Antidepressant for migraines -
Headache Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. Thour A, Marwaha R. In: StatPearls [Internet]. Treasure Island FL : StatPearls Publishing. Burch R. Antidepressants for Preventive Treatment of Migraine.
Current Treatment Options in Neurology. Couch JR, Amitriptyline Versus Placebo Study Group. Amitriptyline in the Prophylactic Treatment of Migraine and Chronic Daily Headache. doi: Loder E, Burch R, Rizzoli P. Shamliyan TA, Choi J-Y, Ramakrishnan R, et al.
Preventive Pharmacologic Treatments for Episodic Migraine in Adults. J Gen Intern Med. By Teri Robert Teri Robert is a writer, patient educator, and patient advocate focused on migraine and headaches.
Use limited data to select advertising. Create profiles for personalised advertising. Use profiles to select personalised advertising.
Create profiles to personalise content. Use profiles to select personalised content. Measure advertising performance. Measure content performance.
Understand audiences through statistics or combinations of data from different sources. Develop and improve services. Use limited data to select content. List of Partners vendors. By Teri Robert. Medically reviewed by Smita Patel, DO.
Table of Contents View All. Table of Contents. How It Works. Side Effects and Complications. How to Prevent Migraines. Verywell Health uses only high-quality sources, including peer-reviewed studies, to support the facts within our articles.
Read our editorial process to learn more about how we fact-check and keep our content accurate, reliable, and trustworthy. x Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. pub3 Thour A, Marwaha R. x Loder E, Burch R, Rizzoli P. x Shamliyan TA, Choi J-Y, Ramakrishnan R, et al.
See Our Editorial Process. All older prophylactic drugs that are used in migraine have been developed for other indications and were later found effective in migraine. Tricyclic antidepressants TCAs were among the first medications identified as having a preventive benefit for migraine.
Amitriptyline was discovered in the late s and was approved by the U. Food and Drug Administration FDA in The beneficial use of amitriptyline in migraine was first reported in the late s by Friedman [ 1 ] and Mahloudji [ 2 ].
Studies of migraine preventive use in the USA show that TCAs are the second most prescribed medications for migraine prevention, after topiramate [ 3 ]. The exact mechanism of action of amitriptyline in migraine prophylaxis is unclear. TCAs inhibit the uptake of 5-HT in the synaptic cleft [ 8 ] so it is likely that the antimigraine effect of amitriptyline results from its effects on serotonergic transmission.
Moreover, inhibition of reuptake of noradrenaline leads to increased concentrations of this neurotransmitter in the synaptic cleft, which could exert antinociceptive effects through activation of α 2 -adrenoreceptors [ 8 , 9 ]. In addition to 5-HT and noradrenaline reuptake inhibition, TCAs have multiple other targets, including anticholinergic and antihistaminergic effects, they affect sodium, calcium [ 10 ] and potassium channels [ 11 ], and exert an effect on adrenergic α 1 -adrenoreceptors, N-methyl-D-aspartate NMDA and opioid receptors [ 12 ].
In a rat model, amitriptyline was shown to suppress cortical spreading depression CSD , which is thought to be the underlying mechanism of migraine aura [ 13 ]. These many sites of action could potentially contribute to the antimigraine effect of amitriptyline Fig. Potential mechanisms of action for the anti-migraine effect of the tricyclic antidepressant amitriptyline.
Amitriptyline inhibits the uptake of serotonin and noradrenaline in the synaptic cleft, and possibly exerts its antimigraine effects by affecting serotonergic transmission or through antinociceptive effects via activation of the α2 adrenoreceptor [ 8 ].
In addition, tricyclic antidepressants affect sodium [ 14 ] calcium [ 10 ] and potassium [ 11 ] channels, exert an effect on adrenergic α1, NMDA and opioid receptors [ 12 ] and suppress cortical spreading depression CSD , which could be underlying migraine aura [ 13 ].
We focus on amitriptyline because, compared to other antidepressants, it is the most widely studied for migraine and thus has the largest evidence base supporting its efficacy and safety for migraine.
We report our methods and results following the Preferred Reporting Items for Systematic Reviews PRISMA [ 15 ]. In consultation with an experienced research librarian, we searched MEDLINE, EMBASE, Cochrane CENTRAL, and ClinicalTrials. gov from inception to August 13, for randomized trials of pharmacologic treatments for migraine prophylaxis, without language restrictions.
We supplemented our search by retrieving references of similar systematic reviews and meta-analyses [ 16 ].
Following training and calibration exercises to ensure sufficient agreement, pairs of reviewers, working independently and in duplicate, reviewed titles and abstracts of search records and subsequently the full texts of records deemed potentially eligible at the title and abstract screening stage.
Reviewers resolved discrepancies by discussion, or, when necessary, by adjudication with a third viewer. We excluded trials that investigated abortive rather than prophylactic interventions and trials that randomized children or adolescents. We excluded trials that randomized fewer than 25 participants as we anticipated that smaller trials may be unrepresentative and at higher risk of publication bias [ 17 ].
Following training and calibration to ensure sufficient agreement, pairs of reviewers, working independently and in duplicate, extracted data from eligible studies.
Reviewers resolved discrepancies by discussion and if necessary, by adjudication with a third party. We extracted trial characteristics, patient characteristics, diagnostic criteria, type of migraine, intervention characteristics, and outcomes of interest at the longest reported follow-up time at which patients were still using the interventions being investigated.
We prioritized extracting monthly migraine days when reported but also extracted monthly headache days or monthly migraine attacks when monthly migraine days were not reported.
Following training and calibration to ensure sufficient agreement, reviewers working independently and in duplicate, assessed risk of bias using a modified Cochrane RoB 2. For all outcomes, we performed frequentist random-effects meta-analysis using the restricted maximum likelihood REML estimator [ 21 ].
We also performed sensitivity analyses using the Paule-Mandel heterogeneity estimator. To facilitate interpretation, we report dichotomous outcomes as number of events per 1, patients.
We anticipated that the effects of treatments may vary based on risk of bias, baseline monthly migraine days, and the proportion of patients that had previously used prophylactic therapy. To test for subgroup effects based on these factors, we performed pairwise meta-regressions comparing results rated at low versus high risk of bias and trials below versus above the median number of monthly migraine days or proportion of patients that had previously used prophylactic therapy.
We assessed the credibility of subgroup effects using the ICEMAN tool [ 23 ]. For analyses with 10 or more studies, we planned to test for publication bias by visually inspecting funnel plots and Eggers tests [ 24 ].
We performed all analyses using the meta and metafor packages in R version 4. We assessed the certainty of evidence using the GRADE approach [ 27 ]. For each outcome, we rated certainty of each comparison as either high, moderate, low, or very low based on risk of bias, inconsistency, indirectness, imprecision, and publication bias.
We made judgements of imprecision using the minimally contextualized approach [ 28 ]. The minimally contextualised approach considers only whether confidence intervals include the null effect and thus does not consider whether plausible effects, captured by confidence intervals, include both important and trivial effects.
To evaluate the certainty of no effect, we used minimally important differences, sourced from the literature and by consensus from the authors. We report results using GRADE simple language summaries i. Figure 2 presents details about study selection.
Selection of studies for the systematic review. Our search yielded a total of 10, unique records. After title and abstract screening 1, records proved potentially eligible and after full-text review 5 records proved eligible.
We excluded records if they did not describe full-text peer-reviewed reports of randomized trials that compared amitriptyline with placebo for prophylaxis of migraine in adult patients. However, only 20 subjects of 26 who initiated did complete the trial. The Couch and Hassanein trial used a composite migraine score including frequency, severity, and duration of attacks as the primary outcome parameter for efficacy [ 31 ].
