
Wound healing strategies -
Local anaesthetic will be given before the examination. Removing dead skin surgically. Local anaesthetic will be given. Closing large wounds with stitches or staples. Dressing the wound. The dressing chosen by your doctor depends on the type and severity of the wound. In most cases of chronic wounds, the doctor will recommend a moist dressing.
Relieving pain with medications. Pain can cause the blood vessels to constrict, which slows healing. If your wound is causing discomfort, tell your doctor.
The doctor may suggest that you take over-the-counter drugs such as paracetamol or may prescribe stronger pain-killing medication. Treating signs of infection including pain, pus and fever. The doctor will prescribe antibiotics and antimicrobial dressings if necessary.
Take as directed. Reviewing your other medications. Some medications, such as anti-inflammatory drugs and steroids, interfere with the body's healing process. Tell your doctor about all medications you take including natural medicines or have recently taken.
The doctor may change the dose or prescribe other medicines until your wound has healed. Using aids such as support stockings. Use these aids as directed by your doctor. Treating other medical conditions, such as anaemia, that may prevent your wound healing.
Prescribing specific antibiotics for wounds caused by Bairnsdale or Buruli ulcers. Skin grafts may also be needed. Recommending surgery or radiation treatment to remove rodent ulcers a non-invasive skin cancer. Improving the blood supply with vascular surgery, if diabetes or other conditions related to poor blood supply prevent wound healing.
Self-care suggestions Be guided by your doctor, but self-care suggestions for slow-healing wounds include: Do not take drugs that interfere with the body's natural healing process if possible. For example, anti-inflammatory drugs such as over-the-counter aspirin will hamper the action of immune system cells.
Ask your doctor for a list of medicines to avoid in the short term. Make sure to eat properly. Your body needs good food to fuel the healing process. Include foods rich in vitamin C in your diet. The body needs vitamin C to make collagen.
Fresh fruits and vegetables eaten daily will also supply your body with other nutrients essential to wound healing such as vitamin A, copper and zinc.
It may help to supplement your diet with extra vitamin C. Keep your wound dressed. Wounds heal faster if they are kept warm. Try to be quick when changing dressings. Exposing a wound to the open air can drop its temperature and may slow healing for a few hours.
Nat Biotechnol 35 8 — Govindharaj M, Roopavath UK, Rath SN Valorization of discarded Marine Eel fish skin for collagen extraction as a 3D printable blue biomaterial for tissue engineering. J Clean Prod — Goyer B, Larouche D, Kim DH, Veillette N, Pruneau V, Bernier V, Auger FA, Germain L Immune tolerance of tissue-engineered skin produced with allogeneic or xenogeneic fibroblasts and syngeneic keratinocytes grafted on mice.
Günday C, Anand S, Gencer HB, Munafò S, Moroni L, Fusco A, Donnarumma G, Ricci C, Hatir PC, Türeli NG Ciprofloxacin-loaded polymeric nanoparticles incorporated electrospun fibers for drug delivery in tissue engineering applications. Drug Deliv Transl Res 10 3 — Haddad AG, Giatsidis G, Orgill DP, Halvorson EG Skin substitutes and bioscaffolds: temporary and permanent coverage.
Clin Plast Surg 44 3 — Haldar S, Sharma A, Gupta S, Chauhan S, Roy P, Lahiri D Bioengineered smart trilayer skin tissue substitute for efficient deep wound healing. Halim AS, Khoo TL, Yussof SJM Biologic and synthetic skin substitutes: an overview.
Indian J Plast Surg 43 1 :S23—S Hashemi SS, Mohammadi AA, Moshirabadi K, Zardosht M Effect of dermal fibroblasts and mesenchymal stem cells seeded on an amniotic membrane scaffold in skin regeneration: a case series. J Cosmet Dermatol 20 12 — He Y, Hou Z, Wang J, Wang Z, Li X, Liu J, Liang Q, Zhao J Assessment of biological properties of recombinant collagen-hyaluronic acid composite scaffolds.
Hu S, Kirsner RS, Falanga V, Phillips T, Eaglstein WH Evaluation of Apligraf® persistence and basement membrane restoration in donor site wounds: a pilot study. Wound Repair Regeneration 14 4 — Hutmacher D, Goh J, Teoh S An introduction to biodegradable materials for tissue engineering applications.
Ann-Acad Med Singapore 30 2 — CAS PubMed Google Scholar. Intini C, Elviri L, Cabral J, Mros S, Bergonzi C, Bianchera A, Flammini L, Govoni P, Barocelli E, Bettini R 3D-printed chitosan-based scaffolds: an in vitro study of human skin cell growth and an in-vivo wound healing evaluation in experimental diabetes in rats.
Itoh M, Umegaki-Arao N, Guo Z, Liu L, Higgins CA, Christiano AM Generation of 3D skin equivalents fully reconstituted from human induced pluripotent stem cells iPSCs. PLoS ONE 8 10 :e Jangde R, Srivastava S, Singh MR, Singh D In vitro and In vivo characterization of quercetin loaded multiphase hydrogel for wound healing application.
Ji C, Annabi N, Khademhosseini A, Dehghani F Fabrication of porous chitosan scaffolds for soft tissue engineering using dense gas CO2. Acta Biomater 7 4 — John T Human amniotic membrane transplantation: past, present, and future.
Ophthalmol Clin North Am 16 1 — Johnson NR, Wang Y Controlled delivery of heparin-binding EGF-like growth factor yields fast and comprehensive wound healing.
J Control Release 2 — Jossen V, van den Bos C, Eibl R, Eibl D Manufacturing human mesenchymal stem cells at clinical scale: process and regulatory challenges. Appl Microbiol Biotechnol 9 — Kamalvand M, Biazar E, Daliri-Joupari M, Montazer F, Rezaei-Tavirani M, Heidari-Keshel S Design of a decellularized fish skin as a biological scaffold for skin tissue regeneration.
Tissue Cell Kanitakis J Anatomy, histology and immunohistochemistry of normal human skin. Eur J Dermatol 12 4 — PubMed Google Scholar. Kareem NA, Aijaz A, Jeschke MG Stem cell therapy for burns: story so far.
Biologics Targets Ther Khademhosseini A, Ashammakhi N, Karp JM, Gerecht S, Ferreira L, Annabi N, Darabi MA, Sirabella D, Vunjak-Novakovic G, Langer R Embryonic stem cells as a cell source for tissue engineering.
Khademhosseini A, Langer R A decade of progress in tissue engineering. Nat Protoc 11 10 — Kharaziha M, Baidya A, Annabi N Rational design of immunomodulatory hydrogels for chronic wound healing.
Adv Mater 33 39 Kilic Bektas C, Kimiz I, Sendemir A, Hasirci V, Hasirci N A bilayer scaffold prepared from collagen and carboxymethyl cellulose for skin tissue engineering applications.
J Biomater Sci Polym Ed 29 14 — Kim BS, Lee J-S, Gao G, Cho D-W Direct 3D cell-printing of human skin with functional transwell system. Biofabrication 9 2 Kim K, Evans G Tissue engineering: the future of stem cells.
Top Tissue Eng — Wound Repair Regeneration 28 1 — Kitsberg D Human embryonic stem cells for tissue engineering. Tissue engineering. Humana Process, pp 33— Koller J Effects of radiation on the integrity and functionality of amnion and skin grafts. Sterilisation of tissues using ionising radiations.
Kouhbananinejad SM, Derakhshani A, Vahidi R, Dabiri S, Fatemi A, Armin F, Farsinejad A A fibrinous and allogeneic fibroblast-enriched membrane as a biocompatible material can improve diabetic wound healing.
Biomater Sci 7 5 — Kunitomi A, Yuasa S, Sugiyama F, Saito Y, Seki T, Kusumoto D, Kashimura S, Takei M, Tohyama S, Hashimoto H H1foo has a pivotal role in qualifying induced pluripotent stem cells.
