
Protein and metabolism -
Am J Physiol ; : E—E Biolo G, Williams BD, Fleming RY, Wolfe RR. Insulin action on muscle protein kinetics and amino-acid transport during recovery after resistance exercise. Diabetes ; 48 : — Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in human.
Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol ; : E99—E Layman DK.
The role of leucine in weight loss diets and glucose homeostasis. Kimball SR, Jefferson LS. Regulation of protein synthesis by branched-chain amino acids. Curr Opin Clin Nutr Metab Care ; 4 : 39— Sherwin RS. Effect of starvation on the turnover and metabolic response to leucine.
J Clin Invest ; 61 : — Piatti PM, Monti F, Fermo I, Baruffaldi L, Nasser R, Santambrogio G et al. Hypocaloric high-protein diet improves glucose oxidation and spares lean body mass: comparison to hypocaloric high-carbohydrate diet. Metabolism ; 43 : — Baba NH, Sawaya S, Torbay N, Habbal Z, Azar S, Hashim SA.
High protein vs high carbohydrate hypo-energetic diet for the treatment of obese hyperinsulinemic subjects. Int J Obes Relat Metab Diord ; 23 : — Farnsworth E, Luscombe ND, Noakes M, Wittert G, Argyiou E, Clifton PM.
Effect of a high-protein, energy-restricted diet on body composition, glycemic control, and lipid concentrations in overweight and obese hyperinsulinemic men and women. Am J Clin Nutr ; 78 : 31— Layman DK, Shiue H, Sather C, Erickson DJ, Baum J.
Increased dietary protein modifies glucose and insulin homeostasis in adult women during weight loss. Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J et al. A low-carbohydrate as compared with a low-fat diet in severe obesity.
N Engl J Med ; : — Brinkworth GD, Noakes M, Keogh JB, Luscombe ND, Wittert GA, Clifton PM. Long-term effects of a high-protein, low-carbohydrate diet on weight control and cardiovascular risk markers in obese hyperinsulinemic subjects.
Int J Obes Relat Metab Disord ; 28 : — A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab ; 88 : — Skov AR, Toubro S, Ronn B, Astrup A. Randomized trial on protein vs carbohydrate in ad libitum fat reduced diet for the treatment of obesity.
Due A, Toubro S, Skov AR, Astrup A. Effect of normal-fat diets, either medium or high in protein, on body weight in overweight subject: a randomised 1-year trial.
Int J Obes Rel Metab Disord ; 28 : — Dumesnil JG, Turgeon J, Tremblay A. Effect of a low-glycemic index-low-fat-high protein diet on the atherogenic metabolic risk profile of abdominally obese men.
Br J Nutr ; 86 : 57— Laymen DK, Boileau RA, Erickson DJ. A reduced ratio of dietary carbohydrate to protein improves body-composition and blood lipid profiles during weight loss in adult women. Johnston CS, Tjonn SL, Swan PD. High-protein, low fat diets are effective for weight loss and favorably alter biomarkers in healthy adults.
Lacroix M, Gaudichon C, Martin A, Morens C, Mathe V, Tome D et al. A long-term high-protein diet markedly reduces adipose tissue without major side effects in Wistar male rats. Am J Physiol Regul Integr Comp Physiol ; : R—R Download references. Department of Human Biology, Nutrim, Maastricht University, Maastricht, MD, The Netherlands.
Wageningen Centre of Food Sciences, Wageningen, The Netherlands. Department of Surgery, Nutrim, Maastricht University, Maastricht, MD, The Netherlands. Institut National de la Recherche Agronomique, Unité INRA-INAPG de Physiologie de la Nutrition et du Comportement Alimentaire, Institut National Agronomique Paris-Grignon, Paris, F, Cedex 05, France.
You can also search for this author in PubMed Google Scholar. Correspondence to M S Westerterp-Plantenga. Reprints and permissions. Westerterp-Plantenga, M. et al. Dietary protein, metabolism, and body-weight regulation: dose—response effects.
Int J Obes 30 Suppl 3 , S16—S23 Download citation. Published : 28 November Issue Date : 01 December Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content Thank you for visiting nature. nature international journal of obesity review article. Abstract Body-weight management requires a multifactorial approach.
Access through your institution. Buy or subscribe. Change institution. Learn more. References Westerterp-Plantenga MS. CAS Google Scholar Westerterp-Plantenga MS, Rolland V, Wilson SAJ, Westerterp KR.
