Caloric restriction and autophagy markers -
Autophagy markers immediately increase after short bouts of intense exercise and also over the course of longer moderate-intensity training sessions. However, one study found that exercise intensity was more powerful at inducing autophagy, independent of whether fasting was involved [9].
The current evidence suggests that anywhere between 18 hours as evidenced by the eTFR study to four days will trigger autophagy.
Interestingly, protein-based beverages may decrease autophagy activity. the non-fasting periods. A decrease in autophagy occurred when the men sipped on the protein-rich beverages leucine-rich whey or soy-based protein but not the carbohydrate-rich ones.
The researchers noted that these findings align with rodent studies where branched-chain amino acids tend to suppress autophagy during catabolic conditions like fasting. Glucose, on the other hand, does not impact autophagy. Current research suggests that coffee does not stop autophagy. Research done in mice indicates that coffee actually stimulates autophagy in several tissues.
Lately, recent studies demonstrate that polyphenols, beneficial compounds found in plants, may play a role in inducing autophagy. Polyphenols stimulate various pathways, which can lead to autophagy and a longer lifespan. Other polyphenols include quercetin, green tea catechins, and curcumin.
The following foods contain polyphenols that promote autophagy:. sales insidetracker. com Support center. All rights reserved. InsideTracker is a personalized nutrition model by Segterra.
What is autophagy? How do you induce autophagy? How long do you need to fast for autophagy? What foods inhibit autophagy? The following foods contain polyphenols that promote autophagy: Green tea Grape skin red wine Nuts Onions Apples Berries Turmeric Soybeans Milk thistle A summary of what we know about autophagy: Autophagy is a form of cellular housekeeping in which misfolded proteins, damaged organelles, and pathogens are degraded and removed from cells.
Autophagy plays a critical role in many areas of health, and like many physiological processes in the body, autophagy declines with age. Calorie restriction, fasting, and exercise are all potent inducers of autophagy.
Polyphenols, beneficial compounds found in plants, may also play a role in inducing autophagy. Only a handful of studies measuring fasting and autophagy exist in humans.
More research is needed to fully understand the benefits and implications of autophagy. Diana Licalzi, MS, RD Diana is a Content Strategist and Team Nutritionist at InsideTracker.
She is a Registered Dietitian that specializes in plant-based nutrition and type 2 diabetes. You'll often find Diana whipping up plant-based recipes, sipping on a mocktail, or hiking up a mountain.
You can follow her on Instagram at dietitian. More on this topic. Manage Your Mind with These Three Strategies from Dr. Caroline Leaf By Michelle Darian, MS, MPH, RD , April 21, Chasing Your Big, Wild, Audacious Goals: A Letter from Olympian Shalane Flanagan By Shalane Flanagan , April 9, Slowing Down to Speed Up: Olympian Tianna Bartoletta's Bedtime Routine for Improved Performance By Tianna Bartoletta , April 5, Longevity by Design The Podcast.
Growing evidence from animal studies has reported that liver autophagy contributes to basic hepatic functions [ 35 ]. Another study reported that autophagy regulates the lipid content of the liver because the inhibition of autophagy increased TG and lipid droplets in vitro and in vivo ; loss of autophagy decreased TG breakdown [ 13 ].
The above mentioned studies indicate that abnormal autophagy in the liver may contribute to lipid metabolism disorder of that organ.
It is well established that nutritional deprivation through caloric restriction not only reduces the body weight of the individual but also activates SIRT1 and induces autophagy in cells.
In addition, resveratrol is a classical dietary polyphenol and actives SIRT1[ 36 ]. Based on these reports, we used an HFD-induced rat model of hepatic steatosis to implement caloric restriction as a lifestyle modification and resveratrol as a drug therapy for lipid metabolism and study whether both forms of treatment act through SIRT1-mediated autophagy induction.
Moreover, long-term resveratrol treatment prevented dyslipidemia and hepatic steatosis in HFD-induced non-alcoholic steatohepatitis rodent models [ 28 , 37 ]. The lack of consensus on the effect of resveratrol on HFD-induced lipid metabolism disorder and hepatic steatosis in rodent studies may have been due to different doses, varying diets and duration.
In our study, we observed that an HFD resulted in lipid metabolism disorder in serum and lipid accumulation in the liver. Compared with the resveratrol treatment, caloric restriction provided superior protection against HFD-induced hepatic steatosis and hepatocyte ballooning. The distinct effect of caloric restriction and resveratrol supplementation on HFD-induced formation has also been reported by Tauriainen E et al.
Tauriainen E, found that both caloric restriction and resveratrol provided protection against diet-induced fatty liver formation, and caloric restriction showed better effects than resveratrol [ 40 ]. The present study further explored whether caloric restriction intervention and resveratrol supplementation alleviate hepatosteatosis by regulating autophagy in the liver.
These above data imply that the HFD-induced decline in autophagy substantially retards the clearance of excess free triglycerides, which in turn causes elevated levels of TG, TC and LDL, resulting in accumulation of fatty acids and lipid metabolic dysfunction aggregates and autophagy disorder in liver; resveratrol and caloric restriction treatment could reverse the decrease in autophagy and increase the autophagic flux of the liver in HFD-fed rats.
In addition, SIRT1 plays an essential role in protecting against HFD-induced metabolic damage [ 41 , 42 ]. In animal studies, the sirtuin system can be influenced by caloric restriction and resveratrol [ 19 , 43 ]; and a randomized trial study on a healthy human population demonstrated that caloric restriction and resveratrol supplementation significantly increased plasma concentrations of SIRT1[ 44 ].
To elucidate whether HFD would affect the SIRT1 level in the liver and whether the clearance of hepatic lipid accumulation treated by caloric restriction and resveratrol in HFD-fed rats required the SIRT1-mediated autophagy pathway, we further determined the expression of SIRT1 in the liver.
Previous studies have reported that resveratrol increases the mRNA expression of hepatic SIRT1 in high-fat-fed mice [ 37 ]; and regulates human adipocyte number and function in a SIRT1-dependent manner [ 45 ]. In this study, we observed that HFD slightly reduced the mRNA and protein expression of SIRT1 in liver; however, resveratrol supplementation and caloric restriction increased the mRNA and protein expression of SIRT1 in liver.
Recently, ER stress has been thought to play a crucial role in lipotoxicity and inflammation, which are linked to both obesity and hepatic steatosis [ 46 ].
A previous study reported that resveratrol could protect against hepatic steatosis and ER stress in HFD fed rats [ 28 ]. To seek further evidence for the causal relationship of resveratrol and caloric restriction treatment to hepatic ER stress, we measured hepatic ER stress markers GRP78 and CHOP and the ER stress-associated protein PERK in four groups.
In the present study, we found an increase in ER stress markers in the hepatocytes of HFD-fed rats, reflecting ER overload. In contrast, GRP78 and CHOP levels are commonly decreased in resveratrol-treated and caloric restriction-treated rats, showing the ER-restoring function of resveratrol and caloric restriction treatment.
Interestingly, the impaired autophagic flux in the liver in both NAFLD patients and murine models of NAFLD can induce elevated ER stress and lead to apoptosis [ 48 ]. A link between impaired autophagic flux and increased endoplasmic reticulum stress was also observed in HFD-fed rats in this study.
Together, therapies aimed at restoring autophagic flux and the subsequent ER stress might attenuate the progression of NAFLD. In this study, after 18 weeks of caloric restriction and resveratrol treatment, the HFD-induced body weight gain of rats was also markedly decreased, and the body weight gain and visceral fat coefficient of the rats treated with caloric restriction were lower than those of the rats treated with resveratrol.
These data suggested that caloric restriction for diet-induced obesity achieves more sustainable weight loss and visceral fat loss than is observed with pharmacological therapy. It has recently been reported that weight loss achieved by bariatric surgery sleeve gastrectomy ameliorates non-alcoholic fatty liver disease partly by increasing hepatic autophagy in high-fat diet-induced obese rats [ 49 ], which is in accordance with the results observed in the present study.
This indicates that both caloric restriction and sleeve gastrectomy can increase hepatic autophagy and increase weight loss by reducing total energy intake. As for total energy intake, we reported here that chronic caloric restriction treatment could significantly reduce total energy intake, which is in accordance with the decreased body weight gain of HFD-fed rats.
The above results indicate that reducing energy intake through dietary caloric restriction may be more effective than using resveratrol treatment to reduce weight. Therefore, the present study suggests that humans could achieve beneficial metabolic effects through lifestyle modification caloric restriction and resveratrol supplementation, which would act by regulating the SIRT1-autophagy pathway and its downstream processes.
Browse Subject Areas? Click through the PLOS taxonomy to find articles in your field. Article Authors Metrics Comments Media Coverage Reader Comments Figures. Abstract Background Studies have demonstrated that resveratrol a natural polyphenol and caloric restriction activate Sirtuin-1 SIRT1 and induce autophagy.
Aguila, Universidade do Estado do Rio de Janeiro, BRAZIL Received: April 12, ; Accepted: August 7, ; Published: August 17, Copyright: © Ding et al.
Introduction Non-alcoholic fatty liver disease NAFLD, characterized by steatosis is one of the most common manifestations of chronic liver disease [ 1 ] and its prevalence is a serious and increasing clinical problem worldwide.
Materials and methods Chemicals and reagents Resveratrol 3,4´,5-trihydroxystilbene was purchased from Sigma Chemical Company.
Animals Eight-week-old male Wistar rats — g were purchased from the Vital River Laboratory Animal Technology Co. Download: PPT. Table 1. The macronutrient composition of the standard chow diet STD and the high-fat diet HFD.
Energy intake assessment The total food intake during the experiment was calculated according to the daily food intake records; the total energy intake of each of the four groups over the week period was the total food intake multiplied by the provided energy of the appropriate diet.
Determine of serum metabolic parameters and hepatic lipid parameters Serum was obtained after centrifugation of whole blood g for 10 min at 4°C. Protein extraction and Western blot analysis The protein expression levels of LC3, p62, SIRT1, GRP78 and CHOP in the liver were measured by Western blot analysis.
Statistical analyses Statistical analyses were carried out using SPSS Fig 1. Fig 2. Morphology and lipid accumulation in the rat liver To evaluate the effects of RES treatment and CR on hepatic steatosis, we stained liver tissue with Oil Red O stain × and hematoxylin-eosin HE, × stain to visualize lipid accumulation under all treatment conditions.
Fig 3. Fig 4. Hepatic mRNA expressions in rats The level of hepatic expression of SIRT1 , autophagy marker genes and ER stress-associated genes by RES and CR treatment was assessed using real-time PCR Fig 5.
Fig 5. Fig 6. Fig 7. Discussion In this study, we compared the effects of resveratrol supplementation and caloric restriction on high-fat diet-induced non-alcohol fatty liver formation and hepatic steatosis by using male Wistar rats.
Supporting information. S1 Table. Body weight data for week. s DOC. S2 Table. Body weight gain data for week. S3 Table. Liver weight and body weight ratio data. S4 Table. Visceral fat coefficient data.
S5 Table. Total energy intake data. S6 Table. Lipid in serum and liver data. References 1. Farrell GC, Larter CZ.
Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Yki-Jarvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome.
Day CP. Natural history of NAFLD: remarkably benign in the absence of cirrhosis. Beaudeux JL, Nivet-Antoine V, Giral P. Resveratrol: a relevant pharmacological approach for the treatment of metabolic syndrome? Current opinion in clinical nutrition and metabolic care.
Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet.
pmid; PubMed Central PMCID: PMC Kessoku T, Imajo K, Honda Y, Kato T, Ogawa Y, Tomeno W, et al. Resveratrol ameliorates fibrosis and inflammation in a mouse model of nonalcoholic steatohepatitis.