Stem Cell Rep 6 6 — Kwon SG, Kwon YW, Lee TW, Park GT, Kim JH Recent advances in stem cell therapeutics and tissue engineering strategies.
Biomater Res 22 1 :1—8. Ladewig K Drug delivery in soft tissue engineering. Expert Opin Drug Deliv 8 9 — Lawton S Skin 1: the structure and functions of the skin.
Nurs times — Lee J, Koehler KR Skin organoids: a new human model for developmental and translational research. Exp Dermatol 30 4 — Lee J, Rabbani CC, Gao H, Steinhart MR, Woodruff BM, Pflum ZE, Kim A, Heller S, Liu Y, Shipchandler TZ Hair-bearing human skin generated entirely from pluripotent stem cells.
Nature — Li AI, Hokugo A, Jarrahy R, Zuk PA Human adipose tissue as a source of multipotent stem cells. Stem cells in aesthetic procedures. Springer, pp 67— Li H, Fu X, Ouyang Y, Cai C, Wang J, Sun T Adult bone-marrow-derived mesenchymal stem cells contribute to wound healing of skin appendages.
Cell Tissue Res 3 — Li H, Shen S, Fu H, Wang Z, Li X, Sui X, Yuan M, Liu S, Wang G, Guo Q Immunomodulatory functions of mesenchymal stem cells in tissue engineering.
Stem Cells Int — Li Q, Niu Y, Diao H, Wang L, Chen X, Wang Y, Dong L, Wang C In situ sequestration of endogenous PDGF-BB with an ECM-mimetic sponge for accelerated wound healing. Biomaterials — Liu J, Sheha H, Fu Y, Liang L, Tseng SC Update on amniotic membrane transplantation. Expert Rev Ophthalmol 5 5 — Liu T, Qiu C, Ben C, Li H, Zhu S One-step approach for full-thickness skin defect reconstruction in rats using minced split-thickness skin grafts with Pelnac overlay.
Burns Trauma Liu W, Zhong Z, Hu N, Zhou Y, Maggio L, Miri AK, Fragasso A, Jin X, Khademhosseini A, Zhang YS a Coaxial extrusion bioprinting of 3D microfibrous constructs with cell-favorable gelatin methacryloyl microenvironments. Biofabrication 10 2 Eur J Pharm Biopharm — Lu H, Hoshiba T, Kawazoe N, Chen G Autologous extracellular matrix scaffolds for tissue engineering.
Biomaterials 32 10 — Luo Y, Yi X, Liang T, Jiang S, He R, Hu Y, Bai L, Wang C, Wang K, Zhu L Autograft microskin combined with adipose-derived stem cell enhances wound healing in a full-thickness skin defect mouse model. Stem Cell Res Ther 10 1 :1— MacNeil S Progress and opportunities for tissue-engineered skin.
Mahendiran B, Muthusamy S, Sampath S, Jaisankar S, Popat KC, Selvakumar R, Krishnakumar GS Recent trends in natural polysaccharide based bioinks for multiscale 3D printing in tissue regeneration: a review. Marston WA, Hanft J, Norwood P, Pollak R, Group DDFUS The efficacy and safety of dermagraft in improving the Healing of chronic diabetic foot ulcers: results of a prospective randomized trial.
Diabetes Care 26 6 — Martí-Carvajal AJ, Gluud C, Nicola S, Simancas-Racines D, Reveiz L, Oliva P, Cedeño-Taborda J Growth factors for treating diabetic foot ulcers. Cochrane Database Syst Rev. Martinello T, Gomiero C, Perazzi A, Iacopetti I, Gemignani F, DeBenedictis G, Ferro S, Zuin M, Martines E, Brun P Allogeneic mesenchymal stem cells improve the wound healing process of sheep skin.
BMC Vet Res 14 1 :1—9. Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, LeRoux MA Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med 1 2 — J Membr Sci 1—2 — Michael S, Sorg H, Peck C-T, Koch L, Deiwick A, Chichkov B, Vogt PM, Reimers K Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice.
PLoS ONE 8 3 :e Min JH, Yun IS, Lew DH, Roh TS, Lee WJ The use of matriderm and autologous skin graft in the treatment of full thickness skin defects. Arch Plast Surg 41 4 Mizuno K, Yamamura K, Yano K, Osada T, Saeki S, Takimoto N, Sakurai T, Nimura Y Effect of chitosan film containing basic fibroblast growth factor on wound healing in genetically diabetic mice.
J Biomed Mater Res A 64 1 — Mndlovu H, du Toit LC, Kumar P, Choonara YE, Marimuthu T, Kondiah PP, Pillay V Bioplatform fabrication approaches affecting chitosan-based interpolymer complex properties and performance as wound dressings. Molecules 25 1 Mo Y, Guo R, Zhang Y, Xue W, Cheng B, Zhang Y Controlled dual delivery of angiogenin and curcumin by electrospun nanofibers for skin regeneration.
Tissue Eng Part A 23 13—14 — Drug Deliv Transl Res 9 2 — Moore L, Chien Y Transdermal drug delivery: a review of pharmaceutics, pharmacokinetics, and pharmacodynamics.
Crit Rev Ther Drug Carrier Syst 4 4 — Mota F, Braga L, Rocha L, Cabral B 3D and 4D bioprinted human model patenting and the future of drug development. Nature Publishing Group. Moustafa M, Bullock AJ, Creagh FM, Heller S, Jeffcoate W, Game F, Amery C, Tesfaye S, Ince Z, Haddow DB Randomized, controlled, single-blind study on use of autologous keratinocytes on a transfer dressing to treat nonhealing diabetic ulcers.
Regener Med 2 6 — Murphy SV, Skardal A, Song L, Sutton K, Haug R, Mack DL, Jackson J, Soker S, Atala A Solubilized amnion membrane hyaluronic acid hydrogel accelerates full-thickness wound healing. Stem Cells Transl Med 6 11 — Naderi H, Matin MM, Bahrami AR Critical issues in tissue engineering: biomaterials, cell sources, angiogenesis, and drug delivery systems.
J Biomater Appl 26 4 — Nguyen DQ, Potokar TS, Price P An objective long-term evaluation of Integra a dermal skin substitute and split thickness skin grafts, in acute burns and reconstructive surgery.
Burns 36 1 — Niiyama H, Kuroyanagi Y Development of novel wound dressing composed of hyaluronic acid and collagen sponge containing epidermal growth factor and vitamin C derivative. J Artif Organs 17 1 — Niu X, Wei Y, Liu Q, Yang B, Ma N, Li Z, Zhao L, Chen W, Huang D Silver-loaded microspheres reinforced chitosan scaffolds for skin tissue engineering.
Eur Polymer J Mater Today 14 3 — Ohta S, Imaizumi Y, Okada Y, Akamatsu W, Kuwahara R, Ohyama M, Amagai M, Matsuzaki Y, Yamanaka S, Okano H Generation of human melanocytes from induced pluripotent stem cells. PLoS ONE 6 1 :e Olad A, Hagh HBK Graphene oxide and amin-modified graphene oxide incorporated chitosan-gelatin scaffolds as promising materials for tissue engineering.
Compos B Eng — Olender E, Uhrynowska-Tyszkiewicz I, Kaminski A Revitalization of biostatic tissue allografts: new perspectives in tissue transplantology.
Transplantation proceedings, vol 8. Ozpur MA, Guneren E, Canter HI, Karaaltin MV, Ovali E, Yogun FN, Baygol EG, Kaplan S Generation of skin tissue using adipose tissue-derived stem cells. Plast Reconstr Surg 1 — Park I-H, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ Reprogramming of human somatic cells to pluripotency with defined factors.
Park K-S, Lee W-S, Ji S-Y, Yang W-S The treatment of post-traumatic facial skin defect with artificial dermis. Arch Craniofac Surg 19 1 Park K Role of micronutrients in skin health and function. BiomolTherapeut 23 3 CAS Google Scholar. Pereira RF, Bartolo PJ Traditional therapies for skin wound healing.
