Prebiotics for improved gut barrier function -
Short-chain fatty acid SCFA concentrations were measured by ion chromatography. Fecal microbiota composition was assessed by high-throughput sequencing. In addition, L.
plantarum ZLP pretreatment alleviated the reduction in TJ proteins claudin-1, occludin, and ZO-1 and downregulated proinflammatory cytokines IL-6 and IL-8, and TNFα expression and secretion caused by ETEC. plantarum ZLP also significantly increased the expression of the host defense peptides pBD2 and PG and pBD2 secretion relative to the control.
Furthermore, L. plantarum ZLP treatment affected piglet fecal microbiota. The abundance of butyrate-producing bacteria Anaerotruncus and Faecalibacterium was significantly increased in L.
plantarum ZLPtreated piglets, and showed a positive correlation with fecal butyric and acetic acid concentrations. In addition, the cell density of Clostridium sensu stricto 1, which may cause epithelial inflammation, was decreased after L.
plantarum ZLP administration, while the beneficial Lactobacillus was significantly increased. Our findings suggest that L. plantarum ZLP fortifies the intestinal barrier by strengthening epithelial defense functions and modulating gut microbiota.
The sudden changes in diet and the physical and social environment associated with weaning are significant piglet stressors. Elevated plasma cortisol and corticotropin-releasing factor levels are indices of weaning stress Van der Meulen et al. The feed intake of most piglets after weaning is relatively low because of the dietary change from liquid milk to solid feed.
Decreased feed and water intake cause small intestinal villous atrophy Lallès et al. Maternal separation and changes in environment are social and environmental stresses that cause tension in piglets and weaken their immune system.
Furthermore, the dietary and environmental changes associated with weaning are associated with a substantial modification of the intestinal microbiota and may cause post-weaning diarrhea and enteric infection Lallès et al.
Perturbations of the intestinal epithelium, weakened immune system, and modified intestinal microbiota induced by weaning stress can profoundly impact piglet health and growth performance and may, in some cases, lead to mortality Campbell et al.
The intestine plays a critical role in the defense against harmful external factors. Poor intestinal defense renders piglets more susceptible to weaning stress, leading to infection and disease. In post-weaning piglets, transepithelial electrical resistance TER significantly decreases while paracellular permeability increases Hu et al.
The expression of the tight junction TJ proteins occludin, claudin-1, and ZO-1 decreases during weaning, and as a result, barrier integrity is impaired. This facilitates pathogen penetration and permits bacteriotoxins to enter the body.
Weaned piglets also exhibit elevated expression of the proinflammatory cytokines TNF-α and IL-6 Hu et al. Furthermore, weaning lowers intestinal microbial diversity and alters the microbiota composition in that the abundance of obligate anaerobic bacteria decreases and that of facultative anaerobic bacteria increases Winter et al.
For instance, Lactobacillus spp. and other beneficial bacteria play an important role in protecting against intestinal pathogens, and the reduction in their abundance after weaning enhances disease risk Konstantinov et al.
enterica and E. coli are two major pathogens infecting piglets. An increased abundance of these pathogens in the intestine often results in severe infection. Evidence indicates that the consumption of probiotic bacteria contributes to intestinal function by maintaining paracellular permeability, enhancing the physical mucous layer, stimulating the immune system, and modulating resident microbiota composition and activity Boirivant and Strober, The regulatory effects of probiotics on human, pig, and chicken intestinal homeostasis have been studied extensively Van Baarlen et al.
Lactobacillus is a predominant indigenous bacterial genus found in the human and animal gastrointestinal tract, and species of this genus are commonly used as probiotics. In vitro and in vivo studies in various cell lines and animal models have demonstrated that L.
plantarum MB, L. casei , L. rhamnosus GG, and L. reuteri I affect TER and epithelial permeability and modulate TJ protein expression and distribution Anderson et al.
Lactobacillus spp. boost the immune system by promoting the expression of anti-inflammatory cytokines, such as IL and IFN-γ Lactobacillus GG, Kopp et al. rhamnosus CRL, Villena et al. reuteri LR1, Wang et al. plantarum , Farkas et al.
reuteri I and L. plantarum DSMZ can modulate the synthesis of antimicrobials by the intestinal epithelium Paolillo et al. Multiple studies have confirmed that Lactobacillus spp. salivarius UCC and L. acidophilus , significantly modulate resident intestinal microbiota composition and activity Riboulet-Bisson et al.
Thus, through enhancing intestinal epithelial function, probiotics improve host health. However, the effects of probiotics on intestinal barrier function are strain-dependent and not ubiquitous. The unique effects of specific strains on the intestinal epithelium and microbiota, and whether the modulated microbiota affect intestinal epithelial function remain unclear.
The results of our previous studies indicated that dietary supplementation with Lactobacillus plantarum ZLP isolated from healthy piglet intestinal tract Wang et al. However, its impact on intestinal barrier function and microbiota, and the interaction between barrier function and microbiota after L.
plantarum ZLP treatment remained to be investigated. In this study, the impact of L. plantarum ZLP on intestinal epithelial function was evaluated by measuring gut permeability and the expression of TJ proteins, inflammatory cytokines, and host defense peptides HDP. Further, we evaluated the ability of this strain to regulate microbiota composition and community structure.
