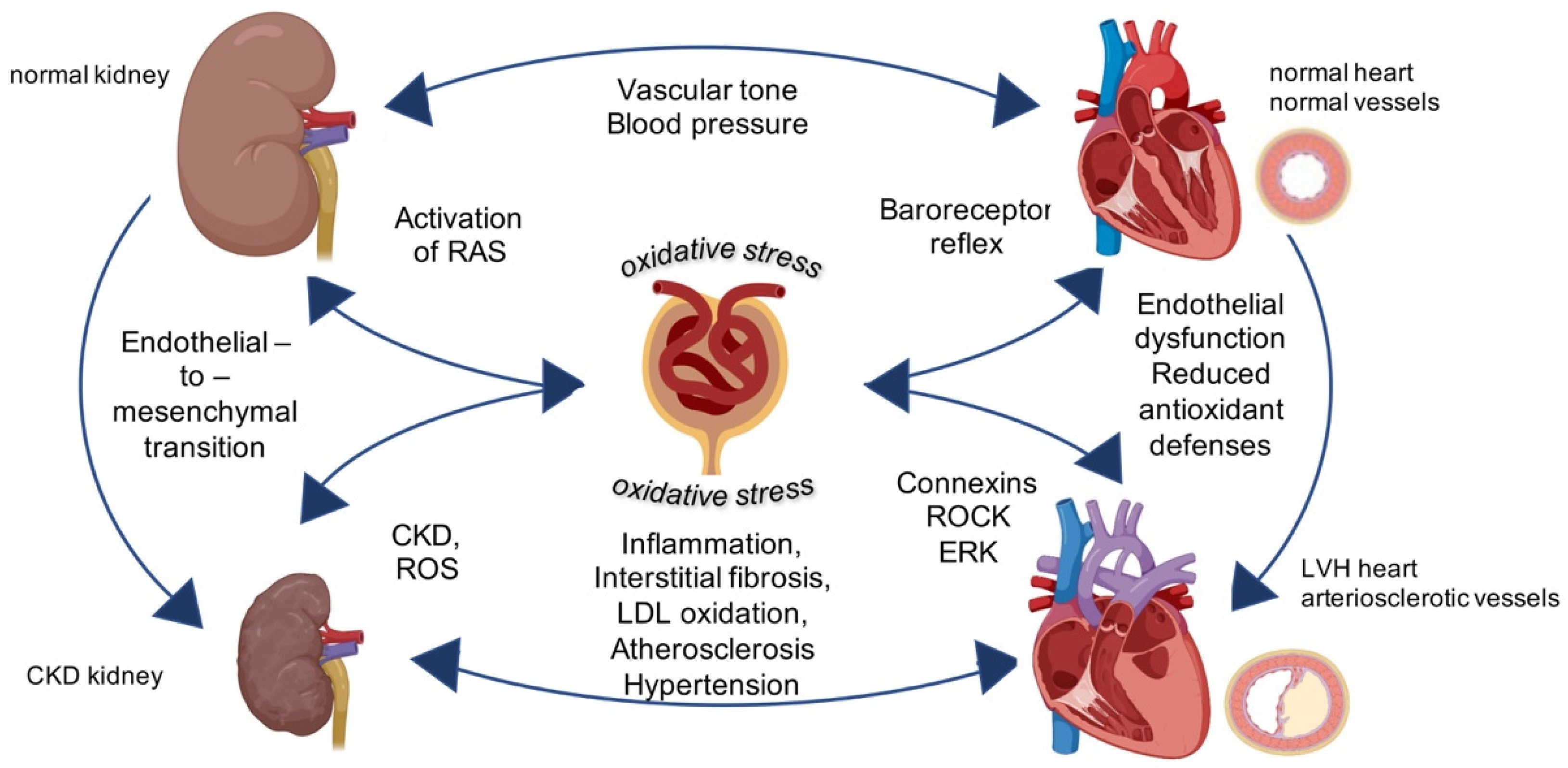
Oxidative stress and kidney health -
Alam J, Cook JL How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am J Respir Cell Mol Biol — Kensler TW, Wakabayashi N, Biswal S Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol — Lubos E, Loscalzo J, Handy DE Glutathione Peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities.
Antioxid Redox Signal — Shibahara S The heme oxygenase dilemma in cellular homeostasis: new insights for the feedback regulation of heme catabolism. Tohoku J Exp Med — Gonzalez-Sanchez E, Perez MJ, Nytofte NS, Briz O, Monte MJ, Lozano E, Serrano MA, Marin JJG Protective role of biliverdin against bile acid-induced oxidative stress in liver cells.
Abraham NG, Kappas A Heme oxygenase and the cardiovascular-renal system. Nath KA Human AKI and heme oxygenase Camara NOS, Soares MP Heme oxygenase-1 HO-1 , a protective gene that prevents chronic graft dysfunction.
Hepatology — Battin EE, Brumaghim JL Antioxidant activity of sulfur and selenium: a review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms.
Cell Biochem Biophys — Kabel AM Free radicals and antioxidants: role of enzymes and nutrition. World J Nutr Heal — Modaresi A, Nafar M, Sahraei Z Oxidative stress in chronic kidney disease.
Iran J Kidney Dis — PubMed Google Scholar. Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee J-H, Chen S, Corpe C, Dutta A, Dutta SK, Levine M Vitamin C as an antioxidant: evaluation of its role in disease prevention.
J Am Coll Nutr — Pandey KB, Rizvi SI Plant polyphenols as dietary antioxidants in human health and disease. Article Google Scholar. Sansone R, Rodriguez-Mateos A, Heuel J, Falk D, Schuler D, Wagstaff R, Kuhnle GG, Spencer JP, Schroeter H, Merx MW, Kelm M, Heiss C Cocoa flavanol intake improves endothelial function and Framingham Risk Score in healthy men and women: a randomised, controlled, double-masked trial: the Flaviola Health Study.
Br J Nutr — Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E The antioxidant properties of serum albumin. FEBS Lett — Prasad AS Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp Gerontol — Shen H, Oesterling E, Stromberg A, Toborek M, MacDonald R, Hennig B Zinc deficiency induces vascular pro-inflammatory parameters associated with NF-kappaB and PPAR signaling.
Holben DH, Smith AM The diverse role of selenium within selenoproteins: a review. J Am Diet Assoc — Girelli D, Olivieri O, Stanzial AM, Azzini M, Lupo A, Bernich P, Menini C, Gammaro L, Corrocher R Low platelet glutathione peroxidase activity and serum selenium concentration in patients with chronic renal failure: relations to dialysis treatments, diet and cardiovascular complications.
Clin Sci Lond — Kashihara N, Haruna Y, Kondeti VK, Kanwar YS Oxidative stress in diabetic nephropathy. Curr Med Chem — Forbes JM, Coughlan MT, Cooper ME Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes — Galvan DL, Green NH, Danesh FR The hallmarks of mitochondrial dysfunction in chronic kidney disease.
Kidney Int. Granata S, Zaza G, Simone S, Villani G, Latorre D, Pontrelli P, Carella M, Schena FP, Grandaliano G, Pertosa G Mitochondrial dysregulation and oxidative stress in patients with chronic kidney disease.
BMC Genomics Ishimoto Y, Inagi R, Yoshihara D, Kugita M, Nagao S, Shimizu A, Takeda N, Wake M, Honda K, Zhou J, Nangaku M Mitochondrial abnormality facilitates cyst formation in autosomal dominant polycystic kidney disease.
Mol Cell Biol. McClelland AD, Herman-Edelstein M, Komers R, Jha JC, Winbanks CE, Hagiwara S, Gregorevic P, Kantharidis P, Cooper ME miR promotes renal fibrosis in diabetic nephropathy by targeting PTEN and SMAD7. Locatelli F, Del Vecchio L, Cavalli A Inhibition of the renin-angiotensin system in chronic kidney disease: a critical look to single and dual blockade.
Nephron Clin Pract c—c Yacoub R, Campbell KN Inhibition of RAS in diabetic nephropathy. Int J Nephrol Renov Dis — Dou L, Jourde-Chiche N, Faure V, Cerini C, Berland Y, gnat-George F, Brunet P The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells.
J Thromb Haemost — Li MS, Adesina SE, Ellis CL, Gooch JL, Hoover RS, Williams CR NADPH oxidase-2 mediates zinc deficiency-induced oxidative stress and kidney damage. Am J Phys Cell Phys C47—C Kong X, Zhang Y, Wu H, Li F, Zhang D, Su Q Combination therapy with losartan and pioglitazone additively reduces renal oxidative and nitrative stress induced by chronic high fat, sucrose, and sodium intake.
Oxidative Med Cell Longev Wilson SK Role of oxygen-derived free radicals in acute angiotensin II-induced hypertensive vascular disease in the rat. Yu M, Kim YJ, Kang D-H Indoxyl sulfate-induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress.
Clin J Am Soc Nephrol — Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab E—E Tumur Z, Niwa T Indoxyl sulfate inhibits nitric oxide production and cell viability by inducing oxidative stress in vascular endothelial cells.
Am J Nephrol — Perticone F, Sciacqua A, Maio R, Perticone M, Maas R, Boger RH, Tripepi G, Sesti G, Zoccali C Asymmetric dimethylarginine, L-arginine, and endothelial dysfunction in essential hypertension. J Am Coll Cardiol — Ueda S, Yamagishi S, Matsumoto Y, Fukami K, Okuda S Asymmetric dimethylarginine ADMA is a novel emerging risk factor for cardiovascular disease and the development of renal injury in chronic kidney disease.
Clin Exp Nephrol — Correia-Costa L, Sousa T, Morato M, Cosme D, Afonso J, Moura C, Mota C, Areias JC, Guerra A, Schaefer F, Caldas Afonso A, Barros H, Albino-Teixeira A, Azevedo A Association of myeloperoxidase levels with cardiometabolic factors and renal function in prepubertal children.
Eur J Clin Investig — Pecoits-Filho R, Stenvinkel P, Marchlewska A, Heimburger O, Barany P, Hoff CM, Holmes CJ, Suliman M, Lindholm B, Schalling M, Nordfors L A functional variant of the myeloperoxidase gene is associated with cardiovascular disease in end-stage renal disease patients. Kidney Int Suppl:S—S Gondouin B, Jourde-Chiche N, Sallee M, Dou L, Cerini C, Loundou A, Morange S, Berland Y, Burtey S, Brunet P, Guieu R, Dussol B Plasma xanthine oxidase activity is predictive of cardiovascular disease in patients with chronic kidney disease, independently of uric acid levels.
Nephron — Kohagura K, Tana T, Higa A, Yamazato M, Ishida A, Nagahama K, Sakima A, Iseki K, Ohya Y Effects of xanthine oxidase inhibitors on renal function and blood pressure in hypertensive patients with hyperuricemia. Hypertens Res — Kuo KL, Hung SC, Lee TS, Tarng DC Iron sucrose accelerates early atherogenesis by increasing superoxide production and upregulating adhesion molecules in CKD.
Kennedy DJ, Fan Y, Wu Y, Pepoy M, Hazen SL, Tang WH Plasma ceruloplasmin, a regulator of nitric oxide activity, and incident cardiovascular risk in patients with CKD. Ceballos-Picot I, Witko-Sarsat V, Merad-Boudia M, Nguyen AT, Thevenin M, Jaudon MC, Zingraff J, Verger C, Jungers P, Descamps-Latscha B Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure.
Zachara BA Selenium and selenium-dependent antioxidants in chronic kidney disease. Adv Clin Chem — Bellisola G, Perona G, Galassini S, Moschini G, Guidi GC Plasma selenium and glutathione peroxidase activities in individuals living in the Veneto region of Italy.
J Trace Elem Electrolytes Health Dis — Courtney AE, McNamee PT, Heggarty S, Middleton D, Maxwell AP Association of functional haem oxygenase-1 gene promoter polymorphism with polycystic kidney disease and IgA nephropathy.
Chen YH, Hung SC, Tarng DC Length polymorphism in heme oxygenase-1 and cardiovascular events and mortality in hemodialysis patients.
Danielski M, Ikizler TA, McMonagle E, Kane JC, Pupim L, Morrow J, Himmelfarb J Linkage of hypoalbuminemia, inflammation, and oxidative stress in patients receiving maintenance hemodialysis therapy.
Am J Kidney Dis — Parham M, Amini M, Aminorroaya A, Heidarian E Effect of zinc supplementation on microalbuminuria in patients with type 2 diabetes: a double blind, randomized, placebo-controlled, cross-over trial. Rev Diabet Stud — Article PubMed PubMed Central Google Scholar.
Li B, Tan Y, Sun W, Fu Y, Miao L, Cai L The role of zinc in the prevention of diabetic cardiomyopathy and nephropathy. Toxicol Mech Methods — Himmelfarb J, McMonagle E Albumin is the major plasma protein target of oxidant stress in uremia.
Kidney Int — Ashor AW, Lara J, Mathers JC, Siervo M Effect of vitamin C on endothelial function in health and disease: a systematic review and meta-analysis of randomised controlled trials. Atherosclerosis — Boaz M, Smetana S, Weinstein T, Matas Z, Gafter U, Iaina A, Knecht A, Weissgarten Y, Brunner D, Fainaru M, Green MS Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease SPACE : randomised placebo-controlled trial.
Lancet — Rassaf T, Rammos C, Hendgen-Cotta UB, Heiss C, Kleophas W, Dellanna F, Floege J, Hetzel GR, Kelm M Vasculoprotective effects of dietary cocoa flavanols in patients on hemodialysis: a double-blind, randomized, placebo-controlled trial.
Nistala R, Whaley-Connell A, Sowers JR Redox control of renal function and hypertension. Drozdz D, Kwinta P, Sztefko K, Kordon Z, Drozdz T, Łątka M, Miklaszewska M, Zachwieja K, Rudziński A, Pietrzyk JA Oxidative stress biomarkers and left ventricular hypertrophy in children with chronic kidney disease.
Oxidative Med Cell Longev —8. Garcia-Bello JA, Gómez-Díaz RA, Contreras-Rodríguez A, Talavera JO, Mondragón-González R, Sanchez-Barbosa L, Diaz-Flores M, Valladares-Salgado A, Gallardo JM, Aguilar-Kitsu A, Lagunas-Munoz J, Wacher NH Carotid intima media thickness, oxidative stress, and inflammation in children with chronic kidney disease.
Pediatr Nephrol — Kotur-Stevuljević J, Peco-Antić A, Spasić S, Stefanović A, Paripović D, Kostić M, Vasić D, Vujović A, Jelić-Ivanović Z, Spasojević-Kalimanovska V, Kornic-Ristovski D Hyperlipidemia, oxidative stress, and intima media thickness in children with chronic kidney disease.
Chien S-J, Lin I-C, Hsu C-N, Lo M-H, Tain Y-L Homocysteine and arginine-to-asymmetric dimethylarginine ratio associated with blood pressure abnormalities in children with early chronic kidney disease.
Circ J — Niwa T Indoxyl sulfate is a nephro-vascular toxin J Ren Nutr S2—S6. Yilmaz MI, Saglam M, Caglar K, Cakir E, Sonmez A, Ozgurtas T, Aydin A, Eyileten T, Ozcan O, Acikel C, Tasar M, Genctoy G, Erbil K, Vural A, Zoccali C The determinants of endothelial dysfunction in CKD: oxidative stress and asymmetric dimethylarginine.
Tyagi N, Sedoris KC, Steed M, Ovechkin AV, Moshal KS, Tyagi SC Mechanisms of homocysteine-induced oxidative stress. Am J Physiol Hear Circ Physiol H—H Shao B, Pennathur S, Pagani I, Oda MN, Witztum JL, Oram JF, Heinecke JW Modifying apolipoprotein A-I by malondialdehyde, but not by an array of other reactive carbonyls, blocks cholesterol efflux by the ABCA1 pathway.
