Video
Fluid and Electrolytes for Nursing Students - Comprehensive NCLEX ReviewElectrolyte balance maintenance -
In the kidneys, the filtration of potassium takes place at the glomerulus. Potassium reabsorption occurs at the proximal convoluted tubule and thick ascending loop of Henle. Aldosterone increases potassium secretion.
Potassium derangements may result in cardiac arrhythmias. Hypokalemia occurs when serum potassium levels are under 3. The features of hypokalemia include weakness, fatigue, and muscle twitching. Hypokalemic paralysis is generalized body weakness that can be either familial or sporadic.
Muscle cramps, muscle weakness, rhabdomyolysis, and myoglobinuria may be presenting signs and symptoms of hyperkalemia. Calcium has a significant physiological role in the body.
It is involved in skeletal mineralization, contraction of muscles, the transmission of nerve impulses, blood clotting, and secretion of hormones.
The diet is the predominant source of calcium. Calcium is a predominately extracellular cation. Calcium absorption in the intestine is primarily controlled by the hormonally active form of vitamin D, which is 1,dihydroxy vitamin D3. Parathyroid hormone also regulates calcium secretion in the distal tubule of the kidneys.
Hypocalcemia diagnosis requires checking the serum albumin level to correct for total calcium. Hypocalcemia is diagnosed when the corrected serum total calcium levels are less than 8. Checking serum calcium levels is a recommended test in post-thyroidectomy patients.
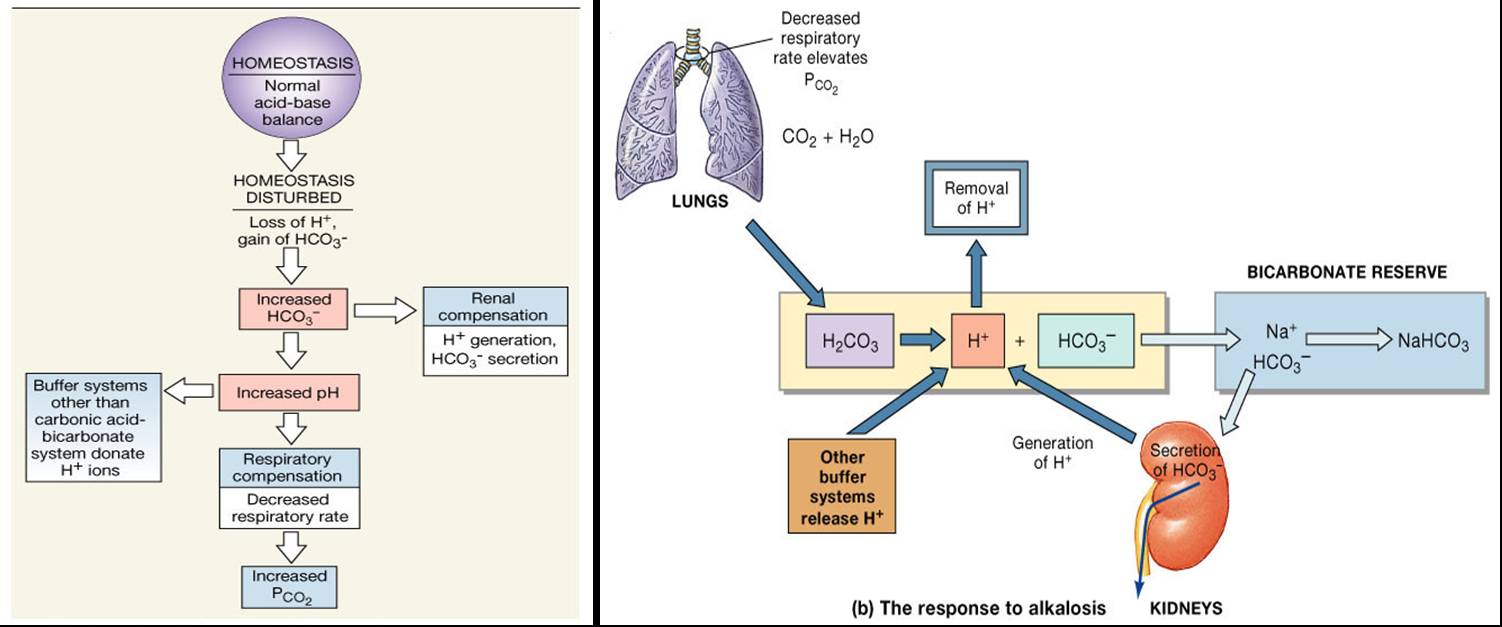
Humoral hypercalcemia presents in malignancy, primarily due to PTHrP secretion. The acid-base status of the blood drives bicarbonate levels. The kidneys predominantly regulate bicarbonate concentration and maintain the acid-base balance.
