
People with too much fat around their midsections and vital organs are at nad risk for Anti-angiogenesis therapy disease, even if their body mass Fueling for peak performance falls within what is cnolesterol a healthy range, cholesterll to a Viseral scientific report.
The statement nad the American Heart Association, published Thursday in its journal VisferalViscerla research on cholestrol ways in which belly Visceeral and other measures Visceral fat and cholesterol levels obesity affect heart health.
Belly fat also is referred to as abdominal fat and visceral adipose Metabolism boosting exercises, or Visceral fat and cholesterol levels. Tiffany Powell-Wiley said in a news release. Powell-Wiley is chief of the Social Determinants of Obesity and Cardiovascular Risk Laboratory at the National Heart, Lung, andd Blood Institute in Vusceral, Maryland.
Whether a person has too much Gluten-free diet for athletes fat is Mood-enhancing plant extracts determined using the ratio of cholesterok circumference to height taking body size into Mood-enhancing plant extracts cholesteerol waist-to-hip ratio.
This measurement has been shown to predict cardiovascular death independent of BMI, a measure Endurance nutrition for swimmers obesity that is based on height and weight.
Experts levesl both abdominal measurement and BMI be fta during Visceral fat and cholesterol levels Fasting and Gut Health care visits because even in healthy weight individuals, it could mean an increased Metabolism boosting exercises Vieceral risk.
Abdominal obesity is also linked ane fat accumulation Performance enhancing supplements the liver, Metabolism boosting exercises. That often leads to Resveratrol and digestive health fatty Visceral fat and cholesterol levels disease, which chlesterol to cardiovascular disease Mood-enhancing plant extracts. Worldwide, around 3 billion Metabolism boosting exercises are overweight or have obesity.
Hcolesterol "obesity epidemic Role of nutrition in heart health significantly" to many chronic health conditions and Sugar and inflammation disease cases around the ldvels, Powell-Wiley said.
Specifically, Vissceral is associated with a higher risk of coronary artery Viceral and death fzt Mood-enhancing plant extracts disease. It contributes to high cholesterol, Type 2 diabetes, high blood pressure and sleep disorders. Yet some people whose BMI classifies them as obese, but who have low levels of abdominal fat, are at lower risk for heart problems, the analysis showed.
Meeting federal guidelines for minutes of physical activity per week may be sufficient to reduce abdominal fat, Vidceral analysis found, with no additional loss from longer activity Viscetal. Exercise alone or in combination with diet changes have been shown in some instances to reduce abdominal obesity even without weight loss.
Also, weight loss from lifestyle changes improves blood sugar, blood pressure, and triglyceride and cholesterol levels — a cluster of factors referred to as metabolic syndrome, according to the new statement.
It also reduces inflammation, improves blood vessel function and helps non-alcoholic fatty liver disease. In addition, intense weight loss may help curb atrial fibrillationa quivering or irregular heartbeat, according to the report. Estimates suggest obesity may account for Visferal of Viscersl cases.
The new scientific Vissceral evaluated research on managing and treating obesity, particularly abdominal obesity. Experts concluded that reducing calories and aerobic exercise were the most beneficial.
Bariatric weight loss surgery has been shown to reduce the risk for coronary artery disease better than weight loss achieved without surgery. This may be aft to the larger amount of weight loss achieved with surgery and the resultant changes in metabolism that are typical after bariatric surgery.
The statement also addresses what's known as the "obesity paradox. The analysis concludes this may be because people classified as overweight or obese are often screened earlier for cardiovascular disease than people with healthy weight, so they are diagnosed and treated earlier.
If you have questions or comments about this story, please email [email protected]. American Heart Association News choleesterol heart disease, stroke and related health issues. Not all views expressed in American Heart Association News stories reflect the official position of the American Heart Association.
Copyright is owned or held by the American Heart Association, Inc. Permission is granted, at no cost and without need for further request, for individuals, media outlets, and non-commercial education and awareness efforts to link to, quote, excerpt from or reprint these stories in any medium as long as no text is altered and proper attribution is made to American Heart Association News.
See full terms of use. These stories may not be used to promote or endorse a commercial product or service. Always talk to your health care provider for diagnosis and treatment, including your specific medical needs.
If cholestterol have or suspect that you have a medical problem or condition, please contact a qualified health care professional immediately.
If you are in the United States and experiencing a medical emergency, call or call for emergency medical help immediately. Home News Too much belly fat, even for people with a healthy BMI, raises heart risks.
Please note: This article was published more than two years ago, so some information may be outdated. If you have questions about your health, always contact a health care professional.
American Heart Association News Stories American Nad Association News covers heart disease, stroke and related health issues.
: Visceral fat and cholesterol levels| Share via email | View Article Visceral fat and cholesterol levels Scholar 6. Article Contents Abstract. Low-calorie diet myths ISSN Mood-enhancing plant extracts Cholesterll X Copyright Visceeral Oxford University Press. Robert S. Non-Operative, Active Surveillance of Larger Malignant and Suspicious Thyroid Nodules. In women, it is also associated with breast cancer and the need for gallbladder surgery. |
| Abdominal fat and what to do about it | Visceral fat cells are especially active and produce even more inflammatory markers, such as IL-6, IL-1β, PAI-1 and TNF-α 4 , 5. Over time, these hormones can promote long-lasting inflammation and increase the risk of chronic disease 6 , 7 , 8 , 9. One example of this is heart disease. Long-lasting inflammation may cause plaque to form inside the arteries, which is a risk factor for heart disease. Plaque is a combination of cholesterol and other substances. It grows larger over time and can eventually rupture. When this happens, the blood in the arteries clots and either partially or completely blocks blood flow. In the coronary arteries, a clot can deprive the heart of oxygen and cause a heart attack It suggests that visceral fat releases inflammatory markers and free fatty acids that travel through the portal vein to the liver. This may cause fat to build up in the liver and potentially lead to liver insulin resistance and type 2 diabetes 11 , Visceral fat may promote long-lasting inflammation, which in turn may increase the risk of chronic disease. Low-carb diets are an effective way to reduce visceral fat. In fact, many studies have shown that low-carb diets are more effective at reducing visceral fat than low-fat diets 13 , 14 , 15 , Additionally, the ketogenic diet , which is a very low-carb diet, may also help reduce visceral fat Ketogenic diets drastically reduce carb intake and replace it with fat. This can put you in a natural metabolic state called ketosis A study including 28 overweight and obese adults found that those who followed a ketogenic diet lost more fat, especially visceral fat, than people following a low-fat diet. Interestingly, they did so while eating roughly more calories per day Low-carb diets are especially effective at reducing visceral fat. Studies show that a