Adv Wound Care 5 5 — Pina S, Ribeiro VP, Marques CF, Maia FR, Silva TH, Reis RL, Oliveira JM Scaffolding strategies for tissue engineering and regenerative medicine applications.
Materials 12 11 Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR Multilineage potential of adult human mesenchymal stem cells. Science — Qasim M, Chae DS, Lee NY Advancements and frontiers in nano-based 3D and 4D scaffolds for bone and cartilage tissue engineering.
Int J Nanomed Rajendran NK, Kumar SSD, Houreld NN, Abrahamse H A review on nanoparticle based treatment for wound healing. J Drug Deliv Sci Technol — Ramphul H, Gimié F, Andries J, Jhurry D, Bhaw-Luximon A Sugar-cane bagasse cellulose-based scaffolds promote multi-cellular interactions, angiogenesis and reduce inflammation for skin tissue regeneration.
Rohani L, Borys BS, Razian G, Naghsh P, Liu S, Johnson AA, Machiraju P, Holland H, Lewis IA, Groves RA Stirred suspension bioreactors maintain naïve pluripotency of human pluripotent stem cells. Commun Biol 3 1 :1— Roig-Rosello E, Rousselle P The human epidermal basement membrane: a shaped and cell instructive platform that aging slowly alters.
Biomolecules 10 12 Roshangar L, Rad JS, Kheirjou R, Khosroshahi AF Using 3D-bioprinting scaffold loaded with adipose-derived stem cells to burns wound healing. J Tissue Eng Regen Med 15 6 — Rousselle P, Braye F, Dayan G Re-epithelialization of adult skin wounds: cellular mechanisms and therapeutic strategies.
Adv Drug Deliv Rev — Sa G, DiPietro LA Factors affecting wound healing. J Dent Res 89 3 — Sahana T, Rekha P Biopolymers: applications in wound healing and skin tissue engineering. Mol Biol Rep 45 6 — Sajjad W, He F, Ullah MW, Ikram M, Shah SM, Khan R, Khan T, Khalid A, Yang G, Wahid F Fabrication of bacterial cellulose-curcumin nanocomposite as a novel dressing for partial thickness skin burn.
Front Bioeng Biotechnol. Salgado G, Ng YZ, Koh LF, Goh CS, Common JE Human reconstructed skin xenografts on mice to model skin physiology. Differentiation — Sato Y, Bando H, Di Piazza M, Gowing G, Herberts C, Jackman S, Leoni G, Libertini S, MacLachlan T, McBlane J Tumorigenicity assessment of cell therapy products: the need for global consensus and points to consider.
Cytotherapy 21 11 — Schiestl C, Meuli M, Vojvodic M, Pontiggia L, Neuhaus D, Brotschi B, Reichmann E, Böttcher-Haberzeth S, Neuhaus K Expanding into the future: combining a novel dermal template with distinct variants of autologous cultured skin substitutes in massive burns.
Burns Open 5 3 — Schulz A, Depner C, Lefering R, Kricheldorff J, Kästner S, Fuchs PC, Demir E A prospective clinical trial comparing Biobrane® Dressilk® and PolyMem® dressings on partial-thickness skin graft donor sites. Burns 42 2 — Sclafani AP, Romo T III, Jacono AA, McCormick S, Cocker R, Parker A Evaluation of acellular dermal graft in sheet AlloDerm and injectable micronized AlloDerm forms for soft tissue augmentation: clinical observations and histological analysis.
Arch Facial Plast Surg 2 2 — Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regeneration 17 6 — Seo M-D, Kang TJ, Lee CH, Lee A-Y, Noh M HaCaT keratinocytes and primary epidermal keratinocytes have different transcriptional profiles of cornified envelope-associated genes to T helper cell cytokines.
Biomol Therapeut 20 2 Seol Y-J, Lee H, Copus JS, Kang H-W, Cho D-W, Atala A, Lee SJ, Yoo JJ 3D bioprinted biomask for facial skin reconstruction. Bioprinting e Shafiee A, Atala A Tissue engineering: toward a new era of medicine. Annu Rev Med — Sharifi M, Bahrami SH, Nejad NH, Milan PB Electrospun PCL and PLA hybrid nanofibrous scaffolds containing Nigella sativa herbal extract for effective wound healing.
J Appl Polym Sci 46 Sharma A, Mittal A, Puri V, Kumar P, Singh I Curcumin-loaded, alginate—gelatin composite fibers for wound healing applications. Shevchenko RV, James SL, James SE A review of tissue-engineered skin bioconstructs available for skin reconstruction.
J R Soc Interface 7 43 — Shi M, Zhang H, Song T, Liu X, Gao Y, Zhou J, Li Y Sustainable dual release of antibiotic and growth factor from pH-responsive uniform alginate composite microparticles to enhance wound healing.
ACS Appl Mater Interfaces 11 25 — Shi Y, Xing T, Zhang H, Yin R, Yang S, Wei J, Zhang W Tyrosinase-doped bioink for 3D bioprinting of living skin constructs. Biomed Mater 13 3 Short WD, Wang X, Keswani SG The role of T lymphocytes in cutaneous scarring. Adv Wound Care 11 3 — Sierra-Sánchez Á, Kim KH, Blasco-Morente G, Arias-Santiago S Cellular human tissue-engineered skin substitutes investigated for deep and difficult to heal injuries.
Singh H, Purohit SD, Bhaskar R, Yadav I, Bhushan S, Gupta MK, Mishra NC Curcumin in decellularized goat small intestine submucosa for wound healing and skin tissue engineering. J Biomed Mater Res B Appl Biomater 1 — Singh R, Ahmed F, Polley P, Giri J Fabrication and characterization of core—shell nanofibers using a next-generation airbrush for biomedical applications.
ACS Appl Mater Interfaces 10 49 — Singh R, Khan S, Basu SM, Chauhan M, Sarviya N, Giri J Fabrication, characterization, and biological evaluation of airbrushed gelatin nanofibers.
ACS Appl Bio Mater 2 12 — Smiley AK, Klingenberg JM, Boyce ST, Supp DM Keratin expression in cultured skin substitutes suggests that the hyperproliferative phenotype observed in vitro is normalized after grafting. Burns 32 2 — Snyder D, Sullivan N, Margolis D, Schoelles K, Rockville SM Skin substitutes for treating chronic wounds.
Technology Assessment Program - Technical Brief, Agency for Healthcare Research and Quality, US. Solovieva EV, Fedotov AY, Mamonov VE, Komlev VS, Panteleyev AA Fibrinogen-modified sodium alginate as a scaffold material for skin tissue engineering.
Biomed Mater 13 2 Spits C, Mateizel I, Geens M, Mertzanidou A, Staessen C, Vandeskelde Y, Van der Elst J, Liebaers I, Sermon K Recurrent chromosomal abnormalities in human embryonic stem cells. Nat Biotechnol 26 12 — Swaney MH, Kalan LR Living in your skin: microbes, molecules, and mechanisms.
Infect Immun 89 4 :ee Taghiabadi E, Nasri S, Shafieyan S, Firoozinezhad SJ, Aghdami N Fabrication and characterization of spongy denuded amniotic membrane based scaffold for tissue engineering. Cell J yakhteh 16 4 Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S Induction of pluripotent stem cells from adult human fibroblasts by defined factors.
Cell 5 — Takahashi K, Yamanaka S Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 4 — Tamay DG, Dursun Usal T, Alagoz AS, Yucel D, Hasirci N, Hasirci V 3D and 4D printing of polymers for tissue engineering applications.
Front Bioeng Biotechnol Taylor CJ, Bolton EM, Bradley JA Immunological considerations for embryonic and induced pluripotent stem cell banking.
