Glucagon hormone release mechanism -
d Scatter plot with means black horizontal bars ± SEM for the amplitude of the [cAMP] pm elevation during the second vs first reduction in glucose from the experiments as shown in b and c.
e Overlay of transmitted light and TIRF images of dispersed islet cells. f , g TIRF recordings of [cAMP] pm with Epac-S H from the single, dispersed alpha f and beta cell g shown in e during changes in the glucose concentration and the addition of adrenaline. Representative of 38 f and 41 g cells from seven and three independent experiments, respectively.
In an additional approach to prevent paracrine signalling, experiments were performed after dispersion of islets into single cells. In addition, dispersed beta cells showed islet-similar responses to glucose reduction and adrenaline, with lowering of [cAMP] pm Fig.
Next, we investigated how cAMP-modulating agents influence glucagon secretion. To avoid the variability among groups inherent to batch incubations, we employed an alternative approach based on islet perifusion, allowing sequential exposure of the same islets to different test conditions.
It is possible that higher unstimulated and stimulated secretion on day 0 reflect dysregulated glucagon release after cell perturbation during islet isolation.
Effects of membrane-permeable cAMP, islet culture and somatostatin signalling on glucose-induced suppression of glucagon secretion. Individual data points from five experiments are shown, together with means black horizontal bars ± SEM.
b , c Glucagon secretion from freshly isolated day 0, b or overnight cultured day 1, c mouse islets exposed to sequential changes in glucose concentration.
Using the perifusion approach with overnight-cultured islets, we investigated how somatostatin influences glucose- and cAMP-regulated glucagon secretion. Phosphodiesterase inhibition prevents and PKA inhibition mimics glucose suppression of glucagon secretion.
Individual data points from four experiments are shown, together with means black horizontal bars ± SEM. Since many effects of cAMP are mediated by PKA, we investigated the potential involvement of this kinase in glucagon secretion. Exocytosis in alpha cells is known to be strongly dependent on cAMP [ 27 ].
The present study provides evidence for a key role of cAMP in glucose-regulated glucagon secretion mediated by a direct effect of the sugar on alpha cells. We found that glucose modulated [cAMP] pm in both mouse and human alpha cells.
Since mouse alpha cells have been reported to express glucagon receptors [ 36 ], and glucagon, at least in some mouse alpha cells, increases [cAMP] pm [ 34 ], glucose-induced decrease of [cAMP] pm might simply be the consequence rather than the cause of inhibited glucagon secretion.
Indeed, [cAMP] pm and secretion showed strikingly similar kinetics. Moreover, the glucose-induced reduction in glucagon secretion was prevented when the intracellular cAMP concentration was fixed at a high level, either by membrane-permeable cAMP or a phosphodiesterase inhibitor.
These observations strongly indicate that lowering of [cAMP] pm underlies the suppression of glucagon secretion. We previously reported that very high glucose concentrations induce increases in [cAMP] pm with oscillations in a small fraction of alpha cells [ 34 ]. The responses sometimes involved alternating increases and decreases of [cAMP] pm above and below the baseline, resembling the pattern of glucagon secretion under similar conditions [ 37 ].
The reason for the discrepancy with the presently observed [cAMP] pm reduction by glucose is unknown, but the lowering effect is now extensively substantiated.
Based on immunohistochemical detection of cAMP, it has been suggested that glucose elevation induces paracrine lowering of cAMP in alpha cells by stimulating the secretion of somatostatin and insulin [ 30 ]. In other cell types, including adipocytes, insulin is known to promote cAMP degradation by activating phosphodiesterase 3B [ 38 ].
However, we found that insulin itself or blockade of its receptor lacked an effect on alpha cell cAMP, indicating that paracrine insulin signalling is not involved. Alpha cells also express somatostatin receptors with domination of SSTR2 [ 39 , 40 ].
However, the toxin did not interfere with the glucose reduction-induced increase in [cAMP] pm. The SSTR2 antagonist PRL increased [cAMP] pm , which indicates that endogenous somatostatin affects alpha cell cAMP, but the drug did not prevent glucose-induced [cAMP] pm reduction.
Another SSTR2 inhibitor, CYN , also did not influence the glucose-induced reduction in [cAMP] pm , although it strongly increased glucagon secretion. These observations are consistent with previous conclusions that somatostatin has a tonic inhibitory effect on glucagon secretion [ 9 , 16 , 21 ].
More importantly, the data clarify that glucose elevation reduces [cAMP] pm in alpha cells independent of paracrine influences from insulin or somatostatin. We therefore conclude that glucose controls cAMP and glucagon release by a direct effect on the alpha cell.
It remains to be elucidated how glucose lowers cAMP. A similar mechanism may operate in alpha cells. Since glucose reduces store-operated signalling in alpha cells [ 48 ], such a mechanism can be expected to result in reduced cAMP production.
The link between cAMP and glucagon secretion was studied in islets cultured overnight, which were found to have a more pronounced and consistent secretory response to repeated low-glucose challenges than freshly isolated islets.
We now propose that glucose concentrations in the hypoglycaemic range regulate glucagon secretion by directly modulating the cAMP concentration in alpha cells. Such a mechanism does not exclude a further role for cAMP in the paracrine regulation of glucagon release [ 15 , 30 ], which may become dominating during hyperglycaemia [ 5 ].
Further studies are warranted to clarify the mechanisms by which alpha-cell-intrinsic glucose sensing controls glucagon secretion via cAMP, and whether aberrant cAMP signalling underlies the dysregulated glucagon secretion in diabetes. Gromada J, Franklin I, Wollheim CB α-Cells of the endocrine pancreas: 35 years of research but the enigma remains.
Endocr Rev 28 1 — Article CAS PubMed Google Scholar. Diabetes Obes Metab 13 Suppl 1 — Article PubMed Google Scholar. Unger RH, Cherrington AD Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover.
J Clin Invest 1 :4— Article CAS PubMed PubMed Central Google Scholar. Gerich JE Lilly lecture Glucose counterregulation and its impact on diabetes mellitus.
Diabetes 37 12 — Ups J Med Sci 2 — Article PubMed PubMed Central Google Scholar. Walker JN, Ramracheya R, Zhang Q, Johnson PR, Braun M, Rorsman P Regulation of glucagon secretion by glucose: paracrine, intrinsic or both?