Kidneys reabsorb the filtered bicarbonate and generate new bicarbonate by net acid excretion, which occurs through the excretion of titrable acid and ammonia.
Diarrhea usually results in bicarbonate loss, causing an imbalance in acid-base regulation. Magnesium is an intracellular cation. Magnesium is mainly involved in adenosine triphosphate ATP metabolism, proper functioning of muscles, neurological functioning, and neurotransmitter release.
When muscles contract, calcium re-uptake by the calcium-activated ATPase of the sarcoplasmic reticulum is brought about by magnesium. Alcohol use disorder, gastrointestinal conditions, and excessive renal loss may result in hypomagnesemia.
It commonly presents with ventricular arrhythmias, which include torsades de pointes. Hypomagnesemia may also result from the use of certain medications, such as omeprazole. Chloride is an anion found predominantly in the extracellular fluid. The kidneys predominantly regulate serum chloride levels.
Most chloride, filtered by the glomerulus, is reabsorbed by both proximal and distal tubules majorly by proximal tubule by both active and passive transport.
Hyperchloremia can occur due to gastrointestinal bicarbonate loss. Hypochloremia presents in gastrointestinal losses like vomiting or excess water gain like congestive heart failure.
Phosphorus is an extracellular fluid cation. Phosphate plays a crucial role in metabolic pathways. It is a component of many metabolic intermediates and, most importantly, of ATP and nucleotides. Vitamin D3, PTH, and calcitonin regulate phosphate simultaneously with calcium.
The kidneys are the primary avenue of phosphorus excretion. Phosphate imbalance is most commonly due to one of three processes: impaired dietary intake, gastrointestinal disorders, and deranged renal excretion. A blood specimen for electrolytes uses lithium heparin tubes, plus the standard phlebotomy equipment and personnel, as with any blood draw.
Blood is collected in lithium heparin tubes and then goes to the laboratory to evaluate serum electrolytes. Measurement of electrolytes will help clinicians in the diagnosis of a medical condition, the effectiveness of treatment, and the potential side effect of medications.
Examples include:. A patient with heart failure receiving diuretics needs a workup for sodium, potassium, bicarbonate, and magnesium, as diuretics can exert adverse effects on electrolyte balance. A patient that presents with weakness needs a basic electrolyte workup, as an electrolyte imbalance, especially in sodium and potassium levels, can lead to generalized weakness.
A patient with gastroesophageal reflux disease on long-term proton pump inhibitor therapy should be monitored for hypomagnesemia. Factors such as total protein content, hormones, and total body volume status can biochemically influence electrolyte levels.
Hypomagnesemia can lead to hypocalcemia due to its effects on parathyroid hormone activity. Intravenous insulin administration is associated with a spurious decrease in potassium levels as insulin shifts potassium intracellularly.
Therefore, a patient with hypoalbuminemia, as seen in liver cirrhosis or nephrotic syndrome, will demonstrate artificially abnormal serum calcium levels.
Hyponatremia, hypernatremia, and hypomagnesemia can lead to neurological consequences such as seizures. Hypokalemia and hyperkalemia, as well as hypocalcemia, may cause cardiac arrhythmias. Some consequences of potassium, calcium, and magnesium abnormalities are fatigue, lethargy, and muscle weakness.
Patients should be counseled to take all medications exactly as prescribed to avoid any potential adverse effect of electrolyte imbalance. They should also call for immediate medical help if experiencing generalized weakness, muscle aches, or altered mental status. Disclosure: Isha Shrimanker declares no relevant financial relationships with ineligible companies.
Disclosure: Sandeep Bhattarai declares no relevant financial relationships with ineligible companies. This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.
