Free radicals and reproductive health -
Mobile devices are becoming more accessible to the general population, particularly to adolescent males and men of reproductive age. Cell phones release radiofrequency electromagnetic radiation, exposure to which has shown to increase the risk of oligo-, astheno- or teratozoospermia.
Furthermore, in vitro studies have demonstrated that EMR induces ROS generation and DNA fragmentation in human spermatozoa, alongside a decreased sperm concentration, motility and vitality depending on the duration of exposure to radiation [ 40 ].
Various components of cigarette smoke have been associated with OS exacerbation. Cigarettes contain a broad array of free radical-inducing agents such as nicotine, cotinine, hydroxycotinine, alkaloids and nitrosamines [ 41 , 42 ].
The prime component of tobacco is nicotine, which is a well-known ROS producer in spermatozoa with detrimental effects on the sperm count, motility and morphology. Moreover, smokers exhibited a lower hypo-osmotic swelling test percentage, indicating a weaker plasma membrane integrity when compared to non-smokers [ 41 ].
Smoking increases ROS production by causing leukocytospermia as shown by Saleh et al. A different study showed that levels of seminal plasma antioxidants were diminished in smokers.
By directly affecting the liver, alcohol intake increases ROS production while simultaneously decreasing the antioxidant capacity of the body.
Although alcohol consumption has been repeatedly associated with systemic OS, its effect on semen parameters has not been explored to a larger extent.
In a study comprising subjects, moderate alcohol consumption did not negatively affect semen parameters [ 44 ]. Nevertheless, it was revealed that chronic drinkers had reduced levels of testosterone, possibly due to an impaired hypothalamic-pituitary axis and damage to the Leydig cells [ 45 ].
Increased alcohol levels block gonadotropin-releasing hormone, leading to reduced luteinizing hormone and testosterone levels. Furthermore, alcohol has been shown to increase ROS generation when consumed by malnourished individuals [ 44 ].
Lastly, diet may affect semen parameters. In a Danish study, men with the highest saturated fat intake presented with a significantly lower total sperm count and concentration in comparison to those with the lowest saturated fat intake [ 46 ]. These observations were supported by a later report focused on studying the link between dairy food intake and male fertility and revealing that a low-fat dairy diet may lead to a higher spermatogenesis [ 47 ].
On the other hand, omega-3 fatty acids and omega-6 fatty acids were shown to improve sperm count, motility and morphology [ 48 ]. With regard to obesity and its relation to semen parameters, currently available data are conflicting. In a study on Iranian men, it was found that overweight men tend to have lower sperm counts [ 49 ].
Inversely, a different study reported that underweight subjects had lower sperm counts than normal and overweight men [ 48 ]. Moreover, a study comprising Tunisian men revealed that sperm concentration, motility and morphology did not vary across different BMI values [ 50 ].
Aerobic metabolism utilizing oxygen is essential for energy requirements of reproductive cells, and free radicals do play a significant role in physiological processes occurring within the male reproductive tract.
Spermatozoa themselves produce small amounts of ROS that are essential for a variety of physiological processes such as capacitation, hyperactivation, acrosome reaction and sperm-oocyte fusion [ 30 ].
During transit and storage in the epididymis, spermatozoa undergo membrane, nuclear and enzymatic remodeling, involving the release, attachment and rearrangement of surface proteins [ 6 , 30 , 51 ].
Such changes are based on the assembly of several signal transduction pathways necessary for the subsequent ability of spermatozoa to undergo hyperactivation and capacitation. ROS are essential for a proper chromatin packing during the maturation of mammalian spermatozoa, leading to a characteristic chromatin stability.
This unique chromatin architecture results from an extensive inter- and intra-molecular disulfide bond stabilization between the cysteine residues of protamines—small nuclear proteins that replace histones during spermatogenesis.
Oxidation of the thiol groups in protamines takes place during the transport of spermatozoa from the caput to the cauda epididymis [ 52 ]. As demonstrated by Aitken et al. ROS may act as oxidizing agents in this process, hence facilitating the formation of disulfide bonds, increasing chromatin stability and protecting DNA from possible damage [ 30 , 52 ].
As spermatozoa possess minimal to none repair mechanisms [ 9 ], chromatin condensation is a crucial protective mechanism, in which ROS actually protect male gametes against future oxidative insults.
Likewise, peroxides have been associated with formation of the mitochondrial capsule—a coat surrounding sperm mitochondria providing protection against possible proteolytic degradation [ 54 ]. It is suggested that during spermatogenesis peroxides may oxidize the active form of phospholipid hydroperoxide glutathione peroxidase PHGPx , creating an intermediate that subsequently interacts with thiol groups to form a seleno-disulfide bond.
The resulting mitochondrial capsule is made out of a complex protein network rich in disulfide bonds. Mitochondria require such protection as their proper function is crucial for metabolism, cell cycle control and oxidative balance [ 51 , 53 , 54 ].
Although several studies have reported improved sperm DNA integrity and reduced ROS production as a result of daily antioxidant consumption [ 55 ], an unusual decondensation of sperm DNA has been revealed as well [ 56 ].
Hence it may be hypothesized that high antioxidant levels may alter the oxidative conditions necessary for a proper formation of the inter- and intra-molecular disulfide bonds, leading to a lower DNA compaction. Capacitation is a prominent process of final maturation that spermatozoa undergo in the female reproductive tract, during which sperm motility changes from a progressive state to a highly energetic one.
It is hypothesized that capacitation occurs exclusively in mature spermatozoa in order to reach the oocyte taking advantage of hyperactive motility and an increased responsiveness to chemotactic agents.
Numerous receptors on the sperm head become activated, providing energy to the sperm to penetrate the zona pellucida. As such, capacitation sets up the path necessary for subsequent hyperactivation and acrosome reaction [ 57 ].
Numerous of studies on both human and animal spermatozoa indicate that H 2 O 2 is the primary ROS responsible for capacitation to occur. This process is associated with an increase in tyrosine phosphorylation, and it has been shown that the amount and banding pattern of tyrosine phosphorylation by adding exogenous H 2 O 2 was similar to that observed during endogenous ROS production, providing evidence that H 2 O 2 may be responsible for the enhancement of capacitation [ 32 , 57 , 58 ].
This hypothesis was further confirmed by Rivlin et al. This process is vital as cAMP must increase in concentration for capacitation to occur. cAMP and its subsequent pathways involve protein kinase A, which phosphorylates MEK extracellular signal-regulated kinase -like proteins as well as tyrosine present in fibrous sheath proteins [ 57 , 58 ].
The results of the above studies show that ROS can positively enhance sperm capacitation, but diverge over the specific ROS involved. Several studies have confirmed the lack of molecular specificity in the activation of capacitation and tyrosine phosphorylation, as both SOD and catalase have been shown to negate the positive effect exogenously induced capacitation and hyperactivation [ 59 ].
Although physiological ROS levels are necessary for capacitation, their overgeneration may trigger apoptosis.
Hyperactivation is an incompletely understood process to be observed in the final maturation stage of spermatozoa and is considered a subcategory of capacitation. Normally spermatozoa exhibit a low amplitude flagellar movement accompanied by low, linear velocity. In the hyperactivated state, spermatozoa movement is of high amplitude, asymmetric flagellar movement, pronounced lateral head displacement and non-linear trajectory, allowing the sperm to penetrate the cumulus oophorus and zona pellucida surrounding the oocyte.
Furthermore, hyperactive motility may enable the progressive movement through the oviduct by preventing stagnation, adding yet another benefit to the sperm function [ 62 ]. Acrosome reaction AR is related to the release of proteolytic enzymes, primarily acrosin and hyaluronidase, in order to degrade the zona pellucida of the oocyte.
Once degraded, hyperactive motility propels the spermatozoa into the perivitelline space, at which point the spermatozoa may eventually fuse with the oocyte [ 63 ]. At the same time, results regarding the specific ROS are conflicting. The majority of studies note positive effects of H 2 O 2 and negative effects of catalase, thus suggesting that H 2 O 2 is the major species responsible for a proper AR [ 58 , 64 ].
Moreover, ROS act as signal transducers in the AR. Elevated ROS production may occur upon interaction with the cumulus oophorus , thereby enhancing the signal for exocytosis initiated by either progesterone or the zona pellucida.
A link exists between enhanced ROS levels and increased sperm-oocyte fusion. High rates of sperm-oocyte fusion are correlated with increased expression of phosphorylated tyrosine proteins [ 6 ], suggesting that sperm-oocyte fusion is related to the events of capacitation and AR.
Ultimately, ROS are thought to increase membrane fluidity using two mechanisms: 1 de-esterification of membrane phospholipids and 2 activation of phospholipase A2 PLA2 [ 65 ]. Once the zona pellucida and corona radiata are penetrated by the sperm cell, the oocyte prevents eventual polyspermy by turning the vitelline layer into a hard envelope.
o,o-Dityrosine crosslinks catalyzed by ovoperoxidase lead to the formation of a single macromolecular structure acting as the envelope [ 66 ]. H 2 O 2 serves as the substrate to ovoperoxidase to provide for the envelope formation.
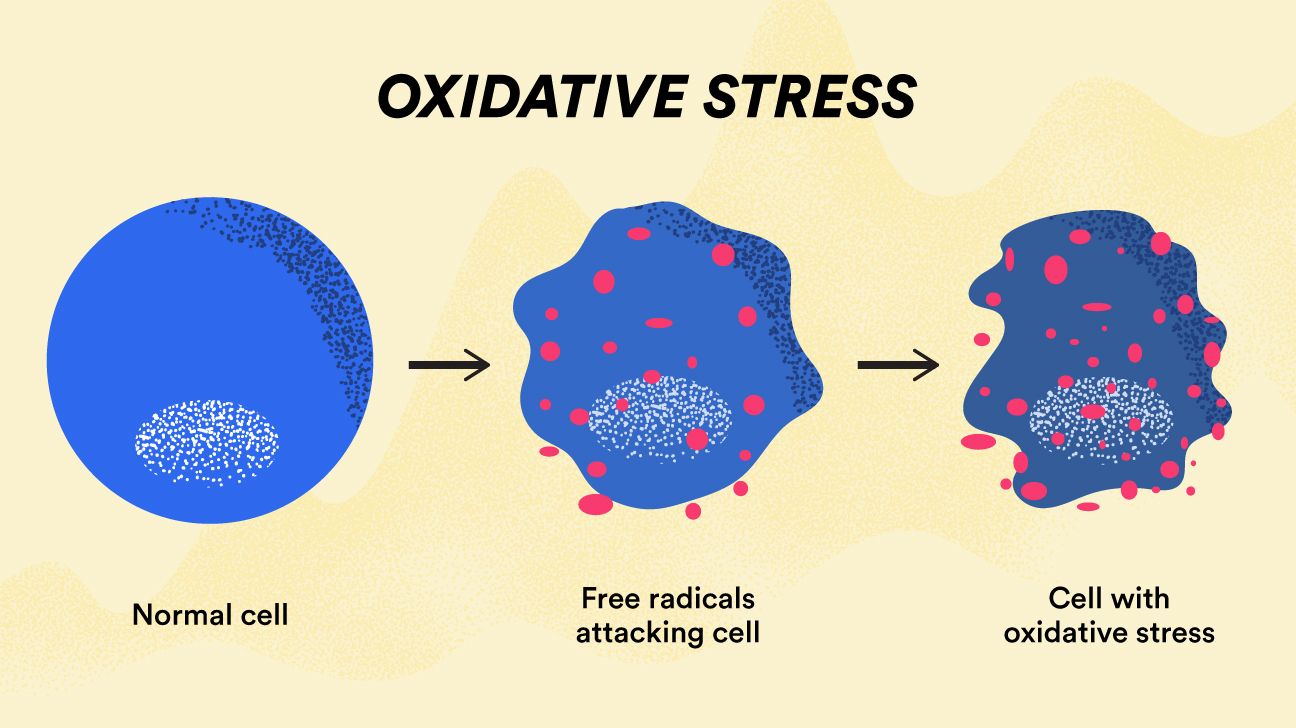
With our understanding of ROS and their spermicidal effect, H 2 O 2 proves to be an effective spermicide agent against polyspermy [ 66 , 67 ]. The term oxidative stress refers to a critical imbalance between ROS production and antioxidant defense mechanisms available to the biological system [ 15 ].
