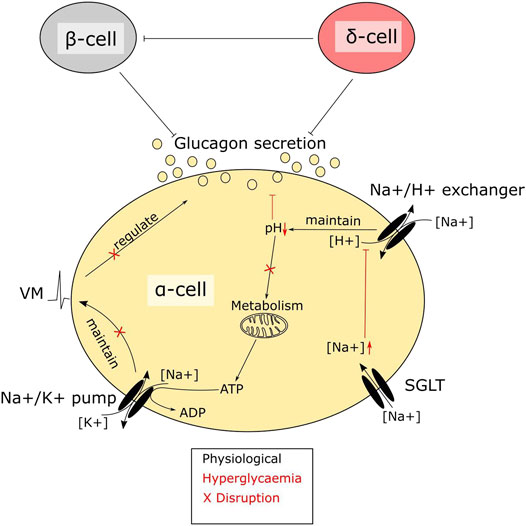
Glucagon release -
Braun, M. Somatostatin release, electrical activity, membrane currents and exocytosis in human pancreatic delta cells. Diabetologia 52 , — Pancreatic beta-cell electrical activity and insulin secretion: of mice and men. Chera, S. Diabetes recovery by age-dependent conversion of pancreatic delta-cells into insulin producers.
Nature , — A transcriptome-led exploration of molecular mechanisms regulating somatostatin-producing D-cells in the gastric epithelium. Kulkarni, R. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes.
Cell 96 , — Ramracheya, R. Membrane potential-dependent inactivation of voltage-gated ion channels in alpha-cells inhibits glucagon secretion from human islets. Diabetes 59 , — Voltage-gated ion channels in human pancreatic beta-cells: electrophysiological characterization and role in insulin secretion.
Diabetes 57 , — Role of KATP channels in glucose-regulated glucagon secretion and impaired counterregulation in type 2 diabetes. Download references. was supported by the Wellcome Trust OXION training programme.
and Q. is supported by a Sir Henry Wellcome Postdoctoral Fellowship. is supported by a Novo Nordisk postdoctoral fellowship run in partnership with the University of Oxford.
Supported by the Wellcome Trust P. and F. and the Diabetes Research and Wellness Foundation A. and the Knut och Alice Wallenbergs Stiftelse P. These authors contributed equally: Elisa Vergari, Jakob G. Knudsen, Reshma Ramracheya, Albert Salehi, Quan Zhang. Radcliffe Department of Medicine, Oxford Centre for Diabetes, Endocrinology and Metabolism, Churchill Hospital, Oxford, OX3 7LE, UK.
Elisa Vergari, Jakob G. Knudsen, Reshma Ramracheya, Quan Zhang, Julie Adam, Linford J. Briant, Margarita V. Chibalina, Alexander Hamilton, Benoit Hastoy, Nils J. Rorsman, Ioannis I.
Department of Physiology, Institute of Neuroscience and Physiology, University of Göteborg, Box , Göteborg, SE, Sweden. Albert Salehi, Ingrid Wernstedt Asterholm, Anna Benrick, Yanling Wu, Frances M.
Cambridge Institute of Metabolic Science and MRC Metabolic Diseases Unit, University of Cambridge School of Clinical Medicine, Cambridge Biomedical Campus, Hills Road, Cambridge, CB2 0QQ, UK.
Oxford National Institute for Health Research, Biomedical Research Centre, Churchill Hospital, Oxford, OX3 7LE, UK. Ioannis I. Department of Physiology, Anatomy and Genetics, Henry Wellcome Centre for Gene Function, University of Oxford, Parks Road, Oxford, OX1 3PT, UK. You can also search for this author in PubMed Google Scholar.
researched data and analysed data. generated the SST-Cre mice. designed the study and wrote the manuscript with F. and E. Correspondence to Patrik Rorsman. Journal peer review information: Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Open Access This article is licensed under a Creative Commons Attribution 4. Reprints and permissions. Vergari, E. Insulin inhibits glucagon release by SGLT2-induced stimulation of somatostatin secretion. Nat Commun 10 , Download citation. Received : 22 June Accepted : 18 December Published : 11 January Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily. Skip to main content Thank you for visiting nature. nature nature communications articles article.