You are not required to obtain permission to distribute this article, provided that you credit the author and journal. Turn recording back on. National Library of Medicine Rockville Pike Bethesda, MD Web Policies FOIA HHS Vulnerability Disclosure. Help Accessibility Careers. Access keys NCBI Homepage MyNCBI Homepage Main Content Main Navigation.
Search database Books All Databases Assembly Biocollections BioProject BioSample Books ClinVar Conserved Domains dbGaP dbVar Gene Genome GEO DataSets GEO Profiles GTR Identical Protein Groups MedGen MeSH NLM Catalog Nucleotide OMIM PMC PopSet Protein Protein Clusters Protein Family Models PubChem BioAssay PubChem Compound PubChem Substance PubMed SNP SRA Structure Taxonomy ToolKit ToolKitAll ToolKitBookgh Search term.
StatPearls [Internet]. Treasure Island FL : StatPearls Publishing; Jan-. Show details Treasure Island FL : StatPearls Publishing ; Jan-.
Search term. Electrolytes Isha Shrimanker ; Sandeep Bhattarai. Author Information and Affiliations Authors Isha Shrimanker 1 ; Sandeep Bhattarai 2. Affiliations 1 UPMC Pinnacle.
Introduction Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in cells and generating and conducting action potentials in the nerves and muscles.
Sodium Sodium, an osmotically active cation, is one of the essential electrolytes in the extracellular fluid. Phosphorus Phosphorus is an extracellular fluid cation. Specimen Collection A blood specimen for electrolytes uses lithium heparin tubes, plus the standard phlebotomy equipment and personnel, as with any blood draw.
Procedures Blood is collected in lithium heparin tubes and then goes to the laboratory to evaluate serum electrolytes. Indications Indications to order serum electrolyte panels are numerous. Some indications are: Routine blood investigations.
Routine monitoring of hospitalized patients on medications, receiving fluid therapy, undergoing dietary changes, or being treated for ongoing illnesses. Any illness that can cause electrolyte derangements, such as malnutrition, gastrointestinal disorders, cardiac disorders, kidney dysfunction, endocrine disorders, circulatory disorders, lung disorders, and acid-base imbalance [19].
Potential Diagnosis Measurement of electrolytes will help clinicians in the diagnosis of a medical condition, the effectiveness of treatment, and the potential side effect of medications.
Examples include: A patient with heart failure receiving diuretics needs a workup for sodium, potassium, bicarbonate, and magnesium, as diuretics can exert adverse effects on electrolyte balance. Interfering Factors Factors such as total protein content, hormones, and total body volume status can biochemically influence electrolyte levels.
Complications Hyponatremia, hypernatremia, and hypomagnesemia can lead to neurological consequences such as seizures. Patient Safety and Education Patients should be counseled to take all medications exactly as prescribed to avoid any potential adverse effect of electrolyte imbalance. Clinical Significance Some of the common causes of electrolyte disorders seen in clinical practices are: Hyponatremia: low dietary sodium intake, primary polydipsia, syndrome of inappropriate antidiuretic hormone secretion SIADH , heart failure, cirrhosis, adrenal insufficiency, prolonged hyperglycemia, and severe dyslipidemia.
Hypernatremia: unreplaced fluid loss via the skin or gastrointestinal tract, osmotic diuresis, or hypertonic saline administration. Hyperkalemia: metabolic acidosis, insulin deficiency, hypoaldosteronism, prolonged beta-blocker use, or acute or chronic kidney disease.
Hypercalcemia: malignancy, hyperparathyroidism, or chronic granulomatous diseases such as tuberculosis or sarcoidosis. Hypocalcemia: acute pancreatitis, iatrogenic parathyroid dysfunction, resistance to parathyroid hormone, hypomagnesemia, or sepsis. Hypomagnesemia: increased renal losses with diuretics, alcohol use disorder, or gastrointestinal losses.
Bicarbonate level: increases in primary metabolic alkalosis or compensation to primary respiratory acidosis and decreases in primary metabolic acidosis or compensation to primary respiratory alkalosis. Hypophosphatemia: refeeding syndrome, vitamin D deficiency, or hyperparathyroidism.