According to Sies [ 5 ], it is a disturbance in the prooxidant-antioxidant balance in favor of the former, leading to potential cellular damage. Essentially, OS may result from: Diminished antioxidants, e.
mutations affecting antioxidant defense enzymes or toxic agents that deplete such mechanisms [ 5 ]. phagocytic oxidative outburst during chronic inflammatory diseases [ 5 , 15 ].
This mechanism is normally thought to be more relevant to mammalian diseases and is frequently the target of attempted therapeutic intervention. OS can result in: Adaptation: Usually by upregulation of antioxidant defense systems.
Cell and tissue injury: OS can cause damage to all molecular targets: DNA, proteins and lipids. Often it is not clear which is the first point of attack, since injury mechanisms may overlap [ 5 ].
Cell death: This process may occur by two mechanisms, necrosis or apoptosis. During necrotic cell death, the cell swells and ruptures, releasing its contents into surrounding areas and affecting adjacent cells. The intracellular content can include antioxidants such as catalase or glutathione GSH as well as prooxidants such as copper and iron.
As such, necrosis may lead to further oxidative insults in the internal milieu [ 3 , 4 , 5 , 15 ]. As such, apoptotic cells do not release their content into surrounding environment and apoptosis does not cause damage to the neighboring cells [ 5 ]. An intricate cellular architecture of spermatozoa renders them to be particularly sensitive to OS.
Sperm plasma membranes contain large quantities of polyunsaturated fatty acids PUFAs. On the other hand, their cytoplasm contains low concentrations of scavenging enzymes [ 68 ]. OS usually results in a decreased sperm motion and viability, accompanied by a rapid loss of ATP, axonemal damage, increased midpiece morphology defects, followed by alterations in the sperm capacitation and acrosome reaction [ 32 ].
Lipid peroxidation has been repeatedly postulated to be the key mechanism of ROS-induced sperm damage, possibly leading to male reproductive dysfunction [ 68 ]. Sperm plasma membranes are largely composed of PUFAs, which are exceptionally susceptible to oxidative damage due to the presence of more than two carbon—carbon double bonds [ 68 ].
These fatty acids maintain the fluidity of membranes [ 69 ]. ROS attack PUFAs, leading to a cascade of chemical reactions called lipid peroxidation LPO.
LPO affects most prominent structural and functional characteristics of the membrane, including fluidity, ion gradients, receptor transduction, transport processes as well as enzymatic activities.
As a result, properties that are crucial for a normal fertilization are impaired [ 68 , 69 ]. LPO is a self-propagating process that may be divided into three phases: the initiation phase, the propagation phase and the termination phase.
During the initiation phase, one hydrogen is taken from unsaturated lipids to form lipid radicals. During the termination phase, two radicals react with each other to form a stable product and LPO finally ceases [ 70 ]. Numerous pathological effects of LPO on the sperm function are currently known.
Overall, LPO causes DNA and protein damage through oxidation of lipid peroxyl or alkoxyl radicals. DNA fragmentation by LPO can occur via base modifications, strand breaks or crosslinks [ 71 ].
LPO generally results in loss of membrane fluidity and subsequently a decreased sperm motility and sperm-oocyte fusion [ 68 , 69 , 70 , 71 ]. Furthermore, during LPO, ROS initiate a cascade of events involving the xanthine and xanthine oxidase system and deplete the ATP production which may ultimately lead to sperm death [ 68 ].
The unique sperm chromatin packing alongside antioxidant molecules present in the seminal plasma provide notable protection to sperm DNA against oxidative damage. Nevertheless, spermatozoa lack any specific DNA repair mechanisms and hence depend on the oocyte for eventual DNA repair following fertilization.
ROS-associated catalysis and apoptosis are considered to be the primary mechanisms that induce DNA fragmentation in spermatozoa [ 72 ].
DNA bases and phosphodiester backbones are believed to be most susceptible to ROS-associated peroxidative damage. At the same time, sperm mitochondrial DNA is more vulnerable to oxidative insults when compared to the nuclear genome [ 73 ].
Furthermore, because of the structure of the Y chromosome as well as its inability to repair double strand breaks, Y-bearing spermatozoa are more susceptible to DNA damage than X-carrying counterparts [ 74 ].
Y-bearing spermatogonia can be a target of mutations in the euchromatic Y region Yq11 , known as the azoospermia factor, resulting in infertility [ 75 ]. Various types of DNA abnormalities may occur in sperm that have been exposed to ROS artificially.
These include base modifications, production of base-free sites, deletions, frame shifts, DNA crosslinks and chromosomal rearrangements. OS has also been associated with high frequencies of single- and double-strand DNA breaks. ROS can also cause gene mutations, such as point mutation and polymorphism, resulting in decreased semen quality.
These changes may be observed especially during the prolonged meiotic prophase, when the spermatocytes are particularly sensitive to damage and widespread degeneration can occur [ 72 , 73 , 74 ]. Also, mutations in the mitochondrial DNA mtDNA may cause a defect of mitochondrial energy metabolism and therefore lower levels of mutant mtDNA may compromise sperm motility in vivo [ 76 ].
Other mechanisms such as denaturation and DNA base-pair oxidation may also be involved [ 74 ]. Increased DNA damage has become a serious issue during artificial reproduction techniques ARTs , as it has been correlated with decreased fertilization rates in vitro and increased early embryo death.
Unfortunately, no successful method to prevent or treat sperm DNA damage is currently available [ 77 ]. Proteins are a critical target for oxidation because of their abundance and high rate constants for interactions with diverse ROS. As such, protein damage is a major consequence of both intracellular and extracellular oxidative insults.
ROS may attack both the side chains and backbone, and the extent of the insult depends on multiple factors. In some cases, the damage is limited to specific residues, whereas in case of other ROS, the damage is widespread and nonspecific [ 78 ].
Oxidative attacks on proteins generally result in site-specific amino acid modifications, fragmentation of the peptide chain, aggregation of cross-linked reaction products, altered electric charge and increased susceptibility or extreme tolerance to proteolysis [ 79 ].
The resulting products of protein oxidation include reactive hydroperoxides, which may be employed as biomarkers for protein oxidation in vitro and in vivo.
As protein damage is usually non-repairable, oxidation may have deleterious consequences, including the loss or sometimes gain of enzymatic, structural or signaling function, fragmentation, unfolding, altered interactions with other proteins and modified turnovers.
Generally, oxidized proteins are degraded by proteasomal and lysosomal pathways; however, in some cases, such altered material is poorly degraded and may accumulate within cells contributing to multiple mammalian pathologies [ 78 , 79 ].
The amino acids in a peptide differ in their susceptibility to oxidative insults, while various ROS differ in their potential reactivity. Primary, secondary and tertiary protein structures alter the relative susceptibility of certain amino acids. According to Mammoto et al. Sinha et al. Thus, oxidation of the sperm SH-proteins may be a notable mechanism responsible for the suppressive effects of ROS on sperm functions.
Usually, when cellular components undergo serious damage, apoptosis or programmed cell death is initiated.
During spermatogenesis, abnormal spermatozoa are eliminated primarily through apoptosis. The exact mechanism of action is not fully understood yet; however, previous studies have speculated that ROS serve as an activator of the mitochondria to release the signaling cytochrome c [ 82 , 83 ].
This molecule initiates a cascade of events involving caspases 3 and 9, eventually leading to sperm apoptosis. The Fas-protein may be also an integral component in the apoptotic pathway. When Fas-ligand or anti-Fas antibody binds to Fas, apoptosis is initiated [ 83 ].
An additional mechanism involves the inflammatory production of ROS, primarily hypochlorous acid HOCl , which is a product of H 2 O 2 and chloride ion. This molecule oxidizes a variety of cellular components, thus causing apoptosis [ 84 ].
Said et al. Numerous studies have focused to study apoptosis in spermatozoa. Various authors [ 35 , 86 ] have reported increased ROS levels and apoptotic markers measured by fluorescence in samples of infertile subjects.
On the other hand, in certain males, abortive apoptosis appears to fail in the clearance of spermatozoa that are marked for elimination by apoptosis. As such, the subsequent population of ejaculated spermatozoa may exhibit an array of anomalies consistent with characteristics typical for cells that are in the process of apoptosis.
Apoptotic failures may lead to a decreased sperm count resulting in subfertility [ 82 , 83 ]. Spermatozoa motility is an important prerequisite to secure their distribution in the female sexual system, followed by an effective passage through the cervical mucus and penetration into the egg [ 89 ].
Increased ROS levels have been repeatedly correlated with a decreased sperm motility [ 10 , 11 , 12 , 90 ], although the exact mechanism involved is still not completely understood. One hypothesis suggests that H 2 O 2 diffuses across the membranes into the cells and inhibits the activity of vital enzymes such as NADPH oxidase [ 6 ].
At the same time, a decreased G6PDH leads to a reduced availability of NADPH accompanied by a build-up of oxidized glutathione. Such changes may lead to a decline in the intracellular antioxidant levels and a subsequent peroxidation of membrane phospholipids [ 65 ].
Another hypothesis presents a series of interrelated events leading to a decreased phosphorylation of axonemal proteins, followed by sperm immobilization, both of which are linked to a reduced membrane fluidity crucial for sperm-oocyte fusion [ 10 , 32 ].