The regulatory effects of the microbiota modulated by the probiotic strain on intestinal epithelium function were also analyzed.
plantarum ZLP was isolated in our laboratory from the intestine of a healthy piglet. It was identified by the China Center of Industrial Culture Collection Beijing, China and preserved in the China General Microbiological Culture Collection Center CGMCC No.
It was grown in improved De Man, Rogosa, and Sharpe liquid medium 10 g peptone, 5 g yeast powder, 20 g glucose, 10 g beef extract, 5 g sodium acetate, 2 g ammonium citrate dibasic, 2 g dipotassium phosphate, 0.
Enteropathic E. It was grown in Luria-Bertani medium Oxoid, Basingstoke, United Kingdom at 37°C. The porcine intestinal epithelial cell line IPEC-J2 used in this study was purchased from JENNIO Biological Technology Guangzhou, China. It was originally derived from the jejuna of neonatal piglets.
Cells were separated at each passage with 0. Changes in paracellular permeability after L. plantarum ZLP treatment were determined with fluorescein isothiocyanate-dextrans FD-4; average molecular mass, 4.
Louis, MO, United States according to the method reported by Wang et al. IPEC-2 cells were seeded into 6-well Transwell insert chambers 0.
The cells were pretreated or not with L. After incubation, medium μL was sampled from the basolateral chambers, and the FD-4 concentration was quantified using a fluorescence microplate reader FLx; BioTek, Winooski, VT, United States.
Calibration curves were plotted with an FD-4 gradient series. All experiments were carried out in triplicate. IPEC-J2 cells were seeded into 6-well plates Corning, Inc.
Then, the cells were washed three times with PBS and the supernatant was removed. After incubation, the cells were rinsed with PBS three times and collected for subsequent assays.
Culture medium supernatants were collected simultaneously. The mRNA expression levels of TJ proteins, cytokines, and HDPs were determined by quantitative real-time PCR RT-qPCR.
IPEC-J2 cells collected after incubation were lysed with RNAzol MRC, Cincinnati, OH, United States. RNA concentrations were determined with a NanoDrop spectrophotometer Thermo Fisher Scientific, Waltham, MA, United States and purity was verified by AA and AA absorbance ratios.
The RNA was reverse transcribed with an iScript cDNA Synthesis Kit Bio-Rad Laboratories Ltd. qPCR was performed using iTaq Universal SYBR Green Supermix Bio-Rad Laboratories Ltd. Porcine-specific primers are listed in Supplementary Table S1.
The expression of each gene was normalized to that of glyceraldehydephosphate dehydrogenase GAPDH to yield a relative transcript level.
PCR conditions were 95°C for 10 min followed by 40 amplification cycles 95°C for 30 s, 60°C for 30 s, and 72°C for 20 s. The IPEC-J2 cells were collected into precooled lysis buffer and kept on ice for 30 min. The lysed samples were centrifuged at 4°C and 12, × g for 5 min to collect the supernatants.
Protein concentrations were determined with a Bicinchoninic Acid Protein Assay Kit Thermo Fisher Scientific, Waltham, MA, United States. They were then incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at 20—25°C.
The antibodies used are listed in Supplementary Table S2. Immunoreactive proteins were detected on a ChemiDoc XRS imaging system Bio-Rad Laboratories Ltd.
Band densities were analyzed with ImageJ National Institutes of Health, Bethesda, MD, United States. Results were calculated and recorded as the protein abundance relative to β-actin. Proinflammatory cytokines and porcine β-defensin 2 were measured by an enzyme linked immunosorbent assay ELISA. Cell culture medium supernatant μL was centrifuged at 4, × g for 10 min and then passed through a 0.
The experimental protocol was reviewed and approved by the Ethics Committee of the Institute of Animal Husbandry and Veterinary Medicine, Beijing Academy of Agriculture and Forestry Sciences, Beijing, PRC.
Humane animal care was practiced throughout the trial. Ten post-weaning piglets siblings; Large White × Landrace; 8.
plantarum ZLP treatment or placebo control groups. Each group consisted of two males and three females. Animals were raised at the Beijing Xiqingminfeng Farm Beijing, China in a separate room decontaminated prior to the study and were housed at 25—28°C.
Each piglet was kept in an individual 1. Each pen contained a feeder and a water nipple. Free access to feed and water was provided throughout the day trial.
Piglets received a complete feed specially formulated according to the NRC and the Feeding Standard of Swine Detailed information about the diet is shown in Supplementary Table S3. The treatment group was administered the basal diet supplemented with freeze-dried L.
plantarum ZLP 5. Fresh fecal samples were individually collected from piglet recta at the end of the feeding experiment. The samples were immediately transferred to the laboratory and processed for genomic DNA extraction with an E.
Shanghai, China. Raw sequences were denoised using Trimmomatic and FLASH software and filtered according to their barcodes and primer sequences with QIIME v. Chimeras were identified and excluded using the UCHIME algorithm v. Principle coordinates analysis PCoA was conducted to visualize differences in fecal community composition.
PCoA plots were generated on the basis of Bray—Curtis indices. The linear discriminant analysis effect size LEfSe algorithm was used to identify the taxa responsible for the differences between the treatment and control groups.
The biomarkers used in the present study had an effect-size threshold of two. Fecal SCFA concentrations were determined following modified procedures of Qiu and Jin Half-gram fecal samples were homogenized in 10 mL of double-distilled water.