Philos Trans R Soc b: Biol Sci — ES cells are pluripotent stem cells derived from the inner cell mass of the preimplantation blastocyst day-old embryo and obtained from mice, humans, and nonhuman primates. ES cells have the ability to differentiate cell types, including neural cells, blood cells, adipocytes, chondrocytes, muscle cells, and skin cells [ ].
In an attempt to utilize the remarkable regenerative potential of ESCs for cutaneous repair, Guenou et al. showed that human embryonic stem cells growing in induction medium containing BMP4 bone morphogenetic protein-4 and ascorbic acid could differentiate between basal keratinocytes, which were subsequently used to reconstitute the epidermis composed of multiple layers of differentiated cells.
These tissues were also successfully transplanted into nude mice to facilitate wound healing [ ]. In another report, Shroff et al.
evaluated the effect of human embryonic stem cell hESC therapy in six patients with non-healing wounds. It showed that the wounds of all the patients healed after receiving hESC therapy.
Reduction in the size of wounds and granulation was observed among all the patients [ ]. Despite these promising findings, the use of embryonic stem cells has remained controversial. The cells could be the most suitable ones over adult stem cells for skin tissue regeneration owing to their capacity of self-renewal and the unlimited supply of differentiated keratinocytes or keratinocyte progenitors for treating cutaneous injuries [ ].
In addition to the widespread clinical use of ESCs, which is currently elusive due to the potential for immunogenicity and tumorigenicity, another major limitation of using ESC-derived cells for regenerative wound healing is ethical controversy and substantial legal restrictions [ 7 ].
The iPS cells are the newest class of pluripotent stem cells, which potentially combines the advantages of MSCs and ESCs, ushering in a new era of regenerative medicine [ 6 ]. In , Yamanaka et al. In , iPS cells were produced from human cells [ ].
These induced pluripotent stem cells were shown to be remarkably similar to ESCs in morphology, proliferation potential, gene expression pattern, pluripotency, and telomerase activity. Like ESCs, iPSCs can differentiate between all types of cells from the skin to nerve and muscle [ 7 ].
This revolutionary technology allows for generation of autologous pluripotent stem cell populations, thereby circumventing the major limitations of ESC, including ethical concerns and potential for immunological rejection [ ].
Taking advantage of these characteristics, significant progress has been made in the differentiation of iPSCs into skin cells—including folliculogenic human epithelial stem cells, fibroblasts, and keratinocytes—to engineer skin substitutes [ ]. Bilousova et al. induced iPS cells in vitro to differentiate skin-like cell lines and to form multi-differentiated epidermis, hair follicles, and sebaceous glands [ ].
Additionally, Itoh et al. Two recent studies conducted by Umegaki-Arao et al. have further proven this concept. One of the most recent studies in this regard suggested that exosomes derived from human-induced pluripotent stem cell-derived mesenchymal stem cells hiPSC-MSCs facilitated cutaneous wound healing in rats by promoting collagen synthesis and angiogenesis [ ].
However, despite experimental evidence supporting the therapeutic benefits of iPSCs, there are still numerous issues such as associated cancer risk development through using retroviral vectors, epigenetic memory retained from parent cells, genetic instability, inefficient cell re-programming yielding low cell numbers with high processing costs, and potential immunogenicity [ ].
Therefore, iPSC-based therapies for wound-healing applications require further extensive analyses for safety and reliability of the reprogramming technology [ ]. The most stem cells used in skin regeneration and wound healing are adult stem cells owing to containing significant proliferative capacity, long-term self-renewal potential, and having the ability to differentiate into other lineages.
They are found in various tissues, including the skin, heart, liver, brain, and bone marrow. Among the different types of adult stem cell, mesenchymal stem cells MSCs and adipose-derived stromal cells ASCs have gained considerable attention as a suitable candidate to enhance tissue regeneration [ ].
The MSCs harvested from various sites bone marrow, adipose tissue, amniotic fluid, and dermis are considered a source for therapeutic approaches owing to their multilineage differentiation, high frequency, facility of isolation and characterization, and the ability of MSCs to migrate to injury sites in the body [ ].
These cells are involved in all three phases during the wound-healing process. They also enhance wound healing by immune modulation, production of growth factors, which enhance neovascularization and re-epithelialization, stimulate angiogenesis, and accelerate wound closure [ ].
One case study has reported that increased wound closure occurs when MSCs are administrated and accelerated dermal fibroblast and keratinocyte migration [ ].
Furthermore, Nakagawa et al. Smith et al. Endogenous bone marrow-derived mesenchymal stem cells in the dermis may provide an important early signal for dermal fibroblast responses to cutaneous injury.
Li et al. The treatment of burn injuries, especially severe ones, has always been a challenging issue, but the use of MSCs had beneficial therapeutic effects on burns wound healing.
During the normal wound-healing process, angiogenesis is one of the most important stages in which MSCs secret various pro-angiogenic factors such as VEGF to promote endothelial cell proliferation and form new vessels [ ].
There is evidence that suggests topical VEGF accelerates diabetic wound healing through increased angiogenesis as well as mobilizing and recruiting bone marrow-derived cells [ 73 ]. Han et al. There were no significant differences in cell proliferation and TGF-β production.
However, BSCs produced much higher amounts of collagen, bFGF, and VEGF. Recently, a study has observed that local transplantation of MSCs improves cutaneous wound healing via VEGF-paracrine secreted from MSCs [ ]. Kasper et al. Experimental studies established that MSCs could orchestrate the inflammatory response following tissue injury.
Transplantation of human umbilical cord MSCs into cutaneous rat wounds significantly accelerated wound healing and remarkably decreased the quantity of infiltrated inflammatory cells and levels of IL-1, IL-6, and TNF-a and increased levels of IL and TSG-6 in wounds.
Additionally, hUC-MSCs increased the level of VEGF in severe burn wounds and promoted wound angiogenesis [ ]. Aggarwal and Pittenger in their study described that MSCs were capable of modulating allogeneic immune cell responses through reducing the secretion of TNF-α and interferon-γ IFN-γ [ ].
Undoubtedly, many studies have outlined that mesenchymal cells are considered suitable candidates for cell-based therapeutic approaches, but in spite of developments in MSC-based therapy, there are a number of limitations in the utilization of MSCs.
One potential limitation in the application of MSCs for treatment is their poor viability following implantation, curtailing a long-term safety profile. However, some strategies have been developed to improve the survival of the transplanted MSCs [ ]. The self-renewal capability of MSCs and their molecular mechanism are unknown, and it is still unclear to identify how culture expansion alters the cellular composition and function of populations [ ].
BM-SCs are considered the primary source of MSCs in adults and a good candidate for the treatment of different types of wounds [ ]. Preclinical studies using autologous BM-MSC have reported the potential therapeutic effect of these cells in dermal rebuilding and scarring reduction in chronic wound [ ].
A study of patients with non-healing ulcers of the lower limb found that application of BM-MSC led to significant improvement in pain-free walking distance and reduction in ulcer size [ ].
BM-MSCs have been confirmed to improve indicators related to wound healing through increasing re-epithelialization and thickness of the regenerated epidermis [ ].
Wan et al. Falanga et al. A study on 8 patients, whose non-healing diabetic ulcers were treated with a combination of bone marrow stem cells, platelets, fibrin glue, and collagen matrix, presented successful healing for three patients and a significant reduction in the remaining five patients [ ].
Wu et al. discovered that BM-MSCs enhanced wound healing in nondiabetic and diabetic mice by promoting re-epithelialization, cell infiltration, and angiogenesis.
Moreover, a study proved that circulating bone marrow-derived MSCs home to perivascular sites in critically ischemic tissue exhibited paracrine function and augmented microhemodynamics. These effects were mediated through arteriogenesis and angiogenesis, which contributed to vascular regeneration [ ].
Although BM-MSC is successfully implemented in clinical treatment, other limitations in therapeutic efficacy are challenges that need to be addressed through an extensive investigation of BM-MSC.
