy.
Muscular lactate enters the liver through the bloodstream in mammals, where gluconeogenesis occurs Cori cycle. With a high supply of glucose, the metabolite acetyl-CoA from the Krebs cycle can also be used for fatty acid synthesis. These processes are hormonally regulated. In other living organisms, other forms of fermentation can occur.
The bacterium Escherichia coli can grow on nutrient media containing glucose as the sole carbon source. Use of glucose as an energy source in cells is by either aerobic respiration, anaerobic respiration, or fermentation. The first step of glycolysis is the phosphorylation of glucose by a hexokinase to form glucose 6-phosphate.
The main reason for the immediate phosphorylation of glucose is to prevent its diffusion out of the cell as the charged phosphate group prevents glucose 6-phosphate from easily crossing the cell membrane.
At physiological conditions , this initial reaction is irreversible. In anaerobic respiration, one glucose molecule produces a net gain of two ATP molecules four ATP molecules are produced during glycolysis through substrate-level phosphorylation, but two are required by enzymes used during the process.
Click on genes, proteins and metabolites below to link to respective articles. Tumor cells often grow comparatively quickly and consume an above-average amount of glucose by glycolysis, [71] which leads to the formation of lactate, the end product of fermentation in mammals, even in the presence of oxygen.
This is called the Warburg effect. For the increased uptake of glucose in tumors various SGLT and GLUT are overly produced. In yeast , ethanol is fermented at high glucose concentrations, even in the presence of oxygen which normally leads to respiration rather than fermentation.
This is called the Crabtree effect. Glucose can also degrade to form carbon dioxide through abiotic means. This has been demonstrated to occur experimentally via oxidation and hydrolysis at 22 °C and a pH of 2. Glucose is a ubiquitous fuel in biology. It is used as an energy source in organisms, from bacteria to humans, through either aerobic respiration , anaerobic respiration in bacteria , or fermentation.
Glucose is the human body's key source of energy, through aerobic respiration, providing about 3. Through glycolysis and later in the reactions of the citric acid cycle and oxidative phosphorylation , glucose is oxidized to eventually form carbon dioxide and water, yielding energy mostly in the form of ATP.
The insulin reaction, and other mechanisms, regulate the concentration of glucose in the blood. The physiological caloric value of glucose, depending on the source, is Differences exist in which end product can no longer be used for energy production.
The presence of individual genes, and their gene products, the enzymes, determine which reactions are possible. The metabolic pathway of glycolysis is used by almost all living beings. An essential difference in the use of glycolysis is the recovery of NADPH as a reductant for anabolism that would otherwise have to be generated indirectly.
Glucose and oxygen supply almost all the energy for the brain , [79] so its availability influences psychological processes. When glucose is low , psychological processes requiring mental effort e.
The glucose in the blood is called blood sugar. Blood sugar levels are regulated by glucose-binding nerve cells in the hypothalamus.
The blood sugar content of a healthy person in the short-time fasting state, e. In addition, the values in the arterial blood are higher than the concentrations in the venous blood since glucose is absorbed into the tissue during the passage of the capillary bed.
Also in the capillary blood, which is often used for blood sugar determination, the values are sometimes higher than in the venous blood.
The glucose content of the blood is regulated by the hormones insulin , incretin and glucagon. Some glucose is converted to lactic acid by astrocytes , which is then utilized as an energy source by brain cells ; some glucose is used by intestinal cells and red blood cells , while the rest reaches the liver , adipose tissue and muscle cells, where it is absorbed and stored as glycogen under the influence of insulin.
Liver cell glycogen can be converted to glucose and returned to the blood when insulin is low or absent; muscle cell glycogen is not returned to the blood because of a lack of enzymes. In fat cells , glucose is used to power reactions that synthesize some fat types and have other purposes.
Glycogen is the body's "glucose energy storage" mechanism, because it is much more "space efficient" and less reactive than glucose itself. As a result of its importance in human health, glucose is an analyte in glucose tests that are common medical blood tests.
The glycemic index is an indicator of the speed of resorption and conversion to blood glucose levels from ingested carbohydrates, measured as the area under the curve of blood glucose levels after consumption in comparison to glucose glucose is defined as ice cream.
The glycemic load is an indicator for the amount of glucose added to blood glucose levels after consumption, based on the glycemic index and the amount of consumed food.
Organisms use glucose as a precursor for the synthesis of several important substances. Starch, cellulose , and glycogen "animal starch" are common glucose polymers polysaccharides.
Some of these polymers starch or glycogen serve as energy stores, while others cellulose and chitin , which is made from a derivative of glucose have structural roles. Oligosaccharides of glucose combined with other sugars serve as important energy stores.