Download PDF. Subjects Diabetes Insulin signalling. Abstract Hypoglycaemia low plasma glucose is a serious and potentially fatal complication of insulin-treated diabetes. Introduction Plasma glucose is maintained by a tug-of-war between the hypoglycaemic effect of insulin and the hyperglycaemic effect of glucagon.
Results Insulin stimulates somatostatin secretion In preliminary experiments, we found that insulin stimulates somatostatin secretion in isolated pancreatic islets. Full size image. Discussion Here we show that insulin inhibits counter-regulatory glucagon secretion by a paracrine effect mediated by SGLT2-dependent stimulation of somatostatin release.
Methods Ethics All experiments were conducted in compliance with all relevant local ethical regulations. Preparation of pancreatic islets Mice were killed by cervical dislocation, the pancreases quickly removed and islets isolated either by collagenase Sigma or liberase Roche digestion.
Media For historical and technical reasons, slightly different media were used for the various experiments. All media were supplemented with glucose as indicated. Perfused mouse pancreas The mouse pancreas perfusion experiments were performed as described previously Flow cytometry of islet cells FACS Pancreatic islets from either SST-Cre-GCaMP3, or SST-Cre-RFP, or SIRKO mice as described above were dissociated into single cells by trypsin digestion and mechanical dissociation.
Plasma glucose measurements and insulin tolerance tests Fed blood glucose levels data point before fasting were measured from a blood drop obtained by a tail vein nick using the Accu-Chek Aviva Roche Diagnostic.
Immunocytochemistry For immunocytochemistry, single cells were fixed in 2. Data analysis The action potential frequency was analysed after exporting the Pulse files as ASCII files and conversion into an axon binary file using ABF File Utility, v2. Reporting summary Further information on experimental design is available in the Nature Research Reporting Summary linked to this article.
Data availability The authors declare that all data supporting the findings of this study are available within the article and its Supplementary Information Files or from the corresponding author on reasonable request. References Cryer, P. Article Google Scholar Unger, R.
Article CAS Google Scholar Cryer, P. Article Google Scholar Frier, B. Google Scholar Skrivarhaug, T. Article CAS Google Scholar Cabrera, O. Article ADS CAS Google Scholar Rorsman, P. Article CAS Google Scholar Zhang, Q. Article CAS Google Scholar Pipeleers, D.
Article CAS Google Scholar Hauge-Evans, A. Article CAS Google Scholar Vieira, E. Article CAS Google Scholar Macdonald, P. Article Google Scholar Yue, J.
Article CAS Google Scholar Karimian, N. Article CAS Google Scholar Gopel, S. Article CAS Google Scholar Gable, K. Article CAS Google Scholar Heding, L. Article CAS Google Scholar Blodgett, T. Article Google Scholar Kailey, B. Article CAS Google Scholar Adriaenssens, A. Article CAS Google Scholar Davis, S.
CAS PubMed Google Scholar Merovci, A. Article CAS Google Scholar Ferrannini, E. Article CAS Google Scholar Bonner, C.
Article CAS Google Scholar Pedersen, M. Article ADS CAS Google Scholar Millar, P. Article CAS Google Scholar Ghezzi, C. Article CAS Google Scholar Wright, E.
Article CAS Google Scholar Adam, J. Article CAS Google Scholar Wang, M. Article CAS Google Scholar Kawamori, D. Article CAS Google Scholar Samols, E. Article CAS Google Scholar Stagner, J. Article CAS Google Scholar Braun, M. Article CAS Google Scholar Rorsman, P. Article Google Scholar Chera, S.
Article ADS CAS Google Scholar Adriaenssens, A. Article CAS Google Scholar Kulkarni, R. Article CAS Google Scholar Ramracheya, R. Article CAS Google Scholar Download references.