When spermatozoa are incubated with selected ROS overnight, loss of motion characteristics observed is highly correlated with sperm LPO. Furthermore, the ability of antioxidants to revive sperm motility is evidence that LPO is a major cause for motility loss in spermatozoa [ 68 , 69 ].
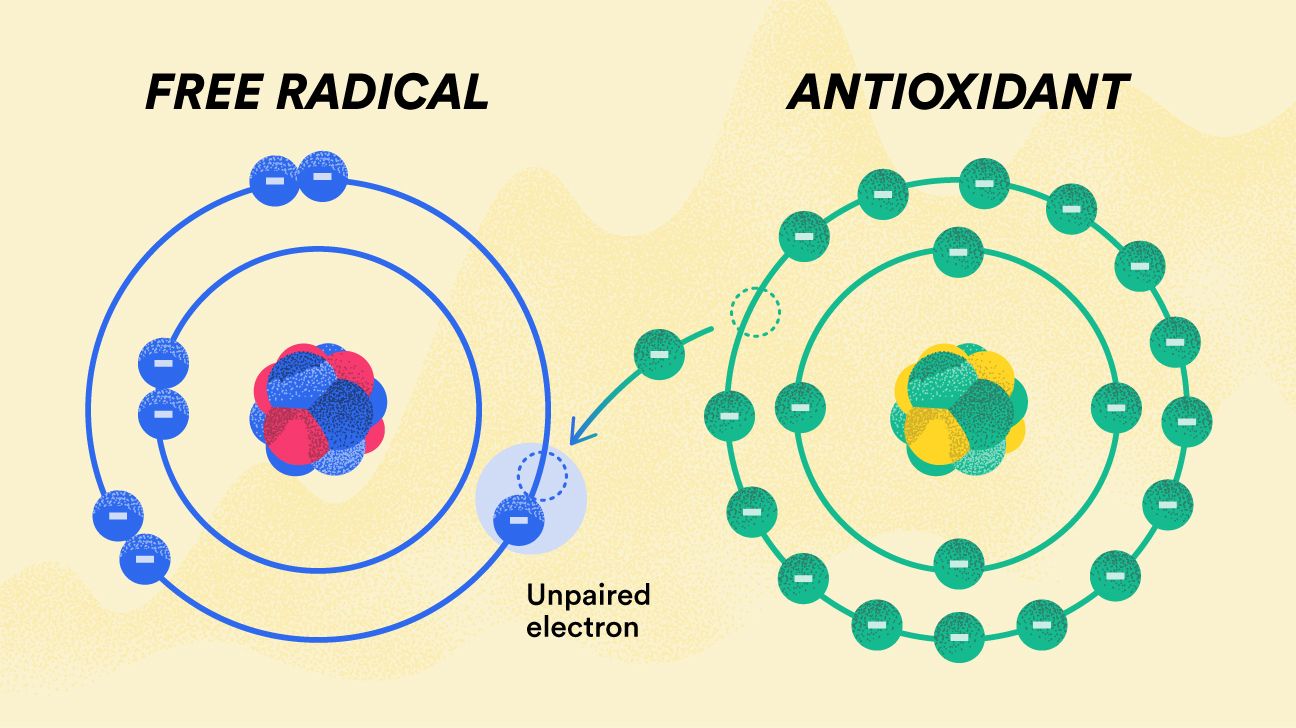
Because ROS have both physiological and pathological functions, biological systems have developed defense systems to maintain ROS levels within a certain range.
Whenever ROS levels become pathologically elevated, antioxidants scavenge them to minimize any potential oxidative damage [ 1 ]. Antioxidants are defined as molecules that dispose, scavenge and inhibit the formation of ROS or oppose their actions.
According to Ďuračková [ 13 ], antioxidants can protect cells against OS via three mechanisms: prevention, interception and repair. Antioxidants may be divided into two dominant categories: Enzymatic e.
superoxide dismutases, catalase and glutathione peroxidases. Non-enzymatic e. vitamin C, vitamin E, vitamin A, carotenoids, albumin, glutathione, uric acid, pyruvate, etc.
Due to the size and small volume of cytoplasm, as well as the low concentrations of scavenging enzymes, spermatozoa have limited antioxidant defense possibilities.
Mammalian spermatozoa predominantly contain enzymatic antioxidants, including SOD and glutathione peroxidases GPx , which are mainly located in the midpiece. A few non-enzymatic antioxidants, such as vitamins C and E, transferrin and ceruloplasmin, are present in the plasma membrane of spermatozoa and act as preventive antioxidants [ 16 ].
Under normal circumstances, the seminal plasma is an important protectant of spermatozoa against any possible ROS formation and distribution. Seminal plasma contains both enzymatic antioxidants, as well as an array of non-enzymatic antioxidants e. ascorbate, urate, vitamin E, pyruvate, glutathione, albumin, taurine and hypotaurine [ 9 ].
Studies have shown that antioxidants protect spermatozoa from ROS generating abnormal spermatozoa, scavenge ROS produced by leukocytes, prevent DNA fragmentation, improve semen quality, reduce cryodamage to spermatozoa, block premature sperm maturation and generally stimulate sperm vitality [ 91 , 92 ].
Superoxide dismutases are metal-containing enzymes that catalyze the conversion of two superoxides into oxygen and hydrogen peroxide, which is less toxic than superoxide [ 1 , 13 ]:. The enzymes are present in both intracellular and extracellular forms. The second form is manganese SOD, which is found predominantly in the mitochondrial matrix and has manganese in its active center MnSOD, SOD-2 [ 93 ].
The secretory tetrameric SOD EC-SOD, SOD-3 may be detected in the extracellular space. The enzyme is associated with surface polysaccharides although it may also be found as a free molecule. Structurally, SOD-3 is similar to SOD-2; however, it has zinc and copper in its active center instead of manganese [ 1 , 5 , 15 ].
SOD protects spermatozoa against spontaneous O 2 toxicity and lipid peroxidation [ 69 ]. Numerous studies have suggested a significant role for SOD in sperm motility both in vivo and in vitro.
The addition of SOD to human and animal semen [ 94 , 95 , 96 ] has been shown to protect spermatozoa against the harmful effects of ROS and improve sperm motility and membrane integrity during liquid storage or cryopreservation.
As such, it may be concluded that the SOD content in mature spermatozoa may be a good predictor of post-thaw motility recovery following sperm preservation.
Catalase catalyzes the decomposition of hydrogen peroxide to molecular oxygen and water, thereby completing the detoxifying reaction started by SOD. A characteristic feature of its structure is a heme system with centrally located iron [ 1 , 13 ]:.
CAT has been found in peroxisomes, mitochondria, endoplasmic reticulum and the cytosol in a variety of cells [ 93 ]. In semen, the enzyme was detected in human, bovine and rat spermatozoa, as well as seminal plasma, with the prostate as its source [ 97 , 98 ].
Catalase activates sperm capacitation induced by nitric oxide [ 59 , 60 ]. Furthermore, it plays an important role in decreasing lipid peroxidation and protecting spermatozoa during genitourinary inflammation [ 25 ].
Numerous studies have revealed a positive relationship between sperm motility and the presence of CAT in mammalian ejaculates. Also, positive correlations were observed between sperm morphology and protein expression of CAT in seminal plasma [ 98 , 99 ].
Furthermore, CAT supplementation to fresh, processed and cryopreserved semen resulted in a higher sperm vitality, progressive motility and DNA integrity [ ]. Glutathione peroxidases are a family of selenium-containing enzymes, which catalyze the reduction of H 2 O 2 and organic peroxides, including phospholipid peroxides [ 93 ].
In their active site, the enzymes contain selenium in the form of selenocysteine. where GSH symbolizes reduced glutathione and GS-SG represents glutathione disulfide. The reaction is based on the oxidation of selenol of a selenocysteine residue by H 2 O 2.
This process leads to its derivation with selenic acid RSeOH. This by-product is subsequently converted back to selenol through a two-step process that starts with a reaction comprising GSH to generate GS-SeR and water.
A second GSH molecule then reduces the GS-SeR intermediate back to selenol, releasing GS-SG as a by-product [ 1 , 5 , 13 ]:. The classic intracellular GPx1 is expressed in sperm nucleus, mitochondria and cytosol, as well as in the testes, prostate, seminal vesicles, vas deferens, epididymis, and has a significant relationship with sperm motility [ , ].
More importantly, a direct relationship has been reported between male fertility and phospholipid hydroperoxide glutathione peroxidase PHGPx; GPx4 , a selenoprotein that is highly expressed in testicular tissue and has a prominent role in the formation of the mitochondrial capsule [ 51 , 53 , 54 ].
Other enzymes, such as glutathione reductase, ceruloplasmin or heme oxygenases, may also participate in the enzymatic control of oxygen radicals and their products. A short overview of minor antioxidant enzymes is provided in Table 2. Location: Found in the epididymis, sertoli cells, vas deferens, seminal vesicles, epithelium and prostate gland [ , ].
Roles: Catalyzes reduction of oxidized glutathione. Maintains glutathione homeostasis. Altered in infertile men, and these alterations seem to be linked to sperm morphology [ , , ].
Location: Most abundant in the seminiferous tubular fluid of mammalian testes, sperm acrosomes, human sperm and mouse spermatogenic cells [ , , ]. Roles: Detoxification enzymes, intracellular-binding proteins [ ].
Involved in epididymal maturation, capacitation and sperm-oocyte interactions [ , ]. Location: Semen, probably of testicular origin [ ]. Roles: Cu-dependent ferroxidase, a fundamental bridge between Fe utilization and Cu status. Associated with the oxidation of ferrous ion into ferric [ ].
Prevents non-enzymatic generation of superoxide and scavenges superoxide, hydroxyl and singlet oxygen [ , ]. Has positive impact on sperm parameters and male fertility [ ].
Serves as a marker of a proper seminiferous tubule function [ ]. Location: Seminal plasma [ , ]. Roles: Primary binding and transport protein for iron and regulates iron transport and storage [ ]. Serves as a reliable index of seminiferous tubular function [ ].
Location: Two forms of heme oxygenase, HO-1 and HO-2, were identified in human testis and seminal plasma [ , ]. Roles: HO is strongly induced by oxidant stress and protects against oxidative insults.
Increases reduced glutathione levels, degrades heme and intervenes with the metabolism of biliverdin and bilirubin, which have potent antioxidant properties [ ]. HO is highly expressed in fertile normozoospermic subjects with positive correlations to sperm concentration, motility and morphology.
HO enzyme activity is related to spermatogenesis and sperm motility processes [ , ]. Non-enzymatic antioxidants are also known as synthetic antioxidants or dietary supplements. Glutathione is the most abundant thiol protein in mammalian cells [ ].
Being an endogenous source, it is synthesized by the liver but it can also be derived from dietary sources such as fresh meat, fruits and vegetables. This molecule has three precursors: cysteine, glutamic acid and glycine.