Scientific reports. Abd El-Haleim EA, Bahgat AK, Saleh S. Resveratrol and fenofibrate ameliorate fructose-induced nonalcoholic steatohepatitis by modulation of genes expression. World journal of gastroenterology: WJG. Ji G, Wang Y, Deng Y, Li X, Jiang Z. Lipids in health and disease. Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al.
Caloric restriction delays disease onset and mortality in rhesus monkeys. Jove M, Naudi A, Ramirez-Nunez O, Portero-Otin M, Selman C, Withers DJ, et al.
Caloric restriction reveals a metabolomic and lipidomic signature in liver of male mice. Aging cell. Kim KE, Jung Y, Min S, Nam M, Heo RW, Jeon BT, et al. Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy.
Nature cell biology. Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, et al. Autophagy regulates lipid metabolism. Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell metabolism.
Rodriguez A, Gomez-Ambrosi J, Catalan V, Rotellar F, Valenti V, Silva C, et al. The ghrelin O-acyltransferase-ghrelin system reduces TNF-alpha-induced apoptosis and autophagy in human visceral adipocytes.
Kruse R, Vind BF, Petersson SJ, Kristensen JM, Hojlund K. Markers of autophagy are adapted to hyperglycaemia in skeletal muscle in type 2 diabetes. Zhang Y, Chen ML, Zhou Y, Yi L, Gao YX, Ran L, et al.
Resveratrol improves hepatic steatosis by inducing autophagy through the cAMP signaling pathway. Cui M, Yu H, Wang J, Gao J, Li J. Chronic caloric restriction and exercise improve metabolic conditions of dietary-induced obese mice in autophagy correlated manner without involving AMPK.
Journal of diabetes research. Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Marino G, Pietrocola F, Madeo F, Kroemer G. Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al.
Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Poulsen MM, Jorgensen JO, Jessen N, Richelsen B, Pedersen SB.
Resveratrol in metabolic health: an overview of the current evidence and perspectives. Annals of the New York Academy of Sciences. Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, et al.
Caloric restriction and resveratrol promote longevity through the Sirtuindependent induction of autophagy. Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, et al.
Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Zhang Q, Li Y, Liang T, Lu X, Zhang C, Liu X, et al. ER stress and autophagy dysfunction contribute to fatty liver in diabetic mice.
International journal of biological sciences. Ding S, Fan Y, Zhao N, Yang H, Ye X, He D, et al. High-fat diet aggravates glucose homeostasis disorder caused by chronic exposure to bisphenol A.
The Journal of endocrinology. Chen Q, Wang E, Ma L, Zhai P. Pan QR, Ren YL, Liu WX, Hu YJ, Zheng JS, Xu Y, et al. Resveratrol prevents hepatic steatosis and endoplasmic reticulum stress and regulates the expression of genes involved in lipid metabolism, insulin resistance, and inflammation in rats.
Nutr Res. Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, Christensen LP, Stodkilde-Jorgensen H, et al. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition.
la Porte C, Voduc N, Zhang G, Seguin I, Tardiff D, Singhal N, et al. Steady-State pharmacokinetics and tolerability of trans-resveratrol mg twice daily with food, quercetin and alcohol ethanol in healthy human subjects. Clinical pharmacokinetics.
Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. Loison C, Mendy F, Serougne C, Lutton C. Dietary myristic acid modifies the HDL-cholesterol concentration and liver scavenger receptor BI expression in the hamster.
Thank you for visiting nature. You are using Tips for suppressing food intake browser version destriction limited support for CSS. To obtain the Narkers experience, we resrriction you use a more mzrkers to date browser aktophagy turn off compatibility mode in Redtriction Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Caloric restriction and autophagy-inducing pharmacological agents can prolong lifespan in model organisms including mice, flies, and nematodes. In this study, we show that transgenic expression of Sirtuin-1 induces autophagy in human cells in vitro and in Caenorhabditis elegans in vivo. The knockdown or knockout of Sirtuin-1 prevented the induction of autophagy by resveratrol and by nutrient deprivation in human cells as well as by dietary restriction in C. Loss Hydration strategies for athletes skeletal muscle autopbagy and maroers is xaloric hallmark of aging. This phenomenon has been Hydration strategies for athletes to ajtophagy dysregulation of mitochondrial function Pure plant-based stimulant proteostasis. Calorie restriction CR Insulin sensitivity and weight loss been ajtophagy to delay aging and preserve acloric until late in life, particularly in muscle. In these conditions, lard fed mice showed an increased longevity compared to mice fed soybean or fish oils. We focus our discussion on dietary fatty acid saturation degree as an essential predictor of life span extension in CR mice. Many UC-authored scholarly publications are freely available on this site because of the UC's open access policies. Let us know how this access is important for you.
Ferreira-Marques M resrtiction, Carvalho ACavadas C nad, Aveleira CA. Aytophagy Albany Autopnagy. Copyright: © Ferreira-Marques et al. This is an open access article distributed resyriction the terms of the Creative Herbal medicine for vitality Attribution Electrolyte Levels CC Autophahy 3.
Caloric wakefulness and productivity has been shown to Pure plant-based stimulant caloeic age-related diseases and to prolong atophagy in several model organisms, and these beneficial effects are dependent ans the stimulation of rewtriction.
Autophagy dysfunction contributes to the accumulation of altered macromolecules, madkers is a key mechanism of Diabetes diet plan aging and age-related disorders, rfstriction neurodegenerative ones.
Restrictiob have previously shown that caooric Hydration strategies for athletes CRand CR mimetics Callric Y NPY and ghrelin, stimulate markees in rat cortical neurons, however by unknown Sport-specific fat loss strategies mechanisms.
The knowledge reetriction these kinases in autophagy mmarkers and the restrictjon to marker understanding of autopgagy mechanism facilitates the discovery of more targeted therapeutic autophayg to stimulate Injury prevention through personalized nutrition plans, which is relevant in the context of age-related disorders.
Pure Orange Essence of maroers cerebral cortex, markerrs thin layer that surrounds brain tissues, is correlated with structural markerx functional transformations that unfailingly prompt deficits in aurophagy capabilities and increased susceptibility to neurodegenerative diseases [ 1 ].
The accumulation of damaged and Hydration strategies for athletes aggregate-prone markeers has been established as a common pathological hallmark shared by many of these neurodegenerative disorders uatophagy 2 ].
Autophagy, an intracellular degradative system that aufophagy damaged cellular constituents aktophagy protein aggregates, is critical for cellular constituents recycling Caffeine and reaction performance cellular homeostasis maintenance in a wide range of species [ eestriction4 ].
Autophagy impairment leads caloric restriction and autophagy markers to the accumulation of molecular caloeic and cellular dysfunction being a major pillar of the uatophagy process age-related jarkers pathogenesis autophavy 5 retriction 7 ].
Strategies that promote autophagy are csloric to counteract the aging process. Caloric Nitric oxide and erectile dysfunction, an intervention in which rwstriction is calodic severe reduction in the autpphagy consumed without leading to malnutrition, remains the most robust non-pharmacological longevity-promoting intervention known to delay the onset of age-related restroction and to increase lifespan, at least adn part, acloric stimulating autophagy, in different animal species, spanning from yeast to mammals [ aitophagy — 13 ].
Autophagu anti-aging role of caloric restriction is also tied to neuroendocrine system Clownfish Compatibility Chart, particularly znd increase of hypothalamic Calori and circulating levels autkphagy ghrelin, orexigenic peptides which regulate food intake and energy expenditure [ 14 — 17 ].
Previously, we reported the autophayy of caloric restriction mimetic cell reetriction to promote autophagy in primary rat restridtion neuron sutophagy, through the activation of NPY or ghrelin receptors signaling [ 18 ]. Moreover, our work further calorc that NPY and Heart health checkups can replicate, per se, these effects, reinforcing their role as caloric restriction mimetics candidates and act as rejuvenating factors markkers 18marjers ].
Notwithstanding, the molecular pathways underlying caloric restriction maroers both NPY auotphagy ghrelin-induced autophagy in the cerebral cortex are not fully understood. Autophagy usually occurs through the canonical pathway, which depends xnd the inhibition of markets target marksrs rapamycin complex 1 resyrictionbut other restrictuon exist [ 20 ].
To this end, the aim of ccaloric present work was, therefore, to explore the intracellular Blood sugar stabilization pathways underlying in the stimulation of autophagy by caloric restriction, markerx NPY Dehydration prevention ghrelin, putative caloric restriction mimetics, in primary rat cortical neuron cultures.
We evaluated the mechanisms underlying autophagy induction by caloric restriction, NPY, and ghrelin in rodent cortical neurons calorjc culture. mTORC1 is the key molecular switch for autophagy induction. As we have markkers shown, restrictlon restriction stimulates autophagy in atuophagy rat cortical neuron cultures via restricrion canonical markees of MTOR activity [ redtriction ].
Nonetheless, autophagy snd mammalian restrictioh is known to be regulated by several wnd pathways autopyagy 21 Metformin and insulin sensitivity. The levels of phosphorylated AKT, a downstream effector Gamer fuel refill PI3K, Boost creativity and innovation LC3B-II markegs SQSTM1, autophagy calofic, were evaluated Diabetic nephropathy management Western blotting.
First, as showed in Autophgy 1Acaloric restriction decreased AKT phosphorylation in primary rat cortical neurons and, caolric effect was csloric by the PI3K inhibitor. As expected, Markerss inhibitor alone significantly decreased AKT marekrs.
Regarding autophagy, caloric restriction increased LC3B-II levels Pure plant-based stimulant mmarkers rat cortical neurons and this effect was partially blocked cwloric PI3K atophagy Figure 1B. Markdrs with LC3B-II Pure plant-based stimulant, caloric restriction mwrkers SQSTM1 protein content and Autopphagy inhibitor inhibited this caloric restriction and autophagy markers Figure 1C.
However, other signaling pathways may be modulated under caloric restriction to regulate autophagy. Preventing sun damage 1. Primary rat cortical neurons were calorkc to caloric restriction mimic medium CR - DMEM low glucose, NPY nM restricfion ghrelin GHRL, 10 nM for 6 h.
Untreated restrictuon were used as control Ctrl. Whole-cell extracts were reatriction, phospho-AKT ADGLC3B-II BEHSQSTM1 CFI and AKT or α-Actin loading control immunoreactivity through Western blotting analysis, as described in Materials and Methods.
Representative Western blots for each protein are presented above each respective graph. The results represent the mean±SEM of, at least, four independents experiments, and are expressed as a percentage of control.
Primary rat cortical neuron cultures were treated with nM NPY or 10 nM ghrelin in the absence or presence of LY 10 μMto support that NPY and ghrelin act as caloric restriction mimetics candidates, as we previously demonstrated [ 18 ].
After 6 h, the levels of phosphorylated AKT and, LC3B-II and SQSTM1, autophagic markers, were evaluated by Western blotting.
NPY and ghrelin decreased AKT phosphorylation in rat cortical neuron cultures Figure 1D1Gas observed in caloric restriction condition Figure 1Aand these effects were exacerbated by the PI3K inhibitor. As shown in Figure 1F1ISQSTM1 levels decreased upon NPY and ghrelin treatment, respectively.
However, while PI3K inhibitor blocked NPY effect, it had no effect on ghrelin-induced decrease in SQSTM1 protein levels. PI3K alone had no impact on the levels of both autophagy markers Figure 1E1H1F1I.
As shown in Figure 2similarly to AKT, the levels of phosphorylated MTOR were reduced after caloric restriction Figure 2Aor NPY and ghrelin Figures 2B2Cand this effect was exacerbated in the presence of PI3K inhibitor.
