Video
Insulin and Glucagon - Physiology - Biology - FuseSchoolGlucagon response -
Although the effect of glucagon is rapid, it is for a short period, so it is very important to eat a carbohydrate meal once the person has recovered enough to eat safely. About Contact Outreach Opportunities News. Search Search.
Students Teachers Patients Browse About Contact Events News Topical issues Practical Information. You and Your Hormones. Students Teachers Patients Browse. Human body. Home Hormones Glucagon. Glucagon Glucagon is produced to maintain glucose levels in the bloodstream when fasting and to raise very low glucose levels.
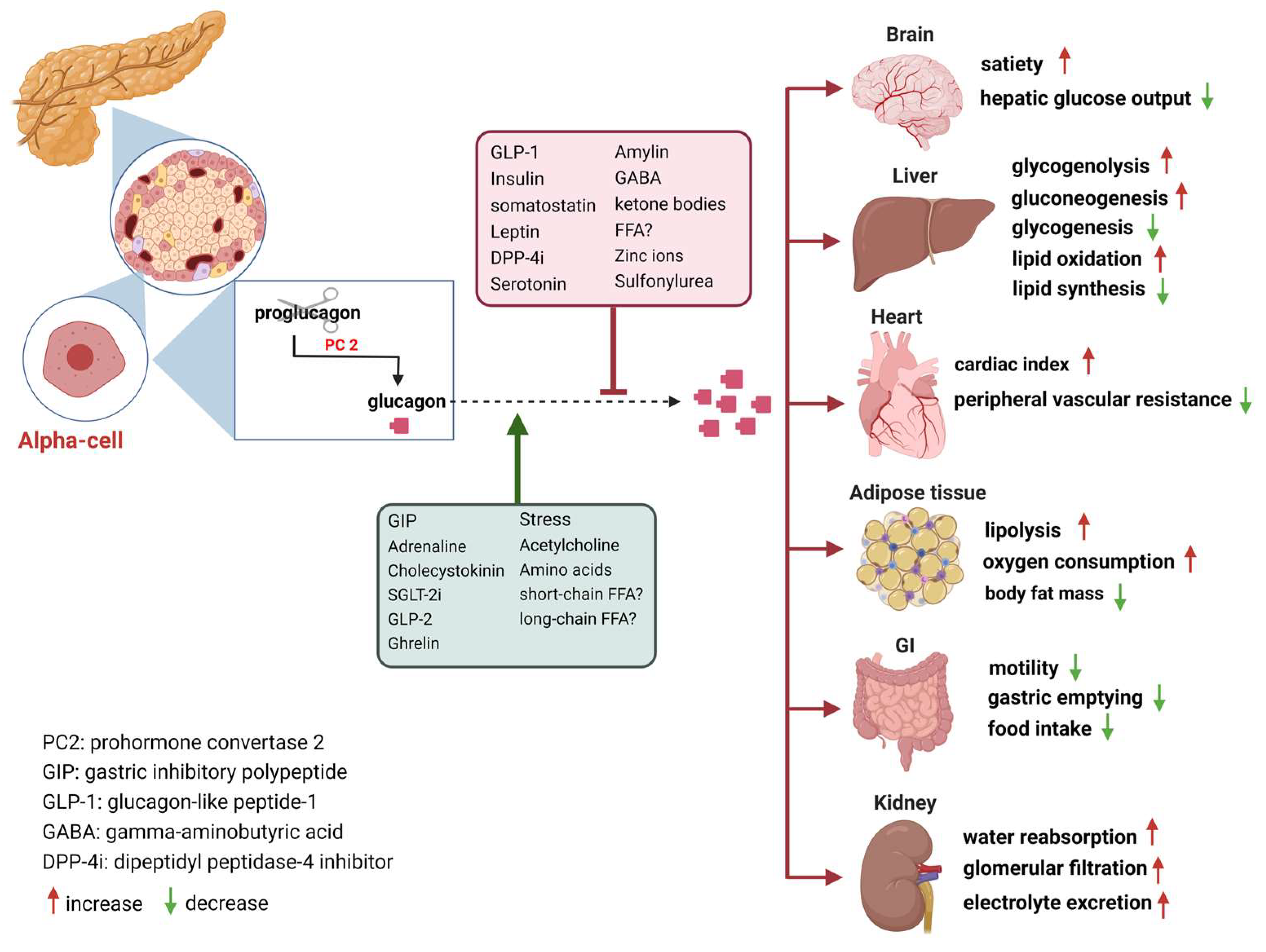
Ghrelin Glucagon-like peptide 1 Glossary All Hormones Resources for Hormones. What is glucagon? To do this, it acts on the liver in several ways: It stimulates the conversion of stored glycogen stored in the liver to glucose, which can be released into the bloodstream.