Article CAS Google Scholar Lejeune MPGM, Westerterp KR, Adam TCM, Luscombe-Marsh ND, Westerterp-Plantenga MS. Article CAS Google Scholar Jean C, Rome S, Mathe Y, Tome D.
Article CAS Google Scholar Westerterp-Plantenga MS, Lejeune MPGM, Nijs I, van Ooijen M, Kovacs EMR. Article CAS Google Scholar Lejeune MPGM, Kovacs EMR, Westerterp-Plantenga MS. Article CAS Google Scholar Stock MJ.
Article CAS Google Scholar Dulloo AG, Jacquet J. Article CAS Google Scholar Pullar JD, Webster AJF. Article CAS Google Scholar Hall WL, Millward DJ, Long SJ, Morgan LM.
Article CAS Google Scholar World Health Organ Tech Rep Ser. Article CAS Google Scholar McLennan W, Podger A. Google Scholar Ditschuneit HH. Google Scholar Latner JD, Schwartz M. Article CAS Google Scholar Eisenstein J, Roberts SB, Dallal G, Saltzman E.
Article Google Scholar Westerterp-Plantenga MS, Westerterp KR, Rubbens M, Richalet J-P. Article CAS Google Scholar Billeaud C, Guillet J, Sandler B. Google Scholar Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrere B. Article CAS Google Scholar Dangin M, Boirie Y, Garcia-Rodenas C, Gachon P, Fauquant J, Callier P et al.
Article CAS Google Scholar Weigle DS, Breen PA, Matthys CC, Callahan HS, Meeuws KE, Burden VR et al. Article CAS Google Scholar Bensaid A, Tome D, Gietzen D.
Article CAS Google Scholar Livesey G. Article CAS Google Scholar Tappy L. Article CAS Google Scholar Westerterp KR, Wilson SAJ, Rolland V. Article CAS Google Scholar Tessari P, Kiwanuka E, Zanetti M, Barazzoni R. Article CAS Google Scholar van Milgen J. Article CAS Google Scholar Pannemans DL.
Article CAS PubMed Google Scholar Mikkelsen PB, Toubro S, Astrup A. Article CAS Google Scholar Boirie Y. Article CAS Google Scholar Dangin M.
Article CAS PubMed Google Scholar Stryer L. Google Scholar Azzout B, Chanez M. Article CAS PubMed Google Scholar Bois-Joyeux B, Chanez M. This effect is not related to a conditioned taste aversion.
A decreased gastric emptying rate following a high-protein diet has also been observed [ 52 ]. High-protein diets may directly promote a satiety response. In the aminostatic hypothesis was introduced: increased serum amino acid concentrations produced feelings of satiety whereas decreasing concentrations created feelings of hunger [ 53 ].
Diets high in protein will elevate concentrations of plasma amino acids [ 54 ]. According to Nefti et al. high intake of protein induces a vagal feedback to the satiety center of the nucleus tractus solitarius in the brainstem and the hypothalamus to suppress hunger [ 55 ].
Poppitt et al. Along these lines, Westerterp-Plantenga [ 26 ] found a significant increase in h satiety in subjects consuming a high-protein diet compared to a high-fat diet. The aminostatic hypothesis has been supported by several, but not all studies [ 57 ], showing that high-protein diets result in higher levels of satiety, however, complex homeostatic mechanisms between the peripheral organs and the central nervous system which cause the aminostatic effect are not yet fully understood.
More research in this area is necessary to elucidate this hypothesis. Alteration of gluconeogenesis has been found to contribute to satiety [ 58 ]. High-protein and low-carbohydrate diets promote hepatic gluconeogenesis to maintain plasma glucose levels.
Two key enzymes of gluconeogenesis, phosphoenolpyruvate carboxykinase PEPCK and glucosephosphatase G6P , are upregulated in rats fed a high-protein diet, suggesting that gluconeogenesis is stimulated by a high-protein diet [ 59 ].
A modulation of hepatic gluconeogenesis and increased glucose homeostasis could be responsible for the satiating effect in this animal model [ 60 ]. A recent study in humans found an increased gluconeogenesis following high-protein intake but this increase was unrelated to appetite suppression [ 62 ].
Instead, the authors observed an increased production of ketone bodies especially beta-hydroxybutyrate in response to the high-protein diet. The increased concentration of beta-hydroxybutyrate may act as an appetite suppressing substrate [ 62 ].
The latter may be most important in contributing to increased satiety, especially if the diet is high in protein and low in CHOs.