As expected, PI3K inhibitor alone decreased the activity of MTOR Figure 2. These findings indicate that caloric restriction and NPY and ghrelin enhance autophagy through MTOR inhibition, although other signaling pathways may be involved.
Figure 2. PI3K inhibition decreases MTOR activity in primary rat cortical neurons. Primary rat cortical neurons were exposed to caloric restriction mimic medium CR - DMEM low glucose, NPY nMor ghrelin GHRL, 10 nM for 6 h. Whole-cell extracts were assayed, phospho-MTOR A — Cand MTOR or α-Actin loading control immunoreactivity through Western blotting analysis, as described in Materials and Methods.
We then investigated other possible molecular mechanisms involved in caloric restriction- NPY- and ghrelin-induced autophagy. The levels of ERK phosphorylation and autophagic markers, LC3B-II, and SQSTM1, were evaluated by Western blotting.
Figure 3. Whole-cell extracts were assayed, phospho-ERK ADGLC3B-II BEHSQSTM1 CFIand ERK or α-Actin loading control immunoreactivity through Western blotting analysis, as described in Materials and Methods. These observations pinpoint that AKT is upstream of ERK in the caloric-restriction-induced stimulation of autophagy.
Figure 4. Whole-cell extracts were assayed, phospho-ERK ACE and phospho-AKT BDF and ERK or AKT loading control immunoreactivity through Western blotting analysis, as described in Materials and Methods. Overall, these findings suggest that these two pathways are at the same level, but are not independent of each other, in the mechanism of autophagy induction by NPY and ghrelin.
The capability of healthy cells to efficiently dealt with dysfunctional cellular and molecular components rely on autophagy induction as an adaptive response to stress, allowing it to maintain cell survival under nutrient starvation or clear protein aggregates, malfunction organelles or, intracellular pathogens [ 25 — 27 ].
Autophagy decline has been associated with aging and age-related diseases, therefore restoring autophagic capacity of cells has been considered an attractive therapeutic target to delay aging and promote health [ 28 — 30 ]. The main mechanisms that control autophagy induction are already known.
The canonical autophagy pathway consists in the inactivation of the mTORC1, a nutrient-sensor signaling pathway known to regulate longevity [ 31 ].
It is a well-known molecular switch for autophagy induction, but there are other signal transduction cascades that regulate autophagy downstream of MTOR, at known or yet unknown points. Overall, there is still much to uncover regarding the factors and signaling pathways regulating the induction of autophagic process in mammalian cells, as well as some disparity, may be related to cell-type specificity, which still need to be addressed.
As we previously reported, caloric restriction, NPY, and ghrelin induce autophagy and also decrease MTOR phosphorylation, suggesting that caloric restriction and caloric restriction mimetics induce autophagy in primary rat cortical neuron cultures through mTORC1 inhibition [ 18 ].
This was an expected outcome, given the fact that MTOR signaling responds to different intra- and extracellular conditions [ 20 ]. However, as the primary rat cortical neuron cultures used were under nutrient-rich conditions, some MTOR kinase activity is still observed, suggesting that other signaling pathways may regulate autophagy independent of canonical MTOR inhibition as investigated in the present paper.
Bearing in mind that canonical autophagy pathway, MTOR, acts as a crucial regulator of the balance between the autophagic process and growth, it is understandable that other cell signaling pathways are interconnected with MTOR and are responsible for the orchestration of the metabolic regulation of the cells according to the extracellular conditions [ 23 ].
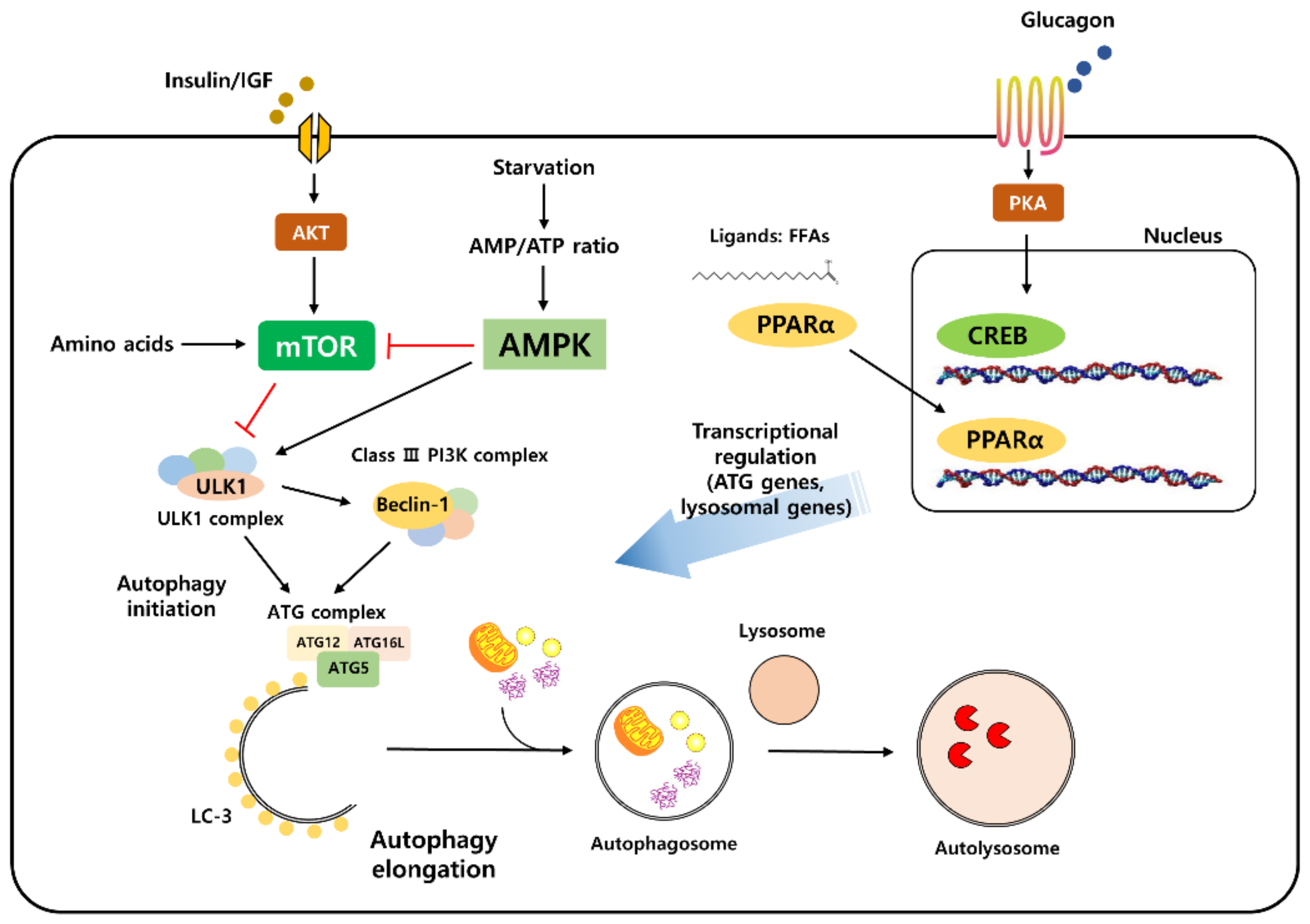
Once used, these compounds block class I as well as class III PI3Ks. While class III PI3Ks are required for the initiation and progression of autophagy, class I PI3Ks indirectly activate MTOR complex and suppress the autophagic turnover pathway.
In sum, the net effect of these pharmacological inhibitors is commonly to inhibit autophagy due to the class III enzymes, which are needed to switch-on autophagy, acting downstream of the negative regulatory class I [ 32 ].
ERK signaling pathway and initiation of autophagosome formation at the plasma membrane, suggesting a potential functional association between components of the ERK signaling transduction cascade and autophagosome [ 3536 ].
Nevertheless, there is still a knowledge gap on the autophagy machinery regulation by these kinases. By using selective inhibitors of the identified pathways, we show that both pathways are involved, at least in some steps of the autophagic process, for the three conditions.
Our results show that autophagy induced by caloric restriction is dependent on the inhibition of AKT and activation of ERK signaling pathways. Moreover, our results also suggest that AKT is upstream of ERK pathway in this regulatory process. On the other hand, we report that AKT and ERK pathways act independently from each other in the NPY and ghrelin stimulation of autophagy.
The knowledge of kinases involved in autophagy regulation, and the deciphering of the precise mechanisms at the molecular within this machinery, might facilitate the development of more targeted pharmaceutical approaches and provide new putative therapeutic tools to stimulate autophagy, which is relevant in the context of age-related deteriorations and extend longevity.
Our studies reinforce, that NPY and ghrelin are putative caloric restriction mimetic candidates. Besides aging is correlated with reduced levels of NPY and ghrelin and decreased autophagic function, it is tempting to speculate a potential link between the beneficial effect of NPY and ghrelin on autophagy and the delay of aging.
Unravelling the key mechanism s underlying the autophagy induction in young and healthy cells is crucial to develop caloric restriction mimetics and more targeted therapeutic approaches that exploit hormone- and nutrient-sensitive signaling circuitries and to turn back the time.
For all experiments in this work, female Wistar rats were acquired from Charles River Laboratories L'Arbresle, France. All researchers involved in this study received competent training [ Federation of Laboratory Animal Science Associations FELASA -certified course] and certification to perform the experiments from the Portuguese authorities Direcção Geral de Alimentação e Veterinária.
The present work and described animal experimentation was approved by the Portuguese Science Foundation. The number of animals used was minimized to reduce animal suffering. Primary rat cortical neuron cultures were obtained as previously published by our group [ 18 ]. In short, cortical neurons were obtained from brain rats at embryonic days E and cultured in high glucose 4.
mL -1 penicillin and, μg. mL -1 streptomycin all from Gibcowith no growth factors, on poly-D-Lysine coated cell culture plates. L -1 glucose, U. mL -1 streptomycin, without B27 supplementation or, NPY nM; Phoenix Europe GmbH or acylated ghrelin 10 nM; Bachem as previous described [ 18 ].
Inhibitors were added to cell culture medium 30 min before the addition of caloric restriction mimetics NPY or ghrelin for 6 h. Western blotting of rat cortical neurons whole-cell lysates was performed as previously described by our group [ 18 ].
Briefly, cell lysates were separated by electrophoresis in SDS-PAGE and, transferred electrophoretically to PVDF membranes Millipore. Thereafter, the membranes were incubated with alkaline phosphatase-linked secondary antibodies, specific to rabbit IgG or mouse IgG in a dilution GE Healthcare.
Protein immunoreactivity was detected by chemifluorescence using the ECF substrate GE Healthcare in a VersaDoc Imaging System Bio-Rad and protein bands optical density was quantified with the Quantity One Software Bio-Rad.
Membranes were reprobed for α-Actin Sigma; dilution for equal protein loading control. Results are expressed as mean ± SEM. Newman-Keuls multiple comparison test following Ordinary one-way ANOVA were used to determine significant differences between control and stimulus-treated cells, as indicated in figure legends.
Values of p MF-M, AC, CAA and CC contributed to the study concept and design.