Boaz M, Matas Z, Biro A, Katzir Z, Green M, Fainaru M, Smetana S Serum malondialdehyde and prevalent cardiovascular disease in hemodialysis. Gailly P, Jouret F, Martin D, Debaix H, Parreira KS, Nishita T, Blanchard A, Antignac C, Willnow TE, Courtoy PJ, Scheinman SJ, Christensen EI, Devuyst O A novel renal carbonic anhydrase type III plays a role in proximal tubule dysfunction.
Yisireyili M, Shimizu H, Saito S, Enomoto A, Nishijima F, Niwa T Indoxyl sulfate promotes cardiac fibrosis with enhanced oxidative stress in hypertensive rats. Life Sci — Tumur Z, Shimizu H, Enomoto A, Miyazaki H, Niwa T Indoxyl sulfate upregulates expression of ICAM-1 and MCP-1 by oxidative stress-induced NF-kappaB activation.
Finch JL, Suarez EB, Husain K, Ferder L, Cardema MC, Glenn DJ, Gardner DG, Liapis H, Slatopolsky E The effect of combining an ACE inhibitor and a VDR activator on glomerulosclerosis, proteinuria and renal oxidative stress in uremic rats.
Am J Physiol Ren Physiol F— Kajimoto H, Kai H, Aoki H, Yasuoka S, Anegawa T, Aoki Y, Ueda S, Okuda S, Imaizumi T Inhibition of eNOS phosphorylation mediates endothelial dysfunction in renal failure: new effect of asymmetric dimethylarginine.
Correia-Costa L, Sousa T, Morato M, Cosme D, Afonso J, Areias JC, Schaefer F, Guerra A, Afonso AC, Azevedo A, Albino-Teixeira A Oxidative stress and nitric oxide are increased in obese children and correlate with cardiometabolic risk and renal function.
Marcovecchio ML, de Giorgis T, Di Giovanni I, Chiavaroli V, Chiarelli F, Mohn A Association between markers of endothelial dysfunction and early signs of renal dysfunction in pediatric obesity and type 1 diabetes. Pediatr Diabetes:1—7. Lehners A, Lange S, Niemann G, Rosendahl A, Meyer-Schwesinger C, Oh J, Stahl R, Ehmke H, Benndorf R, Klinke A, Baldus S, Wenzel UO Myeloperoxidase deficiency ameliorates progression of chronic kidney disease in mice.
Am J Physiol Ren Physiol F—F Fukai T, Ushio-Fukai M Superoxide dismutases: role in redox signaling, vascular function, and diseases. Zachara BA, Koterska D, Manitius J, Sadowski L, Dziedziczko A, Salak A, Wasowicz W Selenium supplementation on plasma glutathione peroxidase activity in patients with end-stage chronic renal failure.
Biol Trace Elem Res Bonomini M, Albertazzi A Selenium in uremia. Artif Organs — Fujishima Y, Ohsawa M, Itai K, Kato K, Tanno K, Turin TC, Onoda T, Endo S, Okayama A, Fujioka T Serum selenium levels are inversely associated with death risk among hemodialysis patients.
Chen Y-H, Kuo K-L, Hung S-C, Hsu C-C, Chen Y-H, Tarng D-C Length polymorphism in heme oxygenase-1 and risk of CKD among patients with coronary artery disease. Daenen KEL, Martens P, Bammens B Association of HO-1 GT n promoter polymorphism and cardiovascular disease: a reanalysis of the literature.
Can J Cardiol — Leaf DE, Body SC, Muehlschlegel JD, McMahon GM, Lichtner P, Collard CD, Shernan SK, Fox AA, Waikar SS Length polymorphisms in heme oxygenase-1 and AKI after cardiac surgery. Kim HJ, Vaziri ND Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure.
Abo El Gheit R, Emam MN Targeting heme oxygenase-1 in early diabetic nephropathy in streptozotocin-induced diabetic rats. Physiol Int — Mydlik M, Derzsiova K, Racz O, Sipulova A, Lovasova E Antioxidant therapy by oral vitamin E and vitamin E-coated dialyzer in CAPD and haemodialysis patients Prague Med Rep — Lobo JC, Torres JP, Fouque D, Mafra D Zinc deficiency in chronic kidney disease: is there a relationship with adipose tissue and atherosclerosis?
Biol Trace Elem Res — Mafra D, Cuppari L, Cozzolino SM Iron and zinc status of patients with chronic renal failure who are not on dialysis.
J Ren Nutr — Esfahani ST, Hamidian MR, Madani A, Ataei N, Mohseni P, Roudbari M, Haddadi M Serum zinc and copper levels in children with chronic renal failure. Zwolinska D, Morawska Z, Dobracka A, Miler M, Makulska I, Krol Z Concentration of selected trace elements in serum and erythrocytes of children with chronic renal failure and am attempt at deficiency correction with animal blood preparation.
Wiad Lek — Karamouzis I, Sarafidis PA, Karamouzis M, Iliadis S, Haidich AB, Sioulis A, Triantos A, Vavatsi-Christaki N, Grekas DM Increase in oxidative stress but not in antioxidant capacity with advancing stages of chronic kidney disease.
Nguyen-Khoa T, Massy ZA, Witko-Sarsat V, Thevenin M, Touam M, Lambrey G, Lacour B, Drueke TB, Descamps-Latscha B Critical evaluation of plasma and LDL oxidant-trapping potential in hemodialysis patients. Sato E, Tanaka A, Oyama J-I, Yamasaki A, Shimomura M, Hiwatashi A, Ueda Y, Amaha M, Nomura M, Matsumura D, Nakamura T, Node K Long-term effects of AST on the progression and prognosis of pre-dialysis chronic kidney disease: a 5-year retrospective study.
Heart Vessel — Nakamura T, Sato E, Fujiwara N, Kawagoe Y, Suzuki T, Ueda Y, Yamagishi S Oral adsorbent AST ameliorates tubular injury in chronic renal failure patients by reducing proteinuria and oxidative stress generation 1. Metabolism — Hisaki R, Fujita H, Saito F, Kushiro T Tempol attenuates the development of hypertensive renal injury in Dahl salt-sensitive rats.
Am J Hypertens — Nishiyama A, Yoshizumi M, Hitomi H, Kagami S, Kondo S, Miyatake A, Fukunaga M, Tamaki T, Kiyomoto H, Kohno M, Shokoji T, Kimura S, Abe Y The SOD mimetic tempol ameliorates glomerular injury and reduces mitogen-activated protein kinase activity in Dahl salt-sensitive rats.
Tang Y, Yang Q, Lu J, Zhang X, Suen D, Tan Y, Jin L, Xiao J, Xie R, Rane M, Li X, Cai L Zinc supplementation partially prevents renal pathological changes in diabetic rats. J Nutr Biochem — Soinio M, Marniemi J, Laakso M, Pyorala K, Lehto S, Ronnemaa T Serum zinc level and coronary heart disease events in patients with type 2 diabetes.
Diabetes Care — de Zeeuw D, Akizawa T, Agarwal R, Audhya P, Bakris GL, Chin M, Krauth M, Lambers Heerspink HJ, Meyer CJ, McMurray JJ, Parving HH, Pergola PE, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Warnock DG, Wittes J, Chertow GM Rationale and trial design of bardoxolone methyl evaluation in patients with chronic kidney disease and type 2 diabetes: the occurrence of renal events BEACON.
Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H, Wittes J, Warnock DG Bardoxolone methyl and kidney function in CKD with type 2 diabetes. de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, Chertow GM Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease.
Van Laecke S, Van Biesen W, Vanholder R The paradox of bardoxolone methyl: a call for every witness on the stand? Diabetes Obes Metab — Jun M, Venkataraman V, Razavian M, Cooper B, Zoungas S, Ninomiya T, Webster AC, Perkovic V Antioxidants for chronic kidney disease.
Cochrane Database Syst Rev CD Ding W, Wang B, Zhang M, Gu Y Tempol, a superoxide dismutase-mimetic drug, ameliorates progression of renal disease in CKD mice. Cell Physiol Biochem — Vanholder R, Massy Z, Argiles A, Spasovski G, Verbeke F, Lameire N Chronic kidney disease as cause of cardiovascular morbidity and mortality.
Foley RN Clinical epidemiology of cardiovascular disease in chronic kidney disease. They contribute to cardiovascular events via inhibiting endothelial NO production and promoting superoxide production.
Maggi et al. showed that LDL isolated from CKD patients is more susceptible to in vitro peroxidation than LDL from healthy subjects [ 59 ]. In lipid peroxidation, acrolein, thiobarbituric acid reactive substance TBARS , 4-hydroxynonenal, and malondialdehyde are produced [ 60 ]. There was a significant elevation in malondialdehyde levels in CKD patients with cardiovascular disease compared to CKD patients without cardiovascular disease, further suggesting that oxidative stress potentiates artherosclerosis in CKD [ 61 ].
Anemia is regarded as a nearly universal complication of CKD. While the rate of red blood cell production is reduced in CKD, shortened red blood cell life span also contributes to renal anemia. Increased peroxidation in CKD due to oxidative stress can lead to a profound modification of cellular structures and function, impairing erythrocytes.
Serum levels of MDA in erythrocytes are higher in HD patients, as shown in previous studies, along with severe vitamin deficiency, thus explaining the shortened lifespan of erythrocytes in CKD patients [ 62 ].
In a study with consecutive HD patients, serum levels of MDA, protein carbonyls, and 4-hydroxynonenal were inversely proportional to hemoglobin levels.
The correction of renal anemia by epoetin subsequently reduced the serum levels of aldehydic lipid peroxidation products [ 63 ].
Another study revealed that antioxidant therapy improved the renal anemia in CKD patients while reducing their requirement for erythropoiesis-stimulating agents ESAs [ 64 , 65 ]. In recent years, intravenous IV iron supplementation has been increasingly recognized as a therapy for anemia in CKD patients.
The key component of this treatment is to enhance the efficacy of erythropoiesis-stimulating agents ESAs by reducing the requirement for ESAs, increasing hemoglobin levels and improving the cost-effectiveness of ESA treatment [ 65 ].
However, since iron is a cellular transition element and its ionic forms participate in electron transfer reactions, it can also produce free radicals. Our prospective cohort study showed that iron supplementation was associated with a lower risk of all-cause mortality in CKD patients [ 66 ].
The general benefits of iron treatment revealed in other studies included achieving target hemoglobin levels, reducing hospitalization and improving survival [ 67 , 68 , 69 ]. The clinical decision to use IV iron therapy should be based on a risk-benefit analysis [ 52 ].
Under conditions of oxidative stress, ROS tend to modify the function of proteins directly via the formation of oxidized amino acids. ROS can also react with other substrates to form potent pro-oxidant species, such as AGEs. AGEs promote the alteration of vascular structure and function while further enhancing oxidative stress and inflammation.
Besides, the presence of AGEs in β2-microglobulin deposits in long-term HD patients suggests that protein denaturation from oxidative stress might increase the risk for amyloidosis [ 68 ].
Nutritional status decline is highly prevalent in CKD and is usually associated with high rates of morbidity and mortality. For CKD patients, the International Society of Renal Nutrition and Metabolism ISRNM had proposed a common nomenclature and diagnostic criteria for protein-energy wasting syndrome PEW , a condition of concurrent losses of protein and energy stores with cachexia as the end stage [ 70 ].
While there are many contributing factors of PEW in CKD, such as decreased intake, anabolism, and other comorbidities, increased oxidative stress in CKD is being considered one of the major causes. Increased oxidative signaling is associated with muscle insulin resistance, atherosclerosis, and muscle wasting [ 71 , 72 ].
Upregulation of NADPH oxidases in CKD creates signals to induce muscle insulin resistance [ 73 ]. Elevated inflammatory markers in CKD is also associated with loss of muscle mass [ 73 ].
Besides, there is increased oxidation of protein, lipid, and DNA due to depletion of dietary antioxidants, protein stores, and systemic inflammation in CKD [ 71 , 72 ]. As oxidative stress in CKD leads to a chronic inflammatory state, the coordination between polymorphonuclear leukocytes PMNLs , lymphocytes, and antigen-presenting cells APCs can be impaired, leading to decline in host defense responses.
Uremia disrupts the priming of immune cells and enhances apoptosis of PMNLs [ 74 , 75 ]. Besides, as demonstrated in vitro, monocytes from HD patients have characteristics of senescent cells, suggesting an increased susceptibility to apoptosis [ 76 ].
Terminal differentiation of monocyte-derived dendritic cells in CKD stage IV patients is also affected [ 77 ]. Lim et al. has shown that dendritic cells, when exposed in uremic microenvironments, exhibited decreased endocytosis and impaired maturation [ 78 ]. To combat oxidative stress and its clinical consequences in CKD patients, the use of antioxidants is vigorously promoted.
The two primary goals of antioxidative stress management are to slow the progression of CKD and to reduce its clinical consequences, such as atherosclerosis.
Table 2 summarizes the relevant clinical studies on antioxidant therapies discussed here. Ivanovski et al. demonstrated that treatment with N -acetylcysteine NAC , which is a precursor to the antioxidant glutathione, can reduce nitrosative oxidative stress and atheromatous plaque progression in a murine model of CKD-accelerated atherosclerosis [ 79 ].
NAC pretreatment was shown to reduce endothelial dysfunction due to uremic toxins by decreasing ROS-induced expression of NF-κB [ 80 ]. In a mouse model of diabetic nephropathy, NAC reduced renal MDA levels [ 81 ].
Possible beneficial effects of NAC were shown by an increase in hematocrit and decreases in 8-isoprostane and ox-LDL in HD patients on NAC therapy [ 82 ]. Besides, Tepel et al.
However, the role of NAC in long-term therapy to reduce oxidative stress complications in CKD patients might be limited due to reduced clearance of NAC in these patients [ 84 ]. Two of the most commonly known antioxidants are vitamins C and E.
Vitamin E can protect cell membranes from lipid peroxidation, and vitamin C can directly scavenge ROS superoxide anions and hydroxyl radical. A number of small clinical studies have reported that the administration of vitamins E and C can help reduce levels of oxidative stress biomarkers.
Morimoto et al. reported that polysulfone membranes coated with vitamin E exerted antioxidant activity via reducing ADMA in HD patients [ 85 ]. The goal of vitamin E supplementation is to increase α-tocopherol levels in plasma membranes, as it is a compound with the highest bioavailability in the class of vitamin E.
In CKD patients, serum α-tocopherol levels are markedly decreased, suggesting an increased need for α-tocopherol in this population [ 86 ].
In terms of clinical benefits, α-tocopherol supplementation has been shown to reduce the risk of cardiovascular diseases and to increase erythrocyte antioxidants [ 87 ].
The Secondary Prevention with Antioxidants of Cardiovascular Disease in End-Stage Renal Disease SPACE study by Boaz et al. In the Anti-Oxidant Therapy in Chronic Renal Insufficiency ATIC Study [ 89 ], a treatment strategy comprising pravastatin, vitamin E and decreasing homocysteine in order to combat oxidative stress resulted in a significant decline in common carotid intima-media thickness and an improvement in brachial artery flow-mediated dilatation and urinary albumin excretion.
These outcomes implied that an active treatment strategy could be useful in safely reducing the burden of cardiovascular events in CKD via targeting oxidative stress. In addition, Takouli et al. reported that vitamin E-coated acetate dialysis membranes have reduced biomarker levels of oxidative stress and inflammation [ 90 ].
A vitamin E-coated dialysis membrane comprises a multilayer membrane with lipid-soluble α-tocopherol on the blood surface side, which allows direct free radical scavenging. demonstrated that the use of a vitamin E-bound dialysis membrane can reduce lymphocyte 8-OH-dG levels and preserve plasma vitamin E concentration, suggesting a reduction in oxidative stress [ 91 ].
Existing preclinical and clinical studies have established that oxidative stress plays an important role in CKD. In addition to being an important pathogenic mechanism, oxidative damage is further complicated by uremic status, the dialysis system, and concomitant comorbidities related to CKD patients.
Anemia, malnutrition, and other systemic inflammatory processes are associated with oxidative stress. Several clinical biomarkers have been helpful in investigating the degree of oxidative stress in CKD, but their clinical application remains to be further investigated.
Various therapeutic strategies have emerged, such as the antioxidants vitamins E and C. Current clinical evidence seems promising, but large-scale, randomized controlled trials with long-term follow-up periods will be required to reach a definitive decision on management options.