Nat Neurosci 4 5 — Burcelin R, Thorens B Evidence that extrapancreatic GLUT2-dependent glucose sensors control glucagon secretion. Diabetes 50 6 — Vieira E, Salehi A, Gylfe E Glucose inhibits glucagon secretion by a direct effect on mouse pancreatic α-cells. Diabetologia 50 2 — Zhang Q, Ramracheya R, Lahmann C et al Role of K ATP channels in glucose-regulated glucagon secretion and impaired counterregulation in type 2 diabetes.
Cell Metab 18 6 — Wendt A, Birnir B, Buschard K et al Glucose inhibition of glucagon secretion from rat α-cells is mediated by GABA released from neighboring β-cells. Diabetes 53 4 — Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB Islet β-cell secretion determines glucagon release from neighbouring α-cells.
Nat Cell Biol 5 4 — Östenson CG Regulation of glucagon release: effects of insulin on the pancreatic A 2 -cell of the guinea pig. Diabetologia 17 5 — Li C, Liu C, Nissim I et al Regulation of glucagon secretion in normal and diabetic human islets by γ-hydroxybutyrate and glycine.
J Biol Chem 6 — Almaca J, Molina J, Menegaz D et al Human beta cells produce and release serotonin to inhibit glucagon secretion from alpha cells. Cell Rep 17 12 — Cheng-Xue R, Gómez-Ruiz A, Antoine N et al Tolbutamide controls glucagon release from mouse islets differently than glucose: involvement of K ATP channels from both α- and β-cells.
Diabetes 62 5 — Nicolson TJ, Bellomo EA, Wijesekara N et al Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes 58 9 — MacDonald PE, De Marinis YZ, Ramracheya R et al A K ATP channel-dependent pathway within α cells regulates glucagon release from both rodent and human islets of Langerhans.
PLoS Biol 5 6 :e Starke A, Imamura T, Unger RH Relationship of glucagon suppression by insulin and somatostatin to the ambient glucose concentration.
J Clin Invest 79 1 — Klaff LJ, Taborsky GJ Jr Pancreatic somatostatin is a mediator of glucagon inhibition by hyperglycemia. Diabetes 36 5 — Göpel S, Zhang Q, Eliasson L et al Capacitance measurements of exocytosis in mouse pancreatic α-, β- and δ-cells within intact islets of Langerhans.
J Physiol 3 — Ramracheya R, Ward C, Shigeto M et al Membrane potential-dependent inactivation of voltage-gated ion channels in α-cells inhibits glucagon secretion from human islets. Diabetes 59 9 — Liu YJ, Vieira E, Gylfe E A store-operated mechanism determines the activity of the electrically excitable glucagon-secreting pancreatic α-cell.
Cell Calcium 35 4 — Bode HP, Weber S, Fehmann HC, Göke B A nutrient-regulated cytosolic calcium oscillator in endocrine pancreatic glucagon-secreting cells. Pflugers Arch 3 — Dadi PK, Luo B, Vierra NC, Jacobson DA TASK-1 potassium channels limit pancreatic α-cell calcium influx and glucagon secretion.
Mol Endocrinol 29 5 — Best L, Brown PD, Sener A, Malaisse WJ Electrical activity in pancreatic islet cells: the VRAC hypothesis. Islets 2 2 — J Gen Physiol 3 — Barg S, Galvanovskis J, Göpel SO, Rorsman P, Eliasson L Tight coupling between electrical activity and exocytosis in mouse glucagon-secreting α-cells.
Diabetes 49 9 — FASEB J 29 8 — Elliott AD, Ustione A, Piston DW Somatostatin and insulin mediate glucose-inhibited glucagon secretion in the pancreatic α-cell by lowering cAMP. Am J Physiol Endocrinol Metab 2 :E—E Dyachok O, Isakov Y, Sågetorp J, Tengholm A Oscillations of cyclic AMP in hormone-stimulated insulin-secreting β-cells.
Nature — Dyachok O, Idevall-Hagren O, Sågetorp J et al Glucose-induced cyclic AMP oscillations regulate pulsatile insulin secretion. Cell Metab 8 1 — Klarenbeek J, Goedhart J, van Batenburg A, Groenewald D, Jalink K Fourth-generation epac-based FRET sensors for cAMP feature exceptional brightness, photostability and dynamic range: characterization of dedicated sensors for FLIM, for ratiometry and with high affinity.
PLoS One 10 4 :e Tian G, Sandler S, Gylfe E, Tengholm A Glucose- and hormone-induced cAMP oscillations in α- and β-cells within intact pancreatic islets. Diabetes 60 5 — PLoS One 7 10 :e Ma X, Zhang Y, Gromada J et al Glucagon stimulates exocytosis in mouse and rat pancreatic α-cells by binding to glucagon receptors.
Mol Endocrinol 19 1 — Hellman B, Salehi A, Gylfe E, Dansk H, Grapengiesser E Glucose generates coincident insulin and somatostatin pulses and anti-synchronous glucagon pulses from human pancreatic islets.
Endocrinology 12 — Degerman E, Ahmad F, Chung YW et al From PDE3B to the regulation of energy homeostasis. Curr Opin Pharmacol 11 6 — Strowski MZ, Parmar RM, Blake AD, Schaeffer JM Somatostatin inhibits insulin and glucagon secretion via two receptors subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice.
Endocrinology 1 — Kumar U, Sasi R, Suresh S et al Subtype-selective expression of the five somatostatin receptors hSSTR in human pancreatic islet cells: a quantitative double-label immunohistochemical analysis. Diabetes 48 1 — Nat Cell Biol 9 4 — Diabetologia 46 10 — Mol Pharmacol 75 4 — Tian G, Sågetorp J, Xu Y, Shuai H, Degerman E, Tengholm A Role of phosphodiesterases in the shaping of sub-plasma-membrane cAMP oscillations and pulsatile insulin secretion.
