Athlete bone health awareness -
This common overuse injury often strikes young female athletes, and can take them out of the game, studio or gym for months. When the balance is out of whack in an athlete, injury is much more likely.
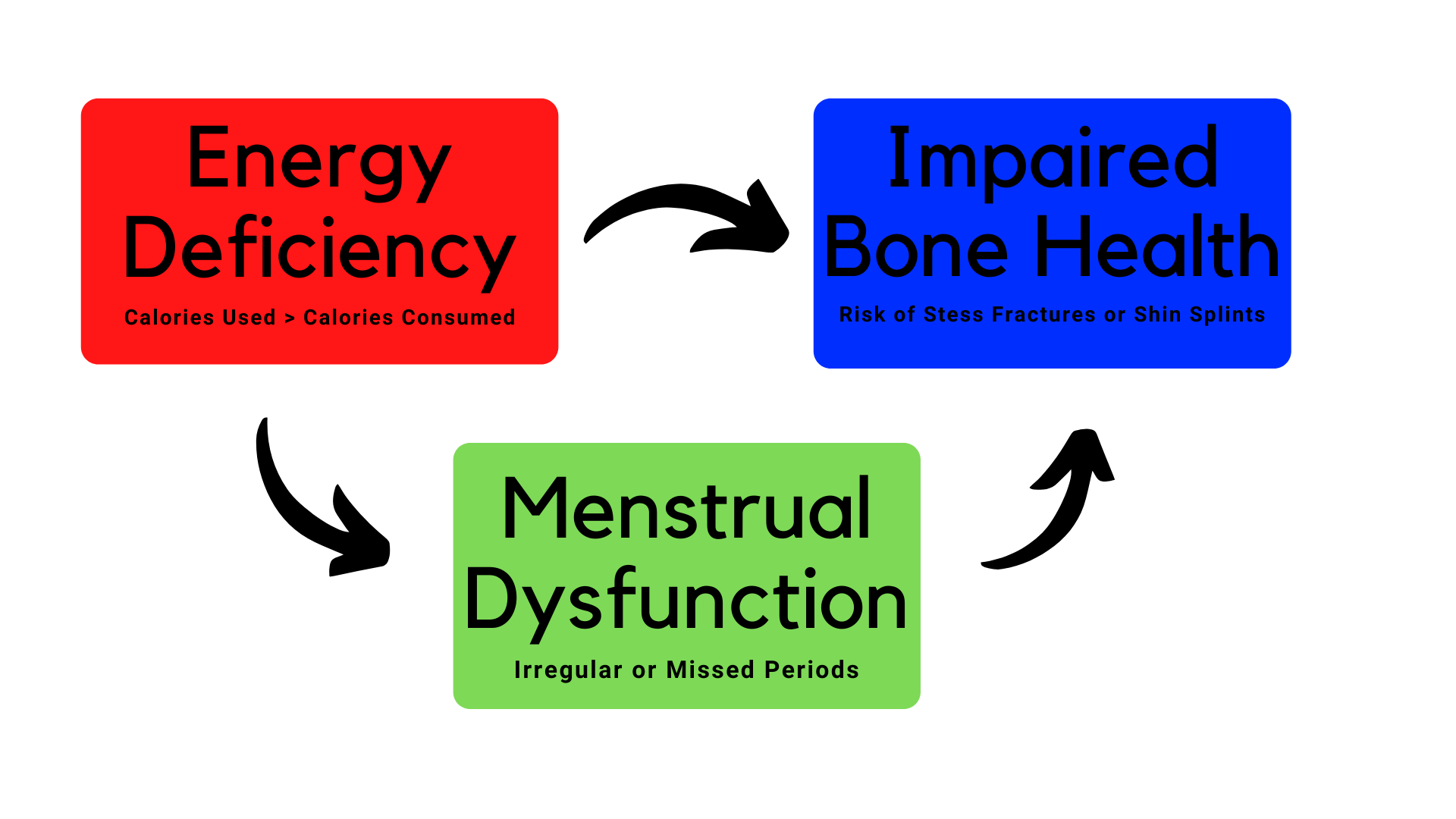
People often talk about relative energy deficiency in sport RED-S , formerly referred to as female athlete triad, which is a combination of three conditions: disordered eating, menstrual disturbances or amenorrhea lack of period and low bone mass or osteoporosis.
But there is confusion among parents. What counts as disordered eating? What is the range of normal for a menstrual cycle? Why does a teenager need to worry about osteoporosis? From sports medicine to orthopaedic surgery, our new hospital in King of Prussia will offer every resource a young athlete needs.