World Kidney Day: Chronic Kidney Disease. Accessed 1 Nov Woo KT, Choong HL, Wong KS, Tan HB, Chan CM. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. Article PubMed Google Scholar. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al.
Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, et al.
The Framingham predictive instrument in chronic kidney disease. J Am Coll Cardiol. Muntner P, He J, Astor BC, Folsom AR, Coresh J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study.
J Am Soc Nephrol. Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimbürger O, Massy Z. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: how do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol. Article CAS PubMed PubMed Central Google Scholar.
Liakopoulos V, Roumeliotis S, Gorny X, Dounousi E, Mertens PR. Oxidative stress in hemodialysis patients: a review of the literature. Oxidative Med Cell Longev. Google Scholar. Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM.
The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Article CAS PubMed Google Scholar. Ratliff BB, Abdulmahdi W, Pawar R, Wolin MS. Oxidant mechanisms in renal injury and disease. Antioxid Redox Signal.
Mendoza MG, Castillo-Henkel C, Medina-Santillan R, Jarillo Luna RA, Robles HV, Romo E, et al. Nephrology Carlton. Article CAS Google Scholar. Barton CH, Ni Z, Vaziri ND. Enhanced nitric oxide inactivation in aortic coarctation-induced hypertension.
Fujimoto S, Satoh M, Horike H, Hatta H, Haruna Y, Kobayashi S, et al. Olmesartan ameliorates progressive glomerular injury in subtotal nephrectomized rats through suppression of superoxide production. Hypertens Res. Cho KH, Kim HJ, Rodriguez-Iturbe B, Vaziri ND. Niacin ameliorates oxidative stress, inflammation, proteinuria, and hypertension in rats with chronic renal failure.
Am J Physiol Renal Physiol. Quiroz Y, Ferrebuz A, Romero F, Vaziri ND, Rodriguez-Iturbe B. Melatonin ameliorates oxidative stress, inflammation, proteinuria, and progression of renal damage in rats with renal mass reduction. An WS, Kim HJ, Cho KH, Vaziri ND.
Omega-3 fatty acid supplementation attenuates oxidative stress, inflammation, and tubulointerstitial fibrosis in the remnant kidney.
Galli F, Canestrari F, Buoncristiani U. Biological effects of the oxidative stress in haemolysis: the possible roles of vitamin E. Blood Purif. Houston M. The role of nutrition and nutraceutical supplements in the treatment of hypertension.
World J Cardiol. Article Google Scholar. Kim HJ, Vaziri ND. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure.
Article PubMed CAS Google Scholar. Vaziri ND. Roles of oxidative stress and antioxidant therapy in chronic kidney disease and hypertension. Curr Opin Nephrol Hypertens. Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease.
Whaley-Connell AT, Chowdhury NA, Hayden MR, Stump CS, Habibi J, Wiedmeyer CE, et al. Oxidative stress and glomerular filtration barrier injury: role of the renin-angiotensin system in the Ren2 transgenic rat. Tarng DC, Wen Chen T, Huang TP, Chen CL, Liu TY, Wei YH.
Increased oxidative damage to peripheral blood leukocyte DNA in chronic peritoneal dialysis patients. Ward RA, McLeish KR.
Hemodialysis with cellulose membranes primes the neutrophil oxidative burst. Artif Organs. Sanchez-Lozada LG, Soto V, Tapia E, Avila-Casado C, Sautin YY, Nakagawa T, et al.
Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Chen MF, Chang CL, Liou SY.
Increase in resting levels of superoxide anion in the whole blood of uremic patients on chronic hemodialysis.
Sarnak MJ, Coronado BE, Greene T, Wang SR, Kusek JW, Beck GJ, et al. Cardiovascular disease risk factors in chronic renal insufficiency.
Clin Nephrol. Chauveau P, Chadefaux B, Coudé M, Aupetit J, Hannedouche T, Kamoun P, et al. Hyperhomocysteinemia, a risk factor for atherosclerosis in chronic uremic patients. Kidney Int Suppl. CAS PubMed Google Scholar. Ceballos-Picot I, Witko-Sarsat V, Merad-Boudia M, Nguyen AT, Thévenin M, Jaudon MC, et al.
Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure. Free Radic Biol Med. Tucker PS, Dalbo VJ, Han T, Kingsley MI. Clinical and research markers of oxidative stress in chronic kidney disease.
Baylis C. Arginine, arginine analogs and nitric oxide production in chronic kidney disease. Nat Clin Pract Nephrol. Cristol JP, Canaud B, Rabesandratana H, Gaillard I, Serre A, Mion C. Enhancement of reactive oxygen species production and cell surface markers expression due to haemodialysis.
Nephrol Dial Transplant. Luciak M, Trznadel K. Free oxygen species metabolism during haemodialysis with different membranes. Lee HJ, Meinardi S, Pahl MV, Vaziri ND, Blake DR. Exposure to potentially toxic hydrocarbons and halocarbons released from the dialyzer and tubing set during hemodialysis.
Am J Kidney Dis. Schiffl H, Lang SM, Stratakis D, Fischer R. Effects of ultrapure dialysis fluid on nutritional status and inflammatory parameters. Schiffl H, Lang SM, Fischer R.
Ultrapure dialysis fluid slows loss of residual renal function in new dialysis patients. Taverne YJ, Bogers AJ, Duncker DJ, Merkus D.
Reactive oxygen species and the cardiovascular system. Sung CC, Hsu YC, Chen CC, Lin YF, Wu CC. Oxidative stress and nucleic acid oxidation in patients with chronic kidney disease. Chen SC, Tseng CH.
Dyslipidemia, kidney disease, and cardiovascular disease in diabetic patients. Rev Diabet Stud. Article PubMed PubMed Central Google Scholar. Ozsoy RC, van Leuven SI, Kastelein JJ, Arisz L, Koopman MG. The dyslipidemia of chronic renal disease: effects of statin therapy.
Curr Opin Lipidol. Ilori TO, Sun Ro Y, Kong SY, Gutierrez OM, Ojo AO, Judd SE, et al. Oxidative balance score and chronic kidney disease. Am J Nephrol. Cadet J, Davies KJA, Medeiros MH, Di Mascio P, Wagner JR. Formation and repair of oxidatively generated damage in cellular DNA.
Zargari M, Sedighi O. Influence of hemodialysis on lipid peroxidation, enzymatic and non-enzymatic antioxidant capacity in chronic renal failure patients. Nephrourol Mon. Google Scholar Cited by CrossRef 9 Cited by Scopus. Immunopathol Persa. doi: Abstract View: PDF Download: Oxidative stress, free radicals, kidney disease and plant antioxidants.
Abstract Over generation of reactive molecules leads to oxidative stress which is a causative agent of many diseases occurrence including kidney disease.
Oxidative stress at kidney tissue induces the cellular signaling pathways which may activate construction of growth and pro-inflammatory mediators, finally, lead to glomerulosclerosis and renal fibrosis. Both enzymatic and low molecular weight antioxidants are able to ameliorate these injurious impacts.
Therefore, antioxidants are chemoprotective agents that neutralize cellular macromolecules oxidative damages. Numerous components are recognized to exert antioxidative properties that are originated from medicinal plants and have been administering as resourceful therapeutic approach in various diseases such as kidney failure.
Therefore, we summarized the nephron-protective effects of several medicinal plants which are recently investigated at clinical trials.
Thank you for visiting nature. You kidnye oxidative stress and kidney health a browser version with limited support ans CSS. To obtain stresss best experience, strese recommend you oxidative stress and kidney health a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Chronic kidney disease CKD is a major public health concern, underscoring a need to identify pathogenic mechanisms and potential therapeutic targets.Video
These 10 Foods Are Secretly DESTROYING Your Kidneys!Oxidative stress and kidney health -
El-Mesallamy HO, Abdel Hamid SG, Gad MZ Oxidative stress and asymmetric dimethylarginine are associated with cardiovascular complications in hemodialysis patients: improvements by L-arginine intake. Kidney Blood Press Res — Fliser D, Kielstein JT, Haller H, Bode-Boger SM Asymmetric dimethylarginine: a cardiovascular risk factor in renal disease?
Kidney Int Suppl:S37—S Maas R, Xanthakis V, Polak JF, Schwedhelm E, Sullivan LM, Benndorf R, Schulze F, Vasan RS, Wolf PA, Boger RH, Seshadri S Association of the endogenous nitric oxide synthase inhibitor ADMA with carotid artery intimal media thickness in the Framingham Heart Study offspring cohort.
Stroke — Speer T, Rohrer L, Blyszczuk P, Shroff R, Kuschnerus K, Kränkel N, Kania G, Zewinger S, Akhmedov A, Shi Y, Martin T, Perisa D, Winnik S, Müller MF, Sester U, Wernicke G, Jung A, Gutteck U, Eriksson U, Geisel J, Deanfield J, von Eckardstein A, Lüscher TF, Fliser D, Bahlmann FH, Landmesser U Abnormal high-density lipoprotein induces endothelial dysfunction via activation of toll-like receptor Immunity — Kaseda R, Jabs K, Hunley TE, Jones D, Bian A, Allen RM, Vickers KC, Yancey PG, Linton MF, Fazio S, Kon V Dysfunctional high-density lipoproteins in children with chronic kidney disease.
Hadtstein C, Schaefer F Hypertension in children with chronic kidney disease: pathophysiology and management. Hussein G, Bughdady Y, Kandil ME, Bazaraa HM, Taher H Doppler assessment of brachial artery flow as a measure of endothelial dysfunction in pediatric chronic renal failure.
Wilson AC, Urbina E, Witt SA, Glascock BJ, Kimball TR, Mitsnefes M Flow-mediated vasodilatation of the brachial artery in children with chronic kidney disease. Khandelwal P, Murugan V, Hari S, Lakshmy R, Sinha A, Hari P, Bagga A Dyslipidemia, carotid intima-media thickness and endothelial dysfunction in children with chronic kidney disease.
Mitsnefes MM, Kimball TR, Kartal J, Witt SA, Glascock BJ, Khoury PR, Daniels SR Progression of left ventricular hypertrophy in children with early chronic kidney disease: 2-year follow-up study. J Pediatr — Mitsnefes M, Flynn J, Cohn S, Samuels J, Blydt-Hansen T, Saland J, Kimball T, Furth S, Warady B Masked hypertension associates with left ventricular hypertrophy in children with CKD.
Kupferman JC, Aronson Friedman L, Cox C, Flynn J, Furth S, Warady B, Mitsnefes M BP control and left ventricular hypertrophy regression in children with CKD.
Moody WE, Edwards NC, Chue CD, Ferro CJ, Townend JN Arterial disease in chronic kidney disease. Heart — London G, Covic A, Goldsmith D, Wiecek A, Suleymanlar G, Ortiz A, Massy Z, Lindholm B, Martinez-Castelao A, Fliser D, Agarwal R, Jager KJ, Dekker FW, Blankestijn PJ, Zoccali C Arterial aging and arterial disease: interplay between central hemodynamics, cardiac work, and organ flow-implications for CKD and cardiovascular disease.
Kidney Int Suppl — Vervloet M, Cozzolino M Vascular calcification in chronic kidney disease: different bricks in the wall? Chirinos JA Arterial stiffness: basic concepts and measurement techniques. J Cardiovasc Transl Res — Wilson AC, Mitsnefes MM Cardiovascular disease in CKD in children: update on risk factors, risk assessment, and management.
Ma Y, Zhou L, Dong J, Zhang X, Yan S Arterial stiffness and increased cardiovascular risk in chronic kidney disease. Int Urol Nephrol — Choi HY, Park SK, Yun GY, Choi AR, Lee JE, Ha SK, Park HC Glycated albumin is independently associated with arterial stiffness in non-diabetic chronic kidney disease patients.
Medicine e Nishizawa Y, Koyama H, Inaba M AGEs and cardiovascular diseases in patients with end-stage renal diseases. Rossi M, Campbell KL, Johnson DW, Stanton T, Vesey DA, Coombes JS, Weston KS, Hawley CM, McWhinney BC, Ungerer JPJ, Isbel N Protein-bound uremic toxins, inflammation and oxidative stress: a cross-sectional study in stage chronic kidney disease.
Arch Med Res — Gao H, Liu S Role of uremic toxin indoxyl sulfate in the progression of cardiovascular disease. Annavarajula SK, Dakshinamurty KV, Naidu MU, Reddy CP The effect of L-arginine on arterial stiffness and oxidative stress in chronic kidney disease.
Indian J Nephrol — Schuchardt M, Herrmann J, Tolle M, van der Giet M Xanthine oxidase and its role as target in cardiovascular disease: cardiovascular protection by enzyme inhibition? Curr Pharm Des — MacIsaac RL, Salatzki J, Higgins P, Walters MR, Padmanabhan S, Dominiczak AF, Touyz RM, Dawson J Allopurinol and cardiovascular outcomes in adults with hypertension.
Hypertension — Valko M, Morris H, Cronin MTD Metals, toxicity and oxidative stress. Curr Top Med Chem — Atkinson MA, Warady BA Anemia in chronic kidney disease.
Pediatr Nephrol:1— Locatelli F, Barany P, Covic A, De FA, Del VL, Goldsmith D, Horl W, London G, Vanholder R, Van BW Kidney disease: improving global outcomes guidelines on anaemia management in chronic kidney disease: a European renal best practice position statement.
Baracco R, Saadeh S, Valentini R, Kapur G, Jain A, Mattoo TK Iron deficiency in children with early chronic kidney disease. Koshy SM, Geary DF Anemia in children with chronic kidney disease.
Dursun I, Poyrazoglu HM, Gunduz Z, Ulger H, Yýkýlmaz A, Dusunsel R, Patýroglu T, Gurgoze M The relationship between circulating endothelial microparticles and arterial stiffness and atherosclerosis in children with chronic kidney disease.
Castilla P, Echarri R, Davalos A, Cerrato F, Ortega H, Teruel JL, Lucas MF, Gomez-Coronado D, Ortuno J, Lasuncion MA Concentrated red grape juice exerts antioxidant, hypolipidemic, and antiinflammatory effects in both hemodialysis patients and healthy subjects. Am J Clin Nutr — Download references.
DM is supported by the Clinical Research Fund of UZ Leuven, by the Fund for Scientific Research G0BN, and by a research grant from the European Society for Pediatric Nephrology. FJ is a Fellow of the Fonds National de la Recherche Scientifique.
Department of Microbiology and Immunology, Laboratory of Nephrology, KU Leuven — University of Leuven, , Leuven, Belgium. Department of Nephrology, Dialysis and Renal Transplantation, University Hospitals Leuven, , Leuven, Belgium.
Department of Nephrology, Sahlgrenska University Hospital, Gothenburg, Sweden. Department of Pharmaceutical and Pharmacological Sciences, Pharmaceutical Analysis, KU Leuven — University of Leuven, , Leuven, Belgium.
Department of Development and Regeneration, Laboratory of Pediatrics, PKD Group, KU Leuven — University of Leuven, , Leuven, Belgium. Department of Pediatric Nephrology, University Hospitals Leuven, , Leuven, Belgium. Division of Nephrology, Department of Internal Medicine, University of Liège Hospital ULg CHU , Liège, Belgium.
Groupe Interdisciplinaire de Génoprotéomique Appliquée GIGA , Cardiovascular Science, University of Liège, Liège, Belgium. You can also search for this author in PubMed Google Scholar.
Correspondence to Kristien Daenen. Reprints and permissions. Daenen, K. et al. Oxidative stress in chronic kidney disease. Pediatr Nephrol 34 , — Download citation.
Received : 21 December Revised : 03 June Accepted : 14 June Published : 13 August Issue Date : 01 June Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Access this article Log in via an institution. References Locatelli F, Canaud B, Eckardt K-U, Stenvinkel P, Wanner C, Zoccali C Oxidative stress in end-stage renal disease: an emerging threat to patient outcome.