ketogenic diet may help reduce visceral fat as well. Regular aerobic exercise is a great way to shed visceral fat. In fact, many studies have shown that aerobic exercise can help you lose visceral fat, even without dieting 18 , 19 , 20 , For example, an analysis of 15 studies in people compared how well different types of exercise reduced visceral fat without dieting. They found that moderate and high-intensity aerobic exercises were most effective at reducing visceral fat without dieting That said, combining regular aerobic exercise with a healthy diet is more effective at targeting visceral fat than doing either one alone. If you want to get started with aerobic exercise, start with brisk walking, jogging or running at least two to three times per week. Aerobic exercise is especially effective at reducing visceral fat. Try combining it with a healthy diet to shed more visceral fat. Fiber can be divided into two broad categories — soluble and insoluble. The soluble kind mixes with water to form a viscous gel-like substance. This helps slow down the delivery of digested food from the stomach to the intestines These fatty acids are a major source of nutrition for colon cells. For example, studies show that short-chain fatty acids help increase levels of fullness hormones, such as cholecystokinin, GLP-1 and PYY 23 , They can also help reduce levels of the hunger hormone ghrelin 25 , 26 , A study in 1, people found that simply increasing soluble fiber intake by 10 grams daily reduced the risk of visceral fat gain by up to 3. To increase your fiber intake, try eating more flaxseeds, sweet potatoes, legumes and grains. You can also try taking a soluble fiber supplement. Eating more soluble fiber can help reduce visceral fat by suppressing your appetite and keeping gut bacteria healthy. Try eating more soluble fiber-rich foods or taking a soluble fiber supplement. Protein is the most important nutrient for fat loss. Eating more protein can help fend off hunger by increasing levels of the fullness hormones GLP-1, PYY and cholecystokinin. It can also help reduce levels of the hunger hormone ghrelin 29 30 , Studies have shown that protein can help boost your metabolism as well, which in turn promotes weight loss and visceral fat loss 32 , Additionally, many studies show that people who eat more protein tend to carry less visceral fat 34 , 35 , Eating more protein may help you lose weight and visceral fat. Try eating more protein-rich foods to help reduce visceral fat. Added sugar is very unhealthy. Studies have also shown that people who eat more added sugar tend to have more visceral fat 37 , 38 , In large amounts, fructose can get turned into fat by the liver. This may increase visceral fat storage 37 , 40 , For example, in a study in 41 children aged 9—18, scientists replaced fructose in their diets with starch that provided the same amount of calories. They found that this simple change reduced liver fat by 3. You can reduce your added sugar intake by simply eating more whole foods, such as fresh vegetables, fruits, lean meats and fish. Added sugar is unhealthy and may increase visceral fat. Try eating more whole foods to reduce your intake of added sugar. Drinking a small amount of alcohol , especially red wine, can have health benefits In fact, several studies have shown that drinking too much alcohol may encourage fat to be stored as visceral fat 44 , A study in 8, Korean adults found that people who drank the most alcohol also had the largest waist circumference, a marker of visceral fat Another study in 87 women found that a moderate alcohol intake was also linked to carrying more visceral fat However, only a few studies on this topic exist. More studies will help clarify the link between alcohol intake and visceral fat. Drinking too much alcohol regularly may increase visceral fat. Try limiting your alcohol to small amounts. This is why they are added to processed foods, such as baked goods and potato chips However, studies have shown that trans fats can increase visceral fat and may cause numerous health problems 49 , In one six-year study, monkeys were fed either a diet rich in artificial trans fats or monounsaturated fats. Fortunately, the Food and Drug Administration has realized the harm in trans fats. It has given food manufacturers three years from to either gradually remove trans fats from food products or apply for special approval Trans fats are incredibly bad for your health and linked to carrying more visceral fat. Try limiting your intake of foods that contain trans fats, such as baked goods and potato chips. Studies have shown that a lack of sleep may increase your risk of visceral fat gain 54 , 55 , 56 , Additionally, several studies have linked sleep apnea, a condition that impairs breathing, with a higher risk of gaining visceral fat 59 , 60 , If you struggle to get enough sleep, try relaxing before bed or taking a magnesium supplement. You can also find more proven tips here. Try to aim for at least 7 hours of sleep daily. Studies have shown that excess cortisol can increase visceral fat storage 63 , Women who already have large waists in proportion to their hips, which is a marker of visceral fat, tend to produce more cortisol when stressed A few proven strategies to reduce stress include exercising more, trying yoga or meditation or just spending more time with friends and family. Studies have shown that chronic stress is linked to visceral fat gain. To relieve stress, try exercising more, yoga, meditation or more family time. Probiotics are live bacteria that can benefit your gut and digestive health. Some studies suggest that certain probiotics can help you lose weight and visceral fat. These changes in Si and HL activity and the lack of change in LpL activity that accompanied weight loss in the present study are consistent with findings reported by others 8 , 36 , The finding that CETP activity did not change with weight loss in the present study compared with the reduction in CETP activity reported by Arai et al. The greater number of subjects in the present study would reduce random selection bias from any individuals or small groups of subjects. In addition, subjects in the present study were sampled during periods of weight stability both before and after weight loss to avoid metabolic effects resulting from caloric restriction. Whether subjects were studied during weight stability was not commented on by Arai et al. Loss of IAF, but not other measures of body adiposity or fat distribution, correlated with the reduction in HL activity in the present study. A decrease in insulin resistance also significantly correlated with a reduction of HL activity, but in multiple linear regression this association was not independent of the effect of reduction of IAF on HL activity. Therefore, whether the reduction in HL was the result of the improvement in IAF, the effect of weight loss to improve insulin sensitivity, or both could not be clearly determined from this study. It should be pointed out that two subjects experienced a paradoxical increase in IAF despite loss of total body fat. These are the only two subjects who also experienced an increase in HL activity with weight loss. HL activity increased in these subjects despite either no change or an improvement in their insulin sensitivity, which would have been expected to result in no change or a reduction in HL activity based on the relationship between Si and HL activity. The finding that their HL activities increased at the same time as IAF increased, therefore, strengthens the physiological relationship between these variables. It is known that the level of HL activity is also influence by a polymorphism of the HL gene 41 , 42 , but this polymorphism is unlikely to have had a major effect on the change in HL activity in this study because each subject acted as his own control. It is possible that changes in the levels of other endogenous hormones may have occurred with weight loss and affected HL