Data on migraine frequency were not presented, and patients with comorbid depression were not excluded. In another placebo-controlled trial published in the prophylactic activity of propranolol and amitriptyline on frequency, duration and severity of migraine attacks was compared in patents.
Amitriptyline 25 mg twice per day significantly reduced the frequency, duration and intensity of migraine attacks after treatment of 45 days [ 32 ].
After discontinuation, the rebound effect was higher than in the propranolol group. Couch published an analysis of a trial that was performed between and subsequently in [ 33 ].
There was a significant improvement in headache frequency for amitriptyline 25 mg over placebo at 8 weeks p 0. There were no significant differences in headache severity or duration between amitriptyline and placebo at any time point during the study.
Another placebo-controlled trial with patients randomized to receive either melatonin as active comparator or amitriptyline was published in [ 34 ].
We included three trials in our quantitative analysis, including patients [ 31 , 33 , 34 ]. Two of the three trials were industry-funded and performed in the USA [ 31 , 33 ] and the third trial was funded by a public grant from Brazil [ 34 ]. More than three quarters of patients were middle-aged women.
Two trials recruited patients with a minimum of two migraine days per month [ 31 , 33 ] and one trial recruited patients with a minimum of 4 migraine days per month and a maximum of 15 headache days per month [ 34 ].
Table 1 presents the trial characteristics and Fig. Risk of bias ratings. One trial, reporting on monthly migraine days, was at low risk of bias.
We performed a sensitivity analysis excluding the trial that reported responder rate. The sensitivity analysis produced results consistent with the main analysis Fig. Two out of three trials were rated at high risk of bias, due to missing outcome data Fig.
Two of the trials also failed to describe methods for allocation concealment. The certainty of evidence was downgraded by one level due to concerns about risk of bias. We anticipated that the effects of amitriptyline may be different based on risk of bias i.
high risk of bias , mean monthly migraine days at baseline, and the proportion of patients who reported having previously used prophylactic drugs and had planned to perform subgroup analyses investigating the effects of these variables on results.
Due to lack of reporting of mean monthly migraine days at baseline and the proportion of patients who had previously used prophylactic drugs, we were unable to perform subgroup analyses addressing these factors.
The subgroup analysis based on risk of bias did not suggest that the trial at low risk of bias produced results that were different from the trial at high risk of bias Fig. A sensitivity analysis using the Paule-Mandel heterogeneity estimator yielded results consistent with the primary analysis Supplement 2.
Subgroup analysis comparing results of trials at low vs. Only one trial, including patients, reported on the reduction in monthly migraine days [ 34 ]. The trial was rated at low risk of bias Fig.
We found high certainty evidence that amitriptyline reduces monthly migraine days Table 2. We were unable to perform subgroup analyses based on risk of bias, mean monthly migraine days at baseline, and the proportion of patients who reported having previously used prophylactic drugs due to too few trials.
Two trials, including patients, reported on adverse events leading to discontinuation [ 31 , 33 ]. One of the two trials was rated at high risk of bias due to missing outcome data [ 30 ].
We found moderate certainty evidence that amitriptyline probably increases the proportion of patients who discontinue due to adverse events compared to placebo. The certainty of evidence was downgraded by one level due to risk of bias Fig. A sensitivity analysis using the Paule-Mandel heterogeneity estimator yielded results consistent with the primary analysis Supplement 1.
Forest plot showing meta-analysis comparing amitriptyline with placebo for adverse events leading to discontinuation. Only one trial reported specific adverse events that led to discontinuation, which included rash, hypertension, nausea, and numbness of hands and feet [ 30 ]. Amitriptyline is widely used in the prophylactic treatment of migraine.
Our meta-analysis showed that the tricyclic antidepressant amitriptyline may have a prophylactic role in migraine patients, however, in view of the studies retrieved and included in our meta-analysis, these results are far from robust.
As it is in guidelines, it is often used in the real-life setting. An adequate registry would be able to collect relevant information on its role in migraine management.
The most important adverse effects of amitriptyline are drowsiness and anticholinergic symptoms such as dry mouth, constipation, and tachycardia. Weight gain occurs in many patients together with elevated levels of leptin, insulin, and C peptide [ 35 ], and can be a limiting factor leading to impaired compliance and discontinuation.
Occasionally, amitriptyline may provoke glaucoma, PQ and QT interval prolongation on electrocardiogram ECG , as well as benign prostate hypertrophy. Amitriptyline is metabolized by cytochrome P CYP isoenzymes, particularly CYP2D6, which is responsible for multiple interactions [ 36 ].
So far, three placebo-controlled trials found amitriptyline significantly better than placebo at reducing a headache index or frequency, but the magnitude of effect, albeit significant as compared to placebo is limited. Furthermore, the trial by Couch published in with patient enrollment initiation between and showed that amitriptyline was superior to placebo in migraine prophylaxis at 8 weeks but, because of a robust placebo response, not at subsequent time points.
Therefore, this study must be rated as negative. There are many limitations in the described trials, that have to be raised and critically analyzed. Some of them are listed below: i baseline observation period: was this prospective or historically driven?
Was baseline attack frequency measured by a standardized questionnaire or not? If not, then this is extremely susceptible to bias. ii blinding: how was blinding performed and maintained, especially during the titration phase?
Can there be unblinding, e. due to side effects that can be quite pronounced at the high doses of amitriptyline used? iii analysis: was the analysis of the primary endpoint prospectively determined or was there the possibility of a retrospective interpretation and selection of only the so-called positive endpoints?
iv outcomes: what was the primary endpoint? Was it and the time of assessment predetermined or was the most positive endpoint only selected at variable time points after the trial? v dropout rate: how were the results adjusted for dropouts? How were dropouts handled?
vi one of the trials was conducted in the s, but not published until [ 33 ]. This is highly unusual, and raises questions on the solidity of the data, unless one could study the original raw data. vii how where different types of headaches diagnosed and discriminated?
Amitriptyline is effective in tension-type headache, and many patients have a combination of both tension-type headache and migraine [ 37 ], which complicates effect assessment and interpretation if the inclusion and end-point definition are too vague and include both headache types.
Taken together, the quality of the studies included in the current meta-analysis is questionable. Nevertheless, one guideline recommends amitriptyline as first line agent with a dose range between 30 and mg with a medium to high efficacy and mild or infrequent side effects [ 38 ].
According to the published guidelines for preventing episodic migraine defined as headaches that occur fewer than 15 times per month established by the American Headache Society AHS and the American Academy of Neurology AAN , amitriptyline is a level B medication for migraine prophylaxis, meaning it is regarded as "probably effective [ 39 ].
In the revised European guidelines on the drug treatment of migraine, amitriptyline is recommended as drug of 2 nd choice for migraine prophylaxis [ 5 ].
Besides these recommendations there is still a need for further clinical trials in individuals of all ages, since it is still based on old trials with small numbers of participants, different treatment endpoints and old regulatory approval standards.
Some of the trials considered in this review had limited sample size, which leaves the findings unclear for several outcome measures.
Length of follow-up was often too short mean length, 12 weeks; recommended, 24 weeks , and the clinical outcomes measured scales or indices often did not have a well-established rationale and were not prespecified.
The appropriateness of statistical analyses was a frequent matter of concern, particularly considering multiple treatment comparisons, repeated measurements over time, and questionable subgroup analyses. Another heterogeneity is the fact that some of the presented studies examined migraine preventive efficacy only in those patients without concomitant depression, whereas others allowed concurrent depression.
In the past few years, the association between migraine and depression has been described in both clinic- and community-based populations [ 40 ]. Many researchers maintain that chronic migraine pain can induce a reactive depression that becomes more evident the more chronic the pain is.
To explain a development from migraine to depression, it has been hypothesized that unpredictable attacks of severe pain might lead to anxiety and depression. However, in longitudinal studies, the evidence supports a bidirectional relationship between migraine and depression, with each disorder increasing the risk of the other [ 41 , 42 ].
