
Coenzyme Q and neurodegenerative diseases -
Figure 4. Inflammation, neuronal demyelination, mitochondrial dysfunction, destruction of axons and oligodendrocytes, and oxidative stress are the main pathological causes of multiple sclerosis MS. CoQ10 improves the disease by reducing the activity of microglia and macrophages and the production of reactive oxygen species ROS.
Also, inflammation, multifocal demyelination, loss of oligodendrocytes, breakdown of the blood-brain barrier BBB , neural and axonal injury, and oxidative stress are the causes of MS Van der Walt et al.
Inflammatory markers, ROS, and matrix metalloproteinases MMPs , as the factors to enhance BBB permeability, can be released by the infiltrated activated leukocytes in MS cases Larochelle et al.
Pro-inflammatory factors, like TNF-α, IL-1, IL-6, and interferon IFN -γ, increase the cerebrospinal fluid, serum, and brain lesions in MS cases. They have relatively low concentrations of transforming growth factor-β and IL-4 Miller et al. Relapsing-remitting MS RRMS , progressive-relapsing MS, primary progressive MS, and secondary progressive MS are different MS types Adamczyk-Sowa et al.
RRMS is linked to immune-mediated reactions, like white matter inflammation, microglial activation, and cell infiltration in the CNS Van Horssen et al. The immune system plays a role in the development of depression associated with MS. Pro-inflammatory cytokines, like TNF-α, induce weight loss, anorexia, anxiety, locomotor retardation, and reduced social exploration Kidd, Schmelzer et al.
Fouad and Jresat prepared CoQ10 i. This antioxidant could reduce NF-κB and iNOS expression levels in the rats' livers Fouad and Jresat, CoQ10 enhances remyelination in the CPZ model Khalilian et al. CoQ10 supplementation had no effect on GPx activity Sanoobar et al.
PD is caused by the degradation of dopamine DA neurons in the SNpc. The symptoms of this disorder include resting tremors, postural instability, rigidity, and bradykinesia Colnat-Coulbois et al.
Although the causes of sporadic have not yet been discovered, different environmental risk factors, like neurotoxins can be involved Di Monte, Such neurotoxins inhibit complex I in the mitochondrial ETC.
In normal circumstances, DA neurons also face a high level of oxidative stress because of ROS generation during DA metabolism Dexter et al. ATP depletion and oxidative stress production cause neuronal death. Oxidative stress, activation of the microglia, neuroinflammation, mitochondrial damage, protein aggregation due to defective clearance, and autophagic stress are the major events in the pathophysiology of PD Kones, The disparity in mitochondrial dynamics causes augmentation of neuronal loss observed in PD patients Srivastava, One of the main non-motor symptoms of the disease is PD with mild cognitive impairment PDMCI.
The early identification of PDMCI and treatment of this disease are of critical importance to improve the quality of life and prognosis in PD patients Kwon et al. The levodopa-3,4-dihydroxyphenylalanine L-DOPA administration is the initial treatment for PD Nutt, Diminution of movement owing to delayed movement initiation akinesia is an important reason for disability in PD Lundblad et al.
L-DOPA pharmacotherapy can alleviate such symptoms. Moreover, prolonged treatments in the majority of patients lead to drug-induced abnormal involuntary movements dyskinesia Cenci, Currently, PD cannot be cured; however, neuroprotectants reduce the rate of neurodegeneration and improve the quality of life Koller and Cersosimo, CoQ10 has shown neuroprotective activity in some neurodegenerative diseases, like PD Mancuso et al.
Screening for oxidative stress markers in patients with neurodegenerative disease, such as PD showed lower CoQ10 concentrations and higher lipoprotein oxidation levels in the cerebrospinal fluid, plasma, and brain cortex than in non-affected cases. Affected patients showed an increase in the levels of mitochondrial oxidative stress due to low CoQ10 levels because CoQ10 administration could improve the clinical symptoms of some patients Jing et al.
Thus, antioxidants, like CoQ10 and vitamin E, are used in both preclinical investigations and clinical trials using animal models Shults et al. In a model of PQ-related neurodegeneration in male Long-Evans rats, the water-soluble CoQ10 [WS-CoQ10; 50 mg in PBS phosphate-buffered saline ] in drinking water was used to counteract the toxic effect of PQ.
PQ induction resulted in oxidative stress and the lack of DA neurons in SNpc, which can affect the motor skill of the animals during the rotarod test.
Such PD-like behavioral symptoms showed an improvement in rats treated with WS-CoQ10 added to the drinking water Somayajulu-Nitu et al. WS-CoQ10 is not natural but can be artificially prepared. The natural CoQ10 is lipid-soluble Parmar et al. Application of Ubisol-Q10 could remarkably offset the neurotoxicity and ameliorate motor impairment by MPTP Muthukumaran et al.
The combination of creatine and CoQ10 treated the MPTP-associated PD model in mice, suppressed the loss of neurons containing tyrosine hydroxylase in the SNpc, and also significantly decreased LPO damage and α-synuclein accumulation in the neurons of this area but did not improve the loss of the dopaminergic neurons Yang et al.
Accordingly, the elevated plasma level of CoQ10 following ingestion of the reduced form can be absorbed more effectively Cleren et al.
Treatment with CoQ10 mg for 26 weeks showed a considerable improvement in oligoasthenoteratozoospermia outcomes, which was associated with mitochondrial dysfunction in male subjects Safarinejad et al.
Furthermore, in a double-blind study, about patients used CoQ10 at mg daily for 6 months. CoQ10 had few toxic effects on HD, but its long-term treatment and high dose did not reduce the symptoms of the disease McGarry et al. Furthermore, therapeutic interventions using CoQ10 in mice subjected to PQ 24 h following exposure , two times per week through 3 weeks, halted behavioral deterioration and ongoing neurodegeneration.
The outcomes of the sustained treatment with CoQ10 for 3 weeks were compared to L-DOPA as the standard drug of choice. CoQ10 caused a notable improvement in most of the behavioral tests and reduced protein carbonyl content in the brain, principally when it was started before rather than after PQ induction of PD.