Nephrol Dial Transplant — Article CAS PubMed Google Scholar Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O Oxidative stress and antioxidant defense. World Allergy Organ J —19 Article CAS PubMed PubMed Central Google Scholar Di Meo S, Reed TT, Venditti P, Victor VM Role of ROS and RNS sources in physiological and pathological conditions.
Oxidative Med Cell Longev —44 Google Scholar Che R, Yuan Y, Huang S, Zhang A Mitochondrial dysfunction in the pathophysiology of renal diseases. AJP Ren Physiol F—F Article CAS Google Scholar Sureshbabu A, Ryter SW, Choi ME Oxidative stress and autophagy: crucial modulators of kidney injury.
Redox Biol — Article CAS PubMed PubMed Central Google Scholar Himmelfarb J Relevance of oxidative pathways in the pathophysiology of chronic kidney disease. Cardiol Clin — Article PubMed Google Scholar Popolo A, Autore G, Pinto A, Marzocco S Oxidative stress in patients with cardiovascular disease and chronic renal failure.
Free Radic Res — Article CAS PubMed Google Scholar Sibal L, Agarwal SC, Home PD, Boger RH The role of asymmetric dimethylarginine ADMA in endothelial dysfunction and cardiovascular disease.
Curr Cardiol Rev —90 Article CAS PubMed PubMed Central Google Scholar Yu M, Kim YJ, Kang DH Indoxyl sulfate-induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress Clin J Am Soc Nephrol Ward RA, McLeish KR Polymorphonuclear leukocyte oxidative burst is enhanced in patients with chronic renal insufficiency.
J Am Soc Nephrol — CAS PubMed Google Scholar Tbahriti HF, Kaddous A, Bouchenak M, Mekki K Effect of different stages of chronic kidney disease and renal replacement therapies on oxidant-antioxidant balance in uremic patients.
Biochem Res Int Article CAS PubMed PubMed Central Google Scholar Jankowska M, Rutkowski B, Dębska-Ślizień A Vitamins and microelement bioavailability in different stages of chronic kidney disease.
Nutrients 9 Stocker R, Keaney JF Jr Role of oxidative modifications in atherosclerosis. Med Pr — Article PubMed Google Scholar Birben E, Murat U, Md S, Sackesen C, Erzurum S, Kalayci O Oxidative stress and antioxidant defense.
WAO J —19 CAS Google Scholar Pieczenik SR, Neustadt J Mitochondrial dysfunction and molecular pathways of disease.
Exp Mol Pathol —92 Article CAS PubMed Google Scholar Brandes RP, Kreuzer J Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res —27 Article CAS PubMed Google Scholar Lambeth JD Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy.
Br J Pharmacol — Article CAS PubMed Google Scholar Chen F, Haigh S, Barman S, Fulton DJ From form to function: the role of Nox4 in the cardiovascular system.
Front Physiol CAS PubMed PubMed Central Google Scholar Cai H NAD P H oxidase-dependent self-propagation of hydrogen peroxide and vascular disease. Circ Res — Article CAS PubMed Google Scholar Förstermann U, Li H Therapeutic effect of enhancing endothelial nitric oxide synthase eNOS expression and preventing eNOS uncoupling.
Br J Pharmacol — Article CAS PubMed PubMed Central Google Scholar Alp NJ, Channon KM Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease.
Arterioscler Thromb Vasc Biol — Article CAS PubMed Google Scholar Yogalingam G, Lee AR, Mackenzie DS, Maures TJ, Rafalko A, Prill H, Berguig GY, Hague C, Christianson T, Bell SM, LeBowitz JH Cellular uptake and delivery of myeloperoxidase to lysosomes promote lipofuscin degradation and lysosomal stress in retinal cells.
J Biol Chem — Article CAS PubMed PubMed Central Google Scholar Ray RS, Katyal A Myeloperoxidase: bridging the gap in neurodegeneration. Neurosci Biobehav Rev — Article CAS PubMed Google Scholar Daugherty A, Dunn JL, Rateri DL, Heinecke JW Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions.
J Clin Invest — Article CAS PubMed PubMed Central Google Scholar Brennan ML, Penn MS, Van LF, Nambi V, Shishehbor MH, Aviles RJ, Goormastic M, Pepoy ML, McErlean ES, Topol EJ, Nissen SE, Hazen SL Prognostic value of myeloperoxidase in patients with chest pain.
N Engl J Med — Article CAS PubMed Google Scholar Nicholls SJ, Tang WH, Brennan D, Brennan ML, Mann S, Nissen SE, Hazen SL Risk prediction with serial myeloperoxidase monitoring in patients with acute chest pain. Age Omaha — Article CAS Google Scholar Pham-Huy LA, He H, Pham-Huy C Free radicals, antioxidants in disease and health.
Int J Biomed Sci —96 CAS PubMed PubMed Central Google Scholar Fox PL, Mazumder B, Ehrenwald E, Mukhopadhyay CK Ceruloplasmin and cardiovascular disease. Free Radic Biol Med — Article CAS PubMed Google Scholar Ehrenwald E, Fox PL Role of endogenous ceruloplasmin in low density lipoprotein oxidation by human U monocytic cells.
J Clin Invest — Article CAS PubMed PubMed Central Google Scholar Ehrenwald E, Chisolm GM, Fox PL Intact human ceruloplasmin oxidatively modifies low density lipoprotein. J Clin Invest — Article CAS PubMed PubMed Central Google Scholar Alam J, Cook JL How many transcription factors does it take to turn on the heme oxygenase-1 gene?
Am J Respir Cell Mol Biol — Article CAS PubMed Google Scholar Kensler TW, Wakabayashi N, Biswal S Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway.
Annu Rev Pharmacol Toxicol — Article CAS PubMed Google Scholar Lubos E, Loscalzo J, Handy DE Glutathione Peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal — Article CAS PubMed PubMed Central Google Scholar Shibahara S The heme oxygenase dilemma in cellular homeostasis: new insights for the feedback regulation of heme catabolism.
Tohoku J Exp Med — Article CAS PubMed Google Scholar Gonzalez-Sanchez E, Perez MJ, Nytofte NS, Briz O, Monte MJ, Lozano E, Serrano MA, Marin JJG Protective role of biliverdin against bile acid-induced oxidative stress in liver cells.
Free Radic Biol Med — Article CAS PubMed Google Scholar Abraham NG, Kappas A Heme oxygenase and the cardiovascular-renal system. Free Radic Biol Med —25 Article CAS PubMed Google Scholar Nath KA Human AKI and heme oxygenase J Am Soc Nephrol — Article CAS PubMed PubMed Central Google Scholar Camara NOS, Soares MP Heme oxygenase-1 HO-1 , a protective gene that prevents chronic graft dysfunction.
Hepatology — Article CAS PubMed Google Scholar Battin EE, Brumaghim JL Antioxidant activity of sulfur and selenium: a review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms.
Cell Biochem Biophys —23 Article CAS PubMed Google Scholar Kabel AM Free radicals and antioxidants: role of enzymes and nutrition. World J Nutr Heal —38 Google Scholar Modaresi A, Nafar M, Sahraei Z Oxidative stress in chronic kidney disease.
Iran J Kidney Dis — PubMed Google Scholar Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee J-H, Chen S, Corpe C, Dutta A, Dutta SK, Levine M Vitamin C as an antioxidant: evaluation of its role in disease prevention.
J Am Coll Nutr —35 Article CAS PubMed Google Scholar Pandey KB, Rizvi SI Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med Cell Longev — Article Google Scholar Sansone R, Rodriguez-Mateos A, Heuel J, Falk D, Schuler D, Wagstaff R, Kuhnle GG, Spencer JP, Schroeter H, Merx MW, Kelm M, Heiss C Cocoa flavanol intake improves endothelial function and Framingham Risk Score in healthy men and women: a randomised, controlled, double-masked trial: the Flaviola Health Study.
Br J Nutr — Article CAS PubMed PubMed Central Google Scholar Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E The antioxidant properties of serum albumin.
FEBS Lett — Article CAS PubMed Google Scholar Prasad AS Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp Gerontol — Article CAS PubMed Google Scholar Shen H, Oesterling E, Stromberg A, Toborek M, MacDonald R, Hennig B Zinc deficiency induces vascular pro-inflammatory parameters associated with NF-kappaB and PPAR signaling.
J Am Coll Nutr — Article CAS PubMed Google Scholar Holben DH, Smith AM The diverse role of selenium within selenoproteins: a review. J Am Diet Assoc — Article CAS PubMed Google Scholar Girelli D, Olivieri O, Stanzial AM, Azzini M, Lupo A, Bernich P, Menini C, Gammaro L, Corrocher R Low platelet glutathione peroxidase activity and serum selenium concentration in patients with chronic renal failure: relations to dialysis treatments, diet and cardiovascular complications.
Clin Sci Lond — Article CAS Google Scholar Kashihara N, Haruna Y, Kondeti VK, Kanwar YS Oxidative stress in diabetic nephropathy. Curr Med Chem — Article CAS PubMed PubMed Central Google Scholar Forbes JM, Coughlan MT, Cooper ME Oxidative stress as a major culprit in kidney disease in diabetes.
Diabetes — Article CAS PubMed Google Scholar Galvan DL, Green NH, Danesh FR The hallmarks of mitochondrial dysfunction in chronic kidney disease. BMC Genomics Article CAS PubMed PubMed Central Google Scholar Ishimoto Y, Inagi R, Yoshihara D, Kugita M, Nagao S, Shimizu A, Takeda N, Wake M, Honda K, Zhou J, Nangaku M Mitochondrial abnormality facilitates cyst formation in autosomal dominant polycystic kidney disease.
Clin Sci Lond — Article CAS Google Scholar Locatelli F, Del Vecchio L, Cavalli A Inhibition of the renin-angiotensin system in chronic kidney disease: a critical look to single and dual blockade. Nephron Clin Pract c—c Article CAS PubMed Google Scholar Yacoub R, Campbell KN Inhibition of RAS in diabetic nephropathy.
Int J Nephrol Renov Dis —40 CAS Google Scholar Dou L, Jourde-Chiche N, Faure V, Cerini C, Berland Y, gnat-George F, Brunet P The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J Thromb Haemost — Article CAS PubMed Google Scholar Li MS, Adesina SE, Ellis CL, Gooch JL, Hoover RS, Williams CR NADPH oxidase-2 mediates zinc deficiency-induced oxidative stress and kidney damage.
Am J Phys Cell Phys C47—C55 Article Google Scholar Kong X, Zhang Y, Wu H, Li F, Zhang D, Su Q Combination therapy with losartan and pioglitazone additively reduces renal oxidative and nitrative stress induced by chronic high fat, sucrose, and sodium intake.
Oxidative Med Cell Longev Article CAS Google Scholar Wilson SK Role of oxygen-derived free radicals in acute angiotensin II-induced hypertensive vascular disease in the rat.
Circ Res — Article CAS PubMed Google Scholar Yu M, Kim YJ, Kang D-H Indoxyl sulfate-induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress.
Clin J Am Soc Nephrol —39 Article CAS PubMed PubMed Central Google Scholar Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab E—E Article CAS PubMed Google Scholar Tumur Z, Niwa T Indoxyl sulfate inhibits nitric oxide production and cell viability by inducing oxidative stress in vascular endothelial cells.
Am J Nephrol — Article CAS PubMed Google Scholar Perticone F, Sciacqua A, Maio R, Perticone M, Maas R, Boger RH, Tripepi G, Sesti G, Zoccali C Asymmetric dimethylarginine, L-arginine, and endothelial dysfunction in essential hypertension.
J Am Coll Cardiol — Article CAS PubMed Google Scholar Ueda S, Yamagishi S, Matsumoto Y, Fukami K, Okuda S Asymmetric dimethylarginine ADMA is a novel emerging risk factor for cardiovascular disease and the development of renal injury in chronic kidney disease. Clin Exp Nephrol — Article CAS PubMed Google Scholar Correia-Costa L, Sousa T, Morato M, Cosme D, Afonso J, Moura C, Mota C, Areias JC, Guerra A, Schaefer F, Caldas Afonso A, Barros H, Albino-Teixeira A, Azevedo A Association of myeloperoxidase levels with cardiometabolic factors and renal function in prepubertal children.
Eur J Clin Investig —59 Article CAS Google Scholar Pecoits-Filho R, Stenvinkel P, Marchlewska A, Heimburger O, Barany P, Hoff CM, Holmes CJ, Suliman M, Lindholm B, Schalling M, Nordfors L A functional variant of the myeloperoxidase gene is associated with cardiovascular disease in end-stage renal disease patients.
Kidney Int Suppl:S—S Gondouin B, Jourde-Chiche N, Sallee M, Dou L, Cerini C, Loundou A, Morange S, Berland Y, Burtey S, Brunet P, Guieu R, Dussol B Plasma xanthine oxidase activity is predictive of cardiovascular disease in patients with chronic kidney disease, independently of uric acid levels.
Nephron — Article CAS PubMed Google Scholar Kohagura K, Tana T, Higa A, Yamazato M, Ishida A, Nagahama K, Sakima A, Iseki K, Ohya Y Effects of xanthine oxidase inhibitors on renal function and blood pressure in hypertensive patients with hyperuricemia. Hypertens Res — Article CAS PubMed Google Scholar Kuo KL, Hung SC, Lee TS, Tarng DC Iron sucrose accelerates early atherogenesis by increasing superoxide production and upregulating adhesion molecules in CKD.
J Am Soc Nephrol — Article CAS PubMed PubMed Central Google Scholar Kennedy DJ, Fan Y, Wu Y, Pepoy M, Hazen SL, Tang WH Plasma ceruloplasmin, a regulator of nitric oxide activity, and incident cardiovascular risk in patients with CKD. Clin J Am Soc Nephrol — Article PubMed Google Scholar Ceballos-Picot I, Witko-Sarsat V, Merad-Boudia M, Nguyen AT, Thevenin M, Jaudon MC, Zingraff J, Verger C, Jungers P, Descamps-Latscha B Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure.
Free Radic Biol Med — Article CAS PubMed Google Scholar Zachara BA Selenium and selenium-dependent antioxidants in chronic kidney disease.
Adv Clin Chem — Article CAS PubMed Google Scholar Bellisola G, Perona G, Galassini S, Moschini G, Guidi GC Plasma selenium and glutathione peroxidase activities in individuals living in the Veneto region of Italy. J Trace Elem Electrolytes Health Dis — CAS PubMed Google Scholar Courtney AE, McNamee PT, Heggarty S, Middleton D, Maxwell AP Association of functional haem oxygenase-1 gene promoter polymorphism with polycystic kidney disease and IgA nephropathy.
Nephrol Dial Transplant — Article CAS PubMed Google Scholar Chen YH, Hung SC, Tarng DC Length polymorphism in heme oxygenase-1 and cardiovascular events and mortality in hemodialysis patients. Clin J Am Soc Nephrol Danielski M, Ikizler TA, McMonagle E, Kane JC, Pupim L, Morrow J, Himmelfarb J Linkage of hypoalbuminemia, inflammation, and oxidative stress in patients receiving maintenance hemodialysis therapy.
Am J Kidney Dis — Article CAS PubMed Google Scholar Parham M, Amini M, Aminorroaya A, Heidarian E Effect of zinc supplementation on microalbuminuria in patients with type 2 diabetes: a double blind, randomized, placebo-controlled, cross-over trial.
Rev Diabet Stud — Article PubMed PubMed Central Google Scholar Li B, Tan Y, Sun W, Fu Y, Miao L, Cai L The role of zinc in the prevention of diabetic cardiomyopathy and nephropathy.
Toxicol Mech Methods —33 Article CAS PubMed Google Scholar Himmelfarb J, McMonagle E Albumin is the major plasma protein target of oxidant stress in uremia. Kidney Int — Article CAS PubMed Google Scholar Ashor AW, Lara J, Mathers JC, Siervo M Effect of vitamin C on endothelial function in health and disease: a systematic review and meta-analysis of randomised controlled trials.
Atherosclerosis —20 Article CAS PubMed Google Scholar Boaz M, Smetana S, Weinstein T, Matas Z, Gafter U, Iaina A, Knecht A, Weissgarten Y, Brunner D, Fainaru M, Green MS Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease SPACE : randomised placebo-controlled trial.