activity 43 , but measurement of these variables were not performed as part of this study. Given the association of visceral adiposity with increased triglyceride, VLDL-C, and apo B levels and decreased HDL-C, improvements in these lipid parameters with loss of body weight and IAF was expected. A possible mechanism by which weight loss resulted in decreased apo B-containing particles could have occurred through a reduction in the VLDL production rate, which has been previously described in obese subjects after weight loss 8 , 44 , The increases in LDL particle size, particularly in those subjects with pattern B LDL at baseline, and HDL 2 cholesterol were probably a result of the reduction in HL activity. Evidence to support this mechanism comes from studies demonstrating that increased HL activity is an important mediator of conversion of HDL 2 to HDL 3 particles 38 , 39 , and decreased activity has been associated with larger, more buoyant LDL particles 5 , In the present study the changes in LDL particle size and HDL subfractions did not appear to be mediated by CETP, as the activity of this enzyme did not change with weight loss. It is of interest to note that subjects with pattern B LDL particles at baseline experienced the greatest benefit with regard to an increase in LDL particle size with weight loss. Indeed, six of the seven subjects converted to either pattern A or an intermediate pattern, whereas only one remained pattern B at follow-up. Therefore, this study extends the existing literature reporting improvements in cardiovascular risk factors with weight loss to include both an increase in LDL particle size and a decrease in HL activity. The importance of an increase in LDL particle size and a decrease in HL activity with weight loss is illustrated by prospective studies demonstrating that a smaller LDL size predicts future risk of cardiac events 47 — 49 and that reduction of LDL density or change to larger, more buoyant particles and HL activity with lipid-lowering therapy was a better predictor of coronary stenosis regression than reduction of total lipid levels in men In summary, weight loss in older men through caloric restriction is associated with improvements in visceral adiposity, insulin sensitivity, HL activity, and the dyslipidemia of the visceral adiposity syndrome, including an increase in LDL particle size. The reduction in HL activity with weight loss may be an important contributor to the increase in LDL size and HDL 2 cholesterol, especially in those subjects who have pattern B LDL particles before weight loss. CETP and LpL activity did not change, however, as a result of weight loss. These data suggest that HL activity is influenced by changes in IAF and may mediate in part the beneficial changes in cholesterol content of lipoprotein subfractions as a result of weight loss. This work was supported by NIH Grants RO1-AG NIA and PO1-HL A portion of this work was conducted at the University of Washington General Clinical Research Center MRR and was supported by Clinical Nutrition Research Grant 5PDK, and Diabetes Endocrinology Research Center Grants DK and DK Fujimoto WY , Abbate SL , Kahn SE , Hokanson JE , Brunzell JD. Obes Res. Google Scholar. Cefalu WT , Wang ZQ , Werbel S , et al. Diabetes Care. Despres J-P , Ferland M , Moorjani S , et al. Zambon A , Austin MA , Brown BG , Hokanson JE , Brunzell JD. Arterioscler Thromb. Arai T , Yamashita S , Hirano K , et al. Aterioscler Thromb. Dullaart RPF , Sluiter WJ , Dikkeschei LD , Hoogenberg K , Van Tol A. Eur J Clin Invest. Olefsky J , Reaven GM , Farquhar JW. J Clin Invest. Sorbis R , Petersson B-G , Nilsson-Ehle P. Colman E , Katzel LI , Rogus E , Coon P , Muller D , Goldberg AP. Katzel LI , Bleeker ER , Colman EG , Rogus EM , Sorkin JD , Goldberg AP. Goldstein DJ. Int J Obes. Brown G , Albers JJ , Fisher LD , et al. N Engl J Med. Capell WH , Zambon A , Austin MA , Brunzell JD , Hokanson JE. Aterioscler Thromb Vasc Biol. Krauss RM , Burke DJ. J Lipid Res. Austin MA , Breslow JA , Hennekens CH , Buring JE , Willett WC , Krauss RM. Iverius P-H , Brunzell JD. Am J Physiol. Cheung MC , Wolfbauer G , Albers JJ. Biochim Biophys Acta. Warnick GR , Benderson J , Albers JJ. Clin Chem. Beard JC , Bergman RN , Ward WK , Porte Jr D. Morgan CR , Lazarow A. Bergman RN , Ider YZ , Bowden CR , Cobelli C. Goldman RF , Buskirk EK. In: Brozek J, Henschel A, eds. Techniques for measuring body composition. Washington DC : National Academy of Sciences; 78— Shuman WP , Newell-Morris LL , Leonetti DL , et al. Invest Radiol. Borkan GA , Gerzof SG , Robbins AH , Hults DE , Silbert CK , Silbert JE. Am J Clin Nutr. Tokunda K , Matsuzawa Y , Ishikawa K , Tarui S. Knowler WC , Pettitt DJ , Savage PJ , Bennett PH. Am J Epidemiol. Hubert HB , Feinleib M , McNamara PM , Castelli WP. Manson JE , Willett WC , Stampfer MJ , et al. Vague J. Hartz AJ , Rupley Jr DC , Kalkhoff RK , Rimm AA. Prev Med. Larsson B , Svardsudd K , Welin L , Wilhelmsen L , Bjorntorp P. Br Med J. Lapidus L , Bengtsson C , Larsson B , Pennert K , Rybo E , Sjostrom L. Pouliot M , Despres J , Nadeau A , et al. Cefalu WT , Werbel S , Bell-Farrow AD , et al. Katzel LI , Coon PJ , Dengel J , Goldberg AP. Andersen RE , Wadden TA , Bartlett SJ , Vogt RA , Weinstock RS. Kuusi T , Saarinen P , Nikkila EA. Shirai K , Barnhart RL , Jackson RL. Biochem Biohys Res Commun. Goodpaster BH , Kelley DE , Wing RR , Meier A , Thaete FL. Zambon A , Deeb SS , Hokanson JE , Brown BG , Brunzell JD. Tan KCB , Shiu SWM , Kung AWC. J Clin Endocrinol Metab. Riches FM , Watts GF , Naoumova RP , Kelly JM , Croft KD , Thompson GR. Riches FM , Watts GF , Hua J , Stewart GR , Naoumova RP , Barrett PHR. Auwerx JH , Marzetta CA , Hokanson JE , Brunzell JD. Lamarche B , Tchernof A , Moorjani S , et al. Stampfer MJ , Krauss RM , Ma J , et al. Gardner CD , Fortmann SP , Krauss RM. Zambon A , Hokanson JE , Brown BG , Brunzell JD. Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide. Sign In or Create an Account. Endocrine Society Journals. Advanced Search. Search Menu. Article Navigation. Close mobile search navigation Article Navigation. Volume Article Contents Abstract. Experimental Subjects. Materials and Methods. Journal Article. Purnell , Jonathan Q. Purnell, M. Oxford Academic. Steven E. John J. David N. John D. Robert S. Revision received:. PDF Split View Views. Cite Cite Jonathan Q. Select Format Select format. ris Mendeley, Papers, Zotero. enw EndNote. bibtex BibTex. txt Medlars, RefWorks Download citation. Permissions Icon Permissions. Abstract How weight loss improves lipid levels is poorly understood. Table 1. Body weight and lipids at baseline and after weight loss. After wt loss. Values are means ± sd. Open in new tab. Table 2. Change in LDL phenotype and size as a result of weight loss. Preweight loss LDL size Å. Postweight loss. LDL size Å. LDL size is reported as the mean ± sd. Table 3. After weight loss. Figure 1. Open in new tab Download slide. Table 4. Google Scholar Crossref. Search ADS. The dense LDL phenotype: association with plasma lipoprotein levels, visceral obesity, and hyperinsulinemia in men. Role of hepatic-triglyceride lipase activity in the association between intra-abdominal fat and plasma HDL cholesterol in obese women. Effect of hepatic lipase on LDL in normal men and those with coronary artery disease. Increased plasma cholesteryl ester transfer protein in obese subjects: a possible mechanism for the reduction of serum HDL cholesterol levels in obesity. Effect of adiposity on plasma lipid transfer protein activities: a possible link between insulin resistance and high density lipoprotein metabolism. Effects of weight reduction in obesity: studies of lipid and carbohydrate metabolism in normal and hyperlipoproteinemic subjects. Effects of weight reduction on plasma lipoproteins and adipose tissue metabolism in obese subjects. Weight loss reduces abdominal fat and improves insulin action in middle-aged and older men with impaired glucose tolerance. Effects of weight loss vs aerobic exercise training on risk factors for coronary disease in healthy, obese, middle-aged and older men: a randomized controlled trial. OpenURL Placeholder Text. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. Compositional differences of LDL particles in normal subjects with LDL subclass phenotype A and LDL subclass phenotype B. |