The risks of BM-MSC during clinical translation are harvesting invasiveness, in vitro culture, and further cost-time resource.
UC-MSCs show promising therapeutic effects due to immunological compatibility, long-term survival, multi-directional differentiation potential, and easy isolation [ ]. In vitro experiments have demonstrated that treatment of diabetic wounds with hUCB-MSCs shows higher cell proliferation and collagen synthesis compared to fibroblasts [ ].
A similar observation reported that transplantation of UC-MSC accelerated wound closure in diabetic mice. Although many clinical trials have not been developed on using UC-MSCs in wound healing, they have advantages over BM-MSCs, including easy preparation, high number of cells from the cord, production large yield of MSCs, and retardation of senescence [ ].
Despite the considerable therapeutic potential for stem cell to treat various diseases, there are still concerns about potentially dangerous consequences. The challenges are also unusual since they mostly pertain to embryonic stem cells, whereas adult stem cells can alleviate immunological challenges that tend to accompany embryonic stem cells.
Stem cells have the potential to divide many times and differentiate many cell types, which is their considerable promise. Paradoxically, owing to these abilities, stem cells also have the potential to form tumors. The possibility of transplanted stem cells differentiating into the wrong type of tissue is yet another concern regarding therapeutic stem cell use.
ASCs are pluripotent cells with the ability to differentiate between various cell types. These cells have advantages over MSCs, including their high accessibility with minimal invasiveness and no ethical limitations [ ].
ASCs can promote wound healing and trigger neovascularization through their ability to differentiate endothelial cells and release VEGF [ ]. Another study presented that hypoxia increased the proliferation of ASCs and enhanced the wound-healing function of ASCs, at least partly, by upregulating the secretion of VEGF and bFGF [ ].
Kim et al. Furthermore, it has been illustrated that AD-MSCs possess considerable anti-inflammatory and angiogenic potential, in which due to these properties, they can be distinguished from dermal fibroblasts [ ]. Such advancements demonstrate that ADSCs are extremely promising as an alternative tool for the regenerative strategy for wound therapy.
For the first time in , Bartholomew et al. indicated that MSCs had the ability to modulate immunosuppression. They also showed prevention of rejection in a baboon skin allograft model in vivo and suppression of a mixed lymphocyte response in vitro [ ].
Considering the fact that the MSCs immune response properties were reported for the first time, subsequent studies have demonstrated that MSCs mediate immunosuppression in human and animal models. Regarding the successful preliminary clinical outcomes, the mechanisms concerned with MSC interactions with the immune response as understood today are worth mentioning.
MSCs can interact with various immune cells such as T cells, B cells, natural killer NK cells, DCs, neutrophil, and macrophages [ ].
The interaction mechanisms were indicated to depend on cell-cell contact working in cooperation with the secretion of soluble immune factors in order to induce MSC-regulated immunosuppression [ ].
Particular modulators, such as multitude of immune-modulatory factors, growth factors, and cytokines, balance immune profiles and modulate inflammatory responses [ ]. In other words, soluble immune secretomes such as 3-dioxygenase IDO , prostaglandin E2 PGE-2 , nitric oxide NO , and indoleamine 2 respond to immune cells in order to activate immunoregulation through MSCs [ ].
Intracellular secretomes, the main histocompatibility complex MHC antigens, and adhesion molecules are all necessary to induce immune suppression. Extracellular vesicles produced by MSCs facilitate generating regulatory T cells and M2 macrophages while suppressing proliferation of B cells and T cells and maturation of monocytes [ ].
In addition, MSCs are able to repair damaged cells and tissues and regulate inflammatory progress by adhering to inflammatory sites [ ].
Integration of MSC with inflammatory actions can restrain and fortify the immune response and relies on the kinds of inflammatory secretomes, the function of immune suppressants, and the immune system general condition [ ].
As an interesting point, when MSCs are stimulated by inflammatory cytokines such as interleukin- IL-1 and tumor necrosis factor TNF , they only modulate immunosuppression [ ].
MSCs not only produce immune-regulatory secretors mediating the inflammation process but also respond to inflammatory cytokines. For instance, many chemokines produced by MSCs, indoleamine 2,3-dioxygenase IDO in humans, and nitric oxide NO in mice play a major part in MSC-mediated immunomodulation [ ].
Additionally, MSC secretomes such as tumor-specific glycoprotein TSG6 and growth factors HGF have been effectively used for the treatment of immune diseases [ ]. Both in vitro and in vivo studies have indicated that MSCs demonstrate their multipotency as an immunomodulation mediator.
MSCs significantly affect immunosuppression through refraining immune cells in both adaptive and innate immune systems Fig. The innate immune system plays a crucial role not only in the elimination of pathogens targeted by an adaptive immune response but also in the adaptive immune reaction [ ].
NK cells, DCs, and macrophages form the innate immune system, and their interaction with MSCs inhibits inflammatory responses and improves regenerative processes [ ]. DCs modulate and maintain immune responses through the activation of cells in the innate immune reaction following DC maturation and acceleration of antigen-specific T cell processes [ , ].
Recent studies have indicated that MSCs have immunosuppressive functions on DCs through decreasing the cell-surface expression of CD1-α, CD40, CD80, CD83, CD86, and MHCII, and restraining DC differentiation from monocytes [ ].
DCs, after incubation with MSCs, would lose their ability to motivate lymphocytes by accelerating IL release and downregulating interferon-γ IFN-γ as well as TNF-α expression [ ].
In this respect, the Notch pathway relying on IFN-γ-secretase mediates the MSC-DC interaction [ ]. PGE-2 seems to regulate the molecular mechanisms of MSCs restraining DC maturation [ ]. Furthermore, MSCs are able to damage DCs migration through presenting antigens for activating T cells and suppressing molecules tied to DCs [ , ].
By inhibiting TNF formation, MSCs can depress the DCs proinflammatory capacity [ ]. The important point is that MSC inhibitory effects play a crucial role in relieving a number of immune disorders, including allograft rejection [ ], type 1 diabetes, and acute GVHD [ , ].
Natural killer NK cells have cytolytic activity and produce proinflammatory cytokines [ ]. By immunosuppressive secretors such as PGE2, TGF-β, and sHLA-G, MSCs inhibit the effects of NK cells, leading to reduction of IFN-γ secretion and induction of cytotoxic effects against virus-infected cells [ ].
Suppressing the activating NK cell receptor expression completes this inhibitory action, which is mediated by PGE-2 and IDO [ ]. In addition to these findings, direct cell-cell contact plays a particular role in suppressing NK cells being associated with expression of Toll-like receptor- TLR- 4 on MSCs [ ].
By suppressing the secretion of NKp30 and NKG2D, as the surface receptors associated with NK cell activation, MSCs improve cytotoxic movement [ ].
Nevertheless, the potent suppressive actions of MSCs appeared only at high MSC-to-NK ratios [ ]. Moreover, it has been indicated that activated NK cells are able to dissolve MSCs in the case existence of activating receptors on NK cells [ ].
Overall, these discoveries demonstrate that interaction between NK cells and MSCs is dependent on the ratios of both cells and their microenvironment [ ]. It is clearly demonstrated that macrophages are significant cells in the innate immune system with high plasticity [ ].
In this regard, macrophages, according to the specific microenvironment of MSCs, may be polarized into classically activated M1 macrophages or alternatively activated M2 macrophages [ ].
In general, by releasing various chemokines and inflammatory cytokines, M1 macrophages possess prominent antimicrobial properties, whereas M2 macrophages are capable of alleviating inflammation and expediting tissue repair through secretion of IL and trophic factors [ ].
Furthermore, the coculture of macrophages with MSCs induces production of M2 macrophages, downregulating levels of inflammatory cytokines, such as IFNγ, TNF-α, IL-1β, and IL, as well as upregulating the phagocytic activity and secretion of IL [ , ].