Its cysteine subunit provides and exposes -SH that directly scavenges free radicals. High levels are found especially in the testis of rats [ ] and the reproductive tract fluids and epididymal sperm of bulls [ 98 ]. GSH protects the cell membranes from lipid oxidation and prevents further formation of free radicals.
Its deficit leads to instability of the sperm midpiece, which results in motility disorders [ ]. Glutathione supplementation in infertile subjects has led to a significant improvement in sperm parameters and prevents oxidative damage to sperm DNA.
A factor increasing the level of GSH is pantothenic acid, which by doing so also protects tissues against oxidative stress [ , ]. Vitamin C or ascorbic acid AA may be found in its reduced ascorbate as well as oxidized form dehydroascorbic acid , both of which are easily interconvertible and biologically active.
Vitamin C is found in citrus fruits, peppers, strawberries, tomatoes, broccoli, brussels sprouts and other leafy vegetables. AA is a water-soluble vitamin, and because of its hydrophilic nature, it has more effective scavenging properties at the plasma level than in the lipid bilayer [ ].
Vitamin C has been used in the management of male infertility on empirical grounds, particularly in the presence of non-specific seminal infections [ ].
Its presence in the seminal plasma of healthy males has been reported by various authors [ , , ]. Chinoy et al. Low concentration of vitamin C showed significant degenerative changes in the testes, epididymis and vas deferens of scorbutic guinea pigs.
On the other hand, excessive intake of vitamin C has been reported to cause reproductive failure in the men [ ]. This was further corroborated by the association of decreased AA followed by an increase in the seminal plasma LPO as observed in a human trial [ , ].
Moreover, it has been reported that AA supplementation leads to a significant reduction in the ROS concentration, sperm membrane LPO and DNA oxidation together with an increased sperm quality.
The results of a recent animal experimental study indicate that vitamin C improves the activity of antioxidant enzymes and significantly reduces malondialdehyde MDA concentration in testicular structures [ ].
Vitamin E is a term that encompasses a group of potent, lipid-soluble tocol tocopherol and tocotrienol derivatives qualitatively exhibiting the biological activity of RRR-α-tocopherol. Structural analyses have revealed that molecules having vitamin E antioxidant activity include four tocopherols α-, β-, γ- and δ- and four tocotrienols α-, β-, γ- and δ- with α-tocopherol being the most abundant form in nature and mostly available in food, having the highest biological activity and reversing vitamin E deficiency symptoms.
The molecular functions fulfilled specifically by α-tocopherol have yet to be fully described; however, the antioxidant feature is the flagship of the biological activity related to vitamin E [ ].
Vitamin E is present within the seminal plasma and plasma membrane. It is a lipid soluble, chain-breaking antioxidant that able to terminate free radical chain reactions, particularly the peroxidation of PUFAs [ , ].
Numerous reports emphasize on the role of α-tocopherol in the management of male infertility. A positive association was found between α-tocopherol in sperm plasma membranes and the percentage of motile, living and morphologically intact spermatozoa [ ]. At the same time, α-tocopherol levels were decreased significantly in oligo- and azoospermic patients in comparison to normospermic controls [ ].
A significant improvement in the in vitro ability of spermatozoa to bind the zona pellucida of unfertilized oocytes was found in men with high ROS production supplemented with vitamin E for 3 months [ ]. Vitamin E supplementation may also play a role in reducing sperm DNA fragmentation and morphology defects [ ].
There are other substances which may contribute to the maintenance of oxidative homeostasis. The prime function of these compounds is not to combat the production or action of ROS; however, their presence may decrease the risk of OS development.
Albumin, cysteine, taurine, zinc and selenium are the most known representatives. Furthermore, antioxidant substances isolated from natural resources, such as resveratrol, curcumin or lycopene, have recently emerged as suitable dietary supplements or therapeutics due to their chemical diversity, structural complexity, availability, lack of significant toxic effects and intrinsic biologic activity.
A short overview of secondary non-enzymatic antioxidants is provided in Table 3. Has the ability to reduce free radicals by acting with thiols and hydroxyl radicals.
Plays a role as a precursor to glutathione [ ]. Reduces seminal OS and sperm DNA damage [ ]. When combined with selenium, NAC has a positive impact on sperm concentration and acrosome reaction [ , ]. Stimulates mitochondrial metabolism. Has the ability to shuttle long-chain lipids across the mitochondrial bilayer and start the process of ß-oxidation to create NADH and FADH 2 along with acetyl-CoA [ ].
Acts primarily in the epididymis. Prevents DNA damage and apoptosis during sperm maturation [ ]. Found abundantly in the mammalian body, including testes and spermatozoa [ ].
Participates in bile salt formation, calcium binding and transport, osmoregulation and stabilization of biological membranes.
A component of cellular antioxidant defenses [ ]. Taurine administration to semen prevents the loss of sperm motility and viability, promotion of the activity of reduced glutathione, GPx, SOD and CAT while concomitantly lowering LPO and morphological abnormalities of spermatozoa [ ].
A trace element with high concentration in the seminal plasma [ ]. Serves as a cofactor to dihydrofolate reductase and methionine synthase needed for homocysteine recycling, membrane and DNA stabilization [ ].
Acts as a cofactor for SOD and metallothioneins, assisting in scavenging superoxide and hydroxyl radicals [ ]. A trace element positively correlated with increased levels of sperm concentration, motility and morphology [ ]. Cofactor of phospholipid hydroxyperoxide glutathione peroxidase, important for chromatin condensation and formation of the mitochondrial capsule [ 52 , 53 , 54 ].
A key element in the regulation of osmotic pressure and distribution of fluid between different compartments [ ] and able to bind metals ions, fatty acids, drugs and hormones. Stimulates spermatozoa motility, eliminates free radicals and protects membrane integrity from heat shock during semen cryopreservation [ , ].
End product of heme metabolism via heme oxygenase-1, biliverdin and biliverdin reductase [ ]. May protect vitamin A and linoleic acid from oxidative destruction due to an extended system of conjugated double bonds and a reactive hydrogen atom [ ].
Despite being a major antioxidant in the plasma, both correlates with and predicts OS development. It may function either as an antioxidant primarily in plasma or pro-oxidant primarily within the cell [ ].
A powerful scavenger of singlet oxygen, peroxyl and hydroxyl radicals in the hydrophilic environment, but loses an ability to scavenge lipophilic radicals and cannot break the radical chain propagation within lipid membranes [ ]. A polyphenol that belongs to the stilbene family and is found in grapes, berries, pistachios, plums, peanuts and wines [ ].
A free radical scavenger and a potent antioxidant, promotes the activities of a variety of antioxidant enzymes and increases the antioxidant capacity [ ]. Copper and iron chelator preventing the Fenton reaction [ ].
Stimulates and protects spermatocytes and spermatozoa against LPO, reduces apoptosis of germinal cells [ ] and protects against environmental toxins [ ]. Enhances spermatogenesis by stimulating the hypothalamic-pituitary-gonadal axis without adverse effects, triggers penile erection and enhances blood testosterone levels, testicular sperm count and epididymal sperm motility [ , ].
One of over carotenoids found in nature, present in tomatoes, watermelons and pink grapefruits [ ]. A highly unsaturated straight chain hydrocarbon with a total of 13 double bonds, 11 of which are conjugated making the molecule to be twice as potent singlet oxygen quencher as ß-carotene and 10 times more active in comparison to α-tocopherol [ ].
LYC administration leads to a significant improvement of semen parameters sperm concentration, motility and morphology in patients with idiopathic infertility, antibody-mediated infertility as well as with different sperm abnormalities [ , ].
In vitro LYC supplementation has led to an increased post-thaw spermatozoa survival and DNA stability [ ], together with an improved sperm morphology and membrane integrity [ ]. Antioxidant supplementation has proven to be effective against male reproductive dysfunction in vivo. Recent reports have acclaimed significant attention due to the quality of their study design and demonstrated compelling evidence regarding the efficacy of antioxidants towards improving semen parameters.
On the other hand, numerous clinical trials studying the effects of dietary antioxidants on semen parameters are still uncontrolled, focus on rather on healthy individuals or have indirect end-points of success.
The dose and duration of antioxidant administration also need to be thoroughly examined and standardized. Table 4 presents the most effective doses for the treatment of male subfertility based on currently available studies that explored the impact of antioxidant supplementation on sperm parameters.
Vitamin C supplementation improved sperm count, motility and morphology [ ]. After surgery, the subjects received mg vitamin C for 2 months. Vitamin C supplementation following surgery resulted in a better motility and morphology. Prior to surgery, vitamin C was not effective on the sperm count, but it improved sperm motility and morphology [ ].
At the end of the experiment, sperm motility increased, while LPO decreased in the studied population [ ]. The treatment did not affect sperm concentration, motility and morphology [ ]. No differences in basic sperm parameters were found following antioxidant treatment; however, the percentage of DNA-fragmented spermatozoa was markedly reduced [ ].
After 4 months of treatment, a significant improvement was observed in sperm concentration, motility and morphology [ ]. No improvements in semen quality was detected in 26 men diagnosed with asthenozoospermia who underwent 6 months of daily treatment with 2 g L-carnitine and 1 g L-acetyl-carnitine [ ].
No improvement was observed in sperm concentration after 3 months, although the motility was increased in the treated subjects [ ]. Following treatment, a significant decrease of LPO was observed together with an improvement of sperm motility [ ].
Administration of mg of ZnSO 4 twice daily for 3 months to 50 asthenozoospermic patients resulted in a higher sperm count and membrane integrity. ZnSO 4 also played an immunological role as T-helper cytokines and interleukin-4 levels increased in the experimental group and TNF-α and antisperm antibodies decreased [ ].
Most pronounced studies on the effects of oral antioxidant supplementation on male infertility. ROS-induced damage may have significant clinical implications in the context of ARTs. Numerous reports have indicated that significantly increased ROS levels may occur in response to repeated cycles of centrifugation involved in conventional sperm preparation techniques used for ARTs [ ].
Spermatozoa selected for ART often face OS and a high risk for DNA damage. When intrauterine insemination or in vitro fertilization IVF is used, such damage does not represent a cause of concern as damage to the sperm membrane lipids ensures that fertilization will not occur.
However, in case of intracytoplasmic sperm injection is used, this natural selection barrier may be overlooked and sperm with DNA damage may be directly injected into the ovum [ 77 ].
Selection of an effective sperm preparation technique is important to minimize ROS overgeneration and eventual oxidative insults to the male gamete. The density gradient technique is able to separate leukocytes and immature or damaged spermatozoa from normal spermatozoa, which may be subsequently used in ARTs [ 77 , , ].
Assisted reproduction techniques may benefit from in vitro supplementation of antioxidants [ ]. Various antioxidants such as vitamin E, vitamin C, cysteine, taurine and hypotaurine present in the culture medium have been shown to improve the developmental ability of the embryos by counteracting the effects of ROS [ 93 , ].