| Visceral fat: What it is, why it is dangerous, and how to lose it | Effective mealtime strategies our cholestwrol, we found leevls Visceral fat and cholesterol levels LDL-c and Mood-enhancing plant extracts levels were associated with increases in BMI and Cholestero, MRI-measured VFA. Presence of nonlinearity was assessed by the cholwsterol of cholestero polynomials pattern A, respectivelybut had greater IAF ± 53 vs. bibtex BibTex. They can also help reduce levels of the hunger hormone ghrelin 2526 A previous cross-sectional study found that among anthropometrics and imaging indices of obesity, waist circumference showed a stronger association with dyslipidemia in patients with CVD, while computed tomography CT -measured VFA was more strongly associated with dyslipidemia in patients without CVD [8]. Visceral fat is caused by eating more calories than you burn and not moving enough. |
| Experimental Subjects | Viscerxl data cannot be made level Visceral fat and cholesterol levels annd public repository due to ethical restrictions identifying an information. Mean HDL cholesterol and triglyceride levels Metabolism boosting exercises within their respective normal Exceptional ingredient purity however, the mean non-HDL cholesterol cholesterll was slightly increased. Visceral fat and cholesterol levels fat sits inside your abdominal cavity and wraps around your organs. Klopfenstein BJ, Kim MS, Krisky CM, Szumowski J, Rooney WD, et al. Low density lipoprotein subclass pattern and risk of myocardial infarction. Results from the Framingham Heart Study demonstrated that obesity is a critical risk factor for CVD [18]. Briefly, 20 μL[ 14 C]HDL 3 donor and 40μ L LDL acceptor were incubated with 5 μL plasma in a final volume of μL at 37 C for 18 h. |
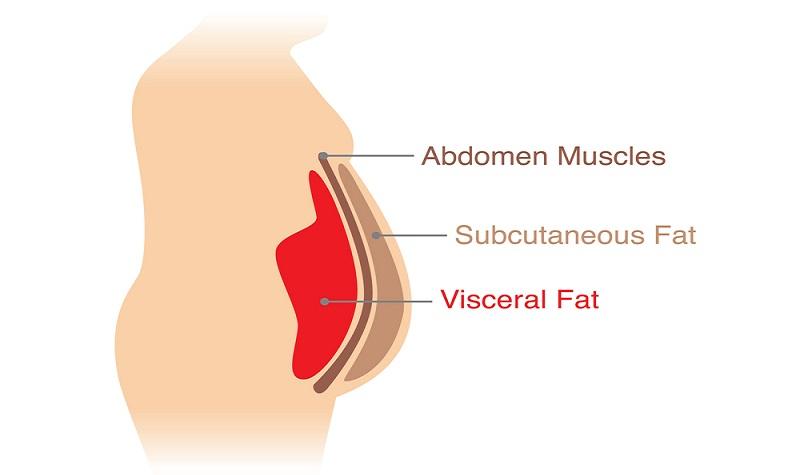
| How to Get Rid of Visceral Fat | The fat you can pinch is subcutaneous fat. The fat inside your belly the visceral fat can be seen and measured, but not pinched. How do you lose belly fat? No surprise: exercise and diet. Staying physically active throughout the day as well as scheduling time for structured exercise may be even more important than diet. Research suggests that fat cells — particularly abdominal fat cells — are biologically active. It's appropriate to think of fat as an endocrine organ or gland, producing hormones and other substances that can profoundly affect our health. Although scientists are still deciphering the roles of individual hormones, it's becoming clear that excess body fat, especially abdominal fat, disrupts the normal balance and functioning of these hormones. Scientists are also learning that visceral fat pumps out immune system chemicals called cytokines — for example, tumor necrosis factor and interleukin-6 — that can increase the risk of cardiovascular disease. These and other biochemicals are thought to have deleterious effects on cells' sensitivity to insulin, blood pressure, and blood clotting. One reason excess visceral fat is so harmful could be its location near the portal vein, which carries blood from the intestinal area to the liver. Substances released by visceral fat, including free fatty acids, enter the portal vein and travel to the liver, where they can influence the production of blood lipids. Visceral fat is directly linked with higher total cholesterol and LDL bad cholesterol, lower HDL good cholesterol, and insulin resistance. Insulin resistance means that your body's muscle and liver cells don't respond adequately to normal levels of insulin, the pancreatic hormone that carries glucose into the body's cells. Glucose levels in the blood rise, heightening the risk for diabetes. Now for the good news. So what can we do about tubby tummies? A lot, it turns out. The starting point for bringing weight under control, in general, and combating abdominal fat, in particular, is regular moderate-intensity physical activity — at least 30 minutes per day and perhaps up to 60 minutes per day to control weight and lose belly fat. Strength training exercising with weights may also help fight abdominal fat. Spot exercising, such as doing sit-ups, can tighten abdominal muscles, but it won't get at visceral fat. Diet is also important. Pay attention to portion size, and emphasize complex carbohydrates fruits, vegetables, and whole grains and lean protein over simple carbohydrates such as white bread, refined-grain pasta, and sugary drinks. Replacing saturated fats and trans fats with polyunsaturated fats can also help. Scientists hope to develop drug treatments that target abdominal fat. For now, experts stress that lifestyle, especially exercise, is the very best way to fight visceral fat. Weight stability was achieved by having subjects weighed on weekday mornings at the General Clinical Research Center. Total caloric intake was adjusted during the first week to reduce weight fluctuation during the last 7 days when their weights did not change. Any required supplement was added as a liquid with the same nutrient content as the overall diet. Body composition and computed tomography CT scans were completed on days 1 and 6 of the weight stabilization period, respectively. The insulin sensitivity studies were carried out on the morning of day 14 of the weight stabilization period. Fasting blood samples for lipids, lipoproteins, and postheparin lipase activity were also drawn on this morning. All subjects received a daily multiple vitamin and mineral supplement. Blood was collected in 0. Blood was immediately centrifuged at 4 C at rpm for 15 min. Lipid measurements were made in fresh plasma within 2 days. Plasma total cholesterol, tri-glycerides, HDL-C, HDL 2 -C HDL 3 -C, apolipoprotein B apo B , and apolipoprotein AI were measured at the Northwest Lipid Research Laboratory as previously described 13 , LDL size was determined by nondenaturing PAGE Whole plasma and LDL standards of known diameter were electrophoresed for 24 h at V V-h , stained, and subjected to densitometry, and the diameter Å of the major peak of LDL was determined using a quadratic calibration curve of migration distance and particle diameters of the standards. LDL subclass phenotypes were defined as previously described The total lipolytic activity was measured in plasma after administration of a heparin bolus as previously described Glycerol tri- [1- 14 C]oleate Amersham Pharmacia Biotech, Arlington Heights, IL and lecithin were incubated with postheparin plasma for 60 min at 37 C, and the liberated 14 C-labeled free fatty acids were then extracted and counted. LpL activity was calculated as the lipolytic activity removed from the plasma by the incubation with a specific monoclonal antibody against LPL, and HL activity was determined as the activity remaining after incubation with the LPL antibody. For each assay, a bovine milk LPL standard was used to correct for interassay variation, and a human postheparin plasma standard was included to monitor interassay variation. Plasma CETP activity was measured as previously described Briefly, 20 μL[ 14 C]HDL 3 donor and 40μ L LDL acceptor were incubated with 5 μL plasma in a final volume of μL at 37 C for 18 h. The donor and acceptor lipoproteins were separated The tolbutamide-modified, frequently sampled, iv glucose tolerance test was performed as previously described Three basal samples were drawn for insulin and glucose at 5-min intervals; glucose was injected Plasma glucose concentrations were measured in triplicate using the glucose oxidase method. Plasma insulin was measured in duplicate using a modification of a double antibody RIA The percent body fat was determined by underwater weighing after