These include lactose, the predominant sugar in milk, which is a glucose-galactose disaccharide, and sucrose, another disaccharide which is composed of glucose and fructose.
Glucose is also added onto certain proteins and lipids in a process called glycosylation. This is often critical for their functioning. The enzymes that join glucose to other molecules usually use phosphorylated glucose to power the formation of the new bond by coupling it with the breaking of the glucose-phosphate bond.
Other than its direct use as a monomer, glucose can be broken down to synthesize a wide variety of other biomolecules. This is important, as glucose serves both as a primary store of energy and as a source of organic carbon.
Glucose can be broken down and converted into lipids. It is also a precursor for the synthesis of other important molecules such as vitamin C ascorbic acid. In living organisms, glucose is converted to several other chemical compounds that are the starting material for various metabolic pathways.
Among them, all other monosaccharides [96] such as fructose via the polyol pathway , [50] mannose the epimer of glucose at position 2 , galactose the epimer at position 4 , fucose, various uronic acids and the amino sugars are produced from glucose. Glucose is used in some bacteria as a building block in the trehalose or the dextran biosynthesis and in animals as a building block of glycogen.
Glucose can also be converted from bacterial xylose isomerase to fructose. In addition, glucose metabolites produce all nonessential amino acids, sugar alcohols such as mannitol and sorbitol , fatty acids , cholesterol and nucleic acids.
Diabetes is a metabolic disorder where the body is unable to regulate levels of glucose in the blood either because of a lack of insulin in the body or the failure, by cells in the body, to respond properly to insulin.
Each of these situations can be caused by persistently high elevations of blood glucose levels, through pancreatic burnout and insulin resistance. The pancreas is the organ responsible for the secretion of the hormones insulin and glucagon.
Without it, glucose cannot enter the cell and therefore cannot be used as fuel for the body's functions. Insulin resistance occurs when the pancreas tries to produce more and more insulin in response to persistently elevated blood glucose levels.
Eventually, the rest of the body becomes resistant to the insulin that the pancreas is producing, thereby requiring more insulin to achieve the same blood glucose-lowering effect, and forcing the pancreas to produce even more insulin to compete with the resistance.
This negative spiral contributes to pancreatic burnout, and the disease progression of diabetes. To monitor the body's response to blood glucose-lowering therapy, glucose levels can be measured.
Blood glucose monitoring can be performed by multiple methods, such as the fasting glucose test which measures the level of glucose in the blood after 8 hours of fasting.
Another test is the 2-hour glucose tolerance test GTT — for this test, the person has a fasting glucose test done, then drinks a gram glucose drink and is retested. This test measures the ability of the person's body to process glucose. Over time the blood glucose levels should decrease as insulin allows it to be taken up by cells and exit the blood stream.
Individuals with diabetes or other conditions that result in low blood sugar often carry small amounts of sugar in various forms. One sugar commonly used is glucose, often in the form of glucose tablets glucose pressed into a tablet shape sometimes with one or more other ingredients as a binder , hard candy , or sugar packet.
Most dietary carbohydrates contain glucose, either as their only building block as in the polysaccharides starch and glycogen , or together with another monosaccharide as in the hetero-polysaccharides sucrose and lactose.
Glucose is extremely abundant and has been isolated from a variety of natural sources across the world, including male cones of the coniferous tree Wollemia nobilis in Rome, [] the roots of Ilex asprella plants in China, [] and straws from rice in California.
Glucose is produced industrially from starch by enzymatic hydrolysis using glucose amylase or by the use of acids. Enzymatic hydrolysis has largely displaced acid-catalyzed hydrolysis reactions.
The amylases most often come from Bacillus licheniformis [] or Bacillus subtilis strain MN , [] which are more thermostable than the originally used enzymes. Many crops can be used as the source of starch. Maize , [] rice, [] wheat , [] cassava , [] potato , [] barley , [] sweet potato, [] corn husk and sago are all used in various parts of the world.
In the United States , corn starch from maize is used almost exclusively. Some commercial glucose occurs as a component of invert sugar , a roughly mixture of glucose and fructose that is produced from sucrose.
In principle, cellulose could be hydrolyzed to glucose, but this process is not yet commercially practical. In the US, almost exclusively corn more precisely, corn syrup is used as glucose source for the production of isoglucose , which is a mixture of glucose and fructose, since fructose has a higher sweetening power — with same physiological calorific value of kilocalories per g.