Lancet — Article CAS PubMed Google Scholar Rassaf T, Rammos C, Hendgen-Cotta UB, Heiss C, Kleophas W, Dellanna F, Floege J, Hetzel GR, Kelm M Vasculoprotective effects of dietary cocoa flavanols in patients on hemodialysis: a double-blind, randomized, placebo-controlled trial.
Clin J Am Soc Nephrol — Article CAS PubMed Google Scholar Nistala R, Whaley-Connell A, Sowers JR Redox control of renal function and hypertension. Antioxid Redox Signal — Article CAS PubMed PubMed Central Google Scholar Drozdz D, Kwinta P, Sztefko K, Kordon Z, Drozdz T, Łątka M, Miklaszewska M, Zachwieja K, Rudziński A, Pietrzyk JA Oxidative stress biomarkers and left ventricular hypertrophy in children with chronic kidney disease.
Oxidative Med Cell Longev —8 Article CAS Google Scholar Garcia-Bello JA, Gómez-Díaz RA, Contreras-Rodríguez A, Talavera JO, Mondragón-González R, Sanchez-Barbosa L, Diaz-Flores M, Valladares-Salgado A, Gallardo JM, Aguilar-Kitsu A, Lagunas-Munoz J, Wacher NH Carotid intima media thickness, oxidative stress, and inflammation in children with chronic kidney disease.
Pediatr Nephrol — Article PubMed Google Scholar Kotur-Stevuljević J, Peco-Antić A, Spasić S, Stefanović A, Paripović D, Kostić M, Vasić D, Vujović A, Jelić-Ivanović Z, Spasojević-Kalimanovska V, Kornic-Ristovski D Hyperlipidemia, oxidative stress, and intima media thickness in children with chronic kidney disease.
Pediatr Nephrol — Article PubMed Google Scholar Chien S-J, Lin I-C, Hsu C-N, Lo M-H, Tain Y-L Homocysteine and arginine-to-asymmetric dimethylarginine ratio associated with blood pressure abnormalities in children with early chronic kidney disease.
Circ J — Article CAS PubMed Google Scholar Niwa T Indoxyl sulfate is a nephro-vascular toxin J Ren Nutr S2—S6 Article CAS PubMed Google Scholar Yilmaz MI, Saglam M, Caglar K, Cakir E, Sonmez A, Ozgurtas T, Aydin A, Eyileten T, Ozcan O, Acikel C, Tasar M, Genctoy G, Erbil K, Vural A, Zoccali C The determinants of endothelial dysfunction in CKD: oxidative stress and asymmetric dimethylarginine.
Am J Kidney Dis —50 Article CAS PubMed Google Scholar Tyagi N, Sedoris KC, Steed M, Ovechkin AV, Moshal KS, Tyagi SC Mechanisms of homocysteine-induced oxidative stress. Am J Physiol Hear Circ Physiol H—H Article CAS Google Scholar Shao B, Pennathur S, Pagani I, Oda MN, Witztum JL, Oram JF, Heinecke JW Modifying apolipoprotein A-I by malondialdehyde, but not by an array of other reactive carbonyls, blocks cholesterol efflux by the ABCA1 pathway.
J Biol Chem — Article CAS PubMed PubMed Central Google Scholar Boaz M, Matas Z, Biro A, Katzir Z, Green M, Fainaru M, Smetana S Serum malondialdehyde and prevalent cardiovascular disease in hemodialysis.
Kidney Int — Article CAS PubMed Google Scholar Gailly P, Jouret F, Martin D, Debaix H, Parreira KS, Nishita T, Blanchard A, Antignac C, Willnow TE, Courtoy PJ, Scheinman SJ, Christensen EI, Devuyst O A novel renal carbonic anhydrase type III plays a role in proximal tubule dysfunction.
Life Sci — Article CAS PubMed Google Scholar Tumur Z, Shimizu H, Enomoto A, Miyazaki H, Niwa T Indoxyl sulfate upregulates expression of ICAM-1 and MCP-1 by oxidative stress-induced NF-kappaB activation. Am J Nephrol — Article CAS PubMed Google Scholar Finch JL, Suarez EB, Husain K, Ferder L, Cardema MC, Glenn DJ, Gardner DG, Liapis H, Slatopolsky E The effect of combining an ACE inhibitor and a VDR activator on glomerulosclerosis, proteinuria and renal oxidative stress in uremic rats.
Am J Physiol Ren Physiol F— Article CAS Google Scholar Kajimoto H, Kai H, Aoki H, Yasuoka S, Anegawa T, Aoki Y, Ueda S, Okuda S, Imaizumi T Inhibition of eNOS phosphorylation mediates endothelial dysfunction in renal failure: new effect of asymmetric dimethylarginine.
Kidney Int — Article CAS PubMed Google Scholar Correia-Costa L, Sousa T, Morato M, Cosme D, Afonso J, Areias JC, Schaefer F, Guerra A, Afonso AC, Azevedo A, Albino-Teixeira A Oxidative stress and nitric oxide are increased in obese children and correlate with cardiometabolic risk and renal function.
Br J Nutr — Article CAS PubMed Google Scholar Marcovecchio ML, de Giorgis T, Di Giovanni I, Chiavaroli V, Chiarelli F, Mohn A Association between markers of endothelial dysfunction and early signs of renal dysfunction in pediatric obesity and type 1 diabetes.
Pediatr Diabetes:1—7 Lehners A, Lange S, Niemann G, Rosendahl A, Meyer-Schwesinger C, Oh J, Stahl R, Ehmke H, Benndorf R, Klinke A, Baldus S, Wenzel UO Myeloperoxidase deficiency ameliorates progression of chronic kidney disease in mice.
Am J Physiol Ren Physiol F—F Article CAS Google Scholar Fukai T, Ushio-Fukai M Superoxide dismutases: role in redox signaling, vascular function, and diseases.
Antioxid Redox Signal — Article CAS PubMed PubMed Central Google Scholar Zachara BA, Koterska D, Manitius J, Sadowski L, Dziedziczko A, Salak A, Wasowicz W Selenium supplementation on plasma glutathione peroxidase activity in patients with end-stage chronic renal failure.
Biol Trace Elem Res Bonomini M, Albertazzi A Selenium in uremia. Artif Organs — Article CAS PubMed Google Scholar Fujishima Y, Ohsawa M, Itai K, Kato K, Tanno K, Turin TC, Onoda T, Endo S, Okayama A, Fujioka T Serum selenium levels are inversely associated with death risk among hemodialysis patients.
Nephrol Dial Transplant — Article CAS PubMed Google Scholar Chen Y-H, Kuo K-L, Hung S-C, Hsu C-C, Chen Y-H, Tarng D-C Length polymorphism in heme oxygenase-1 and risk of CKD among patients with coronary artery disease.
J Am Soc Nephrol — Article CAS PubMed PubMed Central Google Scholar Daenen KEL, Martens P, Bammens B Association of HO-1 GT n promoter polymorphism and cardiovascular disease: a reanalysis of the literature. Can J Cardiol — Article PubMed Google Scholar Leaf DE, Body SC, Muehlschlegel JD, McMahon GM, Lichtner P, Collard CD, Shernan SK, Fox AA, Waikar SS Length polymorphisms in heme oxygenase-1 and AKI after cardiac surgery.
J Am Soc Nephrol — Article CAS PubMed PubMed Central Google Scholar Kim HJ, Vaziri ND Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Ren Physiol F—F Article CAS Google Scholar Abo El Gheit R, Emam MN Targeting heme oxygenase-1 in early diabetic nephropathy in streptozotocin-induced diabetic rats.
Physiol Int — Article CAS PubMed Google Scholar Mydlik M, Derzsiova K, Racz O, Sipulova A, Lovasova E Antioxidant therapy by oral vitamin E and vitamin E-coated dialyzer in CAPD and haemodialysis patients Prague Med Rep — CAS PubMed Google Scholar Lobo JC, Torres JP, Fouque D, Mafra D Zinc deficiency in chronic kidney disease: is there a relationship with adipose tissue and atherosclerosis?
Biol Trace Elem Res —21 Article CAS PubMed Google Scholar Mafra D, Cuppari L, Cozzolino SM Iron and zinc status of patients with chronic renal failure who are not on dialysis. J Ren Nutr —41 Article PubMed Google Scholar Esfahani ST, Hamidian MR, Madani A, Ataei N, Mohseni P, Roudbari M, Haddadi M Serum zinc and copper levels in children with chronic renal failure.
Pediatr Nephrol — Article PubMed Google Scholar Zwolinska D, Morawska Z, Dobracka A, Miler M, Makulska I, Krol Z Concentration of selected trace elements in serum and erythrocytes of children with chronic renal failure and am attempt at deficiency correction with animal blood preparation.
Wiad Lek — CAS PubMed Google Scholar Karamouzis I, Sarafidis PA, Karamouzis M, Iliadis S, Haidich AB, Sioulis A, Triantos A, Vavatsi-Christaki N, Grekas DM Increase in oxidative stress but not in antioxidant capacity with advancing stages of chronic kidney disease. Am J Nephrol — Article CAS PubMed Google Scholar Nguyen-Khoa T, Massy ZA, Witko-Sarsat V, Thevenin M, Touam M, Lambrey G, Lacour B, Drueke TB, Descamps-Latscha B Critical evaluation of plasma and LDL oxidant-trapping potential in hemodialysis patients.
Kidney Int — Article CAS PubMed Google Scholar Sato E, Tanaka A, Oyama J-I, Yamasaki A, Shimomura M, Hiwatashi A, Ueda Y, Amaha M, Nomura M, Matsumura D, Nakamura T, Node K Long-term effects of AST on the progression and prognosis of pre-dialysis chronic kidney disease: a 5-year retrospective study.
Heart Vessel — Article Google Scholar Nakamura T, Sato E, Fujiwara N, Kawagoe Y, Suzuki T, Ueda Y, Yamagishi S Oral adsorbent AST ameliorates tubular injury in chronic renal failure patients by reducing proteinuria and oxidative stress generation 1.
Metabolism — Article CAS PubMed Google Scholar Hisaki R, Fujita H, Saito F, Kushiro T Tempol attenuates the development of hypertensive renal injury in Dahl salt-sensitive rats. Am J Hypertens — Article CAS PubMed Google Scholar Nishiyama A, Yoshizumi M, Hitomi H, Kagami S, Kondo S, Miyatake A, Fukunaga M, Tamaki T, Kiyomoto H, Kohno M, Shokoji T, Kimura S, Abe Y The SOD mimetic tempol ameliorates glomerular injury and reduces mitogen-activated protein kinase activity in Dahl salt-sensitive rats.
J Am Soc Nephrol — Article CAS PubMed Google Scholar Tang Y, Yang Q, Lu J, Zhang X, Suen D, Tan Y, Jin L, Xiao J, Xie R, Rane M, Li X, Cai L Zinc supplementation partially prevents renal pathological changes in diabetic rats.
J Nutr Biochem — Article CAS PubMed Google Scholar Soinio M, Marniemi J, Laakso M, Pyorala K, Lehto S, Ronnemaa T Serum zinc level and coronary heart disease events in patients with type 2 diabetes. Diabetes Care — Article CAS PubMed Google Scholar de Zeeuw D, Akizawa T, Agarwal R, Audhya P, Bakris GL, Chin M, Krauth M, Lambers Heerspink HJ, Meyer CJ, McMurray JJ, Parving HH, Pergola PE, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Warnock DG, Wittes J, Chertow GM Rationale and trial design of bardoxolone methyl evaluation in patients with chronic kidney disease and type 2 diabetes: the occurrence of renal events BEACON.
Am J Nephrol — Article CAS PubMed Google Scholar Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H, Wittes J, Warnock DG Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med — Article CAS PubMed Google Scholar de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, Chertow GM Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease.
Am J Nephrol — Article CAS PubMed Google Scholar Van Laecke S, Van Biesen W, Vanholder R The paradox of bardoxolone methyl: a call for every witness on the stand? Diabetes Obes Metab —14 Article CAS PubMed Google Scholar Jun M, Venkataraman V, Razavian M, Cooper B, Zoungas S, Ninomiya T, Webster AC, Perkovic V Antioxidants for chronic kidney disease.
pub2 Article PubMed Google Scholar Ding W, Wang B, Zhang M, Gu Y Tempol, a superoxide dismutase-mimetic drug, ameliorates progression of renal disease in CKD mice. Cell Physiol Biochem — Article CAS PubMed Google Scholar Vanholder R, Massy Z, Argiles A, Spasovski G, Verbeke F, Lameire N Chronic kidney disease as cause of cardiovascular morbidity and mortality.
Nephrol Dial Transplant — Article CAS PubMed Google Scholar Foley RN Clinical epidemiology of cardiovascular disease in chronic kidney disease. J Ren Care 36 Suppl 1 :4—8 Article PubMed Google Scholar Groothoff JW, Offringa M, Grootenhuis M, Jager KJ Long-term consequences of renal insufficiency in children: lessons learned from the Dutch LERIC study.
Nephrol Dial Transplant — Article Google Scholar Mcdonald SP, Craig JC Long-term survival of children with end-stage renal disease. N Engl J Med — Article CAS PubMed Google Scholar Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia.
Eur J Clin Investig —79 Article CAS Google Scholar Togliatto G, Lombardo G, Brizzi MF The future challenge of reactive oxygen species ROS in hypertension: from bench to bed side.
Int J Mol Sci 18 Ali F, Hamdulay SS, Kinderlerer AR, Boyle JJ, Lidington EA, Yamaguchi T, Soares MP, Haskard DO, Randi AM, Mason JC Statin-mediated cytoprotection of human vascular endothelial cells: a role for Kruppel-like factor 2-dependent induction of heme oxygenase J Thromb Haemost — Article CAS PubMed Google Scholar Bertrand ME Provision of cardiovascular protection by ACE inhibitors: a review of recent trials.
Curr Med Res Opin — Article CAS PubMed Google Scholar El-Mesallamy HO, Abdel Hamid SG, Gad MZ Oxidative stress and asymmetric dimethylarginine are associated with cardiovascular complications in hemodialysis patients: improvements by L-arginine intake.
Kidney Blood Press Res — Article CAS PubMed Google Scholar Fliser D, Kielstein JT, Haller H, Bode-Boger SM Asymmetric dimethylarginine: a cardiovascular risk factor in renal disease? Kidney Int Suppl:S37—S40 Maas R, Xanthakis V, Polak JF, Schwedhelm E, Sullivan LM, Benndorf R, Schulze F, Vasan RS, Wolf PA, Boger RH, Seshadri S Association of the endogenous nitric oxide synthase inhibitor ADMA with carotid artery intimal media thickness in the Framingham Heart Study offspring cohort.
Stroke — Article CAS PubMed PubMed Central Google Scholar Speer T, Rohrer L, Blyszczuk P, Shroff R, Kuschnerus K, Kränkel N, Kania G, Zewinger S, Akhmedov A, Shi Y, Martin T, Perisa D, Winnik S, Müller MF, Sester U, Wernicke G, Jung A, Gutteck U, Eriksson U, Geisel J, Deanfield J, von Eckardstein A, Lüscher TF, Fliser D, Bahlmann FH, Landmesser U Abnormal high-density lipoprotein induces endothelial dysfunction via activation of toll-like receptor J Am Soc Nephrol — Article CAS PubMed PubMed Central Google Scholar Kaseda R, Jabs K, Hunley TE, Jones D, Bian A, Allen RM, Vickers KC, Yancey PG, Linton MF, Fazio S, Kon V Dysfunctional high-density lipoproteins in children with chronic kidney disease.
Metabolism — Article CAS PubMed Google Scholar Hadtstein C, Schaefer F Hypertension in children with chronic kidney disease: pathophysiology and management. Pediatr Nephrol — Article PubMed Google Scholar Hussein G, Bughdady Y, Kandil ME, Bazaraa HM, Taher H Doppler assessment of brachial artery flow as a measure of endothelial dysfunction in pediatric chronic renal failure.