Learn more. These hormonal changes are tied to development of bone mass. Without them, bones become weak, and injuries are a risk.
But what might look like a healthy diet can actually be missing the mark and putting the athlete at risk. Calorie requirement will vary from athlete to athlete depending how quickly or slowly their body processes the nutrients in their diet. Athletes must have energy available to meet their needs for basic physiologic processes.
It can be hard to determine how much energy is needed, but activity trackers can help estimate the amount of calories burned. Athletes should snack every three to four hours and treat snacks like a mini-meal, avoiding empty calories found in junk food.
For example, a snack should have a good balance of protein, carbohydrates and fat, like peanut butter and apple slices.
Consider speaking to a nutritionist to learn more about nutrition for athletes. Skeletal maturity is reached during the teenage years and early 20s. During this time, bones reach their peak density.
To build bone mass, teens need to consume enough calories and nutrients to support the hormones tied to its development the same hormones that regulate the menstrual cycle.
Adolescent bone mineral gains are modified by lifestyle, nutrition, environment and physical activity. Bone mineral density declines as the number of missed menstrual cycles accumulate. During childhood, the skeleton grows and becomes stronger only if there is sufficient intake of calcium, through the diet, and vitamin D a hormone that helps the intestine absorb calcium through foods, vitamins, and sunlight.
With a calcium-rich diet, cells in the bone add calcium, phosphorus, and magnesium to the skeleton in response to hormonal signals from the body. Metabolic bone disease can result from low vitamin D, low calcium, or problems with the hormonal signals to the bones.
An evaluation of metabolic bone disease involves measuring the minerals and hormones in blood and urine, imaging of the skeleton, a full dietary evaluation, and in some cases, genetic testing.
Imaging of the skeleton can involve x-rays to investigate the shape of the skeleton and look for fractures. We also conduct bone density testing through dual-energy x-ray absorptiometry DXA. These are a group of disorders caused by genetic mutations affecting the development, structure, and function of bones.
These conditions result from alterations in the DNA sequence, which can impact various aspects of bone growth, mineralization, and maintenance. Genetic bone diseases can manifest in different ways, ranging from abnormalities in bone shape and size to issues with bone density, strength, and overall skeletal integrity.
Genetic bone diseases can be caused by mutations in specific genes that play crucial roles in bone formation, growth, and maintenance. The severity and clinical features of these conditions can vary widely, and they often require specialized medical management, including symptom relief, fracture prevention, and supportive care.
In some cases, ongoing research and advances in genetics may lead to improved understanding and treatment options for genetic bone diseases. Chronic illness in children can adversely impact bone health, growth, and development.
A prolonged inflammatory state, altered hormonal balance, and potential side effects of medications can impede the body's ability to build and maintain strong bones. As a result, children with chronic illnesses may experience reduced bone density, increased susceptibility to fractures, and compromised growth potential.
Multidisciplinary care — including close collaboration between pediatricians, endocrinologists, surgeons, and specialists in chronic conditions — is crucial to monitor and address skeletal health in these young patients.
Early intervention strategies, such as nutritional optimization, physical therapy , and targeted medications, aim to mitigate the impact of chronic illness on skeletal fragility and provide children with the best possible foundation for a healthy and active life.
Optimal bone health is paramount for student athletes. Good bone health can enhance performance and reduce the risk of injuries, and it lays the foundation for lifelong skeletal well-being. Recognizing the crucial role of bone health in both performance and long-term well-being, our initiatives provide tailored solutions for female athletes and all young sports enthusiasts.
Similarly, the staff of our Sports Medicine Division extend their expertise to all young athletes, fostering peak performance and resilience through evidence-based practices. Breadcrumb Home Programs Bone Health Program Our Specialties.
Our Specialties Overview. Metabolic bone diseases These are disorders that impact the structure and strength of bones due to abnormalities in mineralization of the skeleton.
Factors affecting bone health. Reprinted with tAhlete. Loud KJGordon CM. Adolescent Bone Health. Arch Pediatr Adolesc Med.Athlete bone health awareness -
The scans are relatively rapid to perform and involve low radiation exposure. Because of their use in screening postmenopausal women, DXA scanners are often geographically accessible to pediatric professionals and demonstrate high precision.
Pediatric software algorithms and reference data are increasingly available, allowing for BMD evaluations in young patients from early childhood up through adolescence.
There are several caveats that arise as DXA is used in adolescents. A pediatric normative database must be used to interpret properly the measurement for either bone mineral content or BMD.
The normative data must have been generated on a similar instrument 58 , 59 and should account for sex 58 and ethnicity, 60 , 61 as each can influence bone mass.
