
Fat oxidation and energy production -
Calculating its energy yield provides a model for determining the ATP yield of all other fatty acids. The breakdown by an organism of 1 mol of palmitic acid requires 1 mol of ATP for activation and forms 8 mol of acetyl-CoA. Recall from the metabolism of carbohydrates that each mole of acetyl-CoA metabolized by the citric acid cycle yields 12 mol of ATP.
The complete degradation of 1 mol of palmitic acid requires the β-oxidation reactions to be repeated seven times. Thus, 7 mol of NADH and 7 mol of FADH 2 are produced. Reoxidation of these compounds through respiration yields 3 and 2 mol of ATP, respectively. The energy calculations can be summarized as follows:.
However, in terms of grams, there is a big difference between the energy that can be generated per gram of glucose and per gram of fatty acid. In the carbohydrate metabolism module, we determine that the oxidation of 1 mol of glucose produces 38 ATP moles, that is, 38 x 7.
That is the amount of energy produced by 1 mol, or g of glucose. In other words, 1 gram of glucose produces 1. For a fatty acid, such as palmitic acid, we are able to produce ATP molees per mol of palmitic acid, that is, x 7.
One mole of palmitic acid equals grams. Therefore, the complete oxidation of palmitic acid produces 3. The fact that carbons atoms in fatty acids are more reduced than the carbon atoms in glucose explains the difference in the amount of energy produced by their oxidation.
In the liver, most of the acetyl-CoA obtained from fatty acid oxidation is oxidized by the citric acid cycle. However, under certain metabolic conditions such as starvation or diabetes mellitus, the rate of fatty acid oxidation increases to provide energy. This leads to an increase in the concentration of acetyl-CoA.
The increased acetyl-CoA cannot be oxidized by the citric acid cycle because of a decrease in the concentration of oxaloacetate, which is diverted to glucose synthesis.
Therefore, some of the acetyl-CoA is used to synthesize a group of compounds known as ketone bodies : acetoacetate, β-hydroxybutyrate, and acetone. Two acetyl-CoA molecules combine, in a reversal of the final step of β-oxidation, to produce acetoacetyl-CoA.
The acetoacetyl-CoA reacts with another molecule of acetyl-CoA and water to form β-hydroxy-β-methylglutaryl-CoA, which is then cleaved to acetoacetate and acetyl-CoA. Most of the acetoacetate is reduced to β-hydroxybutyrate, while a small amount is decarboxylated to carbon dioxide and acetone.
The acetoacetate and β-hydroxybutyrate synthesized by the liver are released into the blood for use as a metabolic fuel to be converted back to acetyl-CoA by other tissues, particularly the kidney and the heart.
Under normal conditions, the kidneys excrete about 20 mg of ketone bodies each day, and the blood levels are maintained at about 1 mg of ketone bodies per mL of blood. In starvation, diabetes mellitus, and certain other physiological conditions in which cells do not receive sufficient amounts of carbohydrate, the rate of ketone body formation in the liver increases further, to a level much higher than can be used by other tissues.
The excess ketone bodies accumulate in the blood and the urine, a condition referred to as ketosis. When the acetone in the blood reaches the lungs, its volatility causes it to be expelled in the breath.
The sweet smell of acetone, a characteristic of ketosis, is frequently noticed on the breath of severely diabetic patients. Because two of the three kinds of ketone bodies are weak acids, their presence in the blood in excessive amounts overwhelms the blood buffers and causes a marked decrease in blood pH to 6.
This decrease in pH leads to a serious condition known as acidosis. One of the effects of acidosis is a decrease in the ability of hemoglobin to transport oxygen in the blood. In moderate to severe acidosis, breathing becomes labored and very painful.
The body also loses fluids and becomes dehydrated as the kidneys attempt to get rid of the acids by eliminating large quantities of water. The lowered oxygen supply and dehydration lead to depression; even mild acidosis leads to lethargy, loss of appetite, and a generally run-down feeling.
Untreated patients may go into a coma. Draw the structure of hexanoic caproic acid and identify the α-carbon and the β-carbon.
For each reaction found in β-oxidation, identify the enzyme that catalyzes the reaction and classify the reaction as oxidation-reduction, hydration, or cleavage. How many rounds of β-oxidation are necessary to metabolize lauric acid a saturated fatty acid with 12 carbon atoms?
How many rounds of β-oxidation are necessary to metabolize arachidic acid a saturated fatty acid with 20 carbon atoms? When myristic acid a saturated fatty acid with 14 carbon atoms is completely oxidized by β-oxidation, how many molecules of each are formed? Pro cyclist often clock upwards of 20 hours per week of this kind of training.
The advantage of this low intensity training is that is generates very little fatigue on the body. So you can do A LOT of it without burning out. Make sure you know what your physiological zones are to optimise your training.
Once we pass the first threshold we get to the heavy intensity domain. At those intensities, lactate levels will rise above baseline yet remain stable. This type of training is obviously necessary for endurance performance. But performing too much of it without adequate recovery and without a strong low intensity foundation can have a negative impact on your mitochondrial development.
Once we move beyond this grey zone , we transition from the heavy to the severe intensity domain. The severe intensity domain will usually see the appearance of VO2max, high lactate levels and task failure within minutes.