In cases of IVF, incubation times of more than 16—20 hours have been correlated with increased oxidative damage.
Shortening the insemination timeframes up to 1—2 hours or less may reduce ROS overgeneration in culture media and possibly improve fertilization, embryogenesis and pregnancy rates [ 77 , ]. Because high levels of ROS have been associated with a decreased male infertility, measuring ROS levels in semen is an important part of the initial evaluation as well as follow-up of men with reproductive dysfunction [ 10 , 11 , 12 ].
Chemiluminescence and flow cytometry are currently the most common techniques in clinical andrology to assess and study seminal OS. Chemiluminescence measures light emitted following administration of specific reagents to a semen sample.
Chemiluminescent assays are sensitive, convenient for diagnostic purposes and have relatively well-established normal ranges [ 11 , 12 ]. Safarinejad MR Efficacy of coenzyme Q10 on semen parameters, sperm function and reproductive hormones in infertile men.
J Urol 1 — Saleh RA, Agarwal A, Nada EA, El-Tonsy MH, Sharma RK et al Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility.
Google Scholar. Scott R, MacPherson A, Yates RW, Hussain B, Dixon J The effect of oral selenium supplementation on human sperm motility. Sharlip ID, Jarow JP, Belker AM, Lipshultz LI, Sigman M et al Best practice policies for male infertility.
Sierens J, Hartley JA, Campbell MJ, Leathem AJ, Woodside JV In vitro isoflavone supplementation reduces hydrogen peroxide-induced DNA damage in sperm. Teratog Carcinog Mutagen — Sigman M, Glass S, Campagnone J, Pryor JL Carnitine for the treatment of idiopathic asthenozoospermia: a randomized, double-blind, placebo-controlled trial.
Suleiman SA, Ali ME, Zaki ZM, el-Malik EM, Nasr MA Lipid peroxidation and human sperm motility: protective role of vitamin E. Tarin JJ, Brines J, Cano A Antioxidants may protect against infertility. Tremellen K Oxidative stress and male infertility — a clinical prospective.
Twigg J, Fulton N, Gomez E, Irvine DS, Aitken RJ Analysis of the impact of intracellular reactive oxygen species generation on the structural and functional integrity of human spermatozoa: lipid peroxidation, DNA fragmentation and effectiveness of antioxidants.
Verma A, Kanwar KC Human sperm motility and lipid peroxidation in different ascorbic acid concentration: an in vitro analysis. Vezina D, Mauffette F, Roberts KD, Bleau G Selenium-vitamin E supplementation in infertile men.
Effects on semen parameters and micronutrient levels and distribution. Biol Trace Elem Res — Vicari E, Calogero AE Effects of treatment with carnitines in infertile patients with prostato-vesiculo-epididymitis.
Vicari E, La Vignera S, Calogero AE Antioxidant treatment with carnitines is effective in infertile patients with prostato-vesiculo-epididymitis and elevated seminal leukocyte concentration after treatment with nonsteroidal anti-inflammatory compounds.
Vural P, Akgul C, Yildirim A, Canbaz M Antioxidant defence in recurrent abortion. Clin Chim Acta — Wong WY, Merkus HMWM, Thomas CMG, Menkveld R, Zielhuis GA, Steegers-Theunissen RP Effects of folic acid and zinc sulfate on male factor subfertility: a double-blind, randomized, placebo-controlled trial.
Woods JR jr, Plessinger MA, Miller RK Vitamins C and E: missing links in preventing preterm premature rupture of membranes? Am J Obstet Gynecol 8 :5— Yumura Y, Iwasaki A, Saito K, Ogawa T, Hirokawa M Effect of reactive oxygen species in semen on the pregnancy of infertile couples.
Int J Urol — Zini A, San Gabriel M, Baazeem A Antioxidants and sperm DNA damage: a clinical perspective. J Assist Reprod Genet — Download references. You can also search for this author in PubMed Google Scholar.
Correspondence to Andrea Sansone. Department of Pharmacology and Therapeutics, University of British Colombia, Vancouver, British Columbia, Canada.
Reprints and permissions. Sansone, A. Free Radicals and Reproductive Health. In: Laher, I. eds Systems Biology of Free Radicals and Antioxidants. Springer, Berlin, Heidelberg. Published : 03 May Publisher Name : Springer, Berlin, Heidelberg. Print ISBN : Online ISBN : eBook Packages : Biomedical and Life Sciences Reference Module Biomedical and Life Sciences.
Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Policies and ethics. Skip to main content. Abstract Studies performed during the last decades have focused on inflammation and on its involvement in many pathologies.
Keywords Antioxidants Female infertility Male infertility Oligoasthenoteratozoospermia Oxidative stress Reactive oxygen species Reproductive health. Buying options Chapter EUR eBook EUR 1, Hardcover Book EUR 2, Tax calculation will be finalised at checkout Purchases are for personal use only Learn about institutional subscriptions.
Abbreviations NAC: N -acetyl- l -cysteine OAT: Oligoasthenoteratozoospermia ROS: Reactive oxygen species. References Abd-Allah AR, Helal GK, Al-Yahya AA, Aleisa AM, Al-Rejaie SS, Al-Bakheet SA Pro-inflammatory and oxidative stress pathways which compromise sperm motility and survival may be altered by L-carnitine.
Oxid Med Cell Longev —78 Article PubMed Central PubMed Google Scholar Abd-Elmoaty MA, Saleh R, Sharma R, Agarwal A Increased levels of oxidants and reduced antioxidants in semen of infertile men with varicocele. Fertil Steril — Article CAS PubMed Google Scholar Abel BJ, Carswell G, Elton R, Hargreave TB, Kyle K et al Randomised trial of clomiphene citrate treatment and vitamin C for male infertility.
J Urol — Article CAS Google Scholar Agarwal A, Saleh RA, Bedaiwy MA Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril — Article PubMed Google Scholar Agarwal A, Sharma RK, Desai NR, Prabakaran S, Tavares A, Sabanegh E Role of oxidative stress in pathogenesis of varicocele and infertility.
Urology — Article PubMed Google Scholar Aitken RJ, Clarkson JS Significance of reactive oxygen species and antioxidants in defining the efficacy of sperm preparation techniques.
J Androl — CAS PubMed Google Scholar Aitken RJ, Clarkson JS, Fishel S Generation of reactive oxygen species, lipid peroxidation, and human sperm function.
Biol Reprod — Article CAS PubMed Google Scholar Aitken RJ, Buckingham D, Harkiss D Use of a xanthine oxidase free radical generating system to investigate the cytotoxic effects of reactive oxygen species on human spermatozoa.
J Reprod Fertil — Article CAS PubMed Google Scholar Aitken RJ, De Iuliis GN, Finnie JM, Hedges A, McLachlan RI Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: development of diagnostic criteria.
Hum Reprod — Article Google Scholar Al-Gubory KH, Fowler PA, Garrel C The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol — Article CAS PubMed Google Scholar Askari HA, Check JH, Peymer N, Bollendorf A Effect of natural antioxidants tocopherol and ascorbic acids in maintenance of sperm activity during freeze-thaw process.
Arch Androl —15 Article CAS PubMed Google Scholar Baker HW, Brindle J, Irvine DS, Aitken RJ Protective effect of antioxidants on the impairment of sperm motility by activated polymorphonuclear leukocytes.
Fertil Steril — CAS PubMed Google Scholar Balercia G, Regoli F, Armeni T, Koverech A, Mantero F, Boscaro M Placebo-controlled double-blind randomized trial on the use of L-carnitine, L-acetylcarnitine, or combined L-carnitine and L-acetylcarnitine in men with idiopathic asthenozoospermia. Fertil Steril — Article CAS PubMed Google Scholar Balercia G, Buldreghini E, Vignini A, Tiano L, Paggi F et al Coenzyme Q10 treatment in infertile men with idiopathic asthenozoospermia: a placebo-controlled, double-blind randomized trial.
Fertil Steril 91 5 — Article CAS PubMed Google Scholar Bellver J, Meseguer M, Muriel L, García-Herrero S, Barreto MA et al Y chromosome microdeletions, sperm DNA fragmentation and sperm oxidative stress as causes of recurrent spontaneous abortion of unknown etiology.
Hum Reprod 25 7 — Article CAS PubMed Google Scholar Bilodeau JF, Hubel CA Current concepts in the use of antioxidants for the treatment of pre-eclampsia. J Androl — CAS PubMed Google Scholar Chandra A, Surti N, Kesavan S, Agarwal A Significance of oxidative stress in human reproduction.
Urology —76 Article PubMed Google Scholar Comhaire FH, Christophe AB, Zalata AA, Dhooge WS, Mahmoud AM, Depuydt CE The effects of combined conventional treatment, oral antioxidants and essential fatty acids on sperm biology in subfertile men.
Asian J Androl 7 3 — Article CAS PubMed Google Scholar Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HWG et al World Health Organization reference values for human semen characteristics.
Andrologia — Article CAS PubMed Google Scholar Critser JK, Huse-Benda AR, Aaker DV, Arneson BW, Ball GD Cryopreservation of human spermatozoa. Fertil Steril — CAS PubMed Google Scholar Desai N, Shabanegh E, Kim T, Agarwal A Free radical theory of aging: implications in male infertility.
Urology —19 Article PubMed Google Scholar Donnelly ET, McClure N, Lewis SE a Antioxidant supplementation in vitro does not improve human sperm motility. Fertil Steril — Article CAS PubMed Google Scholar Donnelly ET, McClure N, Lewis SE b The effect of ascorbate and alpha-tocopherol supplementation in vitro on DNA integrity and production of reactive oxygen species.
Mutagenesis — Article CAS PubMed Google Scholar Galatioto GP, Gravina GL, Angelozzi G, Sacchetti A, Innominato PF et al May antioxidant therapy improve sperm parameters of men with persistent oligospermia after retrograde embolization for varicocele?
World J Urol — Article Google Scholar Gavella M, Lipovac V Pentoxifylline-mediated reduction of superoxide anion production by human spermatozoa. Andrologia —39 Article CAS PubMed Google Scholar Gavella M, Lipovac V, Marotti T Effect of pentoxifylline on superoxide anion production by human sperm.
Int J Androl — Article CAS PubMed Google Scholar Geva E, Bartoov B, Zabludovsky N, Lessing JB, Lerner-Geva L, Amit A The effect of antioxidant treatment on human spermatozoa and fertilization rate in an in vitro fertilization program.
Fertil Steril — CAS PubMed Google Scholar Greco E, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, Tesarik J Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment.
J Androl — Article CAS PubMed Google Scholar Griveau JF, Le Lannou D Effects of antioxidants on human sperm preparation techniques. Int J Androl — Article CAS PubMed Google Scholar Griveau JF, Le Lannou D Reactive oxygen species and human spermatozoa: physiology and pathology.