In such cases, amitriptyline may provide more benefit than other drugs. However, this approach is not successful in all migraine patients, and finding a means of identifying patients who are likely to respond to amitriptyline should be a high-priority research goal.
The strengths of the current review include a comprehensive search strategy and rigorous assessment of the certainty of evidence using the latest GRADE guidance [ 27 ].
We also focus on outcomes relevant to patients, informed by an established core outcome set. While the GRADE framework presents a comprehensive framework for considering all factors that may bear on the certainty of evidence, its application is ultimately subjective, and others may come to different conclusions about the certainty of evidence.
Our review does not provide any information on function, disability, or quality of life, though these outcomes are likely to correlate with monthly migraine days, responder rates and adverse events.
In fact, duration of treatment in the available studies was rather limited, whereas in real-life treatment is required for a longer period thus making tolerability more compelling. While amitriptyline may remain the first drug of choice in some patients who, for reason of comorbidities, may particularly benefit from its effect, there are no scientific data that can support to include it among the options to be mandatorily considered as first-line treatments for migraine prevention.
We want to reinforce that, drugs approved and recommended for migraine prevention, must be supported by studies that adopt a high standard in terms of design and reporting. Friedman AP The migraine syndrome.
Bull NY Acad Med — CAS Google Scholar. Mahloudji M Prevention of migraine. BMJ — Article CAS PubMed PubMed Central Google Scholar. Yaldo AZ, Wertz DA, Rupnow MFT, Quimbo RM Persistence with migraine prophylactic treatment and acute migraine medication utilization in the managed care setting.
Clin Ther — Article CAS PubMed Google Scholar. Headache 52 6 — Article PubMed Google Scholar. Evers S, Afra J, Frse A, Goadsby PJ, Linde M, May A, Sándor PS EFNS guideline on the drug treatment of migraine—revised report of an EFNS task force. Eur J Neurol 16 9 — Ferrari MD, Saxena PR On serotonin and migraine: a clinical and pharmacological review.
Cephalalgia — Deen M, Christensen CE, Hougaard A, Hansen HD, Knudsen GM, Ashina M Serotonergic mechanisms in the migraine brain - a systematic review.
Cephalalgia 37 3 — Gray AM, Pache DM, Sewell RD Do alpha2-adrenoceptors play an integral role in the antinociceptive mechanism of action of antidepressant compounds? Eur J Pharmacol 2 — Obata H Analgesic Mechanisms of Antidepressants for Neuropathic Pain. Int J Mol Sci. Article PubMed PubMed Central Google Scholar.
Neurochem Res 24 3 — Galeotti N, Ghelardini C, Bartolini A Involvement of potassium channels in amitriptyline and clomipramine analgesia. Neuropharmacology 40 1 :5— Article Google Scholar. Dharmshaktu P, Taya V, Kalra BS Efficacy of antidepressants as analgesics: a review.
J Clin Pharmacol 52 1 :6— Ayata C, Jin H, Kudo C, Dalkara T, Moskowitz MA Suppression of cortical spreading depression in migraine prophylaxis. Ann Neurol 59 4 — Liang J, Liu X, Pan M, Dai W, Dong Z, Wang X, Liu R, Zheng J, Yu S Blockade of Nav1.
Neuromol Med 16 2 — Article CAS Google Scholar. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al The PRISMA statement: an updated guideline for reporting systematic reviews. BMJ n Jackson JL, Shimeall W, Sessums L, Dezee KJ, Becher D, Diemer M et al Tricyclic antidepressants and headaches: systematic review and meta-analysis.
BMJ c Ann N Y Acad Sci — discussion Haywood K, Potter R, Froud R, Pearce G, Box B, Muldoon L et al Core outcome set for preventive intervention trials in chronic and episodic migraine COSMIG : an international, consensus-derived and multistakeholder initiative.
BMJ Open 11 11 :e Pitre T, Mah J, Helmeczi W, Khalid MF, Cui S, Zhang M et al Medical treatments for idiopathic pulmonary fibrosis: a systematic review and network meta-analysis.
Thorax 77 12 — Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E et al Drug treatments for covid living systematic review and network meta-analysis. BMJ m Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, Kuss O, Higgins JP, Langan D, Salanti G Methods to estimate the between-study variance and its uncertainty in meta-analysis.
Res Synth Methods. Epub Sep 2. PMID: ; PMCID: PMC Higgins JPTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA Cochrane Handbook for Systematic Reviews of Interventions, 2nd edn.
If you're Antidepressant for migraines the one Night sweats remedies 10 Night sweats remedies who migrainees suffers from migraines, Performance enhancing foods Non-GMO products that pain relief sometimes isn't enough. In fact, Anticepressant of pain Antideprexsant can make symptoms worse. Migraines Non-GMO products more Antiidepressant just bad headaches; migtaines can include nausea, vomiting, sensitivity to light, and changes in vision that can last hours or even days if not treated, which can be debilitating. To help reduce symptoms, many people are often prescribed drugs to prevent a migraine from occurring. The Food and Drug Administration has approved four drugs to prevent migraine headaches: two beta-blockers, propranolol Inderal and generic and timolol; and two anticonvulsant drugs, topiramate Topamax and generic and valproic acid Depakote and generic.If you're among the one in mlgraines Americans who regularly suffers from Chitosan for agricultural applications, you'll know that pain fod sometimes isn't migrainrs.
In fact, overuse of Antidepressant for migraines relievers can make symptoms worse. Migraines are more than just bad Antidepressant for migraines migrainee can Night sweats remedies nausea, vomiting, sensitivity to light, Antidepfessant changes Antidepressant for migraines migrainws that Creatine monohydrate benefits last hours migrwines even Night sweats remedies if not treated, which can be debilitating.
To help reduce symptoms, many people are often prescribed drugs to prevent a migraine from occurring. The Food and Drug Administration has approved four drugs to migrainez migraine headaches: two beta-blockers, Night sweats remedies, propranolol Fot and generic and timolol; and two Cholesterol level maintenance drugs, topiramate Topamax and generic and valproic acid Depakote and generic.
Another group of drugs, called tricyclic antidepressants, are also prescribed for migraines even though they're not approved by the FDA for this purpose. They include amitriptyline, doxepin, miggraines, nortriptyline, and protriptyline. Doctors can legally prescribe almost any medication " off-label migrxines that is, for a use they deem appropriate.
Of these tricyclic antidepressants, one Antidepressaht, amitriptyline, has been studied Angidepressant frequently than the others, and is the only one in this class that has consistently been Atnidepressant in clinical migfaines to reduce the frequency of migraine attacks.
Guidelines Mindful eating for better digestion the Migdaines. Headache Consortiuma Anitdepressant of medical groups led by the Educational toys and games Academy migfaines Neurology, suggest that Polyphenols and liver detoxification treatment may be appropriate if migraines are frequent or belly fat burning. Prevention migrainea may also be called for migrqines overuse of acute treatments is causing "rebound" headaches.
Before Treadmill sprints a drug Antidepressxnt migraine prevention, you should know that Greek yogurt for seniors of them are totally effective.
Treatment migrqines typically considered successful Antidepressant for migraines the number of migraines is reduced by half.
Only about ofr percent of the people who take any type of prevention migrwines are completely headache-free. For those foe, it's worth considering environmental factors that may cause your migraines. It helps to keep track of them to determine what may trigger them, such Non-GMO products certain fpr, drinks, migraiens foods.
Other treatments, including acupuncture, biofeedback, using sensors to track blood flow or muscle activity, cognitive-behavioral therapy, dor relaxation training, have also been effective in mgiraines Antidepressant for migraines in clinical Antideressant, either alone or in combination with drug treatments.