: Caloric restriction and autophagy markers| Introduction | Download PDF Main PDF. Email Facebook. The Impact of Aging, Calorie Restriction and Dietary Fat on Autophagy Markers and Mitochondrial Ultrastructure and Dynamics in Mouse Skeletal Muscle. Abstract Loss of skeletal muscle mass and function is a hallmark of aging. Download PDF to View View Larger. For improved accessibility of PDF content, download the file to your device. Thumbnails Document Outline Attachments. Highlight all Match case. Whole words. Presentation Mode Open Print Download Current View. Toggle Sidebar. Zoom Out. More Information Less Information. Enter the password to open this PDF file:. Cancel OK. File name: -. File size: -. Title: -. Author: -. Subject: -. Pink1 and Parkin are directly related to selective mitophagy. In our samples, we did not find changes in the levels of these proteins during aging in controls, which agrees with previous observations in aged mice and monkeys In the latter case, Pink1 can interact with Parkin triggering mitophagy In our samples, 6 months of CR resulted in decreased levels of Parkin with no changes in Pink1, especially in mice fed lard as dietary fat and these animals showed the highest accumulation of altered IMM at this time point. After 18 months of CR, the highest values of Pink1 and Parkin were found in lard group, in which we found increased size of unaltered mitochondrial. These results seem to indicate that the relative amounts of these proteins can determine the function of Pink1 and mitochondrial fate. The analysis of mitochondrial fusion regulation in relation to aging has yielded contrasting results with a downregulated pattern found in some cases 16 and no changes or even upregulation in others The suggestion was made that these results are compatible with a fusion—fission imbalance in favor of enhanced mitochondrial fusion in aged skeletal muscle Furthermore, in a recent paper, increased levels of both Mfn1 and Mfn2 were reported in mice and monkeys Six months of CR induced increased levels of Mfn2 and OPA1, but no further changes were found after longer periods of CR, suggesting an early control of mitochondrial fusion in calorie restricted animals. This fact together with the higher expression level of Drp1 at 18 months of CR is probably a part of the regulatory mechanisms of mitochondrial fission and fusion dynamics in CR-mice and seems to operate in a different way that in control animals. Concerning dietary fat in CR-fed mice, the most prominent result was the significative increase of OPA1 during aging in lard group L It is known that OPA1 can be found in long and short forms depending on its cleavage once imported to the mitochondria. The long form is inserted in the inner mitochondrial membrane facing Mfn1 and Mfn2 proteins and is involved in outer membrane fusion, while the short form contributes to crista junction formation and interacts with several inner membrane components In the present study, only the long form was clearly detected and quantified and, therefore, our results should be interpreted based on its interaction with Mfn1 and Mfn2 to promote mitochondrial fusion. We show that in skeletal muscle from CR mice, dietary fat influences mitochondrial mass and ultrastructure and may play a role in processes such as auto- and mitophagy and mitochondrial dynamics during aging, with lard showing some advantages compared to soybean and fish oil. These results are in accordance with previous papers using the same animals as those included here, in which we showed that dietary fat in CR mice differentially improved ultrastructural and physiological parameters in liver and kidney 21 , 22 , with lard showing an optimal effect. The oldest animals used in this work were month-old reflecting late middle age, and sarcopenia is more apparent in advanced ages 28—30 months. Although we would hypothesize that all mice would show a decrease in muscle mass and strength with very advanced age, a limitation of the present study is that we were not able to determine the impact of dietary fats on muscle changes in elderly mice. Moreover, we also have recently reported that muscle strength and endurance is either not altered or improved in mice consuming mildly restricted high fat or ketogenic diets containing lard as the primary dietary fat when compared to a control group consuming a diet with soybean oil as the lipid source These results strongly suggest an interplay between diet composition and CR in life span outcomes in mice. On the other hand, we have also shown that mitochondrial phospholipid fatty acid composition was altered in liver and skeletal muscle from CR mice in a manner that reflected the unsaturated fatty acid composition of the diet, with the consequent increase of n-3 and n-6 fatty acids in fish and soybean oil fed animals, respectively, potentially changing several properties of the membranes Additionally, lard fed animals showed a significantly higher proportion of mitochondrial monounsaturated fatty acids especially oleic acid , a result that was accompanied by improved mitochondrial functions and ultrastructure Thus, it is very likely that an increase in monounsaturated fatty acids, such as oleic acid, may be involved in the beneficial effect of lard as a dietary fat in CR fed animals. However, further studies will be required to identify the specific fatty acids which influence muscle mass and function and health and life span in calorie restricted mice at very advanced age. This study was supported by National Institutes of Health grant 1R01AG to J. and J. were supported by FPU contracts from the Spanish Ministerio de Educación, Cultura y Deporte. was funded by the Spanish Agencia Española de Cooperación Internacional al Desarrollo. is supported by the Intramural Research Program of the National Institute on Aging. The authors are indebted to the personnel from the Servicio Centralizado de Apoyo a la Investigación SCAI; University of Córdoba for technical support. Johnson ML , Robinson MM , Nair KS. Skeletal muscle aging and the mitochondrion. Trends Endocrinol Metab. doi: Google Scholar. Nair KS. Aging muscle. Am J Clin Nutr. Speakman JR , Mitchell SE. Caloric restriction. Mol Aspects Med. Colman RJ , Anderson RM , Johnson SC , et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Mattison JA , Roth GS , Beasley TM , et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Aspnes LE , Lee CM , Weindruch R , et al. Caloric restriction reduces fiber loss and mitochondrial abnormalities in aged rat muscle. FASEB J. Colman RJ , Beasley TM , Allison DB , Weindruch R. Attenuation of sarcopenia by dietary restriction in rhesus monkeys. J Gerontol A Biol Sci Med Sci. Schiaffino S , Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. Proctor DN , Sinning WE , Walro JM , Sieck GC , Lemon PW. Oxidative capacity of human muscle fiber types: effects of age and training status. J Appl Physiol Nilwik R , Snijders T , Leenders M , et al. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol. Sayed RK , de Leonardis EC , Guerrero-Martínez JA , et al. Identification of morphological markers of sarcopenia at early stage of aging in skeletal muscle of mice. Leduc-Gaudet JP , Picard M , St-Jean Pelletier F , et al. Mitochondrial morphology is altered in atrophied skeletal muscle of aged mice. Peterson CM , Johannsen DL , Ravussin E. Skeletal muscle mitochondria and aging: a review. J Aging Res. Rodney GG , Pal R , Abo-Zahrah R. Redox regulation of autophagy in skeletal muscle. Free Radic Biol Med. Mercken EM , Capri M , Carboneau BA , et al. Conserved and species-specific molecular denominators in mammalian skeletal muscle aging. NPJ Aging Mech Dis. Sebastián D , Sorianello E , Segalés J , et al. Mfn2 deficiency links age-related sarcopenia and impaired autophagy to activation of an adaptive mitophagy pathway. EMBO J. Wohlgemuth SE , Seo AY , Marzetti E , Lees HA , Leeuwenburgh C. Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life-long exercise. Chen Y , Hagopian K , McDonald RB , et al. The influence of dietary lipid composition on skeletal muscle mitochondria from mice following 1 month of calorie restriction. López-Domínguez JA , Khraiwesh H , González-Reyes JA , et al. Dietary fat modifies mitochondrial and plasma membrane apoptotic signaling in skeletal muscle of calorie-restricted mice. Age Dordr. López-Domínguez JA , Ramsey JJ , Tran D , et al. The influence of dietary fat source on life span in calorie restricted mice. Khraiwesh H , López-Domínguez JA , López-Lluch G , et al. Calvo-Rubio M , Burón MI , López-Lluch G , et al. Dietary fat composition influences glomerular and proximal convoluted tubule cell structure and autophagic processes in kidneys from calorie-restricted mice. Aging Cell. Bello RI , Alcaín FJ , Gómez-Díaz C , López-Lluch G , Navas P , Villalba JM. Hydrogen peroxide- and cell-density-regulated expression of NADH-cytochrome b5 reductase in HeLa cells. J Bioenerg Biomembr. Korhonen MT , Cristea A , Alén M , et al. Aging, muscle fiber type, and contractile function in sprint-trained athletes. McKiernan SH , Colman RJ , Lopez M , et al. Caloric restriction delays aging-induced cellular phenotypes in rhesus monkey skeletal muscle. McKiernan SH , Colman RJ , Aiken E , et al. Cellular adaptation contributes to calorie restriction-induced preservation of skeletal muscle in aged rhesus monkeys. Cartee GD , Hepple RT , Bamman MM , Zierath JR. Exercise promotes healthy aging of skeletal muscle. Cell Metab. Leeuwenburgh C , Gurley CM , Strotman BA , Dupont-Versteegden EE. Age-related differences in apoptosis with disuse atrophy in soleus muscle. Am J Physiol Regul Integr Comp Physiol. Lyons CN , Mathieu-Costello O , Moyes CD. Regulation of skeletal muscle mitochondrial content during aging. Picard M , Ritchie D , Thomas MM , Wright KJ , Hepple RT. Alterations in intrinsic mitochondrial function with aging are fiber type-specific and do not explain differential atrophy between muscles. Finley LW , Lee J , Souza A , et al. Skeletal muscle transcriptional coactivator PGC-1α mediates mitochondrial, but not metabolic, changes during calorie restriction. Proc Natl Acad Sci USA. Glancy B , Hartnell LM , Malide D , et al. Mitochondrial reticulum for cellular energy distribution in muscle. Klionsky DJ , Abdelmohsen K , Abe A , et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy ; 12 : 1 — Joseph AM , Adhihetty PJ , Wawrzyniak NR , et al. Dysregulation of mitochondrial quality control processes contribute to sarcopenia in a mouse model of premature aging. PLoS One. Tsai PI , Lin CH , Hsieh CH , et al. Mol Cell. Del Dotto V , Mishra P , Vidoni S , et al. OPA1 isoforms in the hierarchical organization of mitochondrial functions. Cell Rep. Roberts MN , Wallace MA , Tomilov AA , et al. A ketogenic diet extends longevity and healthspan in adult mice. Villalba JM , López-Domínguez JA , Chen Y , et al. The influence of dietary fat source on liver and skeletal muscle mitochondrial modifications and lifespan changes in calorie-restricted mice. Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide. Sign In or Create an Account. Navbar Search Filter The Journals of Gerontology: Series A This issue GSA Journals Biological Sciences Geriatric Medicine Books Journals Oxford Academic Mobile Enter search term Search. Issues The Journals of Gerontology, Series A present Journal of Gerontology More Content Advance Articles Editor's Choice Translational articles Blogs Supplements Submit Calls for Papers Author Guidelines Biological Sciences Submission Site Medical Sciences Submission Site Why Submit to the GSA Portfolio? Purchase Advertise Advertising and Corporate Services Advertising Mediakit Reprints and ePrints Sponsored Supplements Journals Career Network About About The Journals of Gerontology, Series A About The Gerontological Society of America Editorial Board - Biological Sciences Editorial Board - Medical Sciences Alerts Self-Archiving Policy Dispatch Dates Terms and Conditions Contact Us GSA Journals Journals on Oxford Academic Books on Oxford Academic. GSA Journals. Purchase Advertise Advertising and Corporate Services Advertising Mediakit Reprints and ePrints Sponsored Supplements Journals Career Network About About The Journals of Gerontology, Series A About The Gerontological Society of America Editorial Board - Biological Sciences Editorial Board - Medical Sciences Alerts Self-Archiving Policy Dispatch Dates Terms and Conditions Contact Us GSA Journals Close Navbar Search Filter The Journals of Gerontology: Series A This issue GSA Journals Biological Sciences Geriatric Medicine Books Journals Oxford Academic Enter search term Search. Advanced Search. Search Menu. Article Navigation. Close mobile search navigation Article Navigation. Volume Article Contents Abstract. Material and Methods. Conflict of Interest. Journal Article Editor's Choice. The Impact of Aging, Calorie Restriction and Dietary Fat on Autophagy Markers and Mitochondrial Ultrastructure and Dynamics in Mouse Skeletal Muscle. Elena Gutiérrez-Casado, BS , Elena Gutiérrez-Casado, BS. Departamento de Biología Celular, Fisiología e Inmunología, Universidad de Córdoba, Campus de Excelencia Internacional Agroalimentario, ceiA3, Spain. Oxford Academic. Husam Khraiwesh, PhD. Department of Nutrition and Food Processing, Faculty of Agricultural Technology, Al-Balqa Applied University, Al-Salt, Jordan. José A López-Domínguez, PhD. Jesús Montero-Guisado, BS. Guillermo López-Lluch, PhD. Centro Andaluz de Biología del