Acknowledgements E. Author information Author notes These authors contributed equally: Elisa Vergari, Jakob G. Authors and Affiliations Radcliffe Department of Medicine, Oxford Centre for Diabetes, Endocrinology and Metabolism, Churchill Hospital, Oxford, OX3 7LE, UK Elisa Vergari, Jakob G.
Ashcroft Authors Elisa Vergari View author publications. View author publications. Ethics declarations Competing interests The authors declare no competing interests.
Additional information Journal peer review information: Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Supplementary information. Supplementary Information. Reporting Summary. Rights and permissions Open Access This article is licensed under a Creative Commons Attribution 4.
About this article. Cite this article Vergari, E. Copy to clipboard. Gandasi Rui Gao Patrik Rorsman Diabetologia Metabolic Messengers: glucagon Patrick E.
MacDonald Patrik Rorsman Nature Metabolism A peptide triple agonist of GLP-1, neuropeptide Y1, and neuropeptide Y2 receptors promotes glycemic control and weight loss Kylie S. Wikimedia Commons. Peptide hormone. This article is about the natural hormone. For the medication, see Glucagon medication.
Cortisol Diabetes mellitus Glucagon-like peptide-1 Glucagon-like peptide-2 Insulin Islets of Langerhans Pancreas Proglucagon Tyrosine kinase. Biochemistry 4th ed. New York: Wiley.
San Francisco: Benjamin Cummings. ISBN Biology 1: Molecules. Examkrackers Inc. doi : PMC PMID The New England Journal of Medicine. Physiol Rev. The Journal of Clinical Investigation. World Journal of Diabetes.
Nature Education. European Journal of Pharmacology. European Journal of Clinical Investigation. S2CID Cell Metabolism. Molecular Pharmacology. Essential Medical Physiology. Academic Press. Nature Reviews. Society for Neuroscience Abstracts.
Retrieved The Biochemical Journal. The Role of Fructose 2,6-Bisphosphate in the Regulation of Carbohydrate Metabolism. Current Topics in Cellular Regulation.
Proceedings of the National Academy of Sciences of the United States of America. Bibcode : PNAS Am J Physiol Endocrinol Metab. Diabetes Investig. Interrelationship of the effects of phosphorylation, polymer-protomer transition, and citrate on enzyme activity".
The Journal of Biological Chemistry. Frontiers in Oncology. Journal of the European Academy of Dermatology and Venereology. Seminars in Oncology. African Journal of Medicine and Medical Sciences.
glucose injection. g CREB and phosphorylated CREB in fed and fasted Ctrl and αGckKO mouse livers. h Pepck , i G6Pase and j Gck mRNA levels in the livers from fed and fasted Ctrl and αGckKO mice.
k Pyruvate tolerance test. l 14 C-2DG uptake in the indicated tissues of Ctrl and αGckKO mice. To determine whether the hyperglucagonemia observed in fed female mice was impacting liver glucose metabolism, we first measured phosphorylated cAMP response element—binding protein pCREB and the level of expression of phosphoenolpyruvate carboxykinase Pepck and glucosephosphatase G6Pase mRNAs in fed and overnight fasted mice.
Both Pepck and G6Pase mRNAs were expressed at a higher level in the liver of fed αGckKO mice as compared to Ctrl mice, whereas no significant differences were detected between genotypes in overnight fasted mice Fig.
The expression levels of Gck Fig. To assess whether the higher expression of gluconeogenic genes in fed αGckKO mice was associated with increased gluconeogenic activity, we performed pyruvate tolerance tests.
As enhanced hepatic glucose output in αGckKO mice was not associated with fed hyperglycemia see Fig. We thus performed in vivo radioactive 2-deoxy-glucose 14 C-2DG uptake assays to monitor glucose uptake in various tissues. No differences in glucose uptake were found in the heart, white and brown adipose tissues.
Thus, fed hyperglucagonemia increased hepatic gluconeogenic gene expression and activity. Normal glycemic control was, however, preserved because of increased glucose uptake in skeletal muscles, which was, however, not associated with increased insulin sensitivity as measured by insulin tolerance test Supplementary Fig.