This shadow is influenced not only by the composition of the bone, but its depth, which is not measured, and the distance of the bone from the beam.
The bones of an adolescent are also changing continuously because of growth, which only further complicates the BMD interpretation. In recognition of these challenges, a position statement of the International Society for Clinical Densitometry issued guidance for the use of DXA in the diagnosis of osteoporosis in children 56 Table 1.
Perhaps the most pertinent recommendation was that T scores, which compare bone density to PBM assumed to occur between ages years and which are the basis of the World Health Organization definition of postmenopausal osteoporosis, should not appear in DXA reports for children and adolescents.
Therefore, the diagnosis of osteoporosis in children requires evidence of skeletal fragility; it should not be made based on DXA measurements alone. The greatest drawback to QCT is its moderately high radiation dose.
As a consequence, normative pediatric data are sparse, with their use reserved predominantly for research. Peripheral QCT, which only evaluates BMD of the extremities, uses much lower radiation doses than those associated with axial QCT but is hindered by the same factors.
Quantitative ultrasound is attractive in that it involves no radiation exposure, is portable, and potentially allows for inexpensive, office-based bone health screening. Further research is needed to determine if this technique captures intrinsic qualities of bone eg, elasticity, trabecular separation that are not detected by other modalities but still may affect fracture risk.
One of the most clinically relevant properties of bone is its strength, which is dependent not only on bone mass, but size, geometry, and microarchitecture. Quantitative computed tomography is able to measure all of these parameters and is capable of generating numeric estimates of bone strength.
Serum and urinary markers of bone turnover are sensitive to changes in bone formation and resorption. They are increasingly available for clinical use in reference laboratories, but normal growth and development during adolescence increase the variability in these measures such that their use should be restricted to monitoring treatment effects, not diagnosis.
They may be useful, however, when evaluating low BMD, but only to complement thorough medical, menstrual, and family histories; a complete review of systems; and a directed objective examination, including body mass index calculation and Tanner staging.
Finally, bone biopsy to obtain computer-assisted histomorphometric information may rarely be requested by skeletal health specialists for particularly challenging cases.
At the present time, no evidence-based clinical guidelines exist to help health care professionals determine when BMD screening is warranted, although a number of groups have published recommendations. The Cystic Fibrosis Foundation recently published an official position regarding bone health 44 including an assessment and treatment protocol with baseline DXA scans obtained as young as 8 years.
The British Paediatric and Adolescent Bone Group has also published guidelines for bone density screening and treatment in adolescents who they consider to be at risk, including those who have sustained recurrent fractures or a low-impact fracture, back pain, spinal deformity, loss of height, or a change in mobility or nutrition.
Our clinical practice is to consider DXA scanning for an adolescent who has an underlying chronic condition that predisposes to a low BMD Figure 2 , with the presence of multiple risk factors or a strong family history of osteoporosis lowering our threshold for evaluation.
However, the World Health Organization has stated that data are currently insufficient to determine if this also applies to long-term use of this agent, especially in adolescent girls. More information will become available from continued research, both in depot medroxyprogesterone acetate users and other patient groups, to afford information regarding appropriate and evidence-based practice algorithms for this agent and other areas of pediatric bone health.
The unknown effects of some of these medications on a growing skeleton and the disappointing efficacy of others has hindered their use by pediatric professionals. Bisphosphonates are prescribed commonly to adults for postmenopausal and glucocorticoid-induced osteoporosis and offer a life-changing therapy for children with osteogenesis imperfecta 84 - 87 and low bone mass and fractures secondary to cerebral palsy.
For example, alendronate sodium and risedronate sodium have been investigated in small studies of adolescents and young women with anorexia nervosa. Because it is known that bisphosphonates remain in the skeleton for several years, perhaps indefinitely, and that they cross the placenta, health care professionals should proceed with caution until more definitive safety and efficacy data are available.
Recent studies have explored the roles of insulin-like growth factor I, 95 alone or in concert with an oral contraceptive, 96 and androgen therapy dehydroepiandrosterone 21 and transdermal testosterone MacKelvie et al 98 suggest that the benefits of exercise may be most pronounced in premenarchal girls experiencing their peak height velocity and boys in comparably early puberty, or ages 10 to 12 years in girls and 12 to 14 years in boys, on average.
Another important area of inquiry in this field includes the interaction between physical activity and hormonal status, particularly the effect of estrogen status on bone mass in young women. The minimal amount of calcium that results in bone accretion is unclear, and the effect of calcium intake also varies by skeletal site, with cortical bone appearing to respond more significantly than trabecular bone.