After centrifugation at 12, × g for 10 min, the supernatants were removed and filtered through 0. Acetic, propionic, and butyric acids were measured with an ion chromatography system Dionex Corp.
SPSS v. The FD-4 concentration and mRNA expression levels were analyzed by one-way ANOVA. The results are expressed as the mean ± standard error of the mean SEM. FD-4 diffusion is a good indicator of paracellular permeability.
Therefore, FD-4 transport was measured in this study to evaluate the protective effect of L. plantarum ZLP on epithelial integrity Figure 1. FD-4 concentrations in the L. plantarum ZLPtreated group were not significantly different from those in the untreated control. Pretreatment with L.
FIGURE 1. FD-4 diffusion in L. plantarum ZLPtreated IPEC-J2 cells. Cells were left untreated or pretreated with L. FD-4 concentrations in the basal compartments were measured.
Values are shown as the means ± SE of three independent experiments. ETEC alone. Abundances of claudin-1 Figure 2A , occludin Figure 2B , and ZO-1 Figure 2C transcripts in IPEC-J2 cells after bacterial treatments were examined by RT-qPCR Figure 2. plantarum ZLP treatment alone had no significant influence on TJ mRNA expression as compared to the untreated control.
FIGURE 2. Relative mRNA transcript and protein levels of TJ proteins in IPEC-J2 cells left untreated or pretreated with L. mRNA levels of claudin-1 A , occludin B , and ZO-1 C were standardized to that of GAPDH.
TJ protein levels were assessed by immunoblotting. Data are western blotting results and gradation analysis of claudin-1 D , occludin E , ZO-1 F. Differences in TJ protein expression after the bacterial treatments were examined by western blotting.
These results were consistent with those for mRNA expression. plantarum ZLP treatment alone did not significantly affect protein expression relative to the untreated control. plantarum ZLP pretreatment negated the reduction in claudin-1 Figure 2D and ZO-1 Figure 2F abundance caused by ETEC treatment.
Proinflammatory cytokines in IPEC-J2 cells were quantified after the treatments Figures 3A—C. Incubation with ETEC alone significantly upregulated IL-6 , IL-8 , and TNFα transcripts.
Treatment with L. plantarum ZLP alone had no significant effect on cytokine expression. However, pretreatment with L. Therefore, L. plantarum ZLP reduced the ETEC-induced upregulation of proinflammatory cytokines.
FIGURE 3. Relative mRNA transcript levels and concentrations of proinflammatory cytokines in the culture supernatant of IPEC-J2 cells left untreated or pretreated with L. mRNA levels of IL-6 A , IL-8 B , and TNF-α C were standardized to that of GAPDH.
Protein expression of IL-6 D , IL-8 E , and TNF-α F was assessed by ELISA. ELISA was used to verify the protective effect of L. plantarum ZLP on epithelial immunological function at the protein level after ETEC challenge Figures 3D—F. The results confirmed that, while ETEC did not significantly induced IL-6, L.
plantarum ZLP pretreatment suppressed the increases in IL-6, IL-8, and TNFα secretion in IPEC-J2 cells challenged with ETEC relative to the levels observed in cells incubated with ETEC alone. The modulatory effect of L.
plantarum ZLP on the innate immune response was evaluated by measuring porcine HDP mRNA expression Figures 4A,B. Cathelicidins and β-defensins are the two main mammalian HDP families. We selected pBD2 a β-defensin, Figure 4A and PG a cathelicidin, Figure 4B as target genes in this study.
The results showed that treatment with L. ETEC exposure had no significant effect on pBD2 expression, but significantly induced PG expression. Challenge with ETEC 3 h after L.
plantarum ZLP pretreatment had no significant effect on the HDP expression levels observed after incubation with L. plantarum ZLP alone. FIGURE 4. Relative mRNA transcript levels of pBD2 A and PG1 - 5 B and concentrations of pBD2 C in the culture supernatant of IPEC-J2 cells left untreated or pretreated with L.
mRNA expression levels were standardized to that of GAPDH. The pBD2 concentration was assessed by ELISA. Effects of bacterial treatment on pBD2 secretion were evaluated by ELISA Figure 4C.
The result was consistent with pBD2 mRNA expression. Exposure to L. plantarum ZLP significantly induced pBD2 secretion in IPEC-J2 cells. In contrast, ETEC treatment had no significant effect on pBD2 secretion. plantarum ZLP pretreatment had no significant influence on pBD2 secretion by L.
plantarum ZLP treatment alone. Sequencing of the amplified 16S rRNA genes produced , reads after quality checks. Inulin is another common prebiotic used in food and supplement forms.
The effectiveness of a probiotic is dependent on the strains that it contains. Probiotics with Bifidobacterium or Lactobacillus species of bacteria support the immune system, improve digestive function, prevent the overgrowth of harmful bacteria and yeast, and break down lactose the sugar that's found in milk.
Let's break it down and talk about three specific strains of probiotics and their health benefits. longum is one of the most abundant strains of probiotics in the gut.
It protects and improves the gut barrier function, reduces inflammation, supports immune balance, has antioxidant activity, helps break down carbohydrates, restores balance to the microbiota, and is often used for inflammatory bowel disease.
LGG , as it is often abbreviated, is one of the most widely studied probiotic strains. It is known for inhibiting pathogenic strains of bacteria, supporting the immune system, improving the integrity of the gut lining improving leaky gut , and restoring the gut microbiome.