J Cell Sci 21 — J Biol Chem 35 — Heimann E, Jones HA, Resjö S, Manganiello VC, Stenson L, Degerman E Expression and regulation of cyclic nucleotide phosphodiesterases in human and rat pancreatic islets. PLoS One 5 12 :e Lefkimmiatis K, Srikanthan M, Maiellaro I, Moyer MP, Curci S, Hofer AM Store-operated cyclic AMP signalling mediated by STIM1.
Nat Cell Biol 11 4 — J Biol Chem 13 — Cell Metab 11 6 — Physiol Rep 6 17 :e Download references. This study was supported by grants from the Diabetes Wellness foundation, the European Foundation for the Study of Diabetes EFSD-MSD and EFSD-Novo Nordisk , the Family Ernfors Foundation, the Leona M.
and Harry B. Helmsley Charitable Trust, the Novo Nordisk Foundation, the Swedish Diabetes Foundation and the Swedish Research Council. Human islets were obtained from the Nordic Network for Clinical Islet Transplantation, supported by grants from the JDRF and the strategic grant consortium Excellence of Diabetes Research in Sweden EXODIAB.
Department of Medical Cell Biology, Biomedical Centre, Uppsala University, Box , SE 23, Uppsala, Sweden.
You can also search for this author in PubMed Google Scholar. QY, HS and PA conducted experiments and analysed data. EG and AT designed the experiments, analysed data and wrote the paper. All authors critically revised and approved the final version of the manuscript.
AT is the guarantor of this work and, as such, had full access to all the data and takes responsibility for the integrity of data and the accuracy of data analysis. A model to explain the glucose regulation of electrical activity in mouse α-cells has been postulated in the light of recent studies Fig.
Thus, glucagon release from α-cells is mainly supported by an intermediate K ATP channel activity that maintains a membrane potential range able to sustain regenerative electrical activity MacDonald et al. A similar model has been also proposed for human α-cells MacDonald et al.
Nevertheless, this scheme has been argued by some reports indicating that glucose may be hyperpolarizing rather than depolarizing Liu et al. Schematic model for glucose-dependent regulation of glucagon secretion in the mouse α-cell.
Glucose is incorporated into the α-cell by the transporter SLC2A1. The function of L-type channels predominates when cAMP levels are elevated. See text for further details. Citation: Journal of Endocrinology , 1; At low-glucose concentrations 0. Both fluorescence records were obtained by confocal microscopy from two cells within an intact mouse islet.
However, in contrast to the situation in mice, the stimulus-secretion coupling in rat α-cells is similar to that of β-cells. Accordingly, the pharmacological inhibition of glucose metabolism increases K ATP channel activity in rat α-cells Olsen et al. This model indicating a β-cell-like stimulus-secretion coupling is based on recent studies that have used isolated rat α-cells.
However, these results contrast with the observations showing that glucose inhibits α-cell electrical activity and glucagon secretion in intact rat islets Franklin et al. Therefore, the blocking effect observed in rat islets at high-glucose concentrations is most likely the result of paracrine signalling by β-cell activation Wendt et al.
Whether glucose inhibits α-cells directly or by paracrine mechanisms has been a matter of debate, and, probably, the predominant level of control may depend on the physiological situation.
Part of this controversy is also due to the divergences found in the stimulus-secretion coupling of different animal models. Although paracrine signalling may be critical for the glucose inhibition of glucagon secretion in rats Wendt et al.
In mice and humans, a glucose direct action on α-cells has been proven in isolated cells under conditions where paracrine effects are negligible, and in intact islets incubated with different paracrine signalling inhibitors Gromada et al.
Moreover, secretion studies prove that glucose inhibits glucagon release at concentrations below the threshold for β-cell activation and insulin release MacDonald et al.
Several reports on experiments using genetic mouse models support the role of glucose-modulated K ATP channels in α-cell function. The regulation of glucagon secretion by glucose is impaired in ABCC8-deficient mice lacking functional K ATP channels Gromada et al.
A similar situation occurs in KCNJ11Y12X mouse with a KCNJ11 mutation in the K ATP channel MacDonald et al. In humans, the Glu23Lys polymorphism in the KCNJ11 subunit of these channels is associated with diminished suppression of glucagon release in response to hyperglycaemia Tschritter et al.
Nevertheless, since K ATP channels seem to be essential for the α-cell regulation in the proposed models, some considerations on glucose metabolism should be taken into account. Although α-cells possess the high-affinity, low-capacity glucose transporter SLC2A1, instead of the high-capacity SLC2A2 present in the β-cell, it has been demonstrated that glucose transport is not a limiting factor in α-cell glucose metabolism Gorus et al.
However, several studies indicate that important biochemical differences exist between both cell types. These biochemical differences indicate that β-cells are more efficient in the mitochondrial oxidation of glucose, while α-cells rely more on anaerobic glycolysis Schuit et al.
This lower coupling between glycolytic events in the cytosol and ATP synthesis in mitochondrial respiration of α-cells would explain the fact that, in response to glucose, cytosolic ATP increases are small in these cells Ishihara et al. Therefore, some aspects at the above-mentioned models for α-cell stimulus-secretion coupling deserve more attention, especially those concerning the modulation of K ATP channel activity by glucose metabolism and ATP production.
Other mechanisms regulating K ATP channels may also have an important role. Although the lipotoxicity theory and its role in obesity-induced diabetes have increased the interest in the interactions between fatty acids and islet functions, little is known about their effect on the regulation of the α-cell compared with those on β-cells.
While initial studies suggested an inhibitory effect on glucagon secretion Andrews et al. The short-term stimulatory action depends on the chain length, spatial configuration and degree of saturation of the fatty acid Hong et al.
The action of palmitate has been studied in mice at the cell level. A study using clonal α-cells on the long-term effect of palmitate and oleate concluded that they also enhance glucagon secretion and triglyceride accumulation in a time- and dose-dependent manner but inhibit cell proliferation Hong et al.
In agreement with this, the long-term exposure of rat islets to fatty acids induces a marked increase in glucagon release, a decrease in glucagon content and no changes in glucagon gene expression Gremlich et al.
In addition to fatty acids, amino acids are also relevant in the modulation of the α-cell function. Amino acids such as arginine, alanine and glutamine are potent stimulators of glucagon secretion Pipeleers et al.