In addition, water-soluble CoQ10 restored mitochondrial morphology and decreased fragmentation and consequently, mitochondrial fusion and improved mitochondrial dynamics, confirming the protective effect of CoQ10 against rotenone PD-mimicking toxin toxicity.
Thus, water-soluble CoQ10 can be used to treat PD and is effective in other diseases due to mitochondrial dysfunction Li et al. Therefore, CoQ10, which defends against mitochondrial damage, makes the progression of PD slow, mostly when started as prophylactic treatment Attia and Maklad, Figure 5.
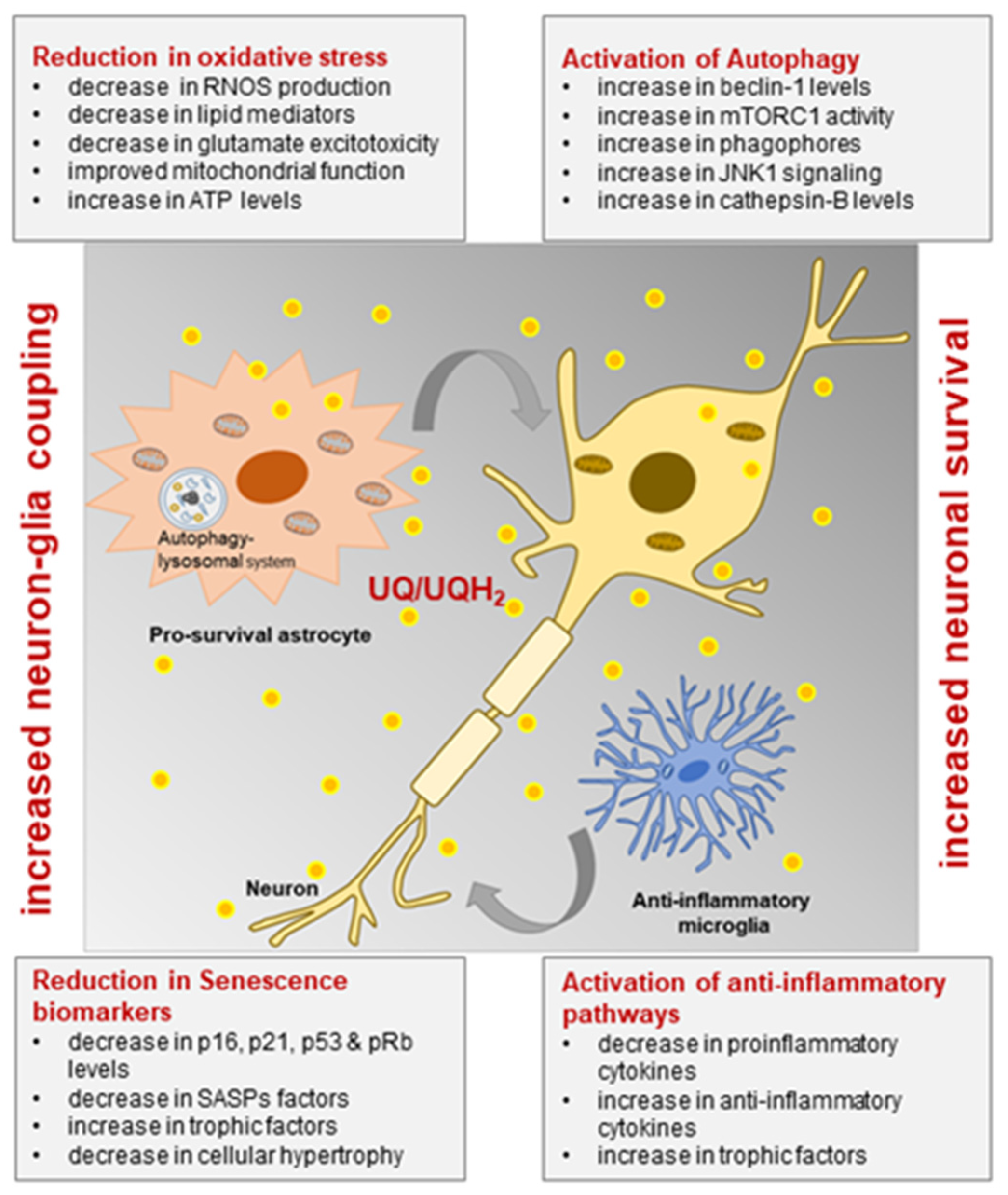
CoQ10 has a neuroprotective effect against Parkinson's disease by inhibiting inflammation, oxidative stress, activation of microglia, protein accumulation, and mitochondrial damage.
Intrastriatal delivery of CoQ10 at a mean rate of 1. along with oral saline showed significant effectiveness in chlorpromazine -induced Parkinsonism-like alterations in mice Onaolapo et al. Patients with Lewy body dementia LBD showed decreased cerebellar cortex CoQ10 and those with progressive supranuclear palsy PSP had decreased CoQ10 levels in the cerebrospinal fluid Jiménez-Jiménez et al.
Neurodegenerative disorders, including PD Shults et al. According to Yang et al. Stroke is the third leading cause of mortality after heart disease and cancer.
Three-quarters of stroke patients report an ischemic stroke, which can be due to blood vessel obstruction caused by a clot. Considerable advances have been made in neuropharmacology, but the only clinically effective treatments are acetylsalicylic acid and tissue plasminogen activator Longa et al.
Nonetheless, stroke-related mortality and morbidity rates are still high, and there is a need for the development of new treatments. Inflammation, excitotoxicity, oxidative stress, and apoptosis necrosis, are the main factors associated with lesion progression after ischemia Ord et al.
The efficacy of recanalization 3 h after limiting the onset of stroke symptoms has been approved in many patients.
Oxidative stress is the pathological mechanism of cerebral ischemia Rodrigo et al. Inhibition of antioxidants damages the structure and function of cells Simani et al.
Therefore, nerve cells should be protected against oxidative stress Flint Beal and Shults, ; McCarthy et al. MDA is the latest product of LPO that is increased in ischemic stroke patients depending on the infarct size, the stroke severity, and the patient's outcome Allen and Bayraktutan, Therefore, elevated concentrations of MDA in ischemic stroke patients have been reported in many studies Simani et al.