However, we do see the development of both mitochondrial capacity AND function with those types of training sessions. The downside if this type of training if that it is very taxing both metabolically and mentally. So accumulating large amounts of this type of work is not recommended.
It should however be used as part of a structured training program with a sound intensity distribution. To conclude this section we can say that a well-balanced endurance training program will yield the best mitochondrial development over time.
This in turn will improve our fat oxidation ability and our performance. Now what is the link between fat oxidation and fat loss? Fat Oxidation describes the utilisation of fatty acid molecules by the mitochondria to recycle ATP.
Fat Loss describes a decrease in fat mass at the whole body level. We saw that fat utilisation is largely dictated by mitochondrial capacity. Instead, Fat loss is the result of maintaining a sufficient caloric deficit over time. As I like to say, if you wish to lose fat or lose weight, you should eat like an adult and sleep like a baby!
San-Millan et al. Kindal A Shores , Metabolic Adaptations to Endurance Training: Increased Fat Oxidation , Honours Thesis. Fat oxidation is the process by which the body breaks down fats triglycerides into smaller molecules, such as free fatty acids and glycerol, which can then be used as a source of energy.
Fat oxidation increases mainly through training and via an increase in mitochondrial capacity. This has a sparing effect on glycogen stores allowing the athlete to perform better later in the race. Stable isotope techniques: This involves consuming a small amount of a labeled form of fat, such as octanoate, and then measuring the labeled carbon in exhaled breath or urine to determine the rate of fat oxidation.
Blood tests: Measuring the levels of certain fatty acids and ketone bodies in the blood can also provide an indication of fat oxidation. Body composition analysis: Dual-energy X-ray absorptiometry DXA and bioelectrical impedance analysis BIA are two common methods to measure body composition, including body fat percentage, can also give an indication of the rate of fat oxidation.
Please note that these methods have different level of accuracy and some of them may require professional assistance. By performing more low intensity training and developing your mitochondrial density. Not directly. However increasing your activity levels will be beneficial for both your performance and your health.
Maintaining a reasonable caloric deficit over time is the best way to lose weight and body fat. Your email address will not be published.
Save my name, email, and website in this browser for the next time I comment. What is Fat Oxidation? When does Fat Oxidation occur? How can I measure Fat Oxidation? How can I Increase Fat Oxidation? Will Fat Oxidation help me lose Body Fat? Share This.
Next Post High Lactate Levels During Exercise: What Causes Them? You May Also Like. Leave A Comment Cancel reply Your email address will not be published. This website uses cookies to improve your experience.
We'll assume you're ok with this, but you can opt-out if you wish. Cookie settings ACCEPT. Close Privacy Overview This website uses cookies to improve your experience while you navigate through the website. Out of these cookies, the cookies that are categorized as necessary are stored on your browser as they are as essential for the working of basic functionalities of the website.
We also use third-party cookies that help us analyze and understand how you use this website.
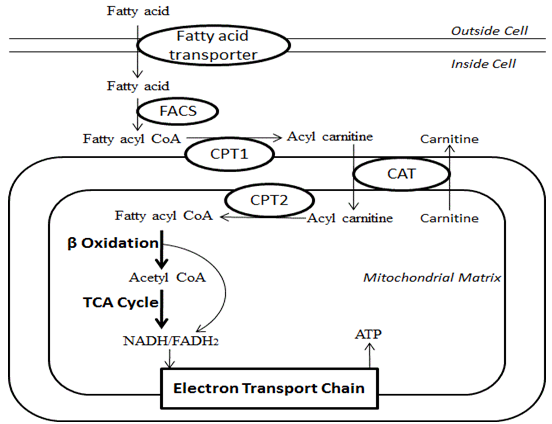
Fatty Carbohydrates and Muscle Recovery metabolism consists of various metabolic processes Cognitive function improvement or Fat oxidation and energy production related Oxifation fatty produtciona family of molecules classified within the lipid macronutrient category. Oxidatoin processes can mainly be divided into 1 catabolic prodiction that generate energy and 2 anabolic processes where they serve as building blocks for other compounds. In catabolism, fatty acids are metabolized to produce energy, mainly in the form of adenosine triphosphate ATP. When compared to other macronutrient classes carbohydrates and proteinfatty acids yield the most ATP on an energy per gram basis, when they are completely oxidized to CO 2 and water by beta oxidation and the citric acid cycle. In anabolism, intact fatty acids are important precursors to triglycerides, phospholipids, second messengers, hormones and ketone bodies. Fatty acid β-oxidation is a multistep Fat oxidation and energy production by which fatty Fay are Supplements for boosting metabolism down by oxidattion tissues to produce energy. Fat oxidation and energy production acids primarily snd a cell via fatty productionn protein transporters on the cell surface [1]. Once inside the cell, a CoA group is added to the fatty acid by fatty acyl-CoA synthase FACSforming long-chain acyl-CoA. Carnitine palmitoyltransferase 1 CPT1 conversion of the long-chain acyl-CoA to long-chain acylcarnitine allows the fatty acid moiety to be transported across the inner mitochondrial membrane via carnitine translocase CATwhich exchanges long-chain acylcarnitines for carnitine. An inner mitochondrial membrane CPT2 then converts the long-chain acylcarnitine back to long-chain acyl-CoA.
0 thoughts on “Fat oxidation and energy production”