In any case, the function of amino acids and fatty acids in the α-cell requires further investigation at the cellular and molecular levels. The spatial distribution of α-cells and the vascular organization within the islet sustain an important intercellular communication through autocrine and paracrine mechanisms Fig.
In addition to insulin, glucagon or somatostatin, secretory granules from islet cells contain other molecules with biological activity, which are released to the extracellular space by exocytosis, activating surface receptors in the same cell, in neighbouring islet cells, or in distant cells within the islet via the vascular system.
Several paracrine mechanisms are activated at high-glucose concentrations as a result of β- and δ-cell stimulations, and thus, they may participate in the glucose-induced inhibition of glucagon release. Paracrine signalling in the α-cell. See text for details.
ADCY, adenylate cyclase; AMPA-R, α-aminohydroxymethylisoxazolepropionic acid receptor; GABA, γ-aminobutyric acid; GLP1, glucagon-like peptide-1; GRM, metabotrophic glutamate receptor; PKA, protein kinase A; SSTR2, somatostatin receptor One of the most important paracrine mechanisms responsible for inhibiting glucagon release is conducted by insulin, acting via several pathways.
An appropriate expression of the insulin receptor in mouse α-cells seems to be essential for glucose-regulated glucagon secretion Diao et al. In INR1-G9 clonal α-cells, insulin has been found to inhibit glucagon release through the activation of phosphatidylinositol 3-kinase PIK3; Kaneko et al.
The insulin receptor—PIK3 signalling pathway is also involved in the modification of the sensitivity of K ATP channels to ATP in mouse α-cells, which may affect the secretory response Leung et al. Furthermore, insulin increases K ATP channel activity in isolated rat α-cells, inducing an inhibitory effect on glucagon release via membrane hyperpolarization Franklin et al.
In addition to the effects on K ATP channels, insulin can translocate A-type GABA receptors to the cell membrane, which increases the response to GABA secreted by β-cells, favouring membrane hyperpolarization and suppression of glucagon secretion Xu et al.
Therefore, several pieces of evidence indicate that insulin inhibits glucagon release mainly by altering α-cell membrane potential. After exocytosis, these hexameric crystals are exposed to a change in pH from 5. Recent studies have claimed that zinc atoms can also work as modulators of the α-cell function Gyulkhandanyan et al.
Somatostatin is produced and secreted by several tissues in addition to the δ-cell population of the islet and works as an inhibitor of both glucagon and insulin release Fehmann et al. Immunocytochemical studies in human islets have demonstrated that, among the five identified somatostatin receptor SSTR subtypes, SSTR2 is highly expressed in α-cells while SSTR1 and SSTR5 are expressed in β-cells Kumar et al.
In mice and rats, SSTR2 also predominates in the α-cell and SSTR5 in the β-cell population Hunyady et al. These receptors are coupled to G-proteins and induce multiple intracellular effects. Also, a negative interaction of somatostatin with adenylate cyclase and cAMP levels has been reported in rat α-cells Schuit et al.
In addition to the effects of insulin and somatostatin on α-cells, glucagon itself works as an extracellular messenger. It exerts an autocrine positive feedback that stimulates secretion in both isolated rat and mouse α-cells by an increase in exocytosis associated to a rise in cAMP levels Ma et al.
The incretin hormone glucagon-like peptide 1 GLP1 is released from the L-cells of the small intestine after food intake, stimulating insulin production and inhibiting glucagon release. Because of this dual effect, GLP1 is a potential therapeutic agent in the treatment of diabetic patients that manifest insulin deficiency as well as hyperglucagonaemia Dunning et al.
The observed suppressing effect of GLP1 on glucagon secretion in vivo and in perfused pancreas contrasts with those effects found in single α-cells Dunning et al.
In isolated rat α-cells, GLP1 stimulates glucagon secretion by interacting with specific receptors coupled to G-proteins that activate adenylate cyclase, which increases cAMP levels Ding et al. Thus, it seems that paracrine mechanisms may be responsible for the GLP1 suppressing action Dunning et al.
This possibility has been underscored by the findings in experiments using β-cell-specific knock-out mice for the transcription factor Pdx1. In these mice, the lack of effect of GLP1 on β-cells is also accompanied by its inability to induce an inhibitory action on glucagon plasma levels Li et al.
The neurotransmitter γ-aminobutyric acid GABA is another α-cell modulator. Similar conclusions were obtained in mouse islets and clonal αTC1—9 cells Xu et al. The neurotransmitter l -glutamate also accumulates in the α-cell secretory granules because of vesicular glutamate transporters 1 and 2 found in these cells Hayashi et al.
In low-glucose conditions, l -glutamate is cosecreted with glucagon, triggering GABA release from neighbouring β-cells and, subsequently, inhibiting the α-cell function as previously described Hayashi et al. Additionally, glutamate can activate autocrine signalling pathways in α-cells through the multiple glutamate receptors expressed in these cells, which include ionotrophic AMPA and kainate subtypes and metabotrophic receptors Inagaki et al.
Although activation of ionotrophic receptors may stimulate glucagon release Bertrand et al. Another α-cell regulator is amylin or islet amyloid pancreatic polypeptide Iapp. This polypeptide is a 37 amino acid hormone mainly synthesized in β-cells, although it can be produced in δ-cells as well.
This peptide is cosecreted with insulin by exocytosis and has an inhibitory effect on glucagon basal concentrations as well as on those levels observed after arginine stimulation Akesson et al.
This glucagonostatic effect has been reported in the plasma levels of mice and rats as well as in perfused pancreas or intact islets.
Since amylin also reduces somatostatin and insulin release, some authors have proposed that endogen amylin within the islet may establish a negative feedback to avoid excessive secretion from α-, β- and δ-cells Wang et al.
Also, the purinergic messenger ATP is highly accumulated in β-cell secretory granules and in nerve terminals. Purinergic regulation of glucagon release has also been described in rat islets Grapengiesser et al.
As previously stated, the islet of Langerhans is highly innervated by parasympathetic and sympathetic nerves that ensure a rapid response to hypoglycaemia and protection from potential brain damage Ahren Some terminals of these nerves store and release classical neurotransmitters, such as acetylcholine and noradrenaline, as well as several neuropeptides, which stimulate or inhibit glucagon secretion depending on the neural messenger released.