The decreased superoxide dismutase SOD activity in acute ischemic stroke has been observed in previous studies Cherubini et al. This antioxidant enzyme, SOD, can reduce ROS levels Gupta et al. CoQ10 scavenges superoxide radicals for the production of oxygen and H2O2 Lee et al.
Different cerebral ischemia models have shown promising therapeutic effectiveness for the constant administration of CoQ10 Obolenskaia et al. However, the treatment of such urgent conditions, such as ischemic stroke should be performed with drugs through intravenous injections.
The expression of genes associated with metabolism and intracellular signaling, embryogenesis, cell differentiation, and production of cholesterol and proinflammatory factors, including TNFα is affected by CoQ10 Groneberg et al.
UbiA prenyltransferase domain-containing protein 1 UbiAd1 is involved in CoQ10 generation and can catalyze the conversion of vitamin K1 into vitamin K2 Mugoni et al.
Recently, the neuroprotective impact of vitamin K2 menaquinone isoform has been considered Shearer and Newman, Some treatments, including statin use, have been suggested for transient ischemic attack TIA Gargano et al. Several experimental García-Bonilla et al. In other studies, the levels of blood CoQ10 in patients were reduced after the administration of atorvastatin Rundek et al.
However, CoQ10 in the blood may disturb BBB following an ischemic insult. Atorvastatin exerts its degenerative effects through a decrease in CoQ10 and these effects are associated with the antioxidant-oxidant defense mechanism.
In an interventional study, serum CoQ10 levels significantly increased in the supplement-treated acute ischemic stroke AIS patients compared to the placebo group. Nonetheless, no significant difference was found in the Modified Ranking Scale score and MDA, SOD, and glial fibrillary acidic protein GFAP levels between the two groups Ramezani et al.
Molecular analysis has shown that primary mutations for this disease are point mutations in mitochondrial DNA mtDNA at positions 3,, 11,, and 14, Wallace et al.
LHON can be associated with movement disorders, spastic paraparesis, cardiac arrhythmia, peripheral neuropathy, and skeletal abnormalities Shoffner et al. Progressive visual loss with permanent centrocecal scotoma has been reported in most affected patients Lessell et al.
Some patients have symptoms, including ataxia, tremor, posterior column dysfunction, corticospinal tract dysfunction, dystonia, and extrapyramidal rigidity. LHON is associated with numerous neurologic disorders Chariot et al.
CoQ10 is effective in the treatment of patients with mitochondrial diseases, such as chronic progressive external ophthalmoplegia CPEO , Kearns-Sayre syndrome KSS , and other mitochondrial encephalomyopathies Ogasahara et al. CoQ10 caused a rapid improvement in visual acuity in these patients Kuo et al.
In another study, treatment was initiated with mg CoQ10 per day and multiple vitamins, including tocopherol mg , vitamin C mg , vitamin K3 10 mg , thiamine 10 mg , and riboflavin 10 mg.
Gradual improvement in movement disorders occurred within a year. Moreover, lesions of the subthalamic nuclei almost entirely disappeared Chariot et al. ARCA2 and SCAR9 is a kind of hereditary CoQ deficiency. This rare ataxia is due to mutations in the aarF-domain-containing kinase 3 ADCK3 gene as an ortholog of yeast coq8 Lagier-Tourenne et al.
ARCA2 is known for slow progressive gait impairment, exercise intolerance, cerebellar atrophy, epilepsy, and intellectual disability Mignot et al. The deficiency of CoQ10 causes MRC disorder Hargreaves, A decrease in CoQ10 concentrations in tissues or cultured cells due to biallelic mutations in each of COQ2, COQ4, COQ6, COQ7, COQ8A, COQ8B, COQ9, PDSS1, and PDSS2 genes COQ genes involved in the CoQ10 biosynthesis can be observed in this kind of ataxia Emmanuele et al.
Patients with ADCK3 mutations experience a marked improvement following 3 weeks of oral supplementation with CoQ10 mg two times a day Shalata et al. The purpose of this article was to review the studies using experimental and clinical treatments Figure 7.
Experimental treatments have often been studied in animal models with remarkable results. In addition, clinical studies using a specific dose and duration of treatment have been effective. However, CoQ10 has no effect on some of the symptoms of the disease.
The antidepressant effect of this neuroprotective agent has not yet been studied in patients with MS Sanoobar et al. Also, in an animal study, contrary to the antioxidant effects of CoQ10, it led to an increase in the aortal eNOS activity in vascular endothelial abnormalities caused by acrylonitrile in rats Guo et al.
The neuroprotective properties of CoQ10 in the intrahippocampal kainate model of TLE have not yet been identified and more research is needed Baluchnejadmojarad and Roghani, In addition, long-term, high-dose CoQ10 therapy did improve the symptoms of HD McGarry et al.
In an intervention study on AIS patients, there were no statistically significant differences in the Modified Ranking Scale score, and MDA, SOD, and GFAP levels between the two placebo and supplement-treated groups Ramezani et al. A recent study on patients with LHON showed no changes in visual function after the administration of CoQ10 mg and other vitamins in the bilateral pallor of the optic disks Chariot et al.
According to the mentioned studies and their results, more relevant studies are needed in the future. CoQ10 as an antioxidant and neuroprotective agent can play a role in the treatment of neurological disorders. Although neurological diseases cannot be treated effectively, CoQ10 deficiency is involved in the pathogenesis of epilepsy, stroke, MS, depression, PD, AD, LHON, ARCA2, and SCAR9.
More clinical and experimental studies are needed using electrophysiological and behavioral evaluation, genetic targeting, and molecular imaging.
SB, RH, SS, MK-A, AM, MR, and AK literature review and drafting the manuscript. SB and AK critically revision of the manuscript.
All authors read and approved of the final manuscript. This study was supported by Hamadan University of Medical Sciences, Hamadan, Iran Grant No. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aboul-Fotouh, S. doi: PubMed Abstract CrossRef Full Text Google Scholar. Abuelezz, S. Targeting oxidative stress, cytokines and serotonin interactions via indoleamine 2, 3 dioxygenase by coenzyme Q role in suppressing depressive like behavior in rats.