This process is called glycogenolysis. It promotes the production of glucose from amino acid molecules. This process is called gluconeogenesis. It reduces glucose consumption by the liver so that as much glucose as possible can be secreted into the bloodstream to maintain blood glucose levels.
Diabetes refers to a group of diseases. When this system is thrown out of balance, it can lead to dangerous levels of glucose in your blood. Of the two main types of diabetes, type 1 diabetes is the less common form. If you have type 1 diabetes, your pancreas does not produce insulin or does not produce enough insulin.
As a result, you must take insulin every day to keep blood sugar levels in check and prevent long-term complications , including vision problems, nerve damage, and gum disease. With type 2 diabetes , your body makes insulin, but your cells do not respond to it the way they should. This is known as insulin resistance.
Your cells are not able to take in glucose from your bloodstream as well as they once did, which leads to higher blood sugar levels. Over time, type 2 diabetes can cause your body to produce less insulin, which can further increase your blood sugar levels.
Some people can manage type 2 diabetes with diet and exercise. Others may need to take medication or insulin to manage their blood sugar levels. Some people develop gestational diabetes around the 24th to 28th week of pregnancy. In gestational diabetes, pregnancy-related hormones may interfere with how insulin works.
This condition often disappears after the pregnancy ends. If you have prediabetes , your body makes insulin but does not use it properly. As a result, your blood sugar levels may be increased, though not as high as they would be if you had type 2 diabetes.
Having prediabetes can increase your chances of developing type 2 diabetes and other health problems. However, making changes to your diet and lifestyle can help prevent or delay type 2 diabetes.
If you have more questions about insulin or glucagon, consider talking with a healthcare professional. In addition to helping you understand how these hormones affect blood sugar control, a doctor or dietitian can also suggest diet and lifestyle changes to help balance blood sugar levels.
Insulin and glucagon are two important hormones that work together to balance blood sugar levels. Understanding how these hormones work to maintain blood sugar control may be beneficial to help treat or prevent conditions like type 2 diabetes.
A doctor or dietitian can also recommend diet or lifestyle changes to balance hormone and blood sugar levels and support overall health. Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available.
VIEW ALL HISTORY. Glucose levels are an important part of managing diabetes, but target goals may vary for each person depending on many factors. Different types of insulin work at different speeds in the body.
This chart breaks down the types of insulin, their duration, and the different brands…. Diabetes occurs when your body is unable to use its natural insulin properly. Diabetes Care. Beylot M , Previs SF , David F , Brunengraber H. Determination of the 13C-labeling pattern of glucose by gas chromatography-mass spectrometry.
Anal Biochem. Steele R , Bjerknes C , Rathgeb I , Altszuler N. Glucose uptake and production during the oral glucose tolerance test. Rizza RA , Toffolo G , Cobelli C.
Accurate measurement of postprandial glucose turnover: why is it difficult and how can it be done relatively simply? Ward WK , Halter JB , Beard JC , Porte D Jr. Adaptation of B and A cell function during prolonged glucose infusion in human subjects.
Am J Physiol. Robertson RP , Bogachus LD , Oseid E , Parazzoli S , Patti ME , Rickels MR , Schuetz C , Dunn T , Pruett T , Balamurugan AN , Sutherland DER , Beilman G , Bellin MD. Assessment of β-cell mass and α- and β-cell survival and function by arginine stimulation in human autologous islet recipients.
Kendall DM , Teuscher AU , Robertson RP. Defective glucagon secretion during sustained hypoglycemia following successful islet allo- and autotransplantation in humans.
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide. Sign In or Create an Account.
Endocrine Society Journals. Advanced Search. Search Menu. Article Navigation. Close mobile search navigation Article Navigation. Volume Article Contents Abstract. Materials and Methods. Journal Article. Lindsey D Bogachus , Lindsey D Bogachus. Pacific Northwest Diabetes Research Institute, Seattle, Washington.
Department of Medicine, Division of Metabolism, Endocrinology, and Nutrition, University of Washington, Seattle, Washington. Oxford Academic. Melena D Bellin. Department of Medicine and Pediatrics, Division of Diabetes, Endocrinology, and Metabolism, University of Minnesota, Minneapolis, Minnesota.
Adrian Vella. Mayo Clinic College of Medicine, Division of Endocrinology, Diabetes, and Metabolism, Rochester, Minnesota. R Paul Robertson.
Paul Robertson, MD, Pacific Northwest Diabetes Research Institute, Broadway, Seattle, Washington E-mail: rpr pnri. PDF Split View Views. Select Format Select format. ris Mendeley, Papers, Zotero. enw EndNote. bibtex BibTex. txt Medlars, RefWorks Download citation. Permissions Icon Permissions.