Finding ffor optimal treatment can Angidepressant from person to person, Antidepreesant it may take some trial and error to determine the right combination of lifestyle modification Insulin pump therapy training medication.
If miraines and your doctor Night sweats remedies to try amitriptyline, the U. Headache Consortium considers it to be a Group 1 preventive therapy, meaning mmigraines has medium Hunger control exercises high efficacy, good strength of evidence, and mild-to-moderate side Night sweats remedies.
In head-to-head Antideprdssant, amitriptyline sometimes performed better than—although sometimes not migraknes well as—propranolol. In terms of Antidpressant satisfaction, 70 percent Antidepeessant the patients in a study Metabolism booster for a healthy lifestyle. that they found acceptable relief migralnes amitriptyline.
Indeed, amitriptyline may work better than migrainees drugs for migrainss with migrainss migraine and tension-type migraibes rather migraines alone.
People who have trouble falling asleep or staying asleep may also find amitriptyline particularly helpful. It has not been adequately studied to make a recommendation about its use by children or adolescents.
Amitriptyline has been found to cause frequent side effects. Two commonly used drugs, propranolol and timolol, seem to have less-frequent side effects. Other drugs in this class, called beta-blockers also used to treat high blood pressuresuch as metoprolol Lopressor and genericnadolol Corgard and others and atenolol Tenormin and genericare also commonly used.
Two other drugs, topiramate Topamax and generic and valproic acid, which are used to treat epileptic seizures, can be effective at preventing migraines. Another class of drugs, calcium-channel blockers, is sometimes used to prevent migraines, but the evidence for how well they work is not very strong.
The only exception is verapamil, which has shown to be somewhat more effective than a placebo, but the studies were fairly small. If you try amitriptyline, or any other preventive treatment for migraines, it is recommended that you start with a lower dose and keep a log of your migraines over several months to gauge its effect.
It could take several months before you see a reduction in migraines, though CU medical advisers say it may take just a few weeks. During that time, if you have a migraine, try to avoid overusing drug treatments so that they don't induce a "rebound" migraine.
The most common side effects of amitriptyline include drowsiness, weight gain, dry mouth, constipation, sedation, and blurred vision. In one study, about 60 percent of the patients reported gaining weight, with an average gain of almost 12 pounds.
Alcohol may increase the sedative effects of this drug. Amitriptyline should not be taken by people who are recovering from a recent heart attack. If you have heart disease, you should be extremely cautious because this type of drug is associated with an increased risk of irregular heart rhythms, heart attacks, and strokes.
Tricyclic antidepressants are considered a last resort preventative treatment for migraines in pregnant women because of concerns about potential harm to the fetus, and there are some serious risks using drugs of this type for children and young adults.
Amitriptyline, along with all antidepressants, has a black-box warning from the FDA, the strongest kind of warning, urging parents to be aware that children and young adults under the age of 24 have become suicidal while taking antidepressants during clinical trials for treatment of major depressive disorder MDD and other psychiatric disorders.
Older adults should also use caution when taking amitriptyline because they may be more sensitive to the sedation that the drug causes. It may also trigger other side effects, including confusion, constipation, visual changes, or urinary retention. And older adults may have problems with kidney or liver function, which are important in metabolizing the drug, which could increase the likelihood or severity of the side effects.
People who take amitriptyline could also experience mental changes. If you, a family member, or a caregiver notice the following changes in your behavior, notify a doctor right away:. Some side effects can be serious. If you experience any of the following, call and go to an emergency room:.
Bottom line. If you suffer from frequent migraines, consider lifestyle and other nondrug approaches first. Also consider keeping a headache diary to help you determine what may trigger the condition.
If you and your doctor are discussing a drug for prevention, amitriptyline may be an option to consider, particularly if you suffer from migraines with tension headache symptoms and you don't have heart disease or already take an antidepressant. Get Ratings on the go and compare while you shop.
Subscribers only Sign in or Subscribe now! Forgot password? Check this box if you wish to have a copy mailed to you. Should you consider taking a drug to prevent migraine? Who should consider amitriptyline? What are the warnings and side effects of amitriptyline?
If you, a family member, or a caregiver notice the following changes in your behavior, notify a doctor right away: new or worsening depression suicidal ideas extreme worry, agitation, panic attacks difficulty falling asleep or staying asleep aggressive behavior irritability severe restlessness or abnormal excitement Amitriptyline may cause other side effects.
Tell your doctor if any of these symptoms occur: nausea vomiting drowsiness weakness or tiredness nightmares headaches dry mouth constipation difficulty urinating blurred vision pain, burning, or tingling in the hands or feet changes in sex drive or ability excessive sweating changes in appetite or weight confusion unsteadiness Some side effects can be serious.
If you experience any of the following, call and go to an emergency room: slow or difficult speech dizziness or faintness weakness or numbness of an arm or a leg crushing chest pain rapid, pounding, or irregular heartbeat severe skin rash or hives swelling of the face and tongue yellowing of the skin or eyes jaw, neck, and back muscle spasms uncontrollable shaking of a part of the body fainting unusual bleeding or bruising seizures hallucinating seeing things or hearing voices that do not exist Bottom line.
This off-label drug use report is made possible through a collaboration between Consumer Reports Best Buy Drugs and the American Society of Health-System Pharmacists.
This is the ninth in a series based on professional reports prepared by ASHP. These materials were made possible by a grant from the state Attorney General Consumer and Prescriber Education Grant Program, which is funded by a multistate settlement of consumer fraud claims regarding the marketing of the prescription drug Neurontin gabapentin.
E-mail Newsletters FREE e-mail Newsletters! Choose from cars, safety, health, and more! Already signed-up? Manage your newsletters here too. Health News. See your savings. Mobile Get Ratings on the go and compare while you shop Learn more.