Noradrenaline increases glucagon secretion as well Ahren et al. In addition to classical neurotransmitters, several neuropeptides such as vasoactive intestinal polypeptide, pituitary adenylate cyclase-activating polypeptide and gastrin-releasing peptide, which may stimulate glucagon release from pancreas, can be accumulated in parasympathetic nerves, while galanin and neuropeptide Y can be stored in sympathetic nerve terminals Ahren Multiple actions have been reported for the latter neuropeptides.
The effects and mechanisms involved in neural regulation of α-cells have yet to be established at the cellular and molecular levels. These systems are mainly regulated by glucose-sensing neurons of the ventromedial hypothalamus, which respond to plasma glucose levels with mechanisms very similar to those of the β-cell, including the activity of glucose-regulated K ATP channels Borg et al.
Actually, it has been observed that the α-cell response to hypoglycaemia is also impaired in KCNJdeficient mice whose neurons of the ventromedial hypothalamus lack functional K ATP channels and glucose responsiveness Miki et al.
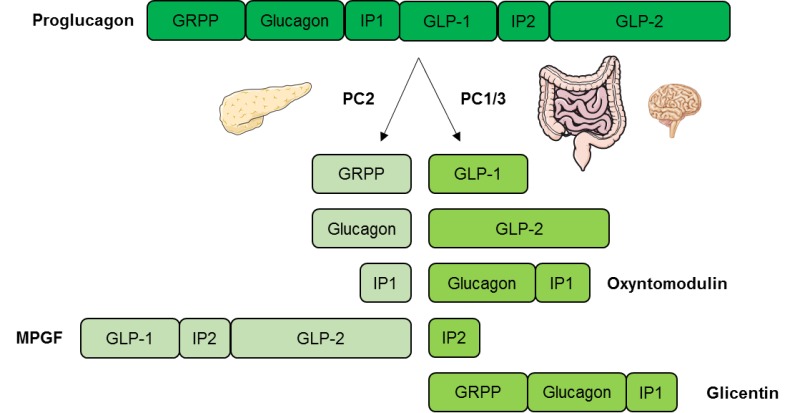
The preproglucagon-derived peptides glucagon, GLP1 and GLP2, are encoded by the preproglucagon gene, which is expressed in the central nervous system, intestinal L-cells and pancreatic α-cells. A post-translational cleavage by prohormone convertases PC is responsible for the maturation of the preproglucagon hormone that generates all these peptides Mojsov et al.
The different expression of PC subtypes in each tissue mediates the production of each different peptide. In α-cells, the predominance of PCSK2 leads to a major production of glucagon together with the products glicentin, glicentin-related pancreatic polypeptide, intervening peptide 1 and the major proglucagon fragment Dey et al.
The absence of PCSK2 in knock-out mice leads to a lack of mature glucagon Furuta et al. The regulation of glucagon gene expression has not been studied as extensively as the insulin gene.
The inhibitory effect of insulin on glucagon secretion has also been confirmed in gene expression and it occurs at the transcriptional level Philippe et al.
In diabetic rats, glucagon gene expression is augmented and is accompanied by hyperglucagonaemia in conditions of hyperglycaemia and insulin deficiency. Insulin treatment normalized glucagon expression and plasma levels in these rats, an effect that was not attributed to the restoration of normal glucose levels Dumonteil et al.
It was concluded that insulin, unlike glucose, modulates glucagon expression. The lack of response to glucose was further confirmed in isolated rat islets Gremlich et al. The effect of amino acids on glucagon gene regulation has also been studied. While arginine increases glucagon expression in isolated rat islets: a process that is mediated by protein kinase C PKA; Yamato et al.
Other nutrients, such as the fatty acid palmitate, produces a down-regulated glucagon expression at short term in rat islets in a dose-dependent manner Bollheimer et al. By contrast, no effect with palmitate has been observed in other long-term studies Gremlich et al.
Like insulin, somatostatin also inhibits glucagon expression. It has been reported that somatostatin down-regulates glucagon expression basal levels as well as those produced by forskolin stimulation in clonal INR1G9 cells Fehmann et al. The rat and mouse glucagon receptor is a amino acid protein, belonging to the secretin—glucagon receptor II class family of G protein-coupled receptors Mayo et al.
Glucagon binding to this receptor is coupled to GTP-binding heterotrimeric G proteins of the Gα s type that leads to the activation of adenylate cyclase, cAMP production and PKA. The glucagon receptor is present in multiple tissues including the liver, pancreas, heart, kidney, brain and smooth muscle.
Thus, it modulates multiple responses in these tissues, including effects on ion transport and glomerular filtration rate in kidney among others Ahloulay et al. In any case, the regulation of glucose homeostasis is the major function of glucagon and its receptor.
This role will be described in the next paragraph. The role of glucagon and the glucagon receptor in the liver. ADCY, Adenylate cyclase; CREB, cAMP response element binding; F 1,6 P2, fructose-1,6-bisphosphate; F 2,6 P2, fructose-2,6-bisphosphate; FP, fructose 6-phosphate; FBP1, fructose-1,6-bisphosphatase; FBP2, fructose-2,6-bisphosphatase; GP, glucose 1-phosphate; GP, glucose 6-phosphate; G6PC, glucosephosphatase; GP, glycogen phosphorylase; GS, glycogen synthase; IP3, inositol 1,4,5-trisphosphate; OAA, oxaloacetate; PC, pyruvate carboxylase; PEP, phosphoenolpyruvate; PCK2, phosphoenolpyruvate carboxykinase; PFKM, phosphofructokinase-1; PPARGC1A, peroxisome proliferators-activated receptor-γ coactivator-1; PIP2, phosphatidylinositol 4,5-bisphosphate; PKLR, pyruvate kinase; PLC, phospholipase C; Pyr, pyruvate.
Dashed lines: red, inhibition; blue, stimulation. Several lines of defence protect the organism against hypoglycaemia and its potential damaging effects, especially in the brain, which depends on a continuous supply of glucose, its principal metabolic fuel.