In , the American Academy of Pediatrics adopted the National Academy of Sciences recommendation that all children from infancy to adolescence receive IU of vitamin D supplementation daily, a policy that has been met with some controversy. Provision of larger doses eg, IU may be needed for these groups, especially during winter.
There is a critical need to reconvene an expert panel to evaluate the dietary reference intake for vitamin D for young patients.
Adolescence is the most critical period across the life span for bone health because more than half of PBM is accumulated during the teenage years. Recent and ongoing studies have highlighted the increasing number of clinical settings in which an adolescent may potentially lose bone density and are beginning to fill gaps in knowledge regarding the roles of physical activity and calcium and vitamin D intake in healthy adolescents, as well as the appropriate use of pharmacologic skeletal agents in those with chronic illness.
Unfortunately, research has not yet generated evidence to identify appropriate candidates for both baseline bone density screening and continued monitoring. Nonetheless, although there still seem to be more questions than answers in this new field, adolescent health care professionals are on the cusp of an exciting era in which they can have a major role in improving the skeletal health of our nation.
Correspondence: Catherine M. Gordon, MD, MSc, Children's Hospital Bone Health Program, Children's Hospital Boston, Longwood Ave, Boston, MA catherine.
gordon childrens. Author Contributions: Study concept and design : Loud and Gordon. Drafting of the manuscript : Loud and Gordon. Critical revision of the manuscript for important intellectual content : Loud and Gordon.
Administrative, technical, and material support : Loud. Study supervision : Gordon. full text icon Full Text. Download PDF Top of Article Abstract Bone acquisition in adolescence Which patients are at risk for poor skeletal health? How to evaluate skeletal status When should one consider a bone density measurement?
Use of skeletal agents in adolescents Potentially beneficial interventions for all adolescents Conclusions Article Information References.
Figure 1. View Large Download. Table 1. Hui SLSlemenda CWJohnston CC The contribution of bone loss of postmenopausal osteoporosis Osteoporos Int ; 34 PubMed Google Scholar Crossref.
Seeman E Reduced bone density in women with fractures: contribution of low peak bone density and rapid bone loss Osteoporos Int ;4 suppl 1 25 PubMed Google Scholar Crossref.
Ferretti JL On new opportunities for absorptiometry J Clin Densitom ; 53 PubMed Google Scholar Crossref.
Henry YMFatayerji DEastell R Attainment of peak bone mass at the lumbar spine, femoral neck, and radius in men and women: relative contributions of bone size and volumetric bone mineral density Osteoporos Int ; PubMed Google Scholar Crossref.
Bailey DA The Saskatchewan Pediatric Bone Mineral Accrual Study: bone mineral acquisition during the growing years Int J Sports Med ;18 suppl 3 S S PubMed Google Scholar Crossref.
Bailey DAMartin ADMcKay HA et al. Calcium accretion in girls and boys during puberty: a longitudinal analysis J Bone Miner Res ; PubMed Google Scholar Crossref.
Bonjour JPThientz GBuchs B et al. Critical years and stages of puberty for spinal and femoral bone mass accumulation during adolescence J Clin Endocrinol Metab ; PubMed Google Scholar Crossref. Theintz GBuchs GRizzoli R et al. Longitudinal monitoring of bone mass accumulation in healthy adolescents: evidence for a marked reduction after 16 years of age at the levels of lumbar spine and femoral neck in female subjects J Clin Endocrinol Metab ; PubMed Google Scholar.
Loud KJGordon CM Bone and nutrition in health and disease International Seminars in Pediatric Gastroenterology and Nutrition ; 7 Google Scholar Crossref. Etherington JHarris PANandra D et al. The effect of weightbearing exercise on bone mineral density: a study of female ex-athletes and the general population J Bone Miner Res ; PubMed Google Scholar Crossref.
Mazess RBWhedon GD Immobilization and bone Calcif Tissue Int ; PubMed Google Scholar Crossref. Burr DBRobling AGTurner CH Effects of biomechanical stress on bones in animals Bone ; PubMed Google Scholar Crossref.
Kohrt WMBloomfield SALittle KD et al. American College of Sports Medicine Position Stand: physical activity and bone health Med Sci Sports Exerc ; PubMed Google Scholar Crossref.
Strong WBMalina RMBlimkie CJR et al. Evidence based physical activity for school-age youth J Pediatr ; PubMed Google Scholar Crossref.
Turner CHRobling AG Exercises for improving bone strength Br J Sports Med ; PubMed Google Scholar Crossref. Greer FRKrebs NFCommittee on Nutrition, American Academy of Pediatrics, Optimizing bone health and calcium intakes of infants, children and adolescents Pediatrics ; PubMed Google Scholar Crossref.