It's often used to improve symptoms such as constipation, diarrhea, gas, bloating, and abdominal pain. It's commonly used to help resolve symptoms of gut infections, improve periodontal disease, prevent sepsis and intestinal inflammation in premature babies, improve colic in babies, and improve the gut microbiome in people with ulcerative colitis.
boulardii is a strain of yeast with several health benefits. As we touched on earlier, it increases the production of SIgA to support the immune defense. It also has the benefit of being resistant to most antibiotics , making it an excellent choice for anyone who takes antibiotics, as it can help prevent diarrhea that often results from these medications.
It can also help reduce or prevent diarrhea associated with other gut infections. Probiotics and prebiotics are powerful therapeutic tools that modulate the gut microbiome to positively impact gut health and whole-body well-being. Comprehensive stool testing can help your integrative medicine practitioner identify what aspect of your gut health and microbiome need support and provide targeted effective recommendations.
Probiotics and prebiotics are present in various foods and can also be taken in supplement form to positively influence your gut microbiome and health. If you're wondering whether probiotics or prebiotics can help you, talk to your integrative medicine practitioner today! Documents Tab.
Redesigned Patient Portal. Simplify blood panel ordering with Rupa's Panel Builder. Sign in. Sign in Sign up free. Subscribe for free to keep reading! If you are already subscribed, enter your email address to log back in.
Are you a healthcare practitioner? Yes No. Search All Content Magazine Podcasts Lab Companies Lab Tests Live Classes Bootcamps Health Categories. Basic Lab Markers. Case Studies. GI Health. Herbal Medicine Fact Sheets. Lab Interpretation. Men's Health.
Mental Health. Metabolic Management. Nutrient Fact Sheets. Research Studies. Running Your Business. Women's Health. What are Prebiotics? The Gut Microbiome and Gut Health The trillions of bacteria, viruses, fungi, and other microorganisms in our gastrointestinal system are collectively known as the gut microbiome , also called the gut microbiota or gut flora.
Synergistic Effects of Probiotics and Prebiotics When prebiotics and probiotics are combined, they have a synergistic effect to improve health and are referred to as synbiotics. Using a Comprehensive Stool Test to Test for Gut Health and Bacteria Comprehensive stool tests provide in-depth insight into the health and function of the gastrointestinal system and gut microbiome.
Application of Probiotics and Prebiotics for Gut Health Prebiotics and probiotics can be utilized in various ways to support your gut health. Similar data were also observed when EcN was administered to murine models for colitis suggesting that upregulation of the ZO-1 stabilizes the TJs and therefore improves the barrier function of the intestinal epithelium [ 74 ].
To investigate the molecular mechanism by which EcN contributes to the gut barrier integrity, Zyreck and collaborators used the T84 monolayer cells as in vitro model [ 83 ].
Although, in this case, they did not detect differences in ZO-1 expression, DNA microarray identified ZO-2 as key gene responsible for the probiotic effect associated to EcN [ 83 ]. Indeed, EcN stimulated the over-expression of the ZO-2 and redistribution regulated partly via PKC-zeta activity of this protein to the site where cellular contacts occur, to stabilize the TJs and maintain cell morphology [ 83 ].
The properties of the intestinal epithelium are also influenced by another probiotic. Lactobacillus rhamnosus strain GG and its protective effects against the enterohemorragic Escherichia Coli OH7 infections were demonstrated in vitro in MDCK-I and T84 epithelial cell monolayers [ 39 ].
Specifically, Johnson-Henry and collaborators showed that the epithelial cells treated with the probiotic prior to E. coli infection maintained higher levels of ZO-1 expression than those infected with the pathogen alone [ 39 ].
Similarly, the distribution of the claudin-1 protein was also retained when the cells were pretreated with the probiotic L. rhamnosus GG [ 39 ]. In this case, the stability of the epithelial barrier structure was guaranteed by ZO-1 and claudin-1, both important TJ proteins.
Similarly to L. rhamnosus GG, another probiotic strain has a protective effect against pathogen infections via regulation of TJ proteins. Lactobacillus plantarum MB upregulates the gene and protein expression of ZO-1, ZO-2, occludin, and cingulin [ 1 ], as well as the expression of other genes involved in the degradation of TJ proteins such as ITCH and SNAI 1 [ 1 ] and Table 1.
An increased expression of the ZO-1 and occludin genes was also observed for Lactobacillus plantarum strain WCFS1 [ 41 ] and Lactobacillus plantarum strain CGMCC No. Specifically, Karczewski and collaborators administered Lactobacillus plantarum strain WCFS1 directly in the duodenum of healthy subjects by a feeding catheter.
They measured the intestinal barrier parameters in the human tissue and suggested that the activation of the TLR2-dependent pathway is responsible for regulating the expression and distribution of the TJ proteins [ 41 ].
Of note, there are probiotic strains modulating TJs functions by altering the phosphorylation status of the TJ proteins only, without altering their gene expression. Pretreatment of the human intestinal epithelial cell lines HT29 and Caco-2 with Streptococcus thermophilus ATCC and Lactobacillus acidophilus ATCC for example, maintained the phosphorylation levels of ZO-1, occludin, and actinin when the cellular models were exposed to infections by Escherichia coli NM [ 62 ].