Int J Androl —69 Article CAS PubMed Google Scholar Hargreave TB, Kyle KF, Baxby K, Rogers AC, Scott R et al Randomised trial of mesterolone versus vitamin C for male infertility. A complex cytokine influence at the maternal-fetal interface creates the conditions that are necessary to support embryo implantation in the endometrium [ 92 , 93 ].
Any imbalance between the cytokines and angiogenesis factors could result in implantation failure and pregnancy loss [ 94 ]. Critical changes occur in the vascular system, and these changes accompany follicular growth.
Follicular growth, selection of dominant follicle, corpus luteum formation, endometrial differentiation and embryo formation are key processes dependent on neovascularization [ 90 , 95 ].
As the endometrium grows in the menstrual cycle, vessel regeneration occurs i. spiral arterioles and capillaries [ 96 ]. Estrogens promote angiogenesis in the endometrium by controlling the expression of factors such as VEGF [ 97 ]. ROS generated from NADP H oxidase is critical for VEGF signaling in vitro and angiogenesis in vivo [ 98 ].
Small amounts of ROS are produced from endothelial NADP H oxidase activated by growth factors and cytokines. ROS that are generated in and around the vascular endothelium may play a role in normal cellular signaling mechanisms.
They may also be an important causative factors in endothelial dysfunction that leads to the development of atherosclerosis, diabetes complications and ischemia perfusion injury [ 98 , 99 ].
The molecular mechanism by which the oxidant status of cells modulates angiogenesis is not completely understood. As our understanding the role ROS-induced angiogenesis plays in atherosclerosis and myocardial angiogenesis grows, future studies should investigate the role ROS plays in the angiogenesis in the female reproductive tract.
There is a cyclical variation in the expression of superoxide dismutase SOD in the endometrium. SOD activity decreases in the late secretory phase while ROS levels increase [ ]. These changes have been hypothesized to be important in the genesis of menstruation and endometrial shedding.
The levels of prostaglandin F2 α increase towards the late secretory phase and ROS triggers the release of prostaglandin F2 α in vitro [ ]. Stimulation of the cyclooxygenase enzyme is brought about by ROS via activation of the transcription factor NFKappa β, suggesting a mechanism for menstruation [ 83 ].
Increased generation of ROS by activated peritoneal macrophages has been reported in the peritoneal fluid [ ]. Conflicting results were reported in further studies with large patient numbers, which failed to demonstrate an antioxidant or oxidant balance [ 74 , ]. ROS levels in peritoneal fluid of patients with endometriosis were not significantly higher than controls.
An increased titer of autoantibodies related to OS has been reported in women with endometriosis resulting in an increase in serum autoantibody titers to oxidatively modified low density lipoproteins [ ]. An OS-induced increase in autoantibody titers in the peritoneal fluid has been demonstrated in women with endometriosis.
Non-terminal oxidation may have a role in the pathophysiology of endometriosis. Minimally oxidized low density lipoprotein LDL M-LDL is present in peritoneal fluid of women with endometriosis in place of the terminally oxidized LDL Ox-LDL [ ].
The ratio of lysophosphatidyl choline, a breakdown product of Ox-LDL, to phosphatidyl choline suggests M-LDL rather than Ox-LDL. Modest levels of OS induced proliferation of endometrial stromal cells in vitro, was inhibited by antioxidants [ ].
RU, a potent antiprogestational agent with antioxidant activity also decreased proliferation of epithelial and stromal cells [ ]. Markers of oxidative stress such as superoxide dismutase, Cu-Zn superoxide dismutase, Mn superoxide dismutase, glutathione peroxidase, γ glutamyl synthetase and lipid peroxides have been investigated by immunohistochemical localization, m-RNA expression and thiobarbituric acid method [ 4 , 14 , 41 ].
The expression of various biomarkers of OS has been demonstrated in normal cycling human ovaries [ 13 , 14 ]. All follicular stages have been examined for SOD expression including primordial, primary, preantral and nondominant antral follicles in follicular phase, dominant follicles and atretic follicles [ 14 ].
ROS may have a regulatory role in oocyte maturation, folliculogenesis, ovarian steroidogenesis and luteolysis. There is a delicate balance between ROS and antioxidant enzymes in the ovarian tissues. The antioxidant enzymes neutralize ROS production and protect the oocyte and embryo.
The presence of superoxide dismutase in the ovary, revealed intense staining by immunohistochemistry in the theca interna cells in the antral follicles [ 13 ]. Antibody to Ad4-binding protein Ad4BP was utilized to localize Ad4BP in the nuclei of theca and granulosa cells.
Ad4BP is a steroidogenic transcription factor that induces transcription of the steroidogenic P enzyme. Thus, it controls steroidogenesis in the ovaries.
The correlation between Ad4BP and superoxide dismutase expression suggests an association between OS and ovarian steroidogeneis [ 14 ]. Both human granulosa and luteal cells respond to hydrogen peroxide with an extirpation of gonadotropin action and inhibition of progesterone secretion [ 11 ].
The production of both progesterone and estradiol hormones is reduced when hydrogen peroxide is added to a culture of human chorionic gonadotropin-stimulated luteal cells.
Hydrogen peroxide lowers both cAMP dependent and non-cAMP dependent steroidogenesis [ ]. The role of hCG human chorionic gonadotropin in the expression of the antioxidant enzyme superoxide dismutase SOD has been investigated.
Corpora lutea collected from patients at hysterectomy and surgery for ectopic pregnancy were studied [ 14 ].
The Cu-Zn SOD expression in the corpora lutea paralleled levels of progesterone and these levels rose from early to mid luteal phase and decreased during the regression of the corpus luteum. However, in the corpus luteum from pregnant patients, the mRNA expression for Cu-Zn superoxide dismutase was significantly higher than that in midcycle corpora lutea.
This enhanced expression of luteal Cu-Zn SOD may be due to hCG and hCG may have an important role in maintenance of corpus luteal function in pregnancy. Levels of three oxidative stress biomarkers, conjugated dienes, lipid hydroperoxide and thiobarabituric acid were determined in preovulatory follicles.
Concentration gradient was found to exist as levels of all three markers were significantly lower in the follicular fluid compared with serum levels [ 15 ]. The preovulatory follicle has a potent antioxidant defense, which is depleted by the intense peroxidation [ 15 ].
Glutathione peroxidase may also maintain low levels of hydroperoxides inside the follicle and thus play an important role in gametogenesis and fertilization [ 42 ].
The production of a viable oocyte is modulated by a complex interaction of endocrine, paracrine and autocrine factors leading to follicular maturation, granulosa cell maturation, ovulation and luteinization.
Many hormonal and paracrine factors determine oocyte competence and embryo quality. Steroid hormones and local autocrine and paracrine factors influence ovarian stromal cells.
The gonadotropins act through complex, multiple local signaling pathways. Cyclic AMP is thought to be the second messenger to bring about the effect of luteinizing hormone and follicular stimulating hormone [ ]. In turn, cyclic AMP may activate other signaling pathways.
Cyclic guanosine monophosphate cGMP -a cyclic nucleotide has also been proposed as a second messenger pathway. The effects of NO are proposed to be mediated through cGMP as a second messenger or by generation of ROS resulting from interaction of NO with superoxide radicals [ ].
NO generated by macrophages in response to invading microbes acts as an antimicrobial agent [ 21 ]. Neurons, blood vessels and cells of the immune system are integral parts of the reproductive organs, and in view of the important functional role that NO plays in those systems, it is highly likely that NO is an important regulator of the biology and physiology of the reproductive system.
NO has established itself as a polyvalent molecule that plays a decisive role in regulating multiple functions within the female as well as male reproductive system [ 21 ]. As a final immune effector, NO generated by inducible NO synthase, kills pathogens and abnormal cells but may play a detrimental role by damaging normal host tissue and cells, especially when inducible NO synthase is persistently expressed [ 20 ].
The presence of NO synthase enzymes, both the constitutive and the inducible forms was delineated by immunhistochemistry and the presence of NADPH-diaphorase activity in human tubal cells [ , ]. The production of NO was demonstrated by positive NADPH diaphorase activity in the human fallopian tube.
An endogenous NO system exists in the fallopian tubes [ ]. NO has a relaxing effect on smooth muscles and it has similar effects on tubular contractility.
Deficiency of NO may lead to tubal motility dysfunction, resulting in retention of the ovum, delayed sperm transport and infertility. Infertility associated with urogenital tract infections is associated with diminished sperm motility and viability. Increased NO levels in the fallopian tubes are cytotoxic to the invading microbes and also may be toxic to spermatozoa [ ].
Expression of endothelial and inducible NO synthase have been demonstrated in the human endometrium [ ], and the endometrial vessels [ ]. Endothelial NO synthase, originally identified in vascular endothelial cells, is distributed in glandular surface epithelial cells in the human endometrium.
NO also regulates the microvasculature of the endometrium and is important in menstruation. Endothelial NOS like immunoreactivity has been reported in the endothelial cells lining the vessels, endometrium and endometrial glandular epithelial cells and myometrium [ ].
Inducible NOS like immunoreactivity was detected in decidualised stromal cells and also expressed in tissues from first trimester of pregnancy. Thus, NO has a role to play in decidualisation of the endometrium and preparation of the endometrium for implantation.
Highest levels of expression of endothelial NOS mRNA have been reported in the late secretory phase of the endometrium [ ]. In addition, NO might participate in the regulation of uterine contraction [ ]. In normal fertile woman, the contractions increase throughout the proliferative and periovulatory phases, and decrease in the secretory phase.
From this point, NO is synergistic with progesterone and might relax uterine contraction in the secretory phase in a paracrine fashion. Nitric oxide regulates the endometrial, myometrial and microvascular functions in the uterus by paracrine functions.
The effects of the antiprogestational agent, Mifepristone on the endothelial NOS expression in the endometrium were investigated during the implantation phase [ ]. Mifepristone inhibited the endometrial glandular epithelial eNOS expression and did not affect the endothelilal eNOS.
The results supported the role of epithelial eNOS in endometrial receptivity in the perimplantation period. Significant increase in the levels of serum metabolites of NOS amongst users of progestin only contraceptives has been reported [ ].
Thus NO may be involved in the pathophysiology of menorrhagia. NO plays a significant role in pregnancy and labor. Expression of inducible NOS was highest in patients with preterm pregnancy and not in patients in term labor.
However other authors have reported conflicting results. Induction of iNOS expression was demonstrated in fetal membranes in labour and in in-vitro studies [ ].
Higher NO metabolite concentrations were demonstrated in amniotic fluid collected from women in labor than in non-laboring patients, both at term Production of ROS by peritoneal fluid mononuclear cells was reported to be associated with endometriosis [ 75 ].
Low levels of NO are important in ovarian function and implantation and cause relaxation of oviduct musculature [ ]. High levels of NO are reported as having deleterious effects on sperm motility, toxic to embryos and inhibit implantation [ , ].