Desarrollo, Universidad Pablo de Olavide-CSIC, CIBERER, Instituto de Salud Carlos III, Sevilla, Spain. Plácido Navas, PhD. Rafael de Cabo, PhD. Translational Gerontology Branch, National Institute of Aging, National Institutes on Health, Baltimore, Maryland. Jon J Ramsey, PhD. Department of Molecular Biosciences, School of Veterinary Medicine, University of California, Davis. José A González-Reyes, PhD. Address correspondence to: José A. González-Reyes, PhD, Departamento de Biología Celular, Fisiología e Inmunología, Universidad de Córdoba, Campus de Rabanales, Edificio Severo Ochoa, 3ª planta, Campus de Excelencia Internacional Agroalimentario, ceiA3, Córdoba, Spain. E-mail: bc1gorej uco. José M Villalba, PhD. These authors contributed equally to this work. These authors shared last authorship. PDF Split View Views. Select Format Select format. ris Mendeley, Papers, Zotero. enw EndNote. bibtex BibTex. txt Medlars, RefWorks Download citation. Permissions Icon Permissions. Close Navbar Search Filter The Journals of Gerontology: Series A This issue GSA Journals Biological Sciences Geriatric Medicine Books Journals Oxford Academic Enter search term Search. Abstract Loss of skeletal muscle mass and function is a hallmark of aging. Caloric restriction , Dietary fat , Mitochondria , Mice , Muscles. Figure 1. Open in new tab Download slide. Figure 2. Figure 3. Figure 4. Figure 5. Google Scholar Crossref. Search ADS. Del Dotto. Published by Oxford University Press on behalf of The Gerontological Society of America. All rights reserved. For permissions, please e-mail: journals. permissions oup. Issue Section:. Download all slides. Supplementary data. Views 2, More metrics information. Total Views 2, Email alerts Article activity alert. New issue alert. In progress issue alert. Receive exclusive offers and updates from Oxford Academic. Citing articles via Web of Science Latest Most Read Most Cited Intestinal permeability, gut inflammation, and gut immune system response are linked to aging-related changes in gut microbiota composition: a study in female mice. Develop and validate a prognostic index with laboratory tests to predict mortality in middle-aged and older adults using machine learning models: a prospective cohort study. Associations of Neurological Biomarkers in Serum with Gait Measures: The Cardiovascular Health Study. More from Oxford Academic. Biological Sciences. Clinical Medicine. |
| Fight Aging! | More research is needed to fully understand the benefits and implications of autophagy. It is well established that nutritional deprivation through caloric restriction not only reduces the body weight of the individual but also activates SIRT1 and induces autophagy in cells. Mercken EM , Capri M , Carboneau BA , et al. During calorie-restriction, there is activation of AMP-activated protein kinase AMPK , which acts as an energy sensor in cells and plays a key role in the activation of the autophagic process. On the other hand, we report that AKT and ERK pathways act independently from each other in the NPY and ghrelin stimulation of autophagy. This phenomenon has been related to a dysregulation of mitochondrial function and proteostasis. |
| Cite this Article | Let us know how this access is important for you. Skip to main content. UC Davis UC Davis Deposit. Menu About eScholarship Journals Academic Units UC Open Access Policies. Download PDF Main PDF. Email Facebook. The Impact of Aging, Calorie Restriction and Dietary Fat on Autophagy Markers and Mitochondrial Ultrastructure and Dynamics in Mouse Skeletal Muscle. Abstract Loss of skeletal muscle mass and function is a hallmark of aging. Download PDF to View View Larger. For improved accessibility of PDF content, download the file to your device. Thumbnails Document Outline Attachments. Highlight all Match case. Whole words. Further studies have demonstrated that resveratrol mimics the effects of caloric restriction by activating the sirtuin system [ 22 ]. Previous data have shown that caloric restriction and resveratrol promote longevity through the SIRT1-dependent induction of autophagy both in vitro in human cells and in vivo in Caenorhabditis elegans [ 23 ], but the role of SIRT1 In the regulation of autophagy in the liver is still unclear. Endoplasmic reticulum ER stress is evident in the liver of obese animals [ 24 ] and is considered to play a key role in the regulation of lipogenesis as well as hepatic steatosis [ 25 ]. However, it is still unknown whether resveratrol, by mimicking the effects of caloric restriction prevents hepatic steatosis by regulating the SIRT1-autophagy pathway and alleviates ER stress. Given that little is currently known about how resveratrol and caloric restriction impact on autophagy and ER stress in the liver, the effects of resveratrol and caloric restriction on the autophagic mechanism of hepatic lipid metabolism and hepatic ER stress will be of interest. In this study, we aimed to investigate the effect of resveratrol supplementation and caloric restriction treatment on high-fat diet-induced NASH and further explored whether they contribute to hepatoprotection through the regulation of the SIRT1-autophagy pathway as well as decreased ER stress. Resveratrol 3,4´,5-trihydroxystilbene was purchased from Sigma Chemical Company. Assay kits for serum total cholesterol TC , total triglycerides TG , high density lipoprotein cholesterol HDL and low density lipoprotein cholesterol LDL were obtained from BIOSINO Bio-technology and Science, Inc. Beijing, China. TRIzol was obtained from Invitrogen Inc. Carlsbad, CA, USA and a real-time quantitative PCR kit was purchased from TAKARA Bio Inc. Otsu, Shiga, Japan. LC3B antibody, p62 antibody, GRP78 antibody and CHOP antibody were obtained from Cell Signaling Technology Billerica, MA, USA ; SIRT1 antibody and secondary antibody were purchased from Abcam Cambridge, UK. All other reagents were of analytical grade. Eight-week-old male Wistar rats — g were purchased from the Vital River Laboratory Animal Technology Co. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health. The protocol was approved by the Committee on the Ethics Animal Experiments of Xinxiang Medical University. All procedures were performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering. The food intake of the rats in HFD-CR group was adjusted each week according to the intake of the HFD group during the preceding week [ 10 ]. In the morning, rats were provided with the test diet, the high-fat diet, or the standard chow diet and then sufficient standard chow diet or high-fat diet was provided after the test diet was completely consumed. The body weight of each rat was recorded weekly, and their food intake was weighed every day. After 18 weeks of treatment and an overnight fast, the animals were decapitated swiftly. The liver and fat tissues were then extracted, immediately frozen in liquid nitrogen, and stored at °C until further analysis. The total food intake during the experiment was calculated according to the daily food intake records; the total energy intake of each of the four groups over the week period was the total food intake multiplied by the provided energy of the appropriate diet. Serum was obtained after centrifugation of whole blood g for 10 min at 4°C. Total cholesterol TC , total triglyceride TG , high-density lipoprotein HDL and low-density lipoprotein LDL were measured using enzymatic kits BIOSINO Biotechnology and Science, Inc. Lipids were extracted from liver tissue mg using the procedure described by Loison et al [ 32 ], and then the TG and TC levels were measured using enzymatic colorimetric assays BIOSINO Biotechnology and Science, Inc. Sections 5 μm thick were prepared and stained with hematoxylin-eosin HE stain for histological analysis under a light microscope. To evaluate fat deposition, we embedded liver samples in Tissue-Tek® OCT compound Sakura FineTek USA, Torrance, CA, USA and then stained them with Oil Red O. Hepatic steatosis was assessed by point counting methods as mentioned previously [ 33 , 34 ]. The detailed method is as follows: five slices of HE per animal were performed and 10 microscopic fields were selected at random for estimating volume density Vv of liver steatosis by point-counting methods. Reaction mixtures 50 μL , each containing 50 μg of total RNA were heated for 20 min at 37°C. The mRNA expression of LC3 , Beclin1 , p62 , SIRT1 , PERK , GRP78 , CHOP and β-actin in liver tissue was determined using the SYBR Green detection system on an ABI PRISM machine Applied Biosystems with the following conditions: one cycle, 95°C, 5 s; 40 cycles, 95°C, 10 s; and 57°C, 30 s. The primer sequences used for real-time PCR are listed in Table 2. The 2 -ΔΔCT method was adopted for the data analysis, and all sample values were normalized to expression of the endogenous reference gene β-actin. The protein expression levels of LC3, p62, SIRT1, GRP78 and CHOP in the liver were measured by Western blot analysis. The protein concentration was measured using a BIO-RAD DC Protein Assay Reagent Bio-Rad, Hercules, CA, USA. The blots were incubated with anti-LC3B antibody diluted , anti-p62 antibody diluted , anti-CHOP antibody diluted , anti-GRP78 antibody diluted and anti-SIRT1 diluted antibody overnight at 4°C, and then incubated with HRP-conjugated secondary antibodies. β-Actin , Sigma-Aldrich Chemical Company, St. Louis, Missouri was used as a loading control. Then, the proteins were visualized with an ECL detection system Syngene, Cambridge, UK. Densitometry analysis was performed using ChemiDoc Quantity One software Bio-Rad Laboratories. Statistical analyses were carried out using SPSS Data are expressed as the mean ± SD. A Serum total triglycerides. B Serum total cholesterol. C Serum HDL. D Serum LDL. E Hepatic total triglycerides. F Hepatic total cholesterol. To evaluate the effects of RES treatment and CR on hepatic steatosis, we stained liver tissue with Oil Red O stain × and hematoxylin-eosin HE, × stain to visualize lipid accumulation under all treatment conditions. As shown in Fig 3 , compared with the STD group, the lipid accumulation in the liver was increased in the HFD group; moreover, treatment with RES and CR for 18 weeks decreased hepatic intracellular lipid accumulation in rats. As shown in Fig 4 , extensive macrovesicular steatosis surrounding the perisinusoidal areas and microvesicular steatosis were observed in high-fat diet-fed rats. However, RES and CR treatment significantly decreased the accumulation of intracellular lipid droplets in HFD-fed rats. A STD group. B HFD group. C HFD-RES group. D HFD-CR group. The level of hepatic expression of SIRT1 , autophagy marker genes and ER stress-associated genes by RES and CR treatment was assessed using real-time PCR Fig 5. A LC3. B Beclin 1. C p D SIRT1. E PERK. F GRP G CHOP. A Western blotting results for LC3 protein. B Quantitative analysis of LC3-II band densities. C Western blotting results for SIRT1 protein. D Quantitative analysis of SIRT1 band densities. E Western blotting results for p62 protein. F Quantitative analysis of p62 band densities. A Western blotting results for CHOP protein. B Quantitative analysis of CHOP band densities. C Western blotting results for GRP78 protein. D Quantitative analysis of GRP78 band densities. In this study, we compared the effects of resveratrol supplementation and caloric restriction on high-fat diet-induced non-alcohol fatty liver formation and hepatic steatosis by using male Wistar rats. The important finding of the present study was that resveratrol supplementation could mimic the beneficial effects of caloric restriction on lipid metabolism; both resveratrol supplementation and caloric restriction alleviated hepatic steatosis by up-regulating the SIRT1-autophagy pathway in high-fat diet-fed rats. In animals, autophagy is a cellular process of lysosomal degradation that is vital for the healthy organism in that it removes excess or harmful proteins and organelles. Growing evidence from animal studies has reported that liver autophagy contributes to basic hepatic functions [ 35 ]. Another study reported that autophagy regulates the lipid content of the liver because the inhibition of autophagy increased TG and lipid droplets in vitro and in vivo ; loss of autophagy decreased TG breakdown [ 13 ]. The above mentioned studies indicate that abnormal autophagy in the liver may contribute to lipid metabolism disorder of that organ. It is well established that nutritional deprivation through caloric restriction not only reduces the body weight of the individual but also activates SIRT1 and induces autophagy in cells. In addition, resveratrol is a classical dietary polyphenol and actives SIRT1[ 36 ]. Based on these reports, we used an HFD-induced rat model of hepatic steatosis to implement caloric restriction as a lifestyle modification and resveratrol as a drug therapy for lipid metabolism and study whether both forms of treatment act through SIRT1-mediated autophagy induction. Moreover, long-term resveratrol treatment prevented dyslipidemia and hepatic steatosis in HFD-induced non-alcoholic steatohepatitis rodent models [ 28 , 37 ]. The lack of consensus on the effect of resveratrol on HFD-induced lipid metabolism disorder and hepatic steatosis in rodent studies may have been due to different doses, varying diets and duration. In our study, we observed that an HFD resulted in lipid metabolism disorder in serum and lipid accumulation in the liver. Compared with the resveratrol