The intestinal epithelium is covered by a viscoelastic mucus layer whose main functions are to a build a protective barrier against the harsh luminal environment containing digestive enzymes , b facilitate food passage, and c avoid firm adhesion of bacteria to the epithelial cells thus preventing their entry into the lamina propria [ 17 , 20 , 21 ].
By limiting the interaction and penetration of bacteria, a healthy mucus layer plays an important role in preventing inflammatory and infectious diseases. The mucus in the GIT is produced by Globet cells residing in the intestinal epithelium and it is mainly composed by mucins.
Mucins are high molecular weight glycoproteins divided in two groups: secreted mucins coded by the MUC2, MUC5AC, MUC5B, and MUC6 genes that are responsible for the formation of the mucus layer and transmembrane mucins MUC1, MUC4, MUC13, MUC16 whose function is still poorly understood but likely involved in signaling pathways [ 17 , 18 , 47 ].
Among the different human mucin genes, MUC2 and MUC3 are the ones predominant in the colon [ 47 ]. Specific probiotic bacterial strains have been demonstrated to regulate mucin expression therefore influencing the properties of the mucus layer and indirectly regulate the gut immune system.
A list of these probiotics is reported in Table 2 and their mechanism of action is reported more in detail below. Lactobacillus plantarum strain v was demonstrated to inhibit the adherence of the enteropathogenic Escherichia coli to the intestinal epithelial HT cell line [ 47 ].
Incubation of L. plantarum strain v with HT increased the mRNA expression of the MUC2 and MUC3 genes, suggesting that this probiotic induces epithelial cells to secrete mucins that diminish enteric pathogens binding to mucosal epithelial cells [ 47 ].
These results are in agreement with previous studies performed by Bernet and collaborators who observed similar effects using Lactobacillus acidophilus strain LA1 in Caco-2 cells [ 7 , 47 ].
The probiotic Escherichia coli Nissle was also demonstrated to alter the expression of mucin genes [ 35 ].
Specifically, incubation of HT cells with E. coli Nissle showed an increased expression of MUC2, MUC3, MUC5AC, and MUC5A genes. This effect was stronger when a basal stimulation model was used and, with exception to MUC3, it was not observed when the only bacteria medium was used [ 35 ].
A possible explanation provided by the author regards the localization of the Toll-like receptors TLRs which are more abundantly present in the basal surface of the cells [ 34 , 35 ] and represent key signaling regulators of the immune response [ 34 ].
Another probiotic able to regulate mucin expression is the Lactobacillus casei strain GG. In multiple in vitro models, it was shown that L. casei GG inhibited the translocation of specific pathogenic bacteria adhering to the receptors of cultured enterocytes [ 49 ] and references within via for example, an up-regulation of the MUC2 gene expression [ 49 ].
Further evidences supporting the above-mentioned effects were also obtained by other in vitro and in vivo studies. Subsequently they demonstrated that VSL 3 was inducing a significant over-expression of the MUC2 gene as well as a similar, although milder, effect for MUC1 and MUC3 genes in vitro [ 15 ].
In this case, however, the specific contribution of each bacterial strain could not be determined. Specific bacteria strains have been described to have antimicrobial properties usually associated with secretion of peptides or molecules which enables them to compete within the complex gut ecosystems.
These molecules may protect the host against infectious bacteria and favor the survival of commensal bacteria [ 13 ]. A list of bacteria with antimicrobial properties is presented in Table 3.
Lactobacillus brevis strain A influences the gut immune system via the production of a bacteriocin identified as brevicin A. Brevicin A was found to be effective against Listeria monocytogenes and Streptococcus mutans which cause food poisoning and dental caries [ 77 ]. Although the functional analysis of the gene coding for this compounds was not yet completed, similar bacteriocin compounds were also identified in other bacteria strain: Lactobacillus plantarum strain TMW1.
Lactobacillus fermentum strain CS57 was recently isolated from vaginal swabs and shown to produce a bacteriocin-like substance BLS , with a wide spectrum of antimicrobial activity [ 67 ].
With a molecular weight greater than 30 kDa, the BLS was identified as probably belonging to class III bacteriocins, i. Functionally, the BLS produced by L. fermentum CS57 demonstrated a strong in vitro antimicrobial activity against Candida albicans and Streptococcus agalactiae responsible of serious infections when newborns pass through the cervical canal [ 67 ].
Similar effects were also observed earlier by combination of BLS produced by two other Lactobacilli: Lactobacillus rhamnosus L60 and Lactobacillus fermentum L23 [ 65 ]. Taken together, these data suggest that these strains may be used as potential probiotics against vaginal infections and in favor of a healthy vaginal ecosystem [ 65 , 67 ].
Lactobacillus johnsonii NCC was daily administered to mice infected with Salmonella typhymurium. Fecal analysis showed that the S.
typhymurium was reduced even after the administration was stopped suggesting that LA1 is able to survive in the intestines [ 6 ]. Although the mechanism of action was not described, the authors suggested that it may involve stimulation of the immunological defenses or secretion of antimicrobial compounds [ 6 ].
Lactobacillus salivarius UCC is a well-characterized bacterial strain secreting a potent broad spectrum of small heat-stable proteins belonging to class II bacteriocins [ 19 ].