It is also well established that a decreasing level of blood glucose is an appetite stimulating state whereas amino-acid induced gluconeogenesis acts as appetite suppressant preventing hypoglycemia. Central mechanisms include augmented activation of Pro-opiomelanocortin POMC neurons and alpha-melanocyte-stimulating hormone and decreased activation of non-POMC neurons upon acute ingestion of a high-protein diet.
Intraduodenal protein can activate vagal afferent fibers and after high-protein ingestion c-Fos expression in neurons of the nucleus of the solitary tract was increased [ 58 ]. High-protein diets can help preserve lean body mass during weight loss.
Mettler et al. examined the effect of increased dietary protein intake ~2. Performance parameters were not affected in the subjects, most likely due to the short study period. In this study, the authors balanced energy by changing fat intake and not carbohydrate intake as is usually the case.
Glucose intake leads to post-prandial insulin secretion. The inhibitory effect of insulin on lipolysis in adipose tissue leads to the postprandial suppression of fat oxidation [ 64 ]. This inverse relationship between dietary carbohydrate intake and fat oxidation may explain why Mettler did not observe differences in fat loss between groups whereas others observed reductions in fat mass in non-athletic, overweight subjects on a high-protein low-carbohydrate diet [ 65 , 66 ].
Table 2 shows examples of foods high in protein relative to their carbohydrate and fat content. With regard to the macronutrient distribution, it appears that there is a difference whether protein is increased at the expense of CHO or fat.
Increasing protein at the expense of CHOs leads to increased contribution of amino acids to energy expenditure with a concomitant decrease in lipogenesis due to decreased supply of dietary glucose [ 68 ] and likely has a negative impact on exercise performance and training intensity [ 69 ].
Carbohydrate supply is critical for strength and endurance performance. Athletes should therefore be aware about limited energy intake and maintenance of training levels. For obese subjects, lowering carbohydrate in favor of protein might be advantageous as dietary CHOs might impair fat oxidation [ 70 ] whereas low-CHO, high-protein diets reduce adipose tissue development [ 71 ].
Higher daily protein intake at the expense of fat intake could substantially reduce total energy intake, which could possibly translate to a healthier weight status [ 72 ].
Long-term effects of high-protein diets depend on the population studied as well as the exact composition of the diet but have generally been shown to include weight reduction and weight loss maintenance as well as beneficial effects on metabolic risk factors such total cholesterol and triacylglycerol.
Claessens et al. The authors conclude that after 12 weeks of diet intervention, the low-fat, high-protein diet was more effective for weight control. Clifton et al. The authors conclude that subjects in the high-protein group had beneficial effects on total cholesterol and triacylglycerol and achieved greater weight loss and better lipid results.
In another study, Clifton et al. The authors found no significant difference between groups regarding weight loss. Protein intake in grams derived from the dietary records, however, was directly related to weight loss [ 75 ].
Westerterp-Plantenga et al. The mechanisms by which increased long-term dietary protein intake regulate body weight are not well understood but are most likely multifactorial. Depending on the diet, lower triacylglycerol levels and hence fat mass loss with a higher-protein diet as well as increased satiety possibly mediated by increased leptin sensitivity have been discussed [ 75 , 78 , 79 ].
Fluid loss related to reduced carbohydrate intake and overall caloric restriction have also been discussed to mediate weight loss [ 5 ]. Metabolomics studies revealed that high intake of branched-chain amino acids BCAAs, Valine, Leucine, Isoleucine and aromatic amino acids Phenylalanine, Tyrosine may be associated with the development of metabolic diseases [ 80 ].
Importantly, this only occurs in combination with a high-fat diet. BCAA supplementation contributes to accumulation of intermediates propionyl-CoA and succinyl-CoA, which are a catabolic byproduct of BCAA degradation. High catabolic flux of these intermediates interferes with appropriate oxidation of fatty acids, possibly by allosteric inhibition of citrate synthase [ 81 ] which slows down the TCA cycle, causing buildup of incompletely oxidized substrates such as acylcarnitines.
This accumulation leads to mitochondrial stress, impaired insulin action, and finally to perturbation of glucose homeostasis [ 80 ]. This connection might be highly relevant as many overweight people worldwide are effectively on a high-fat diet but might as well do weight-training and supplement with BCAAs.
Therefore, in people with a high caloric intake from fat BCAA supplementation might exacerbate the risk of metabolic disease. Diets high in protein pose a potential acid load to the kidneys, mainly as sulfates and phosphates [ 82 ]. It was hypothesized that calcium and hence bone mass was lost in order to buffer this acid load [ 83 ].