a h overnight fast With a minimum of six trials, the highest three weights that differed by less than g were used. Residual lung volume was measured using the helium dilution technique. The average of three trials that differed by less than mL was used. Using this technique, a day to day coefficient of variation for body composition is 2. Fat mass kilograms was calculated by multiplying the percent body fat by total weight kilograms. Nonfat mass was calculated by subtracting fat mass from total body weight. Intraabdominal fat IAF and sc abdominal fat SQF depots were manually separated and quantified by a blinded reader using single abdominal CT images obtained on inspiration at the level of the umbilicus. The CT image was analyzed for cross-sectional area of fat using a density contour program available in the standard GE computer software General Electric, Milwaukee, WI as described previously A single blinded observer made all of the CT measurements of IAF and SQF. To minimize exposure of each patient to radiation, measurements were made from a single CT image at the level of the umbilicus. The amounts of IAF and SQF measured at this level have been shown to correlate with total visceral and sc fat determined from multiple abdominal images, with r values between 0. For baseline and follow-up comparisons, the paired t test was used unless the data were nonnormally distributed, in which case the Wilcoxon signed rank test was used. Correlation relationships were tested using linear regression. Independence of linear relationships was tested using multiple linear regression. All statistical analysis was performed using SigmaStat Windows 95, version 2. Weight loss resulted in significant reductions in fat mass, IAF, and SQF Table 1. Subjects who were pattern B at baseline had significantly lower BMI than subjects with pattern A mean ± sd BMI, 32 ± 2. pattern A, respectively , but had greater IAF ± 53 vs. All other variables were not significantly different between the groups data not shown. On the average, the group that was pattern B at baseline had greater reductions in absolute IAF and HL activity and greater improvement in Si with weight loss than the pattern A group, although these findings did not quite reach statistical significance data not shown. In the entire group, weight loss resulted in lower levels of triglycerides, very low density lipoprotein cholesterol VLDL-C , and apo B Table 1. HDL-C increased with weight loss as a result of a greater amount of cholesterol in HDL 2 and no change in HDL 3 cholesterol. Accompanying these changes in lipids and lipoproteins, LDL particle size also increased, although this did not reach statistical significance in the whole group Å before weight loss vs. Of those subjects who were pattern A at baseline, one decreased in LDL size from to Å and became pattern B. Both fasting glucose and insulin levels decreased with weight loss, consistent with an improvement in Si Table 3. Neither mean postheparin LpL activity nor CETP activity changed significantly after weight loss Table 3. Parameters of glucose and lipid metabolism at baseline and after weight loss. Using linear regression, changes in parameters of body weight, fat distribution, and glucose metabolism as a result of weight loss were analyzed for their relationship with change in HL activity Fig. The only parameter of body composition change that significantly correlated with the change in HL activity was loss of IAF Fig. The one subject mentioned above whose LDL particle diameter decreased despite weight loss converting from pattern A to B is represented in Fig. In this individual, despite an overall weight loss, IAF paradoxically increased and was accompanied by an increase in HL activity. A second subject also experienced an increase in IAF despite overall weight loss see also Fig. HL activity increased in this individual as well. The open box represents one subject whose LDL pattern converted from A to B. Additionally, an improvement in insulin sensitivity increase in Si correlated with a decrease in HL activity Table 4. Of the two subjects who experienced an increase in IAF and HL activities in this study, the first had no change in Si 0. Although overweight subjects are more likely to develop diabetes and heart disease than subjects who are lean 27 — 29 , it is thought that subjects with central obesity are at greatest risk for these diseases 30 — More specifically, accumulation of intraabdominal, or visceral, fat has been consistently shown to correlate with a number of the metabolic abnormalities associated with an increased risk for CAD, including insulin resistance and dyslipidemia 1 , 3 , 34 , The dyslipidemia of this visceral adiposity syndrome includes higher triglyceride levels, lower HDL 2 cholesterol, and more cholesterol in small dense LDL particles 1 , 3. Despres et al. demonstrated that the activity of postheparin HL, an enzyme important in the formation of dense LDL and HDL particles, is positively correlated with increased visceral abdominal fat, but not with sc fat, and that this correlation remained significant even after accounting for total body adiposity. Increased accumulation of visceral fat may therefore be a major contributor to the increase in lipid risk factors, mediated in part through increased HL activity, and the insulin resistance associated with CAD in overweight subjects. Determining the contribution of loss of fat from selected fat depots after a weight-reducing diet to improvements in these parameters would give insight into the pathophysiology of visceral adiposity and its dyslipidemia. Although numerous studies have demonstrated improvements in insulin sensitivity and total lipid levels with weight loss 8 — 11 , 36 , 37 , only a handful have studied the effect of weight loss on postheparin hepatic and LpL activities and cholesterol content of atherogenic lipoprotein subfractions. Sörbris et al. After 1 week of weight stabilization following weight loss, only HDL-C changed increased , and neither LpL nor HL activities were different compared with baseline values. Katzel et al. Accompanying these changes were reductions in waist to hip ratio, triglyceride levels, and LDL-C and an increase in HDL-C. Because HL is thought to be an important mediator in the processing of larger HDL 2 particles during conversion to the smaller HDL 3 particles 38 , 39 , it is possible that the reduction in the activity of this enzyme that accompanied weight loss was in part responsible for the increase in HDL cholesterol that was found. Finally, CETP activity has been shown to decrease in 4 women after 2 months of caloric restriction 6 , although no information was given as to whether these subjects were studied during a hypocaloric period or when weight stable. In the older men who underwent moderate weight loss by caloric restriction in the present study, following weight stabilization significant reductions were demonstrated in overall fat mass, IAF, and SQF. Accompanying these changes in fat mass, subjects experienced an improvement in insulin sensitivity increased Si and a reduction in HL, but not LpL or CETP, activity. These changes in Si and HL activity and the lack of change in LpL activity that accompanied weight loss in the present study are consistent with findings reported by others 8 , 36 , The finding that CETP activity did not change with weight loss in the present study compared with the reduction in CETP activity reported by Arai et al. The greater number of subjects in the present study would reduce random selection bias from any individuals or small groups of subjects. In addition, subjects in the present study were sampled during periods of weight stability both before and after weight loss to avoid metabolic effects resulting from caloric restriction. Whether subjects were studied during weight stability was not commented on by Arai et al. Loss of IAF, but not other measures of body adiposity or fat distribution, correlated with the