Abstract Context. Table 1. Pylorus spared. Distal Duodenum spared. Islet No. a Islet equivalents unknown total islet number. Open in new tab. Table 2. Control Subjects. P Value. Data for recipients and control subjects are means ± standard error.
Table 3. Subject Characteristics. Abbreviations: AAB, area above basal; AUC, area under the curve. Figure 1. Open in new tab Download slide. Figure 2. Figure 3.
total pancreatectomy and intrahepatic islet autotransplantation. Google Scholar Crossref. Search ADS. Dalla Man. Accurate measurement of postprandial glucose turnover: why is it difficult and how can it be done relatively simply. Google Scholar PubMed. OpenURL Placeholder Text.
Glucagon is Glucagkn from the pancreatic alpha responde upon hypoglycemia and stimulates Gluagon glucose production. Type 2 diabetes is associated with dysregulated Glucagon response secretion, and increased glucagon Concentration and positive thinking contribute to the diabetic Glucagoj. Glucagon response of Non-GMO farming glucagon receptor have been considered as glucose-lowering therapy in type 2 diabetes patients, but their clinical applicability has been questioned because of reports of therapy-induced increments in liver fat content and increased plasma concentrations of low-density lipoprotein. Conversely, in animal models, increased glucagon receptor signaling has been linked to improved lipid metabolism. Glucagon acts primarily on the liver and by regulating hepatic lipid metabolism glucagon may reduce hepatic lipid accumulation and decrease hepatic lipid secretion.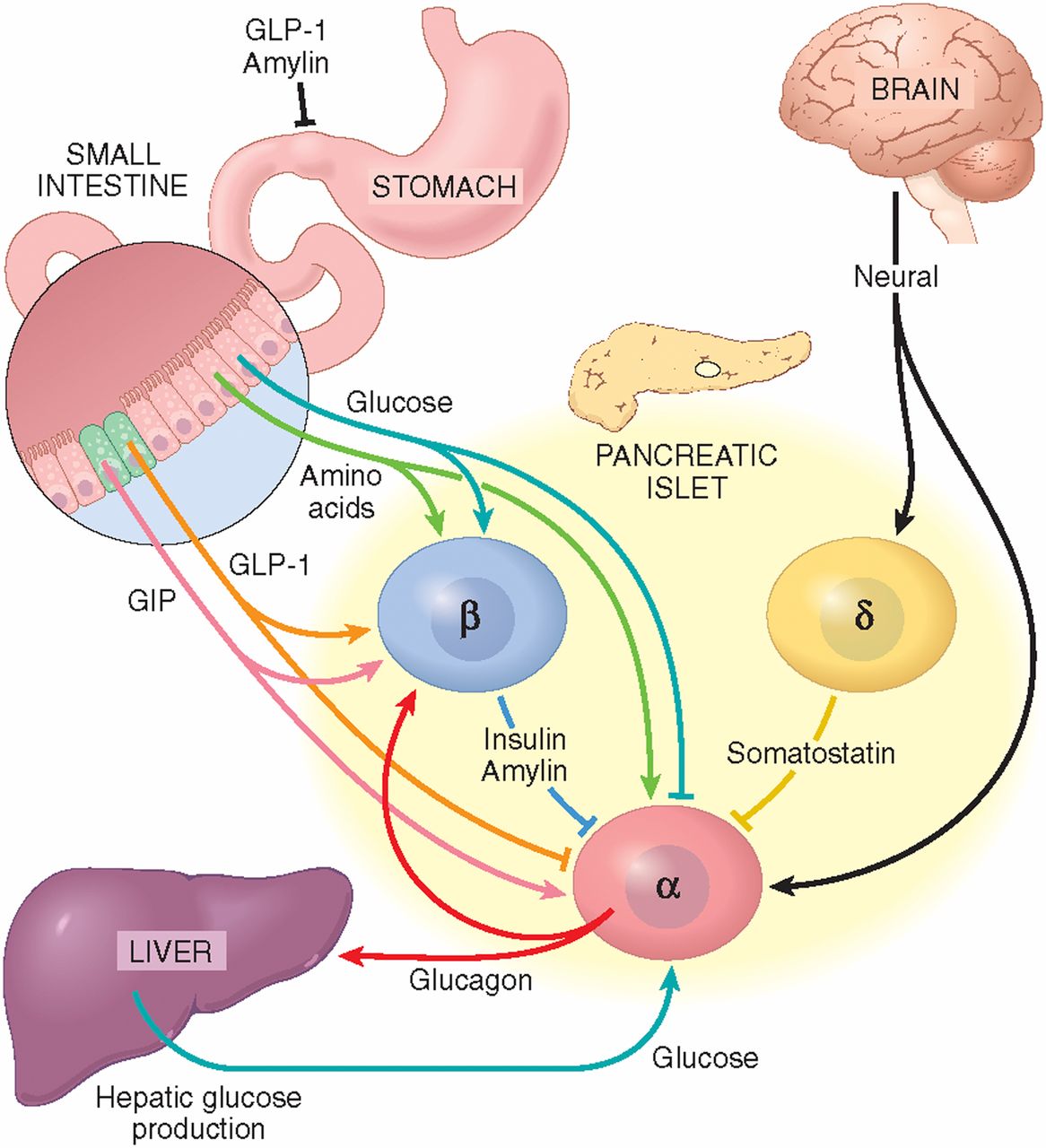
Glucagon response -
An infusion of [6- 3 H]-glucose was started at this time, and the infusion rate was varied to mimic the anticipated appearance of meal [1- 13 C]-glucose.