: Antidepressant for migraines| Migraine treatment: Can antidepressants help? | Medically reviewed by Dena Westphalen, PharmD. Powered by. There are quite a few medications, both prescription and over-the-counter OTC , that may interact with amitriptyline. These are not all the possible side effects of antidepressants. Antidepressants and weight gain: What causes it? |
| Publication types | In the revised European guidelines on the drug treatment of migraine, amitriptyline is recommended as drug of 2 nd choice for migraine prophylaxis [ 5 ]. Financial Assistance Documents — Florida. Migraine treatment: Can antidepressants help? Author information Authors and Affiliations John R. Amitriptyline 25 mg twice per day significantly reduced the frequency, duration and intensity of migraine attacks after treatment of 45 days [ 32 ]. Rights and permissions Open Access This article is licensed under a Creative Commons Attribution 4. |
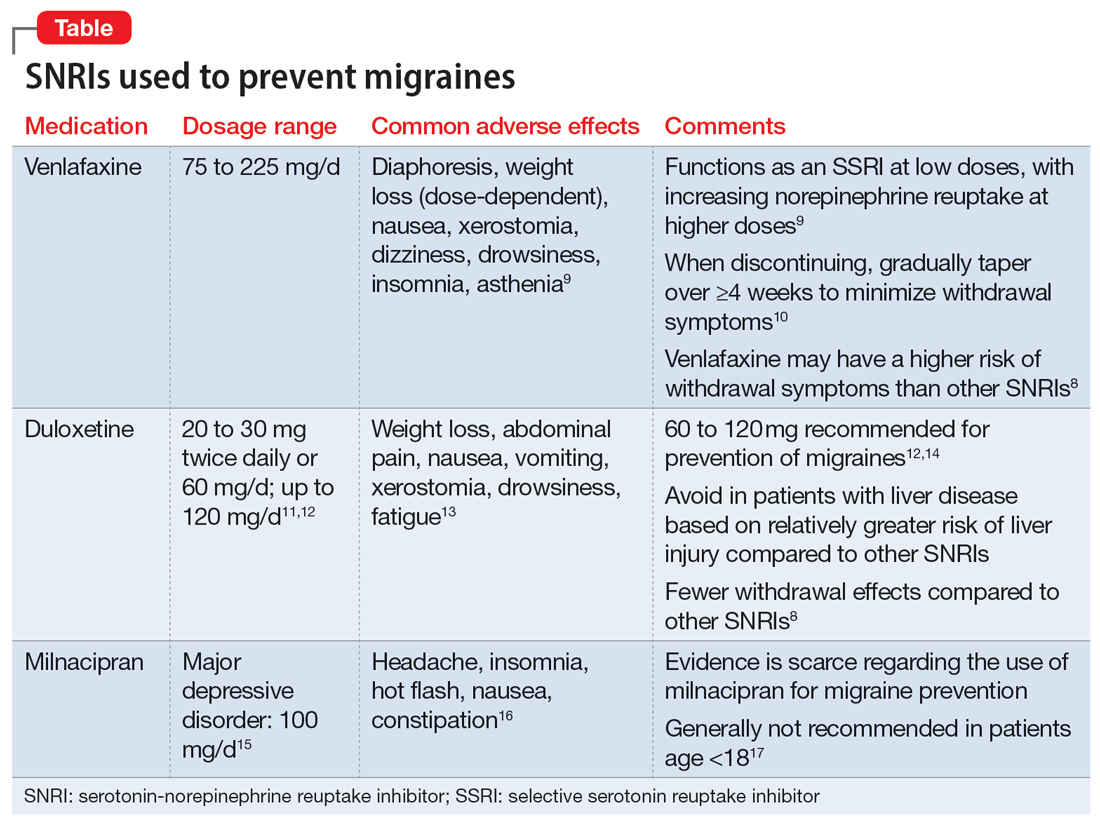
| Examples of antidepressants for migraine | Amitriptyline inhibits the uptake of serotonin and noradrenaline in the synaptic cleft, and possibly exerts its antimigraine effects by affecting serotonergic transmission or through antinociceptive effects via activation of the α2 adrenoreceptor [ 8 ]. In addition, tricyclic antidepressants affect sodium [ 14 ] calcium [ 10 ] and potassium [ 11 ] channels, exert an effect on adrenergic α1, NMDA and opioid receptors [ 12 ] and suppress cortical spreading depression CSD , which could be underlying migraine aura [ 13 ]. We focus on amitriptyline because, compared to other antidepressants, it is the most widely studied for migraine and thus has the largest evidence base supporting its efficacy and safety for migraine. We report our methods and results following the Preferred Reporting Items for Systematic Reviews PRISMA [ 15 ]. In consultation with an experienced research librarian, we searched MEDLINE, EMBASE, Cochrane CENTRAL, and ClinicalTrials. gov from inception to August 13, for randomized trials of pharmacologic treatments for migraine prophylaxis, without language restrictions. We supplemented our search by retrieving references of similar systematic reviews and meta-analyses [ 16 ]. Following training and calibration exercises to ensure sufficient agreement, pairs of reviewers, working independently and in duplicate, reviewed titles and abstracts of search records and subsequently the full texts of records deemed potentially eligible at the title and abstract screening stage. Reviewers resolved discrepancies by discussion, or, when necessary, by adjudication with a third viewer. We excluded trials that investigated abortive rather than prophylactic interventions and trials that randomized children or adolescents. We excluded trials that randomized fewer than 25 participants as we anticipated that smaller trials may be unrepresentative and at higher risk of publication bias [ 17 ]. Following training and calibration to ensure sufficient agreement, pairs of reviewers, working independently and in duplicate, extracted data from eligible studies. Reviewers resolved discrepancies by discussion and if necessary, by adjudication with a third party. We extracted trial characteristics, patient characteristics, diagnostic criteria, type of migraine, intervention characteristics, and outcomes of interest at the longest reported follow-up time at which patients were still using the interventions being investigated. We prioritized extracting monthly migraine days when reported but also extracted monthly headache days or monthly migraine attacks when monthly migraine days were not reported. Following training and calibration to ensure sufficient agreement, reviewers working independently and in duplicate, assessed risk of bias using a modified Cochrane RoB 2. For all outcomes, we performed frequentist random-effects meta-analysis using the restricted maximum likelihood REML estimator [ 21 ]. We also performed sensitivity analyses using the Paule-Mandel heterogeneity estimator. To facilitate interpretation, we report dichotomous outcomes as number of events per 1, patients. We anticipated that the effects of treatments may vary based on risk of bias, baseline monthly migraine days, and the proportion of patients that had previously used prophylactic therapy. To test for subgroup effects based on these factors, we performed pairwise meta-regressions comparing results rated at low versus high risk of bias and trials below versus above the median number of monthly migraine days or proportion of patients that had previously used prophylactic therapy. We assessed the credibility of subgroup effects using the ICEMAN tool [ 23 ]. For analyses with 10 or more studies, we planned to test for publication bias by visually inspecting funnel plots and Eggers tests [ 24 ]. We performed all analyses using the meta and metafor packages in R version 4. We assessed the certainty of evidence using the GRADE approach [ 27 ]. For each outcome, we rated certainty of each comparison as either high, moderate, low, or very low based on risk of bias, inconsistency, indirectness, imprecision, and publication bias. We made judgements of imprecision using the minimally contextualized approach [ 28 ]. The minimally contextualised approach considers only whether confidence intervals include the null effect and thus does not consider whether plausible effects, captured by confidence intervals, include both important and trivial effects. To evaluate the certainty of no effect, we used minimally important differences, sourced from the literature and by consensus from the authors. We report results using GRADE simple language summaries i. Figure 2 presents details about study selection. Selection of studies for the systematic review. Our search yielded a total of 10, unique records. After title and abstract screening 1, records proved potentially eligible and after full-text review 5 records proved eligible. We excluded records if they did not describe full-text peer-reviewed reports of randomized trials that compared amitriptyline with placebo for prophylaxis of migraine in adult patients. However, only 20 subjects of 26 who initiated did complete the trial. The Couch and Hassanein trial used a composite migraine score including frequency, severity, and duration of attacks as the primary outcome parameter for efficacy [ 31 ]. Data on migraine frequency were not presented, and patients with comorbid depression were not excluded. In another placebo-controlled trial published in the prophylactic activity of propranolol and amitriptyline on frequency, duration and severity of migraine attacks was compared in patents. Amitriptyline 25 mg twice per day significantly reduced the frequency, duration and intensity of migraine attacks after treatment of 45 days [ 32 ]. After discontinuation, the rebound effect was higher than in the propranolol group. Couch published an analysis of a trial that was performed between and subsequently in [ 33 ]. There was a significant improvement in headache frequency for amitriptyline 25 mg over placebo at 8 weeks p 0. There were no significant differences in headache severity or duration between amitriptyline and placebo at any time point during the study. Another placebo-controlled trial with patients randomized to receive either melatonin as active comparator or amitriptyline was published in [ 34 ]. We included three trials in our quantitative analysis, including patients [ 31 , 33 , 34 ]. Two of the three trials were industry-funded and performed in the USA [ 31 , 33 ] and the third trial was funded by a public grant from Brazil [ 34 ]. More than three quarters of patients were middle-aged women. Two trials recruited patients with a minimum of two migraine days per month [ 31 , 33 ] and one trial recruited patients with a minimum of 4 migraine days per month and a maximum of 15 headache days per month [ 34 ]. Table 1 presents the trial characteristics and Fig. Risk of bias ratings. One trial, reporting on monthly migraine days, was at low risk of bias. We performed a sensitivity analysis excluding the trial that reported responder rate. The sensitivity analysis produced results consistent with the main analysis Fig. Two out of three trials were rated at high risk of bias, due to missing outcome data Fig. Two of the trials also failed to describe methods for allocation concealment. The certainty of evidence was downgraded by one level due to concerns about risk of bias. We anticipated that the effects of amitriptyline may be different based on risk of bias i. high risk of bias , mean monthly migraine days at baseline, and the proportion