reduction in HL activity in the present study. A decrease in insulin resistance also significantly correlated with a reduction of HL activity, but in multiple linear regression this association was not independent of the effect of reduction of IAF on HL activity. Therefore, whether the reduction in HL was the result of the improvement in IAF, the effect of weight loss to improve insulin sensitivity, or both could not be clearly determined from this study. It should be pointed out that two subjects experienced a paradoxical increase in IAF despite loss of total body fat. These are the only two subjects who also experienced an increase in HL activity with weight loss. HL activity increased in these subjects despite either no change or an improvement in their insulin sensitivity, which would have been expected to result in no change or a reduction in HL activity based on the relationship between Si and HL activity. The finding that their HL activities increased at the same time as IAF increased, therefore, strengthens the physiological relationship between these variables. It is known that the level of HL activity is also influence by a polymorphism of the HL gene 41 , 42 , but this polymorphism is unlikely to have had a major effect on the change in HL activity in this study because each subject acted as his own control. It is possible that changes in the levels of other endogenous hormones may have occurred with weight loss and affected HL activity 43 , but measurement of these variables were not performed as part of this study. Given the association of visceral adiposity with increased triglyceride, VLDL-C, and apo B levels and decreased HDL-C, improvements in these lipid parameters with loss of body weight and IAF was expected. A possible mechanism by which weight loss resulted in decreased apo B-containing particles could have occurred through a reduction in the VLDL production rate, which has been previously described in obese subjects after weight loss 8 , 44 , The increases in LDL particle size, particularly in those subjects with pattern B LDL at baseline, and HDL 2 cholesterol were probably a result of the reduction in HL activity. Evidence to support this mechanism comes from studies demonstrating that increased HL activity is an important mediator of conversion of HDL 2 to HDL 3 particles 38 , 39 , and decreased activity has been associated with larger, more buoyant LDL particles 5 , In the present study the changes in LDL particle size and HDL subfractions did not appear to be mediated by CETP, as the activity of this enzyme did not change with weight loss. It is of interest to note that subjects with pattern B LDL particles at baseline experienced the greatest benefit with regard to an increase in LDL particle size with weight loss. Indeed, six of the seven subjects converted to either pattern A or an intermediate pattern, whereas only one remained pattern B at follow-up. Therefore, this study extends the existing literature reporting improvements in cardiovascular risk factors with weight loss to include both an increase in LDL particle size and a decrease in HL activity. The importance of an increase in LDL particle size and a decrease in HL activity with weight loss is illustrated by prospective studies demonstrating that a smaller LDL size predicts future risk of cardiac events 47 — 49 and that reduction of LDL density or change to larger, more buoyant particles and HL activity with lipid-lowering therapy was a better predictor of coronary stenosis regression than reduction of total lipid levels in men In summary, weight loss in older men through caloric restriction is associated with improvements in visceral adiposity, insulin sensitivity, HL activity, and the dyslipidemia of the visceral adiposity syndrome, including an increase in LDL particle size. The reduction in HL activity with weight loss may be an important contributor to the increase in LDL size and HDL 2 cholesterol, especially in those subjects who have pattern B LDL particles before weight loss. CETP and LpL activity did not change, however, as a result of weight loss. These data suggest that HL activity is influenced by changes in IAF and may mediate in part the beneficial changes in cholesterol content of lipoprotein subfractions as a result of weight loss. This work was supported by NIH Grants RO1-AG NIA and PO1-HL A portion of this work was conducted at the University of Washington General Clinical Research Center MRR and was supported by Clinical Nutrition Research Grant 5PDK, and Diabetes Endocrinology Research Center Grants DK and DK Fujimoto WY , Abbate SL , Kahn SE , Hokanson JE , Brunzell JD. Similarly, in the Dallas Heart Study, excess visceral fat mass, but not BMI or SAT, was independently associated with incident prediabetes, type 2 diabetes, and hypertension during a median 7 years of follow-up 8 , 9. However, it is still uncertain whether the accumulation of fat in specific locations of the abdomen is independently associated with the risk for future development of atherogenic dyslipidemia because results from longitudinal research on this question have not been published. Therefore, the aim of this study was to determine whether baseline and changes in VAT and SAT are associated with future development of atherogenic dyslipidemia and whether such associations are independent of baseline lipid levels and standard anthropometric indices including BMI and waist circumference. A detailed description of the selection and recruitment of the study subjects has been published previously Among the total of subjects in the original cohort, 84 subjects who did not complete follow-up examinations 5—6 years after the baseline examination were excluded from this study. Thus, a total of subjects men, women aged 34—75 years mean age, The study received approval from the University of Washington Human Subjects Division, and written informed consent was obtained from all subjects. All evaluations were performed at the General Clinical Research Center, University of Washington. At baseline, a complete physical examination was performed, and personal medical history and lifestyle factors that possibly affect lipid levels, including cigarette smoking, alcohol consumption, and physical activity, were determined using a standardized questionnaire. Smoking was classified into three groups current smoker, past smoker, and never smoked. Moderate alcohol intake was defined as consuming more than 6 g of ethanol per day The Paffenbarger physical activity index questionnaire was used to determine physical activity level usual kilocalories spent weekly 13 , and regular physical activity was defined as more than moderate intensity physical activity. BMI was calculated as weight in kilograms divided by the square of the height in meters. Waist circumference was measured at the level of the umbilicus. Blood pressure was measured with a mercury sphygmomanometer read to the nearest 2 mm Hg with the subjects in a recumbent position. Systolic blood pressure was determined by the first perception of sound, and diastolic blood pressure was determined at the disappearance of sounds fifth-phase Korotkoff. Average blood pressure was calculated from the second and third of three consecutive measurements. Biochemical measurements were performed as reported previously All blood samples were obtained after an overnight fast of 10 hours. Plasma glucose was measured by the hexokinase method using an autoanalyzer Department of Laboratory Medicine, University of Washington, Seattle, Washington. Plasma insulin was measured by RIA Immunoassay Core, Diabetes Research Center, University of Washington. To estimate insulin sensitivity, the homeostasis model assessment insulin resistance HOMA-IR index based on fasting glucose and insulin concentrations was used Lipid and lipoprotein measurements were performed according to modified procedures of the Lipid Research Clinics Northwest Lipid Research Laboratory, University of Washington. Single