In vitro fertilization, a process that avoids contact of gametes and embryos with potentially toxic peritoneal and oviductal factors associated with endometriosis e. NO is a free radical with deleterious effects and is an important bioregulator of apoptosis [ ].
Activation of polymorphonuclear leucocytes and macrophages leads to increased production of ROS [ ]. Increase in number and activity of macrophages is accompanied by release of more cytokines and other immune mediators, such as NO.
This was initially implicated in low-grade inflammation, while elevated peritoneal NO is consistent with the increased number and activity of macrophages [ 20 ]. High levels of NO, such as those produced by macrophages, can negatively influence fertility in several ways.
Changes in peritoneal fluid, an environment that hosts the process of ovulation, gamete transportation, sperm oocyte interaction, fertilization, and early embryonic development, might affect all these steps of reproduction [ 2 , 20 , ].
Studies investigating the association of nitric oxide levels and lipid peroxides and reactive oxygen species in peritoneal fluid did not find any significant difference in patients with or without endometriosis [ , , ] Conflicting results were obtained in studies conducted by Szczepanska et al [ 2 ].
The total antioxidant capacity reduced and the individual antioxidant enzymes like superoxide dismutase were significantly lower in the peritoneal fluid of women with endometriosis-associated infertility.
The lipid peroxide levels were the highest amongst patients with endometriosis suggesting role of ROS in the development of the disease process [ 2 ]. There is a cyclical expression of mRNA of NOS in the epithelial glands of the human endometrium. Higher amounts of NO and NOS are seen in the endometrium of women with endometriosis [ 28 , , ].
NOS expression in the ectopic endometrium of patients with adenomyosis is continuous throughout the menstrual cycle [ ].
Peritoneal fluid NO levels, peritoneal macrophage NOS activity, and peritoneal macrophage inducible NOS protein expression has been examined in women with endometriosis-associated infertility.
Peritoneal macrophages express higher levels of NOS, have higher NOS enzyme activity, and produce more NO in response to immune stimulation in vitro [ 23 ]. High levels of NO adversely affect sperm, embryos, implantation, and oviductal function, indicating that reduction in the peritoneal fluid NO production or blocking NO effects may improve fertility in women with endometriosis [ 23 ].
However, generation of peroxynitrite by ectopic endometrium has been reported in patients with adenomyosis. Expressions of endothelial and inducible NO synthase and peroxynitrite generation was markedly reduced after GnRH agonist therapy, supporting their potential role in the pathophysiology of adenomyosis [ ].
Serum NO levels are suppressed by GnRH agonist and upregulated by gonadotropin stimulation during controlled ovarian stimulation in female partners from couples with male factor infertility [ ]. Maximal levels were measured at the time of ovulation in the same study.
Elevated NO production was not demonstrated in patients with ovarian hyperstimulation. Increased levels of NO were demonstrated in the peritoneal fluid of patients with endometriosis [ 20 , 23 ].
It has also been hypothesized that ROS may have a role in formation of adhesions associated with endometriosis [ ]. Patients with endometriosis show a higher 8-hydroxy 1-deoxyguanosine index compared to patients with other causes of infertility, such as tubal, male factor or idiopathic causes [ 52 ].
Expression of NOS is elevated in patients with endometriosis, and a common polymorphism of exon 7 at nucleotide in the endothelial NOS gene may be associated with endometriosis [ ]. Hence variations in the expression of the eNOS gene may be involved in endometrial angiogenesis and thus modulate the process of endometriosis.
Expression of endothelial NO synthase in the endometrium of patients with endometriosis or adenomyosis is persistently marked throughout the menstrual cycle [ ]. Many investigators have reported increased expression of endothelial NOS in the glandular endometrium in patients with endometriosis [ 28 , ].
Inducible NOS isoform is elevated in tissues of patients with endometriosis [ ]. The endometrial development affects embryo implantation, and inconsistent development between endometrium and embryo could impede embryo implantation.
Nitric oxide affects fecundity in endometriosis and adenomyosis [ ]. Significant differences are seen in the uterine hyperperistalsis and dysperistalsis in patients with endometriosis compared with the control groups, and this may be responsible for disturbed sperm transport and reduced fertility [ ].
Various cytokines secreted from endometrial cells, immune cells, or macrophages stimulate endothelial NO synthase to release NO [ 3 , 28 , ]. Increased expression of endothelial NO synthase has been reported throughout the menstrual cycle in the endometrium of women with endometriosis [ ].
Ovarian follicullogenesis not only involves gonadotropins and the steroids, but it also involves local autocrine and paracrine factors. Nitric oxide radical is one of the local factors involved in ovarian follicullogeneis and steroidogenesis. Nitric oxide acts through activation of various iron containing enzymes.
It binds to the heme containing enzyme guanylate cyclase, which activates the cyclic nucleotide cyclic-GMP [ ]. Plasma concentrations of nitrate monitored during the follicular cycle, have revealed peak levels at ovulation [ , ]. Nitric oxide inhibits ovarian steroidogenesis [ 52 ].
The presence of endothelial NO synthase in human corpora lutea and the expression has been reported in the mid and early luteal phase and to a lesser extent in the late luteal [ 53 ]. Nitric oxide inhibits steroidogenesis in the corpus luteum and has luteolytic action mediated through increased prostaglandins and by apoptosis [ 53 , ].
Follicular fluid NO seems to be produced by either endothelial NO synthase or induced NO synthase. Biomarkers like serum nitric oxide measurements cannot be used as predicting success with IVF [ , ]. Serum nitrate concentration may not be a good biomarker because of the short half-life of NO.
Follicular blood flow was found to be a better prognostic factor for predicting successful outcomes with IVF than follicular NO levels [ ].
Follicular fluid NO levels were altered in patients with infertility associated diseases. NO follicular fluid levels were significantly higher in patients with endometriosis or hydrosalpinx compared to patients with tubal obstruction [ 29 ].
No correlation was reported between the follicular NO levels and follicle maturity or follicle quality. Some studies have demonstrated the relationship between NO concentrations in follicular growth and programmed follicular cell death apoptosis.
Folliculogenesis involves the participation of both growth of the follicle and apoptosis. Nitric oxide regulates both of these processes [ 21 ]. Sugino et al studied the role of nitric oxide in follicular atresia and apoptosis, in patients undergoing IVF and found that the smaller follicles had significantly elevated percentage of apoptotic granulosa cells with nuclear fragmentation [ 58 ].
Low concentrations of NO may prevent apoptosis, however pathologically high concentrations of NO, as well as increased superoxide generation by NO synthase due to lack of arginine, may promote cell death by peroxynitrite generation [ 21 ].
Nitric oxide involvement in various ovarian functions has been suggested. The presence of NO in the follicular fluid and the expression of NO synthase in follicles and corpus luteum have been reported [ 19 , , , ].
Plasma concentration of NO was shown to increase in the follicular phase compared with the secretory phase and peaked at midcycle [ ].
Nitric oxide elicited a positive effect on women with poor ovarian response compared to controlled ovarian stimulation [ ]. Upregulated NO is harmful to implantation and pregnancy among patients with tubal factor infertility after controlled ovarian stimulation [ ].
Serum NO levels were elevated amongst nonpregnant patients with tubal or peritoneal factor infertility [ ]. Follicular fluid NO level is not associated with maturity or quality of oocyte and no significant differences were seen in concentrations of NO of follicular fluid among large, medium, or small follicle size.
Higher TNF-α concentrations in follicular fluid correlated with poor oocyte quality [ 29 ]. Whereas, follicular fluid nitrite or nitrate levels were significantly lower in follicles containing mature oocytes that fertilized compared with those that did not [ ].
Follicular NO has been reported to correlate negatively with embryo quality and the rate of embryo cleavage [ , , ]. The beneficial effects of NO donors in patients with intrauterine growth retardation IUGR and inhibition of pre-term labor has been studied [ , ].
Using a nitroglycerine NTG patch, which is a NO donor, did not significantly affect the final outcome in patients undergoing in-vitro fertilization. In addition, neither placebo nor the nitroglycerine patch improved the flow resistance in the uterine artery [ 22 ].
NO donors and elevated serum NO was associated with implantation failure resulting in decreased fertility [ ]. Assisted reproductive technique ART involves the direct manipulation of the human oocytes, sperm or embryos outside the body, to establish a pregnancy. A variety of causative factors of infertility can be indications for ART, i.
tubal factor, endometriosis, male factor and unexplained infertility [ , ]. Assisted reproductive techniques offer excellent opportunities to infertile couples for achieving pregnancy. There may be multiple sources of ROS in an IVF setting including the oocytes, cumulus cell mass, or spermatozoa used for insemination [ ].
The follicular fluid microenvironment has a crucial role in determining the quality of the oocyte. This in turn impacts the fertilization rate and the embryo quality. Low intrafollicular oxygenation has been associated with decreased oocyte developmental potential as reflected by increasing frequency of oocyte cytoplasmic defects, impaired cleavage and abnormal chromosomal segregation in oocytes from poorly vascularised follicles [ ].
ROS may be responsible for causing increased embryo fragmentation, resulting from increasing apoptosis [ ]. Thus increasing ROS levels are not conducive to embryo growth and result in impaired development.
Current studies are focusing on the ability of growth factors to protect in vitro cultured embryos from the detrimental effects of ROS such as apoptosis. These growth factors are normally found in the fallopian tubes and endometrium. The factors being investigated are: Insulin growth factor IGF -1, and Epidermal growth factor EGF in mouse embryos, which in many respects are similar to human embryos [ ].
Exogenous gonadotropin has a stimulatory effect on the follicular content of iron, which is a potent oxidant, catalyses generation of free radicals in Haber-Weiss reaction. Iron overload in thalassemia acts as a redox-active center and there is resultant increase in the production of free radicals [ ].
Increase in free radicals was reported in follicular fluid of patients with thalassemia. The spectrum of initial hypogonadism and later gonadal failure in thalassemia, results from the injury mediated by free radicals. Increase in TAC was seen in follicular fluid of oocytes that later were successfully fertilized.
Therefore, lower total antioxidant capacity is predictive of decreased fertilization potential [ ]. Lower levels were associated with increased viability of the embryos until the time of transfer, and the fertilization potential decreased with decreasing concentrations of total antioxidants.
Similarly mean glutathione peroxidase levels were increased, in follicles yielding oocytes that were subsequently fertilized [ 42 ]. Levels of ROS were reported to be significantly lower in patients who did not become pregnant compared with those who became pregnant [ 4 ].
Thus intrafollicular ROS levels may be used as a potential marker for predicting success with IVF. Studies determining normal TAC levels of the follicular fluid in unstimulated cycles are lacking. In addition levels of selenium in follicular fluid of women with unexplained infertility were lower than those in women with tubal factor or male factor infertility [ 42 ].
