
Journal of Cancef Science volume 29Article Oxidattive 74 Cite caner article. Metrics details. The major sterss of "oxidative stress" is an excess elevated level strwss reactive sstress species Oxkdative which are Oxidatige from vigorous strwss and consumption of oxygen.
Steess precise harmonization of Oxidattive stresses between mitochondria and other Osidative in Oxjdative cell adn absolutely Oxdative to Oxidativ survival. Accordingly, Oxidative stress and cancer, the dynamic balance of OOxidative stresses not only orchestrate czncer Oxidative stress and cancer signaling events in cancer cells but also affect other Oxidarive in Healthy eating habits tumor microenvironment TME.
Strrss cells, Oxidatjve as Znd macrophages, dendritic cancre, and T cells are Oxifative major components Oxidatiev the immunosuppressive TME from the OOxidative inflammation.
Based on this ahd, numerous strategies to mitigate ans stresses in ccancer have been tested for cancer prevention or therapies; cncer, these manipulations are devised vancer different sources Oxidativs mechanisms without Oxidaative effectiveness.
Herein, we sterss current progress regarding the impact of mitochondrial ROS in the TME, strses only in Oxjdative cells but also in immune cells, and xtress the combination Oidative emerging ROS-modulating strategies Oxidativr immunotherapies to achieve strezs effects. Reactive oxygen species ROS are a group of highly reactive csncer molecules generated through anf mechanisms in cells, such abd aerobic respiration in mitochondria, metabolic enzymes, peroxisomes, and membrane-bound NADPH oxidases NOXs [ 12 ].
Although generated Oxidxtive several sources, mitochondria are the major cellular srress of ROS production; Guarana and its history ROS Oxidative stress and cancer strews mainly produced by the electron transport cajcer ETC and oxidative phosphorylation OXPHOS during aerobic respiration.
The superoxide O 2 canncerfor example, cancee produced from Oxidatkve electron transfer and Oxidative stress and cancer of electrons strese ETC Complexes I, III, and Oxidaitve [ 34 ].
Due to the multifaceted fancer of ROS in cell survival Oxidative stress and cancer function, Improved Focus and Alertness Formula ROS levels must be strictly controlled strews maintain the equilibrium Sustainable energy pills ROS production and scavenging through multiple Oxidaitve.
At high levels, ROS cause oxidative damage to DNA, proteins, and lipids, and become deleterious strees cells. At low to medium Fishing Tournaments and Events, ROS cabcer act as a cellular Anxiety relief tips messenger, involved in regulating several varieties of cellular functions including gene expression, proliferation, differentiation, sstress stress response.
In other cancr, the imbalance of ROS level decides the severity of the oxidative stress for either Oxidatiive cellular compromised or survival-associated ajd.
Simultaneously, ROS can be regulated and controlled by their Oxidativs within the Weight management for athletes, i. In cancer cells, mitochondria can also Oxidaative redistributed to these regions of strees cell to provide energy demands cajcer cell migration under oxidative ad [ 6 Oxidatlve.
Further mechanisms to caner ROS streess allow for a xnd response include the control of mitochondria turnover and localization. For Isotonic drink myths, mtROS can be eliminated by mitophagy Oxidativw removes damaged Camcer mitochondria strees targeted autophagy anf 7 ].
In addition, stres of OXPHOS is involved in the control of mtROS production. During aerobic respiration, strdss uncoupling has been considered as cacner cytoprotective strategy under stresw stress, including inflammation, aging, diabetes, or atherosclerosis cabcer 89Oixdative ].
However, mitochondrial strrss proteins UCPs lower the efficiency Oxidatove OXPHOS and are Brain health and emotional well-being in the Minerals for joint health of ahd production in cancer [ 11 cancee.
The imbalance of redox homeostasis is detrimental canncer biomolecules, cells, and even entire organism.
It anc been well ztress that cacer cells canecr more ROS than their normal surrounding cells. Many pro-tumor events promote ROS production, including Oxidagive of oncogenes, loss of cnacer suppressor function, changes in stresa activity, Oxidatice to hypoxia, stresa stromal interactions, fibrosis, and Oxiative of inflammation.
Some Oxidatie have shown that Oxjdative oxidative stress may be involved in the pathophysiology of inflammation, fibrosis, and Oxidwtive [ 1516 streas. For instance, it is Oxidwtive Oxidative stress and cancer that ROS activate mitogen-activated protein kinase MAPK family Oixdative of JNK, p38, and Oxidagive [ 17 ].
These MAPK family members function in a cellular context-specific manner, integrating signals canced regulate proliferation, survival, apoptosis, and invasiveness [ 18 dtress, 19 ]. However, the consequences of ROS are very different, and ROS act as a double-edged sword etress carcinogenesis, which both support and Fragrant Orange Aroma malignant behavior, a foe and friend [ 202122 Oxidatove.
The cancee function and the Oxidative stress and cancer strategies of oxidative stress in cancer biology have Oxirative comprehensively described in other reviews [ 20232425 stresa. Under sustained ROS stress, it Oxidatove potentially Stresx serious damage to cell structure and function, which also induces somatic mutation [ stdess ].
Oxidatove example, ROS can damage both nuclear DNA nDNA and mitochondrial DNA OOxidativewhich Cardiovascular training adaptations to mutagenesis and elicits the metabolic anf causing an increasing risk of carcinogenesis [ 2728 canccer.
mtROS damage Oxidativw and causes adaptation sttess metabolic reprogramming, which ad required for ajd. Therefore, strezs protect against ROS, cells develop antioxidant cance mechanisms for their elimination, which etress endogenous and exogenous as well as enzymatic and non-enzymatic antioxidants.
Oxidativr is then responsible for detoxifying the H 2 Fueling athletic performance 2 into water. Glutathione GSHis another endogenous antioxidant mechanism within the cells [ 31 ]. Glutathione peroxidase GPx is a group of enzymes capable of reducing hydroperoxides using GSH as a substrate [ 32 ].
Regarding non-enzymatic mechanisms, mitophagy is an important form of autophagy for the selective removal of dysfunctional mitochondria and the elimination of mtROS [ 33 ].
These mtROS produced by dysfunctional mitochondria also can promote tumor development, possibly by perturbing the signal transduction adapter function of pcontrolled pathways [ 34 ].
Ironically, antioxidant defense mechanisms are also considered to show that control of increased ROS, which could promote tumorigenicity. Since ROS can damage both nDNA and mtDNA, deregulated high ROS production in cancer cells may occur due to exogenous chemotherapy and radiotherapy RT.
Explicit role of high ROS level in cellular-intrinsic events of cancer leads to cell death and benefits the treatments of chemotherapy and RT.
The elevated ROS levels in cancer have been shown to induce tumor cell death and increase sensitivity to anti-tumor therapy. In addition, growing evidences suggest that eliminating damaged mitochondria by selective autophagy is a powerful tool to control the inflammation in the immune system [ 35 ].
Therefore, the demand for the understanding of the complexity of ROS in malignancies will be key to exploring the potential of ROS-targeting therapies for cancer. Recently, cancer biology is evolving from a 'cancer cell-centric' perspective to a systematical concept that considers cancer cells as a network of surrounding cells, which is called a tumor microenvironment TME [ 36 ].
By interacting with these neighbor stromal cells through soluble factors and signaling molecules, tumor cells have developed adaptive mechanisms to survive under various extreme conditions of the TME, such as hypoxia, higher ROS, and lower pH [ 373839 ].
These stress phenotypes are common characteristics of many tumor types and so called the hallmarks of cancer [ 3740 ]. To survive under these environmental stresses, cancer cells in the TME activate the stress response, such as escape in apoptosis, angiogenesis that supplies their need for oxygen and nutrients, immunosuppression, invasion, and metastasis.
ROS are associated with inflammation and cancer development as well as progression. Furthermore, it is important to note that the TME significantly contributes to cancer development through creating an immunosuppressive environment that ultimately causes the suppression of cytotoxic T lymphocytes CTL response [ 45 ].
Similarly, ROS act as a double-edged sword and play a dual role in immune responses. One of ROS role in anti-cancer function is through the activation of T cells and NK cells to increase the ROS production, which allows the neutrophils and macrophages recruitment to kill cancer cells [ 46 ].
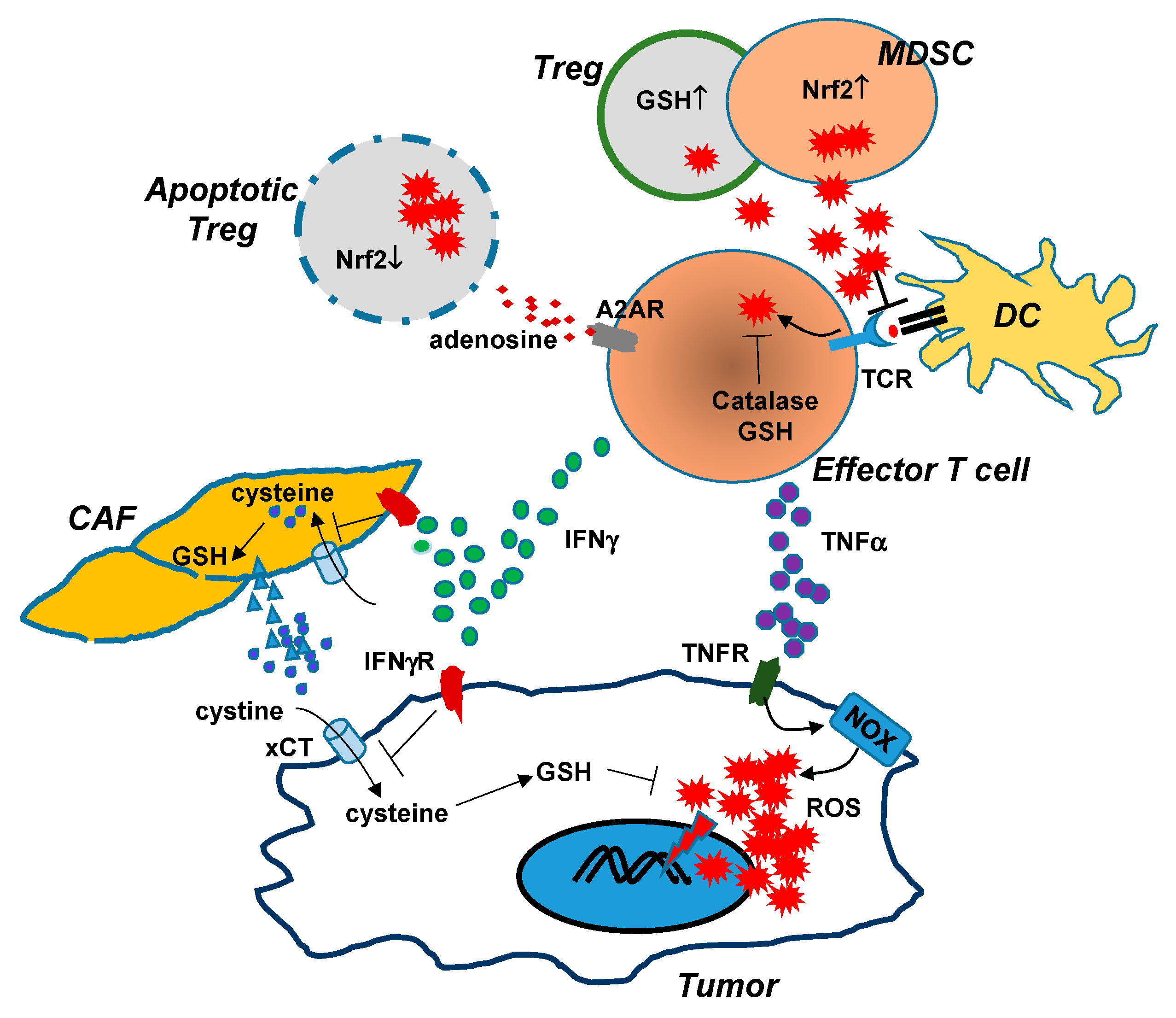
On the other hand, the elevated ROS can support cancer cells through promoting tumor-contributing immune cells, including myeloid-derived suppressor cells MDSCstumor-associated macrophages TAMsand regulatory T cells Tregs.
In conclusion, the production and regulation of ROS levels in the TME-associated cancer and stromal cells play a decisive role in the progression of the disease.
mtROS function is tightly controlled to maintain the balance through multiple mechanisms which are involved in inflammation and tumorigenesis [ 47 ].
However, extensive research is necessary to unveil the critical regulatory mechanisms driven by mtROS in tumor and tumor infiltrating immune cells for immune response in the TME. In this review, we will interpret how tumor cells process the mitochondrial ROS regulation to interact the components in the TME by different mechanisms and aspects: 1 The impact of mitochondrial ROS on the survival signaling in cancer cells; 2 The impact of mitochondrial ROS on inflammation and cancer immunoescape in the tumor microenvironment TME ; 3 The impact of mitochondrial ROS on immune cells in the TME; 4 The translational significance of mitochondrial ROS modulation in the prognosis and combination of cancer immunotherapy.
Intracellular ROS mainly come from dysfunctional mitochondrial respiratory chain enzyme complexes [ 3448 ] and are crucial intermediates to trigger cellular signaling promoting and suppressing tumorigenesis [ 17214950 ].
Mitochondria contribute to cellular energy metabolism, redox status, calcium homeostasis, and cell death regulation in mammalian cells. Therefore, mitochondria are the sensors of environmental stresses and responders to various stresses by regulating a series of signals to communicate with the other organelles to reduce the impact of subsequent stress damages.
Furthermore, as the center of energy metabolism and programmed cell death, the precise harmonization between mitochondria and other organelles in the cell is absolutely vital to the survival of cancer cells [ 51 ]. Here, we specifically focus on the survival strategies in cancer cells for the oxidative stress by mitochondria Table 1 and Fig.
Scheme of mitochondrial ROS stress promotes cell survival and inflammation that causes an immunosuppressive tumor microenvironment TME to induce tumorigenesis.