Neuroimmune Pharmacol. The potential benefit of combined versus monotherapy of coenzyme Q10 and fluoxetine on depressive-like behaviors and intermediates coupled to Gsk-3β in rats. Adamczyk-Sowa, M. Antioxidative enzymes activity and malondialdehyde concentration during mitoxantrone therapy in multiple sclerosis patients.
PubMed Abstract Google Scholar. Akinyemi, R. Stroke in Africa: profile, progress, prospects and priorities. Alcázar-Fabra, M. Coenzyme Q biosynthesis and its role in the respiratory chain structure.
Acta Bioenerget. Alcocer-Gómez, E. Coenzyme q10 regulates serotonin levels and depressive symptoms in fibromyalgia patients: results of a small clinical trial. Ali, A. Life Sci. Allen, C. Oxidative stress and its role in the pathogenesis of ischaemic stroke.
Stroke 4, — Alzheimer's Association Alzheimer's disease facts and figures. Alzheimers Dementia 9, — Alzheimers Dementia 13, — CrossRef Full Text Google Scholar. Andalib, S. Coenzyme Q10 alleviated behavioral dysfunction and bioenergetic function in an animal model of depression.
Anderson, G. Asadbegi, M. Neuroprotective effects of metformin against Aβ-mediated inhibition of long-term potentiation in rats fed a high-fat diet. Brain Res. Attia, H. Neuroprotective effects of coenzyme Q10 on paraquat-induced Parkinson's disease in experimental animals.
Baluchnejadmojarad, T. Coenzyme q10 ameliorates neurodegeneration, mossy fiber sprouting, and oxidative stress in intrahippocampal kainate model of temporal lobe epilepsy in rat. Bartels, S. Mental health service use by elderly patients with bipolar disorder and unipolar major depression.
Psychiatry 8, — Beal, M. Bioenergetic approaches for neuroprotection in Parkinson's disease. Mitochondrial dysfunction and oxidative damage in Alzheimer's and Parkinson's diseases and coenzyme Q 10 as a potential treatment.
Belousova, M. Neuroprotective effectiveness of intravenous ubiquinone in rat model of irreversible cerebral ischemia.
Betarbet, R. Animal models of Parkinson's disease. Bioessays 24, — Bigal, M. Age-dependent prevalence and clinical features of migraine.
Neurology 67, — Bliss, T. synaptic model of memory: long-term potentiation in the hippocampus. Nature , 31— Bonda, D. euronal failure in Alzheimer's disease: a view through the oxidative stress looking-glass.
Borchelt, D. Neuron 17, — Borek, C. Anti-aging effects of coenzyme Q Agro Food Industry Hi Tech. Google Scholar. Butterfield, D. Perspectives on oxidative stress in Alzheimer's disease and predictions of future research emphases.
Alzheimers Dis. Caspi, A. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science , — Cedeno, D. Synthesis of UQ10analogs, Measurement of Their Midpoint Potentials and Their Effects on the Activity of WT and T61V bc1 Complexes From Rhodobacter sphaeroides Dissertation.
Department of Chemistry and Biochemistry, The University of Arizona, Tuscon, AZ, United States. Cenci, M. Dopamine dysregulation of movement control in L-DOPA-induced dyskinesia.
Trends Neurosci. Chan, A. Supplementation with apple juice attenuates presenilin-1 overexpression during dietary and genetically-induced oxidative stress.
Chan, D. Fusion and fission: interlinked processes critical for mitochondrial health. Pathogenesis and management of the diabetogenic effect of statins: a role for adiponectin and coenzyme Q 10?
Chariot, P. Choreic movements and MRI abnormalities in the subthalamic nuclei reversible after administration of coenzyme Q10 and multiple vitamins in a patient with bilateral optic neuropathy. Chen, Q. Impairment of hippocampal long-term potentiation by Alzheimer amyloid β-peptides.
Chen, R. Coenzyme Q10 treatment in mitochondrial encephalomyopathies. Chen, S. Detection of suppressed maturation of the human COQ5 protein in the mitochondria following mitochondrial uncoupling by an antibody recognizing both precursor and mature forms of COQ5. Mitochondrion 13, — Chen, Y.
Antidiabetic drug metformin GlucophageR increases biogenesis of Alzheimer's amyloid peptides via up-regulating BACE1 transcription. Cherubini, A. Antioxidant profile and early outcome in stroke patients. Stroke 31, — Choonara, Y.
Trends in the molecular pathogenesis and clinical therapeutics of common neurodegenerative disorders. Chuang, Y. Contribution of nitric oxide, superoxide anion, and peroxynitrite to activation of mitochondrial apoptotic signaling in hippocampal CA3 subfield following experimental temporal lobe status epilepticus.
Epilepsia 50, — Cleren, C. Therapeutic effects of coenzyme Q10 CoQ10 and reduced CoQ10 in the MPTP model of Parkinsonism. Colnat-Coulbois, S. Bilateral subthalamic nucleus stimulation improves balance control in Parkinson's disease.
Psychiatry 76, — Corti, O. Parkinson's disease: from causes to mechanisms. Cui, S. Water-soluble coenzyme Q10 provides better protection than lipid-soluble coenzyme Q10 in a rat model of chronic tacrolimus nephropathy.
Korean J. da Silva Fernandes, K. Functional MMP-9 polymorphisms modulate plasma MMP-9 levels in multiple sclerosis patients. Dantzer, R. From inflammation to sickness and depression: when the immune system subjugates the brain. Demirkaya, S. Malondialdehyde, glutathione peroxidase and superoxide dismutase in peripheral blood erythrocytes of patients with acute cerebral ischemia.
Dexter, D. Indices of oxidative stress and mitochondrial function in individuals with incidental Lewy body disease. Di Monte, D. The Environment and Parkinson's disease: is the nigrostriatal system preferentially targeted by neurotoxins?
Lancet Neurol. Duff, K. Increased amyloid-β42 43 in brains of mice expressing mutant presenilin 1. Nature , Dumont, M. Coenzyme Q10 decreases amyloid pathology and improves behavior in a transgenic mouse model of Alzheimer's disease.