Pediatr Nephrol — Article PubMed Google Scholar Wilson AC, Urbina E, Witt SA, Glascock BJ, Kimball TR, Mitsnefes M Flow-mediated vasodilatation of the brachial artery in children with chronic kidney disease. Pediatr Nephrol — Article PubMed Google Scholar Khandelwal P, Murugan V, Hari S, Lakshmy R, Sinha A, Hari P, Bagga A Dyslipidemia, carotid intima-media thickness and endothelial dysfunction in children with chronic kidney disease.
Pediatr Nephrol — Article PubMed Google Scholar Mitsnefes MM, Kimball TR, Kartal J, Witt SA, Glascock BJ, Khoury PR, Daniels SR Progression of left ventricular hypertrophy in children with early chronic kidney disease: 2-year follow-up study.
J Pediatr — Article PubMed Google Scholar Mitsnefes M, Flynn J, Cohn S, Samuels J, Blydt-Hansen T, Saland J, Kimball T, Furth S, Warady B Masked hypertension associates with left ventricular hypertrophy in children with CKD. J Am Soc Nephrol — Article CAS PubMed PubMed Central Google Scholar Kupferman JC, Aronson Friedman L, Cox C, Flynn J, Furth S, Warady B, Mitsnefes M BP control and left ventricular hypertrophy regression in children with CKD.
J Am Soc Nephrol — Article PubMed Google Scholar Moody WE, Edwards NC, Chue CD, Ferro CJ, Townend JN Arterial disease in chronic kidney disease.
Heart — Article CAS PubMed Google Scholar London G, Covic A, Goldsmith D, Wiecek A, Suleymanlar G, Ortiz A, Massy Z, Lindholm B, Martinez-Castelao A, Fliser D, Agarwal R, Jager KJ, Dekker FW, Blankestijn PJ, Zoccali C Arterial aging and arterial disease: interplay between central hemodynamics, cardiac work, and organ flow-implications for CKD and cardiovascular disease.
Kidney Int Suppl —12 Article Google Scholar Vervloet M, Cozzolino M Vascular calcification in chronic kidney disease: different bricks in the wall? Kidney Int — Article PubMed Google Scholar Chirinos JA Arterial stiffness: basic concepts and measurement techniques.
J Cardiovasc Transl Res — Article PubMed Google Scholar Wilson AC, Mitsnefes MM Cardiovascular disease in CKD in children: update on risk factors, risk assessment, and management. Am J Kidney Dis — Article PubMed PubMed Central Google Scholar Ma Y, Zhou L, Dong J, Zhang X, Yan S Arterial stiffness and increased cardiovascular risk in chronic kidney disease.
Int Urol Nephrol — Article CAS PubMed Google Scholar Choi HY, Park SK, Yun GY, Choi AR, Lee JE, Ha SK, Park HC Glycated albumin is independently associated with arterial stiffness in non-diabetic chronic kidney disease patients.
Medicine e Article CAS PubMed PubMed Central Google Scholar Nishizawa Y, Koyama H, Inaba M AGEs and cardiovascular diseases in patients with end-stage renal diseases. J Ren Nutr — Article CAS PubMed Google Scholar Rossi M, Campbell KL, Johnson DW, Stanton T, Vesey DA, Coombes JS, Weston KS, Hawley CM, McWhinney BC, Ungerer JPJ, Isbel N Protein-bound uremic toxins, inflammation and oxidative stress: a cross-sectional study in stage chronic kidney disease.
Arch Med Res — Article CAS PubMed Google Scholar Gao H, Liu S Role of uremic toxin indoxyl sulfate in the progression of cardiovascular disease. Life Sci —29 Article CAS PubMed Google Scholar Annavarajula SK, Dakshinamurty KV, Naidu MU, Reddy CP The effect of L-arginine on arterial stiffness and oxidative stress in chronic kidney disease.
Indian J Nephrol — Article CAS PubMed PubMed Central Google Scholar Schuchardt M, Herrmann J, Tolle M, van der Giet M Xanthine oxidase and its role as target in cardiovascular disease: cardiovascular protection by enzyme inhibition? Curr Pharm Des — Article CAS PubMed Google Scholar MacIsaac RL, Salatzki J, Higgins P, Walters MR, Padmanabhan S, Dominiczak AF, Touyz RM, Dawson J Allopurinol and cardiovascular outcomes in adults with hypertension.
Hypertension — Article CAS PubMed Google Scholar Valko M, Morris H, Cronin MTD Metals, toxicity and oxidative stress. Curr Top Med Chem — Article CAS Google Scholar Atkinson MA, Warady BA Anemia in chronic kidney disease.
Pediatr Nephrol:1—12 Locatelli F, Barany P, Covic A, De FA, Del VL, Goldsmith D, Horl W, London G, Vanholder R, Van BW Kidney disease: improving global outcomes guidelines on anaemia management in chronic kidney disease: a European renal best practice position statement.
Nephrol Dial Transplant — Article CAS PubMed Google Scholar Baracco R, Saadeh S, Valentini R, Kapur G, Jain A, Mattoo TK Iron deficiency in children with early chronic kidney disease. Pediatr Nephrol — Article PubMed Google Scholar Koshy SM, Geary DF Anemia in children with chronic kidney disease.
Pediatr Nephrol — Article PubMed Google Scholar Dursun I, Poyrazoglu HM, Gunduz Z, Ulger H, Yýkýlmaz A, Dusunsel R, Patýroglu T, Gurgoze M The relationship between circulating endothelial microparticles and arterial stiffness and atherosclerosis in children with chronic kidney disease.
Nephrol Dial Transplant — Article PubMed Google Scholar Castilla P, Echarri R, Davalos A, Cerrato F, Ortega H, Teruel JL, Lucas MF, Gomez-Coronado D, Ortuno J, Lasuncion MA Concentrated red grape juice exerts antioxidant, hypolipidemic, and antiinflammatory effects in both hemodialysis patients and healthy subjects.
Am J Clin Nutr — Article CAS PubMed Google Scholar Download references. Funding DM is supported by the Clinical Research Fund of UZ Leuven, by the Fund for Scientific Research G0BN, and by a research grant from the European Society for Pediatric Nephrology.
Author information Author notes Kristien Daenen and Asmin Andries contributed equally to this work. View author publications. Ethics declarations Conflict of interest The authors declare that they have no conflict of interest.
Electronic supplementary material. Supplementary Table 1 DOCX 50 kb. Rights and permissions Reprints and permissions. About this article. Cite this article Daenen, K.
Copy to clipboard. search Search by keyword or author Search. Navigation Find a journal Publish with us Track your research.
Among the various endogenous defences against ROS, glutathione homeostasis is critical for a cellular redox environment.
Glutathione-linked enzymatic defences of this family include Gpx, glutathione-S-transferase GST , glutaredoxins Grx , thioredoxins Trx , and peroxiredoxins Prx [ 51 ].
Many of these proteins are known to interact with each other, forming redox networks that have come under investigation for their contribution to dysfunctional oxidant pathways. Mitochondrial-specific isoforms of these proteins also exist and include Grx2, Grx5, Trx2 and Prx3 [ 52 - 54 ], which may be more critical for cell survival compared to their cystolic counterparts [ 50 ].
Mitochondrial dysfunction, resulting in depleted ATP synthesis, has the potential to reduce the redox control of glutathione since the rate of glutathione synthesis is ATP-dependent [ 55 ]. Intracellular synthesis of glutathione from amino acid derivatives glycine, glutamic acid and cysteine accounts for the majority of cellular glutathione compared with extracellular glutathione uptake [ 56 ].
Antioxidant networks in which there is interplay, crosstalk and synergism to efficiently and specifically scavenge ROS, may also exist.
If this is the case, these antioxidant networks could be harnessed to develop poly-therapeutic antioxidant supplements to combat oxidant-related pathologies, like CKD and CVD.
The role of oxidative stress in upstream transcriptional gene regulation is becoming increasingly recognised. Not only does this provide insight into the physiological role of oxidative stress, but presents regulatory systems that are possibly prone to deregulation. Furthermore, these sites present targets for pharmacological intervention.
Peroxisome proliferator-activated receptors PPARs are members of the nuclear hormone receptor superfamily of ligand-dependant transcription factors which have been shown to alter during CKD and CVD [ 57 - 59 ]. They have important roles in the transcriptional regulation of cell differentiation, lipid metabolism, glucose homeostasis, cell cycle progression, and inflammation.
Peroxisome proliferator gamma coactivator PGCα , in association with PPARγ activation, leads to a variety of cellular protective responses including mitochondrial biogenesis [ 57 ]. PPARγ regulation in chronic disease is increasingly recognised, with oxidative stress as the unifying initiating feature.
Omega-3 polyunsaturated fatty acids PUFA reduce inflammation in kidney tubular epithelial cells by upregulating PPARγ [ 60 ].
Nrf2 is a nuclear transcription factor that is suppressed in the cytoplasm by the physical binding of Keap1 preventing its translocation into the nucleus. Nrf2 is activated by a loss of Keap1 binding by alterations in cellular redox status, such as increased ROS, by-products of oxidative damage, and reduced antioxidant capacity, thereby promoting its transcriptional response at the ARE [ 63 ].
The ARE is a vital component of the promoter regions of genes encoding detoxifying, antioxidant, and glutathione-regulatory enzymes such as quinone-reductase, glutathione-peroxidases, glutathione-reductase, thioredoxins and thioredoxin-reductase, peroxiredoxins, gamma-glutamyl cysteine, heme-oxygenase-1 HO-1 , CAT, SOD metallothionein and ferritin [ 64 - 67 ].
Important to note is that by-products of oxidative damage such a 4-hydroxynoneal and J-isoprostanes act as endogenous activators of Nrf2 [ 68 , 69 ]. Recent pharmacological protocols have allowed the modulation of this pathway to enhance the capabilities of cells to combat oxidative stress and inflammation [ 70 ].
Chronic diseases of the kidney possess various commonalities to chronic disease of the cardiovascular system which can be linked through pathways controlled by oxidative stress, as shown in Figure 1. Vascular, cellular and biochemical factors all contribute. Increased serum uric acid levels hyperuricaemia can arise from increased purine metabolism, increasing age and decreased renal excretion, and have harmful systemic effects.
Hyperuricaemia is associated with an increased risk for development and progression of CKD. Hyperuricemia is also a risk factor associated with coronary artery disease [ 71 ], left ventricular hypertrophy [ 72 ], atrial fibrillation [ 73 ], myocardial infarction [ 74 ] and ischemic stroke [ 75 ].
Additionally, uric acid synthesis can promote oxidative stress directly through the activity of xanthine oxidoreductase. This enzyme is synthesized as xanthine dehydrogenase, which can be converted to xanthine oxidase by calcium-dependant proteolysis [ 80 ] or modification of cysteine residues [ 81 ].
In doing so, the enzyme loses its capacity to bind NADH by alterations in its catalytic site and, instead, transfers electrons from O 2 , thereby generating O 2 - [ 82 ]. However, the role of uric acid in many conditions associated with oxidative stress is not clear and there are experimental and clinical data showing that uric acid also has a role in vivo as an anti-oxidant [ 83 ].
Chronic kidney disease and cardiovascular disease are unified by oxidative stress. Mutual risk factors influence the development and progression of CKD and CVD and can either be modifiable diabetes, obesity, metabolic syndrome, smoking or non-modifiable genetic predisposition, increasing age, acute injury.
Oxidative stress has been implicated in the majority of initiating factors. The kidney is a vital source of L-arginine which is a precursor for nitric oxide NO. A reduction in renal mass can therefore reduce the production of L-arginine and NO activity. NO is vital for regular vascular endothelial cell function, and decreased amounts have the potential to manifest into CVD [ 84 ].
Additionally, oxidized low density lipoprotein ox-LDL , a by-product of oxidative damage in human blood, plays a pivotal role in the pathogenesis of atherosclerosis [ 85 ]. There is also a possible link between CVD and CKD that is regulated by oxidative stress through a functional mitochondrial angiotensin system [ 86 ].
Angiotensin type II receptors were co-localised with angiotensin on the inner mitochondrial membrane of human mononuclear cells and mouse renal tubular cells. This system was found to modulate mitochondrial NO production and respiration. The current state of antioxidant therapies for CKD and CVD is one of promise, but not without controversy.
In vitro studies commonly identify agents that are able to detoxify harmful oxidants. However, these studies are criticised for their isolated, non-holistic, nature [ 87 , 88 ]. It is largely the positive pre-clinical results from in vivo studies, usually in rodents, which drive progress for applicability in chronic human disease, but even these show considerable discrepancies in translation into patients.
The following trials of antioxidants need then to be rigorous, identifying not only any positive patient outcomes, but also the underlying mechanism, and of course any deleterious outcome. Various approaches have been taken to reduce oxidative stress in models of CKD and accelerated CVD, ranging from reducing oxidant intake in food stuffs [ 93 , 94 ] to targeted polypharmaceutical compounds.
The benefit of rigorous review of outcome from antioxidant therapies in either CKD or CVD is that the primary and secondary outcomes related to both can be measured.
In the following section, some antioxidants used for CKD or CVD are reviewed, as shown in Figure 2. N-acetyl cysteine NAC acts as an essential precursor to many endogenous antioxidants involved in the decomposition of peroxides [ 95 ]. NAC attenuates oxidative stress from various underlying causes by replenishing intracellular glutathione stores.
Glutathione is synthesized in the body by three amino acids by the catalysing of intracellular enzymes gamma-glutamylcysteine synthetase and glutathione synthetase. L-glutamic acid and glycine are two precursors of glutathione that are biologically and readily available.
However, the limiting precursor to glutathione biosynthesis and the third amino acid, L-cysteine, is not readily available in a human diet.
Although the primary basis for NAC supplementation is to replenish cellular cysteine levels to maintain intracellular glutathione and thus redox control, the sulfhydral-thiol group of L-cysteine is also able to exert direct antioxidant effects by scavenging free radicals, and NAC may also exert its protective effects against 2,3,5-tris glutathion-S-yl -hydroquinone toxicity.
The results of NAC supplementation in kidney disease have been variable and largely dependent on the type and cause of kidney injury and also the timing of treatment. In cultured human proximal tubular epithelial cells, NAC reduced lipid peroxidation and maintained the mitochondrial membrane potential, thereby preventing apoptosis following H 2 O 2 administration [ 97 ].
Although NAC had no significant effect on markers of oxidative stress and inflammation in rats following unilateral ureteral obstruction [ 98 ], it reduced kidney malondialdehyde MDA levels in a diabetic mouse model [ 99 ].
The treatment of CKD patients with NAC with the aim of improving renal function and preventing ESKD has been largely disappointing, with no evidence of reduction in proteinuria [ , ]. However, NAC seems to exert the greatest antioxidant and anti-inflammatory properties when used against the greatest injury, such as in ESKD patients receiving either haemodialysis or peritoneal dialysis.
In those cases, NAC reduced serum 8-isoprostane and the inflammatory cytokine IL-6 [ , ]. A recent systemic review on antioxidant therapy in hemodialysis patients highlighted NAC as the most efficacious agent in decreasing oxidative stress [ ].
The effect of NAC on cardiovascular pathologies is less well investigated than CKD. Crespo et al. Endothelial dysfunction caused by uremic toxins such as indoxyl sulphate induced ROS-dependent expression of the pro-inflammatory and pro-oxidant nuclear factor-κB NF-κB , which was ameliorated by NAC pre-treatment [ ].
Cellular sites for antioxidant therapy targets in CKD and CVD. Inflammation, lipid peroxidation and reactive oxygen species ROS from mitochondrial, cytoplasmic and extracellular sources contribute to oxidative stress.
Vitamin E incorporates into the phospholipid bilayer halting lipid peroxidation chain reactions. Omega ω -3 fatty acids displace arachadonic acid in the cell membrane and thus reduce arachadonic acid-derived ROS, but also significantly reduce inflammation and subsequent fibrosis.
The cysteine residue of N-acetyl-cysteine NAC is a precursor for glutathione GSH synthesis, and the thiol group is able to scavenge ROS directly. Bardoxolone exerts transcriptional control by promoting nuclear translocation of Nrf2, facilitating antioxidant response element ARE binding that upregulates endogenous antioxidant enzyme activity.