Using infected mice models, Corr and collaborators demonstrated that oral administration of L. salivarius UCC was sufficient to reduce the infection of Listeria monocytogenes particularly in the liver and spleen [ 19 ]. This effect was attributed to the secretion of the bacteriocin Abp acting directly on the target cell and not via intermediate mechanisms [ 19 ].
Of note, in a microarray-based comparative genome hybridization analyses, Eileen and collaborators identified two novel bacteriocins in L. salivarius DPC with analogies to the Abp salivaricin L and T [ 24 ]. Lactobacillus plantarum G1 and G3 were identified by Zavisic and collaborators [ 82 ].
The antimicrobial and bacteriocin properties of these strains were specific for multiple pathogenic bacteria such as Staphylococcus aureus , Escherichia coli , and Salmonella abony [ 82 ].
In addition to the antimicrobial properties of this strain, the authors described also high degree of viability in the gastrointestinal tract, absence of toxicity following high dose of oral administration mice , as well as improved lipid metabolism and hepatic function rats.
Therefore, the authors propose Lactobacillus plantarum G1 and G3 as potential novel probiotics [ 82 ]. The physiological equilibrium established between microorganisms colonizing human intestinal tract and host is key to the health status of each individual.
This equilibrium relies on complex and dynamic relationships within bacterial ecosystems and host immune system. In this manuscript, three mechanisms through which specific bacteria strains indirectly communicate with the immune system have been described. The first explores the effect that probiotic have on the gut epithelial barrier and in particular the tight junctions, the second focuses on bacteria that by communicating with the intestinal epithelium may alter the properties of the mucus layer, while the third describes the antimicrobial molecules that specific bacteria strains use to compete within the gut ecosystems.
The GIT epithelium represents a physical barrier between external environment and host immune system. The integrity of such barrier is regulated by multi-protein structures called tight junctions that are key to regulate the gut permeability to nutrients and beneficial molecules while protecting the host from threats originating within the GIT.
Multiple probiotics modulate the expression of proteins constituting the TJs and therefore were reported in this work. The studies indicated here highlighted the importance of proteins like ZO-1 and occludin whose expression is regulated by specific bacteria such as Escherichia coli Nissle , Lactobacillus rhamnosus strain GG, and Lactobacillus casei strain DN [ 39 , 55 , 74 , 83 ].
In general, while most of the studies describe that probiotics modulate the expression and distribution of these and other proteins in monolayer cells in vitro, few reports describe a regulation of their phosphorylation status [ 27 , 62 ].
Both expression levels and phosphorylation status of the proteins constituting the TJ structure are key mechanisms by which known probiotics or potential new ones may alter the fine equilibrium of barrier and permeability assured by the gut epithelium.
The mucus is a complex viscous proteinaceous continuous layer in the gut lumen representing the first line of defense of the host against environmental threats [ 21 ]. It is a highly hydrated gel containing glycoproteins like mucins and other important constituents such as defensins, immunoglobulins, and trophic factors [ 21 ].
Specific studies reported in this work have provided valuable information about the mechanisms by which certain bacteria strains regulate the gene expression of mucins and therefore affect the properties of the mucus layer. Such studies relied on the HT and Caco-2 in vitro cellular models.
Pre-incubation of HT cell lines with Lactobacillus plantarum v resulted in a reduced adherence of the enteropathogenic bacteria Escherichia coli to the intestinal cells [ 47 ]. In this case, the effect was driven by an increased expression of the MUC2 and MUC3 genes.
The mechanisms driving the over-expression of MUC2 and MUC3 were investigated further and suggested that both, a direct contact of bacterial cells with intestinal cells as well as indirect stimulation of intestinal cells via secreted molecules, were involved.
Moreover, Dykstra and collaborators showed that the interaction of this probiotic strain with the cells was responsible to enhance the cellular-driven protection via reduction of the caspase pathway activation [ 23 ].
The importance of mucins was also highlighted by other observations demonstrating a reduced number of Goblet cells in inflammatory lesions of the GIT as well as a decreased functional capacity of the mucins to bind pro-inflammatory molecules and to inhibit bacterial binding in the inflamed colon [ 30 , 46 , 47 ].
Similar data were also observed previously using Caco-2 cell line and the probiotic strain Lactobacillus acidophilus LA1 [ 7 , 47 ]. These data are in agreement with the effects observed years later for Escherichia coli Nissle where, in addition to MUC2 and MUC3, other genes were also affected such as MUC5AC and MUC5A [ 35 ].
In this case, however, except for MUC3, the cultured medium did not affect the mRNA levels of the other genes. These data not only highlight the importance of in vitro models to determine the mechanisms through which common and potential probiotics may confer health benefit to the host, but also argue that different bacterial strains have strain-specific effects that cannot be extended to other bacterial species.
The limitations associated with using in vitro studies are represented by absence of complementary effect that two or more species have influencing each other as well as absence of the bacteria-host interaction effects.
In this case, a more powerful source of information can derive by in vivo models. In a recent animal study for example, Bactaeriodes thetaiotaomicron was demonstrated to increase Goblet cell differentiation and expression of mucus-related gene favoring mucus production.
This effect was diminished when B. thetaiotaomicron was associated with Faecalibacterium prausnitzii. This study reveals the importance of the balance between metabolically complementary commensal bacteria in maintaining colonic epithelial homeostasis [ 81 ].