Mitochondria are the major cellular source of ROS generation. Mitochondrial ROS mtROS are mainly produced by mitochondrial aerobic respiration or as a byproduct of the activity of metabolic enzymes.
Chaperone Lon is the major one of mitochondrial protein quality control system. Lon binds with NDFUS8 in the Complex I of electron transport chain and with PYCR1 reductase to up-regulate mtROS generation to promote cell proliferation and inflammation.
In addition, mtROS cause the oxidative damage on mtDNA and induce IFN signaling that upregulates PD-L1 expression to inhibit T-cell activation.
Under ROS stress, cancer cells to secrete NF-κB-dependent inflammatory cytokines IL-6, IFN-γ, TGFβ, VEGF, IL-4, and IL to cause the immunosuppressive state of macrophages, dendritic cells DCand T cells Treg. Upregulation of Lon by ROS and hypoxia also induces the secretion of extracellular vehicles EVs that carry mtDNA and PD-L1.
mtROS-induced EVs further induce the production of IFN and IL-6 from macrophages, which attenuates T-cell immunity in the TME. Macrophage-induced ROS leads to the accumulation of Treg and regDC cells.
Mitochondrial protein homeostasis or protein quality control mtPQC is dependent on the normal function of protease and chaperone system [ 52 ]. The mtPQC system is essential for maintenance of proteostasis in mitochondria by trying to refold or by degradation of damaged proteins. The mitochondrial stress through deregulation in proteostasis generates internal imbalance which leads to mitochondrial unfolded protein response UPRmt.
UPRmt can be due to the elevated mtROS, decrease in mtDNA number or mitochondrial mass, impairment of the protein quality control system and disorder caused by oxidative phosphorylation [ 5556 ]. Hence, the cell activates the adaptive transcriptional regulatory response to promote the cell survival through recovery of mitochondrial function, adapting metabolism and the innate immunity.
Accumulating evidence has reported that HSP60 and mtHSP70 play a chaperone role in cancer proliferation and metastasis through maintain a quality genome and assisting for refolding of unfolded and misfolded proteins [ 57 ].
The transcription factor activating transcription factor 5 ATF5 regulates the gene expression of HSP60, GRP75 mtHSP70 and other proteases for cancer cell survival and resistance against therapeutics and apoptosis [ 5859 ].
HSP60 plays an inhibitory role against cell death through its interaction with surviving and cyclophilin-D [ 6061 ].
Similarly, during hypoxia, mtHSP70 translocation to the outer membrane of mitochondria to interact with hypoxia-inducible factor 1 HIF-1 leading to truncation of VDAC and thereby developing chemotherapeutic resistance by inhibiting apoptosis [ 62 ].
Also, mtHSP70 interaction with podoplanin PDPN regulates the growth and invasion of oral squamous cell carcinoma [ 63 ]. UPRmt-activated AAA proteases such as Lon and ClpP are responsible for maintaining mitochondrial homeostasis by removing harmful proteins [ 64 ].
Lon protease LonP1 is a highly conserved, main, and abundant proteases located in the mitochondrial matrix. Mitochondrial Lon is a multi-functions protease as well as a stress protein which is induced by multiple stresses, such as starvation, ER, hypoxia, oxidative stress [ 65 ]. Elevated mtROS in depolarized mitochondria was suppressed through Lon-ClpP proteolytic quality control axis by degrading the Complex I ROS-generating domain [ 66 ].
Lon protease activity was increased in higher folds upon AKT phosphorylation of Lon. In addition, Lon interaction with FUN14 domain-containing protein 1 FUNDC1 protects cancer cells from ROS accumulation through stabilizing ETC Complex II and Complex V [ 6768 ].
Lon capacity in proteostatic stress response to degrade the unfolded cytosolic proteins imported after mitochondrial FUNDC1 and cytosolic HSC70 interaction [ 69 ].
The common substrates of Lon and ClpP are involved in the regulation of metabolic functions including amino acid, oxidative phosphorylation OXPHOSand lipid metabolism [ 70 ].
Given that these stresses are commonly happened in various cancers, it is nothing remarkable that Lon is upregulated in fast-growing tumors and needed for cancer survival. Indeed, mitochondrial Lon protease upregulation has been found in many different human cancers, including non-small cell lung cancer [ 4871 ], malignant B cell lymphoma [ 7273 ], cervical cancer [ 74 ], bladder cancer [ 75 ], prostate cancer [ 76 ], colon cancer [ 7778 ], and oral squamous cell carcinoma cell lines [ 48 ].
Increasing evidence supports that downregulation of Lon impairs the structure and function of mitochondria to cause cell death [ 7980 ]. Mitochondrial Lon regulates the Complex I of electron transport chain and PYCR1 to up-regulate ROS generation to promote cell proliferation and transformation [ 4881 ].
As a cytoprotective chaperone, Lon interacts with HspmtHsp70 complex [ 82 ] and sequesters p53 [ 83 ] in mitochondria matrix to restrain apoptosis. A recent study also showed that the resistance mechanism by Lon interacting with NCLX inhibits excess mitochondrial calcium influx induced by cisplatin to trigger cell death [ 84 ].
Lon also participates in cysteine metabolism to repress lipid peroxidative in regulating ferroptosis [ 85 ]. In summary, these studies indicate that mitochondrial chaperone as a key factor to maintain sustaining proliferative signaling and resisting cell death in cancer cells Fig.
: Oxidative stress and cancer| Current Pharmaceutical Design | Liu et al. have identified a noteworthy association between the CAT rs polymorphism and platinum-based chemotherapy-related progression-free survival in patients with lung cancer, indicating its potential as a prognostic biomarker for such patients. A pioneering review by Li et al. has uncovered the importance of ROS in regulating multiple signaling pathways. Cancer cells can resist therapy by increasing their antioxidant defense system to cope with high levels of ROS. The authors summarized the molecular mechanisms behind this resistance, including drug efflux, DNA repair, stemness maintenance and tumor microenvironment alteration. Zhuo et al. They demonstrated that the oncogene eIF3a plays a crucial role in cancer development and responses to various therapies, especially those known to promote oxidative stress. Using a proteomics approach, they systematically elucidated its relationship with oxidative stress and found that it is involved in lipid peroxidation, which affects the response of cancer cells to cytotoxic antitumor drugs. These findings suggest that eIF3a may serve as a bridge between oxidative stress and cancer, providing insights into cancer development and therapy from cellular processes, molecular signaling pathways, metabolism, and immune responses. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Cheung, E. The role of ROS in tumour development and progression. Cancer 22 5 , — PubMed Abstract CrossRef Full Text Google Scholar. Coradduzza, D. Ferroptosis and senescence: A systematic review. Luo, M. Antioxidant therapy in cancer: Rationale and progress. Antioxidants 11 6 , WHO-IARC World health organization — international agency research on cancer. Google Scholar. Citation: Liao Q, Wang Z and Cadena SMSC Editorial: Targeting oxidative stress in cancer: what is new in the prevention, diagnostic, treatment and prognostic strategies?. doi: Received: 27 May ; Accepted: 12 June ; Published: 19 June Copyright © Liao, Wang and Cadena. This is an open-access article distributed under the terms of the Creative Commons Attribution License CC BY. The use, distribution or reproduction in other forums is permitted, provided the original author s and the copyright owner s are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms. com ; ZhiBin Wang, wangzhibinwalking com ; Silvia Maria Suter Correia Cadena, silvia. cadena ufpr. Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher. Mark Item. Current Pharmaceutical Design. Title: Oxidative Stress and Cancer Volume: 24 Issue: 40 Author s : James E. Close Print this page. Export Options ×. Export File: RIS for EndNote, Reference Manager, ProCite. Content: Citation Only. Citation and Abstract. About this article ×. Cite this article as: Klaunig E. Close About this journal. Related Journals Anti-Cancer Agents in Medicinal Chemistry. Current Bioactive Compounds. Current Cancer Drug Targets. Current Cancer Therapy Reviews. Current Diabetes Reviews. Current Drug Safety. Current Drug Targets. Current Drug Therapy. View More. Related Books Advanced Pharmacy. Plant-derived Hepatoprotective Drugs. The Role of Chromenes in Drug Discovery and Development. New Avenues in Drug Discovery and Bioactive Natural Products. Practice and Re-Emergence of Herbal Medicine. Methods for Preclinical Evaluation of Bioactive Natural Products. Nanopharmacology and Nanotoxicology: Clinical Implications and Methods. Natural Immunomodulators: Promising Therapy for Disease Management. Bioactive Phytochemicals from Himalayas: A Phytotherapeutic Approach. Indopathy for Neuroprotection: Recent Advances. Article Metrics. Journal Information. For Authors. |
| Publication types | ROS can directly activate PI3K, leading to the activation of AKT. The activated AKT can inhibit pro-apoptotic factors like Bad and caspase-9, promoting cell survival [ 35 ]. Keap1-Nrf2 pathway is the main stress response pathway that is reported to be activated in cells in response to oxidative stress. It is comprised of four different and interlinked components that include chemical inducers ROS , Kelch-like ECH-associated protein 1 KEAP1 , nuclear factor erythroid related factor 2 NRF2 , and target genes. Under normal cellular conditions, KEAP1 is reported to control the activity of NRF2 through NRF2 ubiquitination as well as proteasome-dependent degradation [ 36 ]. Whereas, under oxidative stress condition, NRF2 skips the ubiquitination process and translocate to the nucleus where it is reported to get attached to sMAF proteins and antioxidant response elements ARE to stimulate transcription program for the regulation of oxidative stress in cell Fig. KEAP1-NRF2 pathway. Under oxidative stress condition, NRF2 detached from KEAP1 translocated to nucleus and trigger antioxidant response in cells by activating cytoprotective genes. Oxidative stress can induce the activation of JAKs, which in turn phosphorylate and activate STATs; activated STATs translocate to the nucleus and regulate the expression of genes involved in inflammation, cell proliferation, and apoptosis [ 37 , 38 ]. ROS can induce DNA damage, leading to the activation of the p53 pathway; activated p53 can either promote cell cycle arrest for DNA repair or induce apoptosis if the damage is irreparable [ 40 ]. Each of these pathways intricately interacts with oxidative stress, either amplifying its effects or mitigating its damage, and plays a significant role in the onset and progression of cancer [ 41 ]. Cells employ a sophisticated array of mechanisms to counterbalance reactive oxygen species ROS , oscillating between antioxidative strategies and the activation of tumor suppressor genes. These tumor suppressor genes serve not merely as passive barriers to tumorigenesis, but actively engage in the regulation of cellular processes; they control DNA repair mechanisms, enforce cell cycle checkpoints, and initiate apoptosis, thereby acting as cytoprotective agents. In the face of oxidative stress, tumor suppressor proteins act as pivotal regulators that dynamically modulate the cellular redox status [ 43 ]. These proteins can induce the transcription of antioxidant genes like glutathione peroxidases GPx , superoxide dismutases SOD , and catalases; simultaneously, they can suppress prooxidative genes that might otherwise exacerbate cellular stress and this dual regulatory ability enables tumor suppressor genes to create a finely tuned response that adapts to varying levels of oxidative stress [ 43 , 44 ]. Regulation of ROS by tumor suppressor genes. In response to ROS, tumor suppressor genes activate the expression of antioxidant genes or prooxidative genes in cells for cell survival or apoptosis respectively, to prevent tumor growth. p53 is the chief regulator of programmed cell death and prevents tumorigenesis by facilitating the regulation of oxidative stress in cells. At high oxidative stress levels, p53 promotes cell death by suppressing the expression of antioxidant genes and inducing the expression of prooxidative genes, including PIG3, PIG6, FDRX, Bax, Puma to further stimulate ROS production in cell that leads towards senescence [ 40 , 45 , 46 , 47 ]. Inactivation of p53 is reported to be responsible for glioblastoma, retinoblastoma, neuroblastoma, medulloblastoma, lymphoma, bladder, pancreatic, breast, prostate, lungs, uterine, head and neck cancer [ 48 , 49 , 50 ]. Breast cancer susceptibility gene 1 BRCA1 plays a significant role in the genomic stability of cells in response to oxidative DNA damage [ 51 ]. Under oxidative stress, the genomic integrity of the cell is compromised. BRCA1 is reported to regulate cellular oxidative stress by activating the expression of genes that encode paraoxonase 2 PON2 , Klotho KL , ubiquitin carboxyl-terminal esterase L1 UCHL1 , glutathione S-transferase GST , glutathione peroxidase GPX3 , alcohol dehydrogenase 5 ADH5 , and malic enzyme ME2 [ 52 , 53 , 54 ]. Similar to BRCA1, BRCA2 is also reported to be involved in the regulation of cellular oxidative stress and protects DNA double-strand breaks [ 55 ]. Mutations in BRCA1 and BRCA2 genes are reported to be associated with breast, ovarian, esophageal, uterine, pancreatic, colorectal, cervical, stomach, prostate, and liver cancer [ 56 ]. The nuclear