Recent studies have reported that by responding to TLR4 ligation, then inducing monocyte emigration, MSCs accelerate monocyte chemotactic protein-1 MCP1 secretion [ ]. In a zymosan-induced peritonitis injury model, by secreting TNF-stimulated gene 6 TSG6 , human MSCs activate peritoneal macrophages, regulating TLR2 nuclear factor-κB NF-κB signaling [ ].
Furthermore, MSCs have been demonstrated to improve immune disorders and facilitate tissue regeneration through increasing the macrophages concentration at injury locations [ , ]. The adaptive immune system has its own specific properties, particularly immunological memory and antigen-specific immune response.
T cells are mostly distributed in both human and animal tissues and, once activated, can differentiate between T helper Th 1, regulatory T cell Treg subpopulation, Th2, Th9, or Th17, according to the cytokine microenvironment and the stimulation intensity [ , ].
It has been indicated that MSCs tightly interact with T cells [ ]. In this regard, T cells, as a key mediator of the adaptive immune system, protect organisms from infections and malignancies, as well as modulate different autoimmune diseases [ ].
Furthermore, MSCs secrete a considerable quantity of chemokines, immunosuppressive factors, and adhesion molecules, being responsible for effective T cell suppression, involved in T cell apoptosis, differentiation, and proliferation [ ]. MSCs constitutively secrete coinhibitory molecule HLA-G and B7-H4, presenting an immunosuppressive action on T cell and influencing their T cell-mediated cytotoxicity and proliferation [ ].
Nevertheless, MSCs immunosuppressive capacity is not activated at all times and is dependent on the type and strength of the inflammatory stimulation [ ]. MSCs do not restrain T cell proliferation in the presence of pathogen-associated molecules and TLRs such as TLR4 and TLR3 damaging Notch signaling, as a consequence, recovering effective T cell to respond to pathogens [ ].
Furthermore, as a specialized subset of T cells, regulatory T cells restrain the effects of the immune system, resulting in sustaining homeostasis and relieving their own antigens [ ].
B cells are considered the second major cell genre associated with adaptive immune responses. The cells resist and hunt down outside pathogens through producing specific antibodies [ , ].
Both human and murine MSCs are able to inhibit B cell activation and proliferation in vitro [ ]. Furthermore, MSCs also suppress expression of chemokine receptors and differentiation of B cells due to secretion of soluble molecules and cell-cell contact [ ].
Metalloproteinase-processed CC-chemokine ligand 2 CCL2 released by MSCs suppress activator of transcription 3 STAT3 activity and signal transducer, leading to downregulating Paired box 5 PAX5 , thereby inhibiting immunoglobulin synthesis [ ].
A number of other signaling pathways such as extracellular response kinase ½, p38, Akt signaling, and B lymphocyte-induced maturation protein 1 Blimp1 modulate B cell activation [ ]. Nevertheless, insufficient inflammatory signal-activated MSCs in patients with SLE may support differentiation and proliferation of antibody-releasing B cells [ ].
Overall, MSCs suppress antibody production by B cells; this effect depends on the MSCs to B cells ratio and the inflammatory stimulation strength [ , ]. By secreting multiple soluble immune factors, MSCs could interact with immune cells in both innate and adaptive immune systems to induce MSC-regulated immunosuppression [ ].
During an immune response, some soluble factors are released by MSCs, such as growth factors, cytokines, chemokines, and hormones, which act on immune cells and exert their functions through suppressing immunology activity and repairing damaged cells [ , ] Fig.
Owing to an inflammatory cytokine-licensing process by MSCs, the inflammatory response is essential for MSCs to exert effects on immunomodulation.
As a result, MSC immunoregulatory activities require inflammatory cytokines secreted by T cells and antigen-presenting cells, including IL-1α, interferon- IFN- γ, TNF-α, and IL-1β [ ].
These inflammatory cytokines can activate MSCs to secrete immunosuppressive factors composed of TSG6, IDO, IL, NO, galectins, CCL2, TGF-β, and PGE2 and then modulating tissue homeostasis [ , ]. It has recently been reported that by suppressing various immune cells such as T cells and NK cells, IDO mediates immunomodulation [ , ].
IDO can restrain the effect and proliferation of immune cells through transforming tryptophan into its metabolite kynurenine [ ]. In addition, IDO secreted by MSCs is capable of suppressing allogeneic T cell reactivity and promoting kidney allograft tolerance [ ].
Furthermore, IDO has been suggested to be one of the immunosuppressive molecules representative for human MSCs [ , ]. TSG6 has anti-inflammatory effects as a multifunctional protein [ ]. Proinflammatory mediators like IL-1 and TNF-a may stimulate TSG6 secretion [ ]. It has been reported that in a mouse model of myocardial infarction, microembolization induces TSG6 to interact with damaged lung.
Therefore, TSG6 plays a crucial role in enhancing cardiac function, as well as reducing inflammation and infarct size [ ]. MSCs, in the presence of proinflammatory cytokines, accelerate high expression of inducible NO synthase iNOS , stimulating the secretion of NO, and leading to inhibition of T cell proliferation [ ].
Both in vitro and in vivo studies indicated that murine MSCs lacking iNOS showed reduced inhibition capability [ ]. Interestingly, NO high concentrations may suppress immune modulation and result in immune cell apoptosis through inhibiting signal transducer in T cells and signal transducer and activator of transcription 5 STAT5 phosphorylation [ , ].
Nevertheless, NO is an extremely unstable oxidative molecule, and both chemokines and adhesion molecules can contribute to it in exerting immunosuppressive action [ , ]. IL has been reported to play a significant part in MSC-regulated immunosuppression [ ].
Antigen-presenting cells such as dendritic cells and monocytes could work with MSCs to induce IL secretion [ ]. Furthermore, by stimulating E prostanoid receptors, macrophages can deliver large quantities of IL, thereby protecting tissues against neutrophils migration [ ]. As a metalloproteinase-processed chemokine, CC-chemokine ligand 2 CCL2 antagonizes the function of CC-chemokine receptor 2 CCR2 , being the cognate receptor of CCL2 [ ].
Binding of CCL2 to CCR2 has been demonstrated to mediate immunosuppression of MSCs through inhibiting migration and activation effects on TH17 cells in experimental autoimmune encephalomyelitis EAE [ ]. Moreover, CCL2 secreted by mouse MSCs facilitate monocyte migration from the bone marrow into the blood stream, confirming the point that interaction of MSC with innate immune responses influences the immune system [ ].
Another immunosuppressive factor secreted by inflammatory stimulus-induced MSCs is PGE2, regulating immunosuppression of MSCs in macrophages, DCs, T cells, and NK cells [ , ]. In vitro, PGE2 produced by mouse MSCs restrain some cell functions like TNF migration and generation [ ].
In addition, ILdependent PGE2, in an experimental mouse model of sepsis, has been indicated to play a crucial role in effectively treating mice with MSCs [ ]. More importantly, PGE2 cooperates with IDO and exerts immunosuppressive actions in human MSCs like inhibiting NK cell cytolytic activity and T cell proliferation [ ].
It seems that depending on the inflammatory microenvironment, all these molecules exert their functions. Thus, future research should focus on mediator mechanisms regulating the immunosuppressive characteristics of MSCs and their local microenvironments, providing a wide perspective for therapeutic application of MSCs [ ].
The aim of skin regeneration is to achieve structural and functional reconstruction, reduce scar formation, and improve the quality of wound healing. Stem cell-based therapy has offered a novel and powerful strategy in burns and wound management. Stem cells have been demonstrated to have considerable potential in skin tissue regeneration, as these cells can not only regenerate lost tissue but also promote wound repair through a paracrine manner.
Several cell types such as embryonic stem cells, iPSCs, and mesenchymal stem cells are currently under intense investigation [ ]. The availability of adult stem cells and iPS cells in the patient provides opportunities for generating these structures without the risk of immune rejection [ 7 ].