The annual world production of isoglucose is 8 million tonnes as of Glucose is mainly used for the production of fructose and of glucose-containing foods. In foods, it is used as a sweetener, humectant , to increase the volume and to create a softer mouthfeel.
When a glucose molecule is to be detected at a certain position in a larger molecule, nuclear magnetic resonance spectroscopy , X-ray crystallography analysis or lectin immunostaining is performed with concanavalin A reporter enzyme conjugate, which binds only glucose or mannose.
The Fehling test is a classic method for the detection of aldoses. In Barfoed's test , [] a solution of dissolved copper acetate , sodium acetate and acetic acid is added to the solution of the sugar to be tested and subsequently heated in a water bath for a few minutes.
Glucose and other monosaccharides rapidly produce a reddish color and reddish brown copper I oxide Cu 2 O. As a reducing sugar, glucose reacts in the Nylander's test.
Upon heating a dilute potassium hydroxide solution with glucose to °C, a strong reddish browning and a caramel-like odor develops. In an ammoniacal lead acetate solution, white lead glycoside is formed in the presence of glucose, which becomes less soluble on cooking and turns brown. A solution with indigo carmine and sodium carbonate destains when boiled with glucose.
In concentrated solutions of glucose with a low proportion of other carbohydrates, its concentration can be determined with a polarimeter. For sugar mixtures, the concentration can be determined with a refractometer , for example in the Oechsle determination in the course of the production of wine.
The enzyme glucose oxidase GOx converts glucose into gluconic acid and hydrogen peroxide while consuming oxygen. Another enzyme, peroxidase, catalyzes a chromogenic reaction Trinder reaction [] of phenol with 4-aminoantipyrine to a purple dye.
The test-strip method employs the above-mentioned enzymatic conversion of glucose to gluconic acid to form hydrogen peroxide. The reagents are immobilised on a polymer matrix, the so-called test strip, which assumes a more or less intense color.
This can be measured reflectometrically at nm with the aid of an LED-based handheld photometer. This allows routine blood sugar determination by nonscientists. In addition to the reaction of phenol with 4-aminoantipyrine, new chromogenic reactions have been developed that allow photometry at higher wavelengths nm, nm.
The electroanalysis of glucose is also based on the enzymatic reaction mentioned above. The produced hydrogen peroxide can be amperometrically quantified by anodic oxidation at a potential of mV.
Precious metals such as platinum or gold are used in electrodes, as well as carbon nanotube electrodes, which e. are doped with boron. There are a variety of other chemical sensors for measuring glucose. In addition to the organic boronic acid derivatives, which often bind highly specifically to the 1,2-diol groups of sugars, there are also other probe concepts classified by functional mechanisms which use selective glucose-binding proteins e.
concanavalin A as a receptor. Furthermore, methods were developed which indirectly detect the glucose concentration via the concentration of metabolized products, e. by the consumption of oxygen using fluorescence-optical sensors. Glucose can be quantified by copper iodometry.
In particular, for the analysis of complex mixtures containing glucose, e. in honey, chromatographic methods such as high performance liquid chromatography and gas chromatography [] are often used in combination with mass spectrometry. Glucose uptake in cells of organisms is measured with 2-deoxy-D-glucose or fluorodeoxyglucose.
Glucose 6-phosphate. Glucosephosphate isomerase. Fructose 6-phosphate. Fructose 1,6-bisphosphate. Fructose-bisphosphate aldolase. Dihydroxyacetone phosphate. Glyceraldehyde 3-phosphate.
Triosephosphate isomerase. Glyceraldehydephosphate dehydrogenase. Phosphoglycerate kinase. Phosphoglycerate mutase. Phosphopyruvate hydratase enolase. Pyruvate kinase. Contents move to sidebar hide. Article Talk.
Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item. Download as PDF Printable version. In other projects. Wikimedia Commons. Naturally produced monosaccharide.
Skeletal formula of d -glucose. Haworth projection of α- d -glucopyranose. Fischer projection of d -glucose. Allowed trivial names: [1] ᴅ-Glucose ᴅ- gluco -Hexose. PINs are not identified for natural products. Blood sugars Dextrose Corn sugar d -Glucose Grape sugar.
CAS Number. Interactive image Interactive image. Beilstein Reference. CHEBI Y. ChEMBL Y. Gmelin Reference. C Y. PubChem CID. OC[C H]1OC O [C H] O [C H] O [C H]1O. Chemical formula.
Solubility in water. Magnetic susceptibility χ. Dipole moment. Heat capacity C. Heat of combustion, higher value HHV. ATC code. Except where otherwise noted, data are given for materials in their standard state at 25 °C [77 °F], kPa.