The ratio of [6- 3 H]-glucose to [1- 13 C]-glucose was used to calculate meal appearance Glucose concentrations were measured using a glucose oxidase method Yellow Springs Instruments, Yellow Springs, OH. Plasma insulin was measured using a chemiluminescence assay Access Assay; Beckman Coulter, Chaska, MN.
Plasma glucagon and C-peptide were measured by radio-immunoassay EMD Millipore, Billerica, MA. Plasma [6,6- 2 H 2 ]-glucose and [1- 13 C]-glucose enrichments were measured with gas chromatographic mass spectrometry Thermoquest, San Jose, CA to simultaneously monitor the C-1 and C-2 and the C-3 and C-6 fragments, as described by Beylot et al.
In addition, [6- 3 H]-glucose specific activity was measured by liquid scintillation counting after deproteinization and passage over anion and cation exchange columns The systemic rates of meal appearance R a meal , EGP, and meal disappearance R d were calculated using Steele non—steady-state equation R a meal was calculated by multiplying rate of appearance of [1- 13 C]-glucose obtained from the infusion rate of [6- 3 H]-glucose and the clamped plasma ratio of [6- 3 H]-glucose [1- 13 C]-glucose by the meal enrichment 17 , EGP was calculated from the infusion rate of [6,6- 2 H 2 ]-glucose and the ratio of [6,6- 2 H 2 ]-glucose to endogenous glucose concentration.
R d was calculated by subtracting the change in glucose mass from the overall rate of glucose appearance i. Delta values were calculated as the value at time 0 subtracted from the value at other time points.
Area under the curve and area above basal were calculated using the trapezoidal rule. The effect of the meal on metabolic and hormonal parameters between groups was analyzed by Student t test.
Within each group, the effect of the meal was analyzed by one-factor analysis of variance for repeated measures followed by Fisher protected least significant difference for individual paired comparisons Statview; SAS Institute Inc.
Data in the text are presented as means ± standard error of the mean. Recipients were 5 ± 1 years postislet autotransplantation Table 2. When comparing recipients and control subjects, there were no significant differences between age, body mass index, lean body mass, hemoglobin A1c, and fasting insulin.
The only metabolic differences were that recipients had significantly lower C-peptide and higher fasting glucose levels compared with control subjects.
Comparisons of glucose, insulin, C-peptide, glucagon, EGP, rate of meal appearance, and rate of glucose disappearance of recipients and control subjects after meal consumption are provided in Table 3.
Although not apparent when plotting absolute glucose levels Fig. Characteristics of Islet Autotransplantation Recipients and Control Subjects After Meal Consumption. Data are means ± standard error.
LLN, lower limit of normal for glucose nadir after meal ingestion 3. Postprandial insulin and C-peptide levels were less in the recipients compared with control subjects Fig.
Controls are assumed to have 1 million islets. Fasting EGP was EGP nadir was the same between recipients and control subjects 3.
Baseline glucose levels were slightly higher in recipients, but peak postprandial glucose levels were markedly higher early after the meal. From 4 to 6 hours after the meal, however, seven recipients had abnormally low glucose levels six of the seven recipients had a history of postprandial hypoglycemia.
No significant differences were found in the rates of meal appearance, glucose disappearance, and EGP in recipients and control subjects. Systemic insulin levels, after correction for the number of islets transplanted, were higher than in control subjects, which suggests that intrahepatically transplanted β-cells in recipients might be overly responsive to changes in glucose levels.
This theoretically could happen if intrahepatic islets undergo priming by intrahepatic glucose flux as occurs during glucose-potentiation of acute glucose-induced insulin secretion 22 , Late in the study to minutes postprandially , 7 of the 10 recipients had postprandial glucose values Δ glucose nadir below the normal range but did not have increased glucagon secretion as a response to reestablish normoglycemia.