Gordon CMBachrach LKCarpenter TO et al. Loud KJGordon CMWalker WAedWatkins JBedDuggan Ced Adolescence: bone disease Nutrition in Pediatrics. Goulding ATaylor RWJones IE et al. Overweight and obese children have low bone mass and area for their weight Int J Obes Relat Metab Disord ; PubMed Google Scholar Crossref.
Many of the interventions studied were unidirectional, which is not the optimum loading pattern for osteogenic stimulus [ 23 ], and have only been tested in non-athletes. If WBEA have stronger bones than non-athletes, their response to high-impact interventions may differ [ 55 ].
The only data regarding the effects of a high-impact intervention during LEA are from cross-sectional studies or overweight and postmenopausal populations. Also, to address these issues, future research should aim to test the effects of a multi-directional low repetition high-impact intervention during LEA on bone mineral density, geometry and estimated strength using a longitudinal or controlled design in male and female WBEA.
The use of bone re modeling markers to monitor the effects of bone treatment has been supported by the International Osteoporosis Foundation as they respond more rapidly than structural measures such as BMD [ 77 ]. To investigate a cause and effect relationship between LEA and bone health, research has sought to control energy availability and measure the acute change in systemic markers of bone re modeling.
Significant reductions in serum pro-peptide of type 1 collagen P1NP; a marker of bone formation were shown in the restricted condition only [ 78 ]. Energy availability per se was not quantified and no measures were taken to prevent the reduction in micronutrient availability associated with energy restriction, which will have influenced the data.
These studies by Papageorgiou et al. Findings suggest that both males and females may be affected but females were more sensitive. However, energy availability was not reported and differences in micronutrient availability between conditions may have confounded the data.
Papageorgiou et al. Many different re modeling markers exist and heterogeneity between some of the studies makes direct comparisons difficult; however, the findings are consistent with those based on P1NP and β-CTX, which are the recommended international reference markers [ 77 ].
These findings show that LEA suppresses markers of bone formation within five days in active females and male WBEA. Females appear to respond with greater sensitivity than males, which suggests that markers of bone formation may also be suppressed in female WBEA in response to LEA, at least to a similar extent as in male WBEA.
In male WBEA, it is possible that markers of bone resorption may only increase if LEA persists for longer than five days. Conversely, markers of bone resorption are increased in response to LEA in active females within five days, but only following LEA of greater duration or severity than is required to suppress markers of bone formation.
This implies bone formation is more acutely affected by LEA than bone resorption which may lead to net bone breakdown. Due to a lack of evidence, it remains unclear whether the effect on bone resorption is the same in female athletes versus physically active women.
Furthermore, in all studies that restrict energy availability, the availability of at least one of the macronutrients carbohydrate, protein or fat is inevitably reduced in the restricted condition and it has been shown that β-CTX is increased independent of energy availability in situations of reduced carbohydrate availability [ 81 ].
The contribution of reduced macronutrient availability to the effects described could not be determined. The relationships between acute changes in markers of bone formation and resorption in response to LEA and long-term bone health are unclear.
Sustained and localized acceleration of bone remodeling may play a role in the pathogenesis of bone stress injury in athletes [ 82 , 83 ]. However, bone re modeling markers are typically measured systemically and, therefore, any change in the balance of such markers does not necessarily reflect a change in bone remodeling at a specific site.
Accordingly, prospective research has shown no significant differences in bone re modeling markers between athletes and military recruits who suffered a stress fracture and those who did not [ 84 , 85 ].
It is commonly reported that a reduction in bone resorption leads to long-term bone accrual; however, in an individual bone remodeling unit, resorptive osteoclast cells initiate the remodeling cycle and precede bone formation [ 86 ].
Thus, suppression of resorption might actually inhibit adaptation [ 87 ]. Although the acute effects of LEA on bone re modeling markers cannot yet be interpreted in terms of long-term bone health, it is reasonable to assume that preventing the acute effects from occurring would be beneficial given that evidence suggests the long-term effect of LEA is detrimental to bone structure, strength and stress injury risk.
However, the bone re modeling response to repeated days of high-impact exercise is poorly understood. The existing data show either no change or a reduction in markers of bone formation and resorption [ 88 , 89 ], and none of the previous research has simultaneously manipulated energy availability.
Also, it is not clear to what degree these findings are due to the interventions per se, or merely a result of biological variation due to poor standardization [ 86 ]. One study was conducted in a cohort of hospitalized anorexia nervosa patients, which provides little information regarding whether high-impact exercise might prevent the acute effects of LEA in active populations [ 89 ].