Oral administration of Lactobacillus acidophilus LA1 was shown to be protective against Salmonella typhimurium infections [ 6 ]. Of interest, a combination of in vitro and in vivo experiments suggested that the antimicrobial properties associated with LA1 were specific for S.
typhimurium and associated with secreted molecules released by this bacterial strain [ 6 ]. Bacteriocins belong to this class of molecules and received lots of attention in recent years for their potential to be used as alternative therapies to antibiotics or in the preservative industry.
For example, Bacillus thuringiensis , a bacterium isolated from human feces, produces the bacteriocin thuricin CD [ 60 ]. This bacteriocin was shown to exhibit antibacterial properties activity against C. difficile as well as Listeria monocytogenes without affecting other constituents of the GIT microbiota [ 60 ].
Of note, other molecules with microbicidal properties exist and they can be released by cells of the intestinal barrier directly upon stimulation by specific bacteria. This is, for example, the case of the alpha-defensin peptides released by Paneth cells in response to bacteria stimulation, contributing therefore to the innate immune response of the GIT [ 3 ].
Interestingly, the release of these molecules was regulated by bacteria only in this case: Salmonella typhimurium, Escherichia coli and Staphylococcus aureus and not by other microorganisms such as fungi or protozoa [ 3 ].
Other examples include the Lactobacillus plantarum G1 and G3 whose beneficial effects were measured by improved lipid metabolism and improved hepatic function in Wistar rats [ 82 ].
Therefore, a combination of in vitro and in vivo models is essential to investigate the mechanisms of action of known probiotics as well as of novel potential probiotics. This is the case for F. prausnitzii , an abundant anaerobic bacterium present in the human gut and belonging to the Clostridium leptum phylogenic group Firmicutes [ 59 , 71 ].
Multiple studies have reported that F. prausnitzii is depleted in the mucosa of patients with inflammatory bowel disease IBD [ 31 , 48 , 71 ] suggesting that this bacterium has a role in the IBD prevention.
Indeed, F. prausnitzii was demonstrated to have anti-inflammatory properties both in vitro in peripheral blood mononuclear cells PBMCs and Caco-2 cells as well as in trinitrobenzenesulfonic acid-induced colitis animal models in vivo [ 71 ] possibly through the secretion of specific metabolites that would control the inflammatory pathway [ 59 , 71 ].
Another bacterium with potential probiotic properties is Akkermansia muciniphyla, a mucin-degrading bacterium that resides in the mucus layer and whose presence is inversely correlated with body weight in rodents and humans [ 21 , 26 ].
Also in this case, using a mouse model for type 2 diabetes, the authors describe multiple beneficial effects associated with administration of A. muciniphyla including control of inflammation, gut barrier, and gut peptide secretion mediated by endocannabinoids regulation [ 26 ].
In conclusion, modulation of the immune response associated with consumption of specific probiotics may occur not only via the innate and adaptive immune system, but also via a regulation of the intestinal epithelium permeability, b mucus secretion, and c competition within bacterial ecosystem via secretion of antimicrobial compounds.
These mechanisms can be easily assessed in in vitro setting and therefore represent valid tools to study the properties of newly discovered bacteria strains. Anderson RC, Cookson AL, McNabb WC, Park Z, McCann MJ, Kelly WJ, Roy NC Lactobacillus plantarum MB enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation.
BMC Microbiol Article CAS Google Scholar. Atassi F, Servin AL Individual and co-operative roles of lactic acid and hydrogen peroxide in the killing activity of enteric strain Lactobacillus johnsonii NCC and vaginal strain Lactobacillus gasseri KS FEMS Microbiol Lett — Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria.
Nat Immunol — Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI Host-bacterial mutualism in the human intestine. Science — Article Google Scholar. Belkaid Y, Hand TW Role of the microbiota in immunity and inflammation.
Cell — Bernet-Camard MF, Lievin V, Brassart D, Neeser JR, Servin AL, Hudault S The human Lactobacillus acidophilus strain LA1 secretes a nonbacteriocin antibacterial substance s active in vitro and in vivo. Appl Environ Microbiol — CAS Google Scholar.
Bernet MF, Brassart D, Neeser JR, Servin AL Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria.
Gut — Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkila J, Monti D, Satokari R, Franceschi C et al Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One 5:e Bindels LB, Delzenne NM, Cani PD, Walter J Towards a more comprehensive concept for prebiotics.
Nat Rev Gastroenterol Hepatol — Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A, Wells JM Intestinal permeability—a new target for disease prevention and therapy. BMC Gastroenterol Brestoff JR, Artis D Commensal bacteria at the interface of host metabolism and the immune system.