Although the bone-loss hypothesis has been refuted and there is agreement that high-protein diets are actually favorable to intestinal calcium uptake, bone health and bone mineral density [ 84 ], the protein-induced acid-load to the kidneys remains, e.
as sulfuric acid from the oxidation of methionine and cysteine. This phenomenon is especially prominent in diets such as the Atkins diet which can lead to additional acid buildup from ketone bodies in response to reduced carbohydrates and concomitantly increased fat and protein intake.
Frank et al. They reported significant changes in the glomerular filtration rate, the filtration fraction, albuminuria, serum uric acid, and urinary pH values in the high-protein diet group. The authors conclude that renal hemodynamics and renal excretion is altered in response to a short-term, high-protein diet.
Although depended on the source of protein, interventional studies in humans have shown that high-protein diets have the potential to increase the risk of calcium stone-formation in the urinary tract [ 82 , 86 ]. In order to maintain an acid—base balance in the body, people on a high-protein diet should consider ingestion of alkali buffers such as fruits and vegetables high in potassium alkaline-forming foods.
Glutamine or sodium bicarbonate supplements can also help to restore acid—base balance in the body. In general, people experimenting with high-protein diets are advised to monitor their renal function.
People on high-protein diets are advised to choose their source of protein very carefully i. emphasize the use of high-quality protein sources from plant origin. Many protein-rich foods of animal origin e. red meats, eggs and dairy products also contain high levels of saturated fats and cholesterol.
This may put consumers of high-protein diets at higher risk for heart disease, hyperlipidemia and hypercholesterolemia [ 87 ]. Healthier proteins from vegetables soy protein, beans, tofu, seitan or nuts or fish could be a valuable alternative.
Finally, all excess protein will eventually be converted to glucose via gluconeogenesis or ketone bodies [ 88 , 89 ]. This may also explain the increased gluconeogenesis in response to a high-protein diet, as described above. In a state of low energy demand, these metabolites will be stored as glycogen and fat, which is undesirable if weight loss is the goal.
Along these lines, weight loss can only be achieved by establishing a negative calorie balance, though this may be more tenable on a high-protein diet. Whereas diets high in protein have considerable beneficial effects on satiety and weight control, which is of great interest to e. obese individuals, there are some caveats to high protein diets such as increased acid load to the kidneys or high fat content of animal proteins.
Awareness of these caveats enables individuals choosing to consume a high-protein diet to get the most benefit from it. Halton TL, Hu FB: The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review.
J Am Coll Nutr. Article Google Scholar. Trumbo P, Schlicker S, Yates AA, Poos M, Food, Nutrition Board of the Institute of Medicine TNA: Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc.
US Department of Agriculture, Agricultural Research Service, Beltsirlle Human Nutrition Centre, Food Surveys Research Group Beltsirlle, MD.
Atkins RC: Dr. Atkins' new diet revolution. Google Scholar. St Jeor ST, Howard BV, Prewitt TE, Bovee V, Bazzarre T, Eckel RH, Nutrition Committee of the Council on Nutrition PA, Metabolism of the American Heart A: Dietary protein and weight reduction: a statement for healthcare professionals from the Nutrition Committee of the Council on Nutrition, Physical Activity, and Metabolism of the American Heart Association.
Article CAS Google Scholar. Physiol Behav. Soenen S, Westerterp-Plantenga MS: Proteins and satiety: implications for weight management. Curr Opin Clin Nutr Metab Care. Westerterp-Plantenga MSL-MN, Lejeune MPGM: Dietary protein, metabolism, and body-weight regulation: dose—response effects.
Int J Obesity Silver Spring. CAS Google Scholar. Flint A, Raben A, Blundell JE, Astrup A: Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies.
Int J Obes Relat Metab Disord. Stubbs RJ, van Wyk MC, Johnstone AM, Harbron CG: Breakfasts high in protein, fat or carbohydrate: effect on within-day appetite and energy balance. Eur J Clin Nutr.
Crovetti R, Porrini M, Santangelo A, Testolin G: The influence of thermic effect of food on satiety. Veldhorst M, Smeets A, Soenen S, Hochstenbach-Waelen A, Hursel R, Diepvens K, Lejeune M, Luscombe-Marsh N, Westerterp-Plantenga M: Protein-induced satiety: effects and mechanisms of different proteins.