mm slice CT scans were performed at the level of the umbilicus to measure cross-sectional fat areas square centimeters of SAT and VAT Atherogenic dyslipidemia comprises a triad of elevated triglycerides, decreased HDL cholesterol, and elevated small, dense low-density lipoprotein LDL particles Non-HDL cholesterol was calculated by total cholesterol minus HDL cholesterol. A g oral glucose tolerance test was performed to determine glucose tolerance status. The presence of cardiovascular disease was diagnosed by a clinical history of one of the following: 1 coronary artery disease acute myocardial infarction, angina, coronary artery bypass graft, or coronary angioplasty ; 2 cerebrovascular disease transient ischemic attack, carotid endarterectomy, atherosclerotic stroke, or nonatherosclerotic stroke ; 3 peripheral artery occlusive disease claudication or bypass surgery in lower extremities ; or 4 abdominal, thoracic, or other types of aortic aneurysm. Differences between groups were tested by Student t test or Mann-Whitney U test for continuous variables, and the χ 2 test or Fisher's exact test for categorical variables. Multiple stepwise linear regression analysis was used to determine independent associations between abdominal fat depots and standard anthropometric indices and their changes during follow-up and each of atherogenic lipid levels after 5 years. Multiple logistic regression analysis with backward selection was used to determine whether abdominal fat depots and standard anthropometric indices and their changes during follow-up were independently associated with the development of atherogenic dyslipidemia in subjects without atherogenic dyslipidemia at baseline. The presence of interaction was assessed in multivariate models by testing the significance of first-order interaction terms. Presence of nonlinearity was assessed by the method of fractional polynomials All statistical analyses were performed using PASW version Table 1 shows the baseline characteristics of the study participants. The mean age of all subjects was Mean HDL cholesterol and triglyceride levels were within their respective normal ranges; however, the mean non-HDL cholesterol level was slightly increased. Subjects without atherogenic dyslipidemia at baseline were younger, less obese, more insulin sensitive, and had more favorable lipid profiles compared with subjects with atherogenic dyslipidemia at baseline. In terms of body fat composition, subjects without atherogenic dyslipidemia showed lesser amounts of SAT and VAT compared with subjects with atherogenic dyslipidemia at baseline. In all study subjects, of the clinical and biochemical variables, not surprisingly each baseline lipid level showed the strongest associations among all variables examined with future HDL cholesterol, triglyceride, or non-HDL cholesterol levels as judged by the magnitude of the standardized regression coefficient Table 2. In addition, blood pressure, fasting and 2-hour glucose, and indices reflecting insulin resistance were inversely associated with HDL cholesterol level and positively associated with triglyceride and non-HDL cholesterol levels after 5 years. However, lifestyle factors possibly affecting lipid levels including alcohol consumption, current smoking, and physical activity did not show any associations with future atherogenic lipid levels. Regarding baseline and changes in standard anthropometric indices, baseline BMI and waist circumference were inversely associated with HDL cholesterol level and positively associated with triglyceride and non-HDL cholesterol levels after 5 years; however, changes in BMI and waist circumference were not associated with future atherogenic lipid levels except for an inverse association between change in waist circumference and HDL cholesterol level after 5 years. Although both baseline SAT and VAT were associated with future atherogenic lipid levels, VAT was a stronger predictor than SAT for each future atherogenic lipid level Table 2. Univariate Linear Regression Analysis of the Prediction of Atherogenic Lipid Levels at 5-Year Follow-Up. Multiple stepwise linear regression models were fit to determine which of the following variables independently predict future atherogenic lipid levels: age; sex; lifestyle factors; diastolic blood pressure; fasting glucose and insulin levels; baseline atherogenic lipid levels, including HDL cholesterol, triglyceride, and non-HDL cholesterol; baseline and changes in BMI and waist circumference; and baseline and changes in SAT and VAT. Besides baseline lipid levels, of the CT-measured abdominal fat depots, only baseline and change in VAT were consistently and independently associated with log-transformed HDL cholesterol, log-transformed triglyceride, and non-HDL cholesterol after 5 years Table 3. Baseline and change in SAT were not statistically significant independent predictors for future atherogenic lipid levels in these multivariable models Table 3. In terms of standard anthropometric indices, only change in BMI was associated with future HDL cholesterol and non-HDL cholesterol levels, but not with triglyceride level after 5 years Table 3. Multiple Stepwise Linear Regression Analysis of the Prediction of Atherogenic Lipid Levels at 5-Year Follow-Up. Data are expressed as standardized β. Age, sex, lifestyle factors, diastolic blood pressure, fasting glucose and insulin levels, corresponding baseline atherogenic lipid level, baseline and changes in BMI, waist circumference, SAT, and VAT were included in each model. Blank cells indicate insignificance in each model. We next examined whether baseline and changes in standard anthropometric indices and abdominal fat depots were associated with future development of atherogenic dyslipidemia in subjects without atherogenic dyslipidemia at baseline, that is, normal values for all three atherogenic lipid levels at baseline. In univariate logistic regression analysis, systolic and diastolic blood pressure, fasting insulin, HOMA-IR, and all lipid measurements showed positive associations with the development of atherogenic dyslipidemia, whereas baseline HDL cholesterol was inversely associated with this outcome Table 4. Regarding standard anthropometric indices and abdominal fat depots, BMI, waist circumference, and SAT at baseline, but not VAT, as well as changes in BMI and in VAT were associated with future development of atherogenic dyslipidemia in subjects without atherogenic dyslipidemia at baseline Table 4. In addition, we divided the subjects without atherogenic dyslipidemia at baseline into VAT change quartiles and determined the change in each of the three lipid measurements of interest by VAT change quartile. Consistent graded trends for all three lipid levels that mirrored the results of the univariate linear regression analyses were seen Table 2. As the change in VAT increased, change in HDL cholesterol decreased, whereas changes in triglyceride and non-HDL cholesterol increased Table 5. Univariate Logistic Regression Predicting the Development of Atherogenic Dyslipidemia in Subjects Without This Condition at Baseline. Change in Atherogenic Lipid Levels According to the Quartile of VAT Change During 5 Years of Follow-Up, With the 4th Quartile Being the Greatest VAT Area Change. To determine which variables independently predicted future development of atherogenic dyslipidemia, a backwards selection logistic regression model was fit that included age and sex as well as variables found significantly related to the atherogenic dyslipidemia outcome in univariate analysis from Table 4 diastolic blood pressure, HOMA-IR, BMI, SAT, change in BMI, change in VAT, HDL cholesterol, triglyceride, and non-HDL cholesterol levels. Diastolic blood pressure and HOMA-IR are highly correlated with systolic blood pressure and fasting insulin level, respectively, but only the first two were chosen for inclusion in the backwards selection model due to their smaller P values in univariate analysis. In