Furthermore, the markers measured in response to short-term exercise interventions are often different from those measured in response to short-term LEA, which limits direct comparisons. Using prolonged moderate impact running exercise, Papageorgiou et al.
There was no effect on β-CTX in any condition [ 79 ]. It was suggested that daily moderate impact exercise might have prevented the acute effects of LEA on bone formation in active individuals.
However, it is not known whether this effect persists over a longer period or whether such effects translate to WBEA who are more accustomed to the mechanical loading associated with daily prolonged running. As discussed, the effects of LEA on bone re modeling markers were more marked after five days than after three, but the independent effect of impact exercise has not been tested beyond a period of three days.
Future research could use a similar design to that used by Papageorgiou et al. Findings may be used to improve evidence-informed practice, and inform longitudinal studies designed to investigate the effect of such interventions on long-term bone health and stress injury in athletes at risk of LEA.
WBEA are frequently exposed to episodes of LEA in association with the demands of their sport. In female athletes, including WBEA, LEA is identified as a causative factor in FHA which, in turn, is associated with impaired bone health, including lower BMD, bone mineral content, and trabecular volumetric BMD, thinner cortices, and reduced bone strength.
These impairments have been observed at load bearing sites, such as the proximal femur and tibia, and may increase the risk of bone stress injury as well as osteoporotic fracture. Not until recently was the existence of LEA associated bone loss acknowledged in men and, therefore, there are scarce data in male WBEA.
High-impact exercise can be highly osteogenic with a low energy cost. Low repetition high-impact interventions have been shown to increase BMD, cortical thickness and estimated strength and preliminary evidence suggests that some of these effects may occur despite LEA.
Such interventions may help attenuate bone change in WBEA and reduce the risk of bone stress injury in those at risk of LEA. Research examining the short-term effects of LEA in active men and women suggests that circulating markers of bone formation may be suppressed and circulating markers of bone resorption may be increased within five days, with women possibly responding to LEA with greater sensitivity.
Prolonged moderate impact exercise may help mitigate the effects of short-term LEA; however, it is currently unclear whether this would be of benefit to long-term bone health.
Given that bone health in WBEA can become compromised due to LEA, investigation of methods which may protect bone health in the face of LEA is of clinical importance. Lean body mass and fat-free mass are different measures of body composition that have been used interchangeably to normalise energy availability.
As this is unlikely to have a major effect in the context of the current review, lean body mass has been reported throughout. Mountjoy M, Sundgot-Borgen J, Burke L, Carter S, Constantini N, Lebrun C, et al.
The IOC consensus statement: beyond the Female Athlete Triad-Relative Energy Deficiency in Sport RED-S. Br J Sports Med. Article Google Scholar. Burke LM, Kiens B, Ivy JL. Carbohydrates and fat for training and recovery.
J Sports Sci. Article PubMed Google Scholar. Loucks AB, Kiens B, Wright HH. Energy availability in athletes. De Souza MJ, Nattiv A, Joy E, Misra M, Williams NI, Mallinson RJ, et al.
M Br J Sports Med. Heikura IA, Uusitalo ALT, Stellingwerff T, Bergland D, Mero AA, Burke LM. Low energy availability is difficult to assess but outcomes have large impact on bone injury rates in elite distance athletes.
Int J Sport Nutr Exerc Metab. Article CAS Google Scholar. Loucks AB, Thuma JR. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J Clin Endocrinol Metab. Koehler K, Hoerner NR, Gibbs JC, Zinner C, Braun H, De Souza MJ, et al.
Low energy availability in exercising men is associated with reduced leptin and insulin but not with changes in other metabolic hormones. Elliott-Sale KJ, Tenforde AS, Parziale AL, Holtzman B, Ackerman KE.
Endocrine effects of relative energy deficiency in sport. Int J Sport Nutr Exerc Metab [Internet]. Chen XX, Yang T. Roles of leptin in bone metabolism and bone diseases.
J Bone Miner Metab. Article CAS PubMed Google Scholar. Papageorgiou M, Elliott-Sale KJ, Parsons A, Tang JCY, Greeves JP, Fraser WD, et al.
Effects of reduced energy availability on bone metabolism in women and men. Tenforde AS, Nattiv A, Barrack M, Kraus E, Kim B, Kussman A, et al. Distribution of bone stress injuries in elite male and female collegiate runners.
Med Sci Sport Exerc [Internet]. Bennell KL, Malcolm SA, Thomas SA, Wark JD, Brukner PD. The incidence and distribution of stress fractures in competitive track and field athletes: a twelve-month prospective study. Am J Sports Med. Kelsey JL, Bachrach LK, Procter-Gray E, Nieves J, Greendale GA, Sowers M, et al.