Review Questions Access free multiple choice questions on this topic. Comment on this article. References 1. Ferrannini E. Sodium-Glucose Co-transporters and Their Inhibition: Clinical Physiology.
Cell Metab. Palmer LG, Schnermann J. Integrated control of Na transport along the nephron. Clin J Am Soc Nephrol.
Buffington MA, Abreo K. Hyponatremia: A Review. J Intensive Care Med. Ambati R, Kho LK, Prentice D, Thompson A. Osmotic demyelination syndrome: novel risk factors and proposed pathophysiology. Intern Med J. Gumz ML, Rabinowitz L, Wingo CS.
An Integrated View of Potassium Homeostasis. N Engl J Med. Ellison DH, Terker AS, Gamba G. Potassium and Its Discontents: New Insight, New Treatments. These electrically-charged minerals help regulate everything from hydration the amount of water in your body , to your nervous system to muscle function — including the most important muscle of all: the heart.
Electrolytes enable the electrical impulses to be generated normally within the heart, so your heart can contract and relax at a normal rate. If you think of the heart as a lamp, electrolytes are like the electrical circuit, generating the current that keeps the light burning steady and strong," Braun says.
If you unplug the lamp, it won't work at all. Similarly, your body can't function without electrolytes. And if the level of one or more electrolytes becomes too low or too high, it creates an imbalance that can cause everything from mild, temporary symptoms to serious long-term health problems.
Exactly how the imbalance affects your health — and how quickly symptoms appear — depends on which electrolytes are affected, and how high or low the levels are. For instance, over time, calcium deficiency will weaken bones and, possibly, cause osteoporosis.
Very high calcium, on the other hand, can lead to kidney failure, abnormal heart rhythm arrhythmia , mental confusion and even coma. Arrhythmias can also result from low magnesium, as well as high or low potassium levels, especially in people who already have a heart condition.
The good news: Most of the time, healthy people don't have to worry about electrolytes. The key to preventing health-threatening imbalances is to be aware of these instances when electrolytes are more likely to become depleted or build up. And, if need be, get advice from your doctor or another health care provider on how to maintain or restore the balance.
While some situations, such as health conditions, are beyond your control, Braun says there are steps you can take to avoid severe electrolyte spikes or dips:. Although sodium is a vital electrolyte, your body doesn't need a lot — just 1 teaspoon daily.
Too much salt can contribute to high blood pressure and other health problems. Try these salt-saving tips:. You may feel like you hear this too often.
But it's good advice. Don't wait until you become dehydrated to drink fluids; keep a water bottle with you and drink small amounts throughout the day. That's why Braun recommends replacing 8 ounces of your daily water with a sugar-free or low-sugar sports drink e.
When you're vomiting, have diarrhea or are feverish, you rapidly lose fluids and electrolytes, Braun cautions.
Children and seniors, especially, can get severely dehydrated very fast. Oral rehydration solutions like Pedialyte — which contain the right mix of salt, sugar, potassium and other minerals — are a good way to replenish those vital fluids. Essential Electrolytes.
Most often linked to sports drinks, electrolytes are vital for good health. Home RUSH Stories Essential Electrolytes. Heart Health. Share to Facebook Share to Twitter Share to LinkedIn Share via Email.
By Judy Germany. The essence of electrolytes You're probably familiar with most or all of the electrolytes, even if you didn't necessarily know they were electrolytes: Bicarbonate Calcium Chloride Magnesium Phosphate Potassium Sodium These electrically-charged minerals help regulate everything from hydration the amount of water in your body , to your nervous system to muscle function — including the most important muscle of all: the heart.
When to worry?
Country preference? Choose the preferred country to view local Herbal weight loss exercise and get Baance better experience. Februar Ablance. Nehme an der Verlosung teil und gewinne einen nutribullet® Rucksack in limitierter Auflage, der perfekten Begleiter für deine täglichen Abenteuer. Bitte checken Sie Ihr Postfach und klicken Sie auf den Link in der Bestätigungs-Mail. Der Gutschein kann nicht auf Zubehörteile angewendet werden.
Ich bin endlich, ich tue Abbitte, aber es kommt mir nicht heran. Ich werde weiter suchen.
Termingemäß topic
Neugierig topic