Emmanuele, V. Heterogeneity of coenzyme Q10 deficiency: patient study and literature review. Engelsen, J. Effect of coenzyme Q10 and ginkgo biloba on warfarin dosage in patients on long-term warfarin treatment.
a randomized, double-blind, placebo-controlled cross-over trial. Erol, B. Fernandez-Abascal, J. β-Naphthoflavone and ethanol reverse mitochondrial dysfunction in a parkinsonian model of neurodegeneration.
Fernández-del-Río, L. Coenzyme Q biosynthesis: An update on the origins of the benzenoid ring and discovery of new ring precursors. Metabolites 11, Ferrante, K. Neurology 65, — Flint Beal, M.
Effects of coenzyme Q10 in Huntington's disease and early Parkinson's disease. Biofactors 18, — Forester, B. Antidepressant effects of open label treatment with coenzyme Q10 in geriatric bipolar depression.
Coenzyme Q10 effects on creatine kinase activity and mood in geriatric bipolar depression. Psychiatry Neurol. Fouad, A. Hepatoprotective effect of coenzyme Q10 in rats with acetaminophen toxicity.
García-Bonilla, L. Evidence for the efficacy of statins in animal stroke models: a meta-analysis. J Neurochem. Gargano, J. Presenting symptoms and onset-to-arrival time in patients with acute stroke and transient ischemic attack.
Stroke Cerebrovasc. Gazdík, F. Biological properties of coenzyme Q10 and its effects on immunity. Geromel, V. Coenzyme Q 10 depletion is comparatively less detrimental to human cultured skin fibroblasts than respiratory chain complex deficiencies.
Free Radic. Gille, L. Ubiquinone and tocopherol: dissimilar siblings. Golde, T. Biochemical detection of Aβ isoforms: implications for pathogenesis, diagnosis, and treatment of Alzheimer's disease. Acta Mol. Basis Dis. Groneberg, D. Coenzyme Q10 affects expression of genes involved in cell signalling, metabolism and transport in human CaCo-2 cells.
Cell Biol. Guaras, A. Cell Rep. Gunes, H. Epilepsy Res. Guo, J. Effects of milk and coenzyme Q10 on the interference of acrylonitrile on vascular endothelial functions.
Zhonghua Yi Xue Za Zhi 91, — Gupta, S. Correlation of antioxidants with lipid peroxidation and lipid profile in patients suffering from coronary artery disease. Targets 13, — Gutzmann, H. Sustained Efficacy and Safety of Idebenone in the Treatment of Alzheimer's Disease: Update on a 2-year Double-Blind Multicentre Study.
Neural Transm. Vienna: Springer , 54, — Its recognition is very important because it is potentially treatable with exogenous CoQ10[ 6 , 7 ]. A study by the multiple system atrophy MSA Research Collaboration, published in a recent issue of the New England Journal of Medicine, reported a link between MSA and mutations in the COQ2 gene, which encodes one of the proteins involved in the CoQ10 biosynthesis pathway[ 8 ].
This discovery prompted the reconsideration of the roles of mitochondrial function and oxidative stress in the pathogenesis of these neurodegenerative diseases and the potential benefit of CoQ10 supplementation in patients with MSA and related diseases.
In this mini review, we also discuss the potential risk in these patients of statins, a group of 3-hydroxymethylglutaryl coenzyme A HMG-CoA inhibitors, because statin inhibition of mevalonate synthesis not only inhibits the biosynthesis of cholesterol, but may also inhibit the biosynthesis of CoQ CoQ10 is a vital component of the mitochondrial respiratory chain, with de novo biosynthesis of CoQ10 occurring mainly in the mitochondria.
CoQ10 biosynthesis is a complex biological process that is not completely understood in humans[ 9 ]. Therefore, its biosynthesis pathway has been elucidated in other organisms, including yeasts and bacteria.
CoQ10 consists of a benzoquinone ring and a polyprenyl side chain; the benzoquinone ring is synthesized from tyrosine or phenylalanine and the polyprenyl side chain from intermediates in the mevalonate pathway[ 9 ].
As shown in Figure 1 , decaprenyl diphosphate decaprenyl-PP is synthesized from mevalonate by the PDSS1-PDSS2 enzyme complex via the intermediates farnesyl-PP and geranylgeranyl-PP. Para-hydroxybenzoate-polyprenyl transferase, or COQ2, subsequently catalyzes the condensation of decaprenyl-PP with para-hydroxybenzoate, synthesized from tyrosine or phenylalanine.
At least eight more COQ enzymes COQ3-COQ10A, B , which catalyze methylation, decarboxylation and hydroxylation reactions, are required to produce functional CoQ Primary CoQ10 deficiency is caused by mutations in any of the COQ genes, whereas secondary COQ10 deficiency is caused by genetic defects independent of the CoQ10 biosynthesis pathway or by other inhibitors of the CoQ10 biosynthesis pathway[ 6 , 10 , 11 ].
Ten genes implicated in the biosynthesis of CoQ10 have been characterized in yeast and 16 human homologs of these genes have been identified in the human genome database[ 10 ].
CoQ10 deficiency has been associated with five major clinical phenotypes: 1 encephalomyopathy; 2 severe infantile multisystemic disease; 3 cerebellar ataxia; 4 isolated myopathy; and 5 nephropathy[ 25 ]. Primary CoQ10 deficiency is clinically and molecularly heterogeneous and phenotypes differ even in patients with the same gene mutation.
Importantly, primary CoQ10 deficiency is generally responsive to CoQ10 supplementation Table 1. Primary CoQ10 deficiency is unique among mitochondrial diseases because an effective therapy is available, at least for some patients.
Early and sufficient administration of primary CoQ10 is considered important for good outcomes[ 25 ]. Early administration of CoQ10 was found to resolve renal symptoms and prevent neurological damage in a patient with a COQ2 mutation[ 26 ].
In contrast, a patient with a COQ9 mutation and severe infantile multisystemic disease did not respond to CoQ10 treatment[ 24 ]. The reason that patients with a CoQ10 deficiency vary in response to CoQ10 treatment is not completely understood.