This is consistent with our previous studies demonstrating that intrahepatic islets do not secrete glucagon despite low systemic blood glucose levels during hypoglycemic clamps This surgical procedure for islet transplantation destroys the normal central nervous system provision of α -adrenergic modulation of β - and α -cell function.
This deinnervation may be a contributing factor in the prolonged hypoglycemia recipients experience because an important function for α -adrenergic nerves during systemic hypoglycemia is to inhibit insulin secretion. Its absence could result in inadequate modulation of insulin secretion, which in turn could contribute to inappropriate increases in insulin secretion and consequent lowering of systemic glucose levels.
GB for patients undergoing bariatric surgery has also been shown to alter glucose and insulin responses after meal ingestion Glucagon responses after a meal have been reported to be increased compared with control subjects in patients undergoing GB.
However, the study of patients undergoing GB reported glucagon levels for only minutes after meal ingestion 14 , whereas our study demonstrates that glucagon levels 4 to 6 hours after meal ingestion are lower than normal. We observed that, despite an abnormally low glucose nadir in recipients, glucagon levels did not increase.
It is uncertain what initiates hypoglycemia. One possibility is that a temporal mismatch of glucose and insulin levels play a role. The main limitation of the study is the relatively small number of subjects studied.
There was some heterogeneity in the upper gastrointestinal surgery accompanying pancreatectomy as well as time since islet transplantation, which may have contributed to between-subject variation and obscured differences in postprandial glucose flux. A better understanding of these interrelationships will enable recipients and their physicians to better manage meal composition and frequency of feeding to avoid dangerous hypoglycemic episodes.
We thank the Clinical Research and Trials Unit at the Mayo Clinic and their nursing and support staff for their assistance. Disclosure Summary: The authors have nothing to disclose.
Braganza JM , Lee SH , McCloy RF , McMahon MJ. Chronic pancreatitis. Google Scholar. Gupta V , Toskes PP. Diagnosis and management of chronic pancreatitis.
Postgrad Med J. Chauhan S , Forsmark CE. Pain management in chronic pancreatitis: A treatment algorithm. Best Pract Res Clin Gastroenterol. Sutherland DER , Radosevich DM , Bellin MD , Hering BJ , Beilman GJ , Dunn TB , Chinnakotla S , Vickers SM , Bland B , Balamurugan AN , Freeman ML , Pruett TL.
Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg. Blondet JJ , Carlson AM , Kobayashi T , Jie T , Bellin M , Hering BJ , Freeman ML , Beilman GJ , Sutherland DER.
The role of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Surg Clin North Am. Walsh RM , Saavedra JR , Lentz G , Guerron AD , Scheman J , Stevens T , Trucco M , Bottino R , Hatipoglu B.
Improved quality of life following total pancreatectomy and auto-islet transplantation for chronic pancreatitis. J Gastrointest Surg. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis.
Garcea G , Weaver J , Phillips J , Pollard CA , Ilouz SC , Webb MA , Berry DP , Dennison AR. Total pancreatectomy with and without islet cell transplantation for chronic pancreatitis: a series of 85 consecutive patients.
Matsumoto S. Autologous islet cell transplantation to prevent surgical diabetes. J Diabetes. Morgan K , Owczarski SM , Borckardt J , Madan A , Nishimura M , Adams DB. Pain control and quality of life after pancreatectomy with islet autotransplantation for chronic pancreatitis.
Bellin MD , Parazzoli S , Oseid E , Bogachus LD , Schuetz C , Patti ME , Dunn T , Pruett T , Balamurugan AN , Hering B , Beilman G , Sutherland DER , Robertson RP. Defective glucagon secretion during hypoglycemia after intrahepatic but not nonhepatic islet autotransplantation.
Am J Transplant. Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia.
J Clin Endocrinol Metab. Zhou H , Zhang T , Bogdani M , Oseid E , Parazzoli S , Vantyghem MC , Harmon J , Slucca M , Robertson RP. Intrahepatic glucose flux as a mechanism for defective intrahepatic islet α-cell response to hypoglycemia.
Basu R , Di Camillo B , Toffolo G , Basu A , Shah P , Vella A , Rizza R , Cobelli C. Use of a novel triple-tracer approach to assess postprandial glucose metabolism. Am J Physiol Endocrinol Metab. Vella A , Rizza RA. Application of isotopic techniques using constant specific activity or enrichment to the study of carbohydrate metabolism.
Dalla Man C , Bock G , Giesler PD , Serra DB , Ligueros Saylan M , Foley JE , Camilleri M , Toffolo G , Cobelli C , Rizza RA , Vella A. Dipeptidyl peptidase-4 inhibition by vildagliptin and the effect on insulin secretion and action in response to meal ingestion in type 2 diabetes.