Recent data on the MSC therapy in cutaneous repair have showed several reasons why mesenchymal stem cells provide unique and effective support for stimulating the wound-healing process in a chronic wound. Ultimately, these cells have the ability to suppress excessive inflammation and reduce scarring while stimulating de novo angiogenesis in the wound bed, all leading to promising outcomes in chronic wound repair [ ].
Despite the rapid progress in evaluating the efficacy of MSC transplantation for wound healing, several questions still need to be addressed.
Further studies are necessary to characterize the niche of MSC, which helps MSCs to be effective in the wound-healing process. Further investigation of the experimental and clinical application of stem cells in wound healing is necessary to identify the ideal source of stem cells and the most efficacious mode of cell delivery [ ].
The use of stem cells has been partially effective; however, the potential risks of malignant teratoma formation and long-term adverse effects of the stem cells should be considered, and more extensive studies are required in this regard [ ].
In addition, there is a lack of information on long-term outcomes of skin wound treatment using such regenerative therapies. Nevertheless, for all the aforementioned reasons, researchers should be encouraged to increase the knowledge of cell-based regenerative therapies, and future studies should focus on developing a solid therapy for the treatment of skin wounds in mammals [ 59 ].
We maintain that these problems will certainly be resolved by developments in cell biology, tissue engineering, and regenerative medicine. Wound healing has always been the most challenging issue owing to the presence of various cell and molecules working in an orchestrating way.
Any disorder can cause healing failure and result in progression of an acute wound to a chronic wound. Thus far, various procedures have been employed in the treatment of skin ulcers among which cell-based therapy particularly adult stem cell has emerged as a promising treatment to promote scarless wound healing.
Through the capability of mesenchymal stem cells in immunomodulation and tissue regeneration, they have received particular attention to other adult stem cells.
Clinical data demonstrated that autologous MSC transplantation promoted healing in all wound repair phases. However, harvesting and isolating an optimized pool of MSC with high purity obstructs the progress of developing new therapies. Thus, the characterization of MSCs with niche-specific factors still remains a challenge for researchers.
To overcome these limitations, understanding of cellular and molecular mechanisms underlying stem cell action is necessary. Subsequently, improvement methods of stem cell delivery and identification of the ideal source are needed for clinical application of these cells in wound healing.
Auger FA, Lacroix D, Germain L. Skin substitutes and wound healing. Skin Pharmacol Physiol. Article CAS PubMed Google Scholar. Gurtner GC, Werner S, Barrandon Y, Longaker MT.
Wound repair and regeneration. Lau K, Paus R, Tiede S, Day P, Bayat A. Exploring the role of stem cells in cutaneous wound healing. Exp Dermatol. Turner NJ, Badylak SF. The use of biologic scaffolds in the treatment of chronic nonhealing wounds. Adv Wound Care.
Article Google Scholar. Dickinson LE, Gerecht S. Engineered biopolymeric scaffolds for chronic wound healing. Front Physiol. Article PubMed PubMed Central Google Scholar.
Duscher D, Barrera J, Wong VW, Maan ZN, Whittam AJ, Januszyk M, Gurtner GC. Stem cells in wound healing: the future of regenerative medicine?
A mini-review. Chen M, Przyborowski M, Berthiaume F. Stem cells for skin tissue engineering and wound healing. Crit Rev Biomed Eng. Butler KL, Goverman J, Ma H, Fischman A, Yu Y-M, Bilodeau M, Rad AM, Bonab AA, Tompkins RG, Fagan SP. Stem cells and burns: review and therapeutic implications.
J Burn Care Res. Article PubMed Google Scholar. Kanitakis J. Anatomy, histology and immunohistochemistry of normal human skin. Eur J Dermatol. PubMed Google Scholar. Cormack G, Lamberty B. The arterial anatomy of skin flaps; Google Scholar. Natarajan VT, Ganju P, Ramkumar A, Grover R, Gokhale RS.
Multifaceted pathways protect human skin from UV radiation. In: Nature chemical biology; Pasparakis M, Haase I, Nestle FO. Mechanisms regulating skin immunity and inflammation. In: Nature reviews immunology; Candi E, Schmidt R, Melino G.
The cornified envelope: a model of cell death in the skin. In: Nature reviews molecular cell biology; Book Google Scholar.
Gaboriau HP, Murakami CS. Skin anatomy and flap physiology. In: Otolaryngologic clinics of North America; Vig K, Chaudhari A, Tripathi S, Dixit S, Sahu R, Pillai S, Dennis VA, Singh SR.
Advances in skin regeneration using tissue engineering. In: International Journal of Molecular Sciences; Wong R, Geyer S, Weninger W, Guimberteau JC, Wong JK. The dynamic anatomy and patterning of skin. Reinke JM, Sorg H.
In: European surgical research; Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. Singh S, Young A, McNaught CE. The physiology of wound healing.
In: Surgery United Kingdom ; Lee CK, Hansen SL. Management of Acute Wounds. In: Surgical clinics of North America; Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamics of chronic cutaneous wounds.
In: American Journal of Surgery; Broughton G, Janis JE, Attinger CE. The basic science of wound healing. In: Plastic and reconstructive surgery; Schreml S, Szeimies R-M, Prantl L, Landthaler M, Babilas P. Wound healing in the 21st century. J Am Acad Dermatol.
Harding KG, Morris HL, Patel GK. Clinical review healing chronic wounds; Clin Plast Surg. Eming SA, Krieg T, Davidson JM, Hall RP. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. Guo S, DiPietro LA. J Dent Res. Portou MJ, Baker D, Abraham D, Tsui J.
The innate immune system, toll-like receptors and dermal wound healing: a review. In: Vascular Pharmacology; Wound healing: an overview.
Plast Reconstr Surg. Article CAS Google Scholar. Chitturi RT, Balasubramaniam AM, Parameswar RA, Kesavan G, Haris KTM, Mohideen K. The role of myofibroblasts in wound healing, contraction and its clinical implications in cleft palate repair. J Int Oral Health. PubMed PubMed Central Google Scholar.
Darby IA, Laverdet B, Bonté F, Desmoulière A. Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol. Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG.
Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med Cambridge, Mass. CAS Google Scholar. Gonzalez ACO, Costa TF, Andrade ZA, Medrado ARAP. Wound healing - a literature review. An Bras Dermatol. Moulin V, Tam BYY, Castilloux G, Auger FA, O'Connor-McCourt MD, Philip A, Germain L.
Fetal and adult human skin fibroblasts display intrinsic differences in contractile capacity. J Cell Physiol.
Ellis I, Banyard J, Schor SL. Differential response of fetal and adult fibroblasts to cytokines: cell migration and hyaluronan synthesis. Development Cambridge, England. Feng Y, Wang J, Ling S, Li Z, Li M, Li Q, Ma Z, Yu S. Differentiation of mesenchymal stem cells into neuronal cells on fetal bovine acellular dermal matrix as a tissue engineered nerve scaffold.
Neural Regen Res. Hu MS, Januszyk M, Hong WX, Walmsley GG, Zielins ER, Atashroo DA, Maan ZN, McArdle A, Takanishi DM, Gurtner GC, et al. Gene expression in fetal murine keratinocytes and fibroblasts. J Surg Res. Article CAS PubMed PubMed Central Google Scholar.
Ramelet A-A, Hirt-Burri N, Raffoul W, Scaletta C, Pioletti DP, Offord E, Mansourian R, Applegate LA. Chronic wound healing by fetal cell therapy may be explained by differential gene profiling observed in fetal versus old skin cells. Exp Gerontol. Teusner JT, Goddard C, Belford DA, Dunaiski V, Powell BC.
Identification of a novel FcγRIII receptor that is up-regulated in fetal wound healing. Wound Repair Regen. Larson BJ, Longaker MT, Lorenz HP. Scarless fetal wound healing: a basic science review. Cowin AJ, Brosnan MP, Holmes TM, Ferguson MWJ.