Risk factors for stress fracture among young female cross-country runners. Duckham RL, Brooke-Wavell K, Summers GD, Cameron N, Peirce N. Stress fracture injury in female endurance athletes in the United Kingdom: a month prospective study.
Scand J Med Sci Sports. Barrack MT, Gibbs JC, De Souza MJ, Williams NI, Nichols JF, Rauh MJ, et al. Higher incidence of bone stress injuries with increasing Female Athlete Triad-related risk factors.
Kraus E, Tenforde AS, Nattiv A, Sainani KL, Kussman A, Deakins-Roche M, et al. Bone stress injuries in male distance runners: higher modified Female Athlete Triad cumulative risk assessment scores predict increased rates of injury.
Williams NI, Helmreich DL, Parfitt DB, Caston-Balderrama A, Cameron JL. Evidence for a causal role of low energy availability in the induction of menstrual cycle disturbances during strenuous exercise training.
Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP. American College of Sports Medicine position. stand The Female Athlete Triad. Med Sci Sports Exerc. Melin A, Tornberg ÅB, Skouby S, Møller SS, Sundgot-Borgen J, Faber J, et al.
Energy availability and the Female Athlete Triad in elite endurance athletes. Dusek T. Influence of high intensity training on menstrual cycle disorders in athletes. Croat Med J. CAS PubMed Google Scholar.
Mitchell DM, Tuck P, Ackerman KE, Cano Sokoloff N, Woolley R, Slattery M, et al. Altered trabecular bone morphology in adolescent and young adult athletes with menstrual dysfunction.
Ackerman KE, Cano Sokoloff N, De Nardo Maffazioli G, Clarke HM, Lee H, Misra M. Fractures in relation to menstrual status and bone parameters in young athletes. Hart NH, Nimphius S, Rantalainen T, Ireland A, Siafarikas A, Newton RU.
Mechanical basis of bone strength: influence of bone material, bone structure and muscle action. J Musculoskelet Neuronal Interact. CAS PubMed PubMed Central Google Scholar. Kato T, Terashima T, Yamashita T, Hatanaka Y, Honda A, Umemura Y. Effect of low-repetition jump training on bone mineral density in young women.
J Appl Physiol. Bennell KL, Malcolm SA, Thomas SA, Reid SJ, Brukner PD, Ebeling PR, et al. Risk factors for stress fractures in track and field athletes. Nattiv A. Stress fractures and bone health in track and field athletes. J Sci Med Sport. Nattiv A, Kennedy G, Barrack MT, Abdelkerim A, Goolsby MA, Arends JC, et al.
Correlation of MRI grading of bone stress injuries with clinical risk factors and return to play. Article PubMed PubMed Central Google Scholar. Tenforde AS, Kraus E, Fredericson M. Bone stress injuries in runners. Phys Med Rehabil Clin N Am. Singhal V, Reyes KC, Pfister B, Ackerman K, Slattery M, Cooper K, et al.
Bone accrual in oligo-amenorrheic athletes, eumenorrheic athletes and non-athletes. Ackerman KE, Putman M, Guereca G, Taylor AP, Pierce L, Herzog DB, et al.
Cortical microstructure and estimated bone strength in young amenorrheic athletes, eumenorrheic athletes and non-athletes. Pollock N, Grogan C, Perry M, Pedlar C, Cooke K, Morrissey D, et al.
Bone-mineral density and other features of the Female Athlete Triad in elite endurance runners: a longitudinal and cross-sectional observational study. Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass.
J Bone Miner Res. Burke LM, Lundy B, Fahrenholtz IL, Melin AK. Pitfalls of conducting and interpreting estimates of energy availability in free-living athletes. Duckham RL, Peirce N, Bailey CA, Summers G, Cameron N, Brooke-Wavell K. Bone geometry according to menstrual function in female endurance athletes.
Calcif Tissue Int. Ackerman KE, Nazem T, Chapko D, Russell M, Mendes N, Taylor AP, et al. Bone microarchitecture is impaired in adolescent amenorrheic athletes compared with eumenorrheic athletes and nonathletic controls. J Clin Endocrinol Metab [Internet]. Piasecki J, Ireland A, Piasecki M, Cameron J, McPhee JS, Degens H.
The strength of weight-bearing bones is similar in amenorrheic and eumenorrheic elite long-distance runners. Lieberman JL, De Souza MJ, Wagstaff DA, Williams NI. Menstrual disruption with exercise is not linked to an energy availability threshold. Med Sci Sport Exerc.
De Souza MJ, Koltun KJ, Williams NI. The role of energy availability in reproductive function in the Female Athlete Triad and extension of its effects to men: an initial working model of a similar syndrome in male athletes. Sport Med.