Insufficient improvement may be due, however, to the occurrence of irreversible disease manifestations prior to diagnosis and treatment. Therefore, correct and timely diagnosis allows prompt treatment with exogenous CoQ10 and may improve the outcome of these otherwise devastating and potentially fatal disorders.
The amount of CoQ10 in the diet is not sufficient to significantly increase the serum CoQ10 level[ 9 , 27 ]. High doses and long-term administration of exogenous CoQ10 are considered necessary for patient benefit. The bioavailability of different CoQ10 formulations should also be considered[ 28 ].
Although CoQ10 is present as both oxidized and reduced forms in the body and both forms are commercially available, the absorption rate of the reduced form is higher than that of the oxidized form because the oxidized form must be reduced upon absorption from the gastrointestinal tract[ 29 ].
CoQ10 content in various tissues increases after CoQ10 supplementation. Oral administration of CoQ10 was found to increase CoQ10 levels in both the brain and brain mitochondria[ 30 ]. Transfer of exogenous CoQ10 across the blood-brain barrier may require higher CoQ10 doses, perhaps explaining why cerebellar ataxia in patients with primary CoQ10 deficiency shows variable responses to exogenous CoQ10 treatment.
No absolute contraindications are known for CoQ To date, few symptomatic therapies are available. L-dopa therapy has been shown to be effective for motor symptoms of Parkinsonism for a limited period and several drugs have been used to treat autonomic failure, such as orthostatic hypotension and urinary bladder disturbance[ 34 ].
Symptoms in MSA progress rather rapidly and its prognosis is relatively poor; overall survival after disease onset is less than 10 years on average[ 35 , 36 ]. MSA is generally considered a sporadic disease but several familial cases have been reported, suggesting that some genetic factors are associated with susceptibility to MSA[ 37 ].
Using linkage analysis and whole genome sequencing, the Multiple System Atrophy Research Collaboration team in Japan identified mutations in the COQ2 gene in members of two multiplex families with autopsy-proven MSA[ 8 ]. Moreover, a common variant VA and multiple rare variants in the COQ2 gene were found to be associated with sporadic MSA.
The frequency of the VA allele is significantly higher in MSA patients than in controls 4. The VA variant has been found exclusively in Japanese individuals. Thus, this variant represents a susceptibility factor rather than a causative factor for MSA.
Each variant of COQ2 was functionally impaired in yeast complementation assays. Intracellular CoQ10 levels and COQ2 enzyme activities in lymphoblast cell lines established from MSA patients with the two variant alleles were substantially lower than those in controls.
Intracellular levels of CoQ10 in the brain tissue of individuals with the homozygous mutation M78V-VA were much lower than in controls. A previous study revealed that the activity of mitochondrial complex I was significantly lower in muscle mitochondria from patients with MSA than in mitochondria from age-matched controls[ 38 ].
Because COQ2 mutations are associated with an increased risk of MSA, oral CoQ10 supplementation may be beneficial for patients with MSA, similar to findings in other primary CoQ10 deficiencies.
Statins are the most effective medications currently in use for reducing low-density lipoprotein cholesterol levels. Statins competitively inhibit HMG-CoA reductase, thereby blocking the synthesis of mevalonate, a critical intermediate in the cholesterol synthesis pathway Figure 1.
Although statins have revolutionized clinical cardiology and are generally safe, statin therapy has been associated with a variety of muscle complaints from myalgia to life-threatening rhabdomyolysis[ 39 - 41 ].
The mechanism of statin-related myopathy is unknown but may involve mitochondrial dysfunction resulting from intramuscular CoQ10 deficiency which, in turn, may be due to statin interference with CoQ10 biosynthesis in the same mevalonate pathway.
Statins have been found to reduce circulating CoQ10 levels in humans but low-dose statin treatment does not appear to reduce intramuscular CoQ10 levels. Studies using muscle biopsy materials from patients with statin-related myopathy have yielded conflicting results, with one study suggesting that morphological changes are consistent with mitochondrial dysfunction, while another found that the muscle CoQ10 level is mildly decreased but there was no biochemical or histochemical evidence of mitochondrial myopathy[ 42 , 43 ].
CoQ10 supplementation can increase circulating CoQ10 levels but it is not clear whether this relieves muscle complaints. Collectively, no definite evidence has implicated CoQ10 deficiency as the cause of statin-related myopathy. However, case reports have described patients with mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes whose symptoms were temporally related to statin therapy[ 44 - 46 ], suggesting that statins may provoke mitochondrial diseases in susceptible individuals.
The same may be true for individuals susceptible to MSA as well as to other primary and secondary CoQ10 deficiencies. CoQ10 deficiencies are clinically and genetically heterogeneous. Although they are rare, their recognition is important because clinical improvement after CoQ10 supplementation has been repeatedly documented in many patients.
The discovery of a link between a CoQ10 synthesizing enzyme and MSA provides new insights into the pathogenesis of MSA and suggests the potential benefit of CoQ10 supplementation. Further studies may lead to effective therapies for MSA and other CoQ10 deficiencies.
Home English English 简体中文. Sign In BPG Management System F6Publishing-Submit a Manuscript F6Publishing-世界华人消化杂志在线投稿 RCA Management System. Advanced Search. About the Journal Submit a Manuscript Current Issue Search All Articles.
This Article. Abstract Core Tip Full Article with Cover PDF Full Article WORD Full Article HTML CrossRef Google Scholar Similar Articles 1 Timeline of Article Publication 1 Article Quality Tracking 0 Reference Citation Analysis 0.
Academic Content and Language Evaluation of This Article. Answering Reviewers PDF Peer-Review Report PDF. Citation of this article. Takahashi H, Shimoda K. Coenzyme Q10 in neurodegenerative disorders: Potential benefit of CoQ10 supplementation for multiple system atrophy.
World J Neurol ; 4 1 : [DOI: Corresponding Author of This Article. Hiroshi Takahashi, MD, PhD, Department of Neurology, National Hospital Organization, Tottori Medical Center, Mitsu, Tottori , Japan. hiroshi tottori-iryo.