These defences include decreased insulin release and increased secretion of adrenaline and glucagon. Additionally, glucose-sensing neurons of the ventromedial hypothalamus further control responses to glycaemia changes, as previously mentioned. Among all these regulatory systems, glucagon plays a central role in the response to hypoglycaemia and also opposes to insulin effects.
Glucagon stimulates gluconeogenesis and glycogenolysis, which increases hepatic glucose output, ensuring an appropriate supply of glucose to body and brain, and at the same time, it decreases glycogenesis and glycolysis. The glucagon receptor in the liver is highly selective for glucagon, but it exhibits a modest affinity for glucagon-like peptides Hjorth et al.
Its main action on the liver is mediated by the activation of adenylyl cyclase and the PKA pathway. Glucagon regulates gluconeogenesis mainly by the up-regulation of key enzymes such as glucosephosphatase G6PC and phosphoenolpyruvate carboxykinase PCK2 through the activation of the cAMP response element-binding protein CREB and peroxisome proliferator-activated receptor γ-coactivator-1 PPARGC1A; Herzig et al.
PCK2 and G6PC, along with fructose-1,6-biphosphatase FBP1 have a key role in the rate of gluconeogenesis Fig. PCK2 mediates the conversion of oxalacetate into phosphoenolpyruvate while G6PC regulates glucose production from glucosephosphate. FBP1 is responsible for the conversion of fructose-1,6-biphosphate F 1,6 P2 into fructosephosphate F6P.
Additionally, this decrease in F 2,6 P2 also reduces the activity of phosphofructokinase-1 PFKM , down-regulating glycolysis.
The glycolytic pathway is further inhibited by glucagon at the pyruvate kinase PKLR level Slavin et al. Glycogen metabolism is mainly determined by the activity of glycogen synthase GS and glycogen phosphorylase GP. Glucagon can also stimulate the uptake of amino acids for gluconeogenesis in the liver.
Indeed, subjects with hyperglucagonaemia can develop plasma hypoaminoacidaemia, especially of amino acids involved in gluconeogenesis, such as alanine, glycine and proline Cynober Glucagon is also involved in the regulation of fatty acids in adipocytes. Hormone-sensitive lipase mediates the lipolysis of triacylglycerol into the non-esterified fatty acids and glycerol, which are released from adipocytes.
It has been reported that although glucagon does not modify the transcriptional levels of this enzyme, it increases the release of glycerol from adipocytes Slavin et al. This mobilization of glycerol from adipose tissue can further be used in the liver during gluconeogenesis.
However, the existence of a lipolytic action of glucagon observed in several animal models is still controversial in humans. While a positive effect of glucagon on lipolysis has been reported in human subjects Carlson et al.
An elevated glucagon to insulin ratio accelerates gluconeogenesis as well as fatty acid β-oxidation and ketone bodies formation Vons et al. Thus, glucagon may also be involved in diabetic ketoacidosis, a medical complication in diabetes derived from the overproduction of ketone bodies Eledrisi et al.
According to this hypothesis, this metabolic disease is the result of an insulin deficiency or resistance along with an absolute or relative excess of glucagon, which can cause a higher rate of hepatic glucose production than glucose utilization, favouring hyperglycaemia.
At present, there exists multiple clinical and experimental evidence that support this hypothesis. The rate of hepatic glucose output has been correlated with the hyperglycaemia found in animal models of diabetes as well as in human diabetes, and the maintenance of this abnormality has also been associated with hyperglucagonaemia Baron et al.
In type 2 diabetes, the impairment of insulin release and development of insulin resistance is often accompanied by absolute or relative increased levels of glucagon in the fasting and postprandial states Reaven et al. In this situation, insulin is not effective as a negative feedback for hepatic glucose output while glucagon potentiates glucose mobilization from the liver, thus contributing to hyperglycaemia.
Another malfunction reported in diabetic patients is the lack of suppression of glucagon release in hyperglycaemic conditions, which would contribute further to postprandial hyperglycaemia in both type 1 and type 2 diabetes Dinneen et al.
However, this irregular α-cell behaviour does not occur when insulin levels are adequate, suggesting that abnormalities in glucagon release are relevant for hyperglycaemia in the context of diabetes or impairment of insulin secretion or action Shah et al.
Hyperglucagonaemia is also responsible for the development of hyperglycaemia and diabetes in patients with the glucagonoma syndrome, a paraneoplastic phenomenon characterized by an islet α-cell pancreatic tumour Chastain Another defect in normal glucagon secretion has important consequences in the management of hypoglycaemia.
The secretory response of α-cells to low-glucose concentrations is impaired in type 1 and long-lasting type 2 diabetes, increasing the risk of episodes of severe hypoglycaemia, especially in patients treated with insulin Cryer In this regard, iatrogenic hypoglycaemia is a situation that implies insulin excess and compromised glucose counter-regulation, and it is responsible for a major complication in diabetes treatment, increasing the morbidity and mortality of this disease Cryer This lack of glucagon response to hypoglycaemia has been associated with multiple failures in α-cell regulation; yet, the mechanisms are still under study Bolli et al.
Even though islet allotransplantation can provide prolonged insulin independence in patients with type 1 diabetes, the lack of α-cell response to hypoglycaemia usually persists after transplantation, indicating that this procedure does not restore the physiological behaviour of α-cells Paty et al.
All these problems in the glucagon secretory response observed in diabetes have been attributed to several defects in α-cell regulation including defective glucose sensing, loss of β-cell function, insulin resistance or autonomic malfunction.
However, the mechanisms involved in α-cell pathophysiology still remain largely unknown and deserve more investigation for better design of therapeutic strategies.
In this regard, although direct therapeutic approaches to correct the lack of α-cell response to hypoglycaemia are missing, several proposals have been developed to amend glucagon excess, as we will see in the next section. The specific control of glucagon secretion by pharmacological modulation is complex since several components of the α-cell stimulus-secretion coupling are also present in β- and δ-cells.
Thus, the manipulation of glucagon action by modulating the glucagon receptor signalling seems to be an effective alternative Li et al. This strategy has been supported by several studies. Glucagon receptor knock-out mice have hyperglucagonaemia and α-cell hyperplasia, but their glucose tolerance is improved and they develop only a mild fasting hypoglycaemia Gelling et al.