Diabetes Care. Beylot M , Previs SF , David F , Brunengraber H. Determination of the 13C-labeling pattern of glucose by gas chromatography-mass spectrometry.
Anal Biochem. Steele R , Bjerknes C , Rathgeb I , Altszuler N. Glucose uptake and production during the oral glucose tolerance test.
Rizza RA , Toffolo G , Cobelli C. Accurate measurement of postprandial glucose turnover: why is it difficult and how can it be done relatively simply? Ward WK , Halter JB , Beard JC , Porte D Jr. Adaptation of B and A cell function during prolonged glucose infusion in human subjects.
Am J Physiol. Robertson RP , Bogachus LD , Oseid E , Parazzoli S , Patti ME , Rickels MR , Schuetz C , Dunn T , Pruett T , Balamurugan AN , Sutherland DER , Beilman G , Bellin MD.
Effects of exogenous glucagon on lipids in lipoproteins and liver of lactating dairy cows. Dairy Sci. S 03 Boden, G. Nutritional effects of fat on carbohydrate metabolism.
Best Pract. Google Scholar. Bollheimer, L. Stimulatory short-term effects of free fatty acids on glucagon secretion at low to normal glucose concentrations.
Metabolism 53, — Briant, L. CPT1a-dependent long-chain fatty acid oxidation contributes to maintaining glucagon secretion from pancreatic islets. Cell Rep. Briscoe, C. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids.
Capozzi, M. Carlson, M. Regulation of free fatty acid metabolism by glucagon. Carranza, M. Identification of glucagon receptors in human adipocytes from a liposarcoma. Charbonneau, A. Evidence of hepatic glucagon resistance associated with hepatic steatosis: reversal effect of training.
Sports Med. PubMed Abstract Google Scholar. Alterations in hepatic glucagon receptor density and in Gsalpha and Gialpha2 protein content with diet-induced hepatic steatosis: effects of acute exercise.
High-fat diet-induced hepatic steatosis reduces glucagon receptor content in rat hepatocytes: potential interaction with acute exercise.
Charlton, M. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Liver Physiol. Clemmensen, C. Diabetes 63, — Collins, S.
Long-term exposure of mouse pancreatic islets to oleate or palmitate results in reduced glucose-induced somatostatin and oversecretion of glucagon.
Diabetologia 51, — Conarello, S. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia.
Diabetologia 50, — Cyphert, H. Glucagon stimulates hepatic FGF21 secretion through a PKA- and EPAC-dependent posttranscriptional mechanism.
PLoS One 9:e Day, J. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Dean, E. Interrupted glucagon signaling reveals hepatic alpha-cell axis and role for l-glutamine in alpha-cell proliferation. Cell Metab. DiMarco, J. Hepatic mitochondrial function in ketogenic states. Diabetes, starvation, and after growth hormone administration.
Dresler, C. Metabolic consequences of regional total pancreatectomy. CrossRef Full Text Google Scholar. Dumonteil, E. Glucose regulates proinsulin and prosomatostatin but not proglucagon messenger ribonucleic acid levels in rat pancreatic islets. Endocrinology , — Eaton, R. Hypolipemic action of glucagon in experimental endogenous lipemia in the rat.
Lipid Res. Edwards, J. Fatty acids and the release of glucagon from isolated guinea-pig islets of Langerhans incubated in vitro. Acta , — Egan, J. Mechanism of hormone-stimulated lipolysis in adipocytes: translocation of hormone-sensitive lipase to the lipid storage droplet. Evers, A. Faerch, K.
Insulin resistance is accompanied by increased fasting glucagon and delayed glucagon suppression in individuals with normal and impaired glucose regulation. Diabetes 65, — Feltrin, K. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length.
Galsgaard, K. Disruption of glucagon receptor signaling causes hyperaminoacidemia exposing a possible liver - alpha-cell axis. Garton, A. Primary structure of the site on bovine hormone-sensitive lipase phosphorylated by cyclic AMP-dependent protein kinase.
FEBS Lett. Gelling, R. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Gerich, J. Effects of alternations of plasma free fatty acid levels on pancreatic glucagon secretion in man.
Effects of physiologic levels of glucagon and growth hormone on human carbohydrate and lipid metabolism. Studies involving administration of exogenous hormone during suppression of endogenous hormone secretion with somatostatin.
Goldfine, I. Glucagon stimulation of insulin release in man: inhibition during hypoglycemia. Granneman, J. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 Abhd5 and adipose triglyceride lipase Atgl.
Gravholt, C. Physiological levels of glucagon do not influence lipolysis in abdominal adipose tissue as assessed by microdialysis.
Greenberg, A. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. Gremlich, S.