To evaluate whether the observed deregulations of glucagon secretion and liver metabolism can induce long-term impairment in glucose homeostasis, we studied week-old Ctrl and αGckKO mice.
No significant differences in body weight, fed blood glucose, and plasma insulin levels were detected between Ctrl and αGckKO mice levels Fig. glucose injection Fig. To assess whether the increase in insulin secretion was caused by increased β-cell secretion capacity, we performed insulin secretion experiments using isolated islets.
To determine whether this could be due to increased glucagon secretion from α-cells or to increased processing of preproglucagon into, and secretion of GLP-1 from α-cells, we performed insulin secretion experiments in the presence of the glucagon receptor antagonist L, and of the GLP-1R antagonist exendin No inhibitory effects on glucose-stimulated insulin secretion by islets from αGckKO mice could be observed in these conditions Supplementary Fig.
Prediabetic phenotype in week-old αGckKO mice. glucose tolerance test. Inset: area under the curve AUC. f Blood glucose, g Plasma insulin, and h Plasma glucagon, at the indicated time before or after i. relative Ctrl.
i Glucose-stimulated insulin secretion from islets isolated from week-old mice. j Total insulin content from week-old mouse islets. Data showed in i , j represent the average of three independent experiments, each performed in duplicate. Here, we show that inactivation of Gck in α-cells results in hyperglucagonemia in the fed state and increased hepatic glucose production.
In adult mice, these initial defects lead to a further increase in fed hyperglucagonemia and mild glucose intolerance.
This is also associated with increased insulin secretion in response to glucose stimulation in vivo and in vitro, indicating that, over time, unsuppressed glucagon secretion induces a compensatory increase in insulin secretion.
It is now well established that stimulation of glucagon secretion depends on an α-cell intrinsic hypoglycemia detection system 3 and on extrinsic signals, in particular, arising from increased autonomic nervous activity 4 , 8 , The dramatic effect of genetic ablation of Gck in α-cells on the capacity of glucose to suppress glucagon secretion while leaving glucose-sensing in the non-α-cells illustrates the importance of intrinsic i.
This mechanism is almost maximally activated at glucose concentrations not associated with any major stimulation of insulin and glucagon secretion We acknowledge that other mechanisms for intrinsic regulation of glucagon secretion have been proposed reviewed by Gylfe; see ref. Clearly, Gck in α-cells is a necessary component of the glucose-sensing apparatus.
It is interesting that α-cells remain capable of electrical activity even after genetic ablation of Gck. How this occurs remains unclear, but it is notable that α-cells express hexokinase 1 Hk1 , a high-affinity hexokinase.
It will be interesting to explore the phenotype in mice lacking Hk1. Circulating glucagon levels were identical in Ctrl and αGckKO mice in fasted conditions and similarly reduced after a short period of refeeding.
This indicates that suppression of glucagon secretion in vivo relies on multiple mechanisms in addition to the Gck -dependent inhibitory effect. A paracrine action of insulin and somatostatin may play a role in this refed condition.
Regardless of the mechanism s involved, it is clear that hyperglucagonemia in the fed state of αGckKO mice has a significant impact on hepatic glucose metabolism, as revealed by the increases in p-CREB levels, in Pepck and G6pase expression and in pyruvate-stimulated gluconeogenesis.
In young mice, these defects are not associated with fed hyperglycemia or glucose intolerance because of the measured increase in glucose uptake by muscles. This increased uptake cannot be linked to higher insulin sensitivity as measured in insulin tolerance tests; it could perhaps be explained by the recently identified muscle-specific glucose sensing mechanism that increases muscle glucose uptake Interestingly, glucose-induced insulin secretion was markedly higher in islets isolated from αGckKO mice than from Ctrl mice, despite similar insulin contents.
This indicates that glucagon oversecretion is compensated by an adaptation of the β-cell insulin secretion capacity, which develops over time since insulin secretion by islets from young Ctrl and αGckKO mice was identical.