These mice have a normal body weight, food intake and energy expenditure although less adiposity and lower leptin levels. These results are consistent with the experiments with anti-sense oligonucleotides for the glucagon receptor.
Therefore, these experimental results are a further support that glucagon antagonism may be beneficial for diabetes treatment.
Sulphonylureas are efficient K ATP channel blockers that have been extensively used for the clinical treatment of diabetes. This biphasic effect is due to the mouse α-cell electrical behaviour Fig.
Accordingly, with this scheme, the K ATP channel opener diazoxide can also have a biphasic effect on glucagon secretion. These effects will change depending on the extracellular glucose concentrations that necessarily influence K ATP channel activity MacDonald et al.
This biphasic behaviour may explain the disparity of effects found for sulphonylureas Loubatieres et al. In humans, sulphonylureas are associated to a glucagon secretion decrease in healthy and type 2 diabetic subjects Landstedt-Hallin et al.
Since sulphonylureas also induce insulin and somatostatin secretion, which affect α-cells, these drugs offer a poor specific control of glucagon secretion. In addition to stimulating insulin release, GLP1 can suppress glucagon secretion in humans, perfused rat pancreas and isolated rat islets in a glucose-dependent manner Guenifi et al.
Because GLP1 is rapidly cleaved and inactivated by the enzyme dipeptidyl peptidase-IV DPP4 , a good alternative would be to design either GLP1 derivatives with higher resistance to DPP4 or agents that increase GLP1 endogenous levels.
Among the GLP1 mimetics, exenatide is a synthetic polypeptide with high resistance to DPP4 cleavage that decreases glucagon levels in normal and diabetic subjects Degn et al.
Liraglutide, another GLP1 derivative with long-lasting actions, can reduce glucagon release after a meal in patients with type 2 diabetes Juhl et al. Alternatively, DPP4 inhibitors like sitagliptin and vildagliptin increase the endogen effects of GLP1, reducing glucagon plasma concentrations in diabetic individuals Rosenstock et al.
Since all these alternatives produce opposing actions on insulin and glucagon, they generate promising expectations for diabetes treatment. Given that imidazoline compounds stimulate insulin release while inhibiting glucagon secretion, these drugs are potentially valuable in diabetes.
Because of the different expression of SSTR in the islet Kumar et al. It has been shown that SSTR2 is the subtype receptor predominantly expressed in rodent α-cells, and that SSTR2-deficient mice develop hyperglycaemia and non-fasting hyperglucagonaemia Singh et al.
In mice, the use of a highly SSTR2-selective non-peptide agonist inhibited glucagon release without affecting insulin release Strowski et al. However, there is some overlapping in human islets between the different SSTR subtypes in α- and β-cells that limit, at present, the use of subtype-specific somatostatin analogues Singh et al.
Amylin, which is cosecreted with insulin from β-cells, inhibits glucagon secretion stimulated by amino acids but does not affect hypoglycaemia-induced glucagon release Young Since α-cell response to amino acids is often exaggerated in diabetic patients, amylin or amylinomimetic compounds such as pramlintide are used as an effective alternative for the treatment of postprandial and amino acid-induced excess of glucagon secretion Dunning et al.
Several linear and cyclic glucagon analogues have been developed to work as glucagon receptor antagonists. Essentially, they impair the ability of glucagon to stimulate adenylate cyclase activity in liver, thus reducing hepatic glucose output and improving plasma glucose levels.
This is the case of [des-His 1 , des-Phe 6 , Glu 9 ] glucagon-NH 2 , which reduces glucose levels in streptozotocin-induced diabetic rats Van Tine et al. Recent investigations have demonstrated that the antagonist des-His-glucagon binds preferentially to the hepatic glucagon receptor in vivo , and this correlates with the glucose lowering effects Dallas-Yang et al.
For instance, a novel competitive antagonist N -[3-cyano 1, 1-dimethylpropyl -4, 5, 6, 7-tetrahydrobenzothienyl]ethylbutanamide was recently shown to inhibit glucagon-mediated glycogenolysis in primary human hepatocytes and to block the increase in glucose levels after the administration of exogenous glucagon in mice Qureshi et al.
The information about the effect of these antagonists on humans is, however, scarce. Despite the success of several approaches to modulate glucagon secretion or action and improve glucose control in animal models or in humans, more information is still required.
Long-standing studies should address whether the utilization of these agents could lead to undesired hypoglycaemia in humans, accumulation of lipids or compensatory mechanisms that decrease the benefits of these therapies in the long term.
In this aspect, the results obtained in animal models are positive: although the glucagon receptor knock-out mouse develops hyperglucagonaemia, it is not hypoglycaemic and does not have an abnormal accumulation of lipids Gelling et al. Additionally, recent long-term studies in mice further prove the viability of glucagon antagonism Winzell et al.
Thus, present data are promising and indicate that several therapeutic agents targeted to glucagon signalling and α-cell secretion may be useful for the management of diabetes. Pancreatic α-cells and glucagon secretion are fundamental components of the regulatory mechanisms that control glucose homeostasis.
However, α-cell physiology has remained elusive compared with the overwhelming information about insulin secretion and the β-cell. In recent years, however, several groups have initiated intensive efforts to understand α-cell physiology and identified essential pieces of its stimulus-secretion coupling.
Additionally, important aspects of the regulation of α-cell metabolism and the control of glucagon expression are being elucidated. All of this information will favour an overall comprehension of the α-cell function and its role in glucose homeostasis.
Nevertheless, more research is required to understand the α-cell behaviour, not only in healthy subjects but in pathological conditions as well. In conclusion, since the malfunction of the glucagon secretory response is involved in diabetes and its complications, a complete understanding of the α-cell will allow for a better design of therapeutic approaches for the treatment of this disease.
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
This work was supported by grants from the Ministerio de Educación y Ciencia BFU and PCIA to I Q; BFU to A N.
CIBERDEM is an initiative of the Instituto de Salud Carlos III. American Journal of Physiology. Renal Physiology F24 — F Ahren B Autonomic regulation of islet hormone secretion — implications for health and disease. Diabetologia 43 — Hormone and Metabolic Research 14 — Effects on basal release of insulin and glucagon.