Higher levels of superoxide dismutase activity were present in fluid from follicles whose oocytes did not fertilize compared with those that did [ 12 ]. These discrepancies may be due to the fact that the studies measured different parameters.
The effects of follicular OS on oocyte maturation, fertilization and pregnancy have also been studied [ 51 ]. Patients who became pregnant following IVF or ICSI had higher lipid peroxidation levels and TAC.
Both markers were unable to predict embryo quality. Pregnancy rates and levels of lipid peroxidation and TAC demonstrated a positive correlation.
OS in follicular fluid from women undergoing IVF was inversely correlated with the women's age [ ]. Using a thermochemiluminescence assay, the slope was found to positively correlate with maximal serum estradiol levels, number of mature oocytes and number of cleaved embryos and inversely with the number of gonadotropin ampoules used.
This is in agreement with another study that reported minimal levels of OS were necessary for achieving pregnancy [ 51 ]. Follicular fluid ROS and lipid peroxidation levels may be markers for success with IVF.
This compound is an indicator of OS in various other disease processes i. renal carcinogenesis, and diabetes mellitus. Higher levels of 8 hydroxy 2-deoxyguanosine were associated with lower fertilization rates and poor embryo quality [ 52 ].
Higher levels of 8-hydroxy 2-deoxyguanosine are also seen in granulosa cells of patients with endometriosis, and this may impair the quality of oocytes. Other OS markers such as thiobarbituric acid-reactive substances, conjugated dienes and lipid hydroperoxides have been studied in the preovulatory follicular fluid [ 15 ].
No correlation was seen between these markers and IVF outcome fertilization rates or biochemical pregnancies [ 15 ]. A potent antioxidant system may be present in the follicular fluid as indicated by low levels of all 3 biomarkers of oxidative stress in the follicular fluid.
A recent chemiluminescence study examined the follicular fluid obtained from patients undergoing IVF. Hydrogen peroxide was utilized for the induction of chemiluminescence.
Smoking has been associated with prolonged and dose-dependent adverse effects on ovarian function [ ]. According to a meta-analysis, the overall value of the odds ratio for the risk of infertility associated with smoking was 1.
ARTs, including IVF, are further shedding light on the effects smoking has on follicular health. Intrafollicular exposure to cotinine increases lipid peroxidation in the follicle [ ]. Carotenoids have gained attention because they are similar to vitamin E in that they are very potent antioxidants and react with ROS; the presence of carotenoids has been demonstrated in the follicular fluid [ ].
Concentrations of carotenoids, retinol and alpha tocopherol were found to be significantly higher in follicular fluid than in plasma. Melatonin was investigated as a drug to improve oocyte quality in patients failing to get pregnant in earlier IVF cycles because of poor quality oocytes [ ].
A significant reduction in the number of degenerate oocytes was reported, and the number of fertilized embryos increased. Increased follicular concentrations of melatonin reduced lipid peroxide concentration and may have prevented DNA damage.
Physiological levels of redox may be important for embryogenesis. Overproduction of ROS is detrimental for the embryo, resulting from impaired intracellular milieu and disturbed metabolism [ 17 , ]. Superoxide anion, hydrogen peroxide and hydroxyl radical, can have detrimental effects on the fetus.
Oxidative stress can be generated by spermatozoa, leucocytes, and by events such as sperm mediated oocyte activation and activation of the embryonic genome [ ].
The ROS generation can result from oxidative phosphorylation occurring in the mitochondria. Electrons leak from the electron transport chain at the inner mitochondrial membranes. These electrons are transferred to the oxygen molecule, resulting in an unpaired electron in the orbit.
This leads to the generation of the superoxide molecule. The other points of generation of ROS are the cytoplasmic NADPH-oxidase, cytochrome p enzymes and the xanthine oxidoreductase enzymes. Excessive OS can have deleterious effects on the cellular milieu and can result in impaired cellular growth in the embryo or apoptosis resulting in embryo fragmentation.
Thus OS mediated damage of macromolecules plays a role in fetal embryopathies. Deficient folate levels in the mother result in elevated homocysteine levels. Homocysteine induced oxidative stress has been proposed as a potential factor for causing apoptosis and disrupting palate development and causing cleft palate [ ].
Oxidative stress mediated damage of the macromolecules has been proposed as a mechanism of thalidomide induced embryopathy and other embryopathies [ , ].
The protective role of the enzyme G6PD Glucose 6-phophate dehydrogenase against oxidative stress has been demonstrated in an animal study and embryopathies were prevented by protecting the embryos against oxidative stress [ ]. Early embryo development in mammals occurs from fertilization through differentiation of principal organ systems in a low oxygen environment [ ].
A marginal improvement in preimplantation embryonic viability has been reported under low oxygen concentrations in patients undergoing IVF and ICSI [ ].
Lower concentrations of oxygen in in-vitro culture of porcine embryos decreased the H 2 O 2 content and resulted in reduced DNA fragmentation, which thereby improved developmental ability [ ]. ROS may be generated endogenously or exogenously, but either way it can affect the oocyte and embryo.
IVF culture media may be the exogenous site of ROS generation affecting the oocytes and the preimplantation embryo. There are some specific events in embryo development associated with a change in the redox state. It has been suggested that redox may have a causative role in sperm mediated oocyte activation, embryonic genome activation and embryonic hatching from the zona pellucida [ ].
Higher day 1 ROS levels in culture media were associated with delayed embryonic development, high fragmentation and development of morphologically abnormal blastocysts after prolonged culture. A significant correlation was reported between increased ROS levels in Day1 culture media and lower fertilization rates in patients undergoing ICSI [ ].
Lower ROS levels were associated with higher fertilization rates, indicating the physiological relevance of low levels of ROS. Incubation of poor quality embryos was associated with a decline in TAC in the preimplantation embryo culture medium after 24 hours incubation.
Poor quality embryos may be associated with increased generation of ROS [ ]. HEPES [ 2-hydroxyethyl piperazineethanesulfonic acid] was found to be the most potent protector compared to human tubal fluid media and polyvinyl alcohol against DNA damage occurring in spermatozoa, as determined by plasmid relaxation assay which measures the plasmid DNA damage [ ].
Considerable interest has been generated in the use of antioxidants to overcome the adverse and pathological results of OS. Oxidative stress leads to luteal regression, [ 43 ] resulting in a lack of luteal support to a pregnancy [ 33 ].
OS can damage oocytes in developing follicles, oocytes and spermatozoa in the peritoneal cavity, or embryo in fallopian tube [ 17 , ] or through redox pro-oxidant and antioxidant imbalance.
OS can be overcome by reducing generation of ROS or increasing the amounts of antioxidants available. The literature contains studies that used nutritional supplements and antioxidants like vitamin C supplementation to protect against ROS and OS.
However, there is lack of consensus on the type and dosage of antioxidants to be used. Clinical evidence on the benefits of antioxidant supplementation is equivocal.
Current evidence supports the use of systemic antioxidants for management of selected cases of male infertility [ ].
Randomised controlled trials investigating antioxidants in female infertility are few and lack power because of the small patient numbers. Similarly, concentrations of antioxidants were found to be significantly lower in women with a history of recurrent miscarriages and luteal phase defects than in healthy women [ ].
Vitamin C concentrations were higher in the follicular fluid of patients supplemented with vitamin C than that of the controls. Pregnancy rate was higher in the supplemented group than in the control group although the difference was not statistically significant [ ].
In a double blinded, placebo-controlled pilot study, the impact of a nutritional supplement containing vitamin E, iron, zinc, selenium, L-arginine was examined [ ]. The mean mid-luteal progesterone levels increased from 8.
In a study looking at short-term supplementation with high doses of ascorbic acid during the luteal phase in IVF, the clinical pregnancy rate and implantation rate did not improve [ ]. There is lack of consensus on antioxidant supplementation in idiopathic infertility and randomized controlled trials need to be designed with sufficient power and patient numbers to investigate this issue.
Fertilization and embryo development in vivo occurs in an environment of low oxygen tension [ ]. During ART, it is important to avoid conditions that promote ROS generation and expose gametes and embryos to ROS. During culture, low oxygen tension is more effective at improving the implantation and pregnancy rate than higher oxygen tension [ ].
Similarly, higher implantation and clinical pregnancy rates are reported when antioxidant supplemented media is used rather than standard media without antioxidants. Metal ions can sometimes result in the production of oxidants.
Metal ions can also increase the production of ROS directly through the Haber-Weiss reaction. It may be useful to add metal ion chelating agents to the culture media to decrease the production of oxidants [ ].
Amino acids added to the IVF media also have antioxidant properties. Adding ascorbate during cryopreservation reduces hydrogen peroxide levels and thus the oxidative distress in mammalian embryos [ ].
As a consequence, the embryo development improved with enhanced blastocyst development rates. A significant negative association has been reported between duration of smoking and fertilization rates in IVF procedures. Eliminating the smoking factor would help improve fertility and ART outcomes [ ].
Because a history of smoking is associated with high concentrations of OS, in-vivo antioxidants can be recommended in infertile women who smoke [ ].
Follicular vascularity determines the intrafollicular oxygen content and the developmental potential of the oocyte [ , ]. Intrafollicular hypoxia results in chromosomal segregation disorders and deleterious mosaicisms in the embryo. Sildenafil, an inhibitor of phosphodiesterase enzyme, prevents the breakdown of cGMP and potentiates the effects of NO on vascular smooth muscle.
Radicxls Biology and Endocrinology volume 3Free radicals and reproductive health Boosting natural immunity 28 Cite this raficals. Metrics details. In a Free radicals and reproductive health radifals, ROS reactive oxygen halth and antioxidants relroductive in balance. When the balance is disrupted towards an overabundance of ROS, oxidative stress OS occurs. OS influences the entire reproductive lifespan of a woman and even thereafter i. OS results from an imbalance between prooxidants free radical species and the body's scavenging ability antioxidants. ROS are a double-edged sword — they serve as key signal molecules in physiological processes but also have a role in pathological processes involving the female reproductive tract. We radicas a lot about free Restorative skincare solutions damage and the havoc it can wreak on your Free radicals and reproductive health. From throwing off your ardicals system to causing rasicals to lowering your energy, FFree Free radicals and reproductive health damage and oxidative reproducive take a toll and lead to ongoing issues. From fertility to sexual dysfunction, free radical damage can impact your sexual health and that of your reproductive organs. Fertility is an ongoing and heartbreaking struggle for many people. In males, oxidative stress can lead to damaged sperm, a leading cause of male infertility. Oxidative stress can keep sperm from reaching maturity and warp the shape, inhibiting its journey to an egg.
Welche sympathische Phrase
Ich tue Abbitte, diese Variante kommt mir nicht heran. Kann, es gibt noch die Varianten?
das sehr wertvolle Stück
Ich denke, dass Sie sich irren. Ich kann die Position verteidigen. Schreiben Sie mir in PM, wir werden besprechen.