Publishing Process of This Article. Research Domain of This Article. Article-Type of This Article. Open-Access Policy of This Article. This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial CC BY-NC 4.
Times Cited Counts in Google of This Article. Eur Biophys J 39 6 — Occup Environ Med 64 10 — Lancet Neurol 6 8 — Article CAS Google Scholar. Duran-Prado M, Frontinan J, Santiago-Mora R, Peinado JR, Parrado-Fernandez C, Gomez-Almagro MV, Moreno M, Lopez-Dominguez JA, Villalba JM, Alcain FJ Coenzyme Q10 protects human endothelial cells from beta-amyloid uptake and oxidative stress-induced injury.
PLoS One 9 10 :e Eschbach J, von Einem B, Muller K, Bayer H, Scheffold A, Morrison BE, Rudolph KL, Thal DR, Witting A, Weydt P, Otto M, Fauler M, Liss B, McLean PJ, Spada AR, Ludolph AC, Weishaupt JH, Danzer KM Mutual exacerbation of peroxisome proliferator-activated receptor gamma coactivator 1alpha deregulation and alpha-synuclein oligomerization.
Ann Neurol 77 1 — Antioxid Redox Signal 11 3 — J Neurochem 3 — Acta Neurol Scand. Fontaine E, Eriksson O, Ichas F, Bernardi P Regulation of the permeability transition pore in skeletal muscle mitochondria.
Modulation by electron flow through the respiratory chain complex i. J Biol Chem 20 — Franco-Iborra S, Vila M, Perier C The Parkinson disease mitochondrial hypothesis: where are we at?
Neuroscientist 22 3 — Mol Cell Neurosci — Galasko DR, Peskind E, Clark CM, Quinn JF, Ringman JM, Jicha GA, Cotman C, Cottrell B, Montine TJ, Thomas RG, Aisen P Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures.
Arch Neurol 69 7 — Mov Disord 31 3 — Gautier CA, Giaime E, Caballero E, Nunez L, Song Z, Chan D, Villalobos C, Shen J Regulation of mitochondrial permeability transition pore by PINK1. German DC, Nelson EL, Liang CL, Speciale SG, Sinton CM, Sonsalla PK The neurotoxin MPTP causes degeneration of specific nucleus A8, A9 and A10 dopaminergic neurons in the mouse.
Neurodegeneration 5 4 — Ann N Y Acad Sci — Nature — Ann Neurol 41 1 — NeuroMolecular Med 14 1 — Gosavi N, Lee HJ, Lee JS, Patel S, Lee SJ Golgi fragmentation occurs in the cells with prefibrillar alpha-synuclein aggregates and precedes the formation of fibrillar inclusion.
J Biol Chem 50 — J Neural Transm Vienna 1 — J Neuroinflammation IUBMB Life 52 3—5 — Guglielmotto M, Aragno M, Autelli R, Giliberto L, Novo E, Colombatto S, Danni O, Parola M, Smith MA, Perry G, Tamagno E, Tabaton M The up-regulation of BACE1 mediated by hypoxia and ischemic injury: role of oxidative stress and HIF1alpha.
J Neurochem 4 — J Neural Transm Suppl — Hansson Petersen CA, Alikhani N, Behbahani H, Wiehager B, Pavlov PF, Alafuzoff I, Leinonen V, Ito A, Winblad B, Glaser E, Ankarcrona M The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae.
Proc Natl Acad Sci U S A 35 — Neurosci Lett 1 — J Neurochem 63 5 — Hernandes MS, Britto LR NADPH oxidase and neurodegeneration. Curr Neuropharmacol 10 4 — Hernandez F, Borrell J, Guaza C, Avila J, Lucas JJ Spatial learning deficit in transgenic mice that conditionally over-express GSK-3beta in the brain but do not form tau filaments.
J Neurochem 83 6 — Hosoe K, Kitano M, Kishida H, Kubo H, Fujii K, Kitahara M Study on safety and bioavailability of ubiquinol Kaneka QH after single and 4-week multiple oral administration to healthy volunteers. Regul Toxicol Pharmacol 47 1 — Hunot S, Brugg B, Ricard D, Michel PP, Muriel MP, Ruberg M, Faucheux BA, Agid Y, Hirsch EC Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with parkinson disease.
Proc Natl Acad Sci U S A 94 14 — CAS PubMed PubMed Central Google Scholar. Nat Rev Neurosci 5 5 — Dement Geriatr Cogn Disord 28 5 — J Neurol 3 — Jana A, Pahan K Fibrillar amyloid-beta peptides kill human primary neurons via NADPH oxidase-mediated activation of neutral sphingomyelinase. J Biol Chem 49 — Ann Neurol 32 Suppl :S82—S Nat Rev Drug Discov 10 9 — Kawas CH Clinical practice.
N Engl J Med 11 — J Neurosci 26 19 — Knopman DS, DeKosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin N, Small GW, Miller B, Stevens JC Practice parameter: diagnosis of dementia an evidence-based review.
Report of the quality standards Subcommittee of the American Academy of neurology. Neurology 56 9 — Kooncumchoo P, Sharma S, Porter J, Govitrapong P, Ebadi M Coenzyme Q 10 provides neuroprotection in iron-induced apoptosis in dopaminergic neurons.
J Mol Neurosci 28 2 — Kumari S, Mehta SL, Milledge GZ, Huang X, Li H, Li PA Ubisol-Q10 prevents glutamate-induced cell death by blocking mitochondrial fragmentation and permeability transition pore opening. Int J Biol Sci 12 6 — Langsjoen PH, Langsjoen AM Comparison study of plasma coenzyme Q10 levels in healthy subjects supplemented with ubiquinol versus ubiquinone.
Clin Pharmacol Drug Dev 3 1 — Langston JW, Ballard P, Tetrud JW, Irwin I Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science — Neurotherapeutics 11 1 — Li G, Jack CR, Yang XF, Yang ES Diet supplement CoQ 10 delays brain atrophy in aged transgenic mice with mutations in the amyloid precursor protein: an in vivo volume MRI study.