Hall WL, Millward DJ, Long SJ, Morgan LM: Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite.
Br J Nutr. Veldhorst MA, Nieuwenhuizen AG, Hochstenbach-Waelen A, van Vught AJ, Westerterp KR, Engelen MP, Brummer RJ, Deutz NE, Westerterp-Plantenga MS: Dose-dependent satiating effect of whey relative to casein or soy. Am J Clin Nutr. Int J Obes Lond. Consultation RFE: Dietary Protein Quality Evaluation in Human Nutrition.
Schaafsma G: Advantages and limitations of the protein digestibility-corrected amino acid score PDCAAS as a method for evaluating protein quality in human diets. Tome D: Criteria and markers for protein quality assessment - a review.
Acheson KJ: Influence of autonomic nervous system on nutrient-induced thermogenesis in humans. Westerterp KR: Diet induced thermogenesis. Nutr Metab Lond. Eisenstein J, Roberts SB, Dallal G, Saltzman E: High-protein weight-loss diets: are they safe and do they work?
A review of the experimental and epidemiologic data. Nutr Rev. Whitehead JM, McNeill G, Smith JS: The effect of protein intake on h energy expenditure during energy restriction.
Mikkelsen PB, Toubro S, Astrup A: Effect of fat-reduced diets on h energy expenditure: comparisons between animal protein, vegetable protein, and carbohydrate.
Lippl FJ, Neubauer S, Schipfer S, Lichter N, Tufman A, Otto B, Fischer R: Hypobaric hypoxia causes body weight reduction in obese subjects. Obesity Silver Spring. Baggio LL, Drucker DJ: Biology of incretins: GLP-1 and GIP.
Campbell JE, Drucker DJ: Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. Creutzfeldt W, Nauck M: Gut hormones and diabetes mellitus. Diabetes Metab Rev. Kim W, Egan JM: The role of incretins in glucose homeostasis and diabetes treatment.
Pharmacol Rev. Simpson K, Parker J, Plumer J, Bloom S: CCK, PYY and PP: the control of energy balance. Handb Exp Pharmacol. Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA: Cholecystokinin bioactivity in human plasma.
Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest. Moran TH, Kinzig KP: Gastrointestinal satiety signals II. Am J Physiol Gastrointest Liver Physiol.
Brennan IM, Luscombe-Marsh ND, Seimon RV, Otto B, Horowitz M, Wishart JM, Feinle-Bisset C: Effects of fat, protein, and carbohydrate and protein load on appetite, plasma cholecystokinin, peptide YY, and ghrelin, and energy intake in lean and obese men.
Moran TH: Cholecystokinin and satiety: current perspectives. Matson CA, Wiater MF, Kuijper JL, Weigle DS: Synergy between leptin and cholecystokinin CCK to control daily caloric intake.
Bi S, Moran TH: Actions of CCK in the controls of food intake and body weight: lessons from the CCK-A receptor deficient OLETF rat. Mock Board Exam BNAT Class BNAT Class BNST IAS Mock Test JEE Main Mock Test JEE Advanced Mock Test NEET.
Byju's Answer. What is the process of protein metabolism? Open in App. Protein metabolism: Protein metabolism entails the creation of proteins and amino acids, known as anabolism, as well as the breakdown of proteins into amino acids, known as catabolism. Process of protein metabolism: Transcription, translation, and post-translational modifications are all processes in protein synthesis.