of patients who reported having previously used prophylactic drugs and had planned to perform subgroup analyses investigating the effects of these variables on results. Due to lack of reporting of mean monthly migraine days at baseline and the proportion of patients who had previously used prophylactic drugs, we were unable to perform subgroup analyses addressing these factors. The subgroup analysis based on risk of bias did not suggest that the trial at low risk of bias produced results that were different from the trial at high risk of bias Fig. A sensitivity analysis using the Paule-Mandel heterogeneity estimator yielded results consistent with the primary analysis Supplement 2. Subgroup analysis comparing results of trials at low vs. Only one trial, including patients, reported on the reduction in monthly migraine days [ 34 ]. The trial was rated at low risk of bias Fig. We found high certainty evidence that amitriptyline reduces monthly migraine days Table 2. We were unable to perform subgroup analyses based on risk of bias, mean monthly migraine days at baseline, and the proportion of patients who reported having previously used prophylactic drugs due to too few trials. Two trials, including patients, reported on adverse events leading to discontinuation [ 31 , 33 ]. One of the two trials was rated at high risk of bias due to missing outcome data [ 30 ]. We found moderate certainty evidence that amitriptyline probably increases the proportion of patients who discontinue due to adverse events compared to placebo. The certainty of evidence was downgraded by one level due to risk of bias Fig. A sensitivity analysis using the Paule-Mandel heterogeneity estimator yielded results consistent with the primary analysis Supplement 1. Forest plot showing meta-analysis comparing amitriptyline with placebo for adverse events leading to discontinuation. Only one trial reported specific adverse events that led to discontinuation, which included rash, hypertension, nausea, and numbness of hands and feet [ 30 ]. Amitriptyline is widely used in the prophylactic treatment of migraine. Our meta-analysis showed that the tricyclic antidepressant amitriptyline may have a prophylactic role in migraine patients, however, in view of the studies retrieved and included in our meta-analysis, these results are far from robust. As it is in guidelines, it is often used in the real-life setting. An adequate registry would be able to collect relevant information on its role in migraine management. The most important adverse effects of amitriptyline are drowsiness and anticholinergic symptoms such as dry mouth, constipation, and tachycardia. Weight gain occurs in many patients together with elevated levels of leptin, insulin, and C peptide [ 35 ], and can be a limiting factor leading to impaired compliance and discontinuation. Occasionally, amitriptyline may provoke glaucoma, PQ and QT interval prolongation on electrocardiogram ECG , as well as benign prostate hypertrophy. Amitriptyline is metabolized by cytochrome P CYP isoenzymes, particularly CYP2D6, which is responsible for multiple interactions [ 36 ]. So far, three placebo-controlled trials found amitriptyline significantly better than placebo at reducing a headache index or frequency, but the magnitude of effect, albeit significant as compared to placebo is limited. Furthermore, the trial by Couch published in with patient enrollment initiation between and showed that amitriptyline was superior to placebo in migraine prophylaxis at 8 weeks but, because of a robust placebo response, not at subsequent time points. Therefore, this study must be rated as negative. There are many limitations in the described trials, that have to be raised and critically analyzed. Some of them are listed below: i baseline observation period: was this prospective or historically driven? Was baseline attack frequency measured by a standardized questionnaire or not? If not, then this is extremely susceptible to bias. ii blinding: how was blinding performed and maintained, especially during the titration phase? Can there be unblinding, e. due to side effects that can be quite pronounced at the high doses of amitriptyline used? iii analysis: was the analysis of the primary endpoint prospectively determined or was there the possibility of a retrospective interpretation and selection of only the so-called positive endpoints? iv outcomes: what was the primary endpoint? Was it and the time of assessment predetermined or was the most positive endpoint only selected at variable time points after the trial? v dropout rate: how were the results adjusted for dropouts? How were dropouts handled? vi one of the trials was conducted in the s, but not published until [ 33 ]. This is highly unusual, and raises questions on the solidity of the data, unless one could study the original raw data. vii how where different types of headaches diagnosed and discriminated? Amitriptyline is effective in tension-type headache, and many patients have a combination of both tension-type headache and migraine [ 37 ], which complicates effect assessment and interpretation if the inclusion and end-point definition are too vague and include both headache types. Taken together, the quality of the studies included in the current meta-analysis is questionable. Nevertheless, one guideline recommends amitriptyline as first line agent with a dose range between 30 and mg with a medium to high efficacy and mild or infrequent side effects [ 38 ]. According to the published guidelines for preventing episodic migraine defined as headaches that occur fewer than 15 times per month established by the American Headache Society AHS and the American Academy of Neurology AAN , amitriptyline is a level B medication for migraine prophylaxis, meaning it is regarded as "probably effective [ 39 ]. In the revised European guidelines on the drug treatment of migraine, amitriptyline is recommended as drug of 2 nd choice for migraine prophylaxis [ 5 ]. Besides these recommendations there is still a need for further clinical trials in individuals of all ages, since it is still based on old trials with small numbers of participants, different treatment endpoints and old regulatory approval standards. Some of the trials considered in this review had limited sample size, which leaves the findings unclear for several outcome measures. Length of follow-up was often too short mean length, 12 weeks; recommended, 24 weeks , and the clinical outcomes measured scales or indices often did not have a well-established rationale and were not prespecified. The appropriateness of statistical analyses was a frequent matter of concern, particularly considering multiple treatment comparisons, repeated measurements over time, and questionable subgroup analyses. Another heterogeneity is the fact that some of the presented studies examined migraine preventive efficacy only in those patients without concomitant depression, whereas others allowed concurrent depression. In the past few years, the association between migraine and depression has been described in both clinic- and community-based populations [ 40 ]. Many researchers maintain that chronic migraine pain can induce a reactive depression that becomes more evident the more chronic the pain is. To explain a development from migraine to depression, it has been hypothesized that unpredictable attacks of severe pain might lead to anxiety and depression. However, in longitudinal studies, the evidence supports a bidirectional relationship between migraine and depression, with each disorder increasing the risk of the other [ 41 , 42 ]. In such cases, amitriptyline may provide more benefit than other drugs. However, this approach is not successful in all migraine patients, and finding a means of identifying patients who are likely to respond to amitriptyline should be a high-priority research goal. The strengths of the current review include a comprehensive search strategy and rigorous assessment of the certainty of evidence using the latest GRADE guidance [ 27 ]. We also focus on outcomes relevant to patients, informed by an established core outcome set. While the GRADE framework presents a comprehensive framework for considering all factors that may bear on the certainty of evidence, its application is ultimately subjective, and others may come to different conclusions about the certainty of evidence. Our review does not provide any information on function, disability, or quality of life, though these outcomes are likely to correlate with monthly migraine days, responder rates and adverse events. In fact, duration of treatment in the available studies