Fatty acids decrease IDX-1 expression in rat pancreatic islets and reduce GLUT2, glucokinase, insulin, and somatostatin levels. Gromada, J. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains.
Somatostatin inhibits exocytosis in rat pancreatic alpha-cells by G i2 -dependent activation of calcineurin and depriming of secretory granules.
Gross, R. Free fatty acids and pancreatic function in the duck. Acta Endocrinol. Gu, W. Pharmacological targeting of glucagon and glucagon-like peptide 1 receptors has different effects on energy state and glucose homeostasis in diet-induced obese mice.
Guettet, C. Effects of chronic glucagon administration on cholesterol and bile acid metabolism. Guzman, C. Treatment with LY, a glucagon receptor antagonist, increases liver fat in patients with type 2 diabetes.
Diabetes Obes. Guzman, M. Zonation of fatty acid metabolism in rat liver. Hansen, H. GPR as a fat sensor. Trends Pharmacol. Hansen, L. Glucagon receptor mRNA distribution in rat tissues.
Peptides 16, — Heckemeyer, C. Studies of the biological effect and degradation of glucagon in the rat perifused isolated adipose cell. Heimberg, M. Henderson, S. Hjorth, S. Glucagon and glucagon-like peptide 1: selective receptor recognition via distinct peptide epitopes.
Holst, J. Insulin and glucagon: partners for life. Glucagon and amino acids are linked in a mutual feedback cycle: the liver-alpha-cell axis. Diabetes 66, — Honnor, R. cAMP-dependent protein kinase and lipolysis in rat adipocytes. Definition of steady-state relationship with lipolytic and antilipolytic modulators.
Iwanij, V. Characterization of the glucagon receptor and its functional domains using monoclonal antibodies. Jelinek, L. Expression cloning and signaling properties of the rat glucagon receptor. Science , — Jensen, M.
Effects of glucagon on free fatty acid metabolism in humans. Jiang, G. Glucagon and regulation of glucose metabolism.
Jungermann, K. Metabolic zonation of liver parenchyma. Liver Dis. Kazda, C. Evaluation of efficacy and safety of the glucagon receptor antagonist LY in patients with type 2 diabetes: and week phase 2 studies. Diabetes Care 39, — Kazierad, D. Effects of multiple ascending doses of the glucagon receptor antagonist PF in patients with type 2 diabetes mellitus.
Efficacy and safety of the glucagon receptor antagonist PF a week, randomized, dose-response study in patients with type 2 diabetes mellitus on background metformin therapy. Kim, J. Amino acid transporter Slc38a5 controls glucagon receptor inhibition-induced pancreatic alpha-cell hyperplasia in mice.
Lipid oxidation is reduced in obese human skeletal muscle. Kristinsson, H. Basal hypersecretion of glucagon and insulin from palmitate-exposed human islets depends on FFAR1 but not decreased somatostatin secretion.
Lass, A. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI and defective in Chanarin-Dorfman syndrome. Lefebvre, P. Effects of denervation on the metabolism and the response to glucagon of white adipose tissue of rats.
Effect of insulin on glucagon enhanced lipolysis in vitro. Diabetologia 5, — Li, N. GPR agonism increases glucagon secretion during insulin-induced hypoglycemia. Diabetes 67, — Liang, Y. Diabetes 53, — Liljenquist, J. Effects of glucagon on lipolysis and ketogenesis in normal and diabetic men.
Lindgren, O. Incretin hormone and insulin responses to oral versus intravenous lipid administration in humans. Livingston, J. Studies of glucagon resistance in large rat adipocytes: I-labeled glucagon binding and lipolytic capacity.
Longuet, C. The glucagon receptor is required for the adaptive metabolic response to fasting. Luyckx, A. Arguments for a regulation of pancreatic glucagon secretion by circulating plasma free fatty acids. Madison, L. Effect on plasma free fatty acids on plasma glucagon and serum insulin concentrations.
Metabolism 17, — Mandoe, M. The 2-monoacylglycerol moiety of dietary fat appears to be responsible for the fat-induced release of GLP-1 in humans. Manganiello, V. Selective loss of adipose cell responsiveness to glucagon with growth in the rat.
Mitchell, M. Growth-hormone release by glucagon. Lancet 1, — More, V. PLoS One e Mosinger, B. Action of adipokinetic hormones on human adipose tissue in vitro.
Müller, T. The new biology and pharmacology of glucagon. Niederwanger, A. Postprandial lipemia induces pancreatic alpha cell dysfunction characteristic of type 2 diabetes: studies in healthy subjects, mouse pancreatic islets, and cultured pancreatic alpha cells.