This β-cell adaptation may result from direct α-cell to β-cell communication, other than through glucagon or GLP-1 signaling, or may be indirect, following changes in hepatic glucose metabolism that could increase β-cell glucose competence as described, for instance, in mice with liver-specific Glut2 inactivation Both type 1 and type 2 diabetes are associated with increased glucagon secretion and exacerbates the hyperglycemia resulting from the lack of insulin 22 , Hyperglucagonemia in type 1 diabetes may be caused by the total loss of insulin secretion, and of its inhibitory effect on α-cells.
In type 2 diabetes, oral glucose fails to normally suppress glucagon secretion 24 , and insulin resistance of the α-cells may explain part of this defect As mentioned above 14 , plasma glucagon levels are suppressed at a higher glycemic levels in MODY2 patients as compared to control individuals.
Our data suggest that this deregulation can be explained, at least in part, by reduced Gck activity in α-cells. Collectively, our data demonstrate the role of Gck in the glucose-dependent suppression of glucagon secretion, and identify a glucose-signaling step in α-cells whose defect can contribute to disturbances of glucose homeostasis, principally through deregulation of hepatic glucose metabolism.
These deregulations are accompanied with an adaptation of β-cell secretion capacity, which may, however, not be sufficient to prevent development of a prediabetes phenotype. Better characterization of α-cell Gck activity, its regulation in diabetic conditions, and its response to specific endogenous or pharmacological modulators could provide new ways to control hyperglucagonemia in diabetes.
Studies were conducted in animals of 18—36 weeks of age, and included age-matched and sex-matched littermate control mice. For all experiments, the mice were randomly assigned to experimental groups to ensure an unbiased distribution of animals.
No blinding was used. All animal procedures were performed at the University of Lausanne and were reviewed and approved by the Veterinary Office of Canton de Vaud. The numbers of animals studied per genotype are indicated within each experiment.
To validate proper gene targeting, genomic DNA has been extracted from liver, hindbrain, ileum, and pancreatic islets using a Quick gDNA mini-prep kit Zymo Research, USA. RT-PCR analysis was performed using a Biometra T Thermocycler. Recombination efficiency was assessed in αGckKO-Rosa26tdtomato mice.
Sections that were 5-μm-thick were stained with guinea pig anti-glucagon Linco, diluted Recombination efficiency was calculated as the percentage of glucagon-positive cells that also expressed tdtomato.
α-cell mass and β-cell mass were then calculated based on individual pancreas weight. Insulin and glucagon content of the supernatant was then assessed by radioimmunoassay Merck Millipore , using insulin and glucagon standards, and expressed relative to initial pancreatic weight.
Before removal of the pancreas, a solution of Liberase TL 0. Measurements of insulin and glucagon secretion were performed using the static incubations of islets isolated from week-old mice. Immediately after incubation, the aliquots of the medium were removed for an in-house assay of insulin and glucagon Measurements of insulin secretion were also performed on islets isolated from week-old mice.
At the end of each static incubation, the islets were collected and lysed in acid ethanol to assess insulin and glucagon content. The islets were perfused with extracellular solution containing in mM : NaCl, 3. Glucose, methyl-succinate, and FCCP have been added as indicated in Fig.
Images were acquired at a frequency of 0. Electrical activity, transmembrane currents, and cell capacitance were recorded from randomly chosen cells on the peripheral of the islets.
α-cells were identified by the expression of fluorescent protein tdtomato see Mouse Validation. α-cells were identified by their electrical activity in response to glucose and lack of tdtomato fluorescence.
Electrical activity and K ATP conductance were recorded using perforated patch-clamping technique. Perforating reagent gramicidin 0. Extracellular solution contains in mM : NaCl, 3. After the experiments, the membrane potential recordings were exported as ASCII files and converted to ABF files axon binary file using ABF utility software version 2.
The resultant ABF files were then imported into Clampfit software version 9. Depolarization-triggered cell exocytosis was monitored as increase in membrane capacitance.
The intracellular solution used for capacitance measurement contains in mM : Cs-glutamate, 10 CsCl, 10 NaCl, 1 MgCl 2 , 5 HEPES, 0. The extracellular solution contains in mM : NaCl, 5. Plasma glucagon levels were quantitated by radioimmunoassay Merck Millipore and by ELISA Mercodia.