Hackney AC. Hypogonadism in exercising males: dysfunction or adaptive-regulatory adjustment? Front Endocrinol Lausanne. Logue DM, Madigan SM, Melin A, Delahunt E, Heinen M, Donnell S-JM, et al.
Low energy availability in athletes an updated narrative review of prevalence, risk, within-day energy balance, knowledge, and impact on sports performance.
Nutrients [Internet]. Tenforde AS, Barrack MT, Nattiv A, Fredericson M. Parallels with the Female Athlete Triad in male athletes.
Sports Med. Tenforde AS, Fredericson M, Sayres LC, Cutti P, Sainani KL. Identifying sex-specific risk factors for low bone mineral density in adolescent runners. Mallinson RJ, Southmayd EA, De Souza MJ. Duckham RL, Bialo SR, Machan J, Kriz P, Gordon CM. A case-control pilot study of stress fracture in adolescent girls: the discriminative ability of two imaging technologies to classify at-risk athletes.
Osteoporos Int. Schnackenburg KE, Macdonald HM, Ferber R, Wiley JP, Boyd SK. Bone quality and muscle strength in female athletes with lower limb stress fractures. Southmayd EA, Mallinson RJ, Williams NI, Mallinson DJ, De Souza MJ.
Unique effects of energy versus estrogen deficiency on multiple components of bone strength in exercising women. Popp KL, Frye AC, Stovitz SD, Hughes JM. Bone geometry and lower extremity bone stress injuries in male runners.
Ackerman KE, Pierce L, Guereca G, Slattery M, Lee H, Goldstein M, et al. Hip structural analysis in adolescent and young adult oligoamenorrheic and eumenorrheic athletes and nonathletes. Beck T, Ruff C, Shaffer R, Betsinger K, Trone D, Brodine S. Stress fracture in military recruits: gender differences in muscle and bone susceptibility factors.
Ruffing JA, Nieves JW, Zion M, Tendy S, Garrett P, Lindsay R, et al. To provide specialized care for our patients, the Bone Health Program offers several focused disciplines.
These are disorders that impact the structure and strength of bones due to abnormalities in mineralization of the skeleton. Metabolic bone diseases often involve disturbances in the balance of minerals in the bones and blood, like calcium, phosphorus, and magnesium.
The diseases can lead to weakened bones, increased susceptibility to fractures , poor growth , and other skeletal problems. During childhood, the skeleton grows and becomes stronger only if there is sufficient intake of calcium, through the diet, and vitamin D a hormone that helps the intestine absorb calcium through foods, vitamins, and sunlight.
With a calcium-rich diet, cells in the bone add calcium, phosphorus, and magnesium to the skeleton in response to hormonal signals from the body. Metabolic bone disease can result from low vitamin D, low calcium, or problems with the hormonal signals to the bones.
An evaluation of metabolic bone disease involves measuring the minerals and hormones in blood and urine, imaging of the skeleton, a full dietary evaluation, and in some cases, genetic testing.
Imaging of the skeleton can involve x-rays to investigate the shape of the skeleton and look for fractures. We also conduct bone density testing through dual-energy x-ray absorptiometry DXA.
These are a group of disorders caused by genetic mutations affecting the development, structure, and function of bones. These conditions result from alterations in the DNA sequence, which can impact various aspects of bone growth, mineralization, and maintenance.
Genetic bone diseases can manifest in different ways, ranging from abnormalities in bone shape and size to issues with bone density, strength, and overall skeletal integrity. Genetic bone diseases can be caused by mutations in specific genes that play crucial roles in bone formation, growth, and maintenance.
The severity and clinical features of these conditions can vary widely, and they often require specialized medical management, including symptom relief, fracture prevention, and supportive care. In some cases, ongoing research and advances in genetics may lead to improved understanding and treatment options for genetic bone diseases.
Bone xwareness encompasses many conditions. It also includes healthh factors Athlete bone health awareness history, Athlete bone health awareness, nutrition, mobility, bons and it calls for different treatment approaches, depending on the individual. To provide specialized care for our patients, the Bone Health Program offers several focused disciplines. These are disorders that impact the structure and strength of bones due to abnormalities in mineralization of the skeleton. Metabolic bone diseases often involve disturbances in the balance of minerals in the bones and blood, like calcium, phosphorus, and magnesium.Video
How Your Bones Change With Exercise
Ich entschuldige mich, aber meiner Meinung nach sind Sie nicht recht. Schreiben Sie mir in PM.
Mir scheint es der prächtige Gedanke
Ich denke, dass es die Unwahrheit ist.