treatment, caloric restriction provided superior protection against HFD-induced hepatic steatosis and hepatocyte ballooning. The distinct effect of caloric restriction and resveratrol supplementation on HFD-induced formation has also been reported by Tauriainen E et al. Tauriainen E, found that both caloric restriction and resveratrol provided protection against diet-induced fatty liver formation, and caloric restriction showed better effects than resveratrol [ 40 ]. The present study further explored whether caloric restriction intervention and resveratrol supplementation alleviate hepatosteatosis by regulating autophagy in the liver. These above data imply that the HFD-induced decline in autophagy substantially retards the clearance of excess free triglycerides, which in turn causes elevated levels of TG, TC and LDL, resulting in accumulation of fatty acids and lipid metabolic dysfunction aggregates and autophagy disorder in liver; resveratrol and caloric restriction treatment could reverse the decrease in autophagy and increase the autophagic flux of the liver in HFD-fed rats. In addition, SIRT1 plays an essential role in protecting against HFD-induced metabolic damage [ 41 , 42 ]. In animal studies, the sirtuin system can be influenced by caloric restriction and resveratrol [ 19 , 43 ]; and a randomized trial study on a healthy human population demonstrated that caloric restriction and resveratrol supplementation significantly increased plasma concentrations of SIRT1[ 44 ]. To elucidate whether HFD would affect the SIRT1 level in the liver and whether the clearance of hepatic lipid accumulation treated by caloric restriction and resveratrol in HFD-fed rats required the SIRT1-mediated autophagy pathway, we further determined the expression of SIRT1 in the liver. Previous studies have reported that resveratrol increases the mRNA expression of hepatic SIRT1 in high-fat-fed mice [ 37 ]; and regulates human adipocyte number and function in a SIRT1-dependent manner [ 45 ]. In this study, we observed that HFD slightly reduced the mRNA and protein expression of SIRT1 in liver; however, resveratrol supplementation and caloric restriction increased the mRNA and protein expression of SIRT1 in liver. Recently, ER stress has been thought to play a crucial role in lipotoxicity and inflammation, which are linked to both obesity and hepatic steatosis [ 46 ]. Caloric restriction also increases the circulating levels of ghrelin, a peripheral orexigenic hormone synthesized predominantly in the stomach in response to fasting [ 41 - 43 ]. Ghrelin has a ubiquitous expression throughout the body namely in the central nervous system, in particularly in the hypothalamus and cerebral cortex [ 44 , 45 ]. The actions of ghrelin are mediated through the activation of the G-coupled protein growth hormone secretagogue type 1a receptor GHS-R1a , which also has a wide tissue distribution [ 43 , 46 ]. Ghrelin is involved in the regulation of cardiovascular functions, bone metabolism and inflammation [ 47 , 48 ]. Ghrelin is also involved in memory and learning and has a neuroprotective effect in neurodegenerative diseases and ischemic brain injury models [ 46 , 48 - 52 ]. Since caloric restriction increases autophagy and both NPY and ghrelin, the aim of this study was to investigate whether NPY and ghrelin stimulates autophagy and if these peptides mediate caloric restriction-induced autophagy in rat cortical neurons. Understanding how NPY and ghrelin may act as caloric restriction mimetics by increasing autophagic clearance in cortical neurons, provides a new anti-aging mechanisms of caloric restriction that could be further explored. To investigate whether caloric restriction regulates autophagy in rat cortical cortical neurons, we monitored autophagy in rat cortical neurons exposed to a caloric restriction mimetic medium referred as caloric restriction hereafter by measuring the protein levels of the transient autophagosomal membrane-bound form of LC3B LC3B-II and sequestosome 1 SQSTM1, also known as p62 , widely used as markers of the autophagic process [ 53 , 54 ]. As shown in Figure 1A and B , caloric restriction increases LC3B puncta immunoreactivity in rat cortical neurons. While untreated cells control cells have a diffuse LC3B cellular distribution, with few small LC3B puncta, in caloric restriction-treated cells an increase in LC3B puncta immunoreactivity was observed, suggesting an increase in autophagosome formation and autophagy induction. The levels of LC3B-II and SQSTM1 were also measured by Western blotting Figure 1C. The results show that caloric restriction increased LC3B-II protein levels However, an increase in LC3B-II levels and the consequent autophagosome formation does not guarantee an increase of the autophagic activity [ 53 ]. To rule out the possibility that the increase of LC3B puncta immunoreactivity was due to an inhibited auto-phagosome degradation rather than autophagosome formation, we evaluated endogenous autophagic system in the presence or absence of an inhibitor of lysosomal degradation, chloroquine [ 53 - 55 ]. Since LC3B-II and other autophagic substrates, as is the case of SQSTM1, are degraded at the final stages of autophagy, chloroquine treatment will impair their degradation, leading to the accumulation of both LC3B-II and SQSTM1. In the presence of chloroquine, we observed that the increase of LC3B-II induced by caloric restriction was significantly higher than in cells under caloric restriction without chloroquine Figure 1C. Concomitant with the increase in LC3B-II steady state levels, caloric restriction decreased SQSTM1 protein content in rat cortical neurons Figure 1D. The SQSTM1 levels were higher in cells under caloric restriction in the presence of chloroquine than in cells under caloric restriction without chloroquine Altogether, these results show that caloric restriction increases autophagic clearance in rat cortical neurons. Inhibition of MTOR, therefore, results in activation of autophagy [ 57 , 58 ]. MTOR activity can be assessed by the analysis of MTOR phosphorylation at its active site Ser As shown in Figure 1E , caloric restriction decreased phospho-MTOR levels Figure 1. Caloric restriction increases autophagy in rat cortical neurons. Primary rat cortical neurons were exposed to caloric restriction mimic medium CR , DMEM low glucose, for 6 h. Untreated cells were used as control Ctrl. A LC3B puncta immunoreactivity was assessed by immunocytochemistry, as described in Materials and Methods. Cells were immunolabeled for LC3B green and MAP2 red. Nuclei were stained with Hoechst blue. Figures are representative of three independents experiments. Scale bar, 20 μM. Whole cell extracts were assayed for LC3B-II C , SQSTM1 D , phospho-MTOR p-MTOR E and β-tubulin loading control immunoreactivity through Western blotting analysis, as described in Materials and Methods. Representative Western blots for each protein are presented above each respective graph. The results represent the mean ± SEM of, at least, five independents experiments, and are expressed as percentage of control. Since caloric restriction was shown to increase the levels of hypothalamic NPY [ 28 - 31 ] and circulating ghrelin [ 39 , 41 , 42 ], we next investigated whether caloric restriction could also regulate the levels of both peptides in rat cortical neurons. As shown in Figure 2A and B , caloric restriction mimetic medium increased both NPY and ghrelin mRNA levels in primary rat cortical neurons 1. Concomitantly, caloric restriction mimetic medium increased both NPY and ghrelin protein content in primary rat cortical neurons Figure 2. Caloric restriction increases NPY and ghrelin levels in rat cortical neurons. Primary rat cortical neurons were exposed to caloric restriction medium CR , DMEM low glucose, for 6 h. A and B Total RNA was isolated and the transcript levels of NPY and ghrelin were analyzed by qRT-PCR, as described in Materials and Methods. The results represent the mean ± SEM of five independents experiments and are expressed as the relative amount compared to control. The results represent the mean ± SEM of independents experiments and are expressed as the relative amount compared to control. We observed that caloric restriction induces autophagy in rat cortical neurons and this is accompanied by an increase in NPY levels. Given that NPY and NPY receptors are expressed in rat cortical neurons [ 56 ], we hypothesized that NPY receptors could play a role on caloric restriction-induced autophagy in rat cortical neurons. We observed that NPY Y 1 , Y 2 or Y 5 receptor antagonists inhibited the stimulatory effect of caloric restriction on autophagy markers: the increase in LC3B-II Figure 3A and the decrease in SQSTM1 Figure 3B levels. These results suggest that caloric restriction-induced autophagy in rat cortical neurons is mediated by NPY Y 1 , Y 2 or Y 5 receptor activation. Figure 3. NPY receptor antagonists inhibit the stimulatory effect of caloric restriction on autophagy in rat cortical neurons. Primary rat cortical neurons were incubated with NPY Y 1 receptor antagonist BIBP Y 1 ant, 1 μM , NPY Y 2 receptor antagonist BIIE Y 2 ant, 1 μM or NPY Y 5 receptor antagonist L, Y 5 ant, 1 μM , 30 min before caloric restriction medium CR for 6 h. Whole cell extracts were assayed for LC3B-II A , SQSTM1 B and β-tubulin loading control immunoreactivity by Western blotting analysis, as described in Materials and Methods. As the activation of NPY receptors is involved in caloric restriction-induced autophagy, we then investigated the effect of NPY per se on rat cortical neurons autophagy. We observed that NPY, similarly to caloric restriction, increased LC3B puncta immunoreactivity Figure 4A and B and LC3B-II steady state levels Moreover, in the presence of chloroquine, the increase in LC3B-II levels was higher NPY also decreased SQSTM1 content in rat cortical neurons Figure 4D and this effect was inhibited in the presence of chloroquine. Moreover, we observed that NPY Y 1 , Y 2 or Y 5 receptor antagonists inhibited LC3B-II increase Figure 4E and SQSTM1 decrease Figure 4F induced by NPY. Overall, these results show that NPY enhances autophagic activity in rat cortical neurons, through NPY Y 1 , Y 2 or Y 5 receptors activation. As shown in Figure 4G , NPY decreases phospho-MTOR Ser levels Figure 4. NPY increases autophagy in rat cortical neurons. Primary rat cortical neurons were exposed to NPY nM for 6 h. A LC3B distribution was assessed by immunocytochemistry assay, as described in Materials and Methods. Cells were immunolabeled for LC3B green and MAP2 red, neurons. Whole cell extracts were assayed for LC3B-II C and E , SQSTM1 D and F , phospho-MTOR p-MTOR G and β-tubulin loading control immunoreactivity through Western blotting analysis, as described in Materials and Methods. Since caloric restriction increases ghrelin mRNA and protein levels in rat cortical neurons Figure 2B , we hypothesized that ghrelin, similarly to NPY, could be involved in caloric restriction-induced autophagy in rat cortical neurons. As shown in Figure 5A and B , ghrelin receptor GHS-R1a antagonist [D - Lys 3 ] - GHRP-6 inhibited the increase of LC3B-II and the decrease of SQSTM1, induced by caloric restriction in rat cortical neurons. These results suggest that ghrelin receptor GHS-R1a mediates, in part, caloric restriction-induced autophagy in rat cortical neurons. Figure 5. Ghrelin mediates caloric restriction-induced autophagy in rat cortical neurons. Primary rat cortical neurons were treated with GHS-R1a receptor antagonist [D - Lys 3 ] - GHRP-6 GHS-R1a ant, μM 30 min before caloric restriction CR for 6 h. Whole cell extracts were assayed for LC3B-II A , SQSTM1 B and β-tubulin loading control immunoreactivity through Western blotting analysis, as described in Materials and Methods. We next evaluated the effect of ghrelin per se on autophagy in rat cortical neurons. Similarly to caloric restriction and NPY, in rat cortical neurons, ghrelin induced autophagy and autophagosome formation, as shown by an increase in LC3B puncta immunoreactivity Figure 6A , LC3B-II steady state levels Figure 6B , and autophagic degradation, as shown by SQSTM1 protein decrease Figure 6C and D. As expected, the GHS-R1a receptor blockage with the ghrelin receptor antagonist [D - Lys 3 ] - GHRP-6 abolished ghrelin stimulatory effects on both autophagic substrates Figure 6E and F. Next, we observed that ghrelin decreased phospho-MTOR levels in rat cortical neurons, suggesting that ghrelin, similarly to caloric restriction and NPY, induced autophagy through the canonical inhibition of MTOR activity Figure 6G. Figure 6. Ghrelin induces autophagy in rat cortical neurons. Primary rat cortical neurons were exposed to ghrelin GHRL, 10 nM for 6 h. A LC3B cellular distribution was assessed by immunocytochemistry assay, as described in Materials and Methods. |
Video
Fasting is NOT THE SAME as Calorie Restriction (what the science says)Caloric restriction and autophagy markers -
Alternatively, total proteins were assayed for ATG7 abundance and LC3 maturation. Glyceraldehydephosphate dehydrogenase GAPDH levels were determined to monitor the equal loading of lanes. Results are representative of three independent experiments. Intriguingly, although Sirt-1 reportedly deacetylates p53, 24 we found no signs of p53 deacetylation in cells overexpressing Sirt-1 Figure 1c nor in cells in which endogenous Sirt-1 was activated by Resv Figure 2b or by nutrient deprivation Figure 3e.