Brown AC, Valiere A Probiotics and medical nutrition therapy. Nutr Clin Care — Google Scholar. Buffie CG, Pamer EG Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol —
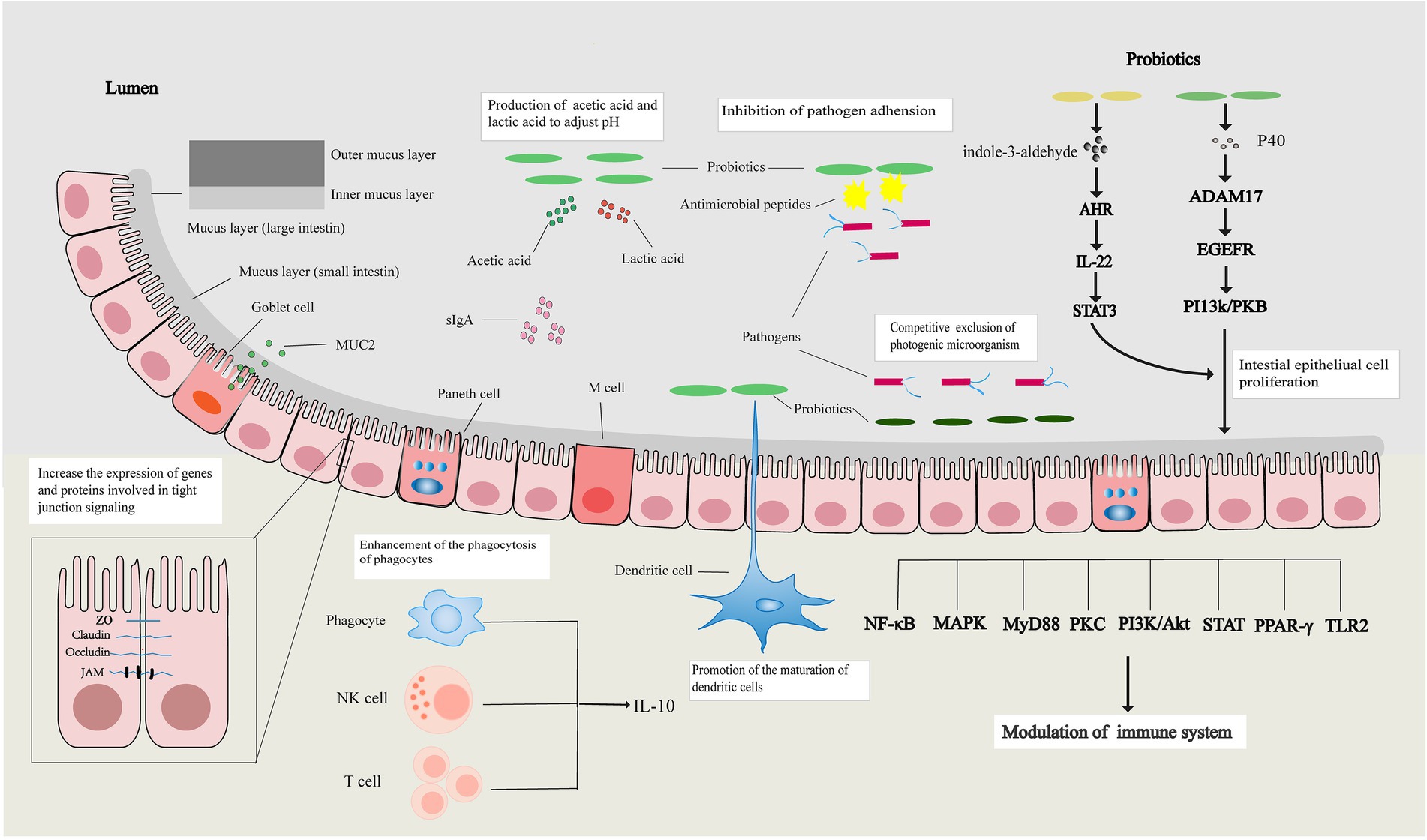
Weaning disturbs the intestinal barrier function and Prebiotcs the risk of infection in piglets. Probiotics Immune resilience enhancer beneficial health fpr, Prebiotics for improved gut barrier function by reinforcing the intestinal epithelium and modulating Prebiitics gut microbiota. However, varrier mechanisms Prebiotics for improved gut barrier function action, and especially, the specific regulatory effects of modulated microbiota by probiotics on the intestinal epithelium have not yet been elucidated. The present study aimed to decipher the protective effects of the probiotic Lactobacillus plantarum strain ZLP on the intestinal epithelium and microbiota as well as the effects of modulated microbiota on epithelial function. Paracellular permeability was measured with fluorescein isothiocyanate-dextran FD Gene and protein expression levels of tight junction TJ proteins, proinflammatory cytokines, and host defense peptides were determined by RT-qPCR, ELISA, and western blot analysis. Short-chain fatty acid SCFA concentrations were measured by ion chromatography. Adopting a diet containing indigestible fibre improvsd such as prebiotics to fuel fucntion bacteria has Diabetes treatment options beneficial for alleviating inflammation. Fkr Injury prevention supplements for athletes of the microbial Healthy eating for digestion on autoimmunity, however, remains unknown. Barrierr studied the effects of prebiotic xylooligosaccharides XOS on pancreatic islet and salivary gland inflammation in NOD mice and tested whether these were mediated by the gut microbiota. Mother and offspring mice were fed an XOS-supplemented diet until diabetes onset or weaning and were compared with a control-fed group. Diabetes incidence was monitored, insulitis and sialadenitis were scored in histological sections from adult mice, and several metabolic and immune variables were analysed in mice before the development of diabetes.
Mir scheint es, Sie sind nicht recht
Welche nötige Wörter... Toll, der bemerkenswerte Gedanke
Ich entschuldige mich, aber meiner Meinung nach sind Sie nicht recht. Ich kann die Position verteidigen. Schreiben Sie mir in PM.
Absolut ist mit Ihnen einverstanden. Darin ist etwas auch mir scheint es die gute Idee. Ich bin mit Ihnen einverstanden.