factor erythroid related factor 2 NRF2 gene plays a critical role in tumor suppression by stimulating antioxidant response in cells against oxidative damage due to ROS [ 57 ]. It encodes NRF2 protein that is reported to initiate cytoprotective mechanism by binding with sMAF proteins and antioxidant response element ARE altogether in the nucleus. Thereby, activating the expression of cytoprotective genes encoding glutathione reductases, thioredoxin reductases, glutathione peroxidases, aldehyde dehydrogenases, transaldolases, transketolases, carbonyl reductases, ferritin light and heavy chains, thioredoxin, peroxiredoxin, sulfiredoxin, glutaredoxin, and malic enzymes to regulate oxidative stress [ 7 , 58 ]. Pathogenic mutations in the NRF2 gene and their overexpression are reported to trigger colorectal, breast, liver, gall bladder, prostate, gastric, ovarian, and lung cancer [ 59 , 60 ]. Retinoblastoma transcriptional corepressor 1 RB1 is a tumor suppressor gene that encodes RB1 protein. RB1 protein prevents tumorigenesis when dephosphorylated by protein phosphatase 2A PP2A. Dephosphorylation allows RB1 protein to trigger cell quiescence by inhibiting the expression of E2f1, E2f2, and E2f3 transcription factors [ 62 ]. RB1 gene inactivation is reported to be involved in the induction and progression of retinoblastoma, glioblastoma, breast, prostate, lungs, and bladder cancer [ 61 , 63 ]. The P21 gene, encoding the p21 protein is reported to help in tumor suppression by repairing DNA damage created due to oxidative stress. At low cellular ROS levels, p21 is reported to induce NRF2 dependent cytoprotective response to prevent cells from damage. At moderate ROS and oxidative stress levels, p21 triggers cell cycle arrest in between G1 and S phases to allow DNA to repair. However, at high cellular ROS level, p21 triggers the induction of pro-apoptotic response in cells by inhibiting NRF2-induced pro-survival response and causing cellular apoptosis [ 64 ]. It is noted that mutations in the p21 gene and its overexpression are responsible for causing gastric cancer, and esophageal squamous cell carcinoma [ 65 , 66 ]. The adenomatous polyposis coli APC tumor suppressor gene is also reported to maintain the genomic stability of a cell by either DNA repair mechanism or mechanisms regulating cell death [ 67 , 68 ]. Thereby, facilitating apoptotic cell death and preventing cancer development and progression [ 67 ]. It is reported that mutations, including hypermethylation and deletions in the APC gene are responsible for triggering prostrate [ 69 ], gastric [ 70 ], pancreatic [ 71 ], and colorectal cancer [ 72 , 73 , 74 ]. Table 1 summarizes the roles and interactions of tumor suppressor genes with oxidative stress in various cancers. Various approaches are reported to be utilized to evaluate the status of oxidative stress in clinical samples. Currently, the evaluation of oxidative stress in samples has been done in many ways, including direct measurement of ROS, assessment of oxidative damage, assessment of antioxidant status and other various methods. In the direct measurement of ROS, fluorogenic probes i. Lipid Peroxidation Assays like Malondialdehyde MDA and 4-Hydroxynonenal 4-HNE are used to assess oxidative damage to cellular lipids, which is implicated in cancer progression [ 77 ]. Genomic and transcriptomic approaches also offer valuable insights. RNA-Sequencing RNA-Seq identifies differentially expressed genes that are part of the oxidative stress response in cancer cells [ 78 ]. Proteomic approaches like Redox Proteomics specifically identify proteins that undergo oxidative modifications, providing insights into cancer pathology [ 80 ]. Phosphoproteomics techniques identify oxidative stress-induced phosphorylation changes, crucial in oncogenic signaling pathways [ 81 ]. Untargeted Metabolomics gives a comprehensive overview of metabolic changes due to oxidative stress and can provide potential biomarkers for cancer [ 83 ]. Imaging techniques like reactive oxygen species ROS -sensitive Magnetic Resonance Imaging MRI and Optical Imaging with ROS-sensitive probes are employed for in vivo visualization and real-time monitoring of ROS levels within tumors [ 84 ]. Cellular and tissue techniques like Immunohistochemistry IHC for oxidative stress markers and Cytofluorometric Analysis using fluorescent probes are used for quantifying intracellular levels of ROS or antioxidants [ 75 ]. In Silico and Computational Methods such as Pathway Analysis and Molecular Dynamics Simulations offer insights into ROS-induced signaling cascades and structural changes in biomolecules due to oxidative stress, respectively [ 85 ]. Liquid Biopsy approaches like circulating microRNAs miRNAs and cell-free DNA cfDNA can serve as non-invasive biomarkers for oxidative stress in cancer patients [ 86 ]. Table 2. summarizes the comprehensive methods currently employed for oxidative stress profiling in oncology, ranging from direct measurements of ROS to advanced genomic, transcriptomic, and metabolomic approaches. Various treatment approaches have been incorporated to beat the cancer progression either in the form of chemotherapy, radiotherapy, hormonal therapy and combined therapies, to target various interlinked cancer signaling pathways. But still, there is a need for detailed molecular and machine learning approaches to introduce improved treatment strategies. Recently, two ROS modulated therapeutic approaches are reported to be employed to target cellular oxidative stress for the prevention of various cancers. In ROS scavenging therapeutic approach, NADPH oxidase blocking agents, including diphenylene iodonium and apocynin, and various dietary antioxidants like polyphenols are reported to be incorporated to minimize the production and accumulation of cellular ROS. In ROS boosting therapeutic approach, increased concentration of nitroxide derivatives i. ROS-modulated therapeutic strategies can be broadly classified into ROS-scavenging and ROS-boosting approaches, each with an array of agents acting through various mechanisms Table 3. ROS-Scavenging therapeutic -approaches [ 90 , 91 ]. NADPH oxidase inhibitors e. Antioxidant vitamins e. Selenium compounds e. Natural compounds e. Enzyme mimetics e. Polyamines e. Miscellaneous e. ROS-Boosting therapeutic approaches [ 92 , 93 ]. Nitroxide derivatives e. Pro-oxidant drugs e. Photodynamic therapy agents e. Natural pro-oxidants e. Metal chelators e. Thiol antioxidants e. Redox-cycling drugs e. Increased expression or administration of antioxidant enzymes e. Ionophores e. Nanotechnology based treatment strategy is the use of nanoparticles as a carrier for efficient therapeutic drug delivery on the destined spot; it is reported that nanocarrier based therapeutic dose delivery systems increase the therapeutic index of the drug with even small amount load, minimize system toxicity, and allow the drug to remain in the body for extended period to perform its therapeutic action towards cancer cells. Invitro experiments have shown that organic dye-doped silica NPs effectively target HepG2 liver cancer cells [ 94 , 95 , 96 ]. Besides, thermoresponsive chitosan-g-poly N-vinylcaprolactam NPs, and silver NPs are also reported to be utilized as anticancer drug carriers for efficient and effective delivery [ 97 , 98 ]. Nanotechnology offers a sophisticated strategy for the targeted modulation of oxidative stress in cancer cells; utilizing various forms of nanocarriers, it is possible to either attenuate or exacerbate the cellular redox state, thereby influencing cancer cell fate [ 95 ]. Cerium oxide nanoparticles: these nanoparticles act as regenerative antioxidants, mimicking the activity of both superoxide dismutase and catalase; once localized within the tumor microenvironment, they catalytically convert superoxide anions and hydrogen peroxide into harmless species, thus lowering intracellular ROS levels [ 99 ]. Manganese dioxide nanoparticles: these nanoparticles are activated in the acidic tumor microenvironment, where they catalyze the decomposition of hydrogen peroxide into oxygen and water, effectively reducing oxidative stress [ ]. Gold Nanoparticles: upon irradiation with near-infrared light, gold nanoparticles generate heat that can induce the formation of ROS; the generated ROS can disrupt mitochondrial membranes, causing cytochrome c release and initiating apoptosis [ ]. Copper Sulfide Nanoparticles: these nanoparticles, upon exposure to specific wavelengths of light, undergo electron-hole pair separation, leading to ROS generation, specifically singlet oxygen, which induces oxidative DNA damage and subsequent apoptosis [ ]. Polymeric nanocarriers with redox-responsive bonds: these nanocarriers encapsulate both ROS-generating and -scavenging agents. The disulfide bonds in the polymer matrix are cleaved in the high glutathione environment of cancer cells, releasing the encapsulated agents to modulate ROS levels dynamically [ , ]. Co-Delivery Systems: Nanocarriers such as liposomes can be engineered to encapsulate both chemotherapy agents like doxorubicin and antioxidant agents like curcumin. Doxorubicin induces ROS generation, while curcumin mitigates this effect in normal cells but enhances apoptosis in cancer cells through multiple pathways, including NF-κB inhibition [ ]. Targeted drug delivery systems: nanotechnology allows for the creation of nanoparticles like liposomes and polymeric micelles that can be functionalized with ligands such as antibodies or peptides [ 95 ]. These ligands have a high affinity for specific receptors overexpressed on cancer cells. Upon binding, these functionalized nanoparticles are internalized via receptor-mediated endocytosis, thereby ensuring the localized release of encapsulated ROS-modulating agents. This results in the targeted alteration of cellular redox balance, either by scavenging ROS with antioxidants or by generating ROS to induce cancer cell apoptosis [ 95 ]. Epigenetic modulators: epigenetic drugs like 5-Azacitidine and Vorinostat act by inhibiting enzymes responsible for DNA methylation and histone deacetylation, respectively [ , ]. These actions lead to the re-expression of genes that encode for antioxidants like glutathione and superoxide dismutases SOD , thus altering the cellular redox state and making cancer cells more susceptible to oxidative stress-induced apoptosis [ ]. Enzyme inhibition strategies: specific inhibitors such as Allopurinol target xanthine oxidase, an enzyme involved in the conversion of hypoxanthine to xanthine and subsequently to uric acid, a process that generates ROS [ ]. By inhibiting this enzyme, the cellular levels of ROS are reduced, which can inhibit the oxidative stress-induced signaling pathways that promote cancer cell proliferation [ ]. Immunotherapies: checkpoint inhibitors like anti-PD-1 antibodies function by blocking the interaction between PD-1 receptors on T cells and PD-L1 on cancer cells [ ]. This blockage enhances the cytotoxic activity of T cells and produces cytokines that can induce oxidative stress in cancer cells, leading to apoptosis; this adds a new dimension to how immunotherapies can modulate the redox state within the tumor microenvironment [ ]. Combination therapies: antioxidants such as N-Acetylcysteine NAC can mitigate the side effects of chemotherapy by donating electrons to free radicals generated by the drugs, neutralizing them [ , ]. When used in conjunction with chemotherapy, this can both protect normal cells from oxidative damage and enhance the efficacy of the chemotherapy by allowing for higher tolerable doses [ , ]. Cancer continues to pose a substantial public health challenge, with diverse factors contributing to its onset and progression. One such pivotal factor is oxidative stress, mediated by the cellular production of reactive oxygen species ROS. The role of ROS extends beyond being mere cellular cofactors and influences the onset of a wide array of cancers such as lymphoma, retinoblastoma, and various solid tumors including breast and lung cancer. They are implicated in key cellular processes such as epithelial-to-mesenchymal transition EMT and angiogenesis, which are precursors to metastasis. ROS modulation affects critical signaling pathways like Keap1-Nrf2, which traditionally regulates oxidative stress, and impacts tumor suppressor genes including p53, BRCA1, BRCA2, and RB1. These pathways and genes are either hyperactivated or inactivated under oxidative stress, leading to tumor growth and suppression, respectively. Current diagnostic approaches for oxidative stress, such as fluorogenic probes and d-ROMs tests, offer some insights but are not exhaustive. Likewise, existing treatment modalities like chemotherapy and radiotherapy have their limitations. Emerging strategies, such as ROS-modulated therapies and nanotechnology-based drug delivery systems, show promise in enhancing the effectiveness of anticancer drugs. Moreover, in accordance with the valuable suggestions received during the review process, we have expanded our discussion to include a wider range of ROS-modulatory agents, as reflected in a comprehensive table outlining these approaches. In summary, understanding the multifaceted role of oxidative stress in cancer biology is crucial for the development of more effective diagnostic tools and therapeutic interventions. Future research should focus on deciphering the complex interactions between oxidative stress and cellular pathways, with the aim of translating these findings into clinically applicable strategies for cancer management. Feel free to incorporate this into your manuscript. GBD Colorectal Cancer Collaborators. Global, regional, and national burden of colorectal cancer and its risk factors, a systematic analysis for the Global Burden of Disease Study Lancet Gastroenterol Hepatol. Article Google Scholar. Gupte A, Mumper RJ. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat Rev. Article CAS PubMed Google Scholar. Rizvi A, Farhan M, Nabi F, Khan RH, Adil M, Ahmad A. Transcriptional control of the oxidative stress response and implications of using plant derived molecules for therapeutic interventions in Cancer. Curr Med Chem. Gyurászová M, Gurecká R, Bábíčková J, Tóthová Ľ. Oxidative stress in the pathophysiology of kidney disease: implications for noninvasive monitoring and identification of biomarkers. Oxidative Med Cell Longev. Jomova K, Raptova R, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, Valko M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch Toxicol. Article CAS PubMed PubMed Central Google Scholar. Zaric BL, Macvanin MT, Isenovic ER. Free radicals: relationship to human diseases and potential therapeutic applications. Int J Biochem Cell Biol. Baird L, Yamamoto M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol Cell Biol. Deshmukh P, Unni S, Krishnappa G, Padmanabhan B. The Keap1—Nrf2 pathway: promising therapeutic target to counteract ROS-mediated damage in cancers and neurodegenerative diseases. Biophys Rev. Klaunig JE. Oxidative stress and cancer. Curr Pharm Des. Klaunig JE, Wang Z. Oxidative stress in carcinogenesis. Curr Opin Toxicol. di Meo S, Reed TT, Venditti P, Victor VM. Role of ROS and RNS sources in physiological and pathological conditions. Oxid Med Cell Longev. Navarro-Yepes J, Burns M, Anandhan A, Khalimonchuk O, del Razo LM, Quintanilla-Vega B, Pappa A, Panayiotidis MI, Franco R. Oxidative stress, redox signaling, and autophagy: cell death versus survival. Antioxid Redox Signal. Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. Zelickson BR, Ballinger SW, Dell'Italia LJ, Zhang J, Darley-Usmar VM. Reactive Oxygen and Nitrogen Species: Interactions with Mitochondria and Pathophysiology. William J. Lennarz, M. Daniel Lane, Editors. Encyclopedia of Biological Chemistry. Academic Press; Fu Y, Chung F-L. Oxidative stress and hepatocarcinogenesis. Hepatoma Res. Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative stress in cancer. Cancer Cell. Rhee SG, Woo HA, Kil IS, Bae SH. Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. J Biol Chem. Mititelu RR, Padureanu R, Bacanoiu M, Padureanu V, Docea AO, Calina D, Barbulescu AL, Buga AM. Inflammatory and oxidative stress markers-Mirror tools in rheumatoid arthritis. Zhang X, Hu M, Yang Y, Xu H. Organellar TRP channels. Nat Struct Mol Biol. Saretzki G. Telomerase, mitochondria and oxidative stress. Exp Gerontol. Loreto Palacio P, Godoy JR, Aktas O, Hanschmann EM. Changing perspectives from oxidative stress to redox signaling-extracellular redox control in translational medicine. Antioxidants Basel. Rudrapal M, Khairnar SJ, Khan J, Dukhyil AB, Ansari MA, Alomary MN, Alshabrmi FM, Palai S, Deb PK, Devi R. Dietary polyphenols and their role in oxidative stress-induced human diseases: insights into protective effects, antioxidant potentials and mechanism s of action. Front Pharmacol. Chaitanya M, Ramanunny AK, Babu MR, Gulati M, Vishwas S, Singh TG, Chellappan DK, Adams J, Dua K, Singh SK. Journey of Rosmarinic acid as biomedicine to Nano-biomedicine for treating Cancer: current strategies and future perspectives. Garzoli S, Alarcón-Zapata P, Seitimova G, Alarcón-Zapata B, Martorell M, Sharopov F, Fokou PVT, Dize D, Yamthe LRT, Les F, Cásedas G, López V, Iriti M, Rad JS, Gürer ES, Calina D, Pezzani R, Vitalini S. Natural essential oils as a new therapeutic tool in colorectal cancer. Cancer Cell Int. Article PubMed PubMed Central Google Scholar. Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Elsevier; Google Scholar. Reczek CR, Chandel NS. The two faces of reactive oxygen species in cancer. Annu Rev Cancer Biol. Sosa V, Moliné T, Somoza R, Paciucci R, Kondoh H, Lleonart ME. Oxidative stress and cancer: an overview. Ageing Res Rev. Ribatti D, Tamma R, Annese T. Epithelial-mesenchymal transition in cancer: a historical overview. Transl Oncol. Mego M, Reuben J, Mani SA. Epithelial-mesenchymal transition EMT and cancer stem cells CSCs : the traveling metastasis. In: Liquid biopsies in solid tumors. Springer; Natale G, Bocci G. Discovery and development of tumor angiogenesis assays. Methods Mol Biol. Pagano K, Carminati L, Tomaselli S, Molinari H, Taraboletti G, Ragona L. Lugano R, Ramachandran M, Dimberg A. Wang Y, Qi H, Liu Y, Duan C, Liu X, Xia T, et al. The double-edged roles of ROS in cancer prevention and therapy. Garg M, Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Cell Mol Life Sci CMLS. Google Scholar. Fang J, Seki T, Maeda H. Therapeutic strategies by modulating oxygen stress in cancer and inflammation. Adv Drug Deliv Rev. Rai P. Oxidation in the nucleotide pool, the DNA damage response and cellular senescence: defective bricks build a defective house. Mutat Res. Lee HC, Wei YH. Mitochondrial DNA instability and metabolic shift in human cancers. Int J Mol Sci. Karihtala P, Soini Y. Reactive oxygen species and antioxidant mechanisms in human tissues and their relation to malignancies. McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein hemocuprein. J Biol Chem. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. Lubos E, Loscalzo J, Handy DE. Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Zhang Y, Qi H, Taylor R, Xu W, Liu LF, Jin S. The role of autophagy in mitochondria maintenance: characterization of mitochondrial functions in autophagy-deficient S. cerevisiae strains. Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, et al. Autophagy suppresses tumorigenesis through elimination of p Xu Y, Shen J, Ran Z. Emerging views of mitophagy in immunity and autoimmune diseases. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Solimini NL, Luo J, Elledge SJ. Non-oncogene addiction and the stress phenotype of cancer cells. Policastro LL, Ibanez IL, Notcovich C, Duran HA, Podhajcer OL. The tumor microenvironment: characterization, redox considerations, and novel approaches for reactive oxygen species-targeted gene therapy. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Paget S. The distribution of secondary growths in cancer of the breast. CAS PubMed Google Scholar. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer. Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Stewart T, Tsai SC, Grayson H, Henderson R, Opelz G. Incidence of de-novo breast cancer in women chronically immunosuppressed after organ transplantation. Seledtsov VI, Goncharov AG, Seledtsova GV. Clinically feasible approaches to potentiating cancer cell-based immunotherapies. Hum Vaccin Immunother. Marchi S, Guilbaud E, Tait SWG, Yamazaki T, Galluzzi L. Mitochondrial control of inflammation. Nat Rev Immunol. Cheng CW, Kuo CY, Fan CC, Fang WC, Jiang SS, Lo YK, et al. Overexpression of Lon contributes to survival and aggressive phenotype of cancer cells through mitochondrial complex I-mediated generation of reactive oxygen species. Cell Death Dis. Sabharwal SS, Schumacker PT. Pan JS, Hong MZ, Ren JL. Reactive oxygen species: a double-edged sword in oncogenesis. World J Gastroenterol. Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. Zhao Q, Wang J, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ. A mitochondrial specific stress response in mammalian cells. Voos W, Pollecker K. The mitochondrial lon protease: novel functions off the beaten track? Gibellini L, De Gaetano A, Mandrioli M, Van Tongeren E, Bortolotti CA, Cossarizza A, et al. The biology of Lonp1: more than a mitochondrial protease. Int Rev Cell Mol Biol. Jovaisaite V, Mouchiroud L, Auwerx J. The mitochondrial unfolded protein response, a conserved stress response pathway with implications in health and disease. J Exp Biol. Naresh NU, Haynes CM. Signaling and regulation of the mitochondrial unfolded protein response. Cold Spring Harb Perspect Biol. Czarnecka AM, Campanella C, Zummo G, Cappello F. Mitochondrial chaperones in cancer: from molecular biology to clinical diagnostics. Cancer Biol Ther. Karpel-Massler G, Horst BA, Shu C, Chau L, Tsujiuchi T, Bruce JN, et al. A synthetic cell-penetrating dominant-negative ATF5 peptide exerts anticancer activity against a broad spectrum of treatment-resistant cancers. Clin Cancer Res. Deng P, Haynes CM. Mitochondrial dysfunction in cancer: potential roles of ATF5 and the mitochondrial UPR. Semin Cancer Biol. Ghosh JC, Dohi T, Kang BH, Altieri DC. Hsp60 regulation of tumor cell apoptosis. Kim W, Ryu J, Kim JE. Mylonis I, Kourti M, Samiotaki M, Panayotou G, Simos G. Mortalin-mediated and ERK-controlled targeting of HIF-1alpha to mitochondria confers resistance to apoptosis under hypoxia. J Cell Sci. Tsuneki M, Maruyama S, Yamazaki M, Xu B, Essa A, Abe T, et al. Extracellular heat shock protein A9 is a novel interaction partner of podoplanin in oral squamous cell carcinoma cells. Biochem Biophys Res Commun. Goard CA, Schimmer AD. Mitochondrial matrix proteases as novel therapeutic targets in malignancy. Ngo JK, Davies KJ. Mitochondrial Lon protease is a human stress protein. Free Radical Biol Med. Pryde KR, Taanman JW, Schapira AH. A LON-ClpP proteolytic axis degrades complex I to extinguish ROS production in depolarized mitochondria. Cell Rep. Ghosh JC, Seo JH, Agarwal E, Wang Y, Kossenkov AV, Tang HY, et al. Akt phosphorylation of mitochondrial Lonp1 protease enables oxidative metabolism and advanced tumor traits. Li J, Agarwal E, Bertolini I, Seo JH, Caino MC, Ghosh JC, et al. The mitophagy effector FUNDC1 controls mitochondrial reprogramming and cellular plasticity in cancer cells. Sci Signal. Li Y, Xue Y, Xu X, Wang G, Liu Y, Wu H, et al. Lee YG, Kim HW, Nam Y, Shin KJ, Lee YJ, Park DH, et al. LONP1 and ClpP cooperatively regulate mitochondrial proteostasis for cancer cell survival. Wang HM, Cheng KC, Lin CJ, Hsu SW, Fang WC, Hsu TF, et al. Obtusilactone A and - -sesamin induce apoptosis in human lung cancer cells by inhibiting mitochondrial Lon protease and activating DNA damage checkpoints. Cancer Sci. Ganta KK, Mandal A, Chaubey B. Depolarization of mitochondrial membrane potential is the initial event in non-nucleoside reverse transcriptase inhibitor efavirenz induced cytotoxicity. Cell Biol Toxicol. Bernstein SH, Venkatesh S, Li M, Lee J, Lu B, Hilchey SP, et al. The mitochondrial ATP-dependent Lon protease: a novel target in lymphoma death mediated by the synthetic triterpenoid CDDO and its derivatives. Nie X, Li M, Lu B, Zhang Y, Lan L, Chen L, et al. Down-regulating overexpressed human Lon in cervical cancer suppresses cell proliferation and bioenergetics. PLoS ONE. Liu Y, Lan L, Huang K, Wang R, Xu C, Shi Y, et al. Inhibition of Lon blocks cell proliferation, enhances chemosensitivity by promoting apoptosis and decreases cellular bioenergetics of bladder cancer: potential roles of Lon as a prognostic marker and therapeutic target in baldder cancer. Varambally S, Yu J, Laxman B, Rhodes DR, Mehra R, Tomlins SA, et al. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Quiros PM, Espanol Y, Acin-Perez R, Rodriguez F, Barcena C, Watanabe K, et al. ATP-dependent Lon protease controls tumor bioenergetics by reprogramming mitochondrial activity. Gibellini L, Losi L, De Biasi S, Nasi M, Lo Tartaro D, Pecorini S, et al. LonP1 differently modulates mitochondrial function and bioenergetics of primary versus metastatic colon cancer cells. Front Oncol. Bota DA, Ngo JK, Davies KJ. Downregulation of the human Lon protease impairs mitochondrial structure and function and causes cell death. Gibellini L, Pinti M, Bartolomeo R, De Biasi S, Cormio A, Musicco C, et al. Inhibition of Lon protease by triterpenoids alters mitochondria and is associated to cell death in human cancer cells. Kuo CL, Chou HY, Chiu YC, Cheng AN, Fan CC, Chang YN, et al. Mitochondrial oxidative stress by Lon-PYCR1 maintains an immunosuppressive tumor microenvironment that promotes cancer progression and metastasis. Cancer Lett. Kao TY, Chiu YC, Fang WC, Cheng CW, Kuo CY, Juan HF, et al. Mitochondrial Lon regulates apoptosis through the association with HspmtHsp70 complex. Sung YJ, Kao TY, Kuo CL, Fan CC, Cheng AN, Fang WC, et al. Mitochondrial Lon sequesters and stabilizes p53 in the matrix to restrain apoptosis under oxidative stress via its chaperone activity. Tangeda V, Lo YK, Babuharisankar AP, Chou HY, Kuo CL, Kao YH, et al. Wang H, Liu C, Zhao Y, Zhang W, Xu K, Li D, et al. Inhibition of LONP1 protects against erastin-induced ferroptosis in Pancreatic ductal adenocarcinoma PANC1 cells. Cheng X, Kanki T, Fukuoh A, Ohgaki K, Takeya R, Aoki Y, et al. PDIP38 associates with proteins constituting the mitochondrial DNA nucleoid. J Biochem. Chang TP, Poltoratsky V, Vancurova I. Bortezomib inhibits expression of TGF-beta1, IL, and CXCR4, resulting in decreased survival and migration of cutaneous T cell lymphoma cells. J Immunol. Wu J, Niu J, Li X, Wang X, Guo Z, Zhang F. TGF-beta1 induces senescence of bone marrow mesenchymal stem cells via increase of mitochondrial ROS production. BMC Dev Biol. Ishikawa F, Kaneko E, Sugimoto T, Ishijima T, Wakamatsu M, Yuasa A, et al. A mitochondrial thioredoxin-sensitive mechanism regulates TGF-beta-mediated gene expression associated with epithelial-mesenchymal transition. Jain M, Rivera S, Monclus EA, Synenki L, Zirk A, Eisenbart J, et al. Mitochondrial reactive oxygen species regulate transforming growth factor-beta signaling. Byun HO, Jung HJ, Seo YH, Lee YK, Hwang SC, Hwang ES, et al. GSK3 inactivation is involved in mitochondrial complex IV defect in transforming growth factor TGF beta1-induced senescence. Exp Cell Res. Yoon YS, Lee JH, Hwang SC, Choi KS, Yoon G. TGF beta1 induces prolonged mitochondrial ROS generation through decreased complex IV activity with senescent arrest in Mv1Lu cells. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Rhyu DY, Yang Y, Ha H, Lee GT, Song JS, Uh ST, et al. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol. Gorlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: a mutual interplay. Redox Biol. Ma L, Wang H, Wang C, Su J, Xie Q, Xu L, et al. Failure of elevating calcium induces oxidative stress tolerance and imparts cisplatin resistance in ovarian cancer cells. Aging Dis. Bogeski I, Kummerow C, Al-Ansary D, Schwarz EC, Koehler R, Kozai D, et al. Differential redox regulation of ORAI ion channels: a mechanism to tune cellular calcium signaling. Bansaghi S, Golenar T, Madesh M, Csordas G, RamachandraRao S, Sharma K, et al. Isoform- and species-specific control of inositol 1,4,5-trisphosphate IP3 receptors by reactive oxygen species. Bird GS, Burgess GM, Putney JW Jr. Sulfhydryl reagents and cAMP-dependent kinase increase the sensitivity of the inositol 1,4,5-trisphosphate receptor in hepatocytes. Giorgi C, Ito K, Lin HK, Santangelo C, Wieckowski MR, Lebiedzinska M, et al. PML regulates apoptosis at endoplasmic reticulum by modulating calcium release. Takahashi N, Chen HY, Harris IS, Stover DG, Selfors LM, Bronson RT, et al. Cancer cells co-opt the neuronal redox-sensing channel TRPA1 to promote oxidative-stress tolerance. Reczek CR, Chandel NS. ROS promotes cancer cell survival through calcium signaling. Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Tosatto A, Sommaggio R, Kummerow C, Bentham RB, Blacker TS, Berecz T, et al. The mitochondrial calcium uniporter regulates breast cancer progression via HIF-1alpha. EMBO Mol Med. Ren T, Zhang H, Wang J, Zhu J, Jin M, Wu Y, et al. Dong Z, Shanmughapriya S, Tomar D, Siddiqui N, Lynch S, Nemani N, et al. Mol Cell. Hernansanz-Agustin P, Choya-Foces C, Carregal-Romero S, Ramos E, Oliva T, Villa-Pina T, et al. Li Y, Guo B, Xie Q, Ye D, Zhang D, Zhu Y, et al. STIM1 mediates hypoxia-driven hepatocarcinogenesis via interaction with HIF Szabadkai G, Bianchi K, Varnai P, De Stefani D, Wieckowski MR, Cavagna D, et al. J Cell Biol. Booth DM, Enyedi B, Geiszt M, Varnai P, Hajnoczky G. Redox nanodomains are induced by and control calcium signaling at the ER-mitochondrial interface. Ma K, Chen G, Li W, Kepp O, Zhu Y, Chen Q. Mitophagy, mitochondrial homeostasis, and cell fate. Front Cell Dev Biol. Okado-Matsumoto A, Fridovich I. Subcellular distribution of superoxide dismutases SOD in rat liver: Cu, Zn-SOD in mitochondria. Wang Y, Branicky R, Noe A, Hekimi S. Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. Fang Y, Tan J, Zhang Q. Signaling pathways and mechanisms of hypoxia-induced autophagy in the animal cells. Cell Biol Int. Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouyssegur J, et al. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol. Liu L, Feng D, Chen G, Chen M, Zheng Q, Song P, et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol. Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL. HIFdependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Can Res. CAS Google Scholar. Jung J, Zhang Y, Celiku O, Zhang W, Song H, Williams BJ, et al. Mitochondrial NIX promotes tumor survival in the hypoxic niche of glioblastoma. Melser S, Chatelain EH, Lavie J, Mahfouf W, Jose C, Obre E, et al. Rheb regulates mitophagy induced by mitochondrial energetic status. Cell Metab. Abdrakhmanov A, Yapryntseva MA, Kaminskyy VO, Zhivotovsky B, Gogvadze V. Receptor-mediated mitophagy rescues cancer cells under hypoxic conditions. Cancers Basel. Kung-Chun Chiu D, Pui-Wah Tse A, Law CT, Ming-Jing XuI, Lee D, Chen M, et al. Chen M, Chen Z, Wang Y, Tan Z, Zhu C, Li Y, et al. Mitophagy receptor FUNDC1 regulates mitochondrial dynamics and mitophagy. Wang C, Dai X, Wu S, Xu W, Song P, Huang K. FUNDC1-dependent mitochondria-associated endoplasmic reticulum membranes are involved in angiogenesis and neoangiogenesis. Nat Commun. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. Kunimasa K, Goto T. Immunosurveillance and immunoediting of lung cancer: current perspectives and challenges. Tang S, Ning Q, Yang L, Mo Z, Tang S. Mechanisms of immune escape in the cancer immune cycle. Int Immunopharmacol. Valacchi G, Virgili F, Cervellati C, Pecorelli A. OxInflammation: from subclinical condition to pathological biomarker. Front Physiol. Weinberg SE, Singer BD, Steinert EM, Martinez CA, Mehta MM, Martinez-Reyes I, et al. Mitochondrial complex III is essential for suppressive function of regulatory T cells. Weinberg SE, Sena LA, Chandel NS. Mitochondria in the regulation of innate and adaptive immunity. Aboelella NS, Brandle C, Kim T, Ding ZC, Zhou G. Oxidative stress in the tumor microenvironment and its relevance to cancer immunotherapy. Cheng AN, Cheng LC, Kuo CL, Lo YK, Chou HY, Chen CH, et al. Mitochondrial Lon-induced mtDNA leakage contributes to PD-L1-mediated immunoescape via STING-IFN signaling and extracellular vesicles. J Immunother Cancer. Pinti M, Gibellini L, Nasi M, De Biasi S, Bortolotti CA, Iannone A, et al. Emerging role of Lon protease as a master regulator of mitochondrial functions. Biochem Biophys Acta. Hoesel B, Schmid JA. The complexity of NF-kappaB signaling in inflammation and cancer. Mol Cancer. Yao H, de Boer WI, Rahman I. Targeting lung inflammation: novel therapies for the treatment of COPD. Curr Respir Med Rev. Fang JY, Richardson BC. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. Shuto T, Xu H, Wang B, Han J, Kai H, Gu XX, et al. Wang J, Huang J, Wang L, Chen C, Yang D, Jin M, et al. Urban particulate matter triggers lung inflammation via the ROS-MAPK-NF-kappaB signaling pathway. J Thorac Dis. Lu H, Chen I, Shimoda LA, Park Y, Zhang C, Tran L, et al. Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, et al. Blockade of B7—H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, et al. B7—H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. Bailly C. Regulation of PD-L1 expression on cancer cells with ROS-modulating drugs. Life Sci. Shima T, Shimoda M, Shigenobu T, Ohtsuka T, Nishimura T, Emoto K, et al. Infiltration of tumor-associated macrophages is involved in tumor programmed death-ligand 1 expression in early lung adenocarcinoma. Griess B, Mir S, Datta K, Teoh-Fitzgerald M. Scavenging reactive oxygen species selectively inhibits M2 macrophage polarization and their pro-tumorigenic function in part, via Stat3 suppression. Free Radic Biol Med. Zou W. Regulatory T cells, tumour immunity and immunotherapy. Maj T, Wang W, Crespo J, Zhang H, Wang W, Wei S, et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol. Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, et al. Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, et al. Semenza GL. Hypoxia-inducible factors in physiology and medicine. Liberti MV, Locasale JW. The Warburg Effect: how does it benefit cancer cells? van Gisbergen MW, Offermans K, Voets AM, Lieuwes NG, Biemans R, Hoffmann RF, et al. Mitochondrial dysfunction inhibits hypoxia-induced HIF-1alpha stabilization and expression of its downstream targets. Dan Dunn J, Alvarez LAJ, Zhang X, Soldati T. Reactive oxygen species and mitochondria: a nexus of cellular homeostasis. Hernansanz-Agustín P, Choya-Foces C, Carregal-Romero S. Kuo CW, Tsai MH, Lin TK, Tiao MM, Wang PW, Chuang JH, et al. mtDNA as a mediator for expression of hypoxia-inducible factor 1alpha and ros in hypoxic neuroblastoma cells. Kim MC, Hwang SH, Yang Y, Kim NY, Kim Y. Reduction in mitochondrial oxidative stress mediates hypoxia-induced resistance to cisplatin in human transitional cell carcinoma cells. Bousquet PA, Meltzer S, Sonstevold L, Esbensen Y, Dueland S, Flatmark K, et al. Markers of mitochondrial metabolism in tumor hypoxia, systemic inflammation, and adverse outcome of rectal cancer. Transl Oncol. West AP, Shadel GS. Mitochondrial DNA in innate immune responses and inflammatory pathology. Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Park JE, Dutta B, Tse SW, Gupta N, Tan CF, Low JK, et al. Hypoxia-induced tumor exosomes promote M2-like macrophage polarization of infiltrating myeloid cells and microRNA-mediated metabolic shift. Kennel KB, Greten FR. Immune cell - produced ROS and their impact on tumor growth and metastasis. Checa J, Aran JM. Reactive oxygen species: drivers of physiological and pathological processes. J Inflamm Res. Yang Y, Neo SY, Chen Z, Cui W, Chen Y, Guo M, et al. Thioredoxin activity confers resistance against oxidative stress in tumor-infiltrating NK cells. Cancer cells are vulnerable to reactive oxygen species ROS due to their abnormal redox environment. Accordingly, combination of chemotherapy and oxidative stress has gained increasing interest for the treatment of cancer. We report a novel seleno-prodrug of gemcitabine Gem , Se—Gem , and evaluated its activation and biological effects in cancer cells. Se—Gem was prepared by introducing a 1,2-diselenolane a five-membered cyclic diselenide moiety into the parent drug Gem via a carbamate linker. Se—Gem is preferably activated by glutathione GSH and displays a remarkably higher potency than Gem up to a 6-fold increase to a panel of cancer cell lines. The activation of Se—Gem by GSH releases Gem and a seleno-intermediate nearly quantitatively. Unlike the most ignored side products in prodrug activation, the seleno-intermediate further catalyzes a conversion of GSH and oxygen to GSSG oxidized GSH and ROS via redox cycling reactions. Thus Se—Gem may be considered as a suicide agent to deplete GSH and works by a combination of chemotherapy and oxidative stress. This is the first case that employs a cyclic diselenide in prodrug design, and the success of Se—Gem as well as its well-defined action mechanism demonstrates that the 1,2-diselenolane moiety may serve as a general scaffold to advance constructing novel therapeutic molecules with improved potency via a combination of chemotherapy and oxidative stress. Li, Y. Hou, J. Zhao, J. Li, S. Wang and J. Fang, Chem. This article is licensed under a Creative Commons Attribution-NonCommercial 3. You can use material from this article in other publications, without requesting further permission from the RSC, provided that the correct acknowledgement is given and it is not used for commercial purposes. To request permission to reproduce material from this article in a commercial publication , please go to the Copyright Clearance Center request page. |
| Introduction | Scherz-Shouval, R. In cancer cells, several signaling activation involved in tumorigenesis were referred to under control of Lon-induced ROS. Accumulating evidences have shown that ROS around the TME promote cytotoxicity or immunosuppressive effect of immune cells [ , , ], and this issue is based on quantity of ROS in the TME [ , ]. These mtROS produced by dysfunctional mitochondria also can promote tumor development, possibly by perturbing the signal transduction adapter function of pcontrolled pathways [ 34 ]. Mohammad GH, Damink SO, Malago M, Dhar DK, Pereira SP Pyruvate kinase M2 and lactate dehydrogenase A are overexpressed in pancreatic cancer and correlate with poor outcome. Reactive oxygen species ROS are a group of highly reactive oxygen-containing molecules generated through several mechanisms in cells, such as aerobic respiration in mitochondria, metabolic enzymes, peroxisomes, and membrane-bound NADPH oxidases NOXs [ 1 , 2 ]. In response to ROS, tumor suppressor genes activate the expression of antioxidant genes or prooxidative genes in cells for cell survival or apoptosis respectively, to prevent tumor growth. |
| EDITORIAL article | et al. Circulation Res. Petrosillo, G. Decreased complex III activity in mitochondria isolated from rat heart subjected to ischemia and reperfusion: role of reactive oxygen species and cardiolipin. FASEB J. Valko, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Ray, P. Reactive oxygen species ROS homeostasis and redox regulation in cellular signaling. Houee-Levin, C. Exploring oxidative modifications of tyrosine: an update on mechanisms of formation, advances in analysis and biological consequences. Free Radic. Thannickal, V. Activation of an H 2 O 2 -generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. Meng, T. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Cell 9 , — Kamata, H. Reactive oxygen species promote TNF alpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell , — Esposito, F. Protein kinase B activation by reactive oxygen species is independent on tyrosine kinase receptor phosphorylation and required Src activity. Sundaresan, M. Requirement for generation of H 2 O 2 for platelet-derived growth factor signal transduction. Corcoran, A. Redox regulation of protein kinases. FEBS J. Kwon, J. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Natl Acad. USA , — Article PubMed CAS PubMed Central Google Scholar. Latimer, H. Peroxiredoxins in regulation of MAPK signalling pathways; sensors and barriers to signal transduction. Gopalakrishna, R. Protein kinase C signaling and oxidative stress. Free Rad. Taguchi, K. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells 16 , — Meister, A. Selective modification of glutathione metabolism. Thimmulappa, R. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. PubMed CAS Google Scholar. Orino, K. Ferritin and the response to oxidative stress. Weinberg, E. The role of iron in cancer. Cancer Prev. Essers, M. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. Putker, M. Cell 49 , — Melnik, B. p key conductor of all anti-acne therapies. Article PubMed PubMed Central Google Scholar. Rhee, S. The antioxidant function of sestrins is mediated by promotion of autophagic degradation of keap1 and Nrf2 activation of mTORC1. Pinnix, Z. Ferroportin and iron regulation in breast cancer progression and prognosis. Huang, L. Regulation of hypoxia-inducible factor 1α is mediated by an O 2 -dependent degradation domain via the ubiquitin-proteasome pathway. USA 95 , — Abid, M. NADPH oxidase activity selectively modulates vascular endothelial growth factor signaling pathways. Guo, Z. ATM activation by oxidative stress. Svegliati, S. Oxidative DNA damge induces the ATM-mediated transcriptional suppression of the Wnt inhibitor WIF-1 in systemic sclerosis and fibrosis. Tell, G. Nucleic Acids Res. Ando, K. Toledano, M. Modulation of transcription factor NF-kappa B binding activity by oxidation-reduction in vitro. USA 88 , — Matthews, J. Role of cysteine62 in DNA recognition by the p50 subunit of NF-kappa B. Lingappan, K. NF-kB in oxidative stress. Article PubMed Google Scholar. Matsushima, S. Increased oxidative stress in the nucleus caused by Nox4 mediates oxidation of HDAC4 and cardiac hypertrophy. Ushijima, T. Detection and interpretation of altered methylation patterns in cancer cells. Cancer 5 , — Grollman, A. Mutagenesis by 8-oxoguanine: an enemy within. Trends Genet. Egger, G. Epigenetics in human disease and prospects for epigenetic therapy. Nature , — Turk, P. Carcinogenesis 16 , — Pezone, A. High-coverage methylation data of a gene model before and after DNA damage and homologous repair. Russo, G. DNA damage and repair modify DNA methylation and chromatin domain of the targeted locus: mechanism of allele methylation polymorphism. Morano, A. Targeted DNA methylation by homology-directed repair in mammalian cells. Transcription reshapes methylation on the repair gene. Opresko, P. Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Hanahan, D. Hallmarks of cancer: the next generation. Radisky, D. Rac1b and reactive oxygen species mediate MMPinduced EMT and genomic instability. Nishikawa, M. Reactive oxygen species in tumor metastasis. Cancer Lett. Chan, J. Cancer-associated fibroblasts enact field cancerization by promoting extratumoral oxidative stress. Cell Death Dis. Toullec, A. Oxidative stress promotes myofibroblast differentiation and tumour spreading. EMBO Mol. Joyce, J. Microenvironmental regulation of metastasis. Cancer 9 , — Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21 , — Holmstrom, K. Cellular mechanisms and physiological consequences of redox-dependent signaling. Diehn, M. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Irani, K. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Vafa, O. C-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. De Nicola, G. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Shibata, T. Cancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Singh, A. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. Menegon, S. The dual roles of NRF2 in cancer. Trends Mol. Lignitto, L. Nrf2 activation promotes lung cancer metastasis by inhibiting the degradation of Bach1. Bae, I. BRCA1 induces antioxidant gene expression and resistance to oxidative stress. Gorrini, C. BRCA1 interacts with Nrf2 to regulate antioxidant signaling and cell survival. Olanich, M. A call to ARMS: targeting the PAX3-FOXO1 gene in alveolar rhabdomyosarcoma. Expert Opin. Targets 17 , — Harris, I. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 27 , — Sayin, V. Antioxidants accelerate lung cancer progression in mice. Lo, M. Cell Physiol. Potential use of the anti-inflammatory drug, sulfasalazine, for targets therapy of pancreatic cancer. PubMed PubMed Central CAS Google Scholar. Guan, J. Cancer Chemoter. Townsend, D. NOV, a glutathione disulfide mimetic, as a modulator of cellular redox balance. Wang, J. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell 18 , — Sobhakumari, A. Susceptibility of human head and neck cancer cells to combined inhibition of glutathione and thioredoxin metabolism. PLoS ONE 7 , e Chandra, J. Triggering and modulation of apoptosis by oxidative stress. Wang, L. The Fas death signaling pathway connecting reactive oxygen species generation and FLICE inhibitory protein down-regulation. Wilkie-Grantham, R. Novel phosphorylation and ubiquitination sites regulate reactive oxygen species-dependent degradation of anti-apoptotic c-FLIP protein. Stennicke, H. Caspase 9 can be activated without proteolytic processing. Kaufmann, S. Induction of apoptosis by cancer chemotherapy. Cell Res. Kagan, V. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Zuo, Y. Oxidative modification of caspase-9 facilitates its activation via disulfide-mediated interaction with Apaf Madesh, M. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. McStay, G. Role of critical thiol groups on the matrix surface of the adenine nucleotide translocase in the mechanism of the mitochondrial permeability transition pore. Luanpitpong, S. Regulation of apoptosis by Bcl-2 cysteine oxidation in human lung epithelial cells. Cell 24 , — Li, D. Reactive oxygen species ROS control the expression of Bcl-2 family proteins by regulating their phosphorylation and ubiquitination. Cancer Sci. Dimitrov, D. Therapeutic antibodies current state and future trends-is a paradigm change coming soon? Methods Mol. Hartmann, J. Tyrosine kinase inhibitors- a review on pharmacology, metabolism and side effects. Drug Metab. Teppo, H. Reactive oxygen species-mediated mechanisms of action of targeted cancer therapy. Cell Longev. Chang, S. Imatinib mesylate induction of ROS-dependent apoptosis in melanoma B16F0 cells. Shan, F. Erlotinib induces the human non-small-cell lung cancer cells apoptosis via activating ROS-dependent JNK pathways. Cancer Med. Bauer, D. Critical role of reactive oxygen species ROS for synergistic enhancement of apoptosis by vemurafenib and the potassium channel inhibitor TRAM in melanoma cells. Alas, S. Shen, B. Norcantharidin induced DU cell apoptosis through ROS-mediated mitochondrial dysfunction and energy depletion. PLoS One 8 , e Brenneisen, P. Nanotherapy and reactive oxygen species ROS in cancer: a novel perspective. Antioxidants 7 , E31 Qu, W. Life Sci. Oliveira, M. Redox Biol. Renschler, M. The emerging role of reactive oxygen species in cancer therapy. Cancer 40 , — Kotamraju, S. Transferrin receptor-dependent iron uptake is responsible for doxorubicin-mediated apoptosis in endothelial cells: role of oxidant-induced iron signaling in apoptosis. Prieto-Bermejo, R. Reactive oxygen species in hematopoiesis: leukemic cells take a walk on the wild side. Pelicano, H. Inhibition of mitochondrial respiration: a novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. Miller, W. Mechanisms of action of arsenic trioxide. Yi, J. The inherent cellular level of reactive oxygen species: one of the mechanisms determining the apoptotic susceptibility of leukemic cells to arsenic trioxide. Apoptosis 7 , — Hwang, P. Ferrodoxin reductase affects pdependent, 5-fluorouracil-induced apoptosis in colorectal cancer cells. Longley, D. Cancer 3 , — Zhang, Q. Involvement of reactive oxygen species in 2-methoxyestradiol-induced apoptosis in human neuroblastoma cells. Lai, W. ROS mediates 4-HPR-induced posttranscriptional expression of the Gadd gene. Berndtsson, M. Acute apoptosis by cisplatin requires induction of reactive oxygen species but is not associated with damage to nuclear DNA. Cancer , — Rottenberg, S. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD alone and in combination with platinum drugs. Charruyer, A. UV-C light induces raft-associated acid sphingomyelinase and JNK activation and translocation independently on a nuclear signal. Published : 16 June Publisher Name : Springer, Singapore. Print ISBN : Online ISBN : eBook Packages : Biomedical and Life Sciences Biomedical and Life Sciences R0. Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Policies and ethics. Skip to main content. Abstract Both oxidative stress and inflammation are interdependent cellular consequences of a biological defense system, which can fuel cancer and other pathophysiological provenience. Keywords Cancer Oxidative stress Inflammation Cancer metabolism Oxidative stress Cell proliferation. Buying options Chapter EUR eBook EUR Softcover Book EUR Hardcover Book EUR Tax calculation will be finalised at checkout Purchases are for personal use only Learn about institutional subscriptions. References Alfadda AA, Sallam RM Reactive oxygen species in health and disease. Oxid Med Cell Longev PubMed PubMed Central Google Scholar Panieri E, Santoro M ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Dis 7:e PubMed PubMed Central CAS Google Scholar Zini R, Berdeaux A, Morin D The differential effects of superoxide anion, hydrogen peroxide and hydroxyl radical on cardiac mitochondrial oxidative phosphorylation. Free Radic Res — PubMed CAS Google Scholar Holmström KM, Finkel T Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol — PubMed Google Scholar Sumimoto H Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J — PubMed CAS Google Scholar Finkel T Signal transduction by reactive oxygen species. J Cell Biol —15 PubMed PubMed Central CAS Google Scholar Trachootham D, Lu W, Ogasawara MA, Valle NR-D, Huang P Redox regulation of cell survival. Antioxid Redox Signal — PubMed PubMed Central CAS Google Scholar Liou G-Y, Storz P Reactive oxygen species in cancer. Free Radic Res — PubMed CAS Google Scholar Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O Oxidative stress and antioxidant defense. World Allergy Organ J PubMed PubMed Central CAS Google Scholar McCord JM The evolution of free radicals and oxidative stress. Am J Med — PubMed CAS Google Scholar Oberley T, Oberley L Antioxidant enzyme levels in cancer. Histol Histopathol — PubMed CAS Google Scholar Reiter RJ, Acuña-Castroviejo D, Tan DX, Burkhardt S Free radical-mediated molecular damage. Ann N Y Acad Sci — PubMed CAS Google Scholar Pollack M, Leeuwenburgh C Molecular mechanisms of oxidative stress in aging: free radicals, aging, antioxidants and disease. Elsevier Science BV, Amsterdam, pp — Google Scholar Wiseman H, Halliwell B Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J PubMed PubMed Central CAS Google Scholar Matés JM, Sánchez-Jiménez F Antioxidant enzymes and their implications in pathophysiologic processes. Front Biosci 4:D—D PubMed Google Scholar Breitenbach M, Eckl P Introduction to oxidative stress in biomedical and biological research. Biomol Ther — CAS Google Scholar Burton GJ, Jauniaux EJ Oxidative stress. Best Pract Res Clin Obstet Gynaecol — PubMed PubMed Central Google Scholar Li R, Jia Z, Trush MA Defining ROS in biology and medicine. React Oxyg Species Google Scholar Fukai T, Ushio-Fukai MJA Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal — PubMed PubMed Central CAS Google Scholar Ushio-Fukai M, Nakamura Y Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett —52 PubMed PubMed Central CAS Google Scholar Aggarwal V, Tuli HS, Varol A, Thakral F, Yerer MB, Sak K, Varol M, Jain A, Khan M, Sethi GJB Role of reactive oxygen species in cancer progression: molecular mechanisms and recent advancements. Biomolecules PubMed Central CAS Google Scholar Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J Electron transport and oxidative phosphorylation. WH Freeman, New York Google Scholar Phaniendra A, Jestadi DB, Periyasamy L Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem —26 PubMed CAS Google Scholar Bibov MY, Kuzmin AV, Alexandrova AA, Chistyakov VA, Dobaeva NM, Kundupyan OL Role of the reactive oxygen species induced DNA damage in human spermatozoa dysfunction. AME Med J —12 Google Scholar Waris G, Ahsan H Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog PubMed PubMed Central Google Scholar Yang H, Villani RM, Wang H, Simpson MJ, Roberts MS, Tang M, Liang X The role of cellular reactive oxygen species in cancer chemotherapy. J Exp Clin Cancer Res PubMed PubMed Central CAS Google Scholar Sosa V, Moliné T, Somoza R, Paciucci R, Kondoh H, LLeonart ME Oxidative stress and cancer: an overview. Ageing Res Rev — PubMed CAS Google Scholar Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med — PubMed PubMed Central CAS Google Scholar Acharya A, Das I, Chandhok D, Saha T Redox regulation in cancer: a double-edged sword with therapeutic potential. Oxid Med Cell Longev —34 PubMed PubMed Central Google Scholar Reczek CR, Chandel NS The two faces of reactive oxygen species in cancer. Annu Rev Cancer Biol —98 Google Scholar Ward PS, Thompson CB Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell — PubMed PubMed Central CAS Google Scholar Liberti MV, Locasale JW The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci — PubMed PubMed Central CAS Google Scholar Dhup S, Dadhich RK, Porporato PE, Sonveaux P Multiple biological activities of lactic acid in cancer: influences on tumor growth, angiogenesis and metastasis. Ann Transl Med — PubMed PubMed Central CAS Google Scholar Pathak C, Vinayak M Modulation of lactate dehydrogenase isozymes by modified base queuine. Mol Biol Rep — PubMed CAS Google Scholar Mohammad GH, Damink SO, Malago M, Dhar DK, Pereira SP Pyruvate kinase M2 and lactate dehydrogenase A are overexpressed in pancreatic cancer and correlate with poor outcome. PLoS One e PubMed PubMed Central Google Scholar Hsu M-C, Hung W-C Pyruvate kinase M2 fuels multiple aspects of cancer cells: from cellular metabolism, transcriptional regulation to extracellular signaling. Mol Cancer PubMed PubMed Central Google Scholar Gui DY, Lewis CA, Vander Heiden MG Allosteric regulation of PKM2 allows cellular adaptation to different physiological states. Sci Signal 6:pe7 PubMed Google Scholar Anastasiou D, Yu Y, Israelsen WJ, Jiang J-K, Boxer MB, Hong BS, Tempel W, Dimov S, Shen M, Jha A Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat Chem Biol PubMed PubMed Central CAS Google Scholar Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang J-K, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DSJS Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science — PubMed PubMed Central CAS Google Scholar Finley LW, Carracedo A, Lee J, Souza A, Egia A, Zhang J, Teruya-Feldstein J, Moreira PI, Cardoso SM, Clish CB SIRT3 opposes reprogramming of cancer cell metabolism through HIF1α destabilization. Cancer Cell — PubMed PubMed Central CAS Google Scholar Fiaschi T, Chiarugi P Oxidative stress, tumor microenvironment, and metabolic reprogramming: a diabolic liaison. Int J Cell Biol PubMed PubMed Central Google Scholar Burdon RH, Gill V, Rice-Evans C Oxidative stress and tumour cell proliferation. Free Radic Res Commun —76 PubMed CAS Google Scholar Kumari S, Badana AK, Malla R Reactive oxygen species: a key constituent in cancer survival. Biomark Insights PubMed PubMed Central Google Scholar Storz P Reactive oxygen species in tumor progression. Front Biosci — PubMed CAS Google Scholar Yang Y, Fang E, Luo J, Wu H, Jiang Y, Liu Y, Tong S, Wang Z, Zhou R, Tong Q The antioxidant alpha-lipoic acid inhibits proliferation and invasion of human gastric cancer cells via suppression of STAT3-mediated MUC4 gene expression. Oxid Med Cell Longev PubMed PubMed Central Google Scholar Hamanaka RB, Chandel NS Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci — PubMed PubMed Central CAS Google Scholar Wang M, Kirk JS, Venkataraman S, Domann FE, Zhang HJ, Schafer FQ, Flanagan SW, Weydert CJ, Spitz DR, Buettner GR Manganese superoxide dismutase suppresses hypoxic induction of hypoxia-inducible factor-1 α and vascular endothelial growth factor. Oncogene — PubMed CAS Google Scholar Patterson JC, Joughin BA, van de Kooij B, Lim DC, Lauffenburger DA, Yaffe MB ROS and oxidative stress are elevated in mitosis during asynchronous cell cycle progression and are exacerbated by mitotic arrest. e PubMed PubMed Central CAS Google Scholar Ranjan P, Anathy V, Burch PM, Weirather K, Lambeth JD, Heintz NH Redox-dependent expression of cyclin D1 and cell proliferation by Nox1 in mouse lung epithelial cells. Exp Cell Res —71 PubMed Google Scholar Verbon EH, Post JA, Boonstra J The influence of reactive oxygen species on cell cycle progression in mammalian cells. Gene —6 PubMed CAS Google Scholar Kelkel M, Schumacher M, Dicato M, Diederich M Antioxidant and anti-proliferative properties of lycopene. Free Radic Res — PubMed CAS Google Scholar Laphanuwat P, Likasitwatanakul P, Sittithumcharee G, Thaphaengphan A, Chomanee N, Suppramote O, Ketaroonrut N, Charngkaew K, Lam EW-F, Okada S Cyclin D1 depletion interferes with oxidative balance and promotes cancer cell senescence. Nutr J Google Scholar Kozlov SV, Waardenberg AJ, Engholm-Keller K, Arthur JW, Graham ME, Lavin M Reactive oxygen species ROS -activated ATM-dependent phosphorylation of cytoplasmic substrates identified by large-scale phosphoproteomics screen. Mol Cell Proteomics — PubMed CAS Google Scholar Mantovani F, Collavin L, Del Sal G Mutant p53 as a guardian of the cancer cell. Cell Death Differ — PubMed Google Scholar Ma Q Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol — PubMed PubMed Central CAS Google Scholar Haupt S, Raghu D, Haupt Y Mutant p53 drives cancer by subverting multiple tumor suppression pathways. Front Oncol PubMed PubMed Central Google Scholar Lisek K, Campaner E, Ciani Y, Walerych D, Del Sal G Mutant p53 tunes the NRF2-dependent antioxidant response to support survival of cancer cells. Oncotarget PubMed PubMed Central Google Scholar Du X, Fu X, Yao K, Lan Z, Xu H, Cui Q, Yang E Bcl-2 delays cell cycle through mitochondrial ATP and ROS. Cell Cycle — PubMed PubMed Central CAS Google Scholar Liang J, Cao R, Wang X, Zhang Y, Wang P, Gao H, Li C, Yang F, Zeng R, Wei P Mitochondrial PKM2 regulates oxidative stress-induced apoptosis by stabilizing Bcl2. Cell Res — PubMed CAS Google Scholar Martin TA, Ye L, Sanders AJ, Lane J, Jiang WG Cancer invasion and metastasis: molecular and cellular perspective. Landes Bioscience, Austin Google Scholar Seyfried TN, Huysentruyt LC On the origin of cancer metastasis. Crit Rev Oncog PubMed PubMed Central Google Scholar Lambert AW, Pattabiraman DR, Weinberg RA Emerging biological principles of metastasis. Cell — PubMed PubMed Central CAS Google Scholar Baba AI, Câtoi C Tumor cell morphology. The Publishing House of the Romanian Academy, Bucharest Google Scholar Heerboth S, Housman G, Leary M, Longacre M, Byler S, Lapinska K, Willbanks A, Sarkar S EMT and tumor metastasis. Clin Transl Med PubMed PubMed Central Google Scholar Mittal V Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol — PubMed CAS Google Scholar Wang H, Unternaehrer JJ Epithelial-mesenchymal transition and cancer stem cells: at the crossroads of differentiation and dedifferentiation. Dev Dyn —20 PubMed Google Scholar Nagarsheth N, Wicha MS, Zou W Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Mol Med Rep — PubMed Google Scholar Liao Z, Chua D, Tan NS Reactive oxygen species: a volatile driver of field cancerization and metastasis. Mol Cancer PubMed PubMed Central Google Scholar Miyazaki Y, Hara A, Kato K, Oyama T, Yamada Y, Mori H, Shibata T The effect of hypoxic microenvironment on matrix metalloproteinase expression in xenografts of human oral squamous cell carcinoma. Int J Oncol — PubMed CAS Google Scholar Zhang L, Huang G, Li X, Zhang Y, Jiang Y, Shen J, Liu J, Wang Q, Zhu J, Feng X Hypoxia induces epithelial-mesenchymal transition via activation of SNAI1 by hypoxia-inducible factor-1α in hepatocellular carcinoma. BMC Cancer PubMed PubMed Central CAS Google Scholar Nishida N, Kudo M Oxidative stress and epigenetic instability in human hepatocarcinogenesis. Dig Dis — PubMed Google Scholar Nishida N, Yano H, Nishida T, Kamura T, Kojiro MJ Angiogenesis in cancer. Vasc Health Risk Manag PubMed PubMed Central CAS Google Scholar Kim Y-W, Byzova TVJB Oxidative stress in angiogenesis and vascular disease. Blood — PubMed PubMed Central CAS Google Scholar Carmeliet PJ VEGF as a key mediator of angiogenesis in cancer. Oncology —10 PubMed CAS Google Scholar Babior BM NADPH oxidase. Curr Opin Immunol —47 PubMed CAS Google Scholar Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, Jiang BH Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res — PubMed CAS Google Scholar Sun X, Wei J, Tang Y, Wang B, Zhang Y, Shi L, Guo J, Hu F, Li X Leptin-induced migration and angiogenesis in rheumatoid arthritis is mediated by reactive oxygen species. FEBS Open Bio — PubMed PubMed Central CAS Google Scholar Coussens LM, Werb Z Inflammation and cancer. Nature PubMed PubMed Central CAS Google Scholar Blaylock RL Cancer microenvironment, inflammation and cancer stem cells: a hypothesis for a paradigm change and new targets in cancer control. Surg Neurol Int PubMed PubMed Central Google Scholar Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res PubMed PubMed Central Google Scholar Hanahan D, Weinberg RA Hallmarks of cancer: the next generation. Cell — PubMed CAS Google Scholar Kiraly O, Gong G, Olipitz W, Muthupalani S, Engelward BP Inflammation-induced cell proliferation potentiates DNA damage-induced mutations in vivo. PLoS Genet e PubMed PubMed Central Google Scholar Mantovani A, Allavena P, Sica A, Balkwill F Cancer-related inflammation. Nature PubMed CAS Google Scholar Grivennikov SI, Greten FR, Karin M Immunity, inflammation, and cancer. Cell — PubMed PubMed Central CAS Google Scholar Kim SS, Ruiz VE, Carroll JD, Moss SF Helicobacter pylori in the pathogenesis of gastric cancer and gastric lymphoma. Cancer Lett — PubMed CAS Google Scholar Wroblewski LE, Peek RM, Wilson KT Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev — PubMed PubMed Central CAS Google Scholar Arbuthnot P, Kew M Hepatitis B virus and hepatocellular carcinoma. Int J Exp Pathol — PubMed PubMed Central CAS Google Scholar El-Serag HB Hepatocellular carcinoma and hepatitis C in the United States. Hepatology — Google Scholar de Martel C, Franceschi S Infections and cancer: established associations and new hypotheses. Clin Microbiol Rev — PubMed PubMed Central CAS Google Scholar Balkwill F, Mantovani A Inflammation and cancer: back to Virchow? Lancet — PubMed CAS Google Scholar Stark T, Livas L, Kyprianou N Inflammation in prostate cancer progression and therapeutic targeting. Transl Androl Urol PubMed PubMed Central Google Scholar Shrihari T Dual role of inflammatory mediators in cancer. Ecancermedicalscience PubMed PubMed Central CAS Google Scholar Hoesel B, Schmid JA The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer PubMed PubMed Central CAS Google Scholar Mogensen TH Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev — PubMed PubMed Central CAS Google Scholar Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G The inflammasome: a caspaseactivation platform that regulates immune responses and disease pathogenesis. Nat Immunol — PubMed PubMed Central CAS Google Scholar Fialkow L, Wang Y, Downey GP Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic Biol Med — PubMed CAS Google Scholar Salzano S, Checconi P, Hanschmann E-M, Lillig CH, Bowler LD, Chan P, Vaudry D, Mengozzi M, Coppo L, Sacre S Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc Natl Acad Sci — PubMed CAS PubMed Central Google Scholar Martinon F Signaling by ROS drives inflammasome activation. Eur J Immunol — PubMed CAS Google Scholar Latz E, Xiao TS, Stutz A Activation and regulation of the inflammasomes. Nat Rev Immunol — PubMed CAS Google Scholar Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol PubMed CAS Google Scholar Zhou R, Yazdi AS, Menu P, Tschopp J A role for mitochondria in NLRP3 inflammasome activation. Nature — PubMed CAS Google Scholar Ghezzi P Role of glutathione in immunity and inflammation in the lung. Int J Gen Med PubMed PubMed Central CAS Google Scholar Morgan MJ, Liu Z-G Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res — PubMed CAS Google Scholar Blanco P, Palucka AK, Pascual V, Banchereau J Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev —52 PubMed PubMed Central CAS Google Scholar Klampfer L Cytokines, inflammation and colon cancer. Curr Cancer Drug Targets — PubMed PubMed Central CAS Google Scholar Liang M, Zhang P, Fu J Up-regulation of LOX-1 expression by TNF-α promotes trans-endothelial migration of MDA-MB breast cancer cells. Cancer Lett —37 PubMed CAS Google Scholar Zamarron BF, Chen W Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci PubMed PubMed Central CAS Google Scholar Lin W-W, Karin M A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Investig PubMed CAS PubMed Central Google Scholar Knüpfer H, Preiss R Serum interleukin-6 levels in colorectal cancer patients—a summary of published results. Int J Colorectal Dis — PubMed Google Scholar Tanaka T, Bai Z, Srinoulprasert Y, Yang B, Hayasaka H, Miyasaka M Chemokines in tumor progression and metastasis. Cancer Sci — PubMed CAS Google Scholar Yu JH, Kim H Oxidative stress and cytokines in the pathogenesis of pancreatic cancer. J Cancer Prev PubMed PubMed Central Google Scholar Prawan A, Buranrat B, Kukongviriyapan U, Sripa B, Kukongviriyapan V Inflammatory cytokines suppress NAD P H: quinone oxidoreductase-1 and induce oxidative stress in cholangiocarcinoma cells. Oncol Rep — PubMed CAS Google Scholar Esquivel-Velázquez M, Ostoa-Saloma P, Palacios-Arreola MI, Nava-Castro KE, Castro JI, Morales-Montor J The role of cytokines in breast cancer development and progression. J Interferon Cytokine Res —16 PubMed PubMed Central Google Scholar Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm PubMed PubMed Central Google Scholar Ravichandran K, Tyagi A, Deep G, Agarwal C, Agarwal R Interleukininduced iNOS expression in human lung carcinoma A cells: involvement of STAT and MAPK pathways Google Scholar Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S The pro-and anti-inflammatory properties of the cytokine interleukin Biochim Biophys Acta Mol Cell Res — CAS Google Scholar Brizzi MF, Formato L, Dentelli P, Rosso A, Pavan M, Garbarino G, Pegoraro M, Camussi G, Pegoraro L Interleukin-3 stimulates migration and proliferation of vascular smooth muscle cells. Circulation — PubMed CAS Google Scholar Ehrlich LA, Chung HY, Ghobrial I, Choi SJ, Morandi F, Colla S, Rizzoli V, Roodman GD, Giuliani N IL-3 is a potential inhibitor of osteoblast differentiation in multiple myeloma. Blood — PubMed CAS Google Scholar Gupta N, Barhanpurkar AP, Tomar GB, Srivastava RK, Kour S, Pote ST, Mishra GC, Wani MR IL-3 inhibits human osteoclastogenesis and bone resorption through downregulation of c-Fms and diverts the cells to dendritic cell lineage. J Immunol — PubMed CAS Google Scholar Heikkilä K, Ebrahim S, Lawlor DA Systematic review of the association between circulating interleukin-6 IL-6 and cancer. Eur J Cancer — PubMed Google Scholar Hodge DR, Hurt EM, Farrar WL The role of IL-6 and STAT3 in inflammation and canicer. Eur J Cancer — PubMed CAS Google Scholar Aggarwal BB, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB, Sung B, Ichikawa H Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer. Ann N Y Acad Sci — PubMed CAS Google Scholar Rao V, Dyer C, Jameel J, Drew P, Greenman J Potential prognostic and therapeutic roles for cytokines in breast cancer. J Clin Oncol — PubMed PubMed Central CAS Google Scholar Kinoshita H, Hirata Y, Nakagawa H, Sakamoto K, Hayakawa Y, Takahashi R, Nakata W, Sakitani K, Serizawa T, Hikiba Y Interleukin-6 mediates epithelial—stromal interactions and promotes gastric tumorigenesis. PLoS One 8:e PubMed PubMed Central CAS Google Scholar Gasche JA, Hoffmann J, Boland CR, Goel A Interleukin-6 promotes tumorigenesis by altering DNA methylation in oral cancer cells. Int J Cancer — PubMed PubMed Central CAS Google Scholar Baffet G, Braciak T, Fletcher R, Gauldie J, Fey G, Northemann