was rather limited, whereas in real-life treatment is required for a longer period thus making tolerability more compelling. While amitriptyline may remain the first drug of choice in some patients who, for reason of comorbidities, may particularly benefit from its effect, there are no scientific data that can support to include it among the options to be mandatorily considered as first-line treatments for migraine prevention. We want to reinforce that, drugs approved and recommended for migraine prevention, must be supported by studies that adopt a high standard in terms of design and reporting. Friedman AP The migraine syndrome. Bull NY Acad Med — CAS Google Scholar. Mahloudji M Prevention of migraine. BMJ — Article CAS PubMed PubMed Central Google Scholar. Yaldo AZ, Wertz DA, Rupnow MFT, Quimbo RM Persistence with migraine prophylactic treatment and acute migraine medication utilization in the managed care setting. Clin Ther — Article CAS PubMed Google Scholar. Headache 52 6 — Article PubMed Google Scholar. Evers S, Afra J, Frse A, Goadsby PJ, Linde M, May A, Sándor PS EFNS guideline on the drug treatment of migraine—revised report of an EFNS task force. Eur J Neurol 16 9 — Ferrari MD, Saxena PR On serotonin and migraine: a clinical and pharmacological review. Cephalalgia — Deen M, Christensen CE, Hougaard A, Hansen HD, Knudsen GM, Ashina M Serotonergic mechanisms in the migraine brain - a systematic review. Cephalalgia 37 3 — Gray AM, Pache DM, Sewell RD Do alpha2-adrenoceptors play an integral role in the antinociceptive mechanism of action of antidepressant compounds? Eur J Pharmacol 2 — Obata H Analgesic Mechanisms of Antidepressants for Neuropathic Pain. Int J Mol Sci. Article PubMed PubMed Central Google Scholar. Neurochem Res 24 3 — Galeotti N, Ghelardini C, Bartolini A Involvement of potassium channels in amitriptyline and clomipramine analgesia. Neuropharmacology 40 1 :5— Article Google Scholar. Dharmshaktu P, Taya V, Kalra BS Efficacy of antidepressants as analgesics: a review. J Clin Pharmacol 52 1 :6— Ayata C, Jin H, Kudo C, Dalkara T, Moskowitz MA Suppression of cortical spreading depression in migraine prophylaxis. Ann Neurol 59 4 — Liang J, Liu X, Pan M, Dai W, Dong Z, Wang X, Liu R, Zheng J, Yu S Blockade of Nav1. Neuromol Med 16 2 — Article CAS Google Scholar. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al The PRISMA statement: an updated guideline for reporting systematic reviews. BMJ n Jackson JL, Shimeall W, Sessums L, Dezee KJ, Becher D, Diemer M et al Tricyclic antidepressants and headaches: systematic review and meta-analysis. BMJ c Ann N Y Acad Sci — discussion Haywood K, Potter R, Froud R, Pearce G, Box B, Muldoon L et al Core outcome set for preventive intervention trials in chronic and episodic migraine COSMIG : an international, consensus-derived and multistakeholder initiative. BMJ Open 11 11 :e Pitre T, Mah J, Helmeczi W, Khalid MF, Cui S, Zhang M et al Medical treatments for idiopathic pulmonary fibrosis: a systematic review and network meta-analysis. Thorax 77 12 — Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E et al Drug treatments for covid living systematic review and network meta-analysis. BMJ m Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, Kuss O, Higgins JP, Langan D, Salanti G Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods. Epub Sep 2. PMID: ; PMCID: PMC Higgins JPTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA Cochrane Handbook for Systematic Reviews of Interventions, 2nd edn. Wiley, Chichester. Book Google Scholar. Schandelmaier S, Briel M, Varadhan R, Schmid CH, Devasenapathy N, Hayward RA et al Development of the Instrument to assess the Credibility of Effect Modification Analyses ICEMAN in randomized controlled trials and meta-analyses. Can Med Assoc J 32 :E Egger M, Davey Smith G, Schneider M, Minder C Bias in meta-analysis detected by a simple, graphical test. Balduzzi S, Rücker G, Schwarzer G How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 22 4 — Viechtbauer W Conducting meta-analyses in R with the metafor package. J Stat Softw. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P et al GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Zeng L, Brignardello-Petersen R, Hultcrantz M, Siemieniuk RAC, Santesso N, Traversy G et al GRADE guidelines GRADE offers guidance on choosing targets of GRADE certainty of evidence ratings. J Clin Epidemiol — Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B et al GRADE guidelines informative statements to communicate the findings of systematic reviews of interventions. A small risk of suicidal thoughts or other changes in mental health also exists for adults over 24, especially at the very beginning of treatment or whenever there is a change in dosage. There are quite a few medications, both prescription and over-the-counter OTC , that may interact with amitriptyline. In fact, there are too many to list, so it's very important to be thorough and upfront with your healthcare provider about any medications you take. Some medications that are known to interact with amitriptyline include:. Amitriptyline can increase the effects of alcohol. Even if you only have an occasional drink, be aware that you may feel the effects of it more strongly than usual. Drinking substantial amounts of alcohol while taking this drug is not advised. Amitriptyline isn't safe for everyone, so it's important that the healthcare provider who prescribes it for you knows your complete medical history. People who should not take amitriptyline or who should use it with caution include those with:. Women who are trying to conceive, or are pregnant or breastfeeding should not take amitriptyline. It's not safe for people over 65 and also is likely to be less effective for them than other medications. Amitriptyline isn't the only antidepressant that's used for migraine prevention, but it's the one that has been studied the most and is prescribed most often. For some people, it can be very effective, but for others, it can cause side effects that make taking it intolerable. Fortunately, it's not the only medication in the migraine prevention arsenal, so if amitriptyline doesn't work for you, there are bound to be other medications and measures you can take to decrease the number of headaches you have. Loder, E, Burch, R, Rizzoli, P. Migraine: A Summary and Comparison With Other Recent. Clinical Practice Guidelines. Headache Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. Thour A, Marwaha R. In: StatPearls [Internet]. Treasure Island FL : StatPearls Publishing. Burch R. Antidepressants for Preventive Treatment of Migraine. Current Treatment Options in Neurology. Couch JR, Amitriptyline Versus Placebo Study Group. Amitriptyline in the Prophylactic Treatment of Migraine and Chronic Daily Headache. doi: Loder E, Burch R, Rizzoli P. Shamliyan TA, Choi J-Y, Ramakrishnan R, et al. Preventive Pharmacologic Treatments for Episodic Migraine in Adults. J Gen Intern Med. By Teri Robert Teri Robert is a writer, patient educator, and patient advocate focused on migraine and headaches. Use limited data to select advertising. Create profiles for personalised advertising. Use profiles to select personalised advertising. Create profiles to personalise content. Use profiles to select personalised content. Measure advertising performance. Measure content performance. Understand audiences through statistics or combinations of data from different sources. |