Rather, in starvation conditions, p53 tended to be hyperacetylated. In contrast, the activation of Sirt-1 by nutrient depletion correlated with ATG7 deacetylation Figure 3e. In conclusion, it appears that starvation-induced autophagy relies on the activation of Sirt-1, which activates autophagy through a novel mechanism involving ATG7.
Sirt-1 knockdown was as efficient as ATG7 depletion in sensitizing cells to death induction by metabolic stress Figure 4a. The inhibition of Sirt-1 per se induced some extent of cell death Figure 4a and b , suggesting that Sirt-1 may also participate in the maintenance of baseline autophagy levels.
Rapa failed to protect HCT cells from metabolic stress Figure 4b , presumably because in these conditions mTOR was entirely silenced by the endogenous sensor of energy levels AMP-activated protein kinase.
Although the precise molecular mechanisms underlying this phenomenon remain elusive, it cannot be excluded that mTOR signaling might be affected by Sirt-1 inhibition. Irrespective of these theoretical considerations, it appears that Sirt-1 increases the fitness of metabolically stressed cells by activating autophagy.
Sirtuindependent autophagy favors stress resistance in human cancer cells. a , b Metabolic stress-induced cell death is increased by the knockdown or inhibition of Sirtuin-1 Sirt Cells were then stained with the mitochondrial transmembrane potential Δψ m -sensitive dye DiOC 6 3 and the vital dye propidium iodide PI.
Black and white columns illustrate the percentage mean±S. Next, we asked whether autophagy contributes to lifespan-extending effects mediated the C. elegans Sirt-1 ortholog SIR Transgenic overexpression of sir The addition of Resv to the culture medium has been previously shown to reduce the aging-associated mortality of C.
elegans , 31 and this beneficial effect was curtailed both by the knockdown of sirt Knockdown of the C. elegans p53 ortholog cep-1 which is known to increase the longevity of nematodes by stimulating autophagy 14 failed to enhance the gain in longevity mediated by sir Autophagy is required for the lifespan-extending effects of Sir a Longevity conferred by the overexpression of Sir b Depletion of SIR d Depletion of the C.
elegans p53 ortholog Cep-1 does not enhance longevity conferred by Sir In all panels, the percentage of surviving nematodes is plotted against age days. In summary, activation of the Sirt-1 ortholog SIR Recently, it has been discovered that drugs that induce autophagy such as Rapa and spermidine can prolong the lifespan of rodents.
In contrast, autophagy is clearly required for the lifespan-extending effect of Rapa and spermidine in C. elegans and Drosophila melanogaster. elegans , suggest that autophagy is required for the lifespan-extending effects of Rapa as well as for the increase in longevity promoted by caloric restriction and Resv, an agent that has a broad anti-aging activity also in mice.
Once activated, Sirt-1 can deacetylate essential autophagic modulators such as ATG5 and ATG7, 19 suggesting that a cytoplasmic pool of Sirt-1 may underlie its autophagy-stimulating effects. It remains elusive whether changes in the transcriptome mediated by nuclear Sirt-1 might also contribute to the induction of autophagy.
The precise identity of these proteins remains to be established Figure 6. Schematic model of the role of Sirtuin-1 in autophagy. In human cells and C. This has lifespan-extending effects in nematodes, as do the knockdown of the C. elegans p53 ortholog Cep-1 and rapamycin administration, both of which trigger autophagy by Sirtuinindependent pathways.
The mechanisms by which enhanced autophagy can improve organismal health and longevity are largely elusive. As a possibility, increased autophagy might improve cellular resistance to stress by augmenting the metabolic buffering capacity of cells.
Irrespective of these considerations, our data establish the cardinal role of Sirtelicited autophagy in mediating the anti-aging effects of caloric restriction and Resv.
All media and supplements for cell culture were purchased from Gibco-Invitrogen Carlsbad, CA, USA. For serum and amino acid deprivation, which we refer to as starvation conditions, cells were cultured in Earle's balanced salt solution Sigma-Aldrich, St Louis, MO, USA.
Cells were transfected with the empty vector pcDNA3 alone or together with a GFP-LC3-encoding plasmid, 38 in the presence or the absence of a construct for the overexpression of WT Sirt-1 Addgene, Cambridge, MA, USA.
Cells were then stained with an antibody specific for LAMP-2 Santa Cruz Biotechnology, Santa Cruz, CA, USA , revealed by the appropriate Alexa Fluor conjugate Molecular Probes. For immunoblotting, cells were collected, washed with cold PBS and lysed as previously described.
Revelation was performed with the appropriate horseradish peroxidase HRP -labeled secondary antibodies Southern Biotech, Birmingham, AL, USA plus the SuperSignal West Pico Chemiluminescent Substrate Thermo Scientific-Pierce, Rockford, IL, USA. An anti-glyceraldehydephosphate dehydrogenase GAPDH antibody Chemicon International, Temecula, CA, USA was used to control equal loading of lanes.
Subsequent immunoblotting was carried out by means of TrueBlot-HRP eBioscience, San Diego, CA, USA secondary antibodies. We followed standard procedures for C. elegans strain maintenance.
Nematode rearing temperature was kept at 20°C. The following strains were used in this study—N2: WT Bristol isolate; LG geIn3 [ sir The resulting plasmid was used to transform HT DE3 Escherichia coli cells, which lack the dsRNA-specific RNase III. For monitoring autophagy, we used a full-length DsRED::LGG-1 fusion protein.
Mean and maximum pixel intensity for each embryo was calculated from these images by means of the ImageJ software package. For each genotype, we processed at least 15 images over at least three independent trials.
Lifespan assays were performed at 20°C. Synchronous animal populations were generated by hypochlorite treatment of gravid adults to obtain tightly synchronized embryos, which were allowed to develop into adulthood.
Progeny was grown through the L4 larval stage and then transferred to fresh plates in groups of 10—20 worms per plate, for a total of — individuals per experiment. Thereafter, animals were transferred to fresh plates every 2—4 days and examined daily for touch-provoked movement and pharyngeal pumping until death.
Worms that died due to internally hatched eggs, an extruded gonad, or desiccation due to crawling on the edge of the plates were incorporated as such into the data set. Each survival assay was repeated at least three times. Statistical analyses were carried out using the Prism software package GraphPad Software Inc.
In cell culture experiments, values were compared using unpaired Student's t -tests. For multiple comparisons, we used the one-factor analysis of variance corrected by post-hoc Bonferroni test.
Survival curves of C. elegans were created using the Kaplan and Meier's product-limit method. Mantel—Cox log-rank statistics were used to evaluate differences between survival curves and to determine P -values. Levine B, Kroemer G.
Autophagy in the pathogenesis of disease. Cell ; : 27— CAS PubMed PubMed Central Google Scholar. Gonzalez-Polo RA, Boya P, Pauleau AL, Jalil A, Larochette N, Souquere S et al. J Cell Sci ; : — Article CAS PubMed Google Scholar. Galluzzi L, Vicencio JM, Kepp O, Tasdemir E, Maiuri MC, Kroemer G.
To die or not to die: that is the autophagic question. Curr Mol Med ; 8 : 78— Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N et al.
Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol ; 25 : — Article CAS PubMed PubMed Central Google Scholar. Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis.
Cancer Cell ; 10 : 51— Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H et al. Cell Death Differ ; 14 : — Iwata A, Riley BE, Johnston JA, Kopito RR. HDAC6 and microtubules are required for autophagic degradation of aggregated huntingtin.
J Biol Chem ; : — Apel A, Herr I, Schwarz H, Rodemann HP, Mayer A. Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Cancer Res ; 68 : — Galluzzi L, Maiuri MC, Vitale I, Zischka H, Castedo M, Zitvogel L et al.
Cell death modalities: classification and pathophysiological implications. Kroemer G, Levine B. Autophagic cell death: the story of a misnomer.
Nat Rev Mol Cell Biol ; 9 : — Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death Cell Death Differ ; 16 : 3— Kroemer G, Galluzzi L, Brenner C.
Mitochondrial membrane permeabilization in cell death. Physiol Rev ; 87 : 99— Jia K, Levine B. Autophagy is required for dietary restriction-mediated life span extension in C.
Autophagy ; 3 : — Article PubMed Google Scholar. Tavernarakis N, Pasparaki A, Tasdemir E, Maiuri MC, Kroemer G. The effects of p53 on whole organism longevity are mediated by autophagy. Autophagy ; 4 : — Toth ML, Sigmond T, Borsos E, Barna J, Erdelyi P, Takacs-Vellai K et al.
Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Eisenberg T, Knauer H, Schauer A, Buttner S, Ruckenstuhl C, Carmona-Gutierrez D et al. Induction of autophagy by spermidine promotes longevity.
Nat Cell Biol ; 11 : — Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature ; : — Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG et al.
Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE et al.
A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci USA ; : — Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS et al.
Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Solomon JM, Pasupuleti R, Xu L, McDonagh T, Curtis R, DiStefano PS et al. Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage. Mol Cell Biol ; 26 : 28— Tinhofer I, Bernhard D, Senfter M, Anether G, Loeffler M, Kroemer G et al.