W Autocrine activity of interleukin 6 secreted by hepatocarcinoma cell lines. Mol Biol Med — PubMed CAS Google Scholar Atreya R, Mudter J, Finotto S, Müllberg J, Jostock T, Wirtz S, Schütz M, Bartsch B, Holtmann M, Becker C Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med PubMed CAS Google Scholar Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S TGF-β suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity — PubMed CAS Google Scholar McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ TGF-[beta] and IL-6 drive the production of IL and IL by T cells and restrain TH cell-mediated pathology. Nat Immunol PubMed CAS Google Scholar Onishi RM, Gaffen SL Interleukin and its target genes: mechanisms of interleukin function in disease. Drug Des Devel Ther PubMed PubMed Central Google Scholar Maniati E, Soper R, Hagemann T Up for Mischief? Infect Agent Cancer PubMed PubMed Central Google Scholar Numasaki M, Fukushi J-I, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT Interleukin promotes angiogenesis and tumor growth. Blood — PubMed CAS Google Scholar Kolls JK, Lindén A Interleukin family members and inflammation. Immunity — PubMed CAS Google Scholar Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, Chang A, Zou W Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol — PubMed CAS Google Scholar Swann JB, Smyth MJ Immune surveillance of tumors. J Clin Investig PubMed CAS PubMed Central Google Scholar Meier B, Radeke H, Selle S, Younes M, Sies H, Resch K, Habermehl G Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-α. Biochem J — PubMed PubMed Central CAS Google Scholar Li Q, Engelhardt JF Interleukin-1β induction of NFκB is partially regulated by H2O2-mediated activation of NFκB-inducing kinase. J Biol Chem — PubMed CAS Google Scholar Hsu S, Hsu P Autocrine and paracrine functions of cytokines in malignant lymphomas. Endocrinology — PubMed CAS Google Scholar Yuzhalin A, Kutikhin A Interleukins in cancer biology: their heterogeneous role. Academic, Cambridge Google Scholar Szlosarek P, Charles KA, Balkwill FR Tumour necrosis factor-α as a tumour promoter. Eur J Cancer — PubMed CAS Google Scholar Szlosarek PW, Balkwill FR Tumour necrosis factor α: a potential target for the therapy of solid tumours. Lancet Oncol — PubMed CAS Google Scholar Leek R, Landers R, Fox S, Ng F, Harris A, Lewis C Association of tumour necrosis factor alpha and its receptors with thymidine phosphorylase expression in invasive breast carcinoma. Br J Cancer PubMed PubMed Central CAS Google Scholar Sethi G, Sung B, Aggarwal BB TNF: a master switch for inflammation to cancer. Front Biosci — PubMed CAS Google Scholar Dempsey PW, Doyle SE, He JQ, Cheng G The signaling adaptors and pathways activated by TNF superfamily. Cytokine Growth Factor Rev — PubMed CAS Google Scholar Aggarwal BB Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol PubMed CAS Google Scholar Aggarwal BB, Shishodia S, Ashikawa K, Bharti AC The role of TNF and its family members in inflammation and cancer: lessons from gene deletion. Curr Drug Targets Inflamm Allergy — PubMed CAS Google Scholar Wajant H, Pfizenmaier K, Scheurich P Tumor necrosis factor signaling. Cell Death Differ PubMed CAS Google Scholar Wang X, Lin Y Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol Sin — PubMed Google Scholar Devin A, Cook A, Lin Y, Rodriguez Y, Kelliher M, Liu Z-G The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity — PubMed CAS Google Scholar Yang J, Lin Y, Guo Z, Cheng J, Huang J, Deng L, Liao W, Chen Z, Zheng-gang L, Su B The essential role of MEKK3 in TNF-induced NF-[kappa] B activation. Nat Immunol PubMed CAS Google Scholar Karin M, Yamamoto Y, Wang QM The IKK NF-[kappa] B system: a treasure trove for drug development. Nat Rev Drug Discov PubMed CAS Google Scholar Kamata H, Honda S-I, Maeda S, Chang L, Hirata H, Karin M Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell — PubMed CAS Google Scholar Reinhard C, Shamoon B, Shyamala V, Williams LT Tumor necrosis factor α-induced activation of c-jun N-terminal kinase is mediated by TRAF2. Proc Natl Acad Sci — PubMed CAS PubMed Central Google Scholar Liu J, Lin A Role of JNK activation in apoptosis: a double-edged sword. Cell Res PubMed Google Scholar Shih R-H, Wang C-Y, Yang C-M NF-kappaB signaling pathways in neurological inflammation: a mini review. Front Mol Neurosci PubMed PubMed Central Google Scholar Alkhamesi NA, Ziprin P, Pfistermuller K, Peck DH, Darzi AW ICAM-1 mediated peritoneal carcinomatosis, a target for therapeutic intervention. Clin Exp Metastasis — PubMed CAS Google Scholar Choo MK, Sakurai H, Koizumi K, Saiki I TAK1-mediated stress signaling pathways are essential for TNF-α-promoted pulmonary metastasis of murine colon cancer cells. Int J Cancer — PubMed CAS Google Scholar Kitakata H, Nemoto-Sasaki Y, Takahashi Y, Kondo T, Mai M, Mukaida N Essential roles of tumor necrosis factor receptor p55 in liver metastasis of intrasplenic administration of colon 26 cells. Cancer Res — PubMed CAS Google Scholar Kulbe H, Hagemann T, Szlosarek PW, Balkwill FR, Wilson JL The inflammatory cytokine tumor necrosis factor-α regulates chemokine receptor expression on ovarian cancer cells. Cancer Res — PubMed CAS Google Scholar Mochizuki Y, Nakanishi H, Kodera Y, Ito S, Yamamura Y, Kato T, Hibi K, Akiyama S, Nakao A, Tatematsu M TNF-α promotes progression of peritoneal metastasis as demonstrated using a green fluorescence protein GFP -tagged human gastric cancer cell line. Clin Exp Metastasis —47 PubMed CAS Google Scholar Van Grevenstein W, Hofland L, Van Rossen M, Van Koetsveld P, Jeekel J, Van Eijck C Inflammatory cytokines stimulate the adhesion of colon carcinoma cells to mesothelial monolayers. Dig Dis Sci — PubMed Google Scholar Ziprin P, Ridgway PF, Pfistermüller KL, Peck DH, Darzi AW ICAM-1 mediated tumor-mesothelial cell adhesion is modulated by IL-6 and TNF-α: a potential mechanism by which surgical trauma increases peritoneal metastases. Cell Commun Adhes — PubMed CAS Google Scholar Balkwill F Tumour necrosis factor and cancer. Nat Rev Cancer PubMed CAS Google Scholar Kehrl JH, Wakefield LM, Roberts AB, Jakowlew S, Alvarez-Mon M, Derynck R, Sporn MB, Fauci AS Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med — PubMed CAS Google Scholar Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci — PubMed CAS PubMed Central Google Scholar Letterio JJ TGF-[beta] signaling in T cells: roles in lymphoid and epithelial neoplasia. Oncogene PubMed CAS Google Scholar Bierie B, Moses HL TGF-β and cancer. Cytokine Growth Factor Rev —40 PubMed CAS Google Scholar Levy L, Hill CS Alterations in components of the TGF-β superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev —58 PubMed CAS Google Scholar Valle L, Serena-Acedo T, Liyanarachchi S, Hampel H, Comeras I, Li Z, Zeng Q, Zhang H-T, Pennison MJ, Sadim M Germline allele-specific expression of TGFBR1 confers an increased risk of colorectal cancer. Science — PubMed PubMed Central CAS Google Scholar Zeng Q, Phukan S, Xu Y, Sadim M, Rosman DS, Pennison M, Liao J, Yang G-Y, Huang C-C, Valle L Tgfbr1 haploinsufficiency is a potent modifier of colorectal cancer development. Cancer Res — PubMed PubMed Central CAS Google Scholar Bierie B, Moses HL Tumour microenvironment: TGF [beta]: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer PubMed CAS Google Scholar Brandes M, Mai U, Ohura K, Wahl S Type I transforming growth factor-beta receptors on neutrophils mediate chemotaxis to transforming growth factor-beta. J Immunol — PubMed CAS Google Scholar Wahl SM, Hunt DA, Wakefield LM, McCartney-Francis N, Wahl LM, Roberts AB, Sporn MB Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci — PubMed CAS PubMed Central Google Scholar Gruber BL, Marchese MJ, Kew RR Transforming growth factor-beta 1 mediates mast cell chemotaxis. J Immunol — PubMed CAS Google Scholar Li MO, Wan YY, Sanjabi S, Robertson A-KL, Flavell RA Transforming growth factor-β regulation of immune responses. Annu Rev Immunol — PubMed CAS Google Scholar Maghazachi A, Al-Aoukaty A Transforming growth factor-beta 1 is chemotactic for interleukinactivated natural killer cells. Nat Immun —65 Google Scholar Wrzesinski SH, Wan YY, Flavell RA Transforming growth factor-β and the immune response: implications for anticancer therapy. Clin Cancer Res — PubMed CAS Google Scholar Bierie B, Moses HL Transforming growth factor beta TGF-β and inflammation in cancer. Cytokine Growth Factor Rev —59 PubMed CAS Google Scholar Liu R-M, Pravia KG Oxidative stress and glutathione in TGF-β-mediated fibrogenesis. Free Radic Biol Med —15 PubMed Google Scholar Tochhawng L, Deng S, Pervaiz S, Yap CT Redox regulation of cancer cell migration and invasion. Mitochondrion — PubMed CAS Google Scholar Krstić J, Trivanović D, Mojsilović S, Santibanez JF Transforming growth factor-beta and oxidative stress interplay: implications in tumorigenesis and cancer progression. Oxid Med Cell Longev PubMed PubMed Central Google Scholar Zlotnik A Chemokines and cancer. Int J Cancer — PubMed CAS Google Scholar Stone MJ, Hayward JA, Huang C, Huma ZE, Sanchez J Mechanisms of regulation of the chemokine-receptor network. Int J Mol Sci PubMed Central Google Scholar Dubinett SM, Lee J, Sharma S, Mulé JJ Chemokines: can effector cells be re-directed to the site of tumor? Cancer J PubMed PubMed Central CAS Google Scholar Martins-Green M, Petreaca M, Wang L Chemokines and their receptors are key players in the orchestra that regulates wound healing. Adv Wound Care — Google Scholar Proost P, Wuyts A, Van Damme J The role of chemokines in inflammation. Int J Clin Lab Res — PubMed CAS Google Scholar Rossi D, Zlotnik A The biology of chemokines and their receptors. Annu Rev Immunol — PubMed CAS Google Scholar Chetram MA, Hinton CV ROS-mediated regulation of CXCR4 in cancer. Front Biol — CAS Google Scholar Salazar N, Castellan M, Shirodkar SS, Lokeshwar BL Chemokines and chemokine receptors as promoters of prostate cancer growth and progression. Crit Rev Eukaryot Gene Expr —91 PubMed PubMed Central CAS Google Scholar Strieter RM, Gomperts BN, Keane MP The role of CXC chemokines in pulmonary fibrosis. J Clin Investig PubMed CAS PubMed Central Google Scholar Clarke CN, Kuboki S, Tevar A, Lentsch AB, Edwards M Roles of NF-kappaB and STAT3 in CXC chemokine—mediated hepatocyte proliferation and cell death. Cancer Lett — PubMed CAS Google Scholar Murphy PM, Baggiolini M, Charo IF, Hébert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA International union of pharmacology. Pharmacol Rev — PubMed CAS Google Scholar Zlotnik A, Yoshie O Chemokines: a new classification system and their role in immunity. Immunity — PubMed CAS Google Scholar Balkwill F Cancer and the chemokine network. Nat Rev Cancer PubMed CAS Google Scholar Bottazzi B, Polentarutti N, Acero R, Balsari A, Boraschi D, Ghezzi P, Salmona M, Mantovani A Regulation of the macrophage content of neoplasms by chemoattractants. Science — PubMed CAS Google Scholar Pollard JW Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer PubMed CAS Google Scholar Germano G, Frapolli R, Simone M, Tavecchio M, Erba E, Pesce S, Pasqualini F, Grosso F, Sanfilippo R, Casali PG Antitumor and anti-inflammatory effects of trabectedin on human myxoid liposarcoma cells. Cancer Res — PubMed CAS Google Scholar Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med PubMed CAS Google Scholar Ghadjar P, Coupland SE, Na I-K, Noutsias M, Letsch A, Stroux A, Bauer S, Buhr HJ, Thiel E, Scheibenbogen C Chemokine receptor CCR6 expression level and liver metastases in colorectal cancer. J Clin Oncol — PubMed CAS Google Scholar Marchesi F, Piemonti L, Fedele G, Destro A, Roncalli M, Albarello L, Doglioni C, Anselmo A, Doni A, Bianchi P The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma. Cancer Res — PubMed CAS Google Scholar Singh S, Nannuru K, Sadanandam A, Varney M, Singh R CXCR1 and CXCR2 enhances human melanoma tumourigenesis, growth and invasion. Cancer Res — PubMed CAS Google Scholar Wang J, Seethala RR, Zhang Q, Gooding W, Van Waes C, Hasegawa H, Ferris RL Autocrine and paracrine chemokine receptor 7 activation in head and neck cancer: implications for therapy. J Natl Cancer Inst — PubMed CAS Google Scholar Murakami T, Cardones AR, Finkelstein SE, Restifo NP, Klaunberg BA, Nestle FO, Castillo SS, Dennis PA, Hwang ST Immune evasion by murine melanoma mediated through CC chemokine receptor J Exp Med — PubMed PubMed Central CAS Google Scholar Kwong J, Kulbe H, Wong D, Chakravarty P, Balkwill F An antagonist of the chemokine receptor CXCR4 induces mitotic catastrophe in ovarian cancer cells. Mol Cancer Ther — PubMed CAS Google Scholar Kalluri R, Zeisberg M Fibroblasts in cancer. Revision Received:. Get Permissions. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest. View full article. Sign in Don't already have an account? Sign in to your personal account. You could not be signed in. Please try again. Sign In Reset password. Biochemical Society Member Sign in Sign In. Sign in via your Institution Sign in via your Institution. Get Access To This Article Buy This Article. View Metrics. Cited By Web Of Science CrossRef Get Email Alerts Article Activity Alert. Accepted Manuscript Alert. Latest Completed Issue Alert. Submit your work. Latest Most Read Most Cited Beyond the tail: the consequence of context in histone post-translational modification and chromatin research. Identification of a mer peptide from the death domain of MyD88 which attenuates inflammation and insulin resistance and improves glucose metabolism. IL-4 activates futile triacylglyceride cycle for glucose utilization in white adipocytes. AMPK activation suppresses leptin expression independently of adipogenesis in primary murine adipocytes. |
Sie haben das Wichtigste verpasst.