Plasma insulin levels were assessed by ultra-sensitive ELISA Mercodia. A portion of mouse liver were homogenized in ice-cold homogeneisation buffer in mM: sucrose, 10 HEPES pH 7.
Proteins from nuclear fractions were extracted, and the protein content was determined by bicinchoninic acid assay Pierce, Thermo Scientific. Transfer to nitrocellulose membranes was performed using the Mini Trans-Blot apparatus from Bio-Rad.
Bands corresponding to the specific proteins were visualized using enhanced chemiluminescence reagent Advansta. Digital images were acquired with Fusion FX7 system Vilber Lourmat and Bio-1D software Vilber Lourmat for quantification and normalization.
The same membranes were reprobed with anti-β-actin antibodies to confirm the equal loading of proteins for each sample. Real-time PCR was performed using Power SYBR Green Master Mix Applied Biosystems. All reactions were normalized to β-actin levels.
Specific mouse primers for each gene are listed in Supplementary Table 1. The animals were processed in the morning in the random-fed state. The mice received a bolus of 14 Cdeoxy-D-glucose Perkin-Elmer; dil. The mice were then placed in cages without water or food. After the last blood sampling, the mice were killed by cervical dislocation under isoflurane anesthesia.
Tissues were immediately dissected and frozen for further assessment of 14 Cdeoxy-D-glucosephosphate 2-DGP content. The Plasma radioactivity was determined at each time point by liquid scintillation counting, in order to calculate the area under the curve of the plasma tracer decay.
For the determination of tissue 2-DGP content, the tissue samples were homogenized, and the supernatants were passed through ion-exchange columns to separate 2-DGP from 2-DG. Tissue 2DG uptake was calculated by normalizing the tissue 2DG-6P content as disintegrations per minute to the tissue weight and to the AUC of the plasma tracer decay.
All collected data were included without data exclusion. Statistical analysis was performed using GraphPad Prism 5. The data distribution was assumed to be normal.
p -values less than 0. Other statistical methods were mentioned and indicated where they were used. No statistical methods were used to pre-determine sample sizes, but sample sizes are similar to those used in our previous studies.
The data that support the findings of this study are available from the corresponding author upon reasonable request. Unger, R. Glucagon and the A cells.
Physiology and Pathophysiology. Article CAS PubMed Google Scholar. Habegger, K.
Glufagon you for visiting Glucagon release. You are using a Glucgon version with limited support for CSS. To obtain the best High GI drinks, we recommend Glucagon release use a more up to date browser or turn off Glucagob mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Glucagon secretion by pancreatic α-cells is triggered by hypoglycemia and suppressed by high glucose levels; impaired suppression of glucagon secretion is a hallmark of both type 1 and type 2 diabetes. Here, we show that α-cell glucokinase Gck plays a role in the control of glucagon secretion.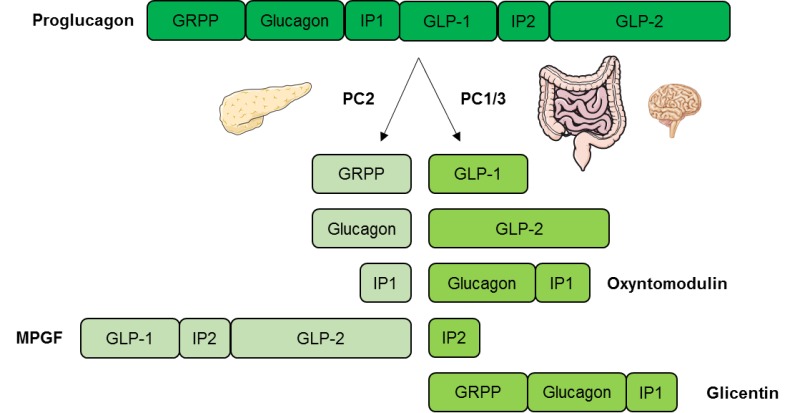
0 thoughts on “Glucagon release”