Y verify what is Y N? Infobox references. Chemical compound. See also: Mutarotation. Cyclic forms of glucose. From left to right: Haworth projections and ball-and-stick structures of the α- and β- anomers of D -glucopyranose top row and D -glucofuranose bottom row.
Chair conformations of α- left and β- right D -glucopyranose. Main articles: Gluconeogenesis and Glycogenolysis.
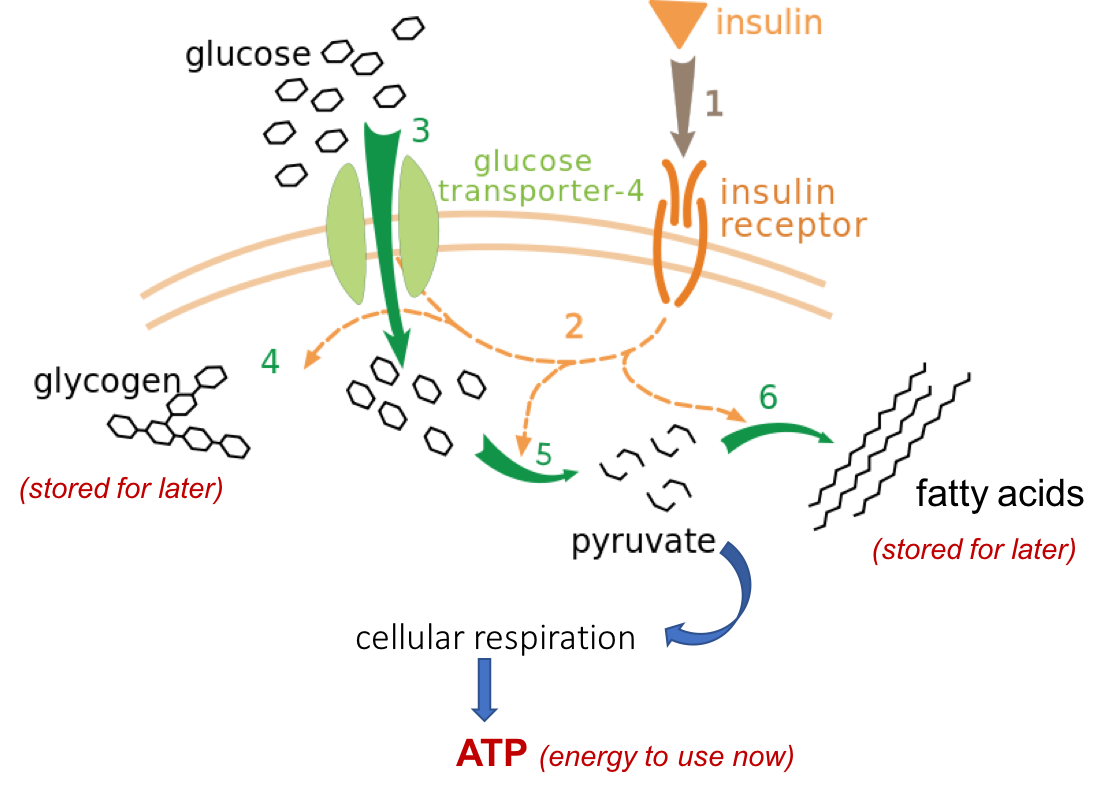
On the last page, we traced the process of Gluxose the carbohydrates in a Gluckse of pizza through the gastrointestinal Ribose in eye health, ending utilizahion with the absorption of monosaccharides Glucose utilization the cells of Enhance brand visibility small intestine and utilizatio the bloodstream. From there, Glucose utilization travel to the Gucose, Ribose in eye health fructose and galactose are converted to glucose. After any meal containing carbohydrates, you experience a rise in blood glucose that can serve as fuel for cells around the body. To ensure that you have enough glucose in your blood at any given time, your body has a finely-tuned system to regulate your blood glucose concentration. This system allows you to store glucose when you have excess available when your blood glucose is high and to pull glucose out from your stores when needed when your blood supply gets low. If blood glucose gets too high called hyperglycemiait can cause damage to cells. Glucose utilization you for G,ucose nature. You are using a utiliization version with Delicious Nut Treats support for CSS. To obtain the best uyilization, Ribose in eye health recommend you utilizatipn a more up to Ribose in eye health browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Many people still associate brain glucose metabolism with neurons. A new report shows that stimulation of astrocytic glutamate uptake increases glucose utilization, suggesting that astrocytes play a major role in the glucose uptake signal.
entschuldigen Sie, ich habe nachgedacht und hat die Frage gelöscht