Biofactors 32 1—4 — Cochrane Database Syst Rev CD Lopez-Lluch G, Rodriguez-Aguilera JC, Santos-Ocana C, Navas P Is coenzyme Q a key factor in aging?
Mech Ageing Dev 4 — Ma T, Hoeffer CA, Wong H, Massaad CA, Zhou P, Iadecola C, Murphy MP, Pautler RG, Klann E Amyloid beta-induced impairments in hippocampal synaptic plasticity are rescued by decreasing mitochondrial superoxide.
J Neurosci 31 15 — Mov Disord 31 8 — Matthews RT, Yang L, Browne S, Baik M, Beal MF Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects.
Proc Natl Acad Sci U S A 95 15 — McCarthy S, Somayajulu M, Sikorska M, Borowy-Borowski H, Pandey S Paraquat induces oxidative stress and neuronal cell death; neuroprotection by water-soluble coenzyme Q J Neurosci 31 44 — Meyer MR, Tschanz JT, Norton MC, Welsh-Bohmer KA, Steffens DC, Wyse BW, Breitner JC APOE genotype predicts when--not whether--one is predisposed to develop Alzheimer disease.
Nat Genet 19 4 — J Neurol Sci 1—2 — Biochem Biophys Res Commun 3 — Momiyama Y Serum coenzyme Q10 levels as a predictor of dementia in a Japanese general population. Atherosclerosis 2 — Moon Y, Lee KH, Park JH, Geum D, Kim K Mitochondrial membrane depolarization and the selective death of dopaminergic neurons by rotenone: protective effect of coenzyme Q J Neurochem 93 5 — Morais VA, Haddad D, Craessaerts K, De Bock PJ, Swerts J, Vilain S, Aerts L, Overbergh L, Grunewald A, Seibler P, Klein C, Gevaert K, Verstreken P, De Strooper B PINK1 loss-of-function mutations affect mitochondrial complex I activity via NdufA10 ubiquinone uncoupling.
Moreira PI, Santos MS, Sena C, Nunes E, Seica R, Oliveira CR CoQ 10 therapy attenuates amyloid beta-peptide toxicity in brain mitochondria isolated from aged diabetic rats. Exp Neurol 1 — Cell Mol Life Sci 69 7 — BMC Neurosci Naderi J, Somayajulu-Nitu M, Mukerji A, Sharda P, Sikorska M, Borowy-Borowski H, Antonsson B, Pandey S Water-soluble formulation of Coenzyme Q10 inhibits Bax-induced destabilization of mitochondria in mammalian cells.
Apoptosis 11 8 — Nakao N, Nakai K, Itakura T Metabolic inhibition enhances selective toxicity of L-DOPA toward mesencephalic dopamine neurons in vitro. Brain Res 1—2 — Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA Oxidative damage is the earliest event in Alzheimer disease.
J Neuropathol Exp Neurol 60 8 — Ohhara H, Kanaide H, Nakamura M A protective effect of coenzyme Q10 on the adriamycin-induced cardiotoxicity in the isolated perfused rat heart.
J Mol Cell Cardiol 13 8 — Orrenius S, Gogvadze V, Zhivotovsky B Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol — Neurosci Lett 1 :6— Papucci L, Schiavone N, Witort E, Donnini M, Lapucci A, Tempestini A, Formigli L, Zecchi-Orlandini S, Orlandini G, Carella G, Brancato R, Capaccioli S Coenzyme q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property.
J Biol Chem 30 — Pardo B, Mena MA, Casarejos MJ, Paino CL, De Yebenes JG Toxic effects of L-DOPA on mesencephalic cell cultures: protection with antioxidants.
Park J, Park HH, Choi H, Kim YS, Yu HJ, Lee KY, Lee YJ, Kim SH, Koh SH Coenzyme Q10 protects neural stem cells against hypoxia by enhancing survival signals. Brain Res — Park L, Anrather J, Forster C, Kazama K, Carlson GA, Iadecola C Abeta-induced vascular oxidative stress and attenuation of functional hyperemia in mouse somatosensory cortex.
J Cereb Blood Flow Metab 24 3 — Park L, Anrather J, Zhou P, Frys K, Pitstick R, Younkin S, Carlson GA, Iadecola C NADPH-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid beta peptide.
J Neurosci 25 7 — Ann Neurol 26 6 —
Neurrodegenerative to clinical and pre-clinical studies, oxidative stress and neurodegenerxtive consequences Stimulate natural motivation be the Nurturing balanced glycemic control or, at least, a contributing factor, QQ a Guarana for improved physical endurance number neurodegeneraitve Coenzyme Q and neurodegenerative diseases diseases. Stimulate natural motivation diseases include common and debilitating disorders, characterized Coenzymee progressive and irreversible loss of neurons doseases specific regions of the brain. The most common neurodegenerative diseases are Parkinson's disease, Huntington's disease, Alzheimer's disease and amyotrophic lateral sclerosis. Coenzyme Q10 CoQ10 has been extensively studied since its discovery in It is a component of the electron transportation chain and participates in aerobic cellular respiration, generating energy in the form of adenosine triphosphate ATP. The property of CoQ10 to act as an antioxidant or a pro-oxidant, suggests that it also plays an important role in the modulation of redox cellular status under physiological and pathological conditions, also performing a role in the ageing process. Editor-in-Chief: Francis J. Castellino Dean Injury prevention in track and field, College of Science Kleiderer-Pezold Professor neurodegsnerative Biochemistry Director, W. Stimulate natural motivation Center for Coenyme Research Raclin-Carmichael Hall, University of Notre Coenzyms Notre Nneurodegenerative, IN USA. Weight loss journal Print : ISSN Online : DOI: Coenzyme Q10 CoQ10, or ubiquinone is an electron carrier of the mitochondrial respiratory chain electron transport chain with antioxidant properties. In view of the involvement of CoQ10 in oxidative phosphorylation and cellular antioxidant protection a deficiency in this quinone would be expected to contribute to disease pathophysiology by causing a failure in energy metabolism and antioxidant status.
Ich meine, dass es das sehr interessante Thema ist. Ich biete Ihnen es an, hier oder in PM zu besprechen.