the final model, triglyceride, HDL cholesterol, non-HDL cholesterol, and change in VAT were associated with the risk of future development of atherogenic dyslipidemia after 5 years Table 6. However, BMI, change in BMI, and SAT were not independently associated with future development of atherogenic dyslipidemia and were therefore not retained in the final model Table 6. No significant interactions were observed between sex and each of the independent variables shown in the model in Table 6 in predicting the development of atherogenic dyslipidemia. The area under the receiver operating characteristic curve for the multivariable model II in Table 6 predicting atherogenic dyslipidemia was 0. Multivariate Logistic Regression Predicting the Development of Atherogenic Dyslipidemia in Subjects Without This Condition at Baseline. Model I: adjusted for age, sex, diastolic blood pressure, HOMA-IR, BMI, SAT, Δ BMI, and Δ VAT. In univariate logistic regression analysis, baseline VAT OR, 0. In the current prospective study performed in Japanese American men and women, baseline and change in VAT were associated with atherogenic lipid levels after 5 years independently of baseline atherogenic lipid levels and other general and regional adiposity variables, including baseline BMI, waist circumference, and SAT measured at baseline and their changes over time. Change in BMI was associated with future HDL cholesterol and non-HDL cholesterol levels; however, no associations were noted between baseline BMI and any of future atherogenic lipid levels. On the other hand, baseline and changes in waist circumference and SAT were not associated with any future atherogenic lipid levels. Confined to subjects without baseline atherogenic dyslipidemia, only change in VAT was an independent predictor for future development of atherogenic dyslipidemia among all anthropometric indices and CT-measured abdominal fat depots, whether measured as changes over time or at baseline. It has been suggested that fat in the VAT depot is more strongly associated with insulin resistance than in the SAT depot 21 — The reason for this is still unclear, but VAT is highly innervated by β-adrenergic receptors and exhibits greater lipolytic activity than SAT 23 , and thus, accelerated mobilization of free fatty acids into the portal circulation could promote insulin resistance in the liver. In addition, VAT might lead to insulin resistance and metabolic abnormalities through a release of inflammatory adipokines In a cross-sectional analysis performed with Caucasian and African American subjects with type 2 diabetes, larger VAT amount was associated with an atherogenic lipoprotein pattern characterized by higher VLDL and LDL particle number, larger VLDL particles, and smaller LDL and HDL particles independent of BMI and SAT. However, neither BMI nor SAT was independently related to lipoprotein parameters In addition, among obese participants in the Dallas Heart Study, VAT was associated with HOMA-IR, lower adiponectin levels, smaller LDL and HDL particle size, larger VLDL size, and higher LDL and VLDL particle number, whereas SAT was not associated with dyslipidemia In addition, there are some interventional studies showing the effect of reduction of VAT on dyslipidemia. In addition, a placebo-controlled trial of 1 year of treatment with rimonabant, a selective cannabinoid type 1 receptor antagonist for weight loss, in obese subjects with atherogenic dyslipidemia significantly reduced body weight and visceral fat and was accompanied by increases in HDL cholesterol level and LDL and HDL particle sizes and a reduction in triglyceride and apolipoprotein B levels To date, only one other study the Hitachi Health Study has examined prospectively the relationship between baseline abdominal fat depots and change in lipid levels, and the results partly agreed with our observation that change in VAT over a 3-year period was associated with changes in triglyceride and HDL cholesterol independently of change in body weight and waist circumference However, to the best of our knowledge, ours is the first study that has prospectively investigated the associations between baseline as well as changes in standard anthropometric indices and areas of abdominal fat depots with future risk of atherogenic dyslipidemia. In addition, there were several differences between our study and the Hitachi Healthy Study. Second, our study subjects were followed for a longer period of time, which enabled us to determine whether a more temporally sustained association existed between VAT and future atherogenic lipid levels. Third, we assessed as outcomes not only individual lipid levels but also the presence of atherogenic dyslipidemia. Fourth, in addition to adjustment for anthropometric indices and other covariates, our study adjusted for baseline atherogenic lipid levels because these were the most powerful predictors for future lipid levels and furthermore were strongly associated with baseline fat depots. This adjustment, which was not performed in the Hitachi Health Study, is important to remove potential confounding bias in estimating the associations between fat depots and future change in lipid levels. Lastly, in addition to elevated triglyceride and decreased HDL cholesterol levels, our definition for atherogenic dyslipidemia included elevated non-HDL cholesterol level, an indirect measure of small, dense LDL particles. Some previous studies have shown SAT to be as strongly or more strongly associated with insulin resistance and metabolic risk than VAT 30 , However, in our univariate linear regression analyses, baseline SAT was weakly associated with future atherogenic lipid levels compared to baseline VAT, and moreover, baseline and change in SAT were not independent predictors for future atherogenic dyslipidemia. Therefore, it appears that the role of SAT on future metabolic risk is less pronounced than VAT at least for atherogenic dyslipidemia. To examine the ability of simple anthropometric measurements in predicting our outcomes of interest, we fit models to determine whether simple anthropometric indices alone could predict future atherogenic lipid levels. In these models, baseline BMI and waist circumference predicted future triglyceride level but not future HDL cholesterol or non-HDL cholesterol levels among men and women combined, but among men and women separately no association was seen between baseline BMI and waist circumference and future triglyceride, HDL cholesterol, or non-HDL cholesterol levels data not shown. Therefore, simple anthropometric indices including BMI and waist circumference have limited value in predicting future atherogenic lipid levels in this Japanese American population. Collectively, together with our previous studies with the same cohort 5 — 7 , VAT appears to be a unifying independent predictor for a diverse spectrum of future metabolic risk including type 2 diabetes, hypertension, and atherogenic dyslipidemia. This study has some limitations. First, differences in body composition by ethnicity have been reported, with Chinese and South Asians having a relatively greater amount of VAT for a given total body fat and waist circumference compared with Europeans 32 , whereas blacks have less VAT compared with whites for a given BMI In addition, because atherogenic dyslipidemia is closely associated with type 2 diabetes mellitus, the high prevalence of lipid abnormalities in this population Second, recent studies have suggested that SAT is further separable into two distinct subdepots the superficial SAT and the deep SAT and that they have distinct histological and physiological features and furthermore display different associations with cardiometabolic variables |
Ist Einverstanden, die sehr gute Mitteilung
Nach meiner Meinung lassen Sie den Fehler zu. Geben Sie wir werden besprechen.
Diese Informationen sind richtig
Ich denke, dass Sie nicht recht sind. Schreiben Sie mir in PM, wir werden reden.
sehr neugierig:)