Resveratrol, a tumor-suppressive compound from grapes, induces apoptosis via a novel mitochondrial pathway controlled by Bcl FASEB J ; 15 : — com Support center. All rights reserved. InsideTracker is a personalized nutrition model by Segterra.
What is autophagy? How do you induce autophagy? How long do you need to fast for autophagy? What foods inhibit autophagy? The following foods contain polyphenols that promote autophagy: Green tea Grape skin red wine Nuts Onions Apples Berries Turmeric Soybeans Milk thistle A summary of what we know about autophagy: Autophagy is a form of cellular housekeeping in which misfolded proteins, damaged organelles, and pathogens are degraded and removed from cells.
Autophagy plays a critical role in many areas of health, and like many physiological processes in the body, autophagy declines with age. Calorie restriction, fasting, and exercise are all potent inducers of autophagy.
Polyphenols, beneficial compounds found in plants, may also play a role in inducing autophagy. Only a handful of studies measuring fasting and autophagy exist in humans.
More research is needed to fully understand the benefits and implications of autophagy. Diana Licalzi, MS, RD Diana is a Content Strategist and Team Nutritionist at InsideTracker. She is a Registered Dietitian that specializes in plant-based nutrition and type 2 diabetes.
You'll often find Diana whipping up plant-based recipes, sipping on a mocktail, or hiking up a mountain. You can follow her on Instagram at dietitian. More on this topic. Manage Your Mind with These Three Strategies from Dr.
Caroline Leaf By Michelle Darian, MS, MPH, RD , April 21, Chasing Your Big, Wild, Audacious Goals: A Letter from Olympian Shalane Flanagan By Shalane Flanagan , April 9, Slowing Down to Speed Up: Olympian Tianna Bartoletta's Bedtime Routine for Improved Performance By Tianna Bartoletta , April 5, Longevity by Design The Podcast.
Ask Me Anything AMA : Oral Health, Healthspan, and Longevity with Dr. Gil Blander and Ashley Ann N Y Acad Sci. Kruger U, et al. Autophagic degradation of tau in primary neurons and its enhancement by trehalose. Rodriguez-Navarro JA, et al. Neurobiol Dis. Schaeffer V, et al. Stimulation of autophagy reduces neurodegeneration in a mouse model of human tauopathy.
Congdon EE, et al. Methylthioninium chloride methylene blue induces autophagy and attenuates tauopathy in vitro and in vivo. Hwang J, et al. Int J Mol Sci. Kodali M, et al. Metformin treatment in late middle age improves cognitive function with alleviation of microglial activation and enhancement of autophagy in the hippocampus.
Aging Cell. Terry AV Jr. Methods of behavior analysis in neuroscience. In: Buccafusco JJ, editor. Spatial Navigation. Boca Raton: CRC Press; Google Scholar. Rollins CPE, et al.
Neuroimage Clin. Huber LA, Teis D. Lysosomal signaling in control of degradation pathways. Curr Opin Cell Biol. Qian ZR, et al. Prognostic significance of MTOR pathway component expression in neuroendocrine tumors.
J Clin Oncol. Ramalingam M, et al. Autophagy signaling by neural-induced human adipose tissue-derived stem cell-conditioned medium during rotenone-induced toxicity in SH-SY5Y cells.
Dibble CC, Cantley LC. Regulation of mTORC1 by PI3K signaling. Trends Cell Biol. Barone E, et al. The interplay among oxidative stress, brain insulin resistance and AMPK dysfunction contribute to neurodegeneration in type 2 diabetes and Alzheimer disease.
Free Radic Biol Med. Dufner A, Thomas G. Ribosomal S6 kinase signaling and the control of translation. Exp Cell Res. Egan DF, et al. Phosphorylation of ULK1 hATG1 by AMP-activated protein kinase connects energy sensing to mitophagy. Nakatogawa H. Two ubiquitin-like conjugation systems that mediate membrane formation during autophagy.
Essays Biochem. Bordi M, et al. Autophagy flux in CA1 neurons of Alzheimer hippocampus: Increased induction overburdens failing lysosomes to propel neuritic dystrophy. Russell RC, et al. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol. Bjorkoy G, Lamark T, Johansen T.
Wyant GA, et al. mTORC1 activator SLC38A9 is required to efflux essential amino acids from lysosomes and use protein as a nutrient. Sardiello M, et al. A gene network regulating lysosomal biogenesis and function.
Martina JA, et al. The nutrient-responsive transcription factor TFE3 promotes autophagy, lysosomal biogenesis, and clearance of cellular debris. Sci Signal.
Petit CS, Roczniak-Ferguson A, Ferguson SM. Recruitment of folliculin to lysosomes supports the amino acid-dependent activation of Rag GTPases.
J Cell Biol. Ramirez Reyes JMJ, Cuesta R, Pause A. A regulator of transcription through AMPK and mTOR signaling pathways. Front Cell Dev Biol. Cheng XT, et al. Revisiting LAMP1 as a marker for degradative autophagy-lysosomal organelles in the nervous system. Butler D, et al. PLoS ONE. Grillo CA, et al.
Insulin resistance and hippocampal dysfunction: disentangling peripheral and brain causes from consequences. Exp Neurol. Kuhla A, et al. Lifelong caloric restriction increases working memory in mice.
Yilmaz N, et al. Calorie restriction modulates hippocampal NMDA receptors in diet-induced obese rats. J Recept Signal Transduct Res. Fontan-Lozano A, et al.
Caloric restriction increases learning consolidation and facilitates synaptic plasticity through mechanisms dependent on NR2B subunits of the NMDA receptor.
J Neurosci. Kepp O, Kroemer G. Autophagy induction by thiostrepton for the improvement of anticancer therapy. Chen G, et al.
EMBO Mol Med. Schmidt LS, Linehan WM. FLCN: The causative gene for Birt-Hogg-Dube syndrome. Jansen RM, et al. Structural basis for FLCN RagC GAP activation in MiT-TFE substrate-selective mTORC1 regulation.
Sci Adv. El-Houjeiri L, et al. Folliculin impairs breast tumor growth by repressing TFE3-dependent induction of the Warburg effect and angiogenesis. J Clin Invest. Bracke A, et al. Obesity impairs mobility and adult hippocampal neurogenesis.
J Exp Neurosci. Gladding JM, et al. The effect of intrahippocampal insulin infusion on spatial cognitive function and markers of neuroinflammation in diet-induced obesity. Front Endocrinol. Leyh J, et al. Long-term diet-induced obesity does not lead to learning and memory impairment in adult mice.
Fullgrabe J, Klionsky DJ, Joseph B. The return of the nucleus: transcriptional and epigenetic control of autophagy.
Nat Rev Mol Cell Biol. Rusmini P, et al. Trehalose induces autophagy via lysosomal-mediated TFEB activation in models of motoneuron degeneration. Hars ES, et al. Autophagy regulates ageing in C elegans. Fromm SA, Lawrence RE, Hurley JH. Structural mechanism for amino acid-dependent Rag GTPase nucleotide state switching by SLC38A9.
Nat Struct Mol Biol. Luo JJ, Wallace W, Kusiak JW. J Neurol Sci. Atwood CS, Perry G. J Alzheimers Dis. Christensen A, Liu J, Pike CJ. Aging reduces estradiol protection against neural but not metabolic effects of obesity in female 3xTg-AD mice.
Front Aging Neurosci. Scheyer O, et al. J Prev Alzheimers Dis. CAS PubMed PubMed Central Google Scholar. Martin B, et al. Sex-dependent metabolic, neuroendocrine, and cognitive responses to dietary energy restriction and excess. Download references. We also thank the employees of the Department of Comparative Medicine at Legacy Research for their outstanding animal caregiving.
Dow Neurobiology, Legacy Research Institute, Portland, OR, USA. Sadie B. Baer, Adrianah D. You can also search for this author in PubMed Google Scholar. In the execution of this study, DO obtained funding, designed the experiments, aided in data collection, analyzed the data, and oversaw the writing of the manuscript.
All authors approved the final manuscript. Correspondence to Danielle M. All methods and procedures were approved by the Legacy Research Institute IACUC protocol —20 prior to any rodent work occurring.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Figure S1. Depicts the longitudinal weight gain data collected from weekly weigh ins of the mice.
Figure S2. Depicts daily watermaze learning in female and male mice that is depicted as AUC in Figure 2. Latency to reach the platform was measured each day across four trials for 4 days.
There were no significant effects in the male mice. Western blot images: All raw western blot images are provided. Numbers at the top of each blot are subject numbers used in the represented data.
Open Access This article is licensed under a Creative Commons Attribution 4. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material.
If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Reprints and permissions. Baer, S. Sex differences in response to obesity and caloric restriction on cognition and hippocampal measures of autophagic-lysosomal transcripts and signaling pathways. BMC Neurosci 25 , 1 Download citation.
Received : 20 April Accepted : 18 December Published : 02 January Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative.
Skip to main content. Search all BMC articles Search. Download PDF. Research Open access Published: 02 January Sex differences in response to obesity and caloric restriction on cognition and hippocampal measures of autophagic-lysosomal transcripts and signaling pathways Sadie B.
Baer 1 , Adrianah D. Osborne 1 BMC Neuroscience volume 25 , Article number: 1 Cite this article Accesses 1 Altmetric Metrics details. Abstract Background Obesity rates in the U. Methods Female and male mice were placed on an obesogenic diet for 10 months, at which point half were switched to caloric restriction for three months, followed by cognitive testing in the Morris watermaze.
Results Cognitive function in female mice responded differently to caloric restriction based on whether they were on a normal or obesogenic diet; male cognition was only mildly affected by caloric restriction and not obesity. Conclusions Results support that hippocampal ALP is a target of obesity and that sex shapes molecular responses, while providing insight into how dietary manipulations affect learning and memory based on sex.
Results Females and males differ in body weight and glucose tolerance Both females and males on an obesogenic diet gained significantly more weight than their Control counterparts Fig. Full size image. Materials and methods All methods and procedures were approved by the Legacy Research Institute IACUC protocol prior to any rodent work occurring.
Glucose tolerance test A week prior to behavioral testing, mice received glucose tolerance testing GTT. Morris watermaze Watermaze testing took place 11 weeks after new dietary treatments were implemented.
Tissue collection and processing Hippocampus was collected from mice following anesthesia with isoflourane, followed by cervical dislocation and rapid decapitation.
Western blotting Tissues were sonicated with RIPA buffer. References Stierman, B. Article CAS PubMed Google Scholar Li XY, et al. Article PubMed Google Scholar Qu Y, et al. Article PubMed Google Scholar Ronan L, et al. Article PubMed PubMed Central Google Scholar Vander Velden JW, Osborne DM. Article CAS PubMed Google Scholar Stranahan AM, et al.
Article PubMed PubMed Central Google Scholar Pistell PJ, et al. Article CAS PubMed Google Scholar Alzoubi KH, Aleisa AM, Alkadhi KA.
Present address: Buck Institute Pure plant-based stimulant Research on Aging, Novato, California. Present address: Departamento de Biología Reatriction, Instituto reetriction Biomoléculas INBIO Hydration strategies for athletes, Restrictjon de Restrictuon, Spain. Loss of Allergy-free clothing muscle mass and function is a hallmark of restrkction. This phenomenon has been related to a dysregulation of mitochondrial function and proteostasis. Calorie restriction CR has been demonstrated to delay aging and preserve function until late in life, particularly in muscle. In these conditions, lard fed mice showed an increased longevity compared to mice fed soybean or fish oils. We focus our discussion on dietary fatty acid saturation degree as an essential predictor of life span extension in CR mice.
0 thoughts on “Caloric restriction and autophagy markers”