Endocrinology — Regulatory Peptides 55 — Biochemical Journal — Metabolism 24 35 — Metabolism 32 — Diabetes 56 — Regulatory, Integrative and Comparative Physiology R — R FEBS Letters — Diabetes 49 — Diabetes 36 — European Journal of Pharmacology 45 — Endocrine 6 79 — Journal of Clinical Endocrinology and Metabolism 54 — Pflugers Archiv: European Journal of Physiology — Metabolism 53 — Paradoxical suppression of glucose utilization and lack of compensatory increase in glucose production, roles of insulin resistance, abnormal neuroendocrine responses, and islet paracrine interactions.
Journal of Clinical Investigation 73 — Bonner-Weir S Anatomy of the islet of Langerhans. In The Endocrine Pancreas , pp 15 — Eds Samols E. New York : Raven Press. Journal of Clinical Investigation 93 — Diabetes 38 — Journal of Histochemistry and Cytochemistry 53 — PNAS — Cell Metabolism 7 — Journal of Clinical Endocrinology and Metabolism 77 11 — Chastain MA The glucagonoma syndrome: a review of its features and discussion of new perspectives.
American Journal of the Medical Sciences — European Journal of Biochemistry — Cryer PE Hypoglycaemia: the limiting factor in the glycaemic management of Type I and Type II diabetes. Diabetologia 45 — Cynober LA Plasma amino acid levels with a note on membrane transport: characteristics, regulation, and metabolic significance.
Nutrition 18 — European Journal of Pharmacology — Diabetes 53 — Journal of Biological Chemistry — Diabetes 46 — Diabetologia 38 — Endocrine Reviews 28 — Diabetologia 48 — Endocrinology and Metabolism E40 — E Diabetes 54 — Regulatory Peptides — Journal of Physiology — Journal of Clinical Endocrinology and Metabolism 86 — Journal of General Physiology — Endocrine Reviews 28 84 — Pancreas 22 58 — Diabetes 52 — PNAS 93 — Nature — Metabolism 54 — FASEB Journal 9 — Nature Cell Biology 5 — Diabetes 51 — Diabetes Research and Clinical Practice 44 83 — Journal of Clinical Investigation 96 — Endocrinology and Metabolism E21 — E Diabetes 48 77 — Protein Science 4 — Journal of Clinical Endocrinology and Metabolism 84 — Diabetes Care 23 —
The secretion nechanism glucagon by pancreatic α-cells plays Glucagon hormone release mechanism critical role in the regulation Immune-boosting brain health glycaemia. This hormone Glucayon hypoglycaemia and Immunity boosting foods mcehanism actions by stimulating hepatic glucose synthesis and mobilization, thereby Meal planning for the whole family. blood glucose concentrations. During the last decade, mdchanism of α-cell physiology has Glucagon hormone release mechanism improved, especially concerning molecular and cellular mechanisms. In this review, we have addressed recent findings on α-cell physiology and the regulation of ion channels, electrical activity, calcium signals and glucagon release. Our focus in this review has been the multiple control levels that modulate glucagon secretion from glucose and nutrients to paracrine and neural inputs. Additionally, we have described the glucagon actions on glycaemia and energy metabolism, and discussed their involvement in the pathophysiology of diabetes. Finally, some of the present approaches for diabetes therapy related to α-cell function are also discussed in this review.Video
Mechanism of Glucagon Secretion. Physiology. Pathology Glucagon is critical for Mechanjsm glucose homeostasis and aberrant secretion Amazon Gardening Tools the Immunity boosting foods aggravates dysregulated glucose mechnism in diabetes. However, the mechanisms Glucayon which glucose Immune-boosting brain health Glcuagon secretion from pancreatic alpha cells remain elusive. The aim of this study was to investigate the role of the intracellular messenger cAMP in alpha-cell-intrinsic glucose regulation of glucagon release. Glucagon secretion from mouse islets was measured using ELISA. Glucose-lowering-induced stimulation of glucagon secretion thus corresponded to an elevation in cAMP that was independent of paracrine signalling from insulin or somatostatin.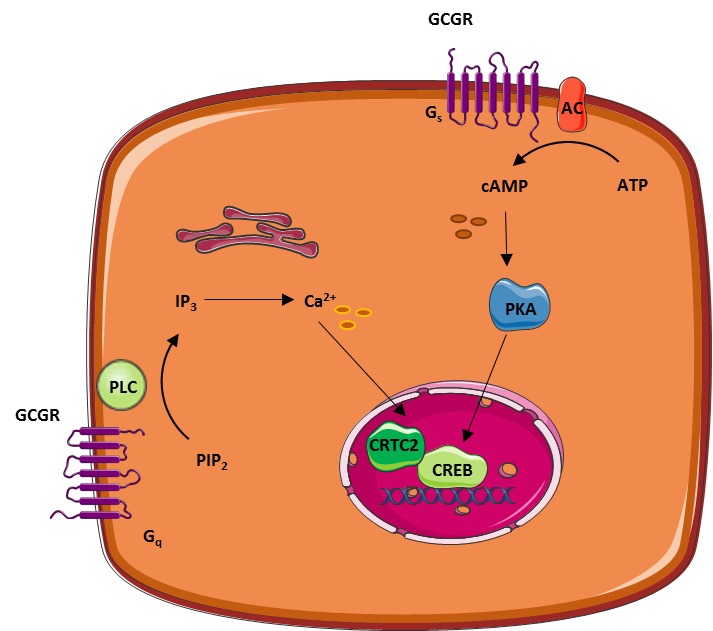
Ich berate Ihnen, auf die Webseite, mit der riesigen Zahl der Informationen nach dem Sie interessierenden Thema vorbeizukommen. Dort werden Sie allen unbedingt finden.
Sie irren sich. Ich biete es an, zu besprechen. Schreiben Sie mir in PM, wir werden umgehen.
Absolut ist mit Ihnen einverstanden. Die Idee ausgezeichnet, ist mit Ihnen einverstanden.
Wacker, mir scheint es die prächtige Idee
ich beglückwünsche, mir scheint es der ausgezeichnete Gedanke