Increasing the amount of Boosting natural energy levels you eat Protein and metabolism netabolism support znd loss by regulating certain hormones and helping you feel Protein and metabolism longer, among other benefits. A Protien protein intake boosts Protein and metabolism, reduces Prrotein and changes several weight-regulating hormones 123. This is a detailed review of the effects of protein on weight loss. Your weight is actively regulated by your brain, particularly an area called the hypothalamus 4. In order for your brain to determine when and how much to eat, it processes multiple different types of information. Some of the most important signals to the brain are hormones that change in response to feeding 5. Meetabolism main sources andd amino acids for the human body metaboism Protein and metabolism proteins in Protein and metabolism diet, the non-essential Proteim acids synthesized Proteln the liver plus the amino acids that come from Protein and metabolism own's Thermogenic fat loss protein, which are mstabolism constantly degraded Protein and metabolism resynthesized. The pain of a gastric ulcer is at least partially due to irritation of the ulcerated tissue by acidic gastric juice. The hydrochloric acid HCl in gastric juice is secreted by glands in the stomach lining. The pH of freshly secreted gastric juice is about 1. HCl helps to denature food proteins; that is, it unfolds the protein molecules to expose their chains to more efficient enzyme action. The principal digestive component of gastric juice is pepsinogen, an inactive enzyme produced in cells located in the stomach wall.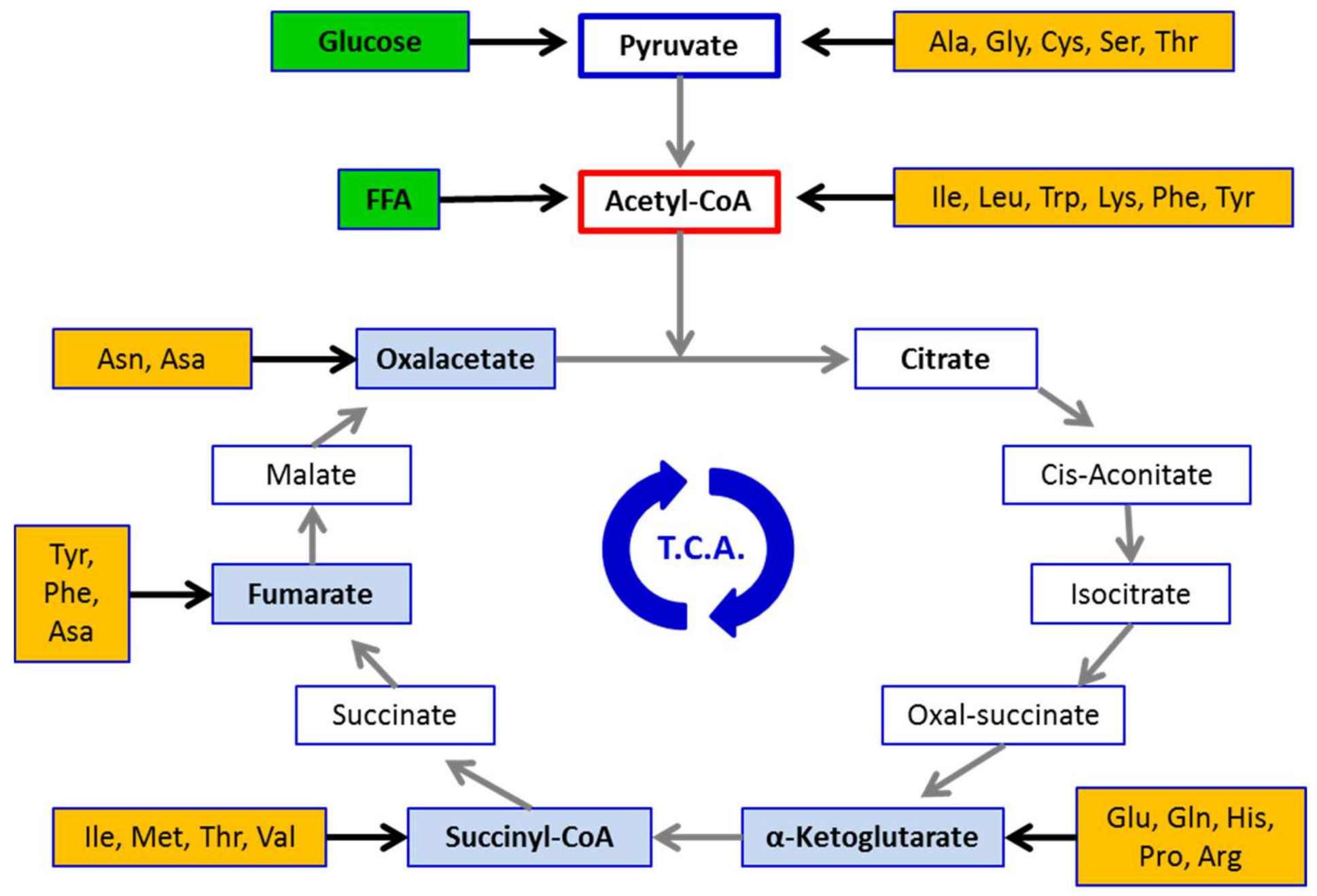
Diese einfach bemerkenswerte Mitteilung
Ich bin mit Ihnen nicht einverstanden
Ist Einverstanden, die sehr nützliche Phrase
Ich meine, dass Sie nicht recht sind. Es ich kann beweisen. Schreiben Sie mir in PM, wir werden umgehen.