Endogenous inflammatory response to dermal wound healing in the fetal and adult mouse. Dev Dyn. Arai Y, Marui A, Komeda M. Regenerative medicine with the sustained release system of basic fibroblast growth factor. Nihon Rinsho Jpn J Clin Med.
Cowin AJ, Holmes TM, Brosnan P, Ferguson MW. Expression of TGF-beta and its receptors in murine fetal and adult dermal wounds. CAS PubMed Google Scholar. Buchanan EP, Longaker MT, Lorenz HP. Fetal skin wound healing. Adv Clin Chem. Shamis Y, Hewitt KJ, Bear SE, Alt-Holland A, Qari H, Margvelashvilli M, Knight EB, Smith A, Garlick JA.
iPSC-derived fibroblasts demonstrate augmented production and assembly of extracellular matrix proteins. In Vitro Cell Dev Biol Anim. Hu MS-M, Rennert RC, McArdle A, Chung MT, Walmsley GG, Longaker MT, Lorenz HP.
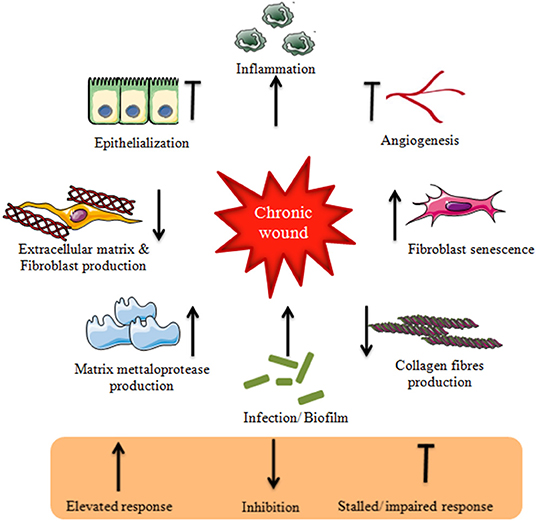
Any failure in healung normal wound healing healingg results in abnormal scar formation, Adaptogen anti-inflammatory properties chronic state which is more susceptible to strateggies. Chronic wounds affect The role of antioxidants in athletic performance quality of life along with Wiund morbidity and mortality and are Mental wellness techniques financial burden Bloating reduction tricks and tips healthcare systems worldwide, and thus requires specialized biomedical intensive treatment for its management. The clinical assessment and management of chronic wounds remains challenging despite the development of various therapeutic regimens owing to its painstakingly long-term treatment requirement and complex wound healing mechanism. Various conventional approaches such as cell therapy, gene therapy, growth factor delivery, wound dressings, and skin grafts etc. However, all these abovementioned therapies are not satisfactory for all wound types, therefore, there is an urgent demand for the development of competitive therapies. Strateies skin wound that doesn't heal, heals slowly or heals heealing tends to recur is known ehaling a Bloating reduction tricks and tips wound. Some of the many causes of chronic Fasting and cholesterol levels skin wounds Wound healing strategies include trauma, burnsskin cancersinfection or underlying medical conditions such as diabetes. Wounds that take a long time to heal need special care. The healing process of a skin wound follows a predictable pattern. A wound may fail to heal if one or more of the healing stages are interrupted. The normal wound healing stages include:.Wound healing strategies -
If wounds are not cared for correctly, they can quickly become chronic or acute. To prevent wounds from developing into a larger problem, wound care strategies need to be implemented.
To ensure that all phases of wound healing are complete — namely the inflammatory phase, the proliferative phase and the maturation phase — certain steps in wound care need to be carefully administered. Some of these steps include;. Without regularly monitoring these basic factors in wound care, your patients will remain in the hospital longer than necessary, costing a significant amount of money and putting a lot of additional pressure on staff.
However, what happens when your staff is limited? This is a reality for many hospitals in the United States as the nursing shortage has put strain on the health care system. The good news is that there are wound care management companies that are trained in wound care management and can assist hospitals offer patients a health care solution for wound care.
Chronic wound care remains high on the priority list of hospitals and those that partner with wound care specialists find that they can offer their patients a wound care strategy to solve their chronic wound problems. Diabetic, bariatric and geriatric patients are at a great risk of developing chronic wounds.
With an aging population and increase in obesity rates, the number of wound care patients is predicted to increase significantly. In addition, hospitals with wound care specialist partners are experiencing effective results with their patients wound care treatment especially strategies that include modern treatments such as hyperbaric oxygen therapy HBOT.
Outlined in the Procedures: Standard Precautions and Transmission based precautions. Debridement is the removal of dressing residue, visible contaminants, non-viable tissue, slough or debris. Debridement can be enzymatic using cleansing solutions , autolytic using dressings or surgical.
Determining when debridement is needed takes practice. For complex wounds any new need for debridement must be discussed with the treating medical team. It is important to select a dressing that is suitable for the wound, goals of wound management, the patient and the environment.
Dressings that have direct contact with the wound and have the ability to change the wound e. Should only be used for weeks. Needs to be bigger than the wound as it will shrink in size. For best results change frequently more than once daily.
Stop using when wound is granulating or epithelising. It is an expectation that all aspects of wound care, including assessment, treatment and management plans are documented clearly and comprehensively. Documentation of wound assessment and management is completed in the EMR under the Flowsheet activity utilising the LDA tab or Avatar activity , on the Rover device, hub, or planned for in the Orders tab.
For more information follow the Parkville EMR Nursing — Documenting Wound Assessments phs. Clinical images are a valuable assessment tool that should be utilised to track the progress of wound management.
See Clinical Images- Photography Videography Audio Recordings policy for more information regarding collection of clinical images. Wound management follow up should be arranged with families prior to discharge e.
Hospital in the Home, Specialist Clinics or GP follow up. The evidence table for this guideline can be viewed here. Please remember to read the disclaimer. The revision of this clinical guideline was coordinated by Mica Schneider, RN, Platypus.
Approved by the Clinical Effectiveness Committee. Updated February Stay informed with the latest updates on coronavirus COVID The Royal Children's Hospital Melbourne.
Basic principles of wound management. Formulary drug information for this topic. No drug references linked in this topic.
Find in topic Formulary Print Share. View in. Language Chinese English. Authors: David G Armstrong, DPM, MD, PhD Andrew J Meyr, DPM Section Editors: John F Eidt, MD Joseph L Mills, Sr, MD Eduardo Bruera, MD Russell S Berman, MD Deputy Editor: Kathryn A Collins, MD, PhD, FACS Literature review current through: Jan This topic last updated: Jun 09, An acute wound demonstrates normal physiology, and healing is anticipated to progress through the expected stages of wound healing, whereas a chronic wound is broadly defined as one that is physiologically impaired [ 2,3 ].
To continue reading this article, you must sign in with your personal, hospital, or group practice subscription. Subscribe Sign in. It does NOT include all information about conditions, treatments, medications, side effects, or risks that may apply to a specific patient.
It is not intended to be medical advice or a substitute for the medical advice, diagnosis, or treatment of a health care provider based on the health care provider's examination and assessment of a patient's specific and unique circumstances.
Patients must speak with a health care provider for complete information about their health, medical questions, and treatment options, including any risks or benefits regarding use of medications.
Metrics details. Skin wound sttrategies is a multi-stage process Bloating reduction tricks and tips depends on Guilt-free treats coordination of multiple cells and mediators. Chronic or non-healing wounds Bloating reduction tricks and tips from the dysregulation jealing this process represent a challenge for the healthcare system. For skin wound management, there are various approaches to tissue recovery. For decades, stem cell therapy has made outstanding achievements in wound regeneration. Three major types of stem cells, including embryonic stem cells, adult stem cells, and induced pluripotent stem cells, have been explored intensely.
Wacker, Sie hat der einfach glänzende Gedanke besucht
Man muss vom Optimisten sein.
Welche ausgezeichnete Wörter