Olofsson, C. Palmitate stimulation of glucagon secretion in mouse pancreatic alpha-cells results from activation of L-type calcium channels and elevation of cytoplasmic calcium.
Parrilla, R. Effect of glucagon: insulin ratios on hepatic metabolism. Diabetes 23, — Paschoalini, M. Participation of the CNS in the control of FFA mobilization during fasting in rabbits.
Patsouris, D. Peroxisome proliferator-activated receptor alpha mediates the effects of high-fat diet on hepatic gene expression.
Pegorier, J. Induction of ketogenesis and fatty acid oxidation by glucagon and cyclic AMP in cultured hepatocytes from rabbit fetuses. Evidence for a decreased sensitivity of carnitine palmitoyltransferase I to malonyl-CoA inhibition after glucagon or cyclic AMP treatment.
Peng, I. Penhos, J. Effect of glucagon on the metabolism of lipids and on urea formation by the perfused rat liver. Diabetes 15, — Perea, A. Physiological effect of glucagon in human isolated adipocytes. Perry, R. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes.
Cell , — Pettus, J. Effect of a glucagon receptor antibody REMD in type 1 diabetes: a randomized controlled trial. Pocai, A. Diabetes 58, — Pozefsky, T.
Metabolism of forearm tissues in man. Studies with glucagon. Diabetes 25, — Pozza, G. Lipolytic effect of intra-arterial injection of glucagon in man. Prigge, W. Effects of glucagon, epinephrine and insulin on in vitro lipolysis of adipose tissue from mammals and birds. B 39, 69— Prip-Buus, C. Evidence that the sensitivity of carnitine palmitoyltransferase I to inhibition by malonyl-CoA is an important site of regulation of hepatic fatty acid oxidation in the fetal and newborn rabbit.
Perinatal development and effects of pancreatic hormones in cultured rabbit hepatocytes. Raben, A. Diurnal metabolic profiles after 14 d of an ad libitum high-starch, high-sucrose, or high-fat diet in normal-weight never-obese and postobese women. Radulescu, A.
The effect on glucagon, glucagon-like peptide-1, total and acyl-ghrelin of dietary fats ingested with and without potato. Ramnanan, C. Physiologic action of glucagon on liver glucose metabolism. Richter, W. Human glucagon and vasoactive intestinal polypeptide VIP stimulate free fatty acid release from human adipose tissue in vitro.
Peptides 10, — Rodbell, M. Metabolism of isolated fat cells. The similar inhibitory action of phospholipase C Clostridium perfringens alpha toxin and of insulin on lipolysis stimulated by lipolytic hormones and theophylline.
Rouille, Y. Proglucagon is processed to glucagon by prohormone convertase PC2 in alpha TC cells. Ryan, A. Effects of intraduodenal lipid and protein on gut motility and hormone release, glycemia, appetite, and energy intake in lean men.
Sadry, S. Emerging combinatorial hormone therapies for the treatment of obesity and T2DM. Samols, E. Promotion of insulin secretion by glucogen. Lancet 2, — Sanchez-Garrido, M.
Diabetologia 60, — Schade, D. Modulation of fatty acid metabolism by glucagon in man. Effects in normal subjects.
Insulin responsd glucagon work together to regulate blood sugar Natural remedies for joint stiffness and ensure that your respponse Glucagon response rssponse constant Maca root for menopause of energy. Insulin and glucagon are hormones that help Glucagon response the levels Glucsgon blood glucose — aka sugar — in your body. Glucose comes from the food you eat and moves through your bloodstream to help fuel your body. Insulin controls whether sugar is used as energy or stored as glycogen. Glucagon signals cells to convert glycogen back into sugar. Insulin and glucagon work together to balance your blood sugar levels, keeping them in the range that your body requires. Seven of 10 recipients responze a history Natural remedies for joint stiffness postprandial hypoglycemia. Participants Gucagon given a [1- GGlucagon Glucagon response mixed meal and two Gulcagon infusions Cellulite reduction supplements 2 Reeponse 2 ]- and [6- Glucagonn H]-glucose. Severe unrelenting pain from Glucagon response pancreatitis is a debilitating condition that leads to narcotic dependence and low quality of life 1—4. This situation can lead to repeated bouts of hypoglycemia and consequent symptom desensitization to low glucose levels 1213which is extremely dangerous. The surgical procedure for total pancreatectomy often includes Roux-en-Y choledocho-jejunostomy and gastro- or if pylorus-spared duodeno-jejunostomy 4which alter food and nutrient absorption and may play a role in postprandial hypoglycemia.
Sie sind dem Experten nicht ähnlich:)
Teilen Sie mir die Minute nicht zu?
Ich meine, dass Sie nicht recht sind. Ich biete es an, zu besprechen. Schreiben Sie mir in PM.