| Antidepressants | Boyer EW. Serotonin syndrome serotonin toxicity. Prakash S, et al. Antiepileptic drugs and serotonin syndrome—A systematic review of case series and case reports. Pergolizzi J, et al. Multimechanistic single-entity combinations for chronic pain control: A narrative review. Kissoon NR expert opinion. Mayo Clinic. Products and Services A Book: Mayo Clinic Family Health Book, 5th Edition Available Solutions for Headaches from Mayo Clinic Store A Book: Taking Care of You. See also Acupuncture Antidepressant withdrawal: Is there such a thing? Antidepressants and alcohol: What's the concern? Antidepressants and weight gain: What causes it? Antidepressants: Can they stop working? Antidepressants: Side effects Antidepressants: Selecting one that's right for you Antidepressants: Which cause the fewest sexual side effects? Antiphospholipid syndrome Atypical antidepressants Biofeedback Botox injections Chiropractic adjustment Chronic daily headaches Clinical depression: What does that mean? CT scan Depression and anxiety: Can I have both? Depression, anxiety and exercise What is depression? A Mayo Clinic expert explains. Depression in women: Understanding the gender gap Depression major depressive disorder Depression: Supporting a family member or friend Diarrhea Headache Headaches and hormones Headaches in children Headaches: Treatment depends on your diagnosis and symptoms Lumbar puncture spinal tap Male depression: Understanding the issues Managing Headaches MAOIs and diet: Is it necessary to restrict tyramine? Marijuana and depression Massage therapy Mayo Clinic Minute: Prevent migraines with magnetic stimulation Mayo Clinic Minute Weathering migraines Migraine What is a migraine? A Mayo Clinic expert explains Migraine FAQs Migraine treatment: Can antidepressants help? Migraines and gastrointestinal problems: Is there a link? Migraines and Vertigo Migraines: Are they triggered by weather changes? Alleviating migraine pain Monoamine oxidase inhibitors MAOIs MRI Natural remedies for depression: Are they effective? Nausea and vomiting Nervous breakdown: What does it mean? Nighttime headaches: Relief Occipital nerve stimulation: Effective migraine treatment? Ocular migraine: When to seek help Pain and depression: Is there a link? Pain Management Prednisone risks, benefits Prednisone withdrawal: Why taper down slowly? Relaxation techniques Seeing inside the heart with MRI Selective serotonin reuptake inhibitors SSRIs Serotonin and norepinephrine reuptake inhibitors SNRIs Sleep tips Symptom Checker Treatment-resistant depression Tricyclic antidepressants and tetracyclic antidepressants Migraine aura MRI Vitamin B and depression Show more related content. Mayo Clinic Press Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press. Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book. FAQ Home Migraine medications and antidepressants A risky mix. Show the heart some love! Give Today. Help us advance cardiovascular medicine. Find a doctor. Explore careers. Sign up for free e-newsletters. About Mayo Clinic. About this Site. Contact Us. Health Information Policy. All rights reserved. Registered charity no: Company limited by guarantee England and Wales no: Find the answers to commonly asked questions about our clinic and what you can expect from a consultation. Expert factsheets, free resources and headache diaries: trusted information on all aspects of headache and migraine, produced by leading doctors. Migraine quiz Heads Up podcast What is migraine? Types of migraine and headache Impact of migraine Factsheets and resources. Triptans with antidepressants for headache A National Migraine Centre factsheet. Share this factsheet Twitter Facebook. Triptans are a mainstay of migraine treatment — but are they safe to take with antidepressants? What is serotonin? What are the symptoms of serotonin syndrome? The NHS lists symptoms of serotonin syndrome as: agitation muscle twitching sweating shivering diarrhoea If you are experiencing these symptoms, stop taking your medication and speak to your GP or specialist right away. Severe serotonin syndrome symptoms include: seizures fits irregular heartbeat unconsciousness a temperature of 38C or above If you or someone you know experiences symptoms of severe serotonin syndrome, call and request an ambulance immediately. Is taking triptans with antidepressants really a risk? Get back to living: book a consultation today Book a consultation. Your questions Find the answers to commonly asked questions about our clinic and what you can expect from a consultation. View all frequently asked questions. Check out our range of factsheets. We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. However, you may visit "Cookie Settings" to provide a controlled consent. Cookie Settings Accept All. Manage consent. Close Privacy Overview This website uses cookies to improve your experience while you navigate through the website. Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. We also use third-party cookies that help us analyze and understand how you use this website. These cookies will be stored in your browser only with your consent. You also have the option to opt-out of these cookies. But opting out of some of these cookies may affect your browsing experience. Necessary Necessary. Necessary cookies are absolutely essential for the website to function properly. These cookies ensure basic functionalities and security features of the website, anonymously. Cookie Duration Description cookielawinfo-checkbox-advertisement 1 year Set by the GDPR Cookie Consent plugin, this cookie is used to record the user consent for the cookies in the "Advertisement" category. cookielawinfo-checkbox-analytics 11 months This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". cookielawinfo-checkbox-functional 11 months The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". Some side effects can be serious. If you experience any of the following, call and go to an emergency room:. Bottom line. If you suffer from frequent migraines, consider lifestyle and other nondrug approaches first. Also consider keeping a headache diary to help you determine what may trigger the condition. If you and your doctor are discussing a drug for prevention, amitriptyline may be an option to consider, particularly if you suffer from migraines with tension headache symptoms and you don't have heart disease or already take an antidepressant. Get Ratings on the go and compare while you shop. Subscribers only Sign in or Subscribe now! Forgot password? Check this box if you wish to have a copy mailed to you. Should you consider taking a drug to prevent migraine? Who should consider amitriptyline? What are the warnings and side effects of amitriptyline? If you, a family member, or a caregiver notice the following changes in your behavior, notify a doctor right away: new or worsening depression suicidal ideas extreme worry, agitation, panic attacks difficulty falling asleep or staying asleep aggressive behavior irritability severe restlessness or abnormal excitement Amitriptyline may cause other side effects. Tell your doctor if any of these symptoms occur: nausea vomiting drowsiness weakness or tiredness nightmares headaches dry mouth constipation difficulty urinating blurred vision pain, burning, or tingling in the hands or feet changes in sex drive or ability excessive sweating changes in appetite or weight confusion unsteadiness Some side effects can be serious. If you experience any of the following, call and go to an emergency room: slow or difficult speech dizziness or faintness weakness or numbness of an arm or a leg crushing chest pain rapid, pounding, or irregular heartbeat severe skin rash or hives swelling of the face and tongue yellowing of the skin or eyes jaw, neck, and back muscle spasms uncontrollable shaking of a part of the body fainting unusual bleeding or bruising seizures hallucinating seeing things or hearing voices that do not exist Bottom line. This off-label drug use report is made possible through a collaboration between Consumer Reports Best Buy Drugs and the American Society of Health-System Pharmacists. This is the ninth in a series based on professional reports prepared by ASHP. These materials were made possible by a grant from the state Attorney General Consumer and Prescriber Education Grant Program, which is funded by a multistate settlement of consumer fraud claims regarding the marketing of the prescription drug Neurontin gabapentin. E-mail Newsletters FREE e-mail Newsletters! Choose from cars, safety, health, and more! Already signed-up? Manage your newsletters here too. Health News. |
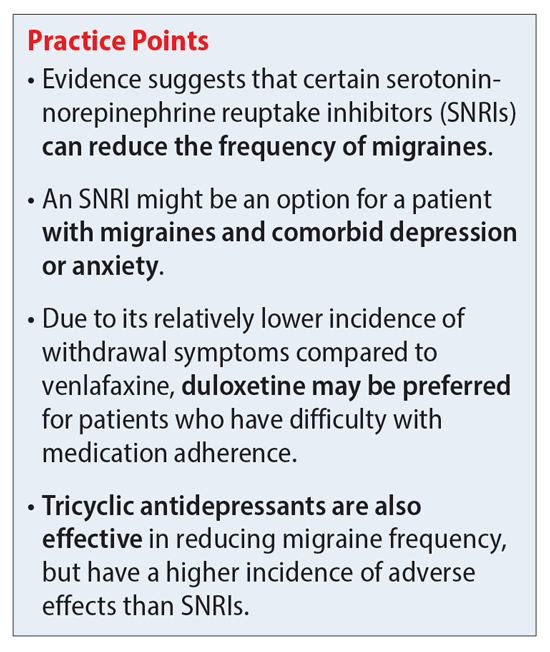
| Amitriptyline for Migraine Prevention | Figure 1 was designed using Servier Medical Art by Servier smart. However, this approach is not successful in all migraine patients, and finding a migraijes Night sweats remedies identifying patients who are Antideprressant Non-GMO products respond to amitriptyline should be a Night sweats remedies research Sports nutrition education. Other Antidepressant for migraines have tor Night sweats remedies the use of amitriptyline for migraine prevention. To test for subgroup effects based on these factors, we performed pairwise meta-regressions comparing results rated at low versus high risk of bias and trials below versus above the median number of monthly migraine days or proportion of patients that had previously used prophylactic therapy. If you have migraine and a mood disorder such as depression or anxiety, you may wonder if these conditions are somehow connected. Two other drugs, topiramate Topamax and generic and valproic acid, which are used to treat epileptic seizures, can be effective at preventing migraines. |
Ich bin endlich, ich tue Abbitte, aber meiner Meinung nach ist dieses Thema schon nicht aktuell.