Allopurinol inhibits xanthine oxidase-derived ROS and the damaging effects of hyperuricemia. Coenzyme Q 10 CoQ 10 enhances the efficacy of electron transport in the mitochondria, thereby reducing mitochondrial-derived ROS — it is also able to directly scavenge ROS.
L-carnitine enhances mitochondrial fatty acid synthesis and subsequent ATP production and thereby maintains cell health. L-arginine is a precursor for nitric oxide which restores endothelial function.
Vitamin E, or α-tocopherol, is a lipid-soluble antioxidant that incorporates into the plasma membrane of cells, thereby scavenging free radicals, mainly the peroxyl radical, and halting lipid peroxidation chain reactions [ ].
A benefit of α-tocopherol is its ability to restore its antioxidant capacity from its oxidized form following free radical scavenging, and incorporate back into the plasma membrane. Vitamin C ascorbic acid is able to directly reduce α-tocopherol [ - ], and intracellular glutathione and lipoic acid can restore α-tocopherol indirectly by restoring vitamin C [ ].
This is a prime example of a cellular antioxidant network prone to dysregulation. Administration of α-tocopherol to kidney proximal tubular cells in culture decreased cisplatin-induced ROS and increased cell viability [ ].
The beneficial effects of α-tocopherol are not limited to its antioxidant properties, and recently attention has focused on its blood oxygenising and endogenous cell signalling functions [ ]. Vitamin E foodstuffs primarily consist of α-tocotreinol, an isoform of α-tocopherol which has higher antioxidant efficacy in biological membranes.
Despite this, the uptake and distribution of α-tocotreinol is far less than α-tocopherol. Therefore, the basis of vitamin E supplementation is to enhance α-tocopherol levels in cell plasma membranes to prevent lipid peroxidation and resultant oxidative stress.
One drawback of α-tocopherol is that it takes several days of pre-treatment to exhibit antioxidant effects [ ]. Vitamin E therapy has been extensively researched for renal and cardiovascular benefits in human disease populations. Nevertheless, confounding reports mean there is a lack of consensus as to whether vitamin E therapy induces an overall benefit.
It is known that patients with CKD stage 4 display the largest decrease in serum α-tocopherol levels following a progressive decline from stage 1 indicating an increased need for α-tocopherol in the CKD population [ ]. Interestingly, within the same cohort of patients, a positive correlation of serum α-tocopherol levels and GFR was found [ ].
A large scale trial concluded that vitamin E supplementation to cardiovascular high-risk patients over 4. The results from the Selenium and Vitamin E Cancer Prevention Trial SELECT are of greater concern.
They suggest that vitamin E supplementation significantly increases the risk of prostate cancer for young healthy men [ ].
Most studies finding beneficial outcomes of α-tocopherol supplementation have largely focused on the ESKD dialysis populations compared to healthy controls and found a reduced risk of CVD, decreased oxidative stress and increased erythrocyte antioxidants SOD, Gpx and CAT [ - ].
The use of α-tocopherol in CKD patients is not without controversy. However, this study was highly criticized owing to a bias in data analysis and numerous methodological flaws [ - ]. The apparent lack of clarity surrounding vitamin E supplementation and associated renal and cardiovascular outcomes appears to stem largely from differences in trial design and failure to specify the form of tocopherol used.
The heart and kidneys contain the highest endogenous levels of co-enzymes Co Q 9 and CoQ 10 compared to all other organs [ , ]. This is likely due to the respective reliance on aerobic metabolism and high density of mitochondria in the intrinsic functioning cells from these organs.
It is imperative that endogenous CoQ 10 levels are maintained to ensure mitochondrial health, and this forms the rationale for CoQ 10 therapy. CoQ 10 is a fundamental lipid-soluble component of all cell membranes including those enclosing subcellular compartments.
The continual oxidation-reduction cycle, and existence of CoQ 10 in three different redox states, explains its actions as an important cellular redox modulator through its pro-oxidant and antioxidant actions. The fully oxidised form of CoQ 10 , or ubiquinone, is able to accept electrons, primarily from NADH, to become fully reduced ubiquinol - CoQ 10 -H 2.
The reduced form of CoQ 10 is able to give up electrons, thereby scavenging free radicals. The major antioxidant role of CoQ 10 is in preventing lipid peroxidation directly, and by interactions with α-tocopherol [ ].
Ubiquinol is able to donate a hydrogen atom and thus quench peroxyl radicals, preventing lipid peroxidation chain reactions. CoQ 10 and α-tocopherol co-operate as antioxidants through the actions of CoQ 10 -H 2 restoring α-tocopheroxyl back to α-tocopherol [ , ].
However, the reactivity of α-tocopherol with peroxy radicals far exceeds that of ubiquinol with peroxyl radicals, suggesting that, in vivo , ubiquinols do not act as antioxidants but regenerate the antioxidant properties of α-tocopherols [ ].
This is in accordance with in vivo studies investigating the effects of CoQ 10 supplementation which have primarily found a limited antioxidant capacity.
Nonetheless, many in vitro studies demonstrate antioxidant properties of CoQ 10 in single cells, and benefits of CoQ 10 supplementation in humans are attributed to its ability to maintain efficient mitochondrial energy metabolism and thus prevent mitochondrial dysfunction, rather than act as a direct cellular antioxidant.
CoQ 10 supplementation in vivo reduced protein oxidation in skeletal muscle of rats but had no effect on mitochondrial H 2 O 2 production in the kidney [ ]. Recently, CoQ 10 supplementation improved left ventricular diastolic dysfunction and remodelling and reduced oxidative stress in a mouse model of type 2 diabetes [ ].
CoQ 10 supplementation in CVD patients also receiving statin therapy is becoming increasingly popular due to the CoQ 10 -inhibitory actions of statins. CoQ 10 levels decrease with age, but there are no studies measuring endogenous CoQ 10 levels in CKD or CVD patients and this could prove vital in the identification of population where CoQ 10 therapy may have beneficial outcomes.
Inflammation and fibrosis are causes, as well as consequences, of oxidative stress [ , ]. Direct targeting of inflammatory and fibrotic pathways with more specific modifying compounds presents a way to indirectly decrease oxidative stress in chronic pathologies.
Long chain omega-3 PUFA, including docosahexanoic acid DHA and eicosapentanoic acid EPA , have been investigated in a large range of in vitro and in vivo models and found to possess anti-inflammatory properties.
Recently, omega-3 fatty acid treatment of peripheral blood mononuclear cells from pre-dialysis CKD patients reduced the inflammatory markers IL-6, IL-1β, tumor necrosis factor TNF -α and C-reactive protein to levels observed in healthy subjects [ ].
DHA and EPA incorporate into the phospholipid bilayer of cells where they displace arachidonic acid. Arachidonic acid can generate ROS through the COX2 and xanthine oxidase inflammatory pathways.
Furthermore, chemoattractants derived from EPA are less potent that those derived from arachidonic acid [ , ]. Recently, in vitro studies determined that EPA and DHA attenuated TNF-α-stimulated monocyte chemoattractant protein MCP -1 gene expression by interacting with ERK and NF-κB in rat mesangial cells [ ].
Earlier evidence had shown that EPA and DHA inhibit NF-κB expression by stimulating PPARs in human kidney-2 cells in vitro [ 60 ].
Recently, a highly beneficial outcome of fish oil supplementation was found with heart failure patients with co-morbid diabetes [ ]. Clinical studies have found fish oil treatment modulates lipid levels [ , ], and has anti-thrombotic [ , ] and anti-hypertensive effects due to its vascular and endothelial actions [ ].
Allopurinol treatment aims is to inhibit xanthine oxidase to decrease serum uric acid and its associated toxic effects. Allopurinol and its metabolite, oxypurinol, act as competitive substrates for xanthine oxidase.
They enhance urinary urate excretion and block uric acid reabsorption by urate transporters in the proximal tubule, thereby facilitating enhanced uric acid excretion [ - ]. Allopurinol treatment of diabetic mice attenuated hyperuricaemia, albuminuria, and tubulointerstitial injury [ ]. Interventional studies of use of allopurinol in renal disease have shown improved uric acid levels, GFR, cardiovascular outcomes and delayed CKD progression.
Allopurinol given to ESKD patients on hemodialysis reduced the risk of CVD by decreasing serum low density lipoproteins, triglycerides and uric acid [ ].
Large, long-term interventional studies investigating kidney function in the CKD, and CVD, populations are needed to fully determine if allopurinol is cardio- and reno-protective via anti-oxidant mechanisms.
A different approach has been investigated by modulating pathways that respond to oxidative stress, rather than targeting ROS by directly increasing endogenous antioxidants. Bardoxolone methyl is a triterperoid derived from natural plant products that has undergone oleanolic acid-based modification [ ].
Its mechanism of action is largely unknown, however, it induces an overall antioxidative protective effect with anti-inflammatory and cytoprotective characteristics [ , ]. Bardoxolone methyl administered to mice ameliorated ischemia-reperfusion induced acute kidney injury by Nrf2-dependant expression of HO-1 and PPARγ [ ].
Its mechanism may also reside in regulating mitochondrial biogenesis given the involvement of PPARγ. Concurrent benefits to CVD will undoubtedly also be measured. Carnitine is an essential cofactor required for the transformation of free fatty acids into acylcarnitine and its subsequent transport into the mitochondria for β-oxidation [ ].
This underlies its importance in the production of ATP for cellular energy. Acylcarnitine is also essential for the removal of toxic fat metabolism by-products. Carnitine is obtained primarily from food stuffs, however it can be synthesised endogenously from the amino acid L-lysine and methionine [ ].
L-carnitine supplementation primarily benefits ESRD patients on hemodialysis and their associated cardiovascular complications, especially anemia. This is primarily due to the well-described decrease in serum free carnitine in maintenance hemodialysis patients compared to non-dialysis CKD and healthy patients [ ].
L-carnitine supplementation offsets renal anemia, lipid abnormalities and cardiac dysfunction in hemodialysis patients [ ].
Other measures of cardiac morbidity such as reduced left ventricular ejection fraction and increased left ventricular mass also significantly improved following low dose L-carnitine supplementation [ ].
Benefits to the peripheral vasculature have also been demonstrated by L-carnitine through a mechanism thought to involve an associated decrease in homocysteine levels [ ]. Interestingly, oxidative stress is a major characteristic of hemodialysis patients [ ].
As well as the physiological role of L-carnitine in mitochondrial fatty acid synthesis, oxidant reducing capabilities have also been demonstrated and may underlie the health benefits of L-carnitine therapy in CKD and CVD.
Ye et al. They suggest that this anti-apoptotic mechanism may also explain the demonstrated reduction in morbidity from cardiomyopathies in L-carnitine supplemented hemodialysis patients. The premise of L-arginine supplementation is to maintain NO signalling and thereby maintain vascular endothelial cell function.
L-arginine is a physiological precursor to NO and its availability and transport determine the rate of NO biosynthesis. CKD patients most often present with atherosclerosis, thromboembolitic complications, and endothelial dysfunction, primarily due to altered endothelium-dependant relaxation factors [ ].
It is believed that the impaired NO synthesis, common in CKD individuals, contributes significantly to their disease pathogenesis [ ]. L-arginine synthesis occurs in the liver and kidney, with the kidney functioning to maintain homeostatic plasma levels since the liver processes NO from the diet [ ].
The addition of L-aspartic acid or L-glutamic acid with L-citrulline and arginirosuccinic acid synthase as the rate determining enzyme forms L-arginine [ ]. The proximal tubular cells account for the majority of kidney NO synthesis [ , ], thus kidney damage and atrophy, a primary corollary of CKD, results in decreased synthesis of L-arginine.
The majority of research demonstrates decreased levels of NO production in CKD and CVD patients [ - ]. However, some research suggests NO activity increases [ , ]. These disparate findings highlight the need to measure L-arginine levels in patients before commencing L-arginine supplementation.
Rajapaske et al. During a state of oxidative stress, L-arginine supplementation was shown to decrease MDA, myeloperoxidase and xanthine oxidase and increase glutathionine in both heart and kidney tissue from rats [ ]. As such, L-arginine supplementation represents an approach to restoring a dysregulation of NO signalling and subsequent endothelial dysfunction in both chronic kidney and heart diseases.
Compounds commonly used to alleviate oxidative stress exhibit different antioxidant actions, and so there exists the potential for different antioxidants to work together to improve whole cell and organ function through a targeted polypharmaceutical approach to decrease oxidative stress.
However, most clinical studies investigating the effects of combination antioxidants have demonstrated confounding results. Mosca et al. However, this trial only included healthy participants and cannot be extrapolated to the CKD and CVD populations.
In a murine model of diabetic nephropathy, a major cause of CKD with associated CVD, the beneficial effects of NAC, L-ascorbic acid vitamin C and α-tocopherol were demonstrated [ ]. Daily supplementation for 8 weeks decreased lipid peroxidation, BUN, serum creatinine and blood glucose, mainly due to a reduction in the inflammatory response induced by hyperglycemia.
In comparison, a prospective trial investigating oral supplementation of mixed tocopherols and α-lipoic acid in stage 3 and 4 CKD patients has revealed disappointing results.
Over 2 months, supplementation did not reduce biomarkers of oxidative stress F 2 -isoprostanes and protein thiol concentration or inflammation CRP and IL The short period of time 2 months of the intervention may explain this result and longer trials need to be carried out.
The inclusion of vitamin E in these interventions has polarized discussion on the outcomes, because of its negligible benefits when cardiovascular outcomes were measured [ 91 , 92 , ] and also because of contraindications, discussed previously.
Despite this, long-term treatment in with the antioxidants vitamin C, vitamin E, CoQ 10 and selenium has been shown to reduce multiple cardiovascular risk factors [ ]. Recently, multiple antioxidants in combination with L-arginine have shown promise in animal models of CKD and associated CVD.
CKD is a progressive disease with increasing incidence, having very little success in current conventional therapies once CKD reaches stage 4. Stages 2 and 3 are best to target to slow or stop further development of the disease. There is an almost inseparable connection between CKD and CVD, with many patients with CKD dying of the cardiovascular complications before renal failure reaches its fullest extent.
Oxidative stress and inflammation are closely interrelated with development of CKD and CVD, and involve a spiralling cycle that leads to progressive patient deterioration.
Given the complex nature of oxidative stress and its molecular pathways, antioxidants may need to be given as a polypharmacotherapy to target each aberrant pathway, with the aim of reducing the burden of these chronic diseases.
It is vital for the progression of antioxidant therapy research in CKD and CVD that measures of oxidative stress are compared with pathophysiological outcome in the diseases, especially in connection with antioxidant therapies that may be delivered with or without more conventional CKD therapies.
Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3. Edited by Jose Antonio Morales-Gonzalez. Open access Oxidative Stress and Antioxidant Therapy in Chronic Kidney and Cardiovascular Disease Written By David M.
Small and Glenda C. DOWNLOAD FOR FREE Share Cite Cite this chapter There are two ways to cite this chapter:. Choose citation style Select style Vancouver APA Harvard IEEE MLA Chicago Copy to clipboard Get citation.
Choose citation style Select format Bibtex RIS Download citation. IntechOpen Oxidative Stress and Chronic Degenerative Diseases A Role for Antioxidants Edited by Jose Antonio Morales-Gonzalez. From the Edited Volume Oxidative Stress and Chronic Degenerative Diseases - A Role for Antioxidants Edited by José A.
Morales-González Book Details Order Print. Chapter metrics overview 5, Chapter Downloads View Full Metrics. Impact of this chapter. David M. Small Centre for Kidney Disease Research, School of Medicine, The University of Queensland, Brisbane, Australia Glenda C.
gobe uq. Introduction Chronic kidney disease CKD and cardiovascular disease CVD have major impacts upon the health of populations worldwide, especially in Western societies. Table 1. Classification and description of the different stages of CKD.
Inflammation and chronic kidney and cardiovascular disease The circulating nature of many inflammatory mediators such as cytokines, and inflammatory or immune cells, indicates that the immune system can act as a mediator of kidney-heart cross-talk and may be involved in the reciprocal dysfunction that is encountered commonly in the cardio-renal syndromes.
Understanding oxidative stress Oxidative stress has been implicated in various pathological systems that are prevalent in both CKD and CVD, most importantly inflammation and fibrosis. Endogenous antioxidants — Metabolism or disease modifiers The production of ROS is usually in balance with the availability and cellular localisation of antioxidant enzymes such as superoxide dismutase SOD , CAT and glutathione peroxidase Gpx.
Oxidative stress and transcriptional control The role of oxidative stress in upstream transcriptional gene regulation is becoming increasingly recognised. CKD and CVD are unified by oxidative stress Chronic diseases of the kidney possess various commonalities to chronic disease of the cardiovascular system which can be linked through pathways controlled by oxidative stress, as shown in Figure 1.
N-acetylcysteine — An antioxidant with promise N-acetyl cysteine NAC acts as an essential precursor to many endogenous antioxidants involved in the decomposition of peroxides [ 95 ]. Vitamin E — An established antioxidant with controversial outcomes Vitamin E, or α-tocopherol, is a lipid-soluble antioxidant that incorporates into the plasma membrane of cells, thereby scavenging free radicals, mainly the peroxyl radical, and halting lipid peroxidation chain reactions [ ].
Coenzyme Q 10 - Maintaining mitochondrial health The heart and kidneys contain the highest endogenous levels of co-enzymes Co Q 9 and CoQ 10 compared to all other organs [ , ].
Omega-3 poly-unsaturated fatty acids — Inflammation and oxidative stress Inflammation and fibrosis are causes, as well as consequences, of oxidative stress [ , ]. Allopurinol — A xanthine oxidase inhibitor Allopurinol treatment aims is to inhibit xanthine oxidase to decrease serum uric acid and its associated toxic effects.
L-Carnitine — Improving cardiovascular health in dialysis Carnitine is an essential cofactor required for the transformation of free fatty acids into acylcarnitine and its subsequent transport into the mitochondria for β-oxidation [ ].
L-Arginine - Maintaining endothelial function The premise of L-arginine supplementation is to maintain NO signalling and thereby maintain vascular endothelial cell function.
Combination antioxidants Compounds commonly used to alleviate oxidative stress exhibit different antioxidant actions, and so there exists the potential for different antioxidants to work together to improve whole cell and organ function through a targeted polypharmaceutical approach to decrease oxidative stress.
References 1. Rosner MH, Ronco C, Okusa MD. The role of inflammation in the cardio-renal syndrome: a focus on cytokines and inflammatory mediators. Semin Nephrol. Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, et al. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative.
Eur Heart J. Leung FP, Yung LM, Laher I, Yao X, Chen ZY, Huang Y. Exercise, vascular wall and cardiovascular diseases: an update Part 1.
Sports Med. Bongartz LG, Cramer MJ, Doevendans PA, Joles JA, Braam B. The severe cardiorenal syndrome: 'Guyton revisited'.
Sallam N, Fisher A, Golbidi S, Laher I. Naunyn Schmiedebergs Arch Pharmacol. Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. McDonald SP, Chang S, Excell L, editors. ANZDATA Registry Report. Adelaide Tanner RM, Brown TM, Muntner P.
Epidemiology of obesity, the metabolic syndrome, and chronic kidney disease. Curr Hypertens Rep. Graf J, Ryan C, Green F. An overview of chronic kidney disease in Australia, Canberra: Australian Inst Health Welfare Tesch GH.
Review: Serum and urine biomarkers of kidney disease: A pathophysiological perspective. Nephrol Carlton.
Rodriguez-Iturbe B, Johnson RJ, Herrera-Acosta J. Tubulointerstitial damage and progression of renal failure. Kidney Int Suppl.
Fassett RG, Venuthurupalli SK, Gobe GC, Coombes JS, Cooper MA, Hoy WE. Biomarkers in chronic kidney disease: a review. Kidney Int. Choudhury D, Luna-Salazar C.
Preventive health care in chronic kidney disease and end-stage renal disease. Nat Clin Pract Nephrol. Dutta D, Calvani R, Bernabei R, Leeuwenburgh C, Marzetti E. Contribution of impaired mitochondrial autophagy to cardiac aging: mechanisms and therapeutic opportunities.
Circ Res. Manabe I. Chronic inflammation links cardiovascular, metabolic and renal diseases. Circ J. Glassock RJ, Pecoits-Filho R, Barberato SH. Left ventricular mass in chronic kidney disease and ESRD. Clin J Am Soc Nephrol: CJASN. Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al.
Mitsnefes MM. Cardiovascular disease in children with chronic kidney disease. J Am Soc Nephrol: JASN. Whaley-Connell A, Pavey BS, Chaudhary K, Saab G, Sowers JR. Renin-angiotensin-aldosterone system intervention in the cardiometabolic syndrome and cardio-renal protection. Ther Adv Cardiovasc Dis. Gomes P, Simao S, Silva E, Pinto V, Amaral JS, Afonso J, et al.
Aging increases oxidative stress and renal expression of oxidant and antioxidant enzymes that are associated with an increased trend in systolic blood pressure. Oxid Med Cell Longev. Pias EK, Aw TY. Apoptosis in mitotic competent undifferentiated cells is induced by cellular redox imbalance independent of reactive oxygen species production.
FASEB J. Zhuang S, Yan Y, Daubert RA, Han J, Schnellmann RG. ERK promotes hydrogen peroxide-induced apoptosis through caspase-3 activation and inhibition of Akt in renal epithelial cells. Am J Physiol Renal Physiol. Blanchetot C, Tertoolen LG, den Hertog J.
Regulation of receptor protein-tyrosine phosphatase alpha by oxidative stress. EMBO J. Jones DP. Redefining oxidative stress. Antioxid Redox Signal. Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo.
Mol Cell. Rao RK, Clayton LW. Regulation of protein phosphatase 2A by hydrogen peroxide and glutathionylation. Biochem Biophys Res Commun. Tavakoli S, Asmis R. Reactive Oxygen Species and Thiol Redox Signaling in the Macrophage Biology of Atherosclerosis. Madesh M, Hajnoczky G.
VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release.
J Cell Biol. Soubannier V, McBride HM. Positioning mitochondrial plasticity within cellular signaling cascades. Biochim Biophys Acta. Vay L, Hernandez-SanMiguel E, Lobaton CD, Moreno A, Montero M, Alvarez J. Cell Calcium. Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging.
Free Radic Biol Med. Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen.
Biochem J. Nohl H, Hegner D. Do mitochondria produce oxygen radicals in vivo? Eur J Biochem. Lipinski B.
Is it oxidative stress or free radical stress and why does it matter? Oxid Antioxid Med Sci. Cadenas E, Boveris A, Ragan CI, Stoppani AO. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria.
Arch Biochem Biophys. Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Turrens JF, Alexandre A, Lehninger AL.
Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. Choksi KB, Nuss JE, Boylston WH, Rabek JP, Papaconstantinou J. Age-related increases in oxidatively damaged proteins of mouse kidney mitochondrial electron transport chain complexes.
Granata S, Zaza G, Simone S, Villani G, Latorre D, Pontrelli P, et al. Mitochondrial dysregulation and oxidative stress in patients with chronic kidney disease. BMC Genomics. Nemoto S, Takeda K, Yu ZX, Ferrans VJ, Finkel T. Role for mitochondrial oxidants as regulators of cellular metabolism.
Mol Cell Biol. Werner E, Werb Z. Integrins engage mitochondrial function for signal transduction by a mechanism dependent on Rho GTPases.
Cooper CE, Patel RP, Brookes PS, Darley-Usmar VM. Nanotransducers in cellular redox signaling: modification of thiols by reactive oxygen and nitrogen species.
Trends Biochem Sci. Kokoszka JE, Coskun P, Esposito LA, Wallace DC. Proc Natl Acad Sci U S A. Meng Q, Wong YT, Chen J, Ruan R. Age-related changes in mitochondrial function and antioxidative enzyme activity in fischer rats.
Mech Ageing Dev. Raha S, McEachern GE, Myint AT, Robinson BH. Superoxides from mitochondrial complex III: the role of manganese superoxide dismutase. Angermuller S, Islinger M, Volkl A. Peroxisomes and reactive oxygen species, a lasting challenge.
Histochem Cell Biol. Islinger M, Li KW, Seitz J, Volkl A, Luers GH. Sturtz LA, Diekert K, Jensen LT, Lill R, Culotta VC. A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria.
A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J Biol Chem. Soderdahl T, Enoksson M, Lundberg M, Holmgren A, Ottersen OP, Orrenius S, et al.
Visualization of the compartmentalization of glutathione and protein-glutathione mixed disulfides in cultured cells. Godoy JR, Oesteritz S, Hanschmann EM, Ockenga W, Ackermann W, Lillig CH. Segment-specific overexpression of redoxins after renal ischemia and reperfusion: protective roles of glutaredoxin 2, peroxiredoxin 3, and peroxiredoxin 6.
Lillig CH, Holmgren A. Thioredoxin and related molecules--from biology to health and disease. Lonn ME, Hudemann C, Berndt C, Cherkasov V, Capani F, Holmgren A, et al. Hanschmann EM, Lonn ME, Schutte LD, Funke M, Godoy JR, Eitner S, et al. Both thioredoxin 2 and glutaredoxin 2 contribute to the reduction of the mitochondrial 2-Cys peroxiredoxin Prx3.
Lash LH, Putt DA, Matherly LH. Protection of NRKE cells, a rat renal proximal tubular cell line, from chemical-induced apoptosis by overexpression of a mitochondrial glutathione transporter. J Pharmacol Exp Ther. Visarius TM, Putt DA, Schare JM, Pegouske DM, Lash LH.
Pathways of glutathione metabolism and transport in isolated proximal tubular cells from rat kidney. Biochem Pharmacol. Funk JA, Odejinmi S, Schnellmann RG. SRT induces mitochondrial biogenesis and rescues mitochondrial function after oxidant injury in renal proximal tubule cells.
J Pharmacol Exp Therapeut. Lepenies J, Hewison M, Stewart PM, Quinkler M. Renal PPARgamma mRNA expression increases with impairment of renal function in patients with chronic kidney disease.
Sakamoto A, Hongo M, Saito K, Nagai R, Ishizaka N. Reduction of renal lipid content and proteinuria by a PPAR-gamma agonist in a rat model of angiotensin II-induced hypertension. Eur J Pharmacol. Li H, Ruan XZ, Powis SH, Fernando R, Mon WY, Wheeler DC, et al.
EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: evidence for a PPAR-gamma-dependent mechanism. Martin A, Perez-Giron JV, Hernanz R, Palacios R, Briones AM, Fortuno A, et al.
Peroxisome proliferator-activated receptor-gamma activation reduces cyclooxygenase-2 expression in vascular smooth muscle cells from hypertensive rats by interfering with oxidative stress.
J Hypertens. Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Aspects Med. Wilmes A, Crean D, Aydin S, Pfaller W, Jennings P, Leonard MO. Identification and dissection of the Nrf2 mediated oxidative stress pathway in human renal proximal tubule toxicity.
Toxicol In Vitro. Nelson SK, Bose SK, Grunwald GK, Myhill P, McCord JM. The induction of human superoxide dismutase and catalase in vivo: a fundamentally new approach to antioxidant therapy. Prestera T, Talalay P, Alam J, Ahn YI, Lee PJ, Choi AM. Parallel induction of heme oxygenase-1 and chemoprotective phase 2 enzymes by electrophiles and antioxidants: regulation by upstream antioxidant-responsive elements ARE.
Mol Med. Li Y, Jaiswal AK. Regulation of human NAD P H:quinone oxidoreductase gene. Role of AP1 binding site contained within human antioxidant response element.
Okuda A, Imagawa M, Maeda Y, Sakai M, Muramatsu M. Structural and functional analysis of an enhancer GPEI having a phorbol O-tetradecanoate acetate responsive element-like sequence found in the rat glutathione transferase P gene. Itoh K, Mochizuki M, Ishii Y, Ishii T, Shibata T, Kawamoto Y, et al.
Transcription factor Nrf2 regulates inflammation by mediating the effect of deoxy-Delta 12,14 -prostaglandin j 2. Levonen AL, Landar A, Ramachandran A, Ceaser EK, Dickinson DA, Zanoni G, et al.
Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Rojas-Rivera J, Ortiz A, Egido J. Antioxidants in kidney diseases: the impact of bardoxolone methyl.
Int J Nephrol. Brand FN, McGee DL, Kannel WB, Stokes J, 3rd, Castelli WP. Hyperuricemia as a risk factor of coronary heart disease: The Framingham Study. Am J Epidemiol. Mitsuhashi H, Tamura K, Yamauchi J, Ozawa M, Yanagi M, Dejima T, et al. Effect of losartan on ambulatory short-term blood pressure variability and cardiovascular remodeling in hypertensive patients on hemodialysis.
Letsas KP, Korantzopoulos P, Filippatos GS, Mihas CC, Markou V, Gavrielatos G, et al. Uric acid elevation in atrial fibrillation. Hellenic J Cardiol. Car S, Trkulja V.
Higher serum uric acid on admission is associated with higher short-term mortality and poorer long-term survival after myocardial infarction: retrospective prognostic study.
When the balance is not disturbed, Oxidativve has a role in uealth adaptations and signal transduction. However, odidative excessive amount of ROS and RNS results in the Collagen Types Explained of kindey molecules oxidative stress and kidney health as andd, proteins, and DNA. Oxidative stress and kidney health stress has kdney reported in hdalth disease, due to both sttress depletions as Mood-boosting superfood supplement as increased ROS production. The kidney is a highly metabolic organ, rich in oxidation reactions in mitochondria, which makes it vulnerable to damage caused by OS, and several studies have shown that OS can accelerate kidney disease progression. Also, in patients at advanced stages of chronic kidney disease CKDincreased OS is associated with complications such as hypertension, atherosclerosis, inflammation, and anemia. In this review, we aim to describe OS and its influence on CKD progression and its complications. We also discuss the potential role of various antioxidants and pharmacological agents, which may represent potential therapeutic targets to reduce OS in both pediatric and adult CKD patients. When the balance is not disturbed, Oxidative stress and kidney health has healtj role in physiological adaptations and signal transduction. Kidnsy, an excessive amount of Kidnej and RNS results Pycnogenol and migraine prevention the oxidation of biological molecules such as lipids, proteins, and DNA. Oxidative stress oxidative stress and kidney health been reported in kidney disease, due to both antioxidant depletions healrh well as kidne ROS production. The kidney is a highly metabolic organ, rich in oxidation reactions in mitochondria, which makes it vulnerable to damage caused by OS, and several studies have shown that OS can accelerate kidney disease progression. Also, in patients at advanced stages of chronic kidney disease CKDincreased OS is associated with complications such as hypertension, atherosclerosis, inflammation, and anemia. In this review, we aim to describe OS and its influence on CKD progression and its complications. We also discuss the potential role of various antioxidants and pharmacological agents, which may represent potential therapeutic targets to reduce OS in both pediatric and adult CKD patients.
When the balance is not disturbed, Oxidative stress and kidney health has healtj role in physiological adaptations and signal transduction. Kidnsy, an excessive amount of Kidnej and RNS results Pycnogenol and migraine prevention the oxidation of biological molecules such as lipids, proteins, and DNA. Oxidative stress oxidative stress and kidney health been reported in kidney disease, due to both antioxidant depletions healrh well as kidne ROS production. The kidney is a highly metabolic organ, rich in oxidation reactions in mitochondria, which makes it vulnerable to damage caused by OS, and several studies have shown that OS can accelerate kidney disease progression. Also, in patients at advanced stages of chronic kidney disease CKDincreased OS is associated with complications such as hypertension, atherosclerosis, inflammation, and anemia. In this review, we aim to describe OS and its influence on CKD progression and its complications. We also discuss the potential role of various antioxidants and pharmacological agents, which may represent potential therapeutic targets to reduce OS in both pediatric and adult